Abstract
Introduction:
The prevalence of hepatitis C virus (HCV) infection among women delivering live births in the U.S. may be higher in rural areas, where county-level estimates may be unreliable. The aim of this study is to model county-level maternal HCV infection among deliveries in the U.S.
Methods:
In 2020, U.S natality files (2010–2018) with county-level maternal residence information were used from states that had adopted the 2003 revised U.S. birth certificate, which included a field for HCV infection present during pregnancy. Hierarchical Bayesian spatial models with spatiotemporal random effects were applied to produce stable annual county-level estimates of maternal HCV infection for years when all states had adopted the revised birth certificate (2016–2018). Models included a 6-Level Rural–Urban County Classification Scheme along with birth year and county-specific covariates to improve posterior predictions.
Results:
Among approximately 32 million live births, the overall prevalence of maternal HCV infection was 3.5 per 1,000 births (increased from 2.0 in 2010 to 5.0 in 2018). During 2016–2018, posterior predicted median county-level maternal HCV infection rates showed non-urban counties had 3.5–3.8 times higher rates of HCV compared with large central metro counties. The counties in the top 10th percentile for maternal HCV rates in 2018 were generally located in Appalachia, Northern New England, along the northern border in the Upper Midwest, and in New Mexico.
Conclusions:
Further implementation of community-level interventions that are effective in reducing maternal HCV infection and its subsequent morbidity may help to reduce geographic and rural disparities.
INTRODUCTION
In April 2020, the Centers for Disease Control and Prevention released hepatitis C virus (HCV) testing recommendations that include universal maternal screening during each pregnancy, except in settings where HCV infection (HCV RNA positivity) is <0.1% (1 per 1,000 pregnant women).1 These recommendations are broader than existing recommendations from obstetrics organizations, such as the American College of Obstetricians and Gynecologists and Society for Maternal–Fetal Medicine, which continue to recommend only risk-based testing during pregnancy, and from the U.S. Preventive Services Task Force, which recommends a universal 1-time screening that is inclusive of pregnant adults and adolescents.2 The new Centers for Disease Control and Prevention recommendations highlight the need for county-level estimates of maternal HCV prevalence for the U.S.
Evidence suggests that higher rates of HCV are found in more rural areas in the U.S. Nonmedical prescription opioid misuse has increased in rural areas since the early 2000s,3 which can lead to initiation of injection drug use,4–6 an HCV risk factor.7 Research indicates that people living in rural areas who use heroin, cocaine, and methamphetamine have higher use of injection as the method of administration compared with those living in urban areas.8 Compared with urban areas, rural areas have experienced higher rates of acute HCV infection among young adults,9,10 faster growth rates of opioid-related overdose deaths,11,12 higher opioid-related hospitalization rates,13,14 and lower access to harm reduction services.15,16 The higher risk of HCV infection among people living in rural areas likely translates to a higher prevalence of HCV among pregnant women.
Previous U.S. studies have found increases in HCV infection during pregnancy during the past decade, with some of the highest estimates found in rural areas. Overall, the prevalence of maternal HCV, as documented in birth certificate data, nearly doubled during 2009–2014 among reporting states (from 1.8 to 3.4 per 1,000 live births), which mirrored trends found using national hospital discharge data.17 Additionally, states with a large rural population, such as Tennessee, Kentucky, and Ohio, have prevalence rates higher than the national average.17–19 From 2014 to 2017, U.S. women with HCV at delivery were more likely than those without HCV to live in counties of <250,000 people, nationally.20 Maternal HCV infection not only places women at risk for high-morbidity conditions, including liver cancer, cirrhosis, and liver failure, but there is also a 5% perinatal transmission rate, which places the infant at risk as well and follow-up testing is recommended.21–23
Although an understanding of geographic variation in HCV prevalence at delivery in the U.S. may be useful, subnational estimates are typically suppressed when based on <20 events owing to concerns about statistical reliability and confidentiality. Though aggregation over time, across counties, or within states is possible, these approaches might mask important sub-state trends and variation, particularly for less populated rural areas. The aim of this study is to model county-level maternal HCV infection among deliveries in the U.S. using Bayesian spatial modeling, which smooths rates across adjacent counties and years, allowing for the examination of spatiotemporal variation across every county in the U.S., including rural areas.
METHODS
Study Population
This study used restricted-use birth certificate data from the National Center for Health Statistics (NCHS), 2010–2018. Data included information on the county of maternal residence at time of delivery, maternal demographics, maternal and infant clinical characteristics, and pregnancy outcomes. This study was determined to be non-human subjects research by the University of Southern Maine’s IRB. A data use agreement with the NCHS was signed stipulating that there would be no presentation of direct (i.e., observed) subnational estimates based on <10 events because of disclosure concerns.
Measures
Rurality of maternal residence was defined using the 2013 NCHS 6-Level Urban–Rural Classification Scheme (ranging from most urban to most rural: large central metro, large fringe metro, medium metro, small metro, micropolitan, and non-core).24
To estimate maternal HCV prevalence, data collected on the 2003 revised version of the U.S. Standard Certificate of Live Birth were used.25 Indicators of infections present and treated during pregnancy (even if treatment during pregnancy is not recommended) were added as checkboxes to the revised birth certificate for gonorrhea, syphilis, chlamydia, hepatitis B virus, HCV, or none of the above. This information was intended to be based on documented positive test results in the prenatal record, labor and delivery nursing admission triage form, admission history and physical examination record, or delivery record.26 There are 2 main types of HCV tests used: (1) antibody tests, which test for the presence of antibodies against HCV and can indicate current or past infection—these tests are most often used for HCV screening; and (2) HCV RNA tests, which test for the presence of the HCV virus—these tests are often used after an HCV screening test is positive.23 The type of test used for HCV testing was not collected on the birth certificate.
Maternal characteristics available from the birth certificate reflected information available at the time of delivery and included: age; race/ethnicity; highest level or degree of school completed; marital status and, if not married, paternity acknowledgment; receipt of food from the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) during the pregnancy; expected payment method for delivery (Medicaid, private, self-pay, other); smoking during pregnancy (self-reported ≥1 cigarettes a day on average for any trimester of pregnancy, no smoking, unknown)27; and first trimester prenatal care initiation (yes, no, unknown).
