Abstract
Objective
To demonstrate the feasibility of percutaneous microwave ablation in desmoid fibromatosis with respect to tumor volume control and improvement in the quality of life.
Materials and Methods
Twelve microwave ablations were performed in 9 patients with a histological diagnosis of desmoid fibromatosis between January 2010 and January 2019. The study population included 6 female and 3 male, with an age range of 21–76 years (mean = 46.6 years; standard deviation [SD] = 19.3 years). The mean major axis of the tumors was 10.9 cm (SD = 5.2 cm) and mean lesion volume was 212.7 cm3 (SD = 213 cm3). Their anatomical distribution was as follows: 3 lesions in the thigh, 2 in the gluteus, 2 in the leg and 2 in the periscapular region. We evaluated the reduction in tumor volume and improvement in the quality of life based on the Eastern Cooperative Oncology Group (ECOG) scale.
Results
An average tumor volume reduction of 70.4% (SD = 24.9) was achieved, while the quality of life (ECOG scale) improved in 88.9% of patients.
Conclusion
Percutaneous microwave ablation may potentially be a safe, effective, and promising technique for controlling tumor volume and improving the quality of life in patients with desmoid fibromatosis.
Keywords: Desmoid; Fibromatosis, Aggressive; Ablation techniques
INTRODUCTION
Desmoid fibromatosis, also known as desmoid tumor and commonly referred to as aggressive fibromatosis, is a relatively rare mesenchymal neoplasm. It is categorized as an intermediate fibroblastic tumor according to the World Health Organization classification. Although it is considered to be a benign tumor, it can be locally aggressive with a high probability of recurrence after treatment [1,2,3,4,5].
Desmoid fibromatosis can occur in any anatomical location but is most common in the limbs and abdominal wall [1,2]. It accounts for 3% of all soft tissue tumors [1], predominantly affects female (80%) and occurs more frequently in the third and fourth decades of life [1,2]. In most cases, its presentation is sporadic with an unknown etiology [1,2,3]. Endocrine factors also play a role in the development and treatment of desmoid fibromatosis. Estrogen receptors have been identified in the lesions, albeit at low levels [3].
There are no universally accepted treatment protocols for desmoid fibromatosis [5,6,7,8], and its management requires a multidisciplinary approach. The treatment decision is based primarily on the growth of the lesion on more than three consecutive magnetic resonance imaging (MRI) controls, symptomatology, and location of the lesion. Various treatment strategies and modalities have been proposed, including expectant management, surgery, radiation therapy, medical therapies, and isolated limb perfusion. Other methods of achieving local control of the disease are currently being investigated, including cryoablation [9,10,11,12,13], radiofrequency ablation [14,15] and high-intensity focused ultrasound ablation [16,17,18]. These therapies are in the early stages of clinical trials and retrospective studies with limited sample sizes that lack comparison with alternative therapies are the best sources of currently available evidence. Microwave ablation has been widely used for the treatment of other tumors and represents a safe and rapid technique that can possibly ablate large areas of tissue, allowing for the removal of almost all types of tumors, irrespective of their nature. This advantage may be particularly useful for the treatment of tumors with adjacent heat dissipaters, such as bone and vascular structures [19].
The objective of this study was to demonstrate the feasibility of percutaneous microwave ablation in desmoid fibromatosis with respect to tumor volume control and improvement in the quality of life.
