Abstract
Preeclampsia, a hypertensive disorder of pregnancy, complicates up to 10% of all pregnancies and increases the risk for perinatal stroke in offspring. The mechanism of this increase is unknown, but may involve vascular dysfunction. The goal of this study was to evaluate the effect of experimental preeclampsia (ePE) on cerebrovascular function in offspring to eludciate a possible mechanism for this association. Dams were fed a high cholesterol diet beginning on day 7 of gestation to induce experimental preeclampsia. Middle cerebral arteries (MCA) and the Vein of Galen (VoG) were isolated from pups from ePE dams and compared to pups from normal pregnant (NP) dams at postnatal days 16, 23, and 30 and studied pressurized in an arteriograph chamber. Markers of inflammation and oxidative stress were measured in serum. Our results suggest altered structure and function in both MCA and VoG of ePE pups. We also found evidence of systemic inflammation and oxidative stress in ePE pups. These findings provide a potential link between preeclampsia and the occurrence or severity of perinatal stroke.
Keywords: Preeclampsia, Offspring, Cerebral Vasculature, Middle Cerebral Artery, Vein of Galen
1. Introduction
Preeclampsia (PE), a hypertensive disorder of pregnancy, complicates 5–10% of pregnancies worldwide1–3. PE creates an unfavorable interauterine environment, exposing the fetus to inflammation, oxidative stress, and hypoxia4. These conditions are known to affect development of multiple organ systems in offspring, including the brain and vasculature.5,6 Infants and children of PE mothers exhibit in increased risk for perinatal and pediatric stroke, early hypertension, endothelial dysfunction, abnormal pulmonary and systemic vascular function, increased aortic intimal thickness, and smaller cardiac size7–13. Many of these features, including the increased risk of stroke, persist into adulthood14. Despite a considerable body of knowledge supporting a life-long increased risk of stroke in offspring of women with PE, little is known about the effects of PE on the development of the cerebral circulation that could contribute to stroke risk later in life.
Human cerebrovascular development begins in utero, before cardiac activity can be detected and when the fetus is just 3 mm in size15. The circle of Willis and major arterial structures of the brain are present at 6–7 weeks of intrauterine life16. From a functional perspective, significant physiologic maturation in the cerebral circulation also occurs in utero. Development of the vascular endothelium, blood-brain barrier, and vascular smooth muscle all occur during gestation16. Vascular smooth muscle differentiates from migratory, to proliferative, to synthetic, to contractile phenotypes during this period17. The immature vessels of the developing cerebrovascular tree are particularly vulnerable to both metabolic and mechanical injury16. In mothers with PE, the abnormal intrauterine environment exposes the fetus to inflammation, oxidative stress, and chronic hypoxia18. Such stimuli could alter cerebrovascular tone and reactivity, endothelial function, and autoregulatory function19–21. If these changes persist, neonates, infants, and/or children may be at increased risk for stroke may be at increased risk for stroke, and when stroke occurs, it may be more severe.
Perinatal stroke occurs between 20 weeks gestation and 28 days of life at a rate of approximately 1:3000 live births22,23. Most perinatal stroke is due to one of two diagnoses: perinatal arterial ischemic stroke (PAIS) or cerebral sinovenous thrombosis (CSVT). Although underlying mechanisms of PAIS and CSVT are distinct, factors that predispose to these conditions are present in offspring of PE mothers. For example, PE exposes the fetus to decreased uteroplacental blood flow24. In this setting, global cerebral hypoperfusion may create a “low-flow” environment in which thrombi or emboli may be able to form, especially in the venous circulation25. Inflammation and oxidative stress, both of which are present in PE, are also known to contribute to the pathogenesis of perinatal stroke, potentially via activation of the coagulation cascade or vasoconstriction26,27. Finally, PE-induced vascular dysfunction in neonates may cause structural and functional changes in both arterial and venous systems that could increase the risk for perinatal stroke and/or worsen stroke outcome.
In the present study, we investigated the effects of experimental preeclampsia (ePE) on the structure and function of both cerebral arteries and veins in rat pups. We hypothesized that pups from ePE dams would have altered function and biomechanical properties in middle cerebral artery (MCA) and Vein of Galen (VoG) when compared to pups born of normal pregnancies. We tested these hypotheses by comparing vessel size, distensibility, stiffness, myogenic reactivity, and response to vasoactive mediators of vasoconstriction and vasodilation. Further, markers of systemic inflammation and oxidative stress were investigated as underlying mechanisms of cerebrovascular dysfunction.
2. METHODS
2.1. Statement of Ethics
All procedures were approved by the Institutional Animal Care and Use Committee at the University of Vermont and complied with the National Institutes of Health guidelines for care and use of laboratory animals. Studies were conducted in accordance with Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
2.2. Animals and Induction of ePE
Pregnant Sprague-Dawley rats were housed singly with environmental enrichment in the University of Vermont Animal Care Facility, an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) accredited facility. Rats were maintained on a 12-h light/dark cycle and allowed access to food and water ad libitum. ePE was induced in dams by administering a high-cholesterol diet beginning on day 7 of gestation as previously described28–31. This model has been previously shown to induce dyslipidemia and cause other features consistent with PE, including maternal endothelial dysfunction and increased blood pressure30,32. Although uteroplacental vascular function was not measured, this model is associated with fetal growth restriction suggesting it encompasses impaired uteroplacental blood flow and/or placental disease associated with PE30.
2.3. Rat Pups
Rat pups of either sex were selected randomly using a coin flip (M vs. F, p23 and p30). Because of their small size, sex was unable to be determined in p16 rat pups. All rat pup experiments were conducted using pups born from normal pregnant (NP) or ePE dams in our facility. Dams were maintained on high-cholesterol diet until weaning. Male and female pups were appropriately weaned from dams at postnatal day (p)21 and were maintained in group housing until experimentation at p16, p23, or p30. Weights and crown-rump length were measured for each pup just prior to experimentation.
2.4. In-vitro Isolated MCA and VoG Experiments
Pups from either NP or ePE dams (n= 5–9/group) at p16, p23, or p30 were decapitated under deep isoflurane anesthesia (3% in oxygen) and brains removed and placed in cold, oxygenated physiologic saline solution (PSS). MCA and VoG were immediately dissected, mounted on glass cannulae and pressurized in an arteriograph chamber, as previously described28,33. MCAs and VoG that did not develop myogenic tone along the length of the vessel segment, indicating damage to the vascular wall, were excluded.
MCAs were equilibrated at an intravascular pressure of 75 mmHg for 1 hour, afterwhich, pressure was first decreased stepwise to measure myogenic vasodilation (50, 25, 15, 5mmHg) and then increased to measure myogenic vasoconstriction (5, 10, 15, 25, 50, 75, 100, 125, 150, 175mmHg). Lumen diameters and wall thicknesses were measured at each pressure once stable, ~ 5 minutes. The vessels were then washed with zero-calcium PSS and diltiazem (10−5M) and papaverine (10−3M) were added to fully relax smooth muscle. Structural and biomechanical measurements of the vascular wall were obtained under passive conditions by increasing pressure stepwise from 5 – 175 mmHg and recording lumen diameters and wall thicknesses.
VoG were equilibrated at the intravascular pressure of 5 mmHg for 1 hour. To measure basal tone, intravascular pressure was increased to 25 mmHg in a stepwise manner and lumen diameters measured at each pressure. Pressure was then decreased to 10 mmHg for the remainder of the experiment. U46619, a thromboxane A2 agonist, was added in increasing concentrations (10−8 – 10−6 M) and lumen diameter measured at each dose. Following a wash, L-NG-Nitro arginine methyl ester (L-NAME; 10−3), a nitric oxide synthase (NOS) inhibitor was added to the bath and the change in lumen diameter was recorded. In the presence of L-NAME, the NO donor sodium nitroprusside (SNP) was added in increasing concentrations (10−8 – 10−6 M) and lumen diameter recorded at each concentration. The bath was then replaced with zero-calcium PSS, and diltiazem and papaverine were added to fully relax the vessels. Passive pressure steps were performed between 5 – 25 mmHg and lumen diameter recorded.
2.6. Rat pup serum samples
Serum samples were obtained via cardiac puncture from rat pups under deep (3%) isoflurane anesthesia and collected in serum separator tubes. After a waiting period of 30 minutes, the tubes were centrifuged for 10 minutes at 2500 revolutions per minute, and the serum immediately aliquoted in 200 μL microcentrifuge tubes. The aliquoted serum was then stored at −80 ºC until measurement.
2.7. Measurement of circulating 8-isoprostane via enzyme-linked immunosorbent assay (ELISA)
To assess a systemic marker of oxidative stress in pups from ePE dams, circulating levels of 8-isoprostane (8-iso) were measured in serum from NP and ePE rat pups at p23 and p30 (n = 8/group) using a commercially available ELISA kit for 8-iso (Cayman Chemical, Ann Arbor, MI, USA). Samples were measured undiluted and in duplicate following manufacturer’s instructions. Serum from p16 pups could not be obtained due to the small amount present at this early stage of development.