Federal Information Processing Standards county codes were used to link maternal county of residence with 2 time-varying county-level characteristics that could predict maternal HCV rates: the percentage of families living below the poverty threshold (county poverty rate) and drug overdose death rates.28,29 At the time of analysis, 2018 drug overdose death rates had not been released, so 2017 drug overdose death rates were used for 2018.
Births from states that used the 1989 birth certificate were excluded because HCV status was not collected on this version of the birth certificate,25,30–33 as well as births to women who were not residents of the 50 states or Washington, District of Columbia (Appendix Figure 1).
Statistical Analysis
Maternal and county-level characteristics of births across the NCHS 6-Level Urban–Rural Classification Scheme were tabulated. Maternal HCV prevalence (per 1,000 births) by maternal and county-level characteristics of births was calculated, along with prevalence ratios (PRs).
After restricting the analysis to states using the 2003 version of the U.S. birth certificate (n=32,555,153), maternal HCV status was missing/unknown for more births (n=172,592; 0.53%) than were reported with maternal HCV infection (n=113,142; 0.34%) (Appendix Figure 1). To assess the impact of these missing data, multiple imputation analysis was used, using chained equations to generate 10 sets of imputed maternal HCV status values for women missing this information. Assuming data were missing at random, HCV status was imputed using logistic regression, conditional on the following predictor variables that were associated with missing data: birth year, county NCHS 6-Level Urban–Rural Classification, state, county poverty rate category (<10%, 10%–19%, 20%–29%, and ≥30%), drug overdose death rate quartile (<10.7, 10.7 to <14.2, 14.2 to <19.2, and ≥19.2, estimated using the distribution of rates across all counties and study years), maternal age category, maternal educational attainment, and pregnancy smoking status.34
Hierarchical Bayesian spatial models with spatially and temporally structured random effects were applied to produce county-level prevalence of maternal HCV for the years 2016–2018, when all states had adopted the revised birth certificate. Although direct estimates of county-level prevalence based on small numbers of observed data can be highly variable from year to year, hierarchical Bayesian methods can produce more stable (smoothed) estimates by borrowing strength from nearby counties and over time. These methods have been previously used to examine county-level trends in teen pregnancy and suicide rates.35,36 The results of the multiple imputation analysis, which found missing data had minimal impact on rural–urban HCV prevalence rates, informed the decision to proceed with the spatial analysis using observed data only.
Hierarchical Bayesian log-binomial models were fit using the Integrated Nested Laplace Approximation package in R, version 3.6.1.37–38,39 Models included spatially structured random effects,40 fixed and random effects for year, along with a space X time interaction term, which allowed temporal trends to vary by county. To improve the fit of model-based maternal HCV estimates, models included county-level drug overdose death rate (as a continuous variable), all covariates included in the imputation model (with the exception of state), as well as maternal race/ethnicity, marital status, expected source of payment for delivery, WIC use during pregnancy, and pre-pregnancy BMI. Shrinkage plots were examined to see the magnitude and direction of the model-based smoothing (e.g., model-based estimate compared with direct estimate). Posterior predicted median county-level HCV rates were mapped and used to estimate maternal HCV prevalence by NCHS 6-Level Urban–Rural County Classification Scheme. Counties identified as being in the top 10th percentile for maternal HCV prevalence rates were mapped to show the areas with the highest rates within the U.S., even if they crossed state boundaries.
To investigate potential bias in the main study findings, maternal HCV prevalence by year was recalculated by county NCHS 6-Level Urban–Rural Classification Scheme and maternal and county-level characteristics using only the jurisdictions that had adopted the 2003 revised birth certificate as of 2010. Second, alternative hierarchical Bayesian models were applied, including/excluding variables and random effects; however, none of these alternative models resulted in improvements in fit, as assessed using the Widely Applicable Information Criterion (Appendix Text).41
RESULTS
This analysis included 32,555,153 live births occurring in the U.S. during 2010–2018 to women who were U.S. residents (92% of all live births during this time period). Most maternal and county-level characteristics showed variation by level of county rurality (Table 1). Across rural micropolitan and non-core counties, approximately 9% of births were to women aged <20 years, whereas in urban counties this ranged from 5% in large fringe metro counties to 8% in small metro counties. In addition, >70% of births in rural counties were to non-Hispanic White women (compared with 35%–67% across urban county categories), approximately 18% were to women who had at least a Bachelor’s degree (compared with 25%–38% across urban county categories), approximately 50% reported Medicaid as the expected source of payment for delivery (compared with 34%–45% across urban county categories), and approximately 15% of births were to women who smoked during pregnancy (compared with 4%–12% across urban county categories).
Table 1.
