Abstract

Cyclocarbonylative Sonogashira reactions of ortho-ethynylbenzamides have been investigated. The process is carried out under CO pressure, in the presence of a very small amount of PdCl2(PPh3)2 (0.4 mol %) as a catalytic precursor and without the need for a Cu salt as the co-catalyst. 2-Ethynylbenzamide reacted successfully with iodoarenes bearing electron-withdrawing and electron-donating groups, giving rise to different classes of compounds depending on the solvent used. On the contrary, N-(4-chlorophenyl)-2-ethynylbenzamide afforded exclusively polyfunctionalized isoindolinones with high stereoselectivity toward (E) isomers.
Introduction
N-containing heterocycles are structural motifs frequently found in a large number of biologically active compounds. For instance, isoindolinone is the core structural unit in several natural products such as chilenine,1 lennoxamine,2 nuevamine,3 chaetosisoindolinone,4 stachybotrisan,5 erinacerin,6 meyeroguilline,7 and caputmedusin.8 In particular, 3-methyleneisoindolin-1-ones have been recognized as nuclei of natural and synthetic compounds such as fumaridine,9 narceine imide,10 stigmalactam,11 magallinesine,12 chartarlactam L,13 aristoyagonine,14 aristolactams,15 and AKS-186.16 These heterocycles have been found to possess antimycobacterial17 and antifungal18 activities and antiplatelet11,19 properties, to act as anti-inflammatory20−22 and neuroprotective23 agents, to inhibit vasoconstriction,24,25 and to show cytotoxic and antitumoral activities.26−30
Owing to their great importance, there has been a continuous interest in developing metal-promoted cyclization methods for the syntheses of 3-methyleneisoindolin-1-ones.31 Transition-metal-catalyzed cyclocarbonylation reaction is a useful approach to the formation of the lactame moiety.32−41 Mancuso and co-workers developed an interesting synthesis of 3-methyleneisoindolin-1-ones based on a PdI2-catalyzed cyclization of 2-alkynylbenzamides with secondary amines under oxidative carbonylation conditions;42,43 Huang44 and Hua45 proposed cyclocarbonylation of ketimines under CO pressure as a valuable approach to isoindolinones; the Wu’s group46 described an elegant procedure based on the cyclization of arylketimine using Mo(CO)6 as a CO source and Jiang and co-workers47 developed a palladium-catalyzed carbonylation reaction of aromatic oxime for the synthesis of isoindolinone derivatives.
In the last years, our research group has acquired a large experience in the synthesis of heterocyclic compounds via transition-metal-promoted cyclocarbonylative coupling.48−53 Because of the large interest of the isoindolinone scaffold, in the present work we explored a new approach for the synthesis of 3-alkylideneisoindolin-1-ones via a copper-free Pd-catalyzed Sonogashira cyclocarbonylative reaction54−58 between 2-ethynylbenzamides and various iodoarenes.
Results and Discussion
We started our study with the synthesis of 2-ethynylbenzamide (1), which was easily obtained from commercially available 2-bromobenzamide according to a sequence of the Sonogashira reaction with trimethylsilylacetylene followed by desilylation process performed with CsF in MeOH (Scheme S1 in Supporting Information). Then, the first cyclocarbonylative Sonogashira reaction was carried out with equimolar quantities of 2-ethynylbenzamide 1 and iodobenzene 2a, in a stainless steel autoclave placed under CO pressure (20 atm) using a very low amount of PdCl2(PPh3) (0.4 mol %), a mixture of CH2Cl2 and triethylamine for 4 h at 100 °C (Table 1, entry 1). The analysis of the 1H NMR spectrum of the crude product showed the partial conversion of precursors and the presence of proton signals that indicated the formation of two different compounds. The first product was the expected 3-(2-oxo-2-phenylethylidene)isoindolin-1-one 3a, recovered chemically pure with a 34% yield. Its structure was confirmed by spectroscopic (1H NMR and 13C NMR), spectrometric (LC–MS), and elemental analysis (see Experimental Section). Moreover, NOE (nuclear Overhauser effect) experiments (Figure 1A) highlighted not only a strong dipolar coupling between the vinyl proton Ha and the aromatic protons Hb and Hc but also the absence of interactions of the amide proton He with other hydrogens. This evidence allowed the attribution of Z configuration to 3a obtained also as a single conformational isomer, the s-cis, probably due to the hydrogen bond between amide proton and carbonyl oxygen (Figure 1A).
Table 1. Optimization Study of the Cyclocarbonylative Sonogashira Reaction Between 2-Ethynylbenzamide 1 and Iodobenzene 2a.
| selectivityc (%) |
|||||||
|---|---|---|---|---|---|---|---|
| entrya | solvent | T (°C) | t (h) | conversionb (%) | 3a | 4a | 5a |
| 1 | CH2Cl2 | 100 | 4 | 80 | 57 (34) | 43 (21) | |
| 2 | CH2Cl2 | 100 | 8 | 78 | 39 | 61 | |
| 3d | CH2Cl2 | 100 | 4 | 69 | 28 | 72 | |
| 4 | CH2Cl2 | 70 | 24 | 79 | 29 | 71 | |
| 5 | CH2Cl2 | 50 | 24 | 78 | 21 | 79 (42) | |
| 6 | THF | 100 | 4 | 94 | 29 | 71 (44) | |
| 7 | THF | 50 | 24 | 79 | 21 | 79 | |
| 8 | THF | 30 | 24 | 16 | 26 | 74 | |
| 9 | CH3CN | 100 | 4 | 85 | 78 | 22 | |
| 10 | CH3CN | 50 | 24 | 83 | 38 | 62 | |
| 11 | DMF | 100 | 4 | 100 | 33 | 32 | 35 |
All reactions were carried with 2-ethynylbenzamide 1 (1.0 mmol), iodobenzene 2a (1.0 mmol), CO (20 atm), PdCl2(PPh3)2 (0.4 mol %), Et3N (1.5 mL), and the solvent (4.0 mL), unless otherwise stated.
Conversion was determined by the 1H NMR peak integration on the crude product.
Selectivity was estimated by 1H NMR spectroscopy; isolated yields of pure products are reported in parentheses.
Reaction performed with 1 mol % of PdCl2(PPh3)2.
Figure 1.
Chemical structure of the products of the cyclocarbonylative Sonogashira reaction between 1 and 2a: (A) 3-(2-oxo-2-phenylethylidene)isoindolin-1-one 3a; (B) 3-amino-2-benzoyl-1H-inden-1-one 4a; and (C) (Z)-2-(1-hydroxy-3-oxo-3-phenylprop-1-en-1-yl)benzonitrile 5a.
The second product (21% yield), required a more in-depth structural study. First, the analysis of the 1H NMR spectrum highlighted the presence of two broad singlet signals at particularly low fields (10.09 and 10.21) which were attributed to amino protons Ha,a′ (Figure 1B). Moreover, the 13C NMR spectrum indicated the presence of two signals corresponding to two carbonyl carbons (186.73 and 190.22 ppm). Two peaks corresponding to a double bond were also detected: the first at 172.21 ppm was related to a carbon atom linked to the NH2 group and the other at 103.02 ppm was due to ≡C–CO. All these data confirmed the formation of 3-amino-2-benzoyl-1H-inden-1-one 4a (Table 1, entry 1). Moreover, NOE experiments conducted on the pure product highlighted a dipolar coupling between the protons Ha, Hb, and Hc and the absence of couplings between Hc′ and Hd (Figure 1B), thus indicating also for product 4a a s-cis conformation.
With the aim to increase the conversion and the selectivity toward desired compound 3a, cyclocarbonylative Sonogashira tests at different reaction times, temperatures, and amounts of the catalytic precursor were performed. As is evident from the results described in Table 1, increasing the reaction time from 4 to 8 h (Table 1, entry 2) or the amount of PdCl2(PPh3)2 (Table 1, entry 3, 1 mol %) did not affect the conversion significantly. On the contrary, an increase in selectivity toward the amino product 4a was observed (up to ∼70%). A similar result was obtained by conducting the reaction at 70 °C for 24 h (Table 1, entry 4).
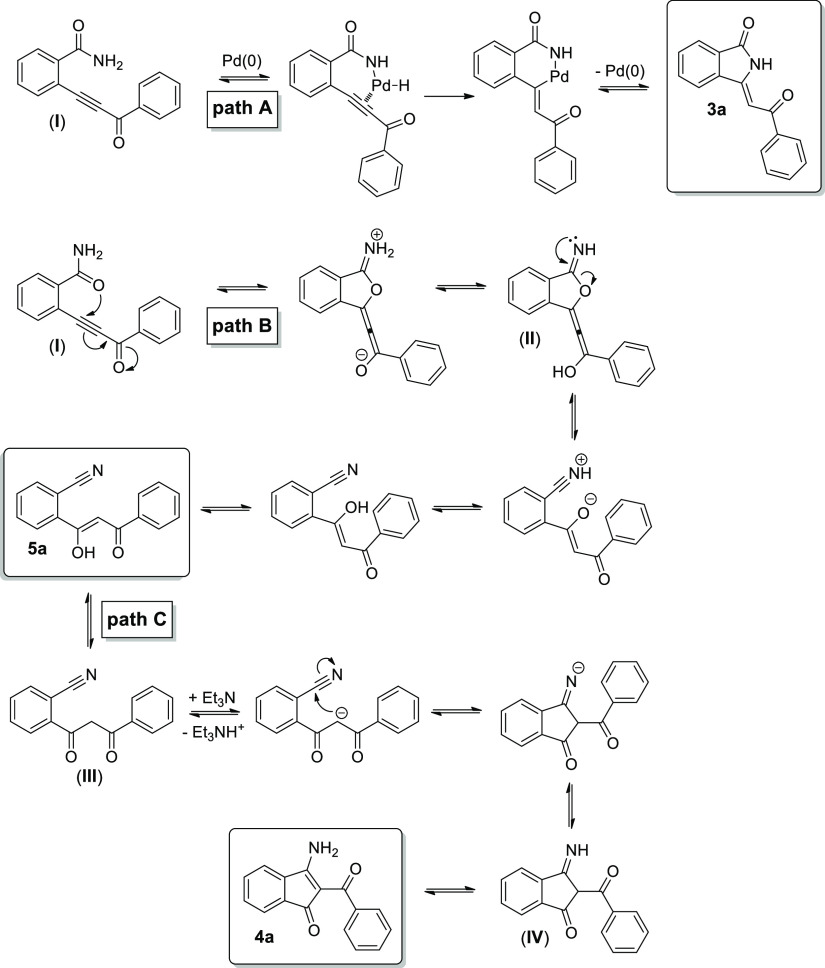
A further reduction in temperature to 50 °C (Table 1, entry 5) gave instead an unexpected result. The analysis of the 1H NMR spectrum of the crude product showed, in addition to the presence of isoindolinonic derivative 3a, the disappearance of the typical signals of 4a and the appearance of a new olefinic proton signal at 6.95 ppm. After purification, the new compound was subjected to 1H NMR, 13C NMR, LC–MS, and elemental analyses in order to determine its exact structure, which resulted to be (Z)-2-(1-hydroxy-3-oxo-3-phenylprop-1-en-1-yl)benzonitrile 5a (Figure 1C). In fact, the analysis of the 1H NMR spectrum indicated the presence of a signal that resonates at very low fields (16.44 ppm), a characteristic of a 1,3-diketonic system in the enolic form.59 Furthermore, in the 13C NMR spectrum, olefinic carbon (96.00 ppm), a signal corresponding to C≡N (118.00 ppm) and two peaks at 183.14 and 186.21 ppm (carbonyl and enolic carbon atoms) were clearly observed, thus confirming the structure of 5a (42% yield of isolated product). The formation of the three products 3a, 4a, and 5a can be tentatively explained by the mechanism described in Scheme 1. First, expected isoindolin-1-one 3a was generated via the initial formation of the Sonogashira product (I), which is in situ cyclized as previously observed (Scheme 1, path A).48,49 On the other hand, in the case of 4a, a process of addition of carbonyl oxygen to the triple bond can be hypothesized with the formation of an allenyl species (II). After prototropic exchange and subsequent opening of the cycle, 1,3-diketone 5a in the enolic form can be generated (Scheme 1, path B). Finally, indenone 4a can derive directly from 5a. Indeed, under experimental conditions (i.e., high temperature and excess of Et3N), the diketone (III) can be deprotonated and the obtained carbanion can attack the −CN functionality forming the cycle. Finally, after protonation (e.g., by Et3NH+) and subsequent imine–enamine rearrangement of (IV), 4a product is formed.
Scheme 1. Plausible Mechanism for the Formation of Products 3a, 4a, and 5a via the Sonogashira Cyclocarbonylative Reaction between 2-Ethynylbenzamide 1 and Iodobenzene 2a.
A confirmation of the above mechanism was given by treating 5a under the cyclocarbonylative Sonogashira conditions of Table 1, entry 1 (0.4 mol % of PdCl2(PPh3)2, CH2Cl2, and Et3N, 20 atm of CO, 100 °C, for 4 h). Indeed, indenone 4a was exclusively formed (Scheme S2 in Supporting Information). In order to obtain more information regarding the reactivity of benzamide 1, further experiments were performed under different experimental conditions.
As reported in Table 1, the nature of the solvents seemed to influence markedly the chemoselectivity of the reactions. In fact, when CH2Cl2 is used as the solvent, the chemoselectivity depends on the experimental conditions (Table 1, entries 1–5). On the contrary, tests carried out in tetrahydrofuran (THF) (Table 1, entries 6–8) afforded 5a with high selectivity (71–79%), regardless of the temperature and duration of the reactions. The particular behavior of THF can be ascribed to a strong coordination effect (hydrogen bond) between ketoenol hydrogen and THF oxygen atoms as depicted in Figure 2.
Figure 2.

Stabilization of (Z)-2-(1-hydroxy-3-oxo-3-phenylprop-1-en-1-yl)benzonitrile (5a) by coordination with THF.
As far as acetonitrile and dimethylformamide (DMF) are concerned, in the first case the preferential formation of 5a was observed at high temperatures (Table 1, entry 9), while the use of DMF involved the formation of a mixture of products.
