Abstract
Tau is a microtubule-stabilizing protein that plays an important role in the formation of axonal microtubules in neurons. Phosphorylated tau (p-Tau) has received great attention in the field of Alzheimer’s disease (AD) as a potential therapeutic target due to its involvement with synaptic damage and neuronal dysfunction. Mounting evidence suggests that amyloid beta (Aβ)-targeted clinical trials continuously failed; therefore, it is important to consider alternative therapeutic strategies such as p-tau-PROTACs targeted small molecules for AD and other tauopathies. The present article describes the characteristics of tau biology, structure, and function in both healthy and pathological states in AD. It also explains data from studies that have identified the involvement of p-tau in neuronal damage and synaptic and cognitive functions in AD. Current article also covers several aspects, including small molecule inhibitors, and the development of p-tau-PROTACs targeted drug molecules to treat patients with AD and other tauopathies.
Keywords: Alzheimer’s disease, tau protein, hyperphosphorylation, neurofibrillary tangles, paired helical filament, PROTACs, tauopathies
1. Introduction
Alzheimer’s disease (AD) is the leading cause of mental illness in the ageing population and it affects over 50 million people worldwide including 5.8 million Americans who suffer from AD [1]. The World Alzheimer Report 2015 states that this number is expected to double by 2030. Major pathological hallmarks of AD include intracellular deposition of neurofibrillary tangles (NFT), which were associated with paired helical filaments (PHF), hyperphosphorylated tau protein and extra cellular accumulation of amyloid beta (Aβ) peptide in the senile plaques. Investigations of postmortem AD brains revealed that loss of synapses, synaptic function, mitochondrial integrity and functional abnormalities, are due to the presence of extracellular amyloid beta (Aβ) plaques and intracellular misfolded tau. Normal tau maintains the structure and function of cytoskeletal neuron in healthy subjects. In AD condition, the normal tau turns to hyperphosphorylation which leads to neurofibrillary tangle (NFT) formation that is associated with the loss of neuron structure in the progression of AD [2–3]. Phosphorylated tau (p-tau) is involved in AD disease progression [4]. Increasing evidence suggests that the role of p-Tau in the AD progression with increased hyperphosphorylation and p-tau aggregation and these are involved in the impairment of axonal transport of mitochondria and nutrients to nerve terminals from the cell soma in AD [5]. In addition to its involvement in AD, p-Tau is also reported to be involved in frontotemporal dementia and other ‘tauopathies’ which are classical neurodegenerative diseases associated with the pathological aggregation of p-tau protein in the human AD postmortem samples [6].
The purpose of this article is to highlight the involvement of p-Tau in AD, hyperphosphorylation conditions, and kinase dependent p-tau regulation in AD and other tauopathies. This article also summarizes the tau structure, and function in healthy and disease states and possible advanced therapeutic approaches.
2. Factors associated with Alzheimer’s disease
Disease progression begin to develop over 20 years earlier than clinical symptoms observed in patients with AD [7]. Both modifiable and non-modifiable factors are associated with AD. Nonmodifiable factors such as age, gender, genetics, family history, and disorders such as Down’s syndrome, are associated with AD.
Increasing evidence suggests that modifiable risk factors contribute significantly to disease progression and pathogenesis of AD. Some of these factors include environmental, exercise, diet, socioeconomic status and educational attainment/awareness about dementia [7–9]. Age-related chronic diseases contributing to the advancement and rapid progression of AD include high cholesterol, diabetes, cardiovascular disease, kidney disease, and cognitive impairment due to brain injury.
In addition, epigenetic factors play a large role in the development and progression of dementia and AD in elderly individuals [10]. Increasing evidence also suggests that environmental contaminants in the etiology of AD [11–12].
3. Tau
Tau is a cytoskeletal protein that binds to neuronal microtubules and regulates the formation and stabilization of the microtubules. Neuronal microtubules are the major component of nerve axons that form the cytoskeleton of cells, involved in nutrient transport and signal neurotransmission [13–15]. The importance of tau as a necessary protein involved in the assembly of microtubules was first elucidated in the 1970s and 1980s [16–20].
Tau proteins perform the function of stabilizing microtubules in nerve cells and are present to a much lesser degree in oligodendrocytes and astrocytes [21]. When tau proteins become defective and fail to adequately stabilize microtubules, pathologies of the nervous system can develop p-tau in AD and other tauopathies [22]. Tau proteins are produced through alternative splicing of a single gene called microtubule-associated protein tau (MAPT) gene. N-tau (normal tau) is an essential for signaling molecules that regulate gene transcription and cell cycle activity. Tau α-β tubulin proteins are the scaffolding proteins that are essential for the formation of the cytoskeletal filament structure [23–24]. MAPT is involved with the coordination of α-β tubulin dimers in polymerization [25–27]. In AD, excessive hyperphosphorylation of tau and aggregations destructs the microtubule structure resulting in neurofibrillary tangles in AD neurons [28, 29]. In normal state, the function of tau helped in the development and maintenance of the cellular cytoskeleton and neuron structure [30–32]. In AD, it has been hypothesized that hyperphosphorylation and an excessive p-Tau aggregation affects cytoskeleton structure, mitochondrial axonal transport, and loss of synapses in AD neuronal cells [33–35].
4. Tau structure and Functions
The tau structure has been hard to characterize due to its alternative splicing, including an immature RNA sequence. The tau protein encompasses 441 amino acid residues in a single gene located on chromosome 17q21 with 16 exons. Of these 16 exons, the first 13 have been successfully transcribed to form the mature sequences 4A, 6, and 8 [36]. Exon 1 to 14 have been transcribed, but they have not been translated to form mature mRNA, whereas exons 1, 4, 5, 7, 9, 11, 12, and 13 have been transcribed and translated. Exons 2, 3, and 10 are responsible for the formation of six tau isoforms in the brain neurons of adult humans [37].
The six isoforms of tau contain 37 to 47 amino acid residues, characterized by conserved repeat regions and 5 to 6 base pairs [38–40]. These repeat isoform residues are mostly the terminals (C and N), where long isoforms repeat. These repeats may play a key role in microtubule stabilization [40, 41]. The (3R) or (4R) tubulin binding domains of amino acids are located in the C- and in the N-terminals [38]. Researchers have hypothesized the terminals contain rich residues of proline, which are amino acids that bind to microtubules regulated by post-translational modifications and phosphorylation [42, 43].
