Abstract

The tandem process of phenol addition to a cyclic α,β-unsaturated ester followed by intramolecular transesterification and [1,5] sigmatropic rearrangement affords a series of helical coumarins based upon a previously unknown 3-amino-7-hydroxybenzo[3,4]cyclohepta[1,2-c]chromen-6-one core. These novel polarized coumarins, possessing a β-ketoester moiety, have been employed to synthesize more rigid and helical coumarin–pyrazolones, which display green fluorescence. The enhanced emission of coumarin–pyrazolones in polar solvents depends on the nature of the S1 state. The coumarin–pyrazolones are predicted to have two vertical states close in energy: a weakly absorbing S1 (1LE) followed by a bright S2 state (1CT). In polar solvents, the 1CT can be stabilized below the 1LE and may become the fluorescent state. Solvatochromism of the fluorescence spectra confirms this theoretical prediction. The presence of an N—H···O=C intramolecular hydrogen bond in these coumarin–pyrazolone hybrids facilitates excited-state intramolecular proton transfer (ESIPT). This process leads to a barrierless conical intersection with the ground electronic state and opens a radiationless deactivation channel effectively competing with fluorescence. Solvent stabilization of the CT state increases the barrier for ESIPT and decreases the efficiency of the nonradiative channel. This results in the observed correlation between solvatochromism and an increase of fluorescence intensity in polar solvents.
Introduction
The rapid progress of modern technologies has relied on the design, creation, and implementation of new materials. Particular attention has been focused on novel organic chromophores as, unlike their inorganic counterparts, they do not contain rare, expensive, or unethically mined elements. Coumarins are opportune fluorophores for many applications as the introduction and modification of substituents on this scaffold has been shown to enable comprehensive control of their photophysical properties.1 Their special features, such as large fluorescence quantum yield, large Stokes shift, high absorption coefficient, and photostability, make them very desirable and allow them to be used as fluorescent probes,2 as dyes for two-photon fluorescence microscopy,3 in OLEDs,4 and as constituents in energy and electron transfer systems.5 Another important modification that allows for coumarins’ bandgap tuning is π-electron core expansion.6 This allows the absorption and fluorescence to be red-shifted, a feature that is particularly important for fluorescence imaging.7 Multiple examples of coumarin chromophore modification have been published recently.8 Among many scaffolds, the V-shaped bis-coumarins, which can be synthesized by the Michael reaction of electron-rich phenols with esters of coumarin-3-carboxylic acids,9 have attracted broader attention.10 Independently, it was reported that coumarins possessing an electron-withdrawing group at position 3 undergo Michael addition with other nucleophiles to form dihydrocoumarins11 or coumarins.12 In both the latter case and in the case of phenol addition,9 a second molecule of coumarin ester acts as an oxidant. In light of these results, we have designed an intramolecular version of such a process. The hypothesis was that, if two α,β-unsaturated ester groups are present within a molecule, one will act as an electrophilic coupling partner and the second will take the role of an oxidant. We envisioned that the replacement of coumarin with ester 1 (Scheme 1) would lead to not only promising dyes containing a coumarin moiety fused with a seven-membered ring but also core-modified [4]helicenes.
Scheme 1. Synthesis of Dye 3 Having a Coumarin Motif Fused to a Seven-Membered Ring and the Proposed Mechanism of This Transformation.
Results and Discussion
Synthesis
Guided by the above-described hypothesis, we designed a reaction between dimethyl 7-oxo-7H-benzo[7]annulene-6,8-dicarboxylate (1)13 and 3-diethylaminophenol (2) as our model system (Scheme 1). Encouraged by our recent discovery of the reaction of electron-rich phenols with esters of coumarin-3-carboxylic acids, leading to conjoined coumarins,9 we used the same conditions herein; i.e., the reaction was performed neat and with indium triflate as a catalyst. These conditions allowed product 3 to be obtained in a satisfactory 51% yield. Replacing In(OTf)3 with other Lewis acids and adding various solvents did not lead to an increase in yield. The potential product from the addition of two molecules of phenol to ester 1 was not detected in the crude reaction mixture as judged by ESI-MS. Attempts to oxidize dye 3 to the corresponding dye bearing a fully conjugated structure with numerous oxidizing agents failed.
The structure of compound 3 was unambiguously confirmed by 1H and 13C NMR experiments, which proved that the C–C double bond is located at the place of the Michael reaction resulting in the formation of the coumarin chromophore. In-depth analysis of the transformation of diester 1 into coumarin 3 led us to the conclusion that the plausible mechanism consists of Michael addition followed by [1,5] sigmatropic rearrangement (Scheme 1). [1,5] Sigmatropic rearrangement is a reaction in which a hydrogen atom migrates from one end of a system of π bonds to the other.14 An S-cis conformation has to be adopted by the molecule, since it has been established that typically a pericyclic mechanism is involved and the hydrogen atom has to, in the transition state, be in contact with both ends of the chain at the same time.15 In our case, the reaction must occur, based on orbital symmetry rules,16 in a suprafacial geometrical pathway since it is a thermal process.17 The driving force for the [1,5] sigmatropic rearrangement ([1,5] hydrogen shift) observed here is plausibly due to a different character of the initially intact olefinic bond versus the coumarin C–C double bond resulting from the presence of the Et2N group.
From the formal point of view, the discovered process is an example of an intramolecular redox process.18 It is noteworthy that coumarins have been previously synthesized by the condensation of alkyl cinnamates with phenols in the presence of transition metal salts.19 Molecule 3 has a [4]helicene architecture, hence axial chirality, which manifests itself through the methylene bridge hydrogen atoms in the seven-membered ring not being equivalent (deduced from the presence of two characteristic doublets in the 1H NMR spectrum, Figure S1a). The presence of the seven-membered ring makes the helical structure more curved.
With this viable methodology, our attention turned to other electron-rich phenols. Using the same reaction conditions, we synthesized derivative 4 bearing a hydroxyjulolidine moiety (Figure 1). The yield of this product was good (77%), but during purification, we noticed that the compound was unstable and decomposed to byproducts observable in the 1H NMR spectrum. Among other starting materials tested, we also used 4-chlororesorcinol (10), which led to compound 5 in 54% yield (Figure 1). The main difference between hydroxy-substituted compound 5 and amino-substituted coumarins 3 and 4 is the fact that the [1,5] sigmatropic rearrangement does not occur at the reaction temperature (140 °C). As a result molecule 5 does not possess a coumarin scaffold (Figure 1). The analysis of the 1H NMR spectrum confirmed this difference. The spectrum for compound 5 showed two single signals at 4.34 and 8.41 ppm, which were assigned to the pyran ring and a double bond in the seven-membered ring, respectively (Figure S1b). This is in contrast to 3 which has two doublets present in the aliphatic region of the spectrum (Figure S1a).
Figure 1.
Structures of dyes 4 and 5.
Using the above-discussed methodology, we decided to extend our studies to molecules possessing a similar structure but with one additional fused benzene ring. Initially, we synthesized the appropriate naphthalene-2,3-dicarbaldehyde (6), starting from phthalaldehyde and dimethoxytetrahydrofuran.20 The next step was a double Knoevenagel condensation of dialdehyde 6 with acetone-1,3-dicarboxylate, which allowed us to obtain compound 8 in 54% yield (Scheme 2). Subsequently, we carried out reactions with 3-diethylaminophenol (2) and 4-chlororesorcinol (10) in the presence of indium triflate, which led to compounds 9 and 11 in 47% and 44% yields, respectively.
Scheme 2. Synthesis of Dyes 9 and 11.
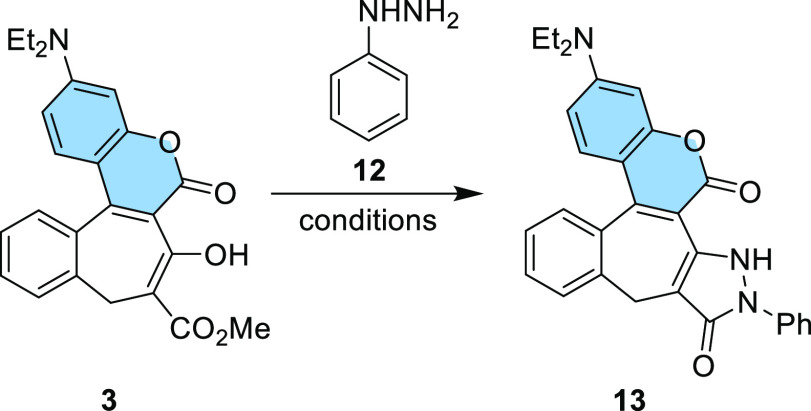
Once we ascertained that cyclic, double α,β-unsaturated esters could be successfully engaged in a coumarin-forming reaction, we decided to capitalize on the fact that the structure of dyes 3, 4, 5, 9, and 11 gives rise to many synthetic opportunities due to their β-ketoester nature. Consequently, we attempted the synthesis of dyes with an additional pyrazolone ring, expecting the appropriate products to possess more rigid geometry. There are many examples described in the literature of pyrazolone ring formation by heating a hydrazine derivative with an appropriate 1,3-dicarbonyl substrate in acetic acid21 or ethanol.22 In order to test the suitability of our dyes for this purpose, we performed the reaction of coumarin 3 with phenylhydrazine (12) by heating the substrates in acetic acid. We obtained the expected product 13, albeit in a very low yield (5%, Table 1). When the reaction was conducted in ethanol, we observed only traces of product. To facilitate the formation of dye 13, we diverged from these traditional conditions, performing a short optimization study using a selection of catalysts (Table 1).
