Abstract
Introduction
Incarceration is associated with decreased cancer screening rates and a higher risk for hospitalisation and death from cancer after release from prison. However, there is a paucity of data on the relationship between incarceration and cancer outcomes and quality of care. In the Incarceration and Cancer-Related Outcomes Study, we aim to develop a nuanced understanding of how incarceration affects cancer incidence, mortality and treatment, and moderates the relationship between socioeconomic status, structural racism and cancer disparities.
Methods and analysis
We will use a sequential explanatory mixed-methods study design. We will create the first comprehensive linkage of data from the Connecticut Department of Correction and the statewide Connecticut Tumour Registry. Using the linked dataset, we will examine differences in cancer incidence and stage at diagnosis between individuals currently incarcerated, formerly incarcerated and never incarcerated in Connecticut from 2005 to 2016. Among individuals with invasive cancer, we will assess relationships among incarceration, quality of cancer care and mortality, and will assess the degree to which incarceration status moderates relationships among race, socioeconomic status, quality of cancer care and cancer mortality. We will use multivariable logistic regression and Cox survival models with interaction terms as appropriate. These results will inform our conduct of in-depth interviews with individuals diagnosed with cancer during or shortly after incarceration regarding their experiences with cancer care in the correctional system and the immediate postrelease period. The results of this qualitative work will help contextualise the results of the data linkage.
Ethics and dissemination
The Yale University Institutional Review Board (#2000022899) and the Connecticut Department of Public Health Human Investigations Committee approved this study. We will disseminate study findings through peer-reviewed publications and academic and community presentations. Access to the deidentified quantitative and qualitative datasets will be made available on review of the request.
Keywords: epidemiology, oncology, protocols & guidelines, public health, epidemiology
Strengths and limitations of this study.
We will use a mixed-methods sequential explanatory design to examine cancer incidence, outcomes and quality of care among individuals currently incarcerated, formerly incarcerated and never incarcerated in Connecticut from 2005 to 2016 and will be the first study to explore the relationship between incarceration and racial and socioeconomic disparities in cancer.
We will devise innovative partnerships among the Connecticut Tumour Registry, the Connecticut Department of Correction and Yale Cancer Centre Rapid Case Ascertainment to create a novel administrative data linkage registry.
Our findings will be based on a single state’s correctional system, and Connecticut has unique state Medicaid policies which may limit the applicability of our findings to other states’ correctional populations.
We rely on registry data rather than self-reported measures of race, ethnicity and socioeconomic status, and attempts to disentangle race from other sociodemographic characteristics may not yield consistent results.
Introduction
The US adult prison population tripled between 1987 and 2015. According to recent data, 2.2 million Americans are incarcerated at any given time,1 and these individuals are disproportionately racial and ethnic minorities and of lower socioeconomic class.2 Incarceration is associated with a higher risk of illness and death after release, including from cancer.3–5 Given the disproportionate impact of mass incarceration on Black and Latinx populations and individuals with lower socioeconomic status, as well as related detrimental health effects, incarceration may also be associated with racial and socioeconomic disparities in cancer outcomes. Past studies documenting the existence of such disparities have focused on assessing the potential influence of biology, health behaviours, bias or access to high-quality care.6 These analyses, however, have largely failed to measure criminal justice exposure directly, or have done so in limited ways.
There are a number of reasons that having been incarcerated would place individuals at higher risk for developing cancer. In 2016, 30.2% of illness-related deaths in US state prisons were attributed to cancer, making it the leading cause of illness-related deaths in the incarcerated population.7 Studies suggest that incarcerated individuals are often at least ten years older in physiological age than chronological age,8 and accelerated ageing predisposes individuals to chronic and geriatric illnesses, including cancer. Researchers have also noted higher rates of self-reported cancer in incarcerated populations9 10 and indirectly found that incarceration increases cancer risk factors.11 Cancer risk factors include smoking, substance use and infectious disease and are more prevalent in incarcerated people compared with the general population.12–14 Additionally, individuals with a history of incarceration have higher rates of comorbidities, including alcohol and substance use disorders and mental health conditions compared with the general population, making treatment management more difficult.15 Moreover, high levels of stress during incarceration have been described in prison ethnographies,16 17 and given that cancers are mediated by inflammatory processes, there may be a higher incidence of cancer in this population.
