Abstract
Objective:
We tested whether substrate concentrations or fatty acid storage proteins predict storage of endogenous lipids in visceral (VAT) and upper body subcutaneous (UBSQ) fat.
Methods:
The day prior to surgery 25 patients undergoing bariatric procedures received an infusion of autologous [1-14C]triolein-labeled VLDL particles and during surgery they received a continuous [U-13C]palmitate infusion/bolus [9,10-3H]palmitate tracer. We collected VAT and UBSQ fat to measure: VLDL-triglyceride (TG) storage, direct free fatty acid (FFA) storage rates, CD36 content, lipoprotein lipase (LPL), acyl-CoA synthetase (ACS), diacylglycerol acetyl-transferase (DGAT) and glycerol-3-phosphate acyltransferase (GPAT) activities.
Results:
Storage of VLDL-TG and FFA-palmitate in UBSQ and VAT was not different. Plasma palmitate concentrations correlated with palmitate storage rates in UBSQ and VAT (r = 0.46, P = 0.02 and r = 0.46, P = 0.02, respectively). In VAT, VLDL-TG storage was correlated with VLDL concentrations (r = 0.53, P< 0.009) and LPL (r = 0.42, P < 0.05). In UBSQ, VLDL-TG storage was correlated with LPL (r = 0.42, P < 0.05). CD36, ACS, GPAT and DGAT were not correlated with VLDL-TG or palmitate storage.
Conclusions:
Adipose storage of VLDL-TG is predicted by VLDL-TG concentrations and LPL; FFA concentrations predict direct adipose tissue FFA storage rates.
Keywords: lipoprotein lipase, acyl-CoA synthetase, diacylglycerol acetyl-transferase, glycerol-3-phosphate acyltransferase, VLDL-triglyceride
Introduction:
Severe obesity is associated with increased morbidity/mortality and can severely impact quality of life. Excess visceral adipose tissue (VAT) is consistently associated with metabolic syndrome (1) and excess FFA release from dysfunctional upper body subcutaneous adipose tissue (UBSQ) can negatively impact insulin sensitivity (2). The relative amounts of VAT and UBSQ appear to be driven, at least in part, by differences in the storage of fatty acids into these depots. However, the contributions of extrinsic vs. intrinsic factors to adipose tissue direct FFA storage in these depots in severe obesity are unclear.
Plasma free fatty acid (FFA) and very low density lipoprotein (VLDL) particles are the main endogenous lipid sources stored in adipose tissue. Plasma FFA that arise from adipose tissue lipolysis can be taken up by the liver and secreted as VLDL-triglycerides (TG) and also can be re-esterified directly in distant adipocytes (3), a process allowing redistribution of FA between adipose tissue depots.
Fatty acid uptake into adipocytes occurs either by “flip-flop” through the plasma membrane or can occur through facilitated transport via a number of proteins, including CD36. In all tissues, extracellular lipoprotein lipase (LPL), which hydrolyzes triglycerides, is necessary for VLDL-TG-FA uptake. However, a series of enzymatic steps are necessary for successful FA storage into adipocyte TG. These enzymes include acyl-CoA synthetase (ACS), which traps FA by forming fatty acyl-CoA’s and allows glycerol-3-phosphate acyltransferases (GPAT(s)) to catalyze the first step in TG synthesis (4). Diacylglycerol acyl transferase (DGAT) catalyzes the final step in this process. We found that in overweight adults, VAT and UBSQ have different enzyme capacities (5,6). Whether this is true in adults with severe obesity and whether these steps are rate limiting for VLDL-TG or direct FFA storage is not known.
This study was undertaken in order to understand the contribution of adipose storage and transport enzymes and substrate availability on direct storage of FFA and VLDL-TG in UBSQ and VAT from adults with severe obesity. We worked with patients scheduled for bariatric surgery because, by coordinating the research with the clinical care, we could obtain the carefully timed and simultaneous intra-operative biopsies of VAT and UBSQ after tracer administration to measure direct FFA storage combined with measures of VLDL-TG storage in adipose tissue. Herein we report the relationships between plasma concentrations of VLDL-TG and FFA, as well as adipose tissue content of LPL, CD36, ACS, GPAT and DGAT, with storage of VLDL-TG and FFA in VAT and UBSQ adipose tissue in adults with severe obesity.
Methods:
Participants:
Twenty-five Caucasian, non-diabetic adults age 18–55 y, scheduled for elective bariatric surgery participated in this Mayo IRB-approved study after signing informed consent; a copy of the informed consent document was provided to participants. All participants were considered to have Class II or Class III obesity. Patient characteristics are provided in Table 1.
Table 1.
Participant demographics, VLDL-TG and plasma palmitate kinetic information.
| N = 25 | |
|---|---|
| Sex (Female/Male) | (15/10) |
| Age (years) | 39 ± 9 |
| BMI (kg/m2) | 47.8 ± 8.2 |
| Body fat (%) | 51 ± 5 |
| Visceral fat area (cm2) | 227 ± 84 |
| Visceral fat (kg) | 7.7 ± 3.4 |
| Upper body subcutaneous fat (kg) | 37.5 ± 10.9 |
| Fasting plasma insulin (mcIU/mL)A | 6.7 ± 4.8 |
| Fasting plasma glucose (mmol/L)A | 5.8 ± 0.8 |
| HOMA-IRA | 3.11 ± 1.45 |
| Fasting plasma HDL-cholesterol (mg/dL)A | 37 ± 8 |
| Fasting plasma triglycerides (mg/dL)A | 158 ± 41 |
| Fasting plasma total cholesterol (mg/dL)A | 135 ± 55 |
| Resting energy expenditure (Kcal/d)A | 2316 ± 438 |
| VLDL-TG concentration (μmol/L)A | 865 ± 395 |
| VLDL oxidation (%)A | 29 ± 8 |
| Palmitate concentration (μmol/L)B | 209 ± 53 |
| Palmitate flux (μmol/min)B | 168 ± 47 |
Data are represented as mean ± SD. BMI, body mass index; VLDL, very-low density lipoprotein; TG, triglycerides.
data from the VLDL infusion study day
data from the day of surgery.
Protocol:
This study has previously been described in detail(7). Approximately one week prior to the inpatient study visit a 100 mL fasting blood sample was obtained for ex-vivo labeling of VLDL-triglyceride (TG) with [1-14C]triolein. Ex-vivo labeling of VLDL particles with [1-14C]triolein has been described in detail (8). At the same visit a single slice computed tomography (CT) scan of the abdomen and a DXA scan (Lunar iDXA, GE Healthcare, Madison, WI) were performed to measure body composition and calculate the mass of upper body subcutaneous and visceral adipose tissue(9). Because of the high BMI of the population, there were 9 volunteers whose abdominal areas slightly exceeded the capacity of the CT image area. To address this we estimated the abdominal subcutaneous fat area for these volunteers using an ellipse measurement tool to extrapolate just beyond the lateral edges of the CT images.
