Abstract
Background and purpose:
The autonomic nervous system has been implicated in stroke and dementia pathophysiology. High resting heart rate (RHR) and low heart rate variability (HRV) indicate the effect of autonomic imbalance on the heart. We examined the associations of RHR and HRV with incident stroke and dementia in a community based cohort of middle and old-aged adults.
Methods:
The study sample included 1,581 participants aged >60y and 3,271 participants aged >45y evaluated for incident dementia and stroke, respectively, who participated in the Framingham Offspring cohort third (1983-1987) examination and had follow-up for neurology events after the seventh (1998-2001) examination. HRV was assessed through the SD of normal-to-normal intervals (SDNN) and the root mean square of successive differences (RMSSD) from 2-hour Holter monitor. Participants were followed-up for stroke and dementia incidence from exam 7 to a maximum of 10 years. Cox regression models were used to assess the link of RHR and HRV with stroke and dementia risk while adjusting for potential confounders, and interactions with age and sex were assessed.
Results:
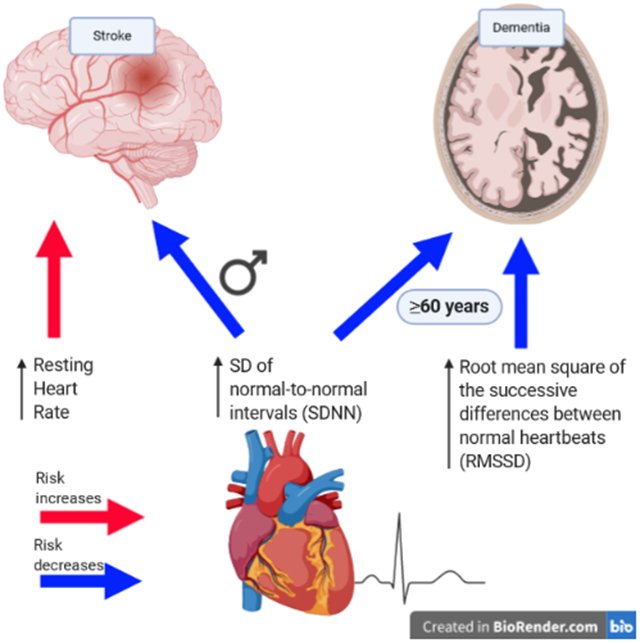
Of the dementia (mean age=55±6y, 46% men) and stroke (mean age=48±9y, 46% men) samples, 133 and 127 developed dementia and stroke, respectively, during the follow-up. Overall, autonomic imbalance was not associated with dementia risk. However, age modified the associations such that SDNN and RMSSD were associated with dementia risk in older persons (HR [95%CI] per 1SD=0.61 [0.38-0.99] and HR [95%CI] per 1SD=0.34 [0.15-0.74], respectively). High RHR was associated with increased stroke risk (HR [95%CI] per 10 bpm=1.18 [1.01-1.39] and high SDNN was associated with lower stroke risk in men (HR [95%CI] per 1SD=0.46 [0.26-0.79]) but not women (HR [95%CI] per 1SD=1.25 [0.88-1.79]; p for interaction=0.003).
Conclusions:
some measures of cardiac autonomic imbalance may precede dementia and stroke occurrence, particularly in older ages and men, respectively.
Keywords: incident stroke, incident dementia, cohort study, risk factors, resting heart rate, heart rate variability
Graphical Abstract
Introduction
The autonomic nervous system regulates multiple involuntary functions in the human body, including autonomic control on cardiac function, through complicated equilibrium between the sympathetic and parasympathetic activities.1 Specifically, evidence supports the use of high resting heart rate (RHR) and low heart rate variability (HRV) (i.e. the change in the time between consecutive heartbeats) as non-invasive indicators of autonomic dysfunction.2 A plethora of evidence exists showing that high RHR3–6 and low HRV7–10 are associated with increased risk for cardiometabolic diseases and mortality. In addition, the autonomic nervous system may be implicated in stroke and dementia through changes in cardiac output alterations and cerebral perfusion.
Accumulating findings point to a link between autonomic imbalance and cognitive performance in dementia-free individuals, thus stressing the potential of autonomic nervous system indicators to serve as early biomarkers for cognitive impairment.11, 12 Yet, prospective studies exploring whether autonomic dysfunction precedes dementia onset include, to our knowledge, only a recently published study from the Atherosclerosis Risk in Communities (ARIC) cohort.13 However, this study was restricted to RHR as an indicator for autonomic function, and the predictive value of HRV is yet to be tested.
While autonomic dysfunction after stroke is common14 and may predict secondary ischemic events,15 little is known about the predictive value of autonomic imbalance in first acute stroke. Although some evidence exists showing that increased RHR may predict incident stroke in the general population,16 studies assessing the relationship between decreased HRV and stroke risk are limited to small sample-sizes, cross-sectional designs and restricted study populations (e.g. patients with atrial fibrillation or renal dysfunction).17 Furthermore, previous literature suggests that the link of HRV and stroke risk may be complexed by effect modification.18
The aim of the current study was to examine whether autonomic imbalance, assessed by RHR and HRV, is an independent predictor of acute stroke and dementia. Findings from this study may translate to stroke and dementia prevention strategies through improvement in autonomic nervous system function.19 In addition, we tested for possible interactions of autonomic function indices with age and sex in order to identify high-risk subpopulations which may particularly benefit from potential interventions.
Methods
Data availability
Because of the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to The Framingham Heart Study at https://framinghamheartstudy.org/.
