Abstract
Glyphosate is currently the most widely used herbicide in the world; however, the zwitterionic and highly polar properties of glyphosate make current pesticide analysis methods unsuitable for its trace analysis in natural waters. Additionally, current glyphosate analysis methods do not account for waters of varying hardness, which is vital as glyphosate can complex with cationic species such as Ca2+ and Mg2+ in the environment. We detail here a robust LC-MS/MS method for the quantitation of glyphosate and its primary transformation product aminomethylphosphonic acid (AMPA) in environmental waters of varying water hardness. Chromatographic separation was achieved with a reversed-phase and weak anion-exchange mixed-mode column. We found that the addition of EDTA into hard water samples increases the response of both glyphosate and AMPA in the mass spectrometer. Limits of detection of 0.23 and 0.30 μg L−1 for glyphosate and AMPA in EDTA-amended hard water were achieved, respectively. We have demonstrated that the accuracy of the method was consistent over a wide range of water hardness levels up to a maximum of ~340 mg mL−1 CaCO3 hardness. We validated the method using matrix fortification of uncontaminated environmental samples from US river water. We then demonstrated that the method was successful at quantifying glyphosate and AMPA across surface and drinking water samples of varying water hardness from North Carolina and Sri Lanka. Measured concentrations of glyphosate and AMPA ranged from 1.6 to 13 μg L−1 and 0.50 to 2.5 μg L−1, respectively. This study represents a significant increase in sensitivity for LC-MS/MS analysis of glyphosate in hard water systems.
Keywords: Glyphosate, Environmental analytical chemistry, Emerging contaminants
Introduction
Glyphosate, commercially known as Roundup®, is a broad-spectrum, non-specific herbicide that is the most widely used herbicide in the modern era [1, 2]. Glyphosate is the only synthetic herbicide that is effective at inhibiting the enzyme 5-enolpyruvyl-shikimate-3-phosphate synthase (EPSPS) of the shikimate pathway [3]. This inhibition prohibits the synthesis of various aromatic acids within plants vital for protein synthesis, ultimately leading to the death of the plants [3–5]. Due to its non-selectivity and ability to inhibit the shikimate pathway, it was named the pesticide of the turn of the millennium and has led to the development of modern sustainable agricultural systems that produce cheap and healthy food [4]. Glyphosate’s vast economic impact can be attributed to the simplicity, price, flexibility, and effectiveness of glyphosate as a whole [1, 5].
As a phosphonate-based herbicide, glyphosate exists in the environment as a zwitterion with pKa values of 0.8 (1st phosphonic), 2.3 (carboxylate), 6.0 (2nd phosphonic), and 11.0 (amine) (Fig. 1) resulting in a net anionic charge [2, 5]. It is thus active as a salt with various cations (e.g., isopropyl amine, sodium, ammonium, and potassium) [5]. Glyphosate readily degrades in the environment: the primary transformation products are AMPA and glyoxylate (Fig. 1a). The degradation occurs when enzymes catalyze the conversion of glyphosate as a potential phosphorous source for microbial processes [6–10]. AMPA is a unique transformation product of the herbicide and can be used as a tracer for glyphosate contamination [6, 7]. Due to glyphosate’s zwitterionic character in the environment, it forms glyphosate-metal complexes in the presence of hard water (Fig. 1b), which increases the half-life of the herbicide from 90 days to 22 years in [11–13]. Specifically, glyphosate forms complexes with various divalent and trivalent cations (e.g., Ca, Mg, Cu, Fe) [12, 13], which reduces the bioavailability of glyphosate for microorganisms to degrade, thus increasing the half-life [14]. These complexes allow glyphosate to accumulate in hard waters in the environment and pose a human exposure risk due to the increased half-life [2].
Fig. 1.
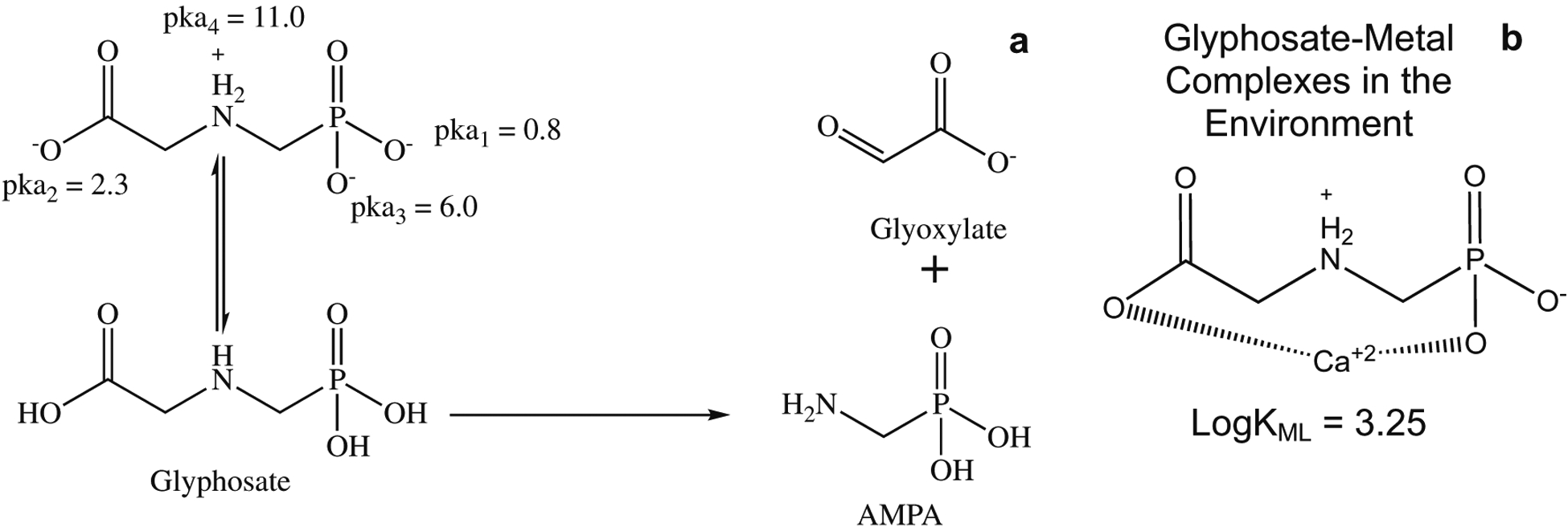
Schematic showing the environmentally relevant form of glyphosate and the biodegradation of glyphosate to AMPA (left). Example of a glyphosate-metal complex that can form in hard water systems in the environment (right)
Recently, the link between glyphosate and chronic toxicity has been called into question after originally being deemed non-toxic [15]. In 2015, the International Agency for Research on Cancer (IARC) concluded that glyphosate and products in its formulations are most likely carcinogenic for humans [16, 17]. Toxicity studies were found that exposure to glyphosate can lead to teratogenic effects and reproductive effects in embryos of Xenopus laevis [18]. Other toxicity studies found glyphosate exposure linked to inhibition of acetylcholinesterase in Cnesterodon decemmaculatus [19] and liver damage in Jenynsia multidentata [20]. Additionally, glyphosate exposure is related to adverse neurodevelopmental effects such as anencephaly in newborns [21]. An extensive review of glyphosate toxicity for animals was conducted by Gill et al. where they found that glyphosate poses a risk to multiple unicellular and multicellular organism including genotoxicity, cytotoxicity, nuclear aberration, hormonal disruption, chromosomal aberrations, and DNA damage [22].
The toxicity of glyphosate in the presence of metals has also been studied since the persistence of glyphosate significantly increases in hard waters and in the presence of metals. One study found that in the presence of glyphosate, the uptake of certain metals (e.g., Hg) was significantly increased, thus increasing the potential for metal toxicity [14]. Additionally, glyphosate has been shown to contribute to kidney damage and kidney developmental effects in zebrafish, Danio rerio, with and without the presence of metals, suggesting a link between kidney health and glyphosate-metal exposure [23]. A current major concern involves glyphosate as a potential cause for kidney disease in humans, where it is hypothesized that co-exposure to glyphosate, hard water, and various metals leads to kidney disease [2, 24–30]. Even though there is strong evidence for the potential health impacts of human exposure glyphosate, there is a lack of sensitive and accurate analytical methods for the determination of glyphosate contamination, especially in hard water systems.
