Abstract
Background:
A better understanding of early pain trajectories (patterns) following scoliosis surgery and how they relate to baseline patient characteristics and functional outcomes may allow for the development of mitigating strategies to improve patient outcomes.
Methods:
This was a prospective cohort study. Adolescents with idiopathic scoliosis were recruited across multiple centers. Latent growth mixture modeling techniques were used to determine pain trajectories over the first postoperative year.
Results:
The median numerical rating scale for pain in the hospital following surgery for adolescent idiopathic scoliosis was 5.0. It improved to 1.0 by 6 weeks, and was maintained at <1 by 3 to 12 months postoperatively. Three trajectories were identified, 2 of which involved moderate acute postoperative pain: 1 with good resolution and 1 with incomplete resolution by 1 year. The third trajectory involved mild acute postoperative pain with good resolution by 1 year. Membership in the “moderate pain with incomplete resolution” trajectory was predicted by higher baseline pain and anxiety, and patients in this trajectory reported worse quality of life than those in the trajectories with good resolution.
Conclusions:
Pain recovery following surgery for idiopathic scoliosis was found to be substantial during the first 6 weeks and continued up to 1 year. We identified 3 main trajectories, 2 with favorable outcomes and 1 with persistent pain and worse quality of life at 1 year postoperatively. The risk factors most associated with the latter trajectory included increased baseline pain and anxiety.
Level of Evidence:
Prognostic Level II. See Instructions for Authors for a complete description of levels of evidence.
Pain following scoliosis surgery has been studied extensively, but most often at discrete, acute, and/or remote time points that do not fully describe the overall pain experience. Trajectory modeling of repeated pain measurements, when used for groups of individuals with the same diagnosis or surgery, can aid in classifying individuals on the basis of different trajectories of pain resolution over time1.
Sieberg and colleagues2 identified 5 highly variable pain trajectories, with 5.8% of their patients following a persistent postoperative pain trajectory, the majority demonstrating good recovery, and 10.5% demonstrating delayed pain. Although that study provides valuable information regarding long-term pain outcomes following surgery for adolescent idiopathic scoliosis (AIS), its measurement schedule was limited to the preoperative baseline followed by 1, 2, and 5 years postoperatively. The transition from acute to chronic postoperative pain has been reported as being between 2 to 3 months, suggesting that measurement during this early period is warranted3.
A better understanding of early pain trajectories following scoliosis surgery and associated functional outcomes would support practitioners in providing valuable clinical information to patients and families. Given the biopsychosocial nature of pain, an examination of modifiable psychological predictors of pain trajectories may allow for the development of a perioperative family-based intervention to improve pediatric patient outcomes4,5. As such, we had 3 aims with the current study: (1) to describe postoperative pain trajectories for patients with AIS, (2) to examine parent and child psychological predictors of trajectory membership, and (3) to compare functional outcomes across identified trajectories.
Materials and Methods
This was a prospective cohort study.
Setting
The data presented in this manuscript are from the Post-Operative Recovery following Spinal Correction: Home Experience (PORSCHE) study, a large multicenter project examining the prevalence, predictors, and consequences of children’s pain following scoliosis surgery.
Participants
Eligible participants included pediatric patients aged 10 to 20 years with AIS who were scheduled to undergo posterior scoliosis correction. This age range is comparable with that reported in similar studies4-6. The indication for surgery was AIS with a progressive curve exceeding 40° to 45° in skeletally immature patients, and exceeding 50° to 55° in skeletally mature patients. Excluded were patients who did not speak English or whose parents did not speak English, who had a developmental delay or non-idiopathic scoliosis diagnosis, or who were classified as American Society of Anesthesiologists (ASA) III or higher or had notable surgical complications. Participants were not excluded for missing data.
This study received Research Ethics Board approval at all study sites.
Variables and Measures
All measures and the data collection schedule are presented in Table I.
TABLE I.
Data Measurement Schedule Depicting When Measures Were Administered to Children and Their Parents
| Measure | T0: Baseline | T1: In-Hospital | T2: 1st Wk at Home | T3: 4-6 Wk | T4: 3 Mo | T5: 6 Mo | T6: 12 Mo |
| Demographic information | X | ||||||
| Numerical rating scale (NRS): average pain in last 24 hr | X | X | X | X | X | X | X |
| State-Trait Anxiety Inventory-Child Form (STAIC) | X | ||||||
| Pain Catastrophizing Scale-Child Form (PCS-C) | X | ||||||
| State-Trait Anxiety Inventory-Parent Form (STAIP) | X | ||||||
| Pain Catastrophizing Scale-Parent Form (PCS-P) | X | ||||||
| Pediatric Quality of Life Inventory-version 4 (PedsQL-4) | X | ||||||
| Functional Disability Index (FDI) | X | ||||||
| Scoliosis Research Society Health-Related Quality of Life Tool (SRS-30) | X |
Baseline demographic data collected included patient sex, age, and length of hospital stay.
