Abstract
Ornithine transcarbamylase (OTC) deficiency is a genetic disorder of the urea cycle characterised by deficiency in the enzyme OTC, resulting in an accumulation of ammonia. Valproic acid (VPA), a commonly used medication in the treatment of neurologic and psychiatric conditions, has been known to cause episodes of acute hyperammonaemia in patients with OTC deficiency. We present the case of a 29-year-old man with a long history of non-specific psychiatric disorders, who suffered from a hyperammonaemic crisis following the administration of VPA, leading to the diagnosis of OTC deficiency. The patient’s hospital course was complicated by progressive cerebral oedema, which resulted in worsening encephalopathy, seizures and death. We discuss the pathophysiology of hyperammonaemia in OTC deficiency, and various management strategies, including lactulose, levocarnitine, scavenger therapy and haemodialysis.
Keywords: neurology (drugs and medicines), adult intensive care, coma and raised intracranial pressure, metabolic disorders
Background
Acute hyperammonaemia episodes, with severe neurological injury, may also be observed in response to metabolic stressors, including infection or with certain medications. Ornithine transcarbamylase (OTC) deficiency is a genetic disorder of the urea cycle characterised by a partial or complete lack of the enzyme OTC with resultant accumulation of ammonia. The accumulation of ammonia is usually precipitated by high-protein diet, medications or catabolic state and result in protean clinical features. The manifestations include anorexia, vomiting, headache, confusion, seizure, behavioural abnormalities, psychiatric disorders and developmental delays among others. OTC deficiency usually manifests in childhood but can also manifest later in life although rare.
We present a fatal case of severe hyperammonaemia-related encephalopathy caused by the administration of valproic acid (VPA), in a young adult who was subsequently diagnosed with OTC deficiency. We also briefly discuss the mechanism of hyperammonaemia related to OTC deficiency and VPA along with diagnosis and treatment.
Case presentation
A 29-year-old Caucasian male with a history of intellectual disability presented to the emergency department requesting for admission for acute psychosis and suicidal ideations. He had a history of recurrent delusions and mood abnormalities, being variably diagnosed with schizophrenia, explosive disorder and depression. His medications included carbamazepine and paliperidone, both of which had been started 2 months prior during a psychiatric hospitalisation for the successful treatment of delusions and suicidal ideation. Physical examination demonstrated normal vital signs, but he was noted to be restless, minimally verbal with monotonous speech, having blunt affect and he reported suicidal ideation. Complete blood count revealed mild anaemia (haemoglobin: 137 g/L; white cell count: 9.51 x 109/L; platelet count: 172 x 109/L); serum chemistry panel was unremarkable (sodium: 143 mmol/L; potassium: 3.7 mmol/L; bicarbonate: 25 mmol/L; chloride: 106 mmol/L; creatinine: 1.2 mg/dL; glucose: 109 mg/dL), as was hepatic panel (alkaline phosphatase: 97 U/L; alanine transaminase: 10 U/L; aspartate transaminase: 23 U/L; total bilirubin: 0.7 mg/dL). Additionally, a complete urine toxicology screen was negative. He was admitted to the psychiatry service, where VPA 500 mg two times per day and escitalopram 10 mg once a day were initiated.
About 36 hours later, he developed nausea, vomiting and diarrhoea. Repeat physical examination again revealed normal vital signs, but he appeared severely dehydrated, pale, lethargic, and he also had mild generalised abdominal tenderness.
Investigations
Repeat laboratory testing was notable for leukocytosis (14.12 K/uL), elevated lactic acidosis (5.9 mmol/L) and elevated ammonia (445 Umol/L). CT of abdominal/pelvis did not demonstrate any acute pathology. VPA toxicity was suspected, prompting the cessation of the medication, and the administration of carnitine, thiamine, as well as intravenous crystalloids after he was transferred to the critical care service.
He subsequently developed generalised tonic-clonic seizure followed by severely depressed mentation. He was intubated and placed on mechanical ventilation with concern for poor airway protection.
