Abstract
We recently developed a recombinant growth factor-free bone regenerative scaffold composed of stoichiometric hydroxyapatite (HA) ceramic particles and human demineralized bone matrix (DBM) particles (HA-DBM). Here, we performed the first pre-clinical comparative evaluation of HA-DBM relative to the industry standard and established positive control, recombinant human bone morphogenetic protein-2 (rhBMP-2), using a rat posterolateral spinal fusion model (PLF). Female Sprague-Dawley rats underwent bilateral L4-L5 PLF with implantation of the HA-DBM scaffold or rhBMP-2. Fusion was evaluated using radiography and blinded manual palpation, while biomechanical testing quantified the segmental flexion-extension range-of-motion (ROM) and stiffness of the fused segments at 8-weeks postoperatively. For mechanistic studies, pro-osteogenic gene and protein expression at 2-days and 1-, 2-, and 8-weeks postoperatively was assessed with another cohort. Unilateral fusion rates did not differ between the HA-DBM (93%) and rhBMP-2 (100%) groups; however, fusion scores were higher with rhBMP-2 (p=0.008). Both treatments resulted in significantly reduced segmental ROM (p<0.001) and greater stiffness (p=0.009) when compared with non-operated controls; however, the degree of stabilization was significantly higher with rhBMP-2 treatment relative to the HA-DBM scaffold. In the mechanistic studies, PLGA and HA scaffolds were used as negative controls. Both rhBMP-2 and HA-DBM treatments resulted in significant elevations of several osteogenesis-associated genes, including Runx2, Osx, and Alp. The rhBMP-2 treatment led to significantly greater early, mid, and late osteogenic markers, which may be the mechanism in which early clinical complications are seen. The HA-DBM scaffold also induced osteogenic gene expression, but primarily at the 2-week postoperative timepoint. Overall, our findings show promise for this 3D-printed composite as a recombinant growth factor-free bone graft substitute for spinal fusion.
Keywords: 3D printing, hydroxyapatite, demineralized bone matrix, bone regeneration, spine fusion
Graphical abstract

1. Introduction
Bone augmentation and regeneration strategies are required in a variety of orthopaedic procedures for conditions such as trauma, disease, deformity or tumor resection [1]. Historically, in spine fusion, autograft (i.e., host-derived bone) has been considered the gold-standard given its proven efficacy in promoting successful bone healing. However, the use of autograft is limited by the requirement for a separate surgical procedure to harvest bone – such as iliac crest bone graft (ICBG) – which can be associated with various donor-site morbidities and complications[2]. Recombinant human bone morphogenetic protein-2 (rhBMP-2) is an osteoinductive growth factor capable of promoting bone regeneration and spinal fusion and clinically available in the form of Infuse™ [3]. However, the supraphysiologic doses required for successful fusion have been associated with significant adverse effects, especially immediately postoperatively [4, 5]. Many authors have postulated that these early complications such as radiculitis, bone resorption, and heterotopic ossification are secondary to the immediate robust osteoinductive response from high doses of rhBMP-2. Nonetheless, rhBMP-2 is undisputed as a positive control in pre-clinical models, with fusion rates much higher than ICBG [6–9]. Development of effective yet safe recombinant growth factor-free approaches for spinal fusion remains an area of active research.
Demineralized bone matrix (DBM) and hydroxyapatite (HA) are both materials that can be used as “bone graft extenders” to minimize the amount of autograft required for successful spinal fusion. However, either of these materials alone are insufficient to promote successful spinal fusion . Ceramics such as HA lack substantial osteoinductivity and have poor mechanical and handling properties [10]. Although DBM posseses osteoinductivity and osteoconductivity, it fails to yield successful spinal fusion clinically when used alone [11].
Towards this end, a plethora of synthetic scaffolds have been proposed for bone regeneration and augmentation [12]. Because bone is a naturally nanostructured ceramic-reinforced composite consisting primarily of a calcium phosphate (CaP) mineral phase – namely, hydroxyapatite (HA) - and type I collagen [13], CaP-based composites have been a material of choice because of the mineral’s similarity to that of bone [14]. A number of such materials have been used clinically for several decades [15–18] in formulations including powders, scaffolds, coatings for prosthetics, and bone cements [19]. However, although they have proven successful for certain applications, conventional synthetic CaP ceramics and composites are typically limited by poor mechanical and handling properties, low biodegradability, and sub-optimal porosity, which may impact bone ingrowth and graft neovascularization [20, 21].
Recently, we developed a 3D-printed ceramic scaffold, comprised of HA, DBM, and polylactide-co-glycolic-acid (PLGA) [22]. This composite addressed several limitations of current materials by harnessing the biological response of each subcomponent: the HA component provided osteoconductivity and the DBM osteoinductivity. The novel HA-DBM scaffold is mechanically flexible and pliant despite being mostly comprised of rigid ceramic, thereby providing excellent surgical handling properties [10, 11]. Additionally, the intrinsic micro- and macro- porosity of the material was exploited to promote cell adhesion, proliferation, and ultimately bone regeneration [10, 11]. Finally, the material can be rapidly 3D-printed with considerable control over size, macrostructure, and architecture [10, 11]. The scaffold formulation [11] and geometry [10] were further advanced to ensure osteointegration and neovascularization in an orthotopic model of rat spine fusion. In previous work, we found that the macroporous structured HA-DBM successfully allows for extensive neovascularization [10].
The rat PLF model is the most well-established small animal model to initially evaluate the efficacy of new materials for the potential to promote bone regeneration and spinal fusion [11, 23, 24]. The present study is the first to assesses the pre-clinical efficacy and osteoinductive potential of this 3D-printed HA-DBM composite in a rat orthotopic model of spine fusion. Its efficacy in promoting spine fusion was compared with that of the industry standard and positive control for this model [rhBMP-2 delivered on an absorbable collagen sponge (ACS); Infuse™], with biomechanical testing as a functional outcome. Pro-osteogenic mechanisms of action at early time points in the bone healing cascade were also investigated.
2. Materials and methods
2.1. Ink preparation and scaffold fabrication
The HA-DBM ink for 3D printing was prepared as we have previously described [10, 11, 24]. Briefly, the ink was composed of 30 vol.% polylactide-co-glycolide (PLGA; 82:18 glycolide to lactide) copolymer (Evonik Cyro) and 70 vol.% of HA+DBM particles (comprising 3 parts of HA:1 part of DBM). Medical-grade stoichiometric HA powder (particle diameter range 1–25 μm) was obtained from Merz Biomaterials. DBM particles (∼100–1000 μm) were acquired from Xtant Medical (Belgrade, MT) and were milled and sieved to a final diameter ranging from 20–80 µm, as previous described [11]. The scaffolds were printed at room temperature using a Manufacturing Series 3D-BioPlotter (EnvisionTEC, Germany). Scaffolds were printed 5 layers thick, with the following previously optimized architectural parameters: each consecutive layer of the ~400 µm-diameter struts was oriented at 45° relative to the underlying layer, and the edge-to-edge strut spacing (i.e., (macropore size) was 1000 μm [10, 11]. The scaffolds were washed with 70% ethanol followed by sterile phosphate-buffered saline and trimmed to uniform size (12 × 4 mm) prior to implantation (Fig. 1). Negative and comparative control scaffolds for the mechanistic studies were printed with the same structure and geometry as the HA-DBM composite scaffolds but contained either PLGA only (100 vol.% PLGA) or PLGA/HA only (30 vol.% PLGA + 70 vol.% HA), respectively.
Figure 1.