Table 1. Optimization of the Synthesis of Pyrazolone 13.
| entry | catalyst | solvent | time [h] | yielda [%] |
|---|---|---|---|---|
| 1 | AcOH | 12b | 5 | |
| 2 | EtOH | 24b | trace | |
| 3 | Zn(NTf2)2 | toluene | 24b | 0 |
| 4 | DMAP | toluene | 24b | 17 |
| 5 | p-TsOH | toluene | 24b | 59 |
| 6 | AgOTf | toluene | 24b | 43 |
| 7 | T3P in DCM | toluene | 24b | 72 |
Isolated yields.
Reflux.
The reaction in the presence of zinc di[bis(trifluoromethylsulfonyl)imide] failed. Using 4-dimethylaminopyridine allowed the final product to be obtained in the still low 17% yield. We obtained a better result when silver triflate23 was used (entry 6). In the presence of p-toluenesulfonic acid,24 we synthesized the product with a moderate to good yield (59%). The best result, however, was obtained when we used propylphosphonic acid cyclic anhydride in dichloromethane25 (T3P in DCM), a well-known reagent widely used in peptide chemistry. The reaction was conducted in toluene under an argon atmosphere, giving product 13 in 72% yield. With the optimized reaction conditions in hand, we decided to study the scope of this methodology. Starting from 4 and 9, we obtained the new dyes containing an additional pyrazolone ring 14 and 15 in the good yields of 79% and 55%, respectively (Scheme 3).
Scheme 3. Synthesis of Coumarins 14 and 15.

We also investigated whether these conditions were suitable for molecules with substituents other than an amino group such as 5. It turned out that, in this case, the conditions were ineffective. Only unreacted substrates were observed. The different outcomes of condensations of ester 1 with 3-dialkylaminophenols and with 4-chlororesorcinol prompted us to attempt to enforce the [1,5] sigmatropic rearrangement reaction in the case of heterocycle 5 by increasing the temperature. We heated compound 5 for 2 h under a vacuum without solvent (Scheme 4). It turned out that, under these conditions, the [1,5] hydrogen shift does occur, and coumarin 16 was obtained in 66% yield. The structure of dye 16 was confirmed by the 1H NMR spectrum.
Scheme 4. Synthesis of Coumarin 16.
Single-Crystal X-ray Diffraction Studies
Compound 15 crystallizes in yellow plates appropriate for X-ray analysis by slow diffusion of hexane into a dichloromethane solution. The crystallographic structure is presented in Figure 2. It belongs to the P21/c space group. This molecule possesses an intramolecular hydrogen bond between the carbonyl group of the pyran-2-one motif and proton from the five-membered pyrazolone ring with a length of 2.353 Å (Figure 2b). The presence of the highly twisted seven-membered ring in compound 15 is the major contributing factor to its lack of planarity. The twist angle between the six-membered aromatic rings on either side of the seven-membered ring is ca. 61 degrees (Figure S4b). The dihedral angle between coumarin moiety and plane of pyrazolone ring is around 31 degrees (Figure S4a). The crystal packing in the unit cell is an antiparallel sandwich-type arrangement (Figure S3).
Figure 2.

X-ray structure of compound 15 with the marked hydrogen bond: (a) front view and (b) side view.
Photophysical Results
Optical studies were performed for dyes 3, 9, 14, 13, and 15 in three solvents: nonpolar toluene (dielectric constant ε = 2.379), dichloromethane (DCM, ε = 8.93), and very polar acetonitrile (ACN, ε = 38.8). Compounds containing a chlorine atom (5 and 11) have a very low solubility in these solvents and were, therefore, only studied in 1,4-dioxane (ε = 2.25). Absorption and fluorescence spectra of all compounds are presented in Figure 3 and Figure S5b, and spectroscopic properties are summarized in Table 2.
Figure 3.
Absorption (solid line) and emission (dashed line) spectra of compounds 3 (excited at 395 nm), 5 (excited at 290 nm), 9 (excited at 395 nm), 13 (excited at 380 nm), 14 (excited at 400 nm), and 15 (excited at 390 nm) in toluene (black), DCM (blue), ACN (red), and dioxane (magenta).
Table 2. Spectroscopic Properties of Compounds 3, 5, 9, 11, 13, 14, 15, and 16 Obtained in Toluene, DCM, ACN, and Dioxane.
| compound | solvent | λabsmax [nm] | λemmax [nm] | ε [M–1 cm–1] | Stokes shift [cm–1] | ΦF | τavr [ns] | krd [ns] | knre [ns] |
|---|---|---|---|---|---|---|---|---|---|
| 3 | toluene | 418 | 492 | 39000 | 3600 | 0.02a | 0.076 | 0.26 | 12.9 |
| DCM | 426 | 501 | 42000 | 3500 | 0.09a | 0.217 | 0.42 | 4.19 | |
| ACN | 423 | 513 | 37100 | 4100 | 0.15a | 0.95 | 0.16 | 0.89 | |
| 5 | dioxane | 295 | 344 | 12900 | 4800 | 0.004b | |||
| 9 | toluene | 423 | 501 | 87400 | 3700 | 0.07a | 0.144 | 0.49 | 6.46 |
| DCM | 432 | 508 | 62400 | 3500 | 0.22a | 0.365 | 0.60 | 2.14 | |
| ACN | 428 | 519 | 28500 | 4100 | 0.32a | 0.645 | 0.50 | 1.05 | |
| 11 | dioxane | 320 | 345 | 17300 | 2300 | 0.002b | |||
| 13 | toluene | 438 | 478 | 46300 | 1900 | 0.006a | 1.23 | 0.005 | 0.81 |
| DCM | 445 | 499 | 59900 | 2400 | 0.23a | 1.79 | 0.13 | 0.43 | |
| ACN | 438 | 505 | 23600 | 3000 | 0.15a | 1.73 | 0.087 | 0.49 | |
| 14 | toluene | 457 | 504 | 50900 | 2000 | 0.01a | 0.85 | 0.012 | 1.17 |
| DCM | 465 | 522 | 30000 | 2300 | 0.20a | 3.56 | 0.056 | 0.23 | |
| ACN | 458 | 556 | 51400 | 3800 | 0.19a | 1.14 | 0.17 | 0.71 | |
| 15 | toluene | 442 | 480 | 22100 | 1800 | 0.003a | 0.94 | 0.003 | 1.06 |
| DCM | 450 | 500 | 33100 | 2200 | 0.17a | 3.52 | 0.048 | 0.24 | |
| ACN | 437 | 513 | 22700 | 3400 | 0.09a | 3.1 | 0.029 | 0.29 | |
| 16 | toluene | 357 | 489 | 21300 | 7600 | 0.0015c | 0.012 | 0.125 | 83.2 |
| DCM | 357 | 483 | 22200 | 7300 | 0.0013c | 0.018 | 0.072 | 55.4 |
Fluorescence quantum yield measured with perylene in cyclohexane (ΦF = 0.94).
Quinine sulfate in 0.5 M aqueous solution of H2SO4 (ΦF = 0.54) as references.
Using an integrating sphere.
kr = ΦF/τavr.
knr = 1/τavr – kr.
Compounds 3 and 9 exhibit maximum absorption and emission in the range of 418–432 nm and 492–519 nm, respectively (Table 2 and Figure 3). These values are similar to the structurally analogous coumarin possessing a diethylamino substituent at position 7 and COCH2CO2Me substituent at position 3 (Cum1) (Figure S2).26 Compounds 5 and 11 possess significantly blue-shifted absorption and emission spectra (Figure 3 and Figure S5b).27 Dyes 13, 14, and 15 containing a pyrazolone ring exhibit bathochromically shifted (only by a few nm) absorption and emission spectra (Table 2 and Figure 3) compared to the model compound (Cum2) (Figure S2).28Cum2 exhibits a strong hypsochromic shift of the absorption band with increasing solvent polarity and slightly weaker shift in the emission spectra. The extension of the π-framework negligibly redshifts absorption/emission bands of 9 with respect to 3. Stopping the rotation of the dialkylamino group (13 → 14) leads to a significant bathochromic shift of both absorption and emission maxima (ca. 20 nm). Interestingly, transforming the β-ketoesters into pyrazol-2-ones with concomitant rigidification of the dye’s architecture (3 → 13, 9 → 15) results in a hypsochromic shift in emission. In our helical coumarins 13, 14, and 15, the solvatochromism in absorption spectra is negligible, while, in the emission spectra, the maxima were bathochromically shifted with increasing solvent polarity, proving that the small ground state dipole moment increases upon optical excitation (Table 2 and Figure 3). Absorption spectra of compounds 3, 9, 13, 14, and 15 are dominated by a strongly allowed band with a maximum at 420–450 nm (2.95–2.75 eV) with molar absorptivity ranging from 30000 to 80000 M–1 cm–1 and exhibiting small solvatochromism (Table 2). Regarding their fluorescence, the solvent polarity dependence increases in the order 3 < 9 < 13 < 15 < 14. These observations are directly correlated to the computed dipole moments for these molecules and explored in the theoretical session. The dyes containing a 1-phenylpyrazol-5-one moiety are significantly more polarized, and consequently, their solvatochromism is significantly more pronounced. The overlap of fluorescence and absorption spectra (Stokes shift ranges from 3000 to 4000 cm–1) visible in Figure 3 confirms that the emission originates from the S1 state. Indeed, the fluorescence quantum yield of all discussed compounds, about 0.01 to 0.2 (ΦF in Table 2), indicates the operation of efficient radiationless processes for this series of compounds. For each given compound, the ΦF is higher in polar solvents than it is in nonpolar toluene. This finding suggests that S1 is significantly stabilized in polar microenvironments. Apparently, this stabilization increases the barrier for the nonradiative process and slows down knr. Inspection of nonradiative rates presented in Table 2 confirms this supposition.