Due to the anionic behavior of glyphosate, typical pesticide analysis methods are not compatible for its detection, since these methods typically rely on reverse-phase chromatography, which is not successful at retaining the highly polar glyphosate and AMPA [2]. Various methods have been developed to detect glyphosate in the environment [31]. Methods have included gas chromatography (GC) [32, 33], high-performance liquid chromatography (HPLC) [34–36], and capillary electrophoresis (CE) [37–39], which use time-consuming derivatization and fluorescence techniques. Ion chromatography (IC) coupled to mass spectrometry has been used as well, in order to eliminate the derivatization step, but chromatographic separation of glyphosate and AMPA is challenging with this technique [40–42]. Most of these methods have detection limits greater than 1 μg L−1 and quantitation limits greater than 10 μg L−1. Method sensitivity must be increased for robust exposure assessment, as glyphosate in the presence of metals has been shown to be toxic at much lower levels [23]. Direct injection LC-MS/MS has been previously utilized for the determination of glyphosate and AMPA in water [43, 44], but these methods did not account for water hardness, rendering it potentially unsuitable for many glyphosate-impacted waters. Since glyphosate is significantly more persistent in the presence of cations, it is essential to develop a robust analytical method to quantify glyphosate and its major transformation product at low levels in all water types. In addition, the sensitivity of one previously reported direct injection LC-MS/MS method (~5 μg L−1) was insufficient for trace analysis [43]. Based on published toxicity studies [23], we believe quantitation levels of < 1 μg L−1 are needed to better assess the risk of potential human toxicity due to glyphosate through drinking water exposure.
Here we present an LC-MS/MS-based direct injection method for the quantitation of glyphosate and its main transformation product AMPA within hard water systems. The method uses reversed-phase and weak anion-exchange mixed-mode column chromatography to analyze both glyphosate and AMPA. Mixed-mode chromatography is successful in retaining glyphosate (and other negatively charged polar compounds) for sensitive and specific quantification using tandem mass spectrometry [43]. Detection is performed using a high-performance, ion funnel–equipped triple quadrupole mass spectrometer. Additionally, this method has been applied to water samples of varying hardness in order to better mimic the hard water systems pertinent and relevant to glyphosate persistence and human exposure. This work represents the most sensitive analytical method available to quantify glyphosate and AMPA in hard water systems.
Materials and methods
Materials
Analytical standards of glyphosate, AMPA, and 13C, 15N-glyphosate (13C, ≥ 99%; 15N, ≥ 98%) were obtained from Sigma-Aldrich (Milwaukee, WI). A stock solution of 13C, 15N, D2-AMPA (13C, 99%;15N, 98%; methylene-D2,98%) was acquired from Cambridge Isotopes Laboratory (Andover, MA). 13C, 15N-glyphosate and 13C, 15N, D2-AMPA were used as internal standards. All samples and calibration standards contained 20 μg L−1 of internal standards to carry out isotope dilution mass spectrometry (IDMS) for both glyphosate and AMPA. Intermediate standard solutions were prepared daily from the analyte stocks. The calibration standards and spiking solutions were then made via serial dilution with NanoPure water to decrease ionic contamination in the standards. Since the analytes can form complexes with metal ions and charged surfaces, plastic labware was used to avoid adsorption to surfaces and loss of analytes.
Methanol (LC/MS Optima) and ammonium acetate (ACS Grade) were purchased from Fisher Scientific (Suwanee, GA). Ethylenediaminetetraacetic acid tetrasodium salt dihydrate (EDTA-Na4 • H2O) was purchased from Sigma-Aldrich (Milwaukee, WI).
Sample collection and storage
Water samples collected in Sri Lanka were stored in 125-mL amber polyethylene bottles and were frozen for transport back to Duke University, Durham, NC. Samples were collected from regions of Sri Lanka experiencing an unknown kidney disease that is hypothesized to be linked to glyphosate, hard water, and metal exposure [27–29] making these samples relevant for method validation. The samples were then stored at − 20 °C for 6 months until used for analysis. Water samples were also collected from the Duke Reclamation Pond (Durham, NC), which collects rain water and runoff across Duke University’s West Campus, the Haw River (North Carolina), which serves as the headwaters for the Cape Fear River Basin, and the Ellerbe Creek, which drains much of urban Durham, NC, and empties into the Neuse River. Samples were collected directly in 15-mL polyethylene centrifuge tubes and stored at − 20 °C for 1 month until used for analysis.
Sample analysis
Samples were allowed to thaw to room temperature before being minimally processed as necessary for analysis by direct injection. Samples were homogenized via manual shaking and 15 mL aliquots of non-analyte-spiked samples were then centrifuged at 15,000 RPM to remove particulate material within the sample. A 480 μL aliquot of sample was transferred to an 800-μL polypropylene HPLC vial. One hundred sixty microliters of each internal standard were added to each vial for an in-vial concentration of 20 μg L−1. Separately, 15 mL standard addition samples were prepared using the same procedure but with initial spike of 20 μg L−1 glyphosate and AMPA. The impacts of EDTA on method performance were evaluated with several sample types: analyte-spiked and 10 μM of EDTA-amended samples, no analyte spike and 10 μM of EDTA-amended, analyte spike and non-EDTA-amended, and no analyte spike and non-EDTA-amended. The LC-MS/MS system used for determination and quantification of the analytes was a Vanquish ultra-high pressure liquid chromatograph (UHPLC, Thermo Fisher, San Jose, CA) coupled to a high-performance, ion funnel TSQ Altis™ Triple Quadrupole Mass Spectrometer (Thermo Fisher, San Jose, CA). Analytes were separated with an Acclaim® Mixed-Mode WAX-1 (reversed-phase/weak anion-exchange, Thermo Fisher, San Jose, CA) 3 μm 50 mm × 3 mm LC column. The column temperature was held at 30 °C and the injection volume was 50 μL. Mobile phases were (A) 50:50 methanol:water and (B) 300 mM ammonium acetate in 50:50 methanol:water with a flow of 0.4 mL/min. The gradient was initially 40% B and raised to 100% B over the first 3 min, maintained for 5 min and then decreased to 40% B over 30 s and maintained for 6.5 min for a total run time of 15 min. This gradient was adapted from Hao et al. [43] and required no chromatographic optimization, given the separation achieved shown in Fig. 2. Between each sample injected, a 10 μL injection of 500 mM EDTA was made on the system to decrease cationic metal contamination within the system and increase peak resolution of the analytes. Multiple reaction monitoring (MRM) in negative ion electrospray mode was used to collect data with the MRM transitions, collision energies, and RF lens voltages for the analytes and internal standards shown in Table 1. Isotope dilution was used for the quantitation of both analytes. The sheath gas, aux gas, sweep gas, ion transfer tube temperature, and vaporizer temperature were set to 35, 18, 2.5, 350 °C, and 300 °C, respectively. Calibration standards were prepared in NanoPure water, EPA moderately hard water [45], and EPA moderately hard water amended with 10 μM EDTA to acquire QA/QC data relevant to the sample compositions acquired from hard water systems.
Fig. 2.
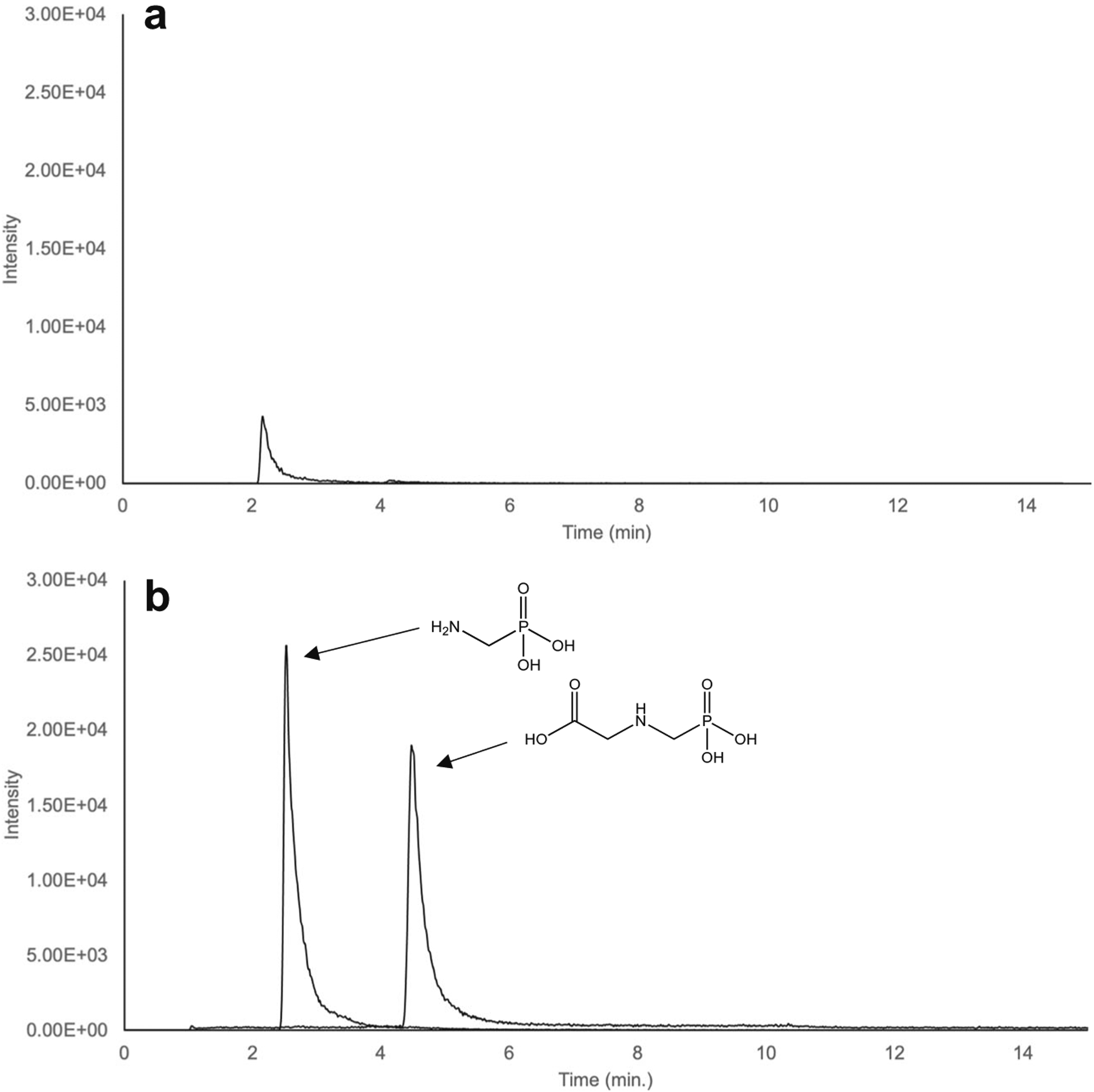
Total ion chromatogram of a 100 μg L−1 glyphosate and AMPA sample pre-EDTA injection (a) and post EDTA injection (b), showing improved peak response after EDTA treatment. Full analyte separation was achieved within a 15-min run time, with AMPA eluting after 2 min, and glyphosate at approximately 5 min
Table 1.