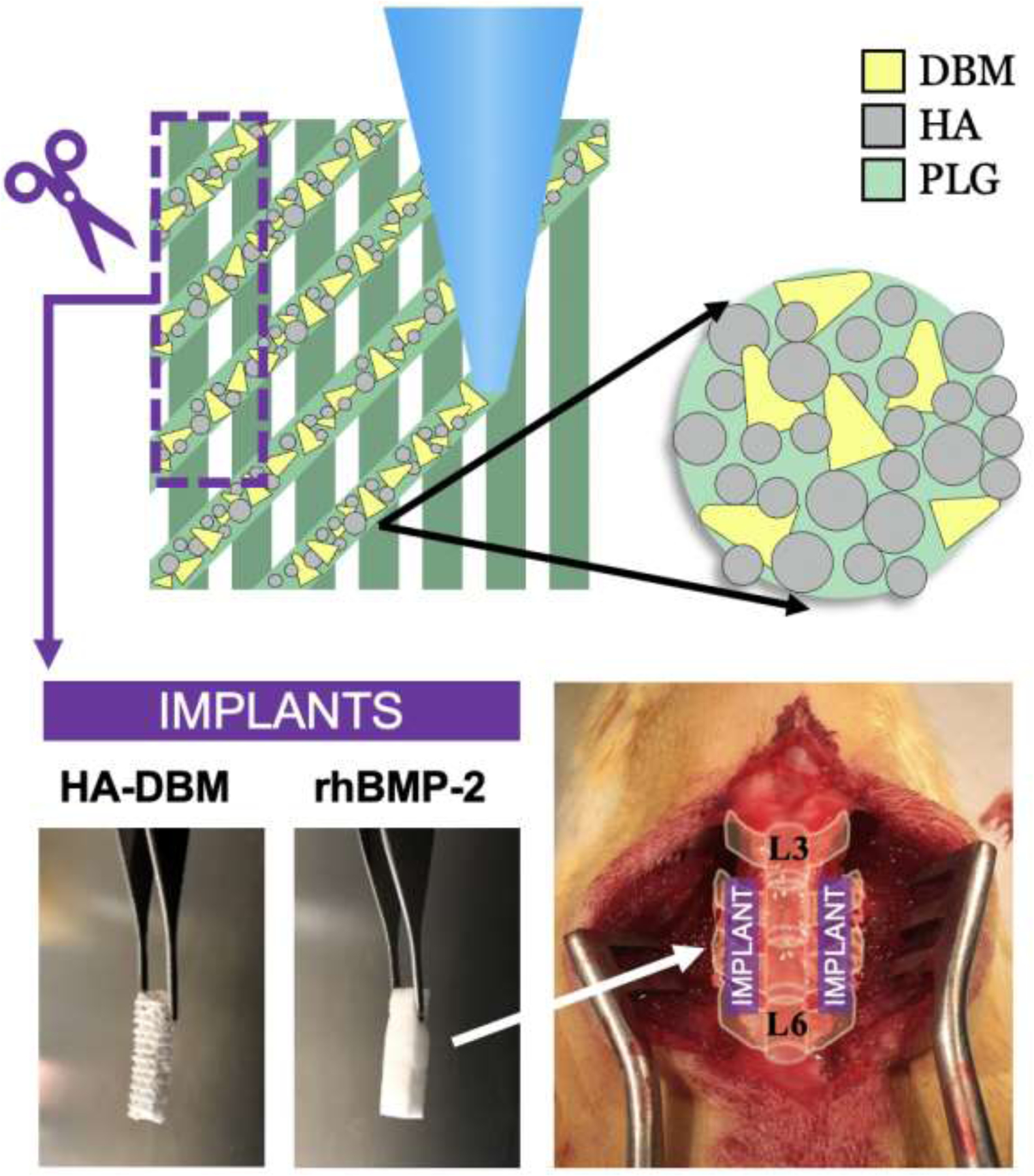
Materials and experimental model overview. The hydroxyapatite-demineralized bone matrix (HA-DBM) composite is 3D-printed and trimmed to size prior to implantation. The HA-DBM group was compared to recombinant human-derived bone morphogenetic protein-2 (rhBMP-2) delivered on a collagen sponge, known to be highly efficacious in yielding successful fusion. An established L4-L5 posterolateral lumbar fusion (PLF) model in rats was used to compare the efficacy of the HA-DBM to rhBMP-2 in achieving spinal fusion and bone regeneration.
2.2. Study design and animal groups
All experiments were approved by the Institutional Animal Care and Use Committee at Northwestern University (protocol IS00002610) and carried out in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
The comparative efficacy study was a randomized control trial with 56 female Sprague-Dawley rats. All animals were randomized using the RAND() function in Microsoft Excel. Forty-eight female Sprague–Dawley rats, aged 12–16 weeks, with body weight 241 ± 25 g (mean ± S.D.) were randomly assigned to one of two treatment groups (n = 24 per group): 1) 10 µg of rhBMP-2 on ACS per animal (5 µg/side; historical positive control [25]; Infuse™ bone graft, Medtronic Sofamor Danek, Memphis, TN); and 2) HA-DBM composite scaffold. An additional 8 age-matched, non-operated control animals, aged 12–16 weeks, with average body weight 245 ± 30 g (mean ± S.D.) were included for baseline assessments in biomechanics testing.
The 10 µg rhBMP-2/ACS implant is the only established positive control for spine fusion in the rat model [6–8, 26–31], and was therefore chosen as positive control. Constructs containing only the DBM particle component were shown in our earlier work to be significantly inferior to the HA-DBM constructs for fusion outcomes [10], so that scaffold was excluded as a comparative control for biomechanical testing. All animals underwent bilateral posterolateral lumbar spinal fusion (PLF) at the L4–L5 level (Fig. 1). At the experimental endpoint (8-weeks postoperatively), animals were randomly assigned to undergo either fusion scoring (n = 14 spines per treatment group) or biomechanical testing (n = 10 spines per treatment group).
The mechanistic efficacy study was a randomized control trial where an additional 80 female Sprague–Dawley rats, aged 12–16 weeks, with body weight 252 ± 10 g (mean ± S.D.) were randomly assigned to one of four treatment groups: (1) PLG-only scaffold [i.e., 3D-printed (3DP) scaffolds devoid of both HA and DBM; negative control]; (2) HA-only scaffold [i.e., 3DP scaffolds composed of only PLG + HA; comparative control]; (3) HA-DBM composite scaffold (test article), and (4) 10 µg rhBMP-2/ACS per animal (Infuse™ clinical comparative and experimental positive control). Animals were randomized using the RAND() function in Microsoft Excel. All animals underwent bilateral posterolateral lumbar spinal fusion (PLF) at the L4–L5 level. Rats were euthanized at 2 days, 1-, 2-, or 8-weeks post-operatively; n = 5 animals (n = 10 implants) per treatment group per timepoint), and fusion beds (the space between the host transverse processes of L4 and L5, where the implant is placed) were harvested bilaterally for analyses.
2.3. Experimental Procedures
Rats were maintained under continuous anesthesia with an isoflurane inhalational anesthetic delivery system. The PLF procedure was performed as previously described [7, 8, 30]. A posterior midline incision was made over the lumbar spinous processes, followed by two separate fascial incisions, each ~4 mm from the midline. The L4 and L5 transverse processes were exposed via blunt dissection to create space for implantation of the materials. The surgical site was irrigated with gentamicin solution, the dorsal surfaces of the transverse processes were decorticated with an oscillating burr, and the graft materials were placed bilaterally to bridge the L4-L5 intertransverse space. Fascial incisions were closed with a 3–0 polyglactin absorbable suture (Vicryl; Ethicon, Inc., Somerville, NJ, USA), and skin incisions were closed with wound clips. Five mL lactated ringer solution was administered intraperitoneally after wound closure to ward off dehydration. Sustained-release buprenorphine (0.03 mg/kg) was administered subcutaneously to provide analgesia for 72 hours, and meloxicam was concurrently administered (1–2 mg/kg) SQ every 24 hours for 3 days. Animals were housed in separate cages and allowed to eat, drink, and bear weight ad libitum.
2.4. Determination of fusion
Animals underwent plain radiography at 10 days postoperative (after the removal of wound clips) and before euthanasia at 8 weeks postoperatively. After euthanasia, lumbar spines were harvested en bloc. Spines undergoing manual palpation-based fusion scoring were fixed in 10% neutral-buffered formalin for 10 days. Specimens were then placed in 50% ethanol (EtOH) for 2 hours followed by 70% EtOH for storage. All spines were scored for fusion via manual palpation by three independent, blinded observers using an established scoring system in which “0” indicates noticeable motion between segments with no bridging bilaterally, “1” indicates no motion between segments unilaterally (i.e., unilateral stabilization), and “2” indicates no motion between segments bilaterally (bilateral stabilization)[11]. As in clinical practice, where unilateral bridging comprises a successful fusion, specimens with an average score of ≥1 were considered successfully fused [8, 30, 32].
2.5. Histological Analysis
After euthanasia and tissue harvest, hemi-sected spines were fixed in 10% neutral buffered formalin for 7 days and then transitioned to 70% ethanol solution in water while awaiting processing. Samples were demineralized in 10% HCl solution for 8h. Samples were then processed and embedded in paraffin by the Northwestern University Mouse Histology & Phenotyping Laboratory (MHPL) core. Seven-micrometer thin sections were cut with a RM2255 microtome (Leica), deparaffinized, and stained with Gill’s hematoxylin and eosin and alcian blue according to the manufacturer’s recommendations (Sigma Aldrich). Stained sections were mounted with Cytoseal XYL mounting medium (Thermo Scientific), imaged on a TissueGnostic histological microscope (Zeiss), and visualized using TissueFAXS software. Additionally, to assess the presence of cells in proximity of the composite struts’ DBM particles, sections of the HA-DBM scaffolds 8 weeks post-operatively were stained with DAPI nuclear stain (Invitrogen), and imaged by confocal laser microscopy, using a Nikon A1 microscope. Both the DAPI nuclear staining and the autofluorescence of DBM in the DAPI field were captured. Images were exported using the NIS Elements Software (Nikon).
