Abstract
Vaccination strategies against mycobacteria, focusing mostly on classical T‐ and B‐cells, have shown limited success, encouraging the addition of alternative targets. Classically restricted T‐cells recognize antigens presented via highly polymorphic HLA class Ia and class II molecules, while donor‐unrestricted T‐cells (DURTs), with few exceptions, recognize ligands via genetically conserved antigen presentation molecules. Consequently, DURTs can respond to the same ligands across diverse human populations. DURTs can be activated either through cognate TCR ligation or via bystander cytokine signaling. TCR‐driven antigen‐specific activation of DURTs occurs upon antigen presentation via non‐polymorphic molecules such as HLA‐E, CD1, MR1, and butyrophilin, leading to the activation of HLA‐E–restricted T‐cells, CD1‐restricted T‐cells, mucosal‐associated invariant T‐cells (MAITs), and TCRγδ T‐cells, respectively. NK cells and innate lymphoid cells (ILCs), which lack rearranged TCRs, are activated through other receptor‐triggering pathways, or can be engaged through bystander cytokines, produced, for example, by activated antigen‐specific T‐cells or phagocytes. NK cells can also develop trained immune memory and thus could represent cells of interest to mobilize by novel vaccines. In this review, we summarize the latest findings regarding the contributions of DURTs, NK cells, and ILCs in anti–M tuberculosis, M leprae, and non‐tuberculous mycobacterial immunity and explore possible ways in which they could be harnessed through vaccines and immunotherapies to improve protection against Mtb.
Keywords: donor‐unrestricted T‐cells, HLA‐E, innate lymphoid cells, Mycobacterium tuberculosis, NK cells, vaccine
1. HLA‐E–RESTRICTED T‐CELLS
The non‐classical HLA class Ib molecule HLA‐E is best known as a ligand for CD94/NKG2A and CD94/NKG2C, which are inhibitory and activating C‐type lectin receptors, respectively, that are expressed by NK cells and some subsets of T‐cells. 1 CD94/NKG2A recognition of HLA‐E–presented self‐peptides, typically derived from HLA class Ia signal sequences, leads to inhibition of NK‐cell cytolytic function. 2 , 3 This interaction operates as a surveillance system for monitoring bonafide surface expression of HLA class Ia, which can be downregulated by tumors or pathogens as an immune escape mechanism. 4 However, HLA‐E molecules have also been shown to present peptides of non–self‐origin, such as the glycoprotein UL40 leader peptide from CMV, which, being identical to HLA class Ia signal peptide, also engages CD94/NKG2A allowing CMV to escape NK‐cell–mediated cytotoxicity. 5 Moreover, HLA‐E–dependent peptide presentation to TCRs on human CD8+ T‐cells has been shown for a variety of bacterial and viral pathogens including Mtb, Salmonella, and CMV. 6 , 7 , 8 , 9 , 10 , 11 , 12 Mtb‐specific HLA‐E–restricted T‐cells were able to control intracellular mycobacterial growth in human macrophages ex vivo, rendering these cells interesting targets for vaccination strategies. 9
In contrast to other HLA molecules, HLA‐E is essentially monomorphic: only one amino acid located outside of the peptide‐binding groove differentiates the two allelic, coding variants in humans, HLA‐E*01:01 (107Arg) and HLA‐E*01:03 (107Gly). 13 These two allelic variants are equally represented in human populations, promoting interest in HLA‐E as a vaccine presentation molecule conserved in otherwise genetically diverse populations. 14 HLA‐E–mediated antigen presentation has attracted particular interest in the context of Mtb infection after several studies showed that HLA‐E, in contrast to classical HLA molecules, was resistant to HIV‐mediated downregulation. 15 Although a recent publication showed that this resistance varied with the HIV strain studied, 16 the observed maintained HLA‐E expression can be of significance when administering vaccines to individuals at risk of Mtb/HIV coinfection. HLA‐E’s function can be studied in mice and non‐human primates, due to the high structural and functional conservation across vertebrates, with Qa‐1b, Mamu‐E, and Mafa‐E being the HLA‐E orthologues in mice, rhesus macaques (RM), and cynomolgus macaques, respectively. 17 , 18 This is particularly relevant for preclinical and translational HLA‐E–oriented vaccine research.
1.1. Functional properties of HLA‐E–restricted T‐cells in an infectious disease: the example of Mtb infection
HLA‐E tetramers loaded with Mtb‐derived peptides were used to detect circulating Mtb‐specific HLA‐E–restricted T‐cells in active TB patients, particularly in TB patients coinfected with HIV, but at lower frequencies also in LTBI. The higher frequencies of these cells in active TB and HIV/TB patients suggest activation during disease, possibly correlating with disease severity. 9 , 19 Functional characterization of these cells ex vivo and of Mtb‐specific HLA‐E–restricted polyclonal and monoclonal T‐cells in vitro revealed an unorthodox Th2 cytokine and effector‐memory profile, with both cytotoxic and regulatory activities. Despite possessing Th2‐like characteristics, these T‐cells were able to inhibit intracellular mycobacterial growth in human Mtb‐infected macrophages. 8 , 9 In mice, balanced activation of Qa‐1b–restricted T‐cells was found to be associated with improved control of bacterial burden, reduced inflammation, and reduced mortality following Mtb infection. 20 In line with these cells’ Th2 characteristics, our own work further demonstrated that Mtb‐specific human HLA‐E–restricted T‐cells could activate B‐cells, 9 which play a key role in T‐cell immunity to Mtb. 21 Mtb‐specific HLA‐E–restricted T‐cells, however, also can express IFN‐γ, a Th1 cytokine, which has been associated with control of Mtb growth. 7 , 11 , 22 Taken together, the polyfunctional nature of Mtb‐specific HLA‐E–restricted T‐cells renders them interesting targets for vaccine‐inducible control of Mtb infection, by mobilizing a number of as yet incompletely resolved functional pathways, which include helping macrophages, promoting B‐cell activation, and regulating excessive inflammatory responses (Table 1).
TABLE 1.
Activation mechanisms and functional relevance of DURTs, NK cells, and ILCs in the context of mycobacterial infections
| HLA‐E–restricted T‐cells | MAIT cells | TCRγδ T‐cells | CD1‐restricted T cells | NK cells | ILCs | |||
|---|---|---|---|---|---|---|---|---|
| Group 1 CD1‐restricted T‐cells | (i)NKT cells | ILC1s | ILC3s | |||||
| Activation via antigen | Peptides: | Metabolic derivatives: | Phosphoantigens: | Lipids: | UL‐16–binding protein (ULBP)1 | |||
| self and pathogen‐derived | 5‐OP‐RU | IPP, HMBPP, mGLP | GMM, MA, SGLs, GroMM | α‐GalCer, PIM | ||||
| Activation via cytokines (TCR‐independent) | IL‐12/IL‐18 | IL‐12/IL‐18 | IL‐12/IL‐18 | IL‐12/IL‐15/IL‐18 | IL‐23 | |||
| Functional relevance | Mtb growth inhibition | Recruitment to site of infection | Mtb growth inhibition | Mtb growth inhibition | Mtb growth inhibition | Mtb growth inhibition | IFN‐γ production | Th17 profile |
| Cytotoxic, regulatory, and helper functions | Regulatory function | Cytotoxic function (perforin and granulysin) | Cytotoxic function (perforin, granzyme B, and granulysin) | Th1 and Th17 cytokine profiles | Trained immunity: IL‐1β, IL‐6, and TNF‐α production | |||
| Th1 and Th2 cytokine profiled | Cytotoxic function (granzyme B) | IFN‐γ, GM‐CSF, IL‐3, TNF‐α production | Th1‐like cytokine profile | Regulatory function | ||||
| Immunoregulatory role (IL‐10) | CD40L, IFN‐γ, IL‐2, TNF‐α, and IL‐17a production | Cytotoxic function | ||||||
| Th17 cytokine production | Memory‐like function | |||||||
| Helper function | IFN‐γ production | |||||||
1.2. Studies on HLA‐E–restricted T‐cells in experimental vaccination studies
The potential of HLA‐E–restricted T‐cells in vaccination strategies is further supported by a series of studies in RM demonstrating the induction of these unconventional T‐cells after vaccination with engineered rhesus cytomegalovirus (RhCMV) vectors. These vectors carried virus or parasite antigens and elicited tissue‐resident and high‐frequency circulating effector‐memory CD4+ and CD8+T‐cell responses specific for the pathogen‐derived antigen. 23 Hansen et al 24 showed that vaccination of RM with RhCMV/simian immunodeficiency virus (SIV) led to complete protection against high‐dose SIV challenge in half of the animals, which was at least partially dependent on Mamu‐E–restricted T‐cell responses. Mamu‐E–restricted T‐cells from these animals were able to recognize SIV‐infected CD4+T‐cells as shown by IFN‐γ and TNF‐α production, a response which could be blocked by the HLA‐E–specific binding VL9 peptide. Similarly, vaccination of RM with RhCMV/hepatitis B virus (HBV) elicited Mamu‐E–restricted T‐cells producing IFN‐γ upon recognition of both RM‐ and human‐derived HBV‐infected primary hepatocytes in vitro. 24 , 25 Plasmodium knowlesi (Pk) challenge of RhCMV/Pk‐vaccinated RM led to delayed parasitemia, again partially dependent on Mamu‐E–restricted T‐cells. 26 A significant reduction in the magnitude of Mtb infection and disease after RhCMV/TB vaccination in RM was observed compared with unvaccinated animals. 27 In this case, Mamu‐E‐ and MHC‐II‐ or MHC‐Ia–restricted Mtb‐specific effector T‐cell responses were induced, partly depending on the vector used, and both may play a role in protection, hinting to diverse and possibly complementary protective mechanisms in the immune response against Mtb. 27 The finding of MHC‐E–restricted SIV epitopes that were recognized by all tested (outbred) animals led to the term “supertopes” and supports the potential of HLA‐E–targeting vaccines to induce effector T‐cell responses regardless of human genetic diversity. 24
Recent insights into HLA‐E peptide‐binding motifs 28 , 29 and the availability of improved peptide/HLA‐E–binding algorithms will allow for better selection of optimal peptide ligands for HLA‐E from diverse pathogens and perhaps even tumors, which could be harnessed in better vaccines and therapeutics. Further research will be necessary to define the influence of peptide‐binding affinity and stability on optimal TCR triggering via HLA‐E. An additional advantage when considering HLA‐E as a vaccine target, especially in the case of intracellular pathogens such as Mtb, is HLA‐E’s expression in the Mtb phagosome of macrophages. 30 Therefore, the Mtb phagosome could be involved in HLA‐E peptide loading and thus be an alternative cytosolic cross‐presentation pathway, although the precise mechanisms involved remain undefined.
2. MUCOSAL‐ASSOCIATED INVARIANT T (MAIT) OR MR1‐RESTRICTED T‐CELLS
MAIT‐cells are a subset of (mostly oligoclonal) TCRαβ T‐cells initially associated with mucosal surfaces, and defined by surface expression of CD161, CD26, and a semi‐invariant TCR. 31 , 32 MAIT‐cells express chemokine receptors CCR9, CCR5, CCR6, and CXCR6, suggesting preferential homing to liver, lung, and intestines. 33 However, MAIT‐cells also circulate in the blood from where they are recruited during bacterial infections such by Mtb. 34 MAIT‐cells have the capacity to detect bacterially infected cells in mice and humans through the recognition of metabolic intermediates presented by the monomorphic MHC‐related protein 1 (MR1). Known MAIT agonists include riboflavin metabolites, which are only present in bacteria and fungi, such as 5‐OE‐RU and 5‐OP‐RU, 35 making them an important component of the mucosal immune response against bacterial infections.
Despite the initially observed conservation of the MAIT TCR repertoire, additional studies using MR1 tetramers discovered more heterogeneous intra‐ and inter‐individual TCR‐V fragment usage. 36 , 37 , 38 Comparison of Mtb‐specific MR1 tetramer–positive and MR1 tetramer–negative MAIT‐cells showed that MAIT‐cells can react to diverse ligands. 39 Nevertheless, the relatively limited MAIT TCR repertoire and its conservation across species are clear indications of underlying conserved specificity and functionality of at least a subset of MAIT‐cells. A recently expanded MAIT‐cell classification has suggested to encompass the apparent increasing diversity of human MAIT‐cell populations, considering MR1 restriction, antigen reactivity, and innate‐like effector function associated with the expression of promyelocytic leukemia zinc finger (PLZF). 31 Classical MAIT‐cells can be identified by the expression of a semi‐invariant TCR encoded by TRAV1‐2 and TRAJ33/12/20 α‐chain, with restricted TCRβ chain usage dominated by TRBV6 and TRBV20. The alternative, more variable expression of TCRA genes in TRAV1‐2–negative MAIT‐cells characterizes non‐classical MAIT‐cells. Atypical MR1‐restricted T‐cells can express variable TCR fragments and are phenotypically distinct from MAIT‐cells as determined by reactivity to alternative antigens and lack of PLZF expression. 31 Therefore, MAIT‐cells are also often referred to as MR1‐restricted T‐cells (MR1T), but it is currently uncertain whether all MR1‐restricted T‐cells classify as MAIT‐cells.
