Abstract
Background:
Cardiac surgery with cardiopulmonary bypass (CPB) is associated with a high risk of postoperative acute kidney injury (AKI). Due to limitations of current diagnostic strategies, we sought to determine whether free hemoglobin (fHb) ratio (i.e., levels of fHb at the end of CPB divided by baseline fHb) could predict AKI after on-pump cardiac surgery.
Methods:
This is a secondary analysis of a randomized controlled trial comparing the effect of nitric oxide (intervention) versus nitrogen (control) on AKI after cardiac surgery (NCT01802619). 110 adult patients in the control arm were included. First, we determined whether fHb ratio was associated with AKI via multivariable analysis. Second, we verified whether fHb ratio could predict AKI and incorporation of fHb ratio could improve predictive performance at an early stage, compared with prediction using urinary biomarkers alone. We conducted restricted cubic spline in logistic regression for model development. We determined the predictive performance, including area under the receiver-operating-characteristics curve (AUC) and calibration (calibration plot and accuracy, i.e., number of correct predictions divided by total number of predictions). We also employed AUC test, likelihood ratio test, and net reclassification index (NRI) to compare the predictive performance between competing models (i.e., fHb ratio vs. NGAL, NAG, and KIM-1, respectively, and incorporation of fHb ratio with NGAL, NAG, and KIM-1 vs. urinary biomarkers alone), if applicable.
Results:
Data stratified by median fHb ratio showed that subjects with an fHb ratio >2.23 presented higher incidence of AKI (80.0% vs. 49.1%, p=0.001), more need of renal replacement therapy (10.9% vs. 0%, p=0.036), and higher in-hospital mortality (10.9% vs. 0%, p=0.036) than subjects with an fHb ratio ≤2.23. fHb ratio was associated with AKI after adjustment for pre-established factors. fHb ratio outperformed urinary biomarkers with the highest AUC of 0.704 (95% CI 0.592-0.804) and accuracy of 0.714 (95% CI 0.579-0.804). Incorporation of fHb ratio achieved better discrimination (AUC test, p= 0.012), calibration [likelihood ratio test, p<0.001; accuracy, 0.740 (95% CI 0.617-0.832) versus 0.632 (95% CI 0.477-0.748)], and significant prediction increment (NRI, 0.638, 95% CI 0.269-1.008, p<0.001) at an early stage, compared with prediction using urinary biomarkers alone.
Conclusions:
Results from this exploratory, hypothesis-generating retrospective, observational study shows that fHb ratio at the end of CPB might be used as a novel, widely applicable biomarker for AKI. The use of fHb ratio might help for an early detection of AKI, compared with prediction based only on urinary biomarkers.
Introduction
Acute kidney injury (AKI) is a common complication among patients undergoing cardiac surgery with cardiopulmonary bypass (CPB) 1, leading to prolonged stay in the intensive care unit (ICU) and higher postoperative mortality 2. Early identification paired with effective intervention has reduced the frequency and severity of postoperative AKI 3, indicating that early diagnosis is extremely warranted.
Currently, AKI diagnosis is based on Kidney Disease Improving Global Outcomes (KDIGO) criteria evaluating serum creatinine (SCr) and urine output 4. However, SCr has been challenged as it rises slowly after an initial insult and is influenced by numerous non-renal factors 5. Urinary biomarkers have been extensively studied as promising strategies for early diagnosis. However, the following issues may limit them from being widely used: (1) The detection needs specific testing facilities, which may not be available in all the hospitals, because a final consensus on which new biomarker is superior has not been reached; (2) The apparent change can only be observed several hours after surgery 6 or even later 7; (3) Prior studies 6, 8-10 have been limited by the absence of statistical methods to account for overfitting due to small sample size 11 and non-linear correlation 12, leading to poor generalizability and flexibility. New biomarkers of AKI following on-pump cardiac surgery are needed.
Intravascular hemolysis is characterized by free hemoglobin (fHb) release from red blood cells (RBCs) into the bloodstream. fHb is suggested as a potential mechanistic contributor of AKI in patients undergoing on-pump cardiac surgery via both molecular 13 and hemodynamic 14 pathway. Levels of fHb peaked 15 min after weaning of CPB 14 and patients with AKI displayed higher fHb 15. In addition, the difference between fHb at the end of CPB and baseline (ΔfHb) was associated with AKI in pediatric patients 16. Whether ΔfHb or fHb ratio, defined as levels of fHb at the end of CPB divided by fHb at baseline, are independent risk factors of AKI in adult patients requires further investigation. Moreover, whether ΔfHb or fHb ratio could predict AKI remains unclear.
Therefore, we performed a secondary analysis on the control arm of a randomized controlled trial 1 to determine whether ΔfHb or fHb ratio, an easily detected surrogate of hemolysis, could be identified as an early biomarker for postoperative AKI after on-pump cardiac surgery and whether incorporation of fHb ratio could improve predictive performance, compared with using urinary biomarkers alone.
Materials and Methods
Study Design
We performed a secondary analysis of a randomized controlled trial conducted in the Departments of Anesthesiology and Cardiovascular Surgery of Xijing Hospital, Xian, China 1 (NCT01802619). The Institutional Review Boards of Xijing Hospital and Massachusetts General Hospital approved our study and waived the requirement for written informed consent. In the primary trial, adult patients undergoing elective multiple valve replacement with CPB and stable pre-operative renal function were included. Patients on dialysis before surgery were excluded. Patients were randomized to receive either nitric oxide (NO) at 80 ppm (intervention arm, 117 subjects) or N2 (control arm, 127 subjects). Treatment gases were commenced at the onset of CPB and lasted for 24 hours or less if patients were ready to be extubated early. In performing this study, we followed the guidelines in Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) 17.
Data Collection
The primary outcome was postoperative AKI, defined by KDIGO SCr criteria 4 as either a 50% increase of SCr within 7 days after surgery or an increase of SCr by 0.3 mg/dL within 2 days after surgery from the pre-operative baseline SCr. The severity of AKI was determined in each case using SCr-based KDIGO definitions 4. Exposure variables were ΔfHb and fHb ratio, which was calculated as the difference and ratio of baseline and post-CBP fHb, respectively.
To determine the association between ΔfHb or fHb ratio at the end of CPB and AKI, data from 110 patients who had available fHb measurements in the control arm 1 were collected. We retrieved the following data for each patient: (1) levels of fHb at baseline and the end of CPB, (2) demographics (i.e., age 18, 19, weight, body mass index [BMI] and gender) as well as smoking 20, (3) comorbidities (i.e., history of diabetes 21, hypertension 19, dyslipidemia, chronic obstructive pulmonary disease [COPD], cardiac surgery, atrial fibrillation [AF], severe heart failure [NYHA III/IV], pulmonary artery hypertension [PAH] 22, and vascular disease), (4) European System for Cardiac Operative Risk Evaluation (EUROscore) II, (5) baseline renal function (i.e., baseline SCr and estimated glomerular filtration rate [eGFR] calculated by CKD-EPI formula), (6) potential intraoperative risk factors (i.e., duration of CPB and cross-aortic clamping, fluid balance 23, and the highest vasoactive/inotropic score [VIS] 24), (7) potential postoperative risk factors (i.e., fluid balance and the highest VIS at the first 24h in the ICU, duration of mechanical ventilation 25 and vasoactive/inotropic drugs 26), and (8) outcomes (i.e., AKI, AKI stage, number of subjects needed renal replacement therapy [RRT], ICU length of stay [LOS], and in-hospital mortality). The detailed description of variables was listed in Supplemental Table 1.