Given the data obtained in the reactions between 2-ethinylbenzamide 1 and iodobenzene 2a, the extension of Sonogashira cyclocarbonylative reactions to iodoarenes having different steric and electronic requirements was subsequently investigated. The reactions were carried out under the reaction conditions which provided the desired isoindolinone 3a with better conversion and chemoselectivity, that is operating in dichloromethane and triethylamine, with 0.4 mol % of PdCl2(PPh3)2, 20 atm of CO, at 100 °C. The main results are described in Table 2. In all cases, a mixture of isoindolinone 3 and indenone 4 was obtained. However, the products could be easily separated and isolated chemically pure with satisfactory yields. As reported in Table 2, compared to preliminary reactions conducted with iodobenzene 2a, the use of p-methoxy derivative 2b showed a similar trend in terms of chemoselectivity and yield. Moreover, increasing the reaction time, an increase in the conversion was observed while the ratio between 3b and 4b remained substantially the same (Table 2, entries 2–4). Instead, when the reaction was performed with the more sterically hindered ortho-methoxyiodobenzene 2c, a decrease in the reaction rate was observed even if the reaction was carried out with 1 mol % of PdCl2(PPh3)2 (Table 2, entry 5, 73% conversion). Finally the reaction could be performed successfully also in the case of iodoarenes characterized by the electron-withdrawing groups such as −Cl and −CN (2d–e) (Table 2, entries 6–7).
Table 2. Cyclocarbonylative Sonogashira Reactions of 2-Ethynylbenzamide 1 with Iodoarenes 2 Performed in CH2Cl2.
| selectivityc (%) |
|||||||
|---|---|---|---|---|---|---|---|
| entrya | Ar | 2 | t (h) | conversionb (%) | 3, 4 | 3 | 4 |
| 1 | Ph | a | 4 | 80 | a | 57 (34) | 43 (21) |
| 2 | 4-OMePh | b | 4 | 79 | b | 55 (38) | 45 (30) |
| 3 | 4-OMePh | b | 8 | 84 | b | 56 | 44 |
| 4 | 4-OMePh | b | 24 | 100 | b | 58 | 42 |
| 5d | 2-OMePh | c | 24 | 73 | c | 50 (37) | 50 (29) |
| 6 | 4-ClPh | d | 4 | 90 | d | 58 (32) | 42 (37) |
| 7 | 4-CNPh | e | 4 | 86 | e | 36 (15) | 64 (44) |
All reactions were carried with 2-ethynylbenzamide 1 (1.0 mmol), iodoarene 2 (1.0 mmol), CO (20 atm), PdCl2(PPh3)2 (0.4 mol %), Et3N (1.5 mL), and CH2Cl2 (4.0 mL) at 100 °C, unless otherwise stated.
Conversion was determined by 1H NMR peak integration on the crude product.
Selectivity was estimated by 1H NMR spectroscopy; isolated yields of pure products are reported in parentheses.
Reaction performed with 1 mol % of PdCl2(PPh3)2.
Considering the interesting synthetic potentialities of compound 5a possessing a diketo group60−66 in the keto-enolic form, a few cyclocarbonylative Sonogashira reactions between 2-ethynylbenzamide 1 and different iodoarenes 2 were also performed in THF. As reported in Table 3, a high conversion of the reactants (75–100%) was observed and the formation of a mixture of isoindolinone 3 and ketoenol 5 was obtained. Nevertheless, compounds 5a–e could be easily isolated chemically pure in moderate yields. The composition of crude products depended on the nature of the functional group on iodoarene 2. Indeed, using 4-iodoanisole 2b, we obtained almost the same result of iodobenzene 2a (Table 3, entries 1–2), while in the reaction between 1 and 1-chloro-4-iodobenzene 2c (Table 3 entry 3) the selectivity changed slightly (40/60).
Table 3. Cyclocarbonylative Sonogashira Reactions of 2-Ethynylbenzamide 1 with Iodoarenes 2 Performed in THF.
| selectivityc (%) |
|||||||
|---|---|---|---|---|---|---|---|
| entrya | Ar | 2 | t (h) | conversionb (%) | 3, 5 | 3 | 5 |
| 1 | Ph | a | 4 | 94 | a | 29 | 71 (44) |
| 2 | 4-OMePh | b | 4 | 75 | b | 29 | 71 (41) |
| 3 | 4-ClPh | d | 4 | 89 | d | 40 | 60 (39) |
| 4 | 4-CNPh | e | 4 | 100 | e | 72 | 28 (22) |
All reactions were carried with 2-ethynylbenzamide 1 (1.0 mmol), iodoarene 2 (1.0 mmol), CO (20 atm), PdCl2(PPh3)2 (0.4 mol %), Et3N (1.5 mL) and CH2Cl2 (4.0 mL) at 100 °C, unless otherwise stated.
Conversion was determined by 1H NMR peak integration on the crude product.
Selectivity was estimated by 1H NMR spectroscopy; isolated yields of pure products are reported in parentheses.
Finally, using 4-iodobenzonitrile 2e as reactant, there was a clear prevalence of the isoindolinone 3e (72%) (Table 3, entry 4). These data clearly showed the need for fine-tuning of the cyclocarbonylation process in the event that keto-enols 5 possessing strong electron-withdrawing groups are desired.
The obtained results described so far indicated that the free −NH2 group is involved in the formation of different products depending on the experimental conditions used. In order to increase the chemoselectivity toward isoindolinone compounds, N-(4-chlorophenyl)-2-ethynylbenzamide 6 was prepared from 2-iodobenzoic acid according to the four-step synthetic procedure depicted in Scheme S3 of Supporting Information.
Initially, N-(4-chlorophenyl)-2-ethynylbenzamide 6 was submitted to a Sonogashira cyclocarbonylation reaction with iodobenzene 2a under experimental conditions which generally favored isoindolinone formation, that is, CH2Cl2, 100 °C, 20 atm CO, 0.4 mol % of PdCl2(PPh3)2 (Table 4, entry 1). To our delight, the analysis of the crude product indicated the complete conversion of starting materials and the formation of two isomers which were identified as (Z)- and (E)-2-(4-chlorophenyl)-3-(2-oxo-2-phenylethylidene)isoindolin-1-one 7a. Moreover the synthesis was highly stereoselective, that is, with a (E)-7a/(Z)-7a molar ratio of 89/11, probably due to the lower steric hindrance of the (E)-isomer. Indeed, when a sample of (Z)-7a in CDCl3 was maintained for 40 h at room temperature, its complete conversion into (E)-isomer was observed (Scheme 2). As previously observed,48 the presence of acid traces in chloroform could cause the interconversion to occur.
Table 4. Cyclocarbonylative Sonogashira Reactions of N-(4-Chlorophenyl)-2-ethynylbenzamide 6 with Iodoarenes 2.
| selectivityc (%) |
|||||||
|---|---|---|---|---|---|---|---|
| entrya | Ar | 2 | t (h) | conversionb (%) | 7 | (E)-7 | (Z)-7 |
| 1 | Ph | a | 4 | 100 | a | 89 (66) | 11 (6) |
| 2d | Ph | a | 24 | 84 | a | 90 | 10 |
| 3 | 4-OMePh | b | 4 | 100 | b | 88 (69) | 12 (10) |
| 4 | 2-OMePh | c | 4 | 100 | c | 93 (74) | 7 (3) |
| 5 | 4-ClPh | d | 4 | 100 | d | 84f (51) | 10f (7) |
| 6e | 4-ClPh | d | 4 | 100 | d | 86f | 10f |
| 7 | 1-Napht | f | 4 | 100 | f | 89 (69) | 11 (5) |
| 8 | 4-MePh | g | 4 | 100 | g | 90 (67) | 10 (7) |
| 9 | 2-MePh | h | 4 | 100 | h | 88 (60) | 12 (5) |
All reactions were carried with N-(4-chlorophenyl)-2-ethynylbenzamide 6 (1.0 mmol), iodoarene 2 (1.0 mmol), CO (20 atm), PdCl2(PPh3)2 (0.4 mol %), Et3N (1.5 mL), and CH2Cl2 (4.0 mL) at 100 °C, unless otherwise stated.
Conversion was determined by 1H NMR peak integration on the crude product.
Selectivity was estimated by 1H NMR spectroscopy; isolated yields of pure products are reported in parentheses.
Reaction performed at 50 °C.
Reaction performed under 40 atm of CO.
The remainder of the product was (Z)-3-(4-chlorobenzylidene)-2-(4-chlorophenyl)isoindolin-1-one 8.
Scheme 2. Interconversion of 2-(4-Chlorophenyl)-3-(2-oxo-2-phenylethylidene)isoindolin-1-one 7a from the (Z)-Isomer to the (E)-Isomer, Performed in CDCl3 at Room Temperature.
When the cyclocarbonylative reaction of amide 6 with iodobenzene 2a was carried out for a longer reaction time (24 h) but at 50 °C, a reduction of conversion was observed (84%) while stereoselectivity resulted in almost the same (Table 4, entry 2, (E)-7a/(Z)-7a molar ratio 90/10). Therefore, all subsequent reactions were carried out at 100 °C for 4 h (Table 4, entries 3–9). In all cases, a quantitative conversion of reagents was observed and generally a mixture of two E/Z isomers (ca. 90/10) was obtained. Both compounds could be easily separated and isolated chemically pure by neutral alumina column chromatography.
The reactions performed between amide 6 and iodoarenes possessing electron-donating groups (i.e., 2b–c and 2f–h) (Table 4, entries 3–4 and 7–9) afforded the (E)-isomers as principle products in good yields (60–74%). In the case of cross-coupling with 1-chloro-4-iodobenzene 2d (Table 4, entry 5), a small amount of (Z)-3-(4-chlorobenzylidene)-2-(4-chlorophenyl)isoindolin-1-one 8 was obtained. Its structure has been assigned by comparison with a pure sample prepared via the cyclic Sonogashira reaction as depicted in Scheme 3. The product composition did not change even performing the cyclocarbonylative reaction of amide 6 with 1-chloro-4-iodobenzene 2d under 40 atm of carbon monoxide pressure (Table 4, entry 6).
Scheme 3. Cyclocarbonylative Sonogashira and Cyclic Sonogashira Reactions of Amide 6 with 1-Chloro-4-iodobenzene 2d.
A cyclocarbonylative Sonogashira reaction of N-(4-chlorophenyl)-2-ethynylbenzamide 6 was also carried out in the presence of the electron-poor 4-iodobenzonitrile 2e: in this case, only small amounts of (E)-4-(2-(2-(4-chlorophenyl)-3-oxoisoindolin-1-ylidene)acetyl)benzonitrile (E)-7e (yield 18%) and (Z)-4-(2-(2-(4-chlorophenyl)-3-oxoisoindolin-1-ylidene)acetyl)benzonitrile (Z)-7e (yield 5%), together with (Z)-4-((2-(4-chlorophenyl)-3-oxoisoindolin-1-ylidene)methyl)benzonitrile 9 (yield 4%) were isolated (Scheme S4 in Supporting Information).
Finally, considering the general toxicity of iodoarenes, a test between 4-bromonitrobenzene 2i and ethynylbenzamide 6 was performed under 20 atm of CO, at 100 °C for 4 h (Scheme 4). Unfortunately, bromoderivative 2i was recovered unreacted, while benzamide 6 was completely consumed. After purification of the crude mixture, 2-(4-chlorophenyl)-3-methyleneisoindolin-1-one (10)45 was isolated in an 85% yield. The formation of cyclisation product 10 could be explained considering the insertion of Pd(0) into the N–H bond, Pd-hydride addition to the triple bond followed by reductive elimination with the generation of methyleneisoindolinone 10 and Pd(0).
Scheme 4. Cyclocarbonylative Sonogashira and Cyclic Sonogashira Reactions of Amide 6 with 1-Chloro-4-iodobenzene 2d.
Conclusions
In conclusion, we have developed an atom-efficient approach to alkylidene isoindolin-1-ones through a Pd-catalyzed copper-free cyclocarbonylative Sonogashira reaction between benzamides and aryl iodides. In particular, when 2-ethynylbenzamide 1 was used in CH2Cl2, the reaction generally afforded (Z)-isoindolinones in a major amount together with indenone derivatives. Changing the solvent to THF determined the preferential formation of keto-enol compounds. On the other hand, the Sonogashira cyclocarbonylative reaction of N-(4-chlorophenyl)-2-ethynylbenzamide 6 with iodoarenes generated almost exclusively the corresponding (E)-isoindolinones in satisfactory yields.
Experimental Section
General Information
Solvents were purified by conventional methods, distilled, and stored over activated molecular sieves under argon. All the chemicals were purchased from commercial sources and used as received without purification. All the operations under an inert atmosphere were carried out using standard Schlenk techniques and employing dried nitrogen. Reactions that required heating were performed in an oil bath. For all reactions, conversion was monitored by thin-layer chromatography analysis on pre-coated silica gel plates (VWR Macherey-Nagel, 0.2 mm thick) or pre-coated neutral alumina plates (Sigma-Aldrich, 0.25 mm thick). Column chromatography was performed with Fluka silica gel, pore size 60 c5, 70–230 mesh, 63–200 μm or Sigma-Aldrich activated, neutral alumina. 1H NMR and 13C NMR spectra were recorded at room temperature in CDCl3 or DMSO-d6 solution with a Varian INOVA-600 spectrometer, operating at a frequency of 600 MHz for 1H and 150 MHz for 13C, using the residual solvent peak as the internal reference; chemical shift (δ) values are given in parts per million (ppm) and coupling constants (J) in Hz. Mass spectra were obtained with an Applied Biosystems-MDS Sciex API 4000 triple quadrupole mass spectrometer (Concord, Ont., Canada), equipped with a Turbo-V ion-spray (TIS) source. Elemental analyses were performed on a Elementar Vario Micro Cube CHN-analyzer.
Synthesis of Ethynylbenzamides
2-((Trimethylsilyl)ethynyl)benzamide (B)67
2-Bromobenzamide (A) (7.00 g, 35.0 mmol), PdCl2(PPh3)2 (983 mg, 1.40 mmol), CuI (267 mg, 1.40 mmol), Et3N (160 mL), and DMF (50 mL) were mixed together, then trimethylsilylacetylene (7.4 mL, 52.5 mmol) was added dropwise. The resulting mixture was refluxed under stirring for 24 h, then it was cooled to room temperature, hydrolyzed with saturated ammonium chloride solution (150 mL) and extracted with CH2Cl2 (3 × 150 mL). The combined organic phases were washed with brine (150 mL), dried over anhydrous Na2SO4, and the solvent was removed under vacuum. The crude product was purified by column chromatography (SiO2, n-hexane/AcOEt 1:1) to give 3.18 g (yield 42%) of 2-((trimethylsilyl)ethynyl)benzamide (B).67
1H NMR (600 MHz, CDCl3) δ (ppm): 0.28 (9H, s), 6.04 (1H, br s), 7.43–7.46 (2H, m), 7.55–7.56 (1H, m), 7.76 (1H, br s), 8.13–8.17 (1H, m). 13C NMR (150 MHz, CDCl3) δ (ppm): −0.2 (3C), 102.3, 104.4, 120.1, 129.3, 130.6, 131.1, 134.1, 134.7, 168.2. LC–MS (APCI+) m/z: 218.1 [M + H]+.
