Abstract
Objective
It is uncertain why a b-value range of 1500–2000 s/mm2 is optimal. This study was aimed at qualitatively and quantitatively analyzing the optimal b-value range of synthetic diffusion-weighted imaging (sDWI) for evaluating prostatic index lesions.
Materials and Methods
This retrospective study included 92 patients who underwent DWI and targeted biopsy for magnetic resonance imaging (MRI)-suggested index lesions. We generated sDWI at a b-value range of 1000–3000 s/mm2 using dedicated software and true DWI data at b-values of 0, 100, and 1000 s/mm2. We hypothesized that lesion conspicuity would be best when the background (i.e., MRI-suggested benign prostatic [bP] and periprostatic [pP] regions) signal intensity (SI) is suppressed and becomes homogeneous. To prove this hypothesis, we performed both qualitative and quantitative analyses. For qualitative analysis, two independent readers analyzed the b-value showing the best visual conspicuity of an MRI-suggested index lesion. For quantitative analysis, the readers assessed the b-value showing the same bP and pP region SI. The 95% confidence interval (CI) or interquartile range of qualitatively and quantitatively selected optimal b-values was assessed, and the mean difference between qualitatively and quantitatively selected b-values was investigated.
Results
The 95% CIs of optimal b-values from qualitative and quantitative analyses were 1761–1805 s/mm2 and 1640–1771 s/mm2 (median, 1790 s/mm2 vs. 1705 s/mm2; p = 0.003) for reader 1, and 1835–1895 s/mm2 and 1705–1841 s/mm2 (median, 1872 s/mm2 vs. 1763 s/mm2; p = 0.022) for reader 2, respectively. Interquartile ranges of qualitatively and quantitatively selected optimal b-values were 1735–1873 s/mm2 and 1573–1867 s/mm2 for reader 1, and 1775–1945 s/mm2 and 1591–1955 s/mm2 for reader 2, respectively. Bland–Altman plots consistently demonstrated a mean difference of less than 100 s/mm2 between qualitatively and quantitatively selected optimal b-values.
Conclusion
b-value range showing a homogeneous background signal may be optimal for evaluating prostatic index lesions on sDWI. Our qualitative and quantitative data consistently recommend b-values of 1500–2000 s/mm2.
Keywords: Prostate, Magnetic resonance imaging, Diffusion, Neoplasm
INTRODUCTION
Prostate Imaging-Reporting and Data System (PI-RADS) currently recommends use of high b-values, of 1400 s/mm2 or greater, to detect clinically significant prostate cancer (csPCa) with diffusion-weighted imaging (DWI) [1,2]. Either actually acquired or calculated high b-value DWI from low (b-value: 0–100 s/mm2) and intermediate b-value (b-value: 800–1000 s/mm2) data is acceptable for evaluating the prostate [2]. Different vendors refer to calculated high b-value DWI using different terms, including calculated, computed, or synthetic DWI [3]. We used the term synthetic DWI (sDWI) in this study. sDWI is advantageous in terms of the contrast-to-noise ratio by suppressing the background noise and maintaining lesion signal intensity (SI) [3,4].
In general, SI of biological tissues on DWI decreases as the b-value increases. Meanwhile, signal decay of benign prostatic (bP) tissues is greater than that of PCa with increasing b-values [3,5,6]. Accordingly, the SI ratio of PCa to bP tissues on sDWI increases with increasing b-values [7]. Therefore, theoretically, lesion conspicuity should be greater at higher b-values. However, Rosenkrantz et al. [5] reported that the sensitivity of sDWI for clinically significant PCa is lower at b-value of greater than 2000–3000 s/mm2 because of the reduced anatomical clarity at higher b-values. Thus, a b-value range of 1500–2000 s/mm2 may be appropriate for prostatic sDWI [3,5,8,9].
Nevertheless, understanding of optimal b-value range and appropriate explanations for it are currently insufficient. A majority of previous studies have investigated prostatic sDWI at discontinuous b-value points (i.e., b-values of 1500, 2000, 2500, or 3000 s/mm2) [4,5,7,8,9], thus, the information of sDWI between the analyzed b-value intervals could not be evaluated. In this study, we hypothesized that PCa would be best visualized when background (i.e., magnetic resonance imaging [MRI]-suggested bP and periprostatic [pP] regions) signals are suppressed and become homogeneous. To prove this hypothesis, we analyzed two types of b-values: 1) visually selected b-value with best lesion conspicuity (i.e., qualitative analysis); and 2) b-value showing same SI between bP and pP regions (i.e., quantitative analysis). Then, we estimated the difference between the two b-values. The purpose of this study was to qualitatively and quantitatively analyze the optimal b-value range of sDWI for evaluating prostatic index lesions.
