Abstract
Divergent total syntheses of 10 yaequinolone-related natural products have been achieved for the first time by late-stage C–H olefination of 3,4-dioxygenated 4-aryl-5-hydroxyquinolin-2(1H)-ones, core structures of this family of natural products. A robust synthetic methodology to construct the core structures has been established, and the C–H olefination reaction has been carried out with synthetically useful yields and high levels of site-selectivity under mild reaction conditions in the presence of a Pd/S,O-ligand catalyst.
Introduction
Yaequinolones and related compounds that comprise 3,4-dioxygenated 4-aryl-quinolin-2(1H)-one cores represent a growing family of biologically active alkaloids isolated from marine and plant fungi (Figure 1a).1 The first two members, NTC-47A and B, were isolated from the second metabolites of Penicillium sp. by Nakaya in 1995 and they showed promising insecticidal activities.2 Since then, many more related compounds have been discovered and their structures, bioactivities, and biosynthetic mechanisms have been intensively studied.3−6 Structurally, the two oxygenated functional groups at 3- and 4-positions have a cis-configuration for most of the members.7 Moreover, the majority of these natural products possess a hydroxyl group at the 5-position and only differ from each other in the olefin moiety present at the 6-position (Figure 1b).
Figure 1.
Structures of yaequinolone-related natural products.
This family of natural products has attracted great attention in the last few years due to their unique structures and biological activities, and the total syntheses of few members of this family of natural products have been reported. The first total synthesis of this family of natural products was the synthesis of (±)-yaequinolone A2, the structurally simplest molecule among the family (Scheme 1a).8 The key step of this approach is the intramolecular aldol reaction of N-glycolated 2-aminobenzophenone to construct the quinolone core, generating the two oxygenated functional groups in a cis-fashion. The first enantioselective total syntheses of (−)-yaequinolones J1 and J2 were reported in 2018 by the group of Hanessian (Scheme 1b).9 The authors used an Evans-type chiral auxiliary to provide the syn-aldol diol as a 1:1 mixture of diastereoisomers that differ in the configuration of the pyranyl tertiary methyl group. The group of Christmann successfully synthesized (±)-peniprequinolone, (±)-aflaquinolones E and F, (±)-6-deoxyaflaquinolone E, (±)-quinolinones A and B, and (±)-aniduquinolone C by developing a general method for the construction of N-glycolated 2-aminobenzophenone via aryne insertions into unsymmetrical imides in flow (Scheme 1c).10
Scheme 1. Total Syntheses of Yaequinolone-Related Natural Products.
In spite of this progress, a general strategy to access these natural products in a direct and divergent manner is still elusive.11 As many members of this family of natural products (>20) only differ from each other in the structure of the olefin at the 6-position, we envisioned that their syntheses can be achieved from a key common intermediate in a divergent manner using late-stage site-selective C–H olefination.12 Herein, we report a new methodology for the late-stage C(6)–H olefination of 3,4-dioxygenated 4-aryl-5-hydroxy-quinolin-2(1H)-ones and its successful application in the divergent total syntheses of 10 yaequinolone-related natural products, which are synthesized for the first time (Scheme 1d).
Results and Discussion
In 2019, we reported a highly selective C(6)–H olefination reaction of tetrahydroquinolines (THQs) in the presence of a Pd/S,O-ligand catalyst (Scheme 2a).13 We hypothesized that late-stage selective C(6)–H olefination of 3,4-dioxygenated 4-aryl-5-hydroxy-quinolin-2(1H)-one cores could be developed in the presence of a Pd/S,O-ligand catalyst to directly access yaequinolone-related natural products in a divergent manner.
Scheme 2. Reactivity Comparison of THQs and 1a.
First, we evaluated the C–H olefination reaction of N-methyl-3,4-dihydroquinolin-2(1H)-one (1a), the simplified backbone of yeaquinolone-related natural products, with ethyl acrylate under reported reaction conditions for the olefination of THQs. As expected, because the aromatic ring is less activated,13,14 the reaction only provided the olefinated product in less than 10% 1H NMR yield (Scheme 2b). When the reaction was performed at 80 °C, a slightly higher yield of 23% was achieved (Scheme 2b).
Because all our target natural products have a hydroxyl group at the 5-position, we decided to investigate the effect of this functional group on the reactivity of the C–H olefination of quinolin-2(1H)-ones. We tested N-methyl-3,4-dihydro-2(1H)-quinolinones bearing an OMe, OEt, or/and OMOM group at the 5-position (2a–4a, Scheme 3). As expected, because these substrates are more activated, synthetically useful yields (45–57%) were observed and the C(6)-olefinated products were exclusively formed. The reaction of the OMe derivative 2a without the S,O-ligand only gave a trace amount of olefinated product, highlighting the key role of the S,O-ligand in this transformation. We also evaluated the olefination reaction of substrate 5a bearing a free OH group at the 5-position, but the olefinated product 5b was obtained only in 27% yield due to its instability under the reaction conditions.15 Having established the beneficial effect of the oxygenated moiety at the 5-position in the C–H olefination reaction, we decided to evaluate other protecting groups at the nitrogen atom. The reaction with the MOM-protected substrate 6a provided the desired olefinated product 6b in 46% yield with slightly lower C(6)-selectivity. When the benzyl-protected substrate 7a was subjected to the standard reaction conditions, the olefinated product was obtained in 48% yield with good C(6)-selectivity.
Scheme 3. C–H Olefination of 5-Oxgenated N-Methyl-3,4-dihydro-2(1H)-quinolinones,b,c.
Reaction conditions: a (0.1 mmol), ethyl acrylate (0.15 mmol), Pd(OAc)2 (0.01 mmol), S,O-ligand (0.01 mmol), and PhCO3tBu (0.1 mmol) in DCE (0.5 mL) at 80 °C for 16 h. bIsolated yield. cRegioselectivity was determined based on the analysis of crude mixture.
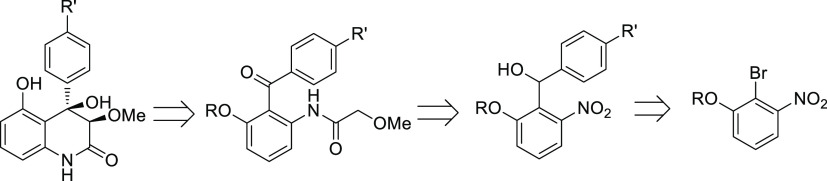
After having proved the suitability of the C–H olefination reaction on model substrates, we proposed the retrosynthetic route for 3,4-dioxygenated 4-aryl-5-hydroxy-quinolin-2(1H)-ones as outlined in Figure 2. 3,4-dioxygenated 4-aryl-5-hydroxy-quinolin-2(1H)-one can be obtained by cyclization as reported by the group of She.8 The starting diarylketone can be synthesized from the nucleophilic addition of the lithiated 3-oxygenated 2-bromonitrobenzene, which can be prepared from commercially available 2-bromo-3-nitrophenol, to para-anisaldehyde, followed by oxidation.
Figure 2.
Retrosynthetic analysis of 3,4-dioxygenated 5-hydroxy-4-aryl-quinolin-2(1H)-ones.
The synthesis of the core structure is shown in Scheme 4. 2-Bromo-3-nitrophenol (8) was first protected with a benzyl group in 91% yield, followed by lithiation at low temperature and reaction with para-anisaldehyde, providing the corresponding alcohol 10 in 86% yield. We then reduced the nitro group using sodium sulfide to form the aniline intermediate 11, followed by amide formation with methoxyacetyl chloride. The formed amide intermediate 12 was oxidized with PCC, providing the ketone 13 in 94% yield. The cyclization of 13 under reported reaction conditions produced (±)-14 in 80% yield as a single diastereoisomer.8 Then, we decided to protect the nitrogen atom with 2-(trimethylsilyl)ethoxymethyl (SEM), as it can be deprotected using mild reagents, such as tetrabutylammonium fluoride (TBAF).16 Then, we introduced the SEM-protecting group to (±)-14 using LiHMDS as the base. SEM-protected 3,4-dihydro-2(1H)-quinolinone derivative (±)-16 was obtained in 95% yield after benzyl deprotection of (±)-15 using Pd/C and H2 (Scheme 4).
Scheme 4. Synthesis of the Core Structure (±)-16.
For the C–H olefination of (±)-16, we slightly optimized the reaction conditions and found that the best conditions were 2.0 equiv of PhCO3tBu in dichloroethane (DCE) at 80 °C for 16 h, providing the olefinated product (±)-17 in 59% isolated yield with a regioselectivity of 6:1 (A/B) together with 5% of the diolefinated product (Table 1). However, the regioisomers were not separable by flash column chromatography. We, therefore, continued the total synthesis with the mixture of regioisomers.
Table 1. Reaction Conditions for the C–H Olefination of (±)-16.
| entry | temp. (°C) | x | time (h) | 1H NMR yielda |
|---|---|---|---|---|
| 1 | 80 | 1.5 | 16 | 49% |
| 2 | 60 | 1.5 | 16 | 31% |
| 3 | 80 | 1.5 | 6 | 45% |
| 4 | 80 | 1.0 | 16 | 42% |
| 5 | 80 | 2.0 | 16 | 54% (59%b,c+ 5%b,d) |
| 6e | 80 | 1.5 | 16 | 55% |
1H NMR yield of the desired regioisomer was determined by using CH2Br2 as the internal standard.
Isolated yield.
Regioselectivity was determined from the crude mixture: A/B = 6:1.
Diolefinated product.
15 mol % of Pd(OAc)2 and S,O-ligand were used.
We performed the deprotection of the SEM group with TBAF, and it was found out that a high concentration of TBAF is required to obtain the desired product in good yield. For instance, when the concentration of TBAF was 0.1 M, no product was observed even after refluxing the reaction for 20 h. When we increased the concentration to 1.0 M, to our delight, the reaction reached full conversion after refluxing for 15 h and (±)-yaequinolone B was obtained in 60% isolated yield as a single regioisomer (Scheme 5). Its 1H and 13C NMR data matched with those reported in the literature.4c Hence, the total synthesis of (±)-yaequinolone B was achieved in 10.6% overall yield in 10 synthetic steps starting from commercially available 2-bromo-3-nitrophenol.
Scheme 5. Synthesis of (±)-Yaequinolone B.
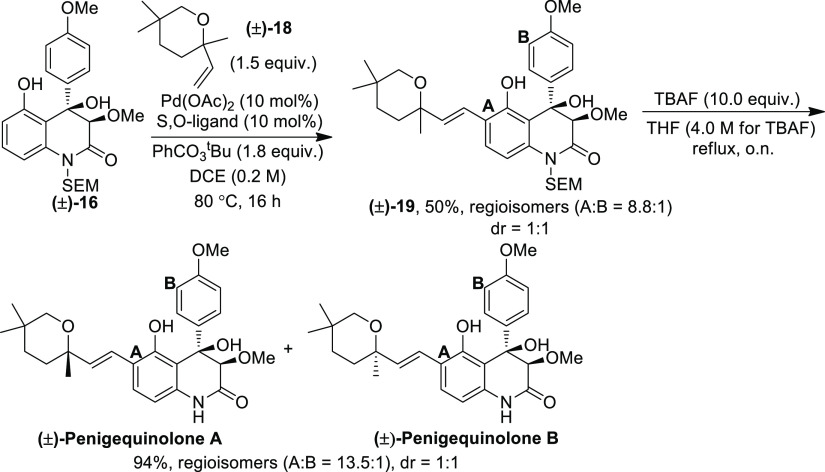
Encouraged by these results, we moved our attention to the total syntheses of (±)-penigequinolones A and B, which can be accomplished by only changing the olefin coupling partner while keeping the same core structure (±)-16. Olefin (±)-18 was prepared in six synthetic steps starting from ethyl isobutyrate (see the Experimental Section). The C–H olefination of (±)-16 using (±)-18 as the olefin under standard reaction conditions furnished the olefinated product (±)-19 in 50% yield with a regioselectivity of 8.8:1 (A/B) and a diastereoselectivity of 1:1. The SEM deprotection of (±)-19 with TBAF (4.0 M) almost quantitatively yielded a mixture of (±)-penigequinolones A and B (dr = 1:1) with improved regioselectivity (13.5:1) (Scheme 6). Their 1H and 13C NMR data matched with those reported in the literature.4c
Scheme 6. Syntheses of (±)-Penigequinolones A and B.
For the synthesis of (±)-yaequinolone C, as the relative stereochemistry of the olefin moiety was not determined in the initial report from the group of O̅mura, we decided to first try the olefin (±)-trans-20 (see the Experimental Section).4c To our delight, the olefinated product (±)-21 was isolated in 52% yield with a regioselectivity of 7:1 (A/B) (Scheme 7a). However, we observed the formation of four diastereoisomers, indicating that racemization of one of the stereocenters of the olefin moiety has occurred. To prove this, we performed the olefination of (±)-16 with a mixture of (±)-trans- and (±)-cis-20 (1:1), and we observed the formation of the same four diastereoisomers (Scheme 7b). Moreover, we further tried the olefination of the model substrate 5a with (±)-trans-20 and with a 1:1 mixture of (±)-trans- and (±)-cis-20 and in both cases, the olefinated product was obtained as a mixture of diastereoisomers in almost 1:1 ratio, confirming that racemization has occurred in one of the stereocenters of the olefin moiety during the C–H olefination (Scheme 7c).17
Scheme 7. C–H Olefination of (±)-16 and 5a with (±)-19.
The deprotection of the olefinated product (±)-21 (A/B = 7:1) using 4.0 M of TBAF reached full conversion after refluxing overnight, providing (±)-yaequinolone C together with three other diastereoisomers in 79% isolated yield with an improved regioselectivity of 10.4:1 (Scheme 8). Based on the results obtained from the reaction of (±)-16 with (±)-trans-20 and with a 1:1 mixture of (±)-trans- and (±)-cis-20, we proposed that the olefin moiety has a trans-configuration (see Supporting Information). Therefore, the structure of (±)-yaequinolone C is either 22-A or 22-B.
Scheme 8. Synthesis of (±)-Yaequinolone C.
In an effort to synthesize (±)-aspoquinolones C and D, we performed the olefination reaction of (±)-16 with (±)-cis-23, but no desired product was observed and (±)-16 was fully recovered (Scheme 9a). Considering that the free hydroxyl group from the olefin might be the problem, we protected the OH of (±)-cis-23 with the TMS group. The reaction of (±)-16 with TMS-protected olefin (±)-cis-24 gave a mixture of TMS-protected and unprotected olefinated products with 50% conversion (Scheme 9b). To simplify the purification, we directly treated the crude mixture with TBAF to deprotect the TMS group.18 After purification, the olefinated product (±)-25 was isolated in 43% yield as a single regioisomer [the other regioisomer was detected by 1H NMR analysis of the crude and separated by preparative thin layer chromatography (TLC)]. Unfortunately, like in the previous case, epimerization also occurred and four diastereoisomers were detected with a ratio of 2.2:2.2:1:1. The SEM deprotection of (±)-25 with TBAF led to full decomposition of the starting material. Then, we performed the deprotection reaction using Me2AlCl.19 This two-step deprotection procedure provided (±)-aspoquinolones C and D together with two other diastereoisomers with diastereoselectivity (2.2:2.2:1:1) remaining in 73% combined yield (Scheme 9c). As the relative stereochemistry of the olefin moieties of aspoquinolones C and D was not determined in the initial report,4b we propose, based on our results, that one has the cis-configuration on the olefin moiety and the other one has the trans-configuration.
Scheme 9. Syntheses of (±)-Aspoquinolones C and D.
After proving the synthetic power of late-stage C–H olefination by the Pd/S,O-ligand catalyst in the divergent synthesis of (±)-yaequinolones B and C, (±)-penigequinolones A and B, and (±)-aspoquinolones C and D, we decided to move our attention to the total syntheses of another class of closely related quinolone natural products, which all share a slightly different backbone (a phenyl group located at the 4-position instead of a 4-methoxyphenyl group). We prepared the corresponding 3,4-dihydro-2(1H)-quinolinone derivative (±)-27 in seven synthetic steps starting from 9 using the same procedure for the preparation of (±)-16 (see Experimental Section) (Scheme 10).
Scheme 10. Syntheses of (±)-Aflaquinolones A, C, and D and (±)-Scopuquinolone B.
To synthesize (±)-aflaquinolones A, C, and D, we prepared olefin (±)-28 as a mixture of diastereoisomers (trans/cis = 5:4) in seven synthetic steps starting from ethyl 4-oxocyclohexanecarboxylate (see the Experimental Section).20 The coupling of this unactivated olefin 28 with (±)-27 gave the olefinated product (±)-29 in 34% yield with perfect regioselectivity (>20:1) and with a diastereoselectivity of 1:1:1.6:1.6. The SEM deprotection of (±)-29 with TBAF failed to give any desired product, while the reaction with Me2AlCl provided (±)-aflaquinolones A, C, and D together with another diastereoisomer in near-quantitative yield with a diastereoselectivity of 1:1:1:1. Thus, epimerization occurred during the deprotection reaction probably at the α-position of the ketone.
Our last target was the synthesis of (±)-scopuquinolone B that can be synthesized by coupling the core structure (±)-27 with the trans-olefin (±)-31. The olefination reaction of (±)-27 with olefin (±)-31 furnished the olefinated product (±)-32 in 39% yield with a diastereoselectivity of 1:1:1:1. The SEM deprotection of (±)-32 with Me2AlCl provided (±)-scopuquinolone B together with three other diastereoisomers in 89% yield with retained diastereoselectivity of 1:1:1:1.
Conclusions
In summary, we have developed novel divergent syntheses of 10 yaequinolone-related natural products, which are synthesized for the first time, by late-stage C–H olefination of 3,4-dioxygenated 4-aryl-5-hydroxyquinolin-2(1H)-ones, core structures of this family of natural products. A robust synthetic methodology is established to construct the core structures and the C–H olefination reaction is efficient and site-selective under mild reaction conditions in the presence of a Pd/S,O-ligand catalyst. The power of the olefination reaction was showcased by successfully using unactivated olefins as coupling partners. Through this synthetic approach, the relative configuration of the olefin moiety of some of these natural products has been elucidated. Our future efforts will be devoted toward the divergent enantioselective total syntheses of this family of natural products.
Experimental Section
General Information
Chromatography: Silicycle Silica Flash P60 size 40–63 μm (230–400 mesh), TLC: Merck silica gel 60 (0.25 mm), and preparative TLC: Analtech silica gel G 1500 μm 20 × 20 cm. Visualization of the TLC plate was performed with phosphomolybdic acid or KMnO4 staining reagent and UV light. Mass spectra were recorded on AccuTOF GC v 4g, JMS-T100GCV mass spectrometers. 1H and 13C were recorded on Bruker 500 AMX, 400 and Bruker DRX 300 using CDCl3 as the solvent otherwise it will be noted. Chemical shift values are reported in ppm with the solvent resonance as the internal standard (CDCl3: δ 7.26 for 1H and δ 77.16 for 13C; dimethyl sulfoxide: δ 2.50 for 1H and δ 39.52 for 13C; and acetone: δ 2.05 for 1H and δ 206.26 for 13C). Data are reported as follows: chemical shifts, multiplicity (s = singlet, d = doublet, dd = doublet of doublets, t = triplet, q = quartet, and m = multiplet), coupling constants (Hz), and integration. IR spectra were recorded on a Bruker Alpha Fourier transform infrared machine and wavelengths are reported in cm–1. The melting point was measured in melting point apparatus Büchi M-565. Tetrahydrofuran (THF) and diethyl ether were dried over Na using benzophenone as the indicator. Dichloromethane was dried over CaH2 and was used freshly after distillation. Anhydrous dimethylformamide (DMF) was purchased from Acros and used as received. Absolute ethanol was purchased from VWR Amsterdam and was used as received. TBAF solution (1.0 M in THF) was purchased from Fluorochem And Pd(OAc)2 was purchased from Strem. The S,O-ligand was prepared using the procedure reported in the literature.13b
Synthesis of Olefins
2,5,5-Trimethyl-2-vinyltetrahydro-2H-pyran (18) was prepared using the following procedures: Step A: In a flame-dried Schlenk flask were added ethyl isobutyrate (5.563 mL, 4.84 g, 41.7 mmol, 1.0 equiv) and anhydrous THF (40 mL) under N2. In another flask, lithium diisopropylamide (LDA) (50 mmol, 1.2 equiv) was prepared and transferred to the substrate flask at −78 °C via a cannula over a period of 0.5 h. The reaction was stirred for 2 h before 1-bromo-2-chloroethane (8.613 mL, 14.34 g, 100 mmol, 2.4 equiv) was added. The reaction was then warmed up to room temperature and stirred overnight. The reaction was quenched by adding saturated aqueous NH4Cl solution and was extracted with EtOAc three times. The combined extracts were washed with brine, dried over Na2SO4, and evaporated in vacuo. The product was purified by flash column chromatography (PE/Et2O, 100:1 to 50:1) giving ethyl 4-chloro-2,2-dimethylbutanoate (Int-A) as a colorless oil (6.10 g, 82%). Its 1H NMR data matched with those reported in the literature.211H NMR (400 MHz): δ 4.16 (q, J = 7.1 Hz, 2H), 3.57–3.48 (m, 2H), 2.12–2.03 (m, 2H), 1.28 (t, J = 7.1 Hz, 3H), 1.24 (s, 6H).
