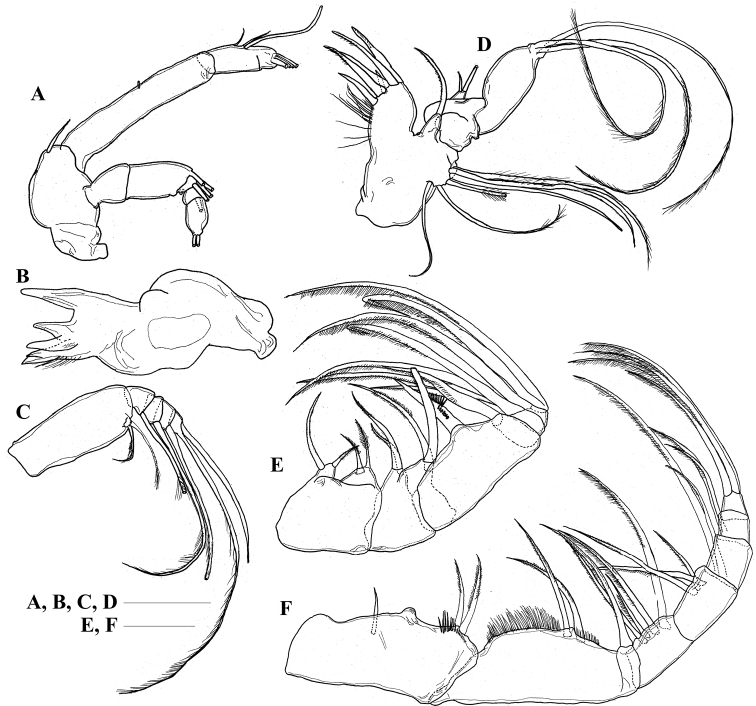Abstract
A new species of the continental shelf hyperbenthic genus Pilarella is described, the first from the Indo-Pacific. This is the second species of Pilarella known, and the first description of a male in the genus. The new species is easily distinguished from other species of Pilarella (P. longicornis) based on: (1) short caudal rami, approximately 1.5 times longer than wide; (2) 2 setae on the mandibular endopod; (3) 6 setae on the maxillular coxal epipodite; and (4) in the female, a short left antennule reaching the posterior border of the genital double-somite. The new diagnosis of Pilarella differs from Metacalanus in the separation of ancestral segments IX–XII and XIV–XV of the antennule, and the presence of 5–6 setae on the maxillular praecoxal arthrite. Pilarella is also separated from Metacalanalis based on the absence of a seta on the third ancestral segment of the antennary exopod, the symmetry of legs 1–3, the presence of a medial basal seta on the female leg 5, and 2 lateral exopodal spines on the female leg 5. A molecular phylogenetic analysis of some representative genera of the family Arietellidae, including the present new species, recovers two arietellid clades (Metacalanus- and Arietellus-clades) as in previous studies. Dichotomous keys for the genera of Arietellidae and the species of Pilarella are included.
Keywords: Arietelloidea, calanoid copepods, colonization, hyperbenthos, molecular phylogeny, Nansei Islands
Introduction
The calanoid family Arietellidae is widely distributed, occurring from littoral caves to the shallow water pelagic realm and deep-sea hyperbenthic layers (Ohtsuka et al. 1993, 1994). Phylogenetic analysis of the family pointed to the existence of two clades, viz. Crassarietellus-Paramisophria-Metacalanus-Pilarella (Metacalanus-clade) and Campaneria-Scutogerulus-Paraugaptilus-Paraugaptiloides-Arietellus-Sarsarietellus (Arietellus-clade) (Ohtsuka et al. 1994). Subsequently, Ohtsuka et al. (2005) and Soh et al. (2013) reconstructed the phylogenetic relationships among genera adding characters of new taxa such as Protoparamisophria, Metacalanalis and Paraugaptiloides. According to Soh et al. (2013), the position of Paramisophria is equivocal, while Crassarietellus-Metacalanalis-Metacalanus-Pilarella-Scutogerulus-Protoparamisophria and Campaneria-Paraugaptilus-Arietellus-Paraugaptiloides-Sarsarietellus belong to Ohtsuka et al.’s (1994)Metacalanus- and Arietellus-clades, respectively. These studies (Ohtsuka et al. 1994, 2005; Ohtsuka 2006; Soh et al. 2013) implied the different colonization routes followed by the family and suggested that pelagic taxa (some species of Metacalanus in Metacalanus-clade, and Arietellus and Paraugaptilus in Arietellus-clade) could have been independently derived from hyperbenthic forms in each clade.
Recently, molecular markers such as ribosomal and mitochondrial DNA sequences have been used as tools for the exploration of phylogenetic relationships among various calanoid taxa. However, only data on one arietellid species (Paraugaptilus buchani) is available in the DDBJ/EMBL/GenBank databases, and no molecular phylogenetic analysis has been conducted yet among arietellid genera.
This study describes a new species of Pilarella collected off the Amami Oshima Island, Kagoshima Prefecture, Japan. This is the second species of Pilarella and the first record of this genus in the Indo-Pacific. The male of Pilarella is described for the first time. Moreover, we explore the phylogenetic relationships among seven arietellid species based on 28S ribosomal DNA. Furthermore, new dichotomous keys to arietellid genera and species of Pilarella are provided.
Materials and methods
Sample collection and morphological observations
The arietellid copepods used in this study were collected off the Nansei Islands in southwest Japan in May 2018 and 2019 by the training and research vessel “Toyoshio-Maru” of Hiroshima University. Sampling dates, localities, and collection gears (cf. Ohtsuka et al. 1992) are shown in Table 1. Specimens were fixed in 99.5% ethanol.