Laboratory microComputed Tomography (microCT) was performed previously on spines containing scaffolds matching those reported here[11]. Synchrotron microCT, as described previously [10, 11], was performed on a very limited number (due to beamtime limitations) of specimens herein, and these results are covered in the Supplemental Materials.
2.6. Biomechanical Evaluation
2.6.1. Biomechanics Specimens
After euthanasia and tissue harvest, spines for biomechanical testing (N=10/treatment group plus 4 non-operative controls) were stored in saline-moistened gauze at –80 °C. After thawing, the musculature was stripped, and 75-gauge (0.53 mm diameter) to 77-gauge (0.46 mm diameter) diameter holes were drilled through the most cranial and caudal vertebral bodies; the drill bits were left in place (Fig. 2A). The drill bits followed a posterolateral to anterior contralateral trajectory through the lamina and through the vertebral body.
Figure 2.

Biomechanics Overview. (A) Schematic of the biomechanics testing apparatus from both a posterior and a left lateral view. Samples of explanted L4-L5 vertebrae were loaded with 5 cycles of flexion-extension using a hybrid control algorithm, namely rotation control with a torque limit of 0.01 Nm. (B) Representative load-displacement curve of moment (Nm) versus rotation angle (°). The range-of-motion (ROM) was measured as the difference in rotation angle at the torque limits (−0.01 Nm and 0.01 Nm) of the flexion and extension cycles. The stiffness (K1) was measured as a linear fit to the load-displacement curve from 0 Nm to the first third of the flexion range-of-motion. The red arrows indicate the bounds of the curve to which the linear fit is made. The slope of the red line yields the stiffness (K1). SP = spinous process, TP = transverse process, PMMA = polymethylmethacrylate
The cranial vertebra was first cemented to the cranial potting platen using polymethylmethacrylate (PMMA); subsequently, the caudal vertebra was cemented to the caudal potting platen on the testing apparatus. The bone cement was sculpted as it cured to prevent fixation directly to the fusion mass or contact between the cement and fusion mass during motion tests. Note that the drill bits were incorporated in the cement volume, effectively locking the vertebral body to the potting platens.
In some specimens, the fusion mass extended beyond a single motion segment. In these cases, three vertebral bodies (2 motion segments) were used during specimen testing. Drill bits were inserted in all three vertebral bodies and two of the vertebral bodies were incorporated into the same PMMA potting mass prior to testing. This technique allowed testing of the specimen without compromising the fused level or allowing the fusion mass to contact the PMMA.
2.6.2. Biomechanics Testing
The specimens were tested in an upright posture. Flexion-extension motion was produced by attaching the caudal platen to a rotation stage oriented to produce sagittal plane rotation (Fig. S1). During potting, the motion segment disc space was aligned with the axis of the rotation stage. The stage was driven by a linear actuator (Model D1, Ultra Motion, Cutchogue, NY). A torque transducer (Model 101-I-MT, CDI Torque, Kenosha, WI) monitored applied torques. A rod (3.2mm diameter) extended vertically from the cranial specimen platen and was constrained between two horizontal bars (3.2mm diameter). The horizontal bars were attached to the torque transducer 57 mm from the transducer axis. Flexion or extension motion of the rotation stage caused the vertical rod to contact the horizontal rods attached to the torque transducer. This arrangement allowed flexion-extension actuation of the cranial vertebral body while allowing freedom of craniocaudal displacement and axial and lateral rotation to normalize soft tissue tension and facet load sharing.
Flexion-extension motion was driven under feedback control using custom software (MATLAB, Mathworks, Inc., Natick, MA). The linear actuator was calibrated to measure stage rotation (flexion-extension angle), and the custom software recorded rotation and load data at 100 Hz. The primary assumption made was that the rotation of the apparatus was the same as the flexion-extension rotation of the functional spinal unit. This was true if (i) the fixation between the apparatus and specimen did not allow any relative motion, and (ii) the spinal unit’s flexion-extension axis is parallel to the flexion-extension axis of the apparatus.
Each specimen was tested with five cycles of flexion-extension using a hybrid control algorithm; namely rotation control with torque limits. The torque limit was 0.01 Nm. The cycle test speed was 10°/min.
2.6.3. Biomechanics Data Analysis
Prior to analysis, angle and torque data were filtered using a 5Hz low-pass zero-phase Butterworth filter. At a rotational velocity of 10°/min, each full flexion-extension cycle was at least one minute long. Therefore, the 5Hz low-pass filter reduced noise without decreasing data fidelity or filtering out features of interest.
Range-of-motion and high flexibility zone stiffness were calculated on the flexion portion of each extension to flexion cycle (Fig. 2B). Range-of-motion was defined as the difference in rotational angle between the start- and end-point of the cycle. The high flexibility zone stiffness (K1), was the linear fit to the load-displacement curve from the 0 Nm crossing to the first third of the flexion range-of-motion [33].
A dead-band of about 0.5° was observed during testing, which was a consequence of the fact that the diameter of the vertical rod (from the cranial potting cup) was slightly smaller than the gap between the two horizontal, constraint bars; this prevented binding of the torque application system and any extraneous forces that could result. To correct for this dead-band, 0.5° was subtracted from the measured range-of-motion data for all specimens.
In the low range-of-motion specimens, the noise and the angular measurements had nearly the same magnitude, and there was a low goodness-of-fit to the data. Consequently, the high flexibility zone stiffness (K1) was less reliably calculated in specimens with ranges-of-motion of one degree or less. Detailed photographs of the biomechanical testing can be found in the supplemental information (Fig. S1).
2.7. Mechanistic Studies
2.7.1. Specimen collection and gene expression analysis
Expression levels of numerous transcripts known to impact bone formation and healing were quantified.
Animals were euthanized at increasing post-operative timepoints for harvest of the fusion bed tissue spanning L4-L5 (including any hematoma/callus). Each fusion bed (2 per animal) was analyzed individually, with the left side reserved for quantitative polymerase chain reaction (qPCR) and Western blot analyses, and the right reserved for histology and immunofluorescence staining (n=5).
Comparative analyses of RNA and protein expression were performed as previously described [34, 35]. Total RNA isolation and complementary DNA (cDNA) synthesis kits were purchased from GenDepot (Barker, TX, USA). Quantitative polymerase chain reaction (qPCR) was performed using a Bio-Rad CFX Connect Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) in the Analytical BioNanoTechnology Equipment Core Facility of the Simpson Querrey Institute at Northwestern University, with implementation of the following program: 94°C denaturation for 5 min followed by 40 repeated cycles of 94°C, 45 s/55°C, 1 min/68°C for 1 min; 79 cycles at 55°C for 30 s each were carried out for generation of melting curves. Expression levels of various osteogenesis-associated marker genes (Table 1) were normalized internally to the glyceraldehyde 3-phosphate dehydrogenase gene (Gapdh). Experimental means were then normalized to that of the control group (PLGA), which was set to a value of 1. Significantly different means among groups were categorized as either <5-fold or >5-fold higher than PLGA controls and summarized in a heat-map.
Table 1. Genes evaluated for differential expression and their corresponding roles in osteogenesis.
