Abstract
Elastic fibers are the main components of the extracellular matrix of the large arterial wall. Elastic fiber remodeling is an intricate process of synthesis and degradation of the core elastin protein and microfibrils accompanied by the assembly and disassembly of accessory proteins. Age-related morphological, structural, and functional proinflammatory remodeling within the elastic fiber has a profound effect upon the integrity, elasticity, calcification, amyloidosis, and stiffness of the large arterial wall. An age-associated increase in arterial stiffness is a major risk factor for the pathogenesis of diseases of the large arteries such as hypertensive and atherosclerotic vasculopathy. This mini review is an update on the key molecular, cellular, functional, and structural mechanisms of elastic fiber proinflammatory remodeling in large arteries with aging. Targeting structural and functional integrity of the elastic fiber may be an effective approach to impede proinflammatory arterial remodeling with advancing age.
Keywords: Aging, Large artery, Elastic fibers, Proinflammation
1. Introduction
Aging is a major risk factor for the development of cardiovascular disease due mainly to age-associated adverse arterial remodeling through proinflammation and stiffening (Gavish and Izzo, 2016; Kohn et al., 2015; Villa-Bellosta, 2020; Wang et al., 2014a; Wang et al., 2020). With advancing age, extensive extracellular matrix (ECM) remodeling, especially elastic fiber degradation and collagen deposition, are commonly observed in the large arterial wall (Wang et al., 2014a; Wang et al., 2015; Wang et al., 2020). In the arterial wall, the elastic fiber provides elasticity and resilience while collagen, especially type I, conveys tensile strength and rigidity. A decrease in the ratio of elastic and collagen fibers is an important determinant of age-associated arterial stiffening (Cuomo et al., 2019; Fhayli et al., 2019a; Halabi and Kozel, 2020; Mariko et al., 2011; Rafuse et al., 2019; Wang et al., 2014a; Wang et al., 2018a).
Elastic fiber degradation causes a decrease in or loss of arterial elasticity (Fhayli et al., 2019a; Rafuse et al., 2019). The degraded elastic fibers are susceptible to being oxidized, calcified, and can even become amyloidogenic (Basalyga et al., 2004; Davies et al., 2019; Davies et al., 2015; Gourgas et al., 2019; Ngai et al., 2018; Pai et al., 2011). Modified and degraded elastic fibers further enhance stiffness and fragility, significantly influencing the behavior of vascular smooth muscle cells (VSMCs) and endothelial cells (ECs) either directly or indirectly (Fhayli et al., 2019a; Karimi and Milewicz, 2016; Maurice et al., 2013; Pai et al., 2011; Qin, 2015; Rafuse et al., 2019).
Notably, elastic degradation in the arterial wall leads to insufficiently conferring the Windkessel effect thereby buffering the stroke volume ejected by the heart during systole and forwarding blood flow to the peripheral circulation during diastole (Humphrey et al., 2016). A common oversimplification is comparing the complicated functional and structural insufficiency of the degraded elastic fibers in the aging large arteries to a repeatedly stretched rubber band, which unavoidably loses its original recoil or elasticity over time but ignores the resultant proinflammation (Gundiah et al., 2009; Rauscher and Pomes, 2012). Notably, arterial stiffening is alleviated through the repair and prevention of the degradation of the elastic fiber with aging (Fhayli et al., 2020; Fhayli et al., 2019b; Wang et al., 2018b).
Arterial stiffening with aging plays a precipitating role in the initiation and progression of hypertension and atherosclerosis (Gavish and Izzo, 2016; Kohn et al., 2015; Villa-Bellosta, 2020; Wang et al., 2014a; Wang et al., 2015). In this mini review, we provide an update on the molecular mechanisms of the age-associated functional and structural elastic fiber remodeling and diseases such as hypertension, atherosclerosis, and aneurysm in large arteries.
2. Elastogenesis in the large arteries
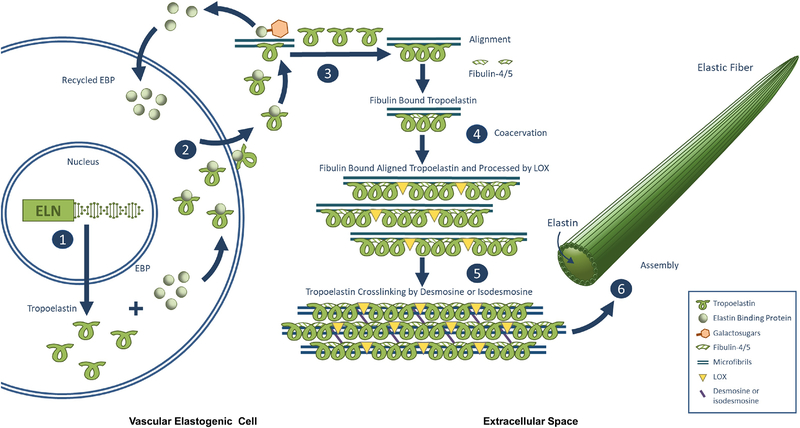
The elastic fiber network consists of ~90% elastin (ELN) and ~10% fibrillin rich microfibrils (Fhayli et al., 2020). ELN is insoluble and is composed of polymerized tropoelastin. Tropoelastin, a soluble protein, with a recyclable ELN binding protein (EBP), is secreted from vascular elastogenic cells, mainly VSMCs (Lannoy et al., 2014; Yanagisawa and Davis, 2010). EBP, a cargo/chaperone protein, has the capacity to bind to the hydrophobic repeats of tropoelastin. The release of tropoelastin from EBP due to competition by unmasking galactosugars on the microfibrils via a series of catalysis of serine carboxypeptidase A on the cell surface (Pshezhetsky and Hinek, 2009). Once separated, EBP is recycled back into the cell; and tropoelastin is then aligned, coacervated, and crosslinked through desmosine and isodesmosine by lysyl oxidases (LOX) catalysis forming polymerized tropoelastin on the microfibrils (Pshezhetsky and Hinek, 2009). Polymerized tropoelastin becomes mature insoluble ELN, which then is embedded on the microfibrils forming elastic fibers (Kothapalli and Ramamurthi, 2009; Yanagisawa and Davis, 2010). Thus, elastic fibers are composite structures, composed of a core ELN protein and an outer layer of fibrillin rich microfibrils (Fhayli et al., 2019a; Lannoy et al., 2014; Wagenseil and Mecham, 2007). In addition, a recent review suggests that potential alternative pathways exist and tropoelastin could be partially crosslinked on the cell surface or outside the cell before interacting with microfibrils and other fiber building components ELN globular protein (Kozel and Mecham, 2019).
Elastogenesis is a very complicated series of molecular events, that still not fully understood. Herein, a stepwise micro-macro-assembly of tropoelastin, microfibrils, and ELN into elastic fibers is briefly illustrated in Figure 1, based on previous reports (NivisoŽ Smith and Weiss, 2011; Pshezhetsky and Hinek, 2009; Schmelzer et al., 2020), as followings: (1) Tropoelastin is transcribed and translated from the ELN gene and is soluble and fragile. (2) Tropoelastin binds to EBP, limiting self-aggregation and degradation, and is secreted from vascular elastogenic cells; (3) Tropoelastin is released from EBP at the cell surface through competition by galactosugars in the microfibrils. Released tropoelastin is aligned, coacervated, and bound to fibulin on the microfibrils while released EBP is eventually recycled back into the cell. (4) Fibulin binds tropoelastin aggregates and stabilize them. (5) LOX on the microfibrils further processes aligned, coacervated the fibulin-stabilized tropoelastin in the extracellular space, which polymerizes tropoelastin to form mature insoluble ELN through desmosine and isodesmosine cross-links. (6) Finally, the elastic fiber is assembled, which consists of a core of ELN surrounded by microfibrils. Notably, aging increases total cell surface glycosaminoglycans (GAGs) including heparan sulfate (HS), chondroitin sulfate, and hyaluronic acid during the first 40 years of life and then decreases in the human aortic wall (Tovar et al., 1998); and tropoelastin interacts with GAG via its c-terminal-domain, i.e., HS is present inside elastic fibers and plays a role in the assembly and stability of elastin coacervates (Annovi et al., 2012; Broekelmann et al., 2005; Pshezhetsky and Hinek, 2009). In addition, EBP with protective protein/cathepsin A and neuraminidase-1 (Neu1) forms a cell surface-targeted complex, known as elastin receptor complex. Notably, Neu1 can desialylate neighboring microfibrillar glycoproteins and facilitate the deposition of insoluble elastin, which also contributes to the maintenance of neighboring cellular quiescence (Hinek et al., 2008).
Figure 1.
Step by step illustration of proposed micro-and macro-assembly of elastic fibers known as elastogenesis, referred from previous reports (NivisoŽ Smith and Weiss, 2011; Pshezhetsky and Hinek, 2009; Schmelzer et al., 2020). (1) Tropoelastin is transcribed and translated from the ELN gene and is soluble and fragile. (2) Tropoelastin binds to EBP, limiting self-aggregation and degradation, and is secreted from vascular elastogenic cells; (3) Tropoelastin is released at the cell surface through competition by galactosugars in the microfibrils. Released tropoelastin is aligned and coacervated on the microfibrils while released EBP is eventually recycled back into the cell. (4) Fibulin binds tropoelastin aggregates and stabilize them. (5) LOX on the microfibrils processes tropoelastin in the extracellular space, which polymerizes tropoelastin to form mature insoluble ELN through desmosine and isodesmosine cross-links. (6) Finally, the elastic fiber is assembled, which consists of a core of ELN surrounded by microfibrils.
The elastic fiber is composed of ELN and microfibrils. ELN appears as slender bundles of polymerized tropoelastin, storing energy, and providing, strength, elasticity, and resilience of the arterial wall. Secreted fibrillin from VSMCs is incorporated into insoluble microfibrils, under the catalysis of transglutaminase 2 (TG2), providing a scaffold for deposition of ELN (Davis and Summers, 2012). Microfibrils appear as a complex 56 nm “beads-on-a-string” structure, which is directed by the fibronectin network (Kielty et al., 2005). Microfibrils not only direct/guide elastogenesis but also provide vascular elasticity and signal molecules such as transforming growth factor-beta 1 (TGF-β1) via a series of cleavage latent TGF binding protein-1 (LTBP-1) as well as cellular signaling via integrin receptors (Davis and Summers, 2012; Halper and Kjaer, 2014; Lannoy et al., 2014).