Table 1.
Collection data of arietellid species used in molecular phylogeny.
| Species | Accession number | Sampling gear | Longitude / Latitude | Depth (m) | Date | Time |
|---|---|---|---|---|---|---|
| Arietellus setosus Giesbrecht, 1892 | LC510290 | ORI net | 29°50.011'N, 130°55.999'E | 0–1000 | May 22, 2019 | 1635–1912 |
| Arietellus simplex Sars, 1905 | LC510291 | ORI net | 29°50.011'N, 130°55.999'E | 0–1000 | May 22, 2019 | 1635–1912 |
| Metacalanus sp. | LC516702 | Conical hand net | 26°23'N, 127°67'E | <10 | May 25, 2019 | Night |
| Paramisophria sp. | LC510294 | Dredge | 26°14.448'N, 127°31.532'E | 53 | May 25, 2019 | 0909–1020 |
| Paraugaptilus buchani Wolfenden, 1904 | LC510293 | ORI net | 30°48.106'N, 131°32.072'E | 0–1000 | May 15, 2018 | 0941–1205 |
| Pilarella compacta sp. nov. | LC510295 | Sledge net | 28°14.023'N, 129°39.559'E | 291–294 | May 18, 2018 | 0816–0826 |
| Sarsarietellus orientalis Soh, Moon, Ohtsuka, Pae & Jeong, 2013 | LC510292 | Beam trawl nets | 28°22.422'N, 129°15.144'E | 315–316 | May 23, 2019 | 1155–1255 |
Specimens of the new species of Pilarella were dissected under a stereomicroscope (SZX7, Olympus), and their appendages and urosome cleared in lactophenol. Illustrations were drawn under a biological microscope (BX53, Olympus) with a drawing tube.
Type specimens (NMST-Cr29010–29012) were deposited at the National Museum of Nature and Science (Tsukuba, Ibaraki Prefecture, Japan). The morphological terminology mainly follows Huys and Boxshall (1991); alternative interpretations of the homology of the maxilla and maxilliped follow Ferrari and Ivanenko (2001, 2008).
DNA extraction, PCR, and sequencing
Total DNA of samples, except of Metacalanus sp., was extracted from the whole body using DNeasy Blood & Tissue kits (Qiagen, Venlo, Netherlands). Total DNA extraction from a small sample of Metacalanus sp. was performed using the whole body according to the method described by Suyama (2011). DNA was quantified using a NanoDrop 2000 instrument (Thermo-Fisher, Waltham, MA, USA) and then adjusted to 1 ng μL– 1 with sterilized water for PCR amplification. The 28S nuclear ribosomal DNA region (28S) was amplified using a T100 Thermal Cycler (Bio-Rad, Hercules, CA, USA) with Taq PCR Master Mix Kit (Qiagen) and primers; 28S-F1a (5ʹ-GCG GAG GAA AAG AAA CTA AC-3ʹ) and 28S-R1a (5ʹ-GCA TAG TTT CAC CAT CTT TCG GG-3ʹ) (Blanco-Bercial et al. 2011). Thermocycling conditions for 28S were 94 °C for 7 min; 35–40 cycles at 94 °C for 45 s, 50 °C for 1 min, and 72 °C for 1 min; and a final extension at 72 °C for 7 min. Amplification results were verified using 2% (w/v) agarose electrophoresis. Excess primers and dNTPs were removed with ExoSAP-IT (Thermo-Fisher, Scientific, Waltham, MA, USA), and the sequencing was performed commercially (Macrogen Japan, Kyoto, Japan).
Seven sequences for 28S (Accession numbers: LC510290–LC510295, LC516702) obtained in this study were deposited in the DNA Data Bank of Japan (DDBJ) and GenBank. Paraheterorhabdus compactus (Sars, 1900) (Accession number: HM997026) was used as the outgroup taxon. Sequence alignment was performed using CLUSTAL W (Thompson et al. 1994) in MEGA 7.026 (Tamura et al. 2011), and final alignments of 649 bp were used for the phylogenetic analysis. Phylogenetic relationships among the partial 28S sequences were inferred using Maximum Likelihood (ML) and Bayesian Inference (BI) analyses. ML analysis was performed using software RAxML 8 (Stamatakis 2006, Stamatakis et al. 2008) under the GTR+Γ model, and the data set was run with 10,000 bootstrap replicates. BI analysis was performed using the software MrBayes 3.2.7 (Ronquist et al. 2012) under the GTR+Γ model. Two parallel analyses of Metropolis-Coupled Markov Chain Monte Carlo (MC3) were conducted for 1,000,000 generations, and topologies were sampled every 100 generations. The convergence of MCMC was checked with the value of the average standard deviation of split frequencies (ASDSF) in MrBayes and trace plots in the software Tracer 1.7.1 (Rambaut et al. 2018). The first 2500 trees (25% of all trees) were discarded as burn-in, and the consensus was estimated by summarizing the remainder 7500 trees. The phylogenetic trees of ML and BI analyses were visualized with Figtree 1.4.4 (Rambaut 2009).
Results
Taxonomy
Order Calanoida Sars, 1901
Family Arietellidae Sars, 1901
Genus. Pilarella
Alvarez, 1985
3C51F783-6E0E-56C4-B823-E4ABEE3A0D85
Diagnosis.
