Abstract
Seagrass ecosystems rank amongst the most efficient natural carbon sinks on earth, sequestering CO2 through photosynthesis and storing organic carbon (Corg) underneath their soils for millennia and thereby, mitigating climate change. However, estimates of Corg stocks and accumulation rates in seagrass meadows (blue carbon) are restricted to few regions, and further information on spatial variability is required to derive robust global estimates. Here we studied soil Corg stocks and accumulation rates in seagrass meadows across the Colombian Caribbean. We estimated that Thalassia testudinum meadows store 241 ± 118 Mg Corg ha−1 (mean ± SD) in the top 1 m-thick soils, accumulated at rates of 122 ± 62 and 15 ± 7 g Corg m−2 year−1 over the last ~ 70 years and up to 2000 years, respectively. The tropical climate of the Caribbean Sea and associated sediment run-off, together with the relatively high primary production of T. testudinum, influencing biotic and abiotic drivers of Corg storage linked to seagrass and soil respiration rates, explains their relatively high Corg stocks and accumulation rates when compared to other meadows globally. Differences in soil Corg storage among Colombian Caribbean regions are largely linked to differences in the relative contribution of Corg sources to the soil Corg pool (seagrass, algae Halimeda tuna, mangrove and seston) and the content of soil particles < 0.016 mm binding Corg and enhancing its preservation. Despite the moderate areal extent of T. testudinum in the Colombian Caribbean (661 km2), it sequesters around 0.3 Tg CO2 year−1, which is equivalent to ~ 0.4% of CO2 emissions from fossil fuels in Colombia. This study adds data from a new region to a growing dataset on seagrass blue carbon and further explores differences in meadow Corg storage based on biotic and abiotic environmental factors, while providing the basis for the implementation of seagrass blue carbon strategies in Colombia.
Subject terms: Ecology, Biogeochemistry, Environmental sciences
Introduction
Seagrass meadows rank amongst the most prized ecosystems on Earth due to the provision of vital ecosystem services such as nutrient cycling, biodiversity and contribution to climate change mitigation and adaption through carbon sequestration and coastal protection despite they occupy less than 0.1% of the global marine surface1,2. Facing the urgency to reduce atmospheric greenhouse gases to mitigate climate change, the strategy known as blue carbon builds up on the conservation and restoration of coastal vegetated ecosystems (mangrove forests, tidal marshes, seagrass meadows and macroalgae beds) to enhance and preserve their capacity to act as natural carbon sinks3. It is estimated that seagrass globally store 140 Mg organic carbon (Corg) per hectare in the top meter of soils, accumulated over centennial-millenial time scales, being up to 40 times more efficient at capturing organic carbon (Corg) than land forests soils4,5.
Despite those benefits and services provided, seagrasses are among the most threatened ecosystems on the planet, and widespread die-off of seagrasses has been estimated at 0.9% year−1, due to a diversity of pressures including coastal development and coupled nutrient and sediment discharges causing eutrophication and siltation, respectively6. More recently, local conservation and management actions resulted in the deceleration and reversal of seagrass declining trends in Europe7 and USA8. However, it has been estimated that 0.15 Pg of CO2 may be released annually from disturbed seagrass ecosystems, which is equivalent to 3% of those from deforestation globally9. The inclusion of seagrass conservation and restoration projects into carbon crediting markets could provide a financial incentive to preserve the ecosystem services they provide, including their role in Corg storage and climate change mitigation and adaptation10.
Seagrass meadows are found along the shores of all continents except Antarctica occupying a global area estimated to range between 0.27 to 1.65 million2,11,12 km2, and encompass about 70 seagrass species13. Seagrass species have a broad dissimilarity in traits including differences in biomass and primary production rates14, that also vary across environmental conditions such as water depth and geomorphology15,16. Such differences in biotic and abiotic habitat characteristics result in up to 18-fold variability in Corg storage capacity across seagrass ecosystems17. An increasing number of studies are reporting seagrass blue carbon stocks and accumulation rates at local, regional and global scales5,18–23. However, there is a scarcity of seagrass blue carbon estimates in American countries, with the exception of Mexico, Canada, the United States in North America, and a few studies in Central and South America (Brazil, Panama and Dutch Caribbean)5,20,23–29. The scarcity of seagrass blue carbon studies in these key regions is limiting our capacity to derive robust global estimates while precluding their incorporation into national carbon accounting and the implementation of blue carbon strategies within Nationally Declared Contributions to mitigate climate change.
The Caribbean Sea, a tropical region with climates ranging from semi-arid to rain forests, provides a case for a region which is currently not represented in global estimates of seagrass blue carbon. Seagrasses grow in reef lagoons between the beaches and coral reefs in the Caribbean, and can form extensive meadows in protected embayment’s and estuaries. Out of the seven seagrass species found within the Caribbean Sea, Thalassia testudinum (turtle grass) is the most abundant species and forms persistent and climax seagrass ecosystems in the region13. The current area of T. testudinum seagrass beds in the Colombian Caribbean has been estimated in 661 km2, but extensive losses have also been documented30. For instance, Cartagena Bay experienced more than 90% reduction in seagrass extent over the past decades (from 2.5 to 0.18 km2) attributed to local anthropogenic disturbances31. Past and current anthropogenic threats to seagrass in the Caribbean region include coastal development, mining, sediment run-off and pollution, while natural threats are mainly related to hurricanes32.
This study aims to provide estimates of seagrass soil Corg stocks and accumulation rates for the underrepresented Colombian Caribbean (Fig. 1), based on biotic and abiotic environmental factors interacting at regional scales. We combine estimates of Corg density in 1 m-thick soils from T. testudinum meadows with soil accumulation rates derived from sediment chronologies determined with 210Pb and 14C to estimate soil Corg stocks within the top meter of soil and short- (last 70 years) and long-term (last 2000 years) soil Corg accumulation rates. We also assess differences in soil Corg storage among habitats based on the contribution of seagrass-Corg and allochthonous-Corg sources to the soil Corg pool, and sediment grain-size. The information gathered provides baseline values for future development of blue carbon strategies in Colombia as well as key information for regional and national marine planning and governance based on seagrass blue carbon ecosystem service.
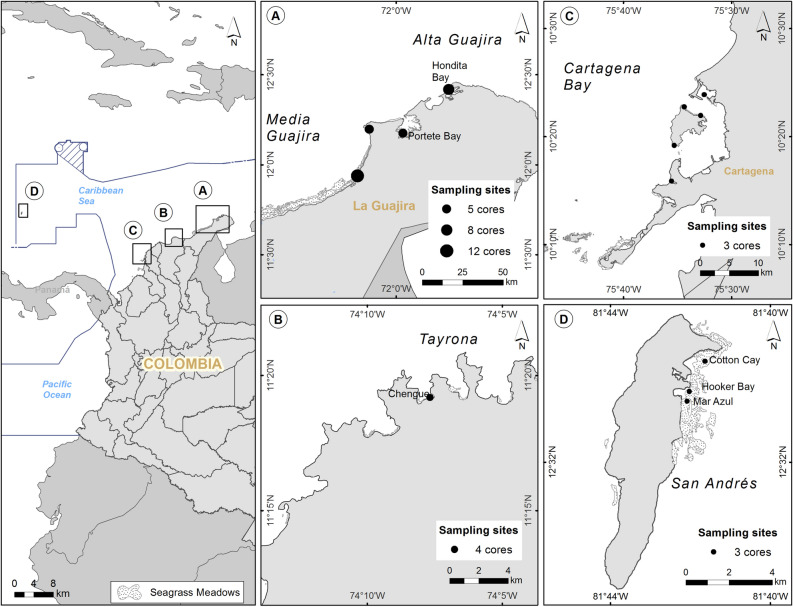
Figure 1.
Seagrass meadow distribution and location of the sampling sites in the Colombian Caribbean. Left panel shows the location of the five regions studied (Alta Guajira, Media Guajira, Cartagena Bay, Tayrona and San Andrés). (A–D) show the sampling sites within each region. The size of the circles indicates the number of cores studied at each sampling site. The maps were created in ArcGis Online (https://www.esri.com/en-us/arcgis/products/arcgis-online/overview).
Results
Compression of seagrass soils during coring operations averaged 37 ± 16% (mean ± SD; Supplementary Information Table A). The top meter of seagrass soils of the Colombian Caribbean had a mean ± SD dry bulk density (DBD) of 0.87 ± 0.32 g cm−3, containing 6.6 ± 4.0% organic matter (OM), 1.2 ± 0.9% organic carbon (Corg) and 7.9 ± 3.5 mg Corg cm−3 (Table 1). Seagrass soils were mainly constituted of particles > 0.016 < 0.125 mm (33 ± 24%), with a relatively high abundance of particles < 0.016 mm (11 ± 14%) and coarse sands > 0.5 mm (21 ± 21%). Soil Corg stocks in the top first meter ranged from 39 to 673 Mg Corg ha−1 (241 ± 118 Mg Corg ha−1; Table 1).
