Abstract
Lower extremity deep vein thrombosis (DVT) is a serious medical condition that can result in local pain and gait disturbance. DVT progression can also lead to death or major disability as a result of pulmonary embolism, postthrombotic syndrome, or limb amputation. However, early thrombus removal can rapidly relieve symptoms and prevent disease progression. Various endovascular procedures have been developed in the recent years to treat DVT, and endovascular treatment has been established as one of the major therapeutic methods to treat lower extremity DVT. However, the treatment of lower extremity DVT varies according to the disease duration, location of affected vessels, and the presence of symptoms. This article reviews and discusses effective endovascular treatment methods for lower extremity DVT.
Keywords: Deep vein thrombosis, Endovascular thrombus removal, Catheter-directed thrombolysis
INTRODUCTION
Thrombosis is a condition in which blood clots within the vascular system. When thrombosis occurs in the deep vein system, it is referred to as deep vein thrombosis (DVT). What then is the deep vein? The extremity venous system is divided into a deep venous system and a superficial venous system depending on their anatomic location. The anatomic border of the lower extremity venous system is the fascial plane. The veins that lie on the subcutaneous fat layer belongs to the superficial venous system, and the veins that lie inside the deep fascia belongs to the deep venous system.
In the lower extremity, the main route of venous flow is the deep veins rather than the superficials. Therefore, cases of DVT may present with severe disorders in the lower extremity venous return, which can cause extremity swelling, discomfort, and gait disorders. If allowed to progress, they may accompany pulmonary embolism (PE) and mortality. Between 2.5% and 5% of the population is affected by DVT at some point in their lives [1]. Within two years of DVT occurrence, over 50% patients develop postthrombotic syndrome (PTS), manifested by leg pain, swelling, skin pigmentation, or venous ulceration, despite the use of anticoagulation treatment [2,3,4,5]. Therefore, adequate treatment is needed for DVT.
Risk Factors of DVT
Thrombosis is essentially caused by the Virchow's triad—alteration in blood flow (i.e., stasis), vascular endothelial injury, and alteration in the constituents of the blood (i.e., hypercoagulation state). The risk factors for DVT are divided into inherited and acquired causes. Inherited causes include inherited thrombophilia, such as Factor V Leiden mutation, prothrombin gene mutation, protein C or protein S deficiency, and antithrombin deficiency. Acquired causes include predisposing conditions that promote thrombosis that satisfy Virchow's triad, such as immobilization, pregnancy, malignancy, use of oral contraceptives, myeloproliferative disorder, antiphospholipid syndrome, placement of central venous catheterization, and other medical conditions that increase the formation of blood clots. In addition, there are some anatomic risk factors, such as May-Thurner syndrome and inferior vena cava (IVC) abnormalities. May-Thurner syndrome is manifested by significant hemodynamic compression of the left common iliac vein by the overlying right common iliac artery and vertebral body. This makes the venous flow extremely slow, eventually causing DVT in the left lower extremity. There are several types of congenital malformations of the IVC, including agenesis, hypoplasia, and malformation. These malformations also slow venous flow in both the lower extremities and may lead to bilateral DVT [6,7,8]. The major risk factors for DVT are summarized in Table 1.
Table 1. Risk Factors of the Development of Deep Vein Thrombosis.
| Inherited causes |
| Factor V Leiden mutation |
| Prothrombin gene mutation |
| Protein C, S deficiency |
| Antithrombin deficiency |
| Acquired causes |
| Motion restriction: trauma, immobilization, surgery (especially, orthopedic) |
| Hormonal effect: pregnancy, use of oral contraceptives, hormone replacement therapy |
| Myeloproliferative disorder, antiphospholipid syndrome |
| Placement of central venous catheterization |
| Anatomic risk factors |
| May-Thurner syndrome |
| IVC abnormality: agenesis, hypoplasia, and malformation |
IVC = inferior vena cava
Category of DVT
Based on the popliteal vein, the DVT occurring in the popliteal vein or above it (i.e., femoral vein, deep femoral vein, common femoral vein, iliac vein, and/or IVC) is called a proximal DVT. Conversely, thrombosis of one or more deep calf veins, including the anterior tibial vein, posterior tibial vein, peroneal vein, and/or deep muscular vein, is called distal DVT. This anatomical classification is not an intuitive classification criterion, but the incidence of PE shows a significant difference according to the DVT location. It is well established that most acute cases of PE is associated with proximal DVT. The reported mortality rate of proximal DVT is higher than that of distal DVT [9,10,11].
Based on symptom duration, DVT can be categorized into acute, subacute, and chronic DVT. Acute DVT refers to the presence of symptoms for less than 14 days or for which imaging studies indicate that thrombosis occurred within the previous 14 days [12]. Subacute DVT refers to cases in which symptoms have been present for 15–28 days, as indicated by clinical history or imaging studies [12]. Finally, chronic DVT refers to cases in which symptoms have been present for more than 28 days, as indicated by clinical history or imaging findings [12]. In some cases, acute DVT presents with subacute or chronic DVT. Symptom duration is an important factor in determining the treatment method for DVT.
The last category is the presence of symptoms. Symptomatic DVT refers to the presence of symptoms, such as leg discomfort or swelling, which usually lead to the radiologic conformation of DVT. In contrast, asymptomatic DVT is diagnosed by incidental radiologic findings. The presence of symptoms is another important factor in determining the treatment method for DVT. There is no doubt that symptomatic proximal DVT should be treated promptly. What about asymptomatic proximal DVTs? Further details are described in the ‘Endovascular Treatment Strategy in DVT’ section.
Radiologic Diagnosis of DVT
Lower extremity DVT may be suspected when clinical symptoms, such as edema, pain, and erythema, appear on the lower extremities, and D-dimer levels rise above normal. However, these are nonspecific indicators; therefore, radiologic imaging is important for diagnosing DVT. Diagnostic imaging methods include ultrasonography (US), CT venography (CT venography), and MR venography (MR venography). In the past, ascending venography was used for DVT diagnosis, but it is no longer being used for diagnostic purposes because of its invasiveness. US is widely used and preferred as the first-choice imaging modality for the diagnosis of proximal DVT [13,14]. Compression US with Doppler is the diagnostic test of choice in patients with suspected DVT, as it shows high sensitivity and high specificity for the diagnosis of symptomatic proximal DVT [15,16,17]. However, this method is limited usefulness to diagnose iliac vein DVT and asymptomatic proximal DVT [17]. In such cases, CT venography can be used, which has a high sensitivity and specificity [18,19,20,21,22,23]. Moreover, CT venography provides additional information about the condition of the iliac vein, which is needed for the planning of surgical or endovascular treatment in DVT patients with or without symptoms [24]. In patients with suspected PE, comprehensive examinations, including pulmonary CT angiography and CT venography, is useful [25,26,27]. CT venography has drawbacks as it requires iodinated contrast media and exposure to ionizing radiation. MR venography has been recently documented as an alternative to US because it avoids exposure to ionizing radiation and iodinated contrast media [28,29]. Some MR techniques, such as time-of-flight or phase-contrast venography, can evaluate DVT without using contrast media [24]. However, the diagnostic accuracy is still higher in contrast-enhanced studies [30]. Studies on MR venography are limited and have been conducted in small patient cohorts, and MR venography requires a high level of patient cooperation with a long examination time. Therefore, MR venography still has limitations for use in all patients.