2-Ethynylbenzamide (1)67
2-((Trimethylsilyl)ethynyl)benzamide (B) (3.18 g, 14.6 mmol), cesium fluoride (3.33 g, 21.9 mmol), and methanol (100 mL) were mixed together. The resulting mixture was left under stirring for 3 h at room temperature, then it was hydrolyzed with brine (100 mL) and extracted with AcOEt (3 × 50 mL). The combined organic phases were washed with brine (100 mL), dried over anhydrous Na2SO4, and the solvent was removed under vacuum. The crude product was purified by column chromatography (SiO2, n-hexane/AcOEt 1:1) to give 1.51 g (yield 71%) of 2-ethynylbenzamide (1).67
1H NMR (600 MHz, CDCl3) δ (ppm): 3.52 (1H, s), 6.10 (1H, br s), 7.34 (1H, br s), 7.45–7.49 (2H, m), 7.59–7.61 (1H, m), 8.08–8.09 (1H, m). 13C NMR (150 MHz, CDCl3) δ (ppm): 82.5, 84.2, 119.1, 129.6, 130.3, 131.2, 134.5, 135.5, 168.3. LC–MS (APCI+) m/z: 146.1 [M + H]+.
2-Iodobenzoyl chloride (D)68
2-Iodobenzoic acid (C) (4.97 g, 20.0 mmol), DMF (46 μL, 0.6 mmol), and CH2Cl2 (50 mL) were mixed together, then oxalyl chloride (3.5 mL, 40.8 mmol) was added dropwise to the solution at 0 °C. The mixture was left under stirring for 2 h at room temperature, then it was evaporated under vacuum to give 2-iodobenzoyl chloride (D)68 (4.66 g, yield 87%), which was used without further purification.
1H NMR (600 MHz, CDCl3) δ (ppm): 7.23–7.26 (1H, m), 7.49–7.51 (1H, m), 8.04–8.05 (1H, m), 8.07–8.08 (1H, m). 13C NMR (150 MHz, CDCl3) δ (ppm): 94.1, 128.2, 133.9, 134.3, 138.2, 141.7, 166.8.
N-(4-Chlorophenyl)-2-iodobenzamide (E)69
4-Chloroaniline (3.19 g, 25.0 mmol), Et3N (3.5 mL, 25.0 mmol), and CH2Cl2 (25 mL) were mixed together, then a solution of 2-iodobenzoyl chloride (D) (6.67 g, 25.0 mmol) in CH2Cl2 (25 mL) was added dropwise to the solution at 0 °C. The mixture was left under stirring for 90 min at room temperature, then it was hydrolyzed with water (100 mL) and extracted with CH2Cl2 (3 × 50 mL). The combined organic phases were washed, in order, with HCl 1 M solution (100 mL), water (100 mL), saturated NaHCO3 solution (100 mL), and brine (100 mL), then dried over anhydrous Na2SO4, and the solvent was removed under vacuum to give N-(4-chlorophenyl)-2-iodobenzamide (E)69 (7.24 g, yield 81%) which was used without further purification.
1H NMR (600 MHz, CDCl3) δ (ppm): 7.13–7.15 (1H, m), 7.32 (2H, d, J = 8.7 Hz), 7.39–7.42 (1H, m), 7.46–7.48 (1H, m), 7.57 (2H, d, J = 8.7 Hz), 7.70 (1H, br s), 7.88–7.89 (1H, m). 13C NMR (150 MHz, CDCl3) δ (ppm): 92.4, 121.4 (2C), 128.2, 128.4, 129.0 (2C), 129.8, 131.5, 136.1, 139.9, 141.6, 167.4. LC–MS (APCI+) m/z: 358.0 [M + H]+.
N-(4-Chlorophenyl)-2-((trimethylsilyl)ethynyl)benzamide (F)70
N-(4-Chlorophenyl)-2-iodobenzamide (E) (6.80 g, 19.0 mmol), PdCl2(PPh3)2 (534 mg, 0.76 mmol), CuI (145 mg, 0.76 mmol), Et3N (5.3 mL, 38.0 mmol), and THF (120 mL) were mixed together, then trimethylsilylacetylene (4.1 mL, 28.9 mmol) was added dropwise. The resulting mixture was left under stirring for 24 h at room temperature, then it was hydrolyzed with saturated ammonium chloride solution (100 mL) and extracted with CH2Cl2 (3 × 100 mL). The combined organic phases were washed with brine (100 mL), dried over anhydrous Na2SO4, and the solvent was removed under vacuum. The crude product was purified by column chromatography (SiO2, n-hexane/AcOEt 6:1) to give 4.95 g (yield 79%) of N-(4-chlorophenyl)-2-((trimethylsilyl)ethynyl)benzamide (F).70
1H NMR (600 MHz, CDCl3) δ (ppm): 0.25 (9H, s), 7.34 (2H, d, J = 9.0 Hz), 7.44–7.48 (2H, m), 7.58–7.59 (1H, m), 7.62 (2H, d, J = 9.0 Hz), 8.10–8.12 (1H, m), 9.33 (1H, br s). 13C NMR (150 MHz, CDCl3) δ (ppm): −0.2 (3C), 102.9, 103.0, 119.3, 121.3 (2C), 129.0 (2C), 129.4, 129.5, 130.3, 130.9, 134.1, 135.4, 136.5, 164.0. LC–MS (APCI+) m/z: 328.1 [M + H]+.
N-(4-Chlorophenyl)-2-ethynylbenzamide (6)
N-(4-Chlorophenyl)-2-((trimethylsilyl)ethynyl)benzamide (F) (3.94 g, 12.0 mmol) and methanol (100 mL) were mixed together, then a solution of tetrabutylammonium fluoride trihydrate (4.54 g, 14.4 mmol) in methanol (100 mL) was added dropwise to the solution. The resulting mixture was left under stirring for 1 h at room temperature, then it was hydrolyzed with brine (200 mL), and extracted with AcOEt (3 × 150 mL). The combined organic phases were washed with brine (100 mL), dried over anhydrous Na2SO4, and the solvent was removed under vacuum. The crude product was purified by column chromatography (SiO2, n-hexane/AcOEt 4:1) to give 1.78 g (yield 58%) of N-(4-chlorophenyl)-2-ethynylbenzamide (6).
1H NMR (600 MHz, CDCl3) δ (ppm): 3.57 (1H, s), 7.27 (2H, d, J = 9.0 Hz), 7.39–7.42 (2H, m), 7.54–7.56 (1H, m), 7.59 (2H, d, J = 9.0 Hz), 7.91–7.92 (1H, m), 9.10 (1H, br s). 13C NMR (150 MHz, CDCl3) δ (ppm): 81.9, 84.4, 118.4, 121.2 (2C), 129.0 (2C), 129.5, 129.6, 129.9, 130.9, 134.2, 136.4, 136.5, 164.3. LC–MS (APCI+) m/z: 256.0 [M + H]+. Anal. Calcd for C15H10ClNO: C, 70.46, H, 3.94, N, 5.48. Found: C, 70.39; H, 3.99; N, 5.47.
Cyclocarbonylative Sonogashira Reactions of 2-Ethynylbenzamide (1)
General Procedure
A Pyrex Schlenk tube under a CO atmosphere was charged with 2-ethynylbenzamide (1) (1.0 mmol), iodoarene (1.0 mmol), Et3N (1.5 mL), and the solvent (4.0 mL). This solution was introduced by a steel siphon into a 25 mL stainless steel autoclave, fitted with a Teflon inner crucible, and a stirring bar, previously carried with PdCl2(PPh3)2 (0.4–1.0 mol %) and placed under vacuum (0.1 Torr). The reactor was pressurized with CO (20 atm) and the mixture was stirred for a selected time at a selected temperature. After removal of excess CO (fume hood), the reaction mixture was diluted with CH2Cl2 (20 mL), washed with brine (15 mL), dried over anhydrous Na2SO4, and the solvent was removed under vacuum. The reagent conversion and the product composition were determined by the 1H NMR spectroscopic analysis. All crude products were purified through column chromatography on silica gel and characterized with 1H NMR, 13C NMR, LC–MS, and elemental analysis techniques.
Cyclocarbonylative Sonogashira of 2-Ethynylbenzamide (1) and Iodobenzene (2a) in CH2Cl2 at 100 °C (Table 1, Entry 1 and Table 2, Entry 1)
Following the general procedure, 2.8 mg (0.004 mmol) of PdCl2(PPh3)2, 145.2 mg (1.0 mmol) of 2-ethynylbenzamide (1), 204.0 mg (1.0 mmol) of iodobenzene (2a), 1.5 mL of Et3N, and 4 mL of CH2Cl2 were put in the autoclave. The resulting mixture was stirred for 4 h at 100 °C. The crude product was purified through column chromatography (SiO2, n-hexane/AcOEt 1:1), obtaining 85 mg (yield 34%) of (Z)-3-(2-oxo-2-phenylethylidene)isoindolin-1-one (3a), and 52 mg (yield 21%) of 3-amino-2-benzoyl-1H-inden-1-one (4a).
3a. 1H NMR (600 MHz, DMSO-d6) δ (ppm): 7.41 (1H, s), 7.58–7.61 (2H, m), 7.67–7.70 (1H, m), 7.72–7.74 (1H, m), 7.81–7.83 (1H, m), 7.85–7.86 (1H, m), 8.20–8.21 (2H, m), 8.35–8.37 (1H, m), 10.92 (1H, br s). 13C NMR (150 MHz, DMSO-d6) δ (ppm): 95.7, 122.6, 123.4, 128.1 (2C), 128.5, 128.8 (2C), 132.1, 133.1, 133.2, 137.2, 137.8, 147.6, 168.8, 189.8. LC–MS (APCI+) m/z: 250.1 [M + H]+. Anal. Calcd for C16H11NO2: C, 77.10, H, 4.45, N, 5.62. Found: C, 77.19; H, 4.41; N, 5.63.
4a. 1H NMR (600 MHz, DMSO-d6) δ (ppm): 7.39–7.42 (2H, m), 7.47–7.51 (2H, m), 7.60–7.62 (2H, m), 7.63–7.67 (2H, m), 8.06–8.10 (1H, m), 10.09 (1H, br s), 10.21 (1H, br s). 13C NMR (150 MHz, DMSO-d6) δ (ppm): 103.0, 121.4, 121.5, 127.2 (2C), 128.5 (2C), 130.5, 132.3, 133.5, 134.9, 135.5, 140.1, 172.2, 186.7, 190.2. LC–MS (APCI+) m/z: 250.1 [M + H]+. Anal. Calcd for C16H11NO2: C, 77.10, H, 4.45, N, 5.62. Found: C, 77.17; H, 4.37; N, 5.61.
Cyclocarbonylative Sonogashira of 2-Ethynylbenzamide (1) and Iodobenzene (2a) in CH2Cl2 at 50 °C (Table 1, Entry 5)
Following the general procedure, 2.8 mg (0.004 mmol) of PdCl2(PPh3)2, 145.2 mg (1.0 mmol) of 2-ethynylbenzamide (1), 204.0 mg (1.0 mmol) of iodobenzene (2a), 1.5 mL of Et3N, and 4 mL of CH2Cl2 were put in the autoclave. The resulting mixture was stirred for 24 h at 50 °C. The crude product was purified through column chromatography (SiO2, CH2Cl2), obtaining 105 mg (yield 42%) of (Z)-2-(1-hydroxy-3-oxo-3-phenylprop-1-en-1-yl)benzonitrile (5a).
5a. 1H NMR (600 MHz, CDCl3) δ (ppm): 6.95 (1H, s), 7.48–7.51 (2H, m), 7.56–7.59 (1H, m), 7.61–7.64 (1H, m), 7.71–7.74 (1H, m), 7.82–7.83 (1H, m), 7.97–8.01 (3H, m), 16.44 (1 H, br s). 13C NMR (150 MHz, CDCl3) δ (ppm): 96.0, 110.6, 118.0, 127.4 (2C), 128.8 (2C), 129.0, 131.5, 132.8, 133.0, 134.7, 134.7, 139.1, 183.1, 186.2. LC–MS (APCI+) m/z: 250.1 [M + H]+. Anal. Calcd for C16H11NO2: C, 77.10, H, 4.45, N, 5.62. Found: C, 77.18; H, 4.39; N, 5.61.
Cyclocarbonylative Sonogashira of 2-Ethynylbenzamide (1) and Iodobenzene (2a) in THF at 100 °C (Table 1, Entry 6 and Table 3, Entry 1)
Following the general procedure, 2.8 mg (0.004 mmol) of PdCl2(PPh3)2, 145.2 mg (1.0 mmol) of 2-ethynylbenzamide (1), 204.0 mg (1.0 mmol) of iodobenzene (2a), 1.5 mL of Et3N, and 4 mL of THF were put in the autoclave. The resulting mixture was stirred for 4 h at 100 °C. The crude product was purified through column chromatography (SiO2, CH2Cl2), obtaining 110 mg (yield 44%) of (Z)-2-(1-hydroxy-3-oxo-3-phenylprop-1-en-1-yl) benzonitrile (5a).
Cyclocarbonylative Sonogashira of 2-Ethynylbenzamide (1) and Iodobenzene (2a) in Acetonitrile at 100 °C (Table 1, Entry 9)
Following the general procedure, 2.8 mg (0.004 mmol) of PdCl2(PPh3)2, 145.2 mg (1.0 mmol) of 2-ethynylbenzamide (1), 204.0 mg (1.0 mmol) of iodobenzene (2a), 1.5 mL of Et3N, and 4 mL of acetonitrile were put in the autoclave. The resulting mixture was stirred for 4 h at 100 °C. The composition of the crude product was determined by the 1H NMR analysis, resulting in a mixture of (Z)-3-(2-oxo-2-phenylethylidene)isoindolin-1-one (3a) and (Z)-2-(1-hydroxy-3-oxo-3-phenylprop-1-en-1-yl) benzonitrile (5a) in the molar ratio 78/22.
Cyclocarbonylative Sonogashira of 2-Ethynylbenzamide (1) and Iodobenzene (2a) in DMF at 100 °C (Table 1, Entry 11)
Following the general procedure, 2.8 mg (0.004 mmol) of PdCl2(PPh3)2, 145.2 mg (1.0 mmol) of 2-ethynylbenzamide (1), 204.0 mg (1.0 mmol) of iodobenzene (2a), 1.5 mL of Et3N, and 4 mL of DMF were put in the autoclave. The resulting mixture was stirred for 4 h at 100 °C. The composition of the crude product was determined by the 1H NMR analysis, resulting in a mixture of (Z)-3-(2-oxo-2-phenylethylidene)isoindolin-1-one (3a), 3-amino-2-benzoyl-1H-inden-1-one (4a) and (Z)-2-(1-hydroxy-3-oxo-3-phenylprop-1-en-1-yl) benzonitrile (5a) in the molar ratio 33/32/35.
