Abstract
Hypertriglyceridemia induced acute pancreatitis (HTGP) was associated with increased risk of local complications, recurrent acute pancreatitis (AP), the frequency of other complications, and its high mortality as compared to other causes. Determining the factors associated with the severity of HTGP was necessary and important in the management of patients with AP.
This study aims to examine the clinical and biochemical characteristics of HTGP patients, and to determine the factors associated with the severity of HTGP according to the revised Atlanta classification.
This retrospective and prospective study enrolled 157 HTGP patients from January 2016 to May 2019 at Cho Ray Hospital who had serum TG levels measured within the first 48 hours of admittance with a TG concentration ≥ 1000 mg/dL and excluded other causes. The clinical features and outcomes of patients with HTGP were determined in terms of demographics, clinical symptoms, laboratory data, system complications, local complications, disease severity, and length of hospital stay. The primary outcome was the severity of HTGP as based according to the revised Atlanta classification. We evaluated the relationship between general information, clinical factors and laboratory data in the study population.
There were 157 HTGP patients participated in this study. Patients with HTGP had evidence of obese or overweight range (61.2%), history of diabetes mellitus (32.5%) or undiagnosed diabetes (28.0%), history of AP (35.7%), alcohol use (23.6%), hypertension (15.9%), dyslipidemia (13.4%). The patients had typical symptoms of AP, including pancreatic abdominal pain (upper abdominal pain) (93%), nausea/vomiting (80.9%), fever (59.2%), distension abdomen (84.7%), and resistance of abdominal wall (24.8%). The severity of HTGP was significantly associated with fever, altered mental status, rapid pulse, and hypotension (P < .05). Patients with severe HTGP had significantly more pancreatic necrosis, higher values of Blood urea nitrogen and creatinine, longer prothrombin time and activated partial thromboplastin time on admission and higher CRP48 than not severe HTGP (P < .05).
The severity of HTGP was significantly related to clinical factors including fever, altered mental status, rapid pulse, hypotension, and pancreatic necrosis. The value of Blood urea nitrogen, creatinine, prothrombin time, and activated partial thromboplastin time at admission is higher and longer in the severe AP group with P < .05.
Keywords: hypertriglyceridemia induced acute pancreatitis, factors related to severity of hypertriglyceridemia-induced acute pancreatitis, pancreatic necrosis, ketoacidosis, splanchnic vein thrombosis, systemic and local complications
1. Introduction
Acute pancreatitis (AP) is one of the most common gastrointestinal diseases in the emergency departments of hospitals. Mild AP, usually self-limited and recovers within 3 to 5 days, has a less than 1% of mortality rate. While in severe AP, the mortality rate can reach 30% to 40%.[1,2] Currently, there are many studies on AP, in general, as well as investigating the value of laboratory tests (procalcitonin, interleukin-6, interleukin-8, ...), the value of many scales (Bedside index of severity in acute pancreatitis, IMRIE Imrie score, systemic inflammatory response syndrome, The Harmless Acute Pancreatitis Score, the CT severity index, ...) in early diagnosis, prognosis, and disease monitoring. Various patient factors may contribute to the severity and outcome of AP depending on the causes. Triglyceride (TG) increase is very common in patients with AP and due to the increased TG in acute pancreatitis, TG tends to further increase. According to several studies,[3–6] AP due to hypertriglyceridemia raises the risk of local complications, recurrence, acute necrotizing pancreatitis, organ failure, and prognosis and mortality scored higher than other causes. In Vietnam, a few studies showing the association of AP and elevated serum TG levels, which have been studied in-depth, but the studies of hypertriglyceridemia induced acute pancreatitis (HTGP) were no more. A few studies were looking for a comparison of HTGP with other causes in Vietnam, but there were no studies examined the factors relating to the severity of HTGP with the value of hypertriglyceridemia above 1000 mg/dL. Furthermore, the identification of factors relating to the severity of HTGP is essential and important to managing patients. Hypertriglyceridemic acute pancreatitis is related to the severity of acute pancreatitis. Therefore, to clarify and contribute more information about the characteristics of the disease and to determine which factors relate to the severity of hypertriglyceridemic acute pancreatitis, according to the 2012 revised Atlanta classification of AP, thereby, can help in its early detection, avoid missing data, and support for better treatment and monitoring of the disease. Therefore, this study aimed to investigate clinical and laboratory characteristics of patients with acute pancreatitis due to hypertriglyceridemia and factors related to severity of HTGP according to the revised Atlanta classification of 2012.
