Abstract
Dental pulp is a highly vascularized tissue, situated in an inextensible environment surrounded by rigid dentinal walls. The pulp receives its blood supply solely from the small apical foramen of a tooth root. Due to the unique anatomy that controls nutrition supply, regeneration of pulp tissue in a full-length tooth root has long been a challenge in regenerative endodontics. In this study, we designed and synthesized a multifunctional peptide-conjugated, pH-sensitive, non-viral gene vector for fast revascularization and pulp regeneration in a full-length human tooth root. The multifunctional peptide was designed to have distinctive features, including a cell-penetrating peptide to enhance cellular uptake, a nuclear localization signal peptide to assist in the translocation of an angiogenic gene into the nucleus, and a fluorescent tryptophan residue to visualize and quantify the transfection efficiency. Furthermore, a pH-sensitive dimethylmaleic anhydride (DMA) was integrated with the multifunctional peptide to enhance the transfected gene complex to escape from endosomes/lysosomes after internalization. In vitro experiments showed that the multifunctional non-viral gene vector significantly increased internalization and gene transfection efficiency as well as reduced cytotoxicity. After dental pulp stem cells (DPSCs) were transfected with the multifunctional gene vector/pVEGF complexes, the expression of VEGF from the DPSCs was upregulated for more than eight folds, which in turn greatly enhanced endothelial cell migration and vascular-like tube formation. Six weeks after implantation, the VEGF-transfected DPSCs accelerated new blood vessel formation and the regenerated pulp tissue occupied most of the area in the canal of a full-length human tooth root. The multifunctional peptide conjugated non-viral gene delivery is a safe and effective approach for regenerative endodontics.
Keywords: revascularization, pulp, tooth root, gene delivery, regenerative endodontics, regeneration, dental pulp stem cells
Graphical Abstract

1. Introduction
Dental caries (tooth decay) affects tooth pulp tissue and leads to irreversible pulpitis if left untreated [1]. Clinically, root canal therapy is a popular method for the treatment of severely infected pulp, which involves pulp extirpation, root canal cleaning and enlargement, and filling with inert biomaterials. While this therapy has relatively high success rates, the repaired tooth is devitalized. In addition, substantial dental structures are removed during root canal treatment, leading to a brittle tooth with the potential of fracture [2]. Therefore, regenerative endodontics has been developed in recent years [3–5].
Anatomically, pulp tissue in a permanent human tooth receives its blood supply only from a small apical foramen (< 1 mm) of the root canal, which limits nutrient diffusion and the ingrowth of new blood vessels [6, 7]. Due to the unique anatomic structure of the tooth root, pulp regeneration in a full-length root canal (11–13 mm) has long been a challenge in regenerative endodontics [8].
Several methods were reported to tackle this challenge. One approach was the incorporation of angiogenic growth factors into scaffolds to enhance angiogenesis during pulp regeneration [9–11]. This method, however, only regenerated vascularized soft connective tissues with a length of less than 4 mm. Another method was the combination of dental pulp stem cells (DPSCs) and human umbilical vein endothelial cells (HUVECs) to enhance angiogenesis [12, 13]. Similarly, this approach regenerated pulp-like tissues up to the lower (3.3 mm) or the middle third (5 mm) of the root canal but could not reach its upper regions. We recently developed a unique hierarchical nanofibrous microsphere delivery system that successfully regenerated pulp tissue that fulfilled the entire apical and middle third root space and reached the coronal third of the canal [14]. However, quantitative measurement indicated that regenerated pulp was 71.3 ± 6.6% of the whole root space, leaving approximately one quarter of the tooth root still void of any tissue. To date, regeneration of vascularized pulp in a full-length human tooth root has not been achieved.
Gene therapy is a promising approach to dental tissue regeneration [15]. In one study, adenovirus vectors were used to overexpress vascular endothelial growth factor (VEGF) and stromal cell derived factor-1α (SDF-1α) in DPSCs to increase vascularized pulp tissue formation [16]. While adenovirus vectors have been widely examined for gene therapy, a number of concerns regarding the use of adenovirus remain, including potential immunogenic and inflammatory responses [17, 18]. As safer alternatives to viral vectors, non-viral vectors have several advantages, including ease of manufacture, stability, low immunogenicity, and low likelihood of being inserted into the host cell genome [19, 20]. Therefore, non-viral vectors for gene therapy have been increasingly explored in recent years [21]. However, low delivery efficiency is the major hurdle for the non-viral vectors. To improve gene transfection and expression efficiencies, an effective non-viral vector has to be developed with several characteristics, including 1) high capability to penetrate through cell membrane barrier, 2) strong intracellular trafficking capability to escape from endosomes after endocytosis, 3) high nuclear import capability to enhance gene expression, and 4) ease of traceability.
In this work, we designed and developed a multifunctional peptide-conjugated, pH-sensitive, non-viral gene delivery system for vascularized dental pulp regeneration (Scheme 1). In this system, the multifunctional peptide C-R9-G-NLSW had several distinctive features. Specifically, cysteine (C) was used to couple the multifunctional peptide to a polymeric non-viral carrier. R9 is a cell-penetrating peptide that possesses the specific ability to cross the cell membrane and enhance the cellular uptake of genes [22, 23]. G is glycine residue and acts as a flexible spacer to regulate the activity of R9 and NLSW. NLS is a nuclear localization signal peptide that assists the translocation of DNAs into the nucleus [24]. W is the tryptophan residue that has a fluorescence emission spectrum to visualize and quantify the multifunctional peptide. We selected poly(L-lysine) (PLL) as the substrate of the non-viral gene carrier, because PLL is biodegradable, has strong DNA binding capability to form a stable complex with pDNA, and has the flexibility of chemical modification [25]. To enhance the escape of genetic cargo from endosomes and avoid lysosomal degradation of loaded pDNA, we further grafted 2,3-dimethylmaleic anhydride (DMA) onto the backbone of the PLL to form dimethylmaleic anhydride-graft PLL (abbreviated as PLD). It is known that endosomes and lysosomes of a cell are more acidic (pH = 5.0–5.5) than in physiological conditions (~7.4). Under an acidic condition, The PLD is hydrolyzed to generate positive charges which increases the zeta potential of the gene carrier, therefore facilitates the gene complex to escape from endosomes and lysosomes (Scheme 1) [26]. Finally, a “thiol-ene” click chemistry reaction was performed to conjugate multifunctional peptide C-R9-G-NLS-W to PLD (abbreviated as PLD-R9-G-NLSW).
Scheme 1.
Schematic illustration of the design and preparation of multifunctional gene delivery carriers for pulp regeneration in a full-length tooth root.
After synthesis of the multifunctional PLD-R9-G-NLSW, pIRES-VEGF plasmids (pVEGF) were condensed with the gene carrier to form a PLD-R9-G-NLSW/pVEGF complex. Next, DPSCs (a primary cell source for pulp regeneration) were transfected with the PLD-R9-G-NLSW/pVEGF complex to induce the over-expression of VEGF. The VEGF-transfected DPSCs were injected in a full-length human tooth root model and were further implanted in vivo. We hypothesized that the DPSCs transfected by the multifunctional peptide-conjugated non-viral gene carrier would induce fast revascularization, leading to pulp tissue regeneration in the full-length human tooth root.
