Abstract

There are myriad ions that are deemed too short-lived to be experimentally accessible. One of them is SF6+. It has never been observed, although not for lack of trying. We demonstrate that long-lived SF6+ can be formed by doping charged helium nanodroplets (HNDs) with sulfur hexafluoride; excess helium is then gently stripped from the doped HNDs by collisions with helium gas. The ion is identified by high-resolution mass spectrometry (resolution m/Δm = 15000), the close agreement between the expected and observed yield of ions that contain minor sulfur isotopes, and collision-induced dissociation in which mass-selected HenSF6+ ions collide with helium gas. Under optimized conditions, the yield of SF6+ exceeds that of SF5+. The procedure is versatile and suitable for stabilizing many other transient molecular ions.
SF6 is an exceptionally stable molecule, but the SF6+ cation has never been observed, in spite of numerous attempts involving electron or photon ionization,1−4 charge transfer,5 photoelectron or photo-ion–photoelectron coincidence spectroscopy,6,7 experiments with ultrashort laser pulses,8 and isolation in a neon matrix.9 Attempts to stabilize SF6+ in SF6 clusters failed, as well.10−12 A reduced level of fragmentation upon ionization has been reported for some species embedded in HNDs, but bond rupture cannot be avoided entirely.13−15 No SF6+ ions appear upon ionization of HNDs doped with SF6.16−18
According to a theoretical study by Bauschlicher and Ricca at the CCSD(T) level of theory, SF6+ consists of a slightly distorted SF5+ and a F atom whose dissociation energy is 62 meV.19 Failure to produce long-lived SF6+ is due to the large (1.2 eV) difference between the vertical and adiabatic ionization energies of SF6.6,20 Kinetic energy release measurements show that ionization leads to impulsive dissociation.4 According to an ab initio dynamics study, vertical ionization of isolated SF6 produces F + SF5+ fragments that will separate within 0.2 ps with ∼1.1 eV channeled into kinetic energy.21 The challenge to the experimentalist is to quench this fierce reaction.
The mass spectra displayed in Figure 1 demonstrate that this can be done by doping preionized HNDs with SF6 in a pick-up cell and then gently stripping the helium matrix in a separate evaporation cell that is filled with low-density helium gas (pressure Pevap) until bare SF6+ or SF6+ complexed with a small number of helium atoms emerges.
Figure 1.
(a) Mass spectrum of charged HNDs doped with SF6 recorded at a low evaporation pressure (Pevap = 0.14 Pa). (b) Expanded view of the spectrum shown in panel a. (c) Mass spectrum recorded with a Pevap of 0.30 Pa. Prominent ion series are labeled. Expected contributions from ions containing the minor isotopes 33S or 34S are marked with asterisks; they agree with observations except for the peak at 147 u in panel c that has contributions from [H32SF6]+.
The spectrum in Figure 1a was recorded with a relatively low pressure Pevap of 0.14 Pa. The ion series Hen+ and HenH2O+ (n ≥ 0) dominate below ≈160 u; their mass peaks are connected by lines. Two singular mass peaks at 127 and 145 u are due to SF5+ and [H2OSF5]+, respectively (fluorine, mass of 19 u, is monoisotopic; the main isotope of sulfur is 32S, with a natural abundance of 94.93%22). Two other ion series commence at 146 and 164 u; they are assigned to HenSF6+ and Hen[H2OSF6]+, respectively.
Figure 1b presents an expanded view of the top spectrum. Mass peaks assigned to SF6+ and HeSF6+ are easily resolved from mass peaks due to He32H2O+ and He33H2O+, respectively, which have the same nominal mass. Possible contributions from another ion at 146 u, [SF5H3O]+, can be safely excluded. It is calculated to be stable, but its mass does not match the spectrum (see the Supporting Information for details).
When the helium evaporation pressure is increased to 0.30 Pa (Figure 1c), the Hen+ and HenH2O+ ion series disappear; the observed ions can be attributed to HenSF5+, Hen[H2OSF5]+, and HenSF6+. SF6+ forms the largest peak. Some mass peaks are due to ions containing 33S or 34S (natural abundance of 0.76% or 4.29%, respectively). Their expected yields, indicated by asterisks, match the observed yields, except for the peak at 147 u that has contributions from He532SF5+, 33SF6+, and H32SF6+ that has been observed previously.23
Many other mass spectra were recorded, with the evaporation pressure ranging from 0.12 to 0.97 Pa. Figure 2 displays the pressure dependence of the ion signal of SF6+, SF5+, and HenSF6+, the latter summed over 1 ≤ n ≤ 200. Between 0.20 and 0.30 Pa, the SF6+ signal exceeds the SF5+ signal. Furthermore, the signal of SF6+ reaches a maximum while that of SF5+ is still increasing. These observations suggest that SF6+ is an intermediate dissociation product
| 1 |
Figure 2.