Cyclocarbonylative Sonogashira of 2-Ethynylbenzamide (1) and 4-Iodoanisole (2b) in CH2Cl2 (Table 2, Entry 2)
Following the general procedure, 2.8 mg (0.004 mmol) of PdCl2(PPh3)2, 145.2 mg (1.0 mmol) of 2-ethynylbenzamide (1), 234.0 mg (1.0 mmol) of 4-iodoanisole (2b), 1.5 mL of Et3N, and 4 mL of CH2Cl2 were put in the autoclave. The resulting mixture was stirred for 4 h at 100 °C. The crude product was purified through column chromatography (SiO2, n-hexane/AcOEt 1:1), obtaining 106 mg (yield 38%) of (Z)-3-(2-(4-methoxyphenyl)-2-oxoethylidene)isoindolin-1-one (3b) and 84 mg (yield 30%) of 3-amino-2-(4-methoxybenzoyl)-1H-inden-1-one (4b).
3b. 1H NMR (600 MHz, CDCl3) δ (ppm): 3.84 (3H, s), 6.79 (1H, s), 6.91–6.93 (2H, m), 7.56–7.58 (1H, m), 7.61–7.64 (1H, m), 7.77–7.78 (1H, m), 7.82–7.83 (1H, m), 7.96–7.99 (2H, m), 10.57 (1H, br s). 13C NMR (150 MHz, CDCl3) δ (ppm): 55.5, 94.75 113.9 (2C), 121.0, 124.0, 129.2, 130.2 (2C), 131.2, 131.7, 132.7, 137.1, 147.6, 163.5, 169.0, 189.4. LC–MS (APCI+) m/z: 280.1 [M + H]+. Anal. Calcd for C17H13NO3: C, 73.11, H, 4.69, N, 5.02. Found: C, 73.07; H, 4.78; N, 5.02.
4b. 1H NMR (600 MHz, DMSO-d6) δ (ppm): 3.82 (3H, s), 6.93–6.95 (2H, m), 7.49–7.51 (1H, m), 7.62–7.66 (4H, m), 8.02–8.05 (1H, m), 9.96 (1H, br s), 10.14 (1H, br s). 13C NMR (150 MHz, DMSO-d6) δ (ppm): 55.3, 103.1, 112.5, 121.3, 121.3 (2C), 130.9 (2C), 132.2, 132.4, 133.4, 135.0, 135.5, 161.4, 172.2, 186.8, 189.18. LC–MS (APCI+) m/z: 280.1 [M + H]+. Anal. Calcd for C17H13NO3: C, 73.11, H, 4.69, N, 5.02. Found: C, 73.05; H, 4.74; N, 5.01.
Cyclocarbonylative Sonogashira of 2-Ethynylbenzamide (1) and 2-Iodoanisole (2c) in CH2Cl2 (Table 2, Entry 5)
Following the general procedure, 7.1 mg (0.01 mmol) of PdCl2(PPh3)2, 145.2 mg (1.0 mmol) of 2-ethynylbenzamide (1), 234.0 mg (1.0 mmol) of 2-iodoanisole (2c), 1.5 mL of Et3N, and 4 mL of CH2Cl2 were put in the autoclave. The resulting mixture was stirred for 24 h at 100 °C. The crude product was purified through column chromatography (SiO2, n-hexane/AcOEt 1:1), obtaining 104 mg (yield 37%) of (Z)-3-(2-(2-methoxyphenyl)-2-oxoethylidene)isoindolin-1-one (3c) and 81 mg (yield 29%) of 3-amino-2-(2-methoxybenzoyl)-1H-inden-1-one (4c).
3c. 1H NMR (600 MHz, CDCl3) δ (ppm): 3.95 (3H, s), 6.94 (1H, s), 7.00–7.01 (1H, m), 7.03–7.06 (1H, m), 7.47–7.50 (1H, m), 7.58–7.64 (2H, m), 7.73–7.76 (2H, m), 7.87–7.88 (1H, m), 10.48 (1H, br s). 13C NMR (150 MHz, CDCl3) δ (ppm): 55.8, 100.2, 111.7, 120.9, 121.1, 123.5, 124.1, 130.6, 131.6, 132.7, 133.5, 135.9, 137.4, 146.5, 158.1, 169.1, 192.3. LC–MS (APCI+) m/z: 280.1 [M + H]+. Anal. Calcd for C17H13NO3: C, 73.11, H, 4.69, N, 5.02. Found: C, 73.22; H, 4.63; N, 5.03.
4c. 1H NMR (600 MHz, DMSO-d6) δ (ppm): 3.64 (3H, s), 6.91–6.93 (1H, m), 7.00–7.01 (1H, m), 7.08–7.10 (1H, m), 7.34–7.36 (1H, m), 7.40–7.42 (1H, m), 7.60–7.62 (2H, m), 8.03–8.04 (1H, m), 9.98 (1H, s), 10.06 (1H, s). 13C NMR (150 MHz, DMSO-d6) δ (ppm): 55.4, 104.5, 111.1, 119.7, 121.2, 121.6, 127.6, 130.0, 131.6, 132.3, 133.4, 135.1, 135.6, 156.4, 170.7, 186.6, 189.2. LC–MS (APCI+) m/z: 280.1 [M + H]+. Anal. Calcd for C17H13NO3: C, 73.11; H, 4.69; N, 5.02. Found: C, 73.18; H, 4.64; N, 5.02.
Cyclocarbonylative Sonogashira of 2-Ethynylbenzamide (1) and 1-Chloro-4-iodobenzene (2d) in CH2Cl2 (Table 2, Entry 6)
Following the general procedure, 2.8 mg (0.004 mmol) of PdCl2(PPh3)2, 145.2 mg (1.0 mmol) of 2-ethynylbenzamide (1), 238.5 mg (1.0 mmol) of 1-chloro-4-iodobenzene (2d), 1.5 mL of Et3N, and 4 mL of CH2Cl2 were put in the autoclave. The resulting mixture was stirred for 4 h at 100 °C. The crude product was purified through column chromatography (SiO2, n-hexane/AcOEt 1:1), obtaining 91 mg (yield 32%) of (Z)-3-(2-(4-chlorophenyl)-2-oxoethylidene)isoindolin-1-one (3d) and 105 mg (yield 37%) of 3-amino-2-(4-chlorobenzoyl)-1H-inden-1-one (4d).
3d. 1H NMR (600 MHz, CDCl3) δ (ppm): 6.81 (1H, s), 7.49 (2H, d, J = 8.7 Hz), 7.64–7.70 (2H, m), 7.82–7.83 (1H, m), 7.90–7.91 (1H, m), 7.98 (2H, d, J = 8.7 Hz), 10.57 (1H, br s). 13C NMR (150 MHz, CDCl3) δ (ppm): 94.2, 121.1, 124.3, 129.0 (2C), 129.3 (2C), 130.6, 132.1, 132.9, 136.7, 137.00, 139.4, 148.9, 169.00, 189.6. LC–MS (APCI+) m/z: 283.9 [M + H]+. Anal. Calcd for C16H10ClNO2: C, 67.74; H, 3.55; N, 4.94. Found: C, 67.68; H, 3.61; N, 4.95.
4d. 1H NMR (600 MHz, DMSO-d6) δ (ppm): 7.46 (2H, d, J = 8.4 Hz), 7.50–7.51 (1H, m), 7.61 (2H, d, J = 8.4 Hz), 7.64–7.66 (2H, m), 8.06–8.08 (1H, m), 10.14 (1H, br s), 10.19 (1H, br s). 13C NMR (150 MHz, DMSO-d6) δ (ppm): 102.9, 121.5, 121.7, 127.3 (2C), 130.4 (2C), 132.5, 133.8, 134.9, 135.2, 135.5, 138.8, 172.2, 186.8, 188.7. LC–MS (APCI+) m/z: 283.9 [M + H]+. Anal. Calcd for C16H10ClNO2: C, 67.74; H, 3.55; N, 4.94. Found: C, 67.63; H, 3.59; N, 4.95.
Cyclocarbonylative Sonogashira of 2-Ethynylbenzamide (1) and 4-Iodobenzonitrile (2e) in CH2Cl2 (Table 2, Entry 7)
Following the general procedure, 2.8 mg (0.004 mmol) of PdCl2(PPh3)2, 145.2 mg (1.0 mmol) of 2-ethynylbenzamide (1), 229.0 mg (1.0 mmol) of 4-iodobenzonitrile (2e), 1.5 mL of Et3N, and 4 mL of CH2Cl2 were put in the autoclave. The resulting mixture was stirred for 4 h at 100 °C. The crude product was purified through column chromatography (SiO2, n-hexane/AcOEt 1:1), obtaining 41 mg (yield 15%) of (Z)-4-(2-(3-oxoisoindolin-1-ylidene) acetyl)benzonitrile (3e) and 121 mg (yield 44%) of 4-(3-amino-1-oxo-1H-indene-2-carbonyl)benzonitrile (4e).
3e. 1H NMR (600 MHz, CDCl3) δ (ppm): 6.81 (1H, s), 7.67–7.73 (2H, m), 7.82–7.85 (3H, m), 7.92–7.94 (1H, m), 8.12 (2H, d, J = 8.4 Hz), 10.56 (1H, br s). 13C NMR (150 MHz, CDCl3) δ (ppm): 93.8, 116.1, 117.9, 121.3, 124.4, 128.3 (2C), 132.4, 132.5 (2C), 132.8, 133.1, 136.8, 141.6, 150.0, 168.9, 189.3. LC–MS (APCI+) m/z: 275.1 [M + H]+. Anal. Calcd for C17H10N2O2: C, 74.44; H, 3.67; N, 10.21. Found: C, 74.52; H, 3.60; N, 10.22.
4e. 1H NMR (600 MHz, DMSO-d6) δ (ppm): 7.50–7.52 (1H, m), 7.66–7.68 (2H, m), 7.70 (2H, d, J = 8.7 Hz), 7.87 (2H, d, J = 8.7 Hz), 8.09–8.11 (1H, m), 10.23 (1H, br s), 10.26 (1H, br s). 13C NMR (150 MHz, DMSO-d6) δ (ppm): 112.4, 118.7, 121.5, 121.9, 128.9 (2C), 131.4 (2C), 132.0, 132.6, 133.8, 134.8, 135.6, 144.3, 172.2, 186.7, 188.3. LC–MS (APCI+) m/z: 275.1 [M + H]+. Anal. Calcd for C17H10N2O2: C, 74.44; H, 3.67; N, 10.21. Found: C, 74.51; H, 3.59; N, 10.21.
Cyclocarbonylative Sonogashira of 2-Ethynylbenzamide (1) and 4-Iodoanisole (2b) in THF (Table 3, Entry 2)
Following the general procedure, 2.8 mg (0.004 mmol) of PdCl2(PPh3)2, 145.2 mg (1.0 mmol) of 2-ethynylbenzamide (1), 234.0 mg (1.0 mmol) of 4-iodoanisole (2b), 1.5 mL of Et3N, and 4 mL of THF were put in the autoclave. The resulting mixture was stirred for 4 h at 100 °C. The crude product was purified through column chromatography (SiO2, CH2Cl2), obtaining 115 mg (yield 41%) of (Z)-2-(1-hydroxy-3-(4-methoxyphenyl)-3-oxoprop-1-en-1-yl) benzonitrile (5b).
5b. 1H NMR (600 MHz, CDCl3) δ (ppm): 3.88 (3H, s), 6.91 (1H, s), 6.98 (2H, d, J = 9.0 Hz), 7.60 (1H, t), 7.71 (1H, t), 7.82 (1H, d, J = 7.8 Hz), 7.96–7.99 (3H, m), 16.59 (1H, br s). 13C NMR (150 MHz, CDCl3) δ (ppm): 55.5, 95.4, 110.4, 114.1 (2C), 118.1, 127.4, 128.9, 129.7 (2C), 131.2, 132.8, 134.7, 139.2, 163.7, 181.2, 186.5. LC–MS (APCI+) m/z: 280.1 [M + H]+. Anal. Calcd for C17H13NO3: C, 73.11; H, 4.69; N, 5.02. Found: C, 73.21; H, 4.62; N, 5.03.
Cyclocarbonylative Sonogashira of 2-Ethynylbenzamide (1) and 1-Chloro-4-iodobenzene (2d) in THF (Table 3, Entry 3)
Following the general procedure, 2.8 mg (0.004 mmol) of PdCl2(PPh3)2, 145.2 mg (1.0 mmol) of 2-ethynylbenzamide (1), 238.5 mg (1.0 mmol) of 1-chloro-4-iodobenzene (2d), 1.5 mL of Et3N, and 4 mL of THF were put in the autoclave. The resulting mixture was stirred for 4 h at 100 °C. The crude product was purified through column chromatography (SiO2, CH2Cl2), obtaining 111 mg (yield 39%) of (Z)-2-(3-(4-chlorophenyl)-1-hydroxy-3-oxoprop-1-en-1-yl)benzonitrile (5d).
5d. 1H NMR (600 MHz, CDCl3) δ (ppm): 6.93 (1H, s), 7.47 (2H, d, J = 8.4 Hz), 7.62–7.65 (1H, m), 7.72–7.75 (1H, m), 7.84 (1H, d, J = 7.8 Hz), 7.94 (2H, d, J = 8.4 Hz), 7.98 (1H, d, J = 7.8 Hz), 16.35 (1H, br s). 13C NMR (150 MHz, CDCl3) δ (ppm): 96.0, 110.6, 118.0, 128.8 (2C), 129.1, 129.2 (2C), 131.6, 132.8, 133.2, 134.8, 138.9, 139.4, 183.0, 185.1. LC–MS (APCI+) m/z: 284.1 [M + H]+. Anal. Calcd for C16H10ClNO2: C, 67.74; H, 3.55; N, 4.94. Found: C, 67.82; H, 3.49; N, 4.93.
Cyclocarbonylative Sonogashira of 2-Ethynylbenzamide (1) and 4-Iodobenzonitrile (2e) in THF (Table 3, Entry 4)
Following the general procedure, 2.8 mg (0.004 mmol) of PdCl2(PPh3)2, 145.2 mg (1.0 mmol) of 2-ethynylbenzamide (1), 229.0 mg (1.0 mmol) of 4-iodobenzonitrile (2e), 1.5 mL of Et3N, and 4 mL of THF were put in the autoclave. The resulting mixture was stirred for 4 h at 100 °C. The crude product was purified through column chromatography (SiO2, CH2Cl2), obtaining 61 mg (yield 22%) of (Z)-2-(3-(4-cyanophenyl)-1-hydroxy-3-oxoprop-1-en-1-yl)benzonitrile (5e).