3. Elastic fiber remodeling in the large arterial wall
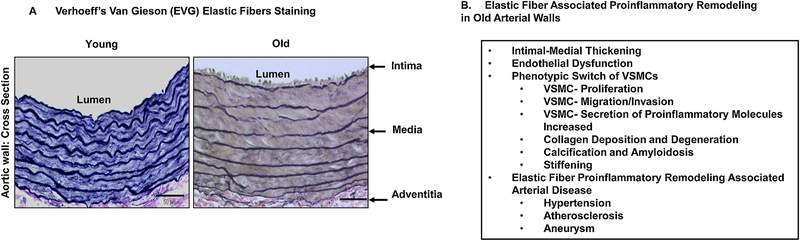
Figure 2 shows that the large arterial elastic laminae (a sheet of concentrated elastic fibers) remodeling and associated adverse cell-matrix events with advancing age. The lumen becomes dilated, the tunica intima, the tunica media, and the tunica adventitia become thickened or enlarged. The elastic laminae become thinned, eroded, fragmented, calcified, and even amyloidogenic (Atanasova et al., 2010; Wang et al., 2014a; Wang et al., 2010; Wang et al., 2015; Wang et al., 2018a; Wang et al., 2012b; Wang et al., 2018b). The VSMCs embedded in the degenerated elastic fiber networks become labile, invasive, and migratory, penetrating the subendothelial space. The invaded intimal cells proliferate and secrete large amounts of ECM and inflammatory factors such as elastase, matrix metalloproteinases, milk fat globule-epidermal growth factor-VIII (MFG-E8), leading to intimal cellularity, thickening, inflammation, stiffening, and promoting endothelial permeability to circulating plasma proteins, such as oxidized low density lipoprotein (oxLDL) and circulating monocytes (Jaffe et al., 2012; Karimi and Milewicz, 2016; Lannoy et al., 2014; Pai et al., 2011; Pustlauk et al., 2020; Rensen et al., 2007; Spofford and Chilian, 2001; Wang et al., 2014a; Wang et al., 2010; Wang et al., 2015; Wang et al., 2018a; Wang et al., 2012b; Wang et al., 2018b). This molecular, cellular, and elastic fiber remodeling is the fertile soil for the development of hypertension, atherosclerosis, and aneurysm (Ailawadi et al., 2009; Chan et al., 2017; Di Gregoli et al., 2017; Harvey et al., 2016; Jacob et al., 2001; Karimi and Milewicz, 2016; Kunecki and Nawrocka, 2001; Mackey et al., 2007; Maurice et al., 2013; Rabkin, 2017; Toma and McCaffrey, 2012; Wagenseil and Mecham, 2012; Wang et al., 2015; Zampetaki et al., 2014). Growing evidence indicates that the disassembly of elastic fibers and the degradation of ELN release elastokines, known as ELN-derived peptides (EDPs), which play a precipitating role in age-associated arterial adverse remodeling such as endothelial dysfunction, fibrosis, calcification, and amyloidosis (Antonicelli et al., 2007; Fhayli et al., 2019a; Fhayli et al., 2020; Fhayli et al., 2019b; Greenwald, 2007; Hirai et al., 2007; Robert, 1998).
Figure 2.
Elastic fiber remodeling and the aging vascular wall. A. Representative photomicrographs of EVG elastic staining in aortic walls from young (2mo, left) and old (30 mo, right) Fisher 344 cross-bred rats (FXBN), modified from previous finding (Wang and Lakatta, 2002). B. A list of adverse remodeling events in large aging arterial walls.
3.1. Tropoelastin
Age related disruption of the elastic fiber laminae is predominantly associated with the suppressed tropoelastin expression and the degradation of ELN (Fhayli et al., 2019a; Fhayli et al., 2020; Fhayli et al., 2019b; Hirai et al., 2007; Robert, 1998; Rosenbloom, 1984; Wang et al., 2018b). Both ECs and VSMCs produce tropoelastin, forming ELN. In large arteries, ELN of the elastic laminae is mainly derived from VSMC production; in small arterioles such as muscular and resistance arteries, ELN of the internal elastic lamina is mainly derived from ECs (Lin et al., 2019). Notably, once ELN has been deposited, ELN synthesis ceases and turnover is close to nil. The half-life of vascular elastin, is ~25 years in mice and ~50 years in human (Arribas et al., 2006; Davis, 1993; Sherratt, 2009).However, a program of neosynthesis of ELN can be activated under certain conditions (Fhayli et al., 2020; Fhayli et al., 2019b).
3.1.1. Transcription factors
Transcription factor specificity protein 1 (SP1) regulates the expression of the ELN gene in VSMCs (Jensen et al., 1995). SP1 contains a zinc finger that binds to the GC-rich motifs of the ELN promoter (Sen et al., 2011; Yeh et al., 1989). ELN is a target of TGF-β1 transcription and an integrated downstream molecule of its SMAD (an acronym from the fusion of Caenorhabditis elegans Sma genes and the Drosophila Mad, Mothers against decapentaplegic proteins) signaling pathway (Davidson, 2002; Jaffe et al., 2012; Sproul and Argraves, 2013). The TGF-β1 downstream SMAD molecule activates SP1, and subsequently activates the ELN gene promoter and or stabilizes ELN mRNA, leading to increases in tropoelastin and ELN formations in both VSMCs and ECs (Ellenrieder, 2008; Sproul and Argraves, 2013). Insulin-like growth factor 1 (IGF-1), like TGF-β1, increases tropoelastin production and ELN formation in elastogenic cells through the activation of SP1 (Davie et al., 2008; Li et al., 2004; Sproul and Argraves, 2013). Notably, with advancing age, intimal TGF-β1 increases in the thickened intima while medial TGF-β1 decreases in the arterial wall (Sauvage et al., 1998; Wang et al., 2006); and aging also decreases IGF-1 abundance, which is closely associated with the downregulation of ELN mRNA expression in the arterial medial wall (Foster et al., 1990).
Fos-related antigen 1 (FRA1) is a leucine zipper protein and dimerizes with oncogene c-Jun forming the transcription factor complex, AP-1. Basic fibroblast growth factor (bFGF) inhibits ELN gene expression through an enhancement of an AP-1/cAMP-response element hybrid site in the distal promoter in VSMCs (Carreras et al., 2002; Rich et al., 1999; Sproul and Argraves, 2013). Tumor necrosis factor-alpha (TNF-α) enhances AP-1 suppression of the ELN promoter and inhibits its activity, leading to decreased tropoelastin expression and ELN formation in VSMCs (Kahari et al., 1992; Spofford and Chilian, 2001).
ELN is a critical regulatory molecule that regulates the phenotypic modulation, proliferation, and migration of VSMCs. ELN expression and VSMCs proliferation are coupled inversely: potent activators such as platelet-derived growth factor-BB (PDGF-BB) of cell proliferation may potentially inhibit ELN expression and potent inhibitors of cell proliferation can stimulate ELN production (Seyama and Wachi, 2004). ELN is expressed maximally at the G0 and minimally at the G2/M phase during the cell cycle, revealing that its expression is tightly regulated by the cell growth rate (Seyama and Wachi, 2004). Notably, retinoblastoma protein (Rb) modulates the inverse relationship between cellular proliferation and elastogenesis, i.e., PDGF-BB increases cellular proliferation but decreases elastic fiber production via mitigation of phosphorylating Rb on Thr-821; IGF decreases cellular proliferation but increases ELN production via enhancement of phosphorylating RB on Thr-821 (Sen et al., 2011).
3.1.2. Post transcriptional/translational modifications
Post-transcriptional/translational modifications are predominant factors for the reduction of ELN levels in the aging arterial wall. Micro-RNA (miR), elastase, matrix metalloproteinases (MMPs) and calpain-1 either directly or indirectly modify intact ELN abundance at the post-transcriptional or post-translational levels.
3.1.2.1. miRs
A miR is a small noncoding RNA molecule that functions in RNA silencing and post-transcriptional regulation of gene expression. miRs effect tropoelastin expression and ELN formation in VSMCs and the arterial wall (Chan et al., 2017; Di Gregoli et al., 2017; Zampetaki et al., 2014). miR-29 downregulates ELN expression, prevents elastic laminae breaks, and VSMC osteoblastic differentiation in the arterial wall (Sudo et al., 2015). miR-181b decreases the expression of ELN expression and promotes elastic fiber destruction in the arterial wall (Di Gregoli et al., 2017). miR-195 reduces ELN expression in VSMCs in vitro while antagomiR-195 upregulates aortic ELN expression in vivo, and silenced miR-195 inactivates MMP-2/9 and subsequently diminishes ELN degradation (Zampetaki et al., 2014). miR-516a-5p regulates MMP-2 and TIMP-1 expressions in human VSMCs, possibly promoting the proteolytic degradation of elastin in the arterial wall (Chan et al., 2017).
3.1.2.2. Elastolysis
Proteases can degrade ELN or elastic fibers, known as elastases. The following elastases, including serine proteases leukocyte and pancreatic elastases, metallopeptidases MMP-2/−7/−9/−12–14, and cysteine proteases cathepsins and calpain-1, directly or indirectly break down elastic fibers and degrade ELN through a process known as elastolysis, that with collagen deposition, predominantly determine the mechanical molecular stiffness properties of the arterial wall with advancing age(Heinz, 2020; Raffetto and Khalil, 2008; Wang et al., 2015). Elastolysis is a very slow enzymatic catalytic process progressing over decades in the aging arterial wall (Heinz, 2020; Wang et al., 2015).
3.1.2.2.1. Serine Proteases
Elastase is a serine endopeptidase, which effectively cleaves tropoelastin, ELN, and elastic fibers in the arterial wall. Leucocyte elastase protein levels increase in aging arterial walls and serum leukocyte elastase activity is increased in old rats (Fornieri et al., 1999; Paczek et al., 2009). Leukocyte and pancreatic elastases cleave the elastic fiber/network and degrades ELN with aging (Antonicelli et al., 2007; Barolet et al., 2001; Fornieri et al., 1999; Paczek et al., 2009; Wang et al., 2015). Notably, calcium-phosphorus products accumulate and bind to degraded elastic fibers, increasing exposed ELN susceptibility to be cleaved in the adverse remodeled arterial wall (Seyama and Wachi, 2004). Importantly, EDPs further enhances the degradation of ELN and elastic fibers via the increase of elastase activity in a feed-forward manner (Hornebeck and Emonard, 2011; Maurice et al., 2013).
3.1.2.2.2. Metallopeptidases
Matrix metalloproteinase types 2/7/9/12/14 (MMP2/9/12) are extracellular proteinases and metalloelastases. They are able to bind to elastic fibers, and in turn degrade the elastic network, and subsequently increase the bioactivity and bioavailability of latent TGF binding proteins (LTBPs), latent associated protein (LAP), and TGF-β1 (Basalyga et al., 2004; Skjot-Arkil et al., 2012; Van Doren, 2015; Wang et al., 2006). Activated MMPs and its activator membrane-type of MMP (MT-MMP, MMP14) effectively cleave elastic fibers and produce EDPs (Robinet et al., 2005; Szychowski et al., 2019). Tissue inhibitors 1/2/3/4 of matrix metalloproteinase (TIMP1/2/3/4) are endogenous inhibitors of MMP activation (Szychowski et al., 2019). Thus, a balance of the MMP/TIMP ratio plays an important role in maintaining the integrity of the elastic laminae or network in the aging arterial wall (Wang et al., 2014a; Wang et al., 2014b; Wang and Khalil, 2018).
3.1.2.2.3. Cysteine proteinases
Cathepsins are cysteine proteases. Normal arteries contained little or no cathepsin K or S, in contrast, intimal smooth muscle cells (SMC), especially those appearing to traverse the internal elastic laminae and macrophages in the atheroma contained abundant immunoreactive cathepsins K and S. These enzymes to effectively degrade elastin and play an elastolytic role during arterial wall remodeling and in the stability of atherosclerotic plaques(Sukhova et al., 1998).
Calpain-1, an intracellular cysteine protease, is increased with age in VSMCs and the arterial wall (Jiang et al., 2008; Jiang et al., 2012). Calpain-1 effectively activates MMP-2 in VSMCs with advancing age (Jiang et al., 2012). The activated MMP-2 degrades elastic fibers (Jiang et al., 2012; Wang et al., 2010). In addition, calpain-1 increases alkaline phosphatase (ALP) levels in cultured VSMCs while decreases the calcification inhibitors, osteopontin and osteonectin, eventually facilitating the calcification of aging VSMCs and the arterial wall (Blewniewski et al., 2011; Jiang et al., 2012). Thus, inhibiting calpain-1 potentially delays the age-related elastic fiber damage and calcification of the arterial wall (Jiang et al., 2012).