Table 1.
Mean (± SD) dry bulk density (g cm−3), organic carbon content (Corg, %), organic matter content (OM, %), δ13C (‰), sediment grain-size < 0.016 mm, > 0.0.016 < 0.125 mm and > 0.5 mm (%) and soil organic carbon stocks (in 1 m-thick, kg Corg m−2) in seagrass soils from all cores studied in the different locations and regions in the Colombian Caribbean.
| Region | Location | Core ID | DBD (g cm−3) | Corg (%) | OM (%) | δ13C (‰) | < 0.016 mm (%) | > 0.016 < 0.125 mm (%) | > 0.5 mm | Soil Corg stock in 1 m-thick | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | (Mg Corg ha−1) | |||
| Alta Guajira | Cabo de la Vela | AGCV1 | 1.0 | 0.2 | 5.0 | 0.8 | 224 | ||||||||||
| AGCV2 | 1.0 | 0.0 | 4.9 | 0.7 | 246 | ||||||||||||
| AGCV3 | 0.9 | 0.0 | 4.2 | 0.6 | 203 | ||||||||||||
| AGCV4 | 1.1 | 0.1 | 4.5 | 1.8 | 279 | ||||||||||||
| AGCV5 | 0.9 | 0.0 | 6.2 | 0.9 | 329 | ||||||||||||
| Portete Bay | AGBP1 | 0.6 | 0.2 | 16.8 | 5.2 | 673 | |||||||||||
| AGBP2 | 0.4 | 0.2 | 14.8 | 4.7 | 344 | ||||||||||||
| AGBP3 | 0.7 | 0.1 | 10.7 | 5.0 | 470 | ||||||||||||
| AGBP4 | 0.6 | 0.1 | 10.3 | 2.8 | 369 | ||||||||||||
| AGBP5 | 0.7 | 0.1 | 5.7 | 0.1 | 235 | ||||||||||||
| Hondita Bay | AGBH1 | 0.8 | 0.0 | 10.3 | 3.6 | 415 | |||||||||||
| AGBH2 | 0.8 | 0.1 | 8.4 | 3.0 | 370 | ||||||||||||
| AGBH3 | 0.8 | 0.3 | 1.3 | 1.1 | 8.9 | 3.2 | − 21.4 | 1.3 | 16.7 | 5.7 | 41.5 | 12.5 | 11.8 | 14.9 | 266 | ||
| AGBH4 | 0.9 | 0.2 | 10.8 | 4.1 | 479 | ||||||||||||
| AGBH5 | 0.8 | 0.2 | 8.2 | 1.7 | 381 | ||||||||||||
| AGBH6 | 0.5 | 0.1 | 1.8 | 0.9 | 11.6 | 3.4 | − 20.3 | 2.0 | 45.6 | 2.0 | 52.0 | 1.9 | 0.0 | 0.0 | 206 | ||
| AGBH7 | 0.5 | 0.0 | 12.1 | 2.6 | 350 | ||||||||||||
| AGBH8 | 0.6 | 0.1 | 15.9 | 3.5 | 522 | ||||||||||||
| Media Guajira | Carrizal | MGCZ1 | 1.2 | 0.1 | 3.3 | 2.0 | 151 | ||||||||||
| MGCZ2 | 1.1 | 0.1 | 6.5 | 1.8 | 357 | ||||||||||||
| MGCZ3 | 1.1 | 0.3 | 5.2 | 1.2 | 258 | ||||||||||||
| MGCZ4 | 1.1 | 0.2 | 0.5 | 0.1 | 2.4 | 0.7 | − 21.5 | 0.6 | 4.8 | 0.1 | 64.9 | 1.9 | 1.2 | 2.3 | 108 | ||
| MGCZ5 | 1.1 | 0.1 | 2.9 | 0.6 | 153 | ||||||||||||
| MGCZ6 | 1.0 | 0.1 | 3.5 | 1.2 | 159 | ||||||||||||
| MGCZ7 | 0.9 | 0.2 | 3.6 | 4.1 | 130 | ||||||||||||
| MGCZ8 | 1.0 | 0.2 | 5.7 | 3.9 | 203 | ||||||||||||
| MGCZ9 | 1.1 | 0.1 | 5.3 | 0.9 | 252 | ||||||||||||
| MGCZ10 | 1.0 | 0.3 | 0.5 | 0.2 | 3.6 | 0.6 | − 20.0 | 0.9 | 3.4 | 1.7 | 71.0 | 18.3 | 2.3 | 6.4 | 145 | ||
| MGCZ11 | 1.0 | 0.0 | 5.6 | 2.5 | 309 | ||||||||||||
| MGCZ12 | 1.1 | 0.1 | 4.7 | 3.6 | 267 | ||||||||||||
| Musichi | MGMC1 | 1.2 | 0.3 | 0.6 | 0.1 | 3.2 | 1.9 | − 22.8 | 1.0 | 3.5 | 0.8 | 64.4 | 21.1 | 6.8 | 10.0 | 139 | |
| MGMC2 | 1.3 | 0.1 | 2.9 | 0.9 | 176 | ||||||||||||
| MGMC3 | 1.1 | 0.3 | 5.2 | 2.6 | 338 | ||||||||||||
| San Andrés | Mar Azul | SAMA1 | 0.6 | 0.1 | 1.5 | 0.5 | 7.6 | 2.8 | − 16.5 | 1.0 | 23.5 | 3.0 | 35.9 | 4.1 | 0.0 | 0.0 | 193 |
| SAMA2 | 0.7 | 0.1 | 7.5 | 0.9 | 251 | ||||||||||||
| SAMA3 | 0.7 | 0.1 | 5.9 | 0.8 | 209 | ||||||||||||
| Hooker Bay | SABH1 | 0.6 | 0.2 | 1.7 | 0.9 | 8.2 | 1.7 | − 15.3 | 1.4 | 10.8 | 3.6 | 33.4 | 6.7 | 17.6 | 11.3 | 206 | |
| SABH2 | 0.8 | 0.2 | 7.4 | 3.2 | 258 | ||||||||||||
| SABH3 | 0.8 | 0.2 | 5.6 | 4.0 | 179 | ||||||||||||
| Cotton Cay | SACC1 | 0.9 | 0.2 | 1.1 | 0.8 | 4.9 | 1.6 | − 17.2 | 1.1 | 5.7 | 5.9 | 15.6 | 11.9 | 41.9 | 19.4 | 197 | |
| SACC2 | 1.0 | 0.1 | 6.5 | 1.2 | 331 | ||||||||||||
| SACC3 | 1.1 | 0.1 | 5.9 | 1.1 | 343 | ||||||||||||
| Tayrona | Chengue Bay | SMCH1 | 0.7 | 0.1 | 6.4 | 3.1 | 217 | ||||||||||
| SMCH2 | 1.0 | 0.1 | 5.0 | 1.4 | 229 | ||||||||||||
| SMCH3 | 1.2 | 0.1 | 5.2 | 1.5 | 240 | ||||||||||||
| SMCH4 | 1.2 | 0.1 | 5.0 | 0.9 | 238 | ||||||||||||
| Cartagena | La Virgen | CLV1 | 0.8 | 0.0 | 5.2 | 0.0 | 2.4 | 0.9 | 11.5 | 1.1 | 44.4 | 8.4 | 148 | ||||
| CLV2 | 0.8 | 0.1 | 7.1 | 3.2 | 3.1 | 1.1 | 12.1 | 1.1 | 38.1 | 10.6 | 219 | ||||||
| CLV3 | 0.7 | 0.0 | 5.2 | 0.3 | 1.6 | 1.0 | 10.0 | 2.1 | 43.6 | 10.0 | 138 | ||||||
| Tierra Bomba | CTTB1 | 0.9 | 0.1 | 5.3 | 0.9 | 8.5 | 5.0 | 28.4 | 14.8 | 25.6 | 12.9 | 168 | |||||
| CTTB2 | 0.9 | 0.0 | 5.8 | 0.8 | 9.5 | 4.8 | 25.8 | 13.3 | 29.8 | 10.7 | 184 | ||||||
| CTTB3 | 1.0 | 0.0 | 4.8 | 1.2 | 6.6 | 5.2 | 21.1 | 16.6 | 28.6 | 16.7 | 162 | ||||||
| Punta Arena | CTPA1 | 0.8 | 0.0 | 4.1 | 0.3 | 0.2 | 0.5 | 6.8 | 3.8 | 47.6 | 7.7 | 119 | |||||
| CTPA2 | 1.1 | 0.0 | 4.4 | 0.3 | 0.9 | 1.2 | 8.6 | 4.4 | 42.8 | 8.4 | 159 | ||||||
| CTPA3 | 0.9 | 0.0 | 4.2 | 0.3 | 0.9 | 1.2 | 8.2 | 3.4 | 44.8 | 2.8 | 142 | ||||||
| Cocon Bay | CTBC1 | 0.9 | 0.1 | 4.5 | 0.8 | 0.0 | 0.0 | 2.7 | 3.2 | 62.9 | 6.7 | 141 | |||||
| CTBC2 | 1.2 | 0.1 | 4.6 | 0.2 | 0.4 | 0.6 | 6.7 | 1.9 | 45.3 | 14.5 | 200 | ||||||
| CTBC3 | 1.4 | 0.0 | 4.4 | 0.8 | 0.5 | 0.5 | 8.2 | 4.2 | 59.0 | 12.5 | 226 | ||||||
| Bocagrande | CTBG1 | 1.3 | 0.0 | 1.4 | 0.2 | 0.0 | 0.0 | 6.4 | 1.4 | 33.0 | 8.3 | 39 | |||||
| CTBG2 | 1.3 | 0.1 | 1.3 | 0.1 | 0.0 | 0.0 | 8.1 | 2.3 | 23.3 | 10.8 | 40 | ||||||
| CTBG3 | 1.9 | 0.5 | 1.0 | 0.2 | 0.0 | 0.0 | 5.1 | 2.9 | 31.0 | 6.5 | 40 | ||||||
| OVERALL | 0.9 | 0.3 | 1.2 | 0.9 | 6.6 | 4.0 | − 19.4 | 2.8 | 11.1 | 13.7 | 33.5 | 23.9 | 21.2 | 21.5 | 241 ± 117 | ||
A total of 11 cores were processed to determine the sedimentation rates during the last decades using the 210Pb dating method. Due to the high degree of mixing presented in five of the cores analyzed, it was only possible to determine sediment accumulation rates in six cores. It was possible to estimate sedimentation rates during the last 500–2000 years using the 14C dating method in five cores, based on one to three radiocarbon results per core (Supplementary Information Table B). Short- and long-term soil accretion rates (SAR) ranged from 0.9 to 7.0 mm year−1 (3.9 ± 0.4 mm year−1) and 0.37 to 3.4 mm year−1 (1.8 ± 0.9 mm year−1), respectively, while short- and long-term Corg accumulation rates (CAR) ranged from 34 to 195 g Corg m−2 year−1 (122 ± 62 g Corg m−2 year−1) and from 2.2 to 27.5 g Corg m−2 year−1 (14.9 ± 7.2 g Corg m−2 year−1), respectively (Table 2).