The tau protein promotes and regulates the assembly of microtubules and the stability of the cellular assembly through the propagation of dendritic signals to a particular axon [44, 45]. These microtubules are formed by the a/β tubulin heterodimer in a head to tail fashion and is intrinsically a polarized molecule, the resulting microtubule is a polarized polymer with the plus end (+) of the β-subunit aligned to the minus end (-) of the next α- subunit. Tau attaches to kinesin proteins, which move along microtubules and affect cell division and organelle transport. As motor proteins, kinesin and dynein play key roles in assisting microtubules by transporting mitochondria through an ATP hydrolysis mechanism with axonal transport considered as microtubule-directed movements were inhibited with excess of tau aggregation [46, 47].
5. What is p-Tau?
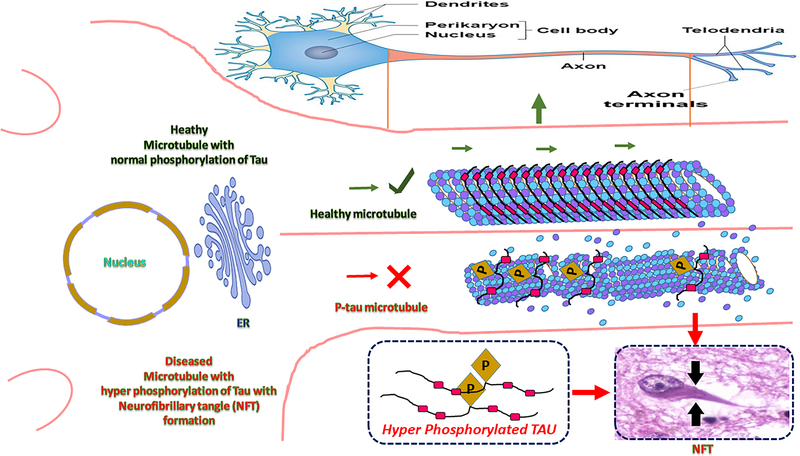
The well-described physiological properties of tau are that of a microtubule associated protein that binds to and stabilizes microtubules, particularly microtubules of neuronal axons [48, 49], Pathological tau can be seen in the brain with the development of phosphorylated pre-tangles and neuropil threads. In the events before the formation of tangles, tau undergoes a series of post-translational modifications, including hyperphosphorylation, acetylation, and truncation, which differentiate it from the normal tau that is seen in healthy brain neurons [50–54]. In AD, tau assumes a number of aberrant features, including, (i) tau mislocalization (ii) hyperphosphorylation [55, 56], and (iii) Tau -Aβ interactions including aggregation/fibrillation [57], leading to neuronal damage in AD (Fig 1).
Fig. 1. Cascade of possible tail events.
Normal phosphoiylated tau maintains the stability of microtubules. Hyperphosphorylated tau may trigger the dissociation of radical tau front the microtubule surface, potentially destabilizing the microtubule or changing the properties of the microtubule surface, which would affect the transport of organelles and lead to the formation of paired helical filaments (PHFs).
6. Mislocalization Neuronal Tau
Tau phosphorylation plays a key role in mediating the mis-localization of p-Tau. The tau protein may cause abnormal interactions between p-tau and other cytosolic/synaptic proteins. Mislocalization of p-Tau may also disrupt axonal transport, leading to synaptic degeneration in AD neurons [58]. Here the serine and threonine residues play a major role in the mis-localization of tau [59]. The experimental validations and reports indicate that healthy neurons maintain the proper special gradient of tau higher in axons and where the somato-dentrites are comparatively exhibited lower [60]. In the studies of wild type and AD mouse models, tau-mediated neurodegeneration has been found to be widespread in the brain, and p-Tau mislocalization has been found to result in synaptic damage (Fig 1).
7. Tau Phosphatase Functions
Microtubule stabilization by tau depends on dimerization of tau flanking regions, including the negatively charged N-terminal domain of one tau molecule interacting with a positively charged neighboring tau molecule [61]. This N-terminal domain is a region where tau regulates phosphosites of tau. These phosphosites of tau are at specific sites and consequently trigger disruption of microtubule organization. Tau phosphosites mostly flank the microtubule-binding region known as a projection domain which plays a key role in structural stabilization [62, 63]. Recent studies have speculated that the tau domains R3 and R4 are adult-specific and responsible for microtubule assembly, these sites may be involved in phosphorylation through the inhibition of phosphatase activities [64].
8. Tau Kinase Regulations
There are 79 to 80 serine, threonine, and tyrosine phosphorylation sites on the tau. All these sites are a variety of Ser, Thr, and Tyr phosphatase residues, such as phosphatase protein1, 2A, 2B, and 2C, all of which are involved in phosphorylation [65, 66]. Nearly 30 to 32 Ser, Thr or Tyr residues are involved in the inhibition of phosphatase 2A in AD neurons. Many kinases, like cyclin-dependent kinases (CDKs), glycogen synthase kinase-3β (GSK3β), protein kinase A (PKA), protein kinase C (PKC), are involved in p-tau phosphorylation regulation in the AD [65–67]. There is a balance between kinase and phosphatase functions in the brain in healthy physiological state in the presence of normal tau. In a disease state such as AD, most of the hyper-phosphorylation regions of p-tau are encoded with three sites Serine/Threonine/Tyrosine at T153 to S253.