MRM transitions determined for glyphosate, AMPA, and respective internal standards
| Compound | Precursor (m/z) | Product (m/z) | Collision energy (V) | RF lens (V) |
|---|---|---|---|---|
| Glyphosate | 168.0 | 124.0 | 12.4 | 20 |
| 168.0 | 150.0 | 10.1 | 20 | |
| 13C,l5N-glyphosate | 170.0 | 152.0 | 10.5 | 42 |
| AMPA | 110.0 | 63.0 | 20.1 | 48 |
| 110.0 | 78.9 | 28.7 | 48 | |
| 13C,15N, D2-aMPA | 114.0 | 67.0 | 20.5 | 18 |
Water hardness measurements
Water hardness measurements were made using a DR2800 Portable Spectrophotometer (Hach). The method used was hardness, calcium, and magnesium, a calmagite colorimetric method (Hach method 8030, Hach Water Analysis Handbook, 3rd ed., Hach Company, Loveland, CO, 1997). The range of this method is 0.00–4.00 mg L−1 of hardness as CaCO3 for both Mg and Ca hardness. For samples that exceed this hardness range, samples were diluted until accurate measurements were recorded.
Results and discussion
Chromatographic separation and detection of analytes
Without the addition of EDTA to samples or pre-injection of EDTA into the chromatographic system, the analyte peaks for both glyphosate and AMPA at 100 μg L−1 had low response areas (~102–103) and reproducibility of peak areas was poor. The observed peaks were only slightly above the noise level in the system, making trace quantification and detection impossible (Fig. 2a). Previous studies have shown similar phenomena when analyzing metal-complexing compounds with WAX columns and have found that trace metal contamination through the LC system was responsible for poor analyte response and peak shape in those cases [46]. EDTA has been shown to decrease the trace metal contamination within the system which aides in improving analyte response in these studies [46]. To increase the response of the analytes, 500 μM of EDTA was injected onto the column before each sample to clear the system of this contamination. The EDTA acts as a chelating agent where it removes the ionic contamination present in the system that decreases peak response areas [46]. EDTA injection prior to sample analysis increased peak area for both glyphosate and AMPA by approximately an order of magnitude relative to measurements without EDTA injection (Fig. 2b).
Utilizing EDTA injection between each sample, the WAX-1 column reproducibly retained and separated glyphosate and AMPA (Fig. 2b). Both glyphosate and AMPA were successfully detected and quantified in standards made in NanoPure water, moderately hard water, and moderately hard water amended with EDTA. The moderately hard water had a total water hardness of 94 mg L−1 CaCO3. After approximately 100 injections, the column was cleaned as previously reported to return the column to optimal selectivity and sensitivity [43]. This involved washing the column with 50 mM disodium EDTA in NanoPure water for 1 h followed by a 3-h wash of 50:50 methanol:water. This wash helped to remove any residual metal buildup in the column that could impact the method performance.
Influence of water chemistry on accuracy and sensitivity
Figures of merit including accuracy and sensitivity for the method were determined for the three water types used in this study: NanoPure water, moderately hard water, and EDTA-amended moderately hard water. Calibration standards were prepared over the range 1–200 μg L−1 for both glyphosate and AMPA. Instrument response for glyphosate correlated well to a linear calibration model over the full calibration range in all water types using 1/x weighting as illustrated by high R2 values obtained for the linear fit generated over 3 separate calibration curves (Supplementary Information (ESM) Fig. S1). AMPA deviated slightly from the linear calibration model (R2 = 0.9904), but this effect was not systematic and was unlikely to significantly impact quantitation for this analyte. Additionally, all predicted concentrations from the calibration samples showed acceptable precision as well as accuracy between 80 and 120% (ESM Table S1). The limits of detection and quantitation of glyphosate were calculated for NanoPure standards, hard water standards, and EDTA-amended hard water standards (Table 2).
Table 2.
Sensitivity for glyphosate and AMPA in analyzed water types
| NanoPure water | Hard water | EDTA-amended hard water | ||
|---|---|---|---|---|
| Glyphosate | LOD (μg L−1) | 0.23(± 0.01)a | 0.30 (± 0.01) | 0.23 (± 0.01) |
| LOQ (μg L−1) | 0.75 (± 0.03) | 1.00 (± 0.03) | 0.75 (± 0.03) | |
| AMPA | LOD (μg L−1) | 0.15 (± 0.02) | 0.50 (± 0.01) | 0.30 (± 0.05) |
| LOQ (μg L−1) | 0.50 (± 0.08) | 1.75 (± 0.01) | 1.05 (± 0.02) |
Data represent mean and standard deviation of triplicate measurements
Limits of detection (LOD) and quantification (LOQ) were determined empirically as described in the literature, where specific accuracy criteria are set for known standards [47, 48]. For this study, LOQ was set as the lowest concentration that can be measured for an analyte with an accuracy between 80 and 120%; the LOD is then mathematically determined via Eq. 1, based on the LOD being 30% less than the LOQ [47, 48].
| (1) |
The glyphosate LOD and LOQ results demonstrate that the hard water system does impact the detection and quantitation limits of glyphosate, but that the addition of EDTA restores full sensitivity. The addition of EDTA also improves the sensitivity for AMPA, demonstrated by the lower LOQ, when compared to sensitivity of AMPA in hard water alone. The lower LOD and LOQ values for both glyphosate and AMPA in EDTA-amended hard water suggest that the addition of a chelating agent suppresses the ionic interference introduced into the mass spectrometer in hard water (ESM Fig. S2 illustrates chromatograms for both analytes at the LOQ as well as associated accuracy). While other methods have been developed for analysis of glyphosate and AMPA with high sensitivity or in complex waters [32–44], the method reported here is one of the first to achieve detection and quantitation limits ≤ 1 μg L−1 across a broad range of water hardness using a rapid, direct injection approach. Both figures of merit for each analyte are over a magnitude of order more sensitive than most previously published methods [32–43]. Additionally, the same sensitivity is achieved with EDTA-amended hard water signifying that we have created a method sensitive enough for use in water systems contaminated by glyphosate.
The precision and recovery of the method was tested using a matrix-matched calibration study with standards of 1, 5, and 10 μg L−1 in each of the 3 types of water (Table 3). Hard water impacted the precision (RSD) and the recovery of both analytes by increasing precision by 4.5–10% and recovery by 2–10% when compared to the NanoPure water. However, adding the EDTA into the samples improved precision by 6–18%. EDTA addition also improved the recovery of measurements by 0.5–8.5%. This result suggests that the addition of EDTA within environmental samples in hard water systems may decrease ionic interference, allowing for more precise detection and quantification of both analytes in hard water system.
Table 3.