Study sample
The study sample consisted of Framingham’s Offspring cohort participants who were recruited in 1971 and have been examined approximately every four years.20 Outcomes including cardiovascular events and dementia are under continuous surveillance. We included participants who had information on RHR (N=3,868) and HRV (N=1,987), measured by electrocardiogram at the 3rd clinic examination (between June 1983 and June 1987), and who were 18 years of age or older at that time. Of them, 3491 with RHR and 1831 with HRV were available for dementia and stroke follow-up initiation at the time of the 7th examination (1998-2001). Of note, we chose to begin the follow-up period at exam 7 because there were very few incident stroke and dementia cases between exams 3 and 7 (due to the young age of the participants) which was reflected by violation of the proportional Hazard assumption in the Cox regression models. We excluded those who at the entrance to follow-up had a history of atrial fibrillation (AF) or congestive heart failure (CHF) (i.e. prevalent cases; N=19). Next, for the dementia sample, we excluded 1,547 participants aged < 60 years at the start of follow-up, 36 individuals with prevalent dementia, 257 with no follow-up information, and 53 with missing education or ApoE ε4 status. Thus, our final sample size for the dementia analysis was 1,579 and 815 in models including RHR and HRV measures as the independent variables, respectively. For stroke, we excluded 103 individuals whose age was <45 years at the start of follow-up, 70 with prevalent stroke, and 30 with no follow-up information. Thus, the final sample for stroke outcome assessment consisted of 3,269 and 1716 individuals who had information on RHR and HRV measures, respectively (figure 1). All participants provided written informed consent, and the study procedures were approved by the Institutional Review Board at Boston University School of Medicine.
Figure 1:
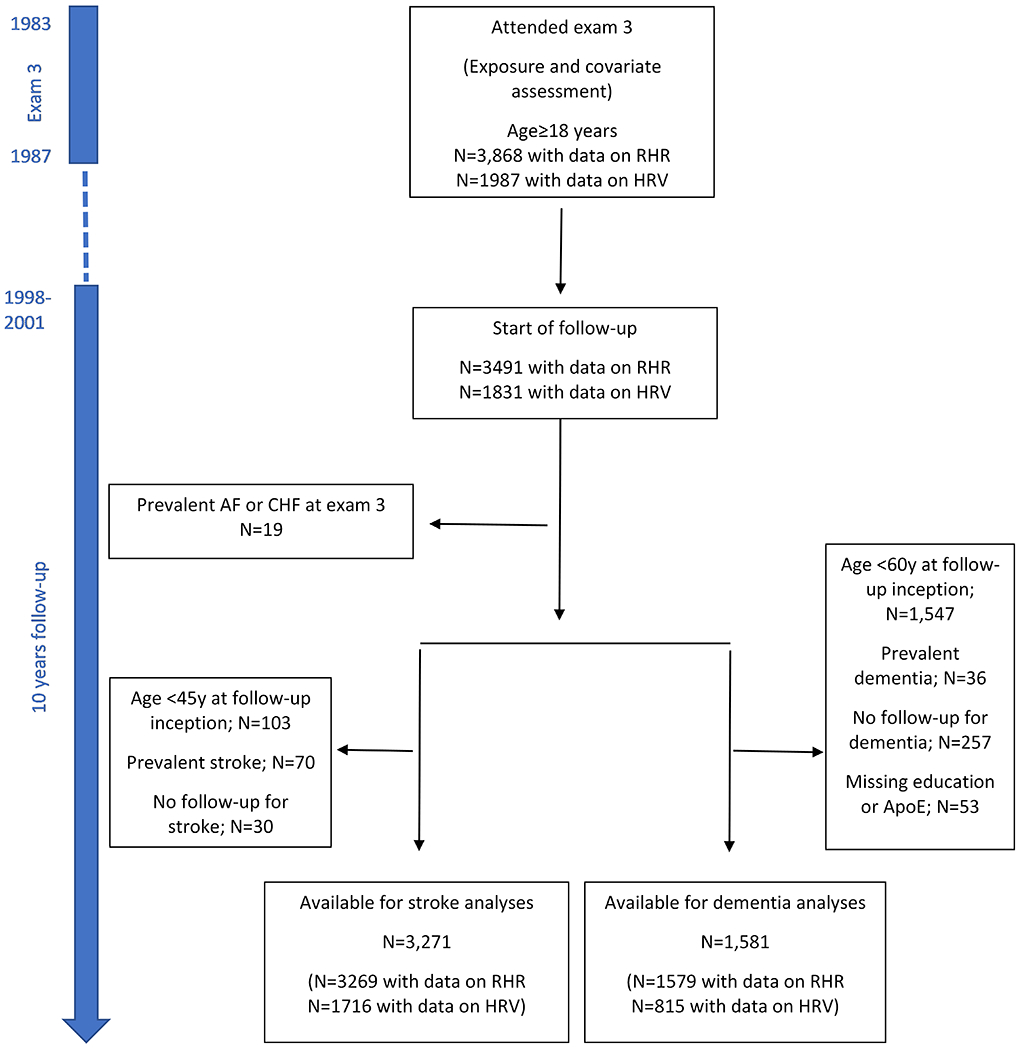
Selection of study participants. The risk of incident dementia and stroke was calculated as the 10-year risk starting from examination cycle 7. AF=atrial fibrillation; CHF=congestive heart failure; ApoE=apolipoprotein E; RHR=Resting heart rate; SDNN= SD of normal-to-normal intervals; RMSSD= square root of the mean of the squared differences.
Assessment of autonomic imbalance
We assessed autonomic imbalance through RHR and HRV conducted in the third examination cycle (1983-1987). These measures are well-established and simple indicators of autonomic dysfunction.2 RHR was documented from electrocardiogram and information on HRV was abstracted from a 2-hour Holter monitor data, and was based on 100s blocks. HRV was assessed by two different indices: 1. the standard deviation of beat-to-beat intervals (SDNN), a commonly-used measure of HRV which represents the total variability and thus joint sympathetic and parasympathetic modulation of HRV. 2. the root mean square of successive differences (RMSSD), which more narrowly represents parasympathetic activity only.