Despite these data, the nature of association between incarceration and cancer outcomes remains unclear (figure 1). When considering screening or prompt diagnosis after symptom onset, incarceration may counterintuitively improve cancer outcomes for minorities and individuals of low socioeconomic status given constitutionally guaranteed access to healthcare during incarceration. This hypothesis is informed by the fact that many adults first engage with the healthcare system during their incarceration, and as a result, approximately 40% receive their first diagnosis of a chronic condition while incarcerated.18 Improved access to healthcare services, reduced access to illicit drugs and alcohol, and enforced adherence to medications may improve overall health while incarcerated.
Figure 1.
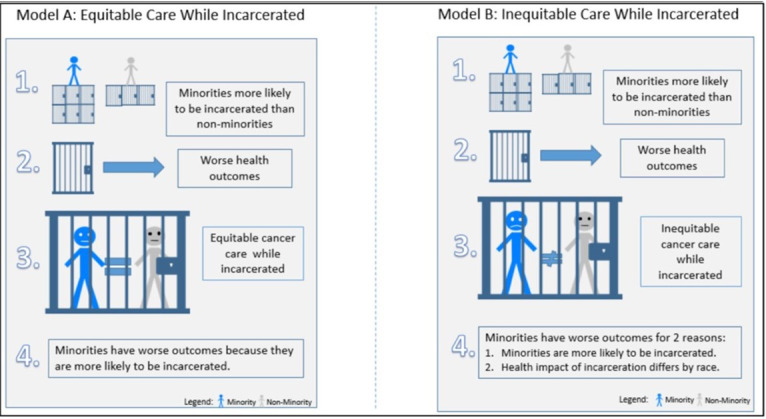
Models regarding incarceration and cancer disparities. There are two potential models for the relationship between criminal justice involvement and disparities in cancer treatment and outcomes. The first (model A; figure 1) involves a causal link between incarceration health outcomes that is independent of race and ethnicity. That is, incarceration might adversely affect cancer care and outcomes, but the effect is similar for minority and non-minority individuals. In this setting, the fact that minority individuals are more likely to be incarcerated is the driver of worse outcomes for minority individuals. In the second model (figure 1; model B), race/ethnicity is an effect modifier in the relation between criminal justice involvement and cancer outcomes, and Black and Latinx communities are disproportionately affected by being incarcerated relative to white communities.
Conversely, it is plausible that incarceration is associated with worse cancer outcomes.19 While research examining quality of care provided to currently or formerly incarcerated individuals is limited,20 21 cost-cutting methods and copayments may limit access to cancer care.22 In one study, researchers noted inadequate pain management among incarcerated individuals with cancer pain but did not assess cancer treatment.23 Another study demonstrated that many individuals do not receive screening during incarceration despite its availability in prison. For instance, of all individuals held in San Francisco jails, only 41% of women older than 40 reported having a mammogram within 2 years, and only 31% of individuals older than 50 reported having a colonoscopy.24 Incarcerated individuals are also likely unaware of cancer screening guidelines, considering the high rates of inadequate health literacy among justice-involved populations.25
Additionally, when individuals are released from a correctional facility, they frequently lose any improvements in health and experience worse health outcomes compared with those never incarcerated. A prior study demonstrated that Medicare beneficiaries recently released from correctional facilities had higher cancer-related hospitalisation rates, along with an increased risk of cancer-related mortality, compared with the general population.26 This result could be due to stressors related to securing housing, food and work post-release—tasks more difficult with a criminal record.27 These barriers may prevent individuals from obtaining primary care and health insurance and resuming their cancer-directed treatments.28 29
Through the Incarceration and Cancer-Related Outcomes (ICRO) study, we will attempt to fill these knowledge gaps and examine the impact of incarceration, independent from socioeconomic differences and other confounding factors, on cancer incidence, quality of care and mortality, and assess the degree to which incarceration status moderates relationships among race, socioeconomic status, quality of cancer care and cancer mortality.
Methods and analysis
Study design
We will use a mixed-methods sequential explanatory design to investigate the relationship between incarceration and cancer outcomes (figure 2). This is a design which includes two distinct consecutive phases: quantitative followed by qualitative, where the second phase builds on the results of the first.30 Specifically, we will create the first comprehensive, population-based linkage of a statewide cancer registry, the Connecticut Tumour Registry (CTR), which includes mortality data and the movement database of the Connecticut Department of Correction (CDOC). Novel data linkages are needed to study the relationship between incarceration and cancer outcomes in part because incarceration status is not addressed by large, national population-based surveys such as the Behavioural Risk Factor Surveillance System or the National Health Interview Survey or by the national cancer registration programmes (the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) programme and the Centers for Disease Control and Prevention’s National Programme of Cancer Registries).