This type of photophysical behavior, i.e., an increase in the fluorescent quantum yield in polar solvents, has been already reported, although only for a rather limited number of dyes such as acridine, pyrene-3-carboxaldehyde, 7-methoxy-4-methylcoumarin, and its π-expanded analogues.29
Compounds 5 and 11, which lack the coumarin chromophore, have much lower molar absorptivity (below 20000 M–1 cm–1, Table 2). Absorption spectra of both molecules are blue-shifted to the UV region, and the maximum of the main spectral band is observed at 295 nm (4.2 eV) and 320 nm (3.87 eV) for 5 and 11, respectively. The observed substantial increase in the excited state energy suggests smaller electronic coupling in these molecular systems. The low-energy shoulder in the absorption spectrum of 11 and low-energy tail in the spectrum of 5 (Figure 3 and Figure S5b) suggest the presence of more than one absorbing species. The fluorescence spectra are also shifted toward higher energy. Two bands are clearly visible in the fluorescence spectrum of 11, which may suggest the presence of two isomers. Fluorescence quantum yields, 0.004 and 0.002 for 5 and 11, respectively, prove that the de-excitation paths in these molecules are dominated by radiationless transitions.
In Figure 4, fluorescence decay traces of 9 recorded at room temperature are depicted along with the excitation pulse curve. Decays in polar solvents are monoexponential. In toluene, the dominant component has a short decay time, and the second component of a much longer decay time is visible. Single exponential decays are also observed in polar solvents for other compounds, with the exception of 13 and 15, for which two exponential decay curves in toluene are also observed (Table 3, Figure S5a). Fast fluorescence decays in toluene and slow decay traces in polar solvents provide more experimental evidence for suppression of the radiationless processes discussed earlier. Nonradiative rate constants, knr, calculated from fluorescence quantum yields (ΦF) and an averaged decay time, τavr, are the highest in toluene for all molecules studied (Table 2). Thus, the supposition that interaction with a polar environment stabilizes the emitting charge transfer excited (1CT) state and inhibits the radiationless process is well-grounded.
Figure 4.

Semilogarithmic plot of fluorescence decays of compound 9 recorded at 21 °C. Excitation pulse 394 nm (black line) and fluorescence decays observed at 500 nm in toluene (blue), DCM (green), and ACN (red). Temporal resolution = 1.02 ps/channel.
Table 3. Fluorescence Decay Times (τ1, τ2) and the Amplitude of Decay Components (A1, A2) of Dyes 3, 9, 13, 14, 15, and 16 in Toluene, DCM, and ACN.
| compound | solvent | τ1 [ns] | τ2 [ns] | A1 | A2 | τaver [ns]a |
|---|---|---|---|---|---|---|
| 3 | toluene | 0.076 | 0.076 | |||
| DCM | 0.195 | 1.46 | 57.2 | 1.0 | 0.217 | |
| ACN | 0.565 | 4.0 | 11.31 | 1.42 | 0.95 | |
| 9 | toluene | 0.144 | 0.144 | |||
| DCM | 0.365 | 0.365 | ||||
| ACN | 0.645 | 0.645 | ||||
| 13 | toluene | 0.271 | 2.34 | 1.4 | 1.2 | 1.23 |
| DCM | 1.790 | 1.79 | ||||
| ACN | 1.730 | 1.73 | ||||
| 14 | toluene | 0.208 | 2.5 | 1.02 | 0.4 | 0.85 |
| DCM | 3.56 | 3.56 | ||||
| ACN | 0.686 | 3.38 | 0.94 | 0.19 | 1.14 | |
| 15 | toluene | 0.335 | 2.55 | 1.16 | 0.436 | 0.94 |
| DCM | 3.52 | 3.52 | ||||
| ACN | 0.322 | 4.06 | 0.32 | 0.92 | 3.1 | |
| 16 | toluene | 0.012 | 0.012 | |||
| DCM | 0.018 | 0.018 |
τaver = (A1τ1 + A2τ2)/(A1 + A2)
To explain the mechanism of this process, however, insight from theoretical calculations is required. Additionally, the absorption and emission spectra were measured for compound 16 in toluene and DCM (Figure S5b). In both cases, we observed significantly red-shifted bands compared to molecule 5 (Figure S5b and Figure 3). Compound 16 exhibited a larger Stokes shift too, which suggests an increase in dipole moment in the excited state as well as higher molar absorptivity resulting from the presence of coumarin chromophore (Table 2 and Scheme 4). Fluorescence quantum yields were also very low: only 0.0015 and 0.0013 for toluene and DCM, respectively. This proves that the relaxation of compound 16 is dominated by radiationless transitions (Table 2).
Computational Results
Molecular model structures of 3, 9, 13, and 15 were constructed with truncated alkyl chains (replacing the amine ethyl groups with methyl groups) to save computational time without significant effect on the electronic properties. The equilibrium geometries of all studied coumarin derivatives in their neutral or ionic closed-shell ground state (S0) were determined with the MP2 method30 without imposing any symmetry constrains. Excitation energies, equilibrium geometries, and corresponding properties of the lowest excited singlet states were calculated using the second-order algebraic diagrammatic construction ADC(2) method.31−33 The correlation–consistent valence double-ζ basis set with polarization functions on all atoms (cc-pVDZ) was used.34 For comparison, the vertical absorption and emission of few selected systems were also computed with the aid of the CC2 method, a simplified version of the coupled-clusters theory with single and double replacements, using the cc-pVDZ basis set.31,33 Additional single-point calculations using the more accurate scaled opposite spin (SOS-CC2) method were also performed.35,36
Coumarins 3, 4, 9, 13, 14, and 15 are predicted to be out of the plane, with a torsion angle between coumarin and benzene ring around 50°. 1H NMR studies have proven that they exist exclusively in the enol forms. For dyes 3, 4, and 9, two rotational isomers are expected to be thermally populated in the ground state (Table S2). Both rotamers are expected to have the labile proton attached to the a oxygen (Figure 5), with the ac structure being more stable and less polarized than ab by ∼0.3 eV and 2 D.
Figure 5.

Chemical structure of the rotamers 3ab and 3ac showing the labels used for the oxygen atoms.
Excitation energies for these derivatives were evaluated at the ADC(2)/cc-pVDZ level of theory using the ground-state equilibrium geometry obtained at the MP2 level with the cc-pVDZ basis set. Inspection of these explorations presented in Table S3 of the ESI leads to the conclusion that the excitation energies computed for ab are around 0.3 eV lower than the respective ac isomers for 3, 4, and 9. All derivatives exhibit strong dipole allowed absorption transitions to the lowest excited singlet state of the vertical excitation energy close to 3 eV. These electronic transitions are of ππ* nature, with transition dipole moments ranging from 13 to 17 D, involving molecular orbitals delocalized over the whole coumarin derivative structure.
Electronic transitions computed at the same level for compounds 13–15 exhibit two vertical excited states in close energy, a weakly absorbing 1LE state of ππ* character around 3 eV, followed by a significantly more polarized and strongly absorbing bright 1CT state (also ππ*), located vertically around 3.2 eV for 13. Thus, we expect that a larger stabilization of the 1CT state in polar solvents would result in enhancement of both the fluorescence and solvatochromism for these compounds. This feature was observed experimentally. Excitation energies of 14 were also computed within CC2 and SOS-CC2 approximations. The latter suggests a significant blueshift of the 0–0 transition energy, as previously observed by Hellweg and co-workers for a series of organic molecules.37
Geometry optimization of the S1 state of 3, 4, and 9 resulted in stable ac structures, which are predicted to fluoresce at about 1.5 eV at the ADC(2) level of theory. As previously observed for related chromophores,36,38−40 ADC(2) tends to underestimate the transition energies below 2 eV; thus, to obtain more accurate emission energies, additional calculations were performed within the scaled opposite spin (SOS-CC2) method (Table S4). Within this approximation, emission energies are predicted to be around 2.3 eV.
To investigate the occurrence of excited-state intramolecular proton transfer41 in this series of compounds, potential energy (PE) profiles along the proton transfer reaction coordinate of the respective ac isomer were performed for selected compounds (3, 13, and 14). The results are presented in Figures S7 and S8. PE profiles were optimized in the excited singlet state (S1), and the vertical energy of the ground state was computed along such determined minimum energy reaction paths. It can be noticed that, upon inspection of Figure S7, for 3, the minimum energy profile of the singlet locally excited (1LE) state, which involves a local excitation within the coumarin core, is an increasing function of the O–H bond distance, with a barrier less than 0.3 eV. However, for the ac isomer, the proton transfer is a nonadiabatic process, most probably induced by charge transfer between the cycloheptatrienol and carbonyl moieties. The electronic structures of the 1LE and 1CT states determined at the same value of the proton transfer coordinate (O–H = 1.3 Å) are shown in Table S5. The proton transfer barrier was also estimated for the coumarin moiety (ab isomer) of compound 3 (Figure S8). In this case, the conical intersection to the ground state would be reached in a barrierless fashion. Summarizing, 3ab would be nonemissive, with ESIPT predicted to be its main deactivation channel, while the emission observed experimentally can be assigned to the population of the isomer 3ac in the excited state.