3.1.2.3. Lysyl oxidase (LOX)
LOX is an extracellular copper-dependent enzyme that catalyzes formation of aldehydes from lysine residues in tropoelastin. The aldehydes are highly reactive and undergo spontaneous chemical reactions with other LOX-derived aldehyde residues, resulting in cross-linking tropoelastin by desmosine or isodesmosine (Gourgas et al., 2019; Kothapalli and Ramamurthi, 2009). LOX deficiency causes a defect in tropoelastin crosslinking and disrupts the elastic fibers or network assembly (Maki et al., 2002). LOX, together with tropoelastin, decreases in the aging arterial wall (Fhayli et al., 2020). Overexpressing or preserving LOX increases the yield of tropoelastin and ELN formation in VSMCs and in the arterial wall with aging (Fhayli et al., 2020; Kothapalli and Ramamurthi, 2009; Oleggini et al., 2007).
3.1.2.4. Glycosylation and Carbamylation
Aging is a progressive chronic process determined by genetic, epigenetic, and acquired factors. The chemical reactions referred to as nonenzymatic posttranslational modifications (NEPTMs) are acquired factors, such as glycosylation and carbamylation, which are responsible for molecular aging. Glycation is associated with aging and has been shown to increase arterial stiffness. Glycation directly effects the mechanical response of intact arteries and the mechanical response and structure of ELN isolated from the arteries (Stephen et al., 2014). Glycation leads to the thinning of elastic fibers and the weakening of the ELN of arteries, contributing to vascular defects in aging (Stephen et al., 2014). Thus, prevention of glycation reactions may be an important consideration for vascular health later in life.
Carbamylation is a more recently described NEPTM that is caused by the nonenzymatic binding of isocyanate derived from urea dissociation or myeloperoxidase-mediated catabolism of thiocyanate to free amino groups of proteins. This modification is considered an adverse reaction because it induces alterations of protein and cell properties. Protein carbamylation may be considered a hallmark of aging in mammalian species that may significantly contribute to the structural and functional adverse remodeling that occurs during aging (Gorisse et al., 2016). Cyanate carbamylation at the N-terminal of amino acids is observed in aortic ELN (Lefevre and Rucker, 1983). Age-related modifications including glycation and carbamylation greatly impact on ECM bioroles and their mechanical properties (Birch, 2018).
3.2. Microfibrils
Microfibrils are composed of fibrillin and are functionally affected by other accessary proteins, including fibulin and emilin. In general, these accessory proteins modify the size and configuration of ELN aggregates on the microfibrils (Yanagisawa and Davis, 2010). The aggregated ELN interacts with the microfibril framework on the cell surface through integrins, inducing cellular remodeling such as the VSMC phenotypic switch (Lannoy et al., 2014; Yanagisawa and Davis, 2010).
3.2.1. Fibrillin
Fibrillin is an essential glycoprotein for the formation of elastic fibers (Davis and Summers, 2012). Secreted fibrillin from VSMCs is incorporated into insoluble microfibrils, providing a scaffold for the deposition of aggregated ELN (Davis and Summers, 2012). Microfibrils not only fulfill the assembly of the elastic fiber but also provide tissue elasticity (Halper and Kjaer, 2014; Lannoy et al., 2014). Fibrillin includes three isoforms: fibrillin-1, −2 and −3. Fibrillin-1 and fibrillin-2 enhances the stability of elastic fibers (Davis and Summers, 2012). Fibrillin-1 also sequesters LTBP and controls the bioavailability of active TGF-β1 (Davis and Summers, 2012). Fibrillin-2 is expressed earlier and is involved in elastic fiber formation in fetal development. Fibrillin-3 is mainly expressed in the brain (Kielty et al., 2005; Kielty et al., 2002). Notably, fibrillin-1 deficient mice have substantial fragmentation and decreased number of elastic lamellae in the aortic wall with aging, which were correlated with an increase in aortic stiffness, a decrease in vasoreactivity, an altered expression of ELN, and a decrease in the relative ratio between tissue ELN and collagen (Mariko et al., 2011).
3.2.2. Fibulin
The fibulin family is a group of glycoproteins involved in elastic fiber formation (Yanagisawa and Davis, 2010). The fibulin family is composed of long fibulins (fibulin-1, −2, −6) and short fibulins (fibulin-3, −4, −5, −7). Fibulin-1 and −2 are mainly produced by VSMCs involved in formation of elastic fibers within the medial layer of the artery (de Vega et al., 2009). Fibulin-4 and −5 participate in elastic fiber assembly and affect the deposition of ELN during elastic fiber generation (Choudhury et al., 2009; Hirai et al., 2007; Yamauchi et al., 2010). Notably, fibulin-5 deficient mice display early vascular aging appearing as marked elastopathy: an increased stiffness of the proximal central arteries due largely to increased wall thickness and collagen–ELN ratio (Cuomo et al., 2019).
3.2.3. Emilin-1
ELN microfibril interface-located protein 1 (Emilin-1) is a glycoprotein that is able to anchor VSMCs to elastic fibers, maintain the quiescence of VSMCs in the normal adult arterial wall and controls the release of the cytokine TGF-β1 (Randell and Daneshtalab, 2017; Zanetti et al., 2004). Emilin-1 is able to bind to ELN and to fibulin-5 stabilizing elastic fibers (Angel et al., 2017; Zanetti et al., 2004). The decrease of Emilin-1 increases TGF-β1 fibrogenesis and inflammation in VSMCs and in the arterial wall with aging (Angel et al., 2017; Randell and Daneshtalab, 2017). Importantly, Emilin-1 deficiency induced the defects of arterial elastogenesis such as elastic fibers fragmentation (Angel et al., 2017).
4. Elastic fiber remodeling associated with other ECM restructuring
Elastic fiber remodeling occurs with the restructuring of other ECM components, such as collagen, fibronectin (FN), laminin (LM), and milk fat globule-epidermal growth factor 8 (MFG-E8) in the arterial wall with aging.
4.1. Collagen
ELN degradation and elastic fiber degeneration dramatically increase activated TGF-β1 bioavailability and release EDPs (Lannoy et al., 2014; Oleggini et al., 2007; Simionescu et al., 2005; Yamashiro and Yanagisawa, 2018) which are powerful peptides that activate TGF-β1 secreted from VSMCs (Simionescu et al., 2005). TGF-β1 signaling potentially increases collagen types I and III production in VSMCs (Wang et al., 2006). Collagen type I is the most abundant collagen in vascular walls and is associated with tensile strength, stiffening, and calcification while collagen type III has a long, inflexible, triple-helical domain, contributing to the elasticity of the vascular wall (Kohn et al., 2015; Wagenseil and Mecham, 2012). Increases in the ratios of collagen/ELN and collagen type I to III in the arterial wall dramatically increase with aging (Kochova et al., 2012; Wang and Lakatta, 2002). Notably, elastolysis often accompanies the degradation of collagen, promoting proinflammation and dramatically increases stiffness in the arterial wall (Mozos et al., 2017; Wang et al., 2020).
4.2. Fibronectin
Fibronectin (FN) is a “master organizer” in matrix assembly and is a downstream molecule of TGF-β1 signaling, which exerts pivotal roles in directing VSMC-mediating remodeling of scaffolds toward the production of a physiological-like, elastin-containing ECM with excellent mechanical properties (Pezzoli et al., 2018; Wang et al., 2006). FN connects with elastic fibers, influencing elastic fiber assembly, deposition, and integrity via the assembly of microfibrils (Pezzoli et al., 2018; Xu and Shi, 2014). With advancing age, FN expression and deposition increases while ELN content declines, elastic fibers degrades, and vascular calcification increases (Zhang et al., 2020). In addition, the functional interplay of EBP and interleukin-1 receptor regulates the production of FN in VSMCs (Hinek et al., 1996).
4.3. Laminin
Activated TGF-β1 triggers the production of laminin (LM) from VSMCs (Basson et al., 1992). LM is a major component of the basal lamina of cells. LM effects on the biosynthesis of ELN and interacts with ELN or EDPs through the transmembrane ELN–LM receptor (ELR) complex, affecting the properties of VSMCs such as proinflammation and proliferation (Kunecki and Nawrocka, 2001; Robert, 1998; Spofford and Chilian, 2001).
4.4. MFG-E8
MFG-E8 is a secreted glycoprotein, which functions as a bridge molecule to connect VSMCs to elastic fibers in the arterial wall(Wang et al., 2012a; Wang et al., 2013). The MFG-E8 fragment, medin, is an amyloidogenic protein and also binds to elastic fibers and VSMCs in the arterial wall (Degenhardt et al., 2020; Migrino et al., 2020; Wang et al., 2012a; Wang et al., 2013; Younger et al., 2020). Aging increases both MFG-E8 and medin in the arterial wall (Wang et al., 2012a; Wang et al., 2013). Both MFG-E8 and medin signaling influence the VSMC phenotypic switch and vessel function (Chiang et al., 2019; Degenhardt et al., 2020; Fu et al., 2009; Migrino et al., 2020; Younger et al., 2020). MFG-E8 increases the invasion, proliferation, and migration of VSMCs; and medin increases the necrosis and MMP-2 activation of VSMCs (Chiang et al., 2019; Fu et al., 2009; Mackey et al., 2007; Wang et al., 2012a; Wang et al., 2013). Notably, MFG-E8, medin fragments, and EDPs all are elements of aortic medial amyloidosis (Boraldi et al., 2018; Davies et al., 2019; Davies et al., 2015; Larsson et al., 2006; Peng et al., 2007).
5. Elastic fiber remodeling effects on VSMC phenotypes in the arterial wall
5.1. Proinflammation
The age-associated inflammation in the arterial wall or VSMCs is a low grade of sterile inflammation where cytokines are secreted mainly by vascular cells rather than traditional immune cells, known as proinflammation. Maintaining stable functional ELN-contractile units is a key to warranting the structural and functional integrity and health of the arterial wall (Karimi and Milewicz, 2016). The integrity of the elastic network has significant effects on VSMC behaviors (Kinnear et al., 2020; Lannoy et al., 2014; Lin et al., 2019; Pai et al., 2011; Sudo et al., 2015). In the arterial wall, VSMCs are generally categorized into contractile and synthetic phenotypes (Rensen et al., 2007). In healthy adult vascular walls, the vast majority of VSMCs are in a quiescent non-proliferative phenotype, known as contractile, while the phenotype of proliferating, invading/migrating, proinflammatory secreting VSMCs in old arterial wall have been widely referred to as synthetic (Wang et al., 2014a). Adverse ELN remodeling, such as releasing EDPs or fragment fibers, plays a permissive role in the phenotypic switching of the contractile into synthetic VSMCs (Kinnear et al., 2020). Synthetic VSMCs exert a proinflammatory role in arterial intimal medial thickening and stiffening with aging (Laurent et al., 2005; Wang et al., 2014a).
