Abstract
Background.
Donor-derived cell-free DNA (dd-cfDNA) has generated interest as a biomarker for kidney injury including transplant (KT) rejection. It is possible that the KT biopsy procedure can cause the release of dd-cfDNA, therefore affecting the reliability of this assay in the postbiopsy period. We evaluated the effect of KT biopsy on the kinetics of dd-cfDNA.
Methods.
We conducted a single-arm prospective study. Samples were collected from 16 adult KT recipients undergoing KT biopsy. All participants had samples drawn within 8 h before the biopsy (prebiopsy), within 20 min (hour 0), 2 h (hour 2), and 24–48 h (hours 24–48) after the biopsy. We evaluated the change in dd-cfDNA from the prebiopsy time point to the following 3 time points after the biopsy.
Results.
At hour 0 and hour 2, there was a significantly larger log dd-cfDNA mean score compared with the prebiopsy score (least square mean estimate 0.4 [0.17-0.63] and 0.39 [0.09-0.68], respectively). By 24–28 h postbiopsy, there was no significant difference in log dd-cfDNA mean score compared with the prebiopsy score (least square mean estimate −0.21 [−0.6 to 0.19]).
Conclusions.
Mechanical injury from a KT biopsy can transiently increase circulating dd-cfDNA. The increase resolves by 24–48 h after the biopsy. Providers should wait 48 h postbiopsy to obtain dd-cfDNA levels to establish the correct baseline to be used for monitoring.
Kidney transplant (KT) rejection causes renal allograft injury and may cause renal allograft loss.1 Early detection and treatment of rejection are essential to prolong renal allograft survival. Renal allograft histology obtained via needle biopsy remains the gold standard for diagnosis of rejection. Because of the potential risks associated with biopsy and possible interobserver variation in assessing histopathology, there has been a quest for noninvasive and more accurate methods to detect KT rejection in the last few decades.2 Plasma donor-derived cell-free DNA (dd-cfDNA) detected in the blood of KT recipients has been proposed as a noninvasive marker for diagnosis of renal allograft rejection.3-11 The Circulating Donor-Derived Cell-Free DNA in Blood for Diagnosing Acute Rejection in Kidney Transplant Recipients (DART) study validated that plasma levels of dd-cfDNA >1% could discriminate active rejection from no rejection with a high negative predictive value of 84% and a positive predictive value of 61%.3 However, the DART and other studies have shown that circulating dd-cfDNA may be increased by other injury such as infection with BK or infection.7,12
Many patients with an elevated plasma dd-cfDNA level undergo a renal allograft biopsy to determine the cause of the injury or confirm the presence and type of rejection. However, there are no data on whether mechanical injury induced by the biopsy itself affects the level of dd-cfDNA. It is possible that the biopsy itself can cause the release of more dd-cfDNA in the blood stream, therefore affecting the reliability of dd-cfDNA measurements for the diagnosis of acute rejection and response to treatment. Determining the effect of the biopsy procedure on dd-cfDNA levels is also important because the sample for dd-cfDNA may have been obtained several days before the biopsy. If the biopsy does cause a release of dd-cfDNA, it would be important to know the extent and duration of this change and whether a new baseline of dd-cfDNA level develops. This information is essential for the clinician managing KT recipients with abnormal dd-cfDNA and/or rejection to assess response to therapy.
We proposed to answer these questions through an evaluation of the kinetics of plasma dd-cfDNA after renal allograft biopsy. We hypothesized that renal allograft biopsy causes an increase in dd-cfDNA level in KT recipients that will be transient but interpretable. Our secondary hypothesis is that in KT recipients undergoing renal allograft biopsy, the level of plasma dd-cfDNA returns to its baseline in a short period after the biopsy.
MATERIALS AND METHODS
Study Cohort
We conducted a single-arm prospective study. The enrollment period was over 10 mo from May 2018 to March 2019. Patients were eligible for the study if they were adult (≥18 y of age) male or female KT recipients undergoing renal allograft biopsy. Written informed consent for study participation was required. Exclusion criteria were as follows: (1) multiple solid organ transplants, (2) pregnancy, (3) history of bone marrow transplant, (4) KT from an identical twin, and (5) KT <2 wk from the time of transplantation.