Step B: In a flame-dried Schlenk flask were added LiBH4 (0.92 g, 42 mmol, 1.5 equiv) and anhydrous DCM (20 mL). To this suspension was then slowly added MeOH (1.7 mL). After the evolution of H2 has ceased, Int-A (5.00 g, 28 mmol, 1.0 equiv) was added. The reaction was then heated under reflux at 45 °C overnight. The reaction was quenched by carefully adding water and the mixture was extracted with EtOAc three times. The combined extracts were washed with brine, dried over Na2SO4, and evaporated in vacuo. The product was purified by flash column chromatography (PE/Et2O, 100:1 to 1:1) giving 4-chloro-2,2-dimethylbutan-1-ol (Int-B) as a colorless oil (2.50 g, 65%). Its 1H NMR data matched with those reported in the literature.221H NMR (300 MHz): δ 3.64–3.51 (m, 2H), 3.35 (d, J = 0.7 Hz, 2H), 1.88–1.75 (m, 2H), 0.93 (s, 6H).
Step C: In a round-bottom flask were successively added Int-B (1.37 g, 10 mmol, 1.0 equiv), DCM (15 mL), and TBSCl (3.01 g, 20 mmol, 2.0 equiv). After stirring the mixture for 5 min, 1-imidazole (1.36 g, 20 mmol, 2.0 equiv) was then added and the stirring was continued for another 2 h. Saturated aqueous NH4Cl solution was added to quench the reaction and the mixture was extracted with EtOAc three times. The combined extracts were washed with brine, dried over Na2SO4, and evaporated in vacuo. The product was purified by flash column chromatography (PE/Et2O, 20:1) giving tert-butyl(4-chloro-2,2-dimethylbutoxy)dimethylsilane (Int-C) as a colorless oil (2.50 g, quantitative yield). 1H NMR (300 MHz): δ 3.62–3.50 (m, 2H), 3.25 (s, 2H), 1.84–1.72 (m, 2H), 0.89 (s, 9H), 0.81 (s, 6H), 0.03 (s, 6H). 13C{H} NMR (75 MHz): δ 71.7, 42.7, 41.8, 36.0, 26.0, 24.3, 18.4, −5.4. HRMS (FD) m/z: [M – tBu]+ calcd for C8H18ClOSi+, 193.0815; found, 193.0854.
Step D: In a flame-dried Schlenk flask were added Int-C (1.50 g, 5.98 mmol, 1.0 equiv), Mg (0.18 g, 7.41 mmol, 1.24 equiv), and anhydrous THF (2 mL) under N2. The mixture was then heated to reflux and a few drops of 1,2-dibromoethane were slowly added to the reaction. The reaction was continued to stir for another 6 h while refluxing at 80 °C. In another flame-dried Schlenk flask were added anhydrous CeCl3 (1.47 g, 5.98 mmol, 1.0 equiv) and anhydrous THF (12 mL), and the suspension was stirred at room temperature for 1 h. The freshly prepared Grignard reagent was transferred to the suspension via a cannula at 0 °C. The reaction was further stirred at 0 °C for 1 h before but-3-en-2-one (1.0 mL, 11.96 mmol, 2.0 equiv) was added. After stirring it at 0 °C for 1 h, the reaction was quenched by adding saturated aqueous NH4Cl solution and the mixture was extracted with EtOAc three times. The combined extracts were washed with brine, dried over Na2SO4, and evaporated in vacuo. The product was purified by flash column chromatography (PE/EtOAc, 15:1) giving 7-[(tert-butyldimethylsilyl)oxy]-3,6,6-trimethylhept-1-en-3-ol (Int-D) as a colorless oil (1.01 g, 59%). 1H NMR (400 MHz): δ 5.89 (dd, J = 17.4, 10.7 Hz, 1H), 5.20 (dd, J = 17.3, 1.4 Hz, 1H), 5.04 (dd, J = 10.8, 1.4 Hz, 1H), 3.22 (s, 2H), 1.51–1.46 (m, 2H), 1.27 (s, 3H), 1.25–1.20 (m, 2H), 0.89 (s, 9H), 0.02 (s, 6H). 13C{H} NMR (75 MHz): δ 145.4, 111.8, 77.4, 73.5, 71.4, 36.5, 35.0, 32.5, 27.8, 26.1, 24.3, 24.3, 18.4, −5.4. HRMS (EI) m/z: [M]+ calcd for C16H34O2Si+, 286.2328; found, 286.2295.
Step E: In a round-bottom flask were added Int-D (1.00 g, 3.49 mmol, 1.0 equiv) and THF (15 mL). TBAF solution (1.0 M in THF, 8.7 mL, 8.7 mmol, 2.5 equiv) was added dropwise. The reaction was left to stir at room temperature for 4 h before it was quenched by adding saturated aqueous NH4Cl solution. The mixture was extracted with EtOAc three times. The combined extracts were washed with brine, dried over Na2SO4, and evaporated in vacuo. The product was purified by flash column chromatography (n-hexane/EtOAc, 11:1) giving 2,2,5-trimethylhept-6-ene-1,5-diol (Int-E) as a white solid (0.50 g, 83%). 1H NMR (300 MHz): δ 5.85 (dd, J = 17.3, 10.7 Hz, 1H), 5.16 (dd, J = 17.4, 1.3 Hz, 1H), 5.01 (dd, J = 10.8, 1.3 Hz, 1H), 3.33–3.18 (m, 2H), 2.76 (br s, 2H), 1.48–1.42 (m, 2H), 1.28–1.20 (m, 2H), 1.25 (s, 3H), 0.82 (s, 3H), 0.81 (s, 3H). 13C{H} NMR (75 MHz): δ 145.2, 111.8, 73.4, 70.7, 35.9, 34.75, 31.6, 27.9, 24.5, 24.2. HRMS (FD) m/z: [M]+ calcd for C10H20O2+, 172.1463; found, 172.1470.
Step F: In a flame-dried Schlenk flask were successively added Int-E (200 mg, 1.16 mmol, 1.0 equiv), DCE (25 mL), and anhydrous ZnCl2 (158 mg, 1.16 mmol, 1.0 equiv) under N2. The reaction was then stirred at 70 °C for 2 h before it was quenched by adding saturated aqueous NH4Cl solution. The mixture was extracted with Et2O three times. The combined extracts were passed through a short plug of silica gel and rinsed with Et2O. The filtrate was evaporated in vacuo giving 2,5,5-trimethyl-2-vinyltetrahydro-2H-pyran (18) as a colorless oil (130 mg, 73%). 1H NMR (400 MHz): δ 5.78 (dd, J = 17.4, 11.4 Hz, 1H), 5.17 (s, 1H), 5.13 (dd, J = 7.9, 1.3 Hz, 1H), 3.33 (d, J = 11.3 Hz, 1H), 3.21 (dd, J = 11.3, 2.0 Hz, 1H), 1.73–1.61 (m, 2H), 1.45–1.37 (m, 1H), 1.35–1.28 (m, 1H), 1.23 (s, 3H), 0.99 (s, 2H), 0.81 (s, 2H). 13C{H} NMR (101 MHz): δ 143.3, 114.3, 74.2, 72.7, 33.5, 30.7, 29.8, 28.7, 26.7, 24.2. HRMS (FD) m/z: [M + Na]+ calcd for C10H18NaO+, 177.1255; found, 177.1268.
trans-Linalool oxide (trans-20) was prepared using the following procedures: Linalool oxide was purchased from TCI as a mixture of diastereoisomers (1:1) not separable by flash column chromatography. To separate the diastereoisomers, the hydroxyl group was first protected with TMS using the following procedure:
In a round bottom flask were successively added linalool oxide (20, dr = 1:1) (1.01 g, 5.92 mmol, 1.0 equiv), DCM (12 mL), 1-imidazole (1.21 g, 17.8 mmol, 3.0 equiv), and TMSCl (1.13 mL, 8.88 mmol, 1.5 equiv). The reaction was then stirred at room temperature for 0.5 h before water was added. The mixture was extracted with Et2O three times. The combined organic extracts were washed with brine, dried over Na2SO4, and evaporated in vacuo. The product TMS-trans-linalool oxide (TMS-trans-20) was obtained as a colorless oil after purification by flash column chromatography using PE as the eluent. Its 1H NMR data matched with those reported in the literature.241H NMR (400 MHz): δ 5.86 (dd, J = 17.2, 10.5 Hz, 1H), 5.16 (d, J = 17.3 Hz, 1H), 4.97 (d, J = 10.6 Hz, 1H), 3.74 (t, J = 6.1 Hz, 1H), 1.94–1.76 (m, 3H), 1.71–1.63 (m, 1H), 1.30 (s, 3H), 1.21 (s, 3H), 1.20 (s, 3H), 0.11 (s, 9H).
TMS-trans-20
The TMS-protecting group was then removed by using the following step: In a round bottom flask were added (2.70 g, 11.13 mmol, 1.0 equiv) and THF (50 mL). TBAF solution (33 mL, 1.0 M in THF, 3.0 equiv) was then added slowly. The reaction was stirred for 1 h before water was added. The mixture was extracted with Et2O three times. The combined organic extracts were washed with brine, dried over Na2SO4, and evaporated in vacuo. The sample was purified by flash column chromatography (n-hexane/EtOAc, 5:1) giving trans-linalool oxide (trans-20) as a colorless oil (1.70 g, 89%). Its 1H NMR data matched with those reported in the literature.231H NMR (400 MHz): δ 5.88 (dd, J = 17.3, 10.6 Hz, 1H), 5.19 (d, J = 17.3 Hz, 1H), 5.00 (d, J = 10.7 Hz, 1H), 3.80 (t, J = 7.1 Hz, 1H), 2.10–1.79 (m, 5H), 1.78–1.67 (m, 1H), 1.32 (s, 3H), 1.23 (s, 3H), 1.14 (s, 3H).
TMS-cis-2,2,6-trimethyl-6-vinyltetrahydropyran-3-ol (cis-24)
2,2,6-Trimethyl-6-vinyltetrahydropyran-3-ol (23) was purchased from TCI as a mixture of diastereoisomers (1:1) not separable by flash column chromatography. The TMS protection and deprotection strategy was used to separate them as presented for the separation of linalool oxide (20). TMS protection was performed using the same procedure as the TMS protection of linalool oxide, and cis-24 was isolated as a colorless oil after purification by flash column chromatography with PE as the eluent. 1H NMR (400 MHz): δ 5.96 (ddd, J = 18.2, 10.9, 1.2 Hz, 1H), 4.99–4.91 (m, 2H), 3.40 (dd, J = 11.3, 4.1 Hz, 1H), 2.10 (dt, J = 12.7, 3.3 Hz, 1H), 1.80–1.65 (m, 1H), 1.62–1.48 (m, 2H), 1.15 (s, 3H), 1.14 (s, 3H), 1.13 (s, 3H), 0.10 (d, J = 1.1 Hz, 9H). 13C{H} NMR (101 MHz): δ 146.6, 110.6, 76.6, 75.7, 73.5, 32.9, 32.2, 30.1, 26.4, 20.9, 0.05. HRMS (FD) m/z: [M – Me]+ calcd for C12H23O2Si+, 227.1467; found, 227.1485.
cis-2,2,6-Trimethyl-6-vinyltetrahydropyran-3-ol (cis-23)
Deprotection of cis-24 (0.60 g, 2.475 mmol) was performed using the same procedure as the deprotection of TMS-trans-linalool oxide (TMS-trans-20). cis-23 was isolated as a wax (0.42 g, quantitative yield) and its 1H NMR data matched with those reported in the literature.241H NMR (400 MHz): δ 5.99 (dd, J = 18.1, 11.0 Hz, 1H), 5.03 (d, J = 5.5 Hz, 1H), 4.99 (d, J = 1.1 Hz, 1H), 3.51–3.43 (m, 1H), 2.15 (dt, J = 13.6, 3.7 Hz, 1H), 1.77–1.71 (m, 2H), 1.64–1.58 (m, 1H), 1.28 (s, 3H), 1.20 (s, 3H), 1.19 (s, 3H).
2,4-Dimethyl-4-vinylcyclohexanone (28) and 2,4-dimethyl-4-vinylcyclohexanol (31) were prepared using the following procedures: Step A: In a round-bottom flask were successively added ethyl 4-oxocyclohexane-1-carboxylate (9.29 mL, 10.00 g, 58.8 mmol, 1.0 equiv), ethylene glycol (11.5 mL, 12.77 g, 205.8 mmol, 3.5 equiv), p-toluenesulfonic acid monohydrate (137 mg, 0.012 equiv), and anhydrous toluene (30 mL). The reaction was then stirred overnight at room temperature. Saturated aqueous Na2CO3 solution was added and the reaction was extracted with EtOAc three times. The combined organic extracts were washed with brine, dried over Na2SO4, and evaporated in vacuo. The product was purified by flash column chromatography (PE/EtOAc, 15:1) giving ethyl 1,4-dioxaspiro[4.5]decane-8-carboxylate as a colorless oil (10.94 g, 87%). Its 1H NMR data matched with those reported in the literature.251H NMR (400 MHz): δ 4.05 (q, J = 7.1 Hz, 2H), 3.86 (s, 4H), 2.29–2.22 (m, 1H), 1.91–1.80 (m, 2H), 1.79–1.64 (m, 4H), 1.54–1.42 (m, 2H), 1.17 (t, J = 7.1 Hz, 3H).
Step B: In a flame-dried Schlenk flask were successively added 1,4-dioxaspiro[4.5]decane-8-carboxylate (10.94 g, 51.1 mmol, 1.0 equiv) and anhydrous THF (70 mL). The solution was slowly transferred to another Schlenk flask containing freshly prepared LDA (66.4 mmol, 1.3 equiv) at −78 °C over a period of 0.5 h. THF (10 mL) was used to rinse the flask and was also transferred. The reaction was then slowly warmed up to room temperature and stirred for 0.5 h before it was cooled to −78 °C again. MeI (6.36 mL, 14.50 g, 102.2 mmol, 2.0 equiv) was added dropwise and the reaction was slowly warmed up to room temperature. After stirring it overnight, the reaction was quenched by adding saturated aqueous NH4Cl solution and was extracted with EtOAc three times. The combined organic extracts were washed with brine, dried over Na2SO4, and evaporated in vacuo. The product was purified by flash column chromatography (PE/EtOAc, 20:1) giving ethyl 8-methyl-1,4-dioxaspiro[4.5]decane-8-carboxylate as a colorless oil (9.68 g, 83%). Its 1H NMR data matched with those reported in the literature.251H NMR (400 MHz): δ 4.02 (q, J = 7.1 Hz, 2H), 3.81 (s, 4H), 2.00 (dt, J = 13.0, 3.6 Hz, 2H), 1.58–1.32 (m, 6H), δ 1.13 (t, J = 7.1 Hz, 3H), 1.06 (s, 3H).
Step C: In a flame-dried Schlenk flask were added ethyl 8-methyl-1,4-dioxaspiro[4.5]decane-8-carboxylate (7.20 g, 31.5 mmol, 1.0 equiv) and anhydrous DCM (70 mL). LAH (2.63 g, 69.4 mmol, 2.2 equiv) was added in small portions at 0 °C. The reaction was stirred at 0 °C for 0.5 h before it was warmed up to room temperature slowly. After the reaction was stirred for 1.5 h, the reaction was diluted with DCM and saturated aqueous potassium sodium tartrate solution was carefully added. The reaction was then stirred vigorously for 1 h. The mixture was extracted with DCM three times. The combined organic extracts were washed with brine, dried over Na2SO4, and evaporated in vacuo to give (8-methyl-1,4-dioxaspiro[4.5]decan-8-yl)methanol as a white solid (5.81 g, 99%), which was pure enough to continue for the next step without further purification. Its 1H NMR data matched with those reported in the literature.251H NMR (400 MHz): δ 3.94 (d, J = 1.7 Hz, 4H), 3.39 (s, 2H), 1.73–1.49 (m, 6H), 1.45–1.34 (m, 3H), 0.96 (s, 3H).
Step D: In a round-bottom flask were successively added (8-methyl-1,4-dioxaspiro[4.5]decan-8-yl)methanol (4.90 g, 26.3 mmol, 1.0 equiv), DCM (60 mL), and PCC (12.48 g, 57.9 mmol, 2.2 equiv). After the reaction was stirred at room temperature for 1 h, it was filtrated through a plug of silica gel and rinsed with EtOAc. The filtrate was evaporated and purified by flash column chromatography (PE/EtOAc, 5:1) giving 8-methyl-1,4-dioxaspiro[4.5]decane-8-carbaldehyde a colorless oil (4.20 g, 87%). Its 1H NMR data matched with those reported in the literature.261H NMR (400 MHz): δ 9.46 (s, 1H), 3.93 (s, 4H), 2.07–1.92 (m, 2H), 1.70–1.49 (m, 6H), 1.04 (s, 3H).
Step E: In a flame-dried Schlenk flask were added methyl triphenylphosphonium bromide (13.44 g, 37.6 mmol, 1.65 equiv) and anhydrous THF (75 mL) at 0 °C under N2. n-BuLi (13.7 mL, 2.5 M in hexanes, 34.25 mmol, 1.5 equiv) was then added dropwise. The reaction was warmed up to room temperature and stirred for 0.5 h before it was cooled to 0 °C again. In another flask, a solution of 8-methyl-1,4-dioxaspiro[4.5]decane-8-carbaldehyde (4.20 g, 22.8 mmol, 1.0 equiv) in anhydrous THF (20 mL) was prepared and the solution was slowly transferred to the Wittig reagent flask via a cannula over a period of 0.5 h. 10 mL of anhydrous THF was used to rinse the flask and was also transferred. The reaction was then stirred at room temperature for 3 h. Acetone (5 mL) was added to quench the Wittig reagent. The mixture was filtrated and washed with Et2O. The filtrate was evaporated and purified by flash column chromatography (n-pentane/Et2O, 15:1) giving 8-methyl-8-vinyl-1,4-dioxaspiro[4.5]decane as a pale yellow oil (3.46 g, 83%). Its 1H NMR data matched with those reported in the literature.261H NMR (400 MHz): δ 5.79 (dd, J = 17.8, 10.7 Hz, 1H), 5.01 (d, J = 4.6 Hz, 1H), 4.98 (s, 1H), 3.93 (s, 4H), 1.69–1.61 (m, 6H), 1.53–1.44 (m, 2H), 1.01 (s, 3H).
Step F: 8-Methyl-8-vinyl-1,4-dioxaspiro[4.5]decane (3.40 g, 18.7 mmol) was dissolved in acetone (60 mL). 10% HCl (24 mL) was then added dropwise. The reaction was stirred for 2.5 h before brine was added. The mixture was extracted with DCM/Et2O (1:2) three times. The volatiles were carefully evaporated in vacuo giving 4-methyl-4-vinylcyclohexan-1-one as a colorless oil (2.57 g, quantitative yield). Its 1H NMR data matched with those reported in the literature.261H NMR (400 MHz): δ 5.89 (dd, J = 17.5, 11.0 Hz, 1H), 5.18–5.08 (m, 2H), 2.45–2.25 (m, 4H), 1.97–1.90 (m, 2H), 1.74–1.67 (m, 2H), 1.12 (d, J = 0.9 Hz, 3H).