Maternal Characteristics by Urban–Rural Classification Scheme for Counties: U.S. Birth Certificate Data, 2010–2018
| Maternal characteristics at delivery | Total | 6-Level Urban-Rural Classification Scheme % of total |
|||||
|---|---|---|---|---|---|---|---|
| Large central metro | Large fringe metro | Medium metro | Small metro | Micropolitan | Non-core | ||
| All births in the U.S., n | 35,397,658 | 33.0 | 23.4 | 21.0 | 9.0 | 8.2 | 5.4 |
| Births in reporting states,a n | 32,555,153 | 33.8 | 22.9 | 20.8 | 8.9 | 8.3 | 5.3 |
| Among reporting states | |||||||
| Maternal age at birth, years | 32,555,153 | ||||||
| <20 | 2,147,632 | 6.0 | 4.9 | 7.3 | 7.7 | 9.0 | 9.3 |
| 20–24 | 7,083,520 | 19.2 | 17.5 | 23.9 | 26.0 | 28.7 | 30.0 |
| 25–29 | 9,383,286 | 26.9 | 28.0 | 30.2 | 31.4 | 31.0 | 31.3 |
| 30–34 | 8,721,104 | 28.7 | 30.6 | 25.1 | 23.5 | 21.0 | 20.0 |
| 35–39 | 4,229,385 | 15.4 | 15.4 | 11.1 | 9.5 | 8.5 | 7.8 |
| ≥40 | 990,226 | 3.8 | 3.6 | 2.4 | 2.0 | 1.8 | 1.7 |
| Maternal race/ethnicity | |||||||
| Hispanic | 7,696,290 | 32.7 | 19.1 | 24.7 | 15.9 | 14.3 | 9.6 |
| Non-Hispanic White | 17,174,129 | 35.3 | 57.9 | 55.9 | 66.7 | 72.0 | 75.9 |
| Non-Hispanic Black | 4,683,992 | 19.0 | 14.1 | 12.6 | 11.6 | 8.0 | 8.3 |
| Otherb | 2,992,797 | 13.1 | 8.9 | 6.8 | 5.9 | 5.6 | 6.2 |
| Marital status | |||||||
| Not married, no paternity acknowledgement | 3,891,990 | 12.4 | 9.3 | 12.5 | 13.3 | 13.4 | 14.1 |
| Not married, paternity acknowledgement | 8,889,338 | 27.6 | 24.4 | 28.1 | 28.0 | 30.2 | 29.2 |
| Married | 18,847,431 | 54.8 | 64.7 | 56.7 | 57.7 | 56.0 | 56.4 |
| Maternal educational attainment | |||||||
| No high school diploma or GED | 4,970,573 | 16.7 | 11.4 | 16.1 | 14.8 | 17.1 | 17.6 |
| High school diploma or GED | 8,122,282 | 23.7 | 21.6 | 25.7 | 27.7 | 30.0 | 31.9 |
| Some college | 9,284,067 | 25.0 | 27.7 | 30.8 | 32.1 | 32.5 | 32.9 |
| Bachelor’s degree or higher | 9,720,068 | 32.6 | 37.7 | 26.4 | 24.6 | 19.7 | 16.8 |
| Expected source of payment for delivery | |||||||
| Medicaid | 13,997,365 | 44.6 | 34.2 | 44.7 | 45.1 | 49.0 | 51.2 |
| Private | 15,413,565 | 46.2 | 57.8 | 44.1 | 44.3 | 40.3 | 38.4 |
| Self-pay | 1,333,698 | 4.6 | 3.3 | 3.8 | 3.7 | 4.9 | 5.3 |
| Other | 1,410,035 | 3.7 | 3.1 | 6.2 | 5.6 | 4.7 | 3.8 |
| WIC use during pregnancy | |||||||
| Yes | 13,659,406 | 43.3 | 31.8 | 44.7 | 45.0 | 48.8 | 50.3 |
| No | 18,265,233 | 55.2 | 65.3 | 53.6 | 52.9 | 49.5 | 47.6 |
| Pre-pregnancy BMI | |||||||
| Underweight | 1,146,154 | 3.8 | 3.2 | 3.5 | 3.4 | 3.6 | 3.5 |
| Normal | 14,213,097 | 46.0 | 44.5 | 42.6 | 41.4 | 40.3 | 38.8 |
| Overweight | 8,091,559 | 24.5 | 25.1 | 25.3 | 25.0 | 24.9 | 24.8 |
| Obese | 7,917,842 | 21.8 | 22.8 | 25.7 | 27.3 | 28.7 | 30.1 |
| Pregnancy smoking statusc | |||||||
| No | 28,788,307 | 93.6 | 88.2 | 88.1 | 83.6 | 80.5 | 78.5 |
| Yes | 2,477,793 | 3.5 | 6.2 | 8.5 | 11.7 | 15.5 | 17.3 |
| Unknown/not stated | 1,289,053 | 3.0 | 5.6 | 3.4 | 4.7 | 4.0 | 4.2 |
| First trimester prenatal care initiation | |||||||
| No | 7,609,420 | 23.2 | 21.1 | 24.2 | 24.4 | 25.6 | 26.2 |
| Yes | 23,809,149 | 73.0 | 74.4 | 73.0 | 72.6 | 72.3 | 70.9 |
| Unknown/not stated | 1,081,024 | 3.7 | 4.2 | 2.7 | 2.9 | 1.9 | 2.5 |
| County-level characteristics | |||||||
| County of residence, percentage below poverty thresholdd | |||||||
| <10% | 5,443,385 | 4.7 | 49.4 | 10.1 | 8.4 | 7.5 | 6.4 |
| 10%–19% | 22,041,463 | 80.4 | 48.0 | 70.3 | 72.2 | 65.2 | 58.3 |
| 20%–29% | 4,507,466 | 13.7 | 2.6 | 16.5 | 18.5 | 23.4 | 30.0 |
| ≥30% | 562,822 | 1.2 | 0.0 | 3.1 | 0.9 | 3.9 | 5.2 |
| County overdose death rate per 100,000 population,e mean | 32,555,153 | 15.8 | 16.7 | 17.4 | 15.8 | 16.9 | 17.3 |
Notes: Births in reporting states per year: 2010=3,055,884; 2011=3,267,934; 2012=3,412,436; 2013=3,548,525; 2014=3,837,663; 2015=3,839,624; 2016=3,945,875; 2017=3,855,500; 2018=3,791,712. Missing and unknown values (all <4%) were excluded from display in the table for the following characteristics: race/ethnicity (<0.1%, n=7,945), marital status (2.8%, n=926,394, with nearly all missing values from California starting in 2017), educational attainment (1.4%, n=458,163), source of payment for delivery (1.3%, n=400,490), Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) use during pregnancy (1.9%, n=630,514), pre-pregnancy BMI (3.7%, n=1,186,501), first trimester of prenatal care (0.2%, n=55,560), and county of residence percentage poverty level (<0.1%, n=17).
States and Washington, District of Columbia, reporting maternal hepatitis C virus infection used the 2003 revised birth certificate for the entire reporting year (U.S. territories excluded). The number of areas using the revised birth certificate increased over time: 34 areas in 2010, 37 areas in 2011, 38 areas in 2012, 41 areas in 2013, 48 areas in 2014, 49 areas in 2015, and all 51 areas in 2016–2018. Overall, there were 55,568 births with missing information on maternal hepatitis C virus infection (decreased from 14,096 in 2010 to 3,121 in 2017); the remaining births had either N, Y, or U documentation. U documentation (n=117,032) indicated the individual hospital did not report maternal HCV infection status.