The institutional review board at our institution approved the study, and all of the patients provided written informed consent. The study was an investigator-initiated trial and funded by CareDx, Inc. (Brisbane, CA).
Donor Derived Cell-free DNA Sampling and Measurement
We used the AlloSure assay (CareDx) for measuring dd-cfDNA. In 2017, the Centers for Medicare and Medicaid Services (CMS) approved the use of AlloSure, a test that measures dd-cfDNA, to be used to assess the probability of renal allograft rejection.13 The AlloSure test is a clinical-grade, targeted, next-generation sequencing assay that measures single-nucleotide polymorphisms to quantify dd-cfDNA in KT recipients. Blood samples for dd-cfDNA measurements were collected from KT recipients undergoing KT biopsy. A percentage of above 1% is associated with a probability of active rejection.3 An increase by >61% in dd-cfDNA from a prior sample exceeds the biological variability observed in the reference population.6 Two samples of blood were collected at the same venipuncture in Streck Cell-Free DNA BCT tubes, stored at room temperature, and shipped to the CLIA-certified laboratory at CareDx, Inc. Participants had an intravenous catheter placed for sample collections. All participants had samples drawn within 8 h before the biopsy (prebiopsy), within 20 min (hour 0), 2 h after (hour 2), and 24–48 h (hours 24–48) after the biopsy. Approximately, 20 mL of blood was collected per draw for a total of 80 mL per patient.
Study Endpoints
The study endpoint was the change in the percentage of dd-cfDNA. The change was assessed over the study time points, which are immediately (within 20 min), hour 2, and hours 24–48 after renal allograft biopsy.
Statistical Analyses
We reported descriptive statistics as means ± SD for normally distributed continuous variables and as median (25th and 75th percentiles [Q25–Q75]) for continuous variables with a skewed distribution. Categorical variables are expressed as frequencies (percentage). Measurements of dd-cfDNA percentages are generally positively skewed. A natural log transform of the percentage dd-cfDNA measurements was performed to ensure the data were more normally distributed and maximally symmetric. Repeated measures ANOVA method was used to compare dd-cfDNA among different time points. We did not impute for missing values (5 missing values). If there is a missing value at some time point for 1 subject, the subject will be still included in the model but only contributes effectively to the nonmissing time points. Bonferroni correction was used to determine the level of significance. Because there are 3 comparisons of interest (immediate, hour 2, and hours 24–48 time points compared to prebiopsy), the correction tested each individual hypothesis at a level of α = 0.05 divided by 3, which is 0.017. All analyses were conducted in SAS, version 9.4 (SAS Institute Inc).
RESULTS
Study Population
Sixteen KT recipients were included. Clinical characteristics are shown in Table 1. Mean age at the time of biopsy was 50.6 ± 7.02 y. A majority of patients were men and Caucasian. Mean serum creatinine at the time of biopsy was 2.24 ± 0.42 mg/dL. The source of renal allograft was deceased donor in all recipients. The maintenance immunosuppression regimen consisted of tacrolimus, mycophenolate, and prednisone in all the recipients. All biopsies were performed for a clinical indication. The most common reason for obtaining biopsy was acute kidney injury in 12 patients, while 4 patients underwent the biopsy due to elevation in dd-cfDNA. None of the patients received treatment for rejection in the first 48 h after the biopsy.
TABLE 1.
Clinical characteristics
| Clinical characteristic (n16) | |
|---|---|
| Mean age (y) | 50.62 ± 7.02 |
| Sex | |
| Male | 12 |
| Female | 4 |
| Race | |
| White | 9 |
| Non-white | 7 |
| Allograft source | |
| Deceased donor | 16 |
| Living donor | 0 |
| Number of HLA antigen mismatches | |
| 6 | 1 |
| 5 | 2 |
| 4 | 10 |
| 3 | 2 |
| 0 | 1 |
| Mean serum creatinine (mg/dL) | 2.24 ± 0.42 |
| Maintenance immunosuppression tacrolimus, mycophenolate, prednisone | 16 |
| Reason for biopsy | |
| Acute kidney injury | 12 |
| Rise in dd-cfDNA | 4 |
| Number of tissue cores obtained | |
| 2 cores | 14 |
| 3 cores | 2 |
| Location of procedure | |
| Outpatient | 14 |
| Inpatient | 2 |
| Postbiopsy complications | |
| Hematoma | 0 |
| Arteriovenous malformation | 0 |
dd-cfDNA, donor-derived cell-free DNA.