2. Methods
2.1. Research design
Descriptive cross-sectional study (retrospective and prospective).
2.2. Research subjects
Eighteen years old or older patients, meeting the diagnostic criteria of acute pancreatitis, diagnosed with TG concentration of more than 1000 mg/dL and excluded other causes. The TG blood test was done within 48 hours of admission. Patients agreed to participate in the study (prospective). The study was conducted on patients with chronic pancreatitis and severe uncontrolled chronic diseases (cirrhosis, malignancies, autoimmune diseases, etc.). Patients who did not have enough necessary information were not admitted to the study.
2.3. Method of implementation
Random, convenient sampling was done on patients who met the research criteria at the Department of Gastroenterology—Cho Ray Hospital from January 2016 to May 2019. Anthropocenmetric characteristics, occupation, history, the reason for admission, vital signs were documented, and Glasgow-scale score, blood oxygen saturation, clinical symptoms were all tested at the time of admission. Follow-up tests were conducted during the hospital confinement period (blood count, prothrombin time (PT), activated partial thromboplastin time (aPTT), aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), creatinine, serum glucose, serum TG, serum bilirubin, ion chart, C-reactive protein (CRP) at 48 hours post-hospitalization (CRP48), serum amylase and lipase, abdominal ultrasound, contrast-enhanced abdominal computed tomography (CT) and chest radiograph, arterial blood gas, blood ketones), local and systemic complications, organ failure, and other clinical outcomes (severe AP, length of hospital stay, death or give up treatment due to disease severity). Atlanta-adjusted severity assessment for hypertriglyceridemia was applied for induced AP. Then, we analyzed the relationship between the above factors and the severity of this disease.
2.4. Criteria and definitions
Diagnosis of acute pancreatitis with at least 2 of the 3 criteria[7,8]:
-
(1)
Acute pancreatic abdominal pain,
-
(2)
serum amylase and/or serum lipase increased to 3 times the upper limit of normal, and
-
(3)
suitable imaging results (abdominal ultrasound, contrast-enhanced abdominal computed tomography and or magnetic contrast abdominal (MRI)).
Acute pancreatitis due to hypertriglyceridemia[9,10]:
Patients with acute pancreatitis have increased blood TG more than 1000 mg/dL and exclude other causes.
Classification of the degree of serum TG according to the 2010 Endocrine Society's Clinical Practice Guidelines: severe increase (1000–1999 mg/dL) and very severe increase in blood TG (≥ 2000 mg/dL).
The 2012 Atlanta-adjusted acute pancreatitis severity classification based on organ failure status, local and/or systemic complications. Mild AP is if and when the patient has no organ failure and no local or systemic complications; moderate AP is if and when the patient has transient organ failure (reversible organ failure within 48 hours) and/or has local or systemic complications; severe acute pancreatitis is if the patient has persistent organ failure (organ failure lasting more than 48 hours). Assessment of organ failure according to the Modified Mashall Scoring System when it is at least 2 points or more, meaning failure of at least one of three organs: renal, respiratory, and cardiovascular organs[7,8]: blood creatinine higher than 1,9 mg/dL or systolic blood pressure lower than 90 mmHg and not a response to rehydration or the ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2) lower than 300 mmHg.
Complications: Local complications include acute peripancreatic fluid accumulation, pancreatic necrosis, infectious necrotizing pancreatitis, pancreatic pseudocysts, impaired gastric drainage, portal vein thrombosis, and intestinal necrosis. Systemic complications are exacerbations of chronic medical conditions associated with acute pancreatitis (coronary artery disease, diabetes, etc.).[7,8]
Criteria for an intensive care unit admission of Cho Ray Hospital: indicated for plasma exchange, continuous dialysis and / or serious illness with organ failure.
2.5. Statistical analysis
The data were processed and analyzed using SPSS 20.0 software (SPSS, Chicago, IL, USA); a P value < .05 was considered to be statistically significant. Confidence intervals are presented at 95%. The quantitative variables are presented as mean ± standard deviation (normal distribution); median and quartile interval (non-normal distribution). Kolmogorov–Smirnov test is used to determine the normal distribution. Qualitative variables are presented as percentages. For comparison between quantitative and qualitative variables, we used chi-square, “t” and Mann–Whitney tests. To analyze the role of each factor related to a variable, we use the multivariate logistic regression method.