A list of the osteogenesis-related genes of interest evaluated in the mechanistic assessment. A brief summary of the role of each gene in osteogenesis, bone regeneration, and/or bone healing are provided.
| Gene | Role in Osteogenesis/Bone Regeneration/Healing |
|---|---|
| Dlx5 | Transcription factor regulating osteogenesis; drives Runx2 expression, a key regulator of osteogenesis[39–41] |
| Runx2 | Transcription factor regulating early osteogenic differentiation[36, 42] |
| Osx | Transcription factor regulating osteoblast differentiation and maturation[43, 44] |
| Cxcl12 | Stem and osteoprogenitor cell homing[45]; regulator of BMP-2-mediated osteogenesis[46–48]; chondrogenic differentiation[49] |
| Alpl | Early/mid osteoblast differentiation marker; hydroxyapatite nucleation[50, 51] |
| Col1a1 | Type 1 collagen in bone matrix; osteoblast differentiation marker, regulated by Runx2[52] |
| Ocn | Osteoblast and bone formation marker; mineral deposition[53] |
| Opn | Osteogenesis marker; mineralization regulator[54, 55] |
| Phex | Marker of fully differentiated osteoblasts and osteocytes; regulates FGF23 activity and phosphate metabolism[56–58] |
| Mmp2 | Bone repair and regeneration, osteoblast differentiation and survival[59, 60] |
| Mmp3 | Bone matrix maturation[59, 60] |
| Mmp7 | Promotes RANKL activity; bone repair and regeneration; bone remodeling[59, 60] |
| Mmp9 | Osteoclast recruitment; bone repair and regeneration; bone remodeling[59, 60] |
| Mmp13 | Chondrocyte differentiation, matrix resorption[59, 60] |
| Mmp14 | Promote RANKL activity, Bone repair and regeneration[59, 60] |
| M-csf | Osteoclast differentiation, maturation, and motility[61] |
| Rankl | Osteoclast regulation, bone homeostasis, bone remodeling[53, 62–64] |
| Opg | Antagonizes RANK signaling; bone remodeling and homeostasis[63, 64] |
| Col2 | Chondrogenic/endochondral marker[65, 66] |
| Col10 | Endochondral marker; chondrocyte hypertrophy[67, 68] |
| Col12a | Osteoblast differentiation and maturation[69]; chondrocyte function and morphogenesis[70] |
| Mmp1 | Marker of chondrogenic differentiation[71] |
2.7.2. Western blotting
Rapid-immunoprecipitation assay (RIPA) buffer, blocking solution, and protease inhibitor were purchased from GenDepot. Anti-RUNX2 and -GAPDH antibodies were purchased from Detroit R&D (Detroit, MI). Anti-DLX5 and -PHEX were purchased from Proteintech (Rosemont, IL, USA). Anti-OCN and -OSX were purchased from Abcam (Cambridge, UK). Cell lysates were prepared from fusion bed tissue using RIPA buffer. Proteins were resolved on SDS-PAGE and transferred to 0.45 μm nitrocellulose blotting membranes (Amersham Protran, Germany). The blots were blocked using superblocking solution (Gengepot) for 1 hour at room temperature and probed with primary antibody overnight at 4°C. Blots were washed with PBST and incubated with an anti-rabbit or –mouse peroxidase-conjugated secondary antibody for 1 hour at room temperature. Signal was visualized by enhanced chemiluminescence (ECL) using Azure300 (Azure Biosystems, Dublin, CA, USA), and intensities were quantified using a computing densitometry program from Image Studio Lite (LI-COR, Lincoln, NE, USA).
2.8. Statistical analyses
All statistical analyses were performed using IBM SPSS Statistics for Windows, version 23 (IBM Corp., Armonk, NY, USA). A p-value <0.05 was considered statistically significant for all analyses (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
2.8.1. Fusion scoring
The average fusion scores of the different treatment groups were compared by using one-way analysis of variance (ANOVA) and Tukey’s HSD (honestly significant difference) test for post hoc analyses. The fusion rate between the different treatment groups were compared using Fisher’s exact test.
2.8.2. Biomechanical assessment
The average stiffness and range-of-motion between the different treatment groups were compared using ANOVA and Tukey’s HSD test for post hoc analyses.
2.8.3. Gene and protein expression analyses
Relative gene and protein expression for various markers at different postoperative time points were compared using ANOVA and Tukey’s HSD test for post hoc analyses.
3. Results
3.1. Assessment of spine fusion
Plain radiographic evaluation of spine specimens 8 weeks after surgery confirmed solid and robust fusion masses at the L4-L5 level in all of the rhBMP-2-treated animals, and these masses often extended proximal to the level of surgery (Fig. 3A). In the HA-DBM group, radiographic imaging showed bilateral placement of the scaffolds across the L4–L5 transverse processes in all animals (Fig. 3A). All 8-week radiographs appear in the supplemental information (Fig. S2). RhBMP-2 treatment produced successful bilateral fusion in all animals from that treatment group, as determined by manual palpation-based fusion scoring (mean fusion score of 2), yielding a 100% rate of fusion as expected in this model (Fig. 3C). The mean fusion score for HA-DBM-treated animals was 1.52 ± 0.57, which was significantly lower than rhBMP-2 (p < 0.05, Fig. 3B). Thirteen out of fourteen animals in this group demonstrated successful fusion overall – a fusion rate of 93% – with a lack of segment motion at the fusion level either unilaterally or bilaterally. This fusion rate did not differ significantly from that of the rhBMP-2 treatment group (Fig. 3C).
Figure 3.

Comparison of the fusion capacity of rhBMP-2 and HA-DBM groups. (A) Representative radiographs of the lumbar spine at 8 weeks postoperatively for the rhBMP-2 and HA-DBM groups. The fusion area is indicated bilaterally with white arrows. (B) Mean fusion score was significantly greater in the rhBMP-2 group relative to the HA-DBM group. (C) The fusion rate was not significantly different between the rhBMP-2 and HA-DBM groups.
* Indicates statistical significance (p<0.05)
3.2. Histological analysis
At 2 weeks post-operative, new tissue formation had already initiated in the rhBMP-2 control group (Fig. 4). The struts (ST) of the PLGA scaffold were not degraded and were mostly intact, but numerous cells were visible within the plane of section (see inset). No evidence of new bone formation was found in the PLGA experimental group, while a new bone-like tissue, rich in type I collagen (blue), formed among the struts of the HA and HA-DBM scaffolds. Intact DBM particles (light pink-orange, indicated by yellow arrows) are found within the struts of the HA-DBM scaffold. By 8 weeks, a large fusion mass (FM) was observed in the rhBMP-2 positive control, consisting of a marrow-like structure surrounded by a thin cortical bone-like layer outside (darker pink to purple) and trabeculae-like structures throughout, as indicated by yellow arrows. No fusion mass was found in neither the PLGA nor the HA group, but only fibrous collagen-rich matrix. The struts of the HA-DBM scaffold were still found mostly intact, with numerous visible DBM particles within (pink-orange, indicated by yellow arrows). New bone-like tissue was observed at the interface of the scaffold and the TPs (TP), and the space between TPs.
Figure 4.

Histological evaluation of representative spines for each experimental group tested, 2 and 8 weeks post-operatively. Sections are stained with Gill’s hematoxylin and eosin and alcian blue. Acronym legend: transverse process (TP), fusion mass (FM), scaffold struts (ST). Scale bars of large images: 1 mm. Yellow arrows in the 8 weeks rhBMP-2 magnified images indicate trabeculae-like structures. Yellow arrows in the 2 and 8 weeks HA-DBM magnified images indicate DBM particles. The yellow arrow in the 8 weeks HA magnified image indicates remaining HA particles (black). Scale bars of magnified images: 500 µm.
Synchrotron microCT (supplemental information, Fig. S3A) shows some DBM particles were at the surface of intact struts, and a fraction of the struts had begun to disintegrate, exposing additional DBM particles to the matrix between the scaffold struts. Through both bright field imaging as well as confocal laser microscopy, 8 weeks post-operatively, cells were found surrounding some of the DBM particles (Fig. S3B–D).
3.3. Biomechanical assessment of segment stabilization
Over the five test cycles, the mean flexion-extension range-of-motion of the non-operated (unfused) control specimens measured 28.4 ± 3.2 degrees (Fig. 5A). Spines treated with rhBMP-2/ACS had a mean flexion-extension range-of-motion of less than 4 degrees (3.7 ± 5.6 degrees, Fig. 5A). Treatment with HA-DBM resulted in a mean flexion-extension range-of-motion of 15.3 ± 3.5 degrees (Fig. 5A). Compared to the unfused controls, both the rhBMP-2 and HA-DBM treatments resulted in significantly reduced range-of-motion at the fused segment (p < 0.05). However, rhBMP-2-treated spines were more solidly fused, with a significantly lower mean flexion-extension range-of-motion than HA-DBM scaffold-treated spines (p < 0.05).
Figure 5.

Biomechanics assessment of explanted specimens. (A) Representative load-displacement curves of a fused and unfused specimen. The stiffness (K1) – determined by the linear fit of the first third of the flexion cycle for each specimen (indicated by red lines on this plot) – was significantly greater in the fused specimen relative to the unfused specimen. Comparison of the (B) range-of-motion (ROM, °) and (C) stiffness between the non-operated control, rhBMP-2, and HA-DBM groups. The rhBMP-2 group had a significantly lower ROM and greater stiffness than both the HA-DBM and control groups, respectively. Additionally, the HA-DBM had a significantly lower ROM and greater stiffness than the non-operated control group. The first stiffness plot demonstrates the data with an unadjusted y-axis. The second stiffness plot utilizes a y-axis break to illustrate representative differences in stiffness between the three groups. * indicates statistical significance (p<0.05).
