Abstract
Postoperative Cognitive Dysfunction (POCD) has been reported with widely varying frequency but appears to be strongly associated with aging. Outside of the surgical arena, chronic and acute cerebral hypoxia may exist as a result of respiratory, cardiovascular, or anemic conditions. Hypoxia has been extensively implicated in cognitive impairment, and disease states associated with hypoxia accompany and progress with aging. Perioperative cerebral hypoxia is likely underdiagnosed and its contribution to POCD underappreciated. Herein we discuss the various disease processes and forms in which hypoxia may contribute to POCD. Further, we outline hypoxia-related mechanisms, such as hypoxia inducible factor activation, cerebral ischemia, cerebrovascular reserve, excitotoxicity, and neuroinflammation, which may contribute to cognitive impairment and how these mechanisms interact with aging. Finally, we discuss opportunities to prevent and manage POCD related to hypoxia.
Introduction
Postoperative Cognitive Dysfunction (POCD) has been reported to occur with widely varying frequency, especially in older adults in the weeks to months following surgery1. Prospective studies with non-surgical control groups suggest the incidence of POCD is actually much lower than originally documented and that lingering effects are either minor or nonexistent2–4. In fact, in a cohort of over 10,000 patients followed over an average of 13 years, age-related cognitive decline following hospital admissions for either non-surgical reasons or stroke was greater than cognitive decline following major surgery5. Nevertheless, anesthetic exposure6 and neuroinflammatory processes7 continue to be frequently investigated as mechanisms for POCD. Several large scale prospective cohort8, multi-center randomized studies9, and meta-analyses10 suggest that, at a minimum, anesthetic exposure by itself does not account for all, or even the majority, of POCD. Surgeries including cardiopulmonary bypass (CPB), associated with a profound inflammatory response, do not confer risk for worse cognitive outcomes than those without CPB11; and studies testing prevention of neuroinflammation using glucocorticoids have not demonstrated effect either7.
While clarity continues to be sought on the role of anesthetics and neuroinflammation in the genesis of POCD, there is consensus that advanced age, especially with pre-existing cognitive impairment and other medical and neurologic comorbidities frequently associated with aging, increases the risk for POCD12,13. Risk factors which normally predispose an elderly individual to cognitive impairment, such as ApoE allelic expression, reduced cerebrovascular reserve, or increased vascular burden (i.e., presence of factors known to impair vascular structure and function, e.g., atherosclerosis, diabetes, hypertension, smoking, obesity, hypercholesterolemia, lack of physical activity, etc.; discussed below), may exacerbate the POCD risk. Coincident cerebral hypoxia is a mechanism common to many medical comorbidities of aging and has been implicated as a risk factor for cognitive decline outside the surgical arena. We propose that cerebral hypoxia from any source should be considered as an important mechanism contributing to POCD.
Perioperative Hypoxia and POCD
Hypoxia has been classified into general categories arising from low oxygen uptake (hypoxic), ischemic, or anemic mechanisms. Each category has been independently linked to cognitive decline in diseases common in the elderly, with accumulating evidence that aging may exacerbate hypoxic stress. Further, major surgery, especially in older adults, involves procedures that decrease oxygen supply, including fluid overload, acute anemia, hypoperfusion, hypoventilation, and atelectasis. Postoperatively, sleep disordered breathing (present in patients with14 and without15 a history of obstructive sleep apnea [OSA]) and narcotics (commonly prescribed for postoperative pain control16) likely contribute to postoperative hypoxemia.
Intraoperative and postoperative hypoxemia occurs with a frequency previously unrecognized. Ehrenfeld et al.17, found that 6.8% of patients experienced an intraoperative hypoxemic event, with a hypoxemic event lasting at least 2 minutes occurring in 3.5% of patients. More recently, Sun et al.18 published the results of a prospective, blinded observational study in which pulse oximetry was recorded continuously in 1500 patients (mean age = 64 years) for 3 consecutive days postoperatively. Twenty-one percent (21%) experienced at least 10 minutes per hour (min/h) of hypoxemia, 8% experienced hypoxemia for at least 20 min/h, and 8% experienced severe hypoxemia (arterial hemoglobin oxygen saturation [SaO2] < 85%). Prolonged hypoxemic episodes were also common, with 37% experiencing at least 1 episode lasting 1 hour minimum, 11% experienced at least 1 episode lasting 6 hours or more, and 3% experienced severe hypoxemia (SaO2< 80%) lasting for 30 minutes or longer. Similar results have been reported by others19.
Regardless of anesthetic approach (general vs regional) and surgical approach (off-CPB vs on-CPB), patients are subject to the same milieu of factors contributing to postoperative hypoxemia or hypoxia which may precede cerebral hypoxia. In fact, cerebral hypoxia has been associated with POCD following hip arthroplasty20, abdominal surgery21 and cardiac surgery15,22.
Hypoxemic Hypoxia
Hypoxemic-hypoxia (HH), due to low oxygen uptake by the pulmonary circulation, is often present prior to surgery due to pathophysiological conditions. Clinically, HH is observed during respiratory failure, as experienced in chronic obstructive pulmonary disease (COPD), interstitial lung disease, OSA, and increased interstitial fluid, or pulmonary edema secondary to heart failure (HF), and pulmonary embolism.
The severity of respiratory disease, such as COPD or OSA, consistently correlates with poor performance on assessments of executive function, processing speed, and attention23. In a multi-center study of individuals over the age of 65 suffering from COPD, cognitive and motor impairment were inversely correlated to resting SaO224. Hypoxemia during sleep also plays a major role in cognition25. Treatment of hypoxemia suggests at least some cognitive function is recoverable25 as observed in children26 and adults with OSA.
Heart failure, in addition to hypoperfusion, may also cause HH secondary to an increased arteriolar-alveolar oxygen gradient with pulmonary edema and is associated with higher risk for cognitive decline27. The Cardiovascular Health Study found that global cognition declined more rapidly following incident HF than in age matched controls without HF28. The REGARDS study, a longitudinal study of racial and geographic disparities in incident HF, also reported that the rate of cognitive failure accelerated following incident HF. Acute decompensated HF, often accompanied by both pulmonary edema and hypoperfusion is associated with acute decline in cognitive performance compared to those with stable HF29. Rarely does HF exist in individuals without attendant atherosclerotic disease, therefore both ischemia and HH likely contribute to cognitive decline.
Ischemic Hypoxia
Ischemic-hypoxia, or ischemia, refers to low tissue oxygenation as a result of reduced blood flow and is the category most well recognized as being associated with cognitive failure. In the surgical arena, ischemia occurs secondary to acute and chronic embolic events or hypoperfusion. Embolic, ischemic, and hemorrhagic strokes have been widely recognized as important contributors to cognitive decline and dementia30. Resulting cognitive and behavioral impairments may be circumscribed or diffuse, as even focal brain damage may disrupt widespread functional networks. The most frequently impaired cognitive domains (and their associated brain regions) following a stroke include executive functions (prefrontal cortex, parietal cortex and underlying white matter), episodic memory (medial temporal lobe and subcortical structures), language/aphasia (dominant [usually left] cerebral hemisphere), attention/hemispatial neglect (brain stem, midbrain, prefrontal cortex, parietal cortex and underlying white matter), and visuospatial/visuo-constructional abilities (parietal cortex, nondominant [usually right] cerebral hemisphere)31,32. Risk factors for cognitive impairment after stroke include older age, prior ischemic lesions, stroke severity (i.e., volume of tissue damaged), location of the stroke, and pre-stroke cognitive impairment31–34.Gradual improvement occurs over time and is most notable in younger patients and within the first six months post-stroke, although many continue to demonstrate residual cognitive impairment years after stroke32.
Patients who suffer from a transient ischemic attack (TIA) also demonstrate a range of cognitive deficits that are observed for months and years beyond the resolution of their focal TIA symptoms35. Individuals with a history of stroke and/or TIAs are at increased risk for future progressive cognitive decline35. Approximately 10% of stroke patients develop some form of dementia in the first year after stroke; which increases to over 30% with recurrent stroke36. Multiple strokes may lead to multi-infarct dementia, characterized by progressive, stepwise decline in cognitive function.