2. Materials and Method
2.1. Materials
ε-Poly(L-lysine) (PLL, M = 4200 Da) was purchased from Bonding Chemical Co., Ltd. (Texas, USA). R9-G-NLSW peptide sequences were synthesized by Biomatik Co. (Wilmington, USA). 2,3-dimethylmaleic anhydride (DMA), diallylcarbamyl chloride (DA), 2,2-dimethoxy-2-phenylacetophenone (DMPA), triethylamine (Et3N), dimethyl sulfoxide (DMSO) and 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) were obtained from Sigma-Aldrich (St. Louis, MO, USA). pIRES-VEGF plasmids (pVEGF) were purchased from Sangon Biotech Co., Ltd. (Shanghai, China). Hoechst 33342 and Alexa Fluor647 labeled-oligonucleotide (AF647-oligonucleotide) were purchased from Thermo Scientific (Rockford, IL, USA). Green DND-26 (LysoTracker) was ordered from Cell Signaling Technology (Danvers, MA). iScript™ gDNA Clear cDNA Synthesis Kit and SsoAdvanced™ Universal SYBR Green Supermix was purchased from Bio-Red Co., Ltd. (Hercules, CA, USA). Human VEGF Quantikine ELISA was purchased from R&D Systems, Inc. (Minneapolis, MN, USA). Matrigel (Cat. Nos. 356234) was obtained from Corning Incorporated (New York, USA). The human dental pulp stem cells (DPSCs) were a gift from Dr. Songtao Shi, University of Pennsylvania School of Dental Medicine. Human umbilical vein endothelial cells (HUVECs) were purchased from the Lonza Company (Allendale, NJ, USA).
2.2. Synthesis and characterization of PLD-R9-G-NLSW
2.2.1. Synthesis of PLD and PLD-DA
PLL (1.0 g, 0.24 mmol) was dissolved in 5.0 mL of DMSO in a dried three-necked flask. Next, 2,3-dimethylmaleic anhydride (DMA) (0.76 g, 6.0 mmol) was dissolved in DMSO (5 mL) in a dried constant pressure drop funnel and the mixed solution was slowly added to a three-necked flask for 2 h. The reaction was carried out at room temperature overnight. The product (abbreviated as PLD) was collected through filtering, dialysis, and lyophilization.
The PLD (0.5 g, 0.07 mmol) was dissolved in 8.0 mL of DMSO in a dried three-necked flask. Et3N (40 μL) was also added to the three-necked flask. Next, 22 μL diallylcarbamyl chloride (DA) DMSO solution (5 wt%) was added slowly to the PLD solution and reacted for 12 h. The product (abbreviated as PLD-DA) was collected through filtering, dialysis, and lyophilization.
FT-IR spectra of the synthetic polymers were obtained using an FT-IR spectrometer (Thermo Scientific Nicolet iS10, USA). Moreover, 1H NMR spectra of the copolymers were recorded with a JEOL Delta NMR spectrometer (JEOL LTD., Akishima, Japan) operating at 500 MHz in DMSO-d6.
2.2.2. Synthesis and characterization of PLD-R9-G-NLSW
PLD-R9-G-NLSW was synthesized by “thiol-ene” click chemistry. Briefly, PLD was dissolved in DMSO and transferred to a petri dish. DMPA and R9-G-NLSW peptide were added to the solution and the mixed solution was treated under a UV light (365 nm) for 10 min to complete the reaction. The product was recovered by dialysis and lyophilization. R9-G-NLSW of two different weights (8.6 mg and 17.4 mg) were conjugated to the PLD and the two final products were abbreviated as PLD-R9-G-NLSW1 and as PLD-R9-G-NLSW2, respectively.
Subsequently, the R9-G-NLSW peptide containing a tryptophan residue was excited at 280 nm and emitted at 348 nm. To quantify the PLD-R9-G-NLSW, a series of R9-G-NLSW peptide solutions (from 5×10−3 to 2×10−1 mg/mL) were prepared to obtain the standard curve. The fluorescence emission spectra of the PLD-R9-G-NLSW (0.3 mg/mL) were measured using a TECAN infinite M200 fluorescence plate reader.
2.3. Preparation and characterization of PLD-R9-G-NLSW/pVEGF complexes
2.3.1. Preparation of polymer/pVEGF complexes
The pVEGF plasmid was diluted to 50 μg/mL with a PBS (pH= 7.4) buffer. PLL/pVEGF, PLD/pVEGF, PLD-R9-G-NLSW1/pVEGF, and PLD-R9-G-NLSW2/pVEGF complexes were prepared by mixing the polymer solution (0.5 mg/mL) separately with plasmid solutions of various weight ratios. The gene complex solutions were stirred for 30 min at room temperature and were used for the following experiments.
2.3.2. Size and zeta potential
The average diameter and zeta potential of the polymer/pVEGF complexes were measured using a Zetasizer Nano ZS (Malvern Instrument, Inc., Worcestershire, UK) with a constant angle of 173°.
2.3.3. Agarose gel electrophoresis
Agarose gel electrophoresis was used to evaluate the condensation of pVEGF and gene carriers to form complexes. Briefly, different ratios of complexes were first prepared as described above. A volume of 2 μL loading buffer (6X) was mixed with 10 μL of complex suspension and was analyzed using 0.8% agarose gel containing 5 μL of EB in 1X TAE buffer at 100 V for 30 min. The pDNA retardation was recorded using a UV illuminator.
2.3.4. In vitro cytotoxicity
An MTT assay was used to evaluate the in vitro cytotoxicity of gene complexes. First, DPSCs were seeded in a 96-well plate with a density of 8 × 103 cells per well and were cultured in α-MEM containing 10% FBS for 24 h. Next, the medium was replaced by a serum-free α-MEM medium followed by adding gene complexes with different concentrations of pVEGF. After the cells were cultured in an incubator for another 24 h, MTT solution was added and cultured for another 4 h. Afterwards, 150 μL of DMSO was added and the optical density (OD) of the formazan solution was acquired using a SpectraMax 250 microplate spectrophotometer (Molecular Devices, USA).
2.4. Cellular uptake and intercellular distribution
DPSCs were seeded in a 24-well plate with a density of 8 × 104 cells per well and cultured in α-MEM containing 10% FBS for 24 h. Before transfection, the medium was replaced with serum-free α-MEM. Then, gene complexes (PLL/AF647-oligonucleotide complexes, PLD/AF647-oligonucleotide complexes, PLD-R9-G-NLSW1/AF647-oligonucleotide complexes and PLD-R9-G-NLSW2/AF647-oligonucleotide complexes) were added and cultured for 4 h. The transfected cells were washed with DPBS and trypsinized with 0.25% trypsin. After centrifuging and re-suspending samples in 300 μL of DPBS (pH = 7.4), the cellular uptake and mean fluorescence intensity (MFI) were evaluated using flow cytometry (BD FACS Canto II Flow Cytometry, BD Biosciences, USA).
For intercellular distribution experiment, 75 nM/well of LysoTracker Green DND-26 was added into the medium and incubated for 1 h. After that, 2 μg/mL Hoechst 33342 was added and incubated for 20 min. The cells were washed with DPBS (pH=7.4) and directly imaged under confocal microscopy (Leica STP6000, Germany). The excitation wavelengths were 649 nm, 504 nm, and 350 nm for AF647 (red), LysoTracker Green (green), and Hoechst 33342 (blue), respectively. The co-localization rate (CLR) of endo/lysosome was calculated using the Image-Pro Plus 6.0 software according to the following equation:
Similarly, the nucleus CLR was calculated using the following equation:
In both equations, red pixels correspond to the AF647-oligonucleotide in cytoplasm, yellow pixels correspond to the AF647-oligonucleotide in endo/lysosomes, and pink pixels corresponds to the AF647-oligonucleotide in nucleus.
2.5. In vitro transfection
To perform in vitro transfection, different complexes containing 3 μg pVEGF were added into each well and cultured for 4 h. Afterwards, the medium was removed and incubated with a complete medium for another 24 h. The expression of green fluorescence protein (GFP) was observed by an inverted fluorescence microscope (Nikon Eclipse TE2000-U microscope system, Japan). The transfected cells were trypsinized, centrifuged, collected, and resuspended in 300 μL DPBS. The transfection efficiency was determined using a flow cytometer (BD FACS Canto II Flow Cytometry, BD Biosciences, USA). Naked pVEGF was used as a negative control.
