Abstract
Recent histological analyses of human brains show that small-vessel type injuries in the setting of type-2 diabetes are colocalized with deposits of amylin, an amyloid-forming hormone secreted by the pancreas. Amylin inclusions are also identified in circulating red blood cells in persons with type-2 diabetes and stroke or cardiovascular disease. In laboratory models of type-2 diabetes, accumulation of aggregated amylin in blood and the cerebral microvasculature induces brain microhemorrhages and reduces cerebral blood flow leading to white matter ischemia and neurological deficits. At the cellular level, aggregated amylin causes cell membrane lipid peroxidation injury, downregulation of tight junction proteins and activation of pro-inflammatory signaling pathways which, in turn, induces macrophage activation and macrophage infiltration in vascular areas positive for amylin deposition. We review each step of this cascade based on experimental and clinical evidence, and propose the hypothesis that systemic amylin dyshomeostasis may underlie the disparity between glycemic control and stroke risk and may be a therapeutic target to reduce the risk of small-vessel ischemic stroke in patients with type-2 diabetes.
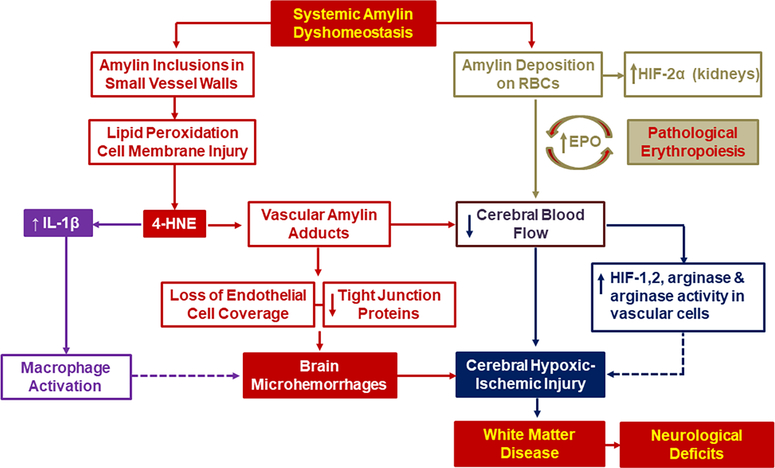
There is a disparity between the level of glycemic control and the risk of macrovascular events such as myocardial infarction and stroke in persons with type-2 diabetes mellitus.1,2 Amylin, an amyloidogenic peptide synthesized and co-secreted with insulin by pancreatic β-cells,3 is overexpressed in individuals with prediabetic insulin resistance,4–6 and forms pancreatic amyloid in those with type-2 diabetes.7–9 Low molecular weight amyloidogenic proteins such as amylin and the Alzheimer’s disease biomarker β-amyloid peptide generate a variety of cytotoxic aggregates.10–13 Studies from multiple research teams find that diabetic states (prediabetic insulin resistance and type-2 diabetes) are associated with increased circulating levels of aggregated amylin14,15 and amylin deposition in extra-pancreatic tissues,14–26. including the brain microvasculature.14,16–20. By using rats with genetically manipulated amylin secretion, our team showed that accumulation of aggregated amylin in the blood and microvasculature causes brain microhemorrhages and reduced cerebral blood flow leading to white matter ischemic changes and neurological deficits.16. At the cellular level, amylin inclusions in vascular walls cause lipid peroxidation-related cell membrane injury, loss of endothelial cell coverage, and downregulation of tight junction proteins (Figure 1, red code pathway). Lipid peroxidation vascular injury contributes to a pro-inflammatory state affecting endothelial cells which, in turn, induces macrophage activation and macrophage infiltration in vascular areas positive for amylin deposition and microhemorrhages (Figure 1, magenta code pathway). Furthermore, amylin inclusions are identified in red blood cells (RBCs) of individuals with type-2 diabetes and stroke or cardiovascular disease.26 Amylin-coated RBCs directly activate hypoxia signaling (Figure 1, green code pathway), have increased adhesion to vascular endothelial cells, and a tendency to aggregate.26 Thus, amylin-mediated microvascular injury, macrophage infiltration, and reduced RBC flux due to amylin deposition on RBCs and endothelial cells may increase the risk of small vessel-type ischemic stroke (Figure 1, navy code pathway). Each step of this cascade is discussed in detail based on experimental and clinical evidence.