Female. Cephalosome separated from first pediger. Fourth and fifth pedigers completely fused. Rostrum produced ventrally, with pair of frontal filaments disposed distally. Genital double-somite symmetrical with paired seminal receptacles and gonopores located ventrolaterally. Antennules asymmetrical, 21-segmented, ancestral segments I-IV and XXIV-XXVIII fused. Left antennule exceeding fifth pediger, approximately 1.5 times longer than right counterpart. Antenna with unarmed coxa; exopod 5-segmented, with ancestral segments II-IV, V-VII and IX-X fused, setal formula as 0, 0-0-1, 1-1-1, 1, 0-2. Mandible with row of setules on dorsal margin of gnathobase; endopod rudimentary, unsegmented, with 2 setae. Maxillule with 5–6 spines on praecoxal arthrite; coxal endite with 1 seta; coxal epipodite with 5–6 setae; proximal and distal basal endites without seta; endopod unsegmented with 2 setae; exopod with 3 setae. Maxilla with 2 setae on all praecoxal and coxal endites; basis having 1 heavily-chitinized spine [Maxilla with 2 setae on all praecoxal, coxal and basal endites; first endopodal segment having 1 heavily-chitinized spine (the homology by Ferrari and Ivanenko 2001, 2008)]. Maxilliped syncoxal endites with 0, 1, 0, and 2 setae, respectively; basis with 2 setae; first to sixth endopodal segments with 1, 4, 3, 2, 2 and 4 setae, respectively [Maxilliped praecoxal endites with 0, 1 and 0 setae, respectively; coxal endite with 2 setae; basis with 2 setae midway and 1 seta distally; first to fifth endopodal segments with 4, 3, 2, 2 and 4 setae, respectively (the homology by Ferrari and Ivanenko 2001, 2008)]. Legs 1–4 symmetrical; Seta and spine formula shown in Table 2, but basal lateral seta of leg 4 reduced in P. longicornis. Leg 5 uniramous, 3-segmented; basis with long medial seta and short lateral seta; exopod unsegmented, with 1 lateral spine and 2 terminal spines.
Table 2.
Setal formula of legs 1–4 of Pilarella compacta sp. nov. Roman numerals: spines, Arabic numerals: setae.
| Exopod | Endopod | |||||||
|---|---|---|---|---|---|---|---|---|
| Coxa | Basis | 1 | 2 | 3 | 1 | 2 | 3 | |
| Leg 1 | 0–1 | 1–1 | I-1; | I-1; | 0, I, 5 | 0–1; | 0–2; | 1, 2, 2 |
| Leg 2 | 0–1 | 0–0 | I-1; | I-1; | III, I, 5 | 0–1; | 0–2; | 2, 2, 4 |
| Leg 3 | 0–1 | 0–0 | I-1; | I-1; | III, I, 5 | 0–1; | 0–2; | 2, 2, 4 |
| Leg 4 | 0–1 | 1–0 | I-1; | I-1; | III, I, 5 | 0–1; | 0–2; | 2, 2, 3 |
Male. Body, antenna, mandible, maxillule, maxilla, maxilliped, and legs 1–4 similar to female counterparts. Antennules asymmetrical; right antennule 21-segmented, ancestral segments I–IV fused, XXIV–XXVIII partly fused, I–VIII with long tape-like aesthetascs. Leg 5 uniramous, 5-segmented; basis with lateral seta; exopod 3-segmented, proximal 2 segments with 1 lateral spine, distal segment with 2 terminal setae.
Type species.
Pilarella longicornis Alvarez, 1985 (by monotypy). Other species: Pilarella compacta sp. nov., described herein.
Remarks.
The diagnosis of Metacalanus from Ohtsuka et al. (1994) differs from Pilarella in the fusion of ancestral segments IX–XII and XIV–XV on the antennule, and the display of 0–2 setae on the maxillular praecoxal arthrite (vs. 5–6 setae in Pilarella). The diagnosis of Metacalanalis from Ohtsuka et al. (2005) differs also from Pilarella in the presence of 1 seta on the third ancestral segment of the antennary exopod, the asymmetry of legs 1–3, the absence of medial basal seta on female leg 5, and the presence of 3 lateral exopodal spines on the female leg 5 (vs. 2 in Pilarella).
Pilarella compacta sp. nov.
DD5DF73D-DA9F-5DFE-88F3-D5F017594A03
http://zoobank.org/CD13E6FD-04D7-42EE-9CDB-571B0E9DB325
Types.
Holotype: ♀ 1.97 mm preserved in vial (NMST-Cr29010). Allotype: ♂ 1.69 mm, appendages mounted on glass slide, body in vial (NMST-Cr29011). Paratype: ♀ 2.01 mm, appendages mounted on glass slide, body in vial (NMST-Cr29012).
Description.
Adult female. Body (Fig. 1A, B) compact; cephalosome separated from first pediger; fourth and fifth pedigers completely fused; posterolateral corners of prosome with small triangular process extending posteriorly. Rostrum (Fig. 1C, D) produced ventrally, with pair of frontal filaments distally. Urosome (Fig. 1E–G) 4-segmented; genital double-somite symmetrical with pair of seminal receptacles; pair of gonopores located ventrolaterally. Caudal rami (Fig. 1F, G) symmetrical, approximately 1.5 times longer than wide; minute seta I placed ventrally, seta II reduced, setae III–VI long, short seta VII dorsally; inner margin of rami furnished with row of fine setules.
Figure 1.
Pilarella compacta sp. nov., adult female A lateral habitus (holotype) B dorsal habitus (holotype) C rostrum, ventral view (paratype) D rostrum, labrum and left paragnath, lateral view (paratype) E genital double-somite, ventral view (paratype) F urosome, ventral view (paratype) G urosome, lateral view (paratype) H right antennule (paratype) I left antennule (paratype). Scale bars: 0.1 mm.