Endovascular Treatment Strategy in DVT
The widely accepted indications for endovascular thrombus removal for lower extremity DVT include massive proximal acute DVT (i.e., iliofemoral or femoropopliteal thrombosis) accompanied by severe symptomatic leg swelling [2,24,31,32,33,34]. Phlegmasia cerulea dolens (PCD) is an uncommon but potentially life-threatening complication of acute DVT characterized by marked swelling of the extremities with pain and cyanosis. This may lead to arterial ischemia and ultimately cause gangrene, with high amputation and mortality rates [35]. Cases of massive DVT with PCD require immediate endovascular thrombus removal [36].
Iliofemoral DVT differs from femoropopliteal DVT in that the fomer is associated with more frequent recurrent DVT, and more frequent and severe PTS [37,38,39]. This may be based on the anatomic characteristics of the lower extremity venous system. Compared to femoropopliteal DVT or distal DVT, which are more easily compensated by collateralization to the deep or superficial vein, an iliofemoral occlusion has poor chances of sufficient collateralization [40]. However, some cases of iliofemoral occlusion develop adequate collateralization, directing the venous return of the obstructed extremity to the ipsilateral and contralateral iliac system. This collateralization provides immediate bypassing of the venous return from the acute obstruction, and symptoms are relieved in a short period. In these cases, patients may remain asymptomatic for many years without treatment [40]. Therefore, subsequently, when chronic iliofemoral obstruction develops, these patients may present with PTS due to valvular reflux in combination with persistent venous flow disturbance. Recanalization of chronic iliofemoral obstruction rarely occurs with conservative or medical treatments. In cases of chronic iliofemoral obstruction with PTS, endovascular recanalization could be helpful in relieving PTS symptoms.
There is no evidence to indicate that endovascular thrombus removal could help to prevent the propagation of DVT in asymptomatic proximal DVT. In asymptomatic proximal DVT, anticoagulation treatment alone is sufficient to reduce the recurrence rate and prevent PE [41]. There is no need for endovascular thrombus removal in patients with isolated distal DVT. The need for anticoagulation treatment for distal DVT has not been established because isolated distal DVT has a low risk of PE. For isolated distal DVT cases, preference for anticoagulation treatment varies among centers and clinicians.
Bleeding complications should also be considered in endovascular treatment. Bleeding risk is definitely higher in patients treated with thrombolytic agents than in those treated with anticoagulants alone. Recently, the CaVenT and the Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis (ATTRACT) studies reported about 3% and 1.7% of major bleeding complications, respectively [5,42]. Bleeding complications were higher with anticoagulation alone (between 0% and 0.3% in each report), although there were no intracranial hemorrhage or mortality cases in the two reports [5,42]. Therefore, endovascular therapy should be performed in selected patients who present a low risk of bleeding. The contraindications to thrombolytic therapy are summarized in Table 2 [12].
Table 2. Contraindications to Thrombolytic Therapy in Deep Vein Thrombolsis.
| Absolute contraindications |
| Active internal bleeding or DIC |
| Recent neurovascular event (< 3 months) |
| CVA (including TIA), neurosurgery (intracranial, spinal), or intracranial trauma |
| Absolute contraindication to anticoagulation |
| Relative contraindications |
| Recent major event (< 7–10 days) |
| CPR, major surgery, cataract surgery, obstetrical delivery, organ biopsy, or major trauma |
| Neurological disorder |
| Intracranial lesion (including tumor) or seizure disorder |
| Uncontrolled hypertension: systolic BP > 180 mm Hg, diastolic BP > 110 mm Hg |
| Recent major gastrointestinal bleeding or internal eye surgery (< 3 months) |
| Serious allergic or other reaction to thrombolytic agent, anticoagulant, or contrast media (not controlled by steroid/antihistamine pretreatment) |
| Severe thrombocytopenia |
| Known right-to-left cardiac or pulmonary shunt or left heart thrombus |
| Severe dyspnea or severe acute medical illness precluding safe procedure performance |
| Suspicion for infected venous thrombus |
| Renal failure (estimated GFR < 60 mL/min) |
| Pregnancy or lactation |
| Severe hepatic dysfunction |
| Bacterial endocarditis |
| Diabetic hemorrhagic retinopathy |
This table was modified from the article by Vedantham et al. J Vasc Interv Radiol 2014;25:1317-1325, with permission of Elsevier Inc. [12].
BP = blood pressure, CPR = cardiopulmonary resuscitation, CVA = cerebrovascular accident, DIC = disseminated intravascular coagulation, GFR = glomerular filtration rate, TIA = transient ischemic attack
Endovascular Thrombus Removal
There are several endovascular techniques for thrombus removal and recanalization: catheter-directed thrombolysis (CDT), pharmacomechanical thrombectomy (PMT) with rheolytic, ultrasound, or rotational device, large-bore catheter aspiration, balloon angioplasty, balloon maceration, and stent placement. Among them, CDT and PMT are the two main treatment methods for thrombus removal, and the other methods are adjunctive techniques for the completion of endovascular thrombus removal. Endovascular thrombus removal is often performed using a combination of two or more methods.
Thrombolytic agents activate serum plasminogen to form plasmin, which accelerates the lysis of thrombus. Direct infusion of a thrombolytic agent into the thrombus, rather than systemic infusion, can accelerate thrombolysis; therefore, systemic thrombolytic therapy is no longer performed. The most representative thrombolytic agent was urokinase, a second-generation plasminogen activator. However, it is no longer used because of discontinuation of production. Recently, alteplase (a tissue plasminogen activator [tPA]), a recombinantly derived analog of human tPA, has predominantly replaced urokinase. Moreover, two tPA variants, reteplase (recombinant plasminogen activator) and tenecteplase (TNK), are also used. Reteplase is a non-glycosylated deletion mutant of the wild-type tPA, is less fibrin selective, and has a longer half-life than alteplase. TNK is a recombinant fibrin-specific plasminogen activator derived from human tPA. It is 14 times more fibrin-specific and has an 80-fold higher resistance to inhibition by plasminogen activator inhibitor-1 than standard tPA, and has a longer plasma half-life, which permits intravenous bolus injection [43]. Of these three types of drugs, only alteplase (Actilyse Injection; Boehringoer Ingelheim Korea) is available in Korea.