MATERIALS AND METHODS
Study Patients
The Institutional Review Board approved this retrospective study, and the requirement for informed consent was waived (IRB No. 2019-12-146). Between January and March 2019, we used the institutional database to screen a consecutive series of 109 patients who had undergone prebiopsy prostate MRI and transrectal ultrasound (TRUS)-guided prostate biopsy (Fig. 1). Of the 109 patients, 17 were excluded due to the following reasons: 1) lack of targeted biopsy for the prostate (n = 13); 2) failure to generate sDWI because the transmission of true DWI data to the software was technically impossible (n = 3); and 3) severe artifact of DWI (n = 1). Therefore, a total of 92 patients (median age, 66.5 years; interquartile range [IQR], 60.0–72.5 years), who had sDWI data and histologic proof from TRUS-guided targeted biopsy for the MRI-suggested index lesion were ultimately included. In these study patients, systematic biopsy, including the contralateral prostatic lobe of the targeted biopsy, was also conducted in the same session as the targeted biopsy (i.e., combined biopsy).
Fig. 1. Flowchart of study patients.
DWI = diffusion-weighted imaging, MRI = magnetic resonance imaging, sDWI = synthetic DWI, TRUS = transrectal ultrasound
DWI Scanning and sDWI Generation
In keeping with the PI-RADS recommendation [1], prostate DWI was performed using a 3T MRI scanner (Intera Archieva, Philips Medical Systems) as follows: imaging direction of axial plane; repetition time greater than 3000 ms; echo time less than 90 ms; slice thickness of 3 mm; field of view of 200 mm; in plane dimension less than 2.5 mm for both phase and frequency directions; and b-values of 0, 100, 1000, and 1500 s/mm2.
We generated sDWI data using non-commercial sDWI analysis software and actual DWI data of b-values 0, 100, and 1000 s/mm2. The software allowed us to synthesize continuous DW images from b-values of 1000 s/mm2 to higher (3000 s/mm2), based on the previously reported algorithm [10,11]. We could also evaluate synthetic DW images at a particular b-value point. The software also provided SI ratio curves between two different regions (i.e., a curve of SI ratio between two different regions of interest [ROIs]), according to the b-value.
Image Analysis
In this study, we assumed that lesion conspicuity would be best when background signals are suppressed and become homogeneous. To prove this hypothesis, we defined a quantitatively selected optimal b-value as the b-value showing same SI between MRI-suggested bP and pP regions on sDWI. We compared the visually selected optimal b-value with the quantitatively selected optimal b-value. We performed qualitative and quantitative image analyses using the dedicated software. For study patients, combined biopsy, comprising targeted biopsy and systematic biopsy, was performed. However, because this research issue was closely related to lesion visibility on sDWI, a lesion-based analysis was required. Therefore, we evaluated only the MRI-suggested index lesion pathologically confirmed by targeted biopsy.
First, the qualitative analysis was performed by two independent readers (reader 1, a faculty of genitourinary radiology with 8 years of experience in prostate MRI; reader 2, a 3rd year radiology resident with experience interpreting PI-RADS of more than 100 cases). To avoid possible discrepancies between histopathologic and MRI-suggested index lesions, the only information provided to the readers was the location of the index lesion based on 12-sector of the prostate. The MRI-suggested index lesion was defined as the prostatic region showing the highest PI-RADS category, where targeted biopsy was performed by one of three radiologists with more than 7 years of experience in prostate MRI interpretation and biopsy. However, the two readers were not provided with this clinical and pathological information. The readers qualitatively selected the b-value that allowed for the best lesion conspicuity for the MRI-suggested index lesion. Continuous sDWI could be evaluated by dragging the b-value control bar in the range of 1000–3000 s/mm2. For each patient, the window width and level were set on sDWI at a b-value of 1000 s/mm2 according to each reader's preference for image interpretation. After this initial setting, the window width or level was not adjusted further during the qualitative analysis for the patient.