Contractile VSMCs are enriched in smooth muscle specific 22 kDa protein (SM22α) and alpha-smooth muscle actin (α-SMA). These cellular markers increase in young arterial walls while decrease in the old (Ailawadi et al., 2009; Martin-Pardillos and Sorribas, 2015). Contractile VSMCs are converted to the synthetic phenotype and are usually accompanied by a decrease in the expression of these contractile proteins and a reduction in ELN production (Ailawadi et al., 2009; Jaffe et al., 2012; Martin-Pardillos and Sorribas, 2015; Rensen et al., 2007). Collapse of the arterial elastic network facilitates the shift from contractile to synthetic phenotypes of VSMCs (Karimi and Milewicz, 2016; Lannoy et al., 2014; Wagenseil and Mecham, 2007; Yamashiro and Yanagisawa, 2018). ELN degradation is associated with synthetic VSMCs, and the increase of ELN-contractile unit damage and modulus, contributes to intimal thickening and arterial stiffening (Barolet et al., 2001; Karimi and Milewicz, 2016). Low levels of elastic fiber deposition around VSMCs coincide with an increase in the proliferation rate, which is reversed by the addition of exogenous ELN (Urban et al., 2002). VSMCs lacking ELN proliferate at a rate greater than that of the wild type cells, fail to form a mature contractile phenotype, and migrate more aggressively to an exogenous chemoattractant than that of the wild type cells. Importantly, these lacking ELN-associated phenotypic shifts in VSMCs are rescued in vitro by the addition of recombinant tropoelastin (Karnik et al., 2003; Li et al., 1998; Seyama and Wachi, 2004). The small, infiltrated synthetic VSMCs have been found in the aged, thickened intimae contain an abundance of angiotensin II and activated MMP-2 which accompanies the destruction of the elastic network, which has evolved into a fertile proinflammatory field (Wang et al., 2003).
Notably, deteriorated elastic fibers release elastokines, or EDPs, that bind to the ELN receptor and other receptors, stimulate the migration and proliferation of VSMCs, and damage ECs (Dale et al., 2016; Kawecki et al., 2014; Maurice et al., 2013; Robinet et al., 2005; Simionescu et al., 2005). In addition, EDPs are involved in thrombosis via increases in platelet aggregation and clotting signaling (Kawecki et al., 2014). These detrimental effects can be largely inhibited by genetic or pharmacological blockades of the ELN receptor complex or through the neutralization of an antibody against EDPs (Qin, 2015). EDPs are chemoattractant proteins and regulates invasion/migration and matrix metalloproteinase expression. EDPs are chemotactic for inflammatory cells such as monocytes, VSMCs, ECs, and myofibroblasts (Annovi et al., 2012; Duca et al., 2016).
5.2. Procalcification
Age-associated arterial proinflammation and profibrosis coupling is the foundation of arterial calcification (Wang et al., 2020). Ectopic micro-and macro-calcification can be detected in either the intimal or medial layers of the arterial wall (Gourgas et al., 2019; Ngai et al., 2018; Pai et al., 2011). Medial calcification appears as linear deposits of calcium along the degraded elastic fibers (Basalyga et al., 2004; Gourgas et al., 2019). The increase in elastic fiber degradation is closely associated with the accumulation of calcification (Basalyga et al., 2004; Maurice et al., 2013). Remarkably, synthetic VSMCs are susceptible to calcification, and are associated with the downregulation of ELN expression and degraded elastic fibers (Maurice et al., 2013; Sudo et al., 2015). Calcium absorbs on ELN-like peptide filaments or fibers, and then evolve upon nucleation, and finally calcium spread out to ELP-like globular structure forming calcification (Gourgas et al., 2019).
Fetuin-A, also known as alpha-2-HS (Heremans-Schmid)-glycoprotein, is a mineral carrier protein and a systemic inhibitor of pathological mineralization (Jahnen-Dechent et al., 2011; Robinson and Teran-Garcia, 2016; Villa-Bellosta, 2020). It carries insoluble calcium phosphate forming soluble complexes that inhibit calcium deposition (Villa-Bellosta, 2020). Loss of fetuin-A increases systemic soft tissue calcification in mice (Jahnen-Dechent et al., 2011; Westenfeld et al., 2009). Fetuin-A decreases with advancing age and thus promotes an age-associated increase in ectopic calcification (Jahnen-Dechent et al., 2011; Robinson and Teran-Garcia, 2016). A significant negative correlation is also observed between plasma fetuin-A concentrations and arterial calcification in humans (Westenfeld et al., 2009).
Transglutaminase-2 (TG-2) is an enzyme that in nature primarily catalyzes the formation of an isopeptide bond between γ-carboxamide groups of glutamine residue side chains and the ε-amino groups of lysine residue side chains and subsequently releases ammonia. TG-2 expression is increased in the aging arterial wall and is abundant in calcified arterial walls (Chabot et al., 2010; Johnson et al., 2008; Lexhaller et al., 2019). TG-2 secreted from VSMCs is a calcium-dependent enzyme that can crosslink most ECM proteins such as ELN and traps the calcium crystals, hydroxyapatites (Chabot et al., 2010; Johnson et al., 2008). An increase in TG-2 expression enhances the chondro-osseous differentiation of VSMCs via the activation of an osseous master Runt-related transcription factor (Runx2)/ core-binding factor subunit alph-1 (Cbf-α−1) and promotes arterial calcification and stiffening (Chabot et al., 2010; Johnson et al., 2008).
Alkaline phosphatase (ALP) is a non-tissue specific enzyme that is detected in the calcified arterial wall (Pustlauk et al., 2020; Tani et al., 2020; Villa-Bellosta, 2020). ALP is a dephosphorylating compound, which converts pyrophosphate (soluble) into phosphate (insoluble), facilitating calcium deposition. A high concentration of ALP is found in the calcified arterial wall. Specifically, aging increases ALP and promotes arterial calcification (Jiang et al., 2012; Simionescu et al., 2005; Sinha and Vyavahare, 2013; Westenfeld et al., 2009).
6. Arterial diseases associated with elastic fiber remodeling
Adverse age-associated elastic fiber remodeling has a significant impact on the pathogenesis of large arterial diseases (Ferrucci, 2008; Fhayli et al., 2019a; Halabi and Kozel, 2020; Robert, 1998; Xu and Shi, 2014).
6.1. Hypertension
Aging increases the prevalence of hypertension, accompanied by the fragmentation of the elastic laminae and the damage of the ELN-contractile unit that links the elastic fibers to the VSMCs in the aortic wall, contributing to an increase in blood pressure (Wagenseil and Mecham, 2012). Proinflammatory signals resulting from the breakdown of elastic fibers are important contributing factors, such as increases in bioactivity and bioavailability of TGF-β1, MFG-E8, medin, and EDPs in the arterial wall, increased vasoconstriction, and elevated progression of hypertension (Faury et al., 2003; Harvey et al., 2016; Wagenseil and Mecham, 2012; Wang et al., 2015). Activated TGF-β1 triggers MMPs activation, elastolysis, fibrosis and arterial stiffening, that promotes or maintains the state of hypertension (Greenwald, 2007; Harvey et al., 2016; Wagenseil and Mecham, 2012; Wang et al., 2011; Zacchigna et al., 2006). Indeed, ELN +/− mice have stable hypertension from birth, with a mean arterial pressure 25 to 30 mm Hg higher than their wildtype counterparts, and further physiologic studies indicated a role for the renin-angiotensin system in maintaining their hypertensive state (Faury et al., 2003). In contrast, the expression of the human ELN transgene reversed the hypertension and cardiovascular changes associated with ELN haploinsufficiency and rescued the perinatal lethality of the ELN-null phenotype (Hirano et al., 2007). In addition, serum levels of glycated ELN derived peptides (AGE-EDP) are increased in arterial hypertension (Wahart et al., 2019). Notably, elastolysis promotes arterial stiffening which precedes hypertension, thus suggesting stiffness is a cause of hypertensin(Humphrey et al., 2016). From a biomechanical perspective, arterial aging or hypertension in mice may not directly mimic human arterial aging or hypertension, but the mouse model which shares similar properties with human cells and extracellular matrix making the mouse a powerful model for studying human aging and hypertension. Analyzing a mouse model is invaluable in understanding the mechanisms of aging and hypertensin (Spronck et al., 2020).Taken together, the association of hypertension with ELN haploinsufficiency in humans and mice strongly suggested that ELN and other proteins of the elastic fiber should be considered as causal genes for essential hypertension.
6.2. Atherosclerosis
Aging increases the arterial elastic network degradation and collapse, which is closely associated with atherogenesis (Maurice et al., 2013; Robert, 1998; Seyama and Wachi, 2004). Aging creates a notable proinflammatory environment that drastically increases elastase and MMP activity, which effectively cleaves ELN, producing elastokine EDPs (Maurice et al., 2013). EDPs also are potent proinflammatory cues to further damage elastic fibers via facilitating oxidation, oxLDL accumulation, calcification, and infiltration of lipids and immune cells into the deep aortic wall, and eventually accelerating the progress of atherosclerotic plaques (Fulop et al., 1998; Toma and McCaffrey, 2012). Fragmented elastic fibers have a high affinity to bind to calcium-phosphorus products and lipids in the arterial wall, promoting the growth and instability of the atherosclerotic plaque (Maurice et al., 2013). Indeed, mice lacking ELN died of an obstructive arterial disease that resulted from subendothelial cell proliferation and reorganization of smooth muscle, which were similar to those seen in atherosclerosis (Li et al., 1998). In a recent study of young (6–15 years-old, n=8) and old rhesus monkeys (16–28 years-old, n=8), fed a high fat and cholesterol diet for two years revealed that aging, per se, predisposes adverse cells and matrix remodeling, including ELN degradation of the arterial wall, to develop atherosclerosis (Wang and Lakatta, 2019). A high cholesterol diet for a fixed time increased atherosclerosis to a markedly greater extent in old rather than in young monkeys. Thus, the risk of atherosclerosis is attributable to age and is not due to longer exposure to dyslipidemia but rather stems from age associated adverse remodeling of the arterial wall, i.e., the soil in which atherosclerosis flourishes (Wang and Lakatta, 2019).
6.3. Aneurysm
With advancing age, ELN is degraded, elastic fibers are degenerated, contributing to arterial inflammation, calcification, and fragility (Kunecki and Nawrocka, 2001; Lavin et al., 2020; Maki et al., 2002). Age increases elastase and MMP activation, which degrade ELN protein and degenerate elastic fibers and the synthetic shift of VSMCs during the pathogenesis of arterial aneurysm (Ailawadi et al., 2009; Antonicelli et al., 2007; Cohen et al., 1992; Lavin et al., 2020; Rabkin, 2017). Destruction of elastic fibers at sites of abdominal aortic aneurysm (AAA) leads to the accumulation of degraded monomeric soluble tropoelastin rather than the cross-linked insoluble ELN (Lavin et al., 2020). Histologic observation indicates that in aneurysm tissue, the number of VSMCs decreases while the elastic network is destroyed, leading to weakness and microcalcification of the ELN-contractile unit (Jacob et al., 2001; Kielty et al., 2005). This weakness of the ELN-contractile unit in the arterial wall is susceptible to being expanded under mechanical and metabolic stress such as hypertension and atherosclerosis forming an opportunistic site for the initiation of arterial aneurysm (Ailawadi et al., 2009; Rabkin, 2017). The ELN associated microcalcification as a novel imaging marker successfully enables monitoring of the local ELN degradation, destruction of elastic fibers and predicts aortic adverse events in aneurysm patients (Wanga et al., 2017).