2.8. Analysis of Serum Cytokine Protein Profile by Quantibody® Rat Cytokine Array 67
The concentration of 67 cytokines in serum (−80°C) of NP or ePE rat pups (n = 8 per group) was determined using RayBiotech’s Quantibody Rat Cytokine Array 6734. Within the array, each individual cytokine is represented four times, along with positive and negative controls which allowed for an analysis of standard deviation. Cytokine analysis of serum was performed by RayBiotech according to their protocol and software analysis35.
2.9. Drugs and Solutions
Papaverine, L-NAME, SNP, and U46619 were purchased from Sigma Aldrich (St. Louis, MO, USA). Diltiazem was purchased from MP Biomedicals (Santa Ana, CA, USA). Isolated MCA and VoG experiments were performed using PSS, the ionic composition of which was (in mM) 119.0 NaCl, 24.0 NaHCO3, 4.7 KCl, 1.18 KH2PO4, 1.17 MgSO4·7H2O, 1.6 CaCl2, 0.026 EDTA, and 5.5 glucose. Glucose was added immediately prior to each experiment. PSS was aerated with 5 % CO2, 10 % O2 and 85 % N2 to maintain pH at 7.40 ± 0.05 and the temperature within the arteriograph chamber was maintained at 37.0 ± 0.2 °C throughout the experiments. Buffers were made each week and stored without glucose at 4°C. Aliquots of U46619 were stored in the −20°C until use. Stock solutions of L-NAME, SNP, diltiazem, and papaverine were made fresh weekly and stored at 4°C.
2.10. Data Calculations
Myogenic tone was calculated as a percent decrease in diameter from the fully relaxed diameter in calcium-free PSS with diltiazem and papaverine by the following equation: [1 − (φtone/φpassive)] × 100%, where φtone is the inner diameter of the vessel with tone, and φpassive is the inner diameter of the vessel under fully relaxed conditions. Percent distensibility was calculated for fully relaxed vessels in calcium-free PSS with diltiazem and papaverine at each pressure by the following equation: (φpressure − φ5mmHg)/(φ5mmHg) × 100%, where φpressure is the passive diameter at a specific pressure, and φ5mmHg is defined as the passive diameter at the lowest pressure (5 mmHg). Percent constriction to L-NAME and U46619 was calculated as a percent change in diameter from baseline by the following equation: [1 − (φdrug/φbaseline)] × 100%, where φdrug is the diameter of vessel in drug, and φbaseline is the diameter before giving drug. Percent reactivity to SNP was calculated from the following equation: [(φdose − φbaseline)/(φpassive − φbaseline)] × 100%, where φdose is the diameter at a specific concentration of drug. Wall tension was calculated across the pressure range 5 – 200 mmHg by converting pressure (mmHg) into dynes/cm2 × (φinner/2). Wall stress was calculated at each pressure by the equation: wall stress = wall tension/wall thickness. Wall strain was calculated by the equation: wall strain = (φpassive – φmmHg)/φmmHg; where φmmHg is the passive diameter at each pressure.
2.11. Statistical Analysis
Data are presented as means ± SEM. Vascular function of MCA and VoG was compared between groups via multiple t-tests followed by Holm-Sidak multiple comparison test. Serum protein levels (NP vs. ePE groups) were analyzed via two-way ANOVA followed by Tukey post-hoc test. Both tests were performed in GraphPad Prism (Version 8.2.1, GraphPad Software, San Diego, CA USA). Within-group analysis of active diameter and myogenic tone used a one-way repeated measure ANOVA followed by a uncorrected Fisher’s LSD were performed in SPSS (Version 26.0, IBM Corp., Armok, NY USA). Differences were considered significant when p < 0.05.
3. RESULTS
3.1. Effect of ePE on Pup Weights and Crown-Rump Length
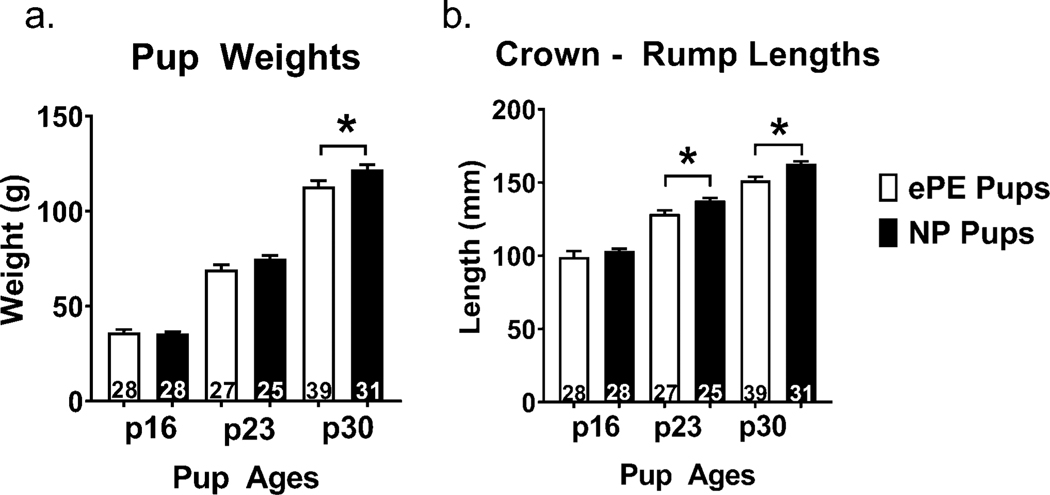
To determine whether ePE caused grown restriction in pups, we measured pup weights and crown-rump lengths in pups from ePE and NP dams at p16, p23, and p30. We found that by p30, rat pups from ePE dams weighed significantly less (Figure 1a), and that at both p23 and p30 pups from ePE dams were significantly shorter (Figure 1b), reflecting growth restriction.
Figure 1. Weight and crown-rump length in NP vs. ePE rat pups.
Body weights were lower at p30 in ePE pups, and crown-rump lengths were less in ePE pups at p23 and p30, suggesting growth restriction in pups from ePE dams. Number of pups (n) embedded in bars; *p < 0.05 NP vs. ePE.
3.2. Effect of ePE on MCA Structure and Function in Offspring
3.2.1. MCAs from ePE pups were smaller and did not mature
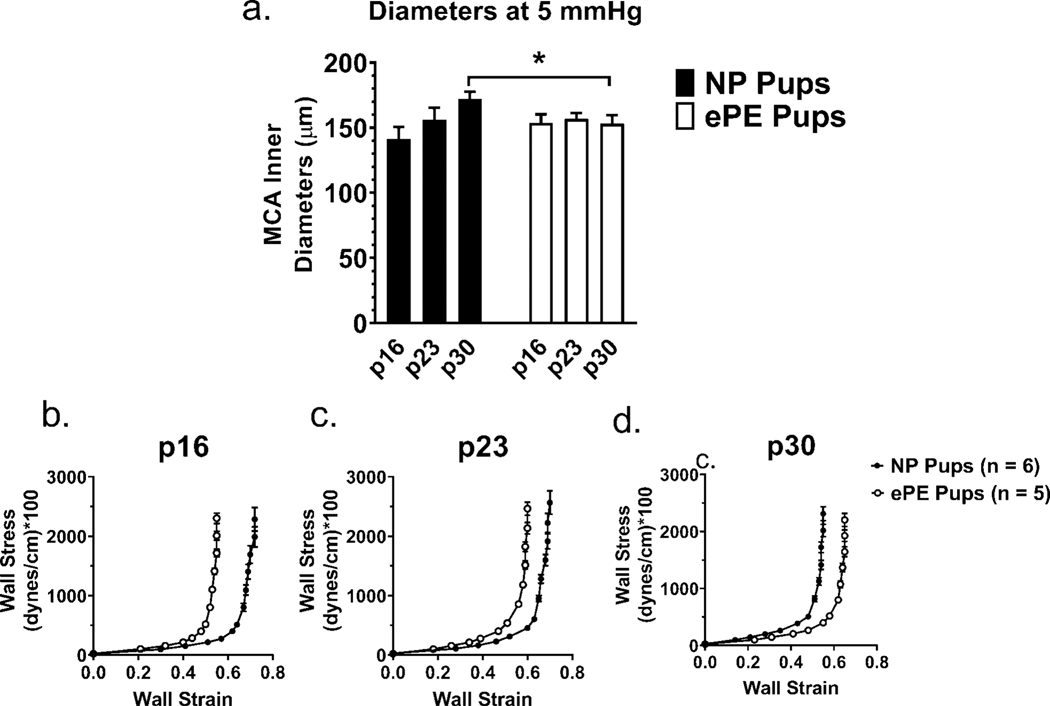
To investigate whether ePE affected the biomechanical and functional properties of MCAs, we studied isolated and pressurized MCAs from pups from NP vs. ePE dams. Figure 2a shows the passive MCA lumen diameter at 5 mmHg to eliminate any differences in distensibility that might influence diameters. While MCAs from NP pups enlarged over maturation, MCAs from ePE pups exhibited no change in lumen diameter, reflecting lack of growth. At p30, MCA diameter was larger in the NP group.