Antennules (Fig. 1H, I) asymmetrical, left antennule approximately 1.5 times longer than right antennule. Right antennule (Fig. 1H) 21-segmented, reaching middle of second pediger, fusion pattern and armature of segments as follows: I-IV–9+2ae, V–1+1ae, VI–2, VII–2+1ae, VIII–2+1ae, IX–1+1ae, X–1+1ae, XI–2+1ae, XII–2+1ae, XIII–2+1ae, XIV–2+1ae, XV–2+1ae, XVI–2+1ae, XVII–2+1ae, XVIII–2+1ae, XIX–1+1ae, XX–2+1ae, XXI–1+1ae, XXII–1, XXIII–1, XXIV-XXVIII–10+1ae. Left antennule (Fig. 1I) 21-segmented, reaching posterior border of genital double-somite, fusion pattern and armature of segments as follows: I-IV–8, V–1, VI–2, VII–1+1ae, VIII–2+1ae, IX–2, X–2, XI–2+1ae, XII–2+1ae, XIII–1+1ae, XIV–2+1ae, XV–2+1ae, XVI–2, XVII–2+1ae, XVIII–2+1ae, XIX–1+1ae, XX–2, XXI–2+1ae, XXII–1, XXIII–1, XXIV-XXVIII–9.
Antenna (Fig. 2A) with unarmed coxa; basis with inner distal seta; exopod 5-segmented; ancestral segments II-IV, V-VII and IX-X fused, setal formula of 0, 0-0-1, 1-1-1, 1, 0-2; endopod indistinctly 3-segmented and distal 2 segments partly fused, proximal segment with seta on middle of inner margin, middle segment with 3 inner setae, distal segment with 4 distal setae.
Figure 2.
Pilarella compacta sp. nov., adult female, paratype A right antenna B coxa of left mandible C palp of left mandible D left maxillule E right maxilla F right maxilliped. Scale bars: 0.1 mm.
Mandible (Fig. 2B, C) gnathobase with 4 well-chitinized teeth, dorsalmost bifurcate; row of setules on dorsal margin of gnathobase; basis unarmed; endopod rudimentary, unsegmented, with 2 setae; exopod 5-segmented, setal formula 1, 1, 1, 1, 1.
Maxillule (Fig. 2D) with 5 long spines, 1 short spine and row of long setules on praecoxal arthrite; coxal endite with 1 seta; coxal epipodite with 6 setae; proximal and distal basal endites without setae; endopod unsegmented with 2 setae; exopod with 3 setae.
Maxilla (Fig. 2E) with praecoxal and coxal endites having 2, 2, 2 and 2 setae, respectively; basis having 1 heavily-chitinized spine with row of long spinules midway; endopod 4-segmented, setal formula 1, 3, 2, 2 [Maxilla with praecoxal and coxal endites having 2, 2 setae, respectively; basal endites having 2, 2 setae, respectively; endopod 5-segmented, setal formula 1, 1, 3, 2, 2; first endopodal segment having 1 heavily-chitinized spine with row of long spinules midway (the homology by Ferrari and Ivanenko 2001, 2008)].
Maxilliped (Fig. 2F) syncoxal endites with 0, 1, 0, and 2 setae, respectively; rows of long setules on inner margin of syncoxa and basis; basis with 2 setae midway; first endopodal segment partly fused to basis; first to sixth endopodal segments with 1, 4, 3, 2, 2, and 4 setae, respectively [Maxilliped praecoxal endites with 0, 1, and 0 setae, respectively; coxal endite with 1 seta; rows of long setules on inner margin of coxa and basis; basis with 2 setae midway, and 1 seta distally; first to fifth endopodal segments with 4, 3, 2, 2, and 4 setae, respectively (the homology by Ferrari and Ivanenko 2001, 2008)].
Seta and spine formula of legs 1–4 as shown in Table 2. Leg 1 (Fig. 3A) with medial setules on proximal 2 segments of exopod; proximal 2 segments of endopod with lateral setules. Legs 2 and 3 (Fig. 3B, C) with medial setules on proximal 2 segments of exopod; distal 2 segments of exopod and all segments of endopod with lateral setules. Leg 4 (Fig. 3D) with medial setules on proximal 2 segments of exopod; distal 2 segments of exopod and proximal 2 segments of endopod with lateral setules.
Figure 3.
Pilarella compacta sp. nov., adult female, paratype A leg 1, posterior side B leg 2, posterior side C leg 3, posterior side D leg 4, posterior side E fifth legs, posterior side. Scale bar: 0.1 mm.
Leg 5 (Fig. 3E) uniramous, 3-segmented; basis broad, approximately 0.6 times as long as wide, with long plumose seta medially and short seta laterally; exopod unsegmented, approximately 3 times longer than wide, with 3 medial setules, 1 lateral spine and 2 terminal spines.