5e. 1H NMR (600 MHz, CDCl3) δ (ppm): 7.00 (1H, s), 7.66–7.69 (1H, m), 7.75–7.77 (1H, m), 7.81 (2H, d, J = 8.4 Hz), 7.86–7.87 (1H, m), 8.01 (1H, d, J = 7.8 Hz), 8.09 (2H, d, J = 8.4 Hz), 16.16 (1H, br s). 13C NMR (150 MHz, CDCl3) δ (ppm): 96.7, 110.6, 116.0, 117.9, 117.9, 127.8 (2C), 129.2, 132.0, 132.5 (2C), 132.9, 134.8, 138.5, 138.6, 182.9, 184.7. LC–MS (APCI+) m/z: 275.1 [M + H]+. Anal. Calcd for C17H10N2O2: C, 74.44; H, 3.67; N, 10.21. Found: C, 74.55; H, 3.61; N, 10.21.
Cyclocarbonylative Sonogashira Reactions of N-(4-Chlorophenyl)-2-ethynylbenzamide (6)
General Procedure
A Pyrex Schlenk tube under a CO atmosphere was charged with N-(4-chlorophenyl)-2-ethynylbenzamide (6) (1.0 mmol), haloarene (1.0 mmol), Et3N (1.5 mL), and CH2Cl2 (4.0 mL). This solution was introduced by a steel siphon into a 25 mL stainless steel autoclave, fitted with a Teflon inner crucible, and a stirring bar, previously carried with PdCl2(PPh3)2 (0.4 mol %) and placed under vacuum (0.1 Torr). The reactor was pressurized with CO (20–40 atm) and the mixture was stirred for a selected time at a selected temperature. After removal of excess CO (fume hood), the reaction mixture was diluted with CH2Cl2 (20 mL), washed with brine (15 mL), dried over anhydrous Na2SO4, and the solvent was removed under vacuum. The reagent conversion and the product composition were determined by 1H NMR spectroscopic analysis. All crude products were purified through column chromatography on neutral alumina and characterized with 1H NMR, 13C NMR, LC–MS, and elemental analysis techniques.
Cyclocarbonylative Sonogashira of N-(4-Chlorophenyl)-2-ethynylbenzamide (6) and Iodobenzene (2a) at 100 °C (Table 4, Entry 1)
Following the general procedure, 2.8 mg (0.004 mmol) of PdCl2(PPh3)2, 255.7 mg (1.0 mmol) of N-(4-chlorophenyl)-2-ethynylbenzamide (6), 204.0 mg (1.0 mmol) of iodobenzene (2a), 1.5 mL of Et3N, and 4 mL of CH2Cl2 were put in the autoclave charged with 20 atm of CO. The resulting mixture was stirred for 4 h at 100 °C. The crude product was purified through column chromatography (neutral Al2O3, n-hexane/AcOEt 4:1), obtaining 238 mg (yield 66%) of (E)-2-(4-chlorophenyl)-3-(2-oxo-2-phenylethylidene)isoindolin-1-one [(E)-7a] and 22 mg (yield 6%) of (Z)-2-(4-chlorophenyl)-3-(2-oxo-2-phenylethylidene)isoindolin-1-one [(Z)-7a].
(E)-7a. 1H NMR (600 MHz, CDCl3) δ (ppm): 6.53 (1H, s), 7.36 (2H, d, J = 7.6 Hz), 7.46 (2H, t, J = 7.8 Hz), 7.55–7.59 (3H, m), 7.67–7.69 (1H, m), 7.72–7.75 (1H, m), 7.84 (2H, d, J = 7.6 Hz), 7.97 (1H, d, J = 7.2 Hz), 8.94 (1H, d, J = 7.8 Hz). 13C NMR (150 MHz, CDCl3) δ (ppm): 105.3, 123.6, 127.3, 128.1 (2C), 128.6 (2C), 129.4, 130.1 (4C), 131.9, 132.3, 132.9, 133.6, 133.8, 135.0, 138.8, 149.2, 166.8, 189.6. LC–MS (APCI+) m/z: 360.1 [M + H]+. Anal. Calcd for C22H14ClNO2: C, 73.44; H, 3.92; N, 3.89. Found: C, 73.41; H, 3.98; N, 3.88.
(Z)-7a. 1H NMR (600 MHz, CDCl3) δ (ppm): 6.61 (1H, s), 6.99 (2H, d, J = 8.4 Hz), 7.15 (2H, d, J = 8.4 Hz), 7.36–7.38 (2H, m), 7.49–7.52 (1H, m), 7.63–7.65 (2H, m), 7.68 (1H, d, J = 7.8 Hz), 7.73–7.76 (1H, m), 7.88 (1H, d, J = 7.8 Hz), 7.96 (1H, d, J = 7.8 Hz). 13C NMR (150 MHz, CDCl3) δ (ppm): 101.6, 105.4, 120.3, 124.3, 128.3 (2C), 128.4 (2C), 128.5 (2C), 128.9 (2C), 130.1, 130.2, 131.2, 133.1, 133.2, 137.2, 138.0, 143.0, 167.5, 191.2. LC–MS (APCI+) m/z: 360.1 [M + H]+. Anal. Calcd for C22H14ClNO2: C, 73.44; H, 3.92; N, 3.89. Found: C, 73.40; H, 3.99; N, 3.88.
Cyclocarbonylative Sonogashira of N-(4-Chlorophenyl)-2-ethynylbenzamide (6) and Iodobenzene (2a) at 50 °C (Table 4, Entry 2)
Following the general procedure, 2.8 mg (0.004 mmol) of PdCl2(PPh3)2, 255.7 mg (1.0 mmol) of N-(4-chlorophenyl)-2-ethynylbenzamide (6), 204.0 mg (1.0 mmol) of iodobenzene (2a), 1.5 mL of Et3N. and 4 mL of CH2Cl2 were put in the autoclave charged with 20 atm of CO. The resulting mixture was stirred for 24 h at 50 °C. The composition of the crude product was determined by the 1H NMR analysis, resulting in a mixture of (E)-2-(4-chlorophenyl)-3-(2-oxo-2-phenylethylidene)isoindolin-1-one [(E)-7a] and (Z)-2-(4-chlorophenyl)-3-(2-oxo-2-phenyl-ethylidene)isoindolin-1-one [(Z)-7a] in the molar ratio 90/10.
Cyclocarbonylative Sonogashira of N-(4-Chlorophenyl)-2-ethynylbenzamide (6) and 4-Iodoanisole (2b) (Table 4, Entry 3)
Following the general procedure, 2.8 mg (0.004 mmol) of PdCl2(PPh3)2, 255.7 mg (1.0 mmol) of N-(4-chlorophenyl)-2-ethynylbenzamide (6), 234.0 mg (1.0 mmol) of 4-iodoanisole (2b), 1.5 mL of Et3N, and 4 mL of CH2Cl2 were put in the autoclave charged with 20 atm of CO. The resulting mixture was stirred for 4 h at 100 °C. The crude product was purified through column chromatography (neutral Al2O3, n-hexane/AcOEt 3:1), obtaining 269 mg (yield 69%) of (E)-2-(4-chlorophenyl)-3-(2-(4-methoxyphenyl)-2-oxoethylidene)isoindolin-1-one [(E)-7b] and 39 mg (yield 10%) of (Z)-2-(4-chlorophenyl)-3-(2-(4-methoxyphenyl)-2-oxoethylidene)isoindolin-1-one [(Z)-7b].
(E)-7b. 1H NMR (600 MHz, CDCl3) δ (ppm): 3.85 (3H, s), 6.49 (1H, s), 6.91 (2H, d, J = 9.0 Hz), 7.36 (2H, d, J = 8.7 Hz), 7.56 (2H, d, J = 8.7 Hz), 7.63–7.65 (1H, m), 7.68–7.71 (1H, m), 7.83 (2H, d, J = 9.0 Hz), 7.94 (1H, d, J = 7.2 Hz), 8.86 (1H, d, J = 7.8 Hz). 13C NMR (150 MHz, CDCl3) δ (ppm): 55.5, 105.7, 113.8 (2C), 123.6, 127.38, 129.5, 130.1 (2C), 130.1 (2C), 130.6 (2C), 131.7, 131.7, 132.5, 133.6, 133.9, 135.0, 148.4, 163.5, 166.8, 188.3. LC–MS (APCI+) m/z: 390.1 [M + H]+. Anal. Calcd for C23H16ClNO3: C, 70.86; H, 4.14; N, 3.59. Found: C, 70.75; H, 4.21; N, 3.59.
(Z)-7b. 1H NMR (600 MHz, CDCl3) δ (ppm): 3.86 (3H, s), 6.58 (1H, s), 6.84 (2H, d, J = 9.0 Hz), 7.00 (2H, d, J = 8.7 Hz), 7.15 (2H, d, J = 8.7 Hz), 7.62 (2H, d, J = 9.0 Hz), 7.66 (1H, d, J = 7.2 Hz), 7.72–7.74 (1H, m), 7.86 (1H, d, J = 7.8 Hz), 7.95 (1H, d, J = 7.8 Hz). 13C NMR (150 MHz, CDCl3) δ (ppm): 55.5, 102.1, 113.5 (2C), 120.2, 124.2, 127.9, 128.6 (2C), 128.8 (2C), 130.8 (2C), 131.0, 131.1, 133.1, 133.3, 134.3, 137.2, 142.0, 163.6, 167.5, 189.8. LC–MS (APCI+) m/z: 390.1 [M + H]+. Anal. Calcd for C23H16ClNO3: C, 70.86; H, 4.14; N, 3.59. Found: C, 70.78; H, 4.22; N, 3.59.
Cyclocarbonylative Sonogashira of N-(4-Chlorophenyl)-2-ethynylbenzamide (6) and 2-Iodoanisole (2c) (Table 4, Entry 4)
Following the general procedure, 2.8 mg (0.004 mmol) of PdCl2(PPh3)2, 255.7 mg (1.0 mmol) of N-(4-chlorophenyl)-2-ethynylbenzamide (6), 234.0 mg (1.0 mmol) of 2-iodoanisole (2c), 1.5 mL of Et3N, and 4 mL of CH2Cl2 were put in the autoclave charged with 20 atm of CO. The resulting mixture was stirred for 4 h at 100 °C. The crude product was purified through column chromatography (neutral Al2O3, n-hexane/AcOEt 3:1), obtaining 289 mg (yield 74%) of (E)-2-(4-chlorophenyl)-3-(2-(2-methoxyphenyl)-2-oxoethylidene)isoindolin-1-one [(E)-7c] and 12 mg (yield 3%) of (Z)-2-(4-chlorophenyl)-3-(2-(2-methoxyphenyl)-2-oxoethylidene)isoindolin-1-one [(Z)-7c].
(E)-7c. 1H NMR (600 MHz, CDCl3) δ (ppm): 3.72 (3H, s), 6.63 (1H, s), 6.91 (1H, d, J = 8.4 Hz), 7.01–7.04 (1H, m), 7.33 (2H, d, J = 8.7 Hz), 7.44–7.47 (1H, m), 7.53 (2H, d, J = 8.7 Hz), 7.66–7.68 (1H, m), 7.73–7.77 (2H, m), 7.96 (1H, d, J = 7.8 Hz), 9.15 (1H, d, J = 8.4 Hz). 13C NMR (150 MHz, CDCl3) δ (ppm): 55.3, 110.5, 111.6, 120.8, 123.6, 127.5, 129.5, 129.6, 129.7 (2C), 130.3 (2C), 130.8, 131.7, 132.7, 133.6, 133.7, 133.9, 134.8, 147.6, 158.1, 167.0, 189.7. LC–MS (APCI+) m/z: 390.1 [M + H]+. Anal. Calcd for C23H16ClNO3: C, 70.86; H, 4.14; N, 3.59. Found: C, 70.77; H, 4.20; N, 3.59.
(Z)-7c. 1H NMR (600 MHz, CDCl3) δ (ppm): 3.89 (3H, s), 6.77 (1H, s), 6.92 (2H, t, J = 7.8 Hz), 7.13 (2H, d, J = 8.7 Hz), 7.25 (2H, d, J = 8.7 Hz), 7.38–7.40 (2H, m), 7.62–7.65 (1H, m), 7.69–7.72 (1H, m), 7.82 (1H, d, J = 7.8 Hz), 7.94 (1H, d, J = 7.8 Hz).
Cyclocarbonylative Sonogashira of N-(4-Chlorophenyl)-2-ethynylbenzamide (6) and 1-Chloro-4-iodobenzene (2d) with 20 atm of CO (Table 4, Entry 5)
Following the general procedure, 2.8 mg (0.004 mmol) of PdCl2(PPh3)2, 255.7 mg (1.0 mmol) of N-(4-chlorophenyl)-2-ethynylbenzamide (6), 238.5 mg (1.0 mmol) of 1-chloro-4-iodobenzene (2d), 1.5 mL of Et3N, and 4 mL of CH2Cl2 were put in the autoclave charged with 20 atm of CO. The resulting mixture was stirred for 4 h at 100 °C. The crude product was purified through column chromatography (neutral Al2O3, n-hexane/AcOEt 3:1), obtaining 201 mg (yield 51%) of (E)-2-(4-chlorophenyl)-3-(2-(4-chlorophenyl)-2-oxoethylidene)isoindolin-1-one [(E)-7d], 28 mg (yield 7%) of (Z)-2-(4-chlorophenyl)-3-(2-(4-chlorophenyl)-2-oxoethylidene)isoindolin-1-one [(Z)-7d], and 11 mg (yield 3%) of (Z)-3-(4-chlorobenzylidene)-2-(4-chlorophenyl)isoindolin-1-one (8).
(E)-7d. 1H NMR (600 MHz, CDCl3) δ (ppm): 6.45 (1H, s), 7.35 (2H, d, J = 9.0 Hz), 7.42 (2H, d, J = 8.4 Hz), 7.57 (2H, d, J = 8.4 Hz), 7.68 (1H, t, J = 7.8 Hz), 7.72–7.77 (3H, m), 7.96 (1H, d, J = 7.2 Hz), 8.94 (1H, d, J = 7.8 Hz). 13C NMR (150 MHz, CDCl3) δ (ppm): 104.7, 123.8, 127.5, 128.9 (2C), 129.6 (2C), 130.1 (2C), 130.2 (2C), 132.1, 132.3, 133.7, 133.8, 134.5, 135.2, 137.2, 139.5, 149.9, 166.9, 188.3. LC–MS (APCI+) m/z: 394.1 [M + H]+. Anal. Calcd for C22H13Cl2NO2: C, 67.02; H, 3.32; N, 3.55. Found: C, 67.08; H, 3.35; N, 3.55.
