Abstract
Purpose.
Biventricular repair of double outlet right ventricle (DORV) necessitates the creation of a complex intracardiac baffle. Creation of the optimal baffle design and placement thereof can be challenging to conceptualize, even with 2-dimensional and 3-dimensional images. We describe a newly developed methodology for creating virtual baffles to inform intraoperative repair.
Description.
Three heart models of DORV were created from cardiac magnetic resonance (CMR) images. Baffles were created and visualized using a custom software.
Evaluation.
We demonstrate application of the novel tool to virtual planning of the baffle for repair of DORV in 3 cases. Models were examined by a multidisciplinary team, on screen and in virtual reality. Baffles could be rapidly created and revised to facilitate planning of the surgical procedure.
Conclusions.
Virtual modeling of the baffle pathway utilizing CMR, creation of physical templates for the baffle, and visualization in virtual reality are feasible and may be beneficial for preoperative planning of complex biventricular repairs in DORV. Further work is needed to demonstrate clinical benefit or improvement in outcomes.
Keywords: Valve replacement, 3D echocardiography, Congenital heart disease
Introduction
Double outlet right ventricle is a complex form of congenital heart disease characterized by both great arteries primarily arising from the right ventricle and a large ventricular septal defect (VSD). When two fully formed ventricles are present, the ideal management strategy is to create a biventricular circulation by creating a pathway from the left ventricle to the native or neo-aortic valve through the VSD using a baffle patch. Preoperative imaging is a critical tool for assessing the feasibility of a biventricular repair and for envisioning the potential baffle pathway. 2-dimensional (2D) echocardiography is routinely utilized, but relies on reader’s ability to reconstruct complex anatomy in 3-dimensions (3D) from 2D images. Cardiac magnetic resonance imaging (CMR) is often used to provide clear images of the entire heart, but is traditionally viewed as a series of 2D slices which similarly rely upon the user to reconstruct the critical internal 3D anatomy. This limitation has led to the exploration of methods to more intuitively comprehend the latent 3D information in CMR images by creating virtual and 3D printed models.
Segmentation and 3D printing of CMR based models has been demonstrated to inform the surgical planning process for DORV.(1–3) Similarly, the segmented virtual 3D models created for 3D printing can also be visualized on screen or in virtual reality. A simulated baffle patch can be placed within these virtual heart models using general-purpose image segmentation tools, but typically requires interpretation and laborious manual segmentation by an experienced and skilled practitioner to create a complex 3D shape using multiple 2D planar views. Further, even for such a skilled practitioner, it can be challenging to precisely craft multiple baffle options in a timely fashion, or edit them in real time based on feedback from a surgeon. Finally, translating the planned optimal virtual model into an actual repair, in a flaccid heart decompressed on bypass, can be challenging.
To elucidate optimal baffle patch planning, we have developed a method for rapid creation and editing of baffle patches within segmented models of CMR images as well as the export of these virtual models to physical templates for the baffle. We demonstrate visualization of these models on screen and in virtual reality.
Technology and Technique
Patient Selection and Image Acquisition
An existing institutional database of patients who had undergone CMR and segmentation for 3D printing for the planning of surgical repair of DORV was queried and 3 representative studies were identified. The CMR studies had been performed on a 1.5 Tesla scanner (Magnetom Avento, Siemens Healthcare) using Ferumoxytol, an iron-based contrast agent. A navigated cardiac gated inversion recovery FLASH sequence was performed to assess the vascular anatomy. The institutional review board at the Children’s Hospital of Philadelphia approved this study.
MRI and 3DE Segmentation
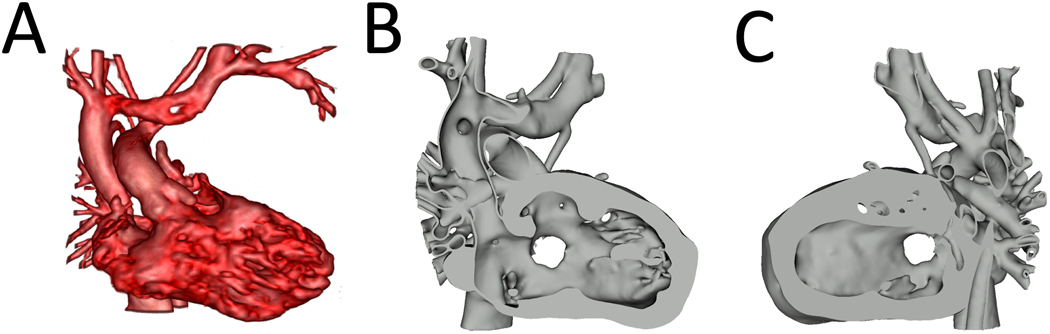
Diastolic CMR images had been previously segmented using Mimics segmentation software (Materialise, Belgium) for the purpose of 3D printing the models. Semi-automatic segmentation techniques were used to create masks and subsequent 3-D volumes of the blood pool and myocardium. The 3-D volumes were post-processed (3matic Design, Materialise, Belgium) to create hollow blood volumes with a uniform 0.75mm thickness that were then merged with the myocardial model. These existing models were imported into 3D Slicer 4.11 (www.slicer.org) in .stl format for baffle placement (Figure 1).