2.6. Quantitative real-time PCR assay
The total RNA was collected 2 days after transfection using a TRIzol reagent, then reverse-transcribed using iScript gDNA Clear cDNA Synthesis Kit (Bio-Rad). The resultant cDNAs were used as templates for quantitative real-time PCR using SsoAdvanced Universal SYBR Green Supermix and performed using Bio-Red CFX96™. The reactions were performed according to manufacturer’s instructions. The primers were VEGF forward 5’-CTACCTCCACCATGCCAAGT-3’, VEGF reverse 5’-AGCTGCGCTGATAGACATCC-3’, GAPDH forward 5’-AAGGTGAAGGTCGGAGTCAA-3’, and GAPDH reverse 5’-AATGAAGGGGTCATTGATGG-3’. The Cq values were generated by CFX™ Manager Software3.1.
2.7. Migration and tube formation assay
2.7.1. Conditioned medium
DPSCs were seeded into a 24-well plate and cultured in α-MEM containing 10% FBS for 24 h. After being transfected with different complexes, the cells were cultured with the α-MEM medium containing 0.1% FBS. Later, after incubation for 48 h, the conditioned medium was harvested and stored at −80 °C.
2.7.2. Enzyme-linked immunosorbent assay (ELISA)
To obtain VEGF concentration released from transfected DPSCs, ELISA was performed using Quantikine ELISA (R&D Systems) according to manufacturer’s instructions. Supernatant was added into each well of a 96-well plate containing an anti-VEGF antibody. OD value was measured using a SpectraMax 250 microplate spectrophotometer at 450 nm.
2.7.3. Wound healing and migration assay
A wound healing assay was performed to investigate the migration ability of HUVECs treated with conditioned medium. HUVECs were seeded in a 24-well plate at a concentration of 8 × 104 cell/mL and cultured for 24 h. A straight line was scratched across each well using a 200 μL micropipettor tip and the medium was replaced with conditioned medium. Cell migration images were obtained with an inverted microscope at 0 and 12 h. The relative recovered area was calculated using Image J software with the following equation.
The migration ability of the transfected cells was also evaluated using trans-well chambers with 8.0 μm pore sized, gelatinized polycarbonate membrane. The upper trans-well chambers were pre-treated with serum-free medium at 37 °C for 2 h. The HUVECs were seeded in the upper trans-well chambers (1.2×105 cells per well). At the same time, the lower trans-well chambers were added with conditioned medium, followed by incubating the trans-well system for 6 h. The upper chambers were washed twice with DPBS and fixed with 4% paraformaldehyde. Sterilized cotton swabs were used to remove the cells inside of the chambers. The cells on the lower surface of the upper chambers were stained with eosin at 37 °C for 8 min. Migrating cells were observed under an inverted fluorescent microscope and the number of migrated cells was counted by Image-Pro Plus 6.0 software.
2.7.4. Tube formation with HUVECs and conditioned medium
Matrigel™ (BD Biosciences) (50 μL) was added into a 96-well plate and pre-incubated for 30 min. HUVECs were resuspended in conditioned medium from transfected DPSCs, and were seeded onto the Matrigel-coated plate at a concentration of 4 × 105 cells/mL. The HUVECs were cultured for 6 h. Images of the formation of capillary-like structure were obtained by using a microscope.
2.8. Pulp regeneration in a full-length human tooth root
2.8.1. Preparation of full-length human tooth root
The roots of freshly extracted human teeth were collected from the Oral and Maxillofacial Surgery Clinic at Texas A&M University College of Dentistry (Protocol # 2015–0464-BCD-EXP) and cut into 11 mm-long segments. The root canals were cleaned and shaped with rotary instruments, and soaked in 17% ethylenediamine tetraacetic acid (EDTA) for 10 min and in 19% citric acid for 1 min to remove the smear layer, followed by treatment with betadine for 30 min and 5.25% NaOCl for 15 min for sterilization. Finally, the roots were rinsed with sterile PBS.
2.8.2. Pulp regeneration in a full-length human tooth root
Transfected DPSCs were trypsinized, centrifuged, and suspended at a concentration of 3 × 106 cells/mL. Cell suspension was encapsulated in a gelatin/tyramine (G/T) hydrogel, which was prepared in our previous study [27]. Briefly, a 100 μL cell suspension was added into the 100 μL 5% G/T hydrogel and mixed well. Next, horseradish peroxidase and H2O2 were added to the hydrogel and cultured for 30 s for gelation. The cell/material constructs were injected into tooth root segments via a syringe (25G) and incubated for 30 min in culture medium. Three experimental groups were included: (1) DPSC-free group, (2) DPSC group, and (3) PLD-R9-G-NLSW2/pVEGF complexes transfected DPSC group. Each group had eight samples (n=8). The animal surgical procedure was approved by the University Committee on Use and Care of Animals (UCUCA) of Texas A&M University Baylor College of Dentistry (Protocol# IACUC 2015–0214-BCD). After the animals (nu/nu mice, 5–6 weeks, Charles River) were anesthetized by an intraperitoneal injection of xylazine/ketamine, the back of each mouse was disinfected with 75% alcohol and the tooth roots were implanted in the dorsal subcutaneous space of nude mice [14]. Each mouse received two implants at random. After 6 weeks, the implants were retrieved, fixed with 4% paraformaldehyde, and decalcified with ethylenediaminetetraacetic acid (EDTA).
2.8.3. Histological and immunohistochemical examination
Samples were embedded in paraffin, sectioned into 5 μm-thick portions, and stained with hematoxylin and eosin (H&E) to study the regenerated pulp tissue. In addition, the sections were immunohistochemically stained with anti-vWF and anti-CD31 antibodies to identify newly formed vascular vessels in the regenerated pulp tissue.
2.9. Statistical analysis
Data were analyzed with SPSS software (version 19.0). All results were expressed as mean ± standard deviation. To test the significance of observed differences between the study groups, an unpaired Student t-test was applied. The significance level was set at p < 0.05.