Figure 2.
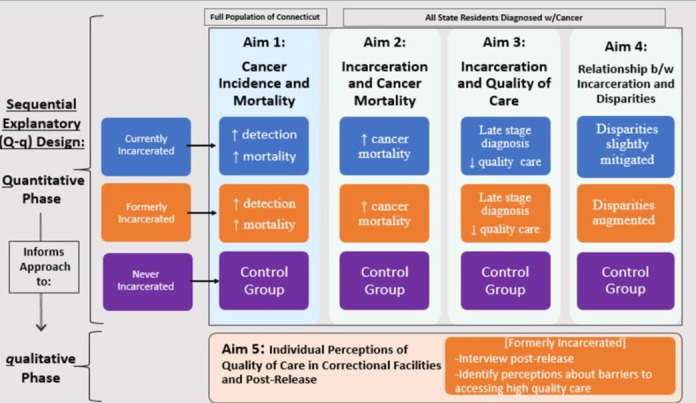
Incarceration and cancer-related outcomes mixed-methods study schema.
We will link these databases based on probabilistic matching of individual cases using name, date of birth, sex and social security number to ensure accuracy. We will then assemble retrospective cohorts with members who are currently, formerly or never incarcerated in Connecticut between 2005 and 2016. Incarceration will be defined as having a history of being admitted to CDOC, whether remanded (admitted to custody, but not yet sentenced) or incarcerated (sentenced to either jail or prison). The ‘never incarcerated’ cohort will be defined as individuals who did not appear in CDOC movement files between 2005 and 2016. Once we have identified these cohorts, we will extract their linked data. We will not track individuals who move out of Connecticut during the study period.
Population-level epidemiological data from our linkage will provide an inroad into understanding the impact of incarceration on cancer outcomes and treatment quality, and into how mass incarceration may contribute to racial and socioeconomic cancer disparities. Yet, only direct narratives from individuals diagnosed with cancer can provide an in-depth understanding of individual and health system factors associated with quality of care during and immediately after release.31 Within our sequential explanatory design, we will use quantitative data from our data linkage to design interview guides that we will use to conduct one-on-one interviews with unique individuals who were released from CDOC within the prior month. This qualitative component will function to refine and explain quantitative results.
Connecticut, a state with the nation’s second highest income gap,32 and wide racial disparities in incarceration,33 is an ideal setting to study the relationship between incarceration, cancer outcomes and racial and socioeconomic disparities. The CTR is the oldest cancer registry in the country and is highly regarded for its quality and long-standing track record of productive academic collaboration. The CDOC also has a combined criminal justice system where jails and prisons are under the authority of a single agency, thus allowing for easier data linkages.
Data sets and linkage
Descriptions of the data sets for linkage in the ICRO study are presented in table 1.
Table 1.
Data sets for linkage
| Database | Description | Key variables |
| The Connecticut Tumour Registry (CTR) | The CTR is a population-based resource for examining cancer patterns in Connecticut which includes all reported cancers diagnosed in Connecticut residents since 1935, as well as follow-up, treatment and survival data. All licensed medical providers, as well as hospitals and private pathology laboratories in the state, are required by law to report cancer cases to the registry, including those that care for incarcerated individuals. The CTR is the oldest population-based cancer registry in the country. Rigorous quality control procedures, stringent requirements in case reporting, and reciprocal cancer reporting agreements with neighbouring states allow the registry to identify cancers among all Connecticut residents even when diagnosed or treated in other states. CTR data have been used widely in research into cancer aetiology, epidemiology and quality of care. | Name*, date of birth*, social security number*, age, race/ethnicity, marital status, sex, residential census tract at time of diagnosis, insurance at time of diagnosis, dates of diagnosis and treatment, vital status, date of last contact, cause of death. |
| Connecticut Department of Correction (CDOC) | The CDOC has an annual population of approximately 15 000 individuals, with disproportionate incarceration of racial and ethnic minorities (demographically similar to rates of incarceration nationwide). CDOC also has a combined criminal justice system, where jails and prisons are under the authority of a single agency. CDOC supports research aimed at improving the health of, and reducing recidivism for, justice involved individuals and has partnered with many academic institutions on federally funded grants.56 | Dates of incarceration, date of release (if applicable), inmate name*, any known alias(es)*, inmate number, place of incarceration, date of birth*, race, social security number*, sex, and place of birth. |
*These variables were used in the record linkage only and were not part of the analytical dataset.