Figure 2. Passive inner diameters of MCAs at 5 mmHg in NP vs. ePE pups and stress-strain curves for MCAs at p16, p23, and p30.
MCAs in NP pups showed enlargement with age that did not occur in ePE pups. MCAs in NP pups were larger at p30 but did not achieve significance. *p<0.05 NP vs. ePE.
To better understand the effect of ePE on biomechanical properties of MCAs, stress-strain curves were compared between groups at each age to determine changes in vascular stiffness across maturation (Figure 2b-d). The stress-strain curve in ePE pups was shifted to the left at p16 (Figure 2b) and p23 (Figure 2c), indicating increased stiffness at these ages. However, this relationship reversed at p30 (Figure 2d), with the stress-strain curve of MCAs from ePE pups being shifted rightward, indicating the MCA were less stiff compared to MCAs from NP dams at this later age. From a maturation standpoint, MCAs from NP pups became stiffer in addition to enlarging from p16 to p30, and these changes were absent in pup MCAs from ePE dams.
3.2.2. Effect of ePE and Maturation on Reactivity and Lumen Diameter of Isolated and Pressurized MCAs
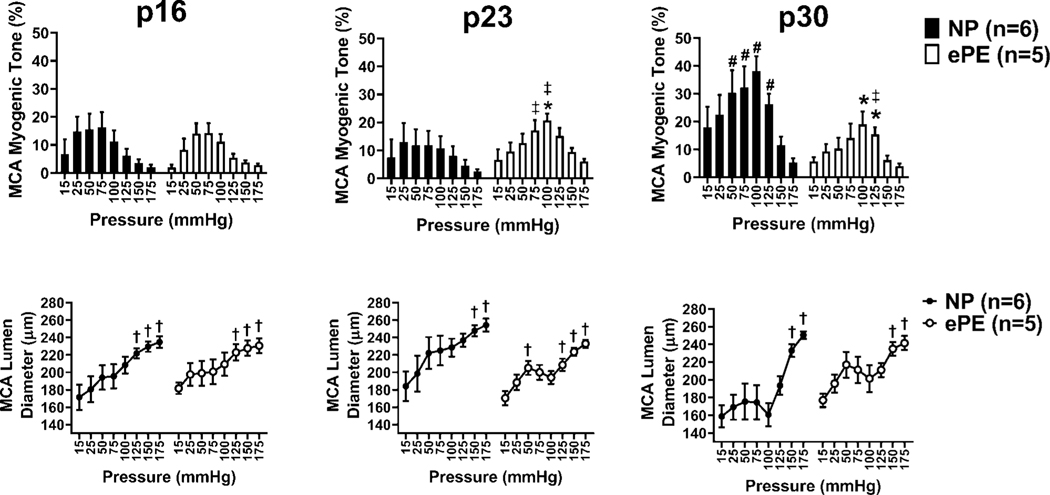
To investigate if ePE affected myogenic reactivity of MCAs, we calculated percent myogenic tone and measured lumen diameter over a range of pressures (15 – 175mmHg; Figure 3). We compared tone between MCAs from NP and ePE pups at each intravascular pressure. We then compared lumen diameters at each pressure to the lumen diameter at 25 mmHg to determine at which pressure(s) lumen diameter became significantly different. Both groups demonstrated tone in response to increasing pressure.
Figure 3. Myogenic tone and lumen diameter for NP vs. ePE pups at p16, p23, and p30.
Figures 3a, c, e show myogenic tone in MCA over a range of intravascular pressures for NP vs. ePE pups at p16, p23, and p30. MCA lumen diameter at each pressure (within groups) was also compared to lumen diameter at 25 mmHg (Figures 3b, d, f). In both NP and ePE pups at all 3 time points, lumen diameter was significantly higher than lumen diameter at 25 mmHg at pressures above 100–125mmHg. *P<0.05 NP vs. ePE; #P<0.05 NP p16 vs. p30, ‡P<0.05 p16 vs p23 and p23 vs. p30; †P<0.05 vs. diameter at 25mmHg within groups.
At p16 (Figure 3a), MCAs from pups from both NP and ePE dams had tone at pressure as low as 15 mmHg that increased at 25, 50 and 75 mmHg, after which tone decreased. When lumen diameters were compared (Figure 3b) to those at 25 mmHg within groups, both NP and ePE pups showed significantly larger lumen diameter at 125, 150, and 175mmHg, suggesting forced dilatation of MCA in both groups occurred at 125mmHg.
At p23 (Figure 3c), NP pup MCAs also had tone at 15 mmHg, and increased tone at pressures from 25–125 mmHg, after which tone decreased. However, MCAs from ePE pups developed greater tone than NP pups that was significantly higher at 100mmHg. MCAs in both groups constricted over the range of vascular pressures. When compared to lumen diameter at 25mmHg, MCAs in NP and ePE pups exhibited forced dilatation at pressures of 125mmHg and above (Figure 3d).
At p30 (Figure 3e), MCAs in NP pups had increased tone compared to MCAs from ePE. In addition, NP pup MCAs at p30 developed myogenic reactivity and maintained diameters over a wide range of pressures (15–100 mmHg) and demonstrated forced dilatation at 125mmHg (Figure 3e). In contrast, MCAs from ePE pups were less constricted and responded to increased pressure only at 75 and 100 mmHg (Figure 3c). In addition, baseline diameter of MCAs from NP pups was smaller than that of MCAs from ePE pups (at 15mmHg, Figure 3f).
To assess the effect of maturation on myogenic tone, we compared tone at each pressure for all time periods (Figure 3a, c, e). In NP pups, MCA tone did not differ significantly at any pressure between p16 and p23. However, when comparing p23 pups to p30 pups, there was a significant increase in tone at 50, 75, 100, and 125mmHg. Thus, in addition to maturing structurally, NP pup MCAs developed more tone with maturation. In ePE pups, MCA tone was significantly higher between p16 and p23 at 100 and 125 mmHg. However, in contrast to NP pups, there was no significant increase in tone between p23 and p30 at any pressure, suggesting lack of maturation in the myogenic response at that age.
3.3. Effect of ePE on VoG Structure and Function in Offspring
Cerebral veins do not have significant myogenic tone and therefore other functional responses were measured. Myogenic tone at p16 was 3.12 ± 1.44% (5 mmHg) and 6.9 ± 1.72% (25 mmHg). At p23, tone was 5.4 ± 1.25% (5 mmHg) and 7.5 ± 1.73% (25 mmHg). Tone at p30 was 3.34 ± 0.74% (5 mmHg) and 7.81 ± 2.09% (25 mmHg). There was no significant difference in myogenic tone between pops from NP vs. ePE dams at any age.
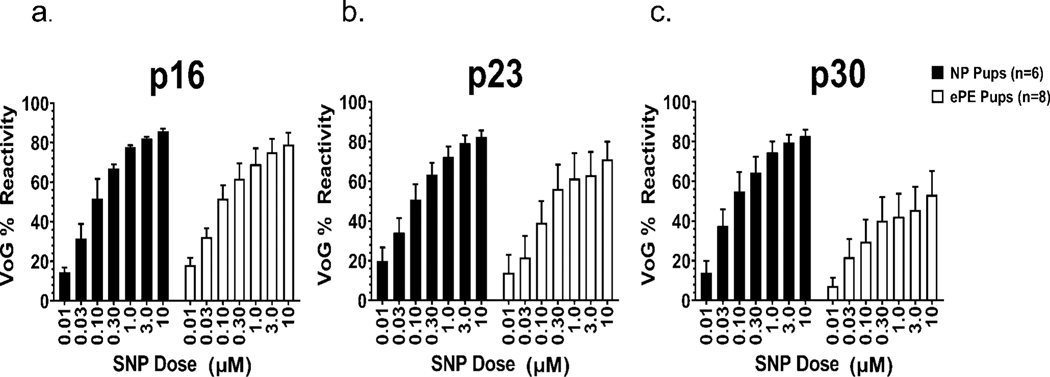
To determine the role of NO in ePE-induced vascular dysfunction in offspring, we exposed veins to sodium nitroprusside (SNP), an NO donor, and measured changes in lumen diameter used to calculate % reactivity. All vessels dilated to SNP in a dose-dependent manner (Figure 4a-c). However, we found no significant difference in reactivity between pups from NP vs. ePE dams at any age when reactivity was compared at each individual SNP dose. We also compared reactivity to SNP within groups at the highest dose (10 μM) over maturation in pups from NP vs. ePE dams, but we found no evidence for an effect of maturation in either group.
Figure 4: Percent reactivity of VoG to sodium nitroprusside (SNP) in NP and ePE pups at p16, p23, and p30.
Graphs showing reactivity to increasing concentration of SNP over age in NP and ePE pups. Maturation had no significant effect on reactivity in VoG.
