The gestational age boundary separating infants considered too immature for intensive care from infants who may benefit has shifted dramatically during the past 50 years. In 1971, a widely used neonatology textbook stated that “The lower limit of viability is probably around 28 weeks, at which time most infants weigh two pounds, four ounces (1000 g).”1 Today, the most immature infants routinely cared for by neonatologists in some parts of the world are born at 22 or 23 weeks of gestation, with many weighing less than a pound.2
Whether to provide intensive care for infants born at ≤23 weeks of gestation remains controversial in much of the world. Ethical and economic issues surrounding this decision have been widely debated.3–6 When a decision to provide intensive care is made, recognizing the particular physiologic and clinical challenges of birth during this period of gestation is critical for improving care processes and outcomes. For this reason, an international group of clinician-researchers from centers in Japan, Sweden, Germany, and the United States (US) with extensive experience providing intensive care for infants born at 22–23 weeks of gestation formed a collaborative to improve research in this area. At several of their centers, survival of >50% of live births at 22 weeks has been reported among hundreds of patients.7–11 Our goal is to summarize unique aspects of the physiology and medical care of infants born near the current limit of viability, specifically highlighting gaps in knowledge and potential opportunities for improvement.
Defining a High-Risk Group at the Margin of Viability
Should infants born at 22–23 weeks of gestation be considered distinct from infants born just weeks later? Fetal development is a continuum. Moreover, for infants born at the same gestational age, factors such as birth weight, infant sex, and antenatal corticosteroid exposure affect the response to intensive care and may reflect differences in developmental maturity. Based on these factors, for some infants born at 22–23 weeks, the likelihood of survival following intensive care is greater than for some infants born weeks later.12 Yet, there are no published reports of infants surviving after birth at 20 weeks of gestation and only a few case reports of infants surviving after birth at 21 weeks.13 By 24 weeks, intensive care in many countries is expected to result in survival for a majority of infants.14 In contemporary clinical practice, infants born between these extremes—at 22–23 weeks of gestation— comprise a particularly vulnerable “grey zone.” For these infants, initiation of intensive care largely depends on the hospital or country where the infant is born.15,16
Existing nomenclature for infants born during various periods of gestation (Table I; available at www.jpeds.com) reflects differences in physiology and outcomes that impact clinical management. This terminology is useful despite the continuous nature of fetal development and the innate variability in maturity observed between similarly aged infants. However, it has not evolved to take into account the changing limit of viability. Although many studies group together infants born at 22 and 26 weeks as “extremely preterm” (<28 weeks),17 this belies the substantial heterogeneity in outcomes and physiology for infants born during this window of fetal development. Whereas clinicians widely recognize that developmental differences of infants born at 32 versus 36 weeks affect respiratory, thermal, and nutritional management, infants born at 22 and 26 weeks are frequently studied together as if their management should be the same. Where a distinguishing line should be drawn (at 236/7 weeks or 246/7 weeks, e.g.) may be to some extent artificial; however, in international guidelines, infants born at 22–23 weeks are often treated as distinct, so this grouping seems practical. In research, distinguishing infants born at ≤23 weeks from somewhat more mature infants may confer improved precision in understanding the outcomes, physiology, and clinical care needed for these patients.
Table 1.
Classification of preterm births by gestational age
| Late Term | 41 weeks |
| Term | 39–40 weeks |
| Early Term | 37–38 weeks |
| Late Preterm | 34–36 weeks |
| Moderately Preterm | 32–33 weeks |
| Very Preterm | 28–31 weeks |
| Extremely Preterm | <28 weeks |
Epidemiology of Births and Intensive Care at 22–23 Weeks
Around the world, births at 22–23 weeks of gestation are relatively infrequent but comprise a large proportion of infant mortality. For example, in 2018 in the US, 1,785 infants were born alive at 22 weeks and 2,645 at 23 weeks of gestation, of whom more than two-thirds (2,985/4,340) died during the first year of life.18 Although births at 22–23 weeks made up only 0.1% of the 3,791,712 live births in the US (1 in 1000 - an incidence similar to fetal alcohol syndrome19 and Down Syndrome20), they accounted for nearly 1 in 7 (2,985/21,498) liveborn infants who died in the first year of life.
The care for infants born at 22–23 weeks varies around the world.16 Clinicians in Japan may have the most experience caring for infants ≤23 weeks; in 1991, the Japanese Society of Pediatrics recommended changing the limit for providing intensive care from 24 weeks to 22 weeks based on the survival of infants at 22 and 23 weeks reported in a national study.21 From 2003 to 2015, among dozens of tertiary perinatal centers in the Japanese Neonatal Research Network, survival for live births exceeded 60% at 22 weeks and 70% at 23 weeks of gestation.22 In 2012, a national survey of a majority of Japanese neonatal intensive care units (NICUs) reported that active resuscitation of infants born at 22 and 23 weeks of gestation occurred in 81% and 85% of NICUs, respectively, and 42% and 75% of these NICUs had a universal resuscitation policy.23 By comparison, in Sweden in 2014, the national board of health and welfare recommended centralization of care for all extremely preterm births at 6 university hospitals with an emphasis on maternal transfer to these hospitals prior to delivery. Subsequently, in 2014–2016 compared with 2004–2007, one year survival for all liveborn infants at 22 weeks of gestation in the nation tripled (from 10% to 30%) and the rate of stillbirths was nearly halved (from 65% to 35%).24 Swedish guidelines published in 2016 recommend that, starting at 22 weeks of gestation, a neonatologist is present at birth and intensive care may be considered; starting at 23 weeks of gestation, intensive care is generally recommended.25
The approaches in Japan and Sweden differ substantially from those of several other countries, such as the Netherlands and Denmark, where intensive care at 22–23 weeks of is not recommended.26,27 Other national guidelines recommend an individualized approach less focused on gestational age. In the US, the American Academy of Pediatrics recommends an individualized approach to decision-making for births at 22–24 weeks of gestation, taking into account known fetal and maternal conditions and risk factors as well as parental beliefs regarding the best interest of the child.28 Research has shown that provision of intensive care at 22–23 weeks vary by US region (with higher rates in the South and Midwest compared with the West and Northeast)29 and by hospital of birth.15 At US hospitals participating in the Vermont Oxford Network (VON), the rate of active treatment for infants born at 22 weeks more than doubled since 2014; in 2019, the majority of infants born at 22 weeks in these hospitals received active treatment (Figure 1). During the same period, the rate of survival after birth at 22 weeks of gestation tripled, with 17% of liveborn infants in 2019 surviving to hospital discharge or 1 postnatal year. Similar to the US, German clinical guidelines support a “gray zone” of practice at 22–23 weeks and individual centers show substantial variation in practice and outcomes.30,31
Figure 1. Active Treatment and Survival for Liveborn Infants at 22 Weeks in the US.