MATERIALS AND METHODS
This retrospective study was approved by the Ethics Committee of our institution (1380-N-18). We included patients with a biopsy-proven diagnosis of desmoid fibromatosis who were treated with percutaneous microwave ablation between January 2010 and January 2019. A total of 12 microwave ablations were performed in 9 patients, of whom 6 were female and 3 were male and their ages ranged between 21–76 years (mean = 46.6 years; standard deviation [SD] = 19.3 years). Patients were followed up clinically and using MRI for 6 months after the intervention. Tumor size (major axis of the tumor) and volume were measured on contrast-enhanced T1-weighted images by two musculoskeletal radiologists with 6 (radiologist 1) and 2 (radiologist 2) years of experience in soft tissue tumor imaging. Both radiologists independently measured the active foci of pre- and post-ablation fibromatosis. The major axis of the lesion was measured by the consensus of both radiologists. A slice-by-slice segmentation tool (Vue PACS Livewire, Carestream) was used to measure the volume. Active or growing areas may coexist with inactive or regression areas in desmoid fibromatosis. Active fibromatosis was defined as nodular tissue with relatively higher signal intensity than that of muscle on fat-suppressed T2-weighted sequences and contrast enhancement on T1-weighted sequences. Enhancement was defined as any increase in the signal intensity of the lesion between the pre- and post-contrast T1-weighted sequences, with or without fat suppression. On the other hand, the foci of inactive or regression fibromatosis were defined as areas of very low signal intensity (i.e., lower than that of muscle) on T2-weighted sequences with fat suppression and without enhancement on T1-weighted sequences. The segmentation process was performed only on post-contrast T1-weighted sequences to simplify the calculation of active tumor volume. The fibromatosis foci that showed contrast enhancement were manually drawn slice by slice (Supplementary Fig. 1).
The mean major axis of the tumors was 10.4 cm (SD = 5.7 cm). The mean volume of the lesions was 212.7 cm3 (SD = 213 cm3), and their anatomical distribution was as follows: 3 lesions were found in the thigh, 2 in the gluteus, 2 in the leg and 2 lesions were found in the periscapular region. The mean distance from the tumor to the skin was 10.3 mm (SD = 7.7 mm) and the proximity to large size nerves (brachial plexus, sciatic nerve, etc.) was 13.9 mm (SD = 22.1) (Tables 1, 2, Supplementary Table 1).
Table 1. Personal and Clinical Data of the Studied Patients.
| Sex, female:male | 6:3 |
| Age, years | Mean = 46.6; SD = 19.3; range = 18–76 |
| Length of major axis, cm | Mean = 10.9; SD = 5.2; range = 20.1–2.5 |
| Volume, cm3 | Mean = 212.7; SD = 213.0; range = 3.1–700.9 |
| Location | Thigh (n = 3), gluteus (n = 2), leg (n = 2), periscapular region (n = 2) |
SD = standard deviation
Table 2. Pre- and Post-Ablation Volume Measurement by Radiologist 1 and Radiologist 2.
| Patient | Pre-Treatment Volume (cm3) | Post-Treatment Volume (cm3) | ||
|---|---|---|---|---|
| Radiologist 1 | Radiologist 2 | Radiologist 1 | Radiologist 2 | |
| 1 | 70.4 | 68.1 | 44.8 | 42.2 |
| 2 | 96.6 | 89.2 | 15.6 | 17.3 |
| 3 | 300.4 | 305.7 | 221.6 | 214.1 |
| 4 | 41.5 | 42.8 | 0 | 0 |
| 5 | 700.9 | 686.3 | 257.3 | 265.9 |
| 6 | 293.0 | 286.4 | 56.9 | 58.7 |
| 7 | 132.8 | 126.1 | 41.6 | 39.8 |
| 8 | 295.2 | 286.9 | 70.9 | 73.2 |
| 9 | 3.3 | 3.1 | 0 | 0 |
The decision to perform percutaneous treatment was based on tumor growth as depicted by three consecutive MRI controls, the patient's symptoms (i.e., pain), and in accordance with the algorithm proposed by the European Organization for the Research and Treatment of Cancer (EORTC) (Supplementary Fig. 2). Surgery was dismissed because of the high anesthetic risk (American Society of Anesthesiologists-III or higher), possibility of considerable mutilation, and the high risk of vascular or nerve injury.
Technical Procedure
All patients underwent preoperative evaluation and provided written informed consent.