Figure 1. Model Visualization.
A. Volume Rendering of CMR of patient with (S, D, D) DORV, Sub-aortic VSD; B. Right ventricular view of segmented model showing VSD; C. Left ventricular view of segmented model showing VSD. CMR= Cardiac Magnetic Resonance, DORV= Double Outlet Right Ventricle, VSD=Ventricular Septal Defect.
Baffle Tool
The baffle was created in two steps using a custom code which we have implemented as two new modules in 3D Slicer: the Baffle Planner module, and the Dynamic Modeler module. Using the Baffle planner module, points were placed on the heart model to define the boundary curve of the baffle patch in the 3D view (Figure 2). These points were made to “snap” to the visible model surface facilitating accurate 3D placement. The boundary curve points were used to warp a flat disk template by thin-plate spline transform, resulting in a curved surface with its circumference points all lying on the boundary curve and having minimal bending energy as implemented in the VTK library.(4) In order to optimally visualize the relevant anatomy we created the Dynamic Modeler module (Video 1). As the boundary points for the baffle were placed, the viewpoint of the clipping plane was rotated to allow visualization and intuitive surface definition (Video 1). Once the boundary curve of the baffle had been placed, surface points were added to create the curvature typical of baffles. These points were used as additional landmark points for guiding the thin-plate spline warping transform.
Figure 2. Novel Baffle Creation Tool.
A. Placement of baffle perimeter contour points; B. Completion of perimeter contour point placement; C. Creation of baffle model from perimeter points; D. Addition of surface points to baffle model; E. Modification of baffle surface points; F. Resulting baffle modification using surface point modification.
Video 1:
Baffle Planning in (S, D, D) DORV with Sub-Aortic VSD and Pulmonary Stenosis
Visualization of Models on Screen and in Virtual Reality
Segmented models were visualized on screen in 3D Slicer (Figure 3). Optimal visualization of the baffle pathway was not always possible using traditional cropping of the model. As such, we demonstrate the application of dynamic cropping using virtual reality (Video 2). We visualized the resulting model using SlicerVR (www.slicer.org) running on a standard PC with a RTX 2080TI graphics processor and an HTC Vive Headset (HTC, Taoyan City, Taiwan).(5)
Figure 3. Creation of LV to Aorta Baffle within Heart Model.
Left: RV view and baffle placement in model of (S, D, D) DORV, Sub-aortic VSD; Middle: Mid baffle view; Right: Left ventricular view
Video 2:
Baffle Planning in (S, D, D) DORV with Sub-Aortic VSD and Prominent Conus
Export of Baffle Shape for 3D Printing and Flattening
We created a tool to export the baffle as a 3D surface mesh in .stl format, to allow for 3D printing of the baffle patch when needed. In addition, the 3D shape can be “flattened” to create a 2D template that can be printed on a standard paper printer and used as a guide for cutting out the surface patch from a flat or cylindrical fabric sheet (Figure 4). Flattening is performed with minimal distortion of the 3D shape, adapted and improved for this purpose from an existing conformal texture mapping algorithm implementation.(6)
Figure 4. Creation of Physical Models from Virtual Baffle.
Left: Virtual 3D baffle model created from baffle planning tool placed within model of (S, D, D) DORV, Sub-aortic VSD case; Top Right: 3D printed baffle model created from virtual model; Bottom Right: Virtual flattened baffle computed virtual model.
Clinical Experience
Study design
Baffles were created in pre-operative CMR based models of 3 patients for demonstration and exploration of planning (Figure 5, Videos 1, 2, and 3). Patient demographics are shown in Table 1.
Figure 5. Complex Surgical Planning Utilizing Novel Baffle Creation Tool.
Left: Apical view of Model of patient with Situs Inversus; (I, D, D) DORV, Valvar PS and Sub-Pulmonary VSD; Top Right: Model with baffle connecting left ventricle to aorta; Bottom Right: Model with baffle connecting left ventricle to pulmonary artery. See video 3 for detailed description of this model.
Video 3:
Baffle Planning in Complex Anatomy; (I, D, D) DORV with Valvar Pulmonary Stenosis and Sub-Pulmonary VSD
Table 1.