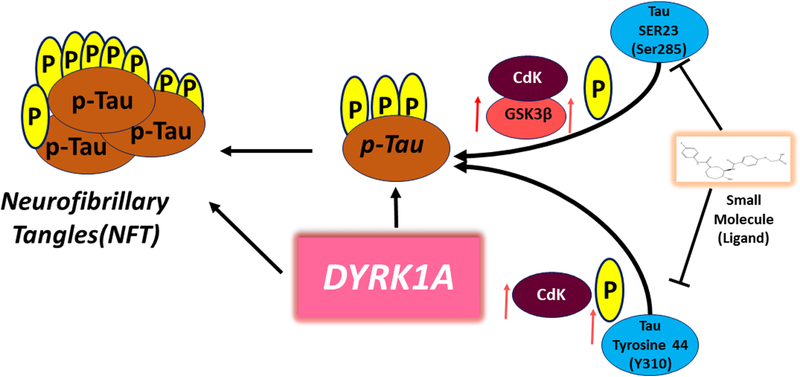
CDKs are at sites S235, S202, and S404. Other examples of phosphorylation sites include Ser214, 324, 356, 409, and 416; Ser214, 324; and Thr 377 by PKC. The Ser-Pro or Thr-Pro sites have been found in regions flanking Ser235 to Ser416. The phosphorylation sites of p-tau are affected by several kinases, such as CDK5 and GSK3β [68]. Lahiri’s group nicely summarized CDK5’s role in the brain [69,70]. Active GSK3β, CDK and its regulator c-JUN N-terminal kinase (JNK) are associated with neurofibrillary pathology and are upregulated in AD by the p-sites of Ser 285 and Tyr 310 (Fig 2).
Fig. 2. Phosphorylated tau sites kinase dependent regulation.
Small molecule for p-tau at Ser 285 and Tyr 310 dual specificity tyrosine-phosphoiylationregulated kinase 1A (DYRK1A) is a new high-potential therapeutic approach for Alzheimer disease. Small molecule inhibits p-tau and DYRK1A-induced Tau phosphorylation by regulating GSK3β, CDK5 including p-tau aggregation.
9. Aβ and tau-mediated disruption
Both intracellular and extracellular pathological proteins Aβ interact with p-Tau and are found to impair cognitive function in the AD brains [63, 64]. Increased Aβ42 expression in AD exacerbates tau-induced axonal transport defects and leads to cytoskeletal breakdown in AD. Studies of postmortem AD brains samples at different stages of AβPP, AβPPxPS1, and 3xTg-AD transgenic mice models, found that Aβ interacts with p-Tau and that these interactions are progressively increased in AD disease progression [71–75]. This increased p-Tau and oligomeric interaction may exacerbate with damaged neuronal structure and therefore contribute to the overall axonal transport dysfunction in AD and thus leading to cognitive decline in AD.
10. Therapeutic Strategies Targeting p-Tau
There are several components such as overexpression of 1) wild type and/or normal tau, oxidative stress exposure and abnormal interactions (with amyloid beta other toxic proteins), are involved in hyperphosphorylation of tau in AD and other tauopathies. Inhibition of normal and p-tau considered as therapeutic strategies (Table 1) [76–78]. However, clinical trials of tau-targeting small molecules for AD and other tauopathies are limited. Therefore, more research on small molecule inhibitors is urgently needed.
Table 1.
Some of the clinical trial studies of tau-targeting small molecules and their trial status in Alzheimer disease.
| Drugs | Clinical trial identifier | Dates | Trial description | Trial status |
|---|---|---|---|---|
| PP2A activators | ||||
| Memantine | NCT03168997 | 2017–2018 | Phase IV open-label, parallel-group interventional study (n = 222) | Not yet recruiting |
| Sodium selenate | ACTRN12613000170729 | 2012–2013 | Open-label extension study (n = 20) | Completed |
| GSK3β inhibitors | ||||
| Tideglusib | 2010-023322-21 | 2013–2015 | Phase II randomized, double-blind, placebo-controlled, four- arm efficacy study in mild to moderate AD (n = 306) | Completed |
| Lithium chloride | NCT02862210 | 2016–2020 | Phase II randomized, double-blind, placebo-controlled low dose efficacy study in FTDP (n = 60) | Not yet recruiting |
| Aggregation inhibitors | ||||
| LMTX | NCT02245568 | 2014–2018 | Phase III open-label extension study for patients in previous trials (n = 1,000) | Terminated |
| Curcumin | NCT00595582 | 2005–2008 | Single-group, open-label, dietary supplement in MCI (n = 10) | Terminated |
| Microtubule stabilizers | ||||
| Epithilone D | NCT01492374 | 2012–2013 | Phase I randomized, double-blind, placebo-controlled trial in mild AD (n = 40) | Completed |
| TPI 287 | NCT02133846 | 2014–2017 | Phase I randomized, double-blind, placebo-controlled, sequential-cohort dose-ranging study in CBD and PSP (n = 44) | Active, not recruiting |
10.1. Hyperphosporylated tau (p-tau) Reduction
Reducing p-tau levels would seem to be a logical therapeutic approach for AD. The reduction of p-tau expression is an important therapeutic strategy in multiple ways [79]. For example, inhibition of tau kinases and/or activation of phosphatases with ligand-based methods with discovery of interest against hyperphosphorylated tau.
10.2. P-tau Targeting Kinase inhibition
Hyperphosphorylation of tau is one of the earliest events in the development of AD, and the degree of p-tau phosphorylation reflects abnormal activity of both protein kinases and phosphatases in AD. Changes in the levels of active kinases in the brains of individuals with AD can be the result of upregulation of the kinase itself or disruption of its regulation. In AD condition, the levels of kinase upregulation are mostly linked with the active glycogen synthase kinase-3β, cyclin-dependent-like kinase 5 and c-JUN N-terminal kinases; if any of these are upregulated that leads to disruption of neuron structure in AD [80]. These kinases are responsible for phosphorylating at different sites of tau, hence the emergence of site-specific p-tau targeting kinase inhibition that might be a promising therapeutic approach with significant kinase inhibition in AD.
10.3. Drug Development
Hyperphosphorylation of the p-tau protein may play a critical role in NFT formation. In studies of drug development for AD, researchers have yet to develop therapeutic targets for preventing the propagation and aggregation of p-Tau with a novel therapeutic strategy [77].
11. Pharmacophore modeling
The pharmacophore virtual screening is the most promising and effective to disclose the disease by extracting common chemical electrostatic features from 3D structures of a set of known ligands representative of essential interactions between the ligands and a specific macromolecular target. This method may allow some sort of decreased state of side effects than the existing drug discovery approaches while discovering of new lead molecules against AD and tauopathies.