Primary Outcome
Pain trajectories were formed on the basis of average pain assessment using a numerical rating scale (NRS). Patients were verbally asked to rate their average pain over the past 24 hours using the NRS (0 to 107, where 0 represents “no pain at all” and 10 represents “the worst pain you can imagine”).
Predictor Variables
Scores for the following measures were used as predictor variables: the State-Trait Anxiety Inventory-Child Form (STAIC)8, used to measure both state and trait anxiety (higher scores indicate greater baseline anxiety8,9); the Pain Catastrophizing Scale-Child Form (PCS-C)10, a questionnaire designed to measure children’s thoughts and feelings in response to pain (higher scores indicate a greater amount of “catastrophizing” in response to pain); the State-Trait Anxiety Inventory-Parent Form (STAIP)11, assessing both the state and trait level of anxiety of the parent (higher scores indicate greater baseline anxiety); and the Pain Catastrophizing Scale-Parent Form (PCS-P)12, a questionnaire designed to measure parents’ thoughts and feelings in response to their child’s pain (higher scores indicate a greater amount of “catastrophizing” in response to pain).
Secondary Outcomes
Secondary outcome measures were the Pediatric Quality of Life Inventory-version 4 (PedsQL-4), the Functional Disability Inventory (FDI), and the Scoliosis Research Society Health-Related Quality of Life Tool (SRS-30). The PedsQL-413 is a questionnaire designed to measure the child’s general quality of life (higher scores indicate greater quality of life). Scores of <78 are consistent with a clinically meaningful chronic health condition14. The FDI15 is used to assess the extent to which children experience difficulties completing specific physical tasks. This instrument has been used for children with chronic pain16-18 and postsurgical pain19. Scores of >12 indicate clinically meaningful disability20. The SRS-30 consists of 30 items that measure scoliosis-specific health-related quality of life21-27. There is no established clinical cutoff score for the SRS-30, but 0.23 is the minimum detectable measurement difference in the total score that indicates clinically meaningful differences between groups28.
Procedure
Participants were identified and informed of the study by their attending surgeon at a preoperative visit, and a research assistant provided further information either in person or at a later time over the telephone, depending on the family’s preference. Baseline measures were completed prior to surgery. Postsurgical measures were completed in-hospital, during the first week at home post-discharge, at 4 to 6 weeks postoperatively, and at 3, 6, and 12 months postoperatively. Data collection procedures were standardized across sites.
Statistical Methods
Preliminary analyses were performed using descriptive statistics to characterize the sample (Table II). Data were tested for normality, and statistical assumptions were inspected.
TABLE II.
Descriptive Data and Correlations Between Baseline Variables*
| Variable | Mean | SD | Range | Correlations with Other Variables: Kendall R | ||||||||
| No. | Description | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||
| 1 | Age in yr | 14.60 | 1.87 | 10-20 | — | −0.19† | −0.01 | 0.03 | 0.08 | 0.14‡ | 0.10 | 0.07 |
| 2 | % female | 86% | — | — | — | −0.02 | 0.09 | −0.05 | 0.02 | 0.05 | −0.11 | |
| 3 | Length of hospital stay in days | 5.8 | 1.4 | 3-12 | — | 0.03 | 0.07 | 0.10 | 0.001 | −0.01 | ||
| 4 | Baseline NRS | 2.8 | 2.3 | 0-9 | — | 0.24† | 0.13‡ | 0.22† | 0.08 | |||
| 5 | PCS-C | 18.7 | 9.6 | 0-44 | — | 0.24† | 0.33† | 0.04 | ||||
| 6 | Child state anxiety | 34.5 | 7.5 | 6-56 | — | 0.37† | 0.04 | |||||
| 7 | Child trait anxiety | 34.9 | 7.3 | 9-57 | — | 0.09 | ||||||
| 8 | PCS-P | 22.2 | 9.1 | 0-52 | — | |||||||
SD = standard deviation, NRS = numerical rating scale, PCS-C = Pain Catastrophizing Scale-Child Form, and PCS-P = Pain Catastrophizing Scale-Parent Form.
P < 0.01.
P < 0.05.