Figure 1. The amylin dyshomeostasis hypothesis of small vessel-type ischemic stroke in the setting of type-2 diabetes mellitus.
Systemic amylin dyshomeostasis is characterized by accumulation of aggregated amylin in blood and microvasculature, brain microhemorrhages and reduced cerebral blood flow leading to white matter injury and neurological deficits. At the cellular level, amylin inclusions in vascular walls generate reactive aldehydes such as 4-hydroxinonenal (4-HNE) and vascular amylin adducts causing loss of endothelial cell coverage and downregulation of tight junction proteins (red code pathway). Increased 4-HNE levels activates pro-inflammatory IL-1β signaling pathways leading to macrophage activation and macrophage infiltration in vascular areas positive for amylin deposition (magenta code pathway). In addition, amylin-coated RBCs directly activate hypoxia signaling (green code pathway), have increased adhesion to vascular endothelial cells and tendency to aggregate exacerbate the risk of small vessel-type ischemic stroke (navy code pathway).
Circulating aggregated amylin induces brain microhemorrhages.
Radiographically-defined brain microhemorrhages (vascular microlesions)27,28 correspond histologically to hemosiderin-laden phagocytic microglia.29–31 They are associated with cerebral amyloid angiopathy, hypertension, and cerebral hypoperfusion associated with arteriolosclerosis, in addition to other conditions.21–34
We found that amylin aggregation in pancreatic islets leads to a feed-forward pathologic process by which aggregated amylin is secreted into the blood, deposits in brain blood vessels, and provokes brain microvascular injury by degrading endothelial cell coverage and tight junctions in rats that express amyloid-forming human amylin within pancreatic β-cells (HIP, human amylin insulin promoter transgenic rats).16 This amylin deposition in cerebral small blood vessels is associated with vessel wall disruption and abnormal surrounding neuropil in patients with type-2 diabetes and vascular dementia,16 in HIP rats,16,35 and in amylin knockout rats infused with aggregated amylin.16 In HIP rats, amylin-mediated injury in the cerebral microvasculature leads to accelerated aging,16 neuroinflammation,35 brain parenchymal loss,16 and impaired neurological functioning.16,35. These data identify amylin deposition in cerebral small vessels as a trigger of brain microhemorrhages and neurologic deficits that are modulated by the circulating level of aggregated amylin.16,35.
Amylin-induced vascular microlesions activate Interleukin-1β (IL-1β) pro-inflammatory pathways.
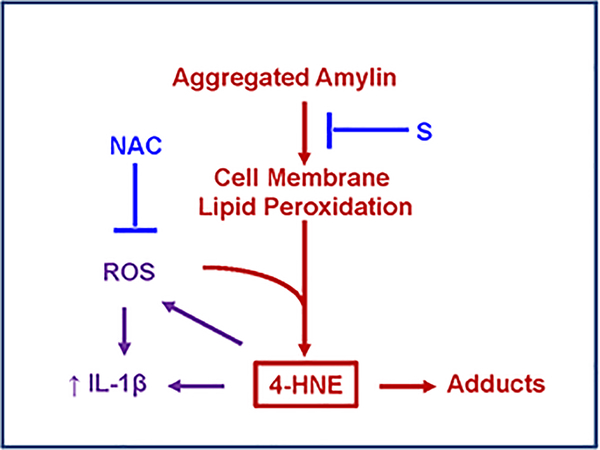
In pancreatic islets, aggregated amylin induces oxidative stress11,12 leading to NLRP3 inflammasome activation and release of IL-1β,36,37 a cytokine involved in a plethora of inflammatory responses.38 In vascular cells, IL-1β is involved in the signaling pathway that mediates leukocyte interactions.38. Histological analyses in human tissues (brain39 and heart15) in addition to in vivo experiments in transgenic animals and cell model systems showed that the interaction between aggregated amylin and cellular membranes destabilizes cellular membranes and generates reactive aldehydes.15,39 The functional effects of these cellular processes include the increased synthesis of IL-1β15,39 in extra-pancreatic tissues, consistent with amylin-mediated injury in pancreatic β-cells and elevated IL-1β in type-2 diabetes.36,37 Thus, exacerbated synthesis of IL-1β may be a critical stress-activated signaling pathway in response to the interaction of aggregated amylin with cellular membranes. Reports from our team also show that generation of reactive aldehydes and increased synthesis of IL-1β are caused by amylin aggregation independent of hyperglycemia.15,39. As proof of concept for the proposed mechanism, we evaluated the effect of combined treatments with N-acetyl cysteine, an antioxidant, and a surfactant membrane stabilizer15,39 and found they synergistically inhibit the lipid peroxidation chain reaction and renormalize IL-1β synthesis15,39 (Figure 2). Thus, IL-1β might function as a sensor of cellular amylin uptake and potential mediator of pro-inflammatory responses to amylin-induced brain microhemorrhages.