General anesthesia was administered in cases with a high level of operational difficulty arising from the tumor's location or large tumor volume. Local anesthesia or anesthetic blocks of the femoral and sciatic nerves were administered along with sedation for tumors located in the lower extremities or for small volume tumors.
The 12 procedures were performed by three different members of the Musculoskeletal Radiology Unit of our institution with 25, 6, and 2 years of experience in tumor ablation. Computed tomography (CT) was used for image guidance and two different CT scanners (LightSpeed VCT 64, General Electric Healthcare; Ingenuity Flex 32, Philips) were used for image acquisition. The initial objective of ablation was to achieve the greatest possible reduction in tumor volume without compromising the adjacent sensitive anatomical structures, especially the nerves. The target area was planned by drawing the regions of fibromatosis on a recent MRI scan (obtained less than two weeks prior to the intervention). Specifically, the contrast-enhanced T1-weighted sequences were evaluated to select the areas of active fibromatosis: the ablation schemes provided by the manufacturer were taken into account. Thus, the variations in time and power resulted in different oval areas of ablation, with subsequent variations in the diameters of the ablation sphere (e.g., from a 23-mm long axis and 17-mm short axis with 20 W for 3 minutes to a 50-mm long axis and 40-mm short axis with 60 W for 10 minutes, respectively). The procedural plan was saved digitally as key images in the Digital Imaging and Communication On Medicine (DICOM) format and subsequently transferred to the CT scanner used in the procedure. Pre-procedural CT examination was performed with an intravenous contrast agent in the portal venous phase for better visualization of the vascular structures and tumor. We marked the entry points on the patient's skin based on the CT images and pre-procedural plan. All procedures were performed under sterile conditions. Due to the consistency and hardness of desmoid fibromatosis, 100-mm long 13-G vertebroplasty needles (Optimed Medical Devices) were used as the introducer trocars. The vertebroplasty needles were progressively advanced with consecutive CT controls till the desired location was reached.
The HS Amica MW & RF generator (Mermaid Medical) microwave system, with an output power of up to 140 W at a continuous wave of 2450 MHz, was used in all cases. A 16-G caliber probe of 200-mm length was used to ensure entry through the vertebroplasty needle, and access to the deeper areas, respectively.
Once the tip of the probe was placed, the difference between the length of the introducer vertebroplasty needle (100 mm) and probe (200 mm) permitted the removal of the introducer, avoiding contact with the active tip of the probe; thus, preventing burns along its path. Once the procedure was completed, a control CT scan was performed for all cases to evaluate the possible complications. Patients were monitored at the day surgery hospital or reanimation unit after the procedure. The occurrence of any adverse events was recorded.
Clinical and Radiological Follow-Up
All patients were referred to the Orthopedic Surgery Tumor Unit for clinical and radiological follow-up. The quality of life before and after the procedure was evaluated using the Eastern Cooperative Oncology Group (ECOG) scale six months after the procedure. The most commonly reported standard follow-up MRI duration for desmoid fibromatosis is 6 months for the first three years and every year in case of lesion stability. The range of the first post-ablation follow-up MRI was 1–6 months and the mean was 3.7 months. The basic MRI protocols recommended for desmoid fibromatosis include T1-weighted and fluid-sensitive fat-suppressed sequences, both of which should be obtained parallel to the long axis of the tumor. T1-weighted and T2 fat-suppressed sequences are also performed in the axial plane at our institution to gain more anatomical information. The acquisition of T1-weighted sequences after intravenous contrast administration in two spatial planes is recommended for better identification of the active foci of fibromatosis [6,7].
The persistence of active fibromatosis foci was evaluated and compared with the pre-treatment MRI scan. The volume of the viable tumor lesion was measured on the pre- and post-treatment MRI scans. Control of the major axis of the lesion was not considered as a criterion for treatment response due to the irregular morphology of this lesion and the fact that this parameter was not useful for differentiating sbetween active and non-active tumor areas [9,10,11,12,13,14,15,16,17,18].