To determine whether early detection of ΔfHb or fHb ratio could predict AKI and improve predictive performance of AKI compared to use of urinary biomarkers alone, we retrieved available data on fHb, urinary creatinine (UCr), and urinary biomarkers (kidney injury molecule-1 [KIM-1], N-acetyl-β-D-glucosaminidase [NAG], and neutrophil gelatinase-associated lipocalin [NGAL]) at (1) baseline, (2) end of CPB, (3) admission to the ICU, and (4) 6 h in the ICU for 107 patients in the control arm. Available data of 105 patients in the intervention arm was also collected to describe the potential relationship between change of fHb and AKI.
Measurements
In the original study, all the measurements of blood and urinary samples were tested prospectively to verify the AKI diagnosis based on KDIGO SCr criteria 4 and effect of NO on urinary biomarkers. Frozen plasma samples were analyzed in Anesthesia Center for Critical Care Research at MGH for fHb with a QuantiChrom Hemoglobin Assay Kit (BioAssay Systems, Hayward, CA), which measures the concentration of hemoglobin (Hb), including all Hb derivatives (expressed as heme) through ultraviolet spectrophotometer. Frozen urine samples were sent to the Renal Division at Brigham and Women’s Hospital (Boston, MA) for measurements of urine creatinine, NAG, NGAL, and KIM-1 27 using specific antibodies through enzyme-linked immunosorbent assay (ELISA). To correct for variations in urine flow, urinary KIM-1, NAG and NGAL were normalized to urinary creatinine concentration and reported in their adjusted forms 8.
Statistical Analysis
To conduct the statistical analysis, we followed the Guidelines for reporting of statistics for clinical research in urology 28.
Descriptive analysis
All the characteristics of the patient population were analyzed descriptively, stratified by the median of fHb ratio. Continuous data were presented as median with interquartile range (IQR, 25–75th percentiles) and compared with the Mann-Whitney U test. Categorical variables are presented as frequencies and compared with a chi-square test or Fisher exact test when appropriate. All analyses were two-sided, and a p value of less than 0.05 was considered statistically significant. All descriptive analyses to compare differences of predictors between groups were conducted in R package “tableone”.
Association analysis
We first employed univariable analysis to decide which variable, ΔfHb or fHb ratio at the end of CPB, should be applied to the subsequent analysis. Since fHb ratio showed stronger relationship with postoperative AKI, we thereby used fHb ratio as our exposure of interest.
Multivariable logistic regression models were used to adjust for potential risk factors of AKI as follows: Model 1 was adjusted for demographics (i.e., age, gender, and BMI); Model 2 was adjusted for comorbidities (i.e., current smoker, hypertension, vascular disease, COPD, severe heart failure, PAH and AF) and baseline renal function (eGFR); Model 3 was adjusted for EUROscore II and intraoperative risk factors (i.e., duration of CPB and cross-aortic clamping, fluid balance, blood transfusion, and highest VIS); Model 4 was adjusted for EUROscore II and postoperative risk factors (i.e., fluid balance and highest VIS at the first 24 hours in the ICU, duration of mechanical ventilation and vasoactive/inotropic drugs). BMI was categorized based on World Health Organization (WHO) BMI classification. To account for nonlinear association of continuous variables with AKI 28, we added quadratic terms to the models (i.e., linear + quadratic terms). If the added quadratic terms were not significant (p>0.05), we then only reported the linear relationship.
Prediction analysis
We first conducted descriptive analysis on patients from the control arm with available measurements of SCr, fHb, NAG, NGAL, KIM-1, and fHb ratio at different time points, if applicable. Data were compared between AKI and non-AKI groups using the Mann-Whitney U test. To further explore the potential relationship between AKI and fHb ratio, we performed following analyses as well: (1) comparison of the fHb ratio among patients without AKI, with AKI stage 1 and high stage (stage 2 and 3) of AKI in the control arm; (2) comparison of the fHb ratio between patients with or without AKI in the intervention arm.
To explore the best prediction strategy to identify patients with high risk of AKI immediately after surgery, we only employed fHb ratio and urinary biomarkers at the end of CPB. For model development, restricted cubic spline in logistic regression was used to account for potential nonlinearity in the association between predictors and AKI using “rcs” function in R packages “rms”. We first performed analyses on every single biomarker. Then we explored whether incorporation of fHb ratio with urinary biomarkers (Model A) could improve predictive performance compared with using urinary biomarkers (Model B) alone.
As the prediction performance, we assessed (1) the area under the receiver-operating-characteristics curve (AUC) generalized to AKI, (2) resampling model calibration, and (3) confusion matrix results [i.e., accuracy (defined as number of correct predictions divided by total number of predictions) 29, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV)]. The calculation of AUC was conducted with “auc” function in R package “pROC”. The 95% confidence interval (CI) for AUC metrics was estimated with the “ci.auc” function in R package “pROC”. Resampling model calibration through calibration curve was plotted with “calibrate” function in R package “rms”. In general, the closer the dots are to the ideal line the better the model. Among all the confusion matrix results, accuracy, sensitivity, specificity, PPV, and NPV were computed with R package “reportROC”. Since models are generated to provide the best fit for the available data, there is the potential that a model originated from small dataset will be overfitted and, hence, we adjusted the optimism/overfitting in measures of AUC and confusion matrix results using bootstrap with 1,000 resamples since it provides unbiased optimism-adjusted estimates 11 in R package “boot”. Cut-off points were calculated with 95%CI to achieve the maximal Youden’s index. The Youden’s index was used to maximize the sum of sensitivity and specificity and identify the best cut-off point for a biomarker. It was calculated as sensiticity+specificity-130), using bootstrap-based method in R package “cutpointr” with 1,000 replicates.
To compare discrimination (i.e., the ability to differentiate between patients who developed AKI and those who did not) between competing models, we conducted AUC test using the “roc.test” function via “bootstrap” method (2000 replicates) in R package “pROC”26. To compare calibration (i.e., the agreement between observed and predicted probability to develop AKI) between nested models (i.e., Model A versus Model B), we performed Likelihood ratio test with “lrtest” function in R package “IMtest” since it was recommended by the Predictive Safety Testing Consortium (PSTC) 31 for examining the predictive performance of new biomarkers. To quantify the prediction increment of a new marker in competing models (either nested or non-nested), we computed net reclassification index (NRI) 32, which can quantify the improvement of risk prediction, using “improveProb” function in R package “Hmisc”.