Figure 2. Proposed mechanism for amylin-mediated lipid peroxidation cell membrane injury and activation of IL-1β pro-inflammatory signaling pathway.
(adapted from Ref. 15). Amylin inclusions in cellular membranes generate reactive aldehydes such as 4-hydroxnonenal (4-HNE) that perturb intracellular homeostasis, leading to increased synthesis of IL-1β. Blocking either cellular amylin uptake (by a surfactant cell membrane stabilizer; S), or the lipid peroxidation chain reaction (by N-acetyl cysteine; NAC), demonstrate that peroxidative membrane injury is upstream of IL-1β increased synthesis.
Amylin-mediated vascular microlesions activate hypoxia signaling.
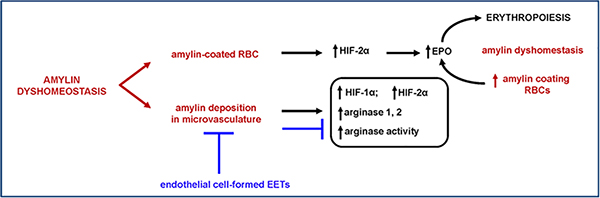
Using biochemical analyses of human blood and blood transfusions in transgenic rats, our team found that amylin accumulation in blood cells and microvasculature activate hypoxia-inducible transcription factors (HIF-1 and HIF-2) in endothelial cells.26 Erythropoietin upregulation, a consequence of hypoxia signaling activation, correlate with lower hematocrit in HIP rats,26 common in pathologic erythropoiesis.40 These effects are present in diabetic HIP rats that express amyloid-forming human amylin in the pancreas, but not in age- and blood glucose-matched rats that express non-amyloid forming rat amylin demonstrating that amylin-induced hypoxia signaling is independent of glucotoxicity.26 Vascular amylin deposition in HIP rats induces arginase dysregulation,26 suggesting subsequent effects on nitric oxide production and endothelium-mediated regulation of vascular smooth muscle cell tone. Nitric oxide production is modulated via regulation of two opposing L-arginine metabolic pathways.41. HIF-1 induces expression of endothelial nitric oxide synthase whereas HIF-2 regulates arginase expression, with both enzymes dependent upon L-arginine as a substrate.41 Increased arginase expression/activity can therefore result in nitric oxide deficiency and deleterious effects on endothelium-mediated regulation of vascular smooth muscle tone, which can exacerbate blood flow impairment in cerebral ischemia. Pharmacological downregulation of adhesion proteins in the vascular endothelium ameliorates the effects of circulating aggregated amylin while also reducing HIF-1,2 and arginase protein expression levels26 (Figure 3). These results suggest that endothelial adhesion proteins are potential therapeutic targets to reduce vascular amylin deposition and pathology.
Figure 3. RBC-vascular endothelium interaction is altered by amylin deposition on RBCs and microvasculature, and is reversed by endothelial cell-secreted epoxyeicosatrienoic acids (EETs).