Table 2.
Mean (± SD) short-term (last decades) and long-term (last 2000 years) sediment accumulation rates (SAR; mm year−1) and organic carbon accumulation rates (CAR; g Corg m−2 year−1) in seagrass soils from all cores successfully dated in the different locations and regions in the Colombian Caribbean.
| Region | Location | Core ID | Short-term SAR | Long-term SAR | Short-term CAR | Long-term CAR |
|---|---|---|---|---|---|---|
| (mm year−1) | (mm year−1) | (g Corg m−2 year−1) | (g Corg m−2 year−1) | |||
| Alta Guajira | Bahia Portete | AGBP2 | 1.8 ± 0.3 | 93.3 ± 17.1 | ||
| Bahia Hondita | AGBH3 | 0.89 ± 0.16 | 0.44 ± 0.09 | 33.6 ± 5.9 | 4.5 ± 0.9 | |
| Media Guajira | Carrizal | MGCZ3 | 3.3 ± 0.8 | 79.5 ± 20.1 | ||
| Musichi | MGMC1 | 0.37 ± 0.17 | 2.20 ± 1.01 | |||
| San Andrés | Mar Azul | SAMA1 | 6.97 ± 0.61 | 3.36 ± 1.35 | 167 ± 15 | 27.5 ± 11.1 |
| Bahia Hooker | SABH1 | 5.97 ± 0.28 | 2.14 ± 1.67 | 164 ± 8 | 19.1 ± 14.9 | |
| Cotton Cay | SACC1 | 4.55 ± 0.15 | 2.84 ± 1.11 | 195 | 21.2 ± 8.2 | |
| OVERALL | 3.9 ± 0.4 | 1.8 ± 0.9 | 122 ± 62 | 14.9 ± 7.2 |
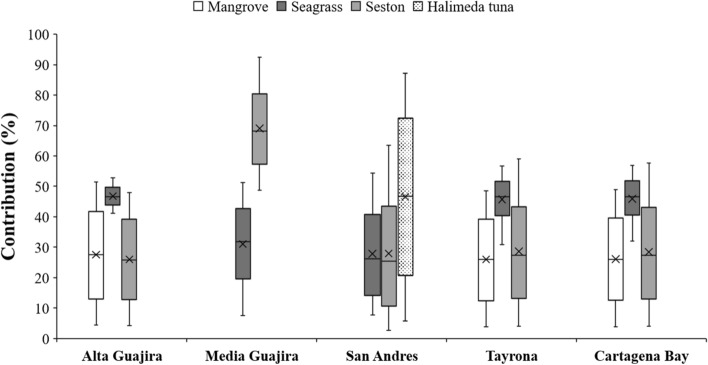
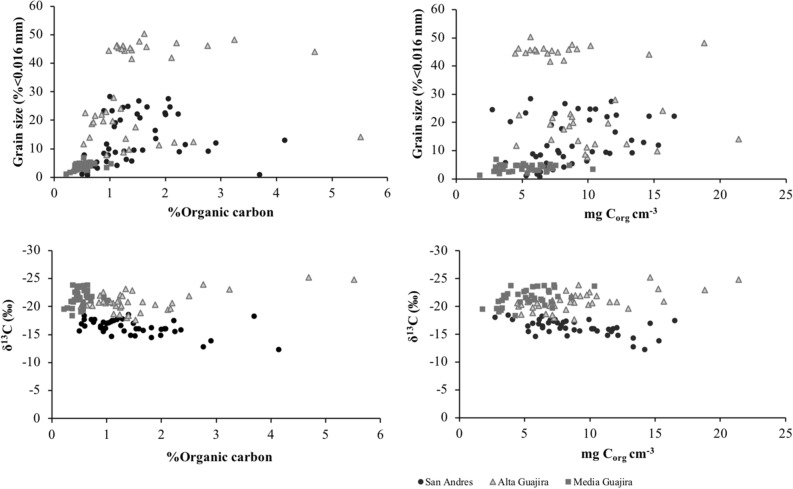
The δ13C values of sedimentary organic Corg in seagrass soils averaged -19.4 ± 2.8‰. The δ13C values of seagrass, mangrove, seston and Halimeda tuna (Supplementary Information Table C) were used to run mixing models to estimate the contribution of potential sources into the sedimentary Corg pool. Seagrass detritus and seston were the most important source of Corg in seagrass soils from the Colombian Caribbean (39 ± 13% and 36 ± 24%, respectively), with the exception of meadows at San Andrés where macroalgae H. tuna contributed 47 ± 29%. Mangrove matter contributed 27 ± 15% across Alta Guajira, Tayrona and Cartagena Bay regions where mangroves are present (Fig. 2). Overall, Corg (% and mg Corg cm−3) contents increased with increasing fine particle contents (% < 0.016 mm) in all regions except Alta Guajira (Fig. 3). There was a lack of relationship between δ13C and Corg (% and mg Corg cm−3) contents.
Figure 2.
Box plots showing the results of the isotope mixing models to determine the contribution of sources (seagrass, seston, Halimeda tuna and mangrove) to the soil organic carbon pool in the five study sites.
Figure 3.
Relationships between soil organic carbon content (% Corg and mg Corg cm−3) and sediment particles < 0.016 mm and δ13C (‰) in the seagrass cores from San Andrés, Cartagena Bay and La Guajira in the Colombian Caribbean.
The DBD increased with soil depth in the seagrass meadows at Alta Guajira, Media Guajira and San Andrés, but remained relatively stable at Tayrona and Cartagena Bay (Supplementary Information Figure A). OM and Corg contents decreased with soil depth at all sites, while δ13C remained stable with soil depth. The content of particles < 0.016 mm remained relatively stable along soil depth, with the exception of higher content below 100 cm depth at Cartagena Bay. Coarse sand (> 0.5 mm) content was relatively higher within the upper 20 to 90 cm at Alta Guajira, Media Guajira and Cartagena Bay, while oscillated between 5 to 40% along the soil cores at San Andrés.