Incident stroke and dementia
The outcome measures for this study were incidence of all-cause dementia and acute stroke after examination cycle 7. Follow-up for those without the outcome were censored at the latest date known free of the event up to a maximum of 10 years. Screening and surveillance methods for the development of stroke and clinical dementia have been outlined previously.21, 22 Dementia was diagnosed according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition.23 Stroke was defined as the rapid onset of focal neurological symptoms of presumed vascular origin, lasting >24 hours or resulting in death. A diagnosis of Alzheimer’s disease (AD) dementia was based on the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s disease and Related Disorders Association for definite, probable, or possible AD.24
Statistical analysis
Multivariable Cox regression models were used to separately examine the associations of RHR and HRV with incident stroke and dementia. Independent variables were examined first as continuous, with Hazard Ratios (HRs) indicating risk for stroke or dementia per 10 beta-per-minute (bpm) higher RHR. SDNN and RMSSD were standardized using the mean and SD of the respective measures in the stroke sample (the larger sample). Mean ± SD used to standardize SDNN and RMSSD were 9.54±2.74 and 3.45±1.68, respectively. When we found evidence for a threshold, we presented the results according to medians in addition to analyses using the independent variable as continuous.
All stroke analyses were adjusted first for age, sex, systolic blood pressure (SBP), prevalent cardiovascular disease (CVD) at exam 3, and for incident congestive heart failure (CHF)25 and atrial fibrillation (AF) occurring after exam 3.26 Next, models were adjusted additionally for smoking status, diabetes, and use of antihypertensives, antiarrhythmics, cardiac glycosides and aspirin at exam 3. Dementia analyses included the following adjustments: first for age at exam 3, sex, education and positivity for an ApoEε4 allele; then additionally for diabetes, smoking status, systolic blood pressure, and use of antihypertensives, antiarrhythmics and cardiac glycosides medications. Both stroke and dementia models were further adjusted for physical activity index27 in a separate model, because information on this variable was available only on a subsample. Because physical activity was not measured at exam 3, we used information from exam 2 or 4 based on availability. If information on physical activity was available in both exams 2 and 4, the average index from these exams was calculated.
For models assessing incident dementia as an outcome, we conducted a sensitivity analysis in which prevalent stroke cases at the 3rd examination were excluded, and incident cases occurring after the 3rd examination were adjusted for. In addition, we conducted analyses restricting to AD dementia as an outcome.
We tested for interaction of the autonomic imbalance measures with age and sex, in determining the risk of stroke and dementia by including interaction terms in the Cox regression models. If a significant interaction was found, we ran a stratified model. A cutoff at 60 years at the time of the electrocardiogram measurement was chosen for age (for both interactions and stratification) because this age was close to the mean and median of the age distribution at this time point.
All models were tested for violation of the proportional hazards assumption using the zph option in PROC PHREG which uses weighted Schoenfeld residuals, and firth correction was applied in instances of small number of events.
A two-sided p-value of <0.05 was considered statistically significant, with the exception of interaction assessment in which case a p-value of ≤0.10 met our threshold for significance, due to low power of the test.
Analyses were performed using Statistical Analyses System software Version 9.4 (SAS Institute, Cary, NC).
Results
The baseline characteristics of the stroke and dementia samples are presented in table 1. Mean age at exam 3 (exposure assessment) was 55±6 and 48±9 years for the dementia and stroke samples, respectively. Mean age when follow-up for dementia and stroke commenced was 69±6 and 63±9 years, respectively. In both samples, ~46% were men.
Table 1:
Baseline characteristics of the study sample
| Study sample for dementia analysis (N=1581) |
Study sample for stroke analysis (N=3271) |
|
|---|---|---|
| Age at exam 3, mean years ± SD | 55.0 ± 6.0 | 48.4 ± 9.2 |
| Age at start of follow-up, mean years ± SD | 69.1 ± 5.9 | 62.7 ± 9.2 |
| Time between exam 3 and start of follow-up (mean ± SD) | 14.1 ± 1.0 | 14.2 ± 1.0 |
| Follow-up duration* | 10.0 [6.9, 10.0] | 10.0 [10.0, 10.0] |
| Men, n (%) | 734 (46.4) | 1489 (45.5) |
| Education, n (%) | ||
| < High-school degree | 116 (7.3) | 175 (5.4) |
| High-school degree | 538 (34.0) | 1016 (31.3) |
| Some college | 459 (29.0) | 946 (29.1) |
| ≥ College graduate | 468 (29.6) | 1113 (34.3) |
| Body mass index, kg/m2* | 26.0 [23.5, 28.7] | 25.5 [22.8, 28.5] |
| Physical Activity Index* | 34.7 [32.2, 38.2] | 34.9 [32.3, 38.2] |
| Smoking, n (%) | 383 (24.2) | 902 (27.6) |
| Diabetes, n (%) | 69 (4.5) | 97 (3.1) |
| Fasting blood glucose, mg/dL* | 93.0 [87.0, 99.0] | 91.0 [85.0, 97.0] |
| Stage 1 hypertension, n (%) | 609 (38.5) | 921 (28.2) |
| Systolic blood pressure, mean mmHg ± SD | 127.3 ± 16.8 | 122.9 ± 16.3 |
| Prevalent CVD, n (%) | 99 (6.3) | 130 (4.0) |
| Total cholesterol, mg/dL* | 218.0 [194.0, 244.0] | 209.0 [183.0, 236.0] |
| HDL-cholesterol, mg/dL* | 49.0 [40.0, 61.0] | 49.0 [41.0, 61.0] |