Precision and recovery measurements for glyphosate and AMPA in NanoPure water, hard water, and EDTA-amended hard water
| NanoPure water | Hard water | EDTA-amended hard water | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Concentration | Avg. calculated amount (μg L−1) | RSDa (%) | Avg. recovery (%) | Avg. calculated amount (μg L−1) | RSD (%) | Avg. recovery (%) | Avg. calculated amount (μg L−1) | RSD (%) | Avg. recovery (%) | |
| Glyphosate | 1 μg L−1 | 0.997 | 7 | 99.7 | 1.1 | 20 | 111 | 0.99 | 10 | 98.5 |
| 5 μg L−1 | 5.1 | 20 | 101 | 4.9 | 10 | 97.5 | 5.1 | 20 | 102 | |
| 10 μg L−1 | 10.1 | 10 | 101 | 10.3 | 20 | 103 | 9.9 | 10 | 99.0 | |
| AMPA | 1 μg L−1 | 0.88 | 4 | 88.1 | 0.84 | 10 | 84.4 | 0.73 | 9 | 72.9 |
| 5 μg L−1 | 4.99 | 9 | 99.8 | 4.8 | 20 | 96.6 | 4.9 | 9 | 98.1 | |
| 10 μg L−1 | 9.9 | 6 | 98.9 | 9.9 | 30 | 99.1 | 10.1 | 8 | 102 | |
RSD values represent instrument precision determined via triplicate measurements of samples with known spiked concentrations of glyphosate and AMPA
The accuracy of the method was tested for all three water types by quantifying the amount of glyphosate and AMPA within single-blind prepared samples. Three sets of samples with 75 μg L−1 of both glyphosate and AMPA were prepared from neat chemicals by a colleague who was not involved with the study. The calibration curve used to quantify these samples was prepared from separate stock than that used for the blind sample preparation. The method is highly accurate for both analytes in each type of water, with EDTA-amended hard water having the best accuracy for both analytes (Table 4).
Table 4.
Accuracy measurements for glyphosate and AMPA in NanoPure Water, hard water, and EDTA-amended hard water
| Water type | Glyphosate | AMPA | ||
|---|---|---|---|---|
| Avg. calculated amount (μg L−1) | Accuracy | Avg. calculated amount (μg L−1) | Accuracy | |
| NanoPure | 76 (± l)a | 101% | 69 (± 3) | 91% |
| Hard | 74.3 (± 0.3) | 99% | 75.8 (± 0.8) | 101% |
| EDTA-amended | 74.5 (± 0.6) | 99% | 76 (± 2) | 101% |
Data represent mean and standard deviation of triplicate measurements
EDTA addition increased response of analytes in hard water systems
The impact of EDTA on analyte response in hard water was assessed via the response factors (RFs) of the calibration curves for NanoPure water, hard water, and EDTA-amended hard water (Fig. 3). The RFs are the measured slopes of the calibration curves. Both analyte response factors decreased in hard water standards compared to NanoPure water samples. However, when EDTA was added into the hard water standards, the response areas of both glyphosate and AMPA increased. Glyphosate response factor increased by 25% while AMPA response factor increased by 32%. The effects of sample matrix on analyte response in the presence of hard water and EDTA-amended hard water were quantified in comparison to analyte response in nanopure water (Fig. 3). Glyphosate instrument response in hard water was 88% of that in the nanopure water, but increased to 109% relative to nanopure water in EDTA-amended hard water. AMPA response in hard water was 51% of that in nanopure water, but increased to 67% relative to nanopure water in EDTA-amended hard water. This suggests that the addition of EDTA into hard water samples improved analyte responses when analyzing hard water samples. It should be noted, however, that isotope dilution mass spectrometry was employed in this method to account for impacts on instrument response for analytes due to matrix-induced ionization suppression/enhancement. This strategy is effective unless the matrix effect is significant enough to nearly eliminate ionization response for analytes, which was not the case in the current study (ESM Fig. S3).
Fig. 3.
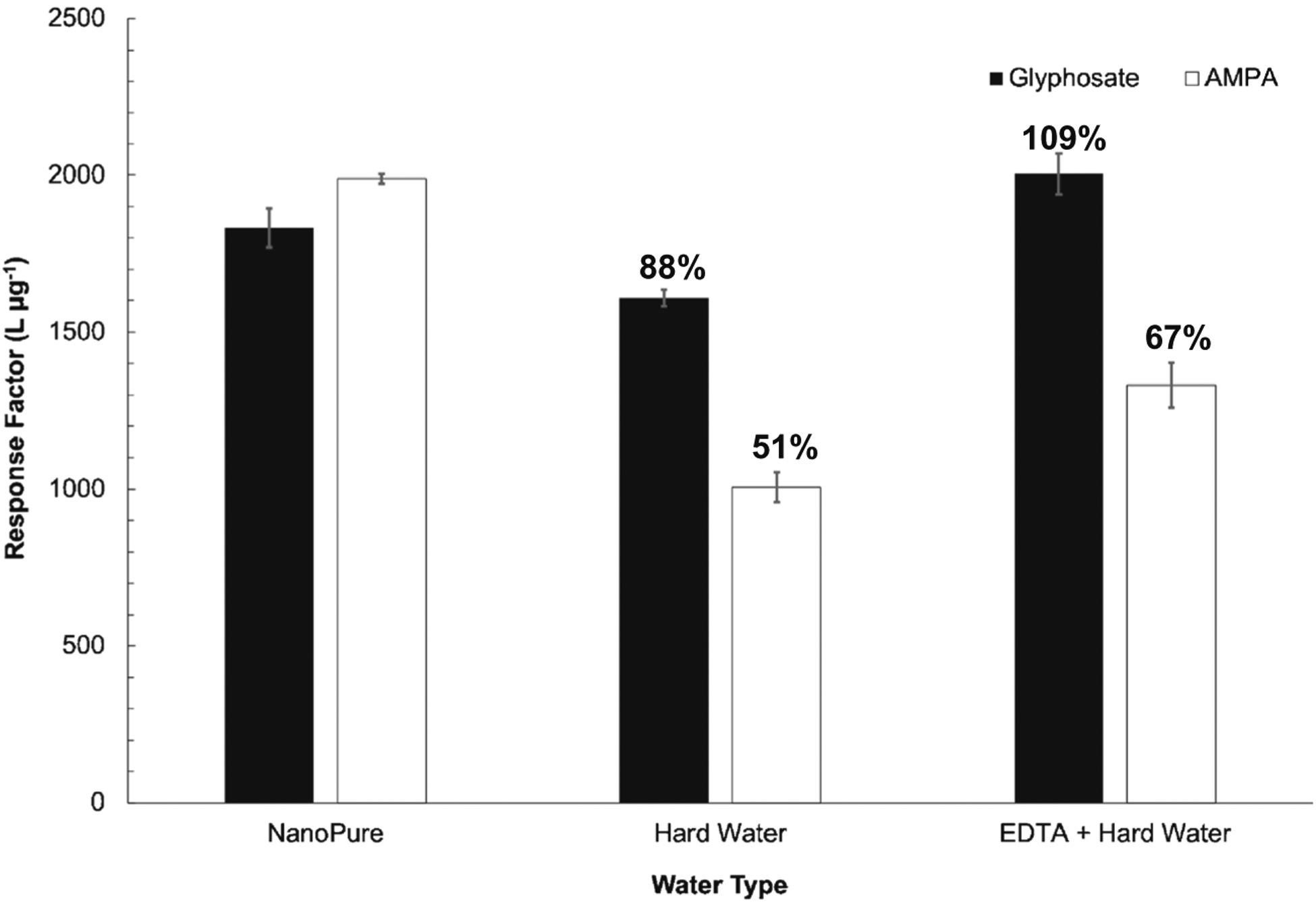
Response factors of glyphosate (black) and AMPA (white) standards in NanoPure water, hard water (94 mg L−1 CaCO3), and EDTA-amended hard water. Error bars represent standard deviation among triplicate measurements. The percentages represent magnitude of observed matrix effects in hard water and EDTA-amended hard water ([EDTA] = 10 μM) with respect to NanoPure water. The data suggest that EDTA-amended samples will increase the response factor of both analytes from hard water
Method robustness
Waters of varying hardness (very soft, soft, moderately hard, hard, very hard, and extremely hard) were prepared according to published methods and water hardness ranges [45] and spiked with 20 μg L−1 of glyphosate and AMPA to investigate the robustness of this method across waters of increasing hardness. Samples were split for analysis with and without addition of EDTA. The accuracy of the measurements was measured to determine whether the method remains robust across all types of water hardness (Fig. 4). Accuracy across all water types ranged between 84 and 91% for glyphosate and 71–91% for AMPA for non-EDTA-amended sample. EDTA-amended samples across all water types had an accuracy range of 99–105% for glyphosate and 98–101% for AMPA. This result demonstrates that addition of EDTA as a metal-complexing agent increases accuracy for the quantification of glyphosate and AMPA, while also increasing robustness across a wide range of water hardness. Matrix effects and freeze/thaw stability of analytes were also investigated (ESM Fig. S3, Fig. S4). Comparisons of response areas of both the 13C, 15N-glyphosate and 13C, 15N, D2-AMPA internal standards for calibration standards and environmental samples with and without EDTA amendment. Similar peak areas were measured for both internal standards for calibration standards and various environmental sample compositions (ESM Fig. S3). Assessment of freeze/thaw stability was performed by repeatedly analyzing a 20 μg L−1 analyte solution, which was frozen and thawed daily over 9 days. No appreciable decrease in measured AMPA or glyphosate concentration was observed over the 9 freeze thawing events (ESM Fig. S4), indicating high freeze/thaw stability of the analytes.