The possibility of the formation of ring-opened photoproducts for 3-amino-7-hydroxybenzo[3,4]cyclohepta[1,2-c]chromen-6-ones (Figure S4) was also considered (Figure S8). The computed PE profile, however, predicts a barrier of 0.4 eV hindering access to ring-open isomers of 3ac species.
Excited-state geometry optimization of 13–15 resulted in a spontaneous ESIPT reaction involving proton transfer from the N–H of the pyrazolone moiety to the carbonyl group of the coumarin (Figure S7 and Figure 6). As a result, the S1–S0 energy gap is drastically reduced, suggesting that the formed 13-OH species would be nonemissive (Figure S6). However, the emission observed experimentally for these compounds is most probably due to the significant stabilization of the S2 (1CT) state (Figure S10) in the presence of polar/polarizable solvents (acetonitrile and dichloromethane/toluene). The effect of solvent on the emission from the 1CT state was evaluated considering two explicit acetonitrile molecules in proximity to the proton transfer site of the compound 14. The resultant microsolvated structure is predicted to emit at lower energy (2.4 eV) than the nonsolvated species (2.9 eV). This trend is consistent with the solvatochromism observed experimentally.
Figure 6.

Summary of active electronic states and photophysical processes for compounds 13, 14, and 15 in polar and nonpolar solvents obtained at the MP2/ADC(2)/cc-pVDZ level of theory. Solid levels denote the adiabatic (optimized) energy of a given state, and dashed levels denote the vertical energy of the absorbing/fluorescing state computed at the optimized geometry of the respective states.
In this contribution, we have made three sets of dyes with remarkably different photophysical properties. For compounds 3 and 9, the isomer called ac is predicted to be more stable than ab; however, this energy difference is rather small. Thus, the fully allowed optical transitions of both species are expected. Only one emissive species was found, ac, which was predicted to fluoresce from the lowest excited ππ* state. The ESIPT reaction path computed for 3ab suggests that the conical intersection would be reached in a barrierless fashion, while the ESIPT of 3ac is predicted to be nonadiabatic. In compounds 13–15, there are two vertical states to be considered, the weakly absorbing 1LE state followed by a bright 1CT state, which strongly absorbs at slightly (0.2 eV) higher energy. The order of these states strongly depends on the polarity of the solvent since 1CT is significantly more polarized than 1LE. These observations are summarized in Figure 6.
Due to solubility issues, only dioxane was used as a solvent for 5 and 11. For these molecules, the absorption is significantly lower than the others. Since a long tail and two distinguished bands can be observed for the absorption and emission spectra of these compounds, respectively, two possible structures were considered, namely, ab and openab, the latter being a rotamer by way of the chlorophenyl OH moiety. In both compounds, the phenyl and naphthyl groups bonded to the cycloheptatrienol moieties are 90° twisted with respect to the coumarin rings. This leads to weak coupling between the moieties, clearly visible in the molecular orbitals of Table S3b, and results in very low oscillator strength (Table S3a), typical for nonemissive compounds. The complexation with two solvent molecules, a polarizable 1,4-dioxane, leads to electronic density delocalization over the whole compound and affects the optical transition moment by a small amount. Indeed, both compounds are weakly absorbing and the lowest molar absorptivity among all molecules studied in this work (Table 2). The twist around the C=C double bond provides an efficient channel for radiationless deactivation, which dominates the radiative transition. In fact, the lowest fluorescence quantum yields are observed for compounds 5 and 11 (Table 2).
Conclusion
Our experiments provide a proof-of-concept demonstration that highly curved, helical coumarins are formed via the Michael addition of aminophenols to cyclic α,β-unsaturated esters. The mechanism consists of the Michael addition, followed by transesterification and [1,5] sigmatropic rearrangement. The net result is that the initially formed C–C single bond becomes a C–C double bond, which leads to the formation of a coumarin ring system. Depending on the strength of the electron-donating group present on the phenol, [1,5] sigmatropic rearrangement occurs either spontaneously at 140 °C (under reaction conditions) or at 220 °C if a dialkylamino group is not present. The addition of phenols to α,β-unsaturated esters leading to coumarins catalyzed by Lewis acids seems to be a general process. The helical coumarins display moderate green emission with a moderate solvatofluorochromic effect and a small effect in absorption spectra. We report de-excitation pathways and mechanisms operating in the electronically excited states, relating the photophysical properties to the molecular structure of the compounds studied. The highly twisted structure of these helical coumarins results in weak electronic coupling that leads to low molar absorptivity in the UV. The less twisted structures of other compounds result in electronic coupling of moieties and strong absorption at a lower energy in the visible blue region. Efficient ESIPT in the case of pyrazolone–coumarins and double bond twist leads to internal conversion to the ground state, providing a nonradiative channel that competes with the radiative transitions in all compounds studied. Our findings hold the potential for an immediate impact on organic synthesis and construction of fluorescent probes.
Experimental Section
General Information
Dry dichloromethane was used for all syntheses. All reported NMR spectra (1H NMR and 13C NMR) were recorded using a Varian 500 or Bruker 500 spectrometer. Chemical shifts (δ ppm) were determined with TMS as the internal reference, and J values are given in hertz (Hz). High-resolution mass spectra (HRMS) were obtained via an electron ionization (EI) source and an EBE double-focusing geometry mass analyzer. Chromatography was performed on silica gel 60 (230–400 mesh), and thin-layer chromatography was performed on TLC plates (Merck, silica gel 60 F254).
Photophysical Measurements
Spectroscopic grade solvents were purchased from Sigma-Aldrich and used as obtained. For optical studies, solutions of molecules at low concentrations, about a few micromoles per liter, were used to avoid dimerization or reabsorption effects. Before measurements, solutions were bubbled with pure nitrogen gas for 20 min. All absorption and fluorescence spectra were taken at room temperature (21 °C). A PerkinElmer UV/vis spectrometer, model Lambda 35, was used for absorption spectra measurement. Fluorescence spectra were recorded with an FLS 1000 spectrofluorometer from Edinburgh Instruments and corrected for the spectral response sensitivity of the photodetector. Fluorescence quantum yields (FQY) of molecules in solvents at 21 °C were determined using 9,10-diphenylanthracene and quinine sulfate as FQY standards. Solutions of low absorbance (A < 0.1) were used to avoid reabsorption or concentration quenching. Corrections for the refractive index of solvents have been performed in the calculations of quantum yields.42 Molar absorptivity (absorption coefficient), ε, was calculated from the absorbance, A, of a solution of the given molar concentration, c, in a cuvette of length l with a well-known formula: A = c × ε × l. Fluorescence kinetics studies were performed with the “time-correlated” single-photon counting technique. Excitation pulses were provided by the second harmonic of a mode-locked Coherent Mira-HP picosecond laser pumped by a Verdi 18 laser. The original 76 MHz repetition rate of a Mira laser was reduced with the aid of APE Pulse Selector to 3.8 MHz. Fluorescence photons were detected with an HMP-100–50 hybrid detector and an SPC-150 module, both from Becker&Hickl GmbH. Fluorescence decays were analyzed with a deconvolution computer program, which uses a nonlinear least-squares procedure with the Marquardt method.43 A standard χ2 test was used along with residual and autocorrelation function plots to judge the quality of a fit. The estimated precision of the decay time determination was 10 ps.
Synthesis
General Procedure for the Synthesis of Compound 3
To the flask was added compound 1 (272 mg, 1 mmol), 3-(diethylamino)phenol (2) (332 mg, 2 mmol), and In(OTf)3 (11 mg, 0.02 mmol). The reaction mixture was stirred at 140 °C (oil bath) for 5 h. Then the mixture was cooled to room temperature, dissolved in a small amount of DCM, and purified by column chromatography (silica, hexane/AcOEt 2:1 as the eluent). Crystallization from MeOH afforded the product in analytical purity.
Compound 3
Yellow precipitate. Yield: 0.206 g (51%). Mp: 165–168 °C. 1H NMR (CD2Cl2, 500 MHz): δ 12.16 (s, 1H), 7.50 (d, 1H, J = 7.7 Hz), 7.47–7.42 (td, 1H, J = 7.5, 1.2 Hz), 7.38–7.30 (m, 3H), 6.58–6.55 (m, 2H), 3.84 (s, 3H), 3.72 (d, 1H, J = 14.0 Hz), 3.45 (q, 4H, J = 7.2 Hz), 2.90 (d, 1H, J = 14.0 Hz), 1.23 (t, 6H, J = 7.2 Hz). 13C{1H} NMR (CD2Cl2, 125 MHz): δ 171.5, 164.8, 159.0, 156.8, 153.5, 151.7, 145.6, 131.4, 131.1, 131.0, 130.9, 126.8, 125.5, 112.6, 109.1, 107.4, 106.6, 97.5, 52.4, 45.2, 30.0, 12.6. HRMS (EI): m/z calcd for C24H23NO5, 405.1576 [M·+]; found, 405.1588.
General Procedure for the Synthesis of Compound 5
To the flask were added compound 1 (272 mg, 1 mmol), 4-chlororesorcinol (10) (290 mg, 2 mmol), and In(OTf)3 (11 mg, 0.02 mmol). The reaction mixture was stirred at 120 °C (oil bath) for 1 h. Then the mixture was cooled to room temperature, and AcOEt was added. The precipitate was filtered and crystallized from MeOH to afford the product in analytical purity.