| Triglycerides, mg/dL* | 107.0 [71.0, 156.0] | 93.0 [63.0, 143.0] |
| ApoE4, n (%) | 344 (21.8) | 690 (22.1) |
| Use of antihypertensive medications, n (%) | 336 (21.3) | 466 (14.3) |
| Use of antiarrhithmics medications, n (%) | 10 (0.6) | 13 (0.4) |
| Use of cardiac glycosides, n (%) | 10 (0.6) | 13 (0.4) |
| Use of beta-blockers, n (%) | 168 (10.6) | 234 (7.2) |
| Use of aspirin, n (%) | 373 (23.7) | 785 (24.1) |
| Resting heart rate (beats/minute) * | 64.0 [58.0, 70.0] | 64.0 [58.0, 72.0] |
| SDNN* | 8.9 [7.3, 10.8] | 9.2 [7.6, 11.2] |
| RMSSD* | 2.8 [2.2, 3.7] | 3.0 [2.3, 4.1] |
CVD=Cardiovascular Disease; HDL=high-density lipoprotein; SDNN=standard deviation of beat-to-beat intervals; RMSSD=root mean square of successive differences
Values are medians [Q1, Q3]
Autonomic balance and the risk of dementia
Overall, none of the assessed autonomic imbalance indices was related to dementia (table 2) as well as AD risk (supplementary table I). However, we found significant interactions between HRV indices and age (p for interaction=0.038 and 0.005 for SDNN and RMSSD, respectively, after controlling for age, sex, education and ApoEɛ4; table 3). In stratified analyses, SDNN and RMSSD were each significantly associated with dementia risk in individuals who were 60 years of age or older at the time of the autonomic balance assessment (HR=0.48; 95% CI: 0.29,0.80, and HR=0.32; 95% CI:0.15,0.71 per 10 bpm increase in RHR and 1 SD increase in SDNN and RMSSD, respectively). After further adjustments for all the study covariates in model 3, the association between SDNN and dementia incidence attenuated, yet remained statistically significant (HR=0.61; 95%CI: 0.38, 0.99), and the association of RMSSD with dementia risk remained similar (HR=0.34; 95%CI: 0.15, 0.74). In addition, sex modified the association between RHR and dementia risk (p for interaction=0.092) such that 10 bpm increase in RHR was associated with 25% increased risk of dementia among women only (HR=1.25; 95% CI:1.01, 1.54). However, these associations were no longer statistically significant after adjustment for additional study covariates (HR=0.97; 95% CI:0.73, 1.28; table 3, models 3). The association of RMSSD with AD in individuals older than 60 years remained statistically significant. Yet, the associations of SDNN and RHR with AD in older adults and females, respectively, were no longer significant, probably due to limited statistical power (supplementary table II).
Table 2:
Association between autonomic balance indices and dementia risk
| Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|
| Events/N | HR (95% CI) | Events/N | HR (95% CI) | Events/N | HR (95% CI) | ||
| RHR | Per 10 bpm | 133/1579 | 1.09 (0.93, 1.29) | 129/1530 | 1.05 (0.88, 1.24) | 128/1498 | 1.02 (0.86, 1.21) |
| SDNN | Per SD | 70/815 | 0.74 (0.56, 0.99) | 68/784 | 0.79 (0.60, 1.06) | 67/767 | 0.81 (0.61, 1.08) |
| RMSSD | Per SD | 70/815 | 0.79 (0.57, 1.09) | 68/784 | 0.77 (0.55, 1.07) | 67/767 | 0.78 s(0.55, 1.08) |
RHR=Resting Heart Rate; SDNN= SD of normal-to-normal intervals; RMSSD= root mean square of successive differences
Model 1 is adjusted for age, sex, education and apoe4
Model 2 is additionally adjusted for smoking status, diabetes, SBP, antihypertensives, antiarrhythmics and cardiac glycosides
Model 3 is additionally adjusted for physical activity
Results were similar after controlling for interim CHF and interim AF and after excluding stroke cases at exam 3
Bold values denote statistical significance at the p ≤ 0.05 level
Table 3:
Association between autonomic balance indices and dementia risk stratified by age and sex
| model | Events/N | HR (95% CI) ‡ | p for interaction | ||
|---|---|---|---|---|---|
| SDNN | 1 | Age< 60y* | 38/653 | 0.99 (0.70, 1.40) | |
| Age≥ 60y* | 32/162 | 0.48 (0.29, 0.80) | 0.038 | ||
| 2 | Age≥ 60y* | 36/630 | 1.04 (0.72, 1.48) | ||
| Age≥ 60y* | 32/154 | 0.56 (0.34, 0.91) | 0.071 | ||
| 3 | Age< 60y* | 36/616 | 1.02 (0.71, 1.47) | ||
| Age≥ 60y* | 31/151 | 0.61 (0.38, 0.99) | 0.158 | ||
| RMSSD | 1 | Age< 60y* | 38/653 | 1.19 (0.82, 1.72) | |
| Age≥ 60y* | 32/162 | 0.32 (0.15, 0.70) | 0.005 | ||
| 2 | Age< 60y* | 36/630 | 1.16 (0.79, 1.70) | ||
| Age≥ 60y* | 32/154 | 0.29 (0.13, 0.64) | 0.003 | ||
| 3 | Age< 60y* | 36/616 | 1.13 (0.77, 1.68) | ||
| Age≥ 60y* | 31/151 | 0.34 (0.15, 0.74) | 0.008 | ||
| RHR | 1 | Females | 85/845 | 1.25 (1.01, 1.54) | |
| Males | 48/734 | 0.92 (0.70, 1.22) | 0.092 | ||
| 2 | Females | 83/809 | 1.14 (0.92, 1.41) | ||
| Males | 46/721 | 0.95 (0.72, 1.24) | 0.293 | ||
| 3 | Females | 82/790 | 1.09 (0.87, 1.35) | ||
| Males | 46/708 | 0.97 (0.73, 1.28) | 0.518 |
RHR=Resting Heart Rate; SDNN= SD of normal-to-normal intervals; RMSSD= root mean square of successive differences
Model 1 is adjusted for age, sex, education and apoe4
Model 2 is additionally adjusted for smoking status, diabetes, SBP, antihypertensives, antiarrhythmics and cardiac glycosides
Model 3 is additionally adjusted for physical activity
At time of electrocardiogram (exam 3)
values are per 10 bpm for RHR and 1SD for SDNN and RMSSD
Bold values denote statistical significance at the p ≤ 0.05 level
Autonomic balance and the risk of stroke
After controlling for all the study covariates, RHR, but not SDNN or RMSSD, was significantly associated with risk of stroke. Each 10 bpm increment in RHR was associated with 18% increase in risk of stroke (HR=1.18; 95% CI: 1.01, 1.39), and RHR above vs. below the median was associated with an increase of ~70% in stroke risk (HR=1.68; 95% CI:1.14, 2.46) (table 4).