Statistical analyses were conducted using R version 3.5.3 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria) with relevant packages.
Results
Clinical characteristics of subjects with available fHb measurements
Clinical characteristics and outcomes from 110 patients (87%) in the control arm with available fHb measurements were listed in Table 1 (for a full list of all included variables, see Supplemental Table 2).
Table 1.
Characteristics and outcomes of subjects in the control arm, stratified by median fHb ratio (N=110)
| Overall (n=110) | fHb ratio ≤2.23 (n=55) | fHb ratio >2.23 (n=55) | p | |
|---|---|---|---|---|
| Age, years | 47.50 [43.25, 53.75] | 46.00 [42.50, 50.00] | 49.00 [45.00, 56.50] | 0.021 |
| Males | 42 (38.2) | 21 (38.2) | 21 (38.2) | 1 |
| BMI, kg/m2 | 22.04 [20.43, 24.08] | 22.04 [20.44, 23.93] | 22.03 [20.38, 24.20] | 0.816 |
| Diagnosis | 0.207 | |||
| Rheumatic valve disease | 104 (94.5) | 54 (98.2) | 50 (90.9) | |
| Congenital valve disease | 2 (1.8) | 0 (0.0) | 2 (3.6) | |
| Infective valve disease | 4 (3.6) | 1 (1.8) | 3 (5.5) | |
| EUROscore II | 1.09 [0.90, 1.52] | 1.09 [0.90, 1.52] | 1.22 [0.90, 1.51] | 0.608 |
| Diabetes | 2 (1.8) | 1 (1.8) | 1 (1.8) | 1 |
| Baseline serum creatinine, mg/dL | 1.07 [0.97, 1.18] | 1.06 [0.98, 1.18] | 1.09 [0.97, 1.18] | 0.931 |
| Baseline eGFR, mL/min/m2 | 67.94 [61.71, 77.63] | 67.25 [62.39, 77.12] | 69.48 [60.21, 77.39] | 0.827 |
| Duration of CPB, min | 131.50 [111.25, 153.75] | 127.00 [109.50, 144.50] | 140.00 [120.00, 161.50] | 0.005 |
| Duration of cross-aortic clamping, min | 74.00 [62.25, 89.50] | 70.00 [60.50, 82.50] | 80.00 [67.00, 101.50] | 0.019 |
| ΔfHb, μM | 38.82 [24.38, 57.20] | 24.25 [11.75, 34.71] | 56.47 [45.27, 73.55] | <0.001 |
| fHb ratio | 2.23 [1.79, 2.99] | 1.78 [1.37, 1.97] | 2.99 [2.54, 3.62] | <0.001 |
| Days on vasoactive/inotropic drugs | 3.00 [2.00, 5.00] | 3.00 [0.50, 5.00] | 4.00 [2.50, 5.00] | 0.023 |
| AKI | 71 (64.5) | 27 (49.1) | 44 (80.0) | 0.001 |
| AKI stage | 0.001 | |||
| 0 | 39 (35.5) | 28 (50.9) | 11 (20.0) | |
| 1 | 60 (54.5) | 24 (43.6) | 36 (65.5) | |
| 2 | 5 (4.5) | 3 (5.5) | 2 (3.6) | |
| 3 | 6 (5.5) | 0 (0.0) | 6 (10.9) | |
| RRT | 6 (5.5) | 0 (0.0) | 6 (10.9) | 0.036 |
| Hours of ICU LOS | 65.29 [44.25, 81.23] | 66.16 [45.79, 85.71] | 62.42 [41.46, 69.08] | 0.064 |
| In-hospital mortality | 6 (5.5) | 0 (0.0) | 6 (10.9) | 0.036 |
Continuous data were presented as median with interquartile range (IQR, 25–75th percentiles) and compared with the Mann-Whitney U test. Categorical variables are presented as frequencies and compared with a chi-square test or Fisher exact test when appropriate. All analyses were two sided. BMI, body mass index; EUROscore II, European System for Cardiac Operative Risk Evaluation II; eGFR, estimated glomerular filtration rate; CPB, cardiopulmonary bypass; fHb, free hemoglobin; AKI, acute kidney injury; RRT, renal replacement therapy; ICU, intensive care unit; LOS, length of stay.
We stratified the cohort by the median of fHb ratio. Subjects with a higher fHb ratio (>2.23) displayed prolonged duration of CPB [140.00 min (120.00, 161.50) versus 127.00 min (109.50, 144.50), p=0.005] and aortic cross-clamping [80.00 min (67.00, 101.50) versus 70.00 min (60.50, 82.50), p=0.019], and more days on vasoactive/inotropic drugs [4.00 d (2.50, 5.00) versus 3.00 d (0.50, 5.00), p=0.023].
Seventy-one of 110 subjects (64.5%) met the criteria for AKI [stage 1 (60/110, 54.5%), stage 2 (5/110, 4.5%), stage 3 (6/110, 5.5%)] as defined by KDIGO guidelines (SCr) 4. Subjects with a higher fHb ratio (>2.23) presented similar baseline SCr [1.09 mg/dL (0.97, 1.18) versus 1.06 mg/dL (0.98, 1.18), p=0.931] and eGFR [69.48 ml/min/m2 (60.21, 77.39) versus 67.25 ml/min/m2 (62.39, 77.12), p=0.827], but higher incidence of AKI [44/55 (80.0%) versus 27/55 (49.1%), p=0.001], more need of RRT [6/55 (10.9%) versus 0/55 (0%), p=0.036], and higher in-hospital mortality [6/55 (10.9%) v.s. 0/55 (0%), p=0.036].
Association between fHb and postoperative AKI
Since fHb ratio had a greater association with AKI than ΔfHb (Supplemental Table 3), we selected fHb ratio as our exposure of interest in subsequent analyses. fHb ratio remained associated with AKI after adjustment for predefined confounders (Table 2 and Supplemental Table 4). The AUCs (95%CI) of Model 1 to Model 4 were 0.742 (0.650-0.834), 0.783 (0.692-0.875), 0.781 (0.687-0.875), and 0.794 (0.708-0.881), respectively (Table 2).
Table 2.