One week later, two days prior to their scheduled surgery, participants were admitted to the Mayo Clinical Research and Trials Unit. The next morning, after an overnight fast, an intravenous forearm vein catheter was placed for tracer infusions and a retrograde intravenous catheter was place in the contralateral hand vein for collection of arterialized blood samples using the heated hand vein technique. Baseline blood samples were collected and a primed, continuous infusion of autologous [1-14C]-triolein-labeled VLDL-TG was started and maintained for 4 hours. Blood samples were collected at regular intervals to measure plasma concentrations and specific activities of VLDL-TG. Resting energy expenditure (REE) was measured using indirect calorimetry at baseline, 2 and 4 hours into the infusion of labeled VLDL. In order to calculate VLDL-FA oxidation, we measured expired breath content of 14CO2 (10,11) at 6 time points during the course of the VLDL infusion. Two participants were unable to complete the VLDL portion of the study. Following completion of the VLDL infusion, participants were provided with self-selected meals from hospital food services for the remainder of the day in order to allow them to continue their habitual diet. Following completion of evening meal at 1830 h participants fasted overnight in preparation for surgery the next day.
The following morning a continuous infusion of [U-13C]palmitate (Isotec, Sigma-Aldrich, Miamisburg, OH) was started ~ 0630 h in the pre-operative holding area and continued during surgery to measure FFA-palmitate flux to allow calculation of tissue palmitate storage rates. A radial artery catheter was placed by the anesthesiologist as part of the surgical procedure and was used for blood collection. Blood samples were drawn just before an intravenous bolus of ~100 μCi of [9,10-3H]palmitate and at 15 min intervals for 30 min. Thirty minutes after the [3H]palmitate bolus the surgeon obtained samples of omental and abdominal subcutaneous adipose tissue in order to measure the storage of [9,10-3H] palmitate from the tracer bolus (6). The rational for the timing of the biopsies relates to the disappearance of FFA tracers from the circulation and their appearance in VLDL-TG-FA (3). These samples were also used to measure the accumulation of VLDL-TG-[1-14C]oleate from the infusion from the previous day (12).
Concentrations and specific activity of plasma and tissue
The VLDL fraction from the plasma samples obtained during the [1-14C]VLDL-TG infusion were isolated by density gradient ultracentrifugation (13) and the specific activity (SA) and TG concentrations (Cobas Integra® 400 plus, Roche Diagnostics, Ltd., Indianapolis, IN) were measured. Plasma concentrations and enrichment of FFA-palmitate were quantified using LC/MS (14).
The adipose tissue biopsy samples were briefly rinsed in ice cold saline to remove any blood, cleared of any visible matrix and aliquoted for cell size determination and enzyme activity assays. An aliquot of each adipose tissue sample was extracted using the Folch technique (15) and the SA of the adipose tissue lipid was measured from an average ~350 mg of lipid from both abdominal subcutaneous and visceral adipose samples using dual-channel liquid scintillation counting set to a counting error of ≤ 2% for both 3H and 14C.
Cell size:
Mean adipocyte cell size (diameter) and μg of lipid per cell were analyzed by collagenase digesting approximately 300 mg of adipose tissue, and measuring cell size using microscopy images (16).
Biochemical Measurements
Total Glycerol-3-Phosphate acyltransferase (GPAT) and GPAT1 activity was measured in UBSQ and VAT samples as previously described (17). We were unable to collect sufficient tissue to perform all assays on all samples; omental GPAT activity was not measured for 2 volunteers and UBSQ GPAT activity was not measured for 5 volunteers. Lipoprotein Lipase (LPL) activity (18), DGAT activity (19,20) and acyl-CoA synthetase (ACS) activity (21) were measured for all samples.
Plasma insulin concentrations were measured by the Mayo Clinic Immunochemical Core Lab at Mayo Clinic using a chemiluminescent sandwich assay.
CD36 content was measured by capillary western blot using the WES SimpleWes System (ProteinSimple, Santa Clara, CA) on the 12–230 kDa separation module (ProteinSimple, Santa Clara, CA) according to manufacturer’s instructions. Briefly, sample and pooled quality control tissue extracts were diluted in 0.1X Sample Buffer to a concentration of 10 ng/uL total protein. Pooled calibrator extract was diluted to a concentration of 50 ng/mL followed by 2X serial dilutions down to 3.125 ng/uL to create a standard curve. Samples were combined with master mix and denatured for 5 minutes at 95°C on a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA). Denatured extracts, anti-human CD36/SR-B3 primary antibody diluted at 1:100 (R&D Systems, Minneapolis, MN), and anti-goat detection module reagents (ProteinSimple, Santa Clara, CA) were loaded on the plate in designated wells and run on a WES Simple Western System. CD36 content was calculated based on the standard curve and expressed as a ratio of sample CD36 to that of a pooled calibrator sample. We have previously validated this approach for measuring CD36 against traditional Western blotting (22).
Calculations and Statistics:
To estimate the storage of VLDL-TG fatty acids in adipose tissue we multiplied the fraction of tracer stored per kg of adipose lipid by the μmol of VLDL-TG fatty acids that transited the circulation over the 4 hours of the infusion. Because loss of fatty acid tracer from adipose tissue is extremely slow in those with large adipose triglyceride depots, we assume for all intents and purposes tracer loss from lipolysis was negligible over 24 h. Plasma FFA-palmitate storage rates were determined as previously described (6), where the fraction of tracer stored in adipose lipid is multiplied by palmitate disappearance rates calculated from the [U-13C]palmitate tracer. The fractional storage of VLDL-TG-14C-oleate and the μmol of VLDL-TG fatty acids stored in adipose tissue was calculated by dividing the 14C dpm/gm lipid in adipose tissue by the steady state plasma VLDL-TG SA (dpm/μmol). To calculate the percent of palmitate and VLDL-TG fatty acids stored in VAT and UBSQ adipose tissue we multiplied the amount per kg adipose lipid times the total kg of adipose lipid in those depots.
The 14CO2 excretion rates from VLDL oxidation over the 4 hours of the VLDL-turnover study were measured as previously described (23). We calculated the percent of the VLDL-TG-[1-14C]oleate that was oxidized as the expired 14CO2 during the 4 hours of the infusion as well as the subsequent 2 hours immediately after divided by the total VLDL-TG-[1-14C]oleate dose infused over 4 h. VLDL-TG-fatty acid oxidation was calculated using an acetate-recovery factor (24).
All data is presented as mean ± SD. Data that was not normally distributed was log transformed before analysis. Student’s t-test was used for comparison between means. We used Pearson correlation analysis to test for associations between variables when the data was normally distributed.
Results:
Subject characteristics
Participant demographic, body composition, REE, plasma VLDL-TG and palmitate kinetics and concentration data are provided in Table 1.