Catheter-Directed Thrombolysis (CDT)
CDT refers to the slow administration of a thrombolytic agent over several hours for intrathrombus infusion via multiple side-holes of an infusion catheter embedded within the thrombosed vein. Catheter insertion is usually performed via a popliteal vein in the affected leg, and US guidance is used to obtain access into the deep venous system. In some cases, the common femoral vein can be used as an alternative access route. Ideally, a vein with blood flow below the thrombosed venous segment should be accessed, if possible. Venous access is usually achieved with a 21-G micropuncture needle and a 0.018-inch diameter hair wire, which is exchanged with a 5-F micropuncture vascular sheath. The venous system is then navigated with a 0.035-inch hydrophilic guidewire and a 5-F catheter. Then, a 5-F multi-side-hole infusion catheter is inserted, traversing the thrombus for infusion of the thrombolytic agent. In some cases, before placing the multiple-side-hole infusion catheter, a bolus dose of the thrombolytic agent is dispersed in the thrombus using a 5-F catheter to elicit thrombus fragmentation and maximize the thrombolytic effect. The bolus of tPA is between 7.5 mg and 10 mg [44]. In most cases of massive proximal DVT, the length of the catheter side-hole does not cover the entire length of the thrombus. To overcome this limitation, multiple side-holds can be created in the vascular sheath. Simultaneously, we can make holes at 1–2 cm intervals along both sides of the vascular sheath using a 25G needle (Fig. 1). If a thrombolytic agent is injected simultaneously through the sidearm of the sheath as well as the multi-side-hole catheter, then the thrombolytic agent can be injected into the entire DVT. To inject the thrombolytic agent through the sidearm of the sheath, a gap between the sheath and the 5F catheter is required. Therefore, a 7- or 8-F vascular sheath is suitable. Although there is no consensus on the optimal thrombolytic agent; the commonly used dosing schemes are alteplase (0.5–1.0 mg/h), reteplase (0.25–0.75 units/h), and TNK (0.25–0.5 mg/h) for CDT [24,45,46]. After 12–24 hours from the initiation of the thrombolytic infusion, follow-up venography is performed, and the operator determines whether to continue or stop the infusion. If venography demonstrates complete lysis, then the infusion is terminated. In case of partial lysis, the catheter is repositioned so that the remaining thrombus is centered, and infusion can be continued or replaced by another technique. If thrombolytic infusion continues, follow-up venography is performed every 12–24 hours. During thrombolytic infusion, fibrinogen values are monitored closely, and the thrombolytic doses are adjusted to maintain fibrinogen levels above 100–150 mg/dL [44].
Fig. 1. Illustration depicting the creation of side-holes in the vascular sheath for catheter-directed thrombolysis.
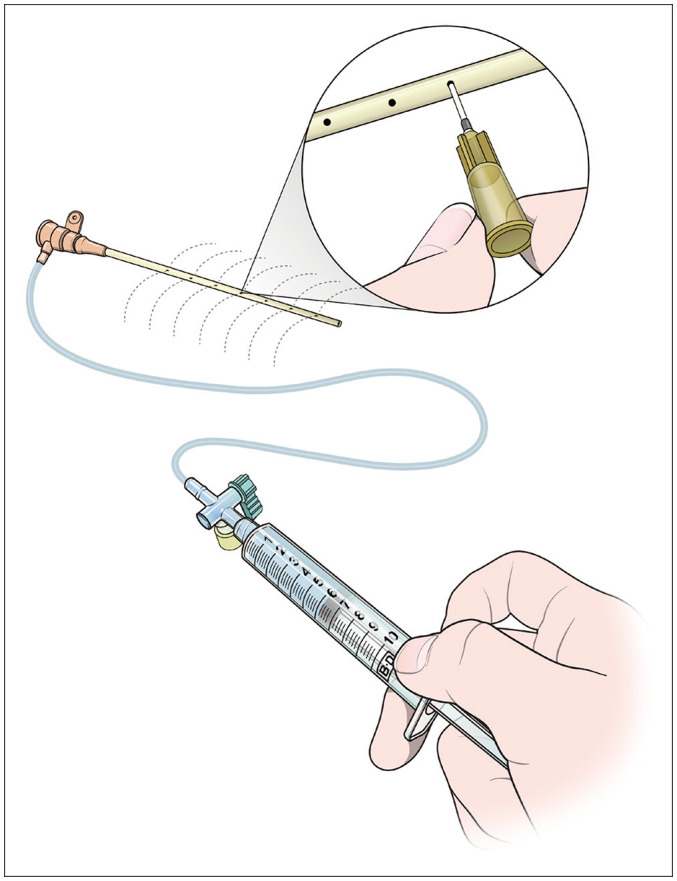
CDT is very effective, especially in cases of acute femoropopliteal DVT; therefore, often, this procedure alone dissolves the thrombus completely. However, a large number of proximal DVTs are accompanied by subacute or chronic DVTs. In such cases, other endovascular techniques should be used in combination to obtain good results.
Stand-Alone Percutaneous Mechanical Thrombectomy (PMT) vs. Pharmacomechanical Catheter-Directed Thrombolysis (PCDT)
PMT refers to the percutaneous use of catheter-based mechanical devices that contribute to thrombus removal via fragmentation, maceration, and/or aspiration, without administering a thrombolytic agent [12,24]. PCDT refers to thrombus dissolution via the concomitant use of pharmacologic CDT and PMT [12]. Both procedures have the advantage of reducing the total volume of the thrombolytic agent required, and the total treatment time is relatively shorter than that required for CDT. The initial steps of the PMT or PCDT procedures resemble CDT—the catheter for mechanical thrombectomy is inserted into an affected vein. A commonly accessed vein in the affected leg is the popliteal vein, with US-guided access. In some cases, the common femoral vein can be used as another access route.
PMT results in finely crushing DVT. Therefore, it is essential to perform an adjunctive endovascular technique, including large-bore catheter aspiration, balloon maceration, and balloon angioplasty, to deal with the remaining DVT. Several mechanical devices can be used for PMT, such as Arrow-Trerotola PTD (Arrow), AngioJet (Boston Scientific), Hydrolyser (Cordis), and EKOS (Ekos Corporation). Arrow-Trerotola PTD is a rotational device for PMT; AngioJet and Hydrolyser are rheolytic recirculation devices; and Ekos is an ultrasound-accelerated thrombolysis device. The latter three are all used for PCDT. Arrow-Trerotola PTD and AngioJet are both available in Korea. If follow-up venography after the PCDT demonstrates complete lysis, the procedure can be terminated. In case of partial lysis, an adjunctive endovascular technique, including large-bore catheter aspiration, balloon maceration, and balloon angioplasty, should be performed to deal with the remaining DVT (Fig. 2).
Fig. 2. Endovascular thrombus removal with pharmacomechanical catheter-directed thrombolysis and additional large-bore catheter aspiration.
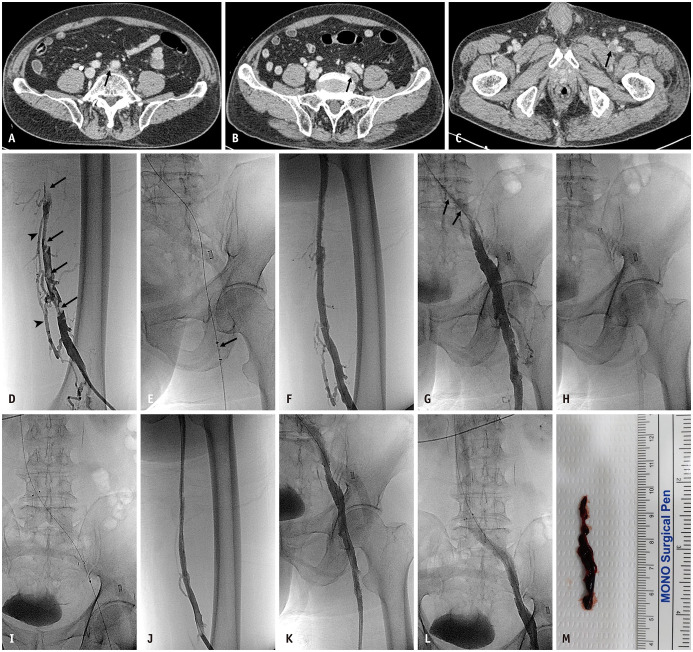
A case of a 76-year-old man who presented with a 10-day history of swelling and pain in his left leg.