Table 4:
Association between autonomic balance indices and stroke risk
| Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|
| Events/N | HR (95% CI) | Events/N | HR (95% CI) | Events/N | HR (95% CI) | ||
| RHR | Per 10 bpm | 127/3269 | 1.15 (0.98,1.35) | 123/3126 | 1.18 (1.00,1.38) | 120/3037 | 1.18 (1.01, 1.39) |
| Below median | 52/1654 | 1.00 (ref.) | 50/1581 | 1.00 (ref.) | 49/1537 | 1.00 (ref.) | |
| Above median | 75/1615 | 1.59 (1.11,2.29) | 73/1545 | 1.71 (1.18,2.50) | 71/1500 | 1.68 (1.14, 2.46) | |
| SDNN | Per SD | 58/1716 | 0.88 (0.67,1.17) | 56/1635 | 0.86 (0.64,1.15) | 56/1586 | 0.85 (0.63,1.15) |
| Below median | 40/858 | 1.00 (ref.) | 39/818 | 1.00 (ref.) | 39/792 | 1.00 (ref.) | |
| Above median | 18/858 | 0.62 (0.35,1.09) | 17/817 | 0.59 (0.33,1.07) | 17/794 | 0.60 (0.33,1.08) | |
| RMSSD | Per SD | 58/1716 | 0.93 (0.69,1.26) | 56/1635 | 0.89 (0.64,1.22) | 56/1586 | 0.89 (0.65,1.23) |
| Below median | 39/858 | 1.00 (ref.) | 38/820 | 1.00 (ref.) | 38/795 | 1.00 (ref.) | |
| Above median | 19/858 | 0.66 (0.38,1.15) | 18/815 | 0.63 (0.36,1.12) | 18/791 | 0.64 (0.36,1.13) | |
RHR=Resting Heart Rate; SDNN= SD of normal-to-normal intervals; RMSSD= root mean square of successive differences
Model 1 is adjusted for age at exam 3, sex, SBP, CVD, interim CHF, and interim AF
Model 2 is additionally adjusted for smoking status, diabetes, antihypertensives, antiarrhythmics, cardiac glycosides, and aspirin
Model 3 is additionally adjusted for physical activity
Bold values denote statistical significance at the p ≤ 0.05 level
There was a significant interaction between SDNN and sex such that SDNN was associated with risk of stroke only among men (HR=0.51; 95% CI:0.30,0.84; p for interaction=0.005 and HR=0.30; 95% CI:0.11,0.81; p for interaction=0.041 per 1SD increased SDNN and top vs. bottom median, respectively). These associations remained similar after additional adjustment for study covariates (HR=0.46; 95% CI:0.26,0.79; p for interaction=0.003 and HR=0.29; 95% CI:0.11, 0.79; p for interaction=0.003 per 1SD increased SDNN and when comparing top vs. bottom medians, respectively (table 5). No effect modification by age or sex was observed in the association of either RHR or RMSSD with stroke incidence.
Table 5:
Association between SDNN and stroke risk stratified by sex
| Model | Events/N | HR (95% CI) | p for interaction | ||
|---|---|---|---|---|---|
| 1 | Per SD | Females | 36/946 | 1.21 (0.88, 1.67) | 0.005 |
| Males | 22/770 | 0.51 (0.30, 0.84) | |||
| Below median | females | 23/497 | 1.00 (ref.) | 0.041 | |
| Above median | 13/449 | 1.01 (0.50, 2.03) | |||
| Below median | Males | 17/361 | 1.00 (ref.) | ||
| Above median | 5/409 | 0.30 (0.11, 0.81) | |||
| 2 | Per SD | Females | 34/882 | 1.25 (0.88, 1.79) | 0.003 |
| Males | 22/753 | 0.47 (0.27, 0.80) | |||
| Below median | females | 23/497 | 1.00 (ref.) | ||
| Above median | 12/415 | 1.08 (0.51, 2.29) | 0.003 | ||
| Below median | Males | 17/361 | 1.00 (ref.) | ||
| Above median | 5/402 | 0.29 (0.11, 0.79) | |||
| 3 | Per SD | Females | 34/851 | 1.25 (0.88, 1.79) | 0.003 |
| Males | 0.46 (0.26, 0.79) | ||||
| Below median | females | 22/450 | 1.00 (ref.) | 0.003 | |
| Above median | 12/401 | 1.08 (0.51, 2.30) | |||
| Below median | Males | 17/342 | 1.00 (ref.) | ||
| Above median | 5/393 | 0.29 (0.11, 0.79) |
SDNN= SD of normal-to-normal intervals
Model 1 is adjusted for age at exam 3, sex, SBP, CVD, interim CHF, and interim AF
Model 2 is additionally adjusted for smoking status, diabetes, antihypertensives, antiarrhythmics, cardiac glycosides, and aspirin
Model 3 is additionally adjusted for physical activity
Bold values denote statistical significance at the p ≤ 0.05 level
Firth correction applied to models comparing above and below SDNN medians
No violation of the proportional hazards assumptions was observed in the cox regression models.