Patient Characteristics and Surgical Question for Modeling
| Native Anatomy | Age (Months) | Weight (kg) | Stage of Repair | Surgical Question |
|---|---|---|---|---|
| (S, D, D) DORV, Sub-Aortic VSD, Pulmonary Stenosis | 10 | 8.0 | S/P BT Shunt | Feasibility of Baffle Path from LV to Ao |
| (S, D, D) DORV, Sub-Aortic VSD, Prominent Conus | 7 | 7.0 | S/P PA Banding | Potential for LVOT obstruction for Baffle Path from LV to Ao pathway, and alternatives such as Baffle Path from LV to PA vs. LV to Ao and Pa (Yasui) |
| Situs Inversus; (I, D, D) DORV, Valvar PS and Sub-Pulmonary VSD | 6 | 7.3 | Unrepaired | Feasibility of Baffle Path from LV to Ao (Need Atrial Switch) vs. LV to PA vs. LV to Ao and PA (Yasui) |
DORV= Double Outlet Right Ventricle, PS= Pulmonary Stenosis, VSD= Ventricular Septal Defect, LV=Left Ventricle, Ao=Aorta, PA= Pulmonary Artery›
Results
The baffle planning tool allowed simpler and rapid interaction and refinement of baffles within 3D models compared to segmentation-based tools historically used to design baffles in 2D views. The average baffle placement time with the new tool was 2 minutes. In addition, baffles could be easily altered and refined rather than revising a significant component of the existing baffle as shown in Video 1. Baffles can be visualized on screen and in virtual reality after placement as shown in Video 2. Virtual baffle templates were successfully transformed into physical templates in the form of both 3D printed shapes and flattened baffle templates (Figure 4).
Comment
We believe this is the first freely-available, open-source dedicated software modeling workflow for the rapid creation of baffles for the planning of biventricular repair of DORV. Based on our initial clinical experience, we assert three main findings: 1) pre-operative image-based virtual modeling holds promise for improved planning of surgical repair of DORV; 2) baffle planning is faster and requires less user experience with this dedicated tool relative to segmentation and commercially available computer-aided design techniques; and 3) creation of physical templates from virtual models is a promising concept for further development and translation to clinical use.
3D modeling holds promise for the planning of cardiac interventions, but the optimal representation (e.g. 3D printed model, virtual) may vary depending on application and situation. Most image based surgical planning in DORV to date has focused on 3D printing. (1–3) 3D printing has the benefit of creating a physical replica, but requires access to a 3D printer, and rapid prototyping of different baffles is hindered by the time for fabrication. Visualization of detailed intracardiac anatomy may also be limited by the physical walls of the structure. Virtual modeling with on screen visualization has been a necessary intermediate step to 3D printing, but in some cases on screen visualization may be sufficient, or even superior to physical models. This is particularly apparent with the development of improved methods of visualization such as virtual reality. Our workflow contributes to both physical and virtual modeling by allowing more rapid creation and editing of baffles, which can become part of both virtual and physical 3D printed models.
Precision at the time of planning may be most helpful if it can be translated into an actual physical baffle at the time of repair. Intraoperatively the heart is arrested and empty. As such, the anatomy seen by the surgeon may appear different than what is visualized pre-operatively, and what will be present post-operatively. We explored the concept of how to best translate an image-derived virtual model into an actual surgical result, and demonstrate the concept of 3D templates and “flattened” baffle shapes to guide the execution of the planned surgery. However, future work is needed to explore the practical application of such techniques as well as whether they can potentially contribute to improved outcomes.
In conclusion, virtual 3D image-based preoperative modeling of baffle planning holds promise for the planning of complex repair of DORV, but will require further optimization and clinical validation. We have implemented the described functionality in the open-source SlicerHeart extension available in the 3D Slicer Extension manager. The 3D Slicer platform is available at http://www.slicer.org. Open-source code and documentation are available at https://github.com/SlicerHeart.
Acknowledgements & Disclosures
C. Pinter is an open-source software developer at Pixel Medical. All other authors declare no relevant conflicts of interest or disclosures. No commercial entity provided any direct support for work. This work was supported by a Children’s Hospital of Philadelphia (CHOP) Cardiac Center Innovation Grant, a CHOP Cardiac Center Research Grant, a CHOP Frontier Grant, NIH R01 HL153166 and T32GM008562, and by CANARIE’s Research Software Program.
References
- 1.Bhatla P, Tretter JT, Chikkabyrappa S, Chakravarti S, Mosca RS. Surgical planning for a complex double-outlet right ventricle using 3d printing. Echocardiography 2017;34(5):802–804. [DOI] [PubMed] [Google Scholar]
- 2.Farooqi KM, Gonzalez-Lengua C, Shenoy R, Sanz J, Nguyen K. Use of a three dimensional printed cardiac model to assess suitability for biventricular repair. World J Pediatr Congenit Heart Surg 2016;7(3):414–416. [DOI] [PubMed] [Google Scholar]
- 3.Valverde I, Gomez-Ciriza G, Hussain T et al. Three-dimensional printed models for surgical planning of complex congenital heart defects: An international multicentre study. Eur J Cardiothorac Surg 2017;52(6):1139–1148. [DOI] [PubMed] [Google Scholar]
- 4.Gobbi DG, Peters TM. Generalized 3d nonlinear transformations for medical imaging: An object-oriented implementation in vtk. Comput Med Imaging Graph 2003;27(4):255–265. [DOI] [PubMed] [Google Scholar]
- 5.Pinter C, Lasso A, Choueib S et al. Slicervr for medical intervention training and planning in immersive virtual reality. IEEE Transactions on Medical Robotics and Bionics 2020;2(2):108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lévy B, Petitjean S, Ray N, Maillot J. Least squares conformal maps for automatic texture atlas generation. ACM Trans Graph 2002;21(3):362–371. [Google Scholar]