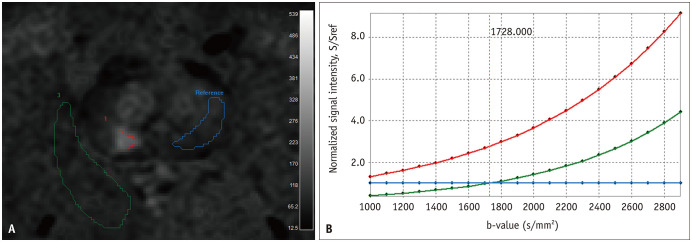
The two independent readers then performed the quantitative analysis (Fig. 2). The readers manually drew three ROIs on sDWI, one each for the MRI-suggested index lesion and bP and pP regions on sDWI. The MRI-suggested bP region was measured in the contralateral peripheral zone (PZ) or transition zone (TZ), depending on the zonal location of the MRI-suggested index lesion (i.e., when the index legion was located in the PZ, the bP region was measured in the contralateral PZ) [12,13,14]. Since the lesion visibility could be closely related to the relative signal difference between the lesion and the benign prostatic region, we measured the SI for the part showing benign MRI findings on the contralateral side of the prostate. Thus, efforts were made to draw ROIs in the contralateral PZ or TZ area with less than moderate diffusion restriction for measuring bP regions. The pP region was measured in the ipsilateral pP region, adjacent to the MRI-suggested index lesion. The rectum and the pelvic bone were excluded from pP region measurement. We drew the ROI to include as large an area of each region as possible. The reference for the SI ratio was the bP region. Thus, we could generate three types of SI ratio curves, as follows: 1) SI ratio curve of MRI-suggested index lesion/bP region; 2) SI ratio curve of pP/bP regions; and 3) SI ratio curve of bP/bP regions. The SI ratio curve of bP/bP regions was a horizontal line with the ratio value of 1. We chose the b-value at the intersection of curves 2) and 3), which means that the averaged SI of both bP and pP regions became the same (i.e., the background signal was homogenized).
Fig. 2. An example showing quantitative analysis in a 73-year-old man with International Society of Urological Pathology grade 3 prostate cancer of right PZ as confirmed by targeted biopsy.
A. Three ROIs were manually drawn: focal cancerous area of diffusion restriction (red ROI), contralateral PZ (blue ROI), and ipsilateral pP region (green ROI). B. From the three ROIs, three types of signal intensity ratio curves were generated with signal intensity of contralateral PZ as the reference (red = cancer/contralateral PZ; green = ipsilateral pP region/contralateral PZ; and blue = contralateral PZ/contralateral PZ). The green and blue curves crossed at b-value of 1728 s/mm2, which means that signals of contralateral PZ and ipsilateral pP region are similar at this b-value (i.e., homogeneous background signal intensity). This b-value was regarded as the quantitatively analyzed optimal b-value of synthetic diffusion-weighted imaging for evaluating the MRI-suggested prostatic index lesion in this patient. pP = periprostatic, PZ = peripheral zone, ROI = region of interest
The formal pathological report for prostatic biopsy specimens was our reference standard. For the targeted or systematic biopsy-proven PCa, the tumor grade (i.e., Gleason score [GS]) was assigned to the corresponding International Society of Urological Pathology (ISUP) grade per patient as follows: grade 1 = GS 3 + 3; grade 2 = GS 3 + 4; grade 3 = GS 4 + 3; grade 4 = GS 8; and grade 5 = GS 9–10 [15]. In this study, we defined clinically significant PCa as ISUP grade 2 or greater.
Statistical Analyses
Due to a lack of well-established methods for setting the optimal b-value range, we analyzed various b-value intervals, as follows: 1) 95% confidence interval (CI) of median of qualitatively or quantitatively selected optimal b-values; and 2) various b-value ranges according to different percentiles (i.e., 5th–95th percentile; 10th–90th percentile; or 25th–75th percentile [IQR]). We also sub-analyzed 95% CI of median b-value according to the location of the MRI-suggested index lesion or presence/absence of csPCa. Because the MRI-suggested bP region might have a risk of MRI-invisible PCa, the 95% CI and IQR of optimal b-values were also analyzed for patients without PCa detected by systematic biopsy of the contralateral lobe of the target biopsy site.
We used the Wilcoxon test to compare the qualitatively and quantitatively selected optimal b-values or ROI sizes for each region. We analyzed Bland–Altman plots to estimate the mean difference between qualitatively and quantitatively selected optimal b-values. We also assessed qualitatively or quantitatively selected optimal b-values and their mean differences according to the zonal location of the MRI-suggested index lesion or the presence/absence of csPCa.
We utilized MedCalc version 19.1 (MedCalc Software) for statistical analyses. A p value < 0.05 was considered statistically significant.
RESULTS
Baseline Characteristics of Study Patients
Table 1 summarizes the baseline characteristics of study patients. The proportion of zonal location of the MRI-suggested index lesion on MRI was 73.9% (68/92) for the PZ and 26.1% (24/92) for the TZ. The proportion of targeted biopsy-proven PCa was 46.7% (43/92) for any PCa and 40.2% (37/92) for csPCa.