12. P-tau Structure Based Pharmacophores
Structure based protein models that enable the steric and electrostatic features of ligand atoms are essential for the optimal supramolecular interactions with 3-D molecular docking. The specific biological 3-D structured p-tau models are responsible for the molecular feature of the proteins. Based on these phenomena blocking those 3-D targeted structures triggers the biological function by blocking/altering the p-tau structures with ligands. The p-tau pharmacophore models and strength of the phenomena depend upon the key functional groups of atoms and a part of a volume associated with functional phosphorylated sites of tau protein. The pharmacophore features include hydrogen bond acceptors (HBA) (red), hydrogen bond donors (Green) and a hydrophobic group (yellow) and mechanically generated excluded volumes represented in (gray) of the model (Fig. 3). This is the most preferred advancement in drug discovery to find the best therapeutics of small molecules in drug discovery when the 3-D structures of proteins are available and characterized [81].
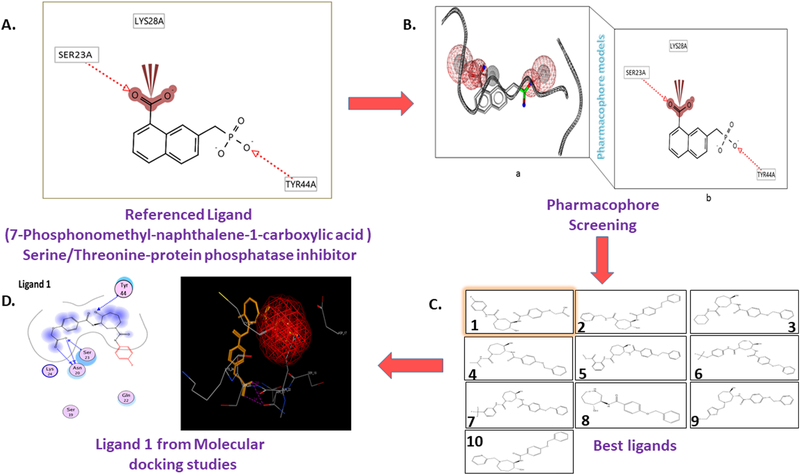
Fig. 3. Pharmacophore models of p-Tau and the drug discovery developmental workflow.
(A) a ligand-based l-D pharmacophore with a known p-tau ligand structure and ligand fingerprints, and (B) 3-D pharmacophore screening with a referenced p-tau ligand. In both cases, pharmacophore query can be used to identify chemical features, such as hydrogen bond acceptors (HBA) and hydrogen bond donors (HBD); and anionic(-), cationic(-l-), hydrophobic (Hi, and aromatic (R) groups for virtual screening. (C) Shows results front virtual screening searches of chemical databases to find the most reliable ligand sets and to filter pharmacophore targets. (D) Final ligand 1 molecule selected based and best docking scores for further validations on p-tau Ser/Thr/Tyr inhibition.
13. Pharmacophore based virtual screening
After the structure-based pharmacophores were generated it can be subjected to 3-D chemical structures imported from the 3-D chemical databases and implies to screen the pharmacophore-based ligands based virtual screening to find the hits based on the template p-tau pharmacophore model with Ser/Thr/Tyr inhibitor. Pharmacophore docking listed the best-fitted lead molecules with best docking scores. These pharmacophore-based molecular docking studies are reliable and are a very time-consuming process to screen the large data sets of chemical libraries to find the best fitted specific Ser/Thr/Tyr targeting p-tau ligand for AD.
14. Molecular Docking
P-tau targeting small molecules molecular docking approach explains the interaction between small molecules (ligands) and the target protein (p-tau) at the atomic level, to characterize the behavior of small molecules in the binding sites. The interaction of p-sites of S/T/Y residues that are involved in the kinase dependent regulation and ligand(s) may elucidate fundamental biochemical processes of a ligand based inhibitory actions in protein biochemistry. In light of the significance of 3D molecular interactions within the active core site of p-Tau is expected to provide the best ligand inhibitory action for better drug development. Small molecules are reported to bind at key hyperphosphorylated sites Ser 285(S23) and Tyr 310 (Y44) regulating the GSK3β and CDK5 of tau like in target and inhibit and/or change the activity of kinase protein function in disease states (Fig 2) [82].
15. PROTAC-based Drug discovery
The success of current targeted drug discovery approaches of p-tau is limited, allowing us to think new and novel approaches such as proteolysis-targeting chimeras (PROTAC). The PROTAC based drug molecules can be synthesized by conjugating ligand molecules with advanced targeted technology proteolysis-targeting chimeras. This is a novel and emerge approach in neurodegenerative diseases such AD. By utilization of this novel approach, new data can be generated in relation to p-tau phosphorylation of at different sites of GSK3-beta and CDK5. Developing multi-targeting small molecule ligands for p-tau is expected to reduce p-tau toxicities and kinase inhibition and protect AD neurons [83–84]. From the last decade to present day, therapeutic developmental approaches have been very limited and mostly unsuccessful. The development of P-tau-PROTACs molecules is the best therapeutic approach for AD and tauopathies diseases.
15.1. P-targeting Small-molecule PROTACs
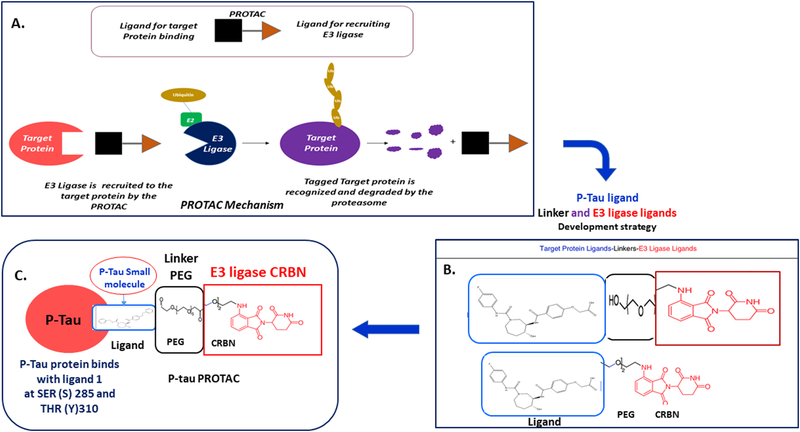
Proteolysis targeting chimeras (PROTACs) are a novel proteasome degradation through the ubiquitin- system is a promising technology for the many unsolved diseases including AD. Selecting and conjugating promising small molecules (ligand) that specifically bind to a target protein (p-tau) at Ser 285(S23) and Tyr 310 (Y44) regulating the GSK3β and CDK5, link this small molecule with a PEG-based linker and binding to E3 ubiquitin Cereblon (CRBN) ligase to develop p-tau PROTAC molecule. These p-tau PROTAC is a chemical knockdown strategy of toxic tau degradation through the ubiquitin-proteasome system [85]. Properties of p-sites phosphorylation and kinase regulation in AD and successful p-tau PROTACs treatments removes 90% pathogenic p-tau in AD (Fig. 4).