2.6. Ethics in research
This study was approved by the Ethics Council in Biomedical Research at the Ho Chi Minh City University of Medicine and Pharmacy.
3. Results
There were 157 patients admitted to the study from January 2016 to May 2019. Of which, 21 patients were in the severe AP group and 136 were in the non-severe group (moderate-severe and mild AP).
3.1. Clinical and demographic data
Baseline characteristics of AP patients are summarized in Table 1. Complications and organ failure rates are shown in Figure 1 and the severity distribution of AP due to hypertriglyceridemia are shown in Figure 2.
Table 1.
Patient characteristics.
| Variables | % (n) |
| Average age∗ | 41.5 ± 9.7 |
| Age group, yr | |
| <20 | 0.6 (1) |
| 20–29 | 7.0 (11) |
| 30–39 | 39.5 (62) |
| 40–49 | 32.5 (51) |
| 50–59 | 16.6 (26) |
| ≥60 | 3.8 (6) |
| Sex Male | 77.7 (122) |
| Female | 22.3 (35) |
| BMI ≥ 23 kg/m2 | 61.2 (96) |
| Pre-existing diabetes | 32.5 (51) |
| Newly diagnosed diabetes mellitus | 28.0 (44) |
| Hypertension | 15.9 (25) |
| Dyslipidemia | 13.4 (21) |
| Alcohol/beer drinking | 23.6 (37) |
| History of acute pancreatitis | 35.7 (56) |
| Estrogen usage | 1.3 (2) |
| Abdominal pain | 100.0 (157) |
| Pancreatic abdominal pain | 93.0 (146) |
| Nausea/vomiting | 80.9 (80,9) |
| Fever | 59.2 (93) |
| Distention | 84.7 (133) |
| Softness of abdominal wall | 96.2 (151) |
| Resistance of the abdominal wall | 24.8 (39) |
| Perceptual changes | 17.2 (27) |
| Tachycardia (>100 beats/min) | 31.2 (49) |
| Hypotension (<90/60mmHg) | 8.3 (13) |
| Triglycerides 1000–1999 mg/dL | 63.7 (100) |
| Triglycerides ≥2000 mg/dL | 36.3 (57) |
| Hospital infection | 12.7 (20) |
| Admission to the ICU department | 19.7 (31) |
| Death | 2.6 (4) |
| Duration of hospitalization† | 8; (6–11) |
Figure 1.
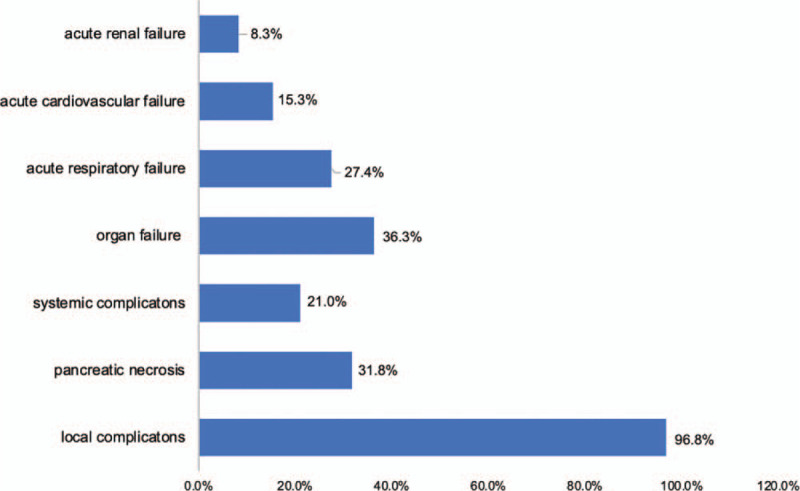
Distribution of complications and organ failure in hypertriglyceridemia induced acute pancreatitis patients.
Figure 2.