(Z)-7d. 1H NMR (600 MHz, CDCl3) δ (ppm): 6.56 (1H, s), 7.00 (2H, d, J = 8.4 Hz), 7.18 (2H, d, J = 8.4 Hz), 7.34 (2H, d, J = 8.4 Hz), 7.58 (2H, d, J = 8.4 Hz), 7.67 (1H, t, J = 7.2 Hz), 7.75 (1H, t, J = 7.2 Hz), 7.87 (1H, d, J = 7.2 Hz), 7.96 (1H, d, J = 7.8 Hz). 13C NMR (150 MHz, CDCl3) δ (ppm): 100.8, 120.3, 124.4, 127.8, 128.4 (2C), 128.6, 128.7 (2C), 129.0 (2C), 129.7 (2C), 131.4, 133.3, 133.5, 134.4, 136.3, 137.1, 139.5, 143.5, 189.8. LC–MS (APCI+) m/z: 394.1 [M + H]+. Anal. Calcd for C22H13Cl2NO2: C, 67.02; H, 3.32; N, 3.55. Found: C, 67.10; H, 3.37; N, 3.55.
8. 1H NMR (600 MHz, CDCl3) δ (ppm): 6.75 (1H, s), 6.80 (2H, d, J = 8.4 Hz), 6.97 (2H, d, J = 8.4 Hz), 7.01 (2H, d, J = 8.7 Hz), 7.11 (2H, d, J = 8.7 Hz), 7.57 (1H, t, J = 7.2 Hz), 7.69 (1H, t, J = 7.2 Hz), 7.84 (1H, d, J = 7.8 Hz), 7.95 (1H, d, J = 7.8 Hz). 13C NMR (150 MHz, CDCl3) δ (ppm): 106.1, 110.6, 119.4, 124.0, 127.4, 127.5 (2C), 128.3 (2C), 128.4 (2C), 129.5, 129.8, 130.3 (2C), 130.7, 132.7, 134.2, 134.7, 138.3, 167.7. LC–MS (APCI+) m/z: 366.0 [M + H]+. Anal. Calcd for C21H13Cl2NO: C, 68.87; H, 3.58; N, 3.82. Found: C, 68.81; H, 3.54; N, 3.81.
Cyclocarbonylative Sonogashira of N-(4-Chlorophenyl)-2-ethynylbenzamide (6) and 1-Chloro-4-iodobenzene (2d) with 40 atm of CO (Table 4, Entry 6)
Following the general procedure, 2.8 mg (0.004 mmol) of PdCl2(PPh3)2, 255.7 mg (1.0 mmol) of N-(4-chlorophenyl)-2-ethynylbenzamide (6), 238.5 mg (1.0 mmol) of 1-chloro-4-iodobenzene (2d), 1.5 mL of Et3N, and 4 mL of CH2Cl2 were put in the autoclave charged with 40 atm of CO. The resulting mixture was stirred for 4 h at 100 °C. The composition of the crude product was determined by 1H NMR analysis, resulting in a mixture of (E)-2-(4-chlorophenyl)-3-(2-(4-chlorophenyl)-2-oxoethylidene)isoindolin-1-one [(E)-7d], (Z)-2-(4-chlorophenyl)-3-(2-(4-chlorophenyl)-2-oxoethylidene)isoindolin-1-one [(Z)-7d], and (Z)-3-(4-chlorobenzylidene)-2-(4-chlorophenyl)isoindolin-1-one (8) in the molar ratio 86/10/4.
Cyclocarbonylative Sonogashira of N-(4-Chlorophenyl)-2-ethynylbenzamide (6) and 1-Iodonaphthalene (2f) (Table 4, Entry 7)
Following the general procedure, 2.8 mg (0.004 mmol) of PdCl2(PPh3)2, 255.7 mg (1.0 mmol) of N-(4-chlorophenyl)-2-ethynylbenzamide (6), 254.1 mg (1.0 mmol) of 1-iodonaphthalene (2f), 1.5 mL of Et3N, and 4 mL of CH2Cl2 were put in the autoclave charged with 20 atm of CO. The resulting mixture was stirred for 4 h at 100 °C. The crude product was purified through column chromatography (neutral Al2O3, n-hexane/AcOEt 6:1), obtaining 283 mg (yield 69%) of (E)-2-(4-chlorophenyl)-3-(2-(naphthalen-1-yl)-2-oxoethylidene)isoindolin-1-one [(E)-7f] and 21 mg (yield 5%) of (Z)-2-(4-chlorophenyl)-3-(2-(naphthalen-1-yl)-2-oxoethylidene)isoindolin-1-one [(Z)-7f].
(E)-7f. 1H NMR (600 MHz, CDCl3) δ (ppm): 6.41 (1H, s), 7.33 (2H, d, J = 8.7 Hz), 7.46 (1H, t, J = 7.8 Hz), 7.49 (2H, d, J = 8.7 Hz), 7.54 (1H, t, J = 7.2 Hz), 7.59 (1H, t, J = 7.2 Hz), 7.69–7.72 (2H, m), 7.76 (1H, t, J = 7.8 Hz), 7.89 (1H, d, J = 8.4 Hz), 7.96–8.00 (2H, m), 8.56 (1H, d, J = 8.4 Hz), 9.11 (1H, d, J = 7.8 Hz). 13C NMR (150 MHz, CDCl3) δ (ppm): 109.0, 123.7, 124.4, 125.4, 126.5, 127.6, 127.7, 127.7, 128.5, 129.1, 129.5, 130.0 (4C), 130.1, 132.0, 132.2, 132.6, 133.8, 133.8, 135.0, 137.7, 149.1, 166.9, 192.9. LC–MS (APCI+) m/z: 410.0 [M + H]+. Anal. Calcd for C26H16ClNO2: C, 76.19; H, 3.93; N, 3.42. Found: C, 76.07; H, 3.99; N, 3.43.
(Z)-7f. 1H NMR (600 MHz, CDCl3) δ (ppm): 6.62 (1H, s), 6.84 (2H, d, J = 8.4 Hz), 6.88 (2H, d, J = 8.4 Hz), 7.40 (1H, t, J = 7.8 Hz), 7.49–7.54 (2H, m), 7.68 (1H, t, J = 7.8 Hz), 7.76 (1H, t, J = 7.8 Hz), 7.79–7.82 (2H, m), 7.89 (1H, d, J = 7.2 Hz), 7.95 (2H, t, J = 6.6 Hz), 8.32–8.33 (1H, m). 13C NMR (150 MHz, CDCl3) δ (ppm): 104.3 120.4, 124.0, 124.3, 125.6, 126.7, 127.5 (2C), 127.7, 127.9, 128.0, 129.1 (2C), 130.1, 130.1, 131.3, 133.0, 133.3, 133.5, 133.9, 135.5, 137.4, 142.8, 167.6, 193.5. LC–MS (APCI+) m/z: 410.0 [M + H]+. Anal. Calcd for C26H16ClNO2: C, 76.19; H, 3.93; N, 3.42. Found: C, 76.11; H, 3.97; N, 3.43.
Cyclocarbonylative Sonogashira of N-(4-Chlorophenyl)-2-ethynylbenzamide (6) and 4-Iodotoluene (2g) (Table 4, Entry 8)
Following the general procedure, 2.8 mg (0.004 mmol) of PdCl2(PPh3)2, 255.7 mg (1.0 mmol) of N-(4-chlorophenyl)-2-ethynylbenzamide (6), 92.1 mg (1.0 mmol) of 4-iodotoluene (2g), 1.5 mL of Et3N, and 4 mL of CH2Cl2 were put in the autoclave charged with 20 atm of CO. The resulting mixture was stirred for 4 h at 100 °C. The crude product was purified through column chromatography (neutral Al2O3, n-hexane/AcOEt 3:1), obtaining 251 mg (yield 67%) of (E)-2-(4-chlorophenyl)-3-(2-oxo-2-(p-tolyl)ethylidene)isoindolin-1-one [(E)-7g] and 26 mg (yield 7%) of (Z)-2-(4-chlorophenyl)-3-(2-oxo-2-(p-tolyl)ethylidene)isoindolin-1-one [(Z)-7g].
(E)-7g. 1H NMR (600 MHz, CDCl3) δ (ppm): 2.39 (3H, s), 6.51 (1H, s), 7.24 (2H, d, J = 8.4 Hz), 7.36 (2H, d, J = 8.4 Hz), 7.56 (2H, d, J = 8.4 Hz), 7.65 (1H, t, J = 7.8 Hz), 7.70 (1H, t, J = 7.8 Hz), 7.74 (2H, d, J = 8.4 Hz), 7.94 (1H, d, J = 7.2 Hz), 8.91 (1H, d, J = 7.8 Hz). 13C NMR (150 MHz, CDCl3) δ (ppm): 21.6, 105.6, 123.6, 127.4, 128.4 (2C), 129.3 (2C), 129.5, 130.1 (2C), 130.1 (2C), 131.8, 132.5, 133.6, 133.9, 135.0, 136.4, 143.9, 148.8, 166.9, 189.4. LC–MS (APCI+) m/z: 374.1 [M + H]+. Anal. Calcd for C23H16ClNO2: C, 73.90; H, 4.31; N, 3.75. Found: C, 74.01; H, 4.25; N, 3.76.
(Z)-7g. 1H NMR (600 MHz, CDCl3) δ (ppm): 2.40 (3H, s), 6.61 (1H, s), 7.01 (2H, d, J = 9.0 Hz), 7.15 (2H, d, J = 9.0 Hz), 7.17 (2H, d, J = 8.1 Hz), 7.56 (2H, d, J = 8.1 Hz), 7.66 (1H, t, J = 7.2 Hz), 7.74 (1H, t, J = 7.8 Hz), 7.87 (1H, d, J = 7.8 Hz), 7.96 (1H, d, J = 7.8 Hz). 13C NMR (150 MHz, CDCl3) δ (ppm): 21.7, 101.8, 120.2, 124.3, 127.9, 128.5 (2C), 128.6 (2C), 128.9 (2C), 129.0 (2C), 131.1, 133.1, 133.3, 134.4, 135.5, 137.3, 142.6, 144.0, 167.5, 190.7. LC–MS (APCI+) m/z: 374.1 [M + H]+. Anal. Calcd for C23H16ClNO2: C, 73.90; H, 4.31; N, 3.75. Found: C, 73.99; H, 4.23; N, 3.76.
Cyclocarbonylative Sonogashira of N-(4-Chlorophenyl)-2-ethynylbenzamide (6) and 2-Iodotoluene (2h) (Table 4, Entry 9)
Following the general procedure, 2.8 mg (0.004 mmol) of PdCl2(PPh3)2, 255.7 mg (1.0 mmol) of N-(4-chlorophenyl)-2-ethynylbenzamide (6), 92.1 mg (1.0 mmol) of 2-iodotoluene (2h), 1.5 mL of Et3N, and 4 mL of CH2Cl2 were put in the autoclave charged with 20 atm of CO. The resulting mixture was stirred for 4 h at 100 °C. The crude product was purified through column chromatography (neutral Al2O3, n-hexane/AcOEt 3:1), obtaining 225 mg (yield 60%) of (E)-2-(4-chlorophenyl)-3-(2-oxo-2-(o-tolyl)ethylidene)isoindolin-1-one [(E)-7h] and 19 mg (yield 5%) of (Z)-2-(4-chlorophenyl)-3-(2-oxo-2-(o-tolyl)ethylidene)isoindolin-1-one [(Z)-7h].
(E)-7h. 1H NMR (600 MHz, CDCl3) δ (ppm): 2.51 (3H, s), 6.24 (1H, s), 7.20–7.26 (2H, m), 7.31 (2H, d, J = 8.4 Hz), 7.36 (1H, t, J = 7.8 Hz), 7.45 (1H, d, J = 7.2 Hz), 7.52 (2H, d, J = 8.4 Hz), 7.69 (1H, t, J = 7.8 Hz), 7.75 (1H, t, J = 7.2 Hz), 7.97 (1H, d, J = 7.2 Hz), 9.00 (1H, d, J = 7.8 Hz). 13C NMR (150 MHz, CDCl3) δ (ppm): 20.7, 108.7, 123.7, 125.8, 127.5, 128.5, 129.6, 130.1 (2C), 130.1 (2C), 131.2, 131.7, 132.0, 132.3, 133.8, 133.9, 135.1, 137.5, 1140.0, 148.8, 166.9, 193.4. LC–MS (APCI+) m/z: 374.1 [M + H]+. Anal. Calcd for C23H16ClNO2: C, 73.90; H, 4.31; N, 3.75. Found: C, 74.00; H, 4.24; N, 3.76.
(Z)-7h. 1H NMR (600 MHz, CDCl3) δ (ppm): 2.24 (3H, s), 6.47 (1H, s), 6.96 (2H, d, J = 9.0 Hz), 7.15 (2H, d, J = 9.0 Hz), 7.18–7.20 (2H, m), 7.56–7.62 (2H, m), 7.66 (1H, t, J = 7.2 Hz), 7.74 (1H, t, J = 7.8 Hz), 7.85 (1H, d, J = 7.8 Hz), 7.95 (1H, d, J = 7.8 Hz).
Cyclocarbonylative Sonogashira of N-(4-Chlorophenyl)-2-ethynylbenzamide (6) and 4-Iodobenzonitrile (2e) (Scheme S4)
Following the general procedure, 2.8 mg (0.004 mmol) of PdCl2(PPh3)2, 255.7 mg (1.0 mmol) of N-(4-chlorophenyl)-2-ethynylbenzamide (6), 229.0 mg (1.0 mmol) of 4-iodobenzonitrile (2e), 1.5 mL of Et3N, and 4 mL of CH2Cl2 were put in the autoclave charged with 20 atm of CO. The resulting mixture was stirred for 4 h at 100 °C. The crude product was purified through column chromatography (neutral Al2O3, n-hexane/AcOEt 3:1), obtaining 70 mg (yield 18%) of (E)-4-(2-(2-(4-chlorophenyl)-3-oxoisoindolin-1-ylidene)acetyl)benzonitrile [(E)-7e], 20 mg (yield 5%) of (Z)-4-(2-(2-(4-chlorophenyl)-3-oxoisoindolin-1-ylidene)acetyl)benzonitrile [(Z)-7e], and 15 mg (yield 4%) of (Z)-4-((2-(4-chlorophenyl)-3-oxoisoindolin-1-ylidene)methyl)benzonitrile (9).