Fig. 4. Schematic representation brief plan for development of p-tan PROTACs strategy. A. general PROTAC Mechanism.
A degrader molecule E3 ligase was designed to preferentially recognize target protein, and simultaneously engage with CKBN in the CRL4CRBN E3 ubiquitin ligase complex. Degrader-mediated association of target protein with CR14CRBN and formation of a ternary complex is predicted to mediate tau ubiquitination and degradation by the proteasome. B. Ptau PROTACs are development strategy. PROTAC require three primary components: First a ligand at one end that targets the protein of interest (POI) i.e. p-tau. Second ligand (small molecule) at die opposite end that binds an E3 ligase Carbon (CRBN), and third a cross linker Polyethylene Glycol (PEG) in the middle that joins the two ends. C. P-tau PROTAC molecule. Small molecule of p-tau target is associated with the key kinases sites of SER (S) 23 (Ser 285) and TYR (Y) 44 (Tyr 310). Target protein is hyperphosphorylated tau (p-tau). Linker- Polyethylene Glycol (PEG) and CRBN E3 ubiquitin ligase complex works as p-tau PROTAC a new bifunctional PROTAC molecules inhibits the p-tau and DYRKIA at specific hyper phosphorylation sites by inhibiting the GSKSβ and CDK kinase upregulation in AD.
Development of small molecules p-tau- PROTACs to reduce p-tau toxicity and improve the synaptic function in AD [86]. These P-targeting Small-molecule PROTACs will provide new information about hyperphosphorylation of tau and kinase dependent regulation in AD, possible p-tau targeted therapeutics development (p-tau-PROTACs) for AD and tauopathies (Fig. 4).
15.2. Challenges and directions of PROTACs
The size of the PROTAC molecules is relatively large and may severely affect their drug-like properties. Currently available PROTAC molecules used for the treatments including Parkinson’s disease, Alzheimer’s disease, Huntington’s disease, and cancers are usually intraperitoneal, subcutaneously, or intravenously administered study for in vivo validation and not orally active PROTAC drugs. If target proteins have low affinities with PROTACs, this can be not effectively degraded molecule while degradation process with low efficacy of PROTACs [87, 88]. Blood–brain barrier (BBB) penetration for PROTACs is a challenging issue with the high molecular weight PROTACs construction (small molecules, E3 ligase and linkers) and development. In order to keep all the possible challenges from 1). Screening and selecting small molecule libraries against proteins with active sites, 2). Low molecular weight small molecules selection, E3 ligases, linkers, and have great potentials in extending the 3). High affinity based PROTAC-based small molecule drugs may offer for many diseases including AD and tauopathies.
16. Concluding Remarks and Future Perspectives
Increasing evidence suggests that tau hyperphosphorylation and free aggregation are toxic to the AD and other tauopathies. Currently available drugs are not capable of reducing p-tau toxicity and protect AD neurons Therefore, there is an urgent need to develop novel and innovative approaches to reduce p-tau toxicity. P-tau PROTAC-based small molecule inhibitors approach is emerging and novel, and results of p-Tau PROTAC based drug discovery are promising. However, more basic research and clinical applications are needed to understand the translational aspects of p-tau PROTAC-based small molecule inhibitors.
Highlights.
Normal tau maintains cytoskeletal stability, transport cargos from the cell soma to nerve terminals and maintain neuronal outgrowth.
Hyperphosphorylated tau causes unstable microtubule binding leading to defective axonal transport.
P-tau sites that activate kinase dependent phosphorylation and activates microtubule instability in Alzheimer’s disease.
Pharmacophore chemical features of p-tau ligands and advanced PROTAC targeted therapies may provide better drug discovery approaches.
Acknowledgments
The authors would like to thank NIH for funding various projects - R01AG042178, R01AG47812, R01NS105473, AG060767, AG069333, and AG066347 (P.H.R).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF COMPETING INTEREST
We would like to inform you that we have a pending patent for Alzheimer’s Disease related to the contents of our manuscript.
Declaration of competing interest
We would like to inform that we have a pending patent on development of p-tau-PROTACs targeted small molecules related to the contents of our manuscript.