A–C. CT venography shows compressed left common iliac vein (arrow, A), and deep vein thrombosis in left external iliac vein (arrow, B) and left femoral vein (arrow, C). These findings are compatible with May-Thurner syndrome. D. Ascending venography shows distended femoral vein with intraluminal thrombosis (arrows) with a few collateral vessels (arrowheads), suggestive of acute deep vein thrombosis. E. Pharmacomechanical catheter-directed thrombolysis was performed using AngioJet (Boston Scientific) (arrow) with urokinase (300k units). F, G. Follow-up venography performed 30 minutes after injection of the thrombolytic agent shows reopened femoral vein, common femoral vein, and external iliac vein with almost completely dissolved deep vein thrombus. However, the compressed left common iliac vein shows obliteration (arrows, G). H, I. Adjunctive endovascular treatment was performed with manual aspiration using a 10-F large-bore catheter (H) and additional stent placement (I) using a 14 mm × 8-cm bare metal stent (E Luminexx; Bard). J–L. On complete angiography, the venous aperture decreases to normal, and the flow from the femoral vein to the inferior vena cava is restored. M. Photography shows a chronic thrombus removed by manual aspiration using a large-bore catheter.
Although the efficiency of mechanical devices is well known, the use of such expensive devices causes an increase in medical expenses. To solve the problem of rising medical expenses, a 5-F catheter and a large-bore catheter can be used to remove the thrombus. First, after inserting the 5-F catheter through the vascular sheath, the catheter is rotated up and down about the entire thrombus area with a simultaneous bolus injection of a thrombolytic agent. A 5-F Imager catheter (Boston Scientific) or a 5-F Omni Flush catheter (Angiodynamics) is used for this step, and the bolus of tPA is between 7.5 mg and 10 mg [44]. After a rest period of about 20–30 minutes, aspiration thrombectomy is performed using a large-bore catheter, by applying negative pressure to the catheter manually using a 50-cc luer-lock syringe (Fig. 3). A large-bore catheter is recommended with a catheter 7-F or larger. These products include a 7-F Envoy guiding catheter (Codman & Shurtleff, Inc.), an 8-F Guider Softip (Boston Scientific), a 10-F or 11-F Super Arrow-Flex (Teleflex, Morrisville) (Supplementary Video).
Fig. 3. Endovascular thrombus removal with large-bore catheter aspiration.
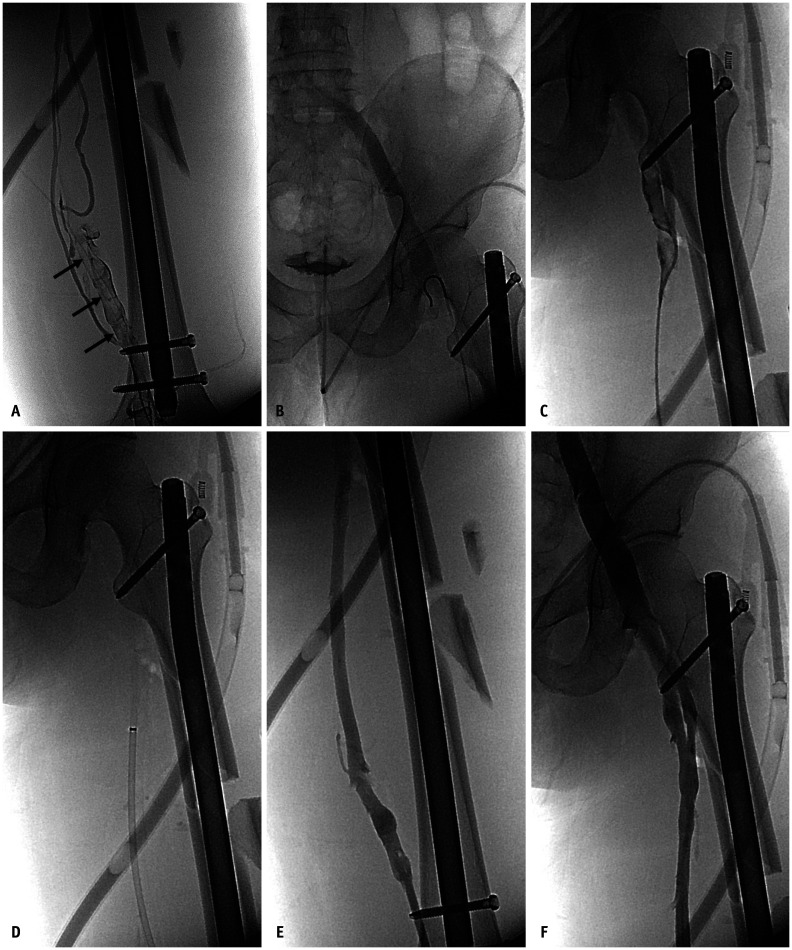
A 52-year-old man presented with acute left leg swelling. He has a left femur fracture in a traffic accident 2 weeks ago.
A, B. Ascending venography shows distended femoral vein with intraluminal filling defects (arrows, A), which is compatible with acute deep vein thrombosis. The common femoral vein and upper vein shows normal (B). C, D. Endovascular thrombus removal was performed using a 5-F Omni Flush catheter (Angiodynamics) (C) and a 10-F large-bore catheter (Super Arrow-Flex; Teleflex) (D) without using a thrombolytic agent. Inferior vena cava filter was inserted before the endovascular procedure began (not shown). E, F. Follow-up venography shows patent venous flow from femoral vein to external iliac vein with complete removal state of deep vein thrombosis.
Indications for adjunctive endovascular treatment include a residual flow-limiting reduction in vein diameter, visible flow stasis during contrast injection, opacification of collateral veins, presence of intraluminal filling defects, and extrinsic compression [47]. The PMT or PCDT methods are generally considered safe, requiring a lower dose of thrombolytic agent and shorter treatment time than other procedures. However, the technique varies according to local institutional resources and expertise, and is not clearly established yet [24].
Stent Placement
Generally, stent placement is the preferred treatment for iliac vein obstruction or IVC obstruction, especially when the affected vein is almost obliterated because of chronic causes. It is difficult to restore the diameter of the affected vein back to normal with only balloon angioplasty because intimal proliferation due to chronic intimal damage of the affected vein often results in elastic recoil after balloon angioplasty. May-Thurner syndrome is one of the representative examples (Fig. 2) (Supplementary Video). Normal blood flow can be restored only when the stent is placed into the affected vein segment to support the venous wall (Fig. 4). Closed-cell stents are characterized by a small free cell area between the structures, whereas open-cell stents have larger gaps uncovered [48]. Logically, stents with a smaller free cell area and hence a greater percentage of wall coverage may better contain the compressed segments to offer sufficient scaffolding [49]. Therefore, in case of May-Thurner syndrome, closed-cell stents are recommended rather than open-cell stents.