All surgeries involve some risk of cerebral ischemia (i.e., “silent” stroke; not accompanied by any observable stroke symptoms and detected only on postoperative diffusion-weighted magnetic resonance imaging [MRI] imaging [DWI]) or clinical stroke (i.e., acute brain lesion with clinical manifestation lasting >24 h)37. Generally, silent strokes are more common than clinical strokes38; and risk is higher in cardiac surgery and in aged individuals. Stroke in nonsurgical populations and perioperative stroke share common patterns of resulting cognitive dysfunction, yet cerebral ischemia is not generally considered in the conceptualization of POCD39. This is particularly surprising, because postoperative MRI studies have demonstrated incidence rates of new ischemic lesions after both cardiac and noncardiac surgery ranging from <1% to 17%37,40,41. A slight increase in perioperative stroke has been reported from 2003 to 201442, which may be partially due to more careful perioperative surveillance. While stroke or TIA was diagnosed after surgical aortic valve replacement in only 7% of patients by the routine clinical care team, 19% were diagnosed when a formal stroke assessment protocol was in place40.
In most studies of POCD, DWI scans are obtained several days after surgery, increasing the likelihood that “acute” lesions reflect irreversibly damaged tissue43. In large prospective studies, perioperative silent stroke on DWI was observed in 7 – 10% following non-cardiac, non-carotid artery surgery38. Silent ischemic lesions are even more common following cardiac surgery and procedures that involve instrumentation of the cerebral vessels or aorta44. After surgical aortic valve replacement, 61% exhibited silent infarcts on postoperative DWI40. Patients experiencing clinically “silent” ischemic events may be at greater risk for future cognitive decline. Cognitive decline one year after surgery was identified in 42% of surgical patients with perioperative silent stroke vs. 29% without38.
Because silent lesions are not accompanied by overt stroke symptoms, the extent to which they contribute to POCD is debatable40. Studies showing no relation between new ischemic lesions and POCD typically include relatively small samples and report relatively small total lesion volumes (e.g., <1,000mm3)45,46. However, a larger study showed that those with POCD had more and larger acute ischemic lesions on DWI five days after surgery, suggesting a threshold effect with poor cognitive outcomes observed only after the burden of multiple infarcts and/or a single large infarct reaches a tipping point2.
Anemic Hypoxia
Anemic-hypoxia results from reduced oxygen carrying capacity of red blood cells. Inadequate oxygen transport may result from low hematocrit, low hemoglobin concentration, or reduced ability of hemoglobin to bind oxygen (sickle cell anemia, carbon monoxide poisoning). Acute anemia elicits cognitive impairment even in healthy individuals47. Anemia that accompanies HF48, COPD49 and lung cancer50 has been associated with cognitive impairment, although the rate of anemia in chronic kidney disease may not contribute to cognitive decline51. Chronic anemia has been associated with impaired cognitive performance, even in otherwise healthy adolescents52 and adults53. Further, worsening white matter lesions54 during chronic anemia are associated with an increased incidence of cognitive impairment in older adults55,56. Finally, anemia prolongs cognitive recovery after stroke57 and erythropoietin (EPO) therapy may improve cognitive performance58.
Surgical candidates frequently present with anemia associated with chronic diseases, and major surgery in all arenas often results in acute and severe anemia. Several studies have demonstrated correlations between lower preoperative hemoglobin levels and adverse postoperative cerebral outcomes59. Mathew et al.60 found that aged subjects randomized to a transfusion hematocrit threshold of 18% experienced a greater degree of cognitive impairment that those randomized to 27%. Finally, the time course of recovery from perioperative anemia61 mimics recovery from POCD.
Finally, hypoxic challenges do not end in the operating room; they do not necessarily end in the recovery room, and they may often extend well beyond hospital discharge. Hypoxia is at the epicenter of the dysfunction of every organ in the perioperative period62 and with aging63, and thus cerebral hypoxia may indeed contribute to POCD.
Mechanisms of Cognitive Decline associated with Chronic & Acute Hypoxia or Hypoxemia
Outside of the surgical arena, diseases associated with acute and chronic cerebral or systemic hypoxia, such as stroke, TIA, cerebrovascular disease, COPD, OSA, lung cancer, congestive heart failure, renal failure, and lack of aerobic fitness are all associated with cognitive impairment63,64. Acute cognitive dysfunction also occurs in patients in the intensive care setting who have not undergone surgery or anesthesia5.
Cellular Hypoxic Response
Protective responses to hypoxia are controlled at the cellular level by hypoxia-inducible factors (HIF)65 (figure 1). Hypoxia immediately stabilizes the various isoforms of HIF-alpha (HIF-1α, HIF-2α, HIF-3α) to regulate acute and chronic responses to hypoxia. HIF-α controls expression of over 600 genes66 including EPO and vascular endothelial growth factor (VEGF), metabolic switching proteins like glucose transporter-1 and lactate dehydrogenase-A, vasoactive nitric oxide, and reactive oxygen species (ROS) generating nicotinamide adenine dinucleotide phosphate oxidase (NOX).
Figure 1: Cellular Hypoxic Response.
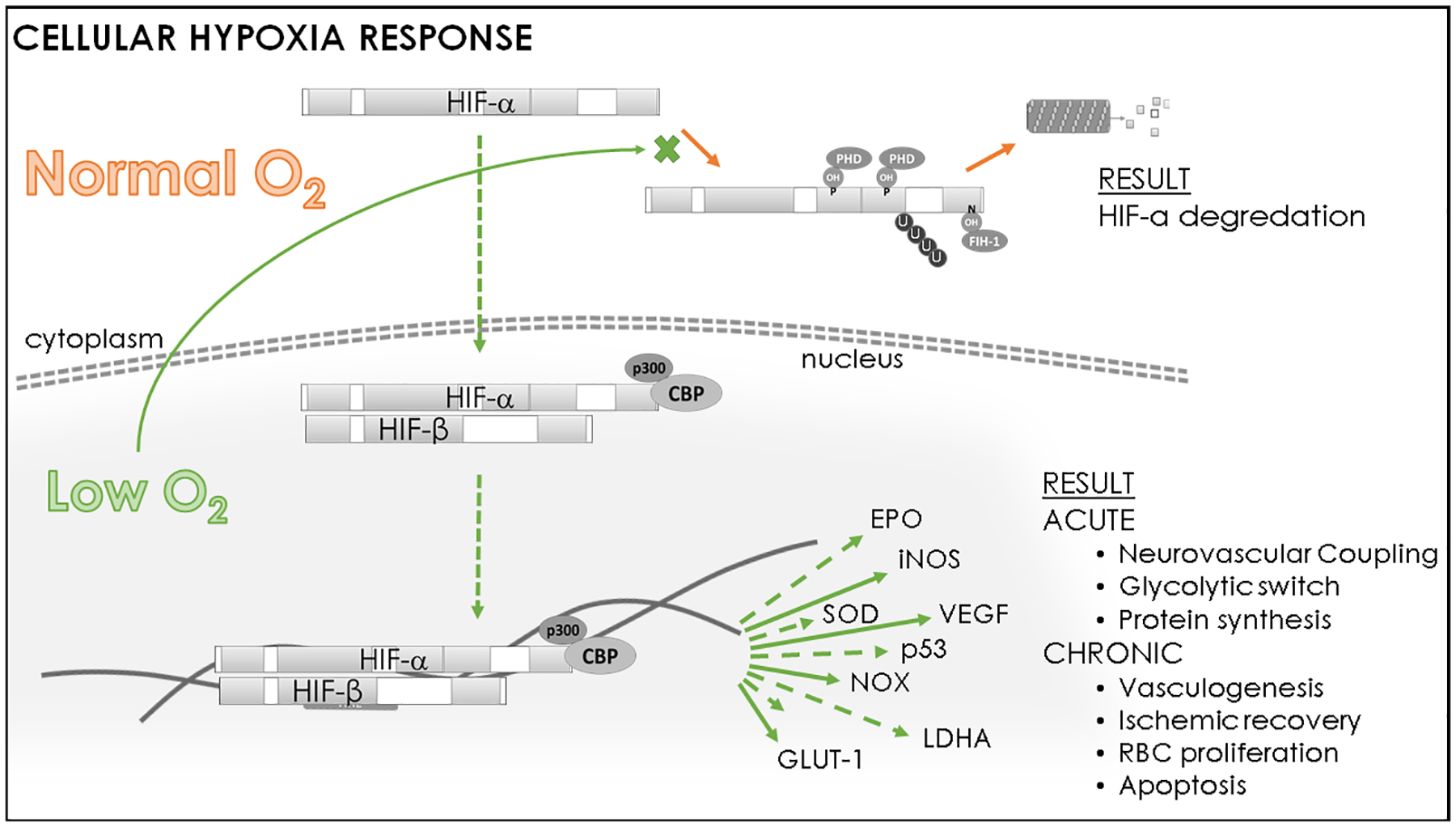
In healthy cells, hypoxia inducible factor alpha (HIF-α) is targeted to degradation under normal oxygenation by prolyl hydroxylases (PHD) and factor-inhibiting HIF protein (FIH-1). However, hypoxia prevents hydroxylation and stabilizes HIF-α, allowing it to enter the nucleus and bind with coactivators (p300 & creb-binding protein [CBP]) to initiate expression of gene products which improve oxygenation and promote cell survival. Representative gene transcription initiated by HIF-α includes: erythropoietin (EPO), inducible nitric oxide synthase (iNOS), superoxide dismutase (SOD), vascular endothelial growth factor (VEGF), nicotinamide adenine dinucleotide phosphate oxidase (NOX), lactose dehydrogenase-A (LDHA), glucose transporter 1 (GLUT-1).