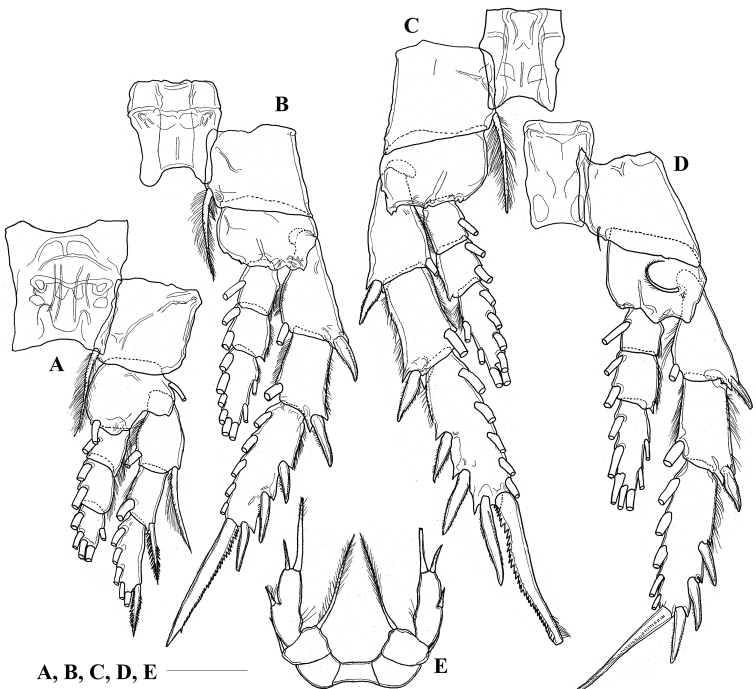
Adult male. Body (Fig. 4A, B) compact; cephalosome separated from first pediger; fourth and fifth pedigers completely fused; posterolateral corners of prosome with small triangular process extending posteriorly. Rostrum similar to that of female. Urosome (Fig. 4C) 5-segmented; gonopore located on right side of genital somite; caudal rami similar to female, except for lacking inner setules.
Figure 4.
Pilarella compacta sp. nov., adult male, allotype A lateral habitus B dorsal habitus C urosome, ventral view D right antennule E left antennule F Fifth legs, anterior view. Scale bars: 0.1 mm.
Antennules (Fig. 4D, E) asymmetrical. Right antennule (Fig. 4D) 21-segmented, ancestral segments XXIV and XXV partly fused, exceeding middle of first pediger, fusion pattern and armature of segments as follows: I-IV–6+4ae, V–1+1ae, VI–2+1ae, VII–1+1ae, VIII–1+1ae, IX–1+1ae, X–1ae, XI–1+1ae, XII–1+1ae, XIII–1+1ae, XIV–1+1ae, XV–1+1ae, XVI–2+1ae, XVII–1+1ae, XVIII–1+1ae, XIX–1+1ae, XX–2+1ae, XXI–1+1ae, XXII–1, XXIII–1, XXIV-XXVIII–7+2ae. Left antennule (Fig. 4E) damaged; ancestral segments XV–XXVIII not observed, fusion pattern and armature of I–XV as follows: I-IV–6+4ae, V–2+1ae, VI–1+1ae, VII–1+1ae, VIII–1+1ae, IX–1+1ae, X–2+1ae, XI–2+1ae, XII–1+1ae, XIII–1+1ae, XIV–2.
Antenna, mandible, maxillule, maxilla, maxilliped and legs 1–4 similar to those of female.
Leg 5 (Fig. 4F) uniramous, 5-segmented; basis with plumose seta laterally; exopod 3-segmented, proximal 2 segments with lateral spine, distal segment with 2 terminal setae.
Etymology.
The specific name of the new species is derived from the Latin adjective compactus meaning “stocky” to denote the habitus of the present new species.
Remarks.
The present new species falls within the diagnosis of the monotypic Pilarella (Alvarez 1985) except for the following features: (1) left antennule not reaching the caudal rami; (2) short caudal rami; (3) 6 spines on the maxillular praecoxal arthrite (5 in the previous diagnosis); and (4) 6 setae on the maxillular coxal epipodite (5 in the previous diagnosis). For the differences from other genera, see generic remarks in the present study.
Pilarella compacta sp. nov. differs from P. longicornis Alvarez, 1985 as follows: (1) the body is more compact (vs. more slender in P. longicornis); (2) the left antennule of the female is short, and reaches the posterior border of genital double-somite (vs. reaching the posterior margin of caudal rami in P. longicornis); (3) the caudal rami are short, approximately 1.5 times longer than wide (vs. long, 4.3 longer than wide in P. longicornis), (4) the mandibular endopod has 2 setae (vs. 1 in P. longicornis), (5) the coxal epipodite of maxillule has 6 setae (vs. 5 in P. longicornis), (6) there is a basal lateral seta on leg 4 (vs. seta absent in P. longicornis).
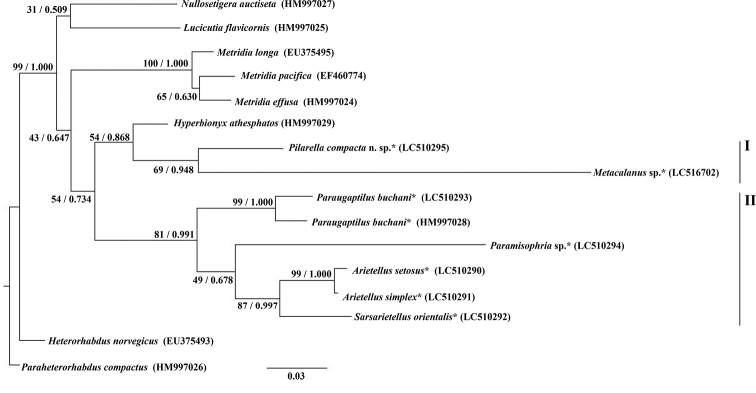
Phylogenetic analysis.
The Maximum Likelihood tree based on 16 species of the superfamily Arietelloidea is shown in Figure 5. Both ML and BI trees showed two clades in Arietellidae. In clade I, Pilarella compacta sp. nov. and Metacalanus sp. were placed in the same clade (BP = 69%; PP = 0.948). Hyperbionyx athesphatos (HM997029) (belonging to the Hyperbionychidae) and clade I formed a cluster with a low bootstrap value (BP = 54%; PP = 0.868). In clade II, four arietellid genera (Paraugaptilus, Paramisophria, Sarsarietellus, and Arietellus) were grouped in the same cluster with high bootstrap value (ML = 81%; PP = 0.991), and Arietellus was placed in the same clade as Sarsarietellus with a high bootstrap value (BP = 87%; PP = 0.997). The sequence of Paraugaptilus buchani (LC510293) in this study showed a lower identity of 96% to P. buchani (HM997028) in the DDBJ/EMBL/GenBank databases, which differed by five gaps and 19 single nucleotide polymorphisms (SNP).