Mean stiffness in the high flexibility zone was measured at 1 × 10−4 ± 3 × 10−5 Nm/degree for the non-operated control spines (Fig. 5B). RhBMP-2-treated spines had a mean stiffness of 2.32 × 10−2 ± 1.87 × 10−2 Nm/degree, which was on the order of 200-fold greater stiffness than unfused controls. The HA-DBM-treated spines had a mean stiffness of 3 × 10−4 ± 9 × 10−5 Nm/degree; although this was significantly stiffer than unfused controls (p < 0.05), the difference was only ~3-fold (Fig. 5B). Therefore, while both treatments resulted in a significant increase in segmental stiffness, rhBMP-2 treatment resulted in much greater stabilization relative to treatment with the HA-DBM scaffolds (p < 0.05).
3.4. HA-DBM mechanistic assessment
3.4.1. Gene expression analyses
The expression levels of the early pro-osteogenic markers, Runx2, Osx, and Alpl were assessed in fusion bed tissues harvested at 2 days post-operative. Although no differences in Runx2 expression were noted among treatment groups at this early time point, there were notable differences in Osx transcript levels. Both HA-DBM and rhBMP-2 treatments resulted in significant induction of Osx relative to PLGA-treated controls, whereas no induction was seen in animals treated with the 3DP HA scaffold (Fig. 6A). On the other hand, Alp expression was mildly but significantly induced in rhBMP-2-treated animals, but not in HA-DBM or HA-only treated animals at this early time point, suggesting that rhBMP-2 may provide a stronger osteoinductive signal early in the regenerative process relative to the HA-DBM scaffold.
Figure 6.

Gene Expression Studies. A. Expression of select early osteogenic markers in fusion bed tissue 2 days after implantation of 3DP PLGA, 3DP HA, 3DP HA-DBM, or rhBMP-2/ACS scaffolds. B. Heat map representation of the relative expression levels of genes important in osteogenic differentiation in explanted fusion bed tissue taken at 1 and 2 weeks post-operative. Light green signifies significantly elevated expression between 2–5-fold higher than PLGA, and dark green signifies >5 fold-higher expression than the PLGA-treated control group. C. Line graphs depicting the relative expression levels of several key early (Runx2, Osx), early/mid (Alp, Col1a), and late osteogenic markers (Opn, Ocn). Statistical significance (p<0.05) is indicated for HA (#), rhBMP-2 (^) and HA-DBM (*), relative to PLGA control explants.
Detailed comparative gene expression analyses in fusion bed tissues at 1- and 2 weeks post-operative are depicted in Fig. 6B. Colored boxes indicate a statistically significant induction (p<0.05) relative to the PLGA control group, with light green depicting expression levels 2–5-fold greater, and dark green representing an induction >5 fold greater than PLGA controls. Line graphs depicting representative early-, mid-, and late osteogenic markers are shown in Fig. 6C; the full data set are shown in the supplemental data (Fig. S4).
Several key genes involved in osteogenesis, chondrogenesis, bone repair and remodeling were elevated in both the rhBMP-2 and HA-DBM groups relative to PLGA control at 1 and/or 2 weeks post-operative. Runx2 and Osx expression were significantly elevated in both the rhBMP-2 and HA-DBM groups at both the 1- and 2-week timepoints. Interestingly, the 3DP HA-only scaffold did not induce Runx2 at either time point, indicating that the DBM component may be responsible for elevating Runx2 expression in vivo. A similar result was seen with Osx, where both HA-DBM and rhBMP-2 implants significantly increased expression levels, but HA-only scaffolds had no such inductive effect. On the other hand, another early marker and pro-osteogenic transcription factor, Dlx5, showed elevated expression in HA-only, HA-DBM, and rhBMP-2-treated tissues relative to PLGA controls at both 1- and 2 weeks post-operative.
Recapitulating the 2-day results, rhBMP-2 appears to induce Alpl both earlier and to a greater degree than HA-DBM scaffolds. At 1-week post-operative, the expression of Alp1 was significantly elevated only in the rhBMP-2 treatment group, and although the HA-DBM did induce Alpl after 2-weeks, the degree of induction was still greater in rhBMP-2-treated animals. Similarly, Col1a1 expression was upregulated in both the rhBMP-2 and HA-DBM groups at the 2-week timepoint but to a greater degree in rhBMP-2-treated animals. Of the late markers, Ocn, Opn, and Phex were all markedly elevated in both the rhBMP-2 and HA-DBM groups at the 2-week timepoint, but levels of all 3 were even greater in rhBMP-2-treated animals.
Of the chondrogenic and other bone repair markers assessed, Col2 was induced earlier and to a greater degree in rhBMP-2 specimens. On the other hand, Col10 was higher in HA-DBM specimens at 1 week and lower at 2 weeks post-operative relative to rhBMP-2 specimens. Other notable differences in transcript levels included Mmp13 and Mmp14, both of which were induced in rhBMP-2 specimens but not in HA-DBM specimens at the 2-week time point. In contrast, induction of Mmp7, Mmp9, M-csf, and Rankl showed similar trends in both HA-DBM and rhBMP-2 -treated animals, especially at the 2-week time point.
The 3DP HA-only treatment group showed upregulation of a few key markers, although substantially less than the rhBMP-2 and HA-DBM groups. In addition to Dlx5 as described earlier, the 3DP HA scaffold promoted expression of Col1a1 and Mmp2 relative to PLGA controls; however, unlike the HA-DBM and rhBMP-2 scaffolds, the HA-only scaffold did not induce Runx2, Osx, Alp, Col10, Opn, Ocn, Phex, or Mmp7 (p>0.05).
3.4.2. Protein expression analyses
To corroborate expression changes seen in several key transcripts, Western blotting was performed on 2-week post-operative explants from each of the four treatment groups. RUNX2, COL1A1, and OPN were all significantly up-regulated in explanted fusion beds from HA-, HA-DBM, and rhBMP-2-treated animals relative to PLGA-treated controls. In contrast, OSX, OCN, and PHEX were induced only in HA-DBM- and rhBMP-2-treated animals, with HA-treated animals showing no elevated expression relative to PLGA controls. There were no significant differences in the relative expression of any of these key transcripts between the HA-DBM and rhBMP-2-treated animals. (Fig. 7).
Figure 7.

Western Blot Analysis. A. Western blot visualization and B. Quantified protein expression of markers of interest– DLX5, RUNX2, OSX, OPN, and PHEX – for PLGA, HA, HA-DBM, and rhBMP-2 treatment groups at 2-weeks postoperatively. * indicates statistical significance relative to PLGA.
4. Discussion
Steady increases in the annual rate and cost of spine fusion procedures highlight the need for more reliable and safer options for bone graft substitute materials [36]. To address the limitations of the currently available materials for bone regeneration and spinal fusion, we have previously reported promising results from a 3D-printed composite material consisting of HA and DBM particles in a PLGA binder [10, 11]. Given that ceramic-based scaffolds generally lack substantive osteoinductivity [37], we hypothesized that the addition of DBM to the 3DP HA scaffold would impart additional bioactivity and promote successful fusion. In this work, we found that using specific ink composition and 3D scaffold geometry, the HA-DBM was able to elicit successful fusion in a rat pre-clinical model [10, 11] In the current study, we employed that composition and geometric shape in a comparative evaluation of the HA-DBM performance alongside an established positive control in the rat posterolateral spine fusion mode – rhBMP-2 [10]. The clinical industry standard bone graft substitute, rhBMP-2/ACS (Infuse™) has been shown in many pre-clinical studies to elicit a fusion rate of 100% at the 10 µg/animal rhBMP-2 dose used in this study, a response that other alternatives cannot match [6–8, 30, 31]. Therefore, notwithstanding its suboptimal safety profile, rhBMP-2/ACS remains a highly osteoinductive scaffold that can be used as a benchmark for the evaluation of newly developed materials for spinal fusion capacity.