Yield of HenSF6+ (summed from n = 1–200), SF6+, and SF5+ ions vs pressure Pevap in the evaporation cell. Lines are drawn to guide the eye.
We have tested the hypothesis by colliding mass-selected HenSF6+ with low-density helium gas in a collision cell located past the evaporation cell. Two representative mass spectra of the products of CID of He832SF6+ at an energy Elab of 20 eV (in the lab frame of reference) are displayed in panels a and b of Figure 3. In panel a, no gas was introduced into the collision cell; 93% of all precursor ions survive the transit through the cell. The value implies a lower limit of 1.0 ms for the lifetime of He8SF6+ (see the Supporting Information). In panel b, the collision gas pressure (Pcoll) was increased to 2.0 mPa. Most of the precursor ions dissociate into SF6+, SF5+, and some HenSF6+ (0 < n < 8). HenSF5+ (0 < n) is virtually absent, thus proving that reaction 1 dominates over the alternative reaction that would bypass formation of SF6+
| 2 |
Figure 3.
(a and b) Collision-induced dissociation of He832SF6+ at a lab energy Elab of 20 eV, at collision gas pressures (Pcoll) of 0 and 2.0 mPa, respectively. The spectrum in panel a was recorded in the absence of a collision gas, at a background pressure of 0.1 mPa. Panels c and d display the relative ion yields vs Elab for He832SF6+ and 32SF6+ precursor ions, respectively.
The energy dependence of the CID fragment ion yield from He8SF6+ versus collision energy is displayed in Figure 3c for a Pcoll of 2.0 mPa. The signal for HenSF6+ represents the sum over 0 < n < 8; the total ion yield is normalized to 100%. At the lowest energy, approximately one-third of the ions have fragmented into SF6+, two-thirds are of the form HenSF6+, but barely any ions have dissociated into HenSF5+ (0 ≤ n). Again, this demonstrates that reaction 1 prevails over reaction 2. HenSF6+ sheds all of its helium atoms before it loses a fluorine. Qualitatively similar results were obtained for CID of He4SF6+.
Note that the ion yield of the precursor He8SF6+ in Figure 3c is close to zero, even when Elab = 0. Why? The initial speed of the HNDs and, more importantly, the thermal motion of the collision gas provide an offset of ∼0.05 eV to the average energy transfer E* per collision24,25 that exceeds the dissociation energy of the parent ion (see below). Values of E* are indicated along the upper abscissa in Figure 3c.
CID data of mass-selected SF6+ are compiled in Figure 3d. SF5+ is the dominant fragment ion. Small amounts of SF4+ and SF3+ appear at high collision energies; no other fragment ions are detected. This observation further strengthens our conclusion that the mass peak at 146 u is due to 32SF6+. First, the mass of the peak assigned to SF6+ in Figure 1 agrees with the expected mass within better than 0.0005 u (see the Supporting Information). Second, the observed yield of ions containing the minor sulfur isotopes 33S and 34S matches the predicted one. Third, the pattern of CID products of ions assumed to be HenSF6+ (n = 0, 4, or 8) is consistent with the assignment, namely loss of neutrals with a mass of either 4 or 19 u.
The prevalence of reaction 1 over reaction 2 suggests that He is less strongly bound to SF6+ than F to SF5+ (assuming that there are no reverse energy barriers for loss of He or F for these reactions). SF6+ is often assumed to be unstable in its electronic ground state,1,9 but some authors have acknowledged that F-SF5+ may form a weakly bonded complex.19,26−28 We considered two isomers of the SF6+ complex (see Figure 4 and the Supporting Information): (a) isomer Ia in which the F atom is dissociated from the structure of the SF6 neutral molecule of Oh symmetry, with the symmetry reduced from C4v to Cs, the F atom being distorted from its original position along the linear F–S–F axis, moving from the equatorial plane of SF6, and (b) isomer Ib with a F atom attached to SF5+ in the D3h bipyramidal structure as a prolongation of a S–F axis, lying 44 meV higher than Ia. The calculated dissociation energy of the F atom from Ia of 87 meV and the S–F distance of 3.21 Å are close to the previously calculated values;19 the difference can be most probably traced to optimization on the B3LYP level in the respective publication. HeSF6+ complexes were then modeled by adding a helium atom to different sites of the Ia isomer, leading to three different structures (IIa–IIc). In the most stable IIa structure, the helium atom lies among three S atoms, on the other side with respect to the weakly bound sulfur atom.