Step G: In a flame-dried Schlenk flask was added LiHMDS (7.6 mL, 1.0 M in THF, 7.6 mmol, 1.05 equiv) under N2 and 4-methyl-4-vinylcyclohexan-1-one (1.00 g, 7.2 mmol, 1.0 equiv) in DMF (4 mL) was added dropwise at −78 °C. The reaction was then left to stir for 1.5 h before MeI (446 μL, 7.2 mmol, 1.0 equiv) was added dropwise. The reaction was slowly warmed up to room temperature and stirred overnight. The reaction was quenched by adding saturated aqueous NH4Cl solution and was extracted with Et2O three times. The combined extracts were washed with water three times and brine, dried over Na2SO4, and evaporated in vacuo. The product was purified by flash column chromatography (n-pentane/Et2O, 20:1) giving 2,4-dimethyl-4-vinylcyclohexan-1-one (28) as a pale yellow oil (0.82 g, 74%, dr: trans/cis = 5:4). 1H NMR (400 MHz): δ 5.98 (dd, J = 17.6, 10.9 Hz, 1Htrans), 5.84 (dd, J = 17.5, 10.7 Hz, 1Hcis), 5.26–5.19 (m, 1Htrans and 1Hcis), 5.04–4.95 (m, 1Htrans and 1Hcis), 2.66–2.41 (m, 2Htrans and 2Hcis), 2.34 (dt, J = 14.4, 3.8 Hz, 1Hcis), 2.25 (ddd, J = 14.0, 4.5, 2.5 Hz, 1Htrans), 2.10–2.01 (m, 1Htrans and 1Hcis), 1.81–1.77 (m, 1Htrans), 1.73–1.65 (m, 1Hcis), 1.51 (t, J = 13.4 Hz, 1Hcis), 1.44 (m, t, J = 13.4 Hz, 1Htrans), 1.33 (s, 3Hcis), 1.06 (s, 3Htrans), 1.04 (d, J = 6.5 Hz, 2Hcis), 1.01 (d, J = 6.5 Hz, 3Htrans). 13C{H} NMR (75 MHz): δ 213.9, 213.5, 148.3, 144.8, 113.5, 110.6, 47.0, 46.4, 41.3, 40.7, 38.5 (trans and cis), 37.9 (trans and cis), 37.7, 37.5, 30.3, 22.1, 14.7, 14.5. HRMS (FD) m/z: [M]+ calcd for C10H16O+, 152.1201; found, 152.1211.
Step H: In a flame-dried Schlenk flask were added 37 (dr = 5:4, 0.36 g, 2.36 mmol, 1.0 equiv) and anhydrous Et2O (70 mL). LAH (0.18 g, 4.74 mmol, 2.0 equiv) was added in small portions at 0 °C. The reaction was stirred for 0.5 h before it was warmed up to room temperature slowly. After the reaction was stirred for 1.5 h, the reaction was diluted with Et2O and saturated aqueous potassium sodium tartrate solution was carefully added. The reaction was then stirred vigorously for 1 h. The mixture was extracted with Et2O three times. The combined organic extracts were washed with brine, dried over Na2SO4, and evaporated in vacuo. The product was purified by flash column chromatography (n-pentane/Et2O, 5:1) giving 2,4-dimethyl-4-vinylcyclohexanol (31) as a pale yellow oil (0.35 g, 96%, dr: A/B = 5:4). 1H NMR (300 MHz): δ 5.78 (dd, J = 17.6, 10.8 Hz, 1HA and 1HB), 5.07–4.84 (m, 2HA and 2HB), 3.14–3.05 (m, 1HA and 1HB), 1.87–1.22 (m, 7HA and 7HB), 1.05 (s, 3HB), 0.99 (d, J = 6.5 Hz, 3HB), 0.97 (d, J = 6.7 Hz, 3HA), 0.95 (s, 3HA). 13C{H} NMR (75 MHz): δ 150.4, 146.2, 112.5, 109.4, 45.4, 44.4, 37.2, 36.5, 36.4, 36.1, 35.5, 35.5, 31.9 (A and B), 31.2, 31.1, 22.4 (A and B), 18.8, 18.7. HRMS (FD) m/z: [M]+ calcd for C10H18O+, 154.1358; found, 154.1360.
Synthesis of Model Substrates
5-Methoxy-N-methyl-3,4-dihydroquinolin-2(1H)-one (2a)
In a flame-dried Schlenk flask were added 5-hydroxy-3,4-dihydroquinolin-2(1H)-one (326 mg, 2.0 mmol, 1.0 equiv) and anhydrous DMF (10 mL) under N2. NaH (240 mg, 60% in mineral oil, 6.0 mmol, 3.0 equiv) was then added at 0 °C and stirred for 0.5 h. MeI (500 μL, 8.0 mmol, 4.0 equiv) was then added dropwise at 0 °C. The reaction was then warmed up to room temperature and stirred overnight. The reaction was quenched by adding water and extracted with EtOAc three times. The combined organic extracts were washed with water three times and brine two times, dried with Na2SO4, filtrated, and concentrated in vacuo to give 2a as a pale yellow solid (380 mg, 99%). Its 1H NMR data matched with those reported in the literature.271H NMR (400 MHz): δ 7.21 (t, J = 8.2 Hz, 1H), 6.64 (d, J = 8.3 Hz, 2H), 3.85 (s, 3H), 3.35 (s, 3H), 2.89 (dd, J = 8.6, 6.4 Hz, 2H), 2.60 (dd, J = 8.6, 6.4 Hz, 2H).
5-Hydroxy-N-methyl-3,4-dihydroquinolin-2(1H)-one (5a)
In a flame-dried Schlenk flask were added 2a (1.15 g, 6.0 mmol, 1.0 equiv) and anhydrous DCM (10 mL) under N2. A solution of BBr3 in DCM (18 mL, 1.0 M, 18.0 mmol, 3.0 equiv) was added dropwise at −78 °C. After stirring the reaction for another 1 h at −78 °C, the reaction was slowly warmed up to room temperature and stirred for additional 3 h. Water was slowly added to quench the reaction and the reaction was extracted with DCM three times. The combined organic extracts were washed with water and brine, dried with MgSO4, filtrated, and concentrated in vacuo to give 5a as a pale yellow solid (1.02 g, 96%). Its 1H NMR data matched with those reported in the literature.281H NMR (400 MHz): δ 7.11 (t, J = 8.2 Hz, 1H), 6.61 (d, J = 8.2 Hz, 1H), 6.54 (d, J = 7.9 Hz, 1H), 3.35 (s, 3H), 2.90 (dd, J = 8.7, 6.3 Hz, 2H), 2.64 (dd, J = 8.5, 6.4 Hz, 2H).
5-(Methoxymethoxy)-N-methyl-3,4-dihydroquinolin-2(1H)-one (4a)
In a flame-dried Schlenk flask were added 5a (532 mg, 3.0 mmol, 1.0 equiv) and freshly distilled THF (10 mL) under N2. NaH (156 mg, 60% in mineral oil, 3.9 mmol, 1.3 equiv) was then added portionwise at 0 °C. After stirring it at room temperature for 0.5 h, MOMCl (1.127 g, 15.1 mmol, 1.2 equiv) was added to the reaction dropwise at 0 °C. After stirring it for 3 h at room temperature, the reaction was quenched by slowly adding water and extracted with EtOAc three times. The combined organic extracts were washed with brine, dried over Na2SO4, and evaporated in vacuo. The product was purified by flash column chromatography (n-hexane/EtOAc, 3:1) giving 4a as a pale yellow solid (500 mg, 75%). 1H NMR (400 MHz): δ 7.18 (t, J = 8.3 Hz, 1H), 6.85 (dd, J = 8.4, 0.9 Hz, 1H), 6.68 (d, J = 8.2 Hz, 1H), 5.21 (s, 2H), 3.49 (s, 3H), 3.35 (s, 3H), 2.93 (dd, J = 8.6, 6.4 Hz, 2H), 2.61 (dd, J = 8.6, 6.4 Hz, 2H). 13C{H} NMR (75 MHz): δ 170.6, 154.1, 141.9, 127.8, 115.5, 109.3, 108.8, 94.8, 56.3, 31.2, 29.9, 18.3. HRMS (FD) m/z: [M]+ calcd for C12H15NO3+, 221.1052; found, 221.1061.
5-Ethoxy-3,4-dihydroquinolin-2(1H)-one
In a flame-dried Schlenk flask were added 5-hydroxy-3,4-dihydroquinolin-2(1H)-one (0.70 g, 4.29 mmol, 1.0 equiv), K2CO3 (1.23 mg, 8.93 mmol, 2.1 equiv), EtBr (0.7 mg, 6.42 mmol, 1.5 equiv), and anhydrous DMF (5 mL) under N2. The reaction was then stirred at 70 °C for 5 h. Water was then added and the mixture was extracted with EtOAc three times. The combined organic extracts were washed with water three times and brine two times, dried with MgSO4, filtrated, and concentrated in vacuo to yield 5-ethoxy-3,4-dihydroquinolin-2(1H)-one as a white solid (800 mg, 98%), which was pure enough to be directly used for the next step. Its 1H NMR data matched with those reported in the literature.291H NMR (400 MHz): δ 7.58 (br s, 1H), 7.10 (t, J = 8.1 Hz, 1H), 6.56 (d, J = 8.2 Hz, 1H), 6.35 (d, J = 7.9 Hz, 1H), 4.05 (q, J = 7.0 Hz, 2H), 2.97 (t, J = 7.7 Hz, 2H), 2.60 (dd, J = 8.4, 6.9 Hz, 2H), 1.42 (t, J = 7.0 Hz, 3H).
5-Ethoxy-N-methyl-3,4-dihydroquinolin-2(1H)-one (3a)
In a flame-dried Schlenk flask were added 5-ethoxy-3,4-dihydroquinolin-2(1H)-one (191 mg, 1.0 mmol, 1.0 equiv) and anhydrous DMF (2 mL) under N2. NaH (60 mg, 60% in mineral oil, 1.5 mmol, 1.5 equiv) was then added at 0 °C and stirred for 0.5 h. MeI (170 mg, 1.2 mmol, 1.2 equiv) was then added dropwise at 0 °C. The reaction was then warmed up to room temperature and stirred overnight. The reaction was quenched by adding water and extracted with EtOAc three times. The combined organic extracts were washed with water three times and brine two times, dried with MgSO4, filtrated, and concentrated in vacuo to give 3a as a white solid (200 mg, 98%). Its 1H NMR data matched with those reported in the literature.291H NMR (400 MHz): δ 7.20–7.15 (m, 1H), 6.63–6.61 (m, 2H), 4.05 (q, J = 7.0 Hz, 2H), 3.34 (s, 3H), 2.93–2.88 (m, 2H), 2.60 (t, J = 7.3 Hz, 2H), 1.42 (t, J = 7.0 Hz, 3H).
5-Ethoxy-N-(methoxymethyl)-3,4-dihydroquinolin-2(1H)-one (6a)
In a flame-dried Schlenk flask were added 5-ethoxy-3,4-dihydroquinolin-2(1H)-one (150 mg, 0.78 mmol, 1.0 equiv) and freshly distilled THF (3 mL) under N2. NaH (47 mg, 60% in mineral oil, 1.17 mmol, 1.5 equiv) was then added at 0 °C. After stirring it for 0.5 h at 0 °C, MOMCl (70 μL, 0.94 mmol, 1.2 equiv) was added to the reaction at 0 °C dropwise. After stirring it for 2 h at room temperature, the reaction was quenched by slowly adding water and extracted with EtOAc three times. The combined organic extracts were washed with brine, dried over Na2SO4, and evaporated in vacuo. The product was purified by flash column chromatography (PE/EtOAc, 4:1) giving 6a as a pale yellow solid (99 mg, 54%). 1H NMR (300 MHz): δ 7.21–7.12 (m, 1H), 6.93 (d, J = 7.9 Hz, 1H), 6.63 (d, J = 8.2 Hz, 1H), 5.30 (s, 2H), 4.05 (q, J = 6.5 Hz, 2H), 3.40 (s, 3H), 2.93 (t, J = 7.5 Hz, 2H), 2.68–2.62 (m, 2H), 1.42 (t, J = 7.0 Hz, 3H). 13C{H} NMR (101 MHz): δ 171.7, 155.7, 140.9, 127.9, 114.7, 108.8, 107.1, 74.2, 64.1, 56.4, 31.5, 18.3, 15.0. HRMS (FD) m/z: [M]+ calcd for C13H17NO3+, 235.1208; found, 235.1219.
4-{[5-Ethoxy-2-oxo-3,4-dihydroquinolin-1(2H)-yl]methyl}phenyl Acetate (7a)
In a flame-dried Schlenk flask were added 5-ethoxy-3,4-dihydroquinolin-2(1H)-one (215 mg, 1.12 mmol, 1.0 equiv) and anhydrous DMF (3 mL) under N2. NaH (54 mg, 60% in mineral oil, 1.35 mmol, 1.2 equiv) was then added at 0 °C and stirred for 0.5 h at 0 °C. 4-(Bromomethyl)phenyl acetate (332 mg, 1.46 mmol, 1.3 equiv) was then added at 0 °C. The reaction was then warmed up to room temperature and stirred overnight. The reaction was quenched by adding water and extracted with EtOAc three times. The combined organic extracts were washed with water three times and brine two times, dried over MgSO4, filtrated, and concentrated in vacuo. The product was purified by flash column chromatography (n-hexane/EtOAc, 3:1) giving 7a as a pale yellow solid (250 mg, 66%). mp 102.0–103.3 °C. 1H NMR (400 MHz): δ 7.22 (d, J = 8.2 Hz, 2H), 7.07–7.01 (m, 3H), 6.58 (d, J = 8.3 Hz, 1H), 6.51 (d, J = 8.2 Hz, 1H), 5.15 (s, 2H), 4.04 (q, J = 6.9 Hz, 2H), 2.98 (dd, J = 8.6, 6.3 Hz, 2H), 2.73 (dd, J = 8.7, 6.3 Hz, 2H), 2.27 (s, 3H), 1.42 (t, J = 6.9, 3H). 13C{H} NMR (101 MHz): δ 170.8, 169.6, 155.9, 149.7, 141.0, 135.0, 127.8, 127.6, 121.9, 114.8, 108.5, 106.8, 64.1, 46.0, 31.5, 21.3, 18.3, 15.0. IR ν: 2977, 1758, 1671, 1594, 1467, 1188, 728 cm–1. HRMS (FD) m/z: [M]+ calcd for C20H21NO4+, 339.1471; found, 339.1471.
Synthesis of Core Structures (±)-16 and (±)-27 of Yaequinolone-Related Natural Products
Synthesis of (±)-16
1-(Benzyloxy)-2-bromo-3-nitrobenzene (9)
In a round-bottom flask were added 2-bromo-3-nitrophenol (8) (2.18 g, 10.0 mmol, 1.0 equiv), K2CO3 (4.15 g, 30.0 mmol, 3.0 equiv), benzyl chloride (2.3 mL, 20 mmol, 2.0 equiv), and EtOH (15 mL) successively. The mixture was then refluxed at 85 °C overnight. The volatiles were removed in vacuo. The product was purified by flash column chromatography (PE/EtOAc, 3:1) giving 9 as a yellow solid (2.80 g, 91%). mp 73.0–74.0 °C. 1H NMR (400 MHz): δ 7.49 (d, J = 7.6 Hz, 2H), 7.48–7.26 (m, 5H), 7.17–7.09 (m, 1H), 5.25 (s, 2H). 13C{H} NMR (101 MHz): δ 156.6, 135.6, 128.9, 128.7, 128.5, 127.2, 117.0, 116.4, 105.5, 71.8. HRMS (FD) m/z: [M]+ calcd for C13H10BrNO3+, 306.9844; found, 306.9816.
[2-(Benzyloxy)-6-nitrophenyl](4-methoxyphenyl)methanol (10)
In a flame-dried Schlenk flask were added 9 (4.00 g, 13.0 mmol, 1.0 equiv) and freshly distilled THF (50 mL) under N2. At −95 °C, n-BuLi (6.24 mL, 2.5 M in hexanes, 15.6 mmol, 1.2 equiv) was then added dropwise. The reaction was continued to stir at −95 °C for 1.5 h before a solution of p-anisaldehyde (3.54 g, 26.0 mmol, 2.0 equiv) in THF (10 mL) was added dropwise. After stirring the reaction for another 2 h, the reaction was quenched by slow addition of saturated solution of NH4Cl and extracted with EtOAc three times. The combined organic extracts were washed with brine, dried over Na2SO4, and concentrated in vacuo. The sample was purified by flash column chromatography (n-hexane/EtOAc, 5:1 to 3:1) giving 10 as a pale yellow oil (2.20 g, 46%). Another reaction using 4.00 g of 9 (13.0 mmol) with 0.95 equiv of n-BuLi and 0.5 equiv of para-anisaldehyde was also performed, and 2.06 g of 10 was obtained (86% based on para-anisaldehyde). 1H NMR (400 MHz): δ 7.44–7.36 (m, 2H), 7.31–7.27 (m, 3H), 7.24–7.18 (m, 3H), 7.02 (dd, J = 7.5, 2.1 Hz, 2H), 6.86–6.82 (m, 2H), 6.23 (d, J = 10.5 Hz, 1H), 5.07 (d, J = 11.5 Hz, 1H), 4.99 (d, J = 11.5 Hz, 1H), 3.81 (s, 3H). 13C{H} NMR (101 MHz): δ 158.8, 157.1, 150.7, 135.1, 134.6, 129.2, 128.6, 128.4, 127.5, 127.1, 126.4, 116.6, 116.5, 113.5, 71.2, 69.4, 55.3. IR ν: 3545, 2934, 1604, 1509, 1356, 1246, 1025, 737 cm–1. HRMS (FD) m/z: [M]+ calcd for C21H19NO5+, 365.1263; found, 365.1248.
[2-Amino-6-(benzyloxy)phenyl](4-methoxyphenyl)methanol (11)
In a round bottom flask were added 10 (2.00 g, 5.5 mmol, 1.0 equiv), EtOH (30 mL), and Na2S (1.28 g, 16.4 mmol, 3.0 equiv) successively. The reaction was then refluxed at 85 °C for 15 min before water was added to quench the reaction. The mixture was then extracted with EtOAc three times. The combined organic extracts were washed with brine, dried over Na2SO4, and evaporated in vacuo. The obtained 11 was directly used for the next step. 1H NMR (400 MHz): δ 7.38–7.30 (m, 8H), 7.04 (t, J = 8.1 Hz, 1H), 6.87–6.82 (m, 2H), 6.49 (s, 1H), 6.45 (dd, J = 8.3, 1.0 Hz, 1H), 6.32 (dd, J = 8.0, 0.9 Hz, 1H), 5.08 (d, J = 11.7 Hz, 1H), 5.02 (d, J = 11.7 Hz, 1H), 3.78 (s, 3H). 13C NMR (101 MHz): δ 158.7, 156.9, 146.4, 137.1, 135.0, 129.1, 128.6, 128.0, 127.5, 127.2, 116.6, 113.7, 111.1, 102.7, 70.6, 68.4, 55.4.
N-{3-(Benzyloxy)-2-[hydroxy(4-methoxyphenyl)methyl]phenyl}-2-methoxyacetamide (12)
In a flame-dried Schlenk flask were added crude 11 obtained from the previous step, iPr2NEt (853 mg, 6.6 mmol, 1.2 equiv), and freshly distilled DCM (25 mL). 2-Methoxyacetyl chloride (657 mg, 6.05 mmol, 1.1 equiv) was then added dropwise at 0 °C. The reaction was stirred for another 40 min at room temperature before it was quenched by adding water. The reaction was extracted with DCM three times and the combined extracts were washed with brine, dried over Na2SO4, and concentrated in vacuo. The product was purified by flash column chromatography (n-hexane/EtOAc, 2:1) giving 12 as a pale yellow oil (1.40 g, 61% over two steps). 1H NMR (400 MHz): δ 10.09 (br s, 1H), 7.95 (d, J = 8.3 Hz, 1H), 7.38–7.22 (m, 8H), 6.82–6.78 (m, 3H), 6.65 (s, 1H), 5.11 (d, J = 11.7 Hz, 1H), 5.04 (d, J = 11.7 Hz, 1H), 3.87 (d, J = 15.4 Hz, 1H), 3.77 (s, 3H), 3.69 (d, J = 15.5 Hz, 1H), 3.28 (s, 3H). 13C{H} NMR (101 MHz): δ 168.2, 158.3, 155.4, 137.6, 136.6, 134.4, 128.5, 128.3, 127.7, 127.1, 127.0, 121.1, 115.2, 113.2, 108.0, 72.0, 70.3, 67.4, 59.1, 54.9. IR ν: 3282, 2934, 2834, 1665, 1593, 1472, 1249, 1116 cm–1. HRMS (FD) m/z: [M]+ calcd for C24H25NO5+, 407.1733; found, 407.1746.