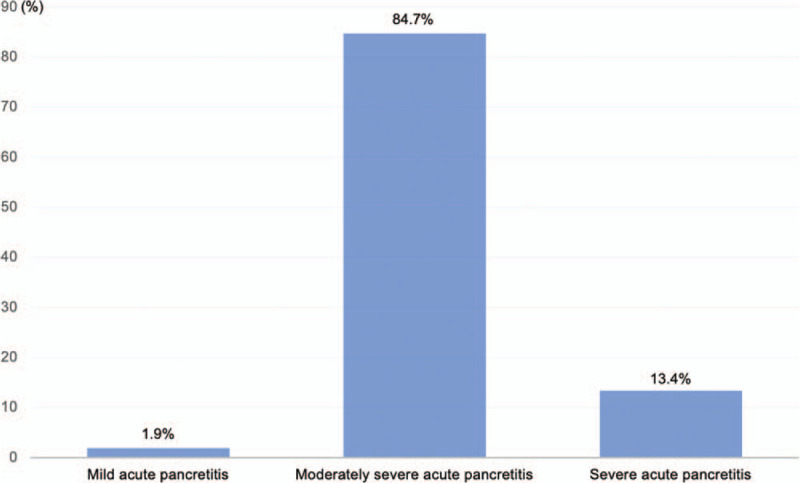
Distribution of severity of acute pancreatitis due to hypertriglyceridemia.
3.1.1. Laboratory characteristics
The value of blood glucose at hospital admission (without glucose transfusion) ≥ 200 mg/dL accounted for 57.3% with the median and interquartile range of 214 and (155 to 291.5), respectively. Blood amylase and blood lipase at admission were performed in 91.1% and 59.2% of cases. The values of blood amylase and lipase ≥ 3 times the upper limit of normal, were 31.2% and 42.7%, respectively. About 58.0% of patients had hyponatremia (serum sodium <135 mg/dL) and the median serum sodium was 134 (interquartile range, 130 to 136). The median of serum TG concentration was 1689.0 (interquartile range, 1209.5 to 2807.0).
3.2. Relationship between clinical and laboratory factors and severity of hypertriglyceridemia induced acute pancreatitis according to adjusted Atlanta standards
Of severe disease in patients, the percentage of pancreatic necrosis (24% (12) in severe HTGP/76% (38) in Mild and moderate HTGP) was higher proportionally in the severe HTGP group than in the non-severe HTGP group (OR = 3.4; 95% CI 1.3–8.8; P = .008) (Table 2). The duration of hospital stay in the severe AP group was longer than the mild and moderate AP group with P = .004 (Table 3). We found no statistically significant association between severity of AP and a group of patients with obesity, male, nausea or vomiting, bloating, history of AP, diabetes, hypertension, dyslipidemia, and alcohol drinking (P > .05). Perhaps a history of AP, diabetes, dyslipidemia and alcohol use were risk factors for AP and hyperglycemic pancreatitis, so when we analyzed these factors in the pancreatitis population, the difference between the severe and non-severe groups was not statistically significant. Besides, Perhaps, the sample distribution of study population at each group have different size that was no accurately evaluation of the gender between the severe AP, and non-severe AP.
Table 2.
Multivariate analysis of several factors associated with severe acute pancreatitis due to increased tryglycerides.
| Severe HTGP (n = 21) | Mild and moderate HTGP (n = 136) | ||||
| Variables | n (%) | n (%) | OR | 95% CI | P value |
| Fever | 19 (20.4) | 74 (79.6) | 8.0 | 1.8–35.5 | .002 |
| Perceptual disturbances | 16 (59.3) | 11 (40.7) | 36.4 | 11.2–118.1 | <.001 |
| Tachycardia | 11 (22.4) | 38 (77.6) | 2.8 | 1.1– 7.2 | .024 |
| Hypotension | 7 (53.8) | 6 (46.2) | 10.8 | 2.6– 44.1 | <.001 |
| Pancreatic necrosis | 12 (24.0) | 38 (76.0) | 3.4 | 1.3– 8.8 | .008 |
| BUN >20 mg/dL∗ | 6 (31.6) | 13 (68.4) | 4.2 | 1.3– 12.9 | .019 |
| Creatinin >1.9 mg/dL | 9 (52.9) | 8 (47.1) | 12.0 | 3.9– 36.8 | <.001 |
| CRP48 >150 mg/L | 20 (15.6) | 108 (84.4) | 5.1 | 0.7– 40.3 | .128 |
| Hct >44%∗ | 2 (13.3) | 13 (86.7) | 0.97 | 0.2– 4.7 | 1 |
Table 3.
Relationship between the severity of acute pancreatitis due to hypertriglyceridemia and the grades of hypertriglyceridemia (HTG).
| The severity of Hypertriglyceride induced acute pancreatitis | ||||||
| The grades of hypertriglyceridemia (mg/dL) | Severe (n = 21) | Mild and moderate (n = 136) | P value | OR (95% CI) | ||
| n | % | n | % | |||
| Very severe | 8 | 14.0 | 49 | 86.0 | .855 | 1.1 (0.4–2.8) |
| Severe | 13 | 13.0 | 87 | 87.0 | ||
We found no statistically significant association between the severity of HTGP and a group of patients with very severe HTG (≥2000 mg/dL) (OR 1.1, 95% CI 0.4–2.8; P = .855) (Table 3).