Disorders of fibrillin-1, emilin-1, fibulin-5, LOX, and ELN-laminin receptor also promote inflammation and the destruction of the ELN-contractile unit and triggers the formation of arterial aneurysm (Karimi and Milewicz, 2016; Kunecki and Nawrocka, 2001). In addition, accumulating evidence indicates that EDPs actively participate in the progression of arterial aneurysm by accelerating inflammation, LDL oxidation, ELN degeneration, calcification, and stiffening of the arterial wall (Antonicelli et al., 2007; Qin, 2015; Wen et al., 2011). EDPs also promote VSMCs proinflammation in a paracrine manner, facilitating ELN degradation and elastic fiber fragmentation and eventual elastolysis in a feed-forward manner, leading to the pathogenesis of arterial aneurysm/dissection (Antonicelli et al., 2007; Qin, 2015; Wen et al., 2011). EDPs create a proinflammatory environment in the aortic wall by inducing macrophage type 1 polarization while neutralizing the EDPs attenuated aortic dilation (Dale et al., 2016.
These pathophysiological effects are mediated by the binding of EDPs on a unique heterotrimeric receptor named ELN receptor complex (ERC) (Maurice et al., 2013).
7. Concluding remarks and perspectives
ELN is a core protein of the elastic fiber. With advancing age, intact ELN protein decreases through transcription, post-transcriptional or post-translational modifications, contributing to the degradation of the arterial extracellular elastic fiber network. These defects lead to the insufficiency and collapse, accompanied by proinflammation, procalcification, amyloidosis, and eventual stiffening in the old arterial wall. Many recent studies indicate that habitual aerobic exercise, dietary calorie restriction, and a healthy lifestyle effectively preserves the integrity, elasticity, resilience of elastic fiber laminae and reduces arterial stiffening and (Alfaras et al., 2016; AlGhatrif et al., 2017; Castello et al., 2005; Seals et al., 2009; Wang et al., 2018b). Age-associated arterial structural and functional declines may not be inevitable (Fhayli et al., 2019b; Foster et al., 1990; Lefevre and Rucker, 1983; Wang et al., 2014b; Wang et al., 2012b; Wang et al., 2018b). This novel conception is robustly supported by the Baltimore Longitudinal Study of Aging (BLSA) of “successful aging in a subset of the human population” (Ferrucci, 2008). This mini review focuses on the repair/prevention of the elastic fiber, which plays a major role in age-associated adverse proinflammatory arterial remodeling, The limitation of the ‘rubber band’ and elastic fiber comparison (cumulative wear and tear over time) still plays an important role since the loss of elasticity not only decreases the Windkessel effect but also leads to age-associated arterial proinflammation (Avolio et al., 1998; Greenwald, 2007; O’Rourke and Hashimoto, 2007). Therefore, targeting the adverse proinflammatory molecular events of elastic fiber remodeling may be a potential fresh therapeutic approach to retard age-associated adverse structural and functional arterial remodeling and diseases such as hypertension, atherosclerosis, and aneurysm.
Highlights.
Elastic laminae are the main components of the extracellular matrix of large arteries.
Elastic lamina remodeling is an intricate process of synthesis and degradation of the core tropoelastin protein accompanied by the assembly and disassembly with accessory proteins.
Age related morphological, structural, and functional proinflammatory remodeling within the elastic laminae have a profound effect upon the integrity, elasticity, calcification, amyloidosis, and stiffness of the large arterial wall.
Age-associated increase in arterial elastic fiber remodeling plays an important role in the pathogenesis of large arterial diseases, e.g., hypertension, atherosclerosis, and aneury
Acknowledgments
Funding sources
This research was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Footnotes
Conflicts of interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ailawadi G, Moehle CW, Pei H, Walton SP, Yang Z, Kron IL, Lau CL, Owens GK, 2009. Smooth muscle phenotypic modulation is an early event in aortic aneurysms. J Thorac Cardiovasc Surg 138, 1392–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaras I, Di Germanio C, Bernier M, Csiszar A, Ungvari Z, Lakatta EG, de Cabo R, 2016. Pharmacological Strategies to Retard Cardiovascular Aging. Circ Res 118, 1626–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlGhatrif M, Wang M, Fedorova OV, Bagrov AY, Lakatta EG, 2017. The Pressure of Aging. Med Clin North Am 101, 81–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel PM, Narmoneva DA, Sewell-Loftin MK, Munjal C, Dupuis L, Landis BJ, Jegga A, Kern CB, Merryman WD, Baldwin HS, Bressan GM, Hinton RB, 2017. Proteomic Alterations Associated with Biomechanical Dysfunction are Early Processes in the Emilin1 Deficient Mouse Model of Aortic Valve Disease. Ann Biomed Eng 45, 2548–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annovi G, Boraldi F, Moscarelli P, Guerra D, Tiozzo R, Parma B, Sommer P, Quaglino D, 2012. Heparan sulfate affects elastin deposition in fibroblasts cultured from donors of different ages. Rejuvenation Res 15, 22–31. [DOI] [PubMed] [Google Scholar]
- Antonicelli F, Bellon G, Debelle L, Hornebeck W, 2007. Elastin-elastases and inflamm-aging. Curr Top Dev Biol 79, 99–155. [DOI] [PubMed] [Google Scholar]
- Arribas SM, Hinek A, Gonzalez MC, 2006. Elastic fibres and vascular structure in hypertension. Pharmacol Ther 111, 771–791. [DOI] [PubMed] [Google Scholar]
- Atanasova M, Konova E, Georgieva M, Dimitrova A, Coquand-Gandit M, Faury G, Baydanoff S, 2010. Age-related changes of anti-elastin antibodies in senescence-accelerated mice. Gerontology 56, 310–318. [DOI] [PubMed] [Google Scholar]
- Avolio A, Jones D, Tafazzoli-Shadpour M, 1998. Quantification of alterations in structure and function of elastin in the arterial media. Hypertension 32, 170–175. [DOI] [PubMed] [Google Scholar]
- Barolet AW, Nili N, Cheema A, Robinson R, Natarajan MK, O’Blenes S, Li J, Eskandarian MR, Sparkes J, Rabinovitch M, Strauss BH, 2001. Arterial elastase activity after balloon angioplasty and effects of elafin, an elastase inhibitor. Arterioscler Thromb Vasc Biol 21, 1269–1274. [DOI] [PubMed] [Google Scholar]
- Basalyga DM, Simionescu DT, Xiong W, Baxter BT, Starcher BC, Vyavahare NR, 2004. Elastin degradation and calcification in an abdominal aorta injury model: role of matrix metalloproteinases. Circulation 110, 3480–3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basson CT, Kocher O, Basson MD, Asis A, Madri JA, 1992. Differential modulation of vascular cell integrin and extracellular matrix expression in vitro by TGF-beta 1 correlates with reciprocal effects on cell migration. J Cell Physiol 153, 118–128. [DOI] [PubMed] [Google Scholar]
- Birch HL, 2018. Extracellular Matrix and Ageing. Subcell Biochem 90, 169–190. [DOI] [PubMed] [Google Scholar]
- Blewniewski M, Forma E, Rozanski W, Brys M, 2011. The calpain system as a potential target for pelvic muscle reinforcement. Cent European J Urol 64, 128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boraldi F, Moscarelli P, Bochicchio B, Pepe A, Salvi AM, Quaglino D, 2018. Heparan sulfates facilitate harmless amyloidogenic fibril formation interacting with elastin-like peptides. Sci Rep 8, 3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekelmann TJ, Kozel BA, Ishibashi H, Werneck CC, Keeley FW, Zhang L, Mecham RP, 2005. Tropoelastin interacts with cell-surface glycosaminoglycans via its COOH-terminal domain. J Biol Chem 280, 40939–40947. [DOI] [PubMed] [Google Scholar]
- Carreras I, Rich CB, Panchenko MP, Foster JA, 2002. Basic fibroblast growth factor decreases elastin gene transcription in aortic smooth muscle cells. J Cell Biochem 85, 592–600. [DOI] [PubMed] [Google Scholar]
- Castello L, Froio T, Cavallini G, Biasi F, Sapino A, Leonarduzzi G, Bergamini E, Poli G, Chiarpotto E, 2005. Calorie restriction protects against age-related rat aorta sclerosis. FASEB J 19, 1863–1865. [DOI] [PubMed] [Google Scholar]
- Chabot N, Moreau S, Mulani A, Moreau P, Keillor JW, 2010. Fluorescent probes of tissue transglutaminase reveal its association with arterial stiffening. Chem Biol 17, 1143–1150. [DOI] [PubMed] [Google Scholar]
- Chan CYT, Cheuk BLY, Cheng SWK, 2017. Abdominal Aortic Aneurysm-Associated MicroRNA-516a-5p Regulates Expressions of Methylenetetrahydrofolate Reductase, Matrix Metalloproteinase-2, and Tissue Inhibitor of Matrix Metalloproteinase-1 in Human Abdominal Aortic Vascular Smooth Muscle Cells. Ann Vasc Surg 42, 263–273. [DOI] [PubMed] [Google Scholar]
- Chiang HY, Chu PH, Lee TH, 2019. MFG-E8 mediates arterial aging by promoting the proinflammatory phenotype of vascular smooth muscle cells. J Biomed Sci 26, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury R, McGovern A, Ridley C, Cain SA, Baldwin A, Wang MC, Guo C, Mironov A Jr., Drymoussi Z, Trump D, Shuttleworth A, Baldock C, Kielty CM, 2009. Differential regulation of elastic fiber formation by fibulin-4 and −5. J Biol Chem 284, 24553–24567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR, Sarfati I, Danna D, Wise L, 1992. Smooth muscle cell elastase, atherosclerosis, and abdominal aortic aneurysms. Ann Surg 216, 327–330; discussion 330–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuomo F, Ferruzzi J, Agarwal P, Li C, Zhuang ZW, Humphrey JD, Figueroa CA, 2019. Sex-dependent differences in central artery haemodynamics in normal and fibulin-5 deficient mice: implications for ageing. Proc Math Phys Eng Sci 475, 20180076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale MA, Xiong W, Carson JS, Suh MK, Karpisek AD, Meisinger TM, Casale GP, Baxter BT, 2016. Elastin-Derived Peptides Promote Abdominal Aortic Aneurysm Formation by Modulating M1/M2 Macrophage Polarization. J Immunol 196, 4536–4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson JM, 2002. Smad about elastin regulation. Am J Respir Cell Mol Biol 26, 164–166. [DOI] [PubMed] [Google Scholar]
- Davie JR, He S, Li L, Sekhavat A, Espino P, Drobic B, Dunn KL, Sun JM, Chen HY, Yu J, Pritchard S, Wang X, 2008. Nuclear organization and chromatin dynamics--Sp1, Sp3 and histone deacetylases. Adv Enzyme Regul 48, 189–208. [DOI] [PubMed] [Google Scholar]