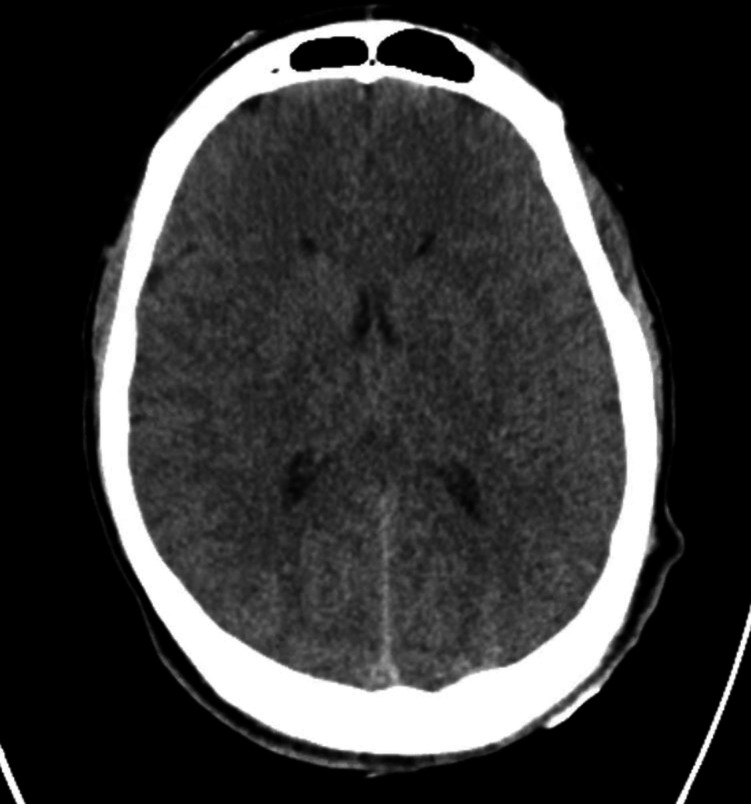
Electroencephalography displayed generalised slowing. CT imaging of the head demonstrated diffuse cerebral oedema with mass effect on the lateral ventricles (figure 1). MRI of the brain showed diffuse areas of diffusion restriction with associated T2/FLAIR signal hyperintensity, suggestive of hyperammonaemia-induced cerebral insult (figure 2).
Figure 1.
CT of the head.
Figure 2.
MRI of the brain.
Treatment
Given persistent hyperammonaemia and cerebral oedema, intermittent haemodialysis was initiated. Hypertonic saline and hyperventilation were used as adjunctive therapy for cerebral oedema. An underlying inborn error of metabolism was suspected prompting testing that revealed elevated urinary glutamic acid (139.2 μmol/g; normal range: 0–83 μmol/g), elevated urinary glycine level (>4000 μmol/g, normal range: 388–2261 μmol/g), elevated urine orotic acid (2.56 mmol/mol creatinine, normal range: 0.5–0.98), depressed plasma citrulline (6.4 μmol/L, normal range: 7–47 μmol/L) and an elevated glutamine:citrulline ratio, a constellation of findings suggestive of OTC deficiency. He was started on protein-restricted tube feeds with arginine supplementation. A combination of haemodialysis and carnitine successfully normalised ammonia levels.
Outcome and follow-up
However, he had persistent encephalopathy, along with progression of cerebral injury on serial CT and MRI imaging. Following a family discussion regarding goals of care, the patient was compassionately extubated and placed on comfort care measures, with demise following shortly thereafter.
Discussion
OTC deficiency is a rare, X-linked recessive disorder of the urea cycle that primarily affects males beginning in the newborn period. More than 230 disease-causing OTC mutations have been identified, with some associated with neonatal disease and others with late manifestations.1 This disorder results from a complete or partial lack of OTC, an enzyme that participates in the urea cycle by catalysing the conversion of carbamyl phosphate and ornithine to citrulline. The deficiency results a failure to detoxify ammonia, leading to the accumulation of ammonia in the blood. Ammonia molecules freely diffuse across cell membranes, including the blood–brain barrier, leading to high concentrations of ammonia within the brain. Astrocytic glutamine synthetase metabolises glutamate and ammonia into glutamine, an intracellular osmole, causing astrocytic swelling.2
Chronic hyperammonaemia is associated with elevated neuronal tryptophan, a precursor for serotonin, the excess of which can contribute to psychiatric manifestations.3 The underlying cause of these manifestations often goes unrecognised making OTC deficiency non-routine consideration in the differential diagnosis of delirium. Patients frequently identify precipitating factors and may demonstrate a pattern of protein avoidance to prevent recurrences of symptoms.4 Our patient had a reported history of developmental delay, intellectual disability and multiple hospitalisations throughout adolescence for acute psychosis and suicidal ideation, leading to the diagnoses of schizophrenia and explosive disorder. In hindsight, his symptoms may have been caused by his underlying metabolic disorder. It is also plausible that his presenting symptoms, including psychosis and mood disorder, were driven by hyperammonaemia, which was then severely exacerbated by the administration of VPA, with resultant cerebral oedema.