The Yale Cancer Centre’s Rapid Case Ascertainment (RCA) Shared Resource, developed in 1986 in response to a Connecticut Hospital Association request to establish a single entity that would be responsible for all aspects of population-based cancer epidemiology studies, will abstract medical records. RCA staff function as agents of the CTR and can conduct record reviews to address information missing from the CTR. RCA can thus abstract patient-specific treatment data including diagnostic, imaging and pathology reports, and clinical notes in each hospital’s electronic medical record or paper charts.
Using data collected between 2005 and 2016, we will use Match*Pro, a probabilistic record linkage software programme available from the National Cancer Institute,34 to link CDOC movement files to CTR data using first name, last name, sex, date of birth and social security number. The linkage methodology is based on the Fellegi and Sunter model.35 We will extract data for matched cases on cancer diagnosis (primary site, date, histology), stage of disease, vital status, date of last contact and cause of death (if deceased) from the CTR. In previous studies, CDOC data has been linked to state health insurance data using sophisticated probabilistic and deterministic algorithms with reported sensitivity and positive predictive values in the mid 90% range.
Patient and public involvement
Our multidisciplinary study team consists of health services researchers, oncologists, primary care doctors, statisticians and individuals with a history of incarceration. For the qualitative component, we will work with individuals with a history of incarceration to design an interview guide, conduct qualitative in-depth interviews and iteratively code and identify themes related to quality of cancer treatment. Community healthcare workers, who have a history of incarceration and are experienced in conducting research with vulnerable populations, will be trained to conduct the interviews. We will convene a Study Advisory Board which consists of correctional providers, oncologists, policy-makers, individuals with cancer and a history of incarceration and community advocates. The board will meet quarterly to provide input on research progress and findings.
Planned analyses
Cancer incidence and mortality analyses
We will use the linked dataset to compare new cancer diagnoses, cancer incidence rate, quality of cancer care and cancer-related deaths among Connecticut residents currently, formerly and never incarcerated. First, for the cancer incidence rate among individuals currently incarcerated, we will divide the number of individuals with new primary cancers diagnosed between the date of admission to custody in 2005 and 31 December 2016, by the person-years at risk of incident cancer, defined as the difference between the date of admission to custody in 2005 and 31 December 2016, death, diagnosis of primary cancer or release (whichever occurred first). If an individual was released and reincarcerated, the period of time between the date of readmission and their death, diagnosis of cancer, or 31 December 2016, will be added to their person-time ‘incarcerated.’ Second, for the released group, we will use CDOC data to estimate the number of formerly incarcerated individuals living in Connecticut in each age/sex strata. In each stratum, we will calculate the number of person-years as the population at risk and employ CTR data to calculate age-adjusted incidence and mortality. Finally, for those never incarcerated between 2005 and 2016, we will divide number of new primary cancers diagnosed by the person-years at risk of incident cancer. The ‘never incarcerated’ group will be estimated by subtracting the currently and formerly incarcerated individuals from the Connecticut population data by age/sex group as obtained from Census data.
To measure the associations among incarceration, cancer incidence and mortality, we will estimate population attributable risk from incarceration with the equation p(ec)x(OR-1)/OR, where p(ec) is the proportion exposed (ie, experience with incarceration) among individuals.28 We will use two tailed χ2 tests to compare cancer incidence and mortality rates among currently incarcerated, ever incarcerated and never incarcerated groups. To detect a difference in incidence and mortality rate equivalent to a medium effect size (OR=3.47 or Cohen’s d=0.5) or a small effect size (OR=1.68 or Cohen’s d=0.2), with alpha=0.05 and power=80%, each group will need N=107 or N=964. Our large sample size will grant more than sufficient statistical power to detect clinical meaningful effect size.