Figure 4.
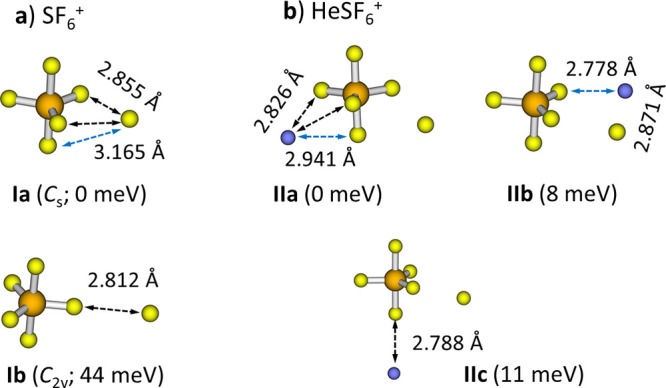
(a) Two isomers of the SF6+ complex. (b) Three isomers of He (blue) bound to SF6+. Relative energies are given, optimized at the CCSD/aug-cc-pVDZ level of theory with single-point recalculation at the CCSD(T)/aug-cc-pVTZ level.
To model dissociation of the HeSF6+ complex (see the Supporting Information), we calculated reaction energies along two different decomposition channels:
| 3a |
| 3b |
| 4a |
| 4b |
The disparity between the energies for reactions 3a and 4a readily explains the low yield of HenSF5+ (n > 0) among the collision products of He8SF6+ (Figure 3). The position of the helium atom with respect to SF6+ plays a rather limited role in energetic considerations (Figure 4). These results are consistent with the experimental observation that, under suitable conditions, SF6+ is the preferred product ion of He8SF6+ and He4SF6+. Furthermore, they suggest that our experimental approach may be used to stabilize any transient molecular ion AB± provided its binding energy A-B± exceeds its binding energy with helium. Species of potential interest include stable compounds of the light noble gases,29 ions whose absorption spectra have so far been measured only in neon or argon matrices,2 or labile organic ions that are important intermediates in atmospheric extraterrestrial chemistry.30,31
The observation of long-lived SF6+ contrasts with previous failures to form these ions by doping neutral HNDs followed by ionization.16−18 It is usually a thankless task to explain past failures, but we believe that we can rationalize the different outcomes.
We start by outlining the likely sequence of events when a charged HND picks up an SF6. In an undoped HND, the charge will be localized on He2+ or He3+.32 Upon pickup of SF6, the dopant and the charge will move toward each other through the superfluid medium; charge transfer will release ∼8.0 eV (see the Supporting Information). Given the large (1.1 eV) energy that is channeled into the F + SF5+ fragments upon vertical ionization,21 and the disparity in the mass of F and He, whether the helium matrix can fully cage the products is questionable. However, all that is needed to stabilize SF6+ is that the fluorine atom is, with some reasonable probability, thermalized and trapped in the HND. The separate F + SF5+ pair can then recombine later, even if a solidlike snowball surrounding SF5+33−35 may delay recombination until most of the helium has been stripped in the evaporation cell.
If the scenario described above applies to charged HNDs, why should not it apply to neutral, doped HNDs that are subsequently ionized? In this situation, the dopant will be ionized either via charge transfer from He2+, which releases 8 eV, or from the primary He+, which releases 10.5 eV (see the Supporting Information). In relative terms, these energies are not that much different.