Despite the fact implants are always placed bilaterally in clinical practice, a posterolateral spine fusion procedure is considered successful when new bone forms to fully bridge the transverse processes unilaterally, which is generally sufficient to stabilize the level. We have shown in previous pre-clinical work that unlike rhBMP-2/ACS, the HA-DBM scaffold does not substantially degrade over the course of the 8-week study, and new bone growth does not extend to fully bridge the L4-L5 gap [10, 11]. Instead, microCT shows bone has grown from the transverse processes into the scaffold micropores and around the cranial and caudal ends of the scaffold [10, 11]; this in confirmed by the present histological evaluation (Fig. 4) and the synchrotron microCT results shown in the supplemental information. Quantitative evaluation of the extent of scaffold osteointegration with the transverse processes (from microCT) showed that composite-bone integration structurally links adjacent spine segments, and manual palpation showed that this structure stabilizes the two segments. Note that, in the context of this composite-based fusion, conventional 3D microCT-derived morphometric quantities are uninformative. Moreover, in a previous study, our microCT work demonstrated that the HA- DBM scaffold allows for extensive neovascularization, and that all macropores were infiltrated with new blood vessels [10]. Based on the designation of unilateral stabilization as a successful fusion, the current study found no significant difference in the rate of successful fusion between HA-DBM and rhBMP-2/ACS (Fig. 3C). Importantly however, comparison of fusion scores provides a more nuanced assessment of fusion response. Here, we found that HA-DBM treatment resulted in unilaterally-fused spines roughly 50% of the time (mean fusion score of 1.52), whereas rhBMP-2/ACS promoted bilateral fusion in all recipients (mean fusion score of 2.0) (Fig. 3B). Therefore, despite the fact the HA-DBM performed extremely well for a recombinant growth factor-free scaffold, its fusion capacity was not equal to that of high-dose rhBMP-2/ACS, which resulted in a much more robust and structured newly formed fusion mass by 8 weeks (Fig. 4).
Given the compositional and structural differences between these two bone grafts substitutes and the resulting differences in mode of fusion, an objective biomechanical assessment was undertaken to compare the mechanical properties of the fused level in rats receiving HA-DBM and rhBMP-2/ACS implants. Our prior work demonstrated that the HA-DBM scaffold provides stabilization of the fused segment via osteointegration—that is, the growth of bony projections from the native transverse processes into the scaffold macropores and around the cranial and caudal ends of the scaffold [10]. Treatment with rhBMP-2, on the other hand, generates substantial bridging bone which spans the L4-L5 intertransverse process space – with new bone often extending into the adjacent level, occasionally producing an unintentional two-level fusion. Despite this robust osteoinductive response, studies suggest that the quality of the bone formed at the fusion site with the use of rhBMP-2 may not be ideal, with the trabecular structure thin and lacelike on histologic appearance as demonstrated previously [38, 39] and in the present histological evaluation (Fig. 4E). The biomechanical significance of this bone morphology is unknown.
In the present study, mechanical testing showed an intermediate range-of-motion in HA-DBM specimens, which was found to be significantly more stabilized than non-operative controls but not as stiff as rhBMP-2-treated spines. The mechanical behavior observed on biomechanical testing seemed to correlate with the results obtained from blinded manual palpation-based fusion scoring; that is, both treatments achieved successful fusion but a more robust response was seen with rhBMP-2. This is likely attributable to differing rates of unilateral versus bilateral fusion success, but also to the difference in fusion mode, i.e., fully bridging bone with rhBMP-2 as also shown by histology (Fig. 4E) versus bony osteointegration at the scaffold-bone interface in HA-DBM fusions as discussed above and visualized by histology (Fig. 4D and H), and as we have shown previously [10]. In a situation where the elastomeric scaffold links the two segments, therefore, lower stiffness is expected.
Results of gene and protein expression analyses largely corresponded with pre-clinical outcomes. Generally, the HA-DBM material elicited pro-osteogenic activity with a similar mechanistic signature to that of rhBMP-2. Histological evaluation showed new bone-like tissue formed among struts of the HA-DBM scaffold by 8 weeks post-operatively. Through gene expression analysis of the fusion mass tissues, both early markers (e.g. Runx2, Osx, and Alp) and late markers (e.g. Opn and Ocn) of osteogenesis were significantly upregulated by rhBMP-2 and HA-DBM in the post-operative period. Some correlation of pro-osteogenic marker expression is not unexpected, considering that DBM contains osteoinductive growth factors—including BMP-2. However, considering that DBM contains far lower concentrations of BMP-2 than what was delivered with rhBMP-2/ACS, as well as the fact that a considerable fraction of the DBM particles were masked within the PLGA binder and within several shells of HA particles, the correlation of response with that of high-dose rhBMP-2 is encouraging. Some DBM particles intersect the surface of the scaffold struts [10, 11] and appear to be the source of activity not entirely masked by the slowly-degrading PLGA binder, especially because HA alone scaffolds did not yield the same induction of pro-osteogenic markers as HA-DBM. In fact, unlike in the HA-DBM-treated spines, the gene expression levels of Runx2, Osx, Alp, Opn, Ocn, and Phex were not significantly upregulated in HA only-treated animals relative to the PGLA-treated controls 2-weeks post-operative. Although Western blotting did show some induction of RUNX2, COL1A, and OPN with the HA scaffolds in addition to the HA-DBM and rhBMP-2/ACS scaffolds, protein expression of OSX, OCN, and PHEX were elevated only in the latter two. These conclusions are further supported by the fact that cells were found around some DBM particles after 8 weeks implantation, most probably exposed DBM particles (Fig. S3). This demonstrates that DBM particles are not always masked by the binder, but some DBM particles become accessible to cells as early as 2 weeks post-operatively within the struts . Hence, it is reasonable to conclude from these results, that the addition of DBM to the HA-based scaffold provided substantial osteoinductivity that may have contributed to its pre-clinical success. Additionally, it is noteworthy that when we previously tested DBM alone within a 3D printed PLGA scaffold (PLGA-DBM), it failed to yield high rates of spinal fusion in the same rat model (~50% efficacy) [10, 11]. Thus, it is reasonable to conclude that the efficacy of HA-DBM observed herein is not due to DBM alone, and that both DBM and HA are needed to achieve it.
Since unlike the HA-DBM, rhBMP-2/ACS does promote robust formation of new bone sufficient to bridge the L4-L5 space, the areas of discordance in gene expression may be functionally important. For example, rhBMP-2/ACS may provide an advantage over HA-DBM by inducing an earlier response in Alpl expression at 2 days post-operative, although levels were only marginally higher relative to PLGA controls and may not be clinically significant. At the 2-week time point, expression of Alpl was significantly induced in both rhBMP-2 and HA-DBM explants relative to PLGA controls, but levels were higher in rhBMP-2 explants (> 5-fold higher than PLGA) relative to HA-DBM explants (<5-fold higher than PLGA). The most striking differences in response to the HA-DBM and rhBMP-2 treatments were noted in mid- and late-markers of osteogenesis at the 2-week time point. In particular, Opn and Ocn expression were markedly higher in the rhBMP-2 specimens relative to HA-DBM. Since these markers are downstream of Runx2, Osx, and Alpl however, it is unclear whether the larger differences in Opn and Ocn are primary responses or an indirect result of the slightly higher Runx2 and Alpl expression[40, 41]. Further complicating this question is the fact that RUNX2 activity is determined not just by its expression level, but also by its phosphorylation state, which was not examined in the current study [42]. Interestingly, despite that transcript levels of Ocn and Opn were higher in rhBMP-2-treated animals, at least at the 2-week time point, protein levels appeared similarly induced relative to PLGA-treated controls. As with RUNX2, however, the activity levels of these proteins can also be influenced by post-translational modifications [43, 44].
Taken together, the results of this study suggest that DBM provides osteoinductivity to the HA-DBM scaffold, but to a lesser degree than rhBMP-2, which more substantially initiates the regenerative process. The differences in preclinical outcomes between the HA-DBM and rhBMP-2/ACS treatment groups—i.e., fusion scores and mechanical stability—may be explained in part by the differential degrees in osteoinductive potency observed. These results are promising; however, the use of a rodent spinal model presents its limitations, including anatomical differences as well as a faster rate of bone healing in rats. While the clinical significance of these differences are unknown, the more robust osteoinductivity from the rhBMP-2 group likely induced newly-formed bone sufficient to fully bridge the intertransverse space but also may explain the mechanism behind some of the reported postoperative complications such as heterotopic ossification. Considering the relatively slow degradation rate of the PLGA binder used in the HA-DBM ink, it is possible that persistence of the elastomer as well as the low solubility of the HA particles may be initially hinder osteoinductivity and the eventual replacement of the scaffold with bone. Modifications enabling faster scaffold degradation and enhanced DBM bioavailability could facilitate a swifter influence of the DBM on local progenitor cells, potentially imparting sufficient osteoinductivity. This could allow for bone to grow in and around the scaffold and linking the adjacent transverse processes with bone, i.e., reproducing the high degree of biomechanical stability achievable with the clinical comparative, rhBMP-2, while still limiting the clinical complications from a rapidly robust response.