A statistically significant association was found between the severity of AP with CRP48, PT, aPTT, BUN, and creatinine. Specifically, the severe AP group had a higher CRP index, longer PT, aPTT time, BUN, and creatinine higher than the non-severe AP group with P, respectively, was .014, .006, .004, .002, and <.001 (Table 4).
Table 4.
Relationship between subclinical characteristics and severity of acute pancreatitis due to hypertriglyceridemia.
| Classification of HTGP | |||
| Variables | Severe (n = 21) | Mild and moderate (n = 136) | P value |
| PT | 104.2 | 75.11 | .006∗ |
| aPTT | 105.6 | 74.9 | .004∗ |
| BUN | 107.2 | 74.6 | .002∗ |
| Creatinine | 116.2 | 73.3 | <.001∗ |
| CRP48 | 297.7 ± 109.2 | 235.5 ± 106.5 | .014† |
| Duration of hospitalization | 105.6 | 75.0 | .004∗ |
A statistically significant relationship between the severity of AP is found with BUN >20 mg/dL and no response with hydration (OR 4.2, 95% CI 1.3–12.9; P = .019) and creatinine >1.9 mg/dL (OR 12, 95% CI 3.9 - 36.8; P < .001) (Table 2).
There was no difference in the values of erythrocytes, Hct, Hb, leukocytes, platelets, sodium, potassium, TG, and glucose between the 2 groups of severe and non-severe acute pancreatitis with P > .05.
4. Discussion
The average age in our study was 41.5 ± 9.7, with the largest proportion of age groups were 30 to 39 and 40 to 49 years old. When compared with other studies,[10,11] we found that the age of 30 to 50 is more common. The ratio of male to female was 3.5/1 and the group of patients with overweight—obesity accounts for the highest proportion. The differences in the above characteristics between studies can be explained by the difference in the study population, living habits, population characteristics, causes of AP, and place of residence. The incidence of clinical history in our study is consistent with Fortson et al[12] on secondary causes in hypertriglyceridemia:
-
(1)
diabetes is common (poor control, unknown), obesity, dyslipidemia;
-
(2)
alcohol;
-
(3)
not related to diabetes, alcohol, and obesity, but because of drugs and diet.
Patients with HTGP have the typical symptoms of AP (Table 1). Abdominal pain is the main symptom of acute pancreatitis, which may not manifest itself in the elderly; however, persistent vomiting is a symptom that is often associated with the disease and plays a role in assessing its severity.[13] Bloating can be caused by decreased intestinal motility or intestinal paralysis, making patients feel uncomfortable; and difficulty in breathing which is possibly due to abdominal fluids. The rate of bloating is higher than Khanna et al[14] which was probably because these studies were carried out in a rural hospital and surgery department. We recorded 17.2% with the changes in perception, most of them were in the group of severe acute pancreatitis (59.3%), tachycardia 31.2%, and blood pressure dropped by 8.3%. There were no death reports; 19.7% transferred to intensive care unit; 2.6% asked to discharge during the treatment period and 97.4% discharge from the hospital. Hospital infection accounts for 12.7%. Duration of hospital stay with a median of 8 days, quartile interval is (6–11). When compared with the study Sheng-Huei Wang et al,[11] we find that the progression of AP due to hypertriglyceridemia is similar to general AP, and will usually be stable in the first week of illness.
There is a statistically significant correlation between the severity of AP due to hypertriglyceridemia and the group of patients with accompanying fever, altered perception, tachycardia, and hypotension. Specifically, the group of severe acute pancreatitis, fever, tachycardia, blood pressure drop and change in perception are a favorable factor in predicting severity with P = .002, respectively (OR = 8.0; 95% CI 1.8–35.5); 0.024 (OR = 2.8; 95% CI 1.1–7.2); <.001 (OR = 10.8; 95% CI 2.6–44.1); and <.001 (OR = 36.4; 95% CI 11.2–118.1). This group had easy recognizable clinical symptoms i.e. showing inflammation, loss of body fluid, pain, and organ failure in acute pancreatitis; so it is necessary to carefully monitor patients with these symptoms.