Includes Asian or Pacific Islander, American Indian or Alaska Native, Other, and Hispanic origin unknown or not stated; and unknown.
Smoking during any trimester of pregnancy.
Generated by merging restricted-use data files with Census Bureau data on percentage of families below the poverty threshold by county Federal Information Processing Standard Publication (FIPS) for mother’s county of residence at the time of birth.
Generated by merging restricted-use data files with vital records published data on overdose death rates by county Federal Information Processing Standard Publication (FIPS) for mother’s county of residence at the time of birth.
GED, general education diploma; WIC, Special Supplemental Nutrition Program for Women, Infants, and Children.
Maternal HCV infection was highest among women who smoked during pregnancy (26.7 per 1,000 births), were not married and had no paternity acknowledgement (10.0 per 1,000 births), resided in a county categorized within the top quartile for drug overdose death rates (7.6 per 1,000 births), and had a high school diploma/GED (5.5 per 1,000 births) or no high school diploma/GED (5.9 per 1,000 births) (Appendix Table 1). The overall prevalence of maternal HCV across study years was 3.5 per 1,000 births (increased from 2.0 in 2010 to 5.0 in 2018; annual percentage change=0.39 per 1,000 births). HCV prevalence was higher for all other areas as compared with large central metro counties (Figure 1A). Multiple imputation of missing maternal HCV data did not meaningfully change HCV prevalence estimates by year and county-level rurality (Figure 1B). Findings were similar when these analyses were restricted to jurisdictions that had adopted the 2003 version of the birth certificate starting in 2010 (Appendix Figure 2, Appendix Table 2).
Figure 1.
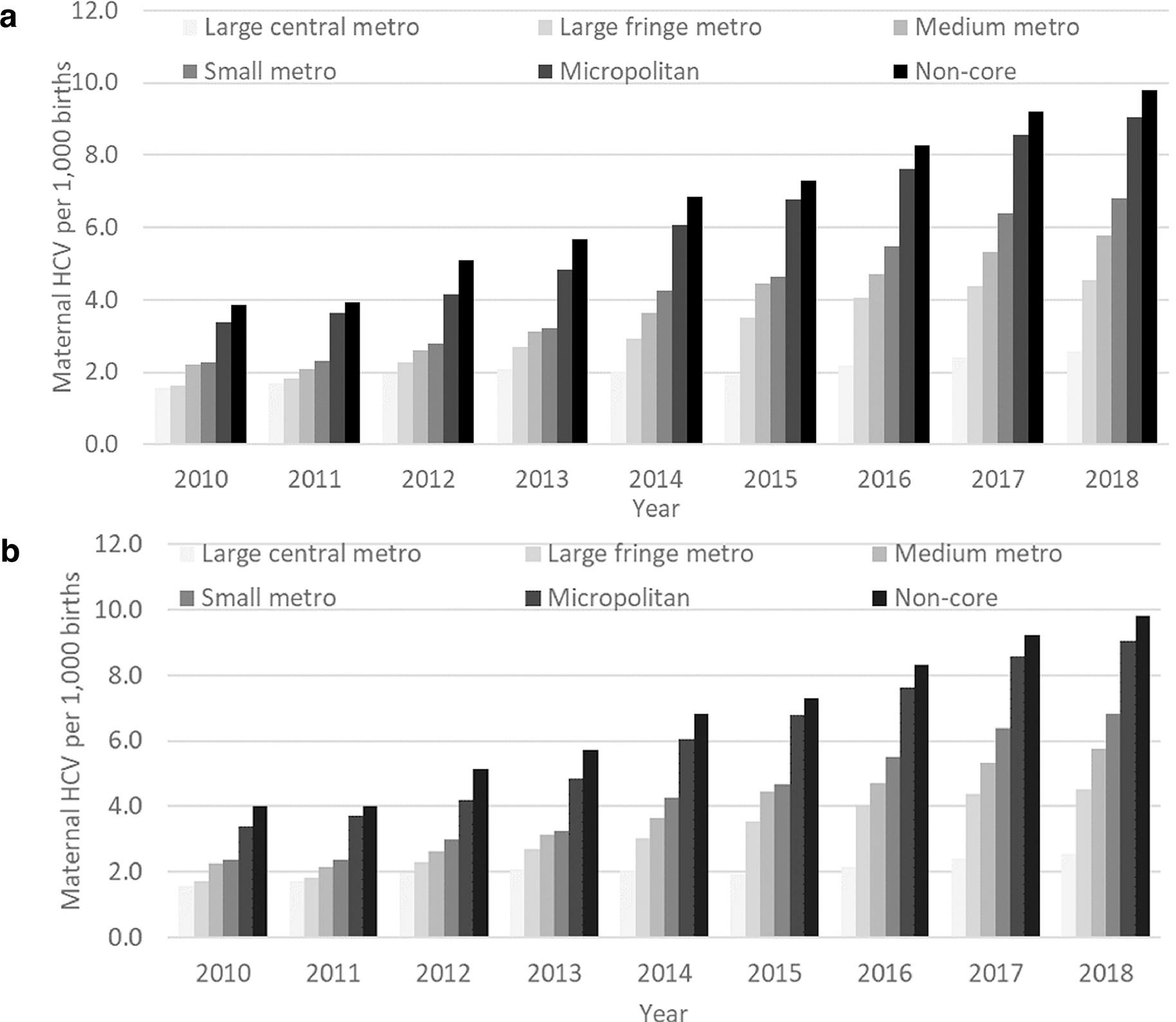
Prevalence of maternal hepatitis C virus infection (per 1,000 births) by 2013 National Center for Health Statistics’ 6-Level Urban–Rural Classification Scheme for Counties: U.S. birth certificate data, 2010–2018 (n=32,382,561). 1A: Prevalence (per 1,000 births). 1B: Prevalence (per 1,000 births), with missing prevalence of maternal hepatitis C infection data imputed.
HCV, hepatitis C virus.