Table 2 provides fat cell size, the fatty acid storage enzyme activity data for VAT and UBSQ adipose tissue. Lipoprotein lipase activity was nearly identical in VAT and UBSQ fat and the values were positively correlated (r = 0.54, P = 0.005). ACS activity was greater in VAT than UBSQ (p < 0.006) and the values tended to correlate (r = 0.38, P = 0.06). DGAT activity was not significantly different between VAT and UBSQ and the values were not correlated. Total GPAT and GPAT1 activities were both greater (P < 0.0001) in VAT than UBSQ. There was not a significant relationship between either total GPAT or GPAT1 activity in omental and subcutaneous adipose tissue. CD36 content was not different between VAT and UBSQ (Table 2) and tended to correlate between depots (r = 0.37, P = 0.07).
Table 2:
Visceral and upper body subcutaneous specific adipose tissue characteristics.
| VAT | UBSQ | |
|---|---|---|
| N = 25ǂ | N = 25֏ | |
| Adipocyte Size (μg lipid/cell) | 0.90 ± 0.32 | 1.22 ± 0.33* |
| Adipose enzyme activity and protein | ||
| LPL activity (μmol FFA•g tissue−1•hour−1) | 0.27 ± 0.22 | 0.27 ± 0.19 |
| Total GPAT activity (pmol TG•mg lipid−1•min−1) | 5.5 ± 1.4 | 2.5 ± 1.3* |
| GPAT1 activity (pmol TG• mg lipid−1•min−1) | 1.8 ± 0.8 | 0.7 ± 0.5* |
| ACS activity (pmol•mg lipid−1•min−1) | 26 ± 13 | 17 ± 9* |
| DGAT activity (pmol TG•mg lipid−1•min−1) | 3.4 ± 1.7 | 2.6 ± 1.8 |
| CD36 protein content (A.U./mg lipid) | 1.8 ± 0.8 | 2.0 ± 0.8 |
| Fatty acid storage | ||
| [9,10-3H] palmitate content (dpm/g lipid)¥ | 383 ± 147 | 488 ± 229 |
| Palmitate storage rate (μmol•kg lipid−1•min−1) | 0.39 ± 0.2 | 0.31 ± 0.17 |
| Percent [9,10-3H] palmitate storage/depot | 1.4 ± 0.78 | 8.9 ± 4.1* |
| [1-14C]VLDL-TG content (dpm/g lipid)¥ | 116 ± 44 | 129 ± 70 |
| Percent [1-14C]VLDL-TG storage/depot | 2.9 ± 1.4 | 16.5 ± 8.1* |
Data are represented as mean ± SD. LPL, Lipoprotein lipase; GPAT, glycerol-3-phosphate acyltransferase FFA; ACS, acetyl-CoA synthetase; DGAT, diacylglycerol acyltransferase; Free Fatty Acid; TG, triglyceride; A.U., arbitrary units; VLDL, very-low density lipoprotein.
dpm/g lipid represented as raw values.
P < 0.05 comparing VAT to UBSQ.
VAT GPAT activity: N = 23.
UBSQ GPAT activity: N = 20.
Storage of VLDL derived fatty acids
The storage of 14C-labeled VLDL-fatty acids in VAT and UBSQ adipose tissue 24 hours after the VLDL tracer study is also provided in Table 2. Storage of VLDL derived fatty acids per gram of lipid in VAT was positively correlated with storage in UBSQ fat (r = 0.77, P <0.0001, Figure 1A). There was no difference in VLDL-TG storage between UBSQ and VAT (0.17 ± 0.1 vs 0.15 ± 0.07 μmol/g lipid, respectively). The percent of VLDL-TG stored in the UBSQ and VAT depots is also provided in Table 2.
Figure 1:
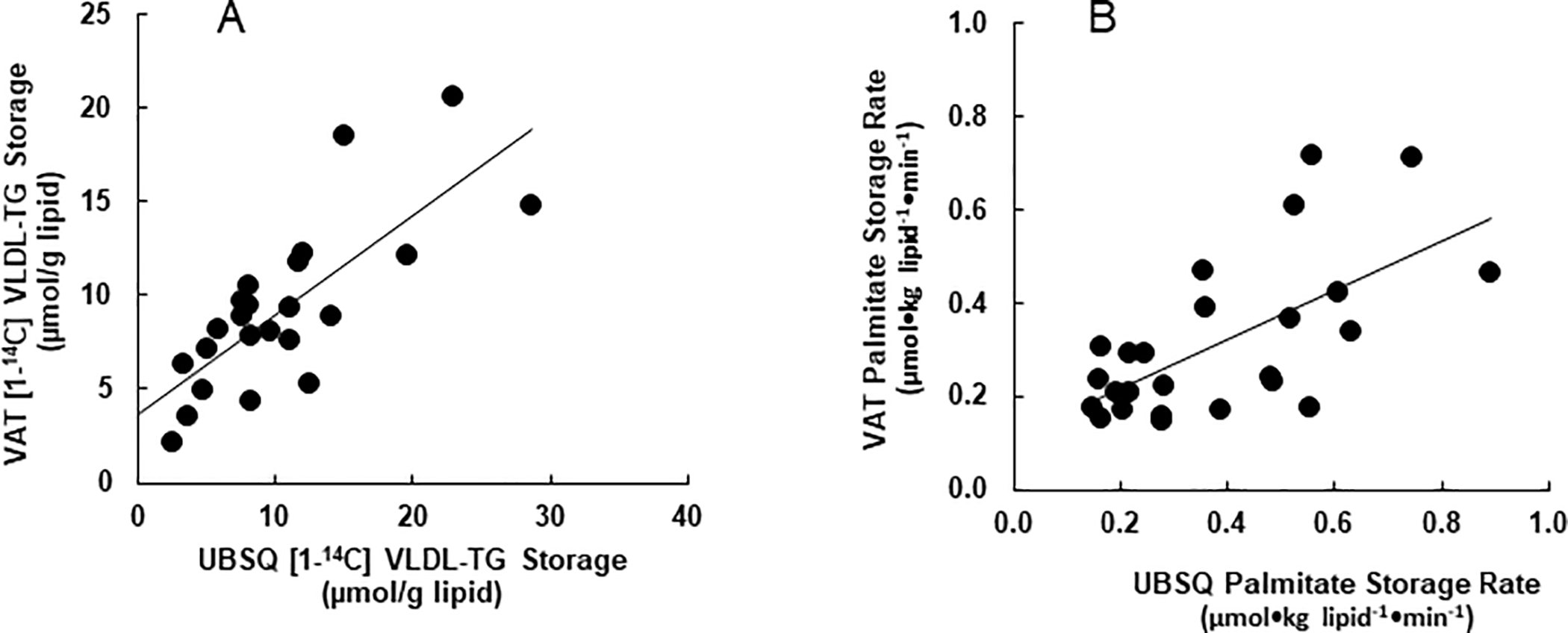
Associations between VAT and UBSQ FFA-palmitate storage rates and quantity of [1-14C]VLDL-TG tissue retention. Tissue quantities of VLDL-TG were determined by dose adjusted tissue specific activity and converted to μmols (A) Association between VAT and UBSQ storage of [1-14C]VLDL-TG (r = 0.77, P <0.0001). (B) Association between storage rates of plasma FFA-palmitate in VAT and UBSQ (r = 0.64, P < 0.006.)