To identify pain trajectories, latent class growth analysis and latent growth mixture modeling (LGMM)29,30 using Mplus (version 8; Muthén & Muthén) were used. These statistical techniques identify multiple homogeneous subpopulations in a heterogenous sample, determining meaningful classes of individual variation over time (i.e., trajectories). The details of these analytic techniques can be found elsewhere29,31. This study utilized the adjusted Bayesian information criterion (BICa) and Akaike information criterion (AIC) as well as entropy values, a measure of probability regarding participant classification, to assess classification accuracy32. Overall fit was assessed with the bootstrapped Lo-Mendell-Rubin test (BLRT). We compared models with 1 to 4 trajectories for NRS ratings over time (in-hospital, first week at home, 4 to 6 weeks, and 3, 6 and 12 months). Because of the variability in data collection time points, baseline pain was not included as a trajectory time point, but given its importance for postsurgical pain, baseline pain was examined as a covariate in the trajectory model.
With respect to predictors of trajectory membership, a multinomial logistic regression analysis was conducted to identify psychological predictors of a persistent pain trajectory. Baseline psychosocial factors (child and parent pain catastrophizing, child state and trait anxiety) were entered as independent variables to predict trajectory class as the dependent variable, accounting for age and sex.
Comparison of Functional Outcomes Across Trajectories
Data for the 12-month functional outcomes were not normally distributed, so transformations were applied, which improved the distribution of the variables and their residuals. A multivariate analysis of variance (MANOVA) was conducted to examine the relationship between trajectory membership and participants’ scores on the 3 functional outcomes (FDI, PedsQL-4, and SRS-30). MANOVA results obtained with the transformed variables were consistent with those obtained using the untransformed variables. Given the missing data in the 12-month outcomes, we conducted multiple imputation modeling with 40 imputations using the Markov chain Monte Carlo algorithm available in SPSS version 25.0 (IBM), with auxiliary variables identified using the Missing Data Analysis function, to validate the MANOVA findings. For interpretability, results of analyses of raw (non-imputed) data are presented.
Results
Participants
Of 267 potential participants, across all 8 participating sites, consent for study participation was received for 246. A total of 220 participants completed at least some part of the baseline (T0) assessment (Fig. 1). The retention rates for each time point are as follows: first week home, 89%; 4 to 6 week follow-up, 89%; 3-month follow-up, 67%; 6-month follow-up, 70%; and 12-month follow-up, 63%. There were no significant differences between the participants who did and did not complete the study at 12 months with respect to baseline measures, with the exception of length of hospital stay: the average hospital stay for those who completed the study was less than that of those who did not complete the study (mean [and standard deviation] of 5.6 ± 1.3 days compared with 6.2 ± 1.6 days).
Fig. 1.
Participant recruitment and study flow. FU = follow-up.
Demographic and Clinical Variables
The final sample included 220 children. Descriptive statistics and correlations between baseline variables are presented in Table II. The median NRS ratings (with the interquartile range) were as follows: baseline, 2.75 (1.00 to 5.00) (n = 202); in-hospital, 5.00 (4.00 to 6.50) (n = 216); first week at home, 3.00 (2.00 to 4.50) (n = 195); 4 to 6 weeks, 1.00 (0.00 to 2.00) (n = 195); 3 months, 0.00 (0.00 to 1.00) (n = 148); 6 months, 0.00 (0.00 to 1.00) (n = 155); and 12 months, 0.00 (0.00 to 1.00) (n = 138).
Determination of Pain Trajectories
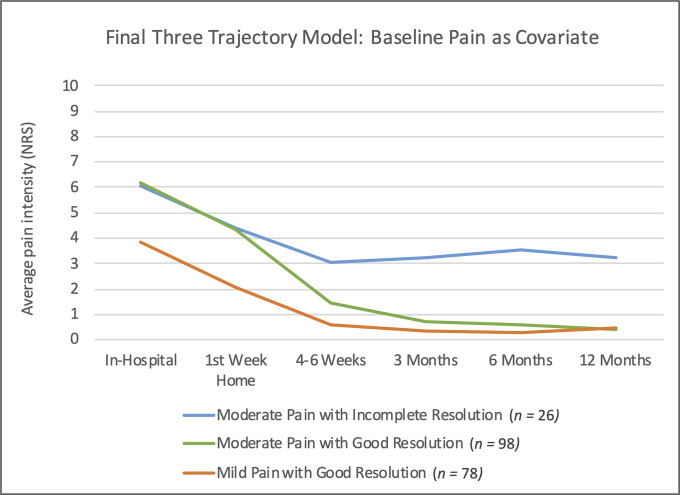
Using iterative LGMM modeling, 4 models were tested with and without a covariate (Table III). The trajectories were named using established adult NRS cutoffs. Because of the improvements observed in the fit indices when compared with other trajectory models without a covariate, baseline pain was retained as a covariate in the final model (Fig. 2). The covariate adjustment resulted in a model with n = 202. Three trajectories were identified, 2 of which involved moderate acute postoperative pain: 1 with good resolution and 1 with incomplete resolution by 1 year. The third trajectory involved mild acute postoperative pain with good resolution by 1 year. Greater baseline pain increased the odds of membership in the “moderate pain with incomplete resolution” trajectory 1.5-fold compared with the “moderate pain with good resolution” trajectory.