Figure 5.
Maximum Likelihood tree based on partial 28S nuclear ribosomal DNA sequences of the superfamily Arietelloidea. Left and right Arabic numerals on nodes indicate bootstrap values and posterior probabilities, respectively. Scale bar shows nucleotide changes per site. Asterisks indicate arietellid species.
Key to genera of the family Arietellidae
The homology of the maxilla and maxilliped by Ferrari and Ivanenko (2001, 2008) is shown in parenthesis.
| 1 | Maxillule located anterior to maxilla; maxillular coxal epipodite with 5–9 setae | 2 |
| – | Maxillule located posterior to maxilla and maxilliped; maxillular coxal epipodite without setae | Griceus Ferrari & Markhaseva, 2000 |
| 2 | Third exopodal segment of leg 1 with 1 outer spine | 3 |
| – | Third exopodal segment of leg 1 with 2 outer spines | 6 |
| 3 | Maxillular praecoxal arthrite with 5–6 elements | 4 |
| – | Maxillular praecoxal arthrite with 0–2 elements | Metacalanus Cleve, 1901 |
| 4 | Distal endite on maxillary praecoxa with 2 setae (coxal endite on maxilla with 2 setae) | 5 |
| – | Distal endite on maxillary praecoxa with 1 seta (coxal endite on maxilla with 1 seta) | Scutogerulus Bradford, 1969 |
| 5 | Third ancestral segment on antennary exopod without seta; legs 1–4 symmetrical; female leg 5 with 1 basal medial seta, 1 exopodal lateral spine and 2 exopodal distal spines | Pilarella Alvarez, 1985 |
| – | Third ancestral segment on antennary exopod with seta; legs 1–4 asymmetrical; female leg 5 with no basal medial seta, 2 exopodal lateral spines and 2 exopodal distal spines | Metacalanalis Ohtsuka, Nishida & Machida, 2005 |
| 6 | Maxillular endopod absent or unsegmented with 0–3 setae | 7 |
| – | Maxillular endopod 3-segmented with 6 setae | Rhapidophorus Edwards, 1891 |
| 7 | Maxillule with 6 setae on coxal epipodite | 8 |
| – | Maxillule with 8–9 setae on coxal epipodite | 9 |
| 8 | Antennary exopod indistinctly 10-segmented; maxillule with strongly serrate spines on coxal arthrite; innermost seta on fifth endopodal segment of maxilliped long; outermost seta on sixth endopodal segment of maxilliped not reduced (innermost seta on fourth endopodal segment of maxilliped long; outermost seta on fifth endopodal segment of maxilliped not reduced) | Crassarietellus Ohtsuka, Boxshall & Roe, 1994 |
| – | Antennary exopod indistinctly 8-segmented; maxillule with weakly serrate spines on coxal arthrite; innermost seta on fifth endopodal segment of maxilliped short; outermost seta on sixth endopod segment of maxilliped reduced (innermost seta on fourth endopodal segment of maxilliped short; outermost seta on fifth endopod segment of maxilliped reduced) | Campaneria Ohtsuka, Boxshall & Roe, 1994 |
| 9 | Innermost seta on fourth and fifth endopodal segments of maxilliped ordinary (Innermost seta on third and fourth endopodal segments of maxilliped ordinary) | 10 |
| – | Innermost seta on fourth and fifth endopodal segments of maxilliped vestigial (Innermost seta on third and fourth endopodal segments of maxilliped vestigial) | Arietellus Giesbrecht, 1892 |
| 10 | Ancestral segment X on antennary exopod with 3 elements | 11 |
| – | Ancestral segment X on antennary exopod unarmed | Paraugaptilus Wolfenden, 1904 |
| 11 | Leg 4 without inner coxal seta; second segment of antennary endopod with 3 inner setae midway | 12 |
| – | Leg 4 with inner coxal seta; second segment of antennary endopod with 2 inner setae midway | Paraugaptiloides Ohtsuka, Boxshall & Roe, 1994 |
| 12 | Antennulary segments XXV and XXVI fused; maxillary basal spine without spinules (spine on first endopodal segment of maxilla without spinules); basal inner process on female leg 5 with 0–2 setae | 13 |
| – | Antennulary segments XXV and XXVI separated; maxillary basal spine with spinules (spine on first endopodal segment of maxilla with spinules); basal inner process on female leg 5 with 4 setae | Sarsarietellus Campaner, 1984 |
| 13 | Female genital double-somite with single copulatory pore; third ancestral segment on exopod of female leg 5 with at most 3 elements and 1 process | Paramisophria T. Scott, 1897 |
| – | Female genital double-somite with paired copulatory pores; third ancestral segment on exopod of female leg 5 with 1 outer, 1 subterminal and 2 terminal elements; male unknown | Protoparamisophria Ohtsuka, Nishida & Machida, 2005 |
Key to species of Pilarella
| 1 | Caudal rami approximately 1.5 times longer than wide; mandibular endopod with 2 setae; maxillular coxal epipodite with 6 setae; female left antennule reaching posterior border of genital double-somite | P. compacta sp. nov. |
| – | Caudal rami approximately 4 times longer than wide; mandibular endopod with 1 seta; maxillular coxal epipodite with 5 setae; female left antennule reaching posterior edge of caudal rami | P. longicornis Alvarez, 1985 |
Discussion
This study is the first report of a male Pilarella. The male antennule of Pilarella compacta sp. nov. has long, tape-like aesthetascs on the basal part. Similar antennules were found in a male Metacalanus acutioperculum and Metacalanus adriaticus (Ohtsuka 1984; Kršinić and Boxshall 2021). The segmentation and setation of the male antennule are similar to those of Crassarietellus and more segments are retained than in the diagnosis of Metacalanus (Ohtsuka et al. 1994). The male leg 5 has the same segmentation as in Metacalanus aurivilli (Chen and Zhang 1965), and corresponds to the Metacalanus-clade in the reduction of endopod (Ohtsuka et al. 1994). These features of the male Pilarella suggest its close relation to Crassarietellus and Metacalanus, which belong to the Metacalanus-clade in Ohtsuka et al. (1994).