Adipose storage of systemic FFA
The storage rates of plasma palmitate per gram of lipid in VAT and UBSQ are provided in Table 2; palmitate storage rates were not different between UBSQ and VAT. Plasma palmitate storage rates in VAT and UBSQ were positively correlated (r = 0.64, P < 0.006, Fig. 1B).
Relationship between intra-depot storage of systemic FFA and VLDL-TG
FFA-palmitate storage rates and 24-hour storage of VLDL-TG-oleate tended to be correlated in VAT (r = 0.39, P = 0.08, Fig. 2A) and were significantly correlated in UBSQ (r = 0.43, P < 0.05, Figure 2B).
Figure 2:
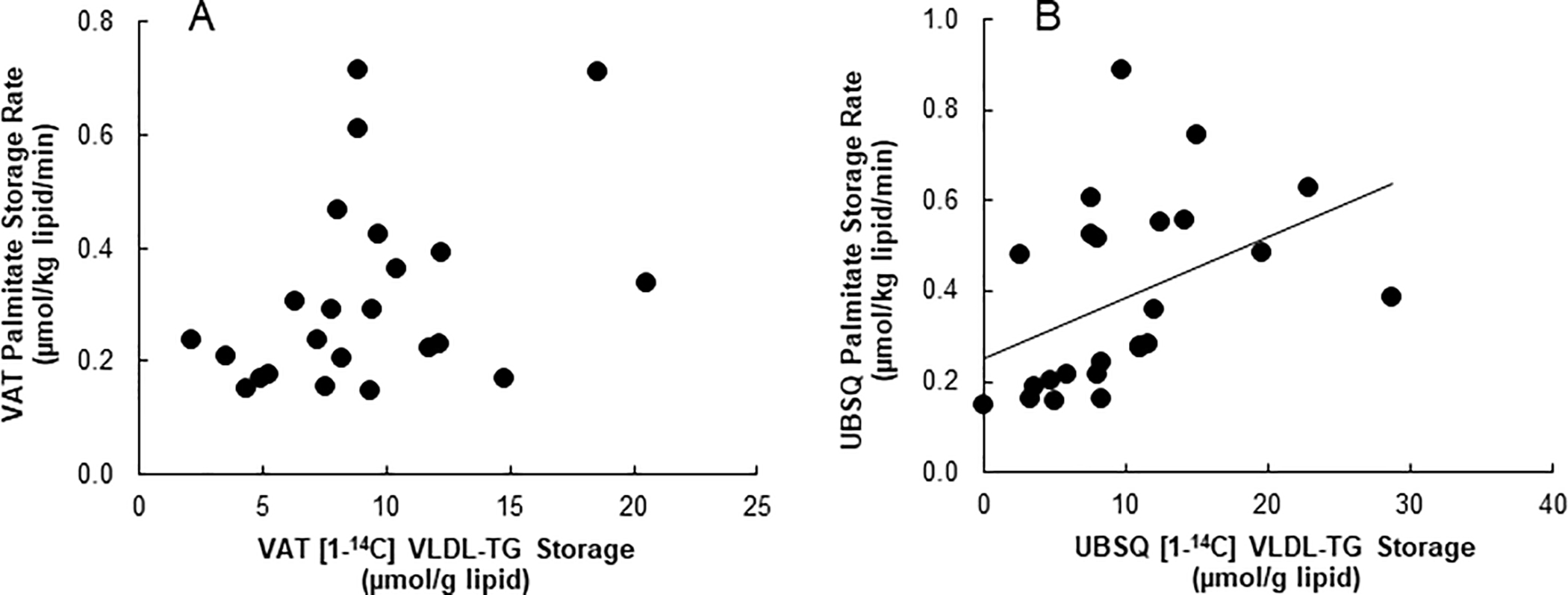
Depot specific correlations between storage of systemic FFA-palmitate as measured by [9,10-3H] palmitate and 24 hours storage of [1-14C]VLDL-TG in (A) VAT (r = 0.36, P= 0.09 (B) and UBSQ (r = 0.43, P < 0.05).
Factors related to adipose tissue VLDL-TG storage
Fasting plasma VLDL-TG concentrations were positively correlated with 24 hour storage of VLDL derived TG in VAT (r = 0.53, P< 0.009, Fig. 3A), and storage in UBSQ fat tended to correlated with VLDL-TG concentrations (r = 0.41, P = 0.054, Fig. 3B).
Figure 3:
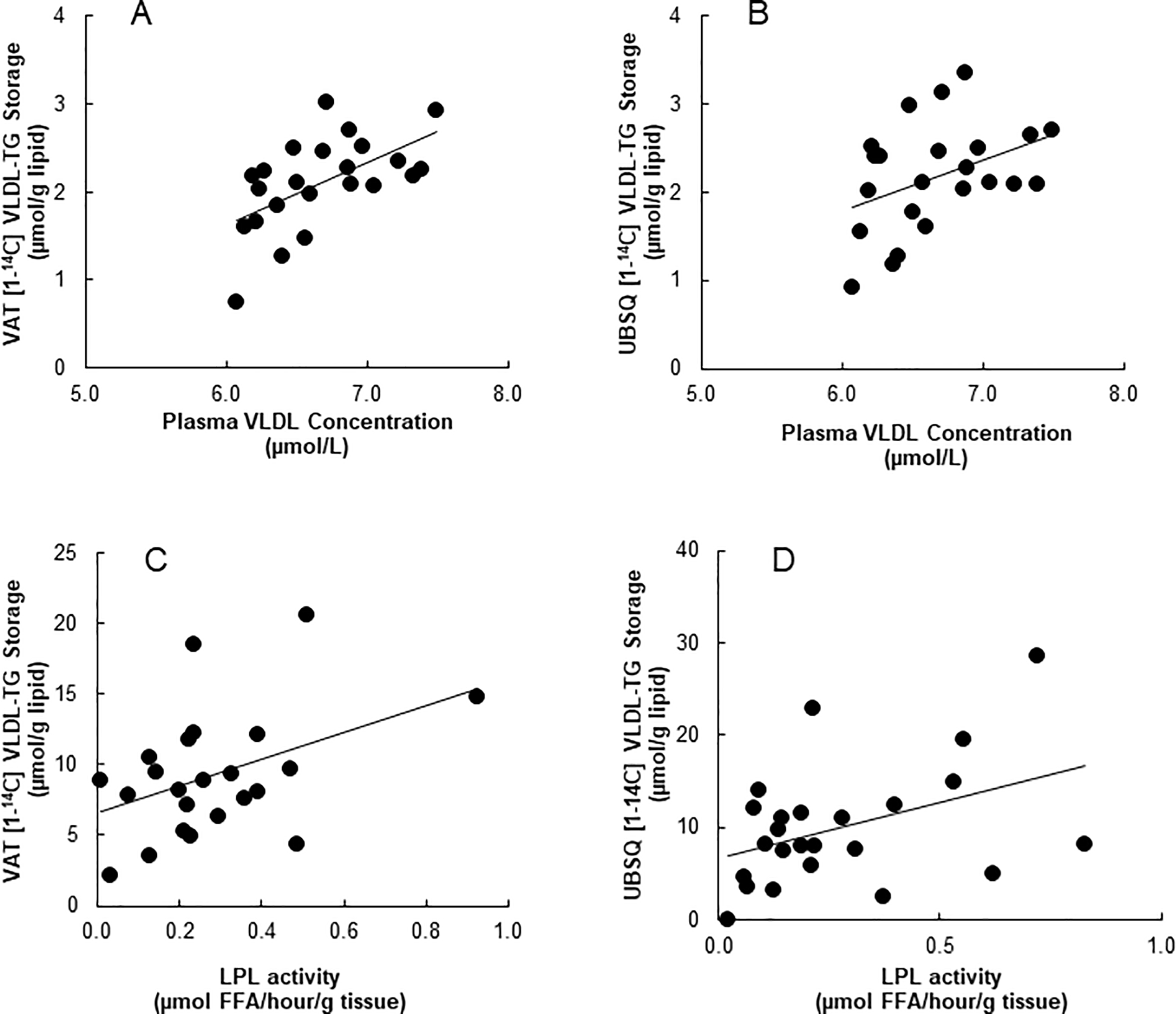
Association between fasting plasma concentrations of VLDL-TG and tracer measured 24 hour storage of VLDL-TG in (A) VAT (r = 0.53, P< 0.009) and (B) UBSQ (r = 0.41, P = 0.054). Data shown in A and B has been log transformed. Relationship between depot specific LPL activity and tracer measured 24 hour storage of VLDL-TG in (C) VAT (r = 0.42, P <0.05) and (D) UBSQ (r = 0.42, P < 0.05).
Omental LPL activity was correlated with storage of VLDL-derived fatty acids in VAT (r = 0.42, P < 0.05, Figure 3C) and UBSQ LPL activity was correlated with storage of VLDL-derived FA in UBSQ (r = 0.42, P < 0.05, Fig. 3D).