Incarceration and mortality
To assess the relationship between incarceration and cancer mortality among Connecticut residents diagnosed with cancer during 2005–2016, we will use Cox regression models to evaluate the independent association of incarceration status and cancer mortality. We will calculate descriptive statistics for each independent variable, stratified by incarceration status. The extended Cox model will use binary incarcerated status as the time-varying covariate as well as other time-fixed covariates. Time-fixed covariates of interest include age, sex, race/ethnicity (categorised into Hispanic, non-Hispanic white, Black and other racial groups), marital status (categorised into single, separated, divorced, widowed and unmarried partner), insurance at the time of diagnosis (no insurance, insurance and other if unknown), mortality, incarceration history prior to the time of diagnosis and socioeconomic states. We will categorise poverty into four levels using the Census Tract Poverty Indicator. We will use a Cox model with time-dependent incarcerated status covariate to assess the association between incarceration status and risk of cancer mortality. We will also evaluate the association between place of diagnosis (ie, during incarceration, postincarceration within a defined time frame of release and never incarcerated) and cancer incidence and risk of mortality. Finally, we will include clinical factors such as late stage of diagnosis to assess whether the relation between incarceration and cancer mortality is mediated by diagnosis stage or treatment timeliness.
To estimate an adequate sample size for this survival analyses, we used Singer and Willett’s sample size table,36 which provides minimum total sample sizes necessary to achieve a reasonable power level based on the ratio of median lifetimes (R=m1/m2) and length of follow-up (F=T/A, where T=total length of follow-up, and A=m1/m2). A previous study found median survival times of 21 months for incarcerated cases and 54 months for a matched SEER cohort, which corresponds to a large effect size of R=2.57.10 When median lifespan in one group is twice as long as median lifespan in the other, the study will have an 80% chance of detecting this difference using only N=100–200 cases. With a more conservative estimate, assuming a minimum detectable effect size of R=1.5–1.75, a significance level of 0.05, and 80% of power, we will need an N=122 or N=296.
Quality of cancer care analyses
We will ascertain quality of care using two approaches. First, we will assess timeliness of care, defined as the system’s capacity to provide care quickly after a need is recognised.37 Guidelines and prior empiric studies have defined treatment delay as the temporal period between diagnosis and definitive cancer treatment and examined the significance of treatment delay for many common cancer types.38–40 We will employ a common definition of delay as >30 days between diagnosis and initial treatment.41 Second, we will assess adherence to care processes recommended for each major cancer type. For instance, we will assess the use of curative cancer therapy among men with intermediate or high grade localised prostate cancer.42
Yale RCA staff will conduct a medical chart review to both validate CTR data and abstract additional information about quality and timeliness of care, including receipt of cancer directed therapy for non-metastatic disease, dates of surgery, radiation therapy and clinicopathological tests for each diagnosis. We will use a rigorous, multistep approach to train abstractors and assure quality of medical record abstraction.43 To measure the association between incarceration and quality of cancer care, we will use descriptive statistics to characterise receipt of care for individuals based on their incarceration status at the time of diagnosis and use χ2 tests to compare. We will use logistic regression to assess the association between incarceration and treatment delay (yes/no) and treatment concordant-care (yes/no). We will adjust for individual characteristics, including age, sex, race, ethnicity, marital status, insurance at time of diagnosis and socioeconomic status (per cent of families living below the poverty level derived from individual census tract.) Poverty, race/ethnicity, marital status and insurance will be grouped as noted above. We will also examine additional variables including place of diagnosis and sex as they are associated with mortality and quality of care. In our analysis of sex as a biological variable, we will focus on cancers that can affect women, excluding cancers that only affect male reproductive organs. Sex can be an important source of variation in detection, quality of care and mortality both because correctional facilities that care for women and the challenges women face on release are unique.44
To estimate the number of participants needed for multivariate regression models, we used a logistic regression sample size estimation method.45 We performed calculations with the following assumptions: (1) the ability to detect an OR of 1.5 (equivalent to a small effect size)46; (2) two-sided 0.05 significance level; (3) adjustment of R-squared of 0.4 (ie, R-squared achieved when the independent variable of interest is regressed on the other covariates in the regression). Given such assumptions, we will need to review medical records from 308 patients from the entire study sample to achieve 80% statistical power. Breast cancer will likely be the smallest number of cancers we identify given the population of incarcerated individuals. We will be able to look at breast cancer outcomes and detect a minimum of small to medium effect size of OR=2.0 if there are at least 86 breast cancers diagnosed in CDOC (assuming 80% statistical power, two-sided test with significance level of 0.05).47
Incarceration and cancer disparities analysis
To assess how incarceration status moderates the relationships between race and ethnicity (as socially, not biologically related variables), socioeconomic status and quality of cancer care and cancer mortality, we will measure Black-white and socioeconomic disparities in cancer treatment and mortality before and after adjusting for incarceration status in a multivariable model. The difference in the race parameter estimate before vs after adjusting for incarceration status will be reported to estimate the degree to which incarceration mediates the relation between race and ethnicity and cancer outcomes. We will also measure the high and low socioeconomic disparity and statistically test whether exposure to incarceration moderates observed associations. In addition to the overall cancer model, we will analyse each primary cancer diagnosis separately when sample size permits.