N-[3-(Benzyloxy)-2-(4-methoxybenzoyl)phenyl]-2-methoxyacetamide (13)
In a round bottom flask were added 12 (1.40 g, 3.44 mmo, 1.0 equiv) and DCM (30 mL). PCC (1.48 g, 6.88 mmol, 2.0 equiv) was then added portionwise. After stirring it at room temperature for 1 h, TLC showed full conversion of the reaction. The reaction was then quenched by a saturated solution of Na2SO3 and extracted with DCM three times. The combined organic extracts were washed with brine, dried over Na2SO4, and evaporated in vacuo. The product was purified by flash column chromatography (n-hexane/EtOAc, 1.5:1) giving 13 as a pale yellow oil (1.30 g, 94%). 1H NMR (400 MHz): δ 9.32 (br s, 1H), 7.99 (d, J = 8.3 Hz, 1H), 7.82–7.75 (m, 2H), 7.37 (t, J = 8.4 Hz, 1H), 7.19–7.06 (m, 3H), 6.91–6.82 (m, 4H), 6.78 (d, J = 8.3 Hz, 1H), 4.89 (s, 2H), 3.87 (s, 2H), 3.78 (s, 3H), 3.32 (s, 3H). 13C{H} NMR (101 MHz): δ 194.9, 168.1, 163.7, 156.6, 136.3, 136.0, 131.7, 131.5, 128.0, 127.4, 126.5, 119.1, 114.8, 113.5, 108.3, 71.9, 70.0, 59.2, 55.3. IR ν: 3359, 2935, 2837, 1692, 1592, 1461, 1253, 927 cm–1. HRMS (FD) m/z: [M]+ calcd for C24H23NO5+, 405.1576; found, 405.1591.
(3R*,4R*)-5-(Benzyloxy)-4-hydroxy-3-methoxy-4-(4-methoxyphenyl)-3,4-dihydroquinolin-2(1H)-one [(±)-14]
In a flame-dried Schlenk flask were added 13 (0.94 g, 2.32 mmol, 1.0 equiv) and freshly distilled THF (50 mL) at 0 °C under N2. A solution of KOtBu (16.2 mL, 1.0 M in THF, 16.2 mmol, 7.0 equiv) was then added dropwise. After stirring it at 0 °C for 2 h, TLC showed full conversion of the reaction. The reaction was quenched by adding water and extracted with EtOAc (three times). The combined organic extracts were washed with brine, dried over Na2SO4, and evaporated in vacuo. The product was purified by flash column chromatography (n-hexane/EtOAc, 1:1.5) giving (±)-14 as a single diastereoisomer: white solid (0.75 g, 80%). 1H NMR (400 MHz): δ 8.80 (br s, 1H), 7.32–7.25 (m, 3H), 7.25–7.16 (m, 3H), 7.12 (dd, J = 6.3, 2.7 Hz, 2H), 6.86–6.77 (m, 2H), 6.73 (d, J = 8.4 Hz, 1H), 6.55 (dd, J = 8.1, 0.9 Hz, 1H), 5.33 (s, 1H), 5.07 (d, J = 11.5 Hz, 1H), 4.99 (d, J = 11.5 Hz, 1H), 3.83 (s, 1H), 3.77 (d, J = 1.0 Hz, 3H), 3.61 (s, 3H). 13C{H} NMR (101 MHz): δ 168.3, 159.7, 158.0, 137.1, 135.7, 133.6, 129.9, 128.7, 128.4, 127.6, 127.5, 115.3, 114.0, 109.6, 108.7, 85.1, 78.1, 71.1, 59.7, 55.3. IR ν: 3509, 2932, 1692, 1607, 1509, 1259, 1103 cm–1. HRMS (FD) m/z: [M]+ calcd for C24H23NO5+, 405.1576; found, 405.1591.
(3R*,4R*)-5-(Benzyloxy)-4-hydroxy-3-methoxy-4-(4-methoxyphenyl)-1-{[2-(trimethylsilyl)ethoxy]methyl}-3,4-dihydroquinolin-2(1H)-one [(±)-15]
In a flame-dried Schlenk flask were added (±)-14 (1.20 g, 2.96 mmol, 1.0 equiv) and anhydrous THF (50 mL) under N2. LiHMDS solution in THF (3.26 mL, 1.0 M, 3.26 mmol, 1.1 equiv) was then added dropwise at 0 °C. After stirring it for 0.5 h, SEMCl (0.59 g, 3.55 mmol, 1.2 equiv) was added dropwise. The reaction was continued to stir at 0 °C for 2 h before water was added to quench the reaction. The mixture was extracted with EtOAc three times. The combined organic extracts were washed with brine, dried over Na2SO4, and evaporated in vacuo. The product was purified by flash column chromatography (PE/EtOAc, 3:1) giving (±)-15 as a white solid (1.40 g, 88%). 1H NMR (400 MHz): δ 7.33 (t, J = 8.3 Hz, 1H), 7.30–7.26 (m, 3H), 7.19–7.11 (m, 5H), 6.84–6.80 (m, 3H), 5.67 (d, J = 11.0 Hz, 1H), 5.49 (s, 1H), 5.10 (d, J = 11.5 Hz, 1H), 5.01 (d, J = 11.5 Hz, 1H), 4.96 (d, J = 11.0 Hz, 1H), 3.92 (s, 1H), 3.78 (s, 3H), 3.67–3.54 (m, 2H), 3.58 (s, 3H), 0.98–0.94 (m, 2H), 0.00 (s, 9H). 13C{H} NMR (101 MHz): δ 167.6, 159.8, 157.4, 139.8, 135.7, 133.5, 129.7, 128.8, 128.4, 127.7, 127.5, 116.9, 114.0, 110.0, 109.3, 85.3, 77.4, 72.2, 71.2, 66.0, 59.4, 55.3, 18.1, −1.3. IR ν: 3508, 2952, 1693, 1594, 1465, 1385, 1247, 1064, 832 cm–1. HRMS (FD) m/z: [M]+ calcd for C30H37NO6Si+, 535.2390; found, 535.2383.
(3R*,4R*)-4,5-Dihydroxy-3-methoxy-4-(4-methoxyphenyl)-1-{[2-(trimethylsilyl)ethoxy]methyl}-3,4-dihydroquinolin-2(1H)-one [(±)-16]
In a Schlenk flask were added (±)-15 (430 mg), EtOH (5 mL), EtOAc (5 mL), and Pd/C (100 mg, 10 wt %) under N2. The flask atmosphere was then changed to H2 by using a balloon filled with H2. The reaction was then stirred for 2 h under H2 (1 atm. pressure). The reaction was filtrated and evaporated in vacuo. The product was purified by flash column chromatography (n-hexane/EtOAc, 3:1) giving (±)-17 as a white solid (446 mg, 95%). 430 mg of (±)-15 (0.80 mmol) and 100 mg Pd/C (10% wt) were used. Purification was performed using PE/EtOAc (3:1) as an eluent. Product (±)-16 was isolated as a white solid in 95% yield (340 mg). 1H NMR (400 MHz): δ 8.86 (s, 1H), 7.28 (t, J = 8.2 Hz, 1H), 7.17–7.15 (m, 2H), 6.98 (d, J = 8.3 Hz, 1H), 6.85–6.82 (m, 2H), 6.74 (d, J = 8.3 Hz, 1H), 5.65 (d, J = 11.0 Hz, 1H), 5.00 (d, J = 10.9 Hz, 1H), 4.53 (s, 1H), 3.85 (s, 1H), 3.77 (s, 3H), 3.63–3.53 (m, 2H), 3.59 (s, 3H), 0.96 (t, J = 8.3 Hz, 2H), 0.00 (s, 9H). 13C{H} NMR (101 MHz): δ 166.1, 160.4, 157.6, 138.1, 130.4, 129.0, 128.2, 114.3, 113.8, 112.3, 107.7, 84.6, 78.1, 72.2, 66.1, 58.8, 55.3, 18.1, −1.3. IR ν: 3353, 2953, 1677, 1613, 1470, 1243, 1073, 834 cm–1. HRMS (FD) m/z: [M]+ calcd for C23H31NO6Si+, 445.1921; found, 445.1939.
Synthesis of (±)-27
[2-(Benzyloxy)-6-nitrophenyl](phenyl)methanol (Int-F)
The procedure for the preparation of 10 was followed. 4.00 g of 9 (13.0 mmol) was used and purification was performed using PE/EtOAc (7:1 to 3:1) as an eluent. Product Int-F was isolated as a yellow oil in 48% yield (2.10 g). Another reaction using 4.00 g of 9 (13.0 mmol) with 0.95 equiv of n-BuLi and 0.5 equiv of anisaldehyde was also performed, and 1.93 g of Int-F was obtained (88% based on anisaldehyde). 1H NMR (400 MHz): δ 7.41–7.36 (m, 2H), 7.34–7.20 (m, 8H), 7.15 (d, J = 7.9 Hz, 1H), 6.94–6.87 (m, 2H), 6.26 (s, 1H), 5.02 (dd, J = 11.6, 2.8 Hz, 1H), 4.92 (d, J = 11.5 Hz, 1H). 13C{H} NMR (101 MHz): δ 157.3, 151.0, 142.6, 135.0, 129.4, 128.8, 128.6, 128.3, 127.6, 127.3, 126.6, 125.8, 116.8, 77.4, 71.5, 69.6. IR ν: 3031, 1528, 1452, 1356, 1023, 737, 697 cm–1. HRMS (EI) m/z: [M – OH]+ calcd for C20H16NO3+, 318.1130; found, 318.1110.
[2-Amino-6-(benzyloxy)phenyl](phenyl)methanol (Int-G)
The procedure for the preparation of 11 was followed. 1.80 g of Int-F (5.4 mmol) was used and the reaction was refluxed for 0.5 h. Product Int-G was used for the next step without purification. 1H NMR (400 MHz): δ 7.46 (d, J = 7.5 Hz, 2H), 7.42–7.16 (m, 8H), 7.08 (t, J = 8.2 Hz, 1H), 6.57 (s, 1H), 6.49 (d, J = 8.3 Hz, 1H), 6.35 (d, J = 7.9 Hz, 1H), 5.17–5.02 (m, 2H). 13C{H} NMR (101 MHz): δ 156.9, 146.1, 143.0, 137.1, 129.2, 128.6, 128.3, 128.0, 127.5, 127.0, 125.9, 116.7, 111.2, 102.9, 70.6, 68.5.
N-{3-(Benzyloxy)-2-[hydroxy(phenyl)methyl]phenyl}-2-methoxyacetamide (Int-H)
The procedure for the preparation of 12 was followed. The crude sample Int-G from the previous step was used and purification was performed using PE/EtOAc (2.5:1) as an eluent. Product Int-H was isolated as a yellow oil in 67% yield (2.10 g) over two steps. 1H NMR (400 MHz): δ 9.76 (s, 1H), 7.89 (d, J = 8.3 Hz, 1H), 7.41–7.16 (m, 11H), 6.82 (d, J = 8.4 Hz, 1H), 6.67 (s, 1H), 5.13 (d, J = 11.6 Hz, 1H), 5.05 (d, J = 11.6 Hz, 1H), 3.89 (d, J = 15.4 Hz, 1H), 3.67 (d, J = 15.3 Hz, 1H), 3.29 (s, 3H). 13C{H} NMR (101 MHz): δ 168.3, 156.0, 142.0, 137.7, 136.7, 129.3, 128.7, 128.2, 128.2, 127.6, 127.2, 125.8, 121.4, 116.1, 108.7, 72.4, 71.0, 68.2, 59.5. IR ν: 3293, 2934, 1665, 1592, 1443, 1261, 1117, 736 cm–1. HRMS (EI) m/z: [M – H2O]+ calcd for C23H21NO3+, 359.1521; found, 359.1513.
N-[2-Benzoyl-3-(benzyloxy)phenyl]-2-methoxyacetamide (Int-I)
The procedure for the preparation of 13 was followed. 1.46 g of Int-H (3.87 mmol) was used and purification was performed using PE/EtOAc (2:1) as eluent. Product Int-I was isolated as a pale yellow solid in 89% yield (1.29 g). 1H NMR (400 MHz): δ 9.53 (s, 1H), 8.04 (d, J = 8.4 Hz, 1H), 7.79 (d, J = 8.5 Hz, 1H), 7.59–7.50 (m, 3H), 7.46–7.40 (m, 3H), 7.20–7.11 (m, 3H), 6.81 (d, J = 8.4 Hz, 1H), 6.76 (d, J = 7.6 Hz, 2H), 4.88 (d, J = 2.0 Hz, 2H), 3.92 (d, J = 1.9 Hz, 2H), 3.39 (d, J = 2.3 Hz, 3H). 13C{H} NMR (101 MHz): δ 197.0, 168.4, 157.3, 139.3, 137.0, 135.9, 133.0, 132.4, 129.2, 128.4, 128.2, 127.7, 126.7, 118.6, 115.0, 108.4, 72.2, 70.3, 59.5. IR ν: 3354, 2936, 1692, 1581, 1466, 1274, 1070, 923, 697 cm–1. HRMS (EI) m/z: [M]+ calcd for C23H21NO4+, 375.1471; found, 375.1464.
(3R*,4R*)-5-(Benzyloxy)-4-hydroxy-3-methoxy-4-phenyl-3,4-dihydroquinolin-2(1H)-one [(±)-Int-J]
The procedure for the preparation of (±)-14 was followed. 1.15 g of Int-I (3.06 mmol) was used and purification was performed using PE/EtOAc (1:1) as an eluent. Product (±)-Int-J was isolated as a white solid in 95% yield (1.10 g). 1H NMR (400 MHz): δ 8.76 (br s, 1H), 7.27–7.18 (m, 9H), 7.06–7.03 (m, 2H), 6.70 (d, J = 8.3 Hz, 1H), 6.55 (d, J = 8.3 Hz, 1H), 5.28 (br s, 1H), 5.03 (d, J = 11.6 Hz, 1H), 4.93 (d, J = 11.6 Hz, 1H), 3.83 (s, 1H), 3.59 (s, 3H). 13C{H} NMR (101 MHz): δ 168.1, 158.0, 141.9, 137.2, 135.7, 130.0, 128.7, 128.7, 128.4, 128.3, 127.4, 126.2, 115.2, 109.6, 108.7, 85.0, 78.3, 71.0, 59.8. Its NMR data matched with those reported in the literature.10a
(3R*,4R*)-5-(Benzyloxy)-4-hydroxy-3-methoxy-4-phenyl-1-{[2-(trimethylsilyl)ethoxy]methyl}-3,4-dihydroquinolin-2(1H)-one [(±)-Int-K]
In a flame-dried Schlenk flask were added (±)-Int-J (0.90 g, 2.40 mmol, 1.0 equiv) and anhydrous THF (35 mL). LiHMDS solution in THF (3.12 mL, 1.0 M, 3.12 mmol, 1.3 equiv) was then added dropwise at 0 °C. After stirring it for 0.5 h, SEMCl (0.60 g, 3.60 mmol, 1.5 equiv) was added dropwise. The reaction was first stirred at 0 °C for 0.5 h and then 2 h at room temperature. Water was added to quench the reaction. The mixture was extracted with EtOAc three times. The combined organic extracts were washed with brine, dried over Na2SO4, and evaporated in vacuo. The sample was purified by flash column chromatography (PE/EtOAc, 4:1) giving (±)-Int-K as a white solid (1.15 g, 94%). 1H NMR (400 MHz): δ 7.31 (t, J = 8.3 Hz, 1H), 7.29–7.20 (m, 8H), 7.16 (d, J = 8.4 Hz, 1H), 7.08–6.99 (m, 2H), 6.80 (d, J = 8.4 Hz, 1H), 5.64 (d, J = 11.0 Hz, 1H), 5.47 (br s, 1H), 5.05 (d, J = 11.5 Hz, 1H), 4.97–4.94 (m, 2H), 3.91 (s, 1H), 3.66–3.53 (m, 2H), 3.56 (s, 3H), 0.98–0.93 (m, 2H), −0.02 (s, 9H). 13C{H} NMR (101 MHz): δ 167.5, 157.4, 141.7, 139.9, 135.7, 129.8, 128.7, 128.6, 128.5, 128.3, 127.4, 126.3, 116.7, 109.9, 109.2, 85.2, 77.6, 72.2, 71.2, 66.0, 59.5, 18.1, −1.3. IR ν: 3507, 2951, 1693, 1593, 1469, 1376, 1247, 1062, 833, 730, 696 cm–1. HRMS (FD) m/z: [M]+ calcd for C29H35NO5Si+, 505.2284; found, 505.2302.
(3R*,4R*)-4,5-Dihydroxy-3-methoxy-4-phenyl-1-{[2-(trimethylsilyl)ethoxy]methyl}-3,4-dihydroquinolin-2(1H)-one [(±)-27]
The procedure for the preparation of (±)-16 was followed. 0.90 g of (±)-Int-K (2.17 mmol) was used and purification was performed using PE/EtOAc (4:1) as an eluent. Product (±)-27 was isolated as a white solid in 94% yield (0.70 g). 1H NMR (400 MHz): δ 8.80 (br s, 1H), 7.32–7.29 (m, 3H), 7.28–7.20 (m, 3H), 6.98 (d, J = 8.2 Hz, 1H), 6.72 (d, J = 8.3 Hz, 1H), 5.65 (d, J = 10.9 Hz, 1H), 5.00 (d, J = 10.9 Hz, 1H), 4.54 (s, 1H), 3.82 (s, 1H), 3.60–3.54 (m, 2H), 3.58 (s, 3H), 0.97–0.92 (m, 2H), −0.02 (s, 9H). 13C{H} NMR (101 MHz): δ 165.9, 157.6, 138.3, 137.3, 130.6, 129.5, 129.0, 126.7, 113.9, 112.2, 107.8, 84.6, 78.3, 72.3, 66.2, 58.9, 18.1, −1.3. IR ν: 3325, 2952, 1680, 1470, 1241, 1070, 834 cm–1. HRMS (FD) m/z: [M]+ calcd for C22H29NO5Si+, 415.1815; found, 415.1836.
C(6)–H Olefination of 3,4-Dihydro-2(1H)-quinolinone Derivatives
General Procedure
In a pressure tube containing a suitable stirring bar were added the corresponding 3,4-dihydro-2(1H)-quinolinone derivative (1.0 equiv), Pd(OAc)2 (10 mol %), PhCO3tBu (1.0–2.0 equiv), olefin (1.5 equiv), S,O-ligand stock solution in DCE (0.1 M, 10 mol %), and DCE. The tube was introduced into an oil bath preheated at 80 °C and was stirred for 16 h. The tube was then removed from the oil bath and cooled to room temperature. The reaction was filtrated through Celite and rinsed with DCM. The filtrate was then washed with a saturated solution of Na2CO3, dried with MgSO4, filtrated, and concentrated in vacuo. The product was purified by flash column chromatography.
(E)-Ethyl 3-(1-Methyl-2-oxo-1,2,3,4-tetrahydroquinolin-6-yl)acrylate (1b)
Substrate 1a (16.1 mg, 0.1 mmol) was olefinated using ethyl acrylate (16.3 μL, 0.15 mmol, 1.5 equiv) and PhCO3tBu (18.6 μL, 0.1 mmol, 1.0 equiv) for 16 h following the general procedure, and the sample was purified by preparative TLC (PE/EtOAc, 5:1) to give 1b as a yellow oil (9.0 mg, 35%) as a mixture of regioisomers (para/others 4.3:1). 1H NMR (400 MHz): δ 7.63 (d, J = 16.1 Hz, 1H), 7.42 (dd, J = 8.4, 2.1 Hz, 1H), 7.35 (d, J = 1.9 Hz, 1H), 6.98 (d, J = 8.4 Hz, 1H), 6.37 (d, J = 16.0 Hz, 1H), 4.26 (q, J = 7.1 Hz, 2H), 3.37 (s, 3H), 2.93 (dd, J = 8.6, 6.1 Hz, 2H), 2.68 (dd, J = 8.7, 6.0 Hz, 3H), 1.34 (t, J = 7.1 Hz, 4H). HRMS (FD) m/z: [M]+ calcd for C15H17NO3+, 259.1208; found, 259.1184.