Fig. 4. Endovascular treatment of chronic deep vein thrombosis from left common iliac vein to left femoral vein.
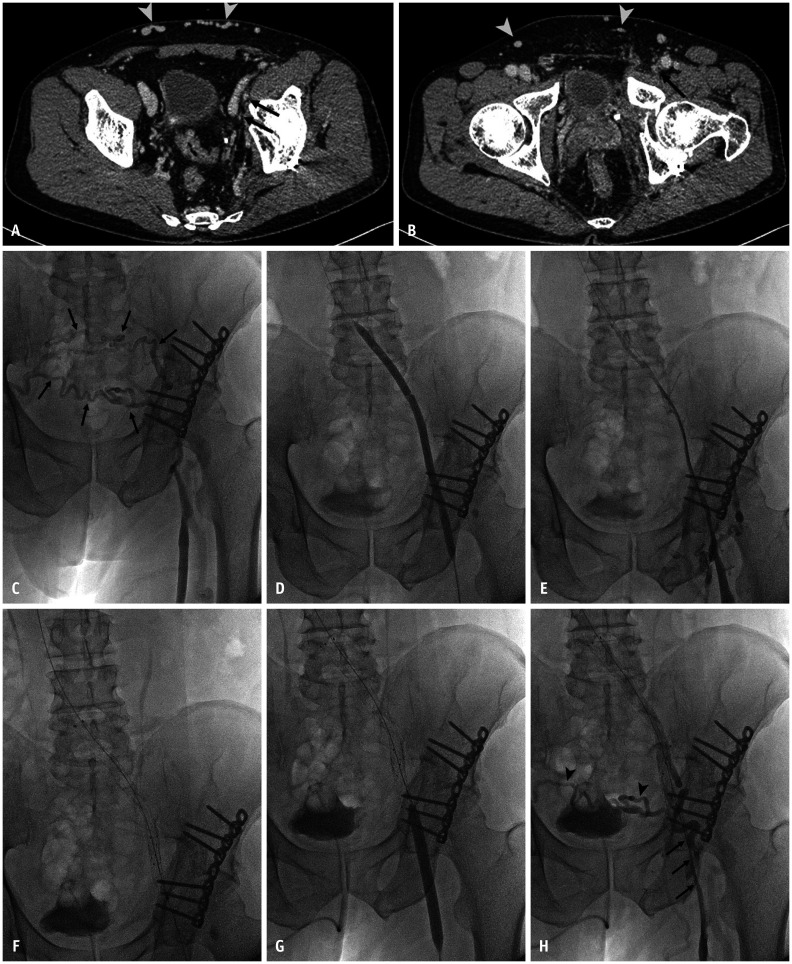
A 60-year-old man presented with left leg swelling and pain in the past year. He had the history of pelvic bone fracture 18 months ago.
A, B. CT shows luminal obliteration of left iliac vein (arrows, A) and left common femoral vein (arrow, B) with multiple dilated collateral vessels along the lower abdominal wall (arrowheads, A, B). C. Venography shows only dilated collateral vessels (arrows) draining into contralateral pelvic vessels, and no deep venous system is observed above the proximal segment of the femoral vein. D. After recanalization of the obliteration segment from the left common iliac vein and femoral vein using a 5-F catheter and a guidewire, sequential balloon angioplasty was performed from left common iliac vein to left femoral vein using a 4 mm × 4-cm balloon catheter (Fortrex; Medtronics) (not shown), and a 7 mm × 20-cm balloon catheter (Mustang; Boston Scientific). E. Follow-up venography shows faint venous flow from the left femoral vein to the left common iliac vein, but this long segmental veins are still collapsed. F, G. Additional stent placement was done for the left common iliac vein and the external iliac vein using two bare metal stents (F), a 12 mm × 10-cm and a 12 mm × 4-cm (E Luminexx; Bard), and balloon angioplasty was done for left common femoral vein and femoral vein using a 8 mm × 8-cm balloon catheter (Conquest; Bard) (G). H. Completion venography shows recovered venous flow from the left femoral vein to inferior vena cava through stents, but the segmental stenosis of the femoral vein and common femoral vein is observed (arrows). Previous noted collateral vessels were reduced but still remain (arrowheads).
Moreover, dedicated venous stents have been developed recently, with features favoring the venous system [50]. There are several commercially available dedicated venous stents, such as sinus-Venous (OptiMed GmbH), VICI (Boston Scientific), Zilver Vena (Cook Medical), and Venovo (Bard). All of these systems are self-expandable, made of nitinol, open-cell stents, except VICI, which is a closed-cell stent. Among them, Venovo is available in Korea. Since these stents have been released very recently, there are no studies comparing them with conventional closed-cell stents. However, the BARD® VENOVO™ Venous Stent Study for Treatment of Iliofemoral Occlusive Disease is a work in progress and is estimated to be completed by October 2020 with promising results.
Stent location is very important, especially in May-Thurner syndrome. If the stenosis is not sufficiently covered, the stent could migrate caudally or be compressed into a tapering cone shape, facilitating restenosis [51,52]. Conversely, if the stent is located overriding the stenosis and extending into the IVC, it impairs the contralateral venous outflow. This consequently increases the risk of contralateral DVT and can also cause intimal hyperplasia of the IVC wall by stent struts [51, 53,54,55]. Therefore, the ideal stent location should sufficiently cover the compressed segment, including the iliocaval confluence, but does not extend into the IVC.
Stent placement is not recommended for femoropopliteal venous stenosis to be flow-limiting, and balloon angioplasty is generally preferred for the lesion, even if the stenotic segment does not return to normal (Fig. 4) [56].
Concomitant Anticoagulation Treatment
Anticoagulation therapy should be continued before, during, and after endovascular thrombus removal treatment. Generally, a subtherapeutic dose of unfractionated heparin is used, and activated partial thromboplastin time should be monitored during the treatment period. The infusion rate is between and 300–500 IU/h during endovascular treatment, and usually, initial bolus injection is not necessary. During thrombolytic infusion, the partial thromboplastin time should be maintained at less than 1.5 times the control level [44].
IVC Filter
Ensuring adequate anticoagulation before, during, and after the endovascular procedure is the best way to prevent procedure-related symptomatic PE [56]. In a multicenter randomized controlled trial in which 92 patients received infusion-only CDT, there were no cases of procedure-related symptomatic PE [5]. The incidence of symptomatic PE during PCDT did not appear to exceed that observed in patients who received anticoagulant therapy alone [5,57,58]. Therefore, routine placement of IVC filters before infusion-only CDT or infusion-first PCDT is not recommended (Fig. 2) [12]. However, placement of a retrieval filter might be effective for certain patients with a particularly high risk of major morbidity due to clinical PE during CDT; these include patients with a poor cardiopulmonary reserve and patients treated with stand-alone PMT without PCDT (Fig. 5) [59]. The complications associated with long-term placement of retrieval IVC filter includes device migration, embolization, fracture, and recurrence of DVT. When PCDT is completed, the IVC filter should be removed as soon as possible to avoid preventable complications.
Fig. 5. IVC filter placement before endovascular thrombus removal.