RESULTS
Before intervention, six patients had ECOG scores of 2 (ambulatory and capable of all selfcare but unable to perform any work activities: up and about more than 50% of waking hours) and three patients had ECOG scores of 1 (restriction in physically strenuous activity but ambulatory and able to perform work of a light or sedentary nature). After intervention, 8 out of 9 patients (88.9%) showed improvement in the ECOG scale scores. Only one patient with an ECOG score of 2 showed no improvement after treatment. The mean pre-procedure active tumor volume was 212.7 cm3 with a SD of 213 cm3. The mean post-procedure tumor volume was 78.9 cm3 with a SD of 95 cm3. The mean tumor volume reduction was 70.4% with a SD of 24.9% from the initial volume; 100% reduction in the active foci was observed in 2 patients. We calculated the intraclass correlation coefficient (ICC) to assess the degree of agreement in the volume measurement between both radiologists. The ICC values for the pre- and post-treatment volume measurement were 0.99 each, an excellent degree of correlation.
A second round of treatment was deemed to be necessary in three patients; one patient required further reduction of tumor volume (which was performed three months later) and the other two patients needed a treatment for ecurred tumor (performed two and three years after the first ablation, respectively), thus bringing the number of total interventions to 12. The following adverse effects occurred during the 12 procedures. One patient developed a post-operative hematoma (grade 2 of the Cardiovascular and Interventional Radiological Society of Europe classification system for complications). Another patient developed a supra-scapular and axillary nerve lesion (grade 4) that caused the inability to raise the arm and showed compatible findings at electromyographic study (Supplementary Table 1, Figs. 1, 2, Supplementary Fig. 3).
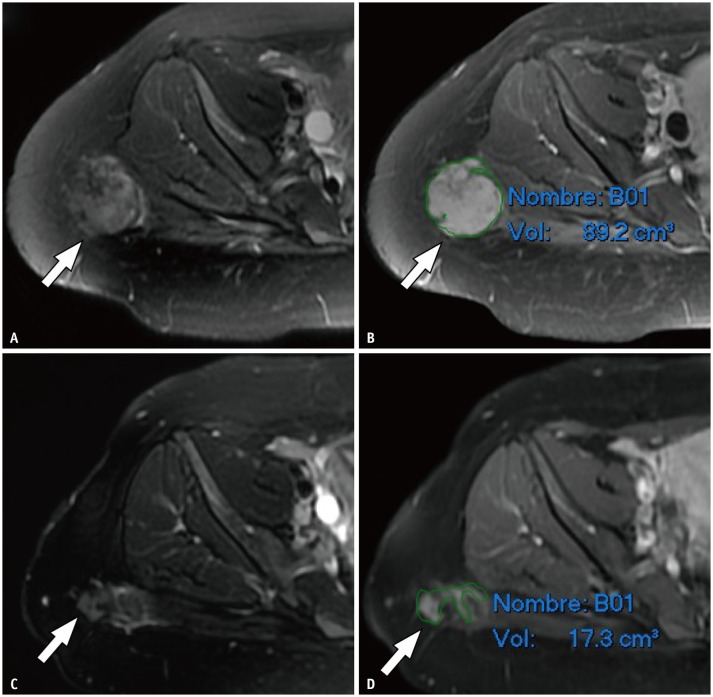
Fig. 1. Magnetic resonance imaging before (A, B) and after (C, D) ablation in a 26-year-old female with desmoid tumor in gluteal region.
A mass with a histological diagnosis of aggressive fibromatosis is observed in the right gluteal region (arrows) on fat-suppressed T2-weighted images (A, C) and contrast-enhanced fat-suppressed T1-weighted images (B, D). Volumetric calculation using a slice-by-slice segmentation allowed the determination of the decrease in tumor volume.