(E)-Ethyl 3-(5-Methoxy-1-methyl-2-oxo-1,2,3,4-tetrahydroquinolin-6-yl)acrylate (2b)
Substrate 2a (19.1 mg, 0.1 mmol) was olefinated using ethyl acrylate (16.3 μL, 0.15 mmol, 1.5 equiv) and PhCO3tBu (18.6 μL, 0.1 mmol, 1.0 equiv) following the general procedure, and the sample was purified by flash column chromatography (PE/EtOAc, 3:1) to give 2b as a yellow oil (13.0 mg, 45%, C6/others > 20:1). 1H NMR (400 MHz): δ 7.88 (d, J = 16.1 Hz, 1H), 7.47 (d, J = 8.6 Hz, 1H), 6.80 (d, J = 8.6 Hz, 1H), 6.45 (d, J = 16.1 Hz, 1H), 4.27 (q, J = 7.1 Hz, 2H), 3.74 (s, 3H), 3.36 (s, 3H), 2.97 (dd, J = 8.6, 6.3 Hz, 2H), 2.63 (dd, J = 8.6, 6.2 Hz, 2H), 1.34 (t, J = 7.1 Hz, 3H). 13C{H} NMR (101 MHz): δ 170.3, 167.4, 156.7, 143.7, 139.0, 127.2, 123.1, 120.1, 118.3, 111.4, 62.0, 60.6, 31.1, 29.9, 18.8, 14.5. IR ν: 2923, 2852, 1675, 1628, 1596, 1265, 1167 cm–1. HRMS (FD) m/z: [M]+ calcd for C16H19NO4+, 289.1314; found, 289.1319. A parallel reaction was performed without using the S,O-ligand and only a trace amount of desired product was detected.
(E)-Ethyl 3-(5-Ethoxy-1-methyl-2-oxo-1,2,3,4-tetrahydroquinolin-6-yl)acrylate (3b)
Substrate 3a (20.5 mg, 0.1 mmol) was olefinated using ethyl acrylate (16.3 μL, 0.15 mmol, 1.5 equiv) and PhCO3tBu (18.6 μL, 0.1 mmol, 1.0 equiv) following the general procedure, and the sample was purified by flash column chromatography (PE/EtOAc, 3:1) to give 3b as a yellow oil (17.3 mg, 57%, C6/others > 20:1). 1H NMR (400 MHz): δ 7.90 (d, J = 16.1 Hz, 1H), 7.47 (d, J = 8.6 Hz, 1H), 6.79 (d, J = 8.6 Hz, 1H), 6.42 (d, J = 16.1 Hz, 1H), 4.25 (q, J = 7.1 Hz, 2H), 3.86 (q, J = 7.0 Hz, 2H), 3.35 (s, 3H), 2.95 (dd, J = 8.6, 6.2 Hz, 2H), 2.61 (dd, J = 8.5, 6.2 Hz, 2H), 1.42 (t, J = 7.0 Hz, 3H), 1.33 (t, J = 7.1 Hz, 3H). 13C{H} NMR (101 MHz): δ 170.3, 167.3, 155.7, 143.7, 139.3, 126.8, 123.3, 120.3, 118.0, 111.3, 70.7, 60.5, 31.2, 29.9, 19.0, 15.6, 14.5. IR ν: 2977, 1679, 1598, 1441, 1352, 1177, 1125, 1044 cm–1. HRMS (FD) m/z: [M]+ calcd for C17H21NO4+, 303.1471; found, 303.1488.
(E)-Ethyl 3-[5-(Methoxymethoxy)-1-methyl-2-oxo-1,2,3,4-tetrahydroquinolin-6-yl]acrylate (4b)
Substrate 4a (22.1 mg, 0.1 mmol) was olefinated using ethyl acrylate (16.3 μL, 0.15 mmol, 1.5 equiv) and PhCO3tBu (18.6 μL, 0.1 mmol, 1.0 equiv) following the general procedure, and the sample was purified by flash column chromatography (PE/EtOAc, 3:1) to give 4b as a yellow oil (15.0 mg, 47%, C6/others > 20:1). 1H NMR (400 MHz): δ 7.93 (d, J = 16.1 Hz, 1H), 7.50 (d, J = 8.5 Hz, 1H), 6.82 (d, J = 8.6 Hz, 1H), 6.38 (d, J = 16.2 Hz, 1H), 4.97 (s, 2H), 4.25 (q, J = 7.2 Hz, 2H), 3.62 (s, 3H), 3.35 (s, 3H), 2.97 (dd, J = 8.6, 6.2 Hz, 2H), 2.61 (dd, J = 8.6, 6.3 Hz, 2H), 1.33 (t, J = 7.1 Hz, 3H). 13C{H} NMR (101 MHz): δ 170.3, 167.2, 154.2, 143.7, 139.4, 126.5, 123.5, 120.5, 118.0, 111.8, 100.7, 60.6, 58.1, 31.2, 30.0, 19.6, 14.5. IR ν: 2852, 1677, 1598, 1354, 1251, 1124, 812 cm–1. HRMS (FD) m/z: [M]+ calcd for C17H21NO5+, 319.1420; found, 319.1415.
(E)-Ethyl 3-(5-Hydroxy-1-methyl-2-oxo-1,2,3,4-tetrahydroquinolin-6-yl)acrylate (5b)
Substrate 5a (17.7 mg, 0.1 mmol) was olefinated using ethyl acrylate (16.3 μL, 0.15 mmol, 1.5 equiv) and PhCO3tBu (18.6 μL, 0.1 mmol, 1.0 equiv) following the general procedure, and the sample was purified by preparative TLC (PE/EtOAc, 2:1) to give 5b as a yellow oil (7.4 mg, 27%). (The product is not stable, as after the sample solution in CDCl3 was stored overnight at room temperature and was measured again, many new peaks appeared.) 1H NMR (300 MHz): δ 7.97 (d, J = 16.0 Hz, 1H), 7.40 (d, J = 8.6 Hz, 1H), 6.64 (d, J = 8.6 Hz, 1H), 6.45 (d, J = 15.9 Hz, 1H), 5.88 (s, 1H), 4.27 (q, J = 7.2 Hz, 2H), 3.36 (s, 3H), 2.96–2.83 (m, 2H), 2.67 (dd, J = 8.8, 6.2 Hz, 2H), 1.34 (t, J = 7.1 Hz, 3H).
(E)-Ethyl 3-[5-Ethoxy-1-(methoxymethyl)-2-oxo-1,2,3,4-tetrahydroquinolin-6-yl]acrylate (6b)
Substrate 6a (81.6 mg, 0.35 mmol) was olefinated using ethyl acrylate (57.0 μL, 0.525 mmol, 1.5 equiv), PhCO3tBu (67.0 μL, 0.35 mmol, 1.0 equiv) following the general procedure, and the sample was purified by flash column chromatography (PE/EtOAc, 3:1) to give a mixture of 6b-a and 6b-b as a yellow oil (50.8 mg, 44%, 6b–a/6b–b = 9.1:1) and 6b–c as a yellow oil (1.7 mg, 1.5%). 1H NMR (400 MHz) (6b–a): δ 7.91 (d, J = 16.2 Hz, 1H), 7.46 (d, J = 8.5 Hz, 1H), 7.11 (d, J = 8.5 Hz, 1H), 6.43 (d, J = 16.1 Hz, 1H), 5.31 (s, 2H), 4.26 (q, J = 7.1 Hz, 2H), 3.86 (q, J = 6.4 Hz, 2H), 3.41 (s, 3H), 2.97 (t, J = 7.2 Hz, 2H), 2.76–2.63 (m, 2H), 1.43 (t, J = 6.3 Hz, 3H), 1.34 (t, J = 7.2 Hz, 3H). 13C{H} NMR (101 MHz) (6b–a): δ 171.1, 167.3, 155.6, 142.6, 139.2, 126.9, 123.9, 120.3, 118.2, 112.5, 74.0, 70.8, 60.5, 56.5, 31.3, 19.1, 15.6, 14.4. IR (6b–a) ν: 2976, 1711, 1680, 1626, 1598, 1393, 1166, 1041 cm–1. HRMS (6b–a) (FD) m/z: [M]+ calcd for C18H23NO5, 333.1576; found, 333.1575. 1H NMR (400 MHz) (6b–c): δ 7.64 (d, J = 16.0 Hz, 1H), 7.12 (d, J = 1.3 Hz, 1H), 6.79 (d, J = 1.4 Hz, 1H), 6.41 (d, J = 15.9 Hz, 1H), 5.31 (s, 2H), 4.27 (q, J = 7.1 Hz, 2H), 4.08 (q, J = 7.0 Hz, 2H), 3.41 (s, 3H), 2.95–2.92 (m, 2H), 2.66 (dd, J = 8.4, 6.3 Hz, 2H), 1.44 (t, J = 7.0 Hz, 3H), 1.34 (t, J = 7.1 Hz, 3H). 13C{H} NMR (101 MHz) (6b-c): δ 171.4, 167.0, 156.0, 144.7, 141.3, 134.5, 118.5, 117.2, 109.0, 106.1, 74.2, 64.2, 60.7, 56.5, 31.2, 18.5, 15.0, 14.5. IR (6b–c) ν: 2979, 2931, 1690, 1601, 1270, 1177 cm–1. HRMS (6b–c) (FD) m/z: [M]+ calcd for C18H23NO5+, 333.1576; found, 333.1575.
(E)-Ethyl 3-[1-(4-Acetoxybenzyl)-5-ethoxy-2-oxo-1,2,3,4-tetrahydroquinolin-6-yl]acrylate (7b)
Substrate 7a (33.9 mg, 0.1 mmol) was olefinated using ethyl acrylate (16.3 μL, 0.15 mmol, 1.5 equiv) and PhCO3tBu (18.6 μL, 0.1 mmol, 1.0 equiv) following the general procedure, and the sample was purified by flash column chromatography (PE/EtOAc, 3:1 to 2:1) to give 7b as a yellow oil (21.0 mg, 48%, C6/others > 20:1). 1H NMR (400 MHz): δ 7.87 (d, J = 16.1 Hz, 1H), 7.34 (d, J = 8.7 Hz, 1H), 7.23–7.21 (m, 2H), 7.05–7.03 (m, 2H), 6.69 (d, J = 8.7 Hz, 1H), 6.37 (d, J = 16.1 Hz, 1H), 5.15 (s, 2H), 4.25 (q, J = 7.1 Hz, 2H), 3.87 (q, J = 7.0 Hz, 2H), 3.02 (dd, J = 8.6, 6.1 Hz, 2H), 2.78–2.72 (m, 2H), 2.28 (s, 3H), 1.44 (t, J = 7.0 Hz, 3H), 1.33 (t, J = 7.1 Hz, 3H). 13C{H} NMR (101 MHz): δ 170.4, 169.6, 167.3, 155.8, 149.9, 142.8, 139.1, 134.4, 127.7, 126.8, 123.6, 122.0, 120.5, 118.1, 112.0, 70.8, 60.6, 45.9, 31.4, 21.3, 19.2, 15.7, 14.5. IR ν: 2927, 1761, 1681, 1371, 1166 cm–1. HRMS (FD) m/z: [M]+ calcd for C25H27NO6+, 437.1838; found, 437.1837.
(E)-5-Hydroxy-6-{2-[5-(2-hydroxypropan-2-yl)-2-methyltetrahydrofuran-2-yl]vinyl}-1-methyl-3,4-dihydroquinolin-2(1H)-one (5c) and 2-[5-(2-Hydroxypropan-2-yl)-2-methyltetrahydrofuran-2-yl]-6-methyl-8,9-dihydrofuro[2,3-f]quinolin-7(6H)-one (5d)
Substrate 5a (44.3 mg, 0.25 mmol) was olefinated using trans-29 (63.8 mg, 0.375 mmol, 1.5 equiv) and PhCO3tBu (86.0 μL, 0.45 mmol, 1.8 equiv) for 16 h following the general procedure, and the sample was purified by preparative TLC (PE/EtOAc, 3:1) to give 5c as a yellow oil (41.4 mg, 48%, dr = 1.3:1) and 5d as a yellow oil (15.4 mg, 18%, dr not determined). Another reaction was also performed using a mixture of trans-29 and cis-29 as the olefin, and the same results were obtained regarding both the yield and diastereoselectivity. 1H NMR (5c) (300 MHz) (two diastereoisomers: A/B = 1:1.3): δ 7.17 (d, J = 8.5 Hz, 1HB), 7.15 (d, J = 8.5 Hz, 1HA), 6.83 (d, J = 16.0 Hz, 1HA), 6.71 (d, J = 15.9 Hz, 1HB), 6.55 (d, J = 8.5 Hz, 1HB), 6.54 (d, J = 8.5 Hz, 1HA), 6.12 (d, J = 15.9 Hz, 1HA), 6.10 (d, J = 15.9 Hz, 1HB), 3.96–3.90 (m, 1HA and 1HB), 3.33 (s, 3HB), 3.33 (s, 3HA), 2.93–2.86 (m, 2HA and 2HB), 2.63–2.57 (m, 2HA and 2HB), 2.04–1.81 (m, 4HA and 4HB), 1.42 (s, 3HB), 1.32 (s, 3HA), 1.18 (s, 3HA), 1.16 (s, 3HB). 13C{H} NMR (5c) (75 MHz): δ 170.6 (A and B), 150.3 (A and B), 141.0 (A and B), 137.7 (A or B), 137.1 (A or B), 125.7 (A or B), 125.6 (A or B), 122.0 (A or B), 121.1 (A or B), 120.3 (A or B), 120.0 (A or B), 113.2 (A and B), 107.5 (A and B), 86.1 (A or B), 86.0 (A or B), 83.6 (A or B), 83.1 (A or B), 72.1 (A or B), 72.0 (A or B), 38.7 (A or B), 38.3 (A or B), 31.2 (A and B), 29.9 (A and B), 27.5 (A and B), 27.0 (A or B), 26.9 (A or B), 26.7 (A or B), 26.5 (A or B), 25.3 (A or B), 23.9 (A or B), 18.4 (A and B). IR (5c) ν: 3306, 2972, 2929, 1652, 1624, 1472, 1372, 1126, 1041, 729 cm–1. HRMS (5c) (FD) m/z: [M]+ calcd for C20H25NO4, 343.1784; found, 343.1771. 1H NMR (5d) (400 MHz) (two diastereoisomers: A and B. Ratio was not determined): δ 7.38 (d, J = 8.5 Hz, 1HA and 1HB), 6.94 (d, J = 8.5 Hz, 1HA and 1HB), 6.57 (s, 1HA and 1HB), 4.05–4.01 (m, 1HA and 1HB), 3.44 (s, 3HA and 3HB), 3.19–3.14 (m, 2HA and 2HB), 2.77–2.72 (m, 2HA and 2HB), 2.52–2.43 (m, 1HA and 1HB), 2.10–1.97 (m, 3HA and 3HB), 1.70 (s, 3HA), 1.69 (s, 3HB), 1.31 (s, 3HA), 1.30 (s, 3HB), 1.21 (s, 3HB), 1.20 (s, 3HA). 13C{H} NMR (5d) (75 MHz): δ 170.2 (A and B), 162.4 (A and B), 152.4 (A or B), 152.2 (A or B), 137.8 (A or B), 137.6 (A or B), 124.0 (A or B), 123.8 (A or B), 119.0 (A or B), 118.9 (A or B), 110.9 (A or B), 110.8 (A or B), 109.7 (A and B), 101.5 (A or B), 101.3 (A or B), 86.7 (A and B), 81.0 (A or B), 80.7 (A or B), 71.5 (A or B), 71.3 (A or B), 38.6 (A or B), 37.5 (A or B), 31.3 (A and B), 30.3 (A and B), 27.9 (A and B), 27.4 (A or B), 27.1 (A or B), 26.7 (A or B), 26.6 (A or B), 24.7 (A or B), 24.4 (A or B), 18.7 (A or B), 18.6 (A or B). IR (5d) ν: 3444, 2975, 2928, 1671, 1632, 1470, 1375, 1127, 1026, 819 cm–1. HRMS (5d) (FD) m/z: [M]+ calcd for C20H27NO4+, 345.1940; found, 345.1944.
(3R*,4R*)-4,5-Dihydroxy-3-methoxy-4-(4-methoxyphenyl)-6-[(E)-3-oxobut-1-en-1-yl]-1-{[2-(Trimethylsilyl)ethoxy]methyl}-3,4-dihydroquinolin-2(1H)-one [(±)-17]
The substrate (±)-16 (44.5 mg, 0.1 mmol) was olefinated using but-3-en-2-one (12.5 μL, 0.15 mmol, 1.5 equiv) and PhCO3tBu (38.0 μL, 0.2 mmol, 2.0 equiv) following the general procedure, and the sample was purified by flash column chromatography (n-hexane/EtOAc, 3:1) to give (±)-17 as a mixture of regioisomers [30.3 mg, 59%, yellow oil, (±)-17-A/(±)-17-B = 6:1], and (±)-17-di (2.9 mg, 5%, yellow oil). 1H NMR (300 MHz) [(±)-17-A]: δ 9.52 (s, 1H), 7.82 (d, J = 16.5 Hz, 1H), 7.55 (d, J = 8.7 Hz, 1H), 7.15–7.10 (m, 2H), 6.99 (d, J = 8.6 Hz, 1H), 6.84–6.78 (m, 2H), 6.72 (d, J = 16.5 Hz, 1H), 5.61 (d, J = 10.9 Hz, 1H), 5.01 (d, J = 11.1 Hz, 1H), 4.62 (s, 1H), 3.84 (s, 1H), 3.75 (s, 3H), 3.58–3.52 (m, 5H), 2.34 (s, 3H), 0.96–0.93 (m, 2H), −0.03 (s, 9H). 13C{H} NMR (75 MHz) [(±)-17-A]: δ 199.3, 165.9, 160.6, 156.9, 139.9, 138.1, 129.5, 128.4, 128.1, 127.0, 119.5, 114.5, 112.6, 108.2, 84.4, 78.2, 72.0, 66.4, 58.9, 55.4, 27.0, 18.1, −1.3. IR [(±)-17-A] ν: 3259, 2953, 2926, 1692, 1605, 1369, 1252, 1081, 834 cm–1. HRMS [(±)-17-A] (FD) m/z: [M]+ calcd for C27H35NO7Si+, 513.2183; found, 513.2208. 1H NMR (400 MHz) [(±)-17-di]: δ 9.41 (s, 1H), 7.82 (d, J = 16.5 Hz, 1H), 7.75 (d, J = 16.5 Hz, 1H), 7.58 (d, J = 8.7 Hz, 1H), 7.53 (d, J = 2.4 Hz, 1H), 7.05 (dd, J = 8.8, 2.4 Hz, 1H), 7.02 (d, J = 8.8 Hz, 1H), 6.81 (d, J = 8.8 Hz, 1H), 6.74 (d, J = 16.5 Hz, 1H), 6.67 (d, J = 16.5 Hz, 1H), 5.64 (d, J = 10.9 Hz, 1H), 5.01 (d, J = 10.8 Hz, 1H), 4.59 (s, 1H), 3.86 (s, 3H), 3.81 (s, 1H), 3.60 (s, 3H), 3.60–3.53 (m, 2H), 2.37 (s, 2H), 2.36 (s, 3H), 0.96–0.92 (m, 2H), −0.03 (s, 9H). 13C{H} NMR (101 MHz) [(±)-17-di]: δ 199.2, 199.1, 165.7, 159.1, 156.8, 139.8, 138.2, 137.9, 129.9, 129.8, 128.9, 128.7, 127.2, 127.2, 124.2, 119.8, 112.1, 111.6, 108.4, 84.3, 78.0, 72.1, 66.4, 59.0, 55.8, 27.4, 27.2, 18.1, −1.3. IR [(±)-17-di] ν: 3251, 2954, 1692, 1604, 1368, 1253, 1082, 836 cm–1. HRMS [(±)-17-di] (FD) m/z: [M]+ calcd for C31H39NO8Si+, 581.2445; found, 581.2443.