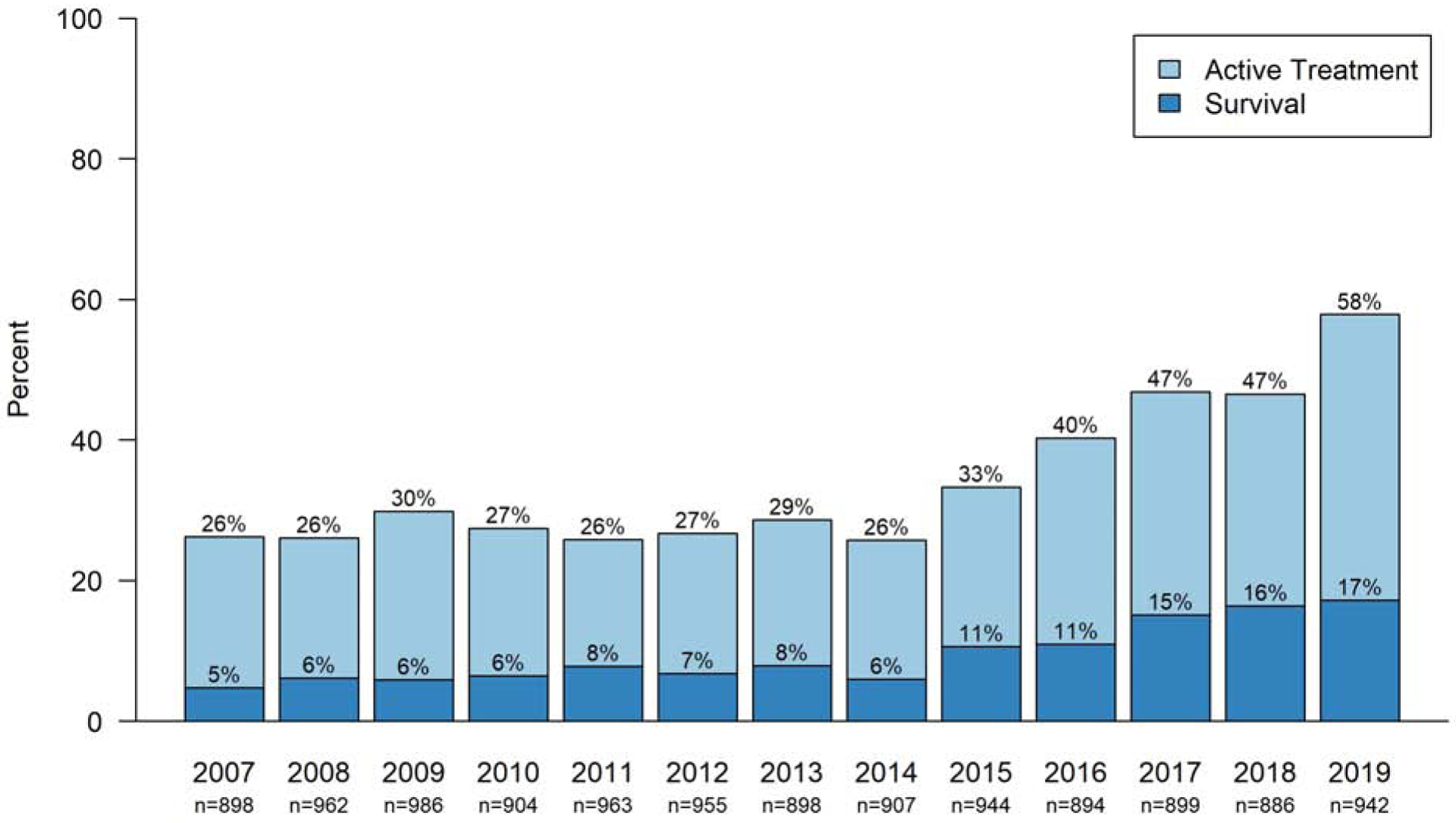
Active treatment is defined as respiratory support (including face mask ventilation, nasal continuous positive airway pressure, endotracheal intubation, surfactant therapy, or mechanical ventilation), chest compressions, or epinephrine. Survival is to hospital discharge or 1 year. The denominator includes all live births, including deaths that occurred in the delivery room. Data are from hospitals participating in the Vermont-Oxford Network.
Despite increasing provision of intensive care and survival for infants born at 22–23 weeks, one recent study using data from infants born at >300 US NICUs from 2006 to 2016 showed that more infants born at 22 and 23 weeks of gestation were exposed to dopamine than caffeine.32 Moreover, amphotericin B was among the top 25 used medications prescribed for infants born at 22 weeks. These patterns were not observed for infants born at 24 weeks. Such data highlight that some clinical characteristics may distinguish infants born at ≤23 weeks from slightly more mature infants and so impact their management. The following section describes several aspects of what is known and not known about such differences in key areas of clinical practice.
Knowns and Unknowns of Clinical Care at 22–23 Weeks
Antenatal Care
Medical care begins prior to preterm birth and includes routine prenatal care and antepartum referral to tertiary perinatal care.17 Rates of morbidity due to extreme prematurity are higher in outborn infants than in infants born at the same hospital where they receive NICU care, even when including only infants transferred on the day of birth and after adjustment for illness severity.33–35
Emerging studies and experience support that antenatal corticosteroid administration for births at ≤23 weeks of gestation reduces infant mortality and morbidity.36,37 A 2016 meta-analysis of observational studies demonstrated a significant reduction of mortality from antenatal steroid exposure among postnatally treated infants at 22–23 weeks,38 which was subsequently supported by an additional large US observational study.37 The absolute reduction of mortality from appropriately timed antenatal corticosteroid administration at early gestational ages may be greater at 22–23 weeks than at older gestational ages (at 23 weeks, the estimated number needed to treat to prevent 1 death is 6).39 This effect may be mediated, in part, by a reduction in intraventricular hemorrhage (IVH) and necrotizing enterocolitis (NEC).40,41 Although nearly all evidence for antenatal corticosteroids for infants born at 22–23 weeks is based on observational data, which has limitations, it is notable that fewer than 50 infants known to have been born <26 weeks were included in clinical trials of antenatal corticosteroids40 and that use of antenatal corticosteroids for infants born at 24–25 weeks is widely recommended. A new large trial of antenatal corticosteroids for births at ≤23 weeks of gestation would better answer questions about efficacy and safety but seems unlikely anytime soon; the only trial of antenatal corticosteroids for deliveries at 22–23 weeks of gestation listed on ClinicalTrials.gov was withdrawn by the sponsor before enrollment.42 In the US, the American College of Obstetricians and Gynecologists (ACOG) recommends against antenatal corticosteroids at 22 weeks of gestation and recommends that clinicians “consider” them at 23 weeks.43 In contrast, antenatal steroids are recommended starting at 22 weeks in national guidelines in Germany31 and the United Kingdom44 when resuscitation is intended. Given important differences in the effects of antenatal corticosteroids and cesarean delivery on the balance of maternal and infant health,45,46 the decision to give antenatal corticosteroids and the decision to perform cesarean delivery should be considered separately.
Delivery Room Intervention
The perinatal transition of infants born at 22–23 weeks reflects the unique physiology and vulnerability of these patients. The ratio of placenta to infant weight is nearly twice as large in infants ≤23 weeks of gestation compared with infants born at term,47 which, together with fragility of blood vessels and immaturity of cardiovascular adaptation and cerebral autoregulation, may differentiate the potential effects of cord management in these immature infants from in their more mature peers. Delayed cord clamping for infants born preterm results in higher survival, fewer red blood cell transfusions, and potentially lower risk of IVH,48 although few studies included infants born at ≤23 weeks.49–51 A recent trial of umbilical cord milking versus delayed cord clamping including infants born at 23 weeks showed a higher rate of severe IVH in the intact cord milking arm (22% vs 6% with cord milking versus delayed cord clamping in infants 23–27 weeks), raising questions about the safety of umbilical cord milking in the most immature infants.52
The incidence of admission hypothermia is inversely proportional to gestational age, due, in part, to high evaporative losses from a larger surface area and less keratinized skin. In some centers, a majority of infants ≤23 weeks are admitted to the NICU with moderate-severe hypothermia.53 International guidelines for postnatal stabilization support a bundle of interventions to avoid hypothermia, including an ambient temperature of 24–26 °C and use of a radiant warmer, thermal mattresses, plastic wraps and hats.54 These interventions are effective in reducing hypothermia in trials among more mature infants.55 The use of heated humidified gas in the delivery room has also been shown to reduce hypothermia56 and may reduce alveolar and distal airway damage.57 This intervention, not currently recommended for the delivery room, deserves further research. Specific data on the optimal means for reducing hypothermia in infants ≤23 weeks are limited; however, an exploratory trial has demonstrated the feasibility of studying optimal thermal management techniques in this unique population.58
Respiratory Management
Throughout history, the limit of viability has been defined, in part, by the inability to provide effective ventilation of the immature infant. However, modern technology together with improved techniques and understanding of respiratory physiology have shifted this limit. Respiratory management of 22–23 week infants is complicated by small size of the mouth, nostrils, pharynx, larynx, and trachea in addition to physiologic immaturity. Although there are scant data in the published literature, a review of practices at the authors’ centers indicates that 2.0-mm internal diameter endotracheal tubes are often necessary for tracheal intubation of infants born at these early gestations and can be effectively used with various ventilator modalities.59 Endotracheal tubes this small are not stocked at many hospitals in the US.60 Notably, the initial depth of endotracheal tube insertion following oral intubation does not follow rules derived for older, larger infants, such as “6 + weight in kg.”61 An appropriate depth for infants ≤23 weeks may be closer to 5.5 cm to the lip.62 When used, the optimal choice of an appropriate noninvasive respiratory support interface is unclear, but should take into account the fragility of the skin, nasal septum, and respiratory mucosa, in addition to the small size of the infant’s nostrils.