Acute episodes of hyperammonaemia can be precipitated by a variety of triggers, including medications, protein intake and catabolic states such as infection, surgery, trauma and pregnancy. Rapid increases in cerebral ammonia and glutamine, and the ensuing osmotic disequilibrium causes the influx of water into the cells, resulting in astrocyte swelling and cerebral oedema. The loss of regulation of vasculature and consequent cerebral hyperaemia may also play a contributory role.2 Findings on neuroimaging depend on the severity and duration of hyperammonaemia; cerebral oedema can be identified in severe acute cases.
Our patient’s hyperammonaemia was likely precipitated by VPA. VPA is a widely used medication in the treatment neurologic and psychiatric illnesses. It has been known to unmask OTC deficiency in previously undiagnosed individuals by, as demonstrated in our case. VPA-associated hyperammonaemia, defined as an ammonia level above 80 mcg/dL, is an adverse effect seen in acute VPA toxicity, or in association with an underlying predisposition to vagaries of ammonia metabolism, such as OTC deficiency. VPA inhibits carbamoyl phosphate synthetase, another enzyme of the urea cycle, which can result in hyperammonaemia, especially in synergism with deficiency of another urea cycle enzyme such as OTC.5 Other mechanisms include increased renal ammonia production, and carnitine depletion, causing omega oxidation and protein metabolism.6–8
The diagnosis of OTC is challenging and requires a high degree of clinical suspicion. Other causes of hyperammonaemia including drug-induced liver failure, infection, viral hepatitis, Reye syndrome, Wilson disease, alcohol cirrhosis and a range of genetic metabolic defects may need to be excluded. Serum amino acid levels can aid in diagnosis specifically; reduced arginine and citrulline levels and increased glutamine levels, in association with high urine orotic acid, which is suggestive of OTC deficiency.1
The treatment of hyperammonaemia is a two-pronged approach, involving the reduction of its synthesis, and the augmentation of its removal. Ammonia should be reduced to <200 μmol/L as early as possible, because levels greater than this threshold are associated with poor neurological outcomes and death. Given that ammonia is a byproduct of protein metabolism in association with catabolic states, dietary protein should be restricted. Intravenous dextrose can be administered to provide an alternative source of calories and reduce protein catabolism, in conjunction with insulin infusion to maintain euglycaemia. Scavenger therapy involves the administration of sodium benzoate and sodium phenylbutyrate to provide an alternative pathway for nitrogen elimination. Lactulose can be employed to reduce gastrointestinal absorption of ammonia. Levocarnitine can be administered to replace or increase carnitine, an essential cofactor of ammonia elimination. Arginine supplementation can help augment the urea cycle and nitrogen metabolism.
Haemodialysis can be initiated in severe cases. While there exist no guidelines regarding the initiation of dialysis in hyperammonaemia-related encephalopathy, the presence of cerebral oedema, ammonia levels above 300 μmol/L and failure of medical therapy could be considered indications for haemodialysis in the appropriate clinical context.9 In the setting of VPA toxicity, guidelines from Extracorporeal Treatments in Poisoning, based on systematic review, suggested extracorporeal removal of ammonia in patients with cerebral oedema or coma. The procedure is also reasonable in patients with hyperammonaemia-related encephalopathy, respiratory depression or blood pH <7.2.10 Haemodialysis successfully normalised ammonia levels in our patient, but ultimately failed to produce a satisfactory resolution of neurotoxicity, resulting in death, thus demonstrating the poor prognostic significance of cerebral oedema.
When cerebral oedema is identified, appropriate treatment should be instituted promptly. Ammonia levels should be normalised aggressively using appropriate therapies. Neuroprotective strategies include osmotic therapy and diuresis with the infusion of hypertonic saline and/or mannitol. Therapeutic hyperventilation causes vasoconstriction thus a reduction in cerebral blood flow and can be used to rapidly but transiently reduce intracranial pressure.11 The safety and utility of therapeutic hypothermia has demonstrated in a small study of neonates with hyperammonaemia-related encephalopathy.12 Induction of barbiturate coma has been used in refractory cases, but its efficacy remains debated.