What, then, causes the difference in observations in experiments with charged and neutral HNDs? The difference is primarily one of perception. There are basically two outcomes when a dopant embedded in a large HND containing N He atoms is ionized: ion ejection and ion trapping. Ejection leads to small ions HenX+ where n ≪ N; trapping leads to large ions HeN–nX+ (X is the dopant or a fragment thereof). The two outcomes have been nicely illustrated in a recent mixed quantum-classical dynamics simulation of He1000Ar4 by Halberstadt and Bonhommeau.36 Experiments on undoped HNDs (where a tightly bound He2+ takes on the role of the dopant) also demonstrate the coexistence of these two channels, although in this situation Coulomb repulsion between multiple charges also plays a role (see the Supporting Information and ref (37)). The relative yields of the two competing channels will depend on the nature of the dopant, the size N, and other factors, but coexist they will.
Conventional experimental setups are blind to ions trapped in large HNDs because the mass of HeN–nX+ exceeds the range of most mass spectrometers; they will detect only ejected ions. Ejection is violent; fragile ions such as SF6+ are unlikely to survive.13,14,38 Conversely, our current approach is blind to small ejected ions because ejection will already happen in the pickup cell; those ions are not guided by electric fields to the mass spectrometer. Only trapped ions will stay on the beam axis. Multiple collisions in the subsequent evaporation cell are needed to gently extract the trapped ions from the HND, unveiling the hitherto unobservable, fragile SF6+.
In conclusion, we have formed long-lived SF6+, previously believed to be a transient ion. The experimental approach, doping ionized HNDs and subsequently stripping excess helium by gentle collisions with helium gas, is generic; many other so-called transient ions can probably be stabilized similarly. The only obvious prerequisite, namely that the binding energy of A-B± exceeds that of He-AB±, should be met for most systems. The long-range interaction between A and B± is the ion-induced dipole interaction that is particularly weak for He-AB± because of the low polarizability of helium.
Experimental and computational methods: SF6+ ions are formed in and gently extracted from HNDs as follows. Neutral HNDs are grown by supersonic expansion of helium through a nozzle (diameter of 5 μm, temperature of 9.8 K, and stagnation pressure of 28 bar) into ultrahigh vacuum. The expanding beam is skimmed and ionized by electrons (energy of 37.7 eV and current of 473 μA). The resulting HeNz+ ions are weakly accelerated into an electrostatic hemispherical deflector set to transmit HNDs with a size-to-charge ratio (N/z) of ≈3.5 × 105. For the sake of simplicity, we discuss ions that are singly charged. The fate of multiply charged HNDs is discussed in the Supporting Information and elsewhere.39
The charged HNDs pass through a pickup cell containing SF6 gas at ∼250 μPa, and an “evaporation cell” that contains helium at low, variable pressure Pevap. Each collision will transfer, on average, 0.05 eV to the HND, ∼80 times the evaporation energy of bulk helium.24,25 Multiple collisions will lead to partial or complete evaporation of helium from the doped HND. The ions are guided by a radiofrequency field into the extraction region of a time-of-flight mass spectrometer (TOFMS) equipped with a reflectron in W configuration.
Dissociation channels are determined by passing the ions that emerge from the evaporation cell through a quadrupole mass filter. The selected precursor ions are accelerated and sent into a cell where they collide with a thermal gas of helium; product ions are analyzed in the TOFMS. Additional experimental details have been published elsewhere.24,39,40
We have calculated SF5+, SF6+, their complexes with a single helium atom, and [SF5H3O]+ using the coupled cluster theory (CC), optimized at the CCSD/aug-cc-pVDZ level of theory with single-point recalculation at the CCSD(T)/aug-cc-pVTZ level, including zero-point correction at the CCSD/aug-cc-pVDZ level; calculations were performed in the Gaussian software.41 “Very tight” optimization criteria were used for complexes with helium; wave function stability was tested for each structure.
Acknowledgments
S.A. and F.L. have received support from K-Regio project “FAENOMENAL”, Project EFRE 2016-4, funded by the Tyrolian Government and the European Regional Development Fund. The work by P.M., S.B., E.G., and O.E. was supported by the FWF, Projects W1259, T1181, and I4130. The computational results have been achieved using the HPC infrastructure LEO of the University of Innsbruck.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jpclett.1c01024.