Discussion
In our community-based cohort, RHR predicted acute stroke in the total sample, and low SDNN preceded the development of stroke in men. In addition, cardiac autonomic measures were not associated with dementia incidence overall, however SDNN and RMSSD, measures of heart rate variability, were associated with dementia risk and specifically with AD, in older individuals. These associations were independent of cardiometabolic risk factors and medication use.
Autonomic dysfunction is common particularly in the Lewy body dementias,28 but is also implicated in all dementia subtypes, including in AD,29 the most common dementia subtype.30 Indeed, one of the most prominent features of AD neuropathology is cholinergic loss,31 which indicates that the sympathetic and parasympathetic systems are affected.32 Additional support for the importance of the cholinergic system in AD comes from findings showing that FDA-approved Cholinesterase inhibitors, which improve cognition and functionality in some patients with AD, are also efficient in increasing autonomic balance.33 In turn, substantial evidence from animal models and post-mortem human studies points to a strong link between cholinergic depletion and other neuropathological features of AD,31 such as promotion of amyloid ß deposition and tau pathology.34 Indeed, AD neuropathology can be found in brain regions responsible for autonomic regulation, including the insula and anterior cingulate cortex and locus coeruleus.35, 36 In addition to markers of neurodegeneration, imbalance between the sympathetic and parasympathetic systems may result in irregular cerebral blood flow and perfusion, thus contributing to brain aging and cognitive impairment.37–39
Our results suggest that indicators of autonomic imbalance may not predict dementia incidence overall. However in a stratified analysis by age, the associations of the two HRV indices (i.e. SDNN and RMSSD) were apparent only when the Holter examination was done after the age of 60 years, implying that autonomic imbalance may have predictive value only after some brain pathology exists during the long prodromal phase of the disease. Despite the rarity of prior prospective studies, it has long been speculated that autonomic dysfunction is present long before clinical symptoms appear, as indeed, brain areas implicated in autonomic regulation are affected early in the disease course.36 Furthermore, studies show that autonomic dysfunction is common in MCI,40, 41 a condition which in many cases precedes the development of clinical dementia.42 Moreover, accumulating literature suggests that decreased HRV is linked with poor cognitive performance in dementia-free individuals, thus stressing the possible role of autonomic dysfunction in dementia prediction.12 Yet, only recently, a prospective cohort study has been conducted on this topic for the first time. In contrast to our findings of no association between RHR and dementia risk, this study demonstrated an association of increased RHR with a greater decline in cognitive performance and in increased dementia risk over a 20 years follow-up.13 This inconsistency may be explained by differences in study design (e.g. follow-up duration), or sample composition (e.g. differences in mean age and ethnicities).Yet, in accordance with our findings, no effect modification by age and sex was observed in this former study with regard to the association between RHR and dementia incidence.
Increased RHR has been linked with stroke risk in the current as well as in other analyses.43 Suggested mechanisms include promotion of endothelial dysfunction, and impairment in cerebral circulation and glucose metabolism.37–39 In addition, high SDNN was related to decreased stroke risk, but only among men. It has been previously hypothesized that autonomic dysfunction may have more impact in populations already at high risk for stroke, such as those with type 2 diabetes.18 Hence, our finding of an association only in men may be explained in light of this prior hypothesis, because men, compared to women, were possibly at higher risk for stroke when autonomic balance was assessed (mean age ~48 years).44
Several prior studies suggested that low HRV may have a predictive value for stroke.17 Yet, studies are heterogeneous in many aspects including measures of HRV used and duration of follow-up.17 Particularly, distribution of the study population characteristics may be important in light of our and other’s18 findings indicating effect modification by various variables.
Strengths of our study include the community-based, prospective design and the careful surveillance for end-points. Several limitations should also be noted: first, measures of autonomic imbalance are associated with multiple risk factors for dementia and stroke, including diabetes, hypertension, depression and inflammation.3, 4, 6, 45–47 While we were able to adjust for many of these factors, the possibility of residual confounding cannot be excluded. Second, due to small numbers of incident events, particularly in subgroup analyses, our results must be considered hypothesis generating and need to be replicated in larger studies. Furthermore, we were underpowered to differentiate between specific subtypes of dementia and stroke. Third, the overwhelmingly European origin of the study sample limits the generalizability of our findings. Forth, autonomic balance was assessed at a single time point, thus may have led to an underestimation of the true associations through non-differential misclassification bias. Lastly, it needs to be acknowledged that the observational nature of the study does not allow causal inferences.
Summary
In conclusion, our study supports previous research showing that high resting heart rate may serve as a risk factor for stroke, and suggests that some indicators of cardiac HRV precede the diagnosis of stroke and dementia in specific subpopulations. RHR and HRV are non-invasive and easy to measure in clinical settings and thus may serve as useful biomarkers for incident dementia and stroke in those who may benefit from preventive interventions. In light of our findings, we suggest that future research exploring the predictive value of autonomic balance to dementia and stroke risk will account for the possible interactions of these measures with characteristics such as age, sex and use of medications. Furthermore, future research is warranted to explore whether improvement of autonomic tone results in reduction of dementia/stroke risk and in slowing of brain aging.