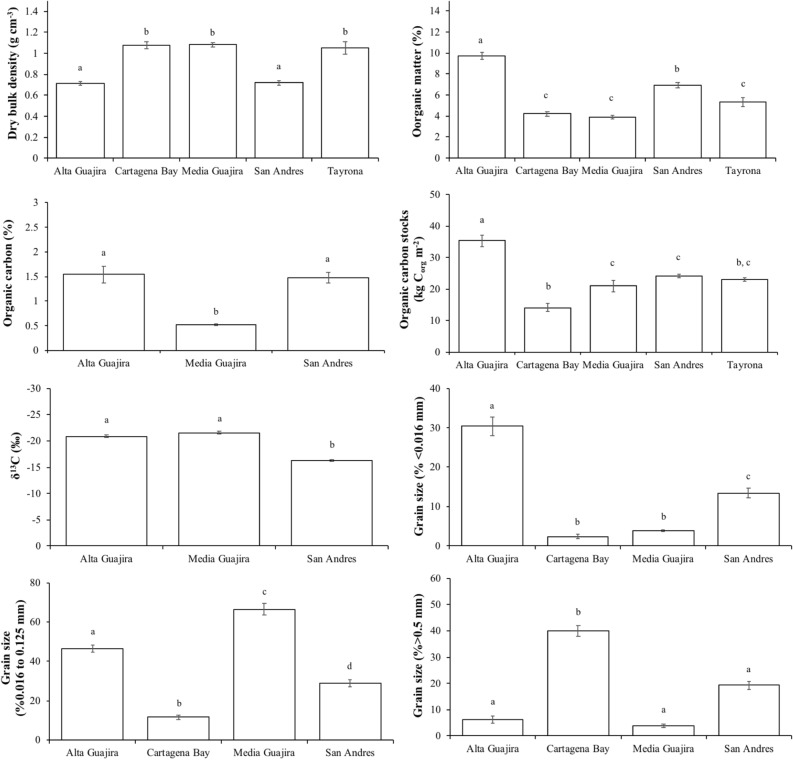
Soil biogeochemical characteristics (DBD, %Corg, %OM, δ13C, and particles < 0.016 mm, > 0.016 < 0.125 mm and > 0.5 mm) and soil Corg stocks were significantly different (P < 0.05) among regions (Alta Guajira, Media Guajira, Tayrona, Cartagena Bay and San Andrés; Table 3). Soil DBD in meadows at Alta Guajira (0.7 ± 0.3 g cm−3) and San Andrés (0.7 ± 0.2 g cm−3) were significantly lower than at the other regions (average ranging from 1.0 to 1.1 g cm−3; Fig. 4). The soil OM content was significantly higher in Alta Guajira (9.7 ± 4.6% OM) compared to meadows at San Andrés (6.9 ± 2.5% OM), while OM content at both Alta Guajira and San Andrés was higher than at Media Guajira, Tayrona and Cartagena Bay (ranging from 3.9 to 5.3%; Table 3). The soil Corg content followed a similar pattern, with significantly higher values at Alta Guajira (1.54 ± 1.02% Corg) and San Andrés (1.47 ± 0.80% Corg) compared to Media Guajira (0.52 ± 0.16% Corg). The Corg stocks within the top meter of soil were up to twofold higher in Alta Guajira (353 ± 125 Mg Corg ha−1) compared to Cartagena Bay (142 ± 61 Mg Corg ha−1), while the other regions had intermediate soil Corg stocks with average values ranging from 210 to 241 Mg Corg ha−1. The lower number of SAR and CAR data across regions precluded comparisons. The δ13C values of soil Corg were significantly lower in Alta and Media Guajira (average ranging from -21 to -22‰) than in San Andrés (-16 ± 1.4‰). Based on isotope mixing models, seagrass was the major contributor to the soil Corg pool in the meadows located at Alta Guajira, Tayrona and Cartagena Bay (46% in all cases), with ~ 25% contributions of each seston and mangrove matter (Fig. 2). At Media Guajira, seston contributed 69%, while seagrass contributed the remaining 31%. The contribution of H. tuna to soil Corg pool at San Andrés was estimated at 47%, with seagrass and seston contributing 27% each. Seagrass soils at Alta Guajira had significantly higher amount of particles < 0.016 mm (30 ± 15%) than the other regions (ranging from 2 to 13%), while particles > 0.016 < 0125 mm were more abundant in Media Guajira (67 ± 17%) and Alta Guajira (46 ± 11%) (ranging from 12 to 29%; Table 1). The concentration of coarse sands > 0.5 mm was significantly higher in Cartagena Bay (40 ± 15%) compared to the other regions (ranging from 4 to 19%).
Table 3.
Results of the Generalized Linear Models (GLM) testing for significant effects of region (Alta Guajira, Media Guajira, San Andrés, Tayrona, Cartagena) on seagrass soil dry bulk density (g cm−3), soil organic carbon content (%), soil organic matter content (%), soil organic carbon stocks (Mg Corg ha−1 in 1-m thick) , δ13C (‰), and content of sediment grain-sizes < 0.016 mm, > 0.0.016 < 0.125 mm and > 0.5 mm (%) in the Colombian Caribbean.
| Variable | Factor | F | df | P value |
|---|---|---|---|---|
| Dry bulk density (g cm−3) | Region | 74.23 | 4 | < 0.001 |
| Organic carbon (%) | Region | 21.25 | 2 | < 0.001 |
| Organic matter (%) | Region | 83.73 | 4 | < 0.001 |
| Corg stocks (Mg Corg ha−1) | Region | 12.47 | 4 | < 0.001 |
| δ13C (‰) | Region | 148.94 | 2 | < 0.001 |
| < 0.016 mm (%) | Region | 93.36 | 3 | < 0.001 |
| > 0.016 < 0.125 mm (%) | Region | 180.86 | 3 | < 0.001 |
| > 0.5 mm (%) | Region | 145.13 | 3 | < 0.001 |
Figure 4.
Mean (± SE) dry bulk density (g cm−3), organic matter (%), organic carbon (% Corg and kg Corg m−2 in 1 m-thick soils), δ13C (‰), and sediment grain-sizes < 0.016 mm, > 0.0.016 < 0.125 mm and > 0.5 mm (%) in seagrass soils from Alta Guajira, Cartagena Bay, Media Guajira and San Andrés in the Colombian Caribbean. Results of GLM tests to assess differences are denoted: different letters (a, b, c) indicate significant differences (P < 0.05).
Discussion
Seagrasses are sparsely distributed along the Colombian Caribbean, but their Corg stocks in 1 m-thick soils (241 Mg Corg ha−1 on average) are well above the values from global estimates (140 Mg Corg ha−1)5 and other tropical regions such as Mexico, US and Indonesia (ranging23,33–35 from 71 to 170 Mg Corg ha−1. Estimates of soil short-term CAR (122 g Corg m−2 year−1) in Colombian seagrass meadows is higher than in the Dutch Caribbean (84 g Corg m−2 year−1)25 and Australian (36 g Corg m−2 year−1)21 meadows. The relatively high Corg sink capacity of Thalassia seagrass meadows in Colombia could be explained by a combination of biogeochemical and environmental processes linked to habitat characteristics. The up to twofold higher above- and below-ground biomass and production of T. testudinum compared to the mean of seagrass species globally14, likely provides a relatively larger amount of seagrass detritus for burial. Indeed, the tropical wet climate in Colombia together with the numerous rivers discharging along the coastline results in the accumulation of fine-sized particles < 0.0125 mm (44 ± 3%) within seagrass meadows (Fig. 4), which have been shown to be conductive of soil Corg preservation36,37. In turn, terrestrial run-off and mangrove vegetation deliver allochthonous Corg into the coastal zone that contributes to the seagrass soil carbon pool (54 ± 0.1%)38. SAR in Colombian seagrass meadows averaged 3.9 ± 0.4 mm year−1 over the last century, which are up to fourfold higher than previously reported for seagrass meadows globally1, and for China, Japan, Mediterranean Sea, Arabian Gulf and Australia (ranging from 0.819,21,39 to 2.1 mm year−1, but similar to Zostera marina meadows20. High SAR entails rapid burial of organic matter in anoxic conditions thereby reducing Corg remineralization rates and enhancing Corg storage15. The lack of excess 210Pb in some of the cores analysed suggests that there is no net accumulation in some areas, or accumulation of reworked materials. Further studies (e.g. deployment of surface elevation tables to measure sediment accretion in situ) are required to overcome the limitations of radioisotopes40. Overall, the high seagrass primary production together with the rapid accumulation of suspended particles results in highly depositional environments that enhance carbon accumulation and preservation, which could explain the relatively high Corg storage capacity of seagrass meadows along the Colombian Caribbean compared to other meadows globally.
Differences in seagrass soil carbon storage across Colombian regions
The up to threefold variability in seagrass soil Corg stocks among regions in the Colombian Caribbean appears to be largely linked to the relative contribution of Corg sources to the soil Corg pool (seagrass, algae, mangrove and seston) and inputs of fine sediment particles binding Corg and enhancing its preservation. The limited number of cores that were successfully dated with 210Pb and/or 14C (N = 6 each) precluded comparison of CAR among regions.