- Davies HA, Caamano-Gutierrez E, Chim YH, Field M, Nawaytou O, Ressel L, Akhtar R, Madine J, 2019. Idiopathic degenerative thoracic aneurysms are associated with increased aortic medial amyloid. Amyloid 26, 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies HA, Phelan MM, Wilkinson MC, Migrino RQ, Truran S, Franco DA, Liu LN, Longmore CJ, Madine J, 2015. Oxidative Stress Alters the Morphology and Toxicity of Aortic Medial Amyloid. Biophys J 109, 2363–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EC, 1993. Stability of elastin in the developing mouse aorta: a quantitative radioautographic study. Histochemistry 100, 17–26. [DOI] [PubMed] [Google Scholar]
- Davis MR, Summers KM, 2012. Structure and function of the mammalian fibrillin gene family: implications for human connective tissue diseases. Mol Genet Metab 107, 635–647. [DOI] [PubMed] [Google Scholar]
- de Vega S, Iwamoto T, Yamada Y, 2009. Fibulins: multiple roles in matrix structures and tissue functions. Cell Mol Life Sci 66, 1890–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt K, Wagner J, Skodras A, Candlish M, Koppelmann AJ, Wild K, Maxwell R, Rotermund C, von Zweydorf F, Gloeckner CJ, Davies HA, Madine J, Del Turco D, Feederle R, Lashley T, Deller T, Kahle P, Hefendehl JK, Jucker M, Neher JJ, 2020. Medin aggregation causes cerebrovascular dysfunction in aging wild-type mice. Proc Natl Acad Sci U S A 117, 23925–23931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Gregoli K, Mohamad Anuar NN, Bianco R, White SJ, Newby AC, George SJ, Johnson JL, 2017. MicroRNA-181b Controls Atherosclerosis and Aneurysms Through Regulation of TIMP-3 and Elastin. Circ Res 120, 49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duca L, Blaise S, Romier B, Laffargue M, Gayral S, El Btaouri H, Kawecki C, Guillot A, Martiny L, Debelle L, Maurice P, 2016. Matrix ageing and vascular impacts: focus on elastin fragmentation. Cardiovasc Res 110, 298–308. [DOI] [PubMed] [Google Scholar]
- Ellenrieder V, 2008. TGFbeta regulated gene expression by Smads and Sp1/KLF-like transcription factors in cancer. Anticancer Res 28, 1531–1539. [PubMed] [Google Scholar]
- Faury G, Pezet M, Knutsen RH, Boyle WA, Heximer SP, McLean SE, Minkes RK, Blumer KJ, Kovacs A, Kelly DP, Li DY, Starcher B, Mecham RP, 2003. Developmental adaptation of the mouse cardiovascular system to elastin haploinsufficiency. J Clin Invest 112, 1419–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, 2008. The Baltimore Longitudinal Study of Aging (BLSA): a 50-year-long journey and plans for the future. J Gerontol A Biol Sci Med Sci 63, 1416–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fhayli W, Boete Q, Harki O, Briancon-Marjollet A, Jacob MP, Faury G, 2019a. Rise and fall of elastic fibers from development to aging. Consequences on arterial structure-function and therapeutical perspectives. Matrix Biol 84, 41–56. [DOI] [PubMed] [Google Scholar]
- Fhayli W, Boete Q, Kihal N, Cenizo V, Sommer P, Boyle WA, Jacob MP, Faury G, 2020. Dill Extract Induces Elastic Fiber Neosynthesis and Functional Improvement in the Ascending Aorta of Aged Mice with Reversal of Age-Dependent Cardiac Hypertrophy and Involvement of Lysyl Oxidase-Like-1. Biomolecules 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fhayli W, Boyer M, Ghandour Z, Jacob MP, Andrieu JP, Starcher BC, Esteve E, Faury G, 2019b. Chronic administration of minoxidil protects elastic fibers and stimulates their neosynthesis with improvement of the aorta mechanics in mice. Cell Signal 62, 109333. [DOI] [PubMed] [Google Scholar]
- Fornieri C, Taparelli F, Quaglino D Jr., Contri MB, Davidson JM, Algeri S, Ronchetti IP, 1999. The effect of caloric restriction on the aortic tissue of aging rats. Connect Tissue Res 40, 131–143. [DOI] [PubMed] [Google Scholar]
- Foster JA, Rich CB, Miller M, Benedict MR, Richman RA, Florini JR, 1990. Effect of age and IGF-I administration on elastin gene expression in rat aorta. J Gerontol 45, B113–118. [DOI] [PubMed] [Google Scholar]
- Fu Z, Wang M, Gucek M, Zhang J, Wu J, Jiang L, Monticone RE, Khazan B, Telljohann R, Mattison J., Sheng S., Cole RN., Spinetti G., Pintus G., Li L., Kolodgie FD., Virmani R., Spurgeon H., Ingram DK., Everett AD., Lakatta EG., Van Eyk JE., 2009. Milk fat globule protein epidermal growth factor-8: a pivotal relay element within the angiotensin II and monocyte chemoattractant protein-1 signaling cascade mediating vascular smooth muscle cells invasion. Circ Res 104, 1337–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T Jr., Jacob MP, Khalil A, Wallach J, Robert L, 1998. Biological effects of elastin peptides. Pathol Biol (Paris) 46, 497–506. [PubMed] [Google Scholar]
- Gavish B, Izzo JL Jr., 2016. Arterial Stiffness: Going a Step Beyond. Am J Hypertens. [DOI] [PubMed] [Google Scholar]
- Gorisse L, Pietrement C, Vuiblet V, Schmelzer CE, Kohler M, Duca L, Debelle L, Fornes P, Jaisson S, Gillery P, 2016. Protein carbamylation is a hallmark of aging. Proc Natl Acad Sci U S A 113, 1191–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourgas O, Muiznieks LD, Bello DG, Nanci A, Sharpe S, Cerruti M, 2019. Cross-Linked Elastin-like Polypeptide Membranes as a Model for Medial Arterial Calcification. Biomacromolecules 20, 2625–2636. [DOI] [PubMed] [Google Scholar]
- Greenwald SE, 2007. Ageing of the conduit arteries. J Pathol 211, 157–172.17200940 [Google Scholar]
- Gundiah N, Ratcliffe MB, Pruitt LA, 2009. The biomechanics of arterial elastin. J Mech Behav Biomed Mater 2, 288–296. [DOI] [PubMed] [Google Scholar]
- Halabi CM, Kozel BA, 2020. Vascular elastic fiber heterogeneity in health and disease. Curr Opin Hematol 27, 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halper J, Kjaer M, 2014. Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv Exp Med Biol 802, 31–47. [DOI] [PubMed] [Google Scholar]
- Harvey A, Montezano AC, Lopes RA, Rios F, Touyz RM, 2016. Vascular Fibrosis in Aging and Hypertension: Molecular Mechanisms and Clinical Implications. Can J Cardiol 32, 659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, 2020. Elastases and elastokines: elastin degradation and its significance in health and disease. Crit Rev Biochem Mol Biol 55, 252–273. [DOI] [PubMed] [Google Scholar]
- Hinek A, Bodnaruk TD, Bunda S, Wang Y, Liu K, 2008. Neuraminidase-1, a subunit of the cell surface elastin receptor, desialylates and functionally inactivates adjacent receptors interacting with the mitogenic growth factors PDGF-BB and IGF-2. Am J Pathol 173, 1042–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinek A, Molossi S, Rabinovitch M, 1996. Functional interplay between interleukin-1 receptor and elastin binding protein regulates fibronectin production in coronary artery smooth muscle cells. Exp Cell Res 225, 122–131. [DOI] [PubMed] [Google Scholar]
- Hirai M, Ohbayashi T, Horiguchi M, Okawa K, Hagiwara A, Chien KR, Kita T, Nakamura T, 2007. Fibulin-5/DANCE has an elastogenic organizer activity that is abrogated by proteolytic cleavage in vivo. J Cell Biol 176, 1061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano E, Knutsen RH, Sugitani H, Ciliberto CH, Mecham RP, 2007. Functional rescue of elastin insufficiency in mice by the human elastin gene: implications for mouse models of human disease. Circ Res 101, 523–531. [DOI] [PubMed] [Google Scholar]
- Hornebeck W, Emonard H, 2011. The cell-elastin-elastase(s) interacting triade directs elastolysis. Front Biosci (Landmark Ed) 16, 707–722. [DOI] [PubMed] [Google Scholar]
- Humphrey JD, Harrison DG, Figueroa CA, Lacolley P, Laurent S, 2016. Central Artery Stiffness in Hypertension and Aging: A Problem With Cause and Consequence. Circ Res 118, 379–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob MP, Badier-Commander C, Fontaine V, Benazzoug Y, Feldman L, Michel JB, 2001. Extracellular matrix remodeling in the vascular wall. Pathol Biol (Paris) 49, 326–332. [DOI] [PubMed] [Google Scholar]
- Jaffe M, Sesti C, Washington IM, Du L, Dronadula N, Chin MT, Stolz DB, Davis EC, Dichek DA, 2012. Transforming growth factor-beta signaling in myogenic cells regulates vascular morphogenesis, differentiation, and matrix synthesis. Arterioscler Thromb Vasc Biol 32, e1–11. [DOI] [PubMed] [Google Scholar]
- Jahnen-Dechent W, Heiss A, Schafer C, Ketteler M, 2011. Fetuin-A regulation of calcified matrix metabolism. Circ Res 108, 1494–1509. [DOI] [PubMed] [Google Scholar]
- Jensen DE, Rich CB, Terpstra AJ, Farmer SR, Foster JA, 1995. Transcriptional regulation of the elastin gene by insulin-like growth factor-I involves disruption of Sp1 binding. Evidence for the role of Rb in mediating Sp1 binding in aortic smooth muscle cells. J Biol Chem 270, 6555–6563. [DOI] [PubMed] [Google Scholar]
- Jiang L, Wang M, Zhang J, Monticone RE, Telljohann R, Spinetti G, Pintus G, Lakatta EG, 2008. Increased aortic calpain-1 activity mediates age-associated angiotensin II signaling of vascular smooth muscle cells. PLoS One 3, e2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Zhang J, Monticone RE, Telljohann R, Wu J, Wang M, Lakatta EG, 2012. Calpain-1 regulation of matrix metalloproteinase 2 activity in vascular smooth muscle cells facilitates age-associated aortic wall calcification and fibrosis. Hypertension 60, 1192–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Polewski M, Terkeltaub RA, 2008. Transglutaminase 2 is central to induction of the arterial calcification program by smooth muscle cells. Circ Res 102, 529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahari VM, Chen YQ, Bashir MM, Rosenbloom J, Uitto J, 1992. Tumor necrosis factor-alpha down-regulates human elastin gene expression. Evidence for the role of AP-1 in the suppression of promoter activity. J Biol Chem 267, 26134–26141. [PubMed] [Google Scholar]
- Karimi A, Milewicz DM, 2016. Structure of the Elastin-Contractile Units in the Thoracic Aorta and How Genes That Cause Thoracic Aortic Aneurysms and Dissections Disrupt This Structure. Can J Cardiol 32, 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnik SK, Brooke BS, Bayes-Genis A, Sorensen L, Wythe JD, Schwartz RS, Keating MT, Li DY, 2003. A critical role for elastin signaling in vascular morphogenesis and disease. Development 130, 411–423. [DOI] [PubMed] [Google Scholar]
- Kawecki C, Hezard N, Bocquet O, Poitevin G, Rabenoelina F, Kauskot A, Duca L, Blaise S, Romier B, Martiny L, Nguyen P, Debelle L, Maurice P, 2014. Elastin-derived peptides are new regulators of thrombosis. Arterioscler Thromb Vasc Biol 34, 2570–2578. [DOI] [PubMed] [Google Scholar]
- Kielty CM, Sherratt MJ, Marson A, Baldock C, 2005. Fibrillin microfibrils. Adv Protein Chem 70, 405–436. [DOI] [PubMed] [Google Scholar]
- Kielty CM, Sherratt MJ, Shuttleworth CA, 2002. Elastic fibres. J Cell Sci 115, 2817–2828. [DOI] [PubMed] [Google Scholar]