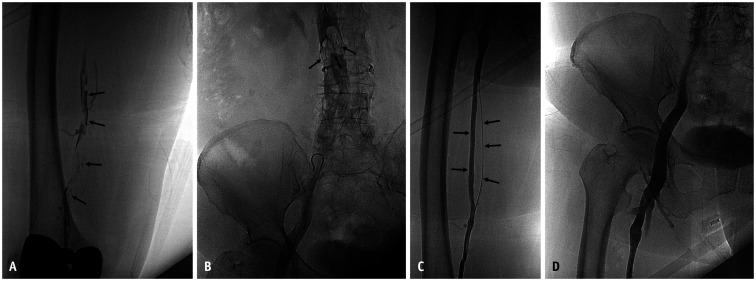
A 76-year-old woman presented with right leg swelling for 3 days ago.
A. Ascending venography shows multiple filling defects along the femoral vein (arrows). The common femoral vein and its upper vein maintain normal blood flow (not shown). B. Follow-up venography performed after catheter maceration using a 5-F catheter had been shown to capture large amounts of thrombus in the IVC filter (arrows). C, D. Completion venography shows recovered venous flow. Femoral vein duplication is observed (arrows, C). The IVC filter was not removed because of bed-ridden state of patient. IVC = inferior vena cava
CONCLUSION
All patients with lower extremity DVT do not need endovascular thrombus removal procedures. However, the results of the ATTRACT Trial shows that endovascular thrombus removal significantly reduced leg-related symptoms earlier and also reduced the severity score of the PTS [42,60]. Moreover, many studies have supported the “open-vein hypothesis,” which states that active removal of acute DVT may preserve venous function and prevent PTS [5,31,32,34,36,38,40,46,57]. Select patients with extensive acute DVT may benefit from relief of early pain and swelling, reducing venous gangrene, and improving quality of life. Therefore, endovascular thrombus removal is expected to play an important role in patient recovery and return to activities of daily living early in patients with acute symptomatic DVT.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Sun Young Choi.
- Data curation: Sun Young Choi, Kyung Ah Kim.
- Formal analysis: Sun Young Choi.
- Investigation: Sun Young Choi.
- Methodology: All authors.
- Project administration: Sun Young Choi.
- Supervision: Sun Young Choi.
- Visualization: Sun Young Choi, Ran Kim.
- Writing—original draft: Sun Young Choi, Kyung Ah Kim.
- Writing—review & editing: Sun Young Choi, Ran Kim.
Supplementary Materials
The Data Supplement is available with this article at https://doi.org/10.3348/kjr.2020.0675.
Video clip for endovascular treatment of May-Thurner syndrome.
A 52-year-old woman presented with left leg swelling that started 3 days prior. Lower extremity CT angiography indicated May-Thurner syndrome (not shown). Endovascular thrombus removal was performed via the left popliteal vein approach. Pharmacomechanical catheter-directed thrombolysis was performed using a 5-F Omni Flush catheter (Angiodynamics) with a bolus injection of urokinase 200 K units. After a rest period of approximately 20 minutes, aspiration thrombectomy was performed using a large-bore catheter, 10-F Super Arrow-Flex (Teleflex), while manually applying negative pressure to the catheter using a 50-cc Luer-lock syringe. After thrombus removal, recanalization was established in the occluded segment of the left common iliac vein using a 5-F KMP Beacon® Tip catheter (Cook Medical) and a 0.036-inch Glidewire® hydrophilic coated guidewire (Terumo). Subsequently, angioplasty was performed using an 8 mm–10-cm balloon catheter (Mustang, Boston Scientific) for pre-dilatation, a 12 mm–10-cm stent (E Luminexx; Bard), and a 12 mm–8 cm balloon catheter (Mustang; Boston Scientific).
References
- 1.White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107:I4–I8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 2.Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–352. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 3.Venous thromboembolic diseases. Nice.org.uk Web site. Published 2012. [Accessed April 4, 2020]. https://www.nice.org.uk/guidance/cg144/documents/cg144-venous-thromboembolic-diseases-evidence-update2.
- 4.Kahn SR, Shrier I, Julian JA, Ducruet T, Arsenault L, Miron MJ, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149:698–707. doi: 10.7326/0003-4819-149-10-200811180-00004. [DOI] [PubMed] [Google Scholar]
- 5.Enden T, Haig Y, Kløw NE, Slagsvold CE, Sandvik L, Ghanima W, et al. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012;379:31–38. doi: 10.1016/S0140-6736(11)61753-4. [DOI] [PubMed] [Google Scholar]
- 6.Chee YL, Culligan DJ, Watson HG. Inferior vena cava malformation as a risk factor for deep venous thrombosis in the young. Br J Haematol. 2001;114:878–880. doi: 10.1046/j.1365-2141.2001.03025.x. [DOI] [PubMed] [Google Scholar]
- 7.Hamoud S, Nitecky S, Engel A, Goldsher D, Hayek T. Hypoplasia of the inferior vena cava with azygous continuation presenting as recurrent leg deep vein thrombosis. Am J Med Sci. 2000;319:414–416. doi: 10.1097/00000441-200006000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Ruggeri M, Tosetto A, Castaman G, Rodeghiero F. Congenital absence of the inferior vena cava: a rare risk factor for idiopathic deep-vein thrombosis. Lancet. 2001;357:441. doi: 10.1016/S0140-6736(00)04010-1. [DOI] [PubMed] [Google Scholar]
- 9.Havig O. Deep vein thrombosis and pulmonary embolism. An autopsy study with multiple regression analysis of possible risk factors. Acta Chir Scand Suppl. 1977;478:1–120. [PubMed] [Google Scholar]
- 10.Browse NL, Thomas ML. Source of non-lethal pulmonary emboli. Lancet. 1974;1:258–259. doi: 10.1016/s0140-6736(74)92559-8. [DOI] [PubMed] [Google Scholar]
- 11.Galanaud JP, Sevestre-Pietri MA, Bosson JL, Laroche JP, Righini M, Brisot D, et al. Comparative study on risk factors and early outcome of symptomatic distal versus proximal deep vein thrombosis: results from the OPTIMEV study. Thromb Haemost. 2009;102:493–500. doi: 10.1160/TH09-01-0053. [DOI] [PubMed] [Google Scholar]
- 12.Vedantham S, Sista AK, Klein SJ, Nayak L, Razavi MK, Kalva SP, et al. Quality improvement guidelines for the treatment of lower-extremity deep vein thrombosis with use of endovascular thrombus removal. J Vasc Interv Radiol. 2014;25:1317–1325. doi: 10.1016/j.jvir.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 13.Konstantinides SV. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3145–3146. doi: 10.1093/eurheartj/ehu393. [DOI] [PubMed] [Google Scholar]
- 14.Ho VB, van Geertruyden PH, Yucel EK, Rybicki FJ, Baum RA, Desjardins B, et al. ACR Appropriateness Criteria® on suspected lower extremity deep vein thrombosis. J Am Coll Radiol. 2011;8:383–387. doi: 10.1016/j.jacr.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 15.Habscheid W, Höhmann M, Wilhelm T, Epping J. Real-time ultrasound in the diagnosis of acute deep venous thrombosis of the lower extremity. Angiology. 1990;41:599–608. doi: 10.1177/000331979004100803. [DOI] [PubMed] [Google Scholar]