Classical MAIT‐cells share high similarity in transcriptional patterns with invariant natural killer T (iNKT)‐cells, which we discuss in detail below, and both types of unconventional T‐cells express a transcriptome distinct from that of conventional CD8+T‐cells. 39 , 40 These shared transcriptional programs are acquired during thymic development and reflect defined effector functions and tissue residency. 41 Upon mycobacterial activation, MAIT‐cell gene expression profiles overlapped also with that of activated NK cells and conventional CD8+T‐cells, further suggesting MAIT‐cells as a functional bridge between innate immunity and adaptive immunity. 42
2.1. Contribution of MAIT‐cells to protective immunity against mycobacterial infections
Circulating MAIT‐cells were decreased in active TB patients compared with healthy controls, a trend also observed in HIV‐ and HIV/TB‐infected patients. In contrast, MAIT‐cells were enriched at the site of infection, suggesting preferential homing from the blood. 43 , 44 , 45 , 46 , 47 , 48 In a recent report, MAIT‐cells were enriched in bronchoalveolar lavage (BAL) fluids of active TB patients compared with uninfected individuals and were reactive to Mycobacterium smegmatis–infected antigen‐presenting cells only when MR1 was expressed. 49 However, a recent study conducted in a high endemic setting detected no differences in circulating MAIT‐cell frequencies when comparing TB patients with LTBI and healthy Mtb–exposed controls. 50 These differential frequencies of MAIT‐cells across different studies suggest a potential effect of (high) Mtb exposure or other environmental factors such as microbiota, nutritional status, co‐infections, or host genetics. In any case, these studies suggest that MAIT‐cells may not be an appropriate correlate of Mtb infection in high‐transmission areas. Moreover, increased susceptibility to meningeal TB in a Vietnamese cohort was associated with a single genetic polymorphism causing increased MR1 expression, while reduced MR1 expression of MR1 was associated with increased mortality. This suggests a role for MR1‐mediated antigen presentation and MAIT‐cell activation in human TB susceptibility, and perhaps also dissemination to the central nervous system. 51
Functionally, MAIT‐cells can be cytotoxic toward bacterially infected MR1‐expressing lung‐epithelial cells. 43 , 52 In recently Mtb‐exposed subjects resistant to infection, MAIT‐cells showed an activated phenotype with expression of granzyme B and IL‐2RA. 53 The reduced frequency of MAIT‐cells in the blood of active TB patients was linked to the elevated expression of exhaustion marker programmed cell death protein 1 (PD‐1) on MAIT‐cells 54 and to deficient MAIT‐cell production of IFN‐γ, TNF‐α, granulysin, granzyme B, and IL‐17F when exposed to antigen, compared with healthy controls. 55 , 56 In contrast, a recent study showed that IFN‐γ production by circulating MAIT‐cells in response to Mtb was higher in active TB patients and LTBI compared with uninfected contacts, although LTBI showed highest IFN‐γ production, suggesting a possible role for MAIT‐cells in infection control. 57 In another study, MAIT‐cells isolated from tuberculous pleural effusions displayed increased cytokine and cytotoxic responses to Mtb‐derived antigens compared with blood MAIT‐cells, mainly mediated by IL‐2, IL‐12, and IL‐18 signaling, supporting the importance of Mtb‐specific and bystander activation of MAIT‐cells at the site of infection. 58 Interestingly, the persistent absence of IFN‐γ release assay (IGRA) conversion in a sizeable fraction of highly Mtb‐exposed household contacts was associated with a reduced proportion of IL‐17–producing circulating MAIT‐cells compared with IGRA converters, an indication of the possible importance of migration of these cells to the infection site for bacterial control, although this could not be proven. 59 In children, where TB disease is usually the result of primary infection as opposed to the reactivation of latent infection predominantly seen in adults, active TB correlated with reduced MAIT‐cell numbers in blood and BAL fluid compared with LTBI and healthy children. 60 Recently, activated, proliferating, granzyme B–producing MAIT‐cells were identified in the blood of NHP after BCG vaccination and Mtb infection, in agreement with observations in humans. 61 In another study, a transient increase in MAIT‐cells was detected in BAL but not peripheral blood after protective intravenous BCG vaccination in NHP, suggesting that MAIT‐cell recruitment to the infection site might be involved in protection against aerosol Mtb challenge. 62 Exploration of lung tissue and TB granulomas of these Mtb‐infected macaques showed an uneven accumulation of activated MAIT‐cells. Kauffman et al found that MAIT‐cells were increased in the lungs of only half of the NHP at late time points of Mtb infection. Moreover, in TB granulomas only few and poorly activated MAIT‐cells were identified, which expressed low levels of granzyme B, suggesting a limited role for MAIT‐cells in Mtb control. 63 Bucsan et al, on the other hand, found increased MAIT‐cell frequencies with Th1 cytokine profiles in blood and BAL fluid of NHP only at early time points after Mtb infection. 64
Altogether, these studies underscore the pertinence of MAIT‐cells for mucosal immunity and the ambiguity of their role in local immune control of Mtb infection and TB disease progression in humans. Importantly, we must consider the spectrum, and the kinetics of Mtb infection and TB disease development when designing strategies to mobilize MAIT‐cells, as they might have an important short term and early role at the site of Mtb infection, with possibly reduced functionality in more advanced TB disease.
2.2. MAIT‐cell activation pathways and potential as vaccine or immunotherapeutic target
Studies in mice have shed further light on the function of MAIT‐cells during infection. MR1‐deficient mice showed increased susceptibility to Mycobacterium abscessus and Escherichia coli infections. 44 Follow‐up studies demonstrated a protective function of mouse MAIT‐cells against infections with Klebsiella pneumoniae, Mycobacterium bovis BCG, Francisella tularensis, and Legionella longbeachae. 65 , 66 , 67 , 68 In vitro activation of human MAIT‐cells primarily depended on MR1 antigen presentation and subsequent TCR signaling at early time points, while combined IL‐12/IL‐18 signaling was shown to be equally important at later time points, supporting a TCR‐independent mechanism of later‐phase MAIT‐cell stimulation. 69 This bystander activation mechanism was also observed in BCG‐specific MAIT‐cells, hinting to a role for these cells in innate immunity cytokine‐driven expansion in the response against Mtb infection. 70
The impaired control of bacterial loads in genetically MR1‐deficient mice compared with wildtype mice at early time points after Francisella tularensis infection provided strong evidence for a role of MAIT‐cells in early innate immunity and in initiating adaptive immune responses to chronic infection through IFN‐γ production and the recruitment of CD4+ and CD8+T‐cells. 67 The importance of MAIT‐cells in protection against bacterial infections was further substantiated by experiments in which MAIT‐cells were adoptively transferred into immunodeficient mice, leading to rescue from lethal Legionella infection. 68 Importantly, in vivo MAIT‐cell priming with 5‐OP‐RU and TLR9/2 agonists CpG or Pam2Lys increased protection against Legionella infection. 68 In contrast, 5‐OP‐RU–mediated expansion of MAIT‐cells prior to Mtb infection in mice was associated with delayed Mtb‐specific CD4+T‐cell priming and lack of protection, suggesting that MAIT‐cell priming might have pathogen‐specific effects. 71 During Mtb infection in vivo, the presence of inhibitory MR1‐ligands such as riboflavin and FO might counteract the effect of MAIT‐cell agonists, 72 resulting in a balanced MAIT‐cell response, which, however, might limit protection against Mtb despite successful vaccine‐mediated priming. In contrast, IL‐17A–mediated control of Mtb in vivo was achieved through administration of MAIT agonist during chronic infection, pointing to differential functions for MAIT‐cells during steady and inflammatory states. 71 In two recent studies, accumulation of MAIT‐cells in BCG‐ and Mtb‐infected mouse lungs was shown to be dependent on exposure to the MAIT‐cell–activating antigen 5‐OP‐RU and TLR2/6 agonist Pam2CSK4 or Pam2Cys. 73 , 74 However, this was associated with a reduced ability to control Mtb, and with increased levels of anti‐inflammatory cytokine IL‐10, adding to the complexity of targeting MAIT‐cells to enhance host protection against Mtb infection. 73 , 74 In the context of Mtb/SIV coinfection in NHP, MAIT‐cells were recruited to granulomas, but were functionally impaired in vitro and in vivo. 75 Impaired MAIT‐cell responses to Mtb were also observed in HIV‐infected patients and could be redressed through IL‐10 signaling blockade. 76 Therefore, a proper balance between induction and regulation of MAIT‐cells linked to the inflammatory environment during MAIT‐cell recruitment seems to be crucial for an optimal response during Mtb infection.
Additionally, host gut microbiota has been shown to contribute to MAIT‐cell function in the early protection of Mtb lung colonization in mice and humans. 53 , 77 Indeed, the development of MAIT‐cells that are relevant for tissue repair depended on the exposure to riboflavin‐derived antigens present in early‐life microbiota. 78 This is further supported by the finding that MAIT‐cell frequencies increased rapidly after birth and with age, regardless of BCG vaccination status, hinting to a role of gut microbiome colonization and increased microbial exposure in MAIT‐cell expansion. 79
In conclusion, although MAIT‐cells have the capacity to eliminate bacteria‐infected cells in vitro, their specific role in overall protection against Mtb in humans and NHP remains unresolved. Notably, MAIT‐cells might be particularly important for mucosal immunity at early time points after primary Mtb infection, by their mobilized effector functions or by regulating other adaptive immune cells (Table 1). Additional larger longitudinal studies will be necessary to unravel MAIT‐cells’ roles, specific effector functions, and homing behavior in host defense against mycobacterial infection, and to identify effective MAIT‐cell–targeting strategies to increase protection at different stages of Mtb infection or TB disease. Moreover, it will be necessary to evaluate MR1 ligands as vaccine components, in particular on the feasibility and potential for MAIT‐cell expansion upon vaccination. Presently, the possibility to target MAIT‐cell responses with other than whole‐cell vaccines hinges upon the development of stable MR1 ligands. Furthermore, enhancing MAIT‐cell priming with cytokine costimulation or blockade, perhaps immune‐checkpoint manipulation, might represent alternatives to improve protection against Mtb, although this remains to be evaluated.
3. TCRγδ T‐CELLS
TCRγδ T‐cells are CD3+T‐cells expressing Vγ and Vδ‐encoded TCR, and are unique in their capacity to recognize non‐peptidic phosphorylated intermediates of metabolic pathways, known as phosphoantigens, presented via butyrophilin (BTN). 80 BTN‐mediated presentation requires binding of phosphoantigen to the intracellular domain of BTN3, known as B30.2, inducing conformational changes in the BTN2/3 extracellular domains recognized by TCRγδ T‐cells. 81 , 82 TCRγδ T‐cells react to pathogen‐derived phosphoantigens such as isopentenyl pyrophosphate (IPP), 83 (E)‐4‐hydroxy‐3‐methyl‐but‐2‐enyl pyrophosphate (HMBPP), 84 and 6‐O‐methylglucose lipopolysaccharides (mGLP), the latter being able to promote Mtb inhibitory TCRγδ T‐cell activity in vitro. 85 Moreover, a small population of TCRγδ T‐cells reactive to CD1d‐presented α‐GalCer has been described 86 ; also, Mtb‐derived protein antigens recognized by TCRγδ T‐cells have been identified, further broadening the possibilities to activate TCRγδ T‐cell in the context of Mtb infection. 87 , 88
TCRγδ T‐cells were more abundant in peripheral blood of TB patients compared with healthy controls and to patients with other lung infections or malignancies. 89 Repertoire sequencing allowed the detection of clonal expansion of TCRγδ T‐cells in blood and lungs of TB patients. 87 , 90 Reactivity of TCRγδ T‐cells to Mtb in vitro was higher in individuals with less severe TB and was associated with the production of anti‐microbial IFN‐γ, GM‐CSF, IL‐3, and TNF‐α. 91 In a different study, TB severity was associated with Vγ9Vδ2+ T‐cell production of IL‐10, suggesting an immunoregulatory role for these cells, which could be unfavorable for protection. 92 A predominant subset of Vγ9Vδ2+ TCRγδ‐expressing cells representing up to 5% of circulating T‐cells in healthy individuals can expand in patients with bacterial and parasitic infections and represent up to 20%‐50%, highlighting their participation in immune responses against infections. 93 Supporting a possibly protective role for TCRγδ T‐cells during Mtb infection, IFN‐γ–producing circulating Vγ9Vδ2+ effector T‐cells were reduced in acute pulmonary TB patients compared with healthy donors, 94 and decreased levels of circulating Vδ2+ TCRγδ T‐cells were correlated with more severe pulmonary lesions in TB patients. 92 , 95 Similarly, hyporesponsive Vγ9Vδ2+ were identified in TB patients’ lungs. 96 A more recent study showed an association between the establishment of latent Mtb infection in exposed household contacts and a defective activation of TCRγδ T‐cells, characterized by impaired upregulation of PD‐1 and CD69, an effect which was not observed in persistently IGRA‐negative household contacts. 53
In NHP, where recognition of phosphoantigens by TCRγδ T‐cells is conserved, 97 protection against Mtb challenge was associated with a rapid and transient expansion of BAL Vγ9+T‐cells after intravenous BCG vaccination. 62 The expansion of Vγ2Vδ2+ TCRγδ T‐cells associated with Mtb clearance and increased survival after challenge of BCG‐vaccinated macaques was linked to Th17 cytokines. 98 In TB patients, frequencies of circulating IL‐17–producing Vγ9Vδ2+ T‐cells were increased compared with controls. 99 Upon BCG vaccination, an increased number of circulating IFN‐γ–producing TCRγδ T‐cells were detected in newborns and infants. 100 , 101 Importantly, TCRγδ T‐cell responses to secondary stimulation were enhanced in adults after BCG vaccination compared with unvaccinated controls. 102 These studies suggest a memory TCRγδ T‐cell function supporting the potential of TCRγδ T‐cells as vaccine targets.
Mechanistically, the coculture of Vγ9Vδ2+ T‐cells with Mtb‐ or BCG‐infected macrophages led to control of intracellular mycobacterial growth and to killing of infected macrophages through perforin and granulysin production in vitro, 103 , 104 or by inducing monocyte TNF‐α production through granzyme A. 105 Moreover, Mtb phosphoantigen–activated Vγ9Vδ2+ T‐cells could induce DC maturation through TNF‐α and IFN‐γ production. 106 The early IFN‐γ production by TCRγδ T‐cells in mice lungs was associated with optimal DC activation in vivo. 107 The helper function of Mtb‐specific Vγ9Vδ2+ T‐cell lines was assessed in vitro, showing that CD40‐CD40L interactions were necessary for Vγ9Vδ2+ T‐cells to promote TCRαβ T‐cell–mediated control of Mtb infection. 108 A regulatory function for TCRγδ T‐cells has also been suggested, as IL‐22–producing T‐cells associated with severe TB were downmodulated by phosphoantigen‐stimulated TCRγδ T‐cells, through an IFN‐γ–mediated mechanism. 109 Thus, in attempts to identify the mechanisms involved in TCRγδ T‐cell–mediated protection against Mtb, these collective studies suggest that cytotoxic, regulatory, and helper effector functions of TCRγδ may all be involved (Table 1).