Fig. 4.
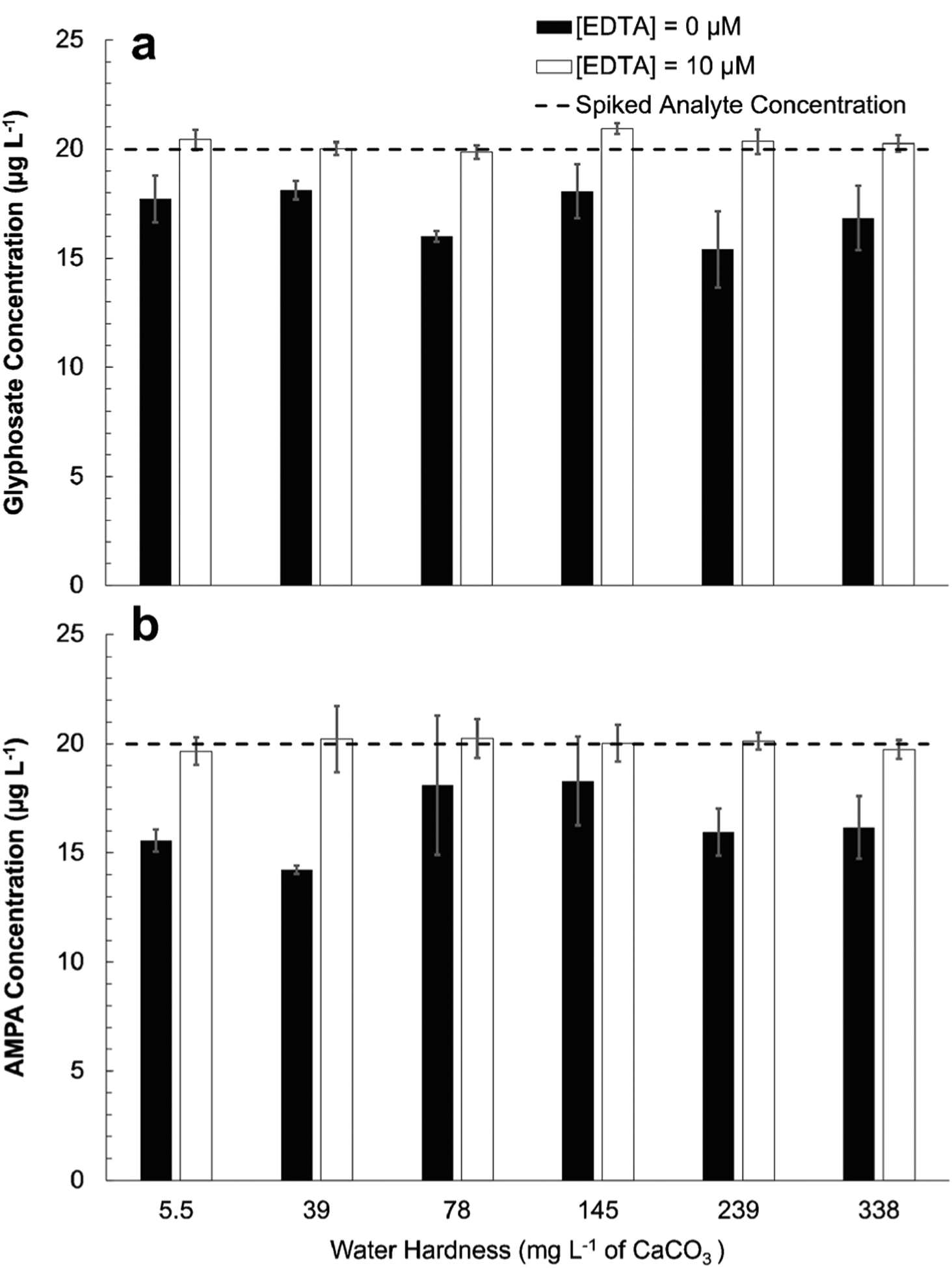
Results of glyphosate (a) and AMPA (b) measurements in water of varying hardness levels. Samples which had no EDTA amendment (black bars, [EDTA] = 0 μM) consistently exhibited analyte measurements below the spiked concentration (dashed line) of 20 μg L−1, while accurate measurements were observed relative to the spiked concentration in EDTA-amended samples (white bars, [EDTA] = 10 μM). Error bars represent standard deviation among triplicate measurements of samples. These results demonstrate that water hardness up to ~340 mg L−1 CaCO3 did not impact the accuracy of the developed method when samples were amended with EDTA
Application to environmental waters
The method was tested on several ambient water samples using (1) a matrix fortification study and (2) a standard addition study. The matrix fortification study was conducted to test whether the method could accurately quantify a spiked concentration of both analytes in environmental samples that had no measurable level of glyphosate or AMPA. The matrix fortification study was conducted with 13 river water samples collected in September, 2020, from the Haw River in North Carolina. Water hardness was measured for these samples ranged from 9.6 mg mL−1 CaCO3 to 19.2 mg mL−1 CaCO3 (Table S2). Initial measurements indicated no detectable glyphosate and/or AMPA present in these waters above the method’s LOD. These river samples were spiked with 20 μg L−1 of both glyphosate and AMPA (Fig. 5). Samples were split for analysis with and without EDTA addition. Results demonstrated that EDTA-amended samples had less variability among AMPA measurements, with a relative standard deviation of 24% in non-EDTA-amended samples and 10% in EDTA-amended samples. EDTA-amended AMPA samples were also more accurate, with an average percent error of 12% versus 42% for non-EDTA-amended samples. EDTA-amended glyphosate samples had a slightly lower average relative standard deviation of 6% versus 8%, while both EDTA and non-EDTA-amended samples had an average percent error of 4% showing no appreciable change in accuracy. Ninety-five percent confidence intervals were calculated for each of the spiked samples to further demonstrate whether EDTA-amended samples or the non-EDTA-amended samples were more accurate (Table S4). The 20 μg L−1 spiked glyphosate concentration was within 92% of the 95% confidence intervals for the samples amended with EDTA but this value was within only 62% of the 95% confidence intervals for samples without EDTA amendment. The 20 μg L−1 spiked AMPA concentration was contained within 62% of the 95% confidence intervals for the samples amended with EDTA versus only in 8% of the 95% confidence intervals spiked only with AMPA. These results demonstrate that samples amended with EDTA more accurately represented the actual concentration of glyphosate and AMPA in water samples.
Fig. 5.