(adapted from Ref. 26). Amylin deposition on RBCs activates HIF-mediated hypoxia signaling pathways in kidneys and downstream upregulation of erythropoietin (EPO). These cellular processes are associated with pathologic erythropoiesis and arginase dysregulation within vascular tissue. EETs reduce this effect by downregulation of adhesion proteins in the vascular endothelium.
Amylin-coated RBCs and vascular amylin deposits promote microthrombi.
RBC amylin content can be a useful maker of abnormally increased secretion of amyloid-forming amylin species from pancreatic islets.26. Because amylin deposition on RBCs directly affects rheological properties of the blood and increases the adhesion of RBCs to endothelium,26 these processes likely contribute to the complex mechanisms underlying diabetic microvascular disease.42,43
RBCs passively incorporate into the growing fibrin network of thrombi via binding to leukocytes and platelets.44 Because the inter-cell interaction appears more important in RBC aggregation than adhesion of RBCs to vascular endothelium,45,46 we hypothesize that the amyloidogenic nature of amylin in amylin-coated RBCs promotes aggregation. Aggregated RBCs could further interact with clotting factors to form microthrombi. Indeed, increased RBC aggregation is common in chronic cerebral ischemia,47,48 acute stroke49,50 and microvascular angina.51 Slowed capillary RBC flow owing to amylin deposition on RBCs and endothelial cells likely leads to impaired neuronal oxygen delivery. Furthermore, because RBCs act as both oxygen carriers and mediators of oxygen sensing and signaling pathways within blood capillary walls,40 amylin-coated RBCs may directly contribute to cerebral hypoxic-ischemic injury.
Clinical perspectives.
Amylin-mediated microvascular injury, macrophage infiltration and reduced RBC flow due to amylin deposition on RBCs and endothelial cells likely exacerbate circulatory disturbances leading to ischemic tissue injury in the setting of type-2 diabetes mellitus. Noteworthy, amylin-positive occluded small blood vessels can be identified both in brain16 and peripheral tissues,23,25 and were also induced in healthy rats by RBC transfusions from diabetic rats expressing amyloid-forming human amylin.26 Because human amylin is amyloidogenic, whereas rodent amylin is not,52 this provides the opportunity to advance mechanistic studies on the potential role of systemic amylin dyshomeostasis in ischemic stroke. Future clinical and experimental studies are needed to: 1, determine the prevalence of amylin-mediated formation of microthrombi in early stages of diabetes-associated ischemic stroke; 2, improve the understanding of whether and how amylin-mediated microthrombi lead to ischemic tissue injury and stroke in patients with type-2 diabetes mellitus; and 3, determine whether lowering amylin accumulation in blood cells and microvasculature could provide a novel strategy for reducing the risk of ischemic stroke and small-vessel tissue injury in patients with diabetes.
Acknowledgments
Sources of Funding
Funding in part by: University of Kentucky Research Alliance to Reduce Diabetes-Associated Microvascular Dysfunction (ADAM) and National Institutes of Health NS116058, AG057290, AG053999.
Non-standard Abbreviations and Acronyms
- HIP rats
Human amylin insulin promoter transgenic rats
- IL-1β
Interleukin-1β
Footnotes
Disclosures
Dr. Goldstein and Dr. Despa report a patent on “Compositions and methods for enhancing neuro-repair” (Amylin skin test, USPTO 16/395,742)
Dr. Despa reports a patent on “Diagnosis of diabetes by detecting aggregated amylin in erythrocytes” (WO 2020/102566 A1)
References