Table 1. Baseline Characteristics of the Study Patients.
| Parameter | Value |
|---|---|
| Age (year) | Median, 66.5 (IQR, 60.0–72.5) |
| PSA (ng/dL) | Median, 5.0 (IQR, 3.8–8.3) |
| Interval between MRI and biopsy (day) | Median, 44.5 (IQR, 24.0–69.5) |
| Core number of targeted biopsy (n) | Median, 3.0 (IQR, 2.0–4.0) |
| Zonal location (%) | |
| PZ | 73.9 (68/92) |
| TZ | 26.1 (24/92) |
| Cancer detection rate of targeted biopsy (%) | |
| Any Pca | 46.7 (43/92) |
| csPCa | 40.2 (37/92) |
| ISUP grade (%) | |
| 1 | 14.0 (6/43) |
| 2 | 44.2 (19/43) |
| 3 | 16.3 (7/43) |
| 4 | 14.0 (6/43) |
| 5 | 11.6 (5/43) |
csPCa = clinically significant PCa, IQR = interquartile range, ISUP = International Society of Urological Pathology, MRI = magnetic resonance imaging, PCa = prostate cancer, PSA = prostate-specific antigen, PZ = peripheral zone, TZ= transition zone
The medians of ROI sizes between readers 1 and 2 were 32.3 mm2 (IQR, 22.6–47.2 mm2) and 39.9 mm2 (IQR, 27.5–65.2 mm2) for the MRI-suggested index lesion (p < 0.001), respectively; 88.1 mm2 (IQR, 63.8–112.8 mm2) and 87.4 mm2 (IQR, 61.1–125.6 mm2) for the bP region (p = 0.825), respectively; and 261.3 mm2 (IQR, 196.4–337.4 mm2) and 287.0 mm2 (IQR, 194.0–385.4 mm2) for the pP region (p = 0.070), respectively.
Optimal b-Value Range from Quantitative or Qualitative Analyses
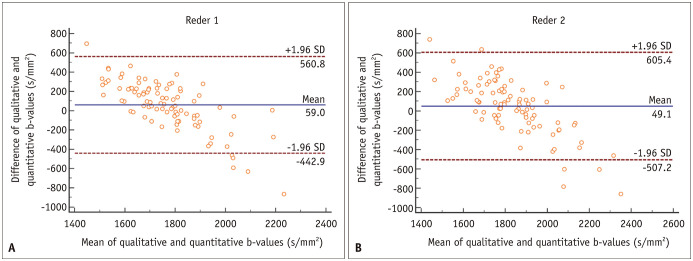
For reader 1, the 95% CI of optimal b-value was 1761–1805 s/mm2 (median, 1790 s/mm2) from the qualitative analysis and 1640–1771 s/mm2 (median, 1705 s/mm2) from the quantitative analysis (p = 0.003). For reader 2, the 95% CI of optimal b-value was 1835–1895 s/mm2 (median, 1872 s/mm2) from the qualitative analysis and 1705–1841 s/mm2 (median, 1763 s/mm2) from the quantitative analysis (p = 0.022). In addition, the IQRs (i.e., 25th–75th percentile range) of both qualitatively and quantitatively selected optimal b-values were within a range of 1500–2000 s/mm2 (Table 2). Bland–Altman plots consistently demonstrated a mean difference of less than 100 s/mm2 between qualitatively and quantitatively selected optimal b-values (Fig. 3).
Table 2. Qualitatively or Quantitatively Selected Optimal b-Value Ranges according to the Percentile of Subjects.
| Percentile | Range of b-Value | |
|---|---|---|
| Qualitative | Quantitative | |
| Reader 1 | ||
| 5th–95th | 1645–1995 | 1377–2273 |
| 10th–90th | 1669–1927 | 1407–2124 |
| 25th–75th (interquartile range) | 1735–1873 | 1573–1867 |
| Reader 2 | ||
| 5th–95th | 1655–2054 | 1397–2379 |
| 10th–90th | 1668–1993 | 1485–2210 |
| 25th–75th (interquartile range) | 1775–1945 | 1591–1955 |
The unit of all b-value data are s/mm2.
Fig. 3. Bland-Altman plots showing the difference between qualitatively and quantitatively selected optimal b-values.