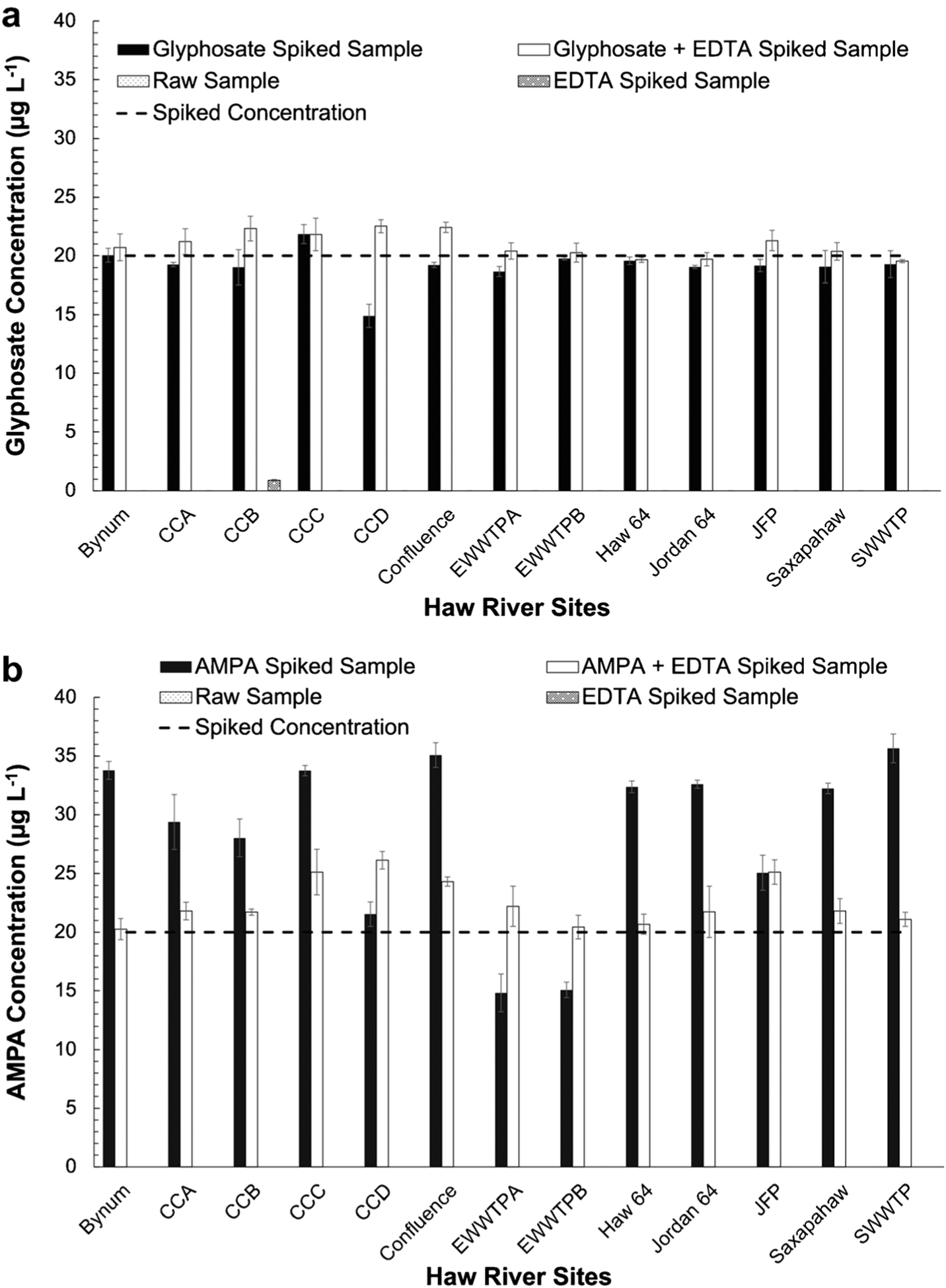
Results of a matrix fortification study with standard addition of glyphosate (a) and AMPA (b) in Haw River samples. Results from analyte-spiked and EDTA-amended samples (white bars, [EDTA] = 10 μM and [Analyte]spiked = 20 μg L−1) and analyte-spiked samples without EDTA amendment (black bars, [EDTA] = 0 μM and [Analyte]spiked = 20 μg L−1) are shown. The dashed line represents the spiked analyte concentration of 20 μg L−1. Error bars represent standard deviation among triplicate analysis of samples. Location CCB was the only river sample with background glyphosate measured at 1.5 μg L−1 (gray bar in panel A), which contributed to the elevated measured concentration of glyphosate in the standard addition sample (22 ± 1 μg L−1). No background AMPA levels were detected in these samples
A standard addition experiment was conducted to quantify the amount of glyphosate and AMPA in potentially contaminated drinking water, well water, and surface waters. Specifically, water samples included a tap water sample from Durham, NC, a sample from the Duke University Stormwater Reclamation Pond, a Sri Lankan drinking water well sample, and 5 samples along the Ellerbe Creek in Durham, NC. The samples were chosen due to an anticipated high probability of glyphosate occurrence, with exception of the tap water. Ellerbe Creek flows through residential and urbanized neighborhoods within Durham and is proximal to a golf course, the Duke University Stormwater Reclamation Pond collects stormwater runoff across all of Duke University’s West Campus, and the Sri Lankan well has historically hard water and is situated in an agricultural area known to use elevated amounts of pesticides. Water hardness measurements were made for all samples (Table S3). Ellerbe Creek and tap water samples were very soft (hardness > 3 mg L−1 CaCO3), the retention pond sample was moderately hard (43 mg L−1 CaCO3), and the Sri Lankan well was hard (163 mg L−1 CaCO3). Samples were split with one sample receiving a 20 μg L−1 addition of both glyphosate and AMPA for standard addition quantification, while the other samples were quantified via reference to internal standard. All samples were amended with EDTA prior to analysis. Varying levels of glyphosate were detected across all samples except the tap water sample; AMPA was only detected and quantified in the Ellerbe Creek samples, which were the overall softer waters (Fig. 6). The standard addition and internal standard sample quantification results agreed within error (p value > 0.05) for all samples analyzed, except for the Creek 4 sample, suggesting that this method can accurately quantify glyphosate and AMPA in realistic environmental water samples.
Fig. 6.
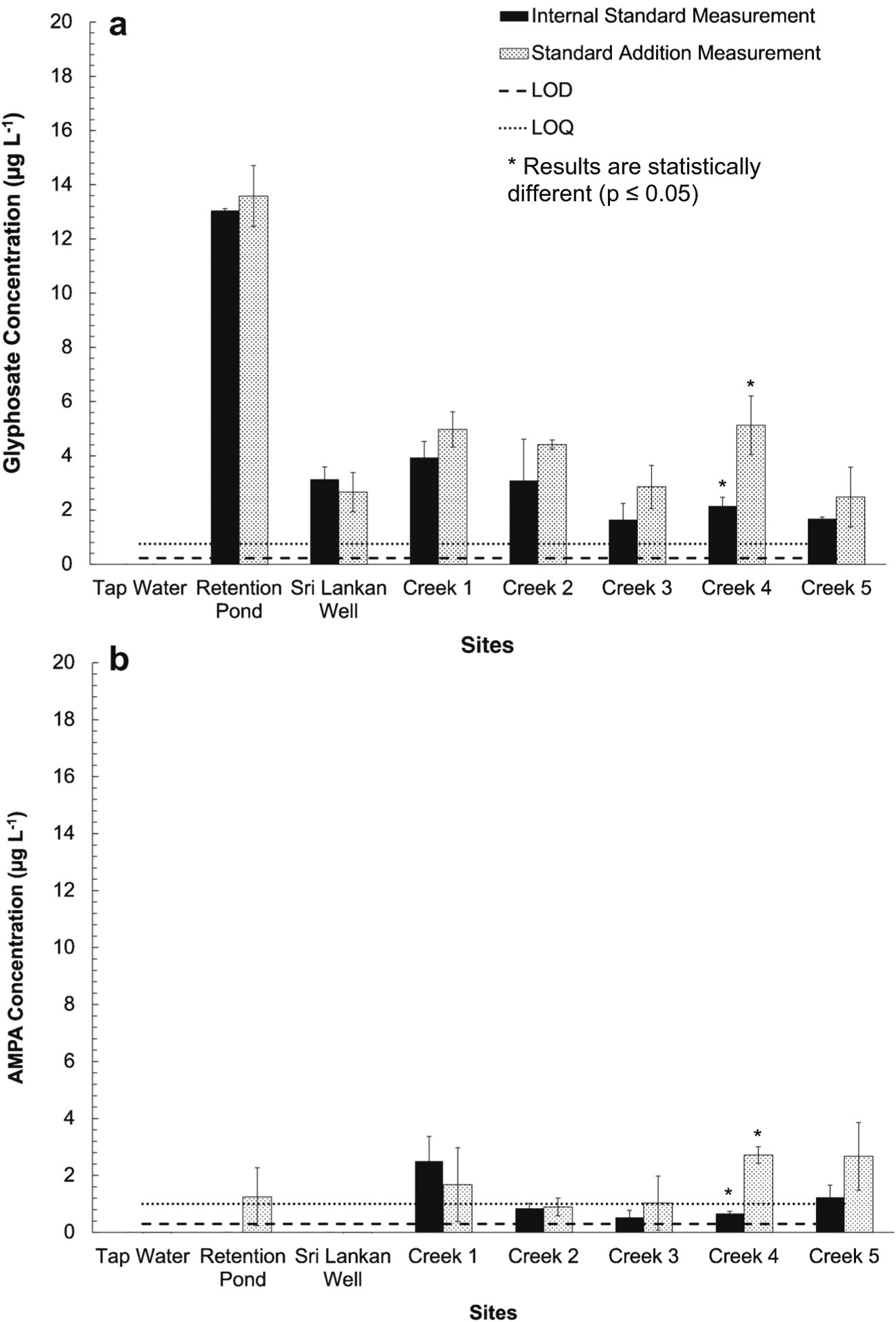
Results of glyphosate (a) and AMPA (b) standard addition experiments in Durham, NC, tap water, Duke University stormwater retention pond water, Sri Lankan well water, and Ellerbe Creek (Durham, NC) water samples. Measurements of samples via internal standard quantitation (black bars, [EDTA] = 10 μM and [Analyte]spiked = 0 μg L−1) and via standard addition (pattern bars, [EDTA] = 10 μM and [Analyte]spiked = 20 μg L−1) are shown. Dashed and dotted lines represent LOD and LOQ of the analytes, respectively. Error bars represent standard deviation among triplicate analyses of samples. Standard addition and internal standard quantified concentrations of analytes agreed within error except for the Creek 4 samples which were significantly different (p value ≤ 0.05) as denoted by the asterisk. Glyphosate was measured above the LOD in all environmental samples except the tap water, while AMPA was found only in Ellerbe creek samples with most of the samples below the LOQ of the method
The reclamation pond sample had the highest levels of glyphosate at 13.05 (± 0.04) μg L−1 and 14 (± 1) μg L−1 (unamended vs. standard addition), which is in general much higher than other studies that have reported glyphosate in stormwater runoff within the USA [49–51]. This may be attributable to the reclamation pond collecting a majority of the stormwater runoff of a 720-acre campus. The levels of glyphosate in the Sri Lankan well and Ellerbe Creek samples were all measured between 1 and 4 μg L−1. The EPA maximum contaminant level for glyphosate was established in 1992 and is 700 μg L−1 [52], which is considerably higher than the concentrations measured. However, this limit does not account for the cancer risk associated with glyphosate or additional considerations for children’s health [53]. The Environmental Working Group has proposed a 5 μg L−1 health guideline, closer to our measured ambient levels [53]. Glyphosate is also noted for its potential kidney toxicity by the EPA [52] and research has shown that adverse kidney development and injury occurs in zebrafish at 10 μg L−1 [23], which suggests that these glyphosate levels, especially in the Sri Lankan well, could be cause for concern for human health.