Although their roles overlap to some degree, the expression ratio of the isoforms modulates acute or chronic hypoxic outcomes to match oxygen supply with metabolic needs. For example, elevated HIF-1α:HIF-2α expression within carotid bodies is an integral component of the hypertensive response to chronic intermittent hypoxia, causing elevated sympathetic excitatory transmission67, whereas HIF-2α is the primary modulator of EPO within astrocytes and is integral to maintaining memory68. Mitochondrial function under hypoxia is mediated by the various HIF-α isoforms, and dysfunction (i.e. impaired membrane potential, lower number, aberrant morphology) is implicated in a number of diseases and cognitive failure69. Cognitive failure associated with hypoxia may be overcome by mimicking the effects of HIF-α, such as by administration of EPO58
Effect of Cerebrovascular Impairment
The cerebrovascular response to hypoxemia is vasodilation to increase cerebral blood flow and oxygen delivery70. In the case of anemia, the marked increase in cerebral blood flow (CBF) is driven primarily by cerebral oxygen demand71. This global response appears to be largely intact with aging72–74. Evidence abounds that limitations in regional cerebrovascular reserve75 could potentially contribute to cerebral hypoxia and/or ischemia under multiple scenarios, to include severe anemia76,77, hypotension, low cardiac output, and intra- and extracranial cerebrovascular occlusive disease78. Conversely, intentional isovolemic hemodilution in the management of acute ischemic stroke does not appear to worsen or improve outcomes79.
Progressive cognitive decline is observed as a consequence of chronic cerebral hypoperfusion, even in the absence of acute stroke/TIA30,31. The effect of chronic diseases of the vasculature (e.g., small vessel disease, carotid disease, atherosclerosis, endothelial dysfunction, deficient cerebral autoregulation,80 amyloidosis81, integrity of the blood brain barrier [BBB]82) on the brain are observed in reliable neuroimaging (MRI) markers, including small punctate lesions, microbleeds, and white matter hyperintensities (WMH, also called leukoarioisis, and lacunes)82. Zhong et al. found that increased severity of carotid disease was associated with higher risk of cognitive impairment during a 10-year follow up83. WMH and lacunes (small subcortical cavities arising from arterial disease) have been associated with general cognitive impairment and decline in information processing speed and executive function82,84 and WMH with increased risk for mild cognitive impairment and dementia82,85.
Similarly, deficient hemoglobin saturation or diminished release of oxygen at tissue sites due to anemia contributes to poor cognitive outcomes47. In fact, multiple hypoxic sources can coexist as evidenced by an elevated risk of developing WMH due to anemia55,86, which is exacerbated by coexistent hypertension77,86. Further, the age dependent elevation of cortical HIF (in spite of preserved tissue oxygenation) in a chronically hypertensive rat model of acute isovolemic hemodilution, suggests hypertension and anemia interact to cause a failure of oxygen delivery equated with cellular hypoxic states77.
The terms vascular cognitive impairment, vascular dementia, and Binswanger’s disease are all used to denote progressive decline in cognition from chronic vascular disease87,88. These disorders are similar to Alzheimer’s disease (AD) in that cognitive impairment progresses slowly over time. However, these disorders differ from AD in that the cognitive impairment profile is notable for deficits in processing speed and executive function, as opposed to episodic memory impairment and the loss of recognition memory observed in AD.
More recent conceptualizations of dementia recognize the complexity of neurodegenerative neuropathology and include a prominent role for vascular pathology/cerebral ischemia89. Current research suggests that cerebrovascular pathology has a dose-dependent effect on cognition82,84,90,91, independent of other pathologies92. These observations have led to development of the concept of “vascular burden”, a general term that refers to the cumulative effect of vascular disorders and risk factors including stroke, hypertension, white matter disease, diabetes mellitus, obesity,30,31,36 as well as vascular reactivity93 on the degree of cognitive impairment and age-related brain atrophy84,94. Consequently, investigators have focused on understanding the role of cerebrovascular pathologies in AD and have discovered that cerebrovascular disease reduces the threshold of AD-specific pathologic burden of cortical pathology (e.g., cortical amyloid plaques, cortical atrophy) needed to produce cognitive impairment84,95.
Impaired Connectivity
Even in the surgical arena, recent studies underscore the relevance of vascular burden for understanding POCD. Accumulating evidence shows that presurgical neuroimaging markers of general vascular health96, cerebral ischemia97, and presurgical cognitive status are all strong predictors of POCD97,98. A recent systematic review of 15 neuroimaging (MRI) studies44 reported that POCD was more frequently associated with presurgical imaging markers of cerebrovascular ischemia (WMH) than neuroimaging markers of neurodegenerative changes (i.e., global and regional brain volumes). Therefore, mechanisms which hinder cerebral vascularization may be of particular interest in future investigations of POCD.
Cerebral white matter is critical for rapid signaling among both close and distant neuronal circuits. Disturbances indicated by WMH disrupt neuronal transmission and functional network connectivity, leading to widespread cerebral dysfunction and cognitive impairment99. For instance, in patients with chronic ischemia due to carotid artery stenosis but without overt indicators of clinical stroke, MRI network analyses show imaging patterns associated with cognitive impairment in the form of impaired cerebral connectivity100 and altered resting state blood oxygen level dependent (BOLD) signal101.
Neurons have a high metabolic rate, resulting in a need for rapid and precise regulation of CBF102 and making them particularly vulnerable to damage from both acute and chronic hypoxia. Oligodendrocytes, glial cells that compose the cerebral white matter, may be even more sensitive than neurons103. Acute and complete oxygen deprivation to cerebral tissue downstream of an ischemic insult, such as a major clinical stroke, can result in relatively focal cell death (infarct) within minutes, leading to disruption of focal and widespread cognitive and sensorimotor functions, dependant upon the infarct size and location102. Astrocytes modulate synapses, neurovascular coupling, and transport of molecules across the BBB and are highly sensitive to hypoxia, altering glucose uptake and BBB integrity to fulfill energetic needs of neurons102,104.
Regional responses to cerebral hypoxia often differ. The severity of OSA has been correlated with hypoperfusion of lateral cortical regions in elderly patients, but hyperperfusion of medial and subcortical regions105 which may be a contributing factor in the loss of cortical and hippocampal gray matter associated with cognitive dysfunction in OSA106. Additionally, sleep disordered breathing damages cerebellar and hypothalamic control of sympathetic tone107 and is associated with diminished working memory108. In HF, low CBF is observed in the posterior hippocampal regions109, resulting in depressed mood and impairment of delayed and immediate recall109,110.
Excitotoxicity
Elevated ROS from hypoxia induces cytokine transcription and elevates intracellular calcium69,111,112. These molecules modulate the composition and number of postsynaptic excitatory and inhibitory receptors107, leading to more frequent excitatory postsynaptic potentials and altered synaptic connectivity. Excitotoxicity induces the neuronal loss associated with cognitive dysfunction observed in hypoxia108,112. Excitotoxicity is reported in the brainstem, cerebellar Purkinje neurons, and memory centers of the central nervous system (CNS) during hypoxia107. Cholinergic neurons (integral to many cognitive pathways) appear to be particularly vulnerable to excitotoxicity as fewer are evident in the forebrain of young adult male rats following as little as fourteen days of chronic intermittent hypoxia and their loss contributes to impairments in spatial working memory113.
Neuroinflammation
Many studies demonstrate that hypoxia induces inflammation both systemically and centrally114,115. Neuroinflammation arising from hypoxia is initiated by HIF-α116, may be of neuronal or glial origin and is correlated with cognitive impairment111,117. Cognitive effects are attenuated by administrated by EPO118. Neuroinflammation is often proposed as a central culprit in the onset of POCD7. These overlaps provide further evidence cerebral hypoxia may be at the epicenter of mechanisms of POCD.