Effective respiration is thought to be possible during the latter part of the canalicular period of fetal lung development (~16–25 weeks of gestation) because thin-walled terminal saccules or primordial alveoli have developed at the ends of the respiratory bronchioles and the lung tissue is adequately vascularized to facilitate gas exchange.63 Lung development is heterogeneous, with cranial segments generally maturing earlier than caudal segments, resulting in areas of the lung apparently mature enough to support gas exchange in some infants born at 22 weeks of gestation. Approaches to ventilating the 22–23 week infant attempt to adequately support the infant while minimizing lung injury. Infants born at 22–23 weeks are particularly susceptible to pulmonary interstitial emphysema and pneumothorax after birth (Figure 2).64 Although evidence to support an optimal approach to initial ventilation for infants born at ≤23 weeks is limited, initial strategies used to limit volutrauma and barotrauma at the authors’ centers include the use of first-intention high-frequency ventilation8 and less-invasive surfactant application (LISA) with early non-invasive ventilation.9,65 Notably, these strategies are not used in isolation and might best be studied as bundles: for example, an approach using LISA and initial non-invasive ventilation requires mitigation of the potential for pulmonary hemorrhage from a large left-to-right shunt through the ductus arteriosus when the pulmonary vascular resistance decreases without the control of invasive ventilation, as well as methods to decrease gastrointestinal insufflation from non-invasive ventilation that may predispose to perforation.66
Figure 2. Morbidities of Infants born Extremely Preterm in the Japanese Neonatal Research Network.
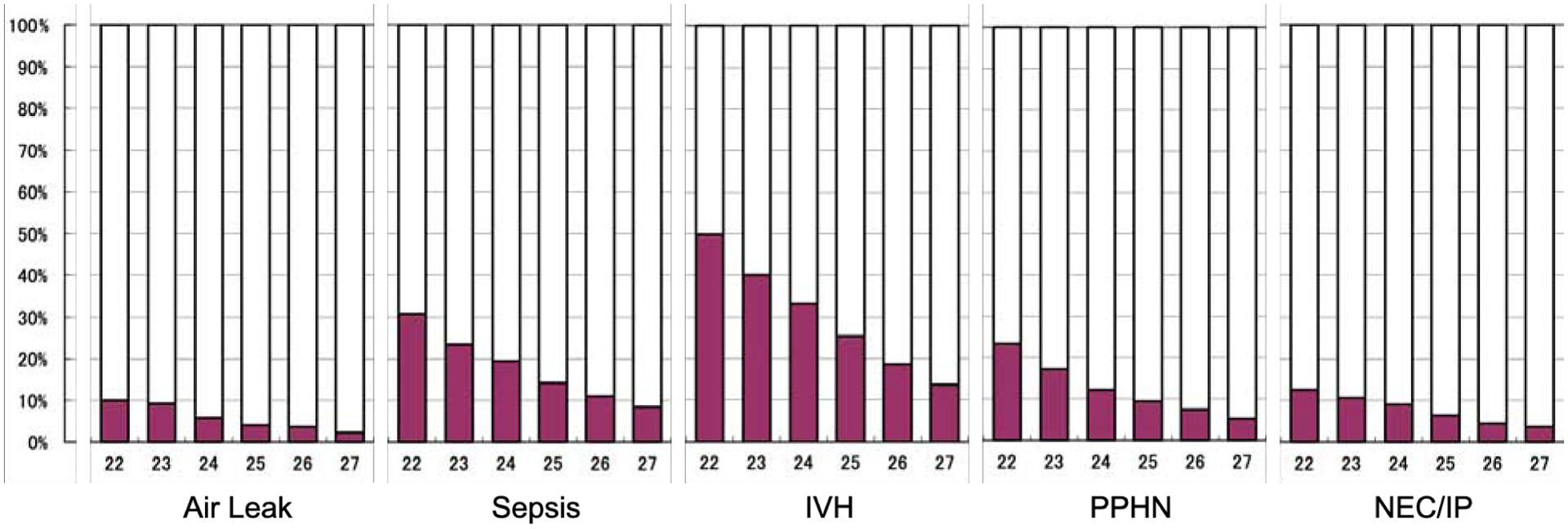
In-hospital morbidities for live born infants with extreme prematurity in the Japanese Neonatal Research Network 2003–2017 by gestational age at birth.64 Air leak includes pneumothorax and pneumomediastinum. Sepsis was defined as a positive blood culture at any time after birth. Intraventricular hemorrhage (IVH) includes all grades. Persistent pulmonary hypertension of the newborn (PPHN) was defined as a right-to-left shunt at the foramen ovale and/or ductus arteriosus, without cardiac anatomical abnormality, diagnosed by echocardiography. Necrotizing enterocolitis (NEC) was defined as Bell stage II or greater; intestinal perforation (IP) was diagnosed if free air in the abdomen for a reason besides NEC. The denominator includes all live births (n=943 at 22 weeks; n=2712 at 23 weeks; n=3764 at 24 weeks; n=4366 at 25 weeks; n=5278 at 26 weeks; n=6161 at 27 weeks).
The initial approach to ventilation should be distinguished from the approach to ventilation for infants at a later postnatal age. Early LISA and non-invasive support results in later periods of invasive ventilation for most infants at 22–23 weeks;67 likewise, conventional (“sigh”) ventilator breaths may be later incorporated into a first-intention high-frequency approach in order to maintain lung recruitment. Infants born at 22–23 weeks often require weeks or months of invasive or non-invasive respiratory support as the lungs develop. There remains much to be learned about the best methods of providing respiratory support throughout the duration of this clinical course in order to ensure optimal infant development, avoid pulmonary and orofacial injury, minimize pain and discomfort, and promote the normal processes of pulmonary development that continue into childhood. Measures of the efficacy and safety of ventilatory intervention in this age group, such as standard definitions of bronchopulmonary dysplasia, which were not developed for infants born this early in gestation, deserve further attention.