- Kinnear C, Agrawal R, Loo C, Pahnke A, Rodrigues DC, Thompson T, Akinrinade O, Ahadian S, Keeley F, Radisic M, Mital S, Ellis J, 2020. Everolimus Rescues the Phenotype of Elastin Insufficiency in Patient Induced Pluripotent Stem Cell-Derived Vascular Smooth Muscle Cells. Arterioscler Thromb Vasc Biol, ATVBAHA119313936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochova P, Kuncova J, Sviglerova J, Cimrman R, Miklikova M, Liska V, Tonar Z, 2012. The contribution of vascular smooth muscle, elastin and collagen on the passive mechanics of porcine carotid arteries. Physiol Meas 33, 1335–1351. [DOI] [PubMed] [Google Scholar]
- Kohn JC, Lampi MC, Reinhart-King CA, 2015. Age-related vascular stiffening: causes and consequences. Front Genet 6, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothapalli CR, Ramamurthi A, 2009. Lysyl oxidase enhances elastin synthesis and matrix formation by vascular smooth muscle cells. J Tissue Eng Regen Med 3, 655–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel BA, Mecham RP, 2019. Elastic fiber ultrastructure and assembly. Matrix Biol 84, 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunecki M, Nawrocka A, 2001. Elastin-laminin receptor and abdominal aortic aneurysms. New subject to study? A review. Pathol Biol (Paris) 49, 333–338. [DOI] [PubMed] [Google Scholar]
- Lannoy M, Slove S, Jacob MP, 2014. The function of elastic fibers in the arteries: beyond elasticity. Pathol Biol (Paris) 62, 79–83. [DOI] [PubMed] [Google Scholar]
- Larsson A, Peng S, Persson H, Rosenbloom J, Abrams WR, Wassberg E, Thelin S, Sletten K, Gerwins P, Westermark P, 2006. Lactadherin binds to elastin--a starting point for medin amyloid formation? Amyloid 13, 78–85. [DOI] [PubMed] [Google Scholar]
- Laurent S, Boutouyrie P, Lacolley P, 2005. Structural and genetic bases of arterial stiffness. Hypertension 45, 1050–1055. [DOI] [PubMed] [Google Scholar]
- Lavin B, Lacerda S, Andia ME, Lorrio S, Bakewell R, Smith A, Rashid I, Botnar RM, Phinikaridou A, 2020. Tropoelastin: an in vivo imaging marker of dysfunctional matrix turnover during abdominal aortic dilation. Cardiovasc Res 116, 995–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre M, Rucker RB, 1983. Modification of arterial elastin in vivo. Effects of age and diet on changes in the N-terminal amino acid content of aorta elastin. Biochim Biophys Acta 743, 338–342. [DOI] [PubMed] [Google Scholar]
- Lexhaller B, Ludwig C, Scherf KA, 2019. Comprehensive Detection of Isopeptides between Human Tissue Transglutaminase and Gluten Peptides. Nutrients 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E, Keating MT, 1998. Elastin is an essential determinant of arterial morphogenesis. Nature 393, 276–280. [DOI] [PubMed] [Google Scholar]
- Li L, He S, Sun JM, Davie JR, 2004. Gene regulation by Sp1 and Sp3. Biochem Cell Biol 82, 460–471. [DOI] [PubMed] [Google Scholar]
- Lin CJ, Staiculescu MC, Hawes JZ, Cocciolone AJ, Hunkins BM, Roth RA, Lin CY, Mecham RP, Wagenseil JE, 2019. Heterogeneous Cellular Contributions to Elastic Laminae Formation in Arterial Wall Development. Circ Res 125, 1006–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey RH, Venkitachalam L, Sutton-Tyrrell K, 2007. Calcifications, arterial stiffness and atherosclerosis. Adv Cardiol 44, 234–244. [DOI] [PubMed] [Google Scholar]
- Maki JM, Rasanen J, Tikkanen H, Sormunen R, Makikallio K, Kivirikko KI, Soininen R, 2002. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation 106, 2503–2509. [DOI] [PubMed] [Google Scholar]
- Mariko B, Pezet M, Escoubet B, Bouillot S, Andrieu JP, Starcher B, Quaglino D, Jacob MP, Huber P, Ramirez F, Faury G, 2011. Fibrillin-1 genetic deficiency leads to pathological ageing of arteries in mice. J Pathol 224, 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Pardillos A, Sorribas V, 2015. Effects of donor age and proliferative aging on the phenotype stability of rat aortic smooth muscle cells. Physiol Rep 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice P, Blaise S, Gayral S, Debelle L, Laffargue M, Hornebeck W, Duca L, 2013. Elastin fragmentation and atherosclerosis progression: the elastokine concept. Trends Cardiovasc Med 23, 211–221. [DOI] [PubMed] [Google Scholar]
- Migrino RQ, Karamanova N, Truran S, Serrano GE, Davies HA, Madine J, Beach TG, 2020. Cerebrovascular medin is associated with Alzheimer’s disease and vascular dementia. Alzheimers Dement (Amst) 12, e12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozos I, Malainer C, Horbanczuk J, Gug C, Stoian D, Luca CT, Atanasov AG, 2017. Inflammatory Markers for Arterial Stiffness in Cardiovascular Diseases. Front Immunol 8, 1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai D, Lino M, Bendeck MP, 2018. Cell-Matrix Interactions and Matricrine Signaling in the Pathogenesis of Vascular Calcification. Front Cardiovasc Med 5, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NivisoŽ Smith L, Weiss A, 2011. Elastin Based Constructs. [Google Scholar]
- O’Rourke MF, Hashimoto J, 2007. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol 50, 1–13. [DOI] [PubMed] [Google Scholar]
- Oleggini R, Gastaldo N, Di Donato A, 2007. Regulation of elastin promoter by lysyl oxidase and growth factors: cross control of lysyl oxidase on TGF-beta1 effects. Matrix Biol 26, 494–505. [DOI] [PubMed] [Google Scholar]
- Paczek L, Michalska W, Bartlomiejczyk I, 2009. Proteolytic enzyme activity as a result of aging. Aging Clin Exp Res 21, 9–13. [DOI] [PubMed] [Google Scholar]
- Pai A, Leaf EM, El-Abbadi M, Giachelli CM, 2011. Elastin degradation and vascular smooth muscle cell phenotype change precede cell loss and arterial medial calcification in a uremic mouse model of chronic kidney disease. Am J Pathol 178, 764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Larsson A, Wassberg E, Gerwins P, Thelin S, Fu X, Westermark P, 2007. Role of aggregated medin in the pathogenesis of thoracic aortic aneurysm and dissection. Lab Invest 87, 1195–1205. [DOI] [PubMed] [Google Scholar]
- Pezzoli D, Di Paolo J, Kumra H, Fois G, Candiani G, Reinhardt DP, Mantovani D, 2018. Fibronectin promotes elastin deposition, elasticity and mechanical strength in cellularised collagen-based scaffolds. Biomaterials 180, 130–142. [DOI] [PubMed] [Google Scholar]
- Pshezhetsky AV, Hinek A, 2009. Serine carboxypeptidases in regulation of vasoconstriction and elastogenesis. Trends Cardiovasc Med 19, 11–17. [DOI] [PubMed] [Google Scholar]
- Pustlauk W, Westhoff TH, Claeys L, Roch T, Geissler S, Babel N, 2020. Induced osteogenic differentiation of human smooth muscle cells as a model of vascular calcification. Sci Rep 10, 5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z, 2015. Soluble elastin peptides in cardiovascular homeostasis: Foe or ally. Peptides 67, 64–73. [DOI] [PubMed] [Google Scholar]
- Rabkin SW, 2017. The Role Matrix Metalloproteinases in the Production of Aortic Aneurysm. Prog Mol Biol Transl Sci 147, 239–265. [DOI] [PubMed] [Google Scholar]
- Raffetto JD, Khalil RA, 2008. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol 75, 346–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafuse M, Xu X, Stenmark K, Neu CP, Yin X, Tan W, 2019. Layer-specific arterial micromechanics and microstructure: Influences of age, anatomical location, and processing technique. J Biomech 88, 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randell A, Daneshtalab N, 2017. Elastin microfibril interface-located protein 1, transforming growth factor beta, and implications on cardiovascular complications. J Am Soc Hypertens 11, 437–448. [DOI] [PubMed] [Google Scholar]
- Rauscher S, Pomes R, 2012. Structural disorder and protein elasticity. Adv Exp Med Biol 725, 159–183. [DOI] [PubMed] [Google Scholar]
- Rensen SS, Doevendans PA, van Eys GJ, 2007. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J 15, 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich CB, Fontanilla MR, Nugent M, Foster JA, 1999. Basic fibroblast growth factor decreases elastin gene transcription through an AP1/cAMP-response element hybrid site in the distal promoter. J Biol Chem 274, 33433–33439. [DOI] [PubMed] [Google Scholar]
- Robert L, 1998. Mechanisms of aging of the extracellular matrix: role of the elastin-laminin receptor. Gerontology 44, 307–317. [DOI] [PubMed] [Google Scholar]
- Robinet A, Fahem A, Cauchard JH, Huet E, Vincent L, Lorimier S, Antonicelli F, Soria C, Crepin M, Hornebeck W, Bellon G, 2005. Elastin-derived peptides enhance angiogenesis by promoting endothelial cell migration and tubulogenesis through upregulation of MT1-MMP. J Cell Sci 118, 343–356. [DOI] [PubMed] [Google Scholar]
- Robinson KN, Teran-Garcia M, 2016. From infancy to aging: Biological and behavioral modifiers of Fetuin-A. Biochimie 124, 141–149. [DOI] [PubMed] [Google Scholar]
- Rosenbloom J, 1984. Elastin: relation of protein and gene structure to disease. Lab Invest 51, 605–623. [PubMed] [Google Scholar]
- Sauvage M, Hinglais N, Mandet C, Badier C, Deslandes F, Michel JB, Jacob MP, 1998. Localization of elastin mRNA and TGF-beta1 in rat aorta and caudal artery as a function of age. Cell Tissue Res 291, 305–314. [DOI] [PubMed] [Google Scholar]
- Schmelzer CEH, Hedtke T, Heinz A, 2020. Unique molecular networks: Formation and role of elastin cross-links. IUBMB Life 72, 842–854. [DOI] [PubMed] [Google Scholar]
- Seals DR, Walker AE, Pierce GL, Lesniewski LA, 2009. Habitual exercise and vascular ageing. J Physiol 587, 5541–5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S, Bunda S, Shi J, Wang A, Mitts TF, Hinek A, 2011. Retinoblastoma protein modulates the inverse relationship between cellular proliferation and elastogenesis. J Biol Chem 286, 36580–36591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyama Y, Wachi H, 2004. Atherosclerosis and matrix dystrophy. J Atheroscler Thromb 11, 236–245. [DOI] [PubMed] [Google Scholar]
- Sherratt MJ, 2009. Tissue elasticity and the ageing elastic fibre. Age (Dordr) 31, 305–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu A, Philips K, Vyavahare N, 2005. Elastin-derived peptides and TGF-beta1 induce osteogenic responses in smooth muscle cells. Biochem Biophys Res Commun 334, 524–532. [DOI] [PubMed] [Google Scholar]