La Guajira contains 88% of the country's T. testudinum seagrass extent (581 km2) and seagrass soil Corg stocks (12.4 Tg Corg). Although Alta Guajira only contains 18 km2 of seagrass meadows, it holds the largest soil Corg stocks per unit area in Colombia (352 Mg Corg ha−1)41, which also rank among the highest values reported globally5. The high soil Corg stocks per unit area in Alta Guajira are likely related to the high depositional nature of their habitats, restricted to enclosed embayments protected from hydrodynamic energy and hurricanes, with the exception of the meadows located in Cabo de la Vela42. The seagrass soil in Alta Guajira are mainly composed of soil inorganic particles < 0.125 mm (76%), which together with the contribution of mangrove and seston matter to the soil Corg pool (28% and 26%, respectively) likely led to enhanced soil Corg accumulation and preservation in seagrass meadows (Figs. 2 and 4). The lack of correlation between particles < 0.016 mm and Corg content in Alta Guajira can be explained by the saturation of particles with adsorbed organic Corg together with the presence of coarse seagrass detritus37. The Alta Guajira region is exposed to extreme climate events driven by tropical storms and hurricanes, which can result in the sudden destruction of seagrass meadows41,42 and the erosion of seagrass soil Corg stocks43. Since the onset of seagrass monitoring at Cabo de la Vela in 2015, one cyclone disruption event resulted in the erosion of > 0.5 ha of seagrass meadows and the top ~ 0.6 m of soils underneath the meadows41, likely resulting in CO2 emissions from perturbed soil Corg stocks. However, seagrasses in the protected embayments of Hondita Bay and Portete Bay within Alta Guajira remained undisturbed41. Further studies are required to assess the risk of tropical storms for blue carbon projects in tropical regions, in particular in the face of increased frequency and intensity of extreme climate events under predicted climate change scenarios44.
The relatively low seagrass soil Corg stocks in Media Guajira (210 Mg Corg ha−1) compared to the Alta Guajira (352 Mg Corg ha−1) is likely related to the severe exposure of meadows to hydrodynamic energy in the former. This region is constantly exposed to the currents and swell generated by strong winds from the East and Northeast, which results in the resuspension of particulate materials and turbid waters that limit the water depth limit for seagrass to up to 7 m. These processes are reflected in the relatively low content of sediment particles < 0.016 mm (4%) and dominance of particles > 0.016 < 0.125 mm (66%), which together with the low seagrass contribution to the soil Corg pool (31%) compared to seston (69%) suggests that meadows in this region are thriving in a highly dynamic environment compared to other Colombian Caribbean regions42 that could be limiting their productivity owing to reduced irradiance.
Meadows along the rocky coastline of Tayrona are restricted to enclosed environments protected from the swell (only 0.9 km2 of meadows mapped)31. The soil Corg stocks at our study site were relatively high (231 Mg Corg ha−1), likely related to the high productivity of the meadows thriving in clear waters, and the input of seston and mangrove detritus from adjacent creeks. However, the lack of stable Corg isotope and grain size analyses in the cores from this region preclude further interpretations.
Seagrass meadows have been severely decimated at Cartagena Bay over the past decades (from 100 km2 in 1930s to 9 km2 in 200145. In turn, only small patches of seagrass meadows (< 0.1 km2 each) remain at Cartagena Bay, and their soil Corg stocks are the lowest found across Colombia (142 Mg Corg ha−1). Previous studies showed that soil Corg stocks are lower in patchy seagrass landscapes compared to continuous and extensive meadows36,46. Indeed, coarse sand and gravel (40%) originated from adjacent corals dominate the soil inorganic particles in Cartagena Bay’ meadows, which together with the high degree of anthropization in the region could explain the weakening of seagrass Corg sinks in this region47.
Seagrass meadows in San Andrés and Providencia Archipelago (5 km2) are located in a coastal lagoon basin protected from currents and waves, and to a lesser degree from tropical storms and cyclones by a reef barrier, which facilitates sedimentation48,49 and likely explains the relatively high soil Corg stocks in this region (241 Mg Corg ha−1). The short-term CAR reported for this region (175 g Corg m−2 year−1) are the highest reported to date for seagrass meadows, with the exception of two P. oceanica meadows from the Mediterranean Sea (ranging from 202 to 249 g Corg m−2 year−1)50. The seagrass soils in San Andrés contain large amounts of H. tuna inorganic and organic (47%) matter. The calcareous macroalgae Halimeda spp. is common in coral reefs and has very high primary production rates51,52 that likely contribute to enhance SAR through high carbonate production, and CAR through rapid burial of Corg in anoxic conditions. Further studies are required to understand the specific role of H. tuna in blue carbon, including the implications of calcification and dissolution in the carbon cycle53 as well as those enhancing CAR.
Seagrass blue carbon in the Colombian Caribbean
Seagrass T. testidinum in the Caribbean region of Colombia occupies 661 km2, with 18 km2 in Alta Guajira, 563 km2 in Media and Baja Guajira, 0.9 km2 in Tayrona, 9 km2 in Cartagena Bay, 5 km2 in San Andrés and surrounding islands, and 65 km2 in the Caribbean coastline west of Cartagena Bay45. Based on the spatially-explicit estimates of seagrass extent and soil Corg stocks and accumulation rates in the five regions studied (Fig. 4), we estimate that seagrass in the Colombian Caribbean contain 14 Tg of Corg in the top meter of soils. This is equivalent to 52 Tg CO2 stored in the soils, which corresponds to 62% of the CO2 emissions from fossil-fuel burning by Colombia in 201454. Based on average CAR estimates (122 g Corg m−2 year−1 over the last ~ 70 years), we estimated that seagrass meadows in the Colombian Caribbean accumulate 0.081 Tg Corg year−1 (equivalent to 0.3 Tg CO2 year−1), which corresponds to ~ 0.4% of CO2 emissions from fossil fuels in Colombia at 2014 rates54. It is important to note that these estimates are likely underestimating seagrass soil Corg storage in the Colombian Caribbean, owing to poor seagrass mapping in some regions and the exclusion of meadows formed by small species meadows of the genera Syringodium, Halophila, Halodule and Ruppia that occur in Colombia. However, owing to the lack of excess 210Pb in some meadows, further studies are required to determine whether some of the meadows studied continue to accumulate soil Corg40. Indeed, carbonate burial in seagrass meadows, which is particularly important in tropical regions, likely offsets a portion of the CO2 sequestered through the burial of Corg53. Saderne et al55 estimated that CaCO3 burial within seagrass meadows may offset ~ 30% of the net CO2 sequestration through photosynthesis, but acknowledged that the origin of CaCO3 stored within seagrass meadows is mostly derived from adjacent ecosystems rather than by calcifying organisms inhabiting or supported by seagrass ecosystems. In addition, the dissolution of CaCO3 linked to seagrass metabolism and associated biota, which is also common in tropical regions, results in the release of alkalinity and thereby CO2 removal55,56. Further studies are required to decipher the role of inorganic carbon processing in the overall CO2 budget in seagrass meadows53,57.
Our study demonstrates that Colombia host some of the largest seagrass soil carbon stocks per unit area globally (241 Mg Corg ha−1 in the top meter of soils), sitting between global median values (140 Mg Corg ha−1) and those of Posidonia oceanica meadows in the Mediterranean Sea (375 Mg Corg ha−1)50. This characteristic in combination with the large losses of seagrass extent over the last decades in Caribbean Coast41,45, places Colombia among the countries that can largely benefit from blue carbon strategies to contribute to mitigate and adapt to climate change while preserving and restoring their natural heritage and enhancing the plethora of ecosystem services that vegetated coastal ecosystems provide2. Colombia hosts one of the few ongoing blue carbon projects in the world, involving carbon trading by protecting 270 km2 of mangrove forests from deforestation58.
In summary, this study demonstrates that seagrass meadows along the Colombian Caribbean are hotspots for carbon sequestration and storage, provides data from a new region to a growing dataset on seagrass blue carbon and further explores differences in soil Corg storage based on biotic and abiotic drivers. The spatially explicit estimates of seagrass soil Corg stocks contribute to identify key regions for conservation and management actions (including restoration) aiming to enhance CO2 sequestration and/or avoid greenhouse gas emissions resulting from seagrass loss, while providing the basis for the implementation of seagrass blue carbon strategies in Colombia. The reversal of historical seagrass losses can be traded in carbon markets and in this study, we provide baseline estimates of soil Corg stocks and accumulation rates in Colombian seagrasses to contribute to the conservation of seagrass ecosystems through seagrass restoration strategies at the national scale.
Materials and methods
Study sites and sampling
This study was conducted in five regions (Alta Guajira, Media Guajira, Tayrona, San Andrés, and Cartagena Bay) along 2250 km of the Colombian Caribbean (Fig. 1). Seagrass species T. testudinum forms the most extensive and apparent meadows along the Colombian coast. More than 85% of seagrass extent in Colombia is located within the Guajira peninsula (562 km2 out of the estimated 661 km2)45.