Several studies have explored a number of different strategies to target TCRγδ T‐cell responses to improve protection against Mtb before and after infection. NHP vaccinated with ESAT6‐Ag85B fusion protein combined with phosphoantigens induced a fast Th1‐like TCRγδ T‐cell expansion upon immunization. 110 Treatment of NHP with HMBPP phosphoantigen in combination with IL‐2 led to a long‐lasting Vγ9Vδ2 effector‐memory T‐cells expansion in the lungs, with the capacity to produce IFN‐γ and perforin upon restimulation. 111 In another study, Vγ9Vδ2+ T‐cells induced by an attenuated HMBPP‐producing L monocytogenes contributed to reduced pulmonary and systemic bacterial burden in Mtb‐challenged NHP. This vaccine elicited Vγ9Vδ2+ T‐cells capable of inhibiting intracellular Mtb growth through IFN‐γ and perforin production. 112 , 113 Postinfection, TCRγδ‐centered therapy has also been explored through the adoptive transfer of Vγ9Vδ2+ T‐cells after Mtb infection in NHP, inducing decreased pulmonary and systemic bacterial burden and attenuated TB morbidity. 114 The possibility of modulating TCRγδ T‐cell responses with cytokines has additionally been explored in different studies. For instance, IL‐15 enhanced the proliferation of TCRγδ T‐cells and together with IL‐12 induced IFN‐γ production in vitro. 115 IL‐12–mediated expansion of Vγ9Vδ2 T‐cells in vitro led to IFN‐γ or TNF‐α–dependent control of intracellular mycobacteria growth. 116 In NHP, phosphoantigen administration in combination with IL‐2 during early pulmonary Mtb infection induced protective multifunctional IFN‐γ, granulysin, and perforin‐producing Vγ9Vδ2+ T‐cells in blood and lungs, with IL‐12 production by these cells possibly further enhancing conventional T‐cell responses. 117 Therefore, diverse strategies have shown promise to target protective TCRγδ T‐cell responses against Mtb, both pre‐infection and postinfection.
4. CD1‐RESTRICTED T‐CELLS
T‐cell recognition of the molecularly highly complex and uniquely structured Mtb envelope is possible through presentation of lipid‐derived antigens via the CD1 family of monomorphic antigen‐presenting molecules. Lipids are essential components of mycobacterial cell envelopes and can therefore represent unique targets for DURT‐mediated immunity against Mtb.
The CD1 family includes CD1a, CD1b, and CD1c (group 1), and CD1d (group 2) 118 , 119 molecules, complemented by the intracellular lipid transfer protein CD1e (group 3), which is not directly involved in antigen presentation. 120 Group 1 and group 2 CD1 molecules can present antigen to CD4+ and CD8+T‐cells (as well as in some cases double‐negative T‐cells), and to both TCRαβ‐ and TCRγδ‐expressing T‐cells. CD1 molecules recycle in the lysosomes where they encounter phagocytosed bacteria, allowing them to bind lipids and return to the cell surface for presentation. 121 CD1a recycles via early endosomes, 122 while cytoplasmic tyrosine motifs localize CD1b to late endosomes and lysosomes for lipid loading. 123 CD1c and CD1d are instead regulated by adapter protein 2 (AP‐2) to survey different intracellular compartments, and localize to early and late endosomes. 124 Group 2 or CD1d‐restricted T‐cells are commonly known as natural killer T (NKT) cells and can be further classified on antigen specificity and on TCR diversity.
Despite their high structural similarity, the presentation of lipid antigens via group 1 and group 2 CD1 molecules activates functionally distinct CD1‐restricted T‐cells (Table 1). The presence of lipid‐specific polycytotoxic T‐cells in peripheral blood and BAL was associated with Mtb infection control. 125 The expression of CD1b and CD1d on DCs and macrophages in human TB granulomas supports a role for lipid‐specific T‐cell responses during TB and hints to the possibility of modulating their response to enhance immunity. 126 , 127
4.1. Function of group 1 CD1‐restricted T‐cells and potential as vaccine targets
CD1a‐restricted T‐cells can recognize Mtb‐derived lipopeptide dideoxymycobactin (DDM). 128 , 129 CD1b‐restricted T‐cell responses have been defined mostly in the context of mycolic acid (MA), 130 glucose monomycolate (GMM), 121 sulfoglycolipids (SGLs, Mtb‐specific), 131 and glycerol monomycolate (GroMM) 132 recognition. GMM‐reactive T‐cells have been specifically associated with active mycobacterial infection, as its synthesis requires mycobacterial mycolate and host glucose. 133 CD1c‐restricted T‐cells can respond to isoprenoid glycolipids and mycoketide antigens. 134 , 135 The development of lipid‐loaded tetramers for studies focused around group 1 CD1‐restricted T‐cells facilitated the demonstration of comparable frequencies of lipid‐reactive, CD1a‐, CD1b‐, and CD1c–restricted T‐cells in TB patients or LTBI. 136 , 137 , 138
Several studies have explored the role of group 1 CD1‐restricted T‐cells in immunity against Mtb. Initial work demonstrated Mtb‐specific Th1‐like cytokines and granulysin‐mediated cytotoxicity by CD1‐restricted T‐cell clones in vitro. 119 , 139 CD1b‐restricted CD8+T‐cells from Mtb‐infected patients, but not uninfected controls, produced IFN‐γ upon SGL stimulation leading to intracellular Mtb killing. 131 Expansion of CD1‐restricted T‐cells was associated with bacterial burden, as MA‐stimulated circulating and BAL T‐cells from TB patients but not BCG‐vaccinated healthy controls produced IFN‐γ and IL‐2, a response which declined following completion of treatment. 140 Similarly, activated GMM‐specific CD1b‐restricted T‐cells produced proinflammatory cytokines IFN‐γ and TNF, supporting their anti‐microbial effector function. 141 On the other hand, CD1b‐restricted GroMM‐specific CD4+T‐cells were detected in BCG‐vaccinated and LTBI but not active TB patients, suggesting that these cells might be functionally relevant during Mtb exposure and disappear from the circulation or become anergic or exhausted during active TB. 132 Similarly, CD1b‐restricted T‐cells were associated with Mtb exposure rather than TB disease as higher frequencies of MA‐ and GMM‐loaded CD1b tetramer+ cells were identified in highly Mtb‐exposed individuals compared with low or unexposed controls, while no differences were observed between TB patients, LTBI, and uninfected individuals. 142 Ex vivo profiling of GMM‐specific T‐cells in healthy and Mtb‐infected individuals showed a polyfunctional CD4+T‐cell phenotype expressing CD40L, IFN‐γ, IL‐2, TNF‐α, and IL‐17a. 143 Moreover, lipid‐reactive polycytotoxic CD8+T‐cells capable of controlling intracellular Mtb growth by expressing perforin, granzyme B, and granulysin were found in BAL‐cells from LTBI. 125 The relevance of CD1‐mediated immunity to Mtb is further supported by population genetic studies in Vietnam in which increased TB susceptibility was associated with an intronic CD1A polymorphism linked to functional deficiency. 144 GMM‐specific T‐cells from LTBI were shown to persist and express the memory marker CD45RO, 145 and MA‐specific T‐cells could be expanded 1‐2 years after curative treatment, indicating memory function 140 and supporting the potential for targeting these cells by vaccination. Altogether, these studies show that lipid‐reactive CD1‐restricted T‐cells are an important component of the cellular immune response to Mtb.
Group 1 CD1 molecules are naturally expressed in guinea pigs, a TB model in which vaccination with mycobacterial lipids led to a reduction in Mtb burden and lung pathology after aerosol challenge. 146 The importance of CD1‐restricted T‐cells for protection against Mtb infection in vivo is further substantiated by studies in transgenic mouse models expressing human CD1 molecules. 147 Adoptive transfer of MA‐specific CD1b‐restricted T‐cells prior to Mtb challenge showed that these cells played a role in protective immunity against Mtb infection through TNF‐α, IFN‐γ, IL‐2 production, and the expression of the cytotoxic granule release–associated membrane marker CD107a. 148 Optimized intracellular delivery of MA through polymeric micellar nanocarriers elicited potent CD1‐restricted T‐cells in mice, although it remains to be evaluated if this increased protection against Mtb. 149 Altogether, these studies show that lipid‐reactive CD1‐restricted T‐cells are important players in immunity to, and in protection against, Mtb infection and that lipid‐based vaccines, or whole‐cell vaccines containing mycobacterial lipids, are promising strategies to improve immune protection against Mtb infection.
Structural analyses have shown that both the antigen‐exposed polar cap and the distal hydrophobic antigen regions buried within the CD1 groove influence TCR recognition. 150 , 151 , 152 Simplified synthetic SGL variants have been proposed as potential vaccine components to prime CD1‐restricted T‐cells. 153 These and future developments for lipid antigen production, together with the understanding of TCR recognition requirements, will support the potential to administer these ligands as candidate vaccines targeting CD1‐restricted T‐cells against Mtb.
4.2. (invariant) NKT‐cells in immunity against Mtb
Type I or invariant NKT (iNKT) cells recognize α‐galactosylceramide (α‐GalCer) or phosphatidylinositol mannoside (PIM) 154 and are defined by conserved TCRα and TCRβ chains predominantly encoded by TRAV10 joint to TRAJ8 and TRBV25. 155 , 156 In contrast, type II NKT‐cells include CD1d‐restricted T‐cells with diverse TCRs that are responsive to Mtb‐derived phosphatidylglycerol, diphosphatidylglycerol, and phosphatidylinositol. 157
Accumulating evidence highlights the importance of iNKT‐cells for immune control of Mtb. Several studies reported reduced numbers of circulating iNKT‐cells in active TB patients compared with LTBI and healthy controls. 158 , 159 , 160 Interestingly, circulating iNKT‐cells in TB patients showed increased CD38, CD69, and HLA‐DR expression compared with healthy controls, suggesting an activated phenotype. 48 , 160 Moreover, iNKT‐cells from active TB patients, contrary to LTBI, showed a higher PD‐1 expression, which was linked to reduced reactivity to α‐GalCer and increased iNKT‐cell apoptosis. 48 , 161 In NHP, which have conserved primate CD1 and TCR sequences, 162 an increased frequency of circulating iNKT‐cells was linked to improved control of Mtb infection and disease. 163 In line with this, at TB diagnosis in humans, higher counts of NKT‐cells, including cells that do not express an invariant TCR, strongly correlated with faster responses to antibiotic treatment. 164 Higher PD‐1 expression on NKT‐cells correlated with increased bacillary loads in TB patients, and PD‐1 blockade was associated with increased NKT‐cell survival and function. 165 Furthermore, NKT‐cells isolated from pleural fluid from TB patients expressed not only Th1 and Th17 cytokines, but also IL‐21 and CXCR5 upon PPD stimulation, and could induce B‐cell function in vitro. 166 Altogether, these studies suggest that iNKT and NKT‐cells could play an important role in the control of Mtb infection through distinct and complementary mechanisms.