The soft water Ellerbe Creek samples were the only samples that contained any detectable and quantifiable levels of the AMPA, which may suggest that the presence of ions allows for glyphosate to chelate to metals and thus avoid degradation to AMPA in the harder systems. This could occur due to the decreased bioavailability of glyphosate when bound to metals in the environment [14]. Additionally, only two samples, A and E, had AMPA levels above the LOQ of the method of 1 μg L−1. All other Ellerbe Creek samples had AMPA measured above the LOD (0.300 μg L−1) of the method but below the LOQ (1.00 μg L−1). The presence of AMPA in this water system indicates that glyphosate contamination in the past has occurred since AMPA is a tracer of glyphosate [6, 7]. There are no current regulatory guidelines for AMPA, and most monitoring studies do not find AMPA at detectable levels [49–51]. After degradation in the environment, AMPA represents about 13 to 50% of applied glyphosate, which can explain why AMPA is usually undetectable [54]. Overall, our developed method was successful at quantifying ambient levels of both glyphosate and AMPA in various water samples of varying hardness suggesting that the method can be used to assess glyphosate and AMPA contamination in hard water systems, where glyphosate contamination is expected to occur, in future studies.
Conclusions
We have developed a novel, sensitive, and accurate direct injection method for the detection of the widely used herbicide glyphosate and its transformation product AMPA in hard water systems. The method was successful at retaining and separating the two anionic analytes prior to tandem mass spectrometric detection, which typical pesticide analysis techniques are unable to achieve. We demonstrated that hard water appears to decrease the response of both glyphosate and AMPA in electrospray mass spectrometry, likely due to trace metal contamination that suppresses ionization of the analytes. However, by addition of EDTA, the response of glyphosate and AMPA significantly improved by decreasing the ionization suppression caused by both the ions present in the hard water and the trace metal contamination throughout the LC system. The developed method is the most sensitive technique available for quantifying glyphosate and AMPA, with LOD values of 0.08 μg L−1 and 0.300 μg L−1 after an EDTA spike, respectively. The method requires minimal sample preparation and consumes < 1 mL of sample. Accuracy of the method was demonstrated through matrix fortification experiments with North Carolina river water. We have successfully applied this method to detect and quantify both glyphosate and AMPA in water samples of varying hardness with no apparent water hardness limit. A standard addition experiment successfully detected both analytes in surface and drinking waters, with AMPA only being detected in softer waters compared to glyphosate being detected in all waters.
Supplementary Material
Funding
Funding support was provided from the National Institutes of Health through the Duke University Superfund Research Center (P42 ES010356) and the Duke Environmental Analysis Laboratory (U2C ES030851).
Footnotes
Competing interests The authors declare no competing interest.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00216-021-03324-5.
Code availability Not applicable.
Data availability Raw data of analyses is available upon request by contacting the corresponding author.
References
- 1.Baylis AD. Why glyphosate is a global herbicide: strengths, weaknesses and prospects. Pest Manag Sci. 2000;56(4):299–308. [Google Scholar]
- 2.Jayasumana C, Gunatilake S, Senanayake P. Glyphosate, hard water and nephrotoxic metals: are they the culprits behind the epidemic of chronic kidney disease of unknown etiology in Sri Lanka? Int J Environ Res Public Health. 2014;11(2):2125–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janke D. P. C. Kearney and D. D. Kaufman (Editors), Herbicides – chemistry, degradation, and mode of action, Volume 3. XIII + 403 S. New York-Basel 1988. Marcel Dekker Inc. ISBN: 0-8247-7804-9. J Basic Microbiol. 1989;29(10):718. [Google Scholar]
- 4.Szekacs A, Darvas B. Forty years with glyphosate. Herbicides—Properties, Synthesis and Control of Weeds. 2012. pp. 247–284. [Google Scholar]
- 5.Duke SO, Powles SB. Glyphosate: a once-in-a-century herbicide. Pest Manag Sci. 2008;64(4):319–25. [DOI] [PubMed] [Google Scholar]
- 6.Sviridov AV, Shushkova TV, Ermakova IT, Ivanova E, Epiktetov DO, Leontievsky AA. Microbial degradation of glyphosate herbicides (review). Appl Biochem Micro. 2015;51:188–95. [DOI] [PubMed] [Google Scholar]
- 7.Dick RE, Quinn JP. Glyphosate-degrading isolates from environmental samples: occurrence and pathways of degradation. Appl Microbiol Biotechnol. 1995;43:545–50. [DOI] [PubMed] [Google Scholar]
- 8.Jacob GS, Garbow JR, Hallas LE, Kimack NM, Kishore GM. Metabloism of glyphosate in Pseudomonas sp. Strain LBr. Appl Environ Microbiol. 1988;54:2953–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lerbs W, Stock M, Parthier B. Physiological aspects of glyphosate degradation in Alcaligenes spec. strain GL. Arch Microbiol. 1990;153:146–50. [Google Scholar]
- 10.Balthazor TM, Hallas LE. Glyphosate-degrading microorganisms from industrial activated sludge. Appl Environ Microbiol. 1986;51: 432–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rueppel ML, Brightwell BB, Schaefer J, Marvel JS. Metabolism and degradation of glyphosate in soil and water. J Agric Food Chem. 1977;25:517–28. [DOI] [PubMed] [Google Scholar]
- 12.Madsen HEL, Christensen HH, Gottlieb-Petersen C. Stability constants of copper(II), zinc, manganese(II), calcium, and magnesium complexes of N-(Phosphonomethyl)glycine (glyphosate). Acta Chemica Scandinavica A. 1978;32:79–83. [Google Scholar]
- 13.Subramaniam V, Hoggard PE. Metal complexes of glyphosate. J Agric Food Chem. 1988;36:1326–9. [Google Scholar]
- 14.Tsui MTK, Wang W, Chu LM. Influence of glyphosate and its formulation (Roundup®) on the toxicity and bioavailability of metals to Ceriodaphnia dubia. Environ Pollut. 2005;138:59–68. [DOI] [PubMed] [Google Scholar]
- 15.Williams GM, Kroes R, Munro IC. Safety evaluation and risk assessment of the herbicide Roundup and its active ingredient, glyphosate, for humans. Regul Toxicol Pharmacol. 2000;31(2):177–65. [DOI] [PubMed] [Google Scholar]
- 16.Benbrook CM. How did the US EPA and IARC reach diametrically opposed conclusions on the genotoxicity of glyphosate-based herbicides? Environ Sci Eur. 2019;31(1). [Google Scholar]
- 17.IARC (2017) IARC Monographs on the evaluation of carcinogenic risks to humans—volume 112: some organophosphate insecticides and herbicides. https://monographs.iarc.fr/wp-content/uploads/2018/07/mono112.pdf. Accessed 6 April 2020.