A. The mean difference was 59.0 s/mm2 for reader 1. B. 49.1 s/mm2 for reader 2. SD = standard deviation
In this study, 21 of 92 patients (22.8%; ISUP grade of 1, n = 13; ISUP grade of 2 or greater, n = 8) had systematic biopsy-proven PCa in the contralateral lobe of the targeted biopsy site. For the remaining 71 patients with no systematic biopsy-proven PCa in the contralateral lobe, the 95% CIs of qualitatively and quantitatively selected optimal b-values were 1755–1801 s/mm2 (median, 1775 s/mm2) and 1640–1774 s/mm2 (median, 1713 s/mm2) for reader 1 (p = 0.110), respectively, and 1808–1885 s/mm2 (median, 1850 s/mm2) and 1708–1856 s/mm2 (median, 1761 s/mm2) for reader 2 (p = 0.119), respectively. The IQRs of qualitatively or quantitatively selected optimal b-values were 1735–1825 s/mm2 and 1580–1903 s/mm2 for reader 1, respectively, and 1758–1918 s/mm2 and 1625–1957 s/mm2 for reader 2, respectively. For this subgroup, Bland–Altman plots analyzed by the two readers demonstrated a mean difference of less than 30 s/mm2 between qualitatively and quantitatively selected optimal b-values (25.8 s/mm2 for reader 1; 24.2 s/mm2 for reader 2).
Sub-Analyses according to the Zonal Location of the MRI-Suggested Index Lesion or Presence of Clinically Significant Prostate Cancer
In terms of the zonal location of an MRI-suggested index lesion, all 95% CIs of the optimal b-value were within a range of 1500–2000 s/mm2 (Figs. 4, 5), except for the quantitatively selected b-value for the TZ, as determined by reader 2 (1687–2020 s/mm2) (Table 3). Bland–Altman plots demonstrated a mean difference of less than 100 s/mm2 between qualitatively and quantitatively selected optimal b-values, except in patients with a PZ index lesion, as determined by reader 1 (102.7 s/mm2).
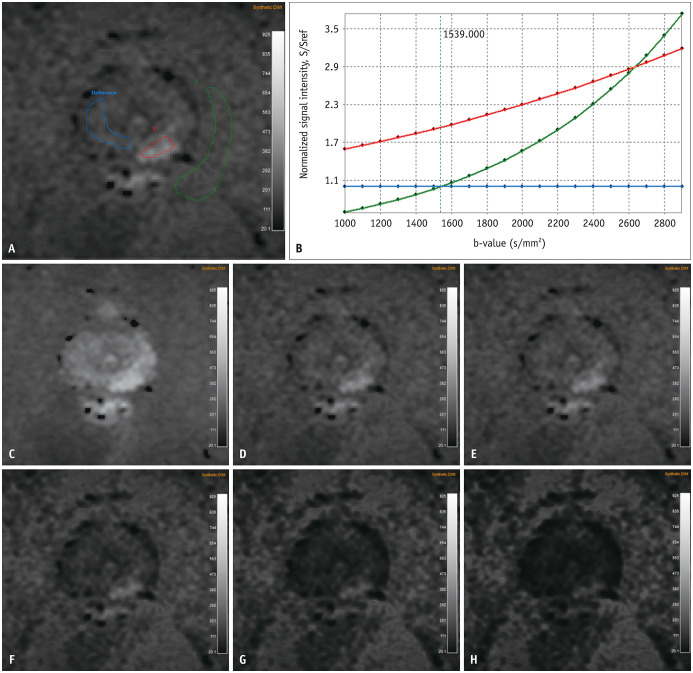
Fig. 4. A 63-year-old man with International Society of Urological Pathology grade 2 prostate cancer of left PZ as confirmed by targeted biopsy.
A. A focal area of diffusion restriction was seen in left PZ on sDWI (red ROI). Three ROIs were manually drawn for three different regions, and the reference for signal intensity ratio was contralateral PZ (blue ROI). B. Quantitatively selected optimal b-value was 1539 s/mm2 (i.e., intersection point of blue and green curves). C–H. In order, sDWI at b-values of 1000, 1500, 1539, 2000, 2500, and 3000 s/mm2. PZ = peripheral zone, ROI = region of interest, sDWI = synthetic diffusion-weighted imaging
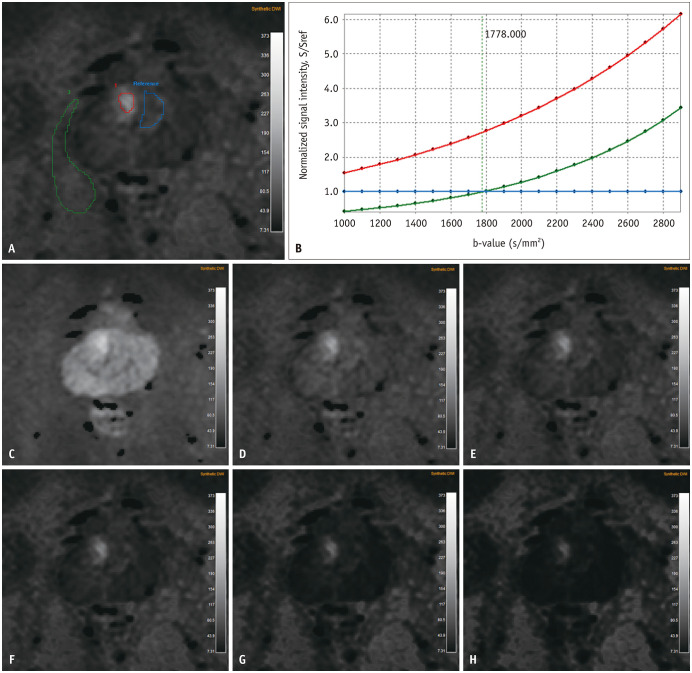
Fig. 5. A 69-year-old man with International Society of Urological Pathology grade 2 prostate cancer of right TZ confirmed by targeted biopsy.