An immunoregulatory role for iNKT‐cells was suggested after BCG‐infected NKT‐cell deficient mice showed no differences in bacterial load but greater lung pathology compared with wildtype mice. 167 Although neither CD1d nor NKT‐cell–deficient mice presented increased susceptibility to Mtb infection, 168 , 169 adoptive transfer of iNKT‐cells demonstrated their contribution to the control of Mtb replication in vivo by recognition of infected macrophages. 170 In this study, iNKT‐cell activation was shown to be TCR‐independent but IL‐12 and IL‐18 induced, which triggered IFN‐γ production. Similarly, induction of NKT‐like cells in humans vaccinated with the subunit vaccine candidate H4:IC31 was associated with TCR‐independent, cytokine‐mediated activation. 171 In the absence of IL‐12/IL‐18, Mtb control by murine iNKT‐cells depended on CD1d and granulocyte‐macrophage‐colony‐stimulating factor (GM‐CSF). GM‐CSF is associated with control of intracellular mycobacterial proliferation in vitro and with protective immunity in mice, 172 , 173 and was shown to be induced by Mtb antigens in in vitro human studies. 174 Anti‐microbial activity of α‐GalCer–stimulated human iNKT‐cell clones in vitro was associated with IFN‐γ and granulysin production. 127
Modulation of iNKT‐cell responses to enhance protection against Mtb was explored through administering mycobacterial‐derived lipids. Reduced bacterial loads and lung pathology were observed after Mtb challenge of guinea pigs vaccinated with PIM and SGL, lipids presented by CD1d and CD1b, respectively. 175 In mice, vaccination with α‐GalCer–containing BCG led to enhanced DC maturation and increased CD8+T‐cell priming, which correlated with increased protection against Mtb compared with BCG alone, suggesting a role for iNKT‐cells in bridging innate and adaptive immunity. 176 Similarly, α‐GalCer–adjuvanted Ag85B and ESAT‐6 subunit vaccines induced stronger CD4+ and CD8+T‐cell responses accompanied by increased local and systemic protection against Mtb, compared with subunit vaccines or BCG alone. 177 α‐GalCer also showed a protective effect against Mtb when administered, alone or in combination with antibiotics, shortly after infection, reminiscent of trained immunity effects likely through activating NKT‐cells. 178 , 179 , 180 However, at later time points, the treatment had no benefits, possibly as a consequence of apoptotic or irresponsive NKT‐cells, underscoring their relevance as an early immune response component against Mtb infection, akin to the above observations for MAITs. Administration of a novel B‐cell targeting vaccine expressing ESAT6 and loaded with α‐GalCer showed both preventive and therapeutic effects in M kansasii infected mice, mediated by enhanced B‐cell and CD4+T‐cell function. 181 Additional efforts are underway to design lipid‐based vaccine strategies able to induce NKT‐cell responses. 182
5. NK CELLS AND INNATE LYMPHOID CELLS (ILCS) IN IMMUNITY AGAINST MTB
5.1. NK cells
NK cells have been observed within granulomatous lesions in humans, suggesting their contribution at the infection site. 183 A large multi‐cohort study showed that circulating NK‐cell frequencies were increased during LTBI, decreased during TB disease, and normalized after treatment, supporting a role for NK cells in Mtb infection control. 184 Importantly, longitudinal analysis identified that circulating NK‐cell frequencies could indeed be a correlate of TB disease progression and an indicator of recovery after treatment. Moreover, functionally impaired NK‐cell function was associated with reactivation of TB disease. 185 NK cells from LTBI showed downregulation of cytotoxic receptor NKp46 compared with uninfected controls. 186 In vitro, the expression of NK‐cell–activating receptors was upregulated upon coculture with Mtb‐infected cells and was associated with antigen‐specific NK‐cell–mediated lysis. 187 , 188 In BCG‐vaccinated infants and children, NK cells were identified as one of the main sources of IFN‐γ, highlighting their functional importance in the early immune responses against Mtb. 101 Although frequencies of peripheral NK cells remained unchanged, BCG vaccination in healthy adults led to increased production of proinflammatory cytokines IL‐1β, IL‐6, and TNF‐α after in vitro exposure to Mtb or unrelated stimuli, reminiscent of trained immunity. 189 Importantly, memory‐like antigen‐specific NK cells isolated from TB patients’ pleural fluid were shown to produce IL‐22 in response to BCG and Mtb antigens, only when expressing CD45RO. 190 In a recent study, supporting the potential for vaccine‐induced memory NK cells, BCG vaccination of mice led to the development of IL‐21–dependent IFN‐γ–producing memory‐like NK cells, which were shown to provide protection against Mtb challenge. 191 Indeed, NK‐cell memory phenotypes and their potential as vaccine target have been described in the context of diverse infectious diseases. 192 In adults revaccinated with BCG, NK‐cell effector responses were long‐lasting and IFN‐γ production by these cells was reported to be dependent on IL‐12 and IL‐18 signaling. 193 Altogether, these studies highlight NK cells as potentially important players not only in the innate but also in trained innate memory immune responses to mycobacteria. However, in a recent study where intravenous administration of BCG was associated with protection against TB in NHP, the lack of innate cytokine production by PBMCs suggested that this response might be redundant for protection. 62
BCG vaccination of mice induced NK‐cell production of IL‐22 and IFN‐γ associated with reduced numbers of immunosuppressive Tregs and reduced bacterial burden after Mtb challenge. 194 NK cells were able to lyse extracellular mycobacteria through the release of cytotoxic granules 195 and to induce phagolysosomal fusion and Mtb growth control in infected phagocytes via IL‐22. 196 NK cells activated by Mtb‐infected monocytes could lyse expanded Tregs in vitro, 197 and multidrug‐resistant TB was associated with decreased NK‐cell function and elevated Treg expansion. 198 Moreover, NK cells induced TCRγδ T‐cell proliferation via cell‐to‐cell contact or TNF‐α, GM‐CSF, and IL‐12 signaling. 199 Furthermore, NK cells were shown to exert regulatory functions on CD8+T‐cells, enhancing their cytotoxicity via IFN‐γ, IL‐15, and IL‐18. 200 In the absence of T‐cell responses, IL‐12–mediated NK‐cell production of IFN‐γ can play an important role in protection against Mtb in mice, a mechanism which might be relevant in patients with genetic or acquired immunodeficiencies. 201 CMV antigen presentation via the non‐classical HLA class Ib molecule HLA‐E has been shown to influence NK‐cell expansion and to induce effector functions in NKG2C‐expressing NK cells in vitro. 202 Differential recognition of HLA‐E–presented peptide variants has been shown to modulate NK‐cell adaptive immune responses, hinting to a possible mechanism through which protective NK cells could be induced. 203 This, together with the potential to target HLA‐E–restricted T‐cells discussed above, further underscores the importance of defining HLA‐E–presented peptides and the requirements for TCR versus NKG2A/C recognition.
Altogether, NK cells present a functional spectrum of cells associated with protection to Mtb, via cytotoxic, regulatory, and memory activities in coordination with or independent from other immune cells (Table 1). The versatility for NK‐cell induction through innate and adaptive mechanisms makes them an appealing cell target for mobilization via new vaccines.
5.2. ILCs
ILCs lack rearranging antigen receptors typically found on T‐cells and B‐cells, and their function, mediated by cytokine stimulation, is important for mucosal immunity. Three different subsets are described based on their cytokine profiles: ILC1s share features with NK cells and respond to IL‐12, IL‐15, and IL‐18 stimulation by producing IFN‐γ; ILC2s are triggered by IL‐25, IL‐33, and thymic stromal lymphopoietin (TSLP) leading to a type 2 cytokine profile; and ILC3s, as the innate counterpart of Th17 cells, respond to IL‐23 and IL‐1β to secrete IL‐17 and IL‐22. 204 ILCs are involved in promoting protective immune responses to pathogens and in maintaining tissue integrity and homeostasis. 205 Circulating ILCs have been shown to be reduced in TB patients compared with healthy controls, and to be restored following treatment. 206 The accumulation of ILC3s in infected lungs, apparently recruited by CXCL13‐CXCR5 signaling to localize to TB granulomas, was associated with Mtb control in humans and mice, mediated by IL‐23–induced IL‐17 and IL‐22. 206 Transfer of ILC3s or IL‐22 treatment reduced inflammation during Mtb infection resulting in increased survival of type 2 diabetic mice, suggesting a role for ILC3s in protection against TB disease. 207 Supporting the possibility of vaccine‐mediated induction of ILCs, IFN‐γ–producing ILC1s and ILC3s were increased in lungs and lymph nodes of mice after BCG vaccination, a response which was more prominent after mucosal BCG administration compared with other routes. 208 Altogether, ILCs, and in particular ILC1s and ILC3s, are recently identified players participating in the human and mouse immune responses to TB, and future studies must delineate their role in vaccine‐induced immunity (Table 1).
6. TARGETING DURTS, NK CELLS, AND ILCS IN PREVENTIVE OR THERAPEUTIC VACCINATION AGAINST MYCOBACTERIA
DURTs, NK cells, and ILCs can help controlling Mtb and may synergize with classical Th1 T‐cells and B‐cells (Table 1). They may therefore be considered as interesting targets for vaccination to prevent infection or disease, or be mobilized via therapeutic strategies in treatment‐resistant TB patients, for example, due to multidrug resistance.
6.1. As vaccine targets to prevent infection (POI) or to prevent disease (POD)
Very recently, BCG revaccination in a phase‐2 clinical trial showed efficacy in protecting Mtb‐uninfected adolescents from acquiring sustained Mtb infection, suggesting POI by vaccination is possible. 209 Whole‐cell vaccines, such as BCG, grant the possibility to target multiple cell populations simultaneously for induction of innate and adaptive immune responses, including DURTs, NK cells, and ILCs, through presenting alternative and diverse antigens. In contrast, subunit or virally vectored vaccines for TB have mostly focused on antigens inducing CD4+T‐cell responses essential to protection, among others through IFN‐γ signaling, 210 although IFN‐γ was not always a correlate of protection against Mtb infection and TB disease in clinical trials. 211 , 212 Recently, a phase 2 clinical trial with the subunit vaccine M72/AS01E reduced progression to TB by 50% in LTBI, although the lack of an adjuvant‐alone control arm precluded the precise definition of the vaccine‐induced mechanisms of protection. 213 Nevertheless, successful recent and future vaccine trials will allow the immunological dissection of protective responses and we strongly support assessment of the various DURT, ILC, and NK‐cell populations in that context.
Advances in our understanding of the role played by DURTs, NK cells, and ILCs in protection against Mtb infection and TB disease now provide new and testable opportunities for vaccine and therapeutic targeting. Considering the effector, regulatory, and memory activities that have been identified for these unconventional immune cells, a whole‐cell vaccine or combination of diverse antigens selected to induce an optimized and balanced response could represent an effective strategy. Nevertheless, the complex interactions occurring in vivo need to be addressed carefully. For instance, in addition to classical immune responses, BCG vaccination could induce trained immunity on NK cells, ILC‐production of IFN‐γ, and an array of DURT‐mediated immune responses. 62 , 101 , 171 , 208 , 214 , 215 The delivery route of the vaccine is an important factor to consider, since intravenous BCG vaccination of NHP induced the transient recruitment of MAIT‐cells and Vγ9+ TCRγδ T‐cells to the lung, and was associated with protection to Mtb challenge. 62 Pulmonary mucosal BCG delivery was also shown to prevent infection following repeated limiting‐dose Mtb challenge, but DURT‐cells were not investigated. 216 Other whole‐cell vaccine candidates currently undergoing clinical testing are the genetically attenuated Mtb vaccine MTBVAC 217 and the recombinant BCG vaccine VPM1002. 218 However, their immunogenicity has been mostly evaluated in the context of Th1 and Th17 cytokine production. The upcoming efficacy trials for these vaccine candidates will likely contribute to the dissection of the immune cells involved in protection against Mtb infection and TB disease, including the role of DURTs, NK cells, and ILCs.
Alternatively to live whole‐cell vaccines, non‐replicating virally vectored vaccines are likely safer and can be designed to express a particular set of relevant antigens. The RhCMV‐based vaccine, as discussed above, induced effector‐memory responses and showed efficacy in preventing Mtb infection and TB disease in NHP. 27 Notably, HLA: classical versus non‐classical (HLA‐E) restriction of immune responses elicited by RhCMV‐vectored vaccines could be selected based on the type of RhCMV strain used. 27 Similarly, Vγ9Vδ2+ effector‐memory T‐cell induction by an attenuated HMBPP‐producing L monocytogenes strain correlated with increased protection in Mtb‐challenged NHP. 113 Further attempts to modify these vaccine delivery modalities and inserted antigens could help inducing increasingly diversified immune responses complementing and enhancing their vaccine efficacy.
Lipid and phosphoantigens inducing CD1‐restricted and TCRγδ T‐cells have been defined and synthesized and, in principle, are available to be included as vaccine components. 85 , 152 , 153 Of particular interest in this context is the design of efficient nanocarrier systems for intracellular delivery of antigens. 149 Moreover, efforts are underway to define novel HLA‐E–presented peptides with optimal capacity to induce T‐cells while simultaneously triggering a favorable NK‐cell response. 219 Furthermore, MR1 ligands for MAIT‐cell induction have also been identified, although their potential to induce protective responses in vivo remains to be elucidated. The lack of stability of MR1 ligands could be counteracted by modulating MAIT‐cell responses through BCG vaccination, 62 via the microbiota, 78 or via TCR‐independent mechanisms. 70 Similarly, iNKT‐cells have been shown to be activated in mice through TCR‐independent mechanisms mediated by IL‐12 and IL‐18 signaling, 170 and protective TCRγδ T‐cells could also be induced with the combination of phosphoantigen and IL‐2 in NHP, 117 supporting the use of cytokines for the induction or modulation of DURT responses against Mtb. Also, ILCs have the potential to be induced through targeted or bystander cytokine signaling. 206 , 208 The induction of protective iNKT‐cells was also possible through α‐GalCer–adjuvanted subunit vaccine 177 or α‐GalCer insert in a modified BCG vaccine, 176 further supporting the combination of antigens for targeting diverse immune responses. Although traditionally considered a component of the innate immune system, NK cells have been shown to have a memory phenotype inducible via trained immunity or antigenic triggering. 189 , 190 Opportunities to target protective NK cells in the context of TB could thus be inspired by the field of cancer immunology and HIV infection, where soluble agents, cytokines, and antibodies are used to modulate NK‐cell function, as well as the design of chimeric antigen receptor (CAR) engineered NK cells. 220 Similar approaches are also being developed to target NKT‐cells against tumors 221 and could potentially be expanded to target these and other DURTs in the context of TB.
Altogether, diverse strategies are currently being investigated to induce immune responses, which could contribute to protection against Mtb via targeting DURTs, NK cells, and ILCs in conjunction with more classical responses (Figure 1). The lack of polymorphism in antigen presentation molecules and the presence of innate‐like features characteristic of these immune cell populations, along with their polyfunctional phenotypes in response to Mtb, represent important advantages and highlight their potential in vaccine and immunotherapeutic strategies.
6.2. TB Treatment
Especially relevant in the context of multidrug‐resistant and extensively multidrug‐resistant TB are vaccine strategies designed to improve treatment outcomes in active TB, such as the liposomal vaccine candidate RUTI, based on detoxified fragments of Mtb‐inducing polyantigenic immune responses. 222 Although knowledge on the efficiency of therapeutic vaccines is limited, the high diversity in the reactivity of DURTs could potentially be exploited to induce protective responses to Mtb antigens that are particularly produced during active TB. This has been explored through vaccination in the context of MAITs in mice, 73 as well as through adoptive transfer of previously expanded TCRγδ T‐cells in NHP. 114 Here too, the design of genetically engineered T‐cells could provide a possibility for DURTs and NK cells to improve treatment outcomes in active TB. Moreover, α‐GalCer administration in combination with antibiotics already has shown to improve outcome in Mtb‐infected mice. 178
7. CONCLUDING REMARKS
Targeting DURTs, NK cells, and ILCs opens a range of possibilities to induce immune responses against Mtb infection and TB disease that complement each other and classical immune responses. These unconventional subsets are considered to contribute important, additional protective immunity in TB and other mycobacterial infectious diseases such as leprosy 159 , 223 and non‐tuberculous mycobacteria infections, which can be difficult to treat. The ongoing challenge to define correlates of protection could be addressed, in part, by exploring the role of these unconventional responses in successful efficacy trials, and bridging human trials to detailed studies in NHP. Whether it is through whole‐cell vaccines, vectored vaccines, subunit vaccines, or a combination of these and other approaches, future studies must consider the contribution of DURTs, NK cells, and ILCs to optimal protective immunity to mycobacterial infectious diseases, a plague to mankind and animals.