- 1.Johnston KC, Bruno A, Pauls Q, Hall CE, Barrett KM, Barsan W, Fansler A, Van de Bruinhorst K, Janis S, Durkalski-Mauldin VL et al. Intensive vs Standard Treatment of Hyperglycemia and Functional Outcome in Patients With Acute Ischemic Stroke: The SHINE Randomized Clinical Trial. JAMA. 2019;322:326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCormick M, Hadley D, McLean JR, Macfarlane JA, Condon B, Muir KW. Randomized, controlled trial of insulin for acute poststroke hyperglycemia. Ann Neurol. 2010; 67:570–578. [DOI] [PubMed] [Google Scholar]
- 3.Kahn SE, D’Alessio DA, Schwartz MW, Fujimoto WY, Ensinck JW, Taborsky GJ Jr, Porte D Jr. Evidence of cosecretion of islet amyloid polypeptide and insulin by beta-cells. Diabetes 1990;39:634–638. [DOI] [PubMed] [Google Scholar]
- 4.Enoki S, Mitsukawa T, Takemura J, Nakazato M, Aburaya J, Toshimori H, Matsukara S. Plasma islet amyloid polypeptide levels in obesity, impaired glucose tolerance and non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1992;15:97–102. [DOI] [PubMed] [Google Scholar]
- 5.Johnson KH, O’Brien TD, Jordan K, Westermark P, Impaired glucose tolerance is associated with increased islet amyloid polypeptide (IAPP) immunoreactivity in pancreatic beta cells. Am J Pathol. 1989;135:245–250. [PMC free article] [PubMed] [Google Scholar]
- 6.Paulsson JF, Ludvigsson J, Carlsson A, Casas R, Forsander G, Ivarsson SA, Kockum I, Lernmark Å, Marcus C, Lindblad B et al. High plasma levels of islet amyloid polypeptide in young with new-onset of type 1 diabetes mellitus. PLoS One. 2014;9:e93053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jurgens CA, Toukatly MN, Fligner CL, Udayasankar J, Subramanian SL, Zraika S, Aston-Mourney K, Carr DB, Westermark P, Westermark GT, et al. β-cell loss and β-cell apoptosis in human type 2 diabetes are related to islet amyloid deposition. Am J Pathol. 2011;178:2632–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westermark P, Andersson A, Westermark GT. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev. 2011;91:795–826. [DOI] [PubMed] [Google Scholar]
- 9.Höppener JWM, Ahren B, Lips CJM: Islet amyloid and type 2 diabetes mellitus. N Engl J Med. 2000: 343:411–419. [DOI] [PubMed] [Google Scholar]
- 10.Janson J, Ashley RH, Harrison D, McIntyre S, Butler PC. The mechanism of islet amyloid polypeptide toxicity is membrane disruption by intermediate-sized toxic amyloid particles. Diabetes. 1999;48:491–498. [DOI] [PubMed] [Google Scholar]
- 11.Zraika S, Hull RL, Udayasankar J, Aston-Mourney K, Subramanian SL, Kisilevsky R, Szarek WA, Kahn SE. Oxidative stress is induced by islet amyloid formation and time-dependently mediates amyloid-induced beta cell apoptosis. Diabetologia. 2009;52:626–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janciauskiene S, Ahrén B. Fibrillar islet amyloid polypeptide differentially affects oxidative mechanisms and lipoprotein uptake in correlation with cytotoxicity in two insulin-producing cell lines. Biochem Biophys Res Commun. 2000;267:619–625. [DOI] [PubMed] [Google Scholar]
- 13.Chiti F, Dobson CM. Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress Over the Last Decade. Ann Rev Biochem. 2017:86:27–68. [DOI] [PubMed] [Google Scholar]
- 14.Jackson K, Barisone GA, Diaz E, Jin L-W, DeCarli C, and Despa F. Amylin deposition in the brain: a second amyloid in Alzheimer’s disease? Ann Neurol. 2013;74:517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu M, Verma N, Peng X, Srodulski S, Morris A, Chow M, Hersh LB, Chen J, Zhu H, Netea M et al. Hyperamylinemia increases IL-1β synthesis in the heart via peroxidative sarcolemmal injury. Diabetes. 2016;65:2772–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ly H, Verma N, Wu F, Liu M, Saatman KE, Nelson PT, Slevin JT, Goldstein LB, Biessels GJ, Despa F. Brain microvascular injury and white matter disease provoked by diabetes-associated hyperamylinemia. Ann Neurol. 2017;82:208–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oskarsson ME, Paulsson JF, Schultz SW, Ingelsson M, Westermark P, Westermark GT, In vivo seeding and cross-seeding of localized amyloidosis: A molecular link between type 2 diabetes and Alzheimer disease. Am J Pathol. 2015;185:834–846. [DOI] [PubMed] [Google Scholar]