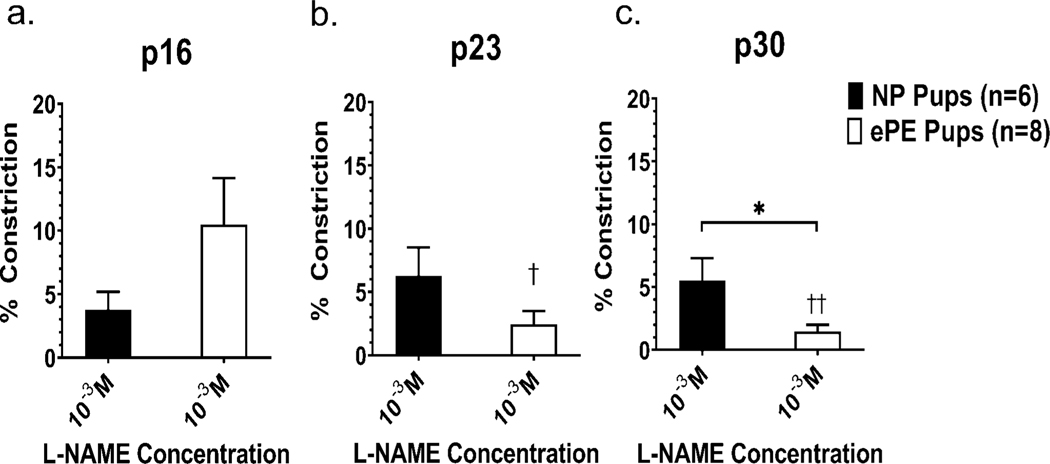
All veins demonstrated constriction to NOS inhibition (L-NAME, Figure 5). No statistically significant difference in L-NAME induced constriction when pups from NP vs. ePE dams were compared at p16 or p23. However, veins from ePE pups were significantly less constricted at p30. Over time, there was no significant change in the % constriction to L-NAME in the NP group. However, in the ePE group, VoG was significantly more responsive to L-NAME when comparing p16 and p23 and p16 and p30.
Figure 5: Percent constriction to L-NAME in VoG at p16, p23 and p30.
Graphs showing constriction to a single concentration (10−3 M) of the NOS inhibitor L-NAME at different ages. Veins from all groups at all ages constricted only ~5–10% to L-NAME. At p30, VoG from NP pups had a significantly lesser constriction to L-NAME compared to ePE. There was no effect of maturation on NP pups, but ePE pups showed significantly more constriction to L-NAME when p16 was compared to both p23 and p30. *P<0.05 ePE vs. NP, †P<0.05 ePE p16 vs. ePE p23; ††P<0.01 ePE p16 vs. ePE p30.
3.5. Effect of ePE on Sensitivity to Thromboxane
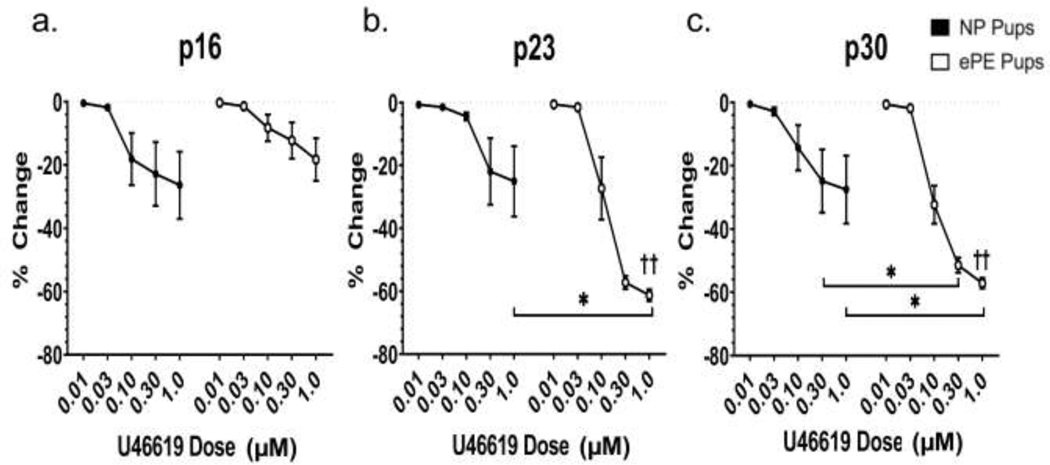
All veins showed dose-dependent constriction to U46619, a thromboxane-A2 receptor-dependent analog (Figure 6). However, veins from ePE animals at p23 (Figure 6b) and p30 (Figure 6c) showed significantly more constriction at higher doses of U46619, suggesting increased sensitivity to thromboxane agonism. To determine the effect of maturation on the response to U46619 in VoG, we compared % change over time at the highest concentration (1 μM). We found no significant differences across time in NP pups. However, VoG from ePE pups constricted significantly more between p16 and p23 and p16 and p30 (p<0.001).
Figure 6. Percent change in VoG lumen diameter to U46619 at p16, p23, and p30.
All veins at all ages constricted to U46619. At p23 and p30, VoG constricted significantly more at higher doses of U46619, compared to ePE. At the highest dose of U46619 (1 μM) maturation did not affect constriction to U46619. However, in ePE pups, 1 μM U46619 caused significantly more constriction over maturation (p16 vs. p23 and p16 vs. p30). *P<0.05 for NP vs. ePE, ††P<0.01 p16 vs. p23 and p16 vs. p30.
3.6. Effect of ePE on Systemic Inflammation, Cytokine Imbalance, and Oxidative Stress in Offspring
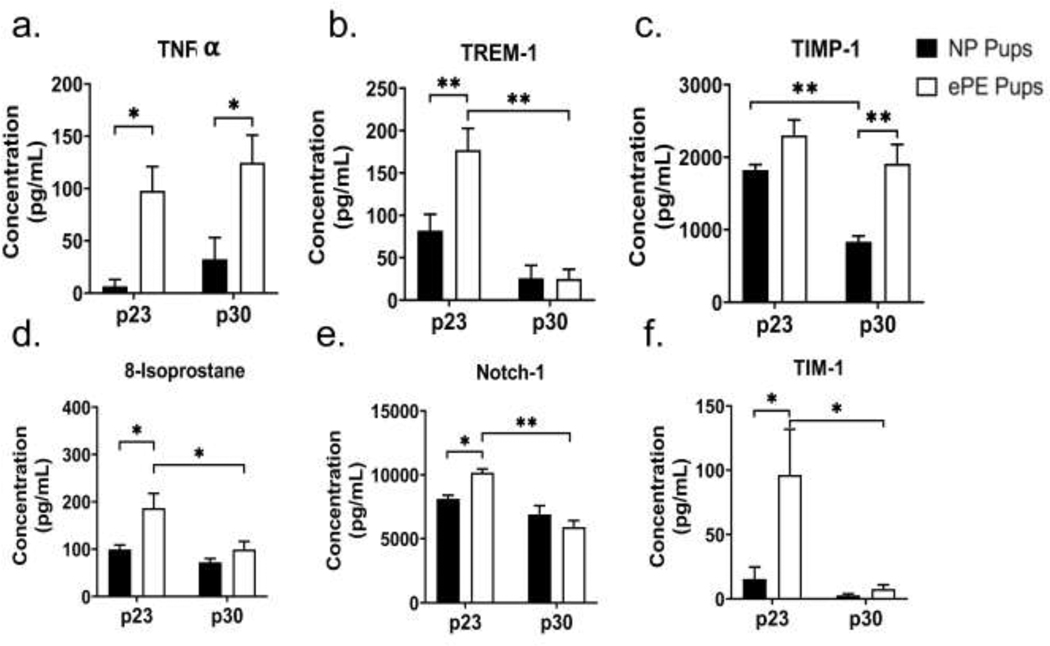
To assess whether ePE caused systemic inflammation in pups that might contribute to vascular dysfunction, we performed Raybiotech Quantibody® Rat Cytokine Array 67 on serum samples from pups of ePE and NP dams. Table 1 shows the results for serum levels for all 67 cytokines in ePE and NP pups at p23 and p30. ePE was associated with a significantly higher serum concentration of a number of proinflammatory cytokines at both p23 and p30 (Figure 7a-e). Notably, serum TNF-α was significantly elevated at both p23 and p30. Additional chemokines/cytokines we found significantly elevated at p23 include TREM-1, TIM-1, and Notch-1. TIMP-1 was significantly elevated in the ePE group at p30.
Table 1.