We record 96.8% of local complications and 21.0% of systemic complications. In the group of systemic complications, only cases of ketoacidosis are recorded in diabetic patients (21.0%) and there is no exacerbation of the chronic medical disease. When doing the chi-square test and calculating the risk ratio, we find a statistically significant relationship between pancreatic necrosis and disease severity (OR = 3.4; 95% CI 1.3–8.8; P = .008). In general, the rate of organ failure is very different depending on the characteristics of the study population, hospital, and the proportion of severe patients in each study. The most common complication is acute respiratory failure, followed by acute renal failure and finally, cardiovascular failure.[11,15]
There had no reported case of splanchnic vein thrombosis in our study. However, we also find the attraction of splanchnic vein thrombosis complicating severe AP. Splanchnic vein thrombosis complicating AP is uncommon and has a reported incidence of 1% to 2%.[1] The splanchnic vessel to thrombose consist of splenic vein (SV), portal vein (PV), and superior mesenteric vein (SMV). On the other hand, splenic vein thrombosis has also been reported in up to 45% of patients with chronic pancreatitis and commonly asymptomatic manifestation.[2] The pathophysiology of splanchnic venous thrombosis may be linked to inherited coagulation disorders such as deficits of protein C, protein S or acquired coagulation disorders such as antithrombin III deficiency. Splanchnic venous thrombosis was associated with pancreatic necrosis and peripancreatic collections, which cause compression and perivascular inflammation. In the reported case,[3] it was to present a patient with splenic vein thrombosis complicating HTGP. About 3.6% of patients had splenic vein thrombosis in pancreatitis due to hypertriglyceridemia.[3] The hypothesis of its complication was associated with the deficiency of coagulation disorders, which vary from mild intravenous thrombosis to disseminated intravenous coagulation. Besides, the state of hypercoagulability is associated with higher plasma levels of fibrinogen and factor VIII.[3] The microvascular complications also related to diabetes mellitus may be present the tendency of thrombosis. In this case report, apheresis is necessary and is an efficient method.
In our study, HTGP patients were divided into two groups according to the grades of hypertriglyceridemia (HTG) by the Endocrine Society[9] called very severe HTG (≥2000 mg/dL) and severe HTG (1000–1999 mg/dL) with the proportion of 36.3% and 63.7%, respectively. We found no statistically significant association between the severity of HTGP and a group of patients with very severe HTG (≥2000 mg/dL) (OR 1.1, 95% CI 0.4–2.8; P = .855). Perhaps, the threshold of TG values (with a cut-off threshold of 2000 mg/dL) to discriminate the above 2 groups with the rising of TG levels, was not accurately evaluated and showed the differences between the two groups, severe HTGP, and non-severe HTGP. When we tested the distribution of TG values that was non-standard and had deviation to the left.
Hyperglycemia is a favorable condition for hyperlipidemia and is common in the early stage in patients with acute pancreatitis.[16] We record that the blood glucose level upon hospital admission >200 mg/dL was quite high at 57.3%. Blood amylase is the most commonly used test in the diagnosis of AP when blood amylase levels are 3 times higher than normal, which has a diagnostic value of acute pancreatitis. In our study, the value of amylase and blood lipase >3 times the upper limit of normal were 31.2% and 42.7%, respectively. This is consistent with possibly artificially or normally low amylase values in AP due to an increased TG.[17] Serum Lipase >3 times the upper limit of normal accounts for 42.7%. The rate of amylase and lipase in the blood is lower than that of Fortson et al [12] (with lipase and amylase >2 times the normal value of 67% and 54%, respectively). The rate of increase in blood amylase and lipase will vary from study to study due to threshold selection, AP population, time of serum amylase and lipase measurement.
CRP value on the second day after admission is useful in assessing the severity of acute pancreatitis, but we should be careful about the limitation of CRP in the early stage.[14,18] In our study, CRP48 > 150 mg/L accounted for 81.5%, higher than that of Khanna et al [14] (41.7%) and Cho et al (14.3%).[18] To explain this, according to Firdous et al,[19] CRP increases with body mass index (> 23 kg/m2 with P < .01) and weakly negative correlation with serum TG. On average, when BMI increased by 1 unit, CRP increased by 0.239 times and CRP increased significantly. While an average increase of 1 unit of lipid causes CRP to decrease 0.006 times but not significantly. In our study, CRP value may be affected by a group of patients with BMI > 23 kg/m2, which is quite high (61.2%). We find a statistically significant association between severity of acute pancreatitis and CRP48, in particular, the severe AP group has a higher mean CRP than the non-severe AP group with P = .014 (similar to Cho et al,[18]P = .026). In our study, a statistically significant relationship is found between the severity of AP and the BUN index at admission (the severe HTGP group had a higher BUN value, P = .002); with long-term BUN > 20 mg/dL and no post-rehydration reduction (OR = 4.2; 95% CI 1.3–12.9; P = .019) and creatinine >1.9 mg/dL (OR = 12; 95% CI 3.9–36.8; P < .001). This is consistent with BUN and creatinine values in predicting the severity of acute pancreatitis.[8] According to Wu et al,[20] a slight increase in TGs has little effect on the severity of AP, but severe hypertriglyceridemia can exacerbate the degree of acute kidney damage in AP.