Odds ratios for AKI according to fHb ratio at the end of CPB (N=110)
| Models | OR (95%CI) | p | AUC (95%CI) |
|---|---|---|---|
| Model 1 | 2.3 (1.3-4.1) | 0.004 | 0.742 (0.650-0.834) |
| Model 2 | 2.6 (1.5-4.8) | 0.001 | 0.783 (0.692-0.875) |
| Model 3 | 2.3 (1.2-4.4) | 0.011 | 0.781 (0.687-0.875) |
| Model 4 | 2.6 (1.4-4.8) | 0.003 | 0.794 (0.708-0.881) |
Model 1: adjustment for demographics (i.e., age, gender, and BMI); Model 2: adjustment for comorbidities (i.e., current smoker, hypertension, vascular disease, COPD, severe heart failure, PAH, and AF) and baseline renal function (eGFR); Model 3: adjustment for EUROscore II and intraoperative risk factors (i.e., time of CPB, time of cross-aortic clamping, fluid balance during operation, blood transfusion, and highest VIS during operation); Model 4: adjustment for EUROscore II and postoperative risk factors (i.e., fluid balance and highest VIS in the first 24 hours, duration of mechanical ventilation, and vasoactive/inotropic drugs). fHb, free hemoglobin; BMI, body mass index; COPD, chronic obstructive pulmonary disease; PAH, pulmonary artery hypertension; AF, atrial fibrillation; eGFR, estimated glomerular filtration rate; EUROscore II, European System for Cardiac Operative Risk Evaluation II; CPB, cardiopulmonary bypass; VIS, vasoactive-inotropic score; OR, odds ratio; CI, confidence interval.
fHb, fHb ratio and urinary biomarkers at different time points
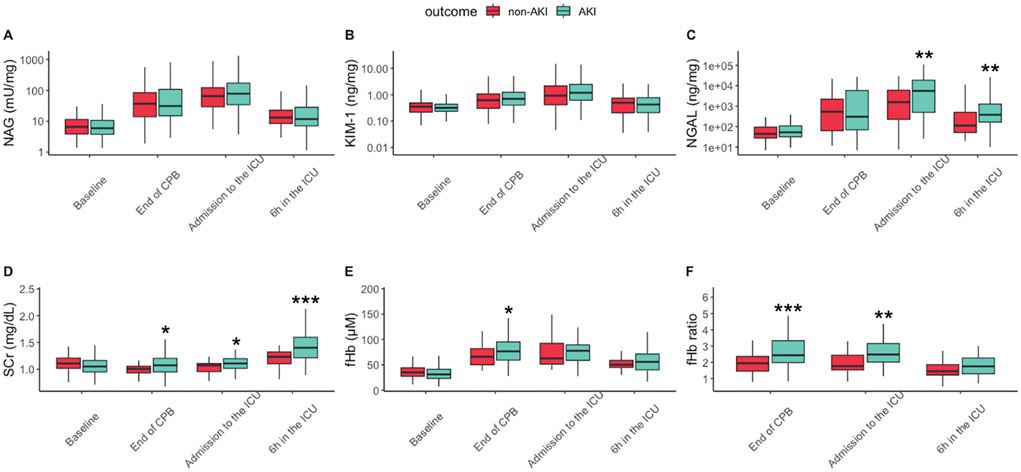
In the control arm, subjects displayed similar levels of SCr and fHb between AKI versus non-AKI groups at baseline. All the biomarkers started to increase at the end of CPB and decreased since 6 h in the ICU (Figure 1).
Figure 1.
Medians with interquartile range (25–75th percentiles) of NAG (mU/mg), KIM-1 (ng/mg), NGAL (ng/mg), SCr (mg/dL), fHb (μM), and fHb ratio at baseline, end of CPB, admission to the ICU and 6 h in the ICU (N=107). Y-axes of NAG, KIM-1 and NGAL were converted into logarithmic scale to accomodate all the values. Samples were compared between AKI and non-AKI group within the same time point using Mann-Whitney U test (*p<0.05, **p<0.01 and ***p<0.001). CPB, cardiopulmonary bypass; ICU, intensive care unit; SCr, serum creatinine; fHb, free hemoglobin; NGAL, neutrophil gelatinase-associated lipocalin; NAG, N-acetyl-β-D-glucosaminidase; KIM-1, kidney injury molecule-1; AKI, acute kidney injury.
Among all the urinary biomarkers, only NGAL showed significant differences between AKI versus non-AKI group at admission to the ICU [5633.76 ng/ml (506.99, 18707.22) versus 1578.34 ng/ml (227.04, 6125.81), p=0.009] and 6 h in the ICU [381.10 ng/ml (166.27, 1272.86) versus 113.24 ng/ml (51.17, 505.77), p=0.005], respectively.
Levels of fHb were higher at the end of CPB, 76.62μM (59.39, 95.24) in AKI group versus 66.01μM (50.32, 82.08) in non-AKI group (p=0.028). Subjects with AKI had higher fHb ratio than subjects without AKI at the end of CPB [2.44 (1.98, 3.33) versus 1.94 (1.46, 2.36), p<0.001] and admission to the ICU [2.48 (2.02, 3.16) versus 1.77 (1.53, 2.44), p=0.001]. All the measurements were shown in Figure 1.
Notably, 10% (11/110) of our cohort showed high stage (stage 2 and 3) of AKI. fHb ratio showed differences among patients without AKI, with AKI stage 1 and high stage (stage 2 and 3) of AKI in the control arm at the end of CPB and admission to the ICU (Supplemental Table 5). We also detected levels of fHb ratio in the intervention arm to explore whether fHb ratio was associated with AKI among patients with NO delivery. fHb ratio remained constant at each time point in the intervention arm and showed no differences between AKI and non-AKI groups (Supplemental Table 6).
Prediction of postoperative AKI in different models
Among all the four biomarkers, fHb ratio showed the highest AUC of 0.703 (0.600-0.806) and bootstrap-adjusted AUC of 0.704 (0.592-0.804) (Supplemental Figure 1, Table 3). AUC test indicated that fHb ratio showed better discrimination of AKI in comparison of NAG (p=0.033), and a trend toward significance in comparison of KIM-1 (p=0.098) but not NGAL (p=0.249). fHb ratio displayed better calibration compared with all the urinary biomarkers (Figure 2). Additionally, compared with all the urinary biomarkers, fHb ratio demonstrated the highest accuracy [0.714(0.579-0.804)], sensitivity [0.730(0.400-0.885)], specificity [0.686(0.474-0.923)], PPV [0.812(0.701-0.932)] and NPV [0.592(0.426-0.762)]. fHb ratio also showed better risk prediction compared to NAG (NRI 0.691, 95%CI 0.328-1.054, p<0.001) and NGAL (NRI 0.662, 95%CI 0.299-1.026, p<0.001) and a trend toward significance in comparison of KIM-1 (NRI 0.336, 95%CI −0.051-0.722, p=0.089). The optimal cut-off point for the fHb ratio to predict AKI was 2.03 (1.96-2.33) (Table 3).
Table 3.
Prediction performance of the models in the control arm (N=107)
| Models | AUC (95% CI) | P* | Cut-off points (95% CI) |
NRI (95% CI) | P# | Accuracy (95% CI) |
Sensitivity (95% CI) |
Specificity (95% CI) |
PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| fHb ratio | 0.70(0.59-0.80) | - | 2.03(1.96-2.33) | - | - | 0.71(0.58-0.80) | 0.73(0.40-0.88) | 0.69(0.47-0.92) | 0.81(0.70-0.93) | 0.59(0.43-0.76) |
| NGAL (ng/mg) | 0.60(0.43-0.73) | 0.249 | 195.96(26.16-1588.61) | 0.66(0.30-1.03)$ | p<0.001$ | 0.64(0.46-0.76) | 0.67(0.21-0.97) | 0.61(0.25-0.97) | 0.77(0.65-0.94) | 0.53(0.32-0.80) |
| NAG (mU/mg) | 0.52(0.40-0.65) | 0.033 | 45.25(10.77-448.83) | 0.69(0.33-1.05)$ | p<0.001$ | 0.58(0.40-0.73) | 0.55(0.11-0.98) | 0.63(0.10-1.00) | 0.77(0.61-1.00) | 0.49(0.32-0.86) |
| KIM-1 (ng/mg) | 0.56(0.43-0.68) | 0.098 | 0.49(0.20-1.25) | 0.34(−0.05-0.72)$ | P=0.089$ | 0.62(0.43-0.74) | 0.63(0.17-0.97) | 0.55(0.15-0.98) | 0.74(0.63-0.95) | 0.51(0.37-0.82) |
| Model A | 0.74(0.64-0.83) | 0.012 | 0.64(0.27-1.01) | p<0.001 | 0.74(0.62-0.83) | 0.76(0.47-0.95) | 0.70(0.44-0.94) | 0.83(0.72-0.95) | 0.64(0.45-0.85) | |
| Model B (Reference) | 0.63(0.51-0.74) | - | - | - | 0.63(0.48-0.75) | 0.60(0.22-0.93) | 0.69(0.30-0.98) | 0.80(0.66-0.97) | 0.51(0.36-0.72) |
Model A: fHb ratio+NGAL+NAG+KIM-1; Model B: NGAL+NAG+KIM-1; NAG, NGAL and KIM-1 were normalized with urinary creatinine concentration. We calculated *p value with “roc.test” function using “bootstrap” method with 2,000 replicates in “pROC” package.
We calculated cut-off points and 95%CI to achieve the maximal Youden’s index, using bootstrap-based method in R package “cutpointr” with 1,000 replicates. We used continuous NRI and its p# value using “improveProb” function in R package “Hmisc”.
indicating NRI and its p-value of fHb ratio when compared with NGAL, NAG, and KIM-1, respectively. We computed AUC and all the confusion matrix results using bootstrap-based method with 1,000 resamples. Accuracy is defined as the number of correct predictions divided by total number of predictions, which can be calculated as follows: Accuracy=. fHb, free hemoglobin; NAG, N-acetyl-β-D-glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; KIM-1, kidney injury molecule-1. AUC, area under the receiver operating characteristic curve; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value; NRI, net reclassification index.
Figure 2.
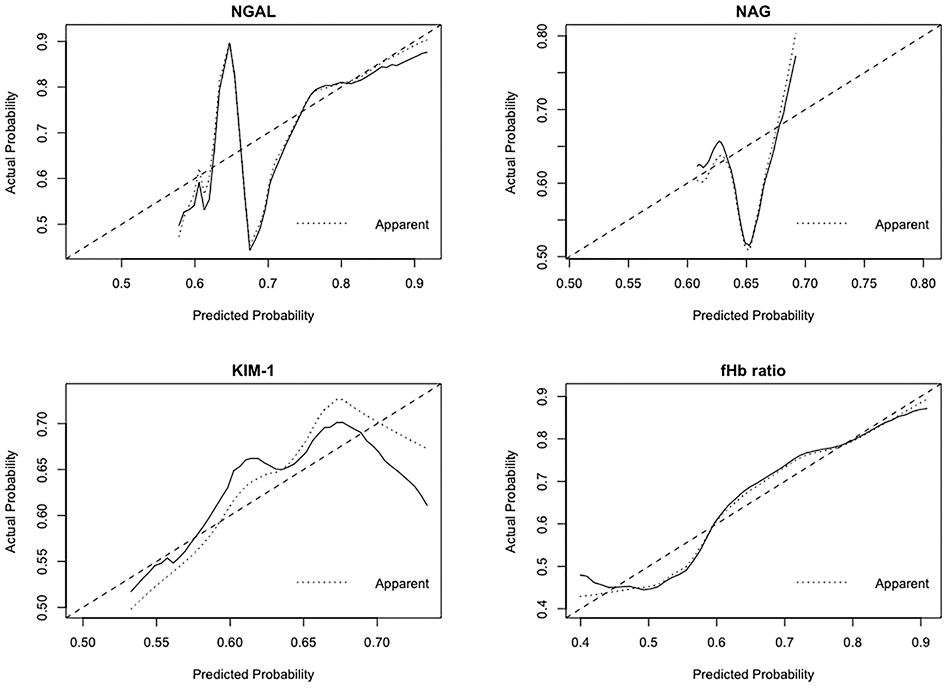
Calibration of fHb ratio, NGAL, NAG, and KIM-1 at the end of CPB in the control arm (N=107). Predicted probability of AKI is plotted on the x-axis; actual probability of AKI is plotted on the y-axis. The ideal calibration line means an intercept of 0 and a slope of 1 for the calibration plot. CPB, cardiopulmonary bypass; fHb, free hemoglobin; NGAL, neutrophil gelatinase-associated lipocalin; NAG, N-acetyl-β-D-glucosaminidase; KIM-1, kidney injury molecule-1; AKI, acute kidney injury.
Model A showed AUCs of 0.771 (0.679-0.856) and 0.653 (0.547-0.760), respectively (Supplemental Figure 2, Table 3). The bootstrap-adjusted AUCs for Models A and B were 0.743 (0.642-0.835) and 0.629 (0.510-0.740) (Table 3). In terms of discrimination, AUC test showed that Model A was better than Model B (p= 0.012). Both calibration curves (Figure 3) and likelihood ratio test (p<0.001) verified that incorporation of fHb ratio improved the calibration against AKI, compared with using urinary biomarkers alone.
Figure 3.
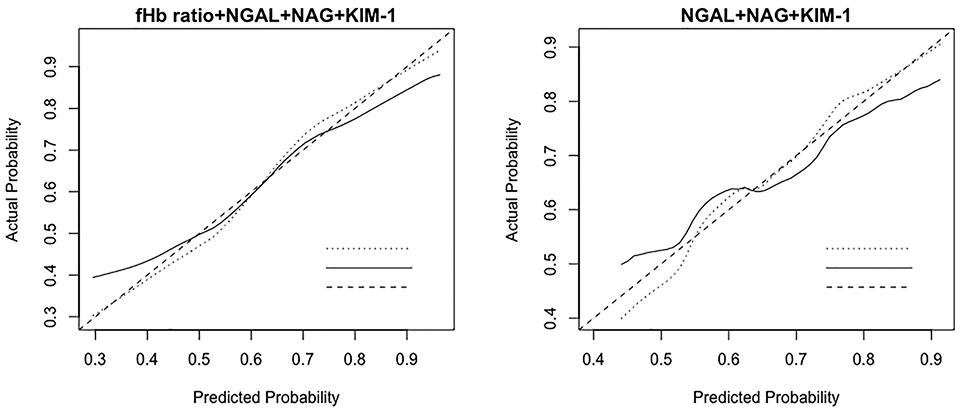
Calibration of Model A (fHb ratio+NGAL+NAG+KIM-1) and Model B (NGAL+NAG+KIM-1) at the end of CPB in the control arm (N=107). Model-predicted probability of AKI is plotted on the x-axis; actual probability of AKI is plotted on the y-axis. The ideal calibration line means an intercept of 0 and a slope of 1 for the calibration plot. CPB, cardiopulmonary bypass; fHb, free hemoglobin; NGAL, neutrophil gelatinase-associated lipocalin; NAG, N-acetyl-β-D-glucosaminidase; KIM-1, kidney injury molecule-1; AKI, acute kidney injury.
Moreover, Model A showed higher accuracy [0.740(0.617-0.832) versus 0.632(0.477-0.748)], sensitivity [0.764(0.472-0.950) versus 0.600(0.225-0.934)], specificity [0.696(0.444-0.944) versus 0.691(0.304-0.978)], PPV [0.828(0.720-0.953) versus 0.799(0.660-0.971)], and NPV [0.642(0.452-0.852) versus 0.513(0.362-0.722)], compared with Model B. In addition, Model A resulted in significant improvements in risk prediction compared to Model B (NRI=0.638, 95% CI 0.269-1.008, p<0.001).
Discussion
In adult patients undergoing on-pump cardiac surgery, fHb ratio at the end of CPB was associated with postoperative AKI. fHb ratio might be used as a novel, widely applicable biomarker for an early detection of AKI, compared with prediction based only on urinary biomarkers. Future prospective studies with large sample size are still needed to verify the predictive value of fHb ratio against AKI.
The incidence of AKI in our cohort seemed to be relatively high. According to prior study in which EUROscore II higher than 3 could predict AKI after cardiac surgery 33, the patients in our cohort should be considered to present a low risk of AKI at baseline since EUROscore II was only 1.09 [0.90, 1.52]. However, the incidence of AKI, need of RRT and in-hospital mortality in our study was similar to the one reported in the PrevAKI study 3, in which only patients with a high risk of AKI (defined by urinary biomarkers recorded 4h after CPB) were included. We speculated that it could be explained by the lower baseline eGFR (69.4±12.4 mL/min/m2 versus 94.4±37.9 mL/min/m2) and longer duration of CPB (131.50 min [111.25, 153.75] versus 117 min [94.0, 155.0]) in our study, compared with the PrevAKI study.
Hemolysis plays a prominent role in AKI after on-pump cardiac surgery 15. During hemolysis, RBCs release fHb into the plasma in the form of ferrous oxyhemoglobin (Oxy-Hb). Oxy-Hb reacts quickly with endogenous NO in a dioxygenation reaction to form ferric methemoglobin (Met-Hb), introducing not only direct tubular injury but also vasoconstriction and endothelial dysfunction due to NO consumption 34. The latter could consequently lead to ischemic injury within corticomedullary junctions and the glomerulus 35, which cannot be thoroughly described by an increase in urinary biomarkers.
In the presence of hemolysis, fHb raises in the bloodstream and this phenomenon is proportionally described – the higher the hemolysis the higher the levels of fHb 36, indicating that fHb is an excellent surrogate of hemolysis. Nahmah et al. found that ΔfHb at the end of CPB was associated with AKI among pediatric patients 16. However, the association among adult patients undergoing on-pump cardiac surgery still needs further investigation in the context of a large number of perioperative risk factors.
In our descriptive and association analysis, levels of fHb and fHb ratio peaked at the end of CPB and showed significant difference between patients with or without AKI, while reduced at 6h in the ICU and showed no difference between groups, which was consistent with prior studies 14, 37. After adjustment for the aforementioned potential confounders, the fHb ratio at the end of CPB remained to be associated with postoperative AKI. Notably, all six patients who needed RRT and/or died within hospitalization after operation presented fHb levels >2.23, and levels of fHb ratio significantly escalated with AKI stage, indicating that the association between fHb ratio and other competing outcomes, such as advanced stage of AKI, need of RRT or survival, are worth further investigation.
AUC test is traditionally utilized to evaluate the prediction increment of a new biomarker. However, it does not adjust for variability in estimated regression coefficients and thereby is too conservative to compare the predictive performance between two competing models 26. Therefore, if statistical testing for improvement in risk prediction is desired, it should rely on likelihood-based measures of model fit (e.g. likelihood ratio test) 32. However, it can only be performed in nested models (i.e., Model A versus Model B). We thereby employed NRI to quantify whether a new marker provides clinically relevant improvements in prediction 32, especially for non-nested models (i.e., fHb ratio versus each urinary biomarkers). In general, the assessment of the model performance indicated that fHb ratio per se yielded a superior performance in predicting postoperative AKI over other urinary biomarkers, and combination of fHb ratio and urinary biomarkers could be promising early diagnostic strategy against postoperative AKI after on-pump cardiac surgery.
Notably, it is extremely appealing that fHb ratio in the intervention arm remained constant between groups at different time points, which was not comparable with that in the control arm. It is mainly attributed to the detective methodology that cannot distinguish Met-Hb from Oxy-Hb and thereby alter the relationship between fHb ratio and postoperative AKI in the intervention arm, indicating that prediction of fHb ratio against AKI can only be employed in subjects without NO administration.
Our study has some strength. First, measurement of fHb can be performed in real time within a few minutes after sample collection. The detection of fHb (using ultraviolet spectrophotometry) is cheaper and faster than the detection of urinary biomarkers (via ELISA). Furthermore, fHb peak anticipates urinary biomarkers peak of some hours providing a leading time that might anticipate potential preventative/therapeutic measures of AKI onset. Therefore, we suggest fHb ratio to be a potentially more feasible measurement for most of the hospitals, compared with urinary biomarkers. Second, our study added novel insights to some relevant publications among patients undergoing ECMO 38, 39 or with sepsis 40 as follows: (1) We performed a prediction analysis to validate discrimination (i.e., how well could fHb distinguish subjects with or without outcome) and calibration (i.e., the agreement between observed and predicted probability to develop AKI) of fHb ratio; (2) we further analyzed the predictive performance of fHb ratio against AKI in the context of three different urinary biomarkers of AKI; and (3) we used appropriate statistical methods to account for non-linearity and overfitting due to small sample size.
We acknowledge limitations in our study. First, the prediction analysis is derived from a limited sample size cohort. Therefore, overfitting cannot be overlooked despite the statistical optimization using the bootstrap adjustment. Second, since the predictors of interest were not planned a priori, our results were exploratory and still need to be verified in a prospective study. Finally, since our derived cohort is a group of Asian patients undergoing multi-valve surgery requiring CPB and the delivery of NO invalidates the prediction of fHb ratio against AKI, the generalizability of our prediction model needs further determination.
Conclusion
In this exploratory, hypothesis-generating retrospective, observational study we found that fHb ratio at the end of CPB yielded a superior performance in predicting postoperative AKI over NGAL, NAG and KIM-1, which might allow the identification of AKI immediately after on-pump cardiac surgery at an earlier stage. However, the results of our study are preliminary and need further determination due to small sample size in the context of an intrinsic biological variability of blood and urine biomarkers. Future prospective studies should be conducted to verify whether fHb ratio alone or with other urinary biomarkers could be employed in clinical practice to predict AKI or other competing outcomes.
Supplementary Material
Supplemental Table 1. Details of the potential risk factors and outcomes.
Supplemental Table 2. Descriptive analyses on all the variables (N=110).
Supplemental Table 3. Univariable analysis of ΔfHb or fHb ratio for AKI in the control arm (N=110).
Supplemental Table 4. Multivariable analysis among patients in the control arm (N=110).
Supplemental Table 5. The comparison of fHb ratio at different time points in the control arm among non-AKI, AKI stage 1, and high stage of AKI (AKI stage 2 and 3 ) groups (N=110).
Supplemental Table 6. The comparison of fHb ratio at different time points between AKI (N=52) and non-AKI (N=53) in the intervention arm.
Supplemental Figure 1. ROC curve analyses of single biomarker at the end of CPB in the control arm (N=107). CPB, cardiopulmonary bypass. ROC, Receiver operating characteristic.
Supplemental Figure 2. ROC curve analyses of prediction models at the end of CPB in the control arm (N=107). CPB, cardiopulmonary bypass. ROC, Receiver operating characteristic.
Key points summary.
Question: Is there a novel, easily detected biomarker to predict postoperative AKI immediately after on-pump cardiac surgery?
Findings: fHb ratio predicted postoperative AKI immediately after on-pump cardiac surgery and incorporation of fHb ratio improved the predictive performance of AKI, compared with prediction using urinary biomarkers alone.
Meaning: fHb ratio might enable perioperative clinicians to identify the patients with high risk of AKI at the end of on-pump cardiac surgery.
Acknowledgments:
The authors thank Brian Healy, Miguel Hernán, Barbra Dickerman, Juan Pedemonte, and Yoshihiko Raita at Harvard Medical School general hospital to help with the statistical analysis and David Imber in Massachusetts General Hospital to review the manuscript.
Funding:
This research was supported by grants from the National Natural Science Foundation of China (NSFC 81501642) and two grants from the administration of Science and Technology in Sanya City, Hainan Province, China (2017YW12 and 2017YD16).
This research was also supported by Department of Anesthesia, Critical Care and Pain Medicine (Massachusetts General Hospital, Boston, Massachusetts, USA) and the National Institutes of Health (National Heart, Lung, and Blood Institute K23 HL128882-01A1).
L.B.’s salary is partially supported by NIH/NHLBI 1 K23 HL128882-01A1. Other authors declare that they have no competing interests.
Glossary of terms
- AKI
acute kidney injury
- CPB
cardiopulmonary bypass
- NO
nitric oxide
- OR
odds ratio
- CI
confidence interval
- ROC
receiver operating characteristic
- AUC
area under the curve
- SCr
serum creatinine
- fHb
free hemoglobin
- NAGL
neutrophil gelatinase-associated lipocalin
- KIM-1
kidney injury molecule-1
- NAG
N-acetyl-β-D-glucosaminidase
- IGFBP7
insulin-like growth factor-binding protein 7
- TIMP-2
tissue inhibitor of metalloproteinases-2
- Oxy-Hb
oxyhemoglobin
- Met-Hb
methemoglobin
- RBCs
red blood cells
- ICU
intensive care unit
- BMI
body mass index
- COPD
chronic obstructive pulmonary disease
- AF
atrial fibrillation
- PAH
pulmonary artery hypertension
- EUROscore
European System for Cardiac Operative Risk Evaluation
- eGFR
estimated glomerular filtration rate
- VIS
vasoactive/inotropic score
- LOS
length of stay
- UCr
urinary creatinine
- N2
nitrogen
- RCT
randomized control trial
- RRT
renal replacement therapy
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- KDIGO
Kidney Disease: Improving Global Outcomes
- ELISA
enzyme-linked immunosorbent assay
- ECMO
extracorporeal membrane oxygenation
- PPV
positive predictive value
- NPV
negative predictive value
- NRI
net reclassification index
Footnotes
Conflicts of Interest:
The authors declare no competing interests.
References:
- 1.Lei C, Berra L, Rezoagli E, et al. Nitric Oxide Decreases Acute Kidney Injury and Stage 3 Chronic Kidney Disease after Cardiac Surgery. Am J Respir Crit Care Med. November 15 2018;198(10):1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hobson CE, Yavas S, Segal MS, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. May 12 2009;119(18):2444–2453. [DOI] [PubMed] [Google Scholar]
- 3.Meersch M, Schmidt C, Hoffmeier A, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med. November 2017;43(11):1551–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney international. 2012;Suppl(2):1–138. [Google Scholar]
- 5.Mehta RL, Chertow GM. Acute renal failure definitions and classification: time for change? J Am Soc Nephrol. August 2003;14(8):2178–2187. [DOI] [PubMed] [Google Scholar]
- 6.Meersch M, Schmidt C, Van Aken H, et al. Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS One. 2014;9(3):e93460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pilarczyk K, Edayadiyil-Dudasova M, Wendt D, et al. Urinary [TIMP-2]*[IGFBP7] for early prediction of acute kidney injury after coronary artery bypass surgery. Ann Intensive Care. December 2015;5(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han WK, Wagener G, Zhu Y, Wang S, Lee HT. Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol. May 2009;4(5):873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu S, Che M, Xue S, et al. Urinary L-FABP and its combination with urinary NGAL in early diagnosis of acute kidney injury after cardiac surgery in adult patients. Biomarkers. February 2013;18(1):95–101. [DOI] [PubMed] [Google Scholar]
- 10.Metzger J, Mullen W, Husi H, et al. Acute kidney injury prediction in cardiac surgery patients by a urinary peptide pattern: a case-control validation study. Crit Care. May 26 2016;20(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith GC, Seaman SR, Wood AM, Royston P, White IR. Correcting for optimistic prediction in small data sets. Am J Epidemiol. August 1 2014;180(3):318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrell Frank E. J. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. 2015. [Google Scholar]
- 13.Deuel JW, Schaer CA, Boretti FS, et al. Hemoglobinuria-related acute kidney injury is driven by intrarenal oxidative reactions triggering a heme toxicity response. Cell Death Dis. January 21 2016;7:e2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rezoagli E, Ichinose F, Strelow S, et al. Pulmonary and Systemic Vascular Resistances After Cardiopulmonary Bypass: Role of Hemolysis. J Cardiothorac Vasc Anesth. April 2017;31(2):505–515. [DOI] [PubMed] [Google Scholar]
- 15.Vermeulen Windsant IC, de Wit NC, Sertorio JT, et al. Hemolysis during cardiac surgery is associated with increased intravascular nitric oxide consumption and perioperative kidney and intestinal tissue damage. Front Physiol. 2014;5:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim-Campbell N, Gretchen C, Callaway C, et al. Cell-Free Plasma Hemoglobin and Male Gender Are Risk Factors for Acute Kidney Injury in Low Risk Children Undergoing Cardiopulmonary Bypass. Crit Care Med. November 2017;45(11):e1123–e1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Bmj. October 20 2007;335(7624):806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreira R, Jacinto T, Neves P, Vouga L, Baeta C. Predictors of Acute Kidney Injury Associated with Cardiopulmonary Bypass. Rev Port Cir Cardiotorac Vasc. Apr-Jun 2019;26(2):109–115. [PubMed] [Google Scholar]
- 19.Yue Z, Yan-Meng G, Ji-Zhuang L. Prediction model for acute kidney injury after coronary artery bypass grafting: a retrospective study. Int Urol Nephrol. September 2019;51(9):1605–1611. [DOI] [PubMed] [Google Scholar]
- 20.Ried M, Puehler T, Haneya A, Schmid C, Diez C. Acute kidney injury in septua- and octogenarians after cardiac surgery. BMC Cardiovasc Disord. August 11 2011;11:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moorthy V, Liu W, Chan SP, Chew STH, Ti LK. Elucidation of the novel role of ethnicity and diabetes in poorer outcomes after cardiac surgery in a multiethnic Southeast Asian cohort. J Diabetes. June 18 2019. [DOI] [PubMed] [Google Scholar]
- 22.Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. January 2005;16(1):162–168. [DOI] [PubMed] [Google Scholar]
- 23.Shen Y, Zhang W, Cheng X, Ying M. Association between postoperative fluid balance and acute kidney injury in patients after cardiac surgery: A retrospective cohort study. J Crit Care. April 2018;44:273–277. [DOI] [PubMed] [Google Scholar]
- 24.Koponen T, Karttunen J, Musialowicz T, Pietiläinen L, Uusaro A, Lahtinen P. Vasoactive-inotropic score and the prediction of morbidity and mortality after cardiac surgery. Br J Anaesth. April 2019;122(4):428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL. Epidemiology of Acute Kidney Injury in Critically Ill Children and Young Adults. N Engl J Med. January 5 2017;376(1):11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Algaze CA, Koth AM, Faberowski LW, Hanley FL, Krawczeski CD, Axelrod DM. Acute Kidney Injury in Patients Undergoing the Extracardiac Fontan Operation With and Without the Use of Cardiopulmonary Bypass. Pediatr Crit Care Med. January 2017;18(1):34–43. [DOI] [PubMed] [Google Scholar]
- 27.Vaidya VS, Waikar SS, Ferguson MA, et al. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin Transl Sci. December 2008;1(3):200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Assel M, Sjoberg D, Elders A, et al. Guidelines for Reporting of Statistics for Clinical Research in Urology. J Urol. March 2019;201(3):595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schemper M Predictive accuracy and explained variation. Stat Med. July 30 2003;22(14):2299–2308. [DOI] [PubMed] [Google Scholar]
- 30.Vetter TR, Schober P, Mascha EJ. Diagnostic Testing and Decision-Making: Beauty Is Not Just in the Eye of the Beholder. Anesth Analg. October 2018;127(4):1085–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pepe MS, Kerr KF, Longton G, Wang Z. Testing for improvement in prediction model performance. Stat Med. April 30 2013;32(9):1467–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas LE, O'Brien EC, Piccini JP, D'Agostino RB, Pencina MJ. Application of net reclassification index to non-nested and point-based risk prediction models: a review. Eur Heart J. June 14 2019;40(23):1880–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen SW, Chang CH, Fan PC, et al. Comparison of contemporary preoperative risk models at predicting acute kidney injury after isolated coronary artery bypass grafting: a retrospective cohort study. BMJ Open. June 27 2016;6(6):e010176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spina S, Lei C, Pinciroli R, Berra L. Hemolysis and Kidney Injury in Cardiac Surgery: The Protective Role of Nitric Oxide Therapy. Semin Nephrol. September 2019;39(5):484–495. [DOI] [PubMed] [Google Scholar]
- 35.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. November 2011;121(11):4210–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donadee C, Raat NJ, Kanias T, et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. July 26 2011;124(4):465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wetz AJ, Richardt EM, Schotola H, Bauer M, Brauer A. Haptoglobin and free haemoglobin during cardiac surgery-is there a link to acute kidney injury? Anaesth Intensive Care. January 2017;45(1):58–66. [DOI] [PubMed] [Google Scholar]
- 38.Lyu L, Long C, Hei F, et al. Plasma Free Hemoglobin Is a Predictor of Acute Renal Failure During Adult Venous-Arterial Extracorporeal Membrane Oxygenation Support. J Cardiothorac Vasc Anesth. August 2016;30(4):891–895. [DOI] [PubMed] [Google Scholar]
- 39.Omar HR, Mirsaeidi M, Socias S, et al. Plasma Free Hemoglobin Is an Independent Predictor of Mortality among Patients on Extracorporeal Membrane Oxygenation Support. PLoS One. 2015;10(4):e0124034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adamzik M, Hamburger T, Petrat F, Peters J, de Groot H, Hartmann M. Free hemoglobin concentration in severe sepsis: methods of measurement and prediction of outcome. Crit Care. July 16 2012;16(4):R125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Details of the potential risk factors and outcomes.
Supplemental Table 2. Descriptive analyses on all the variables (N=110).
Supplemental Table 3. Univariable analysis of ΔfHb or fHb ratio for AKI in the control arm (N=110).
Supplemental Table 4. Multivariable analysis among patients in the control arm (N=110).
Supplemental Table 5. The comparison of fHb ratio at different time points in the control arm among non-AKI, AKI stage 1, and high stage of AKI (AKI stage 2 and 3 ) groups (N=110).
Supplemental Table 6. The comparison of fHb ratio at different time points between AKI (N=52) and non-AKI (N=53) in the intervention arm.
Supplemental Figure 1. ROC curve analyses of single biomarker at the end of CPB in the control arm (N=107). CPB, cardiopulmonary bypass. ROC, Receiver operating characteristic.
Supplemental Figure 2. ROC curve analyses of prediction models at the end of CPB in the control arm (N=107). CPB, cardiopulmonary bypass. ROC, Receiver operating characteristic.