- 18.Robinson C. Teratogenic effects of glyphosate-based herbicides: divergence of regulatory decisions from scientific evidence. J Anal Toxicol. 2012; 01(S4). [Google Scholar]
- 19.Menéndez-Helman RJ, Ferreyroa GV, dos Santos AM, Salibián A. Glyphosate as an acetylcholinesterase inhibitor in Cnesterodon decemmaculatus. Bull Environ Contam Toxicol. 2012;88(1):6–9. [DOI] [PubMed] [Google Scholar]
- 20.Hued AC, Oberhofer S, de los Ángeles Bistoni M. Exposure to a commercial glyphosate formulation (Roundup®) alters normal gill and liver histology and affects male sexual activity of Jenynsia multidentata (Anablepidae, Cyprinodontiformes). Arch Environ Contam Toxicol. 2012;62:107–17. [DOI] [PubMed] [Google Scholar]
- 21.Seneff S, Nigh GL. Glyphosate and anenccephaly: death by a thousand cuts. J Neurol Neurobiol. 2017;3(2):1–15. [Google Scholar]
- 22.Gill JPK, Sethi N, Mohan A, Datta S, Girdhar M. Glyphosate toxicity for animals. Environ Chem Lett. 2017;16(2):401–26. [Google Scholar]
- 23.Babich R, Ulrich JC, Ekanayake D, Massarsky A, De Silva PMCS, Manage P, et al. Kidney developmental effects of metal-herbicide mixtures: implications for chronic kidney disease of unknown etiology. Environ Int. 2020;144:1–15. [DOI] [PubMed] [Google Scholar]
- 24.Jayasumana C, Paranagama P, Agampodi S, Wijeardane C, Gunatilake S, Siribaddana S. Drinking well water and occupational exposure to herbicides is associated with chronic kidney disease, in Padavi-Sripura, Sri Lanka. Environ Health. 2015;14:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aguilar DJ, Madero M. Other potential ckd hotspots in the world: the cases of Mexico and the United States. Semin Nephrol. 2019;39(3):300–7. [DOI] [PubMed] [Google Scholar]
- 26.Kulathunga MRDL, Ayanka Wijayawardena MA, Naidu R, Wijeratne AW. Chronic kidney disease of unknown aetiology in Sri Lanka and the exposure to environmental chemicals: a review of literature. Environ Geochem Health. 2019;41(5):2329–38. [DOI] [PubMed] [Google Scholar]
- 27.Levine KE, Redmon JH, Elledge MF, Wanigasuriya KP, Smith K, Munoz B, et al. Quest to identify geochemical risk factors associated with chronic kidney disease of unknown etiology (CKDu) in an endemic region of Sri Lanka-a multimedia laboratory analysis of biological, food, and environmental samples. Environ Monit Assess. 2016;188(10):548. [DOI] [PubMed] [Google Scholar]
- 28.Ananda Jayalal TB, Jayaruwan Bandara T, Mahawithanage STC, Wansapala MAJ, Galappaththi SPL. A quantitative analysis of chronic exposure of selected heavy metals in a model diet in a CKD hotspot in Sri Lanka. BMC Nephrol. 2019;20(1):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wimalawansa SJ. The role of ions, heavy metals, fluoride, and agrochemicals: critical evaluation of potential aetiological factors of chronic kidney disease of multifactorial origin (CKDmfo/CKDu) and recommendations for its eradication. Environ Geochem Health. 2016;38(3):639–78. [DOI] [PubMed] [Google Scholar]
- 30.Gunatilake S, Seneff S, Orlando L. Glyphosate’s synergistic toxicity in combination with other factors as a cause of chronic kidney disease of unknown origin. Int J Environ Res Public Health. 2019; 16(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stalikas CD, Konidari CN. Analytical methods to determine phosphonic and amino acid group-contaiinng pesticides. J Chromatogr A. 2001;907:1–19. [DOI] [PubMed] [Google Scholar]
- 32.Kudzin ZH, Gralak DK, Drabowicz J, Luczak J. Novel approach for the simultaneous analysis of glyphosate and its metabolites. J Chromatogr A. 2002;947:129–41. [DOI] [PubMed] [Google Scholar]
- 33.Börjesson E, Torstensson L. New methods for determination of glyphosate and (aminomethyl)phosphonic acid in water and soil. J Chromatogr A. 2000;886:207–16. [DOI] [PubMed] [Google Scholar]
- 34.Roseboom H, Berkhoff CJ. Determination of the herbicide glyphosate and its major metabolite aminophosphonic acid by high-performance liquid chromatography after fluorescence labeling. Anal Chim Acta. 1982;135:373–7. [Google Scholar]
- 35.Patsias J, Papadopoulou A, Papadopoulou-Mourkidou E. Automated trace level determination of glyphosate and aminomethylphosphonic acid in water by on-line anion-exchange solid-phase extraction followed by cation-exchange liquid chromatography and post-column derivatization. J Chromatogr A. 2001;932:83–90. [DOI] [PubMed] [Google Scholar]
- 36.Grey L, Nguyen B, Yang P. Liquid chromatography/electrospray ionization/isotopic dilution mass spectrometry analysis of n-(phosphonomethyl) glycine and mass spectrometry analysis of aminomethyl phosphonic acid in environmental water and vegetation matrixes. J AOAC. 2001;84:1770–80. [PubMed] [Google Scholar]
- 37.Cikalo MG, Goodall DM, Matthews W. Analysis of glyphosate using capillary electrophoresis with indirect detection. J Chromatogr A. 1996;745:189–200. [Google Scholar]
- 38.Molina M, Silva M. Simultaneous determination of phosphorus-containing amino acid-herbicides by nonionic surfactant micellar electrokinetic chromatography with laser-induced fluorescence detection. Electrophoresis. 2001;22:1175–81. [DOI] [PubMed] [Google Scholar]
- 39.Chang SY, Liao C. Analysis of glyphosate, glufosinate and aminomethylphosphonic acid by capillary electrophoresis with indirect fluorescence detection. J Chromatogr A. 2002;959(1–2): 309–15. [DOI] [PubMed] [Google Scholar]
- 40.Zhu Y, Zhang F, Tong C, Liu W. Determination of glyphosate by ion chromatography. J Chromatogr A. 1999;850:297–301. [DOI] [PubMed] [Google Scholar]
- 41.Bauer K, Knepper TP, Maes A, Schatz V, Voihsel M. Analysis of polar organic micropollutants in water with ion chromatography-electrospray mass spectrometry. J Chromatogr A. 1999;837:117–28. [DOI] [PubMed] [Google Scholar]
- 42.Qiu HM, Geng JJ, Han C, Ren H-Q. Determination of phosphite, phosphate, glyphosate and aminomethylphosphonic acid by two-dimensional ion chromatography system coupled with capillary ion chromatography. Chin J Anal Chem. 2013;41(12):1910–4. [Google Scholar]
- 43.Hao C, Morse D, Morra F, Zhao X, Yang P, Nunn B. Direct aqueous determination of glyphosate and related compounds by liquid chromatography/tandem mass spectrometry using reversed-phase and weak anion-exchange mixed-mode column. J Chromatogr A. 2011;1218(33):5638–43. [DOI] [PubMed] [Google Scholar]
- 44.Bo P, Lei J, Bingjie W, Yong L. Determination of glyphosate and its main metabolite aminomethyl phosphinc acid in water by two methods: direct injection and pre-column derivatization. Nanjing Nongye Daxue Xuebo. 2020;43:79–87. [Google Scholar]
- 45.Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. Environmental Protection Agency. US Government Printing Office: Washington, DC, 2015. [Google Scholar]
- 46.Myint KT, Uehara T, Aoshima K, Oda Y. Polar anionic metabolome analysis by Nano-LC/MS with a metal chelating agent. Anal Chem. 2009;81:7766–72. [DOI] [PubMed] [Google Scholar]
- 47.Armbruster DA, Tillman MD, Hubbs LM. Limit of detection (lqd)/limit of quantitation (loq): comparison of the empirical and the statistical methods exemplified with gc-ms assays of abused drugs. Clin Chem. 1994;40(7):1233–8. [PubMed] [Google Scholar]
- 48.Lawson GM. Defining limit of detection and limit of quantitation as applied to drug of abuse testing: striving for a consensus. Clin Chem. 1994;40(7):1218–9. [PubMed] [Google Scholar]
- 49.Crawford JK. Water quality of North Carolina streams: water quality of North Carolina streams water-quality characteristics for selected sites on the cape fear river, North Carolina, 1955–30 variability, loads, and trends of selected constituents. United States Geological Survey, 1985;1–51. [Google Scholar]
- 50.Battaglin WA, Thurman EM, Kolpin DW, Scribner EA, Sandstrom MW, Kuivila KM. Work plan for determining the occurrence of glyphosate, its transformation product AMPA, other herbicide compounds, and antibiotics in midwestern United States streams, 2002: U.S. Geological Survey Open-File Report 03–69. 2003;18 [Google Scholar]
- 51.Lee EA, Strahan AP, Thurman EM. Methods of analysis by the U.S. Geological Survey Organic Geochemistry Research Group - determination of glyphosate, aminomethylphosphonic acid, and glufosinate in water using online solid-phase extraction and high-performance liquid chromatography/mass spectrometry: U.S. Geological Survey Open-File Report 01–454. 2002;13. [Google Scholar]
- 52.US Environmental Protection Agency. National primary drinking water regulations. https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations. Accessed 28 Nov 2020.
- 53.Environmental Working Group. EWG’s tap water database: contaminants in your water. https://www.ewg.org/tapwater. Accessed 28 Nov 2020.
- 54.von Mérey G, Manson PS, Mehrsheikh A, Sutton P, Levine SL. Glyphosate and aminomethylphosphonic acid chronic risk assessment for soil biota. Environ Toxicol Chem. 2016;35(11):2742–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