Cardiovascular Support
Aspects of immature cardiovascular physiology affecting the management of infants born at 22–23 weeks include an underdeveloped contractile machinery with disorganized myofibrils, an immature calcium handling system, and inadequately compliant collagen.68,69 These factors predispose the immature heart to diastolic dysfunction, poor tolerance to increased afterload, and an inability to cope with states of reduced preload.70–72 Moreover, animal models demonstrate that adrenergic receptor profiles and the enzyme systems that modify catecholamine response change throughout fetal development; the impact of these changes on clinical management of infants with extreme prematurity is not well understood.73–75
Glucocorticoid production by the immature adrenal cortex also affects cardiovascular performance. The adrenal grand grows throughout fetal life due to the combined actions of adrenocorticotrophic hormone (ACTH) and constant supply of progesterone from the placenta. After birth, the adrenal gland loses approximately 50% of its weight, primarily due to the atrophy associated with a rapid decrease in ACTH concentration.76 Immature function of 3-beta-hydroxysteroid dehydrogenase in infants with extreme prematurity may limit the ability of these infants to produce adequate cortisol once the transplacental supply of both cortisol and progesterone are terminated.77 For these reasons, more immature infants may be at increased risk of adrenal gland-related cardiovascular compromise, potentially explaining the increased efficacy of routine hydrocortisone to decrease mortality and neurodevelopmental impairment in clinical trials among more immature infants versus more mature ones.78,79 The duration of this potential susceptibility is not well known; Japanese neonatologists describe “late-onset circulatory collapse” treated with hydrocortisone in nearly 1 in 4 infants born at 22–23 weeks of gestation,80 although this condition is not well described in medical literature outside of Asia.81
Due to its lack of vasa vasorum, thinner medial wall muscular layer, and fewer intimal convolutions, the ductus arteriosus may be less likely to spontaneously close the earlier in gestation an infant is born.82 Persistent elevation of vasoactive substances that dilate the ductus arteriosus, such as prostaglandin E, nitric oxide, and cytokines induced by inflammation, may also contribute to delayed ductal closure.83,84 Although infants ≤23 weeks may be at risk from prolonged exposure to the hemodynamic consequences of a ductal shunt, evidence of treatment efficacy is currently limited.
The development of pulmonary vasculature is closely associated with airway development. Moreover, animal models suggest that biological mechanisms that reduce pulmonary vascular resistance after birth develop during the second and third trimesters.85 Increased pulmonary arterial resistance in infants born at ≤23 weeks of gestation may lead to impaired oxygenation, decreased right ventricular systolic performance due to elevated afterload, and decreased systemic perfusion. Data from Japan show that the incidence of pulmonary hypertension after birth is inversely associated with gestational age, with more than 1 in 5 infants born at 22 weeks affected (Figure 2).86 The use of selective pulmonary vasodilators such as inhaled nitric oxide for the treatment of acute pulmonary hypertension in this age group is controversial, although is recommended in Japan.87
Due to the high risk of derangements in hemodynamic function (intravascular volume depletion, impaired heart function, patent ductus arteriosus physiology as pulmonary vascular resistance falls), early screening echocardiography (between 12–24 postnatal hours) is often used to inform practice at the authors’ institutions. Longitudinal echocardiography may also be useful to guide the appraisal of response to intervention and to assist weaning of those interventions when no longer needed. Due to the fragility of this patient population, echocardiography evaluations should be performed by highly skilled sonographers who are able to perform a comprehensive and complete assessment (documentation of normal anatomy, assessment of heart function, assessment of pulmonary and systemic hemodynamics, assessment of atrial or ductal shunts) efficiently (e.g., within 15–20 minutes).
Fluids, Nutrition, Skin, and Kidneys
Several characteristics of infants born at ≤23 weeks that have a relatively large importance in their management may be easily overlooked: 1) a total body water content of close to 90% with the relative excess held mostly in the extracellular fluid compartment;88,89 2) an epidermis consisting of only 3–4 cell layers covered by a thin (a few microns) stratum corneum;90 3) a low glomerular filtration rate in combination with renal tubules and collecting ducts that are fewer, functionally immature, and under weak hormonal control, resulting in a limited ability for sodium and water handling and significant risk of acute kidney injury.91,92 For these reasons, infants born at ≤23 weeks of gestation require larger fluid intake volumes in early postnatal life compared with more mature infants.93
The infant born at ≤23-week has high insensible water loss (IWL) when expressed per kg, in part due to the high surface-area-to-mass ratio and the thin barrier to evaporation. Because insensible water loss from the skin and respiratory tract is inversely proportional to the relative humidity close to the air-tissue surface, the care environment has a dramatic impact on IWL and resultant fluid intake needs.92 Although respiratory water loss will be negligible during mechanical ventilation using heated humidified gas, ambient humidity is low under a radiant warmer, resulting in an IWL several-fold higher than what would be the case during care in a highly humidified incubator. By extrapolating from available data, IWL can be estimated to range from 50 mL/kg/day in a maximally humidified incubator (~90% relative humidity) to approximately 170 mL/kg/day during care under a radiant warmer with the use of plastic wrap to promote a humid microenvironment (~40% relative humidity). Depending on the individual urinary output, this huge difference in IWL would translate to an estimated initial fluid requirement ranging from ~100 to ~300 mL/kg/day.94 A mismatch between fluid prescription and care environment could thus easily contribute to rapid dehydration and hypovolemia or, conversely, to fluid overload. Given the difficulty of estimating IWL directly, as well as the immature renal filtration of these infants, clinicians at the authors’ centers pay detailed attention several times daily during early transition to markers of hydration and perfusion, including measurement of serum or whole blood sodium and lactate, urine output, daily weight, or echocardiography indices of adequate preload.
Postnatal exposure to a relatively dry environment induces rapid epidermal proliferation and barrier formation in infants with physiologic immaturity. This results in a gradually reduced cutaneous IWL, and, by one week of age, the fluid lost from the skin is reduced by ~50%.95 However, the clinical relevance of this finding and the optimal trade-off between the skin barrier-promoting benefit of the low-humidity environment and the management of high fluid losses has not been established. Prior to keratinization and thickening, the thin skin barrier of the infants ≤23 is easily compromised by routine care that would pose no problem to the robust skin of a more mature neonate. In particular, stripping of the epidermis by removal of tape or other adhesives is associated with severe barrier disruption and may predispose to fungal or bacterial infection. Although infants at 22–23 weeks have not been subjected to randomized trials of skin care protocols, there is considerable experience to support applying a cautious approach to their skin care, particularly during the first weeks of life, avoiding as much as possible the application of tape and adhesives as well as potentially toxic substances with potential for absorption. The safety of common cutaneous NICU exposures and methods to maintain skin integrity deserve further attention.
Maturation of the most immature infants is only possible if receiving the nutritional building blocks necessary to create lean body, fat, and bone mass and provide the cofactors necessary for appropriate biochemical function. Little has been published regarding the unique nutritional challenges of infants with gestational ages of ≤23 weeks. Using published data regarding the changes in body composition of the fetus over the course of pregnancy, it is possible to identify several nutrients, in addition to water, that are of particular importance for these infants, including protein, phosphorus, and sodium.96 At 22–23 weeks of gestation, the fetus consists predominantly of water and lean body mass with minimal fat mass. As the fetus matures into the third trimester, the rate of lean body mass accretion declines while mineral needs for bone accretion increase, resulting in an increase in the required calcium-to-phosphorus ratio. Although sodium accretion over the course of gestation is relatively steady, immature renal function has been associated with increased sodium requirements especially after postnatal diuresis has been completed and the infant begins to demonstrate anabolism.97 The unique enteral and parenteral nutritional needs of infants born ≤23 weeks require further research.
Protection of the Immature Brain
The brains of infants born at 22–23 weeks of gestation are more immature than those of infants born later, both anatomically and physiologically. There are few gyri visible on the surface of the cerebral cortex, the capillary network of the germinal matrix is very fragile, and there is limited capacity for autoregulation of cerebral blood flow. As a result of these latter factors, infants born at 22–23 weeks appear several-fold more vulnerable to IVH than those born just weeks later (Figure 2).64 Infants born at 22–23 weeks are also at increased risk of adverse neurodevelopmental outcomes. Approximately 1 in 3 survivors at 22 weeks and 1 in 4 survivors at 23 weeks are estimated to have severe cognitive delays, severe cerebral palsy, blindness, or deafness at 2 or 3 years of age. By comparison, the same cohort studies identified these conditions at follow-up in 1 of 10 survivors at 26 weeks.44 In Japan, among 454 infants born at 22 weeks and 1230 at 23 weeks, despite improvements in mortality, visual impairments, and cerebral palsy from 2003–2012, the proportion of survivors at 22–23 weeks affected by cognitive delay at 3 years did not improve.7 Much remains to be learned about the neurodevelopmental needs of infants born so early during gestation, including the management of pain, provision of skin-to-skin care, and use of other developmental supports.