Interaction between Aging and Hypoxia
Older adults are particularly vulnerable to perioperative hypoxia as aging is the leading risk factor for POCD13 and older adults are at highest risk of needing surgical intervention. Dysregulation is observed in cerebrovascular reactivity119, oxygen delivery120–122, neural connectivity82, neuroinflammation123, and responsiveness to hypoxia124 during aging (figure 2). Current literature suggests that protective molecular processes fail to respond to hypoxic conditions during aging and may exacerbate negative cognitive outcomes.
Figure 2: Interaction between age, hypoxia, and protective responses.

Studies have demonstrated age-related impairments in molecular responses to hypoxia and vascular reserve at the same time onset of diseases which cause hypoxia increase. This interaction may predispose aged adults to cognitive impairment when exposed to hypoxic insults. chronic obstructive pulmonary disease (COPD), cerebrovascular disease (CVD) obstructive sleep apnea (OSA) heart failure (HF)
Aging Impairs Oxygenation
Of primary concern is the contribution of hypertension, loss of myogenic tone, accumulated plaque deposition, and deficient response to vasopressin or nitric oxide to reduced cerebrovascular reactivity and reserve119 during aging, in spite of the fact that cerebral metabolic rate of oxygen (CMRO2) remains constant over the lifespan125. Low cerebral oxygenation and worsening cerebral hypoxia is evident even in older adults without diagnosed hypoxia and is associated with memory impairment126. Further, unlike young adults, lower CBF is associated with inattention in older individuals120. Indeed, low resting CBF in older adults is associated with higher WMH volume127 and is a strong predictor of the number of newly developed cortical WMH observed 18 months later128. The loss of cerebrovascular reserve in the elderly may thus impair their ability to recover from perioperative hypoxic stresses.
Preclinical studies indicate the oxygen pressure within cerebral tissue declines in middle age and continues into old age129. The oxygen pressure setpoint in the CNS has a narrow range and extremes lead to vascular dysregulation. Temporary fluctuations in tissue oxygenation early in adulthood have been linked to rapid subsequent vascular dysregulation (e.g., hypertension) and working memory impairment that is not evident until later in life130,131.
In addition to hypoperfusion, aging is associated with more frequent bouts of anemia as nutritional intake declines, gastrointestinal tract disorders and pharmaceutical intake increase, and hormone levels change122. Although Price et al.121 found no difference in circulating EPO in non-anemic individuals of any age, EPO concentration was lower in elderly, but not young, anemic patients. Other studies suggest EPO production slows with age and coincides with lower cerebral metabolism122. Additionally, aged spontaneously hypertensive rats experienced greater memory impairment and evidence of cellular hypoxia (elevated HIF expression) after anemia caused by isovolemic hemodilution which is not evident in their younger adult counterparts77.
Aging Impairs the Hypoxic Response
Elegant studies performed by the LaManna lab demonstrate the normal cortical response to hypoxia stabilizes HIF-α leading to increased capillary density, blood flow, and glycolysis (figure 1)132. Unfortunately, a blunted or, in some cases, nonexistent response to hypoxia mediated by HIF-α is observed in the aged brain66,124,133. Aged rats exhibit low VEGF in carotid bodies under normal oxygen conditions and an attenuated response to 12 hours of 12% oxygen for 12 days than young rats do133. Cortical vascularization and expression of HIF-controlled proteins (e.g. EPO, inducible nitric oxide synthase, and heme oxygenase-1) following hypoxia are lacking in aged models66,133 in combination with fewer and smaller mitochondria in carotid bodies133. Further, prolyl hydroxylases which regulate HIF-α expression also lose responsivity during aging134. Therefore, the primary adaptive hypoxia pathway may be particularly susceptible to acute and chronic hypoxic insults during aging. Investigations using aged pre-clinical models to investigate HIF-α mechanisms in specific brain regions and cell types are scarce and represent an area of active inquiry.
Aging Induces Inflammation and Impairs Neurotransmission
Aged rodent and postmortem human brain samples exhibit low grade inflammation and aberrant glial morphology123,135..Recent studies suggest glial cells are particularly vulnerable to aging and hypoxia104 and typical neuroprotective glial activities136 are diminished while reactive and senescent phenotypes flourish in aged brains137,138. Damage to oligodendrocytes and astrocytes affects both dendritic spine stability and diffuse connectivity. Impaired astrocytic support combined with reduced cardiovascular reserve, vascularization and hypoxic responsiveness renders aged neurons more vulnerable to hypoxic insults than young neurons, as evidenced by diminished excitatory output, smaller post-synaptic density, and lower tissue volume in aged hippocampi137,139.
Management of Hypoxia related POCD
Thankfully, long term cognitive impairment after surgery appears to be a very infrequent phenomenon, with most resolving within weeks to months. Ischemic-hypoxic events may pose the greatest risk for long term effects, while lingering anemia and cardiorespiratory failure in recovery may also contribute. Management of hypoxia-related POCD starts with recognition of the contribution of hypoxia to cognitive performance and identification of those at risk. While aging has been recognized as a major risk factor, older patients with superimposed cerebrovascular disease, chronic anemia, and those with pre-existing cardio-respiratory failure identify subgroups at the highest levels of risk. Those at the highest levels of risk may be further stratified by preoperative cognitive evaluation, as preoperative cognitive status in and of itself appears to be a risk factor for further decline140.
Management of patients at risk for hypoxia-related POCD should start with prevention via optimization of pre-existing disease that carries the hypoxic burden. The strong relationship between cognitive performance, cardiovascular fitness141 and cardiovascular disease in the general population142 clearly demonstrates their interdependence. Prerehabilitation programs have shown benefit in reducing overall and pulmonary morbidity and may therefore impact hypoxia-related cognitive performance after surgery143. Patients at risk with pre-existing anemia may benefit from preoperative supplementation and possibly EPO58,144.
Intraoperative efforts should obviously focus on prevention of hypoxemia and heightened attention to the predisposition of certain high-risk groups such as those with morbid obesity and those undergoing lung transplantation. Intraoperative use of cerebral oximetry has yet to clearly demonstrate benefit in prevention of POCD145. We remain without high quality guidance as to a patient-specific transfusion trigger to guide us either intraoperatively or postoperatively for organ protection, brain or otherwise. Advances in the approach to the heavily calcified aorta146 and use of intra-arterial emboli trapping devices to date have not been as effective as originally hoped in altering the frequency of perioperative stroke and POCD147, likely due to the fact that stroke risk continues beyond the critical period of aortic manipulation.
Postoperatively, it can be assumed that physicians already attempt to optimize cardiopulmonary function and carefully balance the risks and benefits of transfusion in their patients. In the management of anemia, perhaps more aggressive approaches to iron replacement and the use of EPO should be considered to speed the recovery of erythrocyte volume. Enhanced attention to the impact of narcotic use on pulmonary function in those with respiratory compromise from multiple etiologies may include more aggressive and prolonged respiratory monitoring, administration of supplemental oxygen, and advancement of lower or nonnarcotic approaches to pain control148. Earlier and more accurate diagnosis of perioperative stroke may be enhanced by anesthetic techniques which allow for immediate postoperative assessment149, implementation of more frequent and rigorous stroke assessment protocols40, and judicious application of advances in interventional strategies150
Conclusion
There is voluminous evidence that short to intermediate term age-related changes in cognition occur in the perioperative arena. However, the evidence connecting those changes to modern anesthetic use is tenuous. Aging is accompanied by hypoxic conditions which have been firmly established as risk factors for cognitive dysfunction outside the surgical arena. Surgery, as well as critical illness, may elevate hypoxic exposure through hypoperfusion, hypoventilation, pulmonary edema, and blood loss. Given these parallels, it is therefore conceivable that hypoxia, in its many forms, contributes importantly to POCD and may share similar mechanisms with hypoxia-related cognitive failure in the general population. Future studies fully elucidating the role of hypoxia in age-related memory loss in chronic and acute settings may also clarify the role of hypoxia in POCD.