Compound 5
Off-white precipitate. Yield: 0.206 g (54%). Mp: 204–205 °C. 1H NMR (DMSO-d6, 500 MHz): δ 12.21 (s, 1H), 10.78 (s, 1H), 8.41 (s, 1H), 7.73 (d, 1H, J = 8.0 Hz), 7.46 (t, 1H, J = 7.0 Hz), 7.36 (t, 1H, J = 7.5 Hz), 7.20 (s, 1H), 6.82 (s, 1H), 6.52 (d, 1H, J = 7.5 Hz), 4.34 (s, 1H), 3.83 (s, 3H). 13C{1H} NMR (DMSO-d6, 125 MHz): δ 165.6, 164.9, 164.8, 153.6, 149.6, 145.4, 143.6, 132.0, 131.6, 130.4, 130.3, 127.8, 126.6, 123.9, 116.6, 111.1, 104.5, 97.7, 52.6, 37.4. HRMS (EI): m/z calcd for C20H13ClO6, 384.0401 [M·+]; found, 384.0401.
General Procedure for the Synthesis of Compound 8
To the solution of naphthalene-2,3-dicarbaldehyde (6) (1.104 g, 6 mmol) in toluene (100 mL) were added dimethyl-1,3-acetonedicarboxylate (7) (1 mL, 7 mmol), piperidine (0.1 mL, 1 μmol), and acetic acid (0.3 mL, 5 μmol). The reaction mixture was stirred at 130 °C (oil bath) for 12 h using a Dean–Stark apparatus. Then the mixture was cooled to room temperature, most of the toluene was removed under a vacuum, and the remaining precipitate was filtered and purified by column chromatography (silica, DCM/MeOH 99:1 as the eluent). Crystallization from AcOEt–cyclohexane afforded the product in analytical purity.
Compound 8
Yellow precipitate. Yield: 1.040 g (54%). Mp: 212–213 °C. 1H NMR (CDCl3, 500 MHz): δ 8.304 (s, 2H), 8.298 (s, 2H), 8.03–7.97 (m, 2H), 7.72–7.67 (m, 2H), 3.94 (s, 6H). 13C{1H} NMR (CDCl3, 125 MHz): δ 184.2, 166.7, 142.8, 136.1, 134.4, 133.7, 130.4, 129.2, 128.5, 52.9. HRMS (EI): m/z calcd for C19H14O5, 322.0841 [M·+]; found, 322.0833.
General Procedure for the Synthesis of Compound 9
To the flask was added compound 8 (161 mg, 0.5 mmol), 3-(diethylamino)phenol (2) (165 mg, 1 mmol) and In(OTf)3 (5 mg, 0.01 mmol). The reaction mixture was stirred at 140 °C (oil bath) for 1 h. Then the mixture was cooled to room temperature, dissolved in a small amount of DCM, and purified by column chromatography (silica, hexane/AcOEt 2:1 as the eluent). Crystallization from MeOH afforded the product in analytical purity.
Compound 9
Yellow precipitate. Yield: 0.107 g (47%). Mp: 218–220 °C. 1H NMR (CD2Cl2, 500 MHz): δ 12.20 (s, 1H), 7.99 (s, 1H), 7.89 (t, 2H, J = 7.7 Hz), 7.77 (s, 1H), 7.58 (td, 1H, J = 8.0, 1.2 Hz), 7.51 (td, 1H, J = 8.0, 1.1 Hz), 7.36 (d, 1H, J = 9.2 Hz), 6.65–6.57 (m, 2H), 3.91 (d, 1H, J = 14.0 Hz), 3.85 (s, 3H), 3.46 (q, 4H, J = 7.1 Hz), 3.15 (d, 1H, J = 14.0 Hz), 1.24 (t, 6H, J = 7.1 Hz). 13C{1H} NMR (CD2Cl2, 125 MHz): δ 171.6, 165.0, 158.9, 157.0, 153.7, 151.5, 143.1, 134.7, 131.7, 131.0, 130.1, 128.8, 127.9, 127.5, 126.3, 124.2, 112.7, 109.6, 109.0, 108.2, 106.3, 98.0, 52.4, 45.5, 30.4, 12.6. HRMS (EI): m/z calcd for C28H25NO5, 455.1733 [M·+]; found, 455.1743.
General Procedure for the Synthesis of Compound 11
To the flask were added compound 8 (161 mg, 0,5 mmol), 4-chlororesorcinol (10) (145 mg, 1 mmol), and In(OTf)3 (5 mg, 0.01 mmol). The reaction mixture was stirred at 120 °C (oil bath) for 1 h. Then, the mixture was cooled to room temperature, and AcOEt was added. The precipitate was filtered and crystallized from MeOH to afford the product in analytical purity.
Compound 11
Yellow precipitate. Yield: 0.097 g (44%). Mp: 200–201 °C. 1H NMR (DMSO-d6, 500 MHz): δ 12.36 (bs, 1H), 10.80 (s, 1H), 8.55 (s, 1H), 8.38 (s, 1H), 8.02–7.96 (m, 1H), 7.89–7.84 (m, 1H), 7.59–7.52 (m, 2H), 7.27 (s, 1H), 7.00 (s, 1H), 6.89 (s, 1H), 4.69 (s, 1H), 3.88 (s, 3H). 13C{1H} NMR (DMSO-d6, 125 MHz): δ 166.2, 165.1, 165.0, 153.6, 149.7, 145.6, 141.7, 134.0, 131.2, 131.0, 130.5, 130.4, 128.2, 128.0, 127.7, 127.3, 126.6, 122.5, 116.6, 111.4, 104.6, 97.9, 52.6, 37.8. HRMS (EI): m/z calcd for C24H15ClO6, 434.0557 [M·+]; found, 434.0544.
General Procedure for the Synthesis of Compound 13
To a dry Schlenk flask were added compound 3 (41 mg, 0.1 mmol), T3P in DCM (64 mg, 0.2 mmol), and dry toluene (5 mL) under argon. Phenylhydrazine (12) (0.02 mL, 0.2 mmol) was subsequently added, and the reaction mixture was refluxed (oil bath) for 24 h. Then the mixture was cooled to room temperature and evaporated. The crude product was purified by column chromatography (silica, DCM/MeOH 99:1 as the eluent). It was then crystallized from DCM–cyclohexane to afford the product in analytical purity.
Compound 13
Yellow precipitate. Yield: 0.033 g (72%). Mp: 246–247 °C. 1H NMR (CD2Cl2, 500 MHz): δ 9.18 (bs, 1H), 7.78–7.74 (m, 2H), 7.51–7.38 (m, 6H), 7.31 (t, 1H, J = 7.4 Hz), 7.18 (t, 1H, J = 7.4 Hz), 6.67–6.61 (m, 2H), 3.86 (d, 1H, J = 14.4 Hz), 3.48 (q, 4H, J = 7.1 Hz), 3.25 (d, 1H, J = 14.4 Hz), 1.25 (t, 6H, J = 7.1 Hz). 13C{1H} NMR (CD2Cl2, 125 MHz): δ 161.7, 156.2, 154.8, 151.8, 145.6, 143.8, 138.1, 132.3, 132.0, 131.6, 131.2, 129.4, 128.6, 125.7, 125.2, 119.3, 114.6, 109.8, 109.1, 97.7, 45.3, 28.0, 12.6. HRMS (EI): m/z calcd for C29H25N3O3, 463.1896 [M·+]; found, 463.1898.
General Procedure for the Synthesis of Compound 4
To the flask were added compound 1 (816 mg, 3 mmol), 8-hydroxyjulolidine (945 mg, 5 mmol), and In(OTf)3 (34 mg, 0.06 mmol). The reaction mixture was stirred at 140 °C (oil bath) for 5 h. Compound 4 was unstable and was used to the next step without purification
Compound 4
Orange precipitate. Yield: 0.996 g (77%). The structure was confirmed by mass spectrometry analysis in low resolution. LRMS (EI): m/z calcd for C26H23NO5, 429.1576 [M·+]; found, 429.1.
Compounds 14 and 15 were synthesized according to the same procedure as compound 13.
Compound 14
Orange precipitate. Yield: 0.058 g (79%). Mp: 230 °C (decomp). 1H NMR (CD2Cl2, 500 MHz): δ 9.25 (bs, 1H), 7.78–7.74 (m, 2H), 7.50–7.38 (m, 5H), 7.31 (td, 1H, J = 8.0, 1.0 Hz), 7.18 (t, 1H, J = 7.5 Hz), 6.98 (s, 1H), 3.85 (d, 1H, J = 14.0 Hz), 3.39–3.29 (m, 4H), 3.24 (d, 1H, J = 14.0 Hz), 3.02–2.89 (m, 2H), 2.80–2.60 (m, 2H), 2.08–2.01 (m, 2H), 2.01–1.89 (m, 2H). 13C{1H} NMR (CD2Cl2, 125 MHz): δ 161.9, 161.8, 154.9, 151.1, 147.2, 146.0, 143.8, 138.1, 132.5, 132.3, 131.3, 129.3, 128.4, 127.1, 125.6, 125.1, 119.5, 119.3, 114.1, 108.8, 106.6, 105.5, 50.4, 49.9, 28.0, 28.0, 21.7, 20.9, 20.7. HRMS (EI): m/z calcd for C31H25N3O3, 487.1896 [M·+]; found, 487.1897.