(E)-7e. 1H NMR (600 MHz, CDCl3) δ (ppm): 6.45 (1H, s), 7.35 (2H, d, J = 8.4 Hz), 7.59 (2H, d, J = 8.4 Hz), 7.72–7.80 (4H, m), 7.90 (2H, d, J = 8.4 Hz), 8.00 (1H, d, J = 7.8 Hz), 9.04 (1H, d, J = 7.8 Hz). 13C NMR (150 MHz, CDCl3) δ (ppm): 103.7, 116.0, 117.9, 123.9, 127.7, 128.5 (2C), 129.4, 130.0 (2C), 130.3 (2C), 132.1, 132.5 (2C), 132.6, 133.6, 134.0, 135.4, 142.3, 151.3, 166.9, 187.8. LC–MS (APCI+) m/z: 385.2 [M + H]+. Anal. Calcd for C23H13ClN2O2: C, 71.79; H, 3.41; N, 7.28. Found: C, 71.71; H, 3.46; N, 7.27.
(Z)-7e. 1H NMR (600 MHz, CDCl3) δ (ppm): 6.57 (1H, s), 7.00 (2H, d, J = 8.4 Hz), 7.20 (2H, d, J = 8.4 Hz), 7.67 (2H, d, J = 8.4 Hz), 7.70–7.78 (4H, m), 7.88 (1H, d, J = 7.2 Hz), 7.97 (1H, d, J = 7.2 Hz).
9. 1H NMR (600 MHz, CDCl3) δ (ppm): 6.75 (1H, s), 6.97 (2H, d, J = 8.4 Hz), 7.00 (2H, d, J = 8.7 Hz), 7.11 (2H, d, J = 8.7 Hz), 7.27 (2H, d, J = 8.4 Hz), 7.61 (1H, t, J = 7.8 Hz), 7.72 (1H, t, J = 7.2 Hz), 7.86 (1H, d, J = 7.8 Hz), 7.96 (1H, d, J = 7.8 Hz). 13C NMR (150 MHz, CDCl3) δ (ppm): 104.7, 110.1, 118.5, 119.6, 124.2, 127.5, 128.3 (2C), 128.6 (2C), 129.6 (2C), 130.1, 130.9 (2C), 132.0, 133.1, 134.1, 136.5, 138.0, 138.4, 167.6. LC–MS (APCI+) m/z: 357.0 [M + H]+. Anal. Calcd for C22H13ClN2O: C, 74.06; H, 3.67; N, 7.85. Found: C, 73.97; H, 3.74; N, 7.86.
Cyclocarbonylative Sonogashira of N-(4-Chlorophenyl)-2-ethynylbenzamide (6) and 4-Bromonitrobenzene (2i) (Scheme 4)
Following the general procedure, 2.8 mg (0.004 mmol) of PdCl2(PPh3)2, 255.7 mg (1.0 mmol) of N-(4-chlorophenyl)-2-ethynylbenzamide (6), 202.0 mg (1.0 mmol) of 4-bromonitrobenzene (2i), 1.5 mL of Et3N, and 4 mL of CH2Cl2 were put in the autoclave charged with 20 atm of CO. The resulting mixture was stirred for 4 h at 100 °C. The crude product was purified through column chromatography (SiO2, n-hexane/AcOEt 9:1), obtaining 218 mg (yield 85%) of 2-(4-chlorophenyl)-3-methyleneisoindolin-1-one (10).45
1H NMR (600 MHz, CDCl3) δ (ppm): 4.83 (1H, d, J = 2.4 Hz), 5.28 (1H, d, J = 2.4 Hz), 7.36 (2H, d, J = 9.0 Hz), 7.51 (2H, d, J = 9.0 Hz), 7.58–7.61 (1H, m), 7.67–7.69 (1H, m), 7.79 (1H, d, J = 7.6 Hz), 7.94 (1H, d, J = 7.6 Hz). 13C NMR (150 MHz, CDCl3) δ (ppm): 90.4, 120.1, 123.6, 129.3 (2C), 129.6 (2C), 129.9, 132.5, 133.1, 133.8, 135.0, 136.2, 142.8, 166.5. LC–MS (APCI+) m/z: 256.0 [M + H]+. Anal. Calcd for C15H10ClNO: C, 70.46; H, 3.94; N, 5.48. Found: 70.54; H, 3.88; N, 5.47.
Cyclic Sonogashira Reaction of N-(4-Chlorophenyl)-2-ethynylbenzamide (6) and 1-Chloro-4-iodobenzene (2d)
In a 25 mL Carius tube sealed with a Teflon valve, N-(4-chlorophenyl)-2-ethynylbenzamide (6) (255.7 mg, 1.0 mmol), 1-chloro-4-iodobenzene (2d) (238.5 mg, 1.0 mmol), PdCl2(PPh3)2 (2.8 mg, 0.004 mmol), Et3N (1.5 mL), and CH2Cl2 (4 mL) were mixed together. The resulting mixture was left under stirring for 4 h at 100 °C, then it was hydrolyzed with H2O (20 mL) and extracted with CH2Cl2 (3 × 30 mL). The combined organic phases were washed with brine (50 mL), dried over anhydrous Na2SO4 and the solvent was removed under vacuum. The crude product was purified by column chromatography (neutral Al2O3, n-hexane/AcOEt 6:1), to give 319 mg (yield 87%) of (Z)-3-(4-chlorobenzylidene)-2-(4-chlorophenyl)isoindolin-1-one (8).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.0c01282.
Supplementary schemes, 1H NMR and 13C NMR spectra for all the pure compounds (PDF)
Author Present Address
∥ Dipartimento di Chimica, Università degli Studi di Bari “Aldo Moro”, Via Edoardo Orabona 4, 70126 Bari, Italy.
The authors declare no competing financial interest.
Supplementary Material
References
- Leyte-Lugo M.; Britton E. R.; Foil D. H.; Brown A. R.; Todd D. A.; Rivera-Chávez J.; Oberlies N. H.; Cech N. B. Secondary metabolites from the leaves of the medicinal plant goldenseal (Hydrastis canadensis). Phytochem. Lett. 2017, 20, 54. 10.1016/j.phytol.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comins D. L.; Schilling S.; Zhang Y. Asymmetric Synthesis of 3-Substituted Isoindolinones: Application to the Total Synthesis of (+)-Lennoxamine. Org. Lett. 2005, 7, 95. 10.1021/ol047824w. [DOI] [PubMed] [Google Scholar]
- Valencia E.; Freyer A. J.; Shamma M.; Fajardo V. (±)-Nuevamine, an isoindoloisoquinoline alkaloid, and (±)-lennoxamine, an isoindolobenzazepine. Tetrahedron Lett. 1984, 25, 599. 10.1016/s0040-4039(00)99948-9. [DOI] [Google Scholar]
- Narmani A.; Teponno R. B.; Helaly S. E.; Arzanlou M.; Stadler M. Cytotoxic, anti-biofilm and antimicrobial polyketides from the plant associated fungus Chaetosphaeronema achilleae. Fitoterapia 2019, 139, 104390. 10.1016/j.fitote.2019.104390. [DOI] [PubMed] [Google Scholar]
- Zhao J.; Liu J.; Shen Y.; Tan Z.; Zhang M.; Chen R.; Zhao J.; Zhang D.; Yu L.; Dai J. Stachybotrysams A–E, prenylated isoindolinone derivatives with anti-HIV activity from the fungus Stachybotrys chartarum. Phytochem. Lett. 2017, 20, 289. 10.1016/j.phytol.2017.04.031. [DOI] [Google Scholar]
- Wang K.; Bao L.; Qi Q.; Zhao F.; Ma K.; Pei Y.; Liu H. Erinacerins C–L, Isoindolin-1-ones with α-Glucosidase Inhibitory Activity from Cultures of the Medicinal Mushroom Hericium erinaceus. J. Nat. Prod. 2015, 78, 146. 10.1021/np5004388. [DOI] [PubMed] [Google Scholar]
- Chen S.; Liu Z.; Liu Y.; Lu Y.; He L.; She Z. New depsidones and isoindolinones from the mangrove endophytic fungus Meyerozyma guilliermondii (HZ-Y2) isolated from the South China Sea. Beilstein J. Org. Chem. 2015, 11, 1187. 10.3762/bjoc.11.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.; Li Z.-H.; Yao J.-N.; Peng Y.-L.; Huang R.; Feng T.; Liu J.-K. Isoindolinone-containing meroterpenoids with α-glucosidase inhibitory activity from mushroom Hericium caput-medusae. Fitoterapia 2017, 122, 107. 10.1016/j.fitote.2017.08.017. [DOI] [PubMed] [Google Scholar]
- Fazal Hussainz S.; Shamma M. The catabolism of phthalideisoquinoline alkaloids. Tetrahedron Lett. 1980, 21, 1693. 10.1016/s0040-4039(00)77812-9. [DOI] [Google Scholar]
- Lamblin M.; Couture A.; Deniau E.; Grandclaudon P. A concise first total synthesis of narceine imide. Org. Biomol. Chem. 2007, 5, 1466. 10.1039/b701661a. [DOI] [PubMed] [Google Scholar]
- Chia Y.-C.; Chang F.-R.; Teng C.-M.; Wu Y.-C. Aristolactams and Dioxoaporphines from Fissistigma balansae and Fissistigma oldhamii. J. Nat. Prod. 2000, 63, 1160. 10.1021/np000063v. [DOI] [PubMed] [Google Scholar]
- Valencia E.; Fajardo V.; Freyer A. J.; Shamma M. Magallanesine: an isoindolobenzazocine alkaloid. Tetrahedron Lett. 1985, 26, 993. 10.1016/s0040-4039(00)98494-6. [DOI] [Google Scholar]
- Li Y.; Wu C.; Liu D.; Proksch P.; Guo P.; Lin W. Chartarlactams A–P, Phenylspirodrimanes from the Sponge-Associated Fungus Stachybotrys chartarum with Antihyperlipidemic Activities. J. Nat. Prod. 2014, 77, 138. 10.1021/np400824u. [DOI] [PubMed] [Google Scholar]
- Moreau A.; Couture A.; Deniau E.; Grandclaudon P. A New Route to Aristocularine Alkaloids: Total Synthesis of Aristoyagonine. J. Org. Chem. 2004, 69, 4527. 10.1021/jo049869g. [DOI] [PubMed] [Google Scholar]
- Kumar V.; Poonam; Prasad A. K.; Parmar V. S. Naturally occurring aristolactams, aristolochic acids and dioxoaporphines and their biological activities. Nat. Prod. Rep. 2003, 20, 565. 10.1039/b303648k. [DOI] [PubMed] [Google Scholar]
- Hatoum F.; Engler J.; Zelmer C.; Wißen J.; Motti C. A.; Lex J.; Oelgemöller M. Photodecarboxylative addition of carboxylates to phthalimides: a concise access to biologically active 3-(alkyl and aryl)methylene-1H-isoindolin-1-ones. Tetrahedron Lett. 2012, 53, 5573. 10.1016/j.tetlet.2012.07.142. [DOI] [Google Scholar]
- Mata R.; Morales I.; Pérez O.; Rivero-Cruz I.; Acevedo L.; Enriquez-Mendoza I.; Bye R.; Franzblau S.; Timmermann B. Antimycobacterial Compounds from Piper sanctum. J. Nat. Prod. 2004, 67, 1961. 10.1021/np0401260. [DOI] [PubMed] [Google Scholar]
- Tabopda T. K.; Ngoupayo J.; Liu J.; Mitaine-Offer A.-C.; Tanoli S. A. K.; Khan S. N.; Ali M. S.; Ngadjui B. T.; Tsamo E.; Lacaille-Dubois M.-A.; Luu B. Bioactive aristolactams from Piper umbellatum. Phytochemistry 2008, 69, 1726. 10.1016/j.phytochem.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Chen Y.-C.; Chen J.-J.; Chang Y.-L.; Teng C.-M.; Lin W.-Y.; Wu C.-C.; Chen I.-S. A New Aristolactam Alkaloid and Anti-Platelet Aggregation Constituents from Piper taiwanense. Planta Med. 2004, 70, 174. 10.1055/s-2004-815497. [DOI] [PubMed] [Google Scholar]
- Lan Y.-H.; Chia Y.-C.; Chang F.-R.; Hwang T.-L.; Liaw C.-C.; Wu Y.-C. Potential Anti-Inflammatory Activities of Bractelactone and other Compounds Isolated from Fissistigma bracteolatum. Helv. Chim. Acta 2005, 88, 905. 10.1002/hlca.200590068. [DOI] [Google Scholar]
- Zhang Y.-N.; Zhong X.-G.; Zheng Z.-P.; Hu X.-D.; Zuo J.-P.; Hu L.-H. Discovery and synthesis of new immunosuppressive alkaloids from the stem of Fissistigma oldhamii (Hemsl.) Merr.. Bioorg. Med. Chem. 2007, 15, 988. 10.1016/j.bmc.2006.10.034. [DOI] [PubMed] [Google Scholar]
- Ge Y.-W.; Zhu S.; Shang M.-Y.; Zang X.-Y.; Wang X.; Bai Y.-J.; Li L.; Komatsu K.; Cai S.-Q. Aristololactams and aporphines from the stems of Fissistigma oldhamii (Annonaceae). Phytochemistry 2013, 86, 201. 10.1016/j.phytochem.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Kim S. R.; Sung S. H.; Kang S. Y.; Koo K. A.; Kim S. H.; Ma C. J.; Lee H.-S.; Park M. J.; Kim Y. C. Aristolactam BII of Saururus chinensis Attenuates Glutamate-Induced Neurotoxicity in Rat Cortical Cultures Probably by Inhibiting Nitric Oxide Production. Planta Med. 2004, 70, 391. 10.1055/s-2004-818964. [DOI] [PubMed] [Google Scholar]
- Kato Y.; Takemoto M.; Achiwa K. Prostanoids and Related Compounds. VI. Synthesis of Isoindolinone Derivatives Possessing Inhibitory Activity for Thromboxane A2 Analog (U-46619)-Induced Vasoconstriction. Chem. Pharm. Bull. 1993, 41, 2003. 10.1248/cpb.41.2003. [DOI] [PubMed] [Google Scholar]
- Kato Y.; Takemoto M.; Achiwa K. Prostanoids and Related Compounds. VII. Synthesis and Inhibitory Activity of 1-Isoindolinone Derivatives Possessing Inhibitory Activity against Thromboxane A2 Analog (U-46619)-Induced Vasoconstriction. Chem. Pharm. Bull. 1999, 47, 529. 10.1248/cpb.47.529. [DOI] [PubMed] [Google Scholar]
- Couture A.; Deniau E.; Grandclaudon P.; Rybalko-Rosen H.; Léonce S.; Pfeiffer B.; Renard P. Synthesis and biological evaluation of aristolactams. Bioorg. Med. Chem. Lett. 2002, 12, 3557. 10.1016/s0960-894x(02)00794-1. [DOI] [PubMed] [Google Scholar]