Glossary of Terms:
- AD
Alzheimer’s Disease
- BBB
blood brain barrier
- BOLD
blood oxygenation level dependent imaging
- CBF
cerebral blood flow
- CMRO2
cerebral metabolic rate of oxygen
- CNS
central nervous system
- COPD
chronic obstructive pulmonary disease
- CPB
cardiopulmonary bypass
- DWI
diffusion weighted imaging
- EPO
erythropoietin
- HH
hypoxemic hypoxia
- HIF
hypoxic inducible factor
- HF
heart failure
- MRI
magnetic resonance imaging
- NOX
nicotinamide adenine dinucleotide phosphate oxidase
- OSA
obstructive sleep apnea
- POCD
Postoperative Cognitive Dysfunction
- ROS
reactive oxygen species
- SaO2
arterial hemoglobin oxygen saturation
- TIA
transient ischemic attack
- VEGF
vascular endothelial growth factor
- WMH
white matter hyperintensities
Footnotes
Conflicts of Interest: None
References
- 1.Fink HA, Hemmy LS, MacDonald R, et al. Intermediate- and Long-Term Cognitive Outcomes After Cardiovascular Procedures in Older Adults: A Systematic Review. Ann Intern Med. 2015;163(2):107–117. [DOI] [PubMed] [Google Scholar]
- 2.Giovannetti T, Price CC, Fanning M, et al. Cognition and Cerebral Infarction in Older Adults After Surgical Aortic Valve Replacement. Ann Thorac Surg. 2019;107(3):787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dokkedal U, Hansen TG, Rasmussen LS, Mengel-From J, Christensen K. Cognitive Functioning after Surgery in Middle-aged and Elderly Danish Twins. Anesthesiology. 2016;124(2):312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avidan MS, Evers AS. The Fallacy of Persistent Postoperative Cognitive Decline. Anesthesiology. 2016;124(2):255–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krause BM, Sabia S, Manning HJ, Singh-Manoux A, Sanders RD. Association between major surgical admissions and the cognitive trajectory: 19 year follow-up of Whitehall II cohort study. BMJ. 2019;366:l4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avidan MS, Searleman AC, Storandt M, et al. Long-term cognitive decline in older subjects was not attributable to noncardiac surgery or major illness. Anesthesiology. 2009;111(5):964–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safavynia SA, Goldstein PA. The Role of Neuroinflammation in Postoperative Cognitive Dysfunction: Moving From Hypothesis to Treatment. Front Psychiatry. 2018;9(752):752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sprung J, Schulte PJ, Knopman DS, et al. Cognitive function after surgery with regional or general anesthesia: A population-based study. Alzheimers Dement. 2019;15(10):1243–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasmussen LS, Johnson T, Kuipers HM, et al. Does anaesthesia cause postoperative cognitive dysfunction? A randomised study of regional versus general anaesthesia in 438 elderly patients. Acta Anaesthesiol Scand. 2003;47(3):260–266. [DOI] [PubMed] [Google Scholar]
- 10.Miller D, Lewis SR, Pritchard MW, et al. Intravenous versus inhalational maintenance of anaesthesia for postoperative cognitive outcomes in elderly people undergoing non-cardiac surgery. Cochrane Database Syst Rev. 2018;8:CD012317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selnes OA, Gottesman RF, Grega MA, Baumgartner WA, Zeger SL, McKhann GM. Cognitive and neurologic outcomes after coronary-artery bypass surgery. N Engl J Med. 2012;366(3):250–257. [DOI] [PubMed] [Google Scholar]
- 12.Schulte PJ, Roberts RO, Knopman DS, et al. Association between exposure to anaesthesia and surgery and long-term cognitive trajectories in older adults: report from the Mayo Clinic Study of Aging. Br J Anaesth. 2018;121(2):398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351(9106):857–861. [DOI] [PubMed] [Google Scholar]
- 14.Chung F, Liao P, Yegneswaran B, Shapiro CM, Kang W. Postoperative changes in sleep-disordered breathing and sleep architecture in patients with obstructive sleep apnea. Anesthesiology. 2014;120(2):287–298. [DOI] [PubMed] [Google Scholar]
- 15.Chung F, Liao P, Yang Y, et al. Postoperative sleep-disordered breathing in patients without preoperative sleep apnea. Anesth Analg. 2015;120(6):1214–1224. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Sands LP, Vaurio L, Mullen EA, Leung JM. The effects of postoperative pain and its management on postoperative cognitive dysfunction. Am J Geriatr Psychiatry. 2007;15(1):50–59. [DOI] [PubMed] [Google Scholar]
- 17.Ehrenfeld JM, Funk LM, Van Schalkwyk J, Merry AF, Sandberg WS, Gawande A. The incidence of hypoxemia during surgery: evidence from two institutions. Can J Anaesth. 2010;57(10):888–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun Z, Sessler DI, Dalton JE, et al. Postoperative Hypoxemia Is Common and Persistent: A Prospective Blinded Observational Study. Anesth Analg. 2015;121(3):709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moller JT, Johannessen NW, Espersen K, et al. Randomized evaluation of pulse oximetry in 20,802 patients: II. Perioperative events and postoperative complications. Anesthesiology. 1993;78(3):445–453. [DOI] [PubMed] [Google Scholar]
- 20.Lin R, Zhang F, Xue Q, Yu B. Accuracy of regional cerebral oxygen saturation in predicting postoperative cognitive dysfunction after total hip arthroplasty: regional cerebral oxygen saturation predicts POCD. J Arthroplasty. 2013;28(3):494–497. [DOI] [PubMed] [Google Scholar]
- 21.Casati A, Fanelli G, Pietropaoli P, et al. Monitoring cerebral oxygen saturation in elderly patients undergoing general abdominal surgery: a prospective cohort study. Eur J Anaesthesiol. 2007;24(1):59–65. [DOI] [PubMed] [Google Scholar]
- 22.Browne SM, Halligan PW, Wade DT, Taggart DP. Postoperative hypoxia is a contributory factor to cognitive impairment after cardiac surgery. J Thorac Cardiovasc Surg. 2003;126(4):1061–1064. [DOI] [PubMed] [Google Scholar]
- 23.Areza-Fegyveres R, Kairalla RA, Carvalho CRR, Nitrini R. Cognition and chronic hypoxia in pulmonary diseases. Dement Neuropsychol. 2010;4(1):14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grant I, Heaton RK, McSweeny AJ, Adams KM, Timms RM. Neuropsychologic findings in hypoxemic chronic obstructive pulmonary disease. Arch Intern Med. 1982;142(8):1470–1476. [PubMed] [Google Scholar]
- 25.Gagnon K, Baril AA, Gagnon JF, et al. Cognitive impairment in obstructive sleep apnea. Pathologie-biologie. 2014;62(5):233–240. [DOI] [PubMed] [Google Scholar]
- 26.Kheirandish-Gozal L, Philby MF, Alonso-Alvarez ML, Teran-Santos J, Gozal D. Biomarkers of Alzheimer Disease in Children with Obstructive Sleep Apnea: Effect of Adenotonsillectomy. Sleep. 2016;39(6):1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cannon JA, Moffitt P, Perez-Moreno AC, et al. Cognitive Impairment and Heart Failure: Systematic Review and Meta-Analysis. J Card Fail. 2017;23(6):464–475. [DOI] [PubMed] [Google Scholar]
- 28.Hammond CA, Blades NJ, Chaudhry SI, et al. Long-Term Cognitive Decline After Newly Diagnosed Heart Failure: Longitudinal Analysis in the CHS (Cardiovascular Health Study). Circ Heart Fail. 2018;11(3):e004476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kindermann I, Fischer D, Karbach J, et al. Cognitive function in patients with decompensated heart failure: the Cognitive Impairment in Heart Failure (CogImpair-HF) study. Eur J Heart Fail. 2012;14(4):404–413. [DOI] [PubMed] [Google Scholar]
- 30.Sahathevan R, Brodtmann A, Donnan GA. Dementia, stroke, and vascular risk factors; a review. Int J Stroke. 2012;7(1):61–73. [DOI] [PubMed] [Google Scholar]
- 31.Lo Coco D, Lopez G, Corrao S. Cognitive impairment and stroke in elderly patients. Vasc Health Risk Manag. 2016;12:105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jokinen H, Melkas S, Ylikoski R, et al. Post-stroke cognitive impairment is common even after successful clinical recovery. Eur J Neurol. 2015;22(9):1288–1294. [DOI] [PubMed] [Google Scholar]
- 33.Nys GM, van Zandvoort MJ, de Kort PL, Jansen BP, de Haan EH, Kappelle LJ. Cognitive disorders in acute stroke: prevalence and clinical determinants. Cerebrovasc Dis. 2007;23(5–6):408–416. [DOI] [PubMed] [Google Scholar]
- 34.Klimkowicz-Mrowiec A, Dziedzic T, Slowik A, Szczudlik A. Predictors of poststroke dementia: results of a hospital-based study in poland. Dement Geriatr Cogn Disord. 2006;21(5–6):328–334. [DOI] [PubMed] [Google Scholar]
- 35.van Rooij FG, Kessels RP, Richard E, De Leeuw FE, van Dijk EJ. Cognitive Impairment in Transient Ischemic Attack Patients: A Systematic Review. Cerebrovasc Dis. 2016;42(1–2):1–9. [DOI] [PubMed] [Google Scholar]