- 18.Schultz N, Byman E, Fex M, Wennström M. Amylin alters human brain pericyte viability and NG2 expression. J Cereb Blood Flow Metab. 2017:37:1470–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schultz N, Byman E, Bank T and Wennström M. Levels of retinal IAPP are altered in Alzheimer’s disease patients and correlate with vascular changes and hippocampal IAPP levels. Neurobiol. Aging. 2018;69:94–101. [DOI] [PubMed] [Google Scholar]
- 20.Ly H, Verma N, Sharma S, Kotiya D, Despa S, Abner EL, Nelson PT, Jicha GA, Wilcock DM, Goldstein LB et al. The association of circulating amylin with β-amyloid in familial Alzheimer’s disease. Alzheimer’s & Dementia TRCI. 2020; DOI: 10.1002/trc2.12130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fawver JN, Ghiwot Y, Koola C, Carrera W, Rodriguez-Rivera J, Hernandez C, Dineley KT, Kong Y, Li J, Jhamandas J. et al. Islet amyloid polypeptide (IAPP): A second amyloid in Alzheimer’s disease. Curr. Alzheimer. Res 2014;1:928–940. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Valbuena I, Valenti-Azcarate R, Amat-Villegas I, Riverol M, Marcilla I, de Andrea CE, Sánchez-Arias JA, Del Mar Carmona-Abellan M, Marti G, Erro ME et al. Amylin as a potential link between type 2 diabetes and Alzheimer disease. Ann Neurol. 2019;86:539–551. [DOI] [PubMed] [Google Scholar]
- 23.Despa S, Margulies KB, Chen L, Knowlton AA, Havel PJ, Taegtmeyer H, Bers DM, Despa F. Hyperamylinemia contributes to heart dysfunction in obesity and diabetes, a study in humans and rats. Circ Res. 2012:110:598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Despa S, Despa S, Sharma S, Harris TR, Dong H, Li N, Chiamvimonvat N, Taegtmeyer H, Margulies K, Hammock BD et al. Cardioprotection by controlling hyperamylinemia in a “humanized” diabetic rat model. J Am Heart Assoc 2014; 3:e001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong W, ZH Liu, Zeng CH, Peng A, Chen HP, Zhou H and Li LS. Amylin deposition in the kidney of patients with diabetic nephropathy, Kidney Int. 2007;72:213–218. [DOI] [PubMed] [Google Scholar]
- 26.Verma N, Liu M, Ly H, Loria A, Campbell KS, Bush H, Kern PA, Jose PA, Taegtmeyer H, Bers DM et al. Diabetic microcirculatory disturbances and pathologic erythropoiesis are provoked by deposition of amyloid-forming amylin in red blood cells and capillaries. Kidney Int. 2020;97:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, Launer LJ, Van Buchem MA, Breteler MM; Microbleed Study Group. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009; 8:165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fazekas F, Kleinert R, Roob G, Flooh E. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol 1999;20:637–42. [PMC free article] [PubMed] [Google Scholar]
- 29.Hainsworth AH, Minett T, Andoh J, Forster G, Bhide I, Barrick TR, Elderfield K, Jeevahan J, Markus HS, Bridges LR. Neuropathology of White Matter Lesions, Blood-Brain Barrier Dysfunction, and Dementia. Stroke. 2017;48:2799–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shoamanesh A, Kwok CS, Benavente O. Cerebral microbleeds: histopathological correlation of neuroimaging. Cerebrovasc Dis 2011;32:528–34. [DOI] [PubMed] [Google Scholar]
- 31.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology 2001;56:537–39. [DOI] [PubMed] [Google Scholar]
- 32.Fisher CM. Hypertensive cerebral hemorrhage. Demonstration of the source of bleeding. J Neuropathol Exp Neurol 2003;62:104–107. [DOI] [PubMed] [Google Scholar]