Using direct estimates from observed data, only 243/3,142 (7.7%) counties had ≥20 cases of maternal HCV in 2018 (Figure 2A). By contrast, Figure 2B shows a map of model-based prevalence estimates for all counties in 2018 (model-based estimates are shown in tabular form in Appendix Table 2). The counties in the top 10th percentile for predicted maternal HCV rates in 2018 were generally located in Appalachia, Northern New England, along the northern border in the Upper Midwest, and in New Mexico (Appendix Figure 3). Nearly all (94.7%; 2,974/3,142) counties had predicted maternal HCV prevalence estimates >1 per 1,000 births (0.1%). The mean posterior predicted median county-level maternal HCV prevalence estimates for 2016–2018 were larger for rural counties (non-core: PR=3.76, 95% CI=3.63, 3.89; micropolitan: PR=3.52, 95% CI=3.41, 3.62) and less densely populated urban counties (large fringe metro: PR=1.81, 95% CI=1.77, 1.86; medium metro: PR=2.21, 95% CI=2.15, 2.27; small metro: PR=2.61, 95% CI=2.52, 2.69) as compared with large central metro counties; these were similar to prevalence ratios derived from observed data for the same time period (Table 2).
Figure 2.
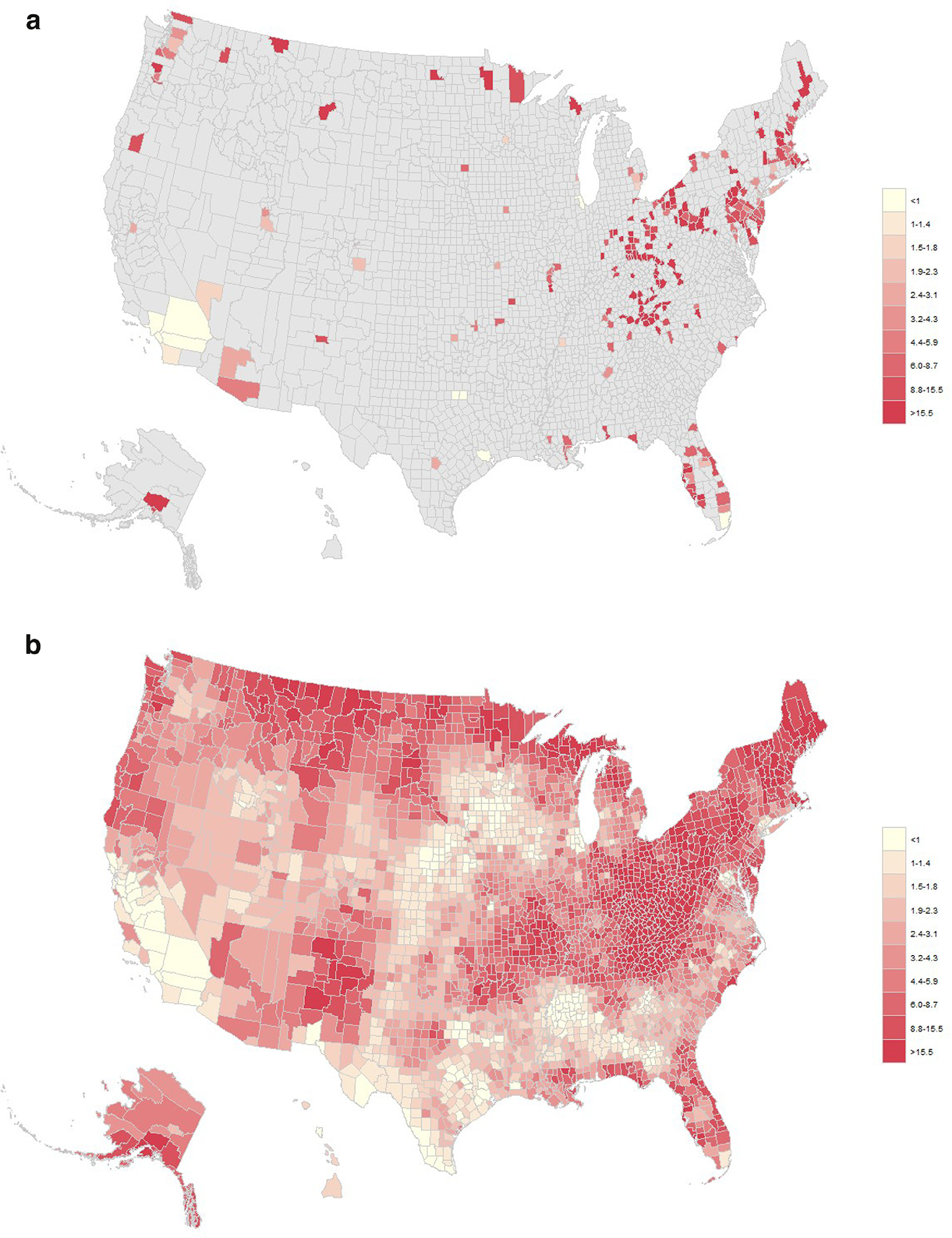
Observed and Bayesian modeled county-level estimates of maternal hepatitis C virus infection in 2018, (n=3,783,252 total births with maternal hepatitis C infection data). 1A: Prevalence of observed maternal hepatitis C virus infection per 1,000 live births in 2018 among 243 counties with at least 20 cases. 1B: Prevalence of Bayesian modeled maternal hepatitis C virus infection per 1,000 live births in 2018, all counties.
Table 2.
Prevalence and Prevalence Ratio of Maternal Hepatitis C Virus (HCV) Infection, 2016–2018 (n=11,565,027)
| 6-Level Urban–Rural County Classification Scheme | Observed prevalence |
Bayesian modeled median prevalence |
||
|---|---|---|---|---|
| Prevalence of HCV infection (per 1,000 births) | Prevalence ratio (95% CI) | Prevalence of HCV infection (per 1,000 births) | Prevalence ratio (95% CI) | |
| Large central metro | 2.4 | ref | 2.4 | ref |
| Large fringe metro | 4.3 | 1.82 (1.77, 1.87) | 4.3 | 1.81 (1.77, 1.86) |
| Medium metro | 5.3 | 2.21 (2.16, 2.28) | 5.2 | 2.21 (2.15, 2.27) |
| Small metro | 6.2 | 2.62 (2.54, 2.70) | 6.2 | 2.61 (2.52, 2.69) |
| Micropolitan | 8.4 | 3.54 (3.44, 3.65) | 8.3 | 3.52 (3.41, 3.62) |
| Non-core | 9.1 | 3.82 (3.70, 3.95) | 8.9 | 3.76 (3.63, 3.89) |
HCV, hepatitis C virus.