Biopsy Technique and Procedural Complications
All participants underwent an ultrasound-guided percutaneous renal allograft biopsy using an 18-gauge automatic spring-loaded biopsy gun. We collected 2 tissue cores in 14 participants and 2 from whom we collected 3 tissue cores. None of the patients developed a hematoma or an arteriovenous malformation (AVM) as a result of the procedure.
Change in dd-cfDNA
Five patients had elevated levels of dd-cf DNA at baseline defined by a percentage equal to 1% or above. Blood sampling was complete across the time points in 11 participants (Figure 1). Five patients had incomplete blood collections. Four had 1 missing value, and 1 had 2 missing values. There were no missing values from the prebiopsy samples but there were 2 missing values from each of the remaining time points’ samples. The reasons for missing values were inability to obtain the sample and/or an inadequate sample as deemed by the processing laboratory.
FIGURE 1.
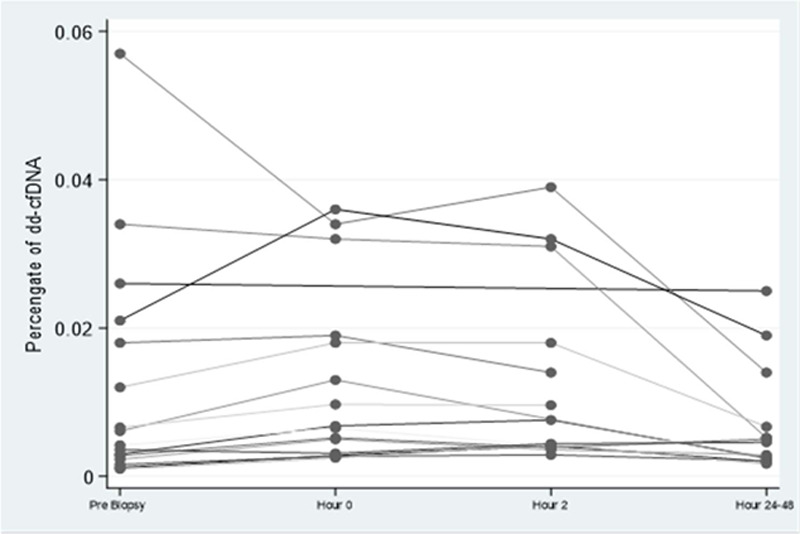
Spaghetti plot of the individual change in dd-cfDNA over time. dd-cfDNA, donor-derived cell-free DNA.
Median percentage of dd-cfDNA was 0.51% (0.25%–1.9%), 0.68% (0.31%–2%), 0.6% (0.41%–1.8%), and 0.48% (0.24%–0.67%) at the prebiopsy, hour 0, hour 2, and hours 24–48 time points, respectively (Figures 1 and 2A). None of the patients had an increase from normal value of dd-cfDNA percentage (≤1%) to above normal (>1%). However, an increase exceeding 61% from baseline was observed in 8 out of the 15 patients who had dd-cfDNA measurement at hour 0.
FIGURE 2.