Limiting Risks of Infection and Necrotizing Enterocolitis
Infants at 22–23 weeks are at higher risk of sepsis and NEC compared with more mature infants, even those at 24–25 weeks of gestation (Figure 2).64 Together with respiratory failure and “immaturity” (which, together, accounted for over half of deaths at 22–23 weeks in some US centers98), sepsis and NEC are common causes of mortality. Rates of invasive fungal infection are also much higher in this population, although optimal prevention strategies remain to be elucidated.99 Based on the authors’ experiences, measures such as human milk feeding,100 probiotics,101 and attention to meconium passage and feeding tolerance102 may mitigate risks of adverse gastrointestinal sequelae, although few infants born at ≤23 weeks were included in the studies that support these interventions.
Outcomes after Birth at ≤23 weeks
Available data suggest that reasons for premature birth may differ by gestational age, such that births of infants at ≤23 weeks are more likely due to chorioamnionitis and less likely due to hypertensive diseases of pregnancy than births later in gestation, possibly influencing infant outcomes.64 Many births at ≤23 weeks result in the fetus dying during labor (intrapartum stillbirth) rather than being born alive. Rates of intrapartum stillbirth at ≤23 weeks vary substantially around the world.103
In the NICU, infants born at ≤23 weeks have higher rates of air leak syndrome, pulmonary hypertension, intraventricular hemorrhage, sepsis, and bowel perforation than infants born just weeks more mature, highlighting the innate vulnerabilities of this population and their distinction from other infants born extremely preterm (Figure 2).64
Based on linked birth certificate and death certificate data from the US Centers for Disease Control as well as data from US Vermont Oxford Network hospitals (Figure 1), between 150 and 250 infants born at 22 weeks of gestation survive in the US each year, and up to 5 times as many survive at 23 weeks of gestation.18 Beyond the early-life neurodevelopmental outcomes noted previously, infants born at ≤23 weeks are at high risk for altered function of other organ systems later in life. However, given the recency of survival after birth at ≤23 weeks in many developed countries, the long-term implications of birth this early in gestation on cardiovascular, pulmonary, neurologic, renal, bone and other diseases of adulthood remain poorly defined.104 Further research on long-term outcomes of survival is critical to improving care throughout the lifespan.
Future Directions
When provided for infants born at ≤23 weeks of gestation, most interventions of intensive care are not currently supported by research. In a systematic review of randomized controlled trials published in 2010–2019 of interventions for infants with extreme prematurity, among 16,287 trial participants, 203 (1.2%) were identifiable as having been born at ≤23 weeks of gestation (n=7 at 22 weeks; n=196 at 23 weeks).105 As shown here, evidence to support clinical management of infants ≤23 weeks of gestation is generally comprised of observational studies, extrapolation from knowledge of physiology, and “adjacent evidence” from research on infants of more mature gestational ages. Although clinical experience has led to improved outcomes for infants born at ≤23 weeks of gestation at the authors’ centers over time, there exist many differences between the centers’ approaches. Much remains to be learned about how to best care for pediatrics’ youngest patients.
Challenges to the study and care of infants born at 22–23 weeks include that: their numbers are often small at any single hospital; their birth is often unplanned and urgent; and their inpatient care is often prolonged. Infants born at 22–23 weeks often require 5 or more months of in-hospital care (a period approximately as long as their duration of in utero gestation) before reaching maturity adequate for discharge, with many events and decisions during their inpatient course impacting their clinical needs and outcomes. Despite the lack of evidence to support many aspects of clinical care, the authors agree that several key factors need to be taken into account at centers considering providing intensive care for infants born at 22–23 weeks (Table II).
Table 2.
Considerations for clinicians offering intensive care for infants ≤ 23 weeks
| How do neonatologists and obstetricians collaborate in the care of the maternal-child dyad? Are decisions about antenatal corticosteroids and c-section considered separately? |
| How do healthcare providers communicate and collaborate with parents and caregivers? Are mechanisms for shared decision-making and ongoing communication in place? |
| Is appropriately sized equipment available for respiratory support and intravenous access? |
| Do all team members in the neonatal intensive care unit (e.g.,physicians, nurse practitioners, nurses, respiratory therapists, nutritionists, and others) agree that intensive care for such immature infants is not futile? |
| Do clinicians recognize the unique physiological challenges and vulnerabilities of infants ≤23 weeks—that they are not just smaller preterm infants? |
| Is multidisciplinary long-term follow-up in place to provide support for these vulnerable patients after they are discharged from the hospital? |
| Are outcomes tracked, benchmarked, and monitored to identify areas for improvement in this nascent area of practice? |
Areas for funding agencies and research institutions to prioritize include prospective registries to better understand outcomes and natural histories as well as collaborative physiology-driven comparative effectiveness research to identify best practices. Analysis of clinical trial data to consider whether there is differential effect from interventions by gestational age should also be considered. The effect of interventions may change as the physiology of the patient changes throughout development: for example, approaches to feeding and thermal management used at 36 weeks may not apply to infants born at 32 weeks; similarly, the effect of interventions at 26 weeks may be different at 22 weeks. Improved reporting practices have been recommended for outcomes of infants born at extremely preterm gestational ages, which may assist in developing better evidence.106 Where reasonable evidence is available, such as regarding the effect of antenatal corticosteroids on reducing mortality at 22 and 23 weeks,37–39 development of consistent medical guidelines43 may save lives and reduce morbidity.36 Moreover, basic and translational research is needed to understand the unique physiology of infants born at ≤23 weeks. These approaches may elucidate better ways to care for this novel group of patients and improve outcomes for them, their families, and society at large.
Conclusions
The provision of intensive care for infants born at 22 and 23 weeks has become commonplace in some hospitals and countries in recent years. Several hospitals around the world now report high rates of survival (>50%) as early as 22 weeks of gestation, but data on long-term outcomes are limited. Although the unique clinical needs of infants born at ≤23 weeks should be distinguished from those of more mature infants, few high-quality sources of clinical evidence exist to guide care for this population, which requires specialized attention. Further research is needed to improve understanding of how to best care for infants born at these early gestations.
Acknowledgements
We are grateful to our colleagues and the families and patients we care for, who informed the content of this manuscript. We thank Drs Yumi Kono, Hidehiko Nakanishi, Tetsuya Isayama, and Christoph Bührer for their thoughtful reviews of the manuscript. We also thank Drs Jeffrey Horbar and Erika Edwards of the Vermont Oxford Network, as well as members of the Japanese Neonatal Research Network, for providing data included in this paper. Vermont Oxford Network and the Japanese Neonatal Research Network played no role in the study design, conduct, analysis, interpretation, or reporting. The views, conclusions, and opinions expressed are solely those of the authors and do not represent those of either network.