8.
FIGURE 1.
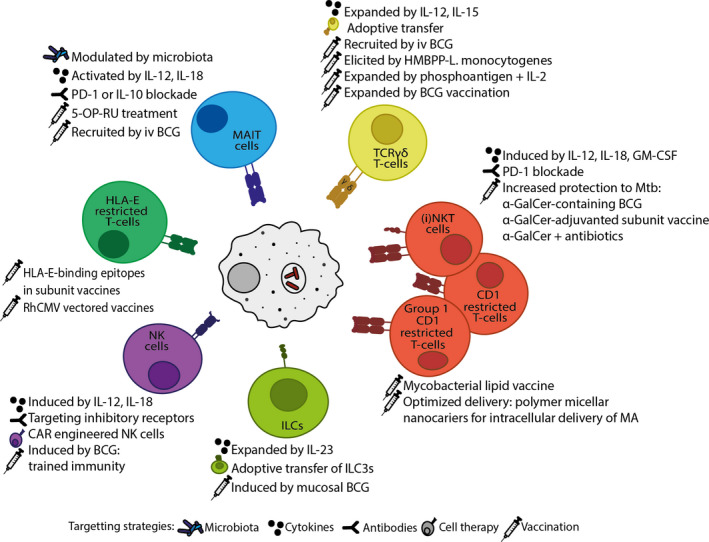
DURTS, NK‐cells and ILCs as unconventional targets in TB. DURTs, NK‐cells and ILCs are important unconventional contributors involved in protective immunity in TB and other mycobacterial infections. Various potential approaches are being investigated to improve targeting DURTs, NK cells and ILCs for increased protection against Mtb‐infection and TB disease. These include vaccine strategies ranging from classical BCG and modified whole cell vaccines to antigen‐expressing viral vectors and subunit vaccines. Treatment with soluble agents such as cytokines and antibodies to increase the immune response can be complemented with the adoptive transfer of certain unconventional immune cells
ACKNOWLEDGMENTS
This study was supported by European Union's Horizon 2020 under the Marie Skłodowska‐Curie Grant Agreement Nos. 707404 and 793027 (to PR); the Netherlands Organization for Scientific Research (NWO‐TOP Grant Agreement No. 91214038); and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Numbers R21AI127133 and R01AI141315. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or any funder. The authors declare no conflict of interest.
Ruibal P, Voogd L, Joosten SA, Ottenhoff THM. The role of donor‐unrestricted t‐cells, innate lymphoid cells, and NK cells in anti‐mycobacterial immunity. Immunol Rev. 2021;301:30–47. 10.1111/imr.12948
This article is part of a series of reviews covering Immunity to Mycobacteria appearing in Volume 301 of Immunological Reviews.
DATA AVAILABILITY STATEMENT
Data discussed were all retrieved from published literature as specified in the reference list.
REFERENCES
- 1. André P, Denis C, Soulas C, et al. Anti‐NKG2A mAb is a checkpoint inhibitor that promotes anti‐tumor immunity by unleashing both T and NK cells. Cell. 2018;175(7):1731‐1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Braud V, Jones EY, McMichael A. The human major histocompatibility complex class Ib molecule HLA‐E binds signal sequence‐derived peptides with primary anchor residues at positions 2 and 9. Eur J Immunol. 1997;27(5):1164‐1169. [DOI] [PubMed] [Google Scholar]
- 3. Lee N, Llano M, Carretero M, et al. HLA‐E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc Natl Acad Sci. 1998;95(9):5199‐5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sharpe HR, Bowyer G, Brackenridge S, Lambe T. HLA‐E: exploiting pathogen‐host interactions for vaccine development. Clin Exp Immunol. 2019;196(2):167‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ulbrecht M, Martinozzi S, Grzeschik M, et al. Cutting edge: the human cytomegalovirus UL40 gene product contains a ligand for HLA‐E and prevents NK cell‐mediated lysis. J Immunol. 2000;164(10):5019‐5022. [DOI] [PubMed] [Google Scholar]
- 6. Pietra G, Romagnani C, Mazzarino P, et al. HLA‐E‐restricted recognition of cytomegalovirus‐derived peptides by human CD8+ cytolytic T lymphocytes. Proc Natl Acad Sci. 2003;100(19):10896‐10901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heinzel AS, Grotzke JE, Lines RA, et al. HLA‐E–dependent Presentation of Mtb‐derived Antigen to Human CD8+ T Cells. J Exp Med. 2002;12900(11):1473‐1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Joosten SA, van Meijgaarden KE, van Weeren PC, et al. Mycobacterium tuberculosis peptides presented by HLA‐E molecules are targets for human CD8+ T‐cells with cytotoxic as well as regulatory activity. PLoS Pathog. 2010;6(2):e1000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Meijgaarden KE, Haks MC, Caccamo N, Dieli F, Ottenhoff THM, Joosten SA. Human CD8+ T‐cells recognizing peptides from Mycobacterium tuberculosis (Mtb) presented by HLA‐E have an unorthodox Th2‐like, multifunctional, Mtb inhibitory phenotype and represent a novel human T‐cell subset. PLoS Pathog. 2015;11(3):1‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salerno‐Gonçalves R, Fernandez‐Viña M, Lewinsohn DM, Sztein MB. Identification of a Human HLA‐E‐Restricted CD8 + T cell subset in volunteers immunized with Salmonella enterica Serovar Typhi strain Ty21a typhoid vaccine. J Immunol. 2004;173(9):5852‐5862. [DOI] [PubMed] [Google Scholar]
- 11. McMurtrey C, Harriff MJ, Swarbrick GM, et al. T cell recognition of Mycobacterium tuberculosis peptides presented by HLA‐E derived from infected human cells. PLoS One. 2017;12(11):e0188288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Joosten SA, Sullivan LC, Ottenhoff THM. Characteristics of HLA‐E restricted T‐Cell responses and their role in infectious diseases. J Immunol Res. 2016;2016:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Strong RK, Holmes MA, Li P, Braun L, Lee N, Geraghty DE. HLA‐E allelic variants: Correlating differential expression, peptide affinities, crystal structures, and thermal stabilities. J Biol Chem. 2003;278(7):5082‐5090. [DOI] [PubMed] [Google Scholar]
- 14. Grimsley C, Ober C. Population genetic studies of HLA‐E: evidence for selection. Hum Immunol. 1997;52(1):33‐40. [DOI] [PubMed] [Google Scholar]
- 15. Nattermann J, Nischalke HD, Hofmeister V, et al. HIV‐1 infection leads to increased HLA‐E expression resulting in impaired function of natural killer cells. Antivi Ther. 2005;10(1):95‐107. [DOI] [PubMed] [Google Scholar]
- 16. van Stigt TT, Akko JI, Niehrs A, et al. Primary HIV‐1 strains use Nef To Downmodulate HLA‐E surface expression. J Virol. 2019;93(20):e00719‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zeng L, Sullivan LC, Vivian JP, et al. A structural basis for antigen presentation by the MHC class Ib molecule, Qa‐1 b. J Immunol. 2012;188(1):302‐310. [DOI] [PubMed] [Google Scholar]
- 18. Wu HL, Wiseman RW, Hughes CM, et al. The role of MHC‐E in T cell immunity is conserved among humans, rhesus macaques, and cynomolgus macaques. J Immunol. 2018;200(1):49‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prezzemolo T, van Meijgaarden KE, Franken KL, et al. Detailed characterization of human Mycobacterium tuberculosis specific HLA‐E restricted CD8+ T cells. Eur J Immunol. 2018;48(2):293‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bian Y, Shang S, Siddiqui S, et al. MHC Ib molecule Qa‐1 presents Mycobacterium tuberculosis peptide antigens to CD8+T cells and contributes to protection against infection. PLoS Pathog. 2017;13(5):1‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Joosten SA, van Meijgaarden KE, del Nonno F, et al. Patients with tuberculosis have a dysfunctional circulating B‐cell compartment, which normalizes following successful treatment. PLOS Pathog. 2016;12(6):e1005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harriff MJ, Wolfe LM, Swarbrick G, et al. HLA‐E presents glycopeptides from the Mycobacterium tuberculosis protein MPT32 to human CD8 + T cells. Sci Rep. 2017;7(1):4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hansen SG, Vieville C, Whizin N, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15(3):293‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hansen SG, Wu HL, Burwitz BJ, et al. Broadly targeted CD8+ T cell responses restricted by major histocompatibility complex‐E. Science. 2016;351(6274):714‐720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burwitz BJ, Hashiguchi PK, Mansouri M, et al. MHC‐E–Restricted CD8 + T cells target hepatitis B virus‐infected human hepatocytes. J Immunol. 2020;204(8):2169‐2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hansen SG, Womack J, Scholz I, et al. Cytomegalovirus vectors expressing Plasmodium knowlesi antigens induce immune responses that delay parasitemia upon sporozoite challenge. PLoS One. 2019;14(1):e0210252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hansen SG, Zak DE, Xu G, et al. Prevention of tuberculosis in rhesus macaques by a cytomegalovirus‐based vaccine. Nat Med. 2018;24(2):130‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ruibal P, Franken KLMC, van Meijgaarden KE, et al. Peptide binding to HLA‐E molecules in humans, nonhuman primates, and mice reveals unique binding peptides but remarkably conserved anchor residues. J Immunol. 2020;205(10):2861‐2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walters LC, McMichael AJ, Gillespie GM. Detailed and atypical HLA‐E peptide binding motifs revealed by a novel peptide exchange binding assay. Eur J Immunol. 2020;50(12):2075–2091. [DOI] [PubMed] [Google Scholar]
- 30. Grotzke JE, Harriff MJ, Siler AC, et al. The Mycobacterium tuberculosis phagosome is a HLA‐I processing competent organelle. PLoS Pathog. 2009;5(4):e1000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Godfrey DI, Koay H‐F, McCluskey J, Gherardin NA. The biology and functional importance of MAIT cells. Nat Immunol. 2019;20(9):1110‐1128. [DOI] [PubMed] [Google Scholar]
- 32. Lepore M, Kalinichenko A, Colone A, et al. Parallel T‐cell cloning and deep sequencing of human MAIT cells reveal stable oligoclonal TCRβ repertoire. Nat Commun. 2014;5(1):3866. [DOI] [PubMed] [Google Scholar]
- 33. Dusseaux M, Martin E, Serriari N, et al. Human MAIT cells are xenobiotic‐resistant, tissue‐targeted, CD161hi IL‐17‐secreting T cells. Blood. 2011;117:1250‐1259. [DOI] [PubMed] [Google Scholar]
- 34. Salou M, Franciszkiewicz K, Lantz O. MAIT cells in infectious diseases. Curr Opin Immunol. 2017;48:7‐14. [DOI] [PubMed] [Google Scholar]
- 35. Kjer‐Nielsen L, Corbett AJ, Chen Z, et al. An overview on the identification of MAIT cell antigens. Immunol Cell Biol. 2018;96(6):573‐587. [DOI] [PubMed] [Google Scholar]
- 36. Reantragoon R, Corbett AJ, Sakala IG, et al. Antigen‐loaded MR1 tetramers define T cell receptor heterogeneity in mucosal‐associated invariant T cells. J Exp Med. 2013;210(11):2305‐2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gherardin N, Keller A, Woolley R, et al. Diversity of T cells restricted by the MHC class I‐related molecule MR1 facilitates differential antigen recognition. Immunity. 2016;44(1):32‐45. [DOI] [PubMed] [Google Scholar]
- 38. Gherardin NA, Souter MNT, Koay H‐F, et al. Human blood MAIT cell subsets defined using MR1 tetramers. Immunol Cell Biol. 2018;96(5):507‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pomaznoy M, Kuan R, Lindvall M, et al. Quantitative and qualitative perturbations of CD8+ MAITs in Healthy Mycobacterium tuberculosis–infected individuals. ImmunoHorizons. 2020;4(6):292‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang H, Sikora MJ, Islam S, et al. Select sequencing of clonally expanded CD8+ T cells reveals limits to clonal expansion. Proc Natl Acad Sci. 2019;116(18):8995‐9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Salou M, Legoux F, Gilet J, et al. A common transcriptomic program acquired in the thymus defines tissue residency of MAIT and NKT subsets. J Exp Med. 2019;216(1):133‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sharma M, Zhang S, Niu L, Lewinsohn DM, Zhang X, Huang S. Mucosal‐associated invariant T cells develop an innate‐like transcriptomic program in anti‐mycobacterial responses. Front Immunol. 2020;11:1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gold MC, Cerri S, Smyk‐Pearson S, et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 2010;8(6):e1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Le Bourhis L, Martin E, Péguillet I, et al. Antimicrobial activity of mucosal‐associated invariant T cells. Nat Immunol. 2010;11(8):701‐708. [DOI] [PubMed] [Google Scholar]
- 45. Wong EB, Akilimali NA, Govender P, et al. Low levels of peripheral CD161++CD8+ mucosal associated invariant T (MAIT) cells are found in HIV and HIV/TB Co‐Infection. PLoS One. 2013;8(12):e83474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sharma PK, Wong EB, Napier RJ, et al. High expression of CD26 accurately identifies human bacteria‐reactive MR1‐restricted MAIT cells. Immunology. 2015;145(3):443‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yang Q, Xu Q, Chen Q, et al. Discriminating Active Tuberculosis from Latent Tuberculosis Infection by flow cytometric measurement of CD161‐expressing T cells. Sci Rep. 2015;5(1):17918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Paquin‐Proulx D, Costa PR, Terrassani Silveira CG, et al. Latent Mycobacterium tuberculosis Infection Is Associated With a Higher Frequency of Mucosal‐Associated Invariant T and Invariant Natural Killer T Cells. Front Immunol. 2018;9:1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wong EB, Gold MC, Meermeier EW, et al. TRAV1‐2+ CD8+ T‐cells including oligoclonal expansions of MAIT cells are enriched in the airways in human tuberculosis. Commun Biol. 2019;2(1):203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Suliman S, Gela A, Mendelsohn SC, et al. Peripheral blood mucosal‐associated invariant T cells in tuberculosis patients and healthy Mycobacterium tuberculosis‐exposed controls. J Infect Dis. 2020;222(6):995‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Seshadri C, Thuong NTT, Mai NTH, et al. A polymorphism in human MR1 is associated with mRNA expression and susceptibility to tuberculosis. Genes Immun. 2017;18(1):8‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Le Bourhis L, Dusseaux M, Bohineust A, et al. MAIT cells detect and efficiently lyse bacterially‐infected epithelial cells. PLoS Pathog. 2013;9(10):e1003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vorkas CK, Wipperman MF, Li K, et al. Mucosal‐associated invariant and γδ T cell subsets respond to initial Mycobacterium tuberculosis infection. JCI insight. 2018;3(19):e121899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Saeidi A, Tien Tien VL, Al‐Batran R, et al. Attrition of TCR Vα7.2+ CD161++ MAIT Cells in HIV‐tuberculosis co‐infection is associated with elevated levels of PD‐1 expression. PLoS One. 2015;10(4):e0124659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kwon YS, Cho YN, Kim MJ, et al. Mucosal‐associated invariant T cells are numerically and functionally deficient in patients with mycobacterial infection and reflect disease activity. Tuberculosis. 2015;95(3):267‐274. [DOI] [PubMed] [Google Scholar]