- 33.Pasi M, Charidimou A, Boulouis G, Auriel E, Ayres A, Schwab KM, Goldstein JN, Rosand J, Viswanathan A, Pantoni L, et al. Mixed-location cerebral hemorrhage/microbleeds: Underlying microangiopathy and recurrence risk. Neurology 2018;90:e119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai HH, Pasi M, Tsai LK, Chen YF, Lee BC, Tang SC, Fotiadis P, Huang CY, Yen RF, Jeng JS et al. Microangiopathy underlying mixed-location intracerebral hemorrhages/microbleeds: A PiB-PET study. Neurology 2019; 92: e774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srodulski S, Savita S, Bachstetter AB, Brelsfoard JM, Pascual V, Xie XS, Saatman KE, Van Eldik LJ, Despa F, Neuroinflammation and neurologic deficits in diabetes linked to brain accumulation of amylin. Mol Neurodegener. 2014;9:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masters SL, Dunne A, Subramanian SL, Hull Rl, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol. 2010;11:897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Westwell-Roper C, Dai DL, Soukhatcheva G, Potter KJ, van Rooijen N, Ehses JA, Verchere CB. IL-1 blockade attenuates islet amyloid polypeptide-induced proinflammatory cytokine release and pancreatic islet graft dysfunction. J Immunol. 2011;187:2755–2765. [DOI] [PubMed] [Google Scholar]
- 38.Netea MG, van de Veerdonk FL, van der Meer JW, Dinarello CA, Joosten LA. Inflammasome-independent regulation of IL-1-family cytokines. Annu Rev Immunol. 2015;33:49–77 [DOI] [PubMed] [Google Scholar]
- 39.Verma N, Ly H, Liu M, Chen J, Zhu H, Chow M, Hersh L, Despa F. Intraneuronal amylin deposition, peroxidative membrane injury and increased IL-1beta synthesis in brains of Alzheimer’s disease patients with type-2 diabetes and in diabetic HIP rats. J Alzheimers Dis. 2016;53:259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Semenza GL. Involvement of oxygen-sensing pathways in physiologic and pathologic erythropoiesis. Blood. 2009;114:2015–2019. [DOI] [PubMed] [Google Scholar]
- 41.Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol. 2007;34:906–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barrett EJ, Liu Z, Khamaisi M, King GL, Klein R, Klein BEK, Hughes TM, Craft S, Freedman BI, Bowden DW et al. Diabetic Microvascular Disease: An Endocrine Society Scientific Statement. J Clin Endocrinol Metab. 2017;102:4343–4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Biessels G, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol. 2018;14:591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walton BL, Byrnes JR, Wolberg AS. Fibrinogen, red blood cells, and factor XIII in venous thrombosis. J Thromb Haemost. 2015;13 Suppl 1:S208–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baskurt OK, Meiselman HJ. Erythrocyte aggregation: basic aspects and clinical importance. Clin Hemorheol Microcirc. 2013;53:23–37 [DOI] [PubMed] [Google Scholar]
- 46.Baskurt OK, Meiselman HJ. RBC aggregation: more important than RBC adhesion to endothelial cells as a determinant of in vivo blood flow in health and disease. Microcirculation. 2008;15:585–590. [DOI] [PubMed] [Google Scholar]
- 47.Pantoni L Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701 [DOI] [PubMed] [Google Scholar]
- 48.Mok V, Kim JS. Prevention and management of cerebral small vessel disease. J Stroke. 2015;17:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szapary L, Horvath B, Marton Z, Alexy T, Demeter N, Szots M, Klabuzai A, Kesmarky G, Juricskay I, Gaal V et al. Hemorheological disturbances in patients with chronic cerebrovascular diseases. Clin Hemorheol Microcirc. 2004;31:1–9 [PubMed] [Google Scholar]
- 50.Kowal P, Marcinkowska-Gapinska A. Hemorheological changes dependent on the time from the onset of ischemic stroke. J Neurol Sci. 2007;258:132–136 [DOI] [PubMed] [Google Scholar]
- 51.Crea F, Camici PG,Bairey Merz CN Coronary microvascular dysfunction: an update. Eur Heart J. 2014;35:1101–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Westermark P, Engstrom U, Johnson KH et al. Islet amyloid polypeptide: Pinpointing amino acid residues linked to amyloid fibril formation. Proc Natl Acad Sci USA. 1990;87:5036–5040 [DOI] [PMC free article] [PubMed] [Google Scholar]