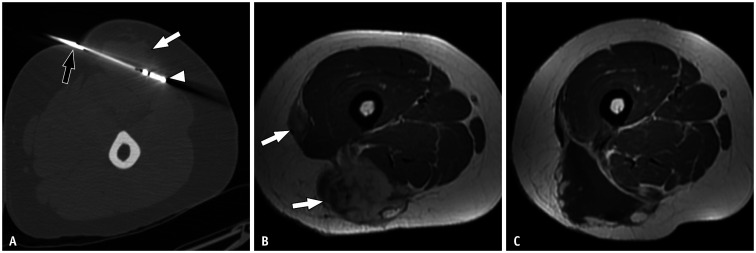
Fig. 2. Ablation technique and outcome in a 33-year-old female with desmoid tumor in the thigh.
A. Computed tomography-guided ablation in prone position. The vertebroplasty needle (black arrow) and microwave probe (arrowhead) are placed inside the tumor (white arrow). B. The axial contrast-enhanced T1-weighted image obtained in supine position before treatment reveals a multifocal desmoid tumor on the posterior lateral aspect of the thigh (arrows). C. Axial contrast-enhanced T1-weighted image obtained in supine position after ablation with microwaves. No enhancement foci that might suggest tumor remnants can be observed at the site of ablation.
DISCUSSION
The treatment of desmoid fibromatosis is challenging at present. Some algorithms have been proposed by scientific societies, such as the EORTC algorithm for bone and soft tissue sarcomas. Management guidelines that prioritize an expectant attitude have been established on the basis of this consensus. The treatment plan may be altered in the event of progression. Historically, surgery has been the initial treatment of choice in case of lesions in the limb; however, expectant management is currently being prioritized due to the high rates of incomplete tumor resection or recurrence after surgery. Treatment should be considered if the “wait-and-see” approach fails, the patient is highly symptomatic, there is a risk of neurovascular involvement, or the esthetic defect is significant [6,7,20]. If surgery is expected to be considerably mutilating, alternative medical therapies can be used: surgery and other investigational therapeutic modalities such as percutaneous ablative treatments form the second line of treatment. These medical therapies include isolated limb perfusion (especially in multifocal disease and involvement of the feet or hands), radiotherapy, chemotherapy and combined hormonal therapy. Finally, the so-called investigational therapies that are also alternative methods for achieving local control of the tumor include cryoablation, radiofrequency ablation, and high-intensity focused ultrasound [9,10,11,12,13,14,15,16,17,18,19]. Cryoablation has been shown to be effective in extra-abdominal fibromatosis [9,10,11,12,13]. Bouhamama et al. [13] conducted the largest case series study on cryoablation: they treated 34 patients with desmoid fibromatosis and achieved resolution in up to 40% of cases and volume reduction in more than 95% of patients. Ablative therapy is another local high-intensity focused ultrasound technique that is usually guided by MRI [16,17,18]. In this procedure, MRI is used to define the area to be treated and the tissues to be avoided, allowing minimally invasive ablation without affecting the surrounding tissues. Bucknor and Rieke [17] described a case series of seven patients with desmoid fibromatosis in the thigh treated with ultrasound, in which a reduction in tumor volume was achieved with varying degrees of success. Radiofrequency ablation is another percutaneous modality that has been used for local tumor control. A few published case reports and case series, the largest of which included five patients, obtained satisfactory results (cure or reduction of the tumor) with radiofrequency ablation [14].
Based on current evidence, cryoablation seems to be the most promising percutaneous treatment modality for local control of desmoid fibromatosis. To date, there is a lack of studies comparing cryoablation with other ablative techniques for desmoid fibromatosis. Recent meta-analyses and clinical trials that compared cryoablation and microwave ablation for small kidney tumors reported no significant differences in the safety or efficacy of the two procedures [21,22]. Niu et al. [23] compared microwave ablation and cryoablation in a non-human liver model, concluding that microwave ablation may be appropriate for larger volumes in simple anatomical structures, while cryoablation may be more suitable for tumors in complex anatomical regions or near sensitive organs. Studies have compared the economic cost of the principal ablative techniques, estimating that cryoablation would be more expensive by 2000 dollars compared to microwave or radiofrequency ablation [24].