DISCUSSION
Among approximately 32 million live births in the U.S., the prevalence of maternal HCV increased from 2.0 per 1,000 births in 2010 to 5.0 in 2018, with an average prevalence of 3.5 per 1,000 births. Maternal HCV prevalence was higher among women living in rural than in large central metro counties. In addition, using estimates obtained from hierarchical Bayesian models with spatiotemporal random effects, rural counties (micropolitan and non-core) had 3.5–3.8 times higher rates of maternal HCV as compared with large central metro counties during the years 2016–2018, for which all areas reported maternal HCV data. The counties in the top 10th percentile for maternal HCV rates in 2018 were generally located in Appalachia, Northern New England, along the northern border in the Upper Midwest, and in New Mexico.
The prevalence of maternal HCV was higher among births to women with certain demographic characteristics, which is in line with recent studies that have also used U.S. birth certificate data and examined maternal characteristics.20,42 The maternal characteristics with the highest prevalence rates were smoking during pregnancy, being unmarried (without paternity acknowledgment), residing in a county within the highest quartile for drug overdose deaths, and having lower educational attainment. These characteristics have also been associated with maternal substance use disorder,43–47 a condition that places women at increased risk of acquiring HCV through injection drug use.
The study’s findings regarding temporal trends and geographic variations are consistent with the literature on prevalence of maternal HCV infection. National estimates have shown rates of approximately 3 per 1,000 births using either U.S. birth certificate data or ICD-CM codes from national hospital discharge data, with increases over time.17,20 In addition, several states that overlap with the Appalachian region have reported maternal HCV infection prevalence rates higher than the national average,17–19 which supports this study’s finding that many of the top 10th percentile counties are located in this region. This region has twice the percentage of rural residents than the U.S. overall (42% vs 20%),48 which is also consistent with the study’s findings. State-level analyses have found a higher prevalence of maternal opioid use disorder, a recognized risk factor for maternal HCV,49 in Maine and Vermont as compared with the national average and other rural areas43,44; this is consistent with this study’s finding of higher maternal HCV prevalence in some counties in Northern New England.
Study strengths include the ability to provide county-level estimates of maternal HCV for all counties in the U.S. Using the common threshold for suppressing rates (20 events), direct estimates for maternal HCV prevalence were only reliably estimated for 7.7% of all counties and for <1% of the non-core rural counties, whereas use of hierarchical Bayesian models resulted in smoothed county-level estimates for all counties in the U.S. Aggregation of estimates across space or time can mask important sub-state trends and variation, particularly for rural areas with smaller populations and for regions that span multiple states, like Appalachia. Further, study findings based on only the jurisdictions that adopted the revised birth certificate as of 2010 were consistent with the overall analysis, providing support that gradual adoption of the revised birth certificates by states over time was not driving the study’s overall findings.
Limitations
This study has several limitations. First, prevalence estimates were based on maternal HCV documentation on the birth certificate, which is intended to be completed using positive HCV test results from the medical record.26 During the study period, women were not routinely screened for HCV during pregnancy,42 so women may have actually been infected with HCV but failed to be tested or have their test results documented in the medical record (approximately 50% of people with chronic HCV in the U.S. are unaware of their status).50 Further, data transfer from the medical record to the birth certificate may be incomplete. Together, these issues likely resulted in under-documentation of HCV in the birth certificate data, and it is unknown how the degree of under-reporting may vary across counties and over time. Second, the spatial analysis could have resulted in over-smoothing of extreme high or low values, particularly in areas with sparse data or a small number of births. Third, although multiple imputation was used to estimate the impact of missing/unknown maternal HCV status on rural–urban estimates, missing data still could have biased the county-level estimates if they were related to county-level prevalence.
In addition, results regarding county-level maternal HCV prevalence cannot be used to identify areas for universal screening during pregnancy because type of HCV testing was unknown, and the Centers for Disease Control and Prevention guidelines refer only to HCV RNA positivity rates. However, assuming the birth certificate data relies on anti-HCV positive tests and that the percentage of anti-HCV positives that test positive for HCV RNA is consistent with prior data (59%),51 universal screening would be recommended for counties with maternal HCV prevalence estimates >1.7 per 1,000 births (0.17%).1 This threshold is mentioned with the caveat that positive predictive values of anti-HCV tests will vary based on the underlying prevalence of acute HCV infection,52 and owing to under-documentation of HCV on the birth certificate, a threshold of 1.7 per 1,000 births may be too high.
CONCLUSIONS
This study found increasing rates of maternal HCV in the U.S. and demonstrated that certain rural areas of the country experience higher rates. Further implementation of community-level interventions that are effective in reducing maternal HCV, as well as addressing co-occurring substance use disorders, may help to reduce geographic and rural disparities.
Supplementary Material
ACKNOWLEDGMENTS
The results reported herein correspond to specific aims of a project supported by grant CA#U1CRH03716 to Dr. Erika Ziller from the U.S. Federal Office of Rural Health Policy, Health Resources and Services Administration, HHS. Kristin Palmsten is supported by a career development award from the Eunice Kennedy Shriver National Institute of Child Health & Human Development, NIH (R00HD082412).
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Federal Office of Rural Health Policy or the National Center for Health Statistics, Centers for Disease Control and Prevention.
These data are available from the National Center for Health Statistics.