- 56. Jiang J, Yang B, An H, et al. Mucosal‐associated invariant T cells from patients with tuberculosis exhibit impaired immune response. J Infect. 2016;72(3):338‐352. [DOI] [PubMed] [Google Scholar]
- 57. Estévez O, Anibarro L, Garet E, et al. Multi‐parameter flow cytometry immunophenotyping distinguishes different stages of tuberculosis infection. J Infect. 2020;81(1):57‐71. [DOI] [PubMed] [Google Scholar]
- 58. Jiang J, Chen X, An H, Yang B, Zhang F, Cheng X. Enhanced immune response of MAIT cells in tuberculous pleural effusions depends on cytokine signaling. Sci Rep. 2016;6(1):32320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Coulter F, Parrish A, Manning D, et al. IL‐17 production from T helper 17, mucosal‐associated invariant T, and γδ Cells in tuberculosis infection and disease. Front Immunol. 2017;8:1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Malka‐Ruimy C, Ben Youssef G, Lambert M, et al. Mucosal‐associated invariant T cell levels are reduced in the peripheral blood and lungs of children with active pulmonary tuberculosis. Front Immunol. 2019;10:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Greene JM, Dash P, Roy S, et al. MR1‐restricted mucosal‐associated invariant T (MAIT) cells respond to mycobacterial vaccination and infection in nonhuman primates. Mucosal Immunol. 2017;10(3):802‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Darrah PA, Zeppa JJ, Maiello P, et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature. 2020;577(7788):95‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kauffman KD, Sallin MA, Hoft SG, et al. Limited pulmonary mucosal‐associated invariant T cell accumulation and activation during Mycobacterium tuberculosis infection in rhesus macaques. Infect Immun. 2018;86(12):e00431‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bucsan AN, Rout N, Foreman TW, Khader SA, Rengarajan J, Kaushal D. Mucosal‐activated invariant T cells do not exhibit significant lung recruitment and proliferation profiles in macaques in response to infection with Mycobacterium tuberculosis CDC1551. Tuberculosis. 2019;116:S11‐S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Georgel P, Radosavljevic M, Macquin C, Bahram S. The non‐conventional MHC class I MR1 molecule controls infection by Klebsiella pneumoniae in mice. Mol Immunol. 2011;48(5):769‐775. [DOI] [PubMed] [Google Scholar]
- 66. Chua WJ, Truscott SM, Eickhoff CS, Blazevic A, Hoft DF, Hansen TH. Polyclonal mucosa‐associated invariant T cells have unique innate functions in bacterial infection. Infect Immun. 2012;80(9):3256‐3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Meierovics A, Yankelevich W‐JC, Cowley SC. MAIT cells are critical for optimal mucosal immune responses during in vivo pulmonary bacterial infection. Proc Natl Acad Sci. 2013;110(33):E3119‐E3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang H, D’Souza C, Lim XY, et al. MAIT cells protect against pulmonary Legionella longbeachae infection. Nat Commun. 2018;9(1):3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ussher JE, Bilton M, Attwod E, et al. CD161++CD8+ T cells, including the MAIT cell subset, are specifically activated by IL‐12+IL‐18 in a TCR‐independent manner. Eur J Immunol. 2014;44(1):195‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Suliman S, Murphy M, Musvosvi M, et al. MR1‐independent activation of human mucosal‐associated invariant T cells by Mycobacteria. J Immunol. 2019;203(11):2917‐2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sakai S, Kauffman KD, Oh S, Nelson CE, Barry CE, Barber DL. MAIT cell‐directed therapy of Mycobacterium tuberculosis infection. Mucosal Immunol. 2020;14(1):199‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Harriff MJ, McMurtrey C, Froyd CA, et al. MR1 displays the microbial metabolome driving selective MR1‐restricted T cell receptor usage. Sci Immunol. 2018;3(25):2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yu H, Yang A, Derrick S, et al. Artificially induced MAIT cells inhibit M. bovis BCG but not M. tuberculosis during in vivo pulmonary infection. Sci Rep. 2020;10(1):13579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Vorkas CK, Levy O, Skular M, Li K, Aubé J, Glickman MS. Efficient 5‐OP‐RU‐Induced Enrichment of Mucosa‐Associated Invariant T Cells in the Murine Lung Does Not Enhance Control of Aerosol Mycobacterium tuberculosis Infection. Infect Immun. 2020;89(1):e00524‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ellis AL, Balgeman AJ, Larson EC, et al. MAIT cells are functionally impaired in a Mauritian cynomolgus macaque model of SIV and Mtb co‐infection. PLoS Pathog. 2020;16(5):e1008585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tang X, Zhang S, Peng Q, et al. Sustained IFN‐I stimulation impairs MAIT cell responses to bacteria by inducing IL‐10 during chronic HIV‐1 infection. Sci Adv. 2020;6(8):eaaz0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dumas A, Corral D, Colom A, et al. The host microbiota contributes to early protection against lung colonization by Mycobacterium tuberculosis . Front Immunol. 2018;9:2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Constantinides MG, Link VM, Tamoutounour S, et al. MAIT cells are imprinted by the microbiota in early life and promote tissue repair. Science. 2019;366(6464):eaax6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Swarbrick GM, Gela A, Cansler ME, et al. Postnatal expansion, maturation, and functionality of MR1T cells in humans. Front Immunol. 2020;11:556695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Harly C, Peigné CM, Scotet E. Molecules and mechanisms implicated in the peculiar antigenic activation process of human Vγ9Vδ2 T cells. Front Immunol. 2015;6:657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gu S, Sachleben JR, Boughter CT, et al. Phosphoantigen‐induced conformational change of butyrophilin 3A1 (BTN3A1) and its implication on Vγ9Vδ2 T cell activation. Proc Natl Acad Sci. 2017;114(35):E7311‐E7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rigau M, Ostrouska S, Fulford TS, et al. Butyrophilin 2A1 is essential for phosphoantigen reactivity by γδ T cells. Science. 2020;367(6478):eaay5516. [DOI] [PubMed] [Google Scholar]
- 83. Tanaka Y, Tanaka Y, Bloom BR, Morita CT, Brenner MB, Nieves E. Natural and synthetic non‐peptide antigens recognized by human γδ T cells. Nature. 1995;375(6527):155‐158. [DOI] [PubMed] [Google Scholar]
- 84. Hintz M, Reichenberg A, Altincicek B, et al. Identification of (E)‐4‐hydroxy‐3‐methyl‐but‐2‐enyl pyrophosphate as a major activator for human γδ T cells in Escherichia coli. FEBS Lett. 2001;509(2):317‐322. [DOI] [PubMed] [Google Scholar]
- 85. Xia M, Hesser DC, De P, et al. A subset of protective γ9δ2 T cells is activated by novel mycobacterial glycolipid components. Infect Immun. 2016;84(9):2449‐2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Uldrich AP, Le Nours J, Pellicci DG, et al. CD1d‐lipid antigen recognition by the γδ TCR. Nat Immunol. 2013;14(11):1137‐1145. [DOI] [PubMed] [Google Scholar]
- 87. Li Y, Wang X, Teng DA, et al. Identification of the ligands of TCRγδ by screening the immune repertoire of γδT cells from patients with tuberculosis. Front Immunol. 2019;10: 2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ding Y, Ma F, Wang Z, Li B. Characteristics of the Vδ2 CDR3 sequence of peripheral γδ T cells in patients with pulmonary tuberculosis and identification of a new tuberculosis‐related antigen peptide. Clin Vaccine Immunol. 2015;22(7):761‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Balbi B, Valle MT, Oddera S, et al. T‐Lymphocytes with γδ + Vδ2+ antigen receptors are present in increased proportions in a fraction of patients with tuberculosis or with sarcoidosis. Am Rev Respir Dis. 1993;148(6 pt. 1):1685‐1690. [DOI] [PubMed] [Google Scholar]
- 90. Ogongo P, Steyn AJC, Karim F, et al. Differential skewing of donor‐unrestricted and γδ T cell repertoires in tuberculosis‐infected human lungs. J Clin Invest. 2020;130(1):214‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Barnes PF, Grisso CL, Abrams JS, Band H, Rea TH, Modlin RL. γδ T Lymphocytes in human tuberculosis. J Infect Dis. 1992;165(3):506‐512. [DOI] [PubMed] [Google Scholar]
- 92. Pinheiro MB, Antonelli LR, Sathler‐Avelar R, et al. CD4‐CD8‐αβ and γδ T cells display inflammatory and regulatory potentials during human tuberculosis. PLoS One. 2012;7(12):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Morita CT, Mariuzza RA, Brenner MB. Antigen recognition by human γδ T cells: Pattern recognition by the adaptive immune system. Springer Semin Immun. 2000;22(3):191‐217. [DOI] [PubMed] [Google Scholar]
- 94. Gioia C, Agrati C, Casetti R, et al. Lack of CD27 − CD45RA − Vγ9Vδ2 + T cell effectors in immunocompromised hosts and during active pulmonary tuberculosis. J Immunol. 2002;168(3):1484‐1489. [DOI] [PubMed] [Google Scholar]
- 95. Yan L, Cui H, Xiao H, Zhang Q. Anergic pulmonary tuberculosis is associated with contraction of the Vd2+T cell population, apoptosis and enhanced inhibitory cytokine production. PLoS One. 2013;8(8):e71245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. El Daker S, Sacchi A, Montesano C, et al. An abnormal phenotype of lung Vγ9Vδ2 T cells impairs their responsiveness in tuberculosis patients. Cell Immunol. 2013;282(2):106‐112. [DOI] [PubMed] [Google Scholar]
- 97. Wang H, Lee HK, Bukowski JF, et al. Conservation of nonpeptide antigen recognition by rhesus monkey Vγ2Vδ2 T cells. J Immunol. 2003;170(7):3696‐3706. [DOI] [PubMed] [Google Scholar]
- 98. Shen H, Wang Y, Chen CY, et al. Th17‐related cytokines contribute to recall‐like expansion/effector function of HMBPP‐specific Vγ2Vδ2 T cells after Mycobacterium tuberculosis infection or vaccination. Eur J Immunol. 2015;45(2):442‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Peng M, Wang Z, Yao C, et al. Interleukin 17‐producing γδ T cells increased in patients with active pulmonary tuberculosis. Cell Mol Immunol. 2008;5(3):203‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Mazzola TN, Da Silva M, Moreno Y, et al. Robust γδ+ T cell expansion in infants immunized at birth with BCG vaccine. Vaccine. 2007;25(34):6313‐6320. [DOI] [PubMed] [Google Scholar]
- 101. Zufferey C, Germano S, Dutta B, Ritz N, Curtis N. The contribution of non‐conventional T cells and NK cells in the mycobacterial‐specific IFNγ response in bacille calmette‐guérin (BCG)‐immunized infants. PLoS One. 2013;8(10):e77334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hoft DF, Brown RM, Roodman ST. Bacille Calmette‐Guérin vaccination enhances human gamma delta T cell responsiveness to mycobacteria suggestive of a memory‐like phenotype. J Immunol. 1998;161(2):1045‐1054. [PubMed] [Google Scholar]
- 103. Dieli F, Troye‐Blomberg M, Ivanyi J, et al. Granulysin‐dependent killing of intracellular and extracellular Mycobacterium tuberculosis by Vγ9/Vδ2 T lymphocytes. J Infect Dis. 2001;184(8):1082‐1085. [DOI] [PubMed] [Google Scholar]
- 104. Spencer C, Abate G, Blazevic A, Hoft DF. Only a subset of phosphoantigen‐responsive γ9δ2 T cells mediate protective TB immunity. J Immunol. 2008;181(7):4471‐4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Spencer CT, Abate G, Sakala IG, et al. Granzyme A produced by γ9δ2 T cells induces human macrophages to inhibit growth of an intracellular pathogen. PLoS Pathog. 2013;9(1):e1003119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Devilder M‐C, Maillet S, Bouyge‐Moreau I, Donnadieu E, Bonneville M, Scotet E. Potentiation of antigen‐stimulated Vγ9Vδ2 T cell cytokine production by immature dendritic cells (DC) and reciprocal effect on DC maturation. J Immunol. 2006;176(3):1386‐1393. [DOI] [PubMed] [Google Scholar]
- 107. Caccamo N, Sireci G, Meraviglia S, Dieli F, Ivanyi J, Salerno A. γδ T cells condition dentritic cells in vivo for priming pulmonary CD8 T cell responses against Mycobacterium tuberculosis . Eur J Immunol. 2006;36(10):2681‐2690. [DOI] [PubMed] [Google Scholar]