Microwaves are non-ionizing waves that lie within the electromagnetic spectrum whose frequency ranges from 900 to 2450 MHz. When microwaves are applied to tissue through an antenna connected to a generator, the polar molecules (mainly water) in the field of action of the antenna are forced to continuously realign themselves with the oscillating electric field generated by the microwaves, which leads to an increase in their kinetic energy and therefore an increase in tissue temperature. Microwaves do not depend on electrical conduction or resistance. Consequently, they can propagate through several tissues, irrespective of their conductivity or impedance and create large areas of ablation. Microwave ablation has several advantages over other techniques, especially its rapidity of action in large areas and its ability to act in high-impedance areas such as lung tissue, carbonized areas, or tissues with adjacent heat sinks [25].
To date, there are no published series or even unique reports suggesting the utility of percutaneous microwave therapy in desmoid fibrosis. We opine that microwave ablation can be a suitable technique because of its proven advantages in other tumors, especially visceral lesions. These include the speed of ablation, possibility of covering large tumor volumes, and therapeutic use of heat, which allow for the treatment of almost all tumor types [16].
Our results suggest that microwave ablation is an effective technique for controlling the tumor volume of desmoid fibromatosis and improving the quality of life. The mean pre-treatment volume of active disease foci in our series was 210.6 cm3, which was significantly higher than that of other published cryoablation studies. We believe that we could have obtained better results with respect to total control of desmoid fibromatosis, if our study sample had contained smaller tumors.
To the best of our knowledge, this is the largest case series study on desmoid fibromatosis treated by microwave ablation. The limitations of this study are its retrospective design, small sample size (and hence number of procedures), non-uniform follow-up durations, heterogeneity of the study population, and lack of comparison with other percutaneous ablative therapies or other treatments. Comparative studies, especially with cryoablation, and prospective studies are needed to assess the actual efficacy of this technique.
Percutaneous microwave ablation could potentially be a safe, effective, and promising technique for controlling tumor volume and improving the quality of life in patients with desmoid fibromatosis. It may especially be useful after failure of other first-line therapies, if cryoablation, which is less accessible and more expensive, is not available, or in large tumors where the application of radiofrequency ablation could be more technically challenging.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: all authors.
- Data curation: Alberto Martínez-Martínez, Jade García-Espinosa.
- Formal analysis: all authors.
- Investigation: all authors.
- Methodology: Alberto Martínez-Martínez, Jade García-Espinosa, Fernando Ruiz Santiago.
- Project administration: Alberto Martínez-Martínez.
- Resources: Alberto Martínez-Martínez, Jade García-Espinosa, Fernando Ruiz Santiago.
- Supervision: Alberto Martínez-Martínez.
- Validation: all authors.
- Writing—original draft: all authors.
- Writing—review & editing: all authors.
Supplement
The Supplement is available with this article at https://doi.org/10.3348/kjr.2020.0768
Patients Characteristics
Fat-suppressed T2-weighted (A), T1-weighted (B) and contrast-enhanced T1-weighted (C) magnetic resonance images of an 18-year-old female with desmoid tumor in the right thigh areas of relatively lower intensity than that of muscle can be seen on T2 and T1-weighted sequences without contrast enhancement within the tumor, which were considered to be the foci of inactive fibromatosis (arrows) and were excluded from the calculation of the tumor volume.
Consensus algorithm.
Ablation technique and outcome in a 76-year-old male with desmoid tumor of the periscapular region.