Compound 15
Yellow precipitate. Yield: 0.037 g (55%). Mp: 268 °C (decomp). 1H NMR (CD2Cl2, 500 MHz): δ 7.97 (s, 1H), 7.90–7.80 (m, 3H), 7.73 (d, 2H, J = 7.8 Hz), 7.55 (t, 1H, J = 7.0 Hz), 7.50 (t, 1H, J = 7.0 Hz), 7.44 (d, 1H, J = 8.9 Hz), 7.40 (t, 2H, J = 8.4 Hz), 7.18 (t, 1H, J = 7.4 Hz), 6.68–6.62 (m, 2H), 4.12 (d, 1H, J = 14.3 Hz), 3.53–3.43 (m, 5H), 1.26 (t, 6H, J = 7.1 Hz). 13C{1H} NMR (CD2Cl2, 125 MHz): δ 161.6, 156.4, 155.1, 151.9, 145.7, 141.6, 137.9, 135.0, 132.8, 131.3, 131.1, 130.8, 129.4, 128.7, 128.1, 127.5, 126.5, 125.4, 119.6, 110.0, 109.6, 97.8, 45.4, 28.4, 12.7. HRMS (EI): m/z calcd for C33H27N3O3, 513.2052 [M·+]; found, 513.2062.
General Procedure for the Synthesis of Compound 16
Compound 5 (250 mg, 0.65 mmol) was added to a round-bottom flask and stirred at 230 °C (oil bath) for 2 h under a vacuum. Then the mixture was cooled to room temperature, and the product was purified by preparative chromatography (silica, DCM/MeOH 95:5 as the eluent). The precipitate was crystallized from MeOH to afford the product in analytical purity.
Compound 16
Yellow precipitate. Yield: 0.164 g (66%). Mp: 225 °C (decomp). 1H NMR (DMSO-d6, 500 MHz): δ 11.80 (s, 1H), 7.61–7.51 (m, 2H), 7.45 (t, 1H, J = 7.6 Hz), 7.41 (d, 1H, J = 7.6 Hz), 7.37 (s, 1H), 7.00 (s, 1H), 3.82 (s, 3H), 3.65 (d, 1H, J = 14.2 Hz), 2.96 (d, 1H, J = 14.2 Hz). 13C{1H} NMR (DMSO-d6, 125 MHz): δ 170.0, 161.8, 157.3, 156.9, 153.3, 151.0, 145.2, 131.2, 130.5, 129.4, 129.3, 126.6, 125.7, 117.1, 115.6, 110.9, 107.3, 103.5, 52.4, 28.9. HRMS (EI): m/z calcd for C20H13ClO6, 384.0401 [M·+]; found, 384.0403.
Acknowledgments
The authors would like to thank the Foundation for Polish Science (TEAM POIR.04.04.00-00-3CF4/16-00), COST (CHAOS CA 15106), Global Research Laboratory Program (2014K1A1A2064569) through the National Research Foundation (NRF) funded by the Ministry of Science, ICT & Future Planning (Korea), and the National Science Centre under the QuantERA program (project 2017/25/Z/ST2/03038). This research was supported in part by PL-Grid Infrastructure. We thank Dr. David C. Young for amending the manuscript. We thank the anonymous referee for pointing out the possibility of the [1,5] sigmatropic rearrangement.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.0c02978.
X-ray crystallographic information; photophysical data; theoretical data; copies of 1H and 13C{1H} NMR data (PDF)
Accession Codes
CCDC 2046086 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
The authors declare no competing financial interest.
Supplementary Material
References
- a Messaoudi S.; Brion J.-D.; Alami M. Palladium-Catalyzed Decarboxylative Coupling of Quinolinone-3-Carboxylic Acids and Related Heterocyclic Carboxylic Acids with (Hetero)aryl Halides. Org. Lett. 2012, 14, 1496–1499. 10.1021/ol300235k. [DOI] [PubMed] [Google Scholar]; b Min M.; Hong S. Regioselective Palladium-Catalyzed Direct Cross-Coupling of Coumarins with Simple Arenes. Chem. Commun. 2012, 48, 9613–9615. 10.1039/c2cc34551j. [DOI] [PubMed] [Google Scholar]; c Jun Y. W.; Kim H. R.; Reo Y. J.; Dai M.; Ahn K. H. Addressing the Autofluorescence Issue in Deep Tissue Imaging by Two-Photon Microscopy: The Significance of Far-Red Emitting Dyes. Chem. Sci. 2017, 8, 7696–7704. 10.1039/C7SC03362A. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Bassolino G.; Nancoz C.; Thiel Z.; Bois E.; Vauthey E.; Rivera-Fuentes P. Photolabile Coumarins with Improved Efficiency Through Azetidinyl Substitution. Chem. Sci. 2018, 9, 387–391. 10.1039/C7SC03627B. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Schill H.; Nizamov S.; Bottanelli F.; Bierwagen J.; Belov V. N.; Hell S. W. 4-Trifluoromethyl-Substituted Coumarins with Large Stokes Shifts: Synthesis, Bioconjugates, and Their Use in Super-Resolution Fluorescence Microscopy. Chem. - Eur. J. 2013, 19, 16556–16565. 10.1002/chem.201302037. [DOI] [PubMed] [Google Scholar]
- a Tsukamoto K.; Shinohara Y.; Iwasaki S.; Maeda H. A Coumarin-Based Fluorescent Probe for Hg2+ and Ag+ with an N’-Acetylthioureido Group as a Fluorescence Switch. Chem. Commun. 2011, 47, 5073–5075. 10.1039/c1cc10933b. [DOI] [PubMed] [Google Scholar]; b Miyaji H.; Kim H.-K.; Sim E.-K.; Lee C.-K.; Cho W.-S.; Sessler J. L.; Lee C.-H. Coumarin-Strapped Calix[4]pyrrole: A Fluorogenic Anion Receptor Modulated by Cation and Anion Binding. J. Am. Chem. Soc. 2005, 127, 12510–12512. 10.1021/ja053612y. [DOI] [PubMed] [Google Scholar]
- a Kim I.; Kim D.; Sambasivan S.; Ahn K. H. Synthesis of π-Extended Coumarins and Evaluation of Their Precursors as Reactive Fluorescent Probes for Mercury Ions. Asian J. Org. Chem. 2012, 1, 60–64. 10.1002/ajoc.201200034. [DOI] [Google Scholar]; b Kim D.; Singha S.; Wang T.; Seo E.; Lee J. H.; Lee S.-J.; Kim K. H.; Ahn K. H. In Vivo Two-Photon Fluorescent Imaging of Fluoride with a Desilylation-Based Reactive Probe. Chem. Commun. 2012, 48, 10243–10245. 10.1039/c2cc35668f. [DOI] [PubMed] [Google Scholar]
- a Liu Z.; Helander M. G.; Wang Z.; Lu Z. Efficient Single-Layer Organic Light-Emitting Diodes Based on C545T-Alq3 System. J. Phys. Chem. C 2010, 114, 11931–11935. 10.1021/jp101269r. [DOI] [Google Scholar]; b Zhang H.; Yu T.; Zhao Y.; Fan D.; Xia D.; Zhang P. Synthesis, Crystal Structure, Photo- and Electroluminescence of 3-(4-(anthracen-10-yl)phenyl)-7-(N,N‘-diethylamino)coumarin. Synth. Met. 2010, 160, 1642–1647. 10.1016/j.synthmet.2010.05.034. [DOI] [Google Scholar]
- a Adronov A.; Gilat S. L.; Fréchet J. M. J.; Ohta K.; Neuwahl F. V. R.; Fleming G. R. Light harvesting and energy transfer in laser- dye-labeled poly (aryl ether) dendrimers. J. Am. Chem. Soc. 2000, 122, 1175–1185. 10.1021/ja993272e. [DOI] [Google Scholar]; b Tasior M.; Gryko D. T.; Pielacińska D.; Zanelli A.; Flamigni L. Trans-A2B-Corroles Bearing a Coumarin Moiety-From Synthesis to Photophysics. Chem. - Asian J. 2010, 5, 130–140. 10.1002/asia.200900345. [DOI] [PubMed] [Google Scholar]
- a Mukhopadhyay A.; Hossen T.; Ghosh I.; Koner A. L.; Nau W. M.; Sahu K.; Moorthy J. N. Helicity-Dependent Regiodifferentiation in the Excited-State Quenching and Chiroptical Properties of Inward/Outward Helical Coumarins. Chem. - Eur. J. 2017, 23, 14797–14805. 10.1002/chem.201701787. [DOI] [PubMed] [Google Scholar]; b Reo Y. J.; Jun Y. W.; Cho S. W.; Jeon J.; Roh H.; Singha S.; Dai M.; Sarkar S.; Kim H. R.; Kim S.; Jin Y.; Jung Y. L.; Yang Y. J.; Ban C.; Joo J.; Ahn K. H. A Systematic Study on the Discrepancy of Fluorescence Properties Between in Solutions and in Cell: Super-Bright, Environment-Insensitive Benzocoumarin Dyes. Chem. Commun. 2020, 56, 10556–10559. 10.1039/D0CC03586F. [DOI] [PubMed] [Google Scholar]; c Nazir R.; Stasyuk A. J.; Gryko D. T. Vertically π-Expanded Coumarins: The Synthesis and Optical Properties. J. Org. Chem. 2016, 81, 11104–11114. 10.1021/acs.joc.6b02094. [DOI] [PubMed] [Google Scholar]; d Tasior M.; Kim D.; Singha S.; Krzeszewski M.; Ahn K. H.; Gryko D. T. π-Expanded Coumarins: Synthesis, Optical Properties and Applications. J. Mater. Chem. C 2015, 3, 1421–1446. 10.1039/C4TC02665A. [DOI] [Google Scholar]; e Zagotto G.; Palumbo M.; Uriarte E.; Bonsignore L.; Delogu G.; Podda G. Synthesis of 2H,9H-naphtho[2,3-b:7,6-b’]dipyran-2,9-diones as Potential DNA – Reactive Agents. Farmaco 1998, 53, 675–679. 10.1016/S0014-827X(98)00085-8. [DOI] [Google Scholar]; f Moorthy J. N.; Venkatakrishnan P.; Sengupta S.; Baidya M. Facile Synthesis, Fluorescence, and Photochromism of Novel Helical Pyrones and Chromenes. Org. Lett. 2006, 8, 4891–4894. 10.1021/ol0619260. [DOI] [PubMed] [Google Scholar]; g Wecławski M. K.; Deperasińska I.; Banasiewicz M.; Young D. C.; Leniak A.; Gryko D. T. Building Molecular Complexity from Quinizarin; Conjoined Coumarins and Coronene Analogs. Chem. - Asian J. 2019, 14, 1763–1770. 10.1002/asia.201800757. [DOI] [PubMed] [Google Scholar]; h Moorthy J. N.; Mandal S.; Mukhopadhyay A.; Samanta S. Helicity as a Steric Force: Stabilization and Helicity-Dependent Reversion of Colored o-Quinonoid Intermediates of Helical Chromenes. J. Am. Chem. Soc. 2013, 135, 6872–6884. 10.1021/ja312027c. [DOI] [PubMed] [Google Scholar]