- 108. Abate G, Spencer CT, Hamzabegovic F, Blazevic A, Xia M, Hoft DF. Mycobacterium‐specific γ9δ2 T cells mediate both pathogen‐inhibitory and CD40 ligand‐dependent antigen presentation effects important for tuberculosis immunity. Infect Immun. 2016;84(2):580‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Yao S, Huang D, Chen CY, et al. Differentiation, distribution and γδ T cell‐driven regulation of IL‐22‐producing T cells in tuberculosis. PLoS Pathog. 2010;6(2):e1000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Cendron D, Ingoure S, Martino A, et al. A tuberculosis vaccine base on phosphoantigens and fusion proteins induces distinct γδ and αβ T cell responses in primates. Eur J Immunol. 2007;37(2):549‐565. [DOI] [PubMed] [Google Scholar]
- 111. Ali Z, Shao L, Halliday L, et al. Prolonged (E)‐4‐Hydroxy‐3‐Methyl‐But‐2‐Enyl pyrophosphate‐driven antimicrobial and cytotoxic responses of pulmonary and systemic Vγ2Vδ2 T cells in macaques. J Immunol. 2007;179(12):8287‐8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ryan‐Payseur B, Frencher J, Shen L, Chen CY, Huang D, Chen ZW. Multieffector‐functional immune responses of HMBPP‐specific Vγ2Vδ2 T cells in nonhuman primates inoculated with listeria monocytogenes ΔactA prfA*. J Immunol. 2012;189(3):1285‐1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Shen L, Frencher J, Huang D, et al. Immunization of Vγ2Vδ2 T cells programs sustained effector memory responses that control tuberculosis in nonhuman primates. Proc Natl Acad Sci. 2019;116(13):6371‐6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Qaqish A, Huang D, Chen CY, et al. Adoptive transfer of phosphoantigen‐specific γδ T cell subset attenuates Mycobacterium tuberculosis infection in nonhuman primates. J Immunol. 2017;198(12):4753‐4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. García VE, Jullien D, Song M, et al. IL‐15 enhances the response of human gamma delta T cells to nonpeptide microbial antigens. J Immunol. 1998;160(9):4322‐4439. [PubMed] [Google Scholar]
- 116. Yang R, Yao L, Shen L, et al. IL‐12 Expands and differentiates human Vγ2Vδ2 T effector cells producing antimicrobial cytokines and inhibiting intracellular mycobacterial growth. Front Immunol. 2019;10:913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Chen CY, Yao S, Huang D, et al. Phosphoantigen/IL2 expansion and differentiation of Vγ2Vδ2 T cells increase resistance to tuberculosis in nonhuman primates. PLoS Pathog. 2013;9(8):e1003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Van Rhijn I, Godfrey DI, Rossjohn J, Moody DB. Lipid and small‐molecule display by CD1 and MR1. Nat Rev Immunol. 2015;15(10):643‐654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Rosat JP, Grant EP, Beckman EM, et al. CD1‐restricted microbial lipid antigen‐specific recognition found in the CD8+αβ T cell pool. J Immunol. 1999;162(1):366‐371. [PubMed] [Google Scholar]
- 120. Garcia‐Alles LF, Giacometti G, Versluis C, et al. Crystal structure of human CD1e reveals a groove suited for lipid‐exchange processes. Proc Natl Acad Sci. 2011;108(32):13230‐13235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Jayawardena‐Wolf J, Benlagha K, Chiu Y‐H, Mehr R, Bendelac A. CD1d endosomal trafficking is independently regulated by an intrinsic CD1d‐encoded tyrosine motif and by the invariant chain. Immunity. 2001;15(6):897‐908. [DOI] [PubMed] [Google Scholar]
- 122. Cernadas M, Cavallari M, Watts G, Mori L, De Libero G, Brenner MB. Early recycling compartment trafficking of CD1a is essential for its intersection and presentation of lipid antigens. J Immunol. 2010;184(3):1235‐1241. [DOI] [PubMed] [Google Scholar]
- 123. Jackman RM, Stenger S, Lee A, et al. The tyrosine‐containing cytoplasmic tail of CD1b is essential for its efficient presentation of bacterial lipid antigens. Immunity. 1998;8(3):341‐351. [DOI] [PubMed] [Google Scholar]
- 124. Sugita M, Peters PJ, Brenner MB. Pathways for lipid antigen presentation by CD1 molecules: Nowhere for intracellular pathogens to hide. Traffic. 2000;1(4):295‐300. [DOI] [PubMed] [Google Scholar]
- 125. Busch M, Herzmann C, Kallert S, et al. Lipoarabinomannan‐responsive polycytotoxic T cells are associated with protection in human tuberculosis. Am J Resp Crit Care. 2016;194(3):345‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Chancellor A, Tocheva AS, Cave‐Ayland C, et al. CD1b‐restricted GEM T cell responses are modulated by Mycobacterium tuberculosis mycolic acid meromycolate chains. Proc Natl Acad Sci. 2017;114(51):E10956‐E10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Gansert JL, Kieβler V, Engele M, et al. Human NKT cells express granulysin and exhibit antimycobacterial activity. J Immunol. 2003;170(6):3154‐3161. [DOI] [PubMed] [Google Scholar]
- 128. Moody DB, Young DC, Cheng TY, et al. T cell activation by lipopeptide antigens. Science. 2004;303(5657):527‐531. [DOI] [PubMed] [Google Scholar]
- 129. Cheng JMH, Liu L, Pellicci DG, et al. Total synthesis of Mycobacterium tuberculosis Dideoxymycobactin‐838 and stereoisomers: diverse CD1a‐restricted T cells display a common hierarchy of lipopeptide recognition. Chemistry. 2017;23(7):1694‐1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Sieling P, Chatterjee D, Porcelli S, et al. CD1‐restricted T cell recognition of microbial lipoglycan antigens. Science. 1995;269(5221):227‐230. [DOI] [PubMed] [Google Scholar]
- 131. Gilleron M, Stenger S, Mazorra Z, et al. Diacylated sulfoglycolipids are novel mycobacterial antigens stimulating CD1‐restricted T cells during Infection with Mycobacterium tuberculosis . J Exp Med. 2004;199(5):649‐659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Layre E, Collmann A, Bastian M, et al. Mycolic acids constitute a scaffold for mycobacterial lipid antigens stimulating CD1‐restricted T cells. Chem Biol. 2009;16(1):82‐92. [DOI] [PubMed] [Google Scholar]
- 133. Moody DB, Guy MR, Grant E, et al. CD1b‐mediated T cell recognition of a glycolipid antigen generated from mycobacterial lipid and host carbohydrate during infection. J Exp Med. 2000;192(7):965‐976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Moody DB, Ulrichs T, Mühlecker W, et al. CD1c‐mediated T‐cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature. 2000;404(6780):884‐888. [DOI] [PubMed] [Google Scholar]
- 135. Matsunaga I, Bhatt A, Young DC, et al. Mycobacterium tuberculosis pks12 produces a novel polyketide presented by CD1c to T cells. J Exp Med. 2004;200(12):1559‐1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Kasmar AG, van Rhijn I, Cheng T‐Y, et al. CD1b tetramers bind αβ T cell receptors to identify a mycobacterial glycolipid‐reactive T cell repertoire in humans. J Exp Med. 2011;208(9):1741‐1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Kasmar AG, Van Rhijn I, Magalhaes KG, et al. Cutting edge: CD1a tetramers and dextramers identify human lipopeptide‐specific T cells Ex vivo. J Immunol. 2013;191(9):4499‐4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Ly D, Kasmar AG, Cheng T‐Y, et al. CD1c tetramers detect ex vivo T cell responses to processed phosphomycoketide antigens. J Exp Med. 2013;210(4):729‐741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Stenger S, Hanson DA, Teitelbaum R, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282(5386):121‐125. [DOI] [PubMed] [Google Scholar]
- 140. Montamat‐Sicotte DJ, Millington KA, Willcox CR, et al. A mycolic acid‐specific CD1‐restricted T cell population contributes to acute and memory immune responses in human tuberculosis infection. J Clin Invest. 2011;121(6):2493‐2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Van Rhijn I, Kasmar A, De Jong A, et al. A conserved human T cell population targets mycobacterial antigens presented by CD1b. Nat Immunol. 2013;14(7):706‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Lopez K, Iwany SK, Suliman S, et al. CD1b Tetramers broadly detect T cells that correlate with mycobacterial exposure but not tuberculosis disease state. Front Immunol. 2020;11:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Seshadri C, Lin L, Scriba TJ, et al. T cell responses against mycobacterial lipids and proteins are poorly correlated in South African adolescents. J Immunol. 2015;195(10):4595‐4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Seshadri C, Thuong NTT, Yen NTB, et al. A polymorphism in human CD1A is associated with susceptibility to tuberculosis. Genes Immun. 2014;15(3):195‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Kasprowicz VO, Cheng TY, Ndung’u T, Sunpath H, Moody DB, Kasmar AG. HIV disrupts human T cells that target mycobacterial glycolipids. J Infect Dis. 2016;213(4):628‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Dascher CC, Hiromatsu K, Xiong X, et al. Immunization with a mycobacterial lipid vaccine improves pulmonary pathology in the guinea pig model of tuberculosis. Int Immunol. 2003;15(8):915‐925. [DOI] [PubMed] [Google Scholar]
- 147. Felio K, Nguyen H, Dascher CC, et al. CD1‐restricted adaptive immune responses to Mycobacteria in human group 1 CD1 transgenic mice. J Exp Med. 2009;206(11):2497‐2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Zhao J, Siddiqui S, Shang S, et al. Mycolic acid‐specific T cells protect against Mycobacterium tuberculosis infection in a humanized transgenic mouse model. Elife. 2015;4:e08525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Shang S, Kats D, Cao L, et al. Induction of mycobacterium tuberculosis lipid‐specific t cell responses by pulmonary delivery of mycolic acid‐loaded polymeric micellar nanocarriers. Front Immunol. 2018;9:2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Grant EP, Beckman EM, Behar SM, et al. Fine specificity of TCR complementarity‐determining region residues and lipid antigen hydrophilic moieties in the recognition of a CD1‐lipid complex. J Immunol. 2002;168(8):3933‐3940. [DOI] [PubMed] [Google Scholar]
- 151. Guiard J, Collmann A, Garcia‐Alles LF, et al. Fatty acyl structures of Mycobacterium tuberculosis sulfoglycolipid govern T cell response. J Immunol. 2009;182(11):7030‐7037. [DOI] [PubMed] [Google Scholar]
- 152. Van Rhijn I, Iwany SK, Fodran P, et al. CD1b‐mycolic acid tetramers demonstrate T‐cell fine specificity for mycobacterial lipid tails. Eur J Immunol. 2017;47(9):1525‐1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. James CA, Yu KKQ, Gilleron M, et al. CD1b tetramers identify T cells that recognize natural and synthetic diacylated sulfoglycolipids from Mycobacterium tuberculosis . Cell Chem Biol. 2018;25(4):392‐402.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: An innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013;13(2):101‐117. [DOI] [PubMed] [Google Scholar]
- 155. Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4−8− αβ T cells demonstrates preferential use of several Vβ genes and an invariant TCR α chain. J Exp Med. 1993;178(1):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Dellabona P, Padovan E, Casorati G, Brockhaus M, Lanzavecchia A. An invariant vα24‐jαQ/vβ11 t cell receptor is expressed in all individuals by clonally expanded CD4−8−t cells. J Exp Med. 1994;180(3):1171‐1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Tatituri RVV, Watts GFM, Bhowruth V, et al. Recognition of microbial and mammalian phospholipid antigens by NKT cells with diverse TCRs. Proc Natl Acad Sci. 2013;110(5):1827‐1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Snyder‐Cappione J, Nixon D, Loo C, et al. Individuals with pulmonary tuberculosis have lower levels of circulating CD1d‐restricted NKT cells. J Infect Dis. 2007;195(9):1361‐1364. [DOI] [PubMed] [Google Scholar]
- 159. Im JS, Kang T‐J, Lee S‐B, et al. Alteration of the relative levels of iNKT cell subsets is associated with chronic mycobacterial infections. Clin Immunol. 2008;127(2):214‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Montoya CJ, Cataño JC, Ramirez Z, Rugeles MT, Wilson SB, Landay AL. Invariant NKT cells from HIV‐1 or Mycobacterium tuberculosis‐infected patients express an activated phenotype. Clin Immunol. 2008;127(1):1‐6. [DOI] [PubMed] [Google Scholar]
- 161. Kee S‐J, Kwon Y‐S, Park Y‐W, et al. Dysfunction of natural killer T cells in patients with active Mycobacterium tuberculosis infection. Infect Immun. 2012;80(6):2100‐2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Yu KKQ, Wilburn DB, Hackney JA, et al. Conservation of molecular and cellular phenotypes of invariant NKT cells between humans and non‐human primates. Immunogenetics. 2019;71(7):465‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Chancellor A, White A, Tocheva AS, et al. Quantitative and qualitative iNKT repertoire associations with disease susceptibility and outcome in macaque tuberculosis infection. Tuberculosis. 2017;105:86‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Veenstra H, Baumann R, Carroll NM, et al. Changes in leucocyte and lymphocyte subsets during tuberculosis treatment; prominence of CD3dimCD56+ natural killer T cells in fast treatment responders. Clin Exp Immunol. 2006;145(2):252‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Singh A, Dey AB, Mohan A, Mitra DK. Programmed death‐1 receptor suppresses γ‐IFN producing NKT cells in human tuberculosis. Tuberculosis. 2014;94(3):197‐206. [DOI] [PubMed] [Google Scholar]