Omental and abdominal subcutaneous CD36 content were not correlated with storage of VLDL-derived FA in VAT or UBSQ fat, respectively. Likewise, the activities of abdominal subcutaneous adipose ACS, DGAT, total GPAT and GPAT1 did not correlate with fractional storage of VLDL-derived TG in UBSQ (data not shown). The storage of VLDL derived TG-FA in VAT did not correlate with omental ACS, DGAT or total GPAT, but did correlate (r = 0.49, P < 0.02) with GPAT 1 activity. This may have been because GPAT1 activity was correlated with omental LPL activity (r = 0.63, P = 0.0014).
Because omental LPL activity, fasting plasma VLDL-TG concentrations and GPAT1 activity were correlated with VLDL storage, we performed multivariate regression that included all of these factors as independent predictors of fractional storage of VLDL-TG in VAT. In this model, both omental LPL activity (P = 0.003) and plasma VLDL-TG concentrations (P = 0.02) were significant independent predictors of VLDL-TG storage in VAT. GPAT1 activity was not significant in this model. In contrast, none of these factors were significant predictors of VLDL-TG fatty acid storage in UBSQ fat when this same model was applied.
Factors related to adipose tissue palmitate storage rates
Plasma palmitate concentrations were positively correlated with palmitate storage rates in both VAT and UBSQ fat (r = 0.46, P = 0.02, and r = 0.46, P = 0.02, respectively, Fig. 4). Neither VAT nor UBSQ palmitate storage rates were correlated with depot specific ACS, DGAT, total GPAT or GPAT1 activities. To assess whether the adipose content of the fatty acid storage factors influenced direct palmitate storage rates independent of plasma palmitate concentrations we performed regression analysis using storage rates as the dependent variable and plasma palmitate concentrations, as well as the enzyme activities and CD36 as independent variables. None of the enzyme activities or CD36 content predicted palmitate storage rates in either VAT or UBSQ in these models.
Figure 4:
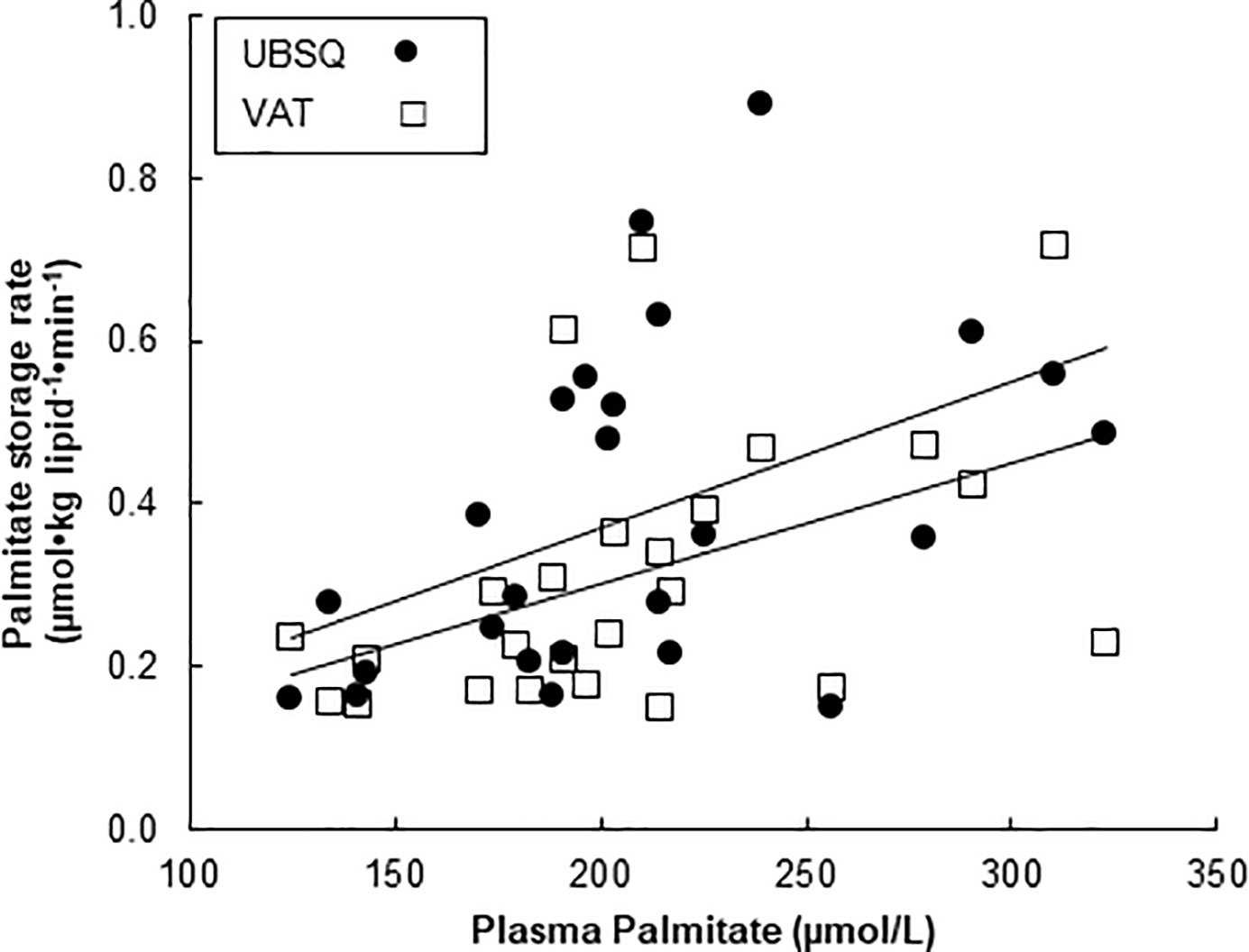
Associations between fasting plasma palmitate concentrations and palmitate storage rates in VAT (r = 0.57, P < 0.03) and UBSQ (r = 0.48, P < 0.03).