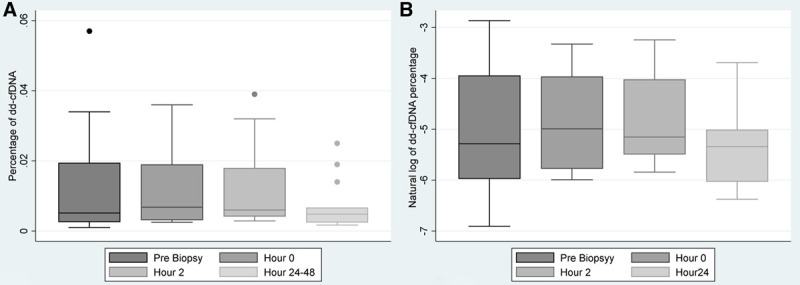
Trend in donor-derived cell-free DNA after kidney transplant biopsy. A, Box plot of the trend in donor-derived cell-free DNA percentage at different time points (prebiopsy, hour 0, hour 2, and hours 24–48 after the biopsy). B, Box plot of the trend in the natural log of donor-derived cell-free DNA percentage at different time points (prebiopsy, hour 0, hour 2, and hours 24–48 after the biopsy).
The longitudinal analysis of the log-transformed percentage of dd-cfDNA measurements demonstrated that there were differences in measurements as a function of time (Figure 2B). The prebiopsy time point was compared against all remaining time points. The natural log-transformed dd-cfDNA at hour 0 and hour 2 were significantly higher than that at prebiopsy (least square mean [LSM] estimate 0.4 [0.17-0.63] for hour 0 and 0.39 [0.09-0.68] for hour 2), while the natural log-transformed dd-cfDNA at hours 24–48 was not significantly higher than prebiopsy (LSM estimate −0.21 [−0.6 to 0.19]) (Table 2). The natural log-transformed dd-cfDNA at hours 24–48 was significantly lower than at hour 0 and hour 2 (LSM estimate −0.6 [−0.94 to −0.27] for hour 0 and −0.59 [−0.97 to −0.22] for hour 2).
TABLE 2.
Differences of dd-cfDNA least square means between 2 time points
| Time point | Reference time point | Estimatea (confidence interval) | P b |
|---|---|---|---|
| Hour 0 | Prebiopsy | 0.4 (0.17 to 0.63) | 0.0022 |
| Hour 2 | Prebiopsy | 0.39 (0.09 to 0.68) | 0.0138 |
| Hours 24–48 | Prebiopsy | −0.21 (−0.6 to 0.19) | 0.2846 |
aChange in natural log of dd-cfDNA.
bBonferroni correction of level of significance is α = 0.017.
dd-cfDNA, donor-derived cell-free DNA.
DISCUSSION
Our study is the first of its kind assessing the kinetics of dd-cfDNA after renal allograft biopsy and fills the gap in knowledge about the effect of biopsy on dd-cfDNA levels. Our study demonstrates that dd-cfDNA rises after renal allograft biopsy; however, the rise is transient and returns to baseline by 24–48 h, confirming that the level of dd-cfDNA does not permanently change following renal allograft biopsy. Our findings can assist the transplant provider managing KT recipients in decision-making regarding time of dd-cfDNA measurement in certain scenarios and allow remeasurement as soon as 48 h after the biopsy. Clinicians have hesitated to obtain dd-cfDNA for several days or even longer after the biopsy due to the assumption that the biopsy may have affected the level. Our findings suggest that the clinician can obtain it any time after the biopsy as long as 48 h have passed. In addition, there are scenarios when a dd-cfDNA measurement is not obtained before the biopsy and the clinician wants to establish a baseline for dd-cfDNA before initiating treatment. In this scenario, our findings suggest that the clinician can obtain a dd-cfDNA as soon as 48 h after the biopsy with confidence that the biopsy has not affected the dd-cfDNA level.
Although renal allograft biopsies are generally considered safe, they are associated with risks such as bleeding, hematoma, and AVM formations. A “liquid biopsy” obtained by measurement of dd-cfDNA can serve as an alternative to the invasive renal allograft biopsy and has evolved to a frequently used tool for surveillance and diagnosing of KT rejection, as well as monitoring of therapy.3,8,10,14
The transient rise in dd-cfDNA in our study can be explained by mechanical injury from the biopsy needle. This injury leads to the release of donor dd-cfDNA into the blood stream. This is a novel and important finding that should inform clinical practice. It is critical to establish the correct baseline of the dd-cfDNA to know whether a patient is responding or not to treatment.