Fig. 2.
The final model with 3 pain trajectories and baseline pain as a covariate. NRS = numerical rating scale.
TABLE III.
Fit Indices Used to Determine the Final Trajectory Model*
| Model | AIC | BICa | BLRT P Value | Latent Class Probability Range | Entropy | Sample Size per Trajectory Based on Most Likely Membership |
| 1 trajectory | 3,622.94 | 3,626.08 | — | 1.0 | — | 220 |
| 2 trajectories | 3,687.19 | 3,690.56 | <0.001 | 0.92-0.98 | 0.902 | 189/31 |
| 3 trajectories | 3,628.61 | 3,632.65 | <0.001 | 0.84-0.90 | 0.700 | 75/28/117 |
| 4 trajectories | 3,594.92 | 3,599.64 | <0.001 | 0.84-0.99 | 0.769 | 71/28/116/5 |
| 3 trajectories with baseline pain covariate†‡ | 3,293.51 | 3,296.59 | <0.001 | 0.87-0.93 | 0.740 | 78/26/98 |
AIC = Akaike information criterion, BICa = adjusted Bayesian information criterion, and BLRT = bootstrapped Lo-Mendell-Rubin test.
Model with the best fit to the data that respected all criteria (lower AIC and BICa values, smallest trajectory with n >5%, adequate classification accuracy).
Addition of baseline pain as a covariate resulted in an adjusted sample size of n = 202.
Prediction of Trajectory Membership
The results of the multinomial regression analysis predicting group membership are shown in Table IV. No differences in predictors were observed between the 2 trajectories with good pain resolution. When using the “mild pain with good resolution” trajectory as the reference class, child trait anxiety increased the odds of being classified in the “moderate pain with incomplete resolution” trajectory, and female sex increased the odds of being classified in the “moderate pain with good resolution” trajectory.
TABLE IV.
Multinomial Logistic Regression Showing the Relationship Between Baseline Predictors and Group Membership in Pain Trajectories*
| “Moderate Pain with Incomplete Resolution” Vs. “Mild Pain with Good Resolution” | “Moderate Pain with Good Resolution” Vs. “Mild Pain with Good Resolution” | |||||
| Predictor | Beta (SE) | Wald | Odds Ratio (95% CI) | Beta (SE) | Wald | Odds Ratio (95% CI) |
| Child age | −0.18 (1.54) | 1.33 | 1.20 (0.88-1.62) | 0.11 (0.11) | 0.92 | 1.11 (0.90-1.38) |
| Child sex | −0.95 (0.87) | 1.21 | 0.39 (0.07-2.11) | −1.1 (0.54)† | 4.14 | 0.33 (0.12-0.96) |
| Child PCS | −0.006 (0.03) | 0.04 | 1.00 (0.93-1.06) | 0.02 (0.02) | 0.98 | 1.02 (0.98-1.07) |
| Child state anxiety | 0.04 (0.05) | 0.61 | 1.04 (0.95-1.13) | −0.05 (0.03) | 2.11 | 0.95 (0.89-1.02) |
| Child trait anxiety | 0.12 (0.05)† | 5.34 | 1.12 (1.02-1.24) | 0.06 (0.03) | 2.78 | 1.06 (1.00-1.13) |
| Parent PCS | −0.06 (0.03) | 3.46 | 0.94 (0.88-1.00) | −0.04 (0.02) | 2.98 | 0.96 (0.92-1.00) |
SE = standard error of beta, and CI = confidence interval. Child sex was coded as 1 for female and 0 for male. R2 = 0.15 (Cox and Snell), 0.18 (Nagelkerke). Model χ2(12) = 26.00; p < 0.05.
P < 0.05.
Functional Outcomes Associated with Each Trajectory
The MANOVA indicated significant differences in functional outcomes between trajectory groups (F[6,246] = 4.34, p < 0.001; Wilk lambda = 0.82, partial eta2 = 0.10, d = 0.98). Group differences in each of the functional outcomes are presented in Figures 3-A, 3-B, and 3-C. Members of the “moderate pain with incomplete resolution” trajectory had significantly worse functioning on all measures compared with the other 2 trajectories, but clinically relevant differences were observed only for the quality-of-life measures (SRS-30, PedsQL-4). While there were significant differences between groups on the FDI, all trajectory groups demonstrated little to no clinically relevant functional disability (FDI) at 12 months.
Figs. 3-A, 3-B, and 3-C Differences between trajectory groups in 12-month functional outcomes as measured using the Functional Disability Index (Fig. 3-A), the Pediatric Quality of Life Inventory (Fig. 3-B), and the Scoliosis Research Society Health-Related Quality of Life Tool (SRS-30) (Fig. 3-C). The dashed lines indicate the clinical cutoff values. CI = confidence interval.