- 36.Pendlebury ST. Dementia in patients hospitalized with stroke: rates, time course, and clinico-pathologic factors. Int J Stroke. 2012;7(7):570–581. [DOI] [PubMed] [Google Scholar]
- 37.Mashour GA, Shanks AM, Kheterpal S. Perioperative stroke and associated mortality after noncardiac, nonneurologic surgery. Anesthesiology. 2011;114(6):1289–1296. [DOI] [PubMed] [Google Scholar]
- 38.NeuroVISION I. Perioperative covert stroke in patients undergoing non-cardiac surgery (NeuroVISION): a prospective cohort study. Lancet. 2019;394(10203):1022–1029. [DOI] [PubMed] [Google Scholar]
- 39.van Dijk D, Keizer AM, Diephuis JC, Durand C, Vos LJ, Hijman R. Neurocognitive dysfunction after coronary artery bypass surgery: a systematic review. J Thorac Cardiovasc Surg. 2000;120(4):632–639. [DOI] [PubMed] [Google Scholar]
- 40.Messe SR, Acker MA, Kasner SE, et al. Stroke after aortic valve surgery: results from a prospective cohort. Circulation. 2014;129(22):2253–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Indja B, Woldendorp K, Vallely MP, Grieve SM. Silent Brain Infarcts Following Cardiac Procedures: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2019;8(9):e010920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smilowitz NR, Gupta N, Ramakrishna H, Guo Y, Berger JS, Bangalore S. Perioperative Major Adverse Cardiovascular and Cerebrovascular Events Associated With Noncardiac Surgery. JAMA Cardiol. 2017;2(2):181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bang OY, Li WY. Applications of diffusion-weighted imaging in diagnosis evaluation, and treatment of acute ischemic stroke. Precision and Future Medicine. 2019;3(2):69–76. [Google Scholar]
- 44.Kant IMJ, de Bresser J, van Montfort SJT, Slooter AJC, Hendrikse J. MRI Markers of Neurodegenerative and Neurovascular Changes in Relation to Postoperative Delirium and Postoperative Cognitive Decline. Am J Geriatr Psychiatry. 2017;25(10):1048–1061. [DOI] [PubMed] [Google Scholar]
- 45.Cook DJ, Huston J 3rd, Trenerry MR, Brown RD Jr., Zehr KJ, Sundt TM 3rd. Postcardiac surgical cognitive impairment in the aged using diffusion-weighted magnetic resonance imaging. Ann Thorac Surg. 2007;83(4):1389–1395. [DOI] [PubMed] [Google Scholar]
- 46.Knipp SC, Matatko N, Wilhelm H, et al. Cognitive outcomes three years after coronary artery bypass surgery: relation to diffusion-weighted magnetic resonance imaging. Ann Thorac Surg. 2008;85(3):872–879. [DOI] [PubMed] [Google Scholar]
- 47.Weiskopf RB, Kramer JH, Viele M, et al. Acute severe isovolemic anemia impairs cognitive function and memory in humans. Anesthesiology. 2000;92(6):1646–1652. [DOI] [PubMed] [Google Scholar]
- 48.Kim HB, Park B, Shim JY. Anemia in Association with Cognitive Impairment: A Systematic Review and Meta-Analysis. J Alzheimers Dis. 2019;72(3):803–814. [DOI] [PubMed] [Google Scholar]
- 49.Padberg I, Schneider A, Rohmann JL, Kelley SW, Grittner U, Siegerink B. Impact of COPD and anemia on motor and cognitive performance in the general older population: results from the English longitudinal study of ageing. Respir Res. 2020;21(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mancuso A, Migliorino M, De Santis S, Saponiero A, De Marinis F. Correlation between anemia and functional/cognitive capacity in elderly lung cancer patients treated with chemotherapy. Ann Oncol. 2006;17(1):146–150. [DOI] [PubMed] [Google Scholar]
- 51.Kurella Tamura M, Vittinghoff E, Yang J, et al. Anemia and risk for cognitive decline in chronic kidney disease. BMC Nephrol. 2016;17:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bahrami A, Khorasanchi Z, Tayefi M, et al. Anemia is associated with cognitive impairment in adolescent girls: A cross-sectional survey. Appl Neuropsychol Child. 2020;9(2):165–171. [DOI] [PubMed] [Google Scholar]
- 53.Agrawal S, Kumar S, Ingole V, et al. Does anemia affects cognitive functions in neurologically intact adult patients: Two year cross sectional study at rural tertiary care hospital. J Family Med Prim Care. 2019;8(9):3005–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Son SJ, Lee KS, Na DL, et al. The effect of anemia and white matter hyperintensities (WMH) on cognitive impairment in patients with amnestic mild cognitive impairment (MCI). Arch Gerontol Geriatr. 2012;55(2):251–256. [DOI] [PubMed] [Google Scholar]
- 55.Deal JA, Carlson MC, Xue QL, Fried LP, Chaves PH. Anemia and 9-year domain-specific cognitive decline in community-dwelling older women: The Women’s Health and Aging Study II. Journal of the American Geriatrics Society. 2009;57(9):1604–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schneider AL, Jonassaint C, Sharrett AR, et al. Hemoglobin, Anemia, and Cognitive Function: The Atherosclerosis Risk in Communities Study. J Gerontol A Biol Sci Med Sci. 2016;71(6):772–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meng F, Zhang S, Yu J, et al. Low Hemoglobin Levels at Admission Are Independently Associated with Cognitive Impairment after Ischemic Stroke: a Multicenter, Population-Based Study. Transl Stroke Res. 2020;11(5):890–899. [DOI] [PubMed] [Google Scholar]
- 58.Hernandez CC, Burgos CF, Gajardo AH, et al. Neuroprotective effects of erythropoietin on neurodegenerative and ischemic brain diseases: the role of erythropoietin receptor. Neural Regen Res. 2017;12(9):1381–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karkouti K, Wijeysundera DN, Beattie WS, Reducing Bleeding in Cardiac Surgery I. Risk associated with preoperative anemia in cardiac surgery: a multicenter cohort study. Circulation. 2008;117(4):478–484. [DOI] [PubMed] [Google Scholar]
- 60.Mathew JP, Mackensen GB, Phillips-Bute B, et al. Effects of extreme hemodilution during cardiac surgery on cognitive function in the elderly. Anesthesiology. 2007;107(4):577–584. [DOI] [PubMed] [Google Scholar]
- 61.Mundy GM, Birtwistle SJ, Power RA. The effect of iron supplementation on the level of haemoglobin after lower limb arthroplasty. J Bone Joint Surg Br. 2005;87(2):213–217. [DOI] [PubMed] [Google Scholar]
- 62.Shoemaker WC, Appel PL, Kram HB. Role of oxygen debt in the development of organ failure sepsis, and death in high-risk surgical patients. Chest. 1992;102(1):208–215. [DOI] [PubMed] [Google Scholar]
- 63.Katschinski DM. Is there a molecular connection between hypoxia and aging? Experimental gerontology. 2006;41(5):482–484. [DOI] [PubMed] [Google Scholar]
- 64.Arjun R, Acharya S, Shender BS, Rorres C, Hrebien L, Kam M. Correlation of Cognitive Scores and the Onset of Hypoxia. Aerosp Med Hum Perform. 2019;90(5):429–439. [DOI] [PubMed] [Google Scholar]
- 65.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anderson J, Sandhir R, Hamilton ES, Berman NE. Impaired expression of neuroprotective molecules in the HIF-1alpha pathway following traumatic brain injury in aged mice. J Neurotrauma. 2009;26(9):1557–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Semenza GL, Prabhakar NR. Neural regulation of hypoxia-inducible factors and redox state drives the pathogenesis of hypertension in a rodent model of sleep apnea. J Appl Physiol (1985). 2015;119(10):1152–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leiton CV, Chen E, Cutrone A, et al. Astrocyte HIF-2alpha supports learning in a passive avoidance paradigm under hypoxic stress. Hypoxia (Auckland, NZ). 2018;6:35–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Herst PM, Rowe MR, Carson GM, Berridge MV. Functional Mitochondria in Health and Disease. Frontiers in endocrinology. 2017;8:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Juttukonda MR, Donahue MJ. Neuroimaging of vascular reserve in patients with cerebrovascular diseases. Neuroimage. 2019;187:192–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Waschke KF, Krieter H, Hagen G, Albrecht DM, Van Ackern K, Kuschinsky W. Lack of dependence of cerebral blood flow on blood viscosity after blood exchange with a Newtonian O2 carrier. J Cereb Blood Flow Metab. 1994;14(5):871–876. [DOI] [PubMed] [Google Scholar]
- 72.Li M, Ratcliffe SJ, Knoll F, et al. Aging: impact upon local cerebral oxygenation and blood flow with acute isovolemic hemodilution. J Neurosurg Anesthesiol. 2006;18(2):125–131. [DOI] [PubMed] [Google Scholar]
- 73.Floyd TF, McGarvey M, Ochroch EA, et al. Perioperative changes in cerebral blood flow after cardiac surgery: influence of anemia and aging. Ann Thorac Surg. 2003;76(6):2037–2042. [DOI] [PubMed] [Google Scholar]