Modifications aimed at tailoring the scaffold degradation to a rate proportional to new bone growth, as well as evaluations to establish the safety of the material for use in the spine fusion settings are areas of ongoing investigation.
5. Conclusion
The HA-DBM composite scaffold represents a recombinant growth factor-free biomaterial that shows potential for promoting bone regeneration and spinal fusion. When compared with the established positive control and industry standard bone graft substitute (rhBMP-2/ACS; Infuse™) in a rat preclinical model, the HA-DBM scaffold shows similar rates of successful spine fusion, but the fusion masses were not as biomechanically strong. Mechanistic analyses supported these pre-clinical findings in that HA-DBM scaffolds show similar mechanisms of action to rhBMP-2, but with some variation in the degree of osteoinductivity. We believe that the reduction in the rapid osteoinductive response with the use of a PLGA binder may provide excellent fusion rates while reducing the complications seen from the use of recombinant growth factors. Future development will focus on enhancing scaffold degradation rate and osteoinductive capacity.
Supplementary Material
Acknowledgments:
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This research used resources of Northwestern University’s Analytical bioNanoTechnology Equipment Core (ANTEC), the Center for Advanced Microscopy (CAM), and the Mouse Histology and Phenotyping Laboratory core facilities. The laboratory microCT data were collected at the Rush University Microcomputed Tomography/Histology Core Facility. This research also used resources of the Musculoskeletal Biomechanics Laboratory of the Edward Hines Jr. VA Hospital. The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility, operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02–06CH11357; and the authors acknowledge the assistance of P. Shevchenko during the imaging experiments at beamline 2-BM.
Funding:
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health [grant number R01AR069580].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interests: M.A.P., S.M., J.G.L., A.G., D.E., M.H., J.Y., S.J., C.Y., K.R.B., R.M.H., M.M., A.G.P., W.K.H., S.R.S., and E.L.H. have nothing to disclose that may create a conflict of interest within this body of work. A.E.J. and R.N.S. are cofounders of the company Dimension Inx, LLC – which aims to create biomaterials, including 3D-printed biologically active materials, that induce tissue regeneration and repair – including some of the materials discussed in this paper. As of October 2020, A.E.J. is the Chief Technology Officer (CTO) and R.N.S. is the Chief Science Officer (CSO) of Dimension Inx, LLC. The trademark for Hyperelastic Bone® is owned by Dimension Inx. The study design, reporting of data, and interpretation of data in this body of work was not influenced by the interests of Dimension Inx LLC.
References
- [1].Minardi S, Taraballi F, Cabrera F, Van Eps J, Wang X, Gazze S, Fernandez-Mourev JS, Tampieri A, Francis L, Weiner B, Biomimetic hydroxyapatite/collagen composite drives bone niche recapitulation in a rabbit orthotopic model, Materials Today Bio 2 (2019) 100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dimar JR 2nd, Glassman SD, Burkus JK, Pryor PW, Hardacker JW, Carreon LY, Two-year fusion and clinical outcomes in 224 patients treated with a single-level instrumented posterolateral fusion with iliac crest bone graft, Spine J 9(11) (2009) 880–5. [DOI] [PubMed] [Google Scholar]
- [3].Kadam A, Millhouse PW, Kepler CK, Radcliff KE, Fehlings MG, Janssen ME, Sasso RC, Benedict JJ, Vaccaro AR, Bone substitutes and expanders in Spine Surgery: A review of their fusion efficacies, Int J Spine Surg 10 (2016) 33–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tannoury CA, An HS, Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery, The spine journal : official journal of the North American Spine Society 14(3) (2014) 552–9. [DOI] [PubMed] [Google Scholar]
- [5].James AW, LaChaud G, Shen J, Asatrian G, Nguyen V, Zhang X, Ting K, Soo C, A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2, Tissue Eng Part B Rev 22(4) (2016) 284–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hsu WK, Polavarapu M, Riaz R, Larson AC, Diegmueller JJ, Ghodasra JH, Hsu EL, Characterizing the host response to rhBMP-2 in a rat spinal arthrodesis model, Spine (Phila Pa 1976) 38(12) (2013) E691–8. [DOI] [PubMed] [Google Scholar]
- [7].Hsu WK, Polavarapu M, Riaz R, Roc GC, Stock SR, Glicksman ZS, Ghodasra JH, Hsu EL, Nanocomposite therapy as a more efficacious and less inflammatory alternative to bone morphogenetic protein-2 in a rodent arthrodesis model, J. Orthop. Res 29(12) (2011) 1812–9. [DOI] [PubMed] [Google Scholar]
- [8].Hsu WK, Wang JC, Liu NQ, Krenek L, Zuk PA, Hedrick MH, Benhaim P, Lieberman JR, Stem cells from human fat as cellular delivery vehicles in an athymic rat posterolateral spine fusion model, J Bone Joint Surg Am 90(5) (2008) 1043–52. [DOI] [PubMed] [Google Scholar]
- [9].Lee SS, Hsu EL, Mendoza M, Ghodasra J, Nickoli MS, Ashtekar A, Polavarapu M, Babu J, Riaz RM, Nicolas JD, Nelson D, Hashmi SZ, Kaltz SR, Earhart JS, Merk BR, McKee JS, Bairstow SF, Shah RN, Hsu WK, Stupp SI, Gel scaffolds of BMP-2-binding peptide amphiphile nanofibers for spinal arthrodesis, Adv Healthc Mater 4(1) (2015) 131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hallman M, Driscoll JA, Lubbe R, Jeong S, Chang K, Haleem M, Jakus A, Pahapill R, Yun C, Shah R, Influence of Geometry and Architecture on the In Vivo Success of 3D-Printed Scaffolds for Spinal Fusion, Tissue Engineering Part A (2020). [DOI] [PMC free article] [PubMed]
- [11].Driscoll JA, Lubbe R, Jakus AE, Chang K, Haleem M, Yun C, Singh G, Schneider AD, Katchko KM, Soriano C, 3D-printed ceramic-demineralized bone matrix hyperelastic bone composite scaffolds for spinal fusion, Tissue Engineering Part A 26(3–4) (2020) 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Alves Cardoso D, Jansen J, Leeuwenburgh SG, Synthesis and application of nanostructured calcium phosphate ceramics for bone regeneration, Journal of Biomedical Materials Research Part B: Applied Biomaterials 100(8) (2012) 2316–2326. [DOI] [PubMed] [Google Scholar]
- [13].Lyons JG, Plantz MA, Hsu WK, Hsu EL, How to grow bone: nanostructured materials for bone regeneration, Frontiers in Bioengineering and Biotechnology 8 (2020) 922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Minardi S, Corradetti B, Taraballi F, Sandri M, Van Eps J, Cabrera FJ, Weiner BK, Tampieri A, Tasciotti E, Evaluation of the osteoinductive potential of a bio-inspired scaffold mimicking the osteogenic niche for bone augmentation, Biomaterials 62 (2015) 128–137. [DOI] [PubMed] [Google Scholar]
- [15].Fernandez de Grado G, Keller L, Idoux-Gillet Y, Wagner Q, Musset A-M, Benkirane-Jessel N, Bornert F, Offner D, Bone substitutes: a review of their characteristics, clinical use, and perspectives for large bone defects management, Journal of tissue engineering 9 (2018) 204173141877681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Szpalski M, Gunzburg R, Applications of calcium phosphate-based cancellous bone void fillers in trauma surgery, Orthopedics 25(5) (2002) S601–S609. [DOI] [PubMed] [Google Scholar]
- [17].Giannoudis PV, Dinopoulos H, Tsiridis E, Bone substitutes: an update, Injury 36(3) (2005) S20–S27. [DOI] [PubMed] [Google Scholar]