(E)-4,5-Dihydroxy-3-methoxy-4-(4-methoxyphenyl)-1-{[2-(trimethylsilyl)ethoxy]methyl}-6-[2-(2,5,5-trimethyltetrahydro-2H-pyran-2-yl)vinyl]-3,4-dihydroquinolin-2(1H)-one (19)
Substrate (±)-16 (111.2 mg, 0.25 mmol) was olefinated using olefin 18 (57.8 mg, 0.375 mmol, 1.5 equiv) and PhCO3tBu (86.0 μL, 0.45 mmol, 1.8 equiv) following the general procedure, and the sample was purified by preparative TLC (n-hexane/acetone, 6:1) to give 19 as a mixture of regioisomers (75.0 mg, 50%, yellow oil, regioselectivity: 8.8:1, dr = 1:1). 1H NMR (400 MHz) (two diastereoisomers: A and B): δ 9.18 (br s, 1HA or 1HB), 9.17 (br s, 1HA or 1HB), 7.48 (d, J = 8.6 Hz, 1HA and 1HB), 7.18–7.13 (m, 2HA and 2HB), 6.96 (d, J = 8.6 Hz, 1HA and 1HB), 6.83–6.80 (m, 2HA and 2HB), 6.77 (d, J = 16.7 Hz, 1HA and 1HB), 6.18 (d, J = 16.7 Hz, 1HA or 1HB), 6.17 (d, J = 16.7 Hz, 1HA or 1HB), 5.63 (d, J = 10.9 Hz, 1HA and 1HB), 4.99 (d, J = 10.9 Hz, 1HA and 1HB), 4.45 (s, 1HA and 1HB), 3.81 (s, 1HA or 1HB), 3.80 (s, 1HA or 1HB), 3.76 (s, 3HA or 3HB), 3.75 (s, 3HA or 3HB), 3.59–3.53 (m, 2HA and 2HB), 3.58 (s, 3HA or 3HB), 3.57 (s, 3HA or 3HB), 3.41–3.36 (m, 1HA and 1HB), 3.25–3.21 (m, 1HA and 1HB), 1.86–1.79 (1HA and 1HB), 1.75–1.67 (1HA and 1HB), 1.52–1.44 (1HA and 1HB), 1.36–1.32 (1HA and 1HB), 1.31 (s, 3HA and 3HB), 1.01 (s, 3HA or 3HB), 1.00 (s, 3HA or 3HB), 0.95–0.91 (2HA and 2HB), 0.79 (s, 3HA and 3HB), −0.03 (s, 9HA and 9HB). 13C{H} NMR (101 MHz): δ 165.9 (A and B), 160.5 (A and B), 154.7 (A and B), 137.1 (A and B), 134.8, 134.7, 128.8 (A and B), 128.2 (A and B), 127.4, 127.3, 123.3 (A and B), 122.4 (A and B), 114.4 (A and B), 112.3 (A and B), 107.7 (A and B), 84.7 (A and B), 78.3 (A and B), 74.5 (A and B), 73.0, 72.9, 72.1 (A and B), 66.2 (A and B), 58.9 (A and B), 55.4 (A and B), 33.7 (A and B), 31.3, 31.1, 29.9 (A and B), 29.4, 29.2, 26.8 (A and B), 24.2, 24.1, 18.2 (A and B), −1.3 (A and B). IR ν: 3314, 2950, 1690, 1611, 1511, 1441, 1390, 1250, 1083, 834 cm–1. HRMS (FD) m/z: [M]+ calcd for C33H47NO7Si+, 597.3122; found, 597.3134.
(E)-4,5-Dihydroxy-6-{2-[5-(2-hydroxypropan-2-yl)-2-methyltetrahydrofuran-2-yl]vinyl}-3-methoxy-4-(4-methoxyphenyl)-1-{[2-(trimethylsilyl)ethoxy]methyl}-3,4-dihydroquinolin-2(1H)-one (21)
Substrate (±)-16 (44.5 mg, 0.1 mmol) was olefinated using trans-20 (25.5 mg, 0.15 mmol, 1.5 equiv) and PhCO3tBu (34.2 μL, 0.18 mmol, 1.8 equiv) following the general procedure, and the sample was purified by flash column chromatography (n-hexane/EtOAc, 3:1) to give 21 as a mixture of regioisomers (32.0 mg, 52%, yellow oil, A/B = 7:1, dr: not determined). From the 1H NMR spectra of 21, we mainly found two groups of peaks. That is because the 1H NMR spectra of (±)-21-A and (±)-21-B were highly identical, while the 1H NMR spectra of (±)-21-C and (±)-21-D were also highly identical. Therefore, we were not able to determine the ratios of (±)-21-A/(±)-21-B and (±)-21-C/(±)-21-D, but we assumed that they were both 1:1. Also, because of overlapping of these two groups of peaks, we were not able to determine their ratio. A reaction using a mixture of trans- and cis-20 (1:1) as the olefin was also performed [0.1 mmol scale for (±)-16], and the product 21 was isolated as a mixture of regioisomers (A/B = 5.3:1) (30.0 mg, 50%, mixture of four diastereoisomers). 1H NMR (400 MHz): δ 9.19 (br s, 1HA, 1HB, 1HC and 1HD), 7.43 (d, J = 8.6 Hz, 1HA and 1HB), 7.41 (d, J = 8.6 Hz, 1HC and 1HD), 7.16–7.11 (m, 2HA, 2HB, 2HC and 2HD), 6.93 (d, J = 8.6 Hz, 1HA, 1HB, 1HC and 1HD), 6.82–6.77 (m, 3HA, 3HB, 3HC and 3HD), 6.33 (d, J = 16.4 Hz, 1HC or 1HD), 6.32 (d, J = 16.4 Hz, 1HC or 1HD), 6.28 (d, J = 16.3 Hz, 1HA or 1HB), 6.27 (d, J = 16.3 Hz, 1HA or 1HB), 5.62 (d, J = 10.9 Hz, 1HA and 1HB), 5.61 (d, J = 10.9 Hz, 1HC and 1HD), 4.98 (d, J = 11.0 Hz, 1HA and 1HB), 4.97 (d, J = 11.0 Hz, 1HC and 1HD), 4.47 (s, 1HA, 1HB, 1HC and 1HD), 3.91–3.82 (m, 1HA, 1HB, 1HC and 1HD), 3.80 (s, 1HA, 1HB, 1HC and 1HD), 3.75 (s, 3HA, 3HB, 3HC and 3HD), 3.59–3.52 (m, 2HA, 2HB, 2HC and 2HD), 3.57 (s, 3HA and 3HB), 3.56 (3HC and 3HD), 2.05–1.73 (m, 4HA, 4HB, 4HC and 4HD), 1.41 (s, 3HC and 3HD), 1.40 (s, 3HA and 3HB), 1.24 (s, 3HA and 3HB), 1.23 (s, 3HC and 3HD), 1.14 (s, 3HC and 3HD), 1.13 (s, 3HA and 3HB), 0.95–0.91 (m, 2HA, 2HB, 2HC and 2HD), −0.03 (s, 9HA, 9HB, 9HC and 9HD). 13C{H} NMR (101 MHz): δ 165.9 (A, B, C, and D), 160.4 (A, B, C, and D), 154.9 (A, B, C, and D), 137.2 (C or D), 137.1 (C or D), 137.0 (A and B), 136.0 (C or D), 135.9 (C or D), 135.6 (A or B), 135.5 (A or B), 128.9 (A and B), 128.8 (C and D), 128.2 (A, B, C, and D), 127.8 (A or B), 127.7 (A or B), 127.6 (C and D), 122.3 (A, B, C, and D), 121.3 (C and D), 120.9 (A and B), 114.4 (A, B, C, and D), 112.2 (A, B, C, and D), 107.7 (A, B, C, and D), 85.8 (C and D), 85.7 (A and B), 84.7 (A, B, C, and D), 83.4 (A and B), 83.2 (C and D), 78.3 (A, B, C, and D), 72.1 (A, B, C, and D), 71.3 (A and B), 71.2 (C and D), 66.2 (A, B, C, and D), 58.9 (A, B, C, and D), 55.4 (A, B, C, and D), 38.7 (C or D), 38.6 (C or D), 38.1 (A and B), 27.5 (A, B, C, and D), 26.6 (A, B, C, and D), 24.3 (A, B, C, and D), 18.1 (A, B, C, and D), −1.3 (A, B, C, and D). IR ν: 3295, 2967, 2929, 1688, 1611, 1511, 1441, 1374, 1082, 835 cm–1. HRMS (FD) m/z: [M]+ calcd for C33H47NO8Si+, 613.3071; found, 613.3064.
(E)-4,5-Dihydroxy-6-[2-(5-hydroxy-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)vinyl]-3-methoxy-4-(4-methoxyphenyl)-1-{[2-(trimethylsilyl)ethoxy]methyl}-3,4-dihydroquinolin-2(1H)-one (25)
Substrate (±)-16 (44.5 mg, 0.1 mmol) was olefinated using olefin cis-24 (36.4 mg, 0.15 mmol, 1.5 equiv) and PhCO3tBu (34.2 μL, 0.18 mmol, 1.8 equiv) following the general procedure.
Before performing purification, the crude sample was deprotected by using the following procedure: The sample was dissolved in THF (2 mL) and TBAF solution (1.0 mL, 1.0 M in THF) was added dropwise. After stirring it for 4 h, the reaction was quenched by adding water. The mixture was extracted with EtOAc three times. The combined extracts were washed with brine, dried with MgSO4, and evaporated in vacuo. the sample was purified by preparative TLC (n-hexane/EtOAc, 4:1) to give 25 as a single regioisomer (26.3 mg, 43%, yellow oil, dr = 2.2:2.2:1:1). 1H NMR (300 MHz) [two groups of diastereoisomers: (A + B)/(C + D), 2.2:1] 9.21–9.19 (m, 1HA, 1HB, 1HC, and 1HD), 7.45 (d, J = 8.6 Hz, 1HA), 7.45 (d, J = 8.6 Hz, 1HA and 1HB), 7.44 (d, J = 8.6 Hz, 1HC and 1HD), 7.19–7.14 (m, 2HA, 2HB, 2HC, and 2HD), 6.96 (d, J = 8.7 Hz, 1HA, 1HB, 1HC, and 1HD), 6.89–6.79 (m, 3HA, 3HB, 3HC, and 3HD), 6.38–6.26 (m, 1HA, 1HB, 1HC, and 1HD), 5.64 (d, J = 10.9 Hz, 1HA, 1HB, 1HC, and 1HD), 5.01 (d, J = 10.9 Hz, 1HA, 1HB, 1HC, and 1HD), 4.48 (s, 1HA and 1HB), 4.47 (s, 1HC and 1HD), 3.95–3.88 (m, 1HA, 1HB, 1HC, and 1HD), 3.78 (s, 3HA, 3HB, 3HC, and 3HD), 3.62–3.54 (m, 2HA, 2HB, 2HC, and 2HD), 3.59 (s, 3HA, 3HB, 3HC, and 3HD), 2.08–1.70 (m, 4HA, 4HB, 4HC, and 4HD), 1.44 (s, 3HC and 3HD), 1.42 (3HA and 3HC), 1.26 (s, 3HA, 3HB, 3HC, and 3HD), 1.16 (s, 3HA, 3HB, 3HC, and 3HD), 0.98–0.92 (m, 2HA, 2HB, 2HC, and 2HD), −0.01 (s, 9HA, 9HB, 9HC, and 9HD). 13C{H} NMR (75 MHz): δ 167.2 (A, B, C, and D), 161.7 (A, B, C, and D), 156.1 (A, B, C, and D), 138.4 (C and D), 136.8 (A and B), 137.3 (C or D), 137.1 (C or D), 136.8 (A and B), 130.1 (A, B, C, and D), 129.4 (A, B, C, and D), 129.1 (A or B), 129.0 (A or B), 128.9 (C or D), 128.8 (C or D), 123.6 (A or B), 123.5 (A or B), 123.51 (C and D), 122.6 (C or D), 122.5 (C or D), 122.2 (A or B), 122.1 (A or B), 115.6 (A, B, C, and D), 113.5 (A, B, C, and D), 109.0 (A, B, C, and D), 87.1 (C and D), 86.9 (A and B), 85.9 (A, B, C, and D), 84.7 (A and B), 84.4 (C and D), 79.5 (A, B, C, and D), 73.4 (A, B, C, and D), 72.6 (A, B, C, and D), 67.5 (A and B), 67.4 (C and D), 60.1 (A, B, C, and D), 56.6 (A, B, C, and D), 40.0 (C or D), 39.9 (C or D), 39.4 (A and B), 29.0 (C or D), 28.9 (C or D), 28.8 (A and B), 28.7 (A, B, C, and D), 28.0 (C and D), 27.8 (A and B), 25.8 (C and D), 25.6 (A and B), 19.4 (A, B, C, and D), 0.0 (A, B, C, and D). IR ν: 3296, 2956, 2927, 1688, 1611, 1511, 1441, 1250, 1082, 834 cm–1. HRMS (FD) m/z: [M]+ calcd for C33H47NO8Si+, 613.3071; found, 613.3084.
(E)-6-[2-(1,3-Dimethyl-4-oxocyclohexyl)vinyl]-4,5-dihydroxy-3-methoxy-4-(4-methoxyphenyl)-1-{[2-(trimethylsilyl)ethoxy]methyl}-3,4-dihydroquinolin-2(1H)-one (29)
Substrate (±)-27 (103.9 mg, 0.25 mmol) was olefinated using olefin 28 (dr = 5:4, 57.1 mg, 0.375 mmol, 1.5 equiv) and PhCO3tBu (86.0 μL, 0.45 mmol, 1.8 equiv) following the general procedure, and the sample was purified by flash column chromatography (n-hexane/EtOAc, 2.5:1) to give 29 as a yellow oil (48.0 mg, 34%, regioselectivity > 20:1, dr = 1:1:1.6:1.6). 1H NMR (400 MHz) [two groups of diastereoisomers: (A + B)/(C + D), 1:1.6]: δ 9.18 (s, 1HA and 1HB), 9.17 (C and D), 7.49 (d, J = 8.7 Hz, 1HA and 1HB), 7.42 (d, J = 8.6 Hz, 1HC and 1HD), 7.31–7.21 (m, 5HA, 5HB, 5HC and 5HD), 6.98 (d, J = 8.7 Hz, 1HA and 1HB), 6.95 (d, J = 8.6 Hz, 1HC and 1HD), 6.82 (d, J = 16.6 Hz, 1HA and 1HB), 6.65 (d, J = 16.5 Hz, 1HC and 1HD), 6.31 (d, J = 16.6 Hz, 1HA and 1HB), 6.18 (d, J = 16.4 Hz, 1HC and 1HD), 5.65 (d, J = 10.9 Hz, 1HA and 1HB), 5.63 (d, J = 10.9 Hz, 1HC and 1HD), 5.02 (d, J = 10.8 Hz, 1HA and 1HB), 5.00 (d, J = 10.8 Hz, 1HC and 1HD), 4.59 (s, 1HA and 1HB), 4.58 (s, 1HC and 1HD), 3.80 (s, 1HA, 1HB, 1HC, and 1HD), 3.59–3.53 (m, 2HA, 2HB, 2HC, and 2HD), 3.58 (s, 3HA and 3HB), 3.57 (s, 3HC and 3HD), 2.66–2.46 (m, 2HA, 2HB, 2HC, and 2HD), 2.37–2.09 (m, 2HA, 2HB, 2HC, and 2HD), 1.91–1.84 (m, 1HA, 1HB, 1HC, and 1HD), 1.76–1.68 (m, 1HC and 1HD), 1.63–1.55 (m, 1HA and 1HB), 1.51–1.43 (m, 1HA, 1HB, 1HC, and 1HD), 1.40 (s, 3HC and 3HD), 1.11 (s, 3HA and 3HB), 1.02 (d, J = 6.4 Hz, 3HC and 3HD), 0.99 (d, J = 6.5 Hz, 3HA and 3HB), 0.95–0.91 (m, 2HA, 2HB, 2HC, and 2HD), −0.03 (s, 9HA, 9HB, 9HC, and 9HD). 13C{H} NMR (75 MHz): δ 213.9 (A and B), 213.5 (C and D), 165.8 (A, B, C, and D), 154.5 (A, B, C, and D), 139.9 (A, B, C, and D), 137.2 (A or B), 137.1 (C or D), 137.1 (A or B), 137.0 (C or D), 136.2 (A, B, C, and D), 129.5 (A and B), 129.0 (C and D), 129.0 (A, B, C, and D), 127.2 (A, B, C, and D), 126.6 (A, B, C, and D), 122.8 (C and D), 122.6 (A and B), 119.8 (A, B, C, and D), 112.3 (A and B), 112.2 (C and D), 107.8 (A, B, C, and D), 84.6 (A, B, C, and D), 78.5 (A, B, C, and D), 72.1 [(A and B) or (C and D)], 71.5 [(A and B) or (C and D)], 66.3 [(A and B) or (C and D)], 66.0 [(A and B) or (C and D)], 58.9 (A, B, C, and D), 47.7 (A and B), 46.9 (C or D), 46.8 (C or D), 41.4 (A and B), 40.8 (C and D), 38.7 (A, B, C, and D), 38.5 (A and B), 38.2 (C or D), 38.1 (C or D), 38.0 (C and D), 37.5 (A and B), 30.6 (A, B, C, and D), 18.2 (A, B, C, and D), 14.7 (C and D), 14.6 (A and B), −1.3 (A, B, C, and D). IR ν: 3294, 2955, 2926, 1689, 1612, 1439, 1376, 1246,1079, 836 cm–1. HRMS (FD) m/z: [M]+ calcd for C32H43NO6Si+, 565.2860; found, 565.2885.
(E)-4,5-Dihydroxy-6-[2-(4-hydroxy-1,3-dimethylcyclohexyl)vinyl]-3-methoxy-4-(4-methoxyphenyl)-1-{[2-(trimethylsilyl)ethoxy]methyl}-3,4-dihydroquinolin-2(1H)-one (32)
Substrate (±)-27 (41.6 mg, 0.1 mmol) was olefinated using olefin 31 (dr = 5:4, 23.1 mg, 0.15 mmol, 1.5 equiv) and PhCO3tBu (34.2 μL, 0.18 mmol, 1.8 equiv) using the following the general procedure, and the sample was purified by flash column chromatography (n-hexane/EtOAc, 3:1 to 1:1) to give 32 as a yellow oil (22.0 mg, 39%, regioselectivity: >20:1, dr = 1:1:1:1). 1H NMR (400 MHz) [two groups of diastereoisomers: (A + B)/(C+ D), 1:1]: δ 9.13 [s, (1HA and 1HB) or (1HC and 1HD)], 9.11 [s, (1HA and 1HB) or (1HC and 1HD)], 7.45 [d, J = 8.6 Hz, (1HA and 1HB) or (1HC and 1HD)], 7.43 [d, J = 8.6 Hz, (1HA and 1HB) or (1HC and 1HD)], 7.31–7.21 (m, 5HA, 5HB, 5HC, and 5HD), 6.95 [d, J = 8.6 Hz, (1HA and 1HB) or (1HC and 1HD)], 6.93 [d, J = 8.6 Hz, (1HA and 1HB) or (1HC and 1HD)], 6.63 [d, J = 16.7 Hz, (1HA and 1HB) or (1HC and 1HD)], 6.59 [d, J = 16.7 Hz, (1HA and 1HB) or (1HC and 1HD)], 6.17 [d, J = 16.4 Hz, (1HA and 1HB) or (1HC and 1HD)], 6.16 [d, J = 8.6 Hz, (1HA and 1HB) or (1HC and 1HD)], 5.63 (d, J = 10.9 Hz, 1HA, 1HB, 1HC, and 1HD), 5.00 (d, J = 10.9 Hz, 1HA, 1HB, 1HC, and 1HD), 4.55 (s, 1HA, 1HB, 1HC, and 1HD), 3.79 (s, 1HA, 1HB, 1HC, and 1HD), 3.57 (s, 3HA, 3HB, 3HC, and 3HD), 3.59–3.53 (m, 2HA, 2HB, 2HC, and 2HD), 3.15–3.06 (m, 1HA, 1HB, 1HC, and 1HD), 1.88–1.73 (m, 3HA, 3HB, 3HC, and 3HD), 1.66–1.20 (m, 4HA, 4HB, 3HC, and 3HD), 1.14 (s, 3HC and 3HD), 1.07 (t, J = 12.9 Hz, 1HA and 1HB), 1.06 (s, 3HA and 3HB), 1.01–0.98 (m, 3HA, 3HB, 3HC, and 3HD). 13C{H} NMR (101 MHz): δ 165.8 (A, B, C, and D), 154.4 [(A and B) or (C and D)], 154.3 [(A and B) or (C and D)], 142.3 (A, B, C, and D), 138.0 (A, B, C, and D), 137.2 (A, B, C, and D), 129.5 (A, B, C, and D), 129.0 (A, B, C, and D), 127.1 [(A and B) or (C and D)], 127.0 [(A and B) or (C and D)], 126.7 (A, B, C, and D), 123.3 (A, B, C, and D),121.6 (A, B, C, and D), 112.1 (A, B, C, and D), 107.8 [(A and B) or (C and D)], 107.7 [(A and B) or (C and D)], 84.6 (A, B, C, and D), 78.5 (A, B, C, and D), 76.3 (A, B, C, and D), 72.1 (A, B, C, and D), 66.2 (A, B, C, and D), 58.9 (A, B, C, and D), 46.0 [(A and B) or (C and D)], 44.8 [(A and B) or (C and D)], 37.4 (A, B, C, and D), 37.4 (A, B, C, and D), 37.2 (A, B, C, and D), 34.0 (C and D), 32.9 (A and B), 32.1 [(A and B) or (C and D)], 32.0 [(A and B) or (C and D)], 18.8 [(A and B) or (C and D)], 18.7 [(A and B) or (C and D)], 18.2 (A, B, C, and D), −1.3 (A, B, C, and D). IR ν: 3338, 2952, 2926, 2855, 1689, 1612, 1440, 1375, 1247, 1080, 1043, 804 cm–1. HRMS (FD) m/z: [M]+ calcd for C32H45NO6Si+, 567.3016; found, 567.3011.