- Sinha A, Vyavahare NR, 2013. High-glucose levels and elastin degradation products accelerate osteogenesis in vascular smooth muscle cells. Diab Vasc Dis Res 10, 410–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjot-Arkil H, Clausen RE, Nguyen QH, Wang Y, Zheng Q, Martinez FJ, Hogaboam CM, Han M, Klickstein LB, Larsen MR, Nawrocki A, Leeming DJ, Karsdal MA, 2012. Measurement of MMP-9 and −12 degraded elastin (ELM) provides unique information on lung tissue degradation. BMC Pulm Med 12, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spofford CM, Chilian WM, 2001. The elastin-laminin receptor functions as a mechanotransducer in vascular smooth muscle. Am J Physiol Heart Circ Physiol 280, H1354–1360. [DOI] [PubMed] [Google Scholar]
- Spronck B, Ferruzzi J, Bellini C, Caulk AW, Murtada SI, Humphrey JD, 2020. Aortic remodeling is modest and sex-independent in mice when hypertension is superimposed on aging. J Hypertens 38, 1312–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sproul EP, Argraves WS, 2013. A cytokine axis regulates elastin formation and degradation. Matrix Biol 32, 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen EA, Venkatasubramaniam A, Good TA, Topoleski LD, 2014. The effect of glycation on arterial microstructure and mechanical response. J Biomed Mater Res A 102, 2565–2572. [DOI] [PubMed] [Google Scholar]
- Sudo R, Sato F, Azechi T, Wachi H, 2015. MiR-29-mediated elastin down-regulation contributes to inorganic phosphorus-induced osteoblastic differentiation in vascular smooth muscle cells. Genes Cells 20, 1077–1087. [DOI] [PubMed] [Google Scholar]
- Sukhova GK, Shi GP, Simon DI, Chapman HA, Libby P, 1998. Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J Clin Invest 102, 576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szychowski KA, Wojtowicz AK, Gminski J, 2019. Impact of Elastin-Derived Peptide VGVAPG on Matrix Metalloprotease-2 and −9 and the Tissue Inhibitor of Metalloproteinase-1, −2, −3 and −4 mRNA Expression in Mouse Cortical Glial Cells In Vitro. Neurotox Res 35, 100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani T, Fujiwara M, Orimo H, Shimizu A, Narisawa S, Pinkerton AB, Millan JL, Tsuruoka S, 2020. Inhibition of tissue-nonspecific alkaline phosphatase protects against medial arterial calcification and improves survival probability in the CKD-MBD mouse model. J Pathol 250, 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma I, McCaffrey TA, 2012. Transforming growth factor-beta and atherosclerosis: interwoven atherogenic and atheroprotective aspects. Cell Tissue Res 347, 155–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar AM, Cesar DC, Leta GC, Mourao PA, 1998. Age-related changes in populations of aortic glycosaminoglycans: species with low affinity for plasma low-density lipoproteins, and not species with high affinity, are preferentially affected. Arterioscler Thromb Vasc Biol 18, 604–614. [DOI] [PubMed] [Google Scholar]
- Urban Z, Riazi S, Seidl TL, Katahira J, Smoot LB, Chitayat D, Boyd CD, Hinek A, 2002. Connection between elastin haploinsufficiency and increased cell proliferation in patients with supravalvular aortic stenosis and Williams-Beuren syndrome. Am J Hum Genet 71, 30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Doren SR, 2015. Matrix metalloproteinase interactions with collagen and elastin. Matrix Biol 44–46, 224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Bellosta R, 2020. New insights into endogenous mechanisms of protection against arterial calcification. Atherosclerosis. [DOI] [PubMed] [Google Scholar]
- Wagenseil JE, Mecham RP, 2007. New insights into elastic fiber assembly. Birth Defects Res C Embryo Today 81, 229–240. [DOI] [PubMed] [Google Scholar]
- Wagenseil JE, Mecham RP, 2012. Elastin in large artery stiffness and hypertension. J Cardiovasc Transl Res 5, 264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahart A, Hocine T, Albrecht C, Henry A, Sarazin T, Martiny L, El Btaouri H, Maurice P, Bennasroune A, Romier-Crouzet B, Blaise S, Duca L, 2019. Role of elastin peptides and elastin receptor complex in metabolic and cardiovascular diseases. FEBS J 286, 2980–2993. [DOI] [PubMed] [Google Scholar]
- Wang M, Fu Z, Wu J, Zhang J, Jiang L, Khazan B, Telljohann R, Zhao M, Krug AW, Pikilidou M, Monticone RE, Wersto R, Van Eyk J, Lakatta EG, 2012a. MFG-E8 activates proliferation of vascular smooth muscle cells via integrin signaling. Aging Cell 11, 500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Jiang L, Monticone RE, Lakatta EG, 2014a. Proinflammation: the key to arterial aging. Trends Endocrinol Metab 25, 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Khazan B, Lakatta EG, 2010. Central Arterial Aging and Angiotensin II Signaling. Curr Hypertens Rev 6, 266–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Kim SH, Monticone RE, Lakatta EG, 2015. Matrix metalloproteinases promote arterial remodeling in aging, hypertension, and atherosclerosis. Hypertension 65, 698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Lakatta EG, 2002. Altered regulation of matrix metalloproteinase-2 in aortic remodeling during aging. Hypertension 39, 865–873. [DOI] [PubMed] [Google Scholar]
- Wang M, Lakatta EG, 2019. Aging, Diet, and Atherogenesis in Nonhuman Primates. Circulation 140, A11637–A11637. [Google Scholar]
- Wang M, Monticone R, McGraw K, 2018a. Proinflammatory Arterial Stiffness Syndrome: A Signature of Large Arterial Aging. J Vasc Res. 55, 210–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Monticone RE, Lakatta EG, 2014b. Proinflammation of aging central arteries: a mini-review. Gerontology 60, 519–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Monticone RE, McGraw KR, 2020. Proinflammation, profibrosis, and arterial aging. Aging Medicine n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Spinetti G, Monticone RE, Zhang J, Wu J, Jiang L, Khazan B, Telljohann R, Lakatta EG, 2011. A local proinflammatory signalling loop facilitates adverse age-associated arterial remodeling. PLoS One 6, e16653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Takagi G, Asai K, Resuello RG, Natividad FF, Vatner DE, Vatner SF, Lakatta EG, 2003. Aging increases aortic MMP-2 activity and angiotensin II in nonhuman primates. Hypertension 41, 1308–1316. [DOI] [PubMed] [Google Scholar]
- Wang M, Wang HH, Lakatta EG, 2013. Milk fat globule epidermal growth factor VIII signaling in arterial wall remodeling. Curr Vasc Pharmacol 11, 768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Zhang J, Telljohann R, Jiang L, Wu J, Monticone RE, Kapoor K, Talan M, Lakatta EG, 2012b. Chronic matrix metalloproteinase inhibition retards age-associated arterial proinflammation and increase in blood pressure. Hypertension 60, 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Zhang L, Zhu W, Zhang J, Kim SH, Wang Y, Ni L, Telljohann R, Monticone RE, McGraw K, Liu L, de Cabo R, Lakatta EG, 2018b. Calorie Restriction Curbs Proinflammation That Accompanies Arterial Aging, Preserving a Youthful Phenotype. J Am Heart Assoc 7, e009112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Zhao D, Spinetti G, Zhang J, Jiang LQ, Pintus G, Monticone R, Lakatta EG, 2006. Matrix metalloproteinase 2 activation of transforming growth factor-beta1 (TGF-beta1) and TGF-beta1-type II receptor signaling within the aged arterial wall. Arterioscler Thromb Vasc Biol 26, 1503–1509. [DOI] [PubMed] [Google Scholar]
- Wang X, Khalil RA, 2018. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv Pharmacol 81, 241–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanga S, Hibender S, Ridwan Y, van Roomen C, Vos M, van der Made I, van Vliet N, Franken R, van Riel LA, Groenink M, Zwinderman AH, Mulder BJ, de Vries CJ, Essers J, de Waard V, 2017. Aortic microcalcification is associated with elastin fragmentation in Marfan syndrome. J Pathol 243, 294–306. [DOI] [PubMed] [Google Scholar]
- Wen D, Zhou XL, Li JJ, Hui RT, 2011. Biomarkers in aortic dissection. Clin Chim Acta 412, 688–695. [DOI] [PubMed] [Google Scholar]
- Westenfeld R, Schafer C, Kruger T, Haarmann C, Schurgers LJ, Reutelingsperger C, Ivanovski O, Drueke T, Massy ZA, Ketteler M, Floege J, Jahnen-Dechent W, 2009. Fetuin-A protects against atherosclerotic calcification in CKD. J Am Soc Nephrol 20, 1264–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Shi GP, 2014. Vascular wall extracellular matrix proteins and vascular diseases. Biochim Biophys Acta 1842, 2106–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro Y, Yanagisawa H, 2018. Crossing Bridges between Extra- and Intra-Cellular Events in Thoracic Aortic Aneurysms. J Atheroscler Thromb 25, 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi Y, Tsuruga E, Nakashima K, Sawa Y, Ishikawa H, 2010. Fibulin-4 and −5, but not Fibulin-2, are Associated with Tropoelastin Deposition in Elastin-Producing Cell Culture. Acta Histochem Cytochem 43, 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa H, Davis EC, 2010. Unraveling the mechanism of elastic fiber assembly: The roles of short fibulins. Int J Biochem Cell Biol 42, 1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh H, Anderson N, Ornstein-Goldstein N, Bashir MM, Rosenbloom JC, Abrams W, Indik Z, Yoon K, Parks W, Mecham R, et al. , 1989. Structure of the bovine elastin gene and S1 nuclease analysis of alternative splicing of elastin mRNA in the bovine nuchal ligament. Biochemistry 28, 2365–2370. [DOI] [PubMed] [Google Scholar]
- Younger S, Jang H, Davies HA, Niemiec MJ, Garcia JGN, Nussinov R, Migrino RQ, Madine J, Arce FT, 2020. Medin Oligomer Membrane Pore Formation: A Potential Mechanism of Vascular Dysfunction. Biophys J 118, 2769–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacchigna L, Vecchione C, Notte A, Cordenonsi M, Dupont S, Maretto S, Cifelli G, Ferrari A, Maffei A, Fabbro C, Braghetta P, Marino G, Selvetella G, Aretini A, Colonnese C, Bettarini U, Russo G, Soligo S, Adorno M, Bonaldo P, Volpin D, Piccolo S, Lembo G, Bressan GM, 2006. Emilin1 links TGF-beta maturation to blood pressure homeostasis. Cell 124, 929–942. [DOI] [PubMed] [Google Scholar]
- Zampetaki A, Attia R, Mayr U, Gomes RS, Phinikaridou A, Yin X, Langley SR, Willeit P, Lu R, Fanshawe B, Fava M, Barallobre-Barreiro J, Molenaar C, So PW, Abbas A, Jahangiri M, Waltham M, Botnar R, Smith A, Mayr M, 2014. Role of miR-195 in aortic aneurysmal disease. Circ Res 115, 857–866. [DOI] [PubMed] [Google Scholar]
- Zanetti M, Braghetta P, Sabatelli P, Mura I, Doliana R, Colombatti A, Volpin D, Bonaldo P, Bressan GM, 2004. EMILIN-1 deficiency induces elastogenesis and vascular cell defects. Mol Cell Biol 24, 638–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lacolley P, Protogerou AD, Safar ME, 2020. Arterial Stiffness in Hypertension and Function of Large Arteries. Am J Hypertens 33, 291–296. [DOI] [PubMed] [Google Scholar]