3. Results
3.1. Synthesis and characterization of PLD-R9-G-NLSW
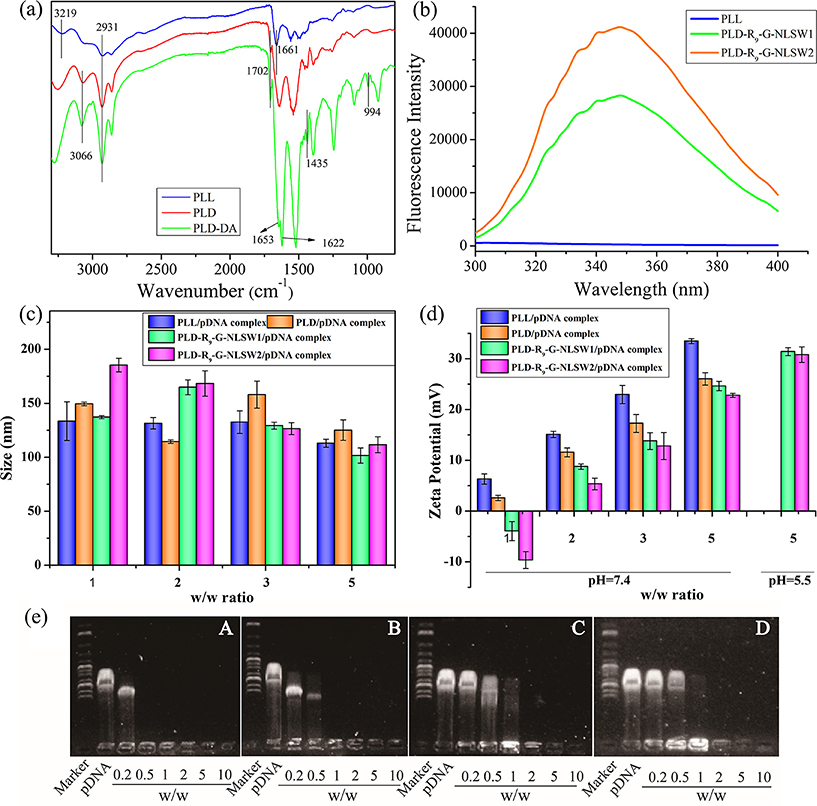
Figure 1 shows the synthesis of PLD-R9-G-NLSW using a three-step process. First, DMA was grafted onto PLL to form PLD. The 1H NMR spectrum of the obtained PLD exhibited a principal peak at 1.87 ppm that was correspondent to the DMA moieties. Additionally, the CH peak belonging to PLL shifted from 3.77 to 4.28 ppm, indicating that DMA was introduced into PLL by an amide linkage (Support Information, Figure S1). Next, DA was coupled onto PLD via an acylation reaction of amines and the peaks at 4.02 ppm (-N-CH2-CH=CH2, 2H), 5.08 ppm (-N-CH2-CH=CH2, 2H), and 5.71ppm (-N-CH2-CH=CH2, H) confirmed the structure of the synthesized PLD-DA. The FT-IR spectra also indicated successful grafting of DMA and DA onto PLL, separately (Figure 2a). Compared with PLL, the appearance of peaks at 3066 cm−1 (-OH) showed the successful introduction of DMA onto PLL. Furthermore, the characteristic peaks of double bounds at 994 cm−1 and 1435 cm−1 and the carbonyl group at 1622 cm−1 confirmed the addition of DA into the PLD-DA. In step three, a “thiol-ene” click chemistry reaction was performed to conjugate multifunctional peptide C-R9-G-NLS-W to PLD-DA. The use of the click reaction precisely tailored the amount of R9-G-NLSW in the PLD-R9-G-NLSW. In our design, a tryptophan residue (which has a specific fluorescence emission spectrum at 348 nm) was introduced to the R9-G-NLSW peptide. Therefore, quantitative evaluation of the PLD-R9-G-NLSW was conveniently performed via measuring the fluorescence intensity of PLD-R9-G-NLSW at 348 nm. Figure 2b shows the fluorescence spectra of PLD-R9-G-NLSW1 and PLD-R9-G-NLSW2, which had 29.2 wt% and 42.8 wt% of the R9-G-NLSW in the PLD-R9-G-NLSW, respectively. Both the PLD-R9-G-NLSW1 and the PLD-R9-G-NLSW2 were tested in the following experiments.
Figure 1.
(a) Synthesis route of multifunctional PLD-R9-G-NLSW. (b) Chemical dissociation of PLD-R9-G-NLSW in an acidic condition assists gene complexes to escape from acidic lysosomes after endocytosis.
Figure 2.
(a) FT-IR spectra of synthesized gene carriers. (b) Fluorescence emission spectra of PLL and PLD-R9-G-NLSWs. (c, d) Sizes and zeta potentials of polymer/pVEGF complexes at various w/w ratios. (e) Agarose gel electrophoresis of gene complexes at various w/w ratios. In (e), (A, B, C, D) are PLL/pVEGF, PLD/pVEGF, PLD-R9-G-NLSW1/pVEGF, and PLD-R9-G-NLSW2/pVEGF complexes, separately.
3.2. Characterization of PLD-R9-G-NLSW/pVEGF complexes
Positively charged PLL, PLD, and PLD-R9-G-NLSW and negatively charged pVEGF plasmid simultaneously formed complexes. As shown in Figure 2c, the sizes of the complexes ranged from 100 to 186 nm and did not vary significantly with the ratios of the pVEGF plasmid in the complexes. Moreover, the addition of DA and R9-G-NLSW to PLL had little effect on the size of the complex. Small sizes of the complexes (< 200 nm) are beneficial for endocytosis.
The zeta potential of the complexes increased with the weight ratio (w/w) of polymer/pVEGF (Figure 2d). The introduction of DMA shielded some positive surface charges on the PLD-R9-G-NLSW. Therefore, the zeta potential of the PLD/pVEGF complex was lower than that of the PLL/pVEGF complex at the same w/w ratio. Due to the conjugation of the R9-G-NLSW peptide on the PLD, the PLD-R9-G-NLSW/pVEGF exhibited a lower surface charge density compared to the PLD/pVEGF. On the other hand, the PLD-R9-G-NLSW is a pH-sensitive carrier. A low pH value results in the removal of DMA and formation of positively charged amino group in the PLD-R9-G-NLSW (Figure 1b), which increases the zeta potential of the PLD-R9-G-NLSW/pVEGF complex. As shown in Figure 2d, when the pH values decreased from 7.4 to 5.5, the zeta potentials of the PLD-R9-G-NLSW1/pVEGF and PLD-R9-G-NLSW2/pVEGF complexes increased from 24.6 mV and 22.8 mV to 31.4 mV and 30.8 mV, respectively. The pH value sensitivity of the PLD-R9-G-NLSW is favorable for the gene complex to escape from endosomes/lysosomes after endocytosis.
The binding and condensing ability of polymer/pVEGF complexes was evaluated using agarose gel electrophoresis. As shown in Figure 2e, negatively charged pVEGF migrated to the positive electrode under an electrophoretic environment. When the pVEGF was condensed with a gene carrier, it formed a complex. The pVEGF was fully retarded by PLL, PLD, PLD-R9-G-NLSW1, and PLD-R9-G-NLSW2 at the w/w ratio of 0.5, 1, 2, and 2, respectively. Generally, a higher positive zeta potential (corresponding to a high ratio of gene carrier/pDNA) is capable of condensing pDNA more efficiently. However, the gene carrier often shows high cytotoxicity when a high amount of positive charges is located on the surface of the carrier. Considering these two factors, the ratio of five in the polymer/pVEGF complex was selected for further studies.
3.3. In vitro cytotoxicity assay, cellular uptake, and intercellular distribution
The cytotoxicity of the polymer/pVEGF complexes was evaluated by an MTT assay. The cell viability of the PLL/pDNA gradually decreased with pDNA concentrations (Support Information, Figure S2). In comparison, the other three groups (PLD/pVEGF, PLD-R9-G-NLSW1/pVEGF, and PLD-R9-G-NLSW2/pVEGF) exhibited relatively lower cytotoxicity and the cell viabilities were higher than 90% at all pDNA concentrations, indicating that the introduction of DMA into PLL reduced cytotoxicity. In addition, the PLD-R9-G-NLSW/pVEGF had relatively higher cell viability than the PLL/pVEGF and PLD/pVEGF groups at the same concentration, indicating that the PLD-R9-G-NLSW was a safe gene carrier.
To evaluate cellular uptakes, the gene complexes were cultured with DPSCs for 4 h and the activity was detected via flow cytometry. As shown in Figure 3, the cellular uptake of the PLD/AF647-oligonucleotide complex was only 33.9% due to the shielding effect of DMA on the PLL. The introduction of R9-G-NLSW into PLD dramatically increased cellular uptake efficiency and the cellular uptake of the PLD-R9-G-NLSW1/AF647-oligonucleotide was 82.5%. In addition, further increasing the ratio of R9-G-NLSW in the PLD improved cellular uptake to 84.8% (PLD-R9-G-NLSW2/AF647-oligonucleotide complex). Similarly, the mean fluorescence intensity (MFI) of the complexes showed the same tendency and increased with the introduction of R9-G-NLSW. Specifically, the MFI value of the PLD-R9-G-NLSW2/AF647-oligonucleotide complex was 21.9 folds to that of the PLL/AF647-oligonucleotide complex. The high MFI ratio indicated that on average many more copies of the gene were transfected into each cell in the PLD-R9-G-NLSW2/AF647-oligonucleotide complex than in the PLL/AF647-oligonucleotide complex. Both of the results confirmed that the R9 in the R9-G-NLSW performed membrane-penetration activity to enhance cellular uptake.
Figure 3.