As regards the molecular analysis, clades I (Metacalanus, Paramisophria, and Pilarella) and II (Arietellus, Paraugaptilus, and Sarsarietellus) in Figure 5 correspond to two lineages, the Metacalanus- and Arietellus-clades, which were discussed by Ohtsuka et al. (1994, 2005) and Soh et al. (2013). Although Ohtsuka et al. (1994, 2005) considered Paramisophria to be in the Metacalanus-clade, Soh et al. (2013) regarded the position of Paramisophria as equivocal based on their cladistic analysis. In the present study, Paramisophria was included in clade II (Arietellus-clade), although with low bootstrap value and posterior probability (BP = 49%, PP = 0.678).
Hyperbionix, belonging to the Hyperbionychidae, was clustered in clade I and included in the lineage of the Arietellidae (Fig. 5). However, estimating the phylogenetic position of the Hyperbionychidae is difficult because the cluster of Hyperbionychidae + clade I was supported by a low bootstrap value (BP = 54%). Blanco-Bercial et al. (2011) indicated the close relationship between Paraugaptilus buchani (Arietellidae) and Hyperbionyx athesphatos (Hyperbionychidae) in their molecular phylogeny. Ohtsuka et al. (1993) described many autapomorphic characteristics in the Hyperbionychidae, and Soh (1998) indicated that the Hyperbionychidae has some plesiomorphic characteristics that are not observed in the Arietellidae. These studies suggested that the Hyperbionychidae is closely related to the Arietellidae but is not derived from the Arietellidae.
In clade II (Arietellus-clade), a comparison of the 28S sequences of Paraugaptilus buchani between the present (material from the western North Pacific) and a previous study (material from the western North Atlantic; Blanco-Bercial et al. 2011) showed a low concordance rate (96%), differing by five gaps and 19 SNPs. However, no differences in morphological characteristics were observed between the Paraugaptilus buchani material collected from the western North Pacific in the present study and the original description of the species based on Atlantic material (Deevey 1973). As a possible explanation of this discordance, we consider it as due to intra-specific variation. Differences in genetic structure among populations of the same species have been reported in many other calanoids (Bucklin et al. 2003). In our case, it may be caused by the significant geographic distance separating the Pacific and the Atlantic locations where the material was collected.
The present study suggests a genetically closer relationship of Arietellus to Sarsarietellus based on high bootstrap value and posterior probability (BP = 87%, PP = 0.997) rather than to Paraugaptilus, as previous studies based on morphological features pointed out (Ohtsuka et al. 1994, 2005; Soh et al. 2013). Furthermore, Arietellus and Sarsarietellus also share a synapomorphic fused right and left copulatory pores (Ohtsuka et al. 1994; Ohtsuka et al. 2004). These two closely related genera, Arietellus and Sarsarietellus, are distributed across the pelagic realm and the deep-sea hyperbenthic layers, respectively (Ohtsuka et al. 1994). Ohtsuka et al. (2005) suggested that, based on morphology, the two pelagic genera (Arietellus and Paraugaptilus) could have diverged from a hyperbenthic ancestor. However, the present study shows that Arietellus (pelagic genus) is closer to Sarsarietellus (hyperbenthic genus) rather than to Paraugaptilus, and that Arietellus and Paraugaptilus could have colonized the pelagic realm independently, or that Sarsarietellus secondarily turned back to the deep-sea hyperbenthic layers. In conclusion, the molecular phylogenetic relationships of the Arietellidae supported the Metacalanus- and Arietellus-clades as depicted by Ohtsuka et al. (1994, 2005) and Soh et al. (2013), and provide new information about other possible colonization routes followed by members of the Arietellus-clade.
Supplementary Material
Acknowledgements
We would like to express our sincere thanks to the captain and crew of TRV Toyoshio-Maru for their help in field samplings. We are also grateful to Dr M. Sano for offering a sample, and associate Prof. A. Kato and assistant Prof. Y. Kondo for providing laboratory equipment. Part of this study was supported by Sasakawa Scientific Research Grant 2019-4074 from the Japan Science Society (JSS) and Research Grant from Hiroshima Earth Environment Information Center.