Results from Raybiotech Quantibody® Rat Cytokine Array 67 on serum samples from p23 and p30 rat pups (NP vs. ePE).
| Concentration (pg/ml) |
Concentration (pg/ml) |
|||||
|---|---|---|---|---|---|---|
| Molecule | NP p23 | ePE p23 | p-value | NP p30 | ePE p30 | p-value |
| 4-1BB | 4241.1 | 20055.5 | 0.13 | 4369.7 | 2837.6 | 1.00 |
| Activin A | 8.5 | 162.5 | 0.48 | 7.4 | 65.7 | 0.95 |
| Adiponectin | 1.8 | 1.7 | >0.99 | 1.5 | 3.9 | 0.87 |
| B7-1 | 1.3 | 4.4 | 0.96 | <LOD | <LOD | n/a |
| B7-2 | 243.1 | 1306.9 | 0.36 | 406 | 206 | 0.99 |
| b-NGF | 90.3 | 390 | 0.44 | 108.9 | 89.9 | 1.0 |
| CD48 | 189.9 | 937.4 | 0.20 | 201.4 | 115.5 | 1.0 |
| CINC-1 | 10.4 | 3.4 | 0.85 | 0.6 | 17.6 | 0.24 |
| CINC-2 | <LOD | <LOD | n/a | <LOD | <LOD | <LOD |
| CINC-3 | <LOD | <LOD | n/a | <LOD | <LOD | n/a |
| CNTF | <LOD | <LOD | n/a | <LOD | <LOD | n/a |
| CTACK | <LOD | <LOD | n/a | <LOD | <LOD | n/a |
| Decorin | 909.3 | 1259.4 | 0.068 | 454.6 | 452.5 | >0.99 |
| Eotaxin | 568.5 | 695.8 | 0.10 | 335.4 | 352.4 | 0.99 |
| EphA5 | <LOD | <LOD | n/a | <LOD | <LOD | n/a |
| Erythropoietin | <LOD | <LOD | n/a | <LOD | <LOD | n/a |
| FGF-BP | <LOD | <LOD | n/a | <LOD | <LOD | n/a |
| Flt-3L | 2568.9 | 3142.3 | 0.49 | 1877 | 1623.5 | 0.92 |
| Fractalkine | 1580 | 1687.5 | 0.98 | 778.2 | 1294.6 | 0.26 |
| Galectin-3 | <LOD | <LOD | <LOD | <LOD | <LOD | n/a |
| Gas 1 | 1486 | 2072.3 | 0.18 | 843.1 | 895.6 | 1.00 |
| GFR alpha-1 | 1284.2 | 3711.6 | 0.15 | 778.1 | 722.1 | >0.99 |
| GM-CSF | <LOD | <LOD | n/a | 0.4 | 0.5 | 1.00 |
| gp130 | 4.7 | 620.9 | 0.22 | <LOD | 115.5 | 0.98 |
| HGF | 5515 | 7680.1 | 0.67 | 4816.3 | 7137.1 | 0.62 |
| ICAM-1 | 2548.5 | 2962 | 0.79 | 1616.8 | 2427.5 | 0.30 |
| IFNg | 0.6 | 1.5 | 0.86 | 2.8 | 2.1 | 0.93 |
| IL-1 R6 | <LOD | <LOD | n/a | <LOD | <LOD | n/a |
| IL-1 ra | 1272.1 | 7055.5 | 0.20 | 1995.4 | 1045.2 | 0.99 |
| IL-1a | <LOD | 1.7 | 1.00 | 11.5 | 1.4 | 0.62 |
| IL-1b | 30 | 22.9 | 0.93 | 22.3 | 52.1 | 0.087 |
| IL-2 | <LOD | 0.3 | 0.17 | <LOD | <LOD | n/a |
| IL-2 Ra | 265.8 | 677.6 | 0.10 | 233.3 | 183.1 | 0.99 |
| IL-3 | 41.5 | 133.7 | 0.24 | 44.7 | 26.9 | 0.98 |
| IL-4 | 1.3 | 4.3 | 0.12 | 3.2 | 3.3 | 1.00 |
| IL-6 | <LOD | <LOD | 0.87 | 5.4 | 3.8 | 0.94 |
| IL-7 | 65.3 | 387.6 | 0.20 | 116.4 | 57.4 | 0.98 |
| IL-10 | 307 | 1077.3 | 0.051 | 452.1 | 605.1 | 0.94 |
| IL-13 | <LOD | 2.7 | 0.48 | 1 | 0.6 | 1.00 |
| IL-17F | 0.9 | 6.5 | 0.13 | 2.6 | 0.7 | 0.85 |
| IL-22 | 172 | 1666.8 | 0.14 | 351.3 | 60.2 | 0.97 |
| LIX | 641.0 | 901 | 0.28 | 400.1 | 700.2 | 0.17 |
| MIP-1a | <LOD | <LOD | n/a | <LOD | <LOD | n/a |
| Neuropilin-1 | 4 | 6.3 | 0.99 | 15.4 | 2.6 | 0.52 |
| Neuropilin-2 | 6272.5 | 8661.7 | 0.39 | 4798.1 | 5762.7 | 0.91 |
| Nope | 13832.7 | 18930.8 | 0.065 | 10267.6 | 9761.3 | 0.99 |
| Notch-2 | 4638.4 | 7071.8 | 0.10 | 2549.2 | 3391.8 | 0.83 |
| P-Cadherin | 314 | 865.5 | 0.28 | 304.6 | 269.3 | 1.00 |
| PDGF-AA | <LOD | <LOD | n/a | <LOD | <LOD | n/a |
| Prolactin | 1198.1 | 2848.5 | 0.080 | 1041.5 | 1291.1 | 0.98 |
| Prolactin R | 72.4 | 660.5 | 0.70 | 956.2 | 746.8 | 0.98 |
| RAGE | <LOD | <LOD | n/a | 0.9 | <LOD | 0.22 |
| RANTES | 81.1 | 142.7 | 0.20 | 93.6 | 132.1 | 0.58 |
| SCF | 121.1 | 340.3 | 0.17 | 110.2 | 86.6 | 1.00 |
| TCK-1 | 4435.9 | 3424.2 | 0.68 | 658.9 | 3001.6 | 0.072 |
| TIMP-2 | <LOD | <LOD | 0.51 | <LOD | 1.4 | 0.99 |
| TWEAK R | 19326 | 37388.4 | 0.069 | 16542.1 | 14742.2 | 0.99 |
| VEGF | <LOD | <LOD | n/a | <LOD | <LOD | n/a |
Figure 7. Markers of systemic inflammation and oxidative stress in pups from ePE dams.
Examination of serum from rat pups at p23 and p30 revealed elevation of proinflammatory cytokines (TNF-α) proteins associated with systemic inflammation (TREM-1, TIMP-1; Notch-1; TIM-1), and oxidative stress (8-isoprostane). * p<0.05; ** p<0.01
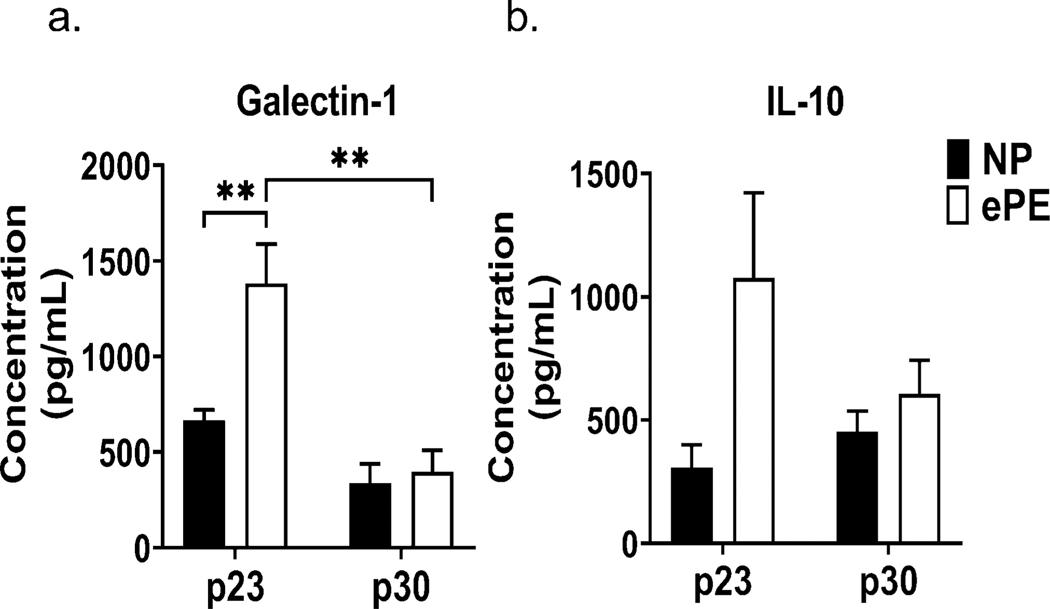
The Quantibody assay measured anti-inflammatory cytokines in addition to proinflammatory cytokines. Figure 8 shows serum concentrations of IL-10 (Figure 8a) and Galectin-1 (Figure 8b). At p23, our analysis showed a significant increase in IL-10 and Galectin-1 in pups from ePE dams, likely reflecting an early response to systemic inflammation. However, the concentrations of both IL-10 and Galectin-1 decreased in ePE pups between p23 and p30, potentially resulting in an imbalance in the pro- and anti-inflammatory milieu in rat pups from ePE dams.
Figure 8. Dysregulation of systemic anti-inflammatory cytokine (IL-10) and anti-inflammatory cytokine-like protein (Galectin-1).