Fate of multiply charged HNDs, the lifetime of He8SF6+, energy release upon charge transfer to SF6, mass analysis, and theoretical results for SF6+, HeSF6+, and [SF5H3O]+ (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Christophorou L. G.; Olthoff J. K. Electron Interactions with SF6. J. Phys. Chem. Ref. Data 2000, 29, 267–330. 10.1063/1.1288407. [DOI] [Google Scholar]
- Jacox M. E. Vibrational and Electronic Energy Levels of Polyatomic Transient Molecules. Supplement B. J. Phys. Chem. Ref. Data 2003, 32, 1–441. 10.1063/1.1497629. [DOI] [Google Scholar]
- Singh R. K.; Hippler R.; Shanker R. Partial Dissociative Ionization of SF6 by Electron Impact Using an Ejected Electron-Ion Coincidence Technique. Phys. Rev. A: At., Mol., Opt. Phys. 2003, 67, 022704. 10.1103/PhysRevA.67.022704. [DOI] [Google Scholar]
- Bull J. N.; Lee J. W. L.; Vallance C. Electron-Impact-Ionization Dynamics of SF6. Phys. Rev. A: At., Mol., Opt. Phys. 2017, 96, 042704. 10.1103/PhysRevA.96.042704. [DOI] [Google Scholar]
- Jarvis G. K.; Kennedy R. A.; Mayhew C. A.; Tuckett R. P. A Selected Ion Flow Tube Study of the Reactions of Several Cations with the Group 6B Hexafluorides SF6, SeF6, and TeF6. J. Phys. Chem. A 2000, 104, 10766–10776. 10.1021/jp002120z. [DOI] [Google Scholar]
- Evans M.; Ng C. Y.; Hsu C. W.; Heimann P. A High Resolution Energy-Selected Kinetic Energy Release Study of the Process SF6 + hv → SF5+ + F + e–: Heat of Formation of SF5+. J. Chem. Phys. 1997, 106, 978–981. 10.1063/1.473963. [DOI] [Google Scholar]
- Kivimaki A.; Ruiz J. A.; Erman P.; Hatherly P.; Garcia E. M.; Rachlew E.; Riu J. R. I.; Stankiewicz M. An Energy Resolved Electron-Ion Coincidence Study near the S 2p Thresholds of the SF6 Molecule. J. Phys. B: At., Mol. Opt. Phys. 2003, 36, 781–791. 10.1088/0953-4075/36/4/310. [DOI] [Google Scholar]
- Dota K.; Dharmadhikari A. K.; Dharmadhikari J. A.; Patra K.; Tiwari A. K.; Mathur D. A Search for the Sulphur Hexafluoride Cation with Intense, Few Cycle Laser Pulses. J. Chem. Phys. 2013, 139, 194302. 10.1063/1.4830222. [DOI] [PubMed] [Google Scholar]
- Lugez C. L.; Jacox M. E.; King R. A.; Schaefer H. F. Experimental and Ab Initio Study of the Infrared Spectra of Ionic Species Derived from SF6 and SF4 and Trapped in Solid Neon. J. Chem. Phys. 1998, 108, 9639–9650. 10.1063/1.476440. [DOI] [Google Scholar]
- Echt O.; Reyes Flotte A.; Knapp M.; Sattler K.; Recknagel E. Magic Numbers in Mass Spectra of Xe, C2F4Cl2 and SF6 Clusters. Ber. Bunsenges. Phys. Chem. 1982, 86, 860–865. 10.1002/bbpc.19820860919. [DOI] [Google Scholar]
- Stamatovic A.; Scheier P.; Märk T. D. Electron Attachment and Electron Impact Ionization of SF6 and SF6/Ar Clusters. J. Chem. Phys. 1988, 88, 6884–6888. 10.1063/1.454385. [DOI] [Google Scholar]
- Toker Y.; Rahinov I.; Schwalm D.; Even U.; Heber O.; Rappaport M. L.; Strasser D.; Zajfman D. Blackbody-Induced Radiative Dissociation of Cationic SF6 Clusters. Phys. Rev. A: At., Mol., Opt. Phys. 2012, 86, 023202. 10.1103/PhysRevA.86.023202. [DOI] [Google Scholar]
- Yang S.; Brereton S. M.; Wheeler M. D.; Ellis A. M. Soft or Hard Ionization of Molecules in Helium Nanodroplets? An Electron Impact Investigation of Alcohols and Ethers. Phys. Chem. Chem. Phys. 2005, 7, 4082–4088. 10.1039/b511628g. [DOI] [PubMed] [Google Scholar]