Supplementary Material
Source of funding
This study was supported by grants from the National Institute on Aging (R01 AG054076, R01 AG049607, R01 AG033040, R01 AG063507, RF1 AG052409, RF1 AG061872, U01 AG049505, AG058589) and the National Institute of Neurological Disorders and Stroke (NS017950 and UH2 NS100605), and the NHLBI contract for the Framingham Heart Study (contract no. N01-HC-25195, HHSN268201500001I, and 75N92019D00031).
Non-standard Abbreviations and Acronyms
- RHR
Resting Heart Rate
- HRV
Heart Rate Variability
- AF
Atrial Fibrillation
- CHF
Congestive Heart Failure
- SDNN
Standard Deviation of beat-to-beat intervals
- RMSSD
Root Mean Square of the Standard Deviation
- HR
Hazard Ratio
- AD
Alzheimer’s Disease
Footnotes
Disclosures:
None
References
- 1.Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task force of the european society of cardiology and the north american society of pacing and electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 2.Freeman R Assessment of cardiovascular autonomic function. Clin Neurophysiol. 2006;117:716–730. [DOI] [PubMed] [Google Scholar]
- 3.Carnethon MR, Yan L, Greenland P, Garside DB, Dyer AR, Metzger B, Daviglus ML. Resting heart rate in middle age and diabetes development in older age. Diabetes Care. 2008;31:335–339. [DOI] [PubMed] [Google Scholar]
- 4.Jiang X, Liu X, Wu S, Zhang GQ, Peng M, Wu Y, Zheng X, Ruan C, Zhang W. Metabolic syndrome is associated with and predicted by resting heart rate: A cross-sectional and longitudinal study. Heart. 2015;101:44–49. [DOI] [PubMed] [Google Scholar]
- 5.Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352:1951–1958. [DOI] [PubMed] [Google Scholar]
- 6.Yang Z, Zhang W, Zhu L, Lin N, Niu Y, Li X, Lu S, Zhang H, Wang X, Wen J, et al. Resting heart rate and impaired glucose regulation in middle-aged and elderly chinese people: A cross-sectional analysis. BMC Cardiovasc Disord. 2017;17:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.La Rovere MT, Bigger JT Jr., Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Atrami (autonomic tone and reflexes after myocardial infarction) investigators. Lancet. 1998;351:478–484. [DOI] [PubMed] [Google Scholar]
- 8.Tsuji H, Larson MG, Venditti FJ Jr., Manders ES, Evans JC, Feldman CL, Levy D. Impact of reduced heart rate variability on risk for cardiac events. The framingham heart study. Circulation. 1996;94:2850–2855. [DOI] [PubMed] [Google Scholar]
- 9.Bigger JT Jr., Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85:164–171. [DOI] [PubMed] [Google Scholar]
- 10.Wulsin LR, Horn PS, Perry JL, Massaro JM, D’Agostino RB. Autonomic imbalance as a predictor of metabolic risks, cardiovascular disease, diabetes, and mortality. J Clin Endocrinol Metab. 2015;100:2443–2448. [DOI] [PubMed] [Google Scholar]
- 11.Elias MF, Torres RV. The renaissance of heart rate variability as a predictor of cognitive functioning. Am J Hypertens. 2017;31:21–23. [DOI] [PubMed] [Google Scholar]
- 12.Forte G, Favieri F, Casagrande M. Heart rate variability and cognitive function: A systematic review. Front Neurosci. 2019;13:710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S, Fashanu OE, Zhao D, Guallar E, Gottesman RF, Schneider ALC, McEvoy JW, Norby FL, Aladin AI, Alonso A, et al. Relation of elevated resting heart rate in mid-life to cognitive decline over 20 years (from the atherosclerosis risk in communities [aric] study). Am J Cardiol. 2019;123:334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mo J, Huang L, Peng J, Ocak U, Zhang J, Zhang JH. Autonomic disturbances in acute cerebrovascular disease. Neurosci Bull. 2019;35:133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan L, Wang Y, Claydon VE, Mazowita G, Wang Y, Brant R, Collet JP. Autonomic parameter and stress profile predict secondary ischemic events after transient ischemic attack or minor stroke. Stroke. 2019;50:2007–2015. [DOI] [PubMed] [Google Scholar]
- 16.Zhang D, Wang W, Li F. Association between resting heart rate and coronary artery disease, stroke, sudden death and noncardiovascular diseases: A meta-analysis. Cmaj. 2016;188:E384–e392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lees T, Shad-Kaneez F, Simpson AM, Nassif NT, Lin Y, Lal S. Heart rate variability as a biomarker for predicting stroke, post-stroke complications and functionality. Biomark Insights. 2018;13:1177271918786931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fyfe-Johnson AL, Muller CJ, Alonso A, Folsom AR, Gottesman RF, Rosamond WD, Whitsel EA, Agarwal SK, MacLehose RF. Heart rate variability and incident stroke: The atherosclerosis risk in communities study. Stroke. 2016;47:1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Raedt S, De Vos A, De Keyser J. Autonomic dysfunction in acute ischemic stroke: An underexplored therapeutic area? J Neurol Sci. 2015;348:24–34. [DOI] [PubMed] [Google Scholar]
- 20.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The framingham offspring study. Design and preliminary data. Prev Med. 1975;4:518–525. [DOI] [PubMed] [Google Scholar]
- 21.McGrath ER, Himali JJ, Levy D, Conner SC, DeCarli CS, Pase MP, Courchesne P, Satizabal CL, Vasan RS, Beiser AS, et al. Circulating igfbp-2: A novel biomarker for incident dementia. Ann Clin Transl Neurol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aparicio HJ, Himali JJ, Satizabal CL, Pase MP, Romero JR, Kase CS, Beiser AS, Seshadri S. Temporal trends in ischemic stroke incidence in younger adults in the framingham study. Stroke. 2019;50:1558–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diagnostic and statistical manual of mental disorders : Dsm-iv. Washington, D.C.: American Psychiatric Association; 1994. [Google Scholar]