Both proteins are elevated at p23 in ePE pups, which may reflect an anti-inflammatory response to systemic inflammatory caused by ePE in dams. This response appears to disappear by p30, which may confer a loss of anti-inflammatory effect by this time point. IL-10 concentration at p23 in ePE pups approached, but did not reach, statistical significance (P=0.05). * p<0.05; ** p<0.01
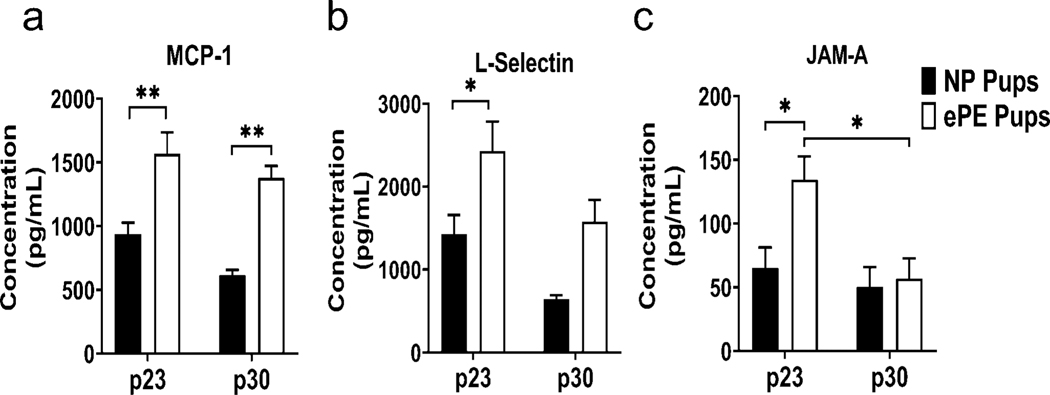
As further evidence of a proinflammatory state in pups from ePE dams, Quantibody analysis revealed an increase in expression in several chemokines that are associated with the systemic inflammatory response (Figure 9). MCP-1 (Figure 9a), a chemokine heavily involved in monocyte trafficking, was significantly increased in serum of ePE pups at both p23 and p30. L-selectin (Figure 9b), a chemokine that is involved in adhesion of leukocytes to vascular endothelium, was significantly elevated in ePE pups at p23 but not p30. Finally, JAM-A (Figure 9c), involved in leukocyte transmigration and platelet adhesion, was significantly elevated in ePE pups at p23, but not p30.
Figure 9. Systemic chemokines in serum of pups from ePE pups at p23 and p30.
Proteins involved in monocyte attraction/vascular inflammation (MCP-1); leukocyte migration at vascular endothelium (L-selectin); and transmigration of leukocytes into vessel walls/vascular inflammation (JAM-A). * p<0.05; ** p<0.01
To assess whether ePE results in oxidative stress in pups, we measured circulating 8-isoprostane levels via an ELISA. Serum 8-iso concentration was higher at p23 (99.2±9.3 μg/mL for NP vs. 186.6±31.1 for ePE; p=0.02) but not p30 (Figure 7d).
4. DISCUSSION
Perinatal and childhood strokes are devastating diseases that result in long-term neurocongnitive and neuromotor consequences. The effects of PE on cerebrovascular development are particularly relevant to the study of perinatal stroke because exposure to PE may create an environment in which stroke may be more likely or stroke may be more severe. A thorough understanfing of the effects of PE on cerebrovascular dysfunction in offspring is critical, because it will provide new insights into the pathogenesis of perinatal stroke as well as suggest potential therapeutic targets in this population.
To our knowledge, this study provides the first evidence that ePE is associated with altered structure and function in both MCA and VoG in offspring. Our study yielded several major findings in offspring from ePE dams: 1) pups from PE dams exhibited growth restriction when compared to NP pups; 2) MCAs from PE pups did not enlarge over maturation and were stiffer biomechanically; 3) MCAs from ePE pups did not increase myogenic tone over maturation while MCAs from NP pups did; 4) VoG from ePE pups showed increasing constriction over the course of maturation, while VoG from NP pups did not; 5) VoG from ePE pups were more constricted when exposed to high doses of thromboxane agonist (at both isolated time points as well as over maturation); 5) VoG in ePE pups showed attenuated reactivity to NO and an increase in basal NO production over the course of maturation; and 6) ePE was associated with evidence of inflammation and oxidative stress.
The MCA is the most common cerebral vessel affected by perinatal stroke, accounting for approximately 70% of cases36. As such, it is a critical target for perinatal stroke research. Our results showed that MCAs in rat pups exposed to ePE did not enlarge over the course of maturation. Postnatally, the rodent cerebrovascular tree undergoes rapid growth and maturation37. PE is known to be associated with disruptions in the postnatal development of multiple organ systems, including the vasculature; however, little is known about the impact on the cerebrovasculature38. Though little is known about growth and maturation of the MCA in neonates, PE is associated with intrauterine growth restriction (IUGR) that may include the cerebral vasculature39,40. Failure of the MCA to enlarge over the course of maturation may predispose to several adverse outcomes for ePE pups, including formation and/or embedding of a thrombus, and limited vasodilatory reserve during hypoxia/ischemia.
We also found differences in stiffness when comparing MCAs from pups from NP dams with those from ePE dams. In early postnatal life, MCAs from ePE were stiffer than those from NP pups, and their stress-strain patterns were relatively similar at p16 and p23. However, by p30 this relationship reversed and MCAs from NP pups became more stiff, and MCAs from ePE pups became more compliant. Preeclampsia is known to cause increased arterial stiffness in pregnant women41. In early life, increased stiffness may impair the ability of the MCA to dilate in response to increased need for blood flow. In addition, the increase in stiffness in MCAs from NP pups may be a normal step in maturation, including vessel wall protein and cellular changes, that was impaired in the setting of ePE. However, our study did not investigate animals beyond the age of 30 days, so results must be interpreted with caution. It is possible that MCAs from ePE pups become similar to those from NP at later ages. A recent study by Trigiani et al. used a mouse model of preeclampsia to show an increase in cerebral blood flow in response to whisker stimulation in 12-month-old offspring from preeclamptic dams.42 These authors theorized that increased capillary density in the ePE pups may be responsible for this finding. Our study did not measure capillary density; however, we found that MCAs were smaller in 30 day old pups from ePE dams compared to pups from NP dams, an age considerably younger than the previous study. It is possible that our study provides insights into early vascular changes in pups from ePE dams that may precede a cerebrovascular phenotype later in life.
Functional changes in the VoG were also observed in rat pups from ePE dams. Overall dilation to SNP, an NO donor, was less in VoG of ePE pups at p30, suggesting attenuated reactivity to NO. Constriciton to NOS inhibition was also significantly affected by maturation in p30 pups from ePE dams. The constriction to NOS inhibiton demonstrates that there was basal NO production in the veins that was inhibiting tone. Figure 5 shows that in veins from p16 pups, the constriction was increased in pups from ePE dams. At the later ages, however, the constriction to NOS inhibiton was less in veins from ePE dams vs. NP. Taken together, these circumstances may create an environment in which decreased reactivity to NO and decreased availability of NO predispose to venous stasis and/or thrombus formation due to low flow in veins from ePE dams. We also assessed constriction to a thromboxane agonist, U46619. We found that constriction to U46619 was significantly higher in VoG from ePE pups at p30, and over the course of maturation, constriction to high-dose U46619 increased. While thromboxane is a vasoconstrictor, one important role is to promote platelet aggregation as part of clot formation. The observed increase in reactivity to U46619 suggests a potential upregulation of thromboxane receptors, which may promote both vasoconstriction and clot formation. It is also possible that diminished basal NO in veins from ePE pups contributes to the increase in constriction to U46619. Further investigation into venous reactivity and coagulation may elucide these changes and their role in venous occlusion over maturation.
PE is known to be a proinflammatory state. Multiple studies have shown that ePE/PE is associated with increased levels of proinflammatory cytokines, including TNF-α and IL-6.11,43 Increased TNF-α and IL-6 both contribute to endothelial dysfunction, which is a hallmark feature of PE and is characterized by increased adhesion molecules and endothelial cell permeability44,45. In addition, an imbalance of pro- and anti-inflammatory cytokines exists in PE, further promoting an inflammatory state.46–48 In the present study, offspring from ePE dams exhibited evidence of systemic inflammation at both p23 and p30, suggesting that maternal inflammation was associated with persistent inflammation in offspring. Interestingly, levels of the anti-inflammatory cytokines IL-10 and Galectin-1 were elevated in ePE pups at p23, potentially suggesting an early response to inflammation. The presence of systemic inflammation that persists until p30 is likely to have significant implications for vascular function in offspring of preeclamptic dams.