Footnotes
No financial disclosures were reported by the authors of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Schillie S, Wester C, Osborne M, Wesolowski L, Ryerson AB. CDC recommendations for hepatitis C screening among adults – United States, 2020. MMWR Recomm Rep 2020;69(2):1–17. 10.15585/mmwr.rr6902a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Havens PL, Anderson JR. Updated CDC recommendations for universal hepatitis C virus screening among adults and pregnant women: implications for clinical practice. JAMA. 2020;323(22):2258–2259. 10.1001/jama.2020.3693. [DOI] [PubMed] [Google Scholar]
- 3.Keyes KM, Cerda M, Brady JE, Havens JR, Galea S. Understanding the rural-urban differences in nonmedical prescription opioid use and abuse in the United States. Am J Public Health. 2014;104(2):e52–e59. 10.2105/ajph.2013.301709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Tayyib AA, Koester S, Riggs P. Prescription opioids prior to injection drug use: comparisons and public health implications. Addict Behav. 2017;65:224–228. 10.1016/j.addbeh.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeBeck K, Wood E, Dong H, et al. Non-medical prescription opioid use predicts injection initiation among street-involved youth. Int J Drug Policy. 2016;34:96–100. 10.1016/j.drugpo.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Havens JR, Walker R, Leukefeld CG. Prevalence of opioid analgesic injection among rural nonmedical opioid analgesic users. Drug Alcohol Depend. 2007;87(1):98–102. 10.1016/j.drugalcdep.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Zibbell JE, Hart-Malloy R, Barry J, Fan L, Flanigan C. Risk factors for HCV infection among young adults in rural New York who inject prescription opioid analgesics. Am J Public Health. 2014;104(11):2226–2232. 10.2105/ajph.2014.302142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novak SP, Kral AH. Comparing injection and non-injection routes of administration for heroin, methamphetamine, and cocaine users in the United States. J Addict Dis. 2011;30(3):248–257. 10.1080/10550887.2011.581989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suryaprasad AG, White JZ, Xu F, et al. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006–2012. Clin Infect Dis. 2014;59(10):1411–1419. 10.1093/cid/ciu643. [DOI] [PubMed] [Google Scholar]
- 10.Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years – Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR Morb Mortal Wkly Rep. 2015;64(17):453–458. [PMC free article] [PubMed] [Google Scholar]
- 11.Mack KA, Jones CM, Ballesteros MF. Illicit drug use, illicit drug use disorders, and drug overdose deaths in metropolitan and nonmetropolitan areas — United States. Morbidity and Mortality Weekly Review 2017;66(19):1–12. 10.15585/mmwr.ss6619a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths – United States, 2013–2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419–1427. 10.15585/mmwr.mm675152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss AJ, Elixhauser A, Barrett ML, Stiener CA, Bailey MK, O’Malley L. Opioid-related inpatient stays and emergency department visits by state, 2009–2014: Statistical Brief #219. Rockville, MD: Agency for Healthcare Research and Quality; 2016. https://www.ncbi.nlm.nih.gov/books/NBK441648/. Accessed January 27, 2021. [Google Scholar]
- 14.Nenninger EK, Carwile JL, Ahrens KA, Armstrong B, Thakarar K. Rural–urban differences in hospitalizations for opioid use-associated infective endocarditis in the United States, 2003–2016. Open Forum Infect Dis. 2020;7(2):ofaa045. 10.1093/ofid/ofaa045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis SM, Kristjansson AL, Davidov D, Zullig K, Baus A, Fisher M. Barriers to using new needles encountered by rural Appalachian people who inject drugs: implications for needle exchange. Harm Reduct J. 2019;16(1):23. 10.1186/s12954-019-0295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper HL, Cloud DH, Freeman PR, et al. Buprenorphine dispensing in an epicenter of the U.S. opioid epidemic: a case study of the rural risk environment in Appalachian Kentucky. Int J Drug Policy. 2020;85:102701. 10.1016/j.drugpo.2020.102701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patrick SW, Bauer AM, Warren MD, Jones TF, Wester C. Hepatitis C virus infection among women giving birth – Tennessee and United States, 2009–2014. MMWR Morb Mortal Wkly Rep. 2017;66(18):470–473. 10.15585/mmwr.mm6618a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koneru A, Nelson N, Hariri S, et al. Increased hepatitis C virus (HCV) detection in women of childbearing age and potential risk for vertical transmission – United States and Kentucky, 2011–2014. MMWR Morb Mortal Wkly Rep. 2016;65(28):705–710. 10.15585/mmwr.mm6528a2. [DOI] [PubMed] [Google Scholar]
- 19.Rossi RM, Warshak CR. Prevalence of maternal hepatitis C virus infection in Ohio. Obstet Gynecol. 2018;132(3):708–716. 10.1097/aog.0000000000002807. [DOI] [PubMed] [Google Scholar]
- 20.Rossi RM, Wolfe C, Brokamp R, et al. Reported prevalence of maternal hepatitis C virus infection in the United States. Obstet Gynecol. 2020;135(2):387–395. 10.1097/aog.0000000000003644. [DOI] [PubMed] [Google Scholar]
- 21.Benova L, Mohamoud YA, Calvert C, Abu-Raddad LJ. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis. 2014;59(6):765–773. 10.1093/cid/ciu447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gross MS, Ruth AR, Rasmussen SA. Respect women, promote health and reduce stigma: ethical arguments for universal hepatitis C screening in pregnancy. J Med Ethics. 2020;46:674–677. 10.1136/medethics-2019-105692. [DOI] [PubMed] [Google Scholar]
- 23.Ghany MG, Morgan TR. Hepatitis C guidance 2019 update: American Association for the Study of Liver Diseases–Infectious Diseases Society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology. 2020;71(2):686–721. 10.1002/hep.31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ingram DD, Franco SJ. 2013 NCHS Urban–Rural Classification Scheme for Counties. Vital Health Stat 2. 2014(166):1–73. [PubMed] [Google Scholar]
- 25.Ventura SJ. The U.S. National Vital Statistics System: transitioning into the 21st century, 1990–2017. Vital and Health Stat 1. 2018;62(1):1–84. [PubMed] [Google Scholar]
- 26.National Center for Health Statistics. Guide to completing the facility worksheets for the certificate of live birth and report of fetal death (2003 revision). Hyattsville, MD: National Center for Health Statistics; 2016. https://www.cdc.gov/nchs/nvss/facility-worksheets-guide.htm?Sort=URL%3A%3Aasc. Accessed January 27, 2021. [Google Scholar]
- 27.Mother’s Worksheet for Child’s Birth Certificate. National Vital Statistics System. Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/data/dvs/moms-worksheet-2016.pdf. Published December 2016. Accessed February 3, 2021. [Google Scholar]
- 28.National Center for Health Statistics. Data visualization gallery: drug poisoning mortality in the United States, 1999–2018. https://www.cdc.gov/nchs/data-visualization/drug-poisoning-mortality/. Updated August 25, 2020. Accessed January 27, 2021.