Fig. 3-A.
Fig. 3-B.
Fig. 3-C.
Discussion
Using LGMM30, we identified 3 distinct postoperative pain trajectories following scoliosis correction for patients with AIS, with baseline pain included as a covariate to improve model fit. Nearly half of the sample followed a postoperative trajectory characterized by moderate to severe pain in the immediate postoperative period that sharply declined over the first few weeks and stabilized to mild or no pain by 12 months (n = 98). A second trajectory was characterized by mild to moderate pain in the immediate postoperative period that gradually declined over the first few weeks and stabilized to mild or no pain by 12 months (n = 78). A small subset of the sample followed a postoperative trajectory characterized by moderate to severe pain in the immediate postoperative period that gradually declined over the first few weeks and then stabilized to moderate pain that remained at 12 months (n = 26). Among potential psychological predictors, anxiety increased the odds of membership in this trajectory, as did greater baseline pain. Patients in this trajectory also reported statistically significant and clinically relevant worse quality of life at 12 months compared with patients in the other trajectories. While significant differences in the FDI were also found between trajectories, it should be noted that all patients reported functional disability ratings suggestive of little to no disability at 12 months.
Baseline pain was found to improve trajectory model fit and increase the odds of following a persistent pain trajectory. However, questions still remain as to whether the pain experienced in this group should be thought of as chronic postsurgical pain or unresolved baseline pain. According to the definition of chronic postsurgical pain as described by Macrae and Davies3, the pain must develop after surgery, and any preexisting problems possibly causing the pain should be excluded. Future studies that utilize a more nuanced measure of pain (e.g., quality, exact location) are needed to differentiate baseline pain from pain reported in the postoperative period as well as to define a threshold for intervention. Nevertheless, findings from this study suggest that the assessment and treatment of baseline pain is important for postoperative outcomes.
Neither parent nor child catastrophizing in response to pain was predictive of trajectory membership. This finding could be related to the measurement tool itself, which does not incorporate a social and/or developmental context of cognition, nor threat appraisal, into its catastrophizing assessment33. In addition, it has been shown that the relationship between catastrophizing and pain intensity is moderated by age, being stronger in adults than in children/adolescents34. It is also possible that baseline catastrophizing is no longer a stable measure in adolescents after experiencing a major surgery35.
In the current study, patients with moderate immediate postoperative pain followed 2 trajectories—good and incomplete resolution—that suggested the influence of factors other than baseline pain and anxiety as being predictive. In particular, it may be important to examine potential risk factors (e.g., return to sports, pain catastrophizing, emotion regulation, and anxiety) and potentially intervene between 4 to 6 weeks, when these trajectories begin to diverge. Katz36 suggested that different factors may contribute to the transition from acute to chronic postsurgical pain than contribute to the persistence of chronic pain, including those that relate to adolescent development (e.g., emotional regulation) or are associated with spinal deformity (e.g., concerns with body image).
Adolescents in the “moderate pain with incomplete resolution” trajectory reported lower quality of life at 12 months compared with participants in the other trajectories. These results are consistent with those of Rabbitts and colleagues4, whose “late recovery” pain trajectory in a mixed orthopaedic sample was associated with lower health-related qualify of life at 12 months. As in their study, our results also support the notion that moderate immediate postsurgical pain alone does not necessarily lead to poor functional outcomes, but the persistence of pain in the longer term may be more salient. Consequently, appropriate pain management over the longer term should also be emphasized.
The findings of the current study should be considered in light of several limitations. First, this study only measured pain up to 12 months postoperatively. Whereas our study demonstrated important changes in pain trajectories over the course of 12 months, the study by Sieberg and colleagues2 suggested that pain trajectories continue to evolve in important ways beyond the first postoperative year. As such, the conclusions drawn in this study can only be applied to pain in the first postoperative year and may not apply to pain or functional outcomes in the longer term.
An additional limitation relates to the labeling of the pain trajectories, as adult NRS cutoffs were used. More research involving pediatric populations is needed to determine the most appropriate cutoffs in a pediatric postsurgical population.
Another important limitation in this study concerns participants who dropped out of the study before the 12-month time point. In order to counteract the effects of missing data, the LGMM data-analysis method used employs a robust full-information maximum-likelihood (FIML) estimation procedure for handling missing data and assumes that the missing data can be predicted from other variables in the model (missing at random)37. LGMM is very well suited to data sets that have missing data because FIML estimation is considered one of the most robust methods for dealing with it. Additionally, multiple imputation modeling was used to verify the results of the MANOVA for functional outcomes38,39.