- Boatwright A.; Jeffs J.; Stace A. J. Ion–Molecule Reactions and Fragmentation Patterns in Helium Nanodroplets. J. Phys. Chem. A 2007, 111, 7481–7488. 10.1021/jp0713965. [DOI] [PubMed] [Google Scholar]
- Bartl P.; Tanzer K.; Mitterdorfer C.; Karolczak S.; Illenberger E.; Denifl S.; Scheier P. Electron Ionization of Different Large Perfluoroethers Embedded in Ultracold Helium Droplets: Effective Freezing of Short-Lived Decomposition Intermediates. Rapid Commun. Mass Spectrom. 2013, 27, 298–304. 10.1002/rcm.6459. [DOI] [PubMed] [Google Scholar]
- Fröchtenicht R.; Henne U.; Toennies J. P.; Ding A.; Fieber-Erdmann M.; Drewello T. The Photoionization of Large Pure and Doped Helium Droplets. J. Chem. Phys. 1996, 104, 2548–2556. 10.1063/1.471009. [DOI] [Google Scholar]
- Peterka D. S.; Kim J. H.; Wang C. C.; Neumark D. M. Photoionization and Photofragmentation of SF6 in Helium Nanodroplets. J. Phys. Chem. B 2006, 110, 19945–19955. 10.1021/jp062195o. [DOI] [PubMed] [Google Scholar]
- Schöbel H.; Dampc M.; Ferreira da Silva F.; Mauracher A.; Zappa M.; Denifl S.; Märk T. D.; Scheier P. Electron Impact Ionization of CCl4 and SF6 Embedded in Superfluid Helium Droplets. Int. J. Mass Spectrom. 2009, 280, 26–31. 10.1016/j.ijms.2008.07.009. [DOI] [Google Scholar]
- Bauschlicher C. W.; Ricca A. Accurate Heats of Formation for SFn, SFn+, and SFn– for n = 1 - 6. J. Phys. Chem. A 1998, 102, 4722–4727. 10.1021/jp981084p. [DOI] [Google Scholar]
- Linstrom P. J.; Mallard W. G.. NIST Chemistry Webbook, NIST Standard Reference Database Number 69; National Institute of Standards and Technology: Gaithersburg, MD, 2019. [Google Scholar]
- Tachikawa H. The Ionization Dynamics of SF6: A Full Dimensional Direct Ab Initio Dynamics Study. J. Phys. B: At., Mol. Opt. Phys. 2000, 33, 1725–1733. 10.1088/0953-4075/33/9/304. [DOI] [Google Scholar]
- Rosman K. J. R.; Taylor P. D. P. Isotopic Compositions of the Elements 1997 (Technical Report). Pure Appl. Chem. 1998, 70, 217–235. 10.1351/pac199870010217. [DOI] [Google Scholar]
- Latimer D. R.; Smith M. A. Direct Observation of HSF6+ and the Bracketing of the SF6 Proton Affinity at 5 K. J. Chem. Phys. 1994, 101, 3410–3411. 10.1063/1.467590. [DOI] [Google Scholar]
- Tiefenthaler L.; Kollotzek S.; Gatchell M.; Hansen K.; Scheier P.; Echt O. Isotope Enrichment in Neon Clusters Grown in Helium Nanodroplets. J. Chem. Phys. 2020, 153, 164305. 10.1063/5.0028056. [DOI] [PubMed] [Google Scholar]
- Liang J.; Kresin V. V. Kinetic Energy Deposited into a Nanodroplet, Cluster, or Molecule in a Sticking Collision with Background Gas. J. Chem. Phys. 2020, 153, 196101. 10.1063/5.0031865. [DOI] [PubMed] [Google Scholar]
- Fisher E. R.; Kickel B. L.; Armentrout P. B. Collision-Induced Dissociation and Charge Transfer Reactions of SFx+ (x = 1–5): Thermochemistry of Sulfur Fluoride Ions and Neutrals. J. Chem. Phys. 1992, 97, 4859–4870. 10.1063/1.463840. [DOI] [Google Scholar]
- Cheung Y. S.; Chen Y. J.; Ng C. Y.; Chiu S. W.; Li W. K. Combining Theory with Experiment: Assessment of the Thermochemistry of SFn, SFn+, and SFn–, n = 1 - 6. J. Am. Chem. Soc. 1995, 117, 9725–9733. 10.1021/ja00143a016. [DOI] [Google Scholar]