Deprotection of SEM to Complete the Total Syntheses of Yaequinolone Natural Products
General Procedure for the Deprotection of SEM with TBAF
In a 10 mL round bottom flask containing the substrate (1.0 equiv) was added a solution of TBAF (1.0 M in THF, 10–15 equiv) (in cases where more concentrated TBAF was required, after mixing TBAF solution with the substrate in the flask, the THF was quickly evaporated using a rotary evaporator and the right amount of THF was then added). The reaction was then left to stir under reflux at 80 °C overnight. The reaction was then quenched by adding water. The mixture was extracted with EtOAc three times. The combined extracts were washed with brine, dried with Na2SO4, and concentrated in vacuo. The sample was purified by flash column chromatography.
General Procedure for the Deprotection of SEM with Me2AlCl
In a flame-dried Schlenk flask were added substrate (1.0 equiv) and anhydrous DCM (0.4–0.5 M) under N2. A solution of Me2AlCl (1.0 in hexanes, 6.0 equiv) was then added dropwise at −78 °C and the reaction was stirred for 1 h before it was warmed up to 0 °C. After stirring it for another 1 h, the reaction was quenched by adding water. The mixture was extracted with EtOAc three times. The combined extracts were washed with brine, dried with Na2SO4, and concentrated in vacuo to give the hydroxymethyl protected intermediate, which was then mixed with MeOH (0.4–0.5 M) and iPr2NEt (7.0 equiv) and heated at 55 °C overnight. The reaction was quenched by adding saturated solution of NH4Cl and extracted with EtOAc three times. The combined extracts were washed with brine, dried with Na2SO4, and concentrated in vacuo. The sample was purified by flash column chromatography.
(±)-Yaequinolone B
SEM Deprotection of (±)-17 with TBAF
20 mg of (±)-17 [regioisomers (6:1), 0.039 mmol, 1.0 equiv] and 600 μL TBAF (1.0 M in THF, 15 equiv) solution were used. Purification was performed using n-hexane/EtOAc (1:1) as an eluent to give (±)-yaequinolone B as a single regioisomer (pale yellow solid, 9.0 mg, 60%). 1H NMR (400 MHz): δ 9.44 (s, 1H), 7.80 (d, J = 16.5 Hz, 1H), 7.70 (br s, 1H), 7.49 (d, J = 8.4 Hz, 1H), 7.20–7.13 (m, 2H), 6.86–6.78 (m, 2H), 6.70 (d, J = 16.5 Hz, 1H), 6.40 (d, J = 8.3 Hz, 1H), 4.62 (s, 1H), 3.77 (s, 3H), 3.73 (s, 1H), 3.62 (s, 3H), 2.35 (s, 3H). 13C{H} NMR (101 MHz): δ 199.3, 165.5, 160.6, 157.5, 138.1, 137.2, 129.8, 128.6, 127.9, 126.8, 119.3, 114.5, 111.2, 107.5, 84.0, 78.8, 59.1, 55.5, 27.1. IR ν: 3251, 2926, 1691, 1599, 1257, 1079, 805 cm–1. Its data matched with those reported in the literature.4c
(±)-Penigequinolones A and B
Deprotection of 19 with TBAF
23 mg of 19 [regioisomers (8.8:1), dr = 1:1, 0.0385 mmol, 1.0 equiv] and 385 μL TBAF (1.0 M in THF, 10 equiv) solution were first mixed in the reaction flask. After quickly removing the THF using a rotary evaporator, 90 μL THF was added. Purification was performed using n-hexane/EtOAc (2:1) as an eluent to give the product as a mixture of regioisomers (A/B = 13.5:1, dr = 1:1) (pale yellow solid, 16.9 mg, 94%). 1H NMR (400 MHz) (Two diastereoisomers): δ 9.12 (br s, 1HA and 1HB), 7.84 (br s, 1HA and 1HB), 7.40 (d, J = 8.3 Hz, 1HA or 1HB), 7.39 (d, J = 8.3 Hz, 1HA or 1HB), 7.21–7.16 (m, 2HA and 2HB), 6.84–6.80 (m, 2HA and 2HB), 6.73 (d, J = 16.3 Hz, 1HA and 1HB), 6.35 (d, J = 8.3 Hz, 1HA or 1HB), 6.34 (d, J = 8.3 Hz, 1HA or 1HB), 6.14 (d, J = 16.7 Hz, 1HA or 1HB), 6.13 (d, J = 16.7 Hz, 1HA or 1HB), 4.56 (br s, 1HA and 1HB), 3.76 (s, 3HA and 3HB), 3.70 (d, J = 1.5 Hz, 1HA or 1HB), 3.69 (d, J = 1.5 Hz, 1HA or 1HB), 3.60 (s, 3HA and 3HB), 3.38 (d, J = 11.3 Hz, 1HA or 1HB), 3.37 (d, J = 11.3 Hz, 1HA or 1HB), 3.24–3.20 (m, 1HA and 1HB), 1.84–1.76 (m, 1HA and 1HB), 1.73–1.66 (m, 1HA and 1HB), 1.51–1.46 (m, 1HA and 1HB), 1.30 (s, 3HA and 3HB), 1.00 (s, 3HA and 3HB), 0.79 (s, 3HA and 3HB). 13C{H} NMR (101 MHz): δ 165.8 (A and B), 160.4 (A and B), 155.3 (A and B), 134.5, 134.43, 134.37 (A and B), 129.1 (A and B), 127.6, 127.5, 123.3 (A and B), 122.1 (A and B), 114.4 (A and B), 110.9 (A and B), 107.0 (A and B), 84.3 (A and B), 74.5 (A and B), 72.9 (A and B), 59.0 (A and B), 55.4 (A and B), 33.7 (A and B), 31.2, 31.1, 29.9 (A and B), 29.3 (A and B), 26.8, 26.7, 24.2 (A and B). IR ν: 3269, 2927, 1686, 1616, 1508, 1256, 1079, 806 cm–1. HRMS (FD) m/z: [M]+ calcd for C27H33NO6+, 467.2308; found, 467.2287. The data matched with those reported in the literature.4c
(±)-Yaequinolone C
Deprotection of 21 with TBAF
24 mg of 21 [regioisomers (6.8:1), dr not determined, 0.039 mmol, 1.0 equiv] and 400 μL TBAF (1.0 M in THF, 10 equiv) solution were first mixed in the reaction flask. After quickly removing the THF using a rotary evaporator, 100 μL THF was added. Purification was performed using n-hexane/EtOAc (1.5:1) as an eluent to give the product 22 as a mixture of regioisomers (10:1, dr = 1:1:0.6:0.6) (pale yellow solid, 15.0 mg, 79%). 1H NMR (400 MHz), two groups of diastereoisomers: (A + B)/(C + D) = 1:0.6. The NMR data of groups A and B matched with those of yaequinolone C reported in the literature.4c Therefore, either (±)-22-A or (±)-22-B is (±)-yaequinolone C. The olefin moiety of (±)-yaequinolone C has a trans-configuration]: δ 9.16 (A and/or B and/or C and/or D), 9.15 (A and/or B and/or C and/or D), 7.84 (A, B, C, and D), 7.37 [d, J = 8.2 Hz, (1HA and 1HB) or (1HC and 1HD)], 7.36 [d, J = 8.2 Hz, (1HA and 1HB) or (1HC and 1HD), 7.22–7.17 (m, 2HA, 2HB, 2HC, and 2HD), 6.85–6.83 (m, 2HA, 2HB, 2HC, and 2HD), 6.79 [d, J = 16.4 Hz, (1HA and 1HB) or (1HC and 1HD)], 6.78 [d, J = 8.2 Hz, (1HA and 1HB) or (1HC and 1HD)], 6.34–6.21 (m, 2HA, 2HB, 2HC, and 2HD), 4.56 [s, (1HA and 1HB) or (1HC and 1HD)], 4.57 [s, (1HA and 1HB) or (1HC and 1HD)], 3.91–3.83 (m, 1HA, 1HB, 1HC, and 1HD), 3.76 [s, (3HA and 3HB) or (3HC and 3HD)], 3.75 [s, (3HA and 3HB) or (3HC and 3HD)], 3.69–3.68 (m, 1HA, 1HB, 1HC, and 1HD), 3.60 (s, 3HA, 3HB, 3HC, and 3HD), 2.04–1.75 (m, 4HA, 4HB, 4HC, and 4HD), 1.40 [s, (3HA and 3HB) or (3HC and 3HD)], 1.39 [s, (3HA and 3HB) or (3HC and 3HD)], 1.24 (s, 3HA, 3HB, 3HC, and 3HD), 1.14 (s, 3HA, 3HB, 3HC, and 3HD). 13C{H} NMR (101 MHz): δ 165.7 (A, B, C, and D), 160.4 (A, B, C, and D), 155.4 (A, B, C, and D), 135.7 (A or B or C or D), 135.6 (A or B or C or D), 135.3 (A and/or B and/or C and/or D), 134.5 (A or B or C or D), 134.4 (A or B or C or D), 134.3 (A and/or B and/or C and/or D), 129.2 [(A and B) or (C and D)], 129.1 [(A and B) or (C and D)], 128.0 (A, B, C, and D), 127.9 [(A and B) or (C and D)], 127.8 [(A and B) or (C and D)], 122.0 [(A and B) or (C and D)], 121.9 [(A and B) or (C and D)], 121.3 [(A and B) or (C and D)], 121.0 (A or B or C or D), 120.9 (A or B or C or D), 114.4 (A, B, C, and D), 110.9 (A, B, C, and D), 107.0 (A, B, C, and D), 85.8 [(A and B) or (C and D)], 85.7 [(A and B) or (C and D)], 84.3 (A, B, C, and D), 83.4 [(A and B) or (C and D)], 83.2 [(A and B) or (C and D)], 78.9 (A, B, C, and D), 71.4 [(A and B) or (C and D)], 71.3 [(A and B) or (C and D)], 59.0 (A, B, C, and D), 55.4 (A, B, C, and D), 38.7 (A or B or C or D), 38.6 (A or B or C or D), 38.1 [(A and B) or (C and D)], 27.5 (A, B, C, and D), 26.7 (A, B, C, and D), 26.6 (A, B, C, and D), 24.6 (A or B or C or D), 24.5 (A or B or C or D), 24.3 [(A and B) or (C and D)]. IR ν: 3249, 2966, 2927, 1686, 1602, 1419, 1373, 1254, 1030, 804 cm–1. HRMS (FD) m/z: [M]+ calcd for C27H33NO7+, 483.2257; found, 483.2262.
(±)-Aspoquinolones C and D
Deprotection of 25 with Me2AlCl
38 mg of 25 (dr = 2.2:2.2:1:1, 0.062 mmol) was used and the product 26 was isolated as a mixture of diastereoisomers (A/B/C/D = 2.2:2.2:1:1) (pale yellow solid, 22.0 mg, 73%) after purification with n-hexane/EtOAc (1:1) as an eluent. 1H NMR (400 MHz), two groups of diastereoisomers: (A + B)/(C + D) = 2.2:1. Aspoquinolones C and D were reported as a mixture and we found out that one of the mixtures matched with the NMR data of group A and the other one matched with those of Group B.4b Therefore, we concluded that one of the natural products has a cis-configuration for the olefin moiety and the other has a trans-configuration for the olefin moiety. δ 9.13 (br s, 1HA or 1HB), 9.13 (br s, 1HC or 1HD), 9.12 (br s, 1HA or 1HB), 9.11 (br s, 1HC or 1HD), 8.12 (br s, 1HC and 1HD), 8.08 (br s, 1HA and 1HB), 7.35–7.31 (m, 1HA, 1HB, 1HC, and 1HD), 7.18–7.14 (m, 2HA, 2HB, 2HC, and 2HD), 6.82–6.73 (m, 3HA, 3HB, 3HC, and 3HD), 6.34–6.21 (m, 2HA, 2HB, 2HC, and 2HD), 4.59–4.57 (m, 1HA, 1HB, 1HC, and 1HD), 3.90–3.83 (m, 1HA, 1HB, 1HC, and 1HD), 3.75–3.74 (m, 3HA, 3HB, 3HC, and 3HD), 3.69–3.68 (m, 1HA, 1HB, 1HC, and 1HD), 3.60–3.59 (m, 3HA, 3HB, 3HC, and 3HD), 2.03–1.74 (m, 4HA, 4HB, 4HC, and 4HD), 1.40–1.38 (m, 3HA, 3HB, 3HC, and 3HD), 1.25–1.23 (m, 3HA, 3HB, 3HC, and 3HD), 1.13 (s, 3HA, 3HB, 3HC, and 3HD). 13C{H} NMR (75 MHz): δ 165.9 (A, B, C, and D), 160.4 (A, B, C, and D), 155.4 (A, B, C, and D), 135.7 (C or D), 135.6 (C or D), 135.3 (A or B), 135.2 (A or B), 134.4 (C and D), 134.4 (C and D), 129.2 (A and B), 129.1 (C and D), 128.0 (A and B), 127.9 (C or D), 127.8 (C or D), 122.0 (C and D), 121.9 (A and B), 121.4 (C or D), 121.3 (C or D), 121.0 (A or B), 120.9 (A or B), 114.4 (A, B, C, and D), 110.9 (A, B, C, and D), 107.0 (A, B, C, and D), 85.8 (C and D), 85.7 (A and B), 84.3 (A, B, C, and D), 83.4 (A and B), 83.2 (C and D), 78.9 (A and B), 78.8 (C and D), 71.4 (C and D), 71.3 (A and B), 59.0 (A, B, C, and D), 55.4 (A, B, C, and D), 38.7 (C and D), 38.6 (A and B), 27.7 (C and D), 27.5 (A, B, C, and D), 27.4 (A and B), 26.8 (C or D), 26.7 (C or D), 26.7 (A or B), 26.6 (A or B), 24.6 (C or D), 24.5 (C or D), 24.3 (A and B). IR ν: 3261, 2924, 1686, 1600, 1377, 1262, 1077, 1026, 734 cm–1. HRMS (FD) m/z: [M]+ calcd for C27H33NO7+, 483.2257; found, 483.2272.
(±)-Aflaquinolones A, C, and D
Deprotection of 29 with Me2AlCl
40 mg of 29 (dr = 1:1:1.6:1.6, 0.071 mmol) was used and the product 30 was isolated as a mixture of diastereoisomers (dr = 1:1:1:1) (pale yellow solid, 30.0 mg, 97%) after purification with n-hexane/EtOAc (1.5:1) as an eluent. 1H NMR (400 MHz) [two groups of diastereoisomers: (A + B)/(C + D) = 1:1, The 1H NMR data of (±)-aflaquinolones A, C, and D matched with those reported in the literature.:5b δ 9.11 [br s, (1HA and 1HB) or (1HC and 1HD)], 9.09 [br s, (1HA and 1HB) or (1HC and 1HD)], 8.35 [br s, (1HA and 1HB) or (1HC and 1HD)], 8.33 [br s, (1HA and 1HB) or (1HC and 1HD)], 7.39 (d, J = 8.3 Hz, 1HA and 1HB), 7.34–7.25 (m, 5HA, 5HB, 6HC, and 6HD), 6.78 (d, J = 16.6 Hz, 1HC and 1HD), 6.61 (d, J = 16.5 Hz, 1HA and 1HB), 6.39 (d, J = 8.1 Hz, 1HC and 1HD), 6.36 (d, J = 8.2 Hz, 1HA and 1HB), 6.27 (d, J = 16.6 Hz, 1HA and 1HB), 6.13 (d, J = 16.4 Hz, 1HC and 1HD), 4.69 [s, (1HA and 1HB) or (1HC and 1HD)], 4.67 [s, (1HA and 1HB) or (1HC and 1HD)], 3.69 (d, J = 1.6 Hz, 1HA, 1HB, 1HC, and 1HD), 3.61 (s, 3HA, 3HB, 3HC, and 3HD), 2.65–2.44 (m, 2HA, 2HB, 2HC, and 2HD), 2.36–2.31 (m, 1HC and 1HD), 2.28–2.21 (m, 1HA and 1HB), 2.21–2.08 (m, 2HA, 2HB, 2HC, and 2HD), 1.76–1.68 (m, 1HA and 1HB), 1.62–1.54 (m, 1HC and 1HD), 1.43–1.39 (m, 1HA, 1HB, 1HC, and 1HD) 1.39 (s, 3HC and 3HD), 1.10 (s, 3HA and 3HB), 1.02 (d, J = 6.5 Hz, 3HC and 3HD), 0.93 (d, J = 6.5 Hz, 3HA or 3HB), 0.92 (d, J = 6.5 Hz, 3HA or 3HB). 1H NMR (400 MHz, acetone-d6): δ 9.62 (br s, 1HA or 1HB or 1HC or 1HD), 9.61 (br s, 1HA or 1HB or 1HC or 1HD), 9.59 [br s, (1HA and 1HB) or (1HC and 1HD)], 9.37 [br s, (1HA and 1HB) or (1HC and 1HD)], 9.36 [br s, (1HA and 1HB) or (1HC and 1HD)], 7.53 (d, J = 8.3 Hz, 1HA or 1HB), 7.52 (d, J = 8.3 Hz, 1HA or 1HB), 7.43 (d, J = 8.4 Hz, 1HC and 1HD), 7.37–7.32 (m, 5HA, 5HB, 5HC, and 5HD), 6.84 (d, J = 16.7 Hz, 1HA and 1HB), 6.65 (d, J = 16.5 Hz, 1HC and 1HD), 6.60 [d, J = 8.6 Hz, (1HA and 1HB) or (1HC and 1HD)], 6.57 [d, J = 8.6 Hz, (1HA and 1HB) or (1HC and 1HD)], 6.44 (d, J = 16.6 Hz, 1HA and 1HB), 6.37 (s, 1HA or 1HB or 1HC or 1HD), 6.36 (s, 1HA or 1HB or 1HC or 1HD), 6.36 [s, (1HA and 1HB) or (1HC and 1HD)], 6.23 (d, J = 16.4 Hz, 1HC and 1HD), 3.68 (d, J = 1.4 Hz, 1HA, 1HB, 1HC and 1HD), 3.52 [s, (3HA and 3HB) or (3HC and 3HD)], 3.51 [s, (3HA and 3HB) or (3HC and 3HD)], 2.73–2.47 (m, 2HA, 2HB, 2HC, and 2HD), 2.22–2.09 (m, 1HA, 1HB, 1HC, and 1HD), 1.91–1.81 (m, 2HA, 2HB, 2HC, and 2HD), 1.75–1.67 (m, 1HA and 1HB), 1.58–1.49 (m, 1HC and 1HD), 1.42 (s, 3HC and 3HD), 1.11 (s, 3HA and 3HB), 0.95 (d, J = 6.5 Hz, 3HC and 3HD), 0.93 (d, J = 6.5 Hz, 3HA or 3HB), 0.92 (d, J = 6.5 Hz, 3HA or 3HB). 13C{H} NMR (101 MHz): δ 214.0 [(A and B) or (C and D)], 213.6 [(A and B) or (C and D)], 165.9 (A, B, C, and D), 155.0 (A, B, C, and D), 137.4 (A, B, C, and D), 136.0 (A, B, C, and D), 134.5 [(A and B) or (C and D)], 134.4 [(A and B) or (C and D)], 129.4 (A, B, C, and D), 129.1 [(A and B) or (C and D)], 129.0 [(A and B) or (C and D)], 127.4 [(A and B) or (C and D)], 127.3 [(A and B) or (C and D)], 126.4 (A, B, C, and D), 122.6 (A, B, C, and D), 122.5 [(A and B) or (C and D)], 122.4 [(A and B) or (C and D)], 110.9 [(A and B) or (C and D)], 110.8 [(A and B) or (C and D)], 107.2 (A, B, C, and D), 84.2 (A, B, C, and D), 79.0 (A, B, C, and D), 59.1 (A, B, C, and D), 47.7 (A or B), 47.6 (A or B), 46.9 (C or D), 46.8 (C or D), 41.5 (A and B), 40.8 (C and D), 38.7 (A, B, C, and D), 38.5 [(A and B) or (C and D)], 38.2 [(A and B) or (C and D)], 38.0 (C and D), 37.4 (A and B), 30.6 (A, B, C, and D), 14.7 (A or B or C or D), 14.6 (A or B or C or D), 14.3 [(A and B) or (C and D)]. IR ν: 3221, 2923, 1689, 1377, 1260, 1080, 1026, 800 cm–1. HRMS (FD) m/z: [M]+ calcd for C26H29NO5+, 435.2046; found, 435.2047.