References
- 1.Otero S, Moskovic EC, Strauss DC, Benson C, Miah AB, Thway K, et al. Desmoid-type fibromatosis. Clin Radiol. 2015;70:1038–1045. doi: 10.1016/j.crad.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Ng E, Tandon AA, Ho BC, Chong BK. Characterising benign fibrous soft-tissue tumours in adults: why is it so difficult and what do we need to know? Clin Radiol. 2015;70:684–697. doi: 10.1016/j.crad.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Janinis J, Patriki M, Vini L, Aravantinos G, Whelan JS. The pharmacological treatment of aggressive fibromatosis: a systematic review. Ann Oncol. 2003;14:181–190. doi: 10.1093/annonc/mdg064. [DOI] [PubMed] [Google Scholar]
- 4.Fletcher C, Bridge J, Antonescu C, Mertens F. WHO classification of tumours: soft tissue and bone tumours. 5th. ed. Lyon: Editorial World Health Organization; 2020. [Google Scholar]
- 5.Smith K, Desai J, Lazarakis S, Gyorki D. Systematic review of clinical outcomes following various treatment options for patients with extraabdominal desmoid tumors. Ann Surg Oncol. 2018;25:1544–1554. doi: 10.1245/s10434-018-6408-7. [DOI] [PubMed] [Google Scholar]
- 6.Kasper B, Baumgarten C, Bonvalot S, Haas R, Haller F, Hohenberger P, et al. Management of sporadic desmoidtype fibromatosis: a European consensus approach based on patients' and professionals' expertise-a sarcoma patients EuroNet and European Organisation for Research and Treatment of Cancer/Soft Tissue and Bone Sarcoma Group initiative. Eur J Cancer. 2015;51:127–136. doi: 10.1016/j.ejca.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Kasper B, Baumgarten C, Garcia J, Bonvalot S, Haas R, Haller F, et al. An update on the management of sporadic desmoid-type fibromatosis: a European Consensus Initiative between Sarcoma PAtients EuroNet (SPAEN) and European Organization for Research and Treatment of Cancer (EORTC)/Soft Tissue and Bone Sarcoma Group (STBSG) Ann Oncol. 2017;28:2399–2408. doi: 10.1093/annonc/mdx323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martínez Trufero J, Pajares Bernad I, Torres Ramón I, Hernando Cubero J, Pazo Cid R. Desmoid-type fibromatosis: who, when, and how to treat. Curr Treat Options Oncol. 2017;18:29. doi: 10.1007/s11864-017-0474-0. [DOI] [PubMed] [Google Scholar]
- 9.Cornelis F, Havez M, Lippa N, Al-Ammari S, Verdier D, Carteret T, et al. Radiologically guided percutaneous cryotherapy for soft tissue tumours: a promising treatment. Diagn Interv Imaging. 2013;94:364–370. doi: 10.1016/j.diii.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Kujak JL, Liu PT, Johnson GB, Callstrom MR. Early experience with percutaneous cryoablation of extra-abdominal desmoid tumors. Skeletal Radiol. 2010;39:175–182. doi: 10.1007/s00256-009-0801-z. [DOI] [PubMed] [Google Scholar]
- 11.Schmitz JJ, Schmit GD, Atwell TD, Callstrom MR, Kurup AN, Weisbrod AJ, et al. Percutaneous cryoablation of extraabdominal desmoid tumors: a 10-year experience. AJR Am J Roentgenol. 2016;207:190–195. doi: 10.2214/AJR.15.14391. [DOI] [PubMed] [Google Scholar]
- 12.Redifer Tremblay K, Lea WB, Neilson JC, King DM, Tutton SM. Percutaneous cryoablation for the treatment of extra-abdominal desmoid tumors. J Surg Oncol. 2019;120:366–375. doi: 10.1002/jso.25597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouhamama A, Lame F, Mastier C, Cuinet M, Thibaut A, Beji H, et al. Local control and analgesic efficacy of percutaneous cryoablation for desmoid tumors. Cardiovasc Intervent Radiol. 2020;43:110–119. doi: 10.1007/s00270-019-02323-5. [DOI] [PubMed] [Google Scholar]