When considering the association of several other laboratory tests, we find a statistical significance between the severity of HTGP and creatinine, PT and aPTT at admission. The severe AP group has a longer PT, aPTT, and higher creatinine than the non-severe AP group with P = .006, P = .004, and P < .001 respectively. Our results are consistent with Wanhang et al[21] on PT, aPTT time in severe acute pancreatitis. According to Wanhang et al,[21] severe AP has a significantly longer PT and aPTT time when compared with the group of mild, moderately severe AP (P < .05). Coagulation dysfunction in patients with severe AP indicates that the activation of the fibrinogen system is believed to be related to altered cytokines in AP and causes impaired microcirculatory disorders to the pancreas and other organ systems. Therefore, the severity of HTGP is associated with fever, altered perception, tachycardia, hypotension, pancreatic necrosis, and CRP48 hours after admission. The value of BUN, creatinine, PT, and aPTT at admission is higher and longer in the severe AP group with P < .05. This is consistent in predicting the severity of AP and HTGP. Patients with HTGP have the common symptoms of AP, which are common in men with young-middle ages and are associated with factors causing secondary hypertriglyceridemia. This may help clinicians to identify and to manage HTGP patients.
In the future, this highlights the need for more prospective research on the HTGP population to confirm the cut-off values for TG levels within 24 hours at admission that can predict the severity and choose the most appropriate TG lowering therapy.
However, there are several limitations to our study. Firstly, this was retrospective research, being a risk of bias in our findings. Secondly, the sample size was small that might not fully be reflected. Thirdly, the data of the study could be insufficient.
Acknowledgments
The authors gratefully acknowledge the Department of Gastroenterology, Cho Ray Hospital and Faculty of Medicine, University of Medicine and Pharmacy at Ho Chi Minh city for kindly providing facilities.
Author contributions
Conceptualization: Vo Duy Thong, Nguyen Thi Mong Trinh.
Data curation: Vo Duy Thong, Nguyen Thi Mong Trinh.
Formal analysis: Vo Duy Thong, Nguyen Thi Mong Trinh.
Investigation: Vo Duy Thong, Ho Tan Phat.
Supervision: Vo Duy Thong.
Validation: Vo Duy Thong, Nguyen Thi Mong Trinh.
Writing – original draft: Vo Duy Thong, Nguyen Thi Mong Trinh.
Writing – review & editing: Vo Duy Thong, Nguyen Thi Mong Trinh.
Footnotes
Abbreviations: AP = acute pancreatitis, aPTT = activated partial thromboplastin time, BUN = blood urea nitrogen, CRP = C-reactive protein, HTGP = hypertriglyceridemia induced acute pancreatitis, PT = prothrombin time, TG = Triglyceride.
How to cite this article: Thong VD, Mong Trinh NT, Phat HT. Factors associated with the severity of hypertriglyceridemia induced acute pancreatitis. Medicine. 2021;100:21(e25983).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
BMI = Body mass index, ICU = intensive care unit, n = number of patients.
Mean ± standard deviation.
Median; quartile.
BUN = blood urea nitrogen, CI = 95% confidence Interval, CRP = C-reactive protein; Hct = hematocrit, HTGP = hypertriglyceridemia induced acute pancreatitis, OR = odds ratio.
no response with hydration.
OR, Odds Ratio; 95% CI, 95% Confidence Interval.
aPTT = activated partial thromboplastin time; BUN = Blood urea nitrogen; CRP48 = C-reactive protein at 48 hours post-hospitalization; HTGP = Hypertriglyceridemia induced acute pancreatitis, n, number of patients, PT = prothrombin time.
U test, Mann-Whitney U.
t test, independent t test.