- Liu J.-J.; Dermatakis A.; Lukacs C.; Konzelmann F.; Chen Y.; Kammlott U.; Depinto W.; Yang H.; Yin X.; Chen Y.; Schutt A.; Simcox M. E.; Luk K.-C. 3,5,6-Trisubstituted naphthostyrils as CDK2 inhibitors. Bioorg. Med. Chem. Lett. 2003, 13, 2465. 10.1016/s0960-894x(03)00488-8. [DOI] [PubMed] [Google Scholar]
- Choi Y. L.; Kim J. K.; Choi S.-U.; Min Y.-K.; Bae M.-A.; Kim B. T.; Heo J.-N. Synthesis of aristolactam analogues and evaluation of their antitumor activity. Bioorg. Med. Chem. Lett. 2009, 19, 3036. 10.1016/j.bmcl.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Nayyatip S.; Thaichana P.; Buayairaksa M.; Tuntiwechapikul W.; Meepowpan P.; Nuntasaen N.; Pompimon W. Aristolactam-Type Alkaloids from Orophea enterocarpa and Their Cytotoxicities. Int. J. Mol. Sci. 2012, 13, 5010. 10.3390/ijms13045010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.; Zhao S.; Li H.; Wang X.; Geng A.; Cui H.; Lu T.; Chen Y.; Zhu Y. Design, synthesis and biological evaluation of novel isoindolinone derivatives as potent histone deacetylase inhibitors. Eur. J. Med. Chem. 2019, 168, 110. 10.1016/j.ejmech.2019.02.032. [DOI] [PubMed] [Google Scholar]
- Youn S. W. Transition-Metal-Catalyzed Annulative Coupling Cascade for the Synthesis of 3-Methyleneisoindolin-1-ones. Synthesis 2020, 52, 807. 10.1055/s-0039-1690046. [DOI] [Google Scholar]
- Kondo Y.; Shiga F.; Murata N.; Sakamoto T.; Yamanaka H. Condensed heteroaromatic ring systems. XXIV. Palladium-catalyzed cyclization of 2-substituted phenylacetylenes in the presence of carbon monoxide. Tetrahedron 1994, 50, 11803. 10.1016/s0040-4020(01)89295-4. [DOI] [Google Scholar]
- Cho C. S.; Shim H. S.; Choi H.-J.; Kim T.-J.; Shim S. C. Palladium-catalysed convenient synthesis of 3-methyleneisoindolin-1-ones. Synth. Commun. 2002, 32, 1821. 10.1081/scc-120004063. [DOI] [Google Scholar]
- Cao H.; McNamee L.; Alper H. Syntheses of Substituted 3-Methyleneisoindolin-1-ones By a Palladium-Catalyzed Sonogashira Coupling–Carbonylation–Hydroamination Sequence in Phosphonium Salt-Based Ionic liquids. Org. Lett. 2008, 10, 5281. 10.1021/ol8021403. [DOI] [PubMed] [Google Scholar]
- Marosvölgyi-Haskó D.; Takács A.; Riedl Z.; Kollár L. High-yielding synthesis of 1-isoindolinone derivatives via palladium-catalysed cycloaminocarbonylation. Tetrahedron 2011, 67, 1036. 10.1016/j.tet.2010.11.099. [DOI] [Google Scholar]
- Xuan Z.; Jung D. J.; Jeon H. J.; Lee S.-g. Pd-Catalyzed Aminocarbonylation of the Blaise Reaction Intermediate: One-Pot Synthesis of (Z)-3-Methyleneisoindolin-1-ones from Nitriles. J. Org. Chem. 2016, 81, 10094. 10.1021/acs.joc.6b02095. [DOI] [PubMed] [Google Scholar]
- Ling F.; Ai C.; Lv Y.; Zhong W. Traceless Directing Group Assisted Cobalt-Catalyzed C–H Carbonylation of Benzylamines. Adv. Synth. Catal. 2017, 359, 3707. 10.1002/adsc.201700780. [DOI] [Google Scholar]
- Fukuyama T.; Bando T.; Ryu I. Electron-Transfer-Induced Intramolecular Heck Carbonylation Reactions Leading to Benzolactones and Benzolactams. Synthesis 2018, 50, 3015. 10.1055/s-0037-1609964. [DOI] [Google Scholar]
- Zhang C.; Ding Y.; Gao Y.; Li S.; Li G. Palladium-Catalyzed Direct C–H Carbonylation of Free Primary Benzylamines: A Synthesis of Benzolactams. Org. Lett. 2018, 20, 2595. 10.1021/acs.orglett.8b00786. [DOI] [PubMed] [Google Scholar]
- Dong W.; Xu G.; Tang W. Enantioselective palladium-catalyzed C(sp2)-H carbamoylation. Tetrahedron 2019, 75, 3239. 10.1016/j.tet.2019.03.038. [DOI] [Google Scholar]
- Fu L.-Y.; Ying J.; Qi X.; Peng J.-B.; Wu X.-F. Palladium-Catalyzed Carbonylative Synthesis of Isoindolinones from Benzylamines with TFBen as the CO Source. J. Org. Chem. 2019, 84, 1421. 10.1021/acs.joc.8b02862. [DOI] [PubMed] [Google Scholar]
- Gabriele B.; Mancuso R.; Ziccarelli I.; Salerno G. A new approach to isoindolinone derivatives by sequential palladium iodide-catalyzed oxidative aminocarbonylation–heterocyclization of 2-ethynylbenzamides. Tetrahedron Lett. 2012, 53, 6694. 10.1016/j.tetlet.2012.09.109. [DOI] [Google Scholar]
- Mancuso R.; Ziccarelli I.; Armentano D.; Marino N.; Giofrè S. V.; Gabriele B. Divergent Palladium Iodide Catalyzed Multicomponent Carbonylative Approaches to Functionalized Isoindolinone and Isobenzofuranimine Derivatives. J. Org. Chem. 2014, 79, 3506. 10.1021/jo500281h. [DOI] [PubMed] [Google Scholar]
- Gao B.; Liu S.; Lan Y.; Huang H. Rhodium-Catalyzed Cyclocarbonylation of Ketimines via C–H Bond Activation. Organometallics 2016, 35, 1480. 10.1021/acs.organomet.6b00072. [DOI] [Google Scholar]
- Ju J.; Qi C.; Zheng L.; Hua R. Synthesis of 3-methyleneisoindolin-1-ones via palladium-catalyzed C–Cl bond cleavage and cyclocarbonylation of ortho-chloro ketimines. Tetrahedron Lett. 2013, 54, 5159. 10.1016/j.tetlet.2013.07.017. [DOI] [Google Scholar]
- Wang Z.; Zhu F.; Li Y.; Wu X.-F. Palladium-Catalyzed Carbonylative Synthesis of 3-Methyleneisoindolin-1-ones from Ketimines with Hexacarbonylmolybdenum(0) as the Carbon Monoxide Source. ChemCatChem 2017, 9, 94. 10.1002/cctc.201601306. [DOI] [Google Scholar]
- Xu Y.; Hu W.; Tang X.; Zhao J.; Wu W.; Jiang H. Palladium-catalyzed Csp2–H carbonylation of aromatic oximes: selective access to benzo[d][1,2]oxazin-1-ones and 3-methyleneisoindolin-1-ones. Chem. Commun. 2015, 51, 6843. 10.1039/c5cc01661d. [DOI] [PubMed] [Google Scholar]
- Aronica L. A.; Giannotti L.; Tuci G.; Zinna F. Cyclocarbonylative Sonogashira Reactions of 1-Ethynylbenzyl Alcohols: Synthesis of 1-Carbonylmethylene-1,3-Dihydroisobenzofurans. Eur. J. Org. Chem. 2015, 2015, 4944. 10.1002/ejoc.201500539. [DOI] [Google Scholar]
- Aronica L. A.; Albano G.; Giannotti L.; Meucci E. Synthesis of N-Heteroaromatic Compounds through Cyclocarbonylative Sonogashira Reactions. Eur. J. Org. Chem. 2017, 2017, 955. 10.1002/ejoc.201601392. [DOI] [Google Scholar]
- Albano G.; Morelli M.; Aronica L. A. Synthesis of Functionalised 3-Isochromanones by Silylcarbocyclisation/Desilylation Reactions. Eur. J. Org. Chem. 2017, 2017, 3473. 10.1002/ejoc.201700455. [DOI] [Google Scholar]
- Albano G.; Aronica L. A. Potentiality and Synthesis of O- and N-Heterocycles: Pd-Catalyzed Cyclocarbonylative Sonogashira Coupling as a Valuable Route to Phthalans, Isochromans, and Isoindolines. Eur. J. Org. Chem. 2017, 2017, 7204. 10.1002/ejoc.201701041. [DOI] [Google Scholar]
- Albano G.; Aronica L. A. Cyclization Reactions for the Synthesis of Phthalans and Isoindolines. Synthesis 2018, 50, 1209. 10.1055/s-0037-1609175. [DOI] [Google Scholar]
- Albano G.; Morelli M.; Lissia M.; Aronica L. A. Synthesis of Functionalised Indoline and Isoquinoline Derivatives through a Silylcarbocyclisation/Desilylation Sequence. ChemistrySelect 2019, 4, 2505. 10.1002/slct.201900524. [DOI] [Google Scholar]
- Wu X.-F.; Neumann H.; Beller M. Convenient and General Palladium-Catalyzed Carbonylative Sonogashira Coupling of Aryl Amines. Angew. Chem., Int. Ed. 2011, 50, 11142. 10.1002/anie.201104653. [DOI] [PubMed] [Google Scholar]
- Qi X.; Jiang L.-B.; Li C.-L.; Li R.; Wu X.-F. Palladium-Catalyzed One-Pot Carbonylative Sonogashira Reaction Employing Formic acid as the CO Source. Chem.—Asian J. 2015, 10, 1870. 10.1002/asia.201500518. [DOI] [PubMed] [Google Scholar]
- Wu F.-P.; Peng J.-B.; Qi X.; Wu X.-F. Palladium-catalyzed carbonylative Sonogashira coupling of aryl diazonium salts with formic acid as the CO source: the effect of 1,3-butadiene. Catal. Sci. Technol. 2017, 7, 4924. 10.1039/c7cy01773a. [DOI] [Google Scholar]
- Li Y.; Hu Y.; Wu X.-F. Non-noble metal-catalysed carbonylative transformations. Chem. Soc. Rev. 2018, 47, 172. 10.1039/c7cs00529f. [DOI] [PubMed] [Google Scholar]
- Peng J.-B.; Wu X.-F. Ligand- and Solvent-Controlled Regio- and Chemodivergent Carbonylative Reactions. Angew. Chem., Int. Ed. 2018, 57, 1152. 10.1002/anie.201709807. [DOI] [PubMed] [Google Scholar]
- Kel’in A. V. Recent Advances in the Synthesis of 1,3-Diketones. Curr. Org. Chem. 2003, 7, 1691. 10.2174/1385272033486233. [DOI] [Google Scholar]
- Kel’in A. V.; Maioli A. Recent Advances in the Chemistry of 1,3-Diketones: Structural Modifications and Synthetic Applications. Curr. Org. Chem. 2003, 7, 1855. 10.2174/1385272033486134. [DOI] [Google Scholar]
- Simon C.; Constantieux T.; Rodriguez J. Utilisation of 1,3-Dicarbonyl Derivatives in Multicomponent Reactions. Eur. J. Org. Chem. 2004, 2004, 4957. 10.1002/ejoc.200400511. [DOI] [Google Scholar]
- Sloop J. C.; Bumgardner C. L.; Washington G.; Loehle W. D.; Sankar S. S.; Lewis A. B. Keto–enol and enol–enol tautomerism in trifluoromethyl-β-diketones. J. Fluorine Chem. 2006, 127, 780. 10.1016/j.jfluchem.2006.02.012. [DOI] [Google Scholar]
- Aromí G.; Gamez P.; Reedijk J. Poly beta-diketones: Prime ligands to generate supramolecular metalloclusters. Coord. Chem. Rev. 2008, 252, 964. 10.1016/j.ccr.2007.07.008. [DOI] [Google Scholar]
- Kavala V.; Wang C.-C.; Barange D. K.; Kuo C.-W.; Lei P.-M.; Yao C.-F. Synthesis of Isocoumarin Derivatives via the Copper-Catalyzed Tandem Sequential Cyclization of 2- Halo-N-phenyl Benzamides and Acyclic 1,3-Diketones. J. Org. Chem. 2012, 77, 5022. 10.1021/jo300501j. [DOI] [PubMed] [Google Scholar]
- Zolotareva N. V.; Semenov V. V. β-Diketones and their derivatives in sol–gel processes. Russ. Chem. Rev. 2013, 82, 964. 10.1070/rc2013v082n10abeh004364. [DOI] [Google Scholar]
- Kljun J.; Turel I. β-Diketones as Scaffolds for Anticancer Drug Design – From Organic Building Blocks to Natural Products and Metallodrug Components. Eur. J. Inorg. Chem. 2017, 1655. 10.1002/ejic.201601314. [DOI] [Google Scholar]
- Long Y.; She Z.; Liu X.; Chen Y. Synthesis of 1-Aminoisoquinolines by Gold(III)-Mediated Domino Reactions from 2-Alkynylbenzamides and Ammonium Acetate. J. Org. Chem. 2013, 78, 2579. 10.1021/jo302794z. [DOI] [PubMed] [Google Scholar]
- Jithunsa M.; Ueda M.; Miyata O. Copper(II)Chloride-Mediated Cyclization Reaction of N-Alkoxy-ortho-alkynylbenzamides. Org. Lett. 2011, 13, 518. 10.1021/ol1029035. [DOI] [PubMed] [Google Scholar]
- Yao B.; Jaccoud C.; Wang Q.; Zhu J. Synergistic Effect of Palladium and Copper Catalysts: Catalytic Cyclizative Dimerization of ortho-(1-Alkynyl)benzamides Leading to Axially Chiral 1,3-Butadienes. Chem.—Eur. J. 2012, 18, 5864. 10.1002/chem.201200215. [DOI] [PubMed] [Google Scholar]
- Kundu N. G.; Khan M. W. Palladium-Catalysed Heteroannulation with Terminal Alkynes: a Highly Regio- and Stereoselective Synthesis of (Z)-3-Aryl(alkyl)idene Isoindolin-1-ones1. Tetrahedron 2000, 56, 4777. 10.1016/s0040-4020(00)00359-8. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.