A final limitation is related to the scope of factors used to predict trajectory membership. We examined parent and child psychological factors and baseline pain as predictors of trajectory membership in order to potentially inform the development of a psychological preoperative intervention. Future studies should examine other factors (e.g., baseline function, return to sports, radiographic details, number of fusion levels, and pain management protocols) as predictors of an unfavorable pain trajectory that could also be used to identify patients and mitigate risk.
In conclusion, pain recovery following scoliosis surgery in adolescents typically occurs within the first 6 weeks and is maintained up to 12 months postoperatively. In this study, we identified 3 trajectories, 2 with favorable outcomes and 1 with persistent pain at 12 months postoperatively. The risk factors most associated with the latter trajectory included increased baseline pain and child anxiety. This trajectory also was associated with worse results on quality-of-life measures. Future research should examine additional risk and protective factors associated with these pain trajectories as well as the functional outcomes for this population, with a view to developing mitigating strategies.
Acknowledgments
Note: The authors acknowledge the Canadian Pediatric Spinal Deformity Study Group and the PORSCHE Study Group for their support of this project. The authors also thank Dr. Sean MacKinnon for consulting on the statistical analyses and Mr. Gilanders Unger for assistance with data management.
Members of the PORSCHE Study Group include: Ron El-Hawary, MD, MS, Kristen M. Bailey, MSc, Jill Chorney, PhD, Benjamin Orlik, MD, FRCS(C), Jennifer K. Hurry, MASc, Yasser AlKhalife, MD, MBBS, Kedar P. Padhye, MD, DNB(Ortho), Vibhu Viswanathan, MBBS, IWK Health Centre, Halifax, Nova Scotia, Canada; Jason J. Howard, MD, FRCS(C), Nemours-Alfred I. duPont Hospital for Children, Wilmington, Delaware; Jean A. Ouellet, MD, FRCS(C), Neil Saran, MD, FRCS(C), Teresa Valois Gomez, Montreal Shriner’s Hospital, Montreal, Quebec, Canada; Edward P. Abraham, MD, FRCS(C), Neil Manson, MD, FRCS(C), Saint John Regional Hospital, Saint John, New Brunswick, Canada; Devin C. Peterson, MD, FRCS(C), Paul Missiuna, MD, FRCS(C), Desi Reddy, Norman Buckley, McMaster University, Hamilton, Ontario, Canada; Douglas M. Hedden, MD, FRCS(C), Stollery Children’s Hospital, Edmonton, Alberta, Canada; David L. Parsons, MD, FRCS(C), Fabio Ferri-de-Barros, MD, FRCS(C), MSc, Kerryn Carter, MD; Alberta Children’s Hospital, Calgary, Alberta, Canada; James G. Jarvis MD, Children’s Hospital of Eastern Ontario, Ottawa, Ontario, Canada; Paul J. Moroz, MD, MS, FRCS(C), Shriners Hospitals for Children-Honolulu, Honolulu, Hawaii; Stefan Parent, MD, PhD, Jean-Marc Mac-Thiong, MD, PhD, Sylvie LeMay, Edith Villeneuve, Ste. Justine Hospital, Montreal, Quebec, Canada.
Footnotes
Investigation performed at the IWK Health Centre, Halifax, Nova Scotia, Canada
Disclosure: The PORSCHE (Post-Operative Recovery following Spinal Correction: Home Experience) study was funded by the Canadian Institutes of Health Research. K.M. Bailey was funded by an award from the Maritime Strategy for Patient Oriented Research Support Unit. On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author had a relevant financial relationship in the biomedical arena outside the submitted work (http://links.lww.com/JBJSOA/A266).