Learning points.
Ornithine transcarbamylase (OTC) deficiency is a challenging diagnosis which often goes unrecognised due to its varied and non-specific presentation; it was not considered early as a differential diagnosis due to the rarity of the condition.
This case highlights that OTC can masquerade for years as merely a psychiatric condition, but can also present as encephalopathy in the context of a hyperammonaemic crisis.
Valproic acid (VPA) can precipitate acute hyperammonaemia in patients with OTC deficiency, and this usually manifests with encephalopathy and seizures due to cerebral oedema.
When recognised, urgent steps should be taken to temporise serum ammonia levels, including administration of lactulose, levocarnitine or scavenger therapy with sodium benzoate or sodium phenylbutryate.
Haemodialysis to lower ammonia levels is a reasonable approach in patients with persistently high-ammonia levels or cerebral oedema.
Footnotes
Contributors: DK contributed to writing case presentation and discussion. NS contributed to writing case presentation and discussion. KO'L helped in editing and discussion. PO helped in supervision of writing the manuscript, editing case presentation and discussion.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Klein OD, Kostiner DR, Weisiger K, et al. Acute fatal presentation of ornithine transcarbamylase deficiency in a previously healthy male. Hepatol Int 2008;2:390–4. 10.1007/s12072-008-9078-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suárez I, Bodega G, Fernández B. Glutamine synthetase in brain: effect of ammonia. Neurochem Int 2002;41:123–42. 10.1016/S0197-0186(02)00033-5 [DOI] [PubMed] [Google Scholar]
- 3.Upadhyay R, Bleck TP, Busl KM. Hyperammonemia: what Urea-lly need to know: case report of severe noncirrhotic hyperammonemic encephalopathy and review of the literature. Case Rep Med 2016;2016:1–10. 10.1155/2016/8512721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lichter-Konecki U, Caldovic L, Morizono H. Ornithine Transcarbamylase Deficiency. : Adam MP, Ardinger HH, Pagon RA, . GeneReviews®. Seattle (WA): University of Washington, 2013. https://www.ncbi.nlm.nih.gov/books/NBK154378/ [Google Scholar]
- 5.Thakur V, Rupar CA, Ramsay DA, et al. Fatal cerebral edema from late-onset ornithine transcarbamylase deficiency in a juvenile male patient receiving valproic acid. Pediatr Crit Care Med 2006;7:273–6. 10.1097/01.PCC.0000216682.56067.23 [DOI] [PubMed] [Google Scholar]
- 6.Gidal BE, Inglese CM, Meyer JF, et al. Diet- and valproate-induced transient hyperammonemia: effect of L-carnitine. Pediatr Neurol 1997;16:301–5. 10.1016/S0887-8994(97)00026-X [DOI] [PubMed] [Google Scholar]
- 7.Duarte J, Macias S, Coria F, et al. Valproate-Induced coma: case report and literature review. Ann Pharmacother 1993;27:582–3. 10.1177/106002809302700510 [DOI] [PubMed] [Google Scholar]
- 8.Marini AM, Zaret BS, Beckner RR. Hepatic and renal contributions to valproic acid-induced hyperammonemia. Neurology 1988;38:365–71. 10.1212/WNL.38.3.365 [DOI] [PubMed] [Google Scholar]
- 9.Gupta S, Fenves AZ, Hootkins R. The role of RRT in hyperammonemic patients. Clin J Am Soc Nephrol 2016;11:1872–8. 10.2215/CJN.01320216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghannoum M, Laliberté M, Nolin TD, et al. Extracorporeal treatment for valproic acid poisoning: systematic review and recommendations from the EXTRIP Workgroup. Clin Toxicol 2015;53:454–65. 10.3109/15563650.2015.1035441 [DOI] [PubMed] [Google Scholar]
- 11.Baddour E, Tewksbury A, Stauner N. Valproic acid–induced hyperammonemia: incidence, clinical significance, and treatment management. Ment Health Clin 2018;8:73–7. 10.9740/mhc.2018.03.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shankaran S. Therapeutic hypothermia for neonatal encephalopathy. Curr Treat Options Neurol 2012;14:608–19. 10.1007/s11940-012-0200-y [DOI] [PMC free article] [PubMed] [Google Scholar]