Our study also demonstrated the presence of systemic oxidative stress in offspring of ePE dams as evidenced by elevated levels of serum 8-isoprostane at both p23 and p30. Typically, pro- and anti-oxidant factors in the body are kept in strict balance to prevent excess formation or reactive oxygen species (ROS), which may be harmful. During PE, however, this strict balance is not maintained. Anti-oxidant mechanisms are overwhelmed and are insufficient in compensating for these pro-oxidant factors, thus resulting in oxidative stress49,50. This oxidative stress contributes to the development of inflammation, which includes the production of inflammatory cytokines, ultimately leading to a state of endothelial dysfunction through recruitment of immune cells that release oxidative stress molecules.51
Our results also show that ePE was associated with increased levels of circulating adhesion molecules (TREM-1, TIM-1, Notch-1) in offspring. Increases in circulating adhesion molecules are known to be induced by inflammation and oxidative stress, and levels of circulating cell adhesion molecules have been shown to be increased in women with PE52. Together, ePE-induced inflammation, oxidative stress, and increases in circulating adhesion molecules provide evidence that the unfavorable intrauterine environment in ePE dams have an impact on offspring. It is unclear how inflammation and oxidative stress in dams leads to similar findings in offspring. This finding may be related to dysfunction of the placental barrier which is known to occur in preeclampsia53. Placental damage could result in an increased transfer of maternal inflammatory factors across the placental barrier into the fetal circulation54. Prior studies have shown that rat fetuses taken from dams with induced placental ischemia exhibit neuroinflammation and cerebral microhemorrhages at embryonic day 1954. Regardless of the source of inflammation and oxidative stress in offspring, it is possible that they contribute to the vascular dysfunction observed in our study.
The upregulation of these circulating adhesion molecules promotes the inflammatory response via a number of different mechanisms. Soluble TREM-1 is known to be upregulated in other obstetric inflammatory states, including obstetric antiphospholipid syndrome and may be a biomarker of thrombosis in pregnant women55. It enhances and amplifies TL-4-mediated inflammation56,57. Circulating TIM-1 stimulates T-cell activation and enhances cytokine production58,59. Notch-1 is a critical mediator of macrophage activation and stimulator of cytokine production60. Elevation of these factors in serum is evidence of systemic inflammation that may involve multiple pathways.
The consequence of elevated circulating adhesion molecules in ePE pups at p23 but not at p30 is unclear. It is possible that this may be related to maturation of the pups over time. This is not to say that the inflammatory response has resolved; indeed TNF-ɑ was still significantly elevated at p30 compared to pups from NP dams. However, we theorize that overall production of these molecules decreases over time such that the net effect is higher production of these proteins that no longer reaches statistical significance. To our knowledge, data do not exist regarding the production of these proteins over the course of development, so this theory is difficult to verify. It is also possible that generation of anti-inflammatory cytokines at p23 (as demonstrated in Fig 8) may have been sufficient to mitigate the inflammatory response in such a way that by p30, the expression of these proteins in the ePE animals has decreased. Additional studies are required to determine the effect of these proteins on vascular function as well as the evolution of their production over the course of development.
It is worth noting that preeclampsia is a syndrome specific to humans. However, the use of animal models of preeclampsia is an important tool to understand this complicated condition, especially when investigating changes in the brain and offspring. In this study, we used a model of preeclampsia that causes hypercholesterolemia and high oxidized low-density lipoprotein (LDL), an important mediator of vascular dysfunction, and similar to the human condition. In addition, this model of preeclampsia has other features of preeclampsia, including maternal hypertension, fetal growth restriction, placental dysfunction, oxidative stress, and endothelial dysfunction29–32,61. While no model perfectly mimics the features of clinical preeclampsia, the model used in this study has a a documented maternal phenotype, and our results suggest an influence of ePE on offspring.
5. CONCLUSIONS
Our study provides the first evidence of structural and functional changes in the cerebrovasculature of young offspring from ePE rats, and in response to maturation. These results have broad clinical implications that are relevant to the practice of clinical pediatrics. Though the effects of PE on many body systems has been well-established, there are limited data studying the how PE affects the development and function of the cerebrovasculature. We demonstrated significant alterations in the growth, structure and function of the MCA, one of the most commonly affected arteries in perinatal stroke. We also found dysfunction in the VoG in pups from ePE dams, one of the most commonly affected veins in perinatal ischemic stroke. The effect of ePE on pup cerebral vasculature may predispose to stroke by enhancing vasoconstriction as well as increase the severity of stroke through limited vasodilatory reserve. These results were obtained in the setting of evidence of systemic inflammation and oxidative stress that may be causal to the vascular dysfunction seen in ePE pups.
The translational relevance of our study is not limited to perinatal stroke. Our results may suggest dysfunction of the blood brain barrier, cerebral blood flow autoregulation, and other features of the cerebral vasculature that are critical to the maintenance of cerebral perfusion. If PE has such effects on the cerebral vasculature in offspring of PE mothers, how physicians care for children during anesthesia and surgery and in critical care settings will fundamentally change. In addition, further study could provide insights as to the predisposition of offspring of PE mothers for adult conditions, including cardiovascular disease.
Highlights.
Preeclampsia increases the risk for perinatal stroke in offspring
The reason for this increase in risk is not known
Preeclampsia induced systemic inflammation and oxidative stress
Preeclampsia altered structure and function of cerebral vessels in offspring
Alterations in vessel tone and response to vasoactive mediators occurred
Acknowledgments
Funding: This study was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke grants R01 NS045940-15; R01 NS108455-02, and R01 NS093289-05, the Totman Medical Research Foundation and the Cardiovascular Research Institute of Vermont.
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol 2009;33:130–7. [DOI] [PubMed] [Google Scholar]
- 2.Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens 2008;21:521–6. [DOI] [PubMed] [Google Scholar]
- 3.Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol 2011;25:391–403. [DOI] [PubMed] [Google Scholar]
- 4.Gumusoglu SB, Chilukuri ASS, Santillan DA, Santillan MK, Stevens HE. Neurodevelopmental Outcomes of Prenatal Preeclampsia Exposure. Trends in Neurosciences 2020;43:253–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dang F, Croy B, Stroman P, Figueiró-Filho E. Impacts of Preeclampsia on the Brain of the Offspring. Revista Brasileira de Ginecologia e Obstetrícia / RBGO Gynecology and Obstetrics 2016;38:416–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giachini FR, Galaviz-Hernandez C, Damiano AE, et al. Vascular Dysfunction in Mother and Offspring During Preeclampsia: Contributions from Latin-American Countries. Current Hypertension Reports 2017;19. [DOI] [PubMed] [Google Scholar]
- 7.Geelhoed JJ, Fraser A, Tilling K, et al. Preeclampsia and gestational hypertension are associated with childhood blood pressure independently of family adiposity measures: the Avon Longitudinal Study of Parents and Children. Circulation 2010;122:1192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oglaend B, Forman MR, Romundstad PR, Nilsen ST, Vatten LJ. Blood pressure in early adolescence in the offspring of preeclamptic and normotensive pregnancies. J Hypertens 2009;27:2051–4. [DOI] [PubMed] [Google Scholar]
- 9.Wu YW, March WM, Croen LA, Grether JK, Escobar GJ, Newman TB. Perinatal stroke in children with motor impairment: a population-based study. Pediatrics 2004;114:612–9. [DOI] [PubMed] [Google Scholar]
- 10.Fugelseth D, Ramstad HB, Kvehaugen AS, Nestaas E, Stoylen A, Staff AC. Myocardial function in offspring 5–8years after pregnancy complicated by preeclampsia. Early Hum Dev 2011;87:531–5. [DOI] [PubMed] [Google Scholar]
- 11.Kvehaugen AS, Dechend R, Ramstad HB, Troisi R, Fugelseth D, Staff AC. Endothelial function and circulating biomarkers are disturbed in women and children after preeclampsia. Hypertension 2011;58:63–9. [DOI] [PubMed] [Google Scholar]
- 12.Aylanc H, Aylanc N, Yildirim S, et al. Relationship between Abdominal Aortic Intima Media Thickness and Central Obesity in Children. Horm Res Paediatr 2016;85:43–8. [DOI] [PubMed] [Google Scholar]
- 13.Jayet PY, Rimoldi SF, Stuber T, et al. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation 2010;122:488–94. [DOI] [PubMed] [Google Scholar]