(a) Cellular uptake of various polymer/AF647-oligonucleotide complexes at 4 h after transfection. (b) Percentages of cellular uptake and mean fluorescence intensity (MFI) values measured by flow cytometry. (A, B, C, D, E) correspond to PLL/AF647-oligonucleotides, PLD/AF647-oligonucleotides, PLD-R9-G-NLSW1/AF647-oligonucleotides, PLD-R9-G-NLSW2/AF647-oligonucleotides, and AF647-oligonucleotides, respectively. N=3, *P < 0.05 vs. group E, #P < 0.05 vs. group A.
The intercellular distribution of AF647-oligonucleotides (red) in transfected DPSCs was evaluated using CLSM. The endosomes/lysosomes and nuclei were stained with LysoTracker Green DND-26 (green) and Hoechst 33342 (blue), respectively. At 4 h, more than 70% of AF647-oligonucleotides remained in the endosomes/lysosomes in all the four groups (Figure 4b). Only a small amount of the AF647-oligonucleotides escaped from endosomes/lysosomes. Among them, the nucleus CLR in the PLD-R9-G-NLSW2/AF647-oligonucleotide group was 5.8%, the highest of all four groups (Figure 4c). At 24 h, more complexes escaped from endosomes/lysosomes into the cytoplasm. Compared with the PLL/AF647-oligonucleotide complex, the endosome/lysosome CLR of PLD/AF647-oligonucleotide complex significantly decreased due to the introduction of the pH-sensitive group DMA. However, the nucleus CLRs between these two groups had no significant difference. In contrast, the nucleus CLR values were greatly increased by introducing NLSW to PLD. As show in Figure 5, the nucleus CLR value of the PLD-R9-G-NLSW1/AF647-oligonucleotide group and the PLD-R9-G-NLSW2/AF647-oligonucleotide group was 17.1±1.4% and 36.2±4.4%, respectively. The high nucleus translocation capacity of the PLD-R9-G-NLSW confirmed the function of NLSW in the gene carrier.
Figure 4.
(a) CLSM images of the intracellular trafficking of various polymer/AF647-oligonucleotide complexes at 4 h after transfection. (b) Endo/lysosome co-localization rate (endo/lysosome CLR) (c) Nucleus CLR. (A, B, C, D) correspond to PLL/AF647-oligonucleotides, PLD/AF647-oligonucleotides, PLD-R9-G-NLSW1/AF647-oligonucleotides, and PLD-R9-G-NLSW2/AF647-oligonucleotides, respectively. N=3 *P < 0.05 vs. group A.
Figure 5.
(a) CLSM images of the intracellular trafficking of various polymer/AF647-oligonucleotide complexes at 24 h after transfection. (b) CLR values between AF647-oligonucleotides and endo/lysosomes. (c) CLR values between AF647-oligonucleotides and the nucleus. (A, B, C, D) correspond to PLL/AF647-oligonucleotides, PLD/AF647-oligonucleotides, PLD-R9-G-NLSW1/AF647-oligonucleotides, and PLD-R9-G-NLSW2/AF647-oligonucleotides, respectively. N=3, *P < 0.05 vs. group A, #P < 0.05 vs. group C).
3.4. Gene delivery and bioactivity assay
The transfection performance was first evaluated by an inverted fluorescent microscope. As shown in Figure 6a, the fluorescence intensities in the PLD-R9-G-NLSW1/pVEGF and PLD-R9-G-NLSW2/pVEGF groups were much stronger than that in the PLL/pVEGF group, indicating that the R9-G-NLSW promoted transfection efficiency. Furthermore, the PLD-R9-G-NLSW2/pVEGF group had stronger fluorescence expression than the PLD-R9-G-NLSW1/pVEGF group. The transfection efficiency of the complexes was further quantified by flow cytometry. As shown in Figure 6b, the transfection efficiency of the PLD-R9-G-NLSW2/pVEGF was 10.5±0.5%, which was 4.8-fold higher than that of the PLL/pVEGF. Meanwhile, the transfection efficiency of the PLD-R9-G-NLSW2/pVEGF was 1.4-fold higher than that of the PLD-R9-G-NLSW1/pVEGF.
Figure 6.
(a) Fluorescence images (dark field and bright field) of DPSCs transfected for 24 h by complexes. (b) The transfection efficiency measured by flow cytometry. (c) Quantitative mRNA expression by RT-PCR assay. (d) The concentration of the VEGF secreted from the DPSCs transfected by different polymer/pZNF580 complexes. In (A, B, C, D), DPSCs were treated with PLL/pVEGF, PLD-R9-G-NLSW1/pVEGF, PLD-R9-G-NLSW2/pVEGF, and pVEGF, respectively. N=3, *P < 0.05 vs. group D, #P < 0.05 vs. group B.
The gene transfection efficiency of the complexes in DPSCs was also evaluated at the transcriptional level using real-time RT-PCR. Figure 6c shows that the mRNA expression of VEGF in the PLD-R9-G-NLSW2/pVEGF was 6.7 folds higher than that in the pVEGF group. In addition, the mRNA expression in the PLD-R9-G-NLSW2/pVEGF group was 2.0 folds and 3.9 folds higher than that in the PLD-R9-G-NLSW1/pVEGF and PLL/pVEGF group, respectively.
The DPSCs transfected with PLD-R9-G-NLSW/pVEGF secreted significantly higher levels of VEGF compared to other groups. As shown in Figure 6d, an average of 2773 pg/mL of VEGF was secreted from the PLD-R9-G-NLSW2/pVEGF group, which was 8.1-fold higher than that from the pVEGF group (341 pg/mL). Moreover, the VEGF secreted from the PLD-R9-G-NLSW2/pVEGF group was 1.5-fold and 4.1-fold higher than that from the PLD-R9-G-NLSW1/pVEGF and the PLL/pVEGF groups, respectively.
An HUVEC migration experiment was used to evaluate the bioactivity of the VEGF secreted from the transfected DPSCs. A wound healing assay and a trans-well migration assay were performed to assess the migration capability of HUVECs. For the wound healing assay, some HUVECs were scratched and the cells that migrated to the scraped area were monitored. As revealed in Figure 7a&c, the HUVECs on the PLD-R9-G-NLSW/pVEGF groups showed higher migration rates than on the two control groups. After 12 h, the relative migration area in the PLD-R9-G-NLSW1/pVEGF and PLD-R9-G-NLSW2/pVEGF groups was 77.3 ± 1.7% and 81.3 ± 1.1%, respectively; while the percentage number was 71.6 ± 3.9% and 58.5 ± 3.1% for the PLD/pVEGF and pVEGF groups, respectively. The trans-well migration assay also indicated that the PLD-R9-G-NLSW2/pVEGF group had the highest migration number, which was nearly two folds to that of the PLL/pVEGF group (Support Information, Figure S3). In addition, the PLD-R9-G-NLSW2/pVEGF group showed a 40% increase of the HUVECs migration number than the PLD-R9-G-NLSW1/pVEGF group.
Figure 7.
(a) A wound healing assay to evaluate HUVEC migration at different time points. (b) Quantitative analysis of the relative recovered area at 12 h. (c) In vitro vascular tube formation after the HUVECs were cultured for 6 h in the conditioned medium from VEGF-transfected DPSCs. (d) Vascular tube numbers formed by the HUVECs that were cultured for 6 h in the conditioned medium from VEGF-transfected DPSCs. In (A, B, C, D), DPSCs were treated with PLL/pVEGF, PLD-R9-G-NLSW1/pVEGF, PLD-R9-G-NLSW2/pVEGF, and pVEGF, respectively. *P < 0.05 vs. group D, #P < 0.05 vs. group B
To examine the functional paracrine effect of the transfected DPSCs on angiogenesis, HUVECs were seeded on Matrigel and treated with the conditioned medium collected from the VEGF-transfected DPSCs. Compared with the negative control group, all treatment groups exhibited stronger vascularization ability. Specifically, the PLD-R9-G-NLSW2/pVEGF treated group displayed ~2-fold vascular ring numbers compared to the PLL/pVEGF treated group (Figure 7d). Together, these results confirmed that the PLD-R9-G-NLSW/pVEGF complex has the ability to transfect DPSCs to secrete a higher level of functional VEGF that promoted the migration and vascularization of HUVECs.