The mean half-life of dd-cfDNA is relatively short at 30 min, but varies from several minutes to 1–2 h.15,16 Clearance of dd-cfDNA depends on the rate of production and elimination. The elimination of dd-cfDNA can occur in multiple sites including the “home” tissue, blood, and other organs (liver, spleen, kidney, and lymph nodes)17,18; hence, there are several factors affecting the efficacy of dd-cfDNA clearance. In the example of biopsy-related tissue damage, the injury, and therefore the rate of production, is transient unless a complication leading to a longer-lasting injury develops. Therefore, the rise is expected to be of short duration as long as mechanisms of elimination are intact. The dd-cfDNA assay we used has high reproducibility within and across runs. The coefficient variation within runs is 4.6–9.2 and across runs is 4.5%–9.9%, making assay variability an unlikely reason for our observations.19
Our study has some limitations. The sample size was relatively small but sufficient enough to show statistical significance. We are pleased that none of our subjects developed a significant complication during the biopsy; however, as a result our findings cannot be generalizable to patients who develop complications such as hematoma, major bleeding, or AVM formation. The levels of dd-cfDNA might vary biologically overtime for factors not related to tissue injury.6 It is possible that perturbations unrelated to direct injuries to the renal allograft, such as the turnover/death rate of cells originating from the recipient’s tissues, could confound the results and interpretation of dd-cfDNA. Finally, this is a single-center study with a relatively small number of patients, but each patient was his own control.
In conclusion, our study showed that mechanical injury from a renal allograft biopsy leads to an increase in dd-cfDNA immediately and 2 h after the procedure. This rise is transient and resolves in 24–48 h. As long as there are no complications related to the biopsy, providers taking care of KT recipients can obtain dd-cfDNA level as soon as after 48 h after biopsy with high confidence that this is a true baseline and that the levels are not affected by the biopsy. Our study also gives insight into new causes of dd-cfDNA release. It is not only released with rejection or other physiological injuries such as infection, but we have shown the novel finding that dd-cfDNA can be released after mechanical injury from a kidney biopsy and may occur after other mechanical injuries and help to explain “false-positive” dd-cfDNA levels.
Footnotes
Published online 25 May, 2021.
This study was an investigator-initiated study supported by CareDx, Inc., Brisbane, CA. The funding body had no role in the designing of study, data collection, interpretation of data, data analysis, or preparing the article.
D.C.B. has received consulting fees from Amplyx, Sanofi, and CareDx; speaker fees from CareDx. Johns Hopkins School of Medicine has received research support (D.C.B.) and fellowship support (A.B. and M.A.) from CareDx. S. Alasfar has received grant support from CareDx, Shire, and NIH. The other authors declare no conflicts of interest.
All authors have read and approved the article. Y.K. participated in writing of the article, performance of research, and samples collection. A.B. participated in sample collection and performance of research. A.P.S. participated in sample collection and writing of the article. Y.J. performed data analysis and writing of the article. S. Alakhdhair participated in sample collection and writing of the article. S.C.O. participated in sample collection and writing of the article. M.A. participated in sample collection and data analysis. D.C.B. participated in research design and writing of the article. S. Alasfar participated in research design, performance of research, writing of the article, and data analysis.
The study was performed in accordance with the Declaration of Helsinki. The study was approved by the Johns Hopkins Hospital Institutional Review Board. Written patient’s consent was required to participate in the study.
All data generated or analyzed during this study are available from the corresponding author on reasonable request and will be saved and available for 5 years after publication.