A. A focal area of diffusion restriction was seen in right TZ on sDWI (red ROI). Three ROIs were manually drawn for three different regions, and the reference for signal intensity ratio was contralateral TZ (blue ROI). B. Quantitatively selected optimal b-value was 1778 s/mm2 (i.e., intersection point of blue and green curves). C–H. In order, sDWI at b-values of 1000, 1500, 1778, 2000, 2500, and 3000 s/mm2. ROI = region of interest, sDWI = synthetic diffusion-weighted imaging, TZ = transition zone
Table 3. Qualitatively or Quantitatively Suggested Optimal b-Values and Their Mean Differences according to the Zonal Location of the MRI-Suggested Index Lesion or Presence of csPCa.
| Parameter | 95% CI of the Median b-Value | Mean Difference of Bland-Altman Plot | |
|---|---|---|---|
| Qualitative | Quantitative | ||
| PZ | |||
| Reader 1 | 1755–1805 | 1596–1715 | 102.7 |
| Reader 2 | 1832–1910 | 1665–1772 | 86.1 |
| TZ | |||
| Reader 1 | 1756–1802 | 1661–1894 | 34.6 |
| Reader 2 | 1759–1888 | 1687–2020 | 19.5 |
| Presence of csPCa | |||
| Reader 1 | 1736–1789 | 1643–1779 | 101.0 |
| Reader 2 | 1870–1932 | 1625–1876 | 96.8 |
| Absence of csPCa | |||
| Reader 1 | 1736–1789 | 1580–1721 | 61.8 |
| Reader 2 | 1790–1875 | 1670–1787 | 46.4 |
The unit of all b-value data are s/mm2. CI= confidence interval, csPCa = clinically significant prostate cancer, MRI = magnetic resonance imaging, PZ = peripheral zone, TZ = transition zone
For the presence or absence of csPCa, all 95% CIs of the optimal b-value were within a range of 1500–2000 s/mm2. Bland–Altman plots demonstrated a mean difference of less than 100 s/mm2 between qualitatively and quantitatively selected optimal b-values, except for the patients with csPCa, as determined by reader 1 (101.0 s/mm2).
DISCUSSION
Theoretically, SI ratio between PCa and the bP region increases continuously with increasing b-value [7]. In a study by Feuerlein et al. [7], quantitatively measured tumor conspicuity serially increased with increasing b-value, up to 4000 s/mm2. They concluded that evaluating conspicuity with very high b-value sDWI (b-value greater than 2000 s/mm2) is better. On the other hand, Rosenkrantz et al. [5] reported that diminished anatomic clarity at very high b-value may be associated with decreased performance for PCa detection. Thus, they recommended the b-value range of 1500–2000 s/mm2 for prostate sDWI. In terms of the SI ratio between PCa and the bP region, our quantitative data, based on SI ratio curves, were in line with the data of Feuerlein et al. [7] As seen in Figures 2, 4, and 5, the SI ratio between the MRI-suggested index lesion and the bP region increases continuously with increasing b-value (red curve). Nevertheless, the range of optimal b-value observed in this study was similar to that of Rosenkrantz et al. [5]. Therefore, the concept of SI ratio between PCa and the bP region alone may not be able to determine the optimal b-value range of sDWI.
In this study, we hypothesized that the prostatic index lesion would be best seen when the suppressed SI of bP and pP regions is homogeneous. The 95% CIs of median b-value showing homogeneous SI between the two regions were between 1600–1900 s/mm2. The IQRs of qualitatively or quantitatively selected optimal b-values were also between 1500–2000 s/mm2. These ranges were similar to those from the qualitatively selected optimal b-value. The mean difference between qualitatively and quantitatively selected optimal b-values was less than 100 s/mm2. In sub-analyses according to zonal location of the MRI-suggested index lesion or the presence or absence of csPCa, the range of optimal b-values was mostly between 1500–2000 s/mm2 in both qualitative and quantitative assessment. The mean difference in Bland–Altman plots between qualitatively and quantitatively selected optimal b-values was also less than 110 s/mm2 in every sub-analysis. Based on the findings of the previous [5] and present studies, setting very high b-values on sDWI to achieve higher SI ratios between MRI-suggested index lesions and bP tissues may not produce the best condition for prostate evaluation. Rather, when the background signals are appropriately suppressed and become homogeneous, the MRI-suggested index lesion of the prostate may be seen more clearly.