References
- 1.Prince M, Herrera C, Knapp M, et al. , World Alzheimer report 2016: improving healthcare for people living with dementia: coverage, quality and costs now and in the future. London, UK: Alzheimer’s Disease International (ADI). 2016:131. [Google Scholar]
- 2.Selkoe DJ. Presenilin, Notch, and the genesis and treatment of Alzheimer’s disease. Proc Natl Acad Sci U S A. 98 (20) (2001) 11039–11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci. 8(7) (2007) 499–509. [DOI] [PubMed] [Google Scholar]
- 4.Pascoal TA, Mathotaarachchi S, Shin M, Benedet AL, Mohades S, Wang S, Beaudry T, Kang MS, Soucy JP, Labbe A, Gauthier S, Rosa-Neto P. Alzheimer’s Disease Neuroimaging Initiative. Synergistic interaction between amyloid and tau predicts the progression to dementia. Alzheimers Dement. 13(6) (2017) 644–653. [DOI] [PubMed] [Google Scholar]
- 5.Reddy PH. Abnormal tau, mitochondrial dysfunction, impaired axonal transport of mitochondria, and synaptic deprivation in Alzheimer’s disease. Brain Res. 1415 (2011) 136–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forner S, Baglietto-Vargas D, Martini AC, Trujillo-Estrada L, LaFerla FM. Synaptic Impairment in Alzheimer’s Disease: A Dysregulated Symphony. Trends Neurosci. 40(6) (2017) 347–357. [DOI] [PubMed] [Google Scholar]
- 7.Sheladia S, Reddy PH. Age-Related Chronic Diseases and Alzheimer’s Disease in Texas: A Hispanic Focused Study. J Alzheimers Dis Rep. 5(1) (2021) 121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.John A, Reddy PH. Synaptic basis of Alzheimer’s disease: Focus on synaptic amyloid beta, P-tau and mitochondria. Ageing Res Rev. 65 (2021) 101208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George EK, Reddy PH. Can Healthy Diets, Regular Exercise, and Better Lifestyle Delay the Progression of Dementia in Elderly Individuals? J Alzheimers Dis. 72(s1) (2019) S37–S58. [DOI] [PubMed] [Google Scholar]
- 10.Maloney B, Lahiri DK. Epigenetics of dementia: understanding the disease as a transformation rather than a state. Lancet Neurol. 15(7) (2016) 760–774. [DOI] [PubMed] [Google Scholar]
- 11.Bakulski KM, Rozek LS, Dolinoy DC, Paulson HL, Hu H. Alzheimer’s disease and environmental exposure to lead: the epidemiologic evidence and potential role of epigenetics. Curr Alzheimer Res. 9(5) (2012) 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yegambaram M, Manivannan B, Beach TG, Halden RU. Role of environmental contaminants in the etiology of Alzheimer’s disease: a review. Curr Alzheimer Res. 12(2) (2015) 116–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dent EW, Baas PW. Microtubules in neurons as information carriers. J. Neurochem. 129(2) (2014) 235–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van de Willige D, Hoogenraad CC, Akhmanova A. Microtubule plus-end tracking proteins in neuronal development. Cell Mol Life Sci. 73(10) (2016) 2053–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones SL, Svitkina TM. Axon Initial Segment Cytoskeleton: Architecture, Development, and Role in Neuron Polarity. Neural Plast. (2016) 6808293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 72(5) (1975) 1858–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabbagh JJ, Dickey CA. The Metamorphic Nature of the Tau Protein: Dynamic Flexibility Comes at a Cost. Front Neurosci. (2016) (10) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmadzadeh H, Smith DH, Shenoy VB. Mechanical Effects of Dynamic Binding between Tau Proteins on Microtubules during Axonal Injury. Biophys J. 109 (11) 2015. 2328–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drubin DG, Feinstein SC, Shooter EM, Kirschner MW. Nerve growth factor-induced neurite outgrowth in PC12 cells involves the coordinate induction of microtubule assembly and assembly-promoting factors. J. Cell Biol. 101(5 Pt 1): 19851799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brandt R, Götz J. Special issue on ’Cytoskeletal proteins in health and neurodegenerative disease’. Brain Res Bull. 126(Pt 3) (2016) 213–216. [DOI] [PubMed] [Google Scholar]
- 21.Orr ME, Sullivan AC, Frost B. A Brief Overview of Tauopathy: Causes, Consequences, and Therapeutic Strategies. Trends Pharmacol Sci. 38 (7) (2017) 637–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spillantini MG, Goedert M. Tau pathology and neurodegeneration. Lancet Neurol. 12(6) (2013) 609–622. [DOI] [PubMed] [Google Scholar]
- 23.Siegel GJ, Agranoff BW, Albers RW, et al. , editors. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th edition. Philadelphia: Lippincott-Raven; 1999. Available from: https://www.ncbi.nlm.nih.gov/books/NBK20385/ [Google Scholar]
- 24.Penazzi L, Bakota L, Brandt R. Microtubule Dynamics in Neuronal Development, Plasticity, and Neurodegeneration. Int Rev Cell Mol Biol. 321 (2016) 89–169. [DOI] [PubMed] [Google Scholar]
- 25.Karki R, Mariani M, Andreoli M, He S, Scambia G, Shahabi S, Ferlini C. ßIII-Tubulin: biomarker of taxane resistance or drug target? Expert Opin Ther Targets. 17(4) (2013) 461–472. [DOI] [PubMed] [Google Scholar]
- 26.Pilhofer M, Ladinsky MS, McDowall AW, Petroni G, Jensen GJ. Microtubules in bacteria: Ancient tubulins build a five-protofilament homolog of the eukaryotic cytoskeleton. PLoS Biol. 9(12) (2011) e1001213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlieper D, Oliva MA, Andreu JM, Löwe J. Structure of bacterial tubulin BtubA/B: evidence for horizontal gene transfer. Proc Natl Acad Sci U S A. 102(26) (2005) 9170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brettschneider J, Del Tredici K, Lee VM, Trojanowski JQ. Spreading of pathology in neurodegenerative diseases: a focus on human studies. Nat Rev Neurosci. 16(2) (2015) 109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee SJ, Lim HS, Masliah E, Lee HJ. Protein aggregate spreading in neurodegenerative diseases: problems and perspectives. Neurosci Res. 70(4) (2011) 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campion D, Pottier C, Nicolas G, Le Guennec K, Rovelet-Lecrux A. Alzheimer disease: modeling an Aβ-centered biological network. Mol Psychiatry. 21(7) (2016) 861–871. [DOI] [PubMed] [Google Scholar]
- 31.Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci. 4(1) (2003) 49–60. [DOI] [PubMed] [Google Scholar]