REFERENCES
- 1.Djamali A, Kaufman DB, Ellis TM, et al. Diagnosis and management of antibody-mediated rejection: current status and novel approaches. Am J Transplant. 2014; 14:255–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgan TA, Chandran S, Burger IM, et al. Complications of ultrasound-guided renal transplant biopsies. Am J Transplant. 2016; 16:1298–1305. [DOI] [PubMed] [Google Scholar]
- 3.Bloom RD, Bromberg JS, Poggio ED, et al. ; Circulating Donor-Derived Cell-Free DNA in Blood for Diagnosing Active Rejection in Kidney Transplant Recipients (DART) Study Investigators. Cell-free DNA and active rejection in kidney allografts. J Am Soc Nephrol. 2017; 28:2221–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jordan SC, Bunnapradist S, Bromberg JS, et al. Donor-derived cell-free DNA identifies antibody-mediated rejection in donor specific antibody positive kidney transplant recipients. Transplant Direct. 2018; 4:e379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H, Zheng C, Li X, et al. Diagnostic performance of donor-derived plasma cell-free DNA fraction for antibody-mediated rejection in post renal transplant recipients: a prospective observational study. Front Immunol. 2020; 11:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bromberg JS, Brennan DC, Poggio E, et al. Biological variation of donor-derived cell-free DNA in renal transplant recipients: clinical implications. J Appl Lab Med. 2017; 2:309–321. [DOI] [PubMed] [Google Scholar]
- 7.García Moreira V, Prieto García B, Baltar Martín JM, et al. Cell-free DNA as a noninvasive acute rejection marker in renal transplantation. Clin Chem. 2009; 55:1958–1966. [DOI] [PubMed] [Google Scholar]
- 8.Beck J, Oellerich M, Schulz U, et al. Donor-derived cell-free DNA is a novel universal biomarker for allograft rejection in solid organ transplantation. Transplant Proc. 2015; 47:2400–2403. [DOI] [PubMed] [Google Scholar]
- 9.Beck J, Bierau S, Balzer S, et al. Digital droplet PCR for rapid quantification of donor DNA in the circulation of transplant recipients as a potential universal biomarker of graft injury. Clin Chem. 2013; 59:1732–1741. [DOI] [PubMed] [Google Scholar]
- 10.Stites E, Kumar D, Olaitan O, et al. High levels of dd-cfDNA identify patients with TCMR 1A and borderline allograft rejection at elevated risk of graft injury. Am J Transplant. 2020; 20:2491–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang E, Sethi S, Peng A, et al. Early clinical experience using donor-derived cell-free DNA to detect rejection in kidney transplant recipients. Am J Transplant. 2019; 19:1663–1670. [DOI] [PubMed] [Google Scholar]
- 12.Kant S, Bromberg J, Haas M, et al. Donor-derived Cell-free DNA and the prediction of BK virus-associated nephropathy. Transplant Direct. 2020; 6:e622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Medicare & Medicaid Services. Local Coverage Determination (LCD): MolDX: AlloSure® Donor-Derived Cell-Free DNA Test (L37303). 2017. Available at https://www.cms.gov/medicare-coverage-database/details/lcd-details.aspx?LCDId=37303&ver=14&articleId=55760&Cntrctr=All&UpdatePeriod=751&bc=AQAAEAAAEAAA&. Accessed November 11, 2020
- 14.Oellerich M, Shipkova M, Asendorf T, et al. Absolute quantification of donor-derived cell-free DNA as a marker of rejection and graft injury in kidney transplantation: results from a prospective observational study. Am J Transplant. 2019; 19:3087–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Celec P, Vlková B, Lauková L, et al. Cell-free DNA: the role in pathophysiology and as a biomarker in kidney diseases. Expert Rev Mol Med. 2018; 20:e1. [DOI] [PubMed] [Google Scholar]
- 16.Yu SC, Lee SW, Jiang P, et al. High-resolution profiling of fetal DNA clearance from maternal plasma by massively parallel sequencing. Clin Chem. 2013; 59:1228–1237. [DOI] [PubMed] [Google Scholar]
- 17.Leung F, Kulasingam V, Diamandis EP, et al. Circulating tumor DNA as a cancer biomarker: fact or fiction? Clin Chem. 2016; 62:1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kustanovich A, Schwartz R, Peretz T, et al. Life and death of circulating cell-free DNA. Cancer Biol Ther. 2019; 20:1057–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grskovic M, Hiller DJ, Eubank LA, et al. Validation of a clinical-grade assay to measure donor-derived cell-free DNA in solid organ transplant recipients. J Mol Diagn. 2016; 18:890–902. [DOI] [PubMed] [Google Scholar]