- 24.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of alzheimer’s disease: Report of the nincds-adrda work group under the auspices of department of health and human services task force on alzheimer’s disease. Neurology. 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 25.Mahmood SS, Wang TJ. The epidemiology of congestive heart failure: The framingham heart study perspective. Global heart. 2013;8:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton-Cheh C, Lubitz SA, Magnani JW, Ellinor PT, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the framingham heart study: A cohort study. Lancet. 2015;386:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan ZS, Spartano NL, Beiser AS, DeCarli C, Auerbach SH, Vasan RS, Seshadri S. Physical activity, brain volume, and dementia risk: The framingham study. J Gerontol A Biol Sci Med Sci. 2017;72:789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allan LM. Diagnosis and management of autonomic dysfunction in dementia syndromes. Curr Treat Options Neurol. 2019;21:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Femminella GD, Rengo G, Komici K, Iacotucci P, Petraglia L, Pagano G, de Lucia C, Canonico V, Bonaduce D, Leosco D, et al. Autonomic dysfunction in alzheimer’s disease: Tools for assessment and review of the literature. J Alzheimers Dis. 2014;42:369–377. [DOI] [PubMed] [Google Scholar]
- 30.Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, Van der Flier WM. Alzheimer’s disease. Lancet. 2016;388:505–517. [DOI] [PubMed] [Google Scholar]
- 31.Hampel H, Mesulam MM, Cuello AC, Farlow MR, Giacobini E, Grossberg GT, Khachaturian AS, Vergallo A, Cavedo E, Snyder PJ, et al. The cholinergic system in the pathophysiology and treatment of alzheimer’s disease. Brain. 2018;141:1917–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giubilei F, Strano S, Imbimbo BP, Tisei P, Calcagnini G, Lino S, Frontoni M, Santini M, Fieschi C. Cardiac autonomic dysfunction in patients with alzheimer disease: Possible pathogenetic mechanisms. Alzheimer Dis Assoc Disord. 1998;12:356–361. [DOI] [PubMed] [Google Scholar]
- 33.da Costa Dias FL, Ferreira Lisboa da Silva RM, de Moraes EN, Caramelli P. Cholinesterase inhibitors modulate autonomic function in patients with alzheimer s disease and mixed dementia. Curr Alzheimer Res. 2013;10:476–481. [DOI] [PubMed] [Google Scholar]
- 34.Ramos-Rodriguez JJ, Pacheco-Herrero M, Thyssen D, Murillo-Carretero MI, Berrocoso E, Spires-Jones TL, Bacskai BJ, Garcia-Alloza M. Rapid beta-amyloid deposition and cognitive impairment after cholinergic denervation in app/ps1 mice. J Neuropathol Exp Neurol. 2013;72:272–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chu CC, Tranel D, Damasio AR, Van Hoesen GW. The autonomic-related cortex: Pathology in alzheimer’s disease. Cereb Cortex. 1997;7:86–95. [DOI] [PubMed] [Google Scholar]
- 36.Braak H, Braak E. Staging of alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–278; discussion 278-284. [DOI] [PubMed] [Google Scholar]
- 37.Hamner JW, Tan CO, Lee K, Cohen MA, Taylor JA. Sympathetic control of the cerebral vasculature in humans. Stroke. 2010;41:102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang R, Zuckerman JH, Iwasaki K, Wilson TE, Crandall CG, Levine BD. Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation. 2002;106:1814–1820. [DOI] [PubMed] [Google Scholar]
- 39.Daulatzai MA. Cerebral hypoperfusion and glucose hypometabolism: Key pathophysiological modulators promote neurodegeneration, cognitive impairment, and alzheimer’s disease. J Neurosci Res. 2017;95:943–972. [DOI] [PubMed] [Google Scholar]
- 40.Collins O, Dillon S, Finucane C, Lawlor B, Kenny RA. Parasympathetic autonomic dysfunction is common in mild cognitive impairment. Neurobiol Aging. 2012;33:2324–2333. [DOI] [PubMed] [Google Scholar]
- 41.Nicolini P, Ciulla MM, Malfatto G, Abbate C, Mari D, Rossi PD, Pettenuzzo E, Magrini F, Consonni D, Lombardi F. Autonomic dysfunction in mild cognitive impairment: Evidence from power spectral analysis of heart rate variability in a cross-sectional case-control study. PLoS One. 2014;9:e96656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med. 2011;364:2227–2234. [DOI] [PubMed] [Google Scholar]
- 43.Kuzma E, Lourida I, Moore SF, Levine DA, Ukoumunne OC, Llewellyn DJ. Stroke and dementia risk: A systematic review and meta-analysis. Alzheimers Dement. 2018;14:1416–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L. Sex differences in stroke: Epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi Y, Zhou W, Liu X, Ping Z, Li YQ, Wang C, Lu J, Mao ZX, Zhao J, Yin L, et al. Resting heart rate and the risk of hypertension and heart failure: A dose-response meta-analysis of prospective studies. J Hypertens. 2017. [DOI] [PubMed] [Google Scholar]
- 46.Nahshoni E, Aravot D, Aizenberg D, Sigler M, Zalsman G, Strasberg B, Imbar S, Adler E, Weizman A. Heart rate variability in patients with major depression. Psychosomatics. 2004;45:129–134. [DOI] [PubMed] [Google Scholar]
- 47.Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Abedini S, Hansen JF. Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. Eur Heart J. 2004;25:363–370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Because of the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to The Framingham Heart Study at https://framinghamheartstudy.org/.