Qualitative investigation of factors associated with cancer care
We will use findings from our quantitative assessments to select cancer types and stages that may be particularly vulnerable to poor quality care among previously incarcerated individuals and to inform the interview guide. Eligible participants must have been released from CDOC within 1 month and diagnosed with cancer. We will use a purposeful sampling strategy to capture diverse perspectives from key groups of interest (gender, race/ethnicity, disease status).48 Multipronged recruitment will include direct engagement at a primary care clinic for individuals with a history of incarceration, participant word of mouth, referral from the CDOC and direct referrals from community health workers. Participants will receive a US$30 gift card as remuneration. We will oversample women to more fully characterise the experience of cancer care for people in the women’s facility and expect to interview close to 20 people. Two members of our research team—one with a history of incarceration who will be trained in qualitative interviewing—will lead semistructured interviews using a standardised interview guide that will include open-ended questions to elucidate how correctional institutions facilitated or constrained management of cancer. For instance, for those who were diagnosed with cancer in prison, sample questions include: ‘What was it like to be diagnosed with cancer in prison?’, ‘What made it easy or hard to manage your cancer in prison?’, or ‘What makes it easy or hard to manage cancer now that you have been released?’ We will design the interview guide in partnership with the Study Advisory Board. Interviews will be audiorecorded, professionally transcribed, and reviewed for accuracy.
Three members of our research team will meet regularly to analyse interviews.49 We will initially review five transcripts to develop a preliminary coding structure through inductive coding. This strategy employs an interpretive description approach to qualitative analysis, allowing themes to emerge inductively from participants rather than from researcher preconceptions.50 51 Code keys will be shared with the full research team and advisory board for feedback periodically. A fourth member of our team will review these transcripts and the preliminary coding structure to assess comprehensiveness and properties of emerging codes. After developing a preliminary code structure, we will code the first five transcripts independently, meeting weekly to negotiate consensus and refine our code structure using constant comparative analysis.52 This iterative process will allow us to refine to our code structure, eliminating or consolidating codes where needed53 until we reach thematic saturation. We will maintain a thorough audit trail of coding decisions. We will then systematically apply the final codes to all transcripts. We will use qualitative analysis software (ATLAS.ti V.8.0) to facilitate data organisation and analysis.
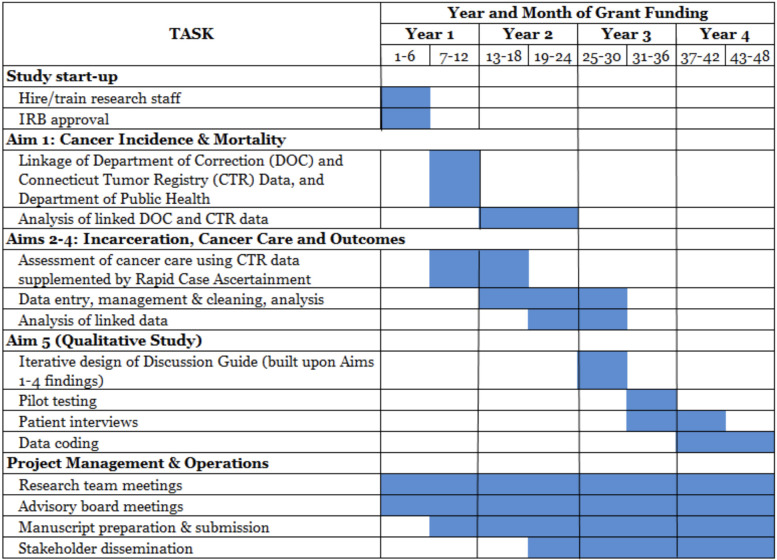
A timeline of the ICRO study is presented in figure 3.
Figure 3.
Timeline of the ICRO study. ICRO, Incarceration and Cancer-Related Outcomes.
Ethics and dissemination
The Yale University Institutional Review Board (#2000022899) approved the entirety of this study and the Connecticut Department of Public Health Institutional Review Board Human Investigations Committee approved the quantitative data matching portion of this study. We will disseminate study findings through peer-reviewed publications and academic and community presentations. The access to the deidentified data set and qualitative interview guides will be made available on review of the request.