- 16.Rose SC, Zwiebel WJ, Nelson BD, Priest DL, Knighton RA, Brown JW, et al. Symptomatic lower extremity deep venous thrombosis: accuracy, limitations, and role of color duplex flow imaging in diagnosis. Radiology. 1990;175:639–644. doi: 10.1148/radiology.175.3.2188293. [DOI] [PubMed] [Google Scholar]
- 17.Rose SC, Zwiebel WJ, Murdock LE, Hofmann AA, Priest DL, Knighton RA, et al. Insensitivity of color Doppler flow imaging for detection of acute calf deep venous thrombosis in asymptomatic postoperative patients. J Vasc Interv Radiol. 1993;4:111–117. doi: 10.1016/s1051-0443(93)71832-1. [DOI] [PubMed] [Google Scholar]
- 18.Kanne JP, Lalani TA. Role of computed tomography and magnetic resonance imaging for deep venous thrombosis and pulmonary embolism. Circulation. 2004;109:I15–I21. doi: 10.1161/01.CIR.0000122871.86662.72. [DOI] [PubMed] [Google Scholar]
- 19.Zierler BK. Ultrasonography and diagnosis of venous thromboembolism. Circulation. 2004;109:I9–I14. doi: 10.1161/01.CIR.0000122870.22669.4a. [DOI] [PubMed] [Google Scholar]
- 20.Cham MD, Yankelevitz DF, Shaham D, Shah AA, Sherman L, Lewis A, et al. Deep venous thrombosis: detection by using indirect CT venography. The Pulmonary Angiography-Indirect CT Venography Cooperative Group. Radiology. 2000;216:744–751. doi: 10.1148/radiology.216.3.r00se44744. [DOI] [PubMed] [Google Scholar]
- 21.Duwe KM, Shiau M, Budorick NE, Austin JH, Berkmen YM. Evaluation of the lower extremity veins in patients with suspected pulmonary embolism: a retrospective comparison of helical CT venography and sonography 2000 ARRS Executive Council Award I. American Roentgen Ray Society. AJR Am J Roentgenol. 2000;175:1525–1531. doi: 10.2214/ajr.175.6.1751525. [DOI] [PubMed] [Google Scholar]
- 22.Byun SS, Kim JH, Kim YJ, Jeon YS, Park CH, Kim WH. Evaluation of deep vein thrombosis with multidetector row CT after orthopedic arthroplasty: a prospective study for comparison with Doppler sonography. Korean J Radiol. 2008;9:59–66. doi: 10.3348/kjr.2008.9.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho ES, Chung JJ, Kim S, Kim JH, Yu JS, Yoon CS. CT venography for deep vein thrombosis using a low tube voltage (100 kVp) setting could increase venous enhancement and reduce the amount of administered iodine. Korean J Radiol. 2013;14:183–193. doi: 10.3348/kjr.2013.14.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Min SK, Kim YH, Joh JH, Kang JM, Park UJ, Kim HK, et al. Diagnosis and treatment of lower extremity deep vein thrombosis: Korean Practice Guidelines. Vasc Specialist Int. 2016;32:77–104. doi: 10.5758/vsi.2016.32.3.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Begemann PG, Bonacker M, Kemper J, Guthoff AE, Hahn KE, Steiner P, et al. Evaluation of the deep venous system in patients with suspected pulmonary embolism with multi-detector CT: a prospective study in comparison to Doppler sonography. J Comput Assist Tomogr. 2003;27:399–409. doi: 10.1097/00004728-200305000-00017. [DOI] [PubMed] [Google Scholar]
- 26.Loud PA, Katz DS, Klippenstein DL, Shah RD, Grossman ZD. Combined CT venography and pulmonary angiography in suspected thromboembolic disease: diagnostic accuracy for deep venous evaluation. AJR Am J Roentgenol. 2000;174:61–65. doi: 10.2214/ajr.174.1.1740061. [DOI] [PubMed] [Google Scholar]
- 27.Thomas SM, Goodacre SW, Sampson FC, van Beek EJ. Diagnostic value of CT for deep vein thrombosis: results of a systematic review and meta-analysis. Clin Radiol. 2008;63:299–304. doi: 10.1016/j.crad.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Evans AJ, Sostman HD, Witty LA, Paulson EK, Spritzer CE, Hertzberg BS, et al. Detection of deep venous thrombosis: prospective comparison of MR imaging and sonography. J Magn Reson Imaging. 1996;6:44–51. doi: 10.1002/jmri.1880060109. [DOI] [PubMed] [Google Scholar]
- 29.Sampson FC, Goodacre SW, Thomas SM, van Beek EJ. The accuracy of MRI in diagnosis of suspected deep vein thrombosis: systematic review and meta-analysis. Eur Radiol. 2007;17:175–181. doi: 10.1007/s00330-006-0178-5. [DOI] [PubMed] [Google Scholar]
- 30.Fraser DG, Moody AR, Davidson IR, Martel AL, Morgan PS. Deep venous thrombosis: diagnosis by using venous enhanced subtracted peak arterial MR venography versus conventional venography. Radiology. 2003;226:812–820. doi: 10.1148/radiol.2263012205. [DOI] [PubMed] [Google Scholar]
- 31.Snow V, Qaseem A, Barry P, Hornbake ER, Rodnick JE, Tobolic T, et al. Management of venous thromboembolism: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Fam Med. 2007;5:74–80. doi: 10.1370/afm.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alesh I, Kayali F, Stein PD. Catheter-directed thrombolysis (intrathrombus injection) in treatment of deep venous thrombosis: a systematic review. Catheter Cardiovasc Interv. 2007;70:143–148. doi: 10.1002/ccd.21079. [DOI] [PubMed] [Google Scholar]
- 33.Forster A, Wells P. Tissue plasminogen activator for the treatment of deep venous thrombosis of the lower extremity: a systematic review. Chest. 2001;119:572–579. doi: 10.1378/chest.119.2.572. [DOI] [PubMed] [Google Scholar]
- 34.Watson L, Broderick C, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev. 2016;11:CD002783. doi: 10.1002/14651858.CD002783.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaochankit W, Akaraborworn O. Phlegmasia cerulea dolens with compartment syndrome. Ann Vasc Dis. 2018;11:355–357. doi: 10.3400/avd.cr.18-00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oguzkurt L, Ozkan U, Demirturk OS, Gur S. Endovascular treatment of phlegmasia cerulea dolens with impending venous gangrene: manual aspiration thrombectomy as the first-line thrombus removal method. Cardiovasc Intervent Radiol. 2011;34:1214–1221. doi: 10.1007/s00270-010-0042-5. [DOI] [PubMed] [Google Scholar]
- 37.Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123:1788–1830. doi: 10.1161/CIR.0b013e318214914f. [DOI] [PubMed] [Google Scholar]