- 74.McKetton L, Cohn M, Tang-Wai DF, et al. Cerebrovascular Resistance in Healthy Aging and Mild Cognitive Impairment. Front Aging Neurosci. 2019;11:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nemoto EM, Yonas H, Kuwabara H, et al. Identification of hemodynamic compromise by cerebrovascular reserve and oxygen extraction fraction in occlusive vascular disease. J Cereb Blood Flow Metab. 2004;24(10):1081–1089. [DOI] [PubMed] [Google Scholar]
- 76.Dexter F, Hindman BJ. Effect of haemoglobin concentration on brain oxygenation in focal stroke: a mathematical modelling study. Br J Anaesth. 1997;79(3):346–351. [DOI] [PubMed] [Google Scholar]
- 77.Li M, Bertout JA, Ratcliffe SJ, Eckenhoff MF, Simon MC, Floyd TF. Acute anemia elicits cognitive dysfunction and evidence of cerebral cellular hypoxia in older rats with systemic hypertension. Anesthesiology. 2010;113(4):845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yonas H, Pindzola RR. Clinical application of cerebrovascular reserve assessment as a strategy for stroke prevention. Keio J Med. 2000;49 Suppl 1:A4–10. [PubMed] [Google Scholar]
- 79.Aichner FT, Fazekas F, Brainin M, Polz W, Mamoli B, Zeiler K. Hypervolemic hemodilution in acute ischemic stroke: the Multicenter Austrian Hemodilution Stroke Trial (MAHST). Stroke. 1998;29(4):743–749. [DOI] [PubMed] [Google Scholar]
- 80.Chang XL, Zhou HQ, Lei CY, et al. Association between asymptomatic carotid stenosis and cognitive function: a systematic review. Neurosci Biobehav Rev. 2013;37(8):1493–1499. [DOI] [PubMed] [Google Scholar]
- 81.Viswanathan A, Greenberg SM. Cerebral amyloid angiopathy in the elderly. Ann Neurol. 2011;70(6):871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: An update. Nat Rev Neurol. 2015;11(3):157–165. [DOI] [PubMed] [Google Scholar]
- 83.Zhong W, Cruickshanks KJ, Schubert CR, et al. Carotid atherosclerosis and 10-year changes in cognitive function. Atherosclerosis. 2012;224(2):506–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.DeCarli C, Villeneuve S, Maillard P, et al. Vascular Burden Score Impacts Cognition Independent of Amyloid PET and MRI Measures of Alzheimer’s Disease and Vascular Brain Injury. J Alzheimers Dis. 2019;68(1):187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bangen KJ, Thomas KR, Weigand AJ, et al. Pattern of regional white matter hyperintensity volume in mild cognitive impairment subtypes and associations with decline in daily functioning. Neurobiol Aging. 2020;86:134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Inzitari M, Studenski S, Rosano C, et al. Anemia is associated with the progression of white matter disease in older adults with high blood pressure: the cardiovascular health study. Journal of the American Geriatrics Society. 2008;56(10):1867–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Libon DJ, Price CC, Davis Garrett K, Giovannetti T. From Binswanger’s disease to leuokoaraiosis: what we have learned about subcortical vascular dementia. Clin Neuropsychol. 2004;18(1):83–100. [DOI] [PubMed] [Google Scholar]
- 88.Roman GC, Sachdev P, Royall DR, et al. Vascular cognitive disorder: a new diagnostic category updating vascular cognitive impairment and vascular dementia. J Neurol Sci. 2004;226(1–2):81–87. [DOI] [PubMed] [Google Scholar]
- 89.2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020;16(3):391–460. [Google Scholar]
- 90.Libon DJ, Price CC, Giovannetti T, et al. Linking MRI hyperintensities with patterns of neuropsychological impairment: evidence for a threshold effect. Stroke. 2008;39(3):806–813. [DOI] [PubMed] [Google Scholar]
- 91.Allan LM, Rowan EN, Firbank MJ, et al. Long term incidence of dementia, predictors of mortality and pathological diagnosis in older stroke survivors. Brain. 2011;134(Pt 12):3716–3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lo RY, Jagust WJ, Alzheimer’s Disease Neuroimaging I. Vascular burden and Alzheimer disease pathologic progression. Neurology. 2012;79(13):1349–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Silvestrini M, Pasqualetti P, Baruffaldi R, et al. Cerebrovascular reactivity and cognitive decline in patients with Alzheimer disease. Stroke. 2006;37(4):1010–1015. [DOI] [PubMed] [Google Scholar]
- 94.O’Brien J, Reisberg B, Erkinjuntti T. Vascular burden of the brain. Int Psychogeriatr. 2003;15 Suppl 1(S1):7–10. [DOI] [PubMed] [Google Scholar]
- 95.Bangen KJ, Nation DA, Delano-Wood L, et al. Aggregate effects of vascular risk factors on cerebrovascular changes in autopsy-confirmed Alzheimer’s disease. Alzheimers Dement. 2015;11(4):394–403 e391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chu Z, Cheng L, Tong Q. Carotid artery calcification score and its association with cognitive impairment. Clin Interv Aging. 2019;14:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maekawa K, Baba T, Otomo S, Morishita S, Tamura N. Low pre-existing gray matter volume in the medial temporal lobe and white matter lesions are associated with postoperative cognitive dysfunction after cardiac surgery. PLoS One. 2014;9(1):e87375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Price CC, Tanner JJ, Schmalfuss I, et al. A pilot study evaluating presurgery neuroanatomical biomarkers for postoperative cognitive decline after total knee arthroplasty in older adults. Anesthesiology. 2014;120(3):601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen X, Huang L, Ye Q, et al. Disrupted functional and structural connectivity within default mode network contribute to WMH-related cognitive impairment. Neuroimage Clin. 2019;24:102088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cheng HL, Lin CJ, Soong BW, et al. Impairments in cognitive function and brain connectivity in severe asymptomatic carotid stenosis. Stroke. 2012;43(10):2567–2573. [DOI] [PubMed] [Google Scholar]
- 101.Xiao F, Wang T, Gao L, et al. Frequency-Dependent Changes of the Resting BOLD Signals Predicts Cognitive Deficits in Asymptomatic Carotid Artery Stenosis. Front Neurosci. 2018;12:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Iadecola C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron. 2017;96(1):17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pantoni L, Garcia JH, Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke. 1996;27(9):1641–1646; discussion 1647. [DOI] [PubMed] [Google Scholar]
- 104.Lourhmati A, Buniatian GH, Paul C, et al. Age-dependent astroglial vulnerability to hypoxia and glutamate: the role for erythropoietin. PLoS One. 2013;8(10):e77182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Baril AA, Gagnon K, Arbour C, et al. Regional Cerebral Blood Flow during Wakeful Rest in Older Subjects with Mild to Severe Obstructive Sleep Apnea. Sleep. 2015;38(9):1439–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Torelli F, Moscufo N, Garreffa G, et al. Cognitive profile and brain morphological changes in obstructive sleep apnea. Neuroimage. 2011;54(2):787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Harper RM, Kumar R, Ogren JA, Macey PM. Sleep-disordered breathing: effects on brain structure and function. Respir Physiol Neurobiol. 2013;188(3):383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21(7):2442–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Suzuki H, Matsumoto Y, Ota H, et al. Hippocampal Blood Flow Abnormality Associated With Depressive Symptoms and Cognitive Impairment in Patients With Chronic Heart Failure. Circ J. 2016;80(8):1773–1780. [DOI] [PubMed] [Google Scholar]
- 110.Frey A, Sell R, Homola GA, et al. Cognitive Deficits and Related Brain Lesions in Patients With Chronic Heart Failure. JACC Heart Fail. 2018;6(7):583–592. [DOI] [PubMed] [Google Scholar]
- 111.Snyder B, Shell B, Cunningham JT, Cunningham RL. Chronic intermittent hypoxia induces oxidative stress and inflammation in brain regions associated with early-stage neurodegeneration. Physiol Rep. 2017;5(9):e13258–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xu W, Chi L, Row BW, et al. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience. 2004;126(2):313–323. [DOI] [PubMed] [Google Scholar]