Sex Differences
Palmitate storage rates were greater in females than males in UBSQ fat (0.47 ± 0.21 vs. 0.26 ± 0.12 μmol•kg lipid−1•min−1, respectively, P < 0.02), but not VAT (0.33 ± 0.21 vs. 0.30 ± 0.09 μmol•kg lipid−1•min−1, respectively, P = NS). Figure 5 depicts the relationships between plasma palmitate concentrations and direct palmitate storage rates for UBSQ (left panel) and VAT (right panel). For UBSQ the relationship appears “up-shifted” in women compared with men, whereas these relationships appear quite similar for storage in VAT (right panel).
Figure 5:
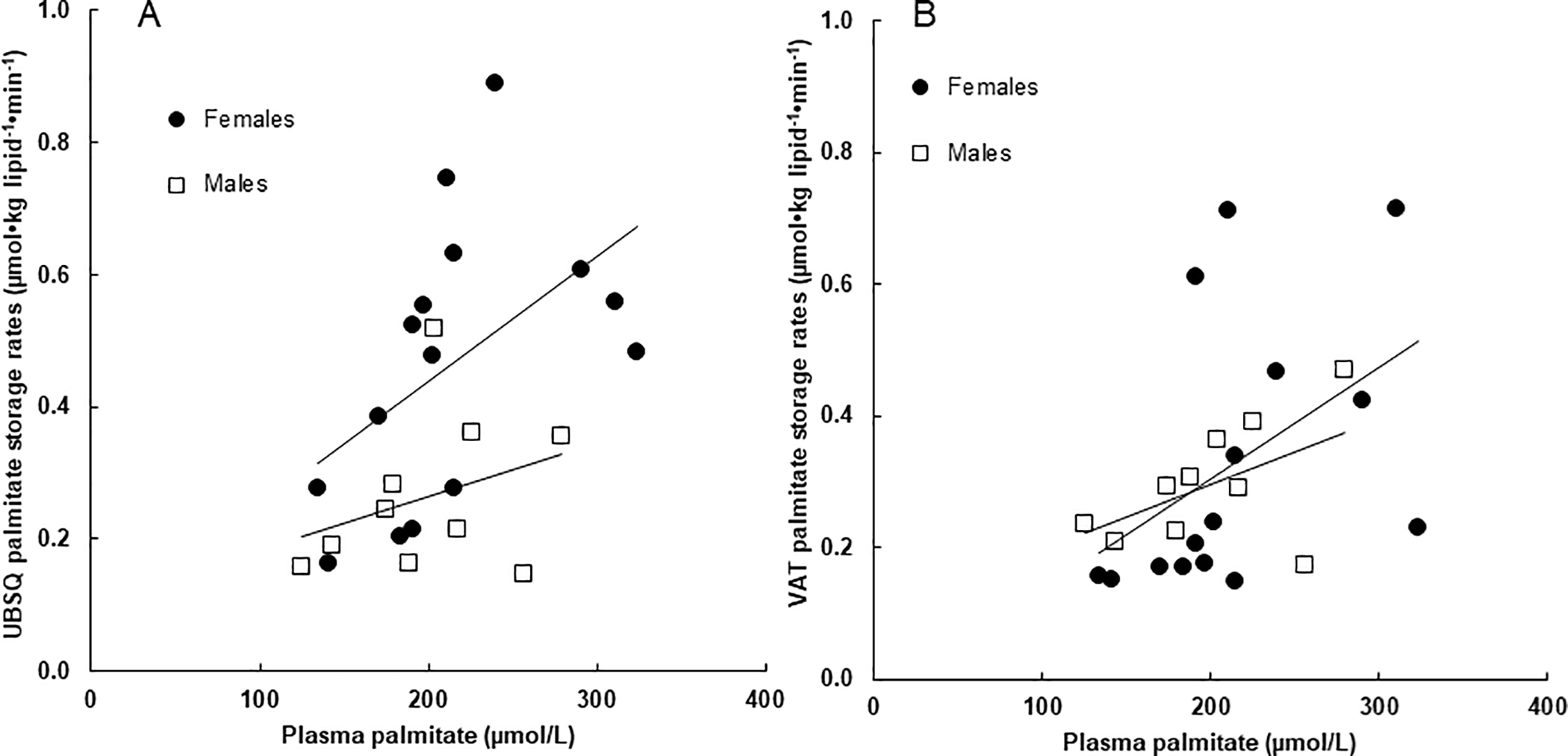
Sex specific associations between fasting plasma palmitate concentrations and palmitate storage in (A) UBSQ and (B) VAT. The correlations between palmitate storage rates in UBSQ adipose tissue (log transformed) and palmitate concentrations were r = 0.55, P = 0.03 and r = 0.33, P = 0.35 for females and males, respectively. The correlations between palmitate storage rates in VAT adipose tissue (log transformed) and palmitate concentrations were r = 0.52, P = 0.047 and r = 0.43, P = 0.22 for females and males, respectively.
There was no difference in fasting plasma VLDL-TG concentrations between females and males (800 ± 386 vs. 981 ± 331 μmol/L, respectively) and there was no sex difference in VLDL-TG storage in UBSQ or VAT.