Seagrass meadows in Alta Guajira and Media Guajira grow not deeper than 7 m water depth on sandy habitats along the shoreline, and are exposed to regular wind-driven waves, with the exception of those meadows inhabiting the few existing embayment’s. The meadows in La Guajira are not exposed to severe anthropogenic disturbances because the remoteness of the region, despite small indigenous and non-indigenous settlements may cause localized impacts31,45. Seagrass meadows in Tayrona National Natural Park grow in shallow sandy areas among corals and within embayment’s, and the study sites are not impacted by direct anthropogenic activities. Cartagena Bay encompasses a wide variety of seagrass habitats with different gradients of anthropogenic disturbances (mainly from industry, boating activities and dredging) and geomorphological settings (open coastlines and embayment’s) 43. Seagrasses in San Andrés grow on shallow soil of weathered coral and the study sites are located near the main touristic village of the island59. Out of the studied regions, mangrove forests are only present in Alta Guajira, Tayrona and Cartagena Bay. La Guajira is characterized by a drier tropical climate (1100 annual precipitation) compared to the other studied regions located further west of the Colombian Caribbean (ranging from 1600 mm per year in Tayrona and Cartagena Bay to 1900 mm per year in San Andrés).
A total of 14 sites across the five regions were sampled in this study (Fig. 1). Sixty-one soil cores were sampled in 0.5 to 3 m-deep meadows dominated by T. testudinum by manual percussion and rotation of PVC pipes (150 cm long, 65 mm inner diameter; Supplementary Information Table A). Three to twelve replicate cores were sampled within 200 m2 of each meadow at each site (61 cores in total). Soil compaction during coring was measured as the difference in surface soil elevation inside and outside the core60. The length of all cores was corrected for compaction (Supplementary Information Table A), and all results presented hereafter refer to the amended ‘uncompressed’ depths. The cores were sealed at both ends, and stored vertically at 4 °C until further processing. Seagrass plants (T. testudinum), mangrove (Rhizophora mangle) leaves, algae (H. tuna) and seston (> 0.7 μm) samples were collected across the study sites to determine of origin of soil Corg in seagrass meadows using stable carbon isotopes (Supplementary Information Table C).
Laboratory procedures
The cores were opened longitudinally using an electric saw and cut at 1 to 15 cm-thick. The samples were dried at 60 °C until constant weight to estimate the dry weight density (g cm−3), and then divided in two by quartering for subsequent analyses. One set of subsamples were milled using an electric mortar and used for soil organic matter and Corg content analyses to estimate soil Corg storage, and stable carbon isotope (δ13C) analysis to determine the sources of soil Corg. The other set of subsamples was used for radioisotope (210Pb and 14C) dating of the cores and soil grain-size analysis to determine distribution of particle sizes. Organic matter analyses were run in all samples from all cores sampled (N = 61), while Corg and grain-size analyses were only conducted in alternate samples along eight and 23 cores, respectively (Table 1).
For organic matter analyses, 4 g of milled sample was combusted at 550 °C for 4 h to estimate the proportionate loss of organic matter (loss on ignition, LOI)61. For the analyses of soil Corg and δ13C, 2 g of ground sample was acidified with 4% HCl to remove inorganic carbon, centrifuged (3400 revolutions per minute, for 5 min), and the supernatant with acid residues was carefully removed by pipette, avoiding resuspension. The sample was then washed with Milli-Q water, centrifuged and the supernatant removed. The residual samples were re-dried and then encapsulated for Corg and δ13C analyses using a Thermo Delta V Conflo III coupled to a Costech 4010 at the UH Hilo Analytical Laboratory (USA). The Corg content was calculated for the bulk (pre-acidified) samples. Macrophyte samples containing inorganic carbon and seston were acidified prior to Corg analysis, following the procedures above for soils. Stable carbon isotope ratios (δ13C) are expressed as δ values in parts per thousand (‰) relative to the Vienna PeeDee Belemnite standard. Replicate assays and standards indicated measurement errors of 0.02% for Corg content and 0.07‰ for δ13C. Sediment grain size was measured with a Coulter LS230 laser-diffraction particle analyzer at the University of Barcelona (Spain), after digestion of organic matter with 30% H2O2. The grain size data was classified in the following fractions: < 0.016 mm, > 0.016 < 0.125 mm and > 0.5 mm.
Concentrations of 210Pb were determined by alpha spectrometry through the measurement of its granddaughter 210Po, assuming radioactive equilibrium between the two radionuclides62. Between 150 and 300 mg of each sample were spiked with known amounts of 209Po and acid digested using a microwave oven. The Po isotopes were plated onto silver discs (99.99% purity) and their emissions were measured by alpha spectrometry using Passivated Implanted Planar Silicon (PIPS) detectors (CANBERRA, Mod. PD-450.18 A.M.) and silicon surface barrier detectors (ORTEC, mod. Ensemble). A selection of samples from some cores was measured for 226Ra by gamma spectrometry using a high-purity Ge well-type detector (CANBERRA, Mod. GCW3523) to determine the concentrations of supported 210Pb, used to calculate the excess 210Pb concentrations were calculated. The 226Ra concentrations were found to be comparable to those of 210Pb at depth in those cores, and thus the later were taken as supported 210Pb for the rest of the cores. The average sediment accumulation rates over the past 100 years could be estimated for six of the eleven dated sediment cores using the Constant Flux:Constant Sedimentation (CF:CS) model below the mixed surface layer when present63. The other cores showed either evidence of intense mixing or negligible presence of excess 210Pb that precluded estimating a sedimentation rate.
A total of 17 radiocarbon analyses were conducted in the bulk soil organic matter in seven cores sampled across the five regions (1–3 14C analyses per core; Supplementary Information Table B) at the AMS Direct Laboratory (USA) following standard procedures64. The 14C age-depth models were produced using the R routine “Bacon” for Bayesian chronology building65, after calibration of 14C ages using the marine13 radiocarbon age calibration curve, considering a local delta R of 25 ± 8 years66, and assuming that the top core corresponded to the year of sampling. In two cores, the 14C results showed that either the core was mixed or that the samples were younger than ~ 400 years and therefore, it was not possible to estimate age-depth chronologies based on 14C in these cores. All 14C ages reported in this study are expressed as radiocarbon calibrated years.
Numerical procedures
A linear regression between % Corg and % LOI (R2 = 0.78) was used to estimate the % Corg content for subsamples that were not analyzed for Corg5. The density of organic carbon (g Corg cm−3) was calculated for each core slice by multiplying the sediment dry bulk density (g cm−3) by the Corg concentration (%). To allow direct comparisons among locations, the soil Corg stocks per unit area (cumulative stocks; Mg Corg ha−1) were standardized to 1 m-thick deposits. When sediment cores (32 out of 61) were shorter than 100 cm (65 to 90 cm), soil Corg stocks in the top 1 m were estimated by extrapolating the integrated values of Corg content (cumulative Corg stock; Mg Corg ha−1) linearly with depth. To validate this extrapolation approach, we assessed the correlation between extrapolated Corg stocks from 65 cm to 1 m and measured Corg stocks in 1 m soil cores across the 29 sampled cores that were equal or longer than 100 cm (r = 0.97; P < 0.001; Supporting Information Figure B). Soil Corg accumulation rates (expressed in g Corg m−2 year−1) for the last century and the last 2500 years were estimated using 210Pb and 14C age-depth models, respectively. Short-term (based on 210Pb) and long-term (based on 14C) soil Corg accumulation were calculated in 6 and 5 out of the 61 cores sampled, respectively, by multiplying the Corg inventories in 1 m-thick soil by the respective average soil accretion rate (cm year−1).
Pearson correlation analyses were run to assess significant relationships between soil Corg storage (stocks in Mg Corg ha−1 and short- and long-term accumulation rates in g Corg m−2 year−1) and soil δ13C and particles < 0.016 mm. Statistical analyses were performed using univariate General Linear Models (GLM) procedures in Statgraphics v16 software. The GLMs were run to test for significant effects of region (Alta Guajira, Media Guajira, San Andrés, Tayrona and Cartagena) on the soil organic carbon content (% Corg), soil Corg stocks (Mg Corg ha−1), short- and long-term soil Corg accumulation rates (g Corg m−2 year−1), stable carbon isotope values of soil Corg (δ13C) , % < 0.016 mm, %0.016 to 0.125 mm and % > 0.5 mm (Table 3). Region was treated as fixed factor and all response variables were square-root transformed prior to analyses to meet homogeneity of variance. Normality and homoscedasticity of model residuals were assessed visually.