M.R. is supported by Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health Award Number F32HD098782. The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schaffer A, Avery ME. Diseases of the Newborn. Philadelphia, PA: Saunders; 1971:23. [Google Scholar]
- 2.Backes CH, Rivera BK, Pavlek L, Beer LJ, Ball MK, Zettler ET, et al. Proactive neonatal treatment at 22 weeks of gestation: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021;224:158–74. [DOI] [PubMed] [Google Scholar]
- 3.Rieder TN. Saving or creating: which are we doing when we resuscitate extremely preterm infants? Am J Bioeth. 2017;17:4–12. [DOI] [PubMed] [Google Scholar]
- 4.Beam AL, Fried I, Palmer N, Agniel D, Brat G, Fox K, et al. Estimates of healthcare spending for preterm and low-birthweight infants in a commercially insured population: 2008–2016. J Perinatol. 2020;40:1091–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hendriks M, Lantos JD. Fragile lives with fragile rights: Justice for babies born at the limit of viability. Bioethics. 2018;32:205–14. [DOI] [PubMed] [Google Scholar]
- 6.Wilkinson D, Petrou S, Savulescu J. Rationing potentially inappropriate treatment in newborn intensive care in developed countries. Sem Fet Neonatal Med. 2018;23:52–8. [DOI] [PubMed] [Google Scholar]
- 7.Kono Y, Yonemoto N, Nakanishi H, Kusuda S, Fujimura M. Changes in survival and neurodevelopmental outcomes of infants born at <25 weeks’ gestation: a retrospective observational study in tertiary centres in Japan. BMJ Paediatr Open. 2018;2:e000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watkins PL, Dagle JM, Bell EF, Colaizy TT. Outcomes at 18 to 22 months of corrected age for infants born at 22 to 25 weeks of gestation in a center practicing active management. J Pediatr. 2020;217:52–58.e1. [DOI] [PubMed] [Google Scholar]
- 9.Mehler K, Oberthuer A, Keller T, Becker I, Valter M, Roth B, Kribs A. Survival among infants born at 22 or 23 weeks’ gestation following active prenatal and postnatal care. JAMA Pediatr. 2016;170:671–7. [DOI] [PubMed] [Google Scholar]
- 10.Backes CH, Söderström F, Ågren J, Sindelar R, Bartlett CW, Rivera BK, et al. Outcomes following a comprehensive versus a selective approach for infants born at 22 weeks of gestation. J Perinatol. 2019;39:39–47. [DOI] [PubMed] [Google Scholar]
- 11.Söderström F, Normann E, Jonsson M, Ågren J. Outcomes of a uniformly active approach to infants born at 22–24 weeks of gestation. Arch Dis Child Fetal Neonatal Ed. 2020; Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Rysavy MA, Horbar JD, Bell EF, Li L, Greenberg LT, Tyson JE, et al. Assessment of an updated Neonatal Research Network Extremely Preterm Birth Outcome Model in the Vermont Oxford Network. JAMA Pediatr. 2020;174:e196294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad KA, Frey CS, Fierro MA, Kenton AB, Placencia FX. Two-year neurodevelopmental outcome of an infant born at 21 weeks’ 4 days’ gestation. Pediatrics. 2017;140:e20170103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel RM, Rysavy MA, Bell EF, Tyson JE. Survival of infants born at periviable gestational ages. Clin Perinatol. 2017;44:287–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rysavy MA, Li L, Bell EF, Das A, Hintz SR, Stoll BJ, et al. Between-hospital variation in treatment and outcomes in extremely preterm infants. N Engl J Med. 2015;372:1801–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guillén Ú, Weiss EM, Munson D, Maton P, Jeffries A, Norman M, et al. Guidelines for the management of extremely premature deliveries: a systematic review. Pediatrics. 2015;136:343–50. [DOI] [PubMed] [Google Scholar]
- 17.Raju TNK, Mercer BM, Burchfield DJ, Joseph GF Jr. Periviable birth: executive summary of a joint workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, American Academy of Pediatrics, and American College of Obstetricians and Gynecologists. Obstet Gynecol. 2014;123:1083–96. [DOI] [PubMed] [Google Scholar]
- 18.United States Department of Health and Human Services (US DHHS), Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS), Linked Birth / Infant Death Records 2017 – 2018, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program, on CDC WONDER Online Database. Available at: http://wonder.cdc.gov/lbd-current-expanded.html [Accessed September 27, 2020].
- 19.Centers for Disease Control and Prevention (CDC). Fetal alcohol syndrome-Alaska, Arizona, Colorado, and New York, 1995–1997. MMWR Morb Mortal Wkly Rep. 2002;51:433–5. [PubMed] [Google Scholar]
- 20.Mai CT, Isenburg JL, Canfield MA, Meyer RE, Correa A, Alverson CJ, et al. National population-based estimates for major birth defects, 2010–2014. Birth Defects Res. 2019;111:1420–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishida H, Ishizuka Y. Survival rate of extremely low birthweight infants and its effect on the amendment of the Eugenic Protection Act in Japan. Acta Paediatr Jpn. 1992;34:612–6. [DOI] [PubMed] [Google Scholar]
- 22.Kono Y Neonatal Research Network of Japan (NRNJ): Prognosis of very low birth weight infants from the database. J Japan Soc Perinat Neonat Med. 2020;56:203–12 (in Japanese). Available at: https://www.jstage.jst.go.jp/article/jjspnm/56/2/56_203/_pdf [Accessed October 24, 2020]. [Google Scholar]
- 23.Isayama T The clinical management and outcomes of extremely preterm infants in Japan: past, present, and future. Transl Pediatr. 2019;8:199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norman M, Hallberg B, Abrahamsson T, Björklund LJ, Domellöf M, Farooqi A, et al. Association between year of birth and 1-year survival among extremely preterm infants in Sweden during 2004–2007 and 2014–2016. JAMA. 2019;321:1188–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Domeloff M, Pattersson K. Riktlinjer vid hotande förtidsbörd ska ge bättre och mer jämlik vård. Läkartidningen. 2017;114:1–3 (in Swedish) [PubMed] [Google Scholar]
- 26.Wilkinson D, Verhagen E, Johansson S. Thresholds for resuscitation of extremely preterm infants in the UK, Sweden, and Netherlands. Pediatrics. 2018;142;S574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lantos JD, Carter B, Garrett J. Do sociocultural factors influence periviability counseling and treatment more than science? Lessons From Scandinavia. Pediatrics. 2018;142;S600. [DOI] [PubMed] [Google Scholar]
- 28.Cummings J, American Academy of Pediatrics Committee on Fetus and Newborn. Antenatal counseling regarding resuscitation and intensive care before 25 weeks of gestation. Pediatrics. 2015;136:588–95. [DOI] [PubMed] [Google Scholar]
- 29.Boghossian NS, Geraci M, Edwards EM, Ehret DEY, Saade GR, Horbar JD. Regional and racial–ethnic differences in perinatal interventions among periviable births. Obstet Gynecol. 2020;135:885–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Humberg A, Härtel C, Rausch TK, Stichtenoth G, Jung P, Wieg C, et al. Active perinatal care of preterm infants in the German Neonatal Network. Arch Dis Child Fetal Neonatal Ed. 2019;0:F1–6. [DOI] [PubMed] [Google Scholar]
- 31.Bührer C, Felderhoff-Müser U, Gembruch U, Hecher K, Kainer F, Kehl S, et al. Frühgeborene an der Grenze der Lebensfähigkeit. Z Geburtshilfe Neonatol. 2020;224:244 (in German). [DOI] [PubMed] [Google Scholar]