Citation
Komeda S, Adachi K, Ohtsuka S (2021) A new species of Pilarella (Copepoda, Calanoida, Arietellidae) from the hyperbenthic layer of Japan, with a molecular phylogenetic analysis of some representative genera of the Arietellidae. ZooKeys 1038: 179–194. https://doi.org/10.3897/zookeys.1038.63170
References
- Alvarez MPJ. (1985) A new arietellid copepod (Crustacea): Pilarella longicornis, gen. n., sp. n., from the Brazilian continental shelf. Revista Brasileira de Zoologia 3: 189–195. 10.1590/S0101-81751985000400005 [DOI] [Google Scholar]
- Blanco-Bercial L, Bradford-Grieve J, Bucklin A. (2011) Molecular phylogeny of the Calanoida (Crustacea: Copepoda). Molecular Phylogenetics and Evolution 59: 103–113. 10.1016/j.ympev.2011.01.008 [DOI] [PubMed] [Google Scholar]
- Bucklin A, Frost BW, Bradford-Grieve J, Allen LD, Copley NJ. (2003) Molecular systematic and phylogenetic assessment of 34 calanoid copepod species of the Calanidae and Clausocalanidae. Marine Biology 142: 333–343. 10.1007/s00227-002-0943-1 [DOI] [Google Scholar]
- Chen QC, Zhang SZ. (1965) The planktonic copepods of the Yellow Sea and the East China Sea. 1. Calanoida. Studia marina Sinica 7: 20–131. [Google Scholar]
- Deevey GB. (1973) Paraugaptilus (Copepoda: Calanoida): two species, one new, from the Sargasso Sea. Proceedings of the Biological Society of Washington 86: 247–260. [Google Scholar]
- Edwards CL. (1891) Beschreibung einiger neuen Copepoden und eines neuen copepodenähnlichen Krebses, Leuckartella paradoxa. Archiv fur Naturgeschichte, Inaugural-Dissertation einer Hohen Philosophischen Faultat der Universität Leipzig, Berlin 57: 75–104. [Google Scholar]
- Ferrari FD. (2000) Griceus buskeyi, a new genus and species of calanoid copepod (Crustacea) from benthopelagic waters off Hawaii. Proceedings of the Biological Society of Washington 113: 77–87. [Google Scholar]
- Ferrari FD, Ivanenko VN. (2001) Interpreting segment homologies of the maxilliped of cyclopoid copepods by comparing stage-specific changes during development. Organisms Diversity and Evolution 1: 113–131. 10.1078/1439-6092-00009 [DOI] [Google Scholar]
- Ferrari FD, Ivanenko VN. (2008) The identity of protopodal segments and the ramus of maxilla 2 of copepods (Copepoda). Crustaceana 81: 823–835. 10.1163/156854008784771702 [DOI] [Google Scholar]
- Huys R, Boxshall GA. (1991) Copepod evolution. The Ray Society, London, 468 pp. 10.4319/lo.1993.38.2.0478 [DOI] [Google Scholar]
- Kršinić F, Boxshall GA. (2021) New arietellid copepods (Calanoida, Arietellidae) from anchialine caves in the Eastern Adriatic Sea. Zootaxa 4951: 107–129. 10.11646/zootaxa.4951.1.4 [DOI] [PubMed] [Google Scholar]
- Ohtsuka S. (1984) Calanoid copepods collected from the near-bottom in Tanabe Bay on the Pacific Coast of the middle Honshu, Japan. I. Arietellidae. Publications of the Seto Marine Biological Laboratory 29: 359–365. 10.5134/176089 [DOI] [Google Scholar]
- Ohtsuka S. (2006) Systematics and evolution of small crustaceans: their diversity and dynamism (in Japanese). TAXA, Proceedings of the Japanese Society of Systematic Zoology 21: 1–12. [Google Scholar]
- Ohtsuka S, Huys R, Boxshall GA, Ito T. (1992) Misophriopsis okinawensis sp. nov. (Crustacea: Copepoda) from hyperbenthic waters off Okinawa, South Japan, with definitions of related genera Misophria Boeck, 1864 and Stygomisophria gen. nov. Zoological science 9: 859–859. [Google Scholar]
- Ohtsuka S, Roe HSJ, Boxshall GA. (1993) A new family of calanoid copepods, the Hyperbionychidae, collected from the deep-sea hyperbenthic community in the Northeastern Atlantic. Sarsia 78: 69–82. 10.1080/00364827.1993.10413523 [DOI] [Google Scholar]
- Ohtsuka S, Boxshall GA, Roe HSJ. (1994) Phylogenetic relationships between arietellid genera (Copepoda: Calanoida), with the establishment of three new genera. Bulletin of the National History Museum (Zoology Series) 60: 105–172. [Google Scholar]
- Ohtsuka S, Nishida S, Machida R. (2005) Systematics and zoogeography of the deep-sea hyperbenthic family Arietellidae (Copepoda: Calanoida) collected from the Sulu Sea. Journal of National History 39: 2483–2514. 10.1080/00222930500087408 [DOI] [Google Scholar]
- Rambaut A. (2009) FigTree version 1.4.4. http://tree.bio.ed.ac.uk/software/figtree/ [accessed August 2019]
- Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA. (2018) Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology 67: 901–904. 10.1093/sysbio/syy032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh HY. (1998) Phylogenetic studies of the calanoid copepod superfamily Arietelloidea, with notes on distribution and feeding habits. PhD thesis, Hiroshima University, Japan.
- Soh HY, Moon SY, Ohtsuka S, Pae SJ, Jeong HG. (2013) Reconstruction of arietellid copepod phylogenetic relationship, with description of a new species of Sarsarietellus (Copepoda, Calanoida, Arietellidae) from Korean waters. Zoological Society of Japan 30: 998–1004. 10.2108/zsj.30.998 [DOI] [PubMed] [Google Scholar]
- Stamatakis A. (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. 10.1093/bioinformatics/btl446 [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. (2008) A Rapid Bootstrap Algorithm for the RAxML Web Servers. Systematic Biology 57: 758–771. 10.1080/10635150802429642 [DOI] [PubMed] [Google Scholar]
- Suyama Y. (2011) Procedure for single-pollen genotyping. In: Isagi Y, Suyama Y (Eds) Single-pollen genotyping. Springer, Tokyo. 10.1007/978-4-431-53901-8 [DOI]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. (2011) MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28: 2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research 22: 4673–4680. 10.1093/nar/22.22.4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.