One-isotope, two- to three-source mixing models were run in R using simmr and rjags packages67,68. The isotope used in this model was δ13C, and the types and values of source end-members used varied across regions based on the presence/absence of potential sources and the specific values obtained for each region. Seagrass, mangrove and seston were included for Alta Guajira, Tayrona and Cartagena Bay, while only seagrass and seston were included for the Media Guajira region, and seagrass, seston and H. tuna for San Andrés (Supplementary Information Table C). The reference isotopic signatures (mean ± SD) used in this model comprised δ13C values from this study.
Supplementary Information
Acknowledgements
The research reported in this paper was supported by MAPCO Action Grant contract for European Union external actions ENV/2016/380-526 cofounded by INVEMAR and Fundación Natura (Colombia,) with the support of ECU (Australia) and CSIC (project COOPB20366, I-COOP+ program, Spain). Its contents are the sole responsibility of the authors and do not necessarily reflect the views of the European Union. O.S. was supported by an ARC DECRA (DE170101524). C.S. was supported by ECU Higher Degree by Research Scholarship. P.M. was supported by the Australian Research Council LIEF Project (LE170100219). The IAEA is grateful for the support provided to its Environment Laboratories by the Government of the Principality of Monaco. This is the Contribution #1306 from INVEMAR. This study complies with national and international guidelines, legislation and permits for the collection of plant and soil specimens. The macrophytes used in this study were identified by D.I.G.-L. and O.S. and have been deposited in the public museum collection at INVEMAR (Santa Marta, Colombia).
Author contributions
O.S drafted the first version of this manuscript. All authors collated and analyzed data and contributed to the writing of this manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-90544-5.
References
- 1.Duarte CM, Losada IJ, Hendriks IE, Mazarrasa I, Marbà N. The role of coastal plant communities for climate change mitigation and adaptation. Nat. Clim. Change. 2013;3:961–968. doi: 10.1038/nclimate1970. [DOI] [Google Scholar]
- 2.Cullen-Unsworth LC, et al. Seagrass meadows globally as a coupled social–ecological system: Implications for human wellbeing. Mar. Pollut. Bull. 2014;83:387–397. doi: 10.1016/j.marpolbul.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Nellemann, C. et al. Blue Carbon: a rapid response assessment. United Nations Environment Programme, GRID-Arendal (2009).
- 4.Mcleod E, et al. A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011;9:552–560. doi: 10.1890/110004. [DOI] [Google Scholar]
- 5.Fourqurean JW, et al. Seagrass ecosystems as a globally significant carbon stock. Nat. Geosci. 2012;5:505–509. doi: 10.1038/ngeo1477. [DOI] [Google Scholar]
- 6.Waycott M, et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. PNAS. 2009;106:12377–12381. doi: 10.1073/pnas.0905620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de los Santos, C. B. et al. Recent trend reversal for declining European seagrass meadows. Nature Commun. 10, 1–8 (2019). [DOI] [PMC free article] [PubMed]
- 8.Lefcheck JS, et al. Long-term nutrient reductions lead to the unprecedented recovery of a temperate coastal region. PNAS. 2018;115:3658–3662. doi: 10.1073/pnas.1715798115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pendleton L, et al. Estimating global “blue carbon” emissions from conversion and degradation of vegetated coastal ecosystems. PLoS ONE. 2012;7:e43542. doi: 10.1371/journal.pone.0043542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macreadie PI, et al. The future of Blue Carbon science. Nat. Commun. 2019;10:1–13. doi: 10.1038/s41467-018-07882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jayathilake DRM, Costello MJ. A modelled global distribution of the seagrass biome. Biol. Conserv. 2018;226:120–126. doi: 10.1016/j.biocon.2018.07.009. [DOI] [Google Scholar]
- 12.McKenzie L, et al. The global distribution of seagrass meadows. Environ. Res. Lett. 2020;15:074041. doi: 10.1088/1748-9326/ab7d06. [DOI] [Google Scholar]
- 13.Den Hartog, C., & Kuo, J. Taxonomy and biogeography of seagrasses. In Seagrasses: biology, ecology and conservation (pp. 1–23). Springer, Dordrecht (2007).
- 14.Duarte CM, Chiscano CL. Seagrass biomass and production: a reassessment. Aquat. Bot. 1999;65:159–174. doi: 10.1016/S0304-3770(99)00038-8. [DOI] [Google Scholar]
- 15.Serrano O, Lavery PS, Rozaimi M, Mateo MA. Influence of water depth on the carbon sequestration capacity of seagrasses. Global Biogeochemic. Cy. 2014;28:950–961. doi: 10.1002/2014GB004872. [DOI] [Google Scholar]
- 16.Gullström M, et al. Blue carbon storage in tropical seagrass meadows relates to carbonate stock dynamics, plant–sediment processes, and landscape context: insights from the western Indian Ocean. Ecosystems. 2018;21:551–566. doi: 10.1007/s10021-017-0170-8. [DOI] [Google Scholar]
- 17.Lavery PS, Mateo MÁ, Serrano O, Rozaimi M. Variability in the carbon storage of seagrass habitats and its implications for global estimates of blue carbon ecosystem service. PLoS ONE. 2013;8:e73748. doi: 10.1371/journal.pone.0073748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell JE, Lacey EA, Decker RA, Crooks S, Fourqurean JW. Carbon storage in seagrass beds of Abu Dhabi United Arab Emirates. Estuaries Coast. 2015;38:242–251. doi: 10.1007/s12237-014-9802-9. [DOI] [Google Scholar]
- 19.Miyajima T, et al. Geographic variability in organic carbon stock and accumulation rate in sediments of East and Southeast Asian seagrass meadows. Global Biogeochemic. Cy. 2015;29:397–415. doi: 10.1002/2014GB004979. [DOI] [Google Scholar]
- 20.Röhr ME, et al. Blue carbon storage capacity of temperate eelgrass (Zostera marina) meadows. Global Biogeochemic. Cy. 2018;32:1457–1475. doi: 10.1029/2018GB005941. [DOI] [Google Scholar]
- 21.Serrano O, et al. Australian vegetated coastal ecosystems as global hotspots for climate change mitigation. Nat. Commun. 2019;10:1–10. doi: 10.1038/s41467-019-12176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prentice, C. et al. A synthesis of blue carbon stocks, sources and accumulation rates in eelgrass (Zostera marina) meadows in the Northeast Pacific. Global Biogeochemic. Cy.34, e2019GB006345 (2020).
- 23.Herrera-Silveira JA, et al. Blue carbon of Mexico, carbon stocks and fluxes: a systematic review. PeerJ. 2020;8:e8790. doi: 10.7717/peerj.8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carruthers TJB, Barnes PA, Jacome G, Fourqurean JW. Lagoon scale processes in a coastally influenced Caribbean system: implications for the seagrass Thalassia testudinum. Caribb. J. Sci. 2005;41:441–455. [Google Scholar]
- 25.Tamis, J. E., & Foekema, E. M. A review of blue carbon in the Netherlands. IMARES Report C151/15. 13 p. (2016).
- 26.Thorhaug AL, et al. Gulf of Mexico estuarine blue carbon stock, extent and flux: Mangroves, marshes, and seagrasses: A North American hotspot. Sci. Total Environ. 2019;653:1253–1261. doi: 10.1016/j.scitotenv.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Novak AB, et al. Factors influencing carbon stocks and accumulation rates in eelgrass meadows across New England, USA. Estuaries Coast. 2020;43:2076–2091. doi: 10.1007/s12237-020-00754-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howard JL, Creed JC, Aguia MV, Fourqurean JW. CO2 released by carbonate sediment production in some coastal areas may offset the benefits of seagrass “Blue Carbon”. Limnol. Oceanogr. 2018;63:160–172. doi: 10.1002/lno.10621. [DOI] [Google Scholar]
- 29.Nóbrega, G. N., Romero, D. J., Otero, X. L., & Ferreira, T. O. Pedological studies of subaqueous soils as a contribution to the protection of seagrass meadows in Brazil. Rev Bras Cienc Solo42 (2018).
- 30.Gómez-Lopez, D., C. et al. Informe técnico Final Proyecto de Actualización cartográfica del atlas de pastos marinos de Colombia: Sectores Guajira, Punta San Bernardo y Chocó: Extensión y estado actual. PRY-BEM-005–13 FONADE-INVEMAR. Santa Marta. 136 pp. (2014).
- 31.Díaz, M., Barrios Suárez, L. M., & Gómez López, D. I. Las praderas de pastos marinos en Colombia: Estructura y distribución de un ecosistema estratégico, Instituto de Investigaciones Marinas y Costeras-INVEMAR (2003).
- 32.Green, E. P., Short, F. T., & Frederick, T. World atlas of seagrasses. Univ of California Press (2003).