References
- 1.Chapman CR, Donaldson GW, Davis JJ, Bradshaw DH. Improving individual measurement of postoperative pain: the pain trajectory. J Pain. 2011. February;12(2):257-62. Epub 2011 Jan 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sieberg CB, Simons LE, Edelstein MR, DeAngelis MR, Pielech M, Sethna N, Hresko MT. Pain prevalence and trajectories following pediatric spinal fusion surgery. J Pain. 2013. December;14(12):1694-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macrae WA, Davies HTO. Chronic postsurgical pain. In: Crombie IK, Linton S, Croft P, Von Knorff M, LeResche L, editors. Epidemiology of pain. Washington: International Association for the Study of Pain; 1999. p. 125-42. [Google Scholar]
- 4.Rabbitts JA, Zhou C, Groenewald CB, Durkin L, Palermo TM. Trajectories of postsurgical pain in children: risk factors and impact of late pain recovery on long-term health outcomes after major surgery. Pain. 2015. November;156(11):2383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenbloom BN, Pagé MG, Isaac L, Campbell F, Stinson JN, Wright JG, Katz J. Pediatric chronic postsurgical pain and functional disability: a prospective study of risk factors up to one year after major surgery. J Pain Res. 2019. November 12;12:3079-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connelly M, Fulmer RD, Prohaska J, Anson L, Dryer L, Thomas V, Ariagno JE, Price N, Schwend R. Predictors of postoperative pain trajectories in adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2014. February 1;39(3):E174-81. [DOI] [PubMed] [Google Scholar]
- 7.von Baeyer CL, Spagrud LJ, McCormick JC, Choo E, Neville K, Connelly MA. Three new datasets supporting use of the Numerical Rating Scale (NRS-11) for children’s self-reports of pain intensity. Pain. 2009. June;143(3):223-7. Epub 2009 Apr 8. [DOI] [PubMed] [Google Scholar]
- 8.Spielberger CD, Edwards D. STAIC preliminary manual for the State-Trait Anxiety for Children (“How I feel questionnaire”). Palo Alto: Consulting Psychologists Press; 1973. [Google Scholar]
- 9.Holmbeck GN, Thill AW, Bachanas P, Garber J, Miller KB, Abad M, Bruno EF, Carter JS, David-Ferdon C, Jandasek B, Mennuti-Washburn JE, O’Mahar K, Zukerman J. Evidence-based assessment in pediatric psychology: measures of psychosocial adjustment and psychopathology. J Pediatr Psychol. 2008. October;33(9):958-80; discussion 981-2. Epub 2007 Aug 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crombez G, Bijttebier P, Eccleston C, Mascagni T, Mertens G, Goubert L, Verstraeten K. The child version of the pain catastrophizing scale (PCS-C): a preliminary validation. Pain. 2003. August;104(3):639-46. [DOI] [PubMed] [Google Scholar]
- 11.Spielberger CD, Gorsuch RL, Lushene RE, Vagg PR, Jacobs GA. State-Trait Anxiety Inventory. Palo Alto; 1970. [Google Scholar]
- 12.Goubert L, Eccleston C, Vervoort T, Jordan A, Crombez G. Parental catastrophizing about their child’s pain. The parent version of the Pain Catastrophizing Scale (PCS-P): a preliminary validation. Pain. 2006. August;123(3):254-63. Epub 2006 Apr 27. [DOI] [PubMed] [Google Scholar]
- 13.Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care. 1999. February;37(2):126-39. [DOI] [PubMed] [Google Scholar]
- 14.Huang IC, Thompson LA, Chi YY, Knapp CA, Revicki DA, Seid M, Shenkman EA. The linkage between pediatric quality of life and health conditions: establishing clinically meaningful cutoff scores for the PedsQL. Value Health. 2009. Jul-Aug;12(5):773-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker LS, Greene JW. The Functional Disability Inventory: measuring a neglected dimension of child health status. J Pediatr Psychol. 1991. February;16(1):39-58. [DOI] [PubMed] [Google Scholar]
- 16.Kashikar-Zuck S, Vaught MH, Goldschneider KR, Graham TB, Miller JC. Depression, coping, and functional disability in juvenile primary fibromyalgia syndrome. J Pain. 2002. October;3(5):412-9. [DOI] [PubMed] [Google Scholar]
- 17.Lynch AM, Kashikar-Zuck S, Goldschneider KR, Jones BA. Psychosocial risks for disability in children with chronic back pain. J Pain. 2006. April;7(4):244-51. [DOI] [PubMed] [Google Scholar]
- 18.Reid GJ, McGrath PJ, Lang BA. Parent-child interactions among children with juvenile fibromyalgia, arthritis, and healthy controls. Pain. 2005. January;113(1-2):201-10. [DOI] [PubMed] [Google Scholar]
- 19.Gidron Y, McGrath PJ, Goodday R. The physical and psychosocial predictors of adolescents’ recovery from oral surgery. J Behav Med. 1995. August;18(4):385-99. [DOI] [PubMed] [Google Scholar]