- a Kim I.; Kim D.; Sambasivan S.; Ahn K. H. Synthesis of π-Extended Coumarins and Evaluation of Their Precursors as Reactive Fluorescent Probes for Mercury Ions. Asian J. Org. Chem. 2012, 1, 60–64. 10.1002/ajoc.201200034. [DOI] [Google Scholar]; b Dai M.; Sarkar S.; Song Ch. W.; Reo Y. J.; Yang Y. J.; Ahn K. H. Bent-Benzocoumarin Dyes that Fluoresce in Solution and in Solid State and Their Application to Bioimaging. Chem. Photo. Chem. 2020, 4, 721–728. 10.1002/cptc.202000059. [DOI] [Google Scholar]
- a Wecławski M. K.; Tasior M.; Hammann T.; Cywiński P. J.; Gryko D. T. From π-Expanded Coumarins to π-Expanded Pentacenes. Chem. Commun. 2014, 50, 9105–9108. 10.1039/C4CC03078H. [DOI] [PubMed] [Google Scholar]; b Poronik Y. M.; Gryko D. T. Pentacyclic Coumarin-Based Blue Emitters-the Case of Bifunctional Nucleophilic Behavior of Amidines. Chem. Commun. 2014, 50, 5688–5690. 10.1039/C4CC01106F. [DOI] [PubMed] [Google Scholar]
- Tasior M.; Poronik Y. M.; Vakuliuk O.; Sadowski B.; Karczewski M.; Gryko D. T. V-Shaped Bis-Coumarins: Synyhesis and Optical Properties. J. Org. Chem. 2014, 79, 8723–8732. 10.1021/jo501565r. [DOI] [PubMed] [Google Scholar]
- a Wang G.; Liu Y.; Zhang L.; An L.; Chen R.; Liu Y.; Luo Q.; Li Y.; Wang H.; Xue Y. Computational Study on the Antioxidant Property of Coumarin-Fused Coumarins. Food Chem. 2020, 304, 125446. 10.1016/j.foodchem.2019.125446. [DOI] [PubMed] [Google Scholar]; b Chen Ch; Zhou L.; Liu W.; Liu W. Coumarinocoumarin-Based Two-Photon Fluorescent Cysteine Biosensor for Targeting Lysosome. Anal. Chem. 2018, 90, 6138–6143. 10.1021/acs.analchem.8b00434. [DOI] [PubMed] [Google Scholar]; c Xi G.-L.; Liu Z.-Q. Coumarin-Fused Coumarin: Antioxidant Story from N,N-Dimethylamino and Hydroxyl Groups. J. Agric. Food Chem. 2015, 63, 3516–3523. 10.1021/acs.jafc.5b00399. [DOI] [PubMed] [Google Scholar]; d Chen Ch; Zhou L.; Liu F.; Li Z.; Liu W.; Liu W. V-Shaped Bis-Coumarin Based Fluorescent Probe for Detecting Palladium in Natural Waters. J. Hazard. Mater. 2020, 386, 121943. 10.1016/j.jhazmat.2019.121943. [DOI] [PubMed] [Google Scholar]
- a Shao Z.; Xu L.; Wang L.; Wei H.; Xiao J. Catalyst – Free Tandem Michael Addition/Decarboxylation of (Thio)coumarin-3-carboxylic Acids with Indoles: Facile Synthesis of Indole-3-substituted 3,4-dihydro(thio)coumarins. Org. Biomol. Chem. 2014, 12, 2185–2188. 10.1039/C3OB42582G. [DOI] [PubMed] [Google Scholar]; b Tang Y.; Ye M.-C.; Yang Y. Y.; Sun X.-L.; Ma Z.; Qin W. M. Diastereoselective Tandem Michael Additions of Indoles to 3-Nitrocoumarin Derivatives and Methyl Vinyl Ketone. Synlett 2006, 8, 1240–1244. 10.1055/s-2006-932472. [DOI] [Google Scholar]
- a Bu F.; Duan R.; Xie Y.; Yi Y.; Peng Q.; Hu R.; Qin A.; Zhao Z.; Tang B. Z. Unusual Aggregation-Induced Emission of a Coumarin Derivative as a Result of the Restriction of an Intramolecular Twisting Motion. Angew. Chem., Int. Ed. 2015, 54, 14492–14497. 10.1002/anie.201506782. [DOI] [PubMed] [Google Scholar]; b Ventura B.; Poronik Y. M.; Deperasińska I.; Gryko D. T. How a Small Structural Difference Can Turn Optical Properties of π-Extended Coumarins Upside Down: The Role of Non-Innocent Saturated Rings. Chem. - Eur. J. 2016, 22, 15380–15388. 10.1002/chem.201603038. [DOI] [PubMed] [Google Scholar]; c Chen J.; Liu W.; Ma J.; Xu H.; Wu J.; Tang X.; Fan Z.; Wang P. Synthesis and Properties of Fluorescence Dyes: Tetracyclic Pyrazolo[3,4-b]Pyridine-Based Coumarin Chromophores with Intramolecular Charge Transfer Character. J. Org. Chem. 2012, 77, 3475–3482. 10.1021/jo3002722. [DOI] [PubMed] [Google Scholar]
- Gawaskar S.; Schepmann D.; Bonifazi A.; Wünsch B. Synthesis, GluN2B Affinity and Selectivity of Benzo[7]annulen-7-amines. Bioorg. Med. Chem. 2014, 22, 6638–6646. 10.1016/j.bmc.2014.10.004. [DOI] [PubMed] [Google Scholar]
- a Gajewski J. J.Hydrocarbon Thermal Isomerizations; Academic Press: NY, 1981. [Google Scholar]; b Spangler C. W. Thermal [1,j] sigmatropic rearrangements. Chem. Rev. 1976, 76, 187–217. 10.1021/cr60300a002. [DOI] [Google Scholar]
- Moss S.; King B. T.; de Meijere A.; Kozhushkov S. I.; Eaton P. E.; Michl J. LiCB11Me12: A Catalyst for Pericyclic Rearrangements. Org. Lett. 2001, 3, 2375–2377. 10.1021/ol0161864. [DOI] [PubMed] [Google Scholar]
- Woodward R. B.; Hoffmann R.. The Conservation of Orbital Symmetry; Academic Press: NY, 1970; pp 114–140. [Google Scholar]
- Hess B. A. Jr.; Baldwin J. E. [1,5] Sigmatropic Hydrogen Shifts in Cyclic 1,3-Dienes. J. Org. Chem. 2002, 67, 6025–6033. 10.1021/jo025917q. [DOI] [PubMed] [Google Scholar]
- a Wróbel Z.; Kwast A. Simple Synthesis of N-Aryl-2-Nitrosoanilines in the Reaction of Nitroarenes with Aniline Anion Derivatives. Synthesis 2010, 22, 3865–3872. 10.1055/s-0030-1258230. [DOI] [Google Scholar]; b Makosza M.; Wojciechowski K. Synthesis of Heterocycles via Nucleophilic Substitution of Hydrogen in Nitroarenes. Heterocycles 2014, 88, 75–101. 10.3987/REV-13-SR(S)1. [DOI] [Google Scholar]
- a Zhang X.-S.; Li Z.-W.; Shi Z.-J. Palladium-Catalyzed Base-Accelerated Direct C-H Bond Alkenylation of Phenols to Synthesize Coumarin Derivatives. Org. Chem. Front. 2014, 1, 44–49. 10.1039/C3QO00010A. [DOI] [Google Scholar]; b Sharma U.; Naveen T.; Maji A.; Manna S.; Maiti D. Palladium-Catalyzed Synthesis of Benzofurans and Coumarins from Phenols and Olefins. Angew. Chem., Int. Ed. 2013, 52, 12669–12673. 10.1002/anie.201305326. [DOI] [PubMed] [Google Scholar]; c Gadakh S. K.; Dey S.; Sudalai A. Rh-Catalyzed Synthesis of Coumarin Derivatives from Phenolic Acetates and Acrylates via C-H Bond Activation. J. Org. Chem. 2015, 80 (0), 11544–11550. 10.1021/acs.joc.5b01713. [DOI] [PubMed] [Google Scholar]
- Mallouli A.; Lepage Y. Convenient Syntheses of Naphthalene,- Anthracene,- and Naphthacene-2,3-dicarboxaldehydes. Synthesis 1980, 1980, 689. 10.1055/s-1980-29170. [DOI] [Google Scholar]
- Alkiş M.; Pekyilmaz D.; Yalçin E.; Aydiner B.; Dede Y.; Seferoğlu Z. H-Bond Stabilization of a Tautomeric Coumarin-Pyrazole-Pyridine Triad Generates a PET Driven, Reversible and Reusable Fluorescent Chemosensor for Anion Detection. Dyes Pigm. 2017, 141, 493–500. 10.1016/j.dyepig.2017.03.011. [DOI] [Google Scholar]