- 14.Ilaslan H, Schils J, Joyce M, Marks K, Sundaram M. Radiofrequency ablation: another treatment option for local control of desmoid tumors. Skeletal Radiol. 2010;39:169–173. doi: 10.1007/s00256-009-0807-6. [DOI] [PubMed] [Google Scholar]
- 15.Cobianchi L, Ravetta V, Viera FT, Filisetti C, Siri B, Segalini E, et al. The challenge of extraabdominal desmoid tumour management in patients with Gardner's syndrome: radiofrequency ablation, a promising option. World J Surg Oncol. 2014;12:361. doi: 10.1186/1477-7819-12-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avedian RS, Bitton R, Gold G, Butts-Pauly K, Ghanouni P. Is MR-guided high-intensity focused ultrasound a feasible treatment modality for desmoid tumors? Clin Orthop Relat Res. 2016;474:697–704. doi: 10.1007/s11999-015-4364-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bucknor MD, Rieke V. MRgFUS for desmoid tumors within the thigh: early clinical experiences. J Ther Ultrasound. 2017;5:4. doi: 10.1186/s40349-017-0081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Wang W, Tang J. Ultrasound-guided high intensity focused ultrasound treatment for extra-abdominal desmoid tumours: preliminary results. Int J Hyperthermia. 2011;27:648–653. doi: 10.3109/02656736.2011.597047. [DOI] [PubMed] [Google Scholar]
- 19.Hinshaw JL, Lubner MG, Ziemlewicz TJ, Lee FT, Jr, Brace CL. Percutaneous tumor ablation tools: microwave, radiofrequency, or cryoablation--what should you use and why? Radiographics. 2014;34:1344–1362. doi: 10.1148/rg.345140054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheth PJ, Del Moral S, Wilky BA, Trent JC, Cohen J, Rosenberg AE, et al. Desmoid fibromatosis: MRI features of response to systemic therapy. Skeletal Radiol. 2016;45:1365–1373. doi: 10.1007/s00256-016-2439-y. [DOI] [PubMed] [Google Scholar]
- 21.Martin J, Athreya S. Meta-analysis of cryoablation versus microwave ablation for small renal masses: is there a difference in outcome? Diagn Interv Radiol. 2013;19:501–507. doi: 10.5152/dir.2013.13070. [DOI] [PubMed] [Google Scholar]
- 22.De Cobelli F, Papa M, Panzeri M, Colombo M, Steidler S, Ambrosi A, et al. Percutaneous microwave ablation versus cryoablation in the treatment of T1a renal tumors. Cardiovasc Intervent Radiol. 2020;43:76–83. doi: 10.1007/s00270-019-02313-7. [DOI] [PubMed] [Google Scholar]
- 23.Niu L, Li J, Zeng J, Zhou L, Wang S, Zhou X, et al. Comparison of percutaneous cryoablation with microwave ablation in a porcine liver model. Cryobiology. 2014;68:194–199. doi: 10.1016/j.cryobiol.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Astani SA, Brown ML, Steusloff K. Comparison of procedure costs of various percutaneous tumor ablation modalities. Radiol Manage. 2014;36:12–17. [PubMed] [Google Scholar]
- 25.Lubner MG, Brace CL, Hinshaw JL, Lee FT., Jr Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol. 2010;21:S192–S203. doi: 10.1016/j.jvir.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patients Characteristics
Fat-suppressed T2-weighted (A), T1-weighted (B) and contrast-enhanced T1-weighted (C) magnetic resonance images of an 18-year-old female with desmoid tumor in the right thigh areas of relatively lower intensity than that of muscle can be seen on T2 and T1-weighted sequences without contrast enhancement within the tumor, which were considered to be the foci of inactive fibromatosis (arrows) and were excluded from the calculation of the tumor volume.
Consensus algorithm.
Ablation technique and outcome in a 76-year-old male with desmoid tumor of the periscapular region.