- 14.Kajantie E, Eriksson JG, Osmond C, Thornburg K, Barker DJ. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki birth cohort study. Stroke 2009;40:1176–80. [DOI] [PubMed] [Google Scholar]
- 15.Plate KH. Mechanisms of angiogenesis in the brain. J Neuropathol Exp Neurol 1999;58:313–20. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro M, Raz E. Cerebrovascular Development and Evolution. Neurovascular Imaging 2015:1–30. [Google Scholar]
- 17.Rensen SS, Doevendans PA, van Eys GJ. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J 2007;15:100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tong W, Giussani DA. Preeclampsia link to gestational hypoxia. J Dev Orig Health Dis 2019;10:322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hubbell MC, Semotiuk AJ, Thorpe RB, et al. Chronic hypoxia and VEGF differentially modulate abundance and organization of myosin heavy chain isoforms in fetal and adult ovine arteries. Am J Physiol Cell Physiol 2012;303:C1090–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lou HC, Tweed WA, Davies JM. Preferential blood flow increase to the brain stem in moderate neonatal hypoxia: reversal by naloxone. Eur J Pediatr 1985;144:225–7. [DOI] [PubMed] [Google Scholar]
- 21.Longo LD, Hull AD, Long DM, Pearce WJ. Cerebrovascular adaptations to high-altitude hypoxemia in fetal and adult sheep. Am J Physiol 1993;264:R65–72. [DOI] [PubMed] [Google Scholar]
- 22.Chabrier S, Husson B, Dinomais M, Landrieu P, Nguyen The Tich S. New insights (and new interrogations) in perinatal arterial ischemic stroke. Thromb Res 2011;127:13–22. [DOI] [PubMed] [Google Scholar]
- 23.Lynch JK, Nelson KB. Epidemiology of perinatal stroke. Curr Opin Pediatr 2001;13:499–505. [DOI] [PubMed] [Google Scholar]
- 24.Roberts JM, Escudero C. The placenta in preeclampsia. Pregnancy Hypertens 2012;2:72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez-Lopez D, Natarajan N, Ashwal S, Vexler ZS. Mechanisms of perinatal arterial ischemic stroke. J Cereb Blood Flow Metab 2014;34:921–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giraud A, Guiraut C, Chevin M, Chabrier S, Sebire G. Role of Perinatal Inflammation in Neonatal Arterial Ischemic Stroke. Front Neurol 2017;8:612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arteaga O, Alvarez A, Revuelta M, Santaolalla F, Urtasun A, Hilario E. Role of Antioxidants in Neonatal Hypoxic-Ischemic Brain Injury: New Therapeutic Approaches. Int J Mol Sci 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson AC, Cipolla MJ. Altered hippocampal arteriole structure and function in a rat model of preeclampsia: Potential role in impaired seizure-induced hyperemia. J Cereb Blood Flow Metab 2017;37:2857–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson AC, Cipolla MJ. Impaired function of cerebral parenchymal arterioles in experimental preeclampsia. Microvasc Res 2018;119:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schreurs MP, Hubel CA, Bernstein IM, Jeyabalan A, Cipolla MJ. Increased oxidized low-density lipoprotein causes blood-brain barrier disruption in early-onset preeclampsia through LOX-1. FASEB J 2013;27:1254–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schreurs MPH, Cipolla MJ. Cerebrovascular Dysfunction and Blood–Brain Barrier Permeability Induced by Oxidized LDL are Prevented by Apocynin and Magnesium Sulfate in Female Rats. Journal of Cardiovascular Pharmacology 2014;63:33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schreurs MP, Cipolla MJ. Pregnancy enhances the effects of hypercholesterolemia on posterior cerebral arteries. Reprod Sci 2013;20:391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cipolla MJ, Sweet JG, Chan SL. Cerebral vascular adaptation to pregnancy and its role in the neurological complications of eclampsia. J Appl Physiol (1985) 2011;110:329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dionne K, Redfern RL, Nichols JJ, Nichols KK. Analysis of tear inflammatory mediators: A comparison between the microarray and Luminex methods. Mol Vis 2016;22:177–88. [PMC free article] [PubMed] [Google Scholar]
- 35.Luck C, DeMarco VG, Mahmood A, Gavini MP, Pulakat L. Differential Regulation of Cardiac Function and Intracardiac Cytokines by Rapamycin in Healthy and Diabetic Rats. Oxid Med Cell Longev 2017;2017:5724046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee C-C, Lin J-J, Lin K-L, et al. Clinical Manifestations, Outcomes, and Etiologies of Perinatal Stroke in Taiwan: Comparisons between Ischemic, and Hemorrhagic Stroke Based on 10-year Experience in A Single Institute. Pediatrics & Neonatology 2017;58:270–7. [DOI] [PubMed] [Google Scholar]
- 37.Coelho‐Santos V, Shih AY. Postnatal development of cerebrovascular structure and the neurogliovascular unit. WIREs Developmental Biology 2019;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stark MJ, Clifton VL, Wright IM. Neonates born to mothers with preeclampsia exhibit sex-specific alterations in microvascular function. Pediatr Res 2009;65:292–5. [DOI] [PubMed] [Google Scholar]
- 39.Alexander BT, Kassab SE, Miller MT, et al. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension 2001;37:1191–5. [DOI] [PubMed] [Google Scholar]
- 40.Srinivas SK, Edlow AG, Neff PM, Sammel MD, Andrela CM, Elovitz MA. Rethinking IUGR in preeclampsia: dependent or independent of maternal hypertension? J Perinatol 2009;29:680–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hausvater A, Giannone T, Sandoval Y-HG, et al. The association between preeclampsia and arterial stiffness. Journal of Hypertension 2012;30:17–33. [DOI] [PubMed] [Google Scholar]
- 42.Trigiani LJ, Lecrux C, Royea J, et al. A Longitudinal Pilot Study on Cognition and Cerebral Hemodynamics in a Mouse Model of Preeclampsia Superimposed on Hypertension: Looking at Mothers and Their Offspring. Frontiers in Physiology 2021;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aggarwal R, Jain AK, Mittal P, Kohli M, Jawanjal P, Rath G. Association of pro- and anti-inflammatory cytokines in preeclampsia. J Clin Lab Anal 2019;33:e22834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dechend R, Homuth V, Wallukat G, et al. Agonistic antibodies directed at the angiotensin II, AT1 receptor in preeclampsia. J Soc Gynecol Investig 2006;13:79–86. [DOI] [PubMed] [Google Scholar]
- 45.Kharfi A, Giguere Y, Sapin V, Masse J, Dastugue B, Forest JC. Trophoblastic remodeling in normal and preeclamptic pregnancies: implication of cytokines. Clin Biochem 2003;36:323–31. [DOI] [PubMed] [Google Scholar]
- 46.Bennett WA, Lagoo-Deenadayalan S, Stopple JA, et al. Cytokine expression by first-trimester human chorionic villi. Am J Reprod Immunol 1998;40:309–18. [DOI] [PubMed] [Google Scholar]
- 47.Hanna N, Hanna I, Hleb M, et al. Gestational age-dependent expression of IL-10 and its receptor in human placental tissues and isolated cytotrophoblasts. J Immunol 2000;164:5721–8. [DOI] [PubMed] [Google Scholar]
- 48.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today 1993;14:353–6. [DOI] [PubMed] [Google Scholar]
- 49.Matsubara K, Matsubara Y, Hyodo S, Katayama T, Ito M. Role of nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. J Obstet Gynaecol Res 2010;36:239–47. [DOI] [PubMed] [Google Scholar]
- 50.Myatt L, Cui X. Oxidative stress in the placenta. Histochem Cell Biol 2004;122:369–82. [DOI] [PubMed] [Google Scholar]
- 51.Yiyenoglu OB, Ugur MG, Ozcan HC, et al. Assessment of oxidative stress markers in recurrent pregnancy loss: a prospective study. Arch Gynecol Obstet 2014;289:1337–40. [DOI] [PubMed] [Google Scholar]
- 52.Szarka A, Rigó J, Lázár L, Bekő G, Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunology 2010;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Luca Brunori I, Battini L, Brunori E, et al. Placental barrier breakage in preeclampsia: ultrastructural evidence. European Journal of Obstetrics & Gynecology and Reproductive Biology 2005;118:182–9. [DOI] [PubMed] [Google Scholar]
- 54.Giambrone AB, Logue OC, Shao Q, Bidwell GL, Warrington JP. Perinatal Micro-Bleeds and Neuroinflammation in E19 Rat Fetuses Exposed to Utero-Placental Ischemia. International Journal of Molecular Sciences 2019;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edel Y, Kliminski V, Pokroy-Shapira E, et al. Elevated plasma level of soluble triggering receptor expressed on myeloid cells-1 is associated with inflammation activity and is a potential biomarker of thrombosis in primary antiphospholipid syndrome. Arthritis research & therapy 2019;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nature Immunology 2006;7:1266–73. [DOI] [PubMed] [Google Scholar]
- 57.Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature 2001;410:1103–7. [DOI] [PubMed] [Google Scholar]
- 58.Ichimura T, Asseldonk EJPv, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule–1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. Journal of Clinical Investigation 2008;118:1657–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Umetsu SE, Lee W-L, McIntire JJ, et al. TIM-1 induces T cell activation and inhibits the development of peripheral tolerance. Nature Immunology 2005;6:447–54. [DOI] [PubMed] [Google Scholar]
- 60.Hamilton Outtz H, Wu JK, Wang X, Kitajewski J. Notch1 Deficiency Results in Decreased Inflammation during Wound Healing and Regulates Vascular Endothelial Growth Factor Receptor-1 and Inflammatory Cytokine Expression in Macrophages. The Journal of Immunology 2010;185:4363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schreurs MPH, Cipolla MJ. Pregnancy Enhances the Effects of Hypercholesterolemia on Posterior Cerebral Arteries. Reproductive Sciences 2012;20:391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]