- 32.Puri R, Suzuki T, Yamakawa K, Ganesh S. Hyperphosphorylation and aggregation of Tau in laforin-deficient mice, an animal model for Lafora disease. J Biol Chem. 284(34) (2009) 22657–22663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conde C, Cáceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci. 10(5) (2009) 319–332. [DOI] [PubMed] [Google Scholar]
- 34.Rajmohan R, Reddy PH. Amyloid-Beta and Phosphorylated Tau Accumulations Cause Abnormalities at Synapses of Alzheimer’s disease Neurons. J Alzheimers Dis. 57(4) (2017) 975–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pozueta J, Lefort R, Shelanski ML. Synaptic changes in Alzheimer’s disease and its models. Neuroscience. 251 (2013) 51–65. [DOI] [PubMed] [Google Scholar]
- 36.Buée L, Bussière T, Buée-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev. 33(1) (2000) 95–130. [DOI] [PubMed] [Google Scholar]
- 37.Pittman AM, Fung HC, de Silva R. Untangling the tau gene association with neurodegenerative disorders. Hum Mol Genet. 15 Spec No 2: 2006R188–95. [DOI] [PubMed] [Google Scholar]
- 38.Maloney B, Lahiri DK. Structural and functional characterization of H2 haplotype MAPT promoter: unique neurospecific domains and a hypoxia-inducible element would enhance rationally targeted tauopathy research for Alzheimer’s disease. Gene. 501(1) (2012) 63–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.West RR, Tenbarge KM, Olmsted JB. A model for microtubule-associated protein 4 structure. Domains defined by comparisons of human, mouse, and bovine sequences. J Biol Chem. 266(32) (1991) 21886–21896. [PubMed] [Google Scholar]
- 40.Lapointe NE, Horowitz PM, Guillozet-Bongaarts AL, Silva A, Andreadis A, Binder LI. Tau 6D and 6P isoforms inhibit polymerization of full-length tau in vitro. Biochemistry. 48 (51) (2009) 12290–12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Csizmok V, Follis AV, Kriwacki RW, Forman-Kay JD. Dynamic Protein Interaction Networks and New Structural Paradigms in Signaling. Chem Rev. 116 (11) (2016) 6424–6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levy SF, Leboeuf AC, Massie MR, Jordan MA, Wilson L, Feinstein SC. Three-and four-repeat tau regulate the dynamic instability of two distinct microtubule subpopulations in qualitatively different manners. Implications for neurodegeneration. J Biol Chem. 280(14) (2005) 13520–13528. [DOI] [PubMed] [Google Scholar]
- 43.Morgan AA, Rubenstein E. Proline: the distribution, frequency, positioning, and common functional roles of proline and polyproline sequences in the human proteome. PLoS One. 8(1) (2013) e53785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Künze G, Barré P, Scheidt HA, Thomas L, Eliezer D, Huster D. Binding of the three-repeat domain of tau to phospholipid membranes induces an aggregated-like state of the protein. Biochim Biophys Acta. 1818(9) (2012) 2302–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sündermann F, Fernandez MP, Morgan RO. An evolutionary roadmap to the microtubule-associated protein MAP Tau. BMC Genomics. 17 (2016) 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Avila J, Jiménez JS, Sayas CL, Bolós M, Zabala JC, Rivas G, Hernández F. Tau Structures. Front Aging Neurosci. 8 (2016) 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neukirchen D, Bradke F. Cytoplasmic linker proteins regulate neuronal polarization through microtubule and growth cone dynamics. J Neurosci. 31(4) (2011) 1528–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sayas CL, Tortosa E, Bollati F, Ramírez-Ríos S, Arnal I, Avila J. Tau regulates the localization and function of End-binding proteins 1 and 3 in developing neuronal cells. J Neurochem. 133(5) (2015) 653–667. [DOI] [PubMed] [Google Scholar]
- 49.Niewidok B, Igaev M, Sündermann F, Janning D, Bakota L, Brandt R. Presence of a carboxy-terminal pseudorepeat and disease-like pseudohyperphosphorylation critically influence tau’s interaction with microtubules in axon-like processes. Mol Biol Cell. 27(22) (2016) 3537–3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dubey J, Ratnakaran N, Koushika SP. Neurodegeneration and microtubule dynamics: death by a thousand cuts. Front Cell Neurosci. 9 (2015) 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murthy MN, Veerappa AM, Seshachalam KB, Ramachandra NB. High-resolution arrays reveal burden of copy number variations on Parkinson disease genes associated with increased disease risk in random cohorts. Neurol Res. 38(9) (2016) 775–85. [DOI] [PubMed] [Google Scholar]
- 52.He RJ, Yu ZH, Zhang RY, Zhang ZY. Protein tyrosine phosphatases as potential therapeutic targets. Acta Pharmacol Sin. 35(10) (2014) 1227–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vassilopoulos A, Fritz KS, Petersen DR, Gius D. The human sirtuin family: evolutionary divergences and functions. Hum Genomics. 5(5) (2011) 485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bodea LG, Eckert A, Ittner LM, Piguet O, Götz J. Tau physiology and pathomechanisms in frontotemporal lobar degeneration. J Neurochem. 138 Suppl 1(Suppl Suppl 1) (2016) 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pedersen JT, Sigurdsson EM. Tau immunotherapy for Alzheimer’s disease. Trends Mol Med. 21(6) (2015) 394–402. [DOI] [PubMed] [Google Scholar]
- 56.Hoover BR, Reed MN, Su J, Penrod RD, Kotilinek LA, Grant MK, Pitstick R, Carlson GA, Lanier LM, Yuan LL, Ashe KH, Liao D. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 68(6) (2010) 1067–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller EC, Teravskis PJ, Dummer BW, Zhao X, Huganir RL, Liao D. Tau phosphorylation and tau mislocalization mediate soluble Aβ oligomer-induced AMPA glutamate receptor signaling deficits. Eur J Neurosci. 39(7) (2014) 1214–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hefti F, Goure WF, Jerecic J, Iverson KS, Walicke PA, Krafft GA. The case for soluble Aβ oligomers as a drug target in Alzheimer’s disease. Trends Pharmacol Sci. 34(5) (2013) 261–266. [DOI] [PubMed] [Google Scholar]
- 59.Brandt R, Hundelt M, Shahani N. Tau alteration and neuronal degeneration in tauopathies: mechanisms and models. Biochim Biophys Acta. 1739(2–3) (2005) 331–354. [DOI] [PubMed] [Google Scholar]