- Irikura K. K. Structure and Thermochemistry of Sulfur Fluorides SFn (n = 1 – 5) and Their Ions SFn+ (n = 1 – 5). J. Chem. Phys. 1995, 102, 5357–5367. 10.1063/1.469263. [DOI] [Google Scholar]
- Khriachtchev L.; Pettersson M.; Runeberg N.; Lundell J.; Rasanen M. A Stable Argon Compound. Nature 2000, 406, 874–876. 10.1038/35022551. [DOI] [PubMed] [Google Scholar]
- Ryazantsev S. V.; Tyurin D. A.; Feldman V. I. Experimental Determination of the Absolute Infrared Absorption Intensities of Formyl Radical HCO. Spectrochim. Acta, Part A 2017, 187, 39–42. 10.1016/j.saa.2017.06.018. [DOI] [PubMed] [Google Scholar]
- Inostroza-Pino N.; Palmer C. Z.; Lee T. J.; Fortenberry R. C. Theoretical Rovibrational Characterization of the cis/trans-HCSH and H2SC Isomers of the Known Interstellar Molecule Thioformaldehyde. J. Mol. Spectrosc. 2020, 369, 111273. 10.1016/j.jms.2020.111273. [DOI] [Google Scholar]
- Marinetti F.; Bodo E.; Gianturco F. A.; Yurtsever E. Energetics and Structures of Charged Helium Clusters: Comparing Stabilities of Dimer and Trimer Cationic Cores. ChemPhysChem 2008, 9, 2618–2624. 10.1002/cphc.200800457. [DOI] [PubMed] [Google Scholar]
- Atkins K. R. Ions in Liquid Helium. Phys. Rev. 1959, 116, 1339–1343. 10.1103/PhysRev.116.1339. [DOI] [Google Scholar]
- Mauracher A.; Echt O.; Ellis A. M.; Yang S.; Bohme D. K.; Postler J.; Kaiser A.; Denifl S.; Scheier P. Cold Physics and Chemistry: Collisions, Ionization and Reactions inside Helium Nanodroplets Close to Zero K. Phys. Rep. 2018, 751, 1–90. 10.1016/j.physrep.2018.05.001. [DOI] [Google Scholar]
- González-Lezana T.; Echt O.; Gatchell M.; Bartolomei M.; Campos-Martínez J.; Scheier P. Solvation of Ions in Helium. Int. Rev. Phys. Chem. 2020, 39, 465–516. 10.1080/0144235X.2020.1794585. [DOI] [Google Scholar]
- Halberstadt N.; Bonhommeau D. A. Fragmentation Dynamics of Ar4He1000 Upon Electron Impact Ionization: Competition between Ion Ejection and Trapping. J. Chem. Phys. 2020, 152, 234305. 10.1063/5.0009363. [DOI] [PubMed] [Google Scholar]
- Laimer F.; Kranabetter L.; Tiefenthaler L.; Albertini S.; Zappa F.; Ellis A. M.; Gatchell M.; Scheier P. Highly Charged Droplets of Superfluid Helium. Phys. Rev. Lett. 2019, 123, 165301. 10.1103/PhysRevLett.123.165301. [DOI] [PubMed] [Google Scholar]
- Bonhommeau D.; Lewerenz M.; Halberstadt N. Fragmentation of Ionized Doped Helium Nanodroplets: Theoretical Evidence for a Dopant Ejection Mechanism. J. Chem. Phys. 2008, 128, 054302. 10.1063/1.2823101. [DOI] [PubMed] [Google Scholar]
- Tiefenthaler L.; Kollotzek S.; Ellis A. M.; Scheier P.; Echt O. Proton Transfer at Subkelvin Temperatures. Phys. Chem. Chem. Phys. 2020, 22, 28165–28172. 10.1039/D0CP05174H. [DOI] [PubMed] [Google Scholar]
- Tiefenthaler L.; Ameixa J.; Martini P.; Albertini S.; Ballauf L.; Zankl M.; Goulart M.; Laimer F.; von Haeften K.; Zappa F.; et al. An Intense Source for Cold Cluster Ions of a Specific Composition. Rev. Sci. Instrum. 2020, 91, 033315. 10.1063/1.5133112. [DOI] [PubMed] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Petersson G. A.; Nakatsuji H.; et al. Gaussian 16, rev. A.03; Gaussian, Inc.: Wallingford, CT, 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