- 113.Row BW, Kheirandish L, Cheng Y, Rowell PP, Gozal D. Impaired spatial working memory and altered choline acetyltransferase (CHAT) immunoreactivity and nicotinic receptor binding in rats exposed to intermittent hypoxia during sleep. Behav Brain Res. 2007;177(2):308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bartels K, Grenz A, Eltzschig HK. Hypoxia and inflammation are two sides of the same coin. Proc Natl Acad Sci U S A. 2013;110(46):18351–18352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364(7):656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Iyalomhe O, Swierczek S, Enwerem N, et al. The Role of Hypoxia-Inducible Factor 1 in Mild Cognitive Impairment. Cell Mol Neurobiol. 2017;37(6):969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li T, Chen Y, Gua C, Wu B. Elevated Oxidative Stress and Inflammation in Hypothalamic Paraventricular Nucleus Are Associated With Sympathetic Excitation and Hypertension in Rats Exposed to Chronic Intermittent Hypoxia. Front Physiol. 2018;9(840):840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dayyat EA, Zhang SX, Wang Y, Cheng ZJ, Gozal D. Exogenous erythropoietin administration attenuates intermittent hypoxia-induced cognitive deficits in a murine model of sleep apnea. BMC Neurosci. 2012;13:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jefferson AL, Cambronero FE, Liu D, et al. Higher Aortic Stiffness Is Related to Lower Cerebral Blood Flow and Preserved Cerebrovascular Reactivity in Older Adults. Circulation. 2018;138(18):1951–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Catchlove SJ, Macpherson H, Hughes ME, Chen Y, Parrish TB, Pipingas A. An investigation of cerebral oxygen utilization, blood flow and cognition in healthy aging. PLoS One. 2018;13(5):e0197055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Price EA. Aging and erythropoiesis: current state of knowledge. Blood cells, molecules & diseases. 2008;41(2):158–165. [DOI] [PubMed] [Google Scholar]
- 122.Stauder R, Valent P, Theurl I. Anemia at older age: etiologies, clinical implications, and management. Blood. 2018;131(5):505–514. [DOI] [PubMed] [Google Scholar]
- 123.Lopez-Valdes HE, Martinez-Coria H. The Role of Neuroinflammation in Age-Related Dementias. Rev Invest Clin. 2016;68(1):40–48. [PubMed] [Google Scholar]
- 124.Benderro GF, Lamanna JC. Hypoxia-induced angiogenesis is delayed in aging mouse brain. Brain research. 2011;1389:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Catchlove SJ, Parrish TB, Chen Y, Macpherson H, Hughes ME, Pipingas A. Regional Cerebrovascular Reactivity and Cognitive Performance in Healthy Aging. J Exp Neurosci. 2018;12:1179069518785151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Carlson BW, Neelon VJ, Carlson JR, Hartman M, Bliwise DL. Cerebral Oxygenation in Wake and During Sleep and Its Relationship to Cognitive Function in Community-Dwelling Older Adults Without Sleep Disordered Breathing. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 2011;66(1):150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bastos-Leite AJ, Kuijer JP, Rombouts SA, et al. Cerebral blood flow by using pulsed arterial spin-labeling in elderly subjects with white matter hyperintensities. AJNR Am J Neuroradiol. 2008;29(7):1296–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bernbaum M, Menon BK, Fick G, et al. Reduced blood flow in normal white matter predicts development of leukoaraiosis. J Cereb Blood Flow Metab. 2015;35(10):1610–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Moeini M, Lu X, Avti PK, et al. Compromised microvascular oxygen delivery increases brain tissue vulnerability with age. Sci Rep. 2018;8(1):8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Weaver J, Jalal FY, Yang Y, Thompson J, Rosenberg GA, Liu KJ. Tissue oxygen is reduced in white matter of spontaneously hypertensive-stroke prone rats: a longitudinal study with electron paramagnetic resonance. J Cereb Blood Flow Metab. 2014;34(5):890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Robertson BA, Clements KM, Wainwright PE. The working memory capabilities of the spontaneously hypertensive rat. Physiol Behav. 2008;94(3):481–486. [DOI] [PubMed] [Google Scholar]
- 132.LaManna JC, Chavez JC, Pichiule P. Structural and functional adaptation to hypoxia in the rat brain. J Exp Biol. 2004;207(Pt 18):3163–3169. [DOI] [PubMed] [Google Scholar]
- 133.Di Giulio C, Bianchi G, Cacchio M, et al. Oxygen and life span: chronic hypoxia as a model for studying HIF-1alpha, VEGF and NOS during aging. Respir Physiol Neurobiol. 2005;147(1):31–38. [DOI] [PubMed] [Google Scholar]
- 134.Ndubuizu OI, Chavez JC, LaManna JC. Increased prolyl 4-hydroxylase expression and differential regulation of hypoxia-inducible factors in the aged rat brain. American journal of physiology Regulatory, integrative and comparative physiology. 2009;297(1):R158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Clarke LE, Liddelow SA, Chakraborty C, Munch AE, Heiman M, Barres BA. Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci U S A. 2018;115(8):E1896–E1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chavez JC, Baranova O, Lin J, Pichiule P. The transcriptional activator hypoxia inducible factor 2 (HIF-2/EPAS-1) regulates the oxygen-dependent expression of erythropoietin in cortical astrocytes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(37):9471–9481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Temido-Ferreira M, Coelho JE, Pousinha PA, Lopes LV. Novel Players in the Aging Synapse: Impact on Cognition. J Caffeine Adenosine Res. 2019;9(3):104–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Soreq L, Consortium UKBE, North American Brain Expression C, et al. Major Shifts in Glial Regional Identity Are a Transcriptional Hallmark of Human Brain Aging. Cell Rep. 2017;18(2):557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rozycka A, Liguz-Lecznar M. The space where aging acts: focus on the GABAergic synapse. Aging Cell. 2017;16(4):634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Adogwa O, Elsamadicy AA, Sergesketter A, et al. Independent Association Between Preoperative Cognitive Status and Discharge Location After Surgery: A Strategy to Reduce Resource Use After Surgery for Deformity. World Neurosurg. 2018;110:e67–e72. [DOI] [PubMed] [Google Scholar]
- 141.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9(1):58–65. [DOI] [PubMed] [Google Scholar]
- 142.An J, Li H, Tang Z, et al. Cognitive Impairment and Risk of All-Cause and Cardiovascular Disease Mortality Over 20-Year Follow-up: Results From the BLSA. J Am Heart Assoc. 2018;7(15):e008252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hughes MJ, Hackney RJ, Lamb PJ, Wigmore SJ, Christopher Deans DA, Skipworth RJE. Prehabilitation Before Major Abdominal Surgery: A Systematic Review and Meta-analysis. World J Surg. 2019;43(7):1661–1668. [DOI] [PubMed] [Google Scholar]
- 144.Smith A, Moon T, Pak T, Park B, Urman RD. Preoperative Anemia Treatment With Intravenous Iron in Patients Undergoing Major Orthopedic Surgery: A Systematic Review. Geriatr Orthop Surg Rehabil. 2020;11:2151459320935094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yu Y, Zhang K, Zhang L, Zong H, Meng L, Han R. Cerebral near-infrared spectroscopy (NIRS) for perioperative monitoring of brain oxygenation in children and adults. Cochrane Database Syst Rev. 2018;1(1):CD010947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gaudino M, Benesch C, Bakaeen F, et al. Considerations for Reduction of Risk of Perioperative Stroke in Adult Patients Undergoing Cardiac and Thoracic Aortic Operations: A Scientific Statement From the American Heart Association. Circulation. 2020;142(14):e193–e209. [DOI] [PubMed] [Google Scholar]
- 147.Mack MJ, Acker MA, Gelijns AC, et al. Effect of Cerebral Embolic Protection Devices on CNS Infarction in Surgical Aortic Valve Replacement: A Randomized Clinical Trial. JAMA. 2017;318(6):536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee LA, Caplan RA, Stephens LS, et al. Postoperative opioid-induced respiratory depression: a closed claims analysis. Anesthesiology. 2015;122(3):659–665. [DOI] [PubMed] [Google Scholar]
- 149.Hyman MC, Vemulapalli S, Szeto WY, et al. Conscious Sedation Versus General Anesthesia for Transcatheter Aortic Valve Replacement: Insights from the National Cardiovascular Data Registry Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Circulation. 2017;136(22):2132–2140. [DOI] [PubMed] [Google Scholar]
- 150.Ko SB. Perioperative stroke: pathophysiology and management. Korean J Anesthesiol. 2018;71(1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]