- 60.Gendron TF, Petrucelli L. The role of tau in neurodegeneration. Mol Neurodegener. 4 (2009) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Winters BD, Jin SX, Ledford KR, Golding NL. Amplitude Normalization of Dendritic EPSPs at the Soma of Binaural Coincidence Detector Neurons of the Medial Superior Olive. J Neurosci. 37(12) (2017) 3138–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Illenberger S, Zheng-Fischhöfer Q, Preuss U, Stamer K, Baumann K, Trinczek B, Biernat J, Godemann R, Mandelkow EM, Mandelkow E. The endogenous and cell cycle-dependent phosphorylation of tau protein in living cells: implications for Alzheimer’s disease. Mol Biol Cell. 9(6) (1998) 1495–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hensley K, Kursula P. Collapsin Response Mediator Protein-2 (CRMP2) is a Plausible Etiological Factor and Potential Therapeutic Target in Alzheimer’s Disease: Comparison and Contrast with Microtubule-Associated Protein Tau. J Alzheimers Dis. 53(1) (2016) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brunden KR, Trojanowski JQ, Lee VM. Advances in tau-focused drug discovery for Alzheimer’s disease and related tauopathies. Nat Rev Drug Discov. 8(10) (2009) 783–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alonso AD, Di Clerico J, Li B, Corbo CP, Alaniz ME, Grundke-Iqbal I, Iqbal K. Phosphorylation of tau at Thr212, Thr231, and Ser262 combined causes neurodegeneration. J Biol Chem. 285(40) (2010. )30851–30860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hosokawa T, Saito T, Asada A, Fukunaga K, Hisanaga S. Quantitative measurement of in vivo phosphorylation states of Cdk5 activator p35 by Phos-tag SDS-PAGE. Mol Cell Proteomics. 9(6) (2010) 1133–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reddy PH, Reddy TP. Mitochondria as a therapeutic target for aging and neurodegenerative diseases. Curr Alzheimer Res. 8(4) (2011) 393–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sheppard O, Plattner F, Rubin A, Slender A, Linehan JM, Brandner S, Tybulewicz VL, Fisher EM, Wiseman FK. Altered regulation of tau phosphorylation in a mouse model of down syndrome aging. Neurobiol Aging. 4 (828) (2012) e31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shah K, Lahiri DK. Cdk5 activity in the brain - multiple paths of regulation. J Cell Sci. 127(Pt 11) (2014) 2391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shah K, Lahiri DK. A Tale of the Good and Bad: Remodeling of the Microtubule Network in the Brain by Cdk5. Mol Neurobiol. 54(3) (2017) 2255–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trinczek B, Biernat J, Baumann K, Mandelkow EM, Mandelkow E. Domains of tau protein, differential phosphorylation, and dynamic instability of microtubules. Mol Biol Cell. 6 (12) (1995)1887–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu D, Pitta M, Lee JH, Ray B, Lahiri DK, Furukawa K, Mughal M, Jiang H, Villarreal J, Cutler RG, Greig NH, Mattson MP. The KATP channel activator diazoxide ameliorates amyloid-β and tau pathologies and improves memory in the 3xTgAD mouse model of Alzheimer’s disease. J Alzheimers Dis. 22(2) (2010) 443–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bloom GS. Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 71(4) (2014) 505–508. [DOI] [PubMed] [Google Scholar]
- 74.Norstrom E. Metabolic processing of the amyloid precursor protein -- new pieces of the Alzheimer’s puzzle. Discov Med. 23(127) (2017) 269–276. [PubMed] [Google Scholar]
- 75.Giacobini E, Gold G. Alzheimer disease therapy--moving from amyloid-β to tau. Nat Rev Neurol. 9(12) (2013) 677–686. [DOI] [PubMed] [Google Scholar]
- 76.Congdon EE, Sigurdsson EM. Tau-targeting therapies for Alzheimer disease. Nat Rev Neurol. 14(7) (2018) 399–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Domínguez JM, Fuertes A, Orozco L, del Monte-Millán M, Delgado E, Medina M. Evidence for irreversible inhibition of glycogen synthase kinase-3β by tideglusib. J Biol Chem. 287(2) (2012) 893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Panza F, Solfrizzi V, Seripa D, Imbimbo BP, Lozupone M, Santamato A, Zecca C, Barulli MR, Bellomo A, Pilotto A, Daniele A, Greco A, Logroscino G. Tau-Centric Targets and Drugs in Clinical Development for the Treatment of Alzheimer’s Disease. Biomed Res Int. (2016) 3245935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Noble W, Jimenez-Sanchez M, Perez-Nievas BG, Hanger DP. Considerations for future tau-targeted therapeutics: can they deliver? Expert Opin Drug Discov. 15(3) (2020) 265–267. [DOI] [PubMed] [Google Scholar]
- 80.Rayasam GV, Tulasi VK, Sodhi R, Davis JA, Ray A. Glycogen synthase kinase 3: more than a namesake. Br J Pharmacol. 156 (6) (2009) 885–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pradeepkiran JA, Reddy PH. Structure Based Design and Molecular Docking Studies for Phosphorylated Tau Inhibitors in Alzheimer’s Disease. Cells. 8(3) (2019) 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pradeepkiran JA, Reddy AP, Reddy PH. Pharmacophore-based models for therapeutic drugs against phosphorylated tau in Alzheimer’s disease. Drug Discov Today. 24(2) (2019) 616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.An S, Fu L. Small-molecule PROTACs: An emerging and promising approach for the development of targeted therapy drugs. EBioMedicine. 36 (2018) 553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Silva MC, Haggarty SJ. Tauopathies: Deciphering Disease Mechanisms to Develop Effective Therapies. Int J Mol Sci. 21(23) (2020) 8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Silva MC, Ferguson FM, Cai Q, Donovan KA, Nandi G, Patnaik D, Zhang T, Huang HT, Lucente DE, Dickerson BC, Mitchison TJ, Fischer ES, Gray NS, Haggarty SJ. Targeted degradation of aberrant tau in frontotemporal dementia patient-derived neuronal cell models. Elife. 8: (2019) e45457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kargbo RB, Treatment of Alzheimer’s by PROTAC-Tau Protein Degradation. ACS Med Chem Lett. 10, (2019) 699–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sun X, PROTACs: great opportunities for academia and industry. Signal Transduct Target Ther. 4, (2019) 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Drummond ML, Improved Accuracy for Modeling PROTAC-Mediated Ternary Complex Formation and Targeted Protein Degradation via New In Silico Methodologies. J. Chem Inf Model. 60, (2020) 5234–5254. [DOI] [PubMed] [Google Scholar]