Discussion
Our ICRO study will create the first comprehensive linkage of a statewide tumour registry that includes vital statistics and correctional system data, integrated with interviews of individuals with cancer. The study will enable us to develop a nuanced understanding of how incarceration affects cancer incidence, mortality, treatment and relates to observed racial and socioeconomic cancer disparities. We anticipate that this state-level study will provide knowledge to identify and develop ways to improve cancer care in correctional settings as well as in the community for people just released from correctional facilities.
There are several methodological limitations to note. First, accurately matching individuals across different data sources is an important challenge for any study involving the creation of a novel, linked data set. Past linkage studies in Connecticut have had 90% success rate in linking data across participants. Second, sample size of those incarcerated while diagnosed with cancer may be small. We have conducted sample size calculations, and estimate sufficient sample size to detect meaningful differences between incarceration exposure and treatment quality, even in cancers most prevalent in women, (eg, breast cancer), despite the low incarceration rate of women. However, for cancers where sample size is small, especially among those diagnosed while incarcerated, we may create a combined variable, ‘history of incarceration,’ to explore differences between those with and without a history of incarceration. Third, the CTR data, although highly reliable in identifying incident cancer diagnoses, may occasionally lack details regarding treatment timeliness and receipt of therapy beyond the peridiagnosis period. Therefore, we will partner with the Yale Cancer Centre RCA programme, which will enable our team to receive detailed cancer treatment information abstracted from hospital medical records. Fourth, our measure of race and ethnicity from the CTR is not self-reported and is derived from the medical record or healthcare provider/system, and we will derive a measure of socioeconomic status from census tract average poverty level. Self-reported race and ethnicity and individual measures of socioeconomic status would be more accurate, but these are limitations from any study using registry data to examine racial, ethnic, and socioeconomic disparities. Fifth, attempts to disentangle race/ethnicity from other sociodemographic characteristics do not always yield consistent results. In some models, socioeconomic status accounts for most of the cancer disparities between whites and non-whites,54 while other studies have found the association between socioeconomic status and racial and ethnic disparities is attenuated but not completely explained.55 For the ICRO study, which would be the first study to explore the relationship between incarceration and racial/ethnic and socioeconomic cancer disparities, we will examine these characteristics separately. Sixth, our interviewees will have been released from a single state’s correctional system. This means our findings may not be transferable to all correctional settings. Similarly, given uniquely stabilising CDOC and state Medicaid policies, many individuals are released in Connecticut with health insurance and are more apt to engage in care following release, again limiting the applicability of the ICRO study to other correctional populations.
Supplementary Material
Footnotes
Twitter: @ewang422
Correction notice: This article has been corrected since it was first published. The name for the author Cary P Gross and Jenerius A Aminawung has been corrected.
Contributors: LP, writing-original draft, investigation, resources. AAH: writing-original draft, JA: methodology, validation, data curation, writing-original draft, project administration. CG: conceptualisation, investigation, LG: software, formal analysis, writing-original draft. DS-G: writing-original draft, methodology, investigation. H-JL: software, validation, methodology, formal analysis, data curation. RM: investigation, writing-origial draft. SM: project administration, writing-original draft, visualisation. OTO: methodology, writing- original draft. CG: conceptualisation, methodology, writing-original draft, supervision. EAW: conceptualisation, methodology, writing-original draft, supervision.
Funding: This work was supported by National Institutes of Health grant number 1R01CA230444-01. The Connecticut Tumour Registry is supported by federal funds from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN261201800002I.