- Seydimemet M.; Ablajan K.; Hamdulla M.; Li W.; Omar A.; Obul M. L-Proline Catalyzed Four-Component One-Pot Synthesis of Coumarin-Containing Dihydropyrano[2,3-c]pyrazoles Under Ultrasonic Irradiation. Tetrahedron 2016, 72, 7599–7605. 10.1016/j.tet.2016.10.016. [DOI] [Google Scholar]
- Pandit R. P.; Lee Y. R. Construction of Multifunctionalized Azopyrazoles by Silver-Catalyzed Cascade Reaction of Diazo Compounds. Adv. Synth. Catal. 2015, 357, 2657–2664. 10.1002/adsc.201500197. [DOI] [Google Scholar]
- a Kagaku O.; Kaisha K. Patent Application US 1997. /005639776A.; b Tada I.; Motoki M.; Takahashi N.; Miyata T.; Takechi T.; Uchida T.; Takagi Y. Synthesis and Structure-Activity Relationships of Miticidal 4,5-Dihydropyrazole-5-thiones. Pestic. Sci. 1996, 48, 165–173. . [DOI] [Google Scholar]
- Desroses M.; Jacques-Cordonnier M.-C.; Llona-Minguez S.; Jacques S.; Koolmeister T.; Helleday T.; Scobie M. A Convenient Microwave-Assisted Propylphosphonic Anhydride (T3P) Mediated One-Pot Pyrazolone Synthesis. Eur. J. Org. Chem. 2013, 2013, 5879–5885. 10.1002/ejoc.201300380. [DOI] [Google Scholar]
- a Rtishchev N. I.; Nosova G. I.; Solovskaya N. A.; Lukyaashina V. A.; Galaktionova E. F.; Kudryavtsev V. V. Photosensitization of Conjugated Bischalcone Derivatives. New T-Sensitizers. Russ. J. Gen. Chem. 2002, 72, 1942–1950. 10.1023/A:1023471514381. [DOI] [Google Scholar]; b Aliaga M. E.; Garcia-Rio L.; Numi A.; Rodriguez A.; Arancibia-Opazo S.; Fierro A.; Cañete A. Controlled Keto-Enol Tautomerism of Coumarin Containing β-Ketodithioester by its Encapsulation in Cucurbit[7]uril. New J. Chem. 2017, 41, 15574–15580. 10.1039/C7NJ03265J. [DOI] [Google Scholar]
- Vitorio F.; Pereira T. M.; Castro R. N.; Guedes G. P.; Graebin C. S.; Kummerle A. E. Synthesis and Mechanism of Novel Fluorescent Coumarin-Dihydropyrimidinone Dyads Obtained by the Biginelli Multicomponent Reaction. New J. Chem. 2015, 39, 2323–2332. 10.1039/C4NJ02155J. [DOI] [Google Scholar]
- Babür B.; Seferoğlu N.; Seferoğlu Z. A Ratiometric Fluorescence Chemosensor Based on a Coumarin-Pyrazolone Hybrid: The Synthesis and an Investigation of the Photophysical, Tautomeric and Anion Binding Properties by Spectroscopic Techniques and DFT Calculations. Tetrahedron Lett. 2015, 56, 2149–2154. 10.1016/j.tetlet.2015.03.014. [DOI] [Google Scholar]
- a Uchiyama S.; Takehira K.; Yoshihara T.; Tobita S.; Ohwada T. Environment-Sensitive Fluorophore Emitting in Protic Environments. Org. Lett. 2006, 8, 5869–5872. 10.1021/ol062490r. [DOI] [PubMed] [Google Scholar]; b Kellmann A. Intersystem Crossing and Internal Conversion Quantum Yields of Acridine in Polar and Nonpolar Solvents. J. Phys. Chem. 1977, 81, 1195–1198. 10.1021/j100527a014. [DOI] [Google Scholar]; c Kalyanasundaram K.; Thomas J. K. Solvent-dependent fluorescence of pyrene-3-carboxaldehyde and its applications in the estimation of polarity at micelle-water interfaces. J. Phys. Chem. 1977, 81, 2176–2180. 10.1021/j100538a008. [DOI] [Google Scholar]; d de Melo J. S. S.; Becker R. S.; Macüanita A. L. Photophysical Behavior of Coumarins as a Function of Substitution and Solvent: Experimental Evidence for the Existence of a Lowest Lying 1(nπ*) State. J. Phys. Chem. 1994, 98, 6054–6058. 10.1021/j100075a002. [DOI] [Google Scholar]
- Weigend F.; Häser M. RI-MP2: First Derivatives and Global Consistency. Theor. Chem. Acc. 1997, 97, 331–340. 10.1007/s002140050269. [DOI] [Google Scholar]
- Schirmer J. Beyond the Random-Phase Approximation: A New Approximation Scheme for the Polarization Propagator. Phys. Rev. A: At., Mol., Opt. Phys. 1982, 26, 2395–2416. 10.1103/PhysRevA.26.2395. [DOI] [Google Scholar]
- Trofimov B.; Schirmer J. An Efficient Polarization Propagator Approach to Valence Electron Excitation Spectra. J. Phys. B: At., Mol. Opt. Phys. 1995, 28, 2299–2324. 10.1088/0953-4075/28/12/003. [DOI] [Google Scholar]
- Hättig Ch Structure Optimizations for Excited States with Correlated Second-Order Methods: CC2 and ADC(2). Adv. Quantum Chem. 2005, 50, 37–60. 10.1016/S0065-3276(05)50003-0. [DOI] [Google Scholar]
- Dunning T. H. Gaussian Basis Sets for Use in Correlated Molecular Calculations. I. The Atoms Boron Through Neon and Hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. 10.1063/1.456153. [DOI] [Google Scholar]
- Christiansen O.; Koch H.; Jørgensen P. The Second-Order Approximate Coupled Cluster Singles and Doubles Model CC2. Chem. Phys. Lett. 1995, 243, 409–418. 10.1016/0009-2614(95)00841-Q. [DOI] [Google Scholar]
- Jung Y.; Lochan R. C.; Dutoi A. D.; Head-Gordon M. Scaled Opposite-Spin Second Order Moller-Plesset Correlation Energy: An Economical Electronic Structure Method. J. Chem. Phys. 2004, 121, 9793–9802. 10.1063/1.1809602. [DOI] [PubMed] [Google Scholar]
- Hellweg A.; Grün S. A.; Hattig C. Benchmarking the Performance of Spin-Component Scaled CC2 in Ground and Electronically Excited States. Phys. Chem. Chem. Phys. 2008, 10, 4119–4127. 10.1039/b803727b. [DOI] [PubMed] [Google Scholar]
- Jacquemin D.; Duchemin I.; Blasé X. 0–0 Energies Using Hybrid Schemes: Benchmarks of TD-DFT, CIS(D), ADC(2), CC2 and BSE/GW Formalisms for 80 Real-Life Compounds. J. Chem. Theory Comput. 2015, 11, 5340–5359. 10.1021/acs.jctc.5b00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vérité P. M.; Hédé S.; Jacquemin D. A Theoretical Elucidation of the Mechanism of Tuneable Fluorescence in a Full-Colour Emissive ESIPT Dye. Phys. Chem. Chem. Phys. 2019, 21, 17400–17409. 10.1039/C9CP03759D. [DOI] [PubMed] [Google Scholar]
- Barboza C. A.; Gawrys P.; Banasiewicz M.; Suwinska K.; Sobolewski A. L. Photophysical Transformations Induced by Chemical Substitution to Salicylaldimines. Phys. Chem. Chem. Phys. 2020, 22, 6698–6705. 10.1039/D0CP00110D. [DOI] [PubMed] [Google Scholar]
- a Kwon J. E.; Park S. Y. Advanced Organic Optoelectronic Materials: Harnessing Excited-State Intramolecular Proton Transfer (ESIPT) Process. Adv. Mater. 2011, 23, 3615–3642. 10.1002/adma.201102046. [DOI] [PubMed] [Google Scholar]; b Barbara P. F.; Brus L. E.; Rentzepis P. M. Intramolecular Proton Transfer and Excited-State Relaxation in 2-(2-Hydroxyphenyl)benzothiazole. J. Am. Chem. Soc. 1980, 102, 5631–5635. 10.1021/ja00537a038. [DOI] [Google Scholar]; c Elsaesser P. T.; Kaiser W. Visible and Infrared Spectroscopy of Intramolecular Proton Transfer Using Picosecond Laser Pulses. Chem. Phys. Lett. 1986, 128, 231–237. 10.1016/0009-2614(86)80331-1. [DOI] [Google Scholar]; d Stasyuk A. J.; Cywiński P.; Gryko D. T. Excited-State Intramolecular Proton Transfer in 2-(2′-Hydroxyphenyl)imidazo[1,2-a]pyridines. J. Photochem. Photobiol., C 2016, 28, 116–137. 10.1016/j.jphotochemrev.2016.05.003. [DOI] [Google Scholar]
- Birks J. B.Photophysics of Aromatic Molecules; Wiley: London, 1979; pp 97–100. [Google Scholar]
- Demas J. N.Excited State Lifetime Measurements; Academic Press: NY, 1983; pp 89–92. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.