- 32.Puia-Dumistresco M, Younge N, Benjamin DK, Lawson K, Hume C, Hill K, et al. Medications and in-hospital outcomes in infants born at 22–24 weeks of gestation. J Perinatol. 2020;40:781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sasaki Y, Ishikawa K, Yokoi A, Ikeda T, Sengoku K, Kusuda S, et al. Short- and long-term outcomes of extremely preterm infants in japan according to outborn/inborn birth status. Pediatr Crit Care Med. 2019;20:963–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Helenius K, Longford N, Lehtonen L, Modi N, Gale C, on behalf of the Neonatal Data Analysis Unit and the United Kingdom Neonatal Collaborative. Association of early postnatal transfer and birth outside a tertiary hospital with mortality and severe brain injury in extremely preterm infants: observational cohort study with propensity score matching. BMJ. 2019;367:I5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang JL, Mara KC, Weaver AL, Clark RH, Carey WA. Outcomes of outborn extremely preterm neonates admitted to a NICU with respiratory distress. Arch Dis Child Fetal Neonatal Ed. 2020;105:33–40. [DOI] [PubMed] [Google Scholar]
- 36.Rysavy MA, Bell EF, Iams JD, Carlo WA, Li L, Mercer BM, et al. Discordance in antenatal corticosteroid use and resuscitation following extremely preterm birth. J Pediatr. 2019;208:156–62.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ehret DEY, Edwards EM, Greenberg LT, Bernstein IM, Buzas JS, Soll RF, et al. association of antenatal steroid exposure with survival among infants receiving postnatal life support at 22 to 25 weeks’ gestation. JAMA Netw Open. 2018;1:e183235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park CK, Isayama T, McDonald SD. Antenatal corticosteroid therapy before 24 weeks of gestation: a systematic review and meta-analysis. Obstet Gynecol. 2016;127:715–25 [DOI] [PubMed] [Google Scholar]
- 39.Travers CP, Clark RH, Spitzer AR, Das A, Garite TJ, Carlo WA. Exposure to any antenatal corticosteroids and outcomes in preterm infants by gestational age: prospective cohort study. BMJ. 2017;356:j1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2017;3:CD004454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chawla S, Natarajan G, Shankaran S, Pappas A, Stoll BJ, Carlo WA, et al. Association of neurodevelopmental outcomes and neonatal morbidities of extremely premature infants with differential exposure to antenatal steroids. JAMA Pediatr. 2016;170:1164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mednax Center for Research, Education, and Quality. Effectiveness of ACS in extreme preemies. Available at: https://clinicaltrials.gov/ct2/show/NCT02351310. [Accessed 3 January 2021]. [Google Scholar]
- 43.Periviable birth. Obstetric Care Consensus No. 6. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2017;130:e187–99. [DOI] [PubMed] [Google Scholar]
- 44.British Association of Perinatal Medicine. Perinatal management of extreme preterm birth before 27 weeks of gestation: a BAPM framerwork for practice. Accessed at: https://www.bapm.org/resources/80-perinatal-management-of-extreme-preterm-birth-before-27-weeks-of-gestation-2019 [Accessed October 24, 2020]. [Google Scholar]
- 45.Mercer BM. Mode of delivery for periviable birth. Semin Perinatol. 2013;37:417–21. [DOI] [PubMed] [Google Scholar]
- 46.Grabovac M, Karim JN, Isayama T, Korale Liyanage S, McDonald SD. What is the safest mode of birth for extremely preterm breech singleton infants who are actively resuscitated? A systematic review and meta-analyses. BJOG. 2018;125:652–63. [DOI] [PubMed] [Google Scholar]
- 47.Macdonald EM, Koval JJ, Natale R, Regnault T, Campbell MK. Population-based placental weight ratio distributions. Int J Pediatr. 2014;2014:291846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fogarty M, Osborn DA, Askie L, Seidler AL, Hunter K, Lui K, et al. Delayed vs early umbilical cord clamping for preterm infants: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218:1–18. [DOI] [PubMed] [Google Scholar]
- 49.Backes CH, Huang H, Iams JD, Bauer JA, Giannone PJ. Timing of umbilical cord clamping among infants born at 22 through 27 weeks’ gestation. 2016;36:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duley L, Dorling J, Pushpa-Rajah A, Oddie SJ, Yoxall CW, Schoonakker B, et al. Randomised trial of cord clamping and initial stabilization at very preterm birth. J Perinatol. Arch Dis Child Fetal Neonatal Ed 2018;103:F6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tarnow-Mordi W, Morris J, Kirby A, Robledo K, Askie L, Brown R, et al. Delayed versus immediate cord clamping in preterm infants. N Engl J Med. 2017;377:2445–55. [DOI] [PubMed] [Google Scholar]
- 52.Katheria A, Reister F, Essers J, Mendler M, Hummler H, Subramaniam A, et al. Association of umbilical cord milking vs delayed umbilical cord clamping with death or severe intraventricular hemorrhage among preterm infants. JAMA. 2019;322:1877–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller SS, Lee HC, Gould JB. Hypothermia in very low birth weight infants: distribution, risk factors and outcomes. J Perinatol. 2011;31 Suppl 1:S49–56. [DOI] [PubMed] [Google Scholar]
- 54.Perlman JM, Wyllie J, Kattwinkel J, Atkins DL, Chameides L, Goldsmith JP, et al. Part 11: Neonatal resuscitation: 2010 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2010;122:S516–38. [DOI] [PubMed] [Google Scholar]
- 55.McCall EM, Alderdice F, Halliday HL, Vohra S, Johnston L. Interventions to prevent hypothermia at birth in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2018;2:CD004210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meyer MP, Owen LS, Te Pas AB. Use of heated humidified gases for early stabilization of preterm infants: a meta-analysis. Front Pediatr. 2018;6:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sottiaux TM. Consequences of under- and over-humification. Respir Care Clin N Am. 2006;12:233–52. [DOI] [PubMed] [Google Scholar]
- 58.Reilly MC, Vohra S, Rac VE, Zayack D, Wimmer J, Vincer M, et al. Parallel exploratory RCT of polyethylene wrap for heat loss prevention in infants born at less than 24 weeks’ gestation. Neonatology. 2019;116:37–41. [DOI] [PubMed] [Google Scholar]
- 59.Neonatal intensive care manual for the infants born less than 28 weeks of gestation, 2019. Neonatal Research Network of Japan. Available from http://plaza.umin.ac.jp/nrndata/ [Accessed October 3, 2020]. [Google Scholar]
- 60.Arbour K, Lindsay E, Laventhal N, Myers P, Andrews B, Klar A, Dunbar AE. Shifting provider attitudes and institutional resources surrounding resuscitation at the limit of gestational viability. Am J Perinatol. 2020. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 61.Pereira SS, Raja E-A, Satas S. Weight-based guide overestimates endotracheal tube tip position in extremely preterm infants. Am J Perinatol. 2019;36:1498–503. [DOI] [PubMed] [Google Scholar]
- 62.Kempley ST, Moreiras JW, Petrone FL. Endotracheal tube length for neonatal intubation. Resuscitation 2008;77:369–73. [DOI] [PubMed] [Google Scholar]
- 63.Moore KL, Persaud T. The Developing Human: Clinically Oriented Embryology. 7th ed. 2002. Philadelphia: WB Saunders. [Google Scholar]
- 64.Neonatal Research Network of Japan Database. Available at: http://plaza.umin.ac.jp/nrndata/indexe.htm [Accessed September 27, 2020].