(±)-Scopuquinolone B
Deprotection of 32 with Me2AlCl
19 mg of 32 (dr = 1:1:1:1, 0.0335 mmol) was used and the product 33 was isolated as a mixture of daistereosiomers (dr = 1:1:1:1) (pale yellow solid, 13.0 mg, 89%) after purification with n-hexane/EtOAc (1:1) as an eluent. 1H NMR (400 MHz, acetone-d6) [two groups of diastereoisomers: (A + B)/(C + D) = 1:1. The NMR data of groups C and D matched with those of scopuquinolone B reported in the literature.:6c δ 9.55 (br s, 1HA, 1HB, 1HC, and 1HD), 9.33 (br s, 1HA, 1HB, 1HC, and 1HD), 7.44 [d, J = 8.3 Hz, (1HA and 1HB) or (1HC and 1HD)], 7.42 [d, J = 8.3 Hz, (1HA and 1HB) or (1HC and 1HD)], 7.37–7.32 (m, 5HA, 5HB, 5HC, and 5HD), 6.65–6.55 (m, 2HA, 2HB, 2HC, and 2HD), 6.20 [d, J = 16.5 Hz, (1HA and 1HB) or (1HC and 1HD)], 6.17 [d, J = 16.5 Hz, (1HA and 1HB) or (1HC and 1HD)], 3.66 (d, J = 1.5 Hz, 1HA, 1HB, 1HC, and 1HD), 3.52 [s, (3HA and 3HB) or (3HC and 3HD)], 3.51 [s, (3HA and 3HB) or (3HC and 3HD)], 3.06–2.97 (m, 1HA, 1HB, 1HC, and 1HD), 1.81–1.68 (m, 3HA, 3HB, 3HC, and 3HD), 1.64–1.27 (m, 3HA, 3HB, 4HC, and 4HD), 1.13 (s, 3HA and 3HB), 1.07 (t, J = 12.9 Hz, 1Hc and 1Hd), 1.01 (s, 3HC and 3HD), 0.98 (d, J = 6.4 Hz, 3HA and 3HB), 0.96 (d, J = 6.4 Hz, 3HA and 3HB). 13C{H} NMR (101 MHz, acetone-d6): δ 166.3 (A, B, C, and D), 156.1 (C and D), 155.9 (A and B), 141.8 (A and B), 140.3 (C and D), 137.6 (A, B, C, and D), 136.9 (A, B, C, and D), 129.7 (A, B, C, and D), 129.6 (A, B, C, and D), 127.5 [(A and B) or (C and D)], 127.5 [(A and B) or (C and D)], 127.4 [(A and B) or (C and D)], 127.3 [(A and B) or (C and D)], 122.6 (A, B, C, and D), 122.3 (A, B, C, and D), 112.1 (A, B, C, and D), 107.7 (A, B, C, and D), 85.8 (A, B, C, and D), 80.0 (A, B, C, and D), 76.8 (A, B, C, and D), 59.0 (A, B, C, and D), 46.9 (C and D), 45.9 (A and B), 37.7 (A, B, C, and D), 37.7 (A, B, C, and D), 37.0 [(A and B) or (C and D)], 36.9 [(A and B) or (C and D)], 34.0 (A and B), 33.1 (C and D), 31.9 [(A and B) or (C and D)], 31.8 [(A and B) or (C and D)], 19.5 [(A and B) or (C and D)], 19.4 [(A and B) or (C and D)]. IR ν: 3274, 2926, 1687, 1620, 1452, 1378, 1261, 1082, 1039, 803 cm–1. HRMS (FD) m/z: [M]+ calcd for C26H31NO5+, 437.2202; found, 437.2198.
Acknowledgments
We acknowledge financial support from NWO through a VIDI grant (723.013.006). W.L.J. gratefully acknowledges financial support from the China Scholarship Council (CSC) (File no. 201606180020).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.1c00042.
General schemes for the synthesis of 18, trans-20, 28, 31, and (±)-27; comparison of the 1H NMR spectra of olefinated product 21 when using trans-20 or a mixture of trans- and cis-20; comparison of the NMR data of natural and synthetic products; and copies of the 1H and 13C NMR spectra for all new compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Simonetti S. O.; Larghi E. L.; Kaufman T. S. The 3,4-dioxygenated 5-hydroxy-4-aryl-quinolin2(1H)-one alkaloids. Results of 20 years of research, uncovering a new family of natural products. Nat. Prod. Rep. 2016, 33, 1425–1446. 10.1039/c6np00064a. [DOI] [PubMed] [Google Scholar]
- Nakaya T. Anti-brine shrimp substances produced by penicillium sp. NTC-47. Seikatsu Eisei 1995, 39, 141–143. [Google Scholar]
- a Kimura Y.; Kusano M.; Koshino H.; Uzawa J.; Fujioka S.; Tani K. Penigequinolones A and B, pollen-growth inhibitors produced by Penicilium sp., No. 410. Tetrahedron Lett. 1996, 37, 4961–4964. 10.1016/0040-4039(96)00978-1. [DOI] [Google Scholar]; b Hayashi H.; Nakatani T.; Inoue Y.; Nakayama M.; Nozaki H. New Dihydroquinolinone Toxic toArtemia salinaProduced byPenicilliumsp. NTC-47. Biosci., Biotechnol., Biochem. 1997, 61, 914–916. 10.1271/bbb.61.914. [DOI] [PubMed] [Google Scholar]; c Kusano M.; Koshino H.; Uzawa J.; Fujioka S.; Kawano T.; Kimura Y. Nematicidal Alkaloids and Related Compounds Produced by the Fungus Penicillium cf. simplicissimum. Biosci., Biotechnol., Biochem. 2000, 64, 2559–2568. 10.1271/bbb.64.2559. [DOI] [PubMed] [Google Scholar]; d He J.; Lion U.; Sattler I.; Gollmick F. A.; Grabley S.; Cai J.; Meiners M.; Schünke H.; Schaumann K.; Dechert U.; Krohn M. Diastereomeric Quinolinone Alkaloids from the Marine-Derived FungusPenicilliumjanczewskii. J. Nat. Prod. 2005, 68, 1397–1399. 10.1021/np058018g. [DOI] [PubMed] [Google Scholar]
- a Uchida R.; Imasato R.; Shiomi K.; Tomoda H.; O̅mura S. Yaequinolones J1 and J2, Novel Insecticidal Antibiotics fromPenicilliumsp. FKI-2140. Org. Lett. 2005, 7, 5701–5704. 10.1021/ol052458h. [DOI] [PubMed] [Google Scholar]; b Scherlach K.; Hertweck C. Discovery of aspoquinolones A-D, prenylated quinoline-2-one alkaloids from Aspergillus nidulans, motivated by genome mining. Org. Biomol. Chem. 2006, 4, 3517–3520. 10.1039/b607011f. [DOI] [PubMed] [Google Scholar]; c Uchida R.; Imasato R.; Yamaguchi Y.; Masuma R.; Shiomi K.; Tomoda H.; O̅mura S. Yaequinolones, new insecticidal antibiotics produced by penicillium sp. FKI-2140. I. Taxonomy, fermentation, isolation and biological activity. J. Antibiot. 2006, 59, 646–651. 10.1038/ja.2006.86. [DOI] [PubMed] [Google Scholar]
- a Uchida R.; Imasato R.; Tomoda H.; O̅mura S. Yaequinolones, new insecticidal antibiotics produced by penicillium sp. FKI-2140. II. Structural elucidation. J. Antibiot. 2006, 59, 652–658. 10.1038/ja.2006.87. [DOI] [PubMed] [Google Scholar]; b Neff S. A.; Lee S. U.; Asami Y.; Ahn J. S.; Oh H.; Baltrusaitis J.; Gloer J. B.; Wicklow D. T. Aflaquinolones A-G: Secondary Metabolites from Marine and Fungicolous Isolates of Aspergillus spp. J. Nat. Prod. 2012, 75, 464–472. 10.1021/np200958r. [DOI] [PMC free article] [PubMed] [Google Scholar]; c An C.-Y.; Li X.-M.; Luo H.; Li C.-S.; Wang M.-H.; Xu G.-M.; Wang B.-G. 4-Phenyl-3,4-dihydroquinolone Derivatives from Aspergillus nidulans MA-143, an Endophytic Fungus Isolated from the Mangrove Plant Rhizophora stylosa. J. Nat. Prod. 2013, 76, 1896–1901. 10.1021/np4004646. [DOI] [PubMed] [Google Scholar]; d Chen M.; Shao C.-L.; Meng H.; She Z.-G.; Wang C.-Y. Anti-Respiratory Syncytial Virus Prenylated Dihydroquinolone Derivatives from the Gorgonian-Derived Fungus Aspergillus sp. XS-20090B15. J. Nat. Prod. 2014, 77, 2720–2724. 10.1021/np500650t. [DOI] [PubMed] [Google Scholar]
- a Shao C.-L.; Xu R.-F.; Wang C.-Y.; Qian P.-Y.; Wang K.-L.; Wei M.-Y. Potent antifouling marine dihydroquinolin-2(1H)-one-containing alkaloids from the gorgonian coral-derived fungus scopulariopsis sp.. Mar. Biotechnol. 2015, 17, 408–415. 10.1007/s10126-015-9628-x. [DOI] [PubMed] [Google Scholar]; b Shao C.-L.; Chao R.; Xu R.-F.; Cao F.; Wei M.-Y. Scopuquinolone A, a new terpenoid dihydroquinolone alkaloid from a gorgonian coral-derived scopulariopsis sp. Fungus. Chin. J. Mar. Drugs 2016, 35, 1–5. [Google Scholar]; c Mou X.-F.; Liu X.; Xu R.-F.; Wei M.-Y.; Fang Y.-W.; Shao C.-L. Scopuquinolone B, a new monoterpenoid dihydroquinolin-2(1H)-one isolated from the coral-derived Scopulariopsis sp. fungus. Nat. Prod. Res. 2018, 32, 773–776. 10.1080/14786419.2017.1359177. [DOI] [PubMed] [Google Scholar]
- Only two members of this family of natural products have a trans-configuration for the two oxygenated functional groups, see: refs (3d) and (5b).
- Li X.; Huo X.; Li J.; She X.; Pan X. A Concise Synthesis of (±)-Yaequinolone A2. Chin. J. Chem. 2009, 27, 1379–1381. 10.1002/cjoc.200990230. [DOI] [Google Scholar]
- Vece V.; Jakkepally S.; Hanessian S. Total synthesis and absolute stereochemical assignment of the insecticidal metabolites yaequinolones J1 and J2. Org. Lett. 2018, 20, 4277–4280. 10.1021/acs.orglett.8b01701. [DOI] [PubMed] [Google Scholar]
- a Schwan J.; Kleoff M.; Hartmayer B.; Heretsch P.; Christmann M. Synthesis of quinolinone alkaloids via aryne insertions into unsymmetric imides in flow. Org. Lett. 2018, 20, 7661–7664. 10.1021/acs.orglett.8b03392. [DOI] [PubMed] [Google Scholar]; b Schwan J.; Kleoff M.; Heretsch P.; Christmann M. Five-step synthesis of yaequinolones J1 and J2. Org. Lett. 2020, 22, 675–678. 10.1021/acs.orglett.9b04455. [DOI] [PubMed] [Google Scholar]
- Li L.; Chen Z.; Zhang X.; Jia Y. Divergent strategy in natural product total synthesis. Chem. Rev. 2018, 118, 3752–3832. 10.1021/acs.chemrev.7b00653. [DOI] [PubMed] [Google Scholar]
- a Lam N. Y. S.; Wu K.; Yu J.-Q. Advancing the logic of chemical synthesis: C-H activation as strategic and tactical disconnections for C-C bond construction. Angew. Chem., Int. Ed. 2021, 60, 2–26. 10.1002/anie.202011901. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Hong B.; Luo T.; Lei X. Late-Stage Diversification of Natural Products. ACS Cent. Sci. 2020, 6, 622–635. 10.1021/acscentsci.9b00916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Jia W.-L.; Westerveld N.; Wong K. M.; Morsch T.; Hakkennes M.; Naksomboon K.; Fernández-Ibáñez M. Á. Selective C-H Olefination of Indolines (C5) and Tetrahydroquinolines (C6) by Pd/S,O-Ligand Catalysis. Org. Lett. 2019, 21, 9339–9342. 10.1021/acs.orglett.9b03505. [DOI] [PMC free article] [PubMed] [Google Scholar]; For other examples of S,O-bidentate ligand, see:; b Naksomboon K.; Valderas C.; Gómez-Martínez M.; Álvarez-Casao Y.; Fernández-Ibáñez M. Á. S,O-Ligand-Promoted Palladium-Catalyzed C-H Functionalization Reactions of Nondirected Arenes. ACS Catal. 2017, 7, 6342–6346. 10.1021/acscatal.7b02356. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Naksomboon K.; Álvarez-Casao Y.; Uiterweerd M.; Westerveld N.; Maciá B.; Fernández-Ibáñez M. Á. S,O-ligand-promoted palladium-catalyzed C-H olefination of arenes with allylic substrates. Tetrahedron Lett. 2018, 59, 379–382. 10.1016/j.tetlet.2017.12.047. [DOI] [Google Scholar]; d Álvarez-Casao Y.; Fernández-Ibáñez M. Á. S,O-Ligand-Promoted Pd-Catalyzed C-H Olefination of Thiophenes. Eur. J. Org. Chem. 2019, 1842–1845. 10.1002/ejoc.201900077. [DOI] [Google Scholar]; e Naksomboon K.; Poater J.; Bickelhaupt F. M.; Fernández-Ibáñez M. Á. para-Selective C-H Olefination of Aniline Derivatives via Pd/S,O-Ligand Catalysis. J. Am. Chem. Soc. 2019, 141, 6719–6725. 10.1021/jacs.9b01908. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jia W.-L.Palladium catalyzed C–H functionalization of amine derivatives and its application in total synthesis. PhD Thesis, University of Amsterdam, 2021. https://hdl.handle.net/11245.1/2726566f-bb12-4ba8-a0ba-104f0985f844. [Google Scholar]
- a Ryabov A. D.; Sakodinskaya I. K.; Yatsimirsky A. K. Kinetics and mechanism of ortho-palladation of ring-substituted NN-dimethylbenzylamines. J. Chem. Soc., Dalton Trans. 1985, 2629–2638. 10.1039/dt9850002629. [DOI] [Google Scholar]; b Labinger J. A.; Bercaw J. E. Understanding and exploiting C-H bond activation. Nature 2002, 417, 507–514. 10.1038/417507a. [DOI] [PubMed] [Google Scholar]
- Cyclized product of 5b was observed after leaving the CDCl3 solution of 5b overnight at room temperature.
- We used MOM as the protecting group for the nitrogen atom. However, although the C–H olefination of the MOM-protected substrate works, we were not able to remove the MOM protecting group after the C–H functionalization step.
- We speculated that the racemization can take place at the allylic position via anti-Tsuji–Trost and Tsuji–Trost processes.
- We tried to deprotect the TMS and SEM at the same time using concentrated TBAF (4.0 M). However, no desired product was detected after refluxing overnight.
- Jaegli S.; Vors J.-P.; Neuville L.; Zhu J. Palladium-catalyzed domino Heck/cyanation: synthesis of 3-cyanomethyloxindoles and their conversion to spirooxoindoles. Tetrahedron 2010, 66, 8911–8921. 10.1016/j.tet.2010.09.056. [DOI] [Google Scholar]
- It was not possible to separate the diastereoisomers by flash column chromatography.
- Castelli R.; Overkleeft H. S.; van der Marel G. A.; Codée J. D. C. 2,2-Dimethyl-4-(4-methoxy-phenoxy) butanoate and 2,2-dimethyl-4-azido butanoate: two new pivaloate-ester-like protecting groups. Org. Lett. 2013, 15, 2270–2273. 10.1021/ol4008475. [DOI] [PubMed] [Google Scholar]
- Oniciu D. C.; Bell R. P. L.; McCosar B. H.; Bisgaier C. L.; Dasseux J. L. H.; Verdijk D.; Relou M.; Smith D.; Regeling H.; Leemhuis F. M. C.; Ebbers E. J.; Mueller R.; Zhang L.; Pop E.; Cramer C. T.; Goetz B.; McKee A.; Pape M. E.; Krause B. R. Syntheses of pantolactone and pantothenic acid derivatives as potential lipid regulating agents. Synth. Commun. 2006, 36, 365–391. 10.1080/00397910500377545. [DOI] [Google Scholar]
- Daub M. E.; Prudhomme J.; Mamoun C. B.; Roch K. G. L.; Vanderwal C. D. Antimalarial properties of simplified kalihinol analogues. ACS Med. Chem. Lett. 2017, 8, 355–360. 10.1021/acsmedchemlett.7b00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidari G.; Di Rosa A.; Zanoni G.; Bicchi C. Enantioselective synthesis of each stereoisomer of the pyranoid linalool oxides: the linalool route. Tetrahedron 1999, 10, 3547–3557. 10.1016/s0957-4166(99)00359-6. [DOI] [Google Scholar]
- Zheng S.; Laxmi Y. R. S.; David E.; Dinkova-Kostova A. T.; Shiavoni K. H.; Ren Y.; Zheng Y.; Trevino I.; Bumeister R.; Ojima I.; Wigley W. C.; Bliska J. B.; Mierke D. F.; Honda T. Synthesis, chemical reactivity as Michael acceptors, and biological potency of monocyclic cyanoenones, novel and highly potent anti-inflammatory and cytoprotective agents. J. Med. Chem. 2012, 55, 4837–4846. 10.1021/jm3003922. [DOI] [PubMed] [Google Scholar]
- Honda T.; David E.; Mierke D.. Monocyclic cyanoenones and methods of use thereof. WO 2010011782 A1, Jan 28, 2010.
- Liu B.; Fan Y.; Gao Y.; Sun C.; Xu C.; Zhu J. Rhodium(III)-Catalyzed N-Nitroso-Directed C-H Olefination of Arenes. High-Yield, Versatile Coupling under Mild Conditions. J. Am. Chem. Soc. 2013, 135, 468–473. 10.1021/ja3099245. [DOI] [PubMed] [Google Scholar]
- Beshore D. C.; Mohanty S. K.; Latthe P. R.; Kuduk S. D.; Hoyt S. B.. Quinazoline compounds useful as m1 receptor positive allosteric modulators. WO 2017155816 A1, Sept 14, 2017.
- Chen W.; Sun C.; Zhang Y.; Hu T.; Zhu F.; Jiang X.; Abame M. A.; Yang F.; Suo J.; Shi J.; Shen J.; Aisa H. A. Oxidative aromatization of 3,4-dihydroquinolin-2(1H)-ones to quinolin-2(1H)-ones using transition-metal-activated persulfate salts. J. Org. Chem. 2019, 84, 8702–8709. 10.1021/acs.joc.9b00756. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.