This study has some limitations. First, this is a retrospective and single institutional study. Thus, there might be a risk of selection bias. However, we tried to avoid selection bias by evaluating a consecutive series of patients who met the inclusion and exclusion criteria. In addition, our data demonstrated a certain degree of consistency with respect to the optimal b-value range (i.e., 1500–2000 s/mm2) and mean difference between qualitatively and quantitatively selected b-values (i.e., less than 100 s/mm2), across two independent readers with different experience levels. The present data need further prospective and external validation. Second, the issue of local staging was not addressed. In addition to lesion detection, prostate MRI also plays a role in tumor staging [16,17]. We could not assess the pathological stage because the reference standard was biopsy results. Thus, the present data may be clinically useful when interpreting prebiopsy MRIs to identify an index lesion and when deciding the conduct of targeted biopsy. In terms of tumor staging, high-resolution T2-weighted imaging is currently pivotal, and is sometimes supplemented by dynamic contrast-enhanced imaging [1,18]. Third, surgical specimens were not evaluated. To overcome this limitation, we performed a sub-analysis involving 71 patients with no PCa detected by systematic biopsy of the contralateral lobe of the target biopsy site. The optimal b-value range was similar as that of all study patients or various sub-groups. Nevertheless, according to the literature, there were risks of measuring cancerous tissues instead of bP tissues because approximately 15% of PCa are invisible on MRI [19]. However, even when prostate MRI is analyzed in a prospective study or clinical practice, a quantitative analysis would be inevitable in a similar way with similar risks as in this study. Validation studies using surgical specimens are required. Fourth, the two readers were aware of the location of the targeted biopsy (i.e., location of the MRI-suggested index lesion). This attempt was required to overcome the possible limitation from the retrospective study design (i.e., discrepancies between the MRI-suspected index lesion and the targeted biopsy site).
In conclusion, the b-value range showing homogeneous background signals may be optimal for evaluating prostatic index lesions on sDWI. Setting very high b-values on sDWI to achieve higher SI ratio between MRI-suggested index lesions and bP tissues may not be optimal. Our qualitative and quantitative data consistently suggest the use of b-values in the range of 1500–2000 s/mm2. Our data also support the PI-RADS recommendation regarding the use of high b-values of 1400 s/mm2 or greater to evaluate prostatic index lesions on sDWI. Based on our data, prospective validation studies are required to confirm whether or not utilizing our method (i.e., a b-value range showing homogeneous background signals) for generating optimal sDWI is helpful to detect csPCa.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Sung Yoon Park.
- Formal analysis: So Yeon Cha, Sung Yoon Park.
- Investigation: So Yeon Cha, Sung Yoon Park.
- Methodology: Sung Yoon Park.
- Software: EunJu Kim, Sung Yoon Park.
- Supervision: Sung Yoon Park.
- Visualization: EunJu Kim, Sung Yoon Park.
- Writing—original draft: all authors.
- Writing—review & editing: Sung Yoon Park.