- 65.Martherus T, Oberthuer A, Dekker J, Kirchgaessner C, van Geloven N, Hooper SB, et al. Comparison of two respiratory support strategies for stabilization of very preterm infants at birth: a matched-pairs analysis. Front Pediatr. 2019;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Herting E, Härtel C, Göpel W. Less invasive surfactant administration (LISA): chances and limitations. Arch Dis Child Fetal Neonatal Ed. 2019;104:F655–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kribs A, Roll C, Göpel W, Wieg C, Groneck P, Laux R, et al. Nonintubated surfactant administration vs conventional therapy in extremely preterm infants. JAMA Pediatr. 2015;169:723–30. [DOI] [PubMed] [Google Scholar]
- 68.Bouvagnet P, Neveu S, Montoya M, Leger JJ. Development changes in the human cardiac isomyosin distribution: an immunohistochemical study using monoclonal antibodies. Circ Res. 1987;61:329–36. [DOI] [PubMed] [Google Scholar]
- 69.Austin A, Fagan DG, Mayhew TM. A stereological method for estimating the total number of ventricular myocyte nuclei in fetal and postnatal hearts. J Anat. 1995;187(Pt 3):641–7. [PMC free article] [PubMed] [Google Scholar]
- 70.Igarashi H, Shiraishi H, Endoh H, Yanagisawa M. Left ventricular contractile state in preterm infants: relation between wall stress and velocity of circumferential fiber shortening. Am Heart J. 1994;127:1336–40. [DOI] [PubMed] [Google Scholar]
- 71.Osborn DA, Evans N, Kluckow M. Left ventricular contractility in extremely premature infants in the first day and response to inotropes. Pediatr Res. 2007;61:335–40. [DOI] [PubMed] [Google Scholar]
- 72.McNamara PJ, Stewart L, Shivananda SP, Stephens D, Sehgal A. Patent ductus arteriosus ligation is associated with impaired left ventricular systolic performance in premature infants weighing less than 1000 g. J Thorac Cardiovasc Surg. 2010;140:150–7. [DOI] [PubMed] [Google Scholar]
- 73.Kojima M, Ishima T, Taniguchi N, Kimura K, Sada H, Sperelakis N. Developmental changes in beta-adrenoceptors, muscarinic cholinoceptors and Ca2+ channels in rat ventricular muscles. Br J Pharmacol. 1990;99:334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chemtob S, Guest I, Potvin W, Varma DR. Ontogeny of responses of rabbit aorta to atrial natriuretic factor and isoproterenol. Dev Phamacol Ther. 1991;16:108–15. [PubMed] [Google Scholar]
- 75.Wilburn LA, Goldsmith PC, Chang KJ, Jaffe RB. Ontogeny of enkephalin and catecholamine-synthesizing enzymes in the primate fetal adrenal medulla. Journal Clin Endocrinol Metab. 1986; 63: 974–80. [DOI] [PubMed] [Google Scholar]
- 76.Ishimoto H, Jaffe RB. Development and function of the human fetal adrenal cortex: a key component in the feto-placental unit. Endocr Rev. 2011;32:317–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Masumoto K, Kusuda S, Aoyagi H, Tamura Y, Obonai T, Yamasaki C, et al. Comparison of serum cortisol concentrations in preterm infants with or without late-onset circulatory collapse due to adrenal insufficiency of prematurity. Pediatr Res. 2008;63:686–90. [DOI] [PubMed] [Google Scholar]
- 78.Onland W, Cools F, Kroon A, Rademaker K, Merkus MP, Dijk PH, et al. Effect of hydrocortisone therapy initiated 7 to 14 days after birth on mortality or bronchopulmonary dysplasia among very preterm infants receiving mechanical ventilation. JAMA. 2019;321:354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baud O, Trousson C, Biran V, Leroy E, Mohamed D, Alberti C. Two-year neurodevelopmental outcomes of extremely preterm infants treated with early hydrocortisone: treatment effect according to gestational age at birth. Arch Dis Child Fetal Neonatal Ed. 2019;104:F30–5. [DOI] [PubMed] [Google Scholar]
- 80.Kawai M Late-onset circulatory collapse of prematurity. Pediatr Int. 2017;59:391–6. [DOI] [PubMed] [Google Scholar]
- 81.Marinelli KC, Lyden ER, Peeples ES. Clinical risk factors for the development of late-onset circulatory collapse in premature infants. Pediatr Res. 2020. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 82.Kluckow M, Evans N. Low systemic blood flow in the preterm infant. Semin Neonatol. 2001;6:75–84. [DOI] [PubMed] [Google Scholar]
- 83.Hundscheid T, van den Broek M, van der Lee R, de Boode WP. Understanding the pathobiology in patent ductus arteriosus in prematurity-beyond prostaglandins and oxygen. Pediatr Res. 2019;86:28–38. [DOI] [PubMed] [Google Scholar]
- 84.Olsson KW, Larsson A, Jonzon A, Sindelar R. Exploration of potential biochemical markers for persistence of patent ductus arteriosus in preterm infants at 22–27 weeks’ gestation. Pediatr Res. 2019;86:333–8. [DOI] [PubMed] [Google Scholar]
- 85.Shaul PW. Ontogeny of nitric oxide in the pulmonary vasculature. Semin Perinatol. 1997;21:381–92. [DOI] [PubMed] [Google Scholar]
- 86.Nakanishi H, Suenaga H, Uchiyama A, Kusuda S. Persistent pulmonary hypertension of the newborn in extremely preterm infants: a Japanese cohort study. Arch Dis Child Fetal Neonatal Ed. 2018;103:F554–61. [DOI] [PubMed] [Google Scholar]
- 87.Shiraishi J, Kusuda S, Cho K, Nakao A, Hiroma T, Sugiura H, et al. Standardization of nitric oxide inhalation in extremely preterm infants in Japan. Pediatr Int. 2019;61:152–7. [DOI] [PubMed] [Google Scholar]
- 88.Friis-Hansen B Water distribution in the foetus and newborn infant. Acta Paediatr. 1983;305:7–11. [DOI] [PubMed] [Google Scholar]
- 89.Hartnoll G, Bétrémieux P, Modi N. Body water content of extremely preterm infants at birth. Arch Dis Child Fetal Neonatal Ed. 2000;83:56–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chiou YB, Blume-Peytavi U. Stratum corneum maturation. A review of neonatal skin function. Skin Pharmacol Physiol. 2004;17:57–66. [DOI] [PubMed] [Google Scholar]
- 91.Jetton JG, Boohaker LJ, Sethi SK, Wazir S, Rohatgi S, Soranno DE, et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health. 2017;1:184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carmody JB, Swanson JR, Rhone ET, Charlton JR. Recognition and reporting of AKI in very low birth weight infants. Clin J Am Soc Nephrol. 2014;9:2036–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wada M, Kusuda S, Takahashi N, Nishida H. Fluid and electrolyte balance in extremely preterm infants <24 weeks of gestation in the first week of life. Pediatr Int. 2008;50:331–6. [DOI] [PubMed] [Google Scholar]
- 94.Costarino AT, Baumgart S. Modern fluid and electrolyte management of the critically ill premature infant. Pediatr Clin North Am. 1986;33:153–78. [DOI] [PubMed] [Google Scholar]
- 95.Ågren J, Sjörs G, Sedin G. Ambient humidity influences the rate of skin barrier maturation in extremely preterm infants. J Pediatr. 2006;148:613–7. [DOI] [PubMed] [Google Scholar]
- 96.Widdowson EM, Spray CM. Chemical development in utero. Arch Dis Child. 1951;26:205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Segar JL. Renal adaptive changes and sodium handling in the fetal-to-newborn transition. Semin Fetal Neonatal Med. 2017;22:76–82. [DOI] [PubMed] [Google Scholar]
- 98.Patel RM, Kandefer S, Walsh MC, Bell EF, Carlo WA, Laptook AR, et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med. 2015;372:331–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Benjamin DK, Stoll BJ, Fanaroff AA, McDonald SA, Oh W, Higgins RD, et al. Neonatal candidiasis among extremely low birth weight infants. Pediatrics. 2006;117;84. [DOI] [PubMed] [Google Scholar]
- 100.Quigley M, Embleton ND, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2019;7:CD002971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sharif S, Meader N, Oddie SJ, Rojas-Reyes MX, McGuire W. Probiotics to prevent necrotising enterocolitis in very preterm or very low birth weight infants. Cochrane Database Syst Rev. 2020;10:CD005496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Siddiqui MMF, Drewett M, Burge DM. Meconium obstruction of prematurity. Arch Dis Child Fetal Neonatal Ed. 2012;97:F147–50. [DOI] [PubMed] [Google Scholar]
- 103.Smith LK, Morisaki N, Morken N-H, Gissler M, Deb-Rinker P, Rouleau J, et al. An international comparison of death classification at 22 to 25 weeks’ gestational age. Pediatrics. 2018;142:e20173324. [DOI] [PubMed] [Google Scholar]
- 104.Raju TNK, Buist AS, Blaisdell CJ, Moxey-Mims M, Saigal S. Adults born preterm: a review of general health and system-specific outcomes. Acta Pediatr. 2017;106:1409–37. [DOI] [PubMed] [Google Scholar]
- 105.Pavlek LR, Rivera BK, Smith CV, Randle J, Hanlon C, Small K, et al. Eligibility criteria and representativeness of randomized clinical trials that include extremely premature infants. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rysavy MA, Marlow N, Doyle LW, Tyson JE, Serenius F, Iams JD, et al. Reporting outcomes of extremely preterm births. Pediatrics. 2016;138:e20160689. [DOI] [PubMed] [Google Scholar]