- [18].Campana V, Milano G, Pagano E, Barba M, Cicione C, Salonna G, Lattanzi W, Logroscino G, Bone substitutes in orthopaedic surgery: from basic science to clinical practice, J Mater Sci Mater Med 25(10) (2014) 2445–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Roberts TT, Rosenbaum AJ, Bone grafts, bone substitutes and orthobiologics: the bridge between basic science and clinical advancements in fracture healing, Organogenesis 8(4) (2012) 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wen Y, Xun S, Haoye M, Baichuan S, Peng C, Xuejian L, Kaihong Z, Xuan Y, Jiang P, Shibi L, 3D printed porous ceramic scaffolds for bone tissue engineering: a review, Biomaterials science 5(9) (2017) 1690–1698. [DOI] [PubMed] [Google Scholar]
- [21].Fielding G, Bose S, SiO2 and ZnO dopants in three-dimensionally printed tricalcium phosphate bone tissue engineering scaffolds enhance osteogenesis and angiogenesis in vivo, Acta biomaterialia 9(11) (2013) 9137–9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jakus AE, Rutz AL, Jordan SW, Kannan A, Mitchell SM, Yun C, Koube KD, Yoo SC, Whiteley HE, Richter CP, Galiano RD, Hsu WK, Stock SR, Hsu EL, Shah RN, Hyperelastic “bone”: A highly versatile, growth factor-free, osteoregenerative, scalable, and surgically friendly biomaterial, Sci Transl Med 8(358) (2016) 358ra127. [DOI] [PubMed] [Google Scholar]
- [23].Hu T, Abbah SA, Toh SY, Wang M, Lam RWM, Naidu M, Bhakta G, Cool SM, Bhakoo K, Li J, Bone marrow-derived mesenchymal stem cells assembled with low-dose BMP-2 in a three-dimensional hybrid construct enhances posterolateral spinal fusion in syngeneic rats, The Spine Journal 15(12) (2015) 2552–2563. [DOI] [PubMed] [Google Scholar]
- [24].Jakus AE, Rutz AL, Jordan SW, Kannan A, Mitchell SM, Yun C, Koube KD, Yoo SC, Whiteley HE, Richter C-P, Hyperelastic “bone”: A highly versatile, growth factor–free, osteoregenerative, scalable, and surgically friendly biomaterial, Science translational medicine 8(358) (2016) 358ra127–358ra127. [DOI] [PubMed] [Google Scholar]
- [25].Lee SS, Hsu EL, Mendoza M, Ghodasra J, Nickoli MS, Ashtekar A, Polavarapu M, Babu J, Riaz RM, Nicolas JD, Gel scaffolds of BMP‐2‐binding peptide amphiphile nanofibers for spinal arthrodesis, Advanced healthcare materials 4(1) (2015) 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mendoza MC, Sonn KA, Kannan AS, Bellary SS, Mitchell SM, Singh G, Park C, Yun C, Stock SR, Hsu EL, The effect of vancomycin powder on bone healing in a rat spinal rhBMP-2 model, Journal of Neurosurgery: Spine 25(2) (2016) 147–153. [DOI] [PubMed] [Google Scholar]
- [27].Wang JC, Kanim LE, Yoo S, Campbell PA, Berk AJ, Lieberman JR, Effect of regional gene therapy with bone morphogenetic protein-2-producing bone marrow cells on spinal fusion in rats, JBJS 85(5) (2003) 905–911. [DOI] [PubMed] [Google Scholar]
- [28].Hsu WK, Polavarapu M, Riaz R, Larson AC, Diegmueller JJ, Ghodasra JH, Hsu EL, Characterizing the host response to rhBMP-2 in a rat spinal arthrodesis model, Spine 38(12) (2013) E691–E698. [DOI] [PubMed] [Google Scholar]
- [29].Lee K-B, Taghavi CE, Song K-J, Sintuu C, Yoo JH, Keorochana G, Tzeng S-T, Fei Z, Liao J-C, Wang JC, Inflammatory characteristics of rhBMP-2 in vitro and in an in vivo rodent model, Spine 36(3) (2011) E149–E154. [DOI] [PubMed] [Google Scholar]
- [30].Lee SS, Hsu EL, Mendoza M, Ghodasra J, Nickoli MS, Ashtekar A, Polavarapu M, Babu J, Riaz RM, Nicolas JD, Nelson D, Hashmi SZ, Kaltz SR, Earhart JS, Merk BR, McKee JS, Bairstow SF, Shah RN, Hsu WK, Stupp SI, Gel scaffolds of BMP-2-binding peptide amphiphile nanofibers for spinal arthrodesis, Adv Healthc Mater 4(1) (2015) 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Miyazaki M, Morishita Y, He W, Hu M, Sintuu C, Hymanson HJ, Falakassa J, Tsumura H, Wang JC, A porcine collagen-derived matrix as a carrier for recombinant human bone morphogenetic protein-2 enhances spinal fusion in rats, Spine J 9(1) (2009) 22–30. [DOI] [PubMed] [Google Scholar]
- [32].Hsu EL, Sonn K, Kannan A, Bellary S, Yun C, Hashmi S, Nelson J, Mendoza M, Nickoli M, Ghodasra J, Park C, Mitchell S, Ashtekar A, Ghosh A, Jain A, Stock SR, Hsu WK, Dioxin Exposure Impairs BMP-2-Mediated Spinal Fusion in a Rat Arthrodesis Model, J Bone Joint Surg Am 97(12) (2015) 1003–10. [DOI] [PubMed] [Google Scholar]
- [33].Patwardhan AG, Havey RM, Carandang G, Simonds J, Voronov LI, Ghanayem AJ, Meade KP, Gavin TM, Paxinos O, Effect of compressive follower preload on the flexion-extension response of the human lumbar spine, J Orthop Res 21(3) (2003) 540–6. [DOI] [PubMed] [Google Scholar]
- [34].Hsu EL, Ghodasra JH, Ashtekar A, Nickoli MS, Lee SS, Stupp SI, Hsu WK, A comparative evaluation of factors influencing osteoinductivity among scaffolds designed for bone regeneration, Tissue Eng Part A 19(15–16) (2013) 1764–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yun C, Katchko KM, Schallmo MS, Jeong S, Yun J, Chen CH, Weiner JA, Park C, George A, Stupp SI, Hsu WK, Hsu EL, Aryl Hydrocarbon Receptor Antagonists Mitigate the Effects of Dioxin on Critical Cellular Functions in Differentiating Human Osteoblast-Like Cells, Int J Mol Sci 19(1) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Martin BI, Mirza SK, Spina N, Spiker WR, Lawrence B, Brodke DS, Trends in Lumbar Fusion Procedure Rates and Associated Hospital Costs for Degenerative Spinal Diseases in the United States, 2004 to 2015, Spine (Phila Pa 1976) 44(5) (2019) 369–376. [DOI] [PubMed] [Google Scholar]
- [37].Chan R, Marino V, Bartold P, The effect of Emdogain® and platelet-derived growth factor on the osteoinductive potential of hydroxyapatite tricalcium phosphate, Clinical oral investigations 16(4) (2012) 1217–1227. [DOI] [PubMed] [Google Scholar]
- [38].Lieberman JR, Daluiski A, Stevenson S, Wu L, McAllister P, Lee YP, Kabo JM, Finerman GA, Berk AJ, Witte ON, The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats, J Bone Joint Surg Am 81(7) (1999) 905–17. [DOI] [PubMed] [Google Scholar]
- [39].Wang JC, Kanim LE, Yoo S, Campbell PA, Berk AJ, Lieberman JR, Effect of regional gene therapy with bone morphogenetic protein-2-producing bone marrow cells on spinal fusion in rats, J Bone Joint Surg Am 85(5) (2003) 905–11. [DOI] [PubMed] [Google Scholar]
- [40].Ducy P, Karsenty G, Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene, Mol. Cell. Biol 15(4) (1995) 1858–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Merriman HL, van Wijnen AJ, Hiebert S, Bidwell JP, Fey E, Lian J, Stein J, Stein GS, The tissue-specific nuclear matrix protein, NMP-2, is a member of the AML/CBF/PEBP2/runt domain transcription factor family: interactions with the osteocalcin gene promoter, Biochemistry 34(40) (1995) 13125–32. [DOI] [PubMed] [Google Scholar]
- [42].Bruderer M, Richards RG, Alini M, Stoddart MJ, Role and regulation of RUNX2 in osteogenesis, European Cells & Materials 28 (2014) 269–86. [DOI] [PubMed] [Google Scholar]
- [43].Al Rifai O, Chow J, Lacombe J, Julien C, Faubert D, Susan-Resiga D, Essalmani R, Creemers JW, Seidah NG, Ferron M, Proprotein convertase furin regulates osteocalcin and bone endocrine function, J. Clin. Invest 127(11) (2017) 4104–4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kazanecki CC, Uzwiak DJ, Denhardt DT, Control of osteopontin signaling and function by post-translational phosphorylation and protein folding, J. Cell. Biochem 102(4) (2007) 912–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