Competing interests: CG has received research funding, though Yale, from the NCCN Foundation (Pfizer/Astra-Zeneca) and Genentech, as well as funding from Johnson & Johnson to help devise and implement new approaches to sharing clinical trial data, and funding from Flatiron for travel to and speaking at a scientific conference. All other authors report no competing interests.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Kaeble D, Glaze L, Tsoutis A. Correctional populations in the United States, 2014. Correctional populations in the United States series. Washington, DC: US Department of Justice, Office of Justice Programs, Bureau of Justice Statistics, 2015. [Google Scholar]
- 2.Carson E. Prisoners in 2014. Washington, DC: US Department of Justice, Office of Justice Programs, Bureau of Justice Statistics, 2015. [Google Scholar]
- 3.Kouyoumdjian FG, Pivnick L, McIsaac KE, et al. Cancer prevalence, incidence and mortality in people who experience incarceration in Ontario, Canada: a population-based retrospective cohort study. PLoS One 2017;12:e0171131. 10.1371/journal.pone.0171131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binswanger IA, Stern MF, Deyo RA, et al. Release from prison — a high risk of death for former inmates. N Engl J Med Overseas Ed 2007;356:157–65. 10.1056/NEJMsa064115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spaulding AC, Seals RM, McCallum VA, et al. Prisoner survival inside and outside of the institution: implications for health-care planning. Am J Epidemiol 2011;173:479–87. 10.1093/aje/kwq422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viswanath K, Emmons KM. Message effects and social determinants of health: its application to cancer disparities. J Commun 2006;56:S238–64. 10.1111/j.1460-2466.2006.00292.x [DOI] [Google Scholar]
- 7.Carson E, Cowhig MP. Mortality in state and federal prisons, 2001-2016 – statistical tables. Washington, DC: US Department of Justice, Office of Justice Programs, Bureau of Justice Statistics, 2020. [Google Scholar]
- 8.Greene M, Ahalt C, Stijacic-Cenzer I, et al. Older adults in jail: high rates and early onset of geriatric conditions. Health Justice 2018;6:3. 10.1186/s40352-018-0062-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Binswanger IA, Krueger PM, Steiner JF. Prevalence of chronic medical conditions among jail and prison inmates in the USA compared with the general population. J Epidemiol Community Health 2009;63:912–9. 10.1136/jech.2009.090662 [DOI] [PubMed] [Google Scholar]
- 10.Mathew P, Elting L, Cooksley C, et al. Cancer in an incarcerated population. Cancer 2005;104:2197–204. 10.1002/cncr.21468 [DOI] [PubMed] [Google Scholar]
- 11.Kouyoumdjian FG, Andreev EM, Borschmann R, et al. Do people who experience incarceration age more quickly? exploratory analyses using retrospective cohort data on mortality from Ontario, Canada. PLoS One 2017;12:e0175837. 10.1371/journal.pone.0175837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cropsey K, Eldridge GD, Ladner T. Smoking among female prisoners: an ignored public health epidemic. Addict Behav 2004;29:425–31. 10.1016/j.addbeh.2003.08.014 [DOI] [PubMed] [Google Scholar]
- 13.Peters RH, Greenbaum PE, Edens JF, et al. Prevalence of DSM-IV substance abuse and dependence disorders among prison inmates. Am J Drug Alcohol Abuse 1998;24:573–87. 10.3109/00952999809019608 [DOI] [PubMed] [Google Scholar]
- 14.Weinbaum CM, Sabin KM, Santibanez SS. Hepatitis B, hepatitis C, and HIV in correctional populations: a review of epidemiology and prevention. AIDS 2005;19:S41–6. 10.1097/01.aids.0000192069.95819.aa [DOI] [PubMed] [Google Scholar]
- 15.Fazel S, Baillargeon J. The health of prisoners. Lancet 2011;377:956–65. 10.1016/S0140-6736(10)61053-7 [DOI] [PubMed] [Google Scholar]
- 16.Sykes GM. The society of captives: a study of a maximum security prison. Princeton University Press: Princeton, NJ, 1958. [Google Scholar]
- 17.Zamble E. Behavior and adaptation in long-term prison inmates: descriptive longitudinal results. Crim Just Behav 1992;19:409–25. [Google Scholar]
- 18.Wang EA, Hong CS, Shavit S, et al. Engaging individuals recently released from prison into primary care: a randomized trial. Am J Public Health 2012;102:e22–9. 10.2105/AJPH.2012.300894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wildeman C, Wang EA. Mass incarceration, public health, and widening inequality in the USA. Lancet 2017;389:1464–74. 10.1016/S0140-6736(17)30259-3 [DOI] [PubMed] [Google Scholar]
- 20.Davies EA, Sehgal A, Linklater KM, et al. Cancer in the London prison population, 1986-2005. J Public Health 2010;32:526–31. 10.1093/pubmed/fdq009 [DOI] [PubMed] [Google Scholar]
- 21.Magee CG, Hult JR, Turalba R, et al. Preventive care for women in prison: a qualitative community health assessment of the Papanicolaou test and follow-up treatment at a California state women’s prison. Am J Public Health 2005;95:1712–7. 10.2105/AJPH.2005.063677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Awofeso N. Prisoner healthcare co-payment policy. Appl Health Econ Health Policy 2005;4:159–64. 10.2165/00148365-200504030-00004 [DOI] [PubMed] [Google Scholar]
- 23.