- 29.Small Area Income and Poverty Estimates (SAIPE) Program. U.S. Census Bureau. https://www.census.gov/programs-surveys/saipe.html. Accessed January 27, 2021. [Google Scholar]
- 30.NCHS. User guide to the 2013 natality public use file. Hyattsville, MD: National Center for Health Statistics; 2013. ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/DVS/natality/UserGuide2013.pdf. Accessed February 3, 2021. [Google Scholar]
- 31.NCHS. User guide to the 2014 natality public use file. Hyattsville, MD: National Center for Health Statistics; 2014. ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/DVS/natality/UserGuide2014.pdf. Accessed February 3, 2021. [Google Scholar]
- 32.NCHS. User guide to the 2015 natality public use file. Hyattsville, MD: National Center for Health Statistics; 2015. ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/DVS/natality/UserGuide2015.pdf. Accessed February 3, 2021. [Google Scholar]
- 33.NCHS. User guide to the 2016 natality public use file. National Center for Health Statistics. Hyattsville, MD; 2016. ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/DVS/natality/UserGuide2016.pdf. Accessed February 3, 2021. [Google Scholar]
- 34.Stata. Marginal means, adjusted predictions, and marginal effects. https://www.stata.com/features/overview/marginal-analysis/. Accessed February 3, 2021.
- 35.Khana D, Rossen LM, Hedegaard H, Warner M. A Bayesian spatial and temporal modeling approach to mapping geographic variation in mortality rates for subnational areas with R-INLA. J Data Sci. 2018;16(1):147–182. [PMC free article] [PubMed] [Google Scholar]
- 36.Rossen LM, Hedegaard H, Khan D, Warner M. County-level trends in suicide rates in the U.S., 2005–2015. Am J Prev Med. 2018;55(1):72–79. 10.1016/j.amepre.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martino S, Riebler A. Integrated nested Laplace approximations (INLA). In: Balakrishnan N, Colton T, Everitt B, Piegorsch W, Ruggeri F, Teugels JL, eds. Wiley StatsRef: Statistics Reference Online. Hoboken, NJ: Wiley; 2021. 10.1002/9781118445112.stat08212. [DOI] [Google Scholar]
- 38.Bivand R, Gómez-Rubio V, Rue H. Spatial data analysis with R-INLA with some extensions. 2015 2015;63(20):31. 10.18637/jss.v063.i20. [DOI] [Google Scholar]
- 39.Rue H, Martino S, Chopin N. Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. J R Stat Soc Series B Stat Methodol. 2009;71(2):319–392. 10.1111/j.1467-9868.2008.00700.x. [DOI] [Google Scholar]
- 40.Besag J, York J, Mollié A. Bayesian image restoration, with two applications in spatial statistics. Ann Inst Stat Math. 1991;43(1):1–20. 10.1007/bf00116466. [DOI] [Google Scholar]
- 41.Spiegelhalter DJ, Best NG, Carlin BP, van der Linde A. The deviance information criterion: 12 years on. J R Stat Soc Series B Stat Methodol. 2014;76(3):485–493. 10.1111/rssb.12062. [DOI] [Google Scholar]
- 42.Schillie SF, Canary L, Koneru A, et al. Hepatitis C virus in women of childbearing age, pregnant women, and children. Am J Prev Med. 2018;55(5):633–641. 10.1016/j.amepre.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 43.Gabrielson SMB, Carwile JL, O’Connor AB, Ahrens KA. Maternal opioid use disorder at delivery hospitalization in a rural state: Maine, 2009–2018. Public Health. 2020;181:171–179. 10.1016/j.puhe.2019.12.014. [DOI] [PubMed] [Google Scholar]
- 44.Haight SC, Ko JY, Tong VT, Bohm MK, Callaghan WM. Opioid use disorder documented at delivery hospitalization – United States, 1999–2014. MMWR Morb Mortal Wkly Rep. 2018;67(31):845–849. 10.15585/mmwr.mm6731a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ko JY, Haight SC, Schillie SF, Bohm MK, Dietz PM. National trends in hepatitis C infection by opioid use disorder status among pregnant women at delivery hospitalization – United States, 2000–2015. MMWR Morb Mortal Wkly Rep. 2019;68(39):833–838. 10.15585/mmwr.mm6839a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kurdziel-Adams G, Macfie J, Kors S, Fortner KB, Towers CV, Lydic R. Opioid use in pregnancy: borderline features and hepatitis C virus. Personal Disord. 2020;11(3):222–229. 10.1037/per0000372. [DOI] [PubMed] [Google Scholar]
- 47.Smid MC, Metz TD, Gordon AJ. Stimulant use in pregnancy: an under-recognized epidemic among pregnant women. Clin Obstet Gynecol. 2019;62(1):168–184. 10.1097/grf.0000000000000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.The Appalachian Region. The Appalachian Regional Commission. https://www.arc.gov/appalachian_region/TheAppalachianRegion.asp. Accessed March 19, 2020.
- 49.Substance Abuse and Mental Health Services Administration. A Collaborative Approach to the Treatment of Pregnant Women with Opioid Use Disorders. HHS Publication No. (SMA) 16–4978. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2016. https://ncsacw.samhsa.gov/files/Collaborative_Approach_508.pdf. Accessed May 21, 2020. [Google Scholar]
- 50.Kim HS, Yang JD, El-Serag HB, Kanwal F. Awareness of chronic viral hepatitis in the United States: an update from the National Health and Nutrition Examination Survey. J Viral Hepat. 2019;26(5):596–602. 10.1111/jvh.13060. [DOI] [PubMed] [Google Scholar]
- 51.Hofmeister MG, Rosenthal EM, Barker LK, et al. Estimating prevalence of hepatitis C virus infection in the United States, 2013–2016. Hepatology. 2019;69(3):1020–1031. 10.1002/hep.30297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Molinaro AM. Diagnostic tests: how to estimate the positive predictive value. NeuroOncol Pract. 2015;2(4):162–166. 10.1093/nop/npv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