- 38.Comerota AJ, Gravett MH. Iliofemoral venous thrombosis. J Vasc Surg. 2007;46:1065–1076. doi: 10.1016/j.jvs.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 39.Delis KT, Bountouroglou D, Mansfield AO. Venous claudication in iliofemoral thrombosis: long-term effects on venous hemodynamics, clinical status, and quality of life. Ann Surg. 2004;239:118–126. doi: 10.1097/01.sla.0000103067.10695.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kölbel T, Gottsäter A, Kühme T, Lindh M, Ivancev K. Endovascular treatment of venous occlusive disease. Ann Vasc Dis. 2008;1:91–101. doi: 10.3400/avd.AVDrev07022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e419S–e496S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vedantham S, Goldhaber SZ, Julian JA, Kahn SR, Jaff MR, Cohen DJ, et al. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med. 2017;377:2240–2252. doi: 10.1056/NEJMoa1615066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keyt BA, Paoni NF, Refino CJ, Berleau L, Nguyen H, Chow A, et al. A faster-acting and more potent form of tissue plasminogen activator. Proc Natl Acad Sci U S A. 1994;91:3670–3674. doi: 10.1073/pnas.91.9.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grunwald MR, Hofmann LV. Comparison of urokinase, alteplase, and reteplase for catheter-directed thrombolysis of deep venous thrombosis. J Vasc Interv Radiol. 2004;15:347–352. doi: 10.1097/01.rvi.0000121407.46920.15. [DOI] [PubMed] [Google Scholar]
- 45.Vedantham S, Thorpe PE, Cardella JF, Grassi CJ, Patel NH, Ferral H, et al. Quality improvement guidelines for the treatment of lower extremity deep vein thrombosis with use of endovascular thrombus removal. J Vasc Interv Radiol. 2006;17:435–447. doi: 10.1097/01.RVI.0000197348.57762.15. quiz 448. [DOI] [PubMed] [Google Scholar]
- 46.Sugimoto K, Hofmann LV, Razavi MK, Kee ST, Sze DY, Dake MD, et al. The safety, efficacy, and pharmacoeconomics of low-dose alteplase compared with urokinase for catheter-directed thrombolysis of arterial and venous occlusions. J Vasc Sur. 2003;37:512–517. doi: 10.1067/mva.2003.41. [DOI] [PubMed] [Google Scholar]
- 47.Raju S, Owen S, Jr, Neglen P. The clinical impact of iliac venous stents in the management of chronic venous insufficiency. J Vasc Surg. 2002;35:8–15. doi: 10.1067/mva.2002.121054. [DOI] [PubMed] [Google Scholar]
- 48.Pierce DS, Rosero EB, Modrall JG, Adams-Huet B, Valentine RJ, Clagett GP, et al. Open-cell versus closed-cell stent design differences in blood flow velocities after carotid stenting. J Vasc Surg. 2009;49:602–606. doi: 10.1016/j.jvs.2008.10.016. discussion 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bosiers M, de Donato G, Deloose K, Verbist J, Peeters P, Castriota F, et al. Does free cell area influence the outcome in carotid artery stenting? Eur J Vasc Endovasc Surg. 2007;33:135–141. doi: 10.1016/j.ejvs.2006.09.019. discussion 142-143. [DOI] [PubMed] [Google Scholar]
- 50.van Vuuren TMAJ, Doganci S, Wittens CHA. Patency rates and clinical outcomes in a cohort of 200 patients treated with a dedicated venous stent. J Vasc Surg Venous Lymphat Disord. 2018;6:321–329. doi: 10.1016/j.jvsv.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 51.Caliste XA, Clark AL, Doyle AJ, Cullen JP, Gillespie DL. The incidence of contralateral iliac venous thrombosis after stenting across the iliocaval confluence in patients with acute or chronic venous outflow obstruction. J Vasc Surg Venous Lymphat Disord. 2014;2:253–259. doi: 10.1016/j.jvsv.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 52.Murphy EH, Johns B, Varney E, Buck W, Jayaraj A, Raju S. Deep venous thrombosis associated with caval extension of iliac stents. J Vasc Surg Venous Lymphat Disord. 2017;5:8–17. doi: 10.1016/j.jvsv.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 53.Neglén P, Hollis KC, Olivier J, Raju S. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg. 2007;46:979–990. doi: 10.1016/j.jvs.2007.06.046. [DOI] [PubMed] [Google Scholar]
- 54.Neglén P, Darcey R, Olivier J, Raju S. Bilateral stenting at the iliocaval confluence. J Vasc Surg. 2010;51:1457–1466. doi: 10.1016/j.jvs.2010.01.056. [DOI] [PubMed] [Google Scholar]
- 55.Le TB, Lee TK, Park KM, Jeon YS, Hong KC, Cho SG. Contralateral deep vein thrombosis after iliac vein stent placement in patients with May-Thurner syndrome. J Vasc Interv Radiol. 2018;29:774–780. doi: 10.1016/j.jvir.2018.01.771. [DOI] [PubMed] [Google Scholar]
- 56.Vedantham S, Thorpe PE, Cardella JF, Grassi CJ, Patel NH, Ferral H, et al. Quality improvement guidelines for the treatment of lower extremity deep vein thrombosis with use of endovascular thrombus removal. J Vasc Interv Radiol. 2009;20:S227–S239. doi: 10.1016/j.jvir.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 57.Mewissen MW, Seabrook GR, Meissner MH, Cynamon J, Labropoulos N, Haughton SH. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry. Radiology. 1999;211:39–49. doi: 10.1148/radiology.211.1.r99ap4739. [DOI] [PubMed] [Google Scholar]
- 58.Sharifi M, Mehdipour M, Bay C, Smith G, Sharifi J. Endovenous therapy for deep venous thrombosis: the TORPEDO trial. Catheter Cardiovasc Interv. 2010;76:316–325. doi: 10.1002/ccd.22638. [DOI] [PubMed] [Google Scholar]
- 59.Imanaka S, Aihara S, Yoshihara K, Kato A, Matsumoto K, Kudo S. Use of a temporary caval filter in a young man with pulmonary embolism to prevent migration of massive caval thrombus during an attempt of caval thrombolysis. J Atheroscler Thromb. 2000;6:18–21. doi: 10.5551/jat1994.6.18. [DOI] [PubMed] [Google Scholar]
- 60.Comerota AJ, Kearon C, Gu CS, Julian JA, Goldhaber SZ, Kahn SR, et al. Endovascular thrombus removal for acute iliofemoral deep vein thrombosis. Circulation. 2019;139:1162–1173. doi: 10.1161/CIRCULATIONAHA.118.037425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video clip for endovascular treatment of May-Thurner syndrome.
A 52-year-old woman presented with left leg swelling that started 3 days prior. Lower extremity CT angiography indicated May-Thurner syndrome (not shown). Endovascular thrombus removal was performed via the left popliteal vein approach. Pharmacomechanical catheter-directed thrombolysis was performed using a 5-F Omni Flush catheter (Angiodynamics) with a bolus injection of urokinase 200 K units. After a rest period of approximately 20 minutes, aspiration thrombectomy was performed using a large-bore catheter, 10-F Super Arrow-Flex (Teleflex), while manually applying negative pressure to the catheter using a 50-cc Luer-lock syringe. After thrombus removal, recanalization was established in the occluded segment of the left common iliac vein using a 5-F KMP Beacon® Tip catheter (Cook Medical) and a 0.036-inch Glidewire® hydrophilic coated guidewire (Terumo). Subsequently, angioplasty was performed using an 8 mm–10-cm balloon catheter (Mustang, Boston Scientific) for pre-dilatation, a 12 mm–10-cm stent (E Luminexx; Bard), and a 12 mm–8 cm balloon catheter (Mustang; Boston Scientific).