References
- 1.Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, et al. PI-RADS prostate imaging–reporting and data system: 2015, version 2. Eur Urol. 2016;69:16–40. doi: 10.1016/j.eururo.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turkbey B, Rosenkrantz AB, Haider MA, Padhani AR, Villeirs G, Macura KJ, et al. Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur Urol. 2019;76:340–335. doi: 10.1016/j.eururo.2019.02.033. [DOI] [PubMed] [Google Scholar]
- 3.Ueno YR, Tamada T, Takahashi S, Tanaka U, Sofue K, Kanda T, et al. Computed diffusion-weighted imaging in prostate cancer: basics, advantages, cautions, and future prospects. Korean J Radiol. 2018;19:832–837. doi: 10.3348/kjr.2018.19.5.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maas MC, Fütterer JJ, Scheenen TW. Quantitative evaluation of computed high B value diffusion-weighted magnetic resonance imaging of the prostate. Invest Radiol. 2013;48:779–786. doi: 10.1097/RLI.0b013e31829705bb. [DOI] [PubMed] [Google Scholar]
- 5.Rosenkrantz AB, Parikh N, Kierans AS, Kong MX, Babb JS, Taneja SS, et al. Prostate cancer detection using computed very high b-value diffusion-weighted imaging: how high should we go? Acad Radiol. 2016;23:704–711. doi: 10.1016/j.acra.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Waseda Y, Yoshida S, Takahara T, Kwee TC, Matsuoka Y, Saito K, et al. Utility of computed diffusion-weighted MRI for predicting aggressiveness of prostate cancer. J Magn Reson Imaging. 2017;46:490–496. doi: 10.1002/jmri.25593. [DOI] [PubMed] [Google Scholar]
- 7.Feuerlein S, Davenport MS, Krishnaraj A, Merkle EM, Gupta RT. Computed high b-value diffusion-weighted imaging improves lesion contrast and conspicuity in prostate cancer. Prostate Cancer Prostatic Dis. 2015;18:155–160. doi: 10.1038/pcan.2015.5. [DOI] [PubMed] [Google Scholar]
- 8.Rosenkrantz AB, Chandarana H, Hindman N, Deng FM, Babb JS, Taneja SS, et al. Computed diffusion-weighted imaging of the prostate at 3 T: impact on image quality and tumour detection. Eur Radiol. 2013;23:3170–3177. doi: 10.1007/s00330-013-2917-8. [DOI] [PubMed] [Google Scholar]
- 9.Ueno Y, Takahashi S, Kitajima K, Kimura T, Aoki I, Kawakami F, et al. Computed diffusion-weighted imaging using 3-T magnetic resonance imaging for prostate cancer diagnosis. Eur Radiol. 2013;23:3509–3516. doi: 10.1007/s00330-013-2958-z. [DOI] [PubMed] [Google Scholar]
- 10.Thörmer G, Otto J, Reiss-Zimmermann M, Seiwerts M, Moche M, Garnov N, et al. Diagnostic value of ADC in patients with prostate cancer: influence of the choice of b values. Eur Radiol. 2012;22:1820–1828. doi: 10.1007/s00330-012-2432-3. [DOI] [PubMed] [Google Scholar]
- 11.Sahoo P, Rockne RC, Jung A, Gupta PK, Rathore RKS, Gupta RK. Synthetic apparent diffusion coefficient for high b-value diffusion-weighted MRI in prostate. Prostate Cancer. 2020;2020:5091218. doi: 10.1155/2020/5091218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SJ, Oh YT, Jung DC, Cho NH, Choi YD, Park SY. Combined analysis of biparametric MRI and prostate-specific antigen density: role in the prebiopsy diagnosis of Gleason score 7 or greater prostate cancer. AJR Am J Roentgenol. 2018;211:W166–W172. doi: 10.2214/AJR.17.19253. [DOI] [PubMed] [Google Scholar]
- 13.Bajgiran AM, Mirak SA, Sung K, Sisk AE, Reiter RE, Raman SS. Apparent diffusion coefficient (ADC) ratio versus conventional ADC for detecting clinically significant prostate cancer with 3-T MRI. AJR Am J Roentgenol. 2019;213:W134–W142. doi: 10.2214/AJR.19.21365. [DOI] [PubMed] [Google Scholar]
- 14.Tamada T, Kanomata N, Sone T, Jo Y, Miyaji Y, Higashi H, et al. High b value (2,000 s/mm2) diffusion-weighted magnetic resonance imaging in prostate cancer at 3 Tesla: comparison with 1,000 s/mm2 for tumor conspicuity and discrimination of aggressiveness. PLoS One. 2014;9:e96619. doi: 10.1371/journal.pone.0096619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierorazio PM, Walsh PC, Partin AW, Epstein JI. Prognostic Gleason grade grouping: data based on the modified Gleason scoring system. BJU Int. 2013;111:753–760. doi: 10.1111/j.1464-410X.2012.11611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71:618–662. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Fulgham PF, Rukstalis DB, Turkbey IB, Rubenstein JN, Taneja S, Carroll PR, et al. AUA policy statement on the use of multiparametric magnetic resonance imaging in the diagnosis, staging and management of prostate cancer. J Urol. 2017;198:832–838. doi: 10.1016/j.juro.2017.04.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta RT, Spilseth B, Patel N, Brown AF, Yu J. Multiparametric prostate MRI: focus on T2-weighted imaging and role in staging of prostate cancer. Abdom Radiol (NY) 2016;41:831–884. doi: 10.1007/s00261-015-0579-5. [DOI] [PubMed] [Google Scholar]
- 19.Borofsky S, George AK, Gaur S, Bernardo M, Greer MD, Mertan FV, et al. What are we missing? False-negative cancers at multiparametric MR imaging of the prostate. Radiology. 2018;286:186–119. doi: 10.1148/radiol.2017152877. [DOI] [PMC free article] [PubMed] [Google Scholar]