3.5. Pulp regeneration in a full-length human tooth root
To mimic the clinical application of our research, a full-length human tooth root model was adapted and VEGF-transfected DPSCs were injected into the root canal to evaluate vascularized pulp regeneration. Six weeks after transplantation, only minimal soft tissue was observed in the root of the DPSC-free control group (Figure 8). The addition of DPSCs regenerated some pulp-like tissue, which was mainly located at the region of the apical third area. By contrast, significantly more pulp-like tissue was regenerated in the VEGF-transfected DPSC group, which occupied most of the area in the canal of an 11-mm length tooth root. In addition, abundant new blood vessels were observed throughout the canals. Furthermore, our quantitative analysis indicated that the regenerated tissue reached only 11.2% and 32.5% of the canals in the DPSC-free and DPSC groups, respectively. In contrast, the regenerated neo-tissue was observed in 68.7% of the canal in the VEGF-transfected DPSCs group (Figure 8c).
Figure 8.
Pulp regeneration in a full-length human tooth root after implantation for 6 weeks. (a) H&E staining images of pulp regeneration after in vivo implantation for six weeks. (b) Immunohistochemical staining images of anti-human vWF and CD31. (c) Quantitative analysis of the regenerated neo-tissues. (d) Quantitative analysis of the number of regenerated blood vessels per 0.16 mm2. (e) Vessel area density. (A, D) DPSC-free group. (B, E) DPSC group. (C, F) PLD-R9-G-NLSW2/pVEGF complexes transfected DPSC group. *P < 0.05 vs. group A and #P < 0.05 vs. group B.
CD31 and vWF, two endothelial cell markers, were used to characterize new blood vessel formation in the regenerated pulp tissue. As shown in Figure 8b, negligible immunohistochemical staining of vWF and CD31 was detected in the DPSC-free group. Weak staining of vWF and CD31 was observed in the DPSC group. In contrast, the staining of vWF and CD31 in the VEGF-transfected DPSC group was very strong and many new blood vessels were detected throughout the regenerated pulp tissue. In addition, the average size of the new blood vessels in the VEGF-transfected DPSC group was much higher than that in the other two groups. Quantitative measurements further indicated that the average number of vascular lumens in the VEGF-transfected DPSC group was 12.7, which is 10.6 folds and 1.8 folds higher than that in the DPSC-free and DPSC groups, respectively (Figure 8d). Overall, 16.6% of the regenerated pulp tissue area in the VEGF-transfected DPSC group was occupied by new blood vessels, while that number was 2.3% and 7.0% in the DPSC-free and DPSC groups, respectively (Figure 8e). The staining of Nestin and DSPP, which are markers of mature odontoblasts [14], showed weak expression in all groups, and no newly formed dentin was found, probably owing to the relatively short observation time (Supporting Information, Fig. S4). Overall, the results indicated that DPSCs transfected with the PLD-R9-G-NLSW2/pVEGF complex successfully regenerated vascularized pulp tissue in a full-length tooth root.
4. Discussion
While pulp regeneration has been explored for over two decades, the regeneration of clinically applicable pulp tissue in a full-length tooth root has never been achieved [11, 28, 29]. The main obstacle is the unique anatomy of the long tooth root that receives its blood supply from only a small apical opening of the root canal, which severely limits nutrient diffusion and the ingrowth of new blood vessels during tissue regeneration. In this study, we designed and synthesized a pH-sensitive, multifunctional, peptide-conjugated, non-viral gene carrier named PLD-R9-G-NLSW to tackle this challenge. Using this gene carrier, we transfected DPSCs with the gene VEGF. Our results show that the DPSCs transfected with the PLD-R9-G-NLSW2/pVEGF vector facilitated fast revascularization and successfully regenerated pulp-like tissue in a full-length human tooth root.
The incorporation of gene transfection into tissue engineering is a promising approach in regenerative dentistry. Electroporation is a physical method for gene transfection and has been tested in regenerative endodontics. For example, Nakashima et al. transferred the growth/differentiation factor 11 (Gdf11) into mesenchymal cells derived from mouse dental papilla by an electroporation-mediated gene delivery process, and showed reparative dentin formation during pulpal wound healing in canine teeth [30]. This method, however, has the disadvantages of potential cell damage and the nonspecific transport of molecules into and out of the cell. Virus-mediated transfections are the most commonly used biological method, because it is highly efficient and is easy to achieve sustainable transgene expression. However, immunogenicity and cytotoxicity are the two concerns of virus-mediated transfection [31]. Non-viral gene delivery has attracted increasing attention because of its lower toxicity, lack of immunogenicity, and ease of production compared to the use of viral vectors. However, the major disadvantage of a non-viral gene delivery approach is its low transfection efficiency that is limited by three transport processes: cellular uptake, endosomal escape, and nuclear translocation. Herein, we designed a multifunctional, peptide-conjugate, non-viral vector to enhance transfection efficiency. PLL was first amidated by DMA to shield some positive charge and facilitate endosomal escape in mildly acidic environment. In addition, the multifunctional peptide R9-G-NLSW was conjugated onto PLL for the enhancement of cellular uptake and nuclear localization. The multifunctional peptide R9-G-NLSW contains the cell-penetrating peptide R9 to enhance cellular uptake and a nuclear localization signal peptide named NLS to assist the translocation of genes into the nucleus. G acts as a flexible spacer to regulate the activity of R9 and NLS, and W provides fluorescence spectrum at 348 nm to trace gene delivery and quantify the multifunctional peptide.
Arginine-rich peptides are one of the most attractive cell-penetrating peptides that can effectively translocate across the cell membrane. R9 contains nine arginine residues and was reported to possess the highest efficiency of translocation through biological membranes among the oligoarginines [32]. R9 passes through the cell membrane via the endocytosis pathway or direct permeation [33]. Studies have shown that the upper size limit for a cargo that can diffuse through the nucleus pore complex (NPC) is 9 nm in diameter, which is markedly smaller than general exogenous genes such as plasmid [34]. One well-known NLS is the Pro-Lys-Lys-Lys-Arg-Lys-Val sequence (PKKKRKV) that is derived from the large T antigen of the SV40 virus and has been reported to improve nuclear access and successfully enhance gene delivery [24]. The NLS is recognized by importin α and linked to β-karyopherin (importin β), which promotes the entrance of plasmid to the nucleus via the interaction between importin β and NPC [35, 36]. The mechanism of DMA to assist with endosome escape is that the amide groups in PLD-R9-G-NLSW are hydrolyzed to generate positive charges in the mild acidic environment of the endosome (Figure 1b). More positive charges in the DMA-containing vector increase zeta potentials of the DMA-containing vector, therefore facilitating endosomal escape [37]. Another benefit is that DMA partially shields the positive charges of PLL to reduce cytotoxicity during the step of endocytosis.
The in vitro cell test confirmed the effectiveness of the above design. The incorporation of cell-penetrating peptide R9 improved the cellular uptake from 33.9% up to 84.8% (Figure 3b). With the addition of DMA, more than 75.5% of the PLD-R9-G-NLSW2/pVEGF escaped from endosomes/lysosomes into the cytoplasm at 24 h, while that number was 44.0% for the PLL/pVEGF (Figure 5b). In addition, the NLS in the PLD-R9-G-NLSW2 promoted the nucleus location to a value of 36.2%, which was 6.8 folds higher than that in the PLL group (Figure 5c).