Lipoprotein lipase activity in VAT was greater in females than males (0.33 ± 0.22 vs 0.18 ± 0.08 μmol FFA•g tissue−1•hour−1, respectively, P < 0.05), whereas the difference in UBSQ did not reach statistical significance (0.33 ± 0.23 vs 0.17 ± 0.16, respectively, P = 0.07)
None of the intracellular enzymes we examined (ACS, DGAT, total GPAT, or GPAT1) or the adipose fatty acid transporter CD36 were different between males and females in either VAT or UBSQ (data not shown).
Discussion:
Because delivery of FFA to the liver from visceral adipose tissue lipolysis increases as a function of visceral fat (25), and increased hepatic uptake of FFA may contribute to adverse metabolic responses, it is important to understand the differences in fatty acid storage characteristics between VAT and UBSQ depots. To address this issue we integrated measures of FA storage from endogenous sources (plasma FFA and VLDL-TG-FA) into adipose tissue in the context of multiple adipocyte FA storage factors. By recruiting patients scheduled for bariatric surgery we could measure these variables in both VAT and UBSQ adipose tissue. Despite the greater activities of ACS and GPAT in VAT than UBSQ fat, the storage of FFA-palmitate and VLDL-TG-FA was not different between these two depots. Overall, our findings indicate that plasma FFA-palmitate and VLDL-TG concentrations are good predictors of adipose tissue storage of FFA and VLDL-TG-FA, respectively, although omental LPL activity appears to be an additional factor predicting VLDL-TG-FA storage in VAT. To our surprise, there are only minor differences between VAT and UBSQ fat in adults with Class III obesity.
The factors driving direct FFA and VLDL-TG storage in adipose tissue of severely obese adults appears to affect VAT and UBSQ equally (Fig. 1 and Table 2). Those with the greatest UBSQ direct FFA and VLDL-TG-FA storage also had high storage of these FA sources in VAT. This could be explained if there were a strong relationship between plasma FFA-palmitate concentrations and VLDL-TG concentrations, however, there was not a statistically significant correlation between these two variables (r = 0.33, P = 0.12). There may be FA storage factors that are expressed similarly in the VAT and UBSQ of adults with severe obesity that modulate adipose FA storage beyond what is driven by substrate concentrations. If this is the case, these fatty acid storage factors are probably not regulated similarly to CD36, ACS, GPAT or DGAT because we failed to detect a relationship between them and storage rates after accounting for substrate concentrations.
We have previously reported significantly greater content/activity of several fatty acid storage factors in VAT than UBSQ adipose tissue (5,17). This was not the case for LPL, DGAT or CD36 in our population of adults with Class III obesity. We don’t believe this relates to an inherent difference in Class III obesity, but instead reflects the phenomenon that fatty acid storage factor expression decreases as a function of increasing omental fat (5), whereas the same is not true for UBSQ fat. Based upon those observations we estimate that humans with 3–5 kg of visceral fat will have the same content of these fatty acid storage factors in subcutaneous and visceral fat (5), and virtually all of our patients met this metric. Despite finding that activities of GPAT and ACS were considerably greater in VAT than UBSQ, VLDL and palmitate storage rates were nearly identical in these two depots. Thus, the amount of GPAT and ACS in UBSQ fat was more than sufficient to store the amount of FA delivered from these sources under the conditions of this study.
Whereas direct palmitate storage in adipose tissue was predicted only by plasma palmitate concentrations, VAT VLDL-TG storage was predicted both by LPL activity and plasma VLDL-TG concentrations. To the extent that LPL activity in some of our patients was in excess of that needed to process the amount of VLDL-TG delivered to the tissue, VLDL-TG concentrations (a surrogate for delivery) would limit the amount of VLDL-TG stored in adipose tissue. Conversely, for other patients the VLDL-TG delivery to adipose tissue may have exceeded the capacity of local LPL activity, making LPL activity a rate-limiting step for VLDL-TG storage in VAT.
The direct palmitate storage rates into adipose tissue in this population of adults with Class III obesity were very close to those we observed in normal weight and adults with Class I obesity (5,6). However, the average amount of body fat in our population was 67 kg. Extrapolating palmitate storage in visceral and UBSQ fat to total body palmitate storage in adipose tissue, we estimate that 14% of palmitate molecules released as a result of lipolysis were recycled back into these adipose tissue depots in the fasting state. The greater amounts of body fat result in a greater proportionate recycling of FFA back into adipose tissue compared with those who have less body fat, consistent with our hypothesis based upon the study of those with lesser degrees of obesity (26).
As with most studies, this study suffers from some limitations. The measures of direct FFA-palmitate storage into adipose tissues were done under general anesthesia, a condition that can create metabolic stress. However, there is no other way to get access to visceral fat and the total FFA concentrations we observed during surgery (855 ± 33 μmol/L) are similar to those previously reported in patients with Class III obesity (27). An additional limitation is that the biopsies to assess VLDL-TG storage in adipose tissue were done 24 h after the tracer infusion and likely reflect events that took place under a mixture of postabsorptive and postprandial conditions. Our measures of adipose LPL were done on tissue obtained in the postabsorptive state. Thus, the lack of association between UBSQ LPL activity (28) and VLDL-TG storage may reflect the fact we don’t know what postprandial LPL activity was in our volunteers. That said, VLDL-TG concentrations were reasonably good predictors of UBSQ VLDL-TG storage. Finally, our volunteers were all Caucasian and thus our findings might not translate to other racial populations.
Conclusions:
In conclusion, we found that VLDL-TG-FA and direct FFA storage was similar in VAT and UBSQ on a per unit lipid weight basis in patients with severe obesity, despite considerable differences in depot size. The activities/amounts of adipocyte fatty acid storage factors were poor predictors of both direct FFA storage and VLDL-TG storage in these depots, whereas plasma concentrations were predictive of direct FFA storage. Both VLDL-TG concentrations and LPL were predictive of VLDL-TG storage in adipose tissue. This suggests that cellular adipocyte fatty acid storage factors are present in excess relative to the demand for storage under these conditions. This is the first study in adults Class III obesity to interrogate paired biopsy samples in order to understand factors that govern fatty acid storage between VAT and UBSQ. Overall we find that plasma concentrations of FA sources predict storage rates. Storage in adults with these greater degrees of adiposity can best be thought about as a continuum of the processes in more mild obesity. Directing treatments toward mitigating high plasma concentrations of fatty acid from all sources to decrease tissue delivery might contribute to reductions in adipose lipid storage. Taken together, our data indicates that there isn’t any specific AT storage abnormality that explains the difference between Class III obesity and adults who are more moderately overweight or obese.
Study Importance Questions.
What is already known about this subject?
VLDL-triglyceride fatty acids are recycled back into adipose tissue
Plasma FFA also are recycled back into adipose tissue
What are the new findings in your manuscript?
VLDL-TG fatty acid storage in VAT and UBSQ fat is related to both concentration and LPL
Direct FFA storage rates are correlated with plasma FFA concentrations in VAT and UBSQ fat
How might your results change the direction of research or the focus of clinical practice?
Modulation of LPL activity has the potential to alter regional fat storage
Modulation of plasma FFA concentrations could modulate fatty acid storage back into UBSQ and VAT
Acknowledgments
Grant support: Supported by grants DK40484, DK45343, DK50456 and the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number 1 UL1 RR024150–01and UL1 TR000135. K.L., N.B. and K.H. received fellowship funding from the NIH training grant T32-DK07352 and the ADA fellowship grant 7–112-MN-36. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations:
- VAT
visceral adipose tissue
- UBSQ
upper body subcutaneous adipose tissue
- FFA
free fatty acid
- VLDL
very low density lipoprotein
- LPL
lipoprotein lipase
- ACS
Acetyl-CoA Synthetase
- GPAT
Glycerol-3-Phosphate Acyltransferases
- DGAT
Diacylglycerol Acyl Transferase
- TG
triglyceride
- CT
computed tomography
- SA
specific activity
Footnotes
Financial disclosure: The authors declare no conflicts of interest.
References:
- 1.Björntorp P Metabolic implications of body fat distribution. Diabetes Care 1991;14:1132–43. [DOI] [PubMed] [Google Scholar]
- 2.Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab 2008;93:S57–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shadid S, Koutsari C, Jensen MD. Direct free fatty acid uptake into human adipocytes in vivo: relation to body fat distribution. Diabetes 2007;56:1369–75. [DOI] [PubMed] [Google Scholar]
- 4.Wendel AA, Lewin TM, Coleman RA. Glycerol-3-phosphate acyltransferases: rate limiting enzymes of triacylglycerol biosynthesis. Biochim Biophys Acta 2009;1791:501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali AH, Koutsari C, Mundi M, Stegall MD, Heimbach JK, Taler SJ et al. Free fatty acid storage in human visceral and subcutaneous adipose tissue: role of adipocyte proteins. Diabetes 2011;60:2300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koutsari C, Ali AH, Mundi MS, Jensen MD. Storage of circulating FFA in adipose tissue of postabsorptive humans: quantitative measures and implications for body fat distribution. Diabetes 2011;60:2032–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lytle KA, Bush NC, Triay JM, Kellogg TA, Kendrick ML, Swain JM et al. Hepatic Fatty Acid Balance and Hepatic Fat Content in Humans With Severe Obesity. J Clin Endocrinol Metab 2019;104:6171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gormsen LC, Nellemann B, Sorensen LP, Jensen MD, Christiansen JS, Nielsen S. Impact of body composition on very-low-density lipoprotein-triglycerides kinetics. Am J Physiol Endocrinol Metab 2009;296:E165–E73. [DOI] [PubMed] [Google Scholar]
- 9.Jensen MD, Kanaley JA, Reed JE, Sheedy PF. Measurement of abdominal and visceral fat with computed tomography and dual-energy x-ray absorptiometry. Am J Clin Nutr 1995;61:274–8. [DOI] [PubMed] [Google Scholar]
- 10.Heiling VJ, Miles JM, Jensen MD. How valid are isotopic measurements of fatty acid oxidation? Am.J.Physiol 1991;261:E572–7. [DOI] [PubMed] [Google Scholar]
- 11.Fromm H, Hofman AF. Breath test for arterial bile acid metabolism. Lancet 1971;2:621–5. [DOI] [PubMed] [Google Scholar]
- 12.Sondergaard E, Rahbek I, Sorensen LP, Christiansen JS, Gormsen LC, Jensen MD et al. Effects of exercise on VLDL-triglyceride oxidation and turnover. Am J Physiol Endocrinol Metab 2011;300:E939–E44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bush NC, Triay JM, Gathaiya NW, Hames KC, Jensen MD. Contribution of very low-density lipoprotein triglyceride fatty acids to postabsorptive free fatty acid flux in obese humans. Metabolism 2014;63:137–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Persson X-MT, Blachnio-Zabielska AU, Jensen MD. Rapid measurement of plasma free fatty acid concentration and isotopic enrichment using LC/MS. J Lipid Res 2010;51:2761–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 1957;226:497–509. [PubMed] [Google Scholar]
- 16.Tchoukalova YD, Harteneck DA, Karwoski RA, Tarara J, Jensen MD. A quick, reliable, and automated method for fat cell sizing. J Lipid Res 2003;44:1795–801. [DOI] [PubMed] [Google Scholar]
- 17.Morgan-Bathke M, Chen L, Oberschneider E, Harteneck DA, Jensen M. Sex and Depot Differences in ex vivo Adipose Tissue Fatty Acid Storage and Glycerol-3-phosphate acyltransferase Activity. Am J Physiol Endocrinol Metab 2015;308:E830–E46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nilsson-Ehle P, Schotz MC. A stable, radioactive substrate emulsion for assay of lipoprotein lipase. J Lipid Res 1976;17:536–41. [PubMed] [Google Scholar]
- 19.Hou XG, Moser S, Sarr MG, Thompson GB, Que FG, Jensen MD. Visceral and subcutaneous adipose tissue diacylglycerol acyltransferase activity in humans. Obesity 2009;17:1129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coleman RA. Diacylglycerol acyltransferase and monoacylglycerol acyltransferase from liver and intestine. Methods Enzymol 1992;209:98–104. [DOI] [PubMed] [Google Scholar]
- 21.Hall AM, Smith AJ, Bernlohr DA. Characterization of the acyl CoA synthetase activity of purified murine fatty acid transport protein 1. J Biol Chem 2003;278:43008–13. [DOI] [PubMed] [Google Scholar]
- 22.Lu J, Allred CC, Jensen MD. Human adipose tissue protein analyses using capillary western blot technology. Nutr Diabetes 2018;8:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorensen LP, Gormsen LC, Nielsen S. VLDL-TG kinetics: a dual isotope study for quantifying VLDL-TG pool size, production rates, and fractional oxidation in humans. Am J Physiol Endocrinol Metab 2009;297:E1324–E30. [DOI] [PubMed] [Google Scholar]
- 24.Koutsari C, Ali AH, Mundi MS, Jensen MD. Measuring plasma fatty acid oxidation with intravenous bolus injection of 3H- and 14C-fatty acid. J Lipid Res 2013;54:254–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen S, Guo ZK, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest 2004;113:1582–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koutsari C, Mundi MS, Ali AH, Jensen MD. Storage rates of circulating free fatty acid into adipose tissue during eating or walking in humans. Diabetes 2012;61:329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lanzi S, Codecasa F, Cornacchia M, Maestrini S, Capodaglio P, Brunani A et al. Short-term HIIT and Fat max training increase aerobic and metabolic fitness in men with class II and III obesity. Obesity (Silver Spring) 2015;23:1987–94. [DOI] [PubMed] [Google Scholar]
- 28.Erskine JM, Jensen DR, Eckel RH. Macronutrient regulation of lipoprotein lipase is posttranslational. J Nutr 1994;124:500–7. [DOI] [PubMed] [Google Scholar]