References
- [1].Krishna SG, Kamboj AK, Hart PA, et al. The changing epidemiology of acute pancreatitis hospitalizations: a decade of trends and the impact of chronic pancreatitis. Pancreas 2017;46:482–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pongprasobchai S, Vibhatavata P, Apisarnthanarak P. Severity, treatment, and outcome of acute pancreatitis in thailand: the first comprehensive review using revised atlanta classification. Gastroenterol Res Pract 2017;2017:3525349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].He WH, Zhu Y, Zhu Y, et al. Comparison of severity and clinical outcomes between hypertriglyceridemic pancreatitis and acute pancreatitis due to other causes. Zhonghua Yi Xue Za Zhi 2016;96:2569–72. [DOI] [PubMed] [Google Scholar]
- [4].Navarro S, Cubiella J, Feu F, et al. Hypertriglyceridemic acute pancreatitis. Is its clinical course different from lithiasic acute pancreatitis? Med Clin (Barc) 2004;123:567–70. [DOI] [PubMed] [Google Scholar]
- [5].Vipperla K, Somerville C, Furlan A, et al. Clinical profile and natural course in a large cohort of patients with hypertriglyceridemia and pancreatitis. J Clin Gastroenterol 2017;51:77–85. [DOI] [PubMed] [Google Scholar]
- [6].Wang Q, Wang G, Qiu Z, et al. Elevated serum triglycerides in the prognostic assessment of acute pancreatitis: a systematic review and meta-analysis of observational studies. J Clin Gastroenterol 2017;51:586–93. [DOI] [PubMed] [Google Scholar]
- [7].Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102–11. [DOI] [PubMed] [Google Scholar]
- [8].Tenner S, Baillie J, DeWitt J, et al. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol 2013;108: 1400-1415; 1416. [DOI] [PubMed] [Google Scholar]
- [9].Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012;97:2969–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang R, Deng L, Jin T, et al. Hypertriglyceridaemia-associated acute pancreatitis: diagnosis and impact on severity. HPB (Oxford) 2019;21:1240–9. [DOI] [PubMed] [Google Scholar]
- [11].Wang SH, Chou YC, Shangkuan WC, et al. Relationship between plasma triglyceride level and severity of hypertriglyceridemic pancreatitis. PLoS One 2016;11:e0163984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fortson MR, Freedman SN, Webster PD, 3rd. Clinical assessment of hyperlipidemic pancreatitis. Am J Gastroenterol 1995;90:2134–9. [PubMed] [Google Scholar]
- [13].Gülen B, Dur A, Serinken M, Karcıoğlu Ö, Sönmez E. Pain treatment in patients with acute pancreatitis: A randomized controlled trial. Turk J Gastroenterol 2016;27(2):192–196. [DOI] [PubMed] [Google Scholar]
- [14].Khanna AK, Meher S, Prakash S, et al. Comparison of Ranson, Glasgow, MOSS, SIRS, BISAP, APACHE-II, CTSI Scores, IL-6, CRP, and procalcitonin in predicting severity, organ failure, pancreatic necrosis, and mortality in acute pancreatitis. HPB Surg 2013;367581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ke L, Tong ZH, Li WQ, et al. Predictors of critical acute pancreatitis: a prospective cohort study. Medicine (Baltimore) 2014;93:e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kikuta K, Masamune A, Shimosegawa T. Impaired glucose tolerance in acute pancreatitis. World J Gastroenterol 2015;21:7367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Scherer J, Singh VP, Pitchumoni CS, et al. Issues in hypertriglyceridemic pancreatitis: an update. J Clin Gastroenterol 2014;48:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cho JH, Kim TN, Chung HH, et al. Comparison of scoring systems in predicting the severity of acute pancreatitis. World J Gastroenterol 2015;21:2387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Firdous S. Correlation of CRP, fasting serum triglycerides and obesity as cardiovascular risk factors. J Coll Physicians Surg Pak 2014;24:308–13. [PubMed] [Google Scholar]
- [20].Wu C, Zou L, Shi S, et al. The role of hypertriglyceridemia for acute kidney injury in the course of acute pancreatitis and an animal model. Pancreatology 2017;17:561–6. [DOI] [PubMed] [Google Scholar]
- [21].Deng W, Zhang D, Liu Q, et al. Changes and significance of early coagulation functions in patients with varying severities of acute pancreatitis. Biomed Res 2017;28:5142–7. [Google Scholar]