While DMA facilitated the escape of the gene complex from endosomes/lysosomes, it did not improve the nucleus accumulation of the escaped gene complex. This phenomenon was confirmed from the result in Figure 5b&c, where the endosome/lysosome CLR of the PLD/AF647-oligonucleotide complex was significantly lower than that of the PLL/AF647-oligonucleotide complex, but the nucleus CLRs between these two groups had no significant difference. Therefore, the incorporation of NLS was an indispensable step to promote nuclear translocation.
Due to the introduction of the multifunctional peptide and DMA, the transfection efficiency of PLD-R9-G-NLSW2/pVEGF was 4.8-fold higher than that of the PLL/pVEGF group (Figure 6b). At the protein level, the VEGF secreted from the PLD-R9-G-NLSW2/pVEGF group was 8.1-fold higher than that from the control group (Figure 6d). These results indicated that the multifunctional peptide gene carriers promoted VEGF transfection efficiency, expression, and protein secretion.
DPSCs are the prime candidate cell type for pulp regeneration. Besides differentiating into pulp cells, DPSCs can secrete pro-angiogenic factors, such as monocyte chemotactic protein-1 and VEGF; therefore, have certain capability of inducing angiogenesis during tissue regeneration [38]. To promote the pro-angiogenic potential of DPSCs, we utilized the multifunctional peptide PLD-R9-G-NLSW2/pVEGF to transfect DPSCs. Next, the VEGF-transfected DPSCs were implanted to regenerate pulp tissue in a full-length tooth root. The VEGF-transfected DPSCs played dual roles during pulp regeneration. On the one hand, the DPSCs overexpressed angiogenic growth factor VEGF to recruit endothelial cells and accelerate new blood vessel formation. On the other hand, the DPSCs themselves differentiated to form pulp fibroblasts and odontoblasts in the root canal. This approach is simple compared to other pulp regeneration methods that involve multiple cell types (e.g. endothelial cells and DPSCs). More importantly, this approach is promising to address the challenge of revascularization of full-length (11–13 mm) tooth root. As shown from the in vivo implantation results, abundant new blood vessels were observed throughout the canal six weeks after the implantation of the VEGF-transfected DPSCs. Due to the fast revascularization, most of the area in the canal of the tooth root was fulfilled with pulp-like tissue. In contrast, new blood vessels were detected only in a small area of the root canal within the DPSCs group, leading to the regeneration of pulp-like tissue that was limited to the lower apical third area. Quantitative data further indicated that the average number and size of neo blood vessels in the VEGF-transfected DPSC group were significantly higher than the controls. Taken together, the multifunctional PLD-R9-G-NLSW was an effective gene carrier and the DPSCs transfected with the PLD-R9-G-NLSW2/pVEGF complex successfully regenerated vascularized pulp tissue in a full-length tooth root.
There were some limitations in this study. First, bubbles in canals were detected in two samples (2 out of 24 samples) extracted six weeks after implantation. Due to the unique structure of a tooth root, the long and narrow root canal tends to trap air during the injection of gene vectors/DPSCs. The appearance of bubbles blocked nutrient diffusion and cell migration, resulting in the obstruction of new blood vessel and tissue formation. Adjustment of the injection procedure (e.g. selection of different needle sizes and optimization of injection speed) may solve this issue in follow-up studies. Second, there were still some areas in the canal that were not fulfilled with pulp-like tissue in the VEGF-transfected DPSCs group six weeks after implantation. We expected that extension of implantation time would regenerate the pulp tissue that covered the entire length of the tooth root. In addition, a complete regenerative endodontics include both pulp and dentin regeneration. No apparent new dentin was found surrounding the canal in the VEGF-transfected DPSCs sample, probably owing to the short implantation time. Future studies are needed to examine formation of the pulp-dentin complex.
5. Conclusion
In this work, we designed and synthesized a multifunctional, pH-sensitive, polymeric, non-viral gene carrier called PLD-R9-G-NLSW via a three-step process. The incorporation of R9, DMA, and NLS into the gene carrier benefited cellular uptake, endosomal escape, and nuclear localization, and significantly increased the transfection efficiency. The VEGF-transfected DPSCs secreted high level of bioactive VEGF that promoted the migration and tube formation of HUVECs. The in vivo study showed that the pH-sensitive, multifunctional, peptide-conjugated system was an excellent carrier for enhanced revascularization and pulp tissue regeneration in a full-length toot root. The pH-sensitive, multifunctional, peptide-conjugated, non-viral gene delivery system is therefore a promising approach for regenerative endodontics.
Supplementary Material
Statement of Significance.
Pulp regeneration in a full-length tooth root canal has long been a challenge in regenerative endodontics. This is due to the unique root anatomy that allows the blood supply of the tooth root only from a small apical foramen (< 1 mm), leading to a severe barrier for revascularization during pulp regeneration. In this work, we designed a multifunctional peptide-conjugated, pH-sensitive, non-viral gene vector to address this challenge. Our work shows that the peptide-conjugated system was an excellent carrier for fast revascularization and pulp tissue regeneration in a full-length toot root. This study will interest the multidisciplinary readership in gene delivery, biomaterials, and dental/craniofacial tissue engineering community.
Acknowledgment
This work was supported by NIH/NIDCR DE024979 and DE029860.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Nakashima M, Iohara K, Murakami M, Nakamura H, Sato Y, Ariji Y, Matsushita K, Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: a pilot clinical study, Stem Cell Res. Ther 8(61) (2017) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Reeh ES, Messer HH, Douglas WH, Reduction in tooth stiffness as a result of endodontic and restorative procedures, J. Endod 15(11) (1989) 512–516. [DOI] [PubMed] [Google Scholar]
- [3].Qu T, Jing J, Jiang Y, Taylor RJ, Feng JQ, Geiger B, Liu X, Magnesium-containing nanostructured hybrid scaffolds for enhanced dentin regeneration, Tissue Eng., Part A 20(17–18) (2014) 2422–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Qu T, Liu X, Nano-structured gelatin/bioactive glass hybrid scaffolds for the enhancement of odontogenic differentiation of human dental pulp stem cells, J. Mater. Chem. B 1(37) (2013) 4764–4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Qu T, Jing J, Ren Y, Ma C, Feng JQ, Yu Q, Liu X, Complete pulpodentin complex regeneration by modulating the stiffness of biomimetic matrix, Acta Biomater. 16 (2015) 60–70. [DOI] [PubMed] [Google Scholar]
- [6].Saghiri MA, Asatourian A, Sorenson CM, Sheibani N, Role of angiogenesis in endodontics: contributions of stem cells and proangiogenic and antiangiogenic factors to dental pulp regeneration, J. Endod 41(6) (2015) 797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yang J, Yuan G, Chen Z, Pulp regeneration: current approaches and future challenges, Front. Physiol 7(58) (2016) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Huang GT, Pulp and dentin tissue engineering and regeneration: current progress, Regen. Med 4(5) (2009) 697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhang R, Xie L, Wu H, Yang T, Zhang Q, Tian Y, Liu Y, Han X, Guo W, He M, Liu S, Tian W, Alginate/laponite hydrogel microspheres co-encapsulating dental pulp stem cells and VEGF for endodontic regeneration, Acta Biomater 113 (2020) 305–316. [DOI] [PubMed] [Google Scholar]