- 20.Kashikar-Zuck S, Flowers SR, Claar RL, Guite JW, Logan DE, Lynch-Jordan AM, Palermo TM, Wilson AC. Clinical utility and validity of the Functional Disability Inventory among a multicenter sample of youth with chronic pain. Pain. 2011. July;152(7):1600-7. Epub 2011 Mar 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bastrom TP, Marks MC, Yaszay B, Newton PO; Harms Study Group. Prevalence of postoperative pain in adolescent idiopathic scoliosis and the association with preoperative pain. Spine (Phila Pa 1976). 2013. October 1;38(21):1848-52. [DOI] [PubMed] [Google Scholar]
- 22.Asher M, Min Lai S, Burton D, Manna B. Discrimination validity of the Scoliosis Research Society-22 patient questionnaire: relationship to idiopathic scoliosis curve pattern and curve size. Spine (Phila Pa 1976). 2003. January 1;28(1):74-8. [DOI] [PubMed] [Google Scholar]
- 23.Bridwell KH, Berven S, Glassman S, Hamill C, Horton WC, 3rd, Lenke LG, Schwab F, Baldus C, Shainline M. Is the SRS-22 instrument responsive to change in adult scoliosis patients having primary spinal deformity surgery? Spine (Phila Pa 1976). 2007. September 15;32(20):2220-5. [DOI] [PubMed] [Google Scholar]
- 24.Adobor RD, Rimeslåtten S, Keller A, Brox JI. Repeatability, reliability, and concurrent validity of the Scoliosis Research Society-22 questionnaire and EuroQol in patients with adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2010. January 15;35(2):206-9. [DOI] [PubMed] [Google Scholar]
- 25.Carreon LY, Sanders JO, Diab M, Polly DW, Diamond BE, Sucato DJ. Discriminative properties of the spinal appearance questionnaire compared with the Scoliosis Research Society-22 revised. Spine Deform. 2013. September;1(5):328-38. Epub 2013 Sep 25. [DOI] [PubMed] [Google Scholar]
- 26.Parent EC, Wong D, Hill D, Mahood J, Moreau M, Raso VJ, Lou E. The association between Scoliosis Research Society-22 scores and scoliosis severity changes at a clinically relevant threshold. Spine (Phila Pa 1976). 2010. February 1;35(3):315-22. [DOI] [PubMed] [Google Scholar]
- 27.Verma K, Lonner B, Hoashi JS, Lafage V, Dean L, Engel I, Goldstein Y. Demographic factors affect Scoliosis Research Society-22 performance in healthy adolescents: a comparative baseline for adolescents with idiopathic scoliosis. Spine (Phila Pa 1976). 2010. November 15;35(24):2134-9. [DOI] [PubMed] [Google Scholar]
- 28.Kelly MP, Lenke LG, Sponseller PD, Pahys JM, Bastrom TP, Lonner BS, Abel MF. The minimum detectable measurement difference for the Scoliosis Research Society-22r in adolescent idiopathic scoliosis: a comparison with the minimum clinically important difference. Spine J. 2019. August;19(8):1319-23. Epub 2019 Apr 12. [DOI] [PubMed] [Google Scholar]
- 29.Jung T, Wickrama KA. An introduction to latent class growth analysis and growth mixture modeling. Soc Personal Psychol Compass. 2008;2(1):302-17. [Google Scholar]
- 30.Muthén B, Muthén LK. Integrating person-centered and variable-centered analyses: growth mixture modeling with latent trajectory classes. Alcohol Clin Exp Res. 2000. June;24(6):882-91. [PubMed] [Google Scholar]
- 31.Muthén B. Latent variable analysis: growth mixture modeling and related techniques for longitudinal data. In: Kaplan D, editor. Handbook of quantitative methodology for the social sciences. Newbury Park: Sage; 2004. p. 345-68. [Google Scholar]
- 32.Nylund KL, Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Struct Equat Modeling. 2007;14(4):535-69. [Google Scholar]
- 33.Eccleston C, Fisher EA, Vervoort T, Crombez G. Worry and catastrophizing about pain in youth: a reappraisal. Pain. 2012. August;153(8):1560-2. Epub 2012 Apr 18. [DOI] [PubMed] [Google Scholar]
- 34.Feinstein AB, Sturgeon JA, Darnall BD, Dunn AL, Rico T, Kao MC, Bhandari RP. The effect of pain catastrophizing on outcomes: a developmental perspective across children, adolescents, and young adults with chronic pain. J Pain. 2017;18(2):144-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katz J. Establishment of a new pain catastrophizing baseline after pediatric major surgery? J Pain. 2015. April;16(4):388. [DOI] [PubMed] [Google Scholar]
- 36.Katz J. One man’s risk factor is another man’s outcome: difference in risk factor profiles for chronic postsurgical pain maintenance vs transition. Pain. 2012. March;153(3):505-6. Epub 2011 Nov 17. [DOI] [PubMed] [Google Scholar]
- 37.McArdle JJ, Nesselroade JR. Growth curve analysis in contemporary research. In: Schinka JA, Velicer WF, Weiner IB, editors. Handbook of psychology: research methods in psychology. 2nd ed. Hoboken: Wiley; 2003. p. 447-80. [Google Scholar]
- 38.Collins LM, Schafer JL, Kam CM. A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychol Methods. 2001. December;6(4):330-51. [PubMed] [Google Scholar]
- 39.Graham JW. Missing data analysis: making it work in the real world. Annu Rev Psychol. 2009;60(1):549-76. [DOI] [PubMed] [Google Scholar]