- 33.Phang VX, Chou LM, Friess DA. Ecosystem carbon stocks across a tropical intertidal habitat mosaic of mangrove forest, seagrass meadow, mudflat and sandbar. Earth Surf Process Landf. 2015;40:1387–1400. doi: 10.1002/esp.3745. [DOI] [Google Scholar]
- 34.Alongi DM, et al. Indonesia’s blue carbon: a globally significant and vulnerable sink for seagrass and mangrove carbon. Wetl. Ecol. Manag. 2016;24:3–13. doi: 10.1007/s11273-015-9446-y. [DOI] [Google Scholar]
- 35.Thorhaug A, Poulos HM, López-Portillo J, Ku TC, Berlyn GP. Seagrass blue carbon dynamics in the Gulf of Mexico: Stocks, losses from anthropogenic disturbance, and gains through seagrass restoration. Sci. Total Environ. 2017;605:626–636. doi: 10.1016/j.scitotenv.2017.06.189. [DOI] [PubMed] [Google Scholar]
- 36.Oreska MP, McGlathery KJ, Porter JH. Seagrass blue carbon spatial patterns at the meadow-scale. PLoS ONE. 2017;12:e0176630. doi: 10.1371/journal.pone.0176630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serrano O, et al. Can mud (silt and clay) concentration be used to predict soil organic carbon content within seagrass ecosystems? Biogeosciences. 2016;17:4915–4926. doi: 10.5194/bg-13-4915-2016. [DOI] [Google Scholar]
- 38.Chen G, et al. Mangroves as a major source of soil carbon storage in adjacent seagrass meadows. Sci. Rep. 2017;7:42406. doi: 10.1038/srep42406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cusack M, et al. Organic carbon sequestration and storage in vegetated coastal habitats along the western coast of the Arabian Gulf. Environ. Res. Lett. 2018;13:074007. doi: 10.1088/1748-9326/aac899. [DOI] [Google Scholar]
- 40.Lafratta A, et al. Challenges to select suitable habitats and demonstrate ‘additionality’ in Blue Carbon projects: A seagrass case study. Ocean Coast. Manag. 2020;197:105295. doi: 10.1016/j.ocecoaman.2020.105295. [DOI] [Google Scholar]
- 41.Gómez-López, D.I. et al. Reporte del estado de los arrecifes coralinos y pastos marinos en Colombia (2016–2017). Serie de publicaciones Generales del Invemar # 101, Santa Marta. 100 pp. (2018).
- 42.Alvarez-León R, Aguilera-Quiñonez J, Andrade-Maya CC, Novak P. Caracterización general de la zona de surgencia en la Guajira Colombiana. Rev. Acad. Colomb. Cienc. 1995;19:679–694. [Google Scholar]
- 43.Wilson SS, Furman BT, Hall MO, Fourqurean JW. Assessment of Hurricane Irma impacts on South Florida seagrass communities using long-term monitoring programs. Estuaries Coast. 2020;43:1119–1132. doi: 10.1007/s12237-019-00623-0. [DOI] [Google Scholar]
- 44.Hiraishi, T. et al. 2013 supplement to the 2006 IPCC guidelines for national greenhouse gas inventories: Wetlands. IPCC, Switzerland (2014).
- 45.Diaz-M JM, Gómez-López DI. Historic changes in the abundance and distribution of seagrass beds in the Cartagena bay and neighbouring areas (Colombia) Bol. Invest. Mar. Cost. 2003;32:57–74. [Google Scholar]
- 46.Ricart AM, et al. High variability of Blue Carbon storage in seagrass meadows at the estuary scale. Sci. Rep. 2020;10:1–12. doi: 10.1038/s41598-020-62639-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macreadie PI, Allen K, Kelaher BP, Ralph PJ, Skilbeck CG. Paleoreconstruction of estuarine sediments reveal human-induced weakening of coastal carbon sinks. Glob. Change Biol. 2012;18:891–901. doi: 10.1111/j.1365-2486.2011.02582.x. [DOI] [Google Scholar]
- 48.Geister, J., & Díaz-Merlano J. M. Reef environments and geology of an oceanic archipelago: San Andrés, Old Providence and Santa Catalina (Caribbean Sea, colombia) with Field Guide. Ingeominas. 104 pp (2007).
- 49.Rodríguez-Ramírez A, et al. Recent dynamics and condition of coral reefs in the Colombian Caribbean. Rev. Biol. Trop. 2010;58:107–131. doi: 10.15517/rbt.v58i1.20027. [DOI] [PubMed] [Google Scholar]
- 50.Serrano O, Lavery PS, Lopez-Merino L, Ballesteros E, Mateo MA. Location and associated carbon storage of erosional escarpments of seagrass Posidonia mats. Front. Mar. Sci. 2016;3:42. doi: 10.3389/fmars.2016.00042. [DOI] [Google Scholar]
- 51.Freile, D., & Hillis, L. Carbonate productivity by Halimeda incrassata land in a proximal lagoon, Pico Feo, San Blas, Panama. In Proceedings 8th International Coral Reef Symposium 1, 767–772 (1997).
- 52.van Tussenbroek BI, van Dijk JK. Spatial and temporal variability in biomass and production of Psammophytic Halimeda incrassata (Bryopsidales, Chlorophyta) in a Caribbean reef lagoon. J. Phycol. 2007;43:69–77. doi: 10.1111/j.1529-8817.2006.00307.x. [DOI] [Google Scholar]
- 53.Macreadie PI, Serrano O, Maher DT, Duarte CM, Beardall J. Addressing calcium carbonate cycling in blue carbon accounting. Limnol. Oceanogr. Lett. 2017;2:195–201. doi: 10.1002/lol2.10052. [DOI] [Google Scholar]
- 54.CDIAC, 2020. Carbon Dioxide Information Analysis Center
- 55.Saderne V, et al. Role of carbonate burial in Blue Carbon budgets. Nat. Commun. 2019;10:1–9. doi: 10.1038/s41467-019-08842-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Challener RC, Robbins LL, McClintock JB. Variability of the carbonate chemistry in a shallow, seagrass-dominated ecosystem: implications for ocean acidification experiments. Mar. Freshw. Res. 2016;67:163–172. doi: 10.1071/MF14219. [DOI] [Google Scholar]
- 57.Gattuso JP, et al. Ocean solutions to address climate change and its effects on marine ecosystems. Front. Mar. Sci. 2018;5:337. doi: 10.3389/fmars.2018.00337. [DOI] [Google Scholar]
- 58.Conservation International (2020). Accesses in November 2020. https://www.conservation.org/stories/critical-investment-in-blue-carbon
- 59.Coralina-Invemar. Gómez-López, D. I., C. Segura-Quintero, P. C. Sierra-Correa y J. Garay-Tinoco (Eds). Atlas de la Reserva de Biósfera Seaflower. Archipiélago de San Andrés, Providencia y Santa Catalina. Instituto de Investigaciones Marinas y Costeras “José Benito Vives De Andréis” -INVEMAR- y Corporación para el Desarrollo Sostenible del Archipiélago de San Andrés, Providencia y Santa Catalina -CORALINA-. Serie de Publicaciones Especiales de INVEMAR # 28. Santa Marta, Colombia 180 p. (2012).
- 60.Glew, J. R., Smol, J. P., & Last, W. M. Sediment Core Collection and Extrusion. In: Tracking Environmental Change UsingLake Sediments. Kluwer Academic Publishers, 73–105 (2005).
- 61.Heiri O, Lotter AF, Lemcke G. Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. J. Paleolimnol. 2001;25:101–110. doi: 10.1023/A:1008119611481. [DOI] [Google Scholar]
- 62.Sanchez-Cabeza JA, Masqué P, Ani-Ragolta I. 210Pb and 210Po analysis in sediments and soils by microwave acid digestion. J. Radioanal. Nucl. Chem. 1998;227:19–22. doi: 10.1007/BF02386425. [DOI] [Google Scholar]
- 63.Krishnaswamy S, Lal D, Martin JM, Meybeck M. Geochronology of lake sediments. Earth Planet. Sci. Lett. 1971;11:407–414. doi: 10.1016/0012-821X(71)90202-0. [DOI] [Google Scholar]
- 64.Stuiver M, Polach HA. Discussion reporting of 14 C data. Radiocarbon. 1977;19:355–363. doi: 10.1017/S0033822200003672. [DOI] [Google Scholar]
- 65.Blaauw M, Christen JA. Flexible paleoclimate age-depth models using an autoregressive gamma process. Bayesian Anal. 2011;6:457–474. doi: 10.1214/ba/1339616472. [DOI] [Google Scholar]
- 66.Reimer PJ, et al. IntCal13 and Marine13 radiocarbon age calibration curves 0–50,000 years cal BP. Radiocarbon. 2013;55:1869–1887. doi: 10.2458/azu_js_rc.55.16947. [DOI] [Google Scholar]
- 67.Parnell AC, et al. Bayesian stable isotope mixing models. Environmetrics. 2013;24:387–399. [Google Scholar]
- 68.Parnell, A. C. Package “simmr”: A Stable Isotope Mixing Model. 10.1371/journal.pone.0009672 (2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.