- [10].Gorin C, Rochefort GY, Bascetin R, Ying H, Lesieur J, Sadoine J, Beckouche N, Berndt S, Novais A, Lesage M, Hosten B, Vercellino L, Merlet P, Le-Denmat D, Marchiol C, Letourneur D, Nicoletti A, Vital SO, Poliard A, Salmon B, Muller L, Chaussain C, Germain S, Priming dental pulp stem cells with fibroblast growth factor-2 increases angiogenesis of implanted tissue-engineered constructs through hepatocyte growth factor and vascular endothelial growth factor secretion, Stem Cells Transl. Med 5(3) (2016) 392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yang JW, Zhang YF, Sun ZY, Song GT, Chen Z, Dental pulp tissue engineering with bFGF-incorporated silk fibroin scaffolds, J. Biomater. Appl 30(2) (2015) 221–229. [DOI] [PubMed] [Google Scholar]
- [12].Khayat A, Monteiro N, Smith EE, Pagni S, Zhang W, Khademhosseini A, Yelick PC, GelMA-encapsulated hDPSCs and HUVECs for dental pulp regeneration, J. Dent. Res 96(2) (2017) 192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dissanayaka WL, Hargreaves KM, Jin L, Samaranayake LP, Zhang C, The interplay of dental pulp stem cells and endothelial cells in an injectable peptide hydrogel on angiogenesis and pulp regeneration in vivo, Tissue Eng., Part A 21(3–4) (2015) 550–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Li X, Ma C, Xie X, Sun H, Liu X, Pulp regeneration in a full-length human tooth root using a hierarchical nanofibrous microsphere system, Acta Biomater. 35 (2016) 57–67. [DOI] [PubMed] [Google Scholar]
- [15].Nussenbaum B, Krebsbach PH, The role of gene therapy for craniofacial and dental tissue engineering, Adv. Drug Delivery Rev 58(4) (2006) 577–591. [DOI] [PubMed] [Google Scholar]
- [16].Zhu L, Dissanayaka WL, Zhang C, Dental pulp stem cells overexpressing stromal-derived factor-1alpha and vascular endothelial growth factor in dental pulp regeneration, Clin. Oral Investig 23(5) (2019) 2497–2509. [DOI] [PubMed] [Google Scholar]
- [17].Rodriguez AL, Wang T-Y, Bruggeman KF, Li R, Williams RJ, Parish CL, Nisbet DR, Tailoring minimalist self-assembling peptides for localized viral vector gene delivery, Nano Res. 9(3) (2015) 674–684. [Google Scholar]
- [18].Rezaee M, Oskuee RK, Nassirli H, Malaekeh-Nikouei B, Progress in the development of lipopolyplexes as efficient non-viral gene delivery systems, J. Controlled Release 236 (2016) 1–14. [DOI] [PubMed] [Google Scholar]
- [19].Franceschi R, Biological approaches to bone regeneration by gene therapy, J. Dent. Res 84(12) (2005) 1093–1103. [DOI] [PubMed] [Google Scholar]
- [20].Lächelt U, Wagner E, Invading target cells: multifunctional polymer conjugates as therapeutic nucleic acid carriers, Front. Chem. Sci. Eng 5(3) (2011) 275–286. [Google Scholar]
- [21].Collet G, Grillon C, Nadim M, Kieda C, Trojan horse at cellular level for tumor gene therapies, Gene 525(2) (2013) 208–216. [DOI] [PubMed] [Google Scholar]
- [22].Ji T, Ding Y, Zhao Y, Wang J, Qin H, Liu X, Lang J, Zhao R, Zhang Y, Shi J, Peptide assembly integration of fibroblast‐targeting and cell‐penetration features for enhanced antitumor drug delivery, Adv. Mater 27(11) (2015) 1865–1873. [DOI] [PubMed] [Google Scholar]
- [23].Wallbrecher R, Ackels T, Olea RA, Klein MJ, Caillon L, Schiller J, Bovée-Geurts PH, van Kuppevelt TH, Ulrich AS, Spehr M, Membrane permeation of arginine-rich cell-penetrating peptides independent of transmembrane potential as a function of lipid composition and membrane fluidity, J. Controlled Release 256 (2017) 68–78. [DOI] [PubMed] [Google Scholar]
- [24].Chen K, Guo L, Zhang J, Chen Q, Wang K, Li C, Li W, Qiao M, Zhao X, Hu H, A gene delivery system containing nuclear localization signal: Increased nucleus import and transfection efficiency with the assistance of RanGAP1, Acta Biomater. 48 (2017) 215–226. [DOI] [PubMed] [Google Scholar]
- [25].Sanjoh M, Hiki S, Lee Y, Oba M, Miyata K, Ishii T, Kataoka K, pDNA/poly(L-lysine) Polyplexes Functionalized with a pH-Sensitive Charge-Conversional Poly(aspartamide) Derivative for Controlled Gene Delivery to Human Umbilical Vein Endothelial Cells, Macromol. Rapid Commun 31(13) (2010) 1181–1186. [DOI] [PubMed] [Google Scholar]
- [26].Jiang Q, Nie Y, Chen X, He Y, Yue D, Gu Z, pH-triggered pinpointed cascading charge-conversion and redox-controlled gene release design: modularized fabrication for nonviral gene transfection, Adv. Funct. Mater 27(26) (2017) 1–12. [Google Scholar]
- [27].Li Z, Qu T, Ding C, Ma C, Sun H, Li S, Liu X, Injectable gelatin derivative hydrogels with sustained vascular endothelial growth factor release for induced angiogenesis, Acta Biomater. 13 (2015) 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dissanayaka WL, Zhu L, Hargreaves KM, Jin L, Zhang C, Scaffold-free Prevascularized Microtissue Spheroids for Pulp Regeneration, J. Dent. Res 93(12) (2014) 1296–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Galler KM, Hartgerink JD, Cavender AC, Schmalz G, D’Souza RN, A customized self-assembling peptide hydrogel for dental pulp tissue engineering, Tissue Eng., Part A 18(1–2) (2012) 176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nakashima M, Mizunuma K, Murakami T, Akamine A, Induction of dental pulp stem cell differentiation into odontoblasts by electroporation-mediated gene delivery of growth/differentiation factor 11 (Gdf11), Gene Ther. 9(12) (2002) 814–818. [DOI] [PubMed] [Google Scholar]
- [31].Della Peruta M, Badar A, Rosales C, Chokshi S, Kia A, Nathwani D, Galante E, Yan R, Arstad E, Davidoff AM, Williams R, Lythgoe MF, Nathwani AC, Preferential targeting of disseminated liver tumors using a recombinant adeno-associated viral vector, Hum. Gene Ther 26(2) (2015) 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mitchell DJ, Steinman L, Kim D, Fathman C, Rothbard J, Polyarginine enters cells more efficiently than other polycationic homopolymers, J. Peptide Res 56(5) (2000) 318–325. [DOI] [PubMed] [Google Scholar]
- [33].Kosuge M, Takeuchi T, Nakase I, Jones AT, Futaki S, Cellular internalization and distribution of arginine-rich peptides as a function of extracellular peptide concentration, serum, and plasma membrane associated proteoglycans, Bioconjug. Chem 19(3) (2008) 656–664. [DOI] [PubMed] [Google Scholar]
- [34].Sun Y, Xian L, Xing H, Yu J, Yang Z, Yang T, Yang L, Ding P, Factors influencing the nuclear targeting ability of nuclear localization signals, J. Drug Target 24(10) (2016) 927–933. [DOI] [PubMed] [Google Scholar]
- [35].Marfori M, Mynott A, Ellis JJ, Mehdi AM, Saunders NF, Curmi PM, Forwood JK, Boden M, Kobe B, Molecular basis for specificity of nuclear import and prediction of nuclear localization, Biochim. Biophys. Acta. Mol. Cell Res 1813(9) (2011) 1562–1577. [DOI] [PubMed] [Google Scholar]
- [36].Mittal A, Chitkara D, Structural modifications in polymeric micelles to impart multifunctionality for improved drug delivery, Ther. Deliv 7(2) (2016) 73–87. [DOI] [PubMed] [Google Scholar]
- [37].Cheng R, Meng F, Deng C, Zhong Z, Bioresponsive polymeric nanotherapeutics for targeted cancer chemotherapy, Nano Today 10(5) (2015) 656–670. [Google Scholar]
- [38].Bronckaers A, Hilkens P, Fanton Y, Struys T, Gervois P, Politis C, Martens W, Lambrichts I, Angiogenic properties of human dental pulp stem cells, PloS One 8(8) (2013) e71104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.