- 166. Wu C, Li Z, Fu X, Yu S, Lao S, Yang B. Antigen‐specific human NKT cells from tuberculosis patients produce IL‐21 to help B cells for the production of immunoglobulins. Oncotarget. 2015;6(30):28633‐28645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Dieli F, Taniguchi M, Kronenberg M, et al. An anti‐inflammatory role for Vα14 NK T cells in Mycobacterium bovis bacillus calmette‐guérin‐infected mice. J Immunol. 2003;171(4):1961‐1968. [DOI] [PubMed] [Google Scholar]
- 168. Behar SM, Dascher CC, Grusby MJ, Wang CR, Brenner MB. Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis . J Exp Med. 1999;189(12):1973‐1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Sugawara I, Yamada H, Mizuno S, Li CY, Nakayama T, Taniguchi M. Mycobacterial infection in natural killer T cell knockout mice. Tuberculosis. 2002;82(2–3):97‐104. [DOI] [PubMed] [Google Scholar]
- 170. Sada‐Ovalle I, Chiba A, Gonzales A, Brenner MB, Behar SM. Innate invariant NKT cells recognize Mycobacterium tuberculosis–Infected macrophages, produce interferon‐γ, and kill intracellular bacteria. PLoS Pathog. 2008;4(12):e1000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Rozot V, Nemes E, Geldenhuys H, et al. Multidimensional analyses reveal modulation of adaptive and innate immune subsets by tuberculosis vaccines. Commun Biol. 2020;3(1):563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Rothchild AC, Jayaraman P, Nunes‐Alves C, Behar SM. iNKT cell production of GM‐CSF controls Mycobacterium tuberculosis . PLoS Pathog. 2014;10(1):e1003805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Rothchild AC, Stowell B, Goyal G, et al. Role of granulocyte‐macrophage colony‐stimulating factor production by T cells during Mycobacterium tuberculosis infection. MBio. 2017;8(5):e01514‐e1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Coppola M, van Meijgaarden KE, Franken KLMC, et al. New genome‐wide algorithm identifies novel in‐vivo expressed mycobacterium tuberculosis antigens inducing human T‐cell responses with classical and unconventional cytokine profiles. Sci Rep. 2016;6(1):37793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Larrouy‐Maumus G, Layre E, Clark S, et al. Protective efficacy of a lipid antigen vaccine in a guinea pig model of tuberculosis. Vaccine. 2017;35(10):1395‐1402. [DOI] [PubMed] [Google Scholar]
- 176. Venkataswamy MM, Baena A, Goldberg MF, et al. Incorporation of NKT cell‐activating glycolipids enhances immunogenicity and vaccine efficacy of Mycobacterium bovis bacillus calmette‐guérin. J Immunol. 2009;183(3):1644‐1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Khan A, Singh S, Galvan G, Jagannath C, Sastry K. Prophylactic sublingual immunization with Mycobacterium tuberculosis subunit vaccine incorporating the natural killer T cell agonist alpha‐galactosylceramide enhances protective immunity to limit pulmonary and extra‐pulmonary bacterial burden in mice. Vaccines. 2017;5(4):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Sada‐Ovalle I, Sköld M, Tian T, Besra GS, Behar SM. α‐galactosylceramide as a therapeutic agent for pulmonary Mycobacterium tuberculosis infection. Am J Respir Crit Care Med. 2010;182(6):841‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Chackerian A, Alt J, Perera V, Behar SM. Activation of NKT cells protects mice from tuberculosis. Infect Immun. 2002;70(11):6302‐6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Chiba A, Dascher CC, Besra GS, Brenner MB. Rapid NKT cell responses are self‐terminating during the course of microbial infection. J Immunol. 2008;181(4):2292‐2302. [DOI] [PubMed] [Google Scholar]
- 181. Kwon BE, Ahn JH, Park EK, et al. B cell‐based vaccine transduced with esat6‐expressing vaccinia virus and presenting α‐galactosylceramide is a novel vaccine candidate against esat6‐expressing mycobacterial diseases. Front Immunol. 2019;10:2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Stolk DA, de Haas A, Vree J, et al. Lipo‐based vaccines as an approach to target dendritic cells for induction of T‐ and iNKT cell responses. Front Immunol. 2020;11:990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Portevin D, Via LE, Eum S, Young D. Natural killer cells are recruited during pulmonary tuberculosis and their ex vivo responses to mycobacteria vary between healthy human donors in association with KIR haplotype. Cell Microbiol. 2012;14(11):1734‐1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Chowdhury RR, Vallania F, Yang Q, et al. A multi‐cohort study of the immune factors associated with M. tuberculosis infection outcomes. Nature. 2018;560(7720):644‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Bozzano F, Costa P, Passalacqua G, et al. Functionally relevant decreases in activatory receptor expression on NK cells are associated with pulmonary tuberculosis in vivo and persist after successful treatment. Int Immunol. 2009;21(7):779‐791. [DOI] [PubMed] [Google Scholar]
- 186. Harris LD, Khayumbi J, Ongalo J, et al. Distinct human NK cell phenotypes and functional responses to Mycobacterium tuberculosis in adults from TB endemic and non‐endemic regions. Front Cell Infect Microbiol. 2020;10:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Vankayalapati R, Garg A, Porgador A, et al. Role of NK Cell‐activating receptors and their ligands in the lysis of mononuclear phagocytes infected with an intracellular bacterium. J Immunol. 2005;175(7):4611‐4617. [DOI] [PubMed] [Google Scholar]
- 188. Vankayalapati R, Wizel B, Weis SE, et al. The NKp46 receptor contributes to NK cell lysis of mononuclear phagocytes infected with an intracellular bacterium. J Immunol. 2002;168(7):3451‐3457. [DOI] [PubMed] [Google Scholar]
- 189. Kleinnijenhuis J, Quintin J, Preijers F, et al. BCG‐induced trained immunity in NK cells: Role for non‐specific protection to infection. Clin Immunol. 2014;155(2):213‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Fu X, Yu S, Yang B, Lao S, Li B, Wu C. Memory‐like antigen‐specific human NK cells from TB pleural fluids produced IL‐22 in response to IL‐15 or Mycobacterium tuberculosis antigens. PLoS One. 2016;11(3):e0151721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Venkatasubramanian S, Cheekatla S, Paidipally P, et al. IL‐21‐dependent expansion of memory‐like NK cells enhances protective immune responses against Mycobacterium tuberculosis. Mucosal Immunol. 2017;10(4):1031‐1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Brillantes M, Beaulieu AM. Memory and memory‐Like NK cell responses to microbial pathogens. Front Cell Infect Microbiol. 2020;10:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Suliman S, Geldenhuys H, Johnson JL, et al. Bacillus calmette‐guérin (BCG) revaccination of adults with latent mycobacterium tuberculosis infection induces long‐lived BCG‐reactive NK cell responses. J Immunol. 2016;197(4):1100‐1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Dhiman R, Periasamy S, Barnes PF, et al. NK1.1 + Cells and IL‐22 regulate vaccine‐induced protective immunity against challenge with Mycobacterium tuberculosis . J Immunol. 2012;189(2):897‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Lu C‐C, Wu T‐S, Hsu Y‐J, et al. NK cells kill mycobacteria directly by releasing perforin and granulysin. J Leukoc Biol. 2014;96(6):1119‐1129. [DOI] [PubMed] [Google Scholar]
- 196. Dhiman R, Indramohan M, Barnes PF, et al. IL‐22 produced by human NK cells inhibits growth of Mycobacterium tuberculosis by enhancing phagolysosomal fusion. J Immunol. 2009;183(10):6639‐6645. [DOI] [PubMed] [Google Scholar]
- 197. Roy S, Barnes PF, Garg A, Wu S, Cosman D, Vankayalapati R. NK cells lyse t regulatory cells that expand in response to an intracellular pathogen. J Immunol. 2008;180(3):1729‐1736. [DOI] [PubMed] [Google Scholar]
- 198. Fan R, Xiang Y, Yang L, et al. Impaired NK cells’ activity and increased numbers of CD4 + CD25+ regulatory T cells in multidrug‐resistant Mycobacterium tuberculosis patients. Tuberculosis. 2016;98:13‐20. [DOI] [PubMed] [Google Scholar]
- 199. Zhang R, Zheng X, Li B, Wei H, Tian Z. Human NK cells positively regulate γδ T cells in response to Mycobacterium tuberculosis . J Immunol. 2006;176(4):2610‐2616. [DOI] [PubMed] [Google Scholar]
- 200. Vankayalapati R, Klucar P, Wizel B, et al. NK cells regulate CD8 + T cell effector function in response to an intracellular pathogen. J Immunol. 2004;172(1):130‐137. [DOI] [PubMed] [Google Scholar]
- 201. Feng CG, Kaviratne M, Rothfuchs AG, et al. NK cell‐derived IFN‐γ differentially regulates innate resistance and neutrophil response in T cell‐deficient hosts infected with Mycobacterium tuberculosis . J Immunol. 2006;177(10):7086‐7093. [DOI] [PubMed] [Google Scholar]
- 202. Rölle A, Pollmann J, Ewen EM, et al. IL‐12‐producing monocytes and HLA‐E control HCMV‐driven NKG2C+ NK cell expansion. J Clin Invest. 2014;124(12):5305‐5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Hammer Q, Rückert T, Borst EM, et al. Peptide‐specific recognition of human cytomegalovirus strains controls adaptive natural killer cells. Nat Immunol. 2018;19(5):453‐463. [DOI] [PubMed] [Google Scholar]
- 204. Panda SK, Colonna M. Innate lymphoid cells in mucosal immunity. Front Immunol. 2019;10:861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Klose CSN, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. 2016;17(7):765‐774. [DOI] [PubMed] [Google Scholar]
- 206. Ardain A, Domingo‐Gonzalez R, Das S, et al. Group 3 innate lymphoid cells mediate early protective immunity against tuberculosis. Nature. 2019;570(7762):528‐532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Tripathi D, Radhakrishnan RK, Sivangala Thandi R, et al. IL‐22 produced by type 3 innate lymphoid cells (ILC3s) reduces the mortality of type 2 diabetes mellitus (T2DM) mice infected with Mycobacterium tuberculosis. PLOS Pathog. 2019;15(12):e1008140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Steigler P, Daniels NJ, McCulloch TR, Ryder BM, Sandford SK, Kirman JR. BCG vaccination drives accumulation and effector function of innate lymphoid cells in murine lungs. Immunol Cell Biol. 2018;96(4):379‐389. [DOI] [PubMed] [Google Scholar]
- 209. Nemes E, Geldenhuys H, Rozot V, et al. Prevention of M Tuberculosis infection with H4:IC31 vaccine or BCG revaccination. N Enl J Med. 2018;379(2):138‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Andersen P, Scriba TJ. Moving tuberculosis vaccines from theory to practice. Nat Rev Immunol. 2019;19(9):550‐562. [DOI] [PubMed] [Google Scholar]
- 211. Tameris MD, Hatherill M, Landry BS, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: A randomised, placebo‐controlled phase 2b trial. Lancet. 2013;381(9871):1021‐1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Fletcher HA, Snowden MA, Landry B, et al. T‐cell activation is an immune correlate of risk in BCG vaccinated infants. Nat Commun. 2016;7(1):11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Tait DR, Hatherill M, Der Meeren O, et al. Final analysis of a trial of M72/AS01E vaccine to prevent tuberculosis. N Engl J Med. 2019;381(25):2429‐2439. [DOI] [PubMed] [Google Scholar]
- 214. Kaufmann E, Sanz J, Dunn JL, et al. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell. 2018;172(1–2):176‐190. [DOI] [PubMed] [Google Scholar]
- 215. Khan N, Downey J, Sanz J, et al. M tuberculosis reprograms hematopoietic stem cells to limit myelopoiesis and impair trained immunity. Cell. 2020;183(3):752‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Dijkman K, Sombroek CC, Vervenne RAW, et al. Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques. Nat Med. 2019;25(2):255‐262. [DOI] [PubMed] [Google Scholar]
- 217. Tameris M, Mearns H, Penn‐Nicholson A, et al. Live‐attenuated Mycobacterium tuberculosis vaccine MTBVAC versus BCG in adults and neonates: a randomised controlled, double‐blind dose‐escalation trial. Lancet Respir Med. 2019;7(9):757‐770. [DOI] [PubMed] [Google Scholar]
- 218. Loxton AG, Knaul JK, Grode L, et al. Safety and immunogenicity of the recombinant Mycobacterium bovis BCG vaccine VPM1002 in HIV‐unexposed newborn infants in South Africa. Clin Vaccine Immunol. 2017;24(2):e00439‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Ottenhoff THM, Joosten SA. Mobilizing unconventional T cells. Science. 2019;366(6463):302‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Alrubayyi A, Ogbe A, Moreno Cubero E, Peppa D. Harnessing natural killer cell innate and adaptive traits in HIV infection. Front Cell Infect Microbiol. 2020;10:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Compton BJ, Farrand KJ, Tang C‐W, et al. Enhancing T cell responses and tumour immunity by vaccination with peptides conjugated to a weak NKT cell agonist. Org Biomol Chem. 2019;17(5):1225‐1237. [DOI] [PubMed] [Google Scholar]
- 222. Nell AS, D’lom E, Bouic P, et al. Safety, tolerability, and immunogenicity of the novel antituberculous vaccine RUTI: Randomized, placebo‐controlled phase II clinical trial in patients with latent tuberculosis infection. PLoS One. 2014;9(2):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Modlin RL, Pirmez C, Hofman FM, et al. Lymphocytes bearing antigen‐specific γδ T‐cell receptors accumulate in human infectious disease lesions. Nature. 1989;339(6225):544‐548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data discussed were all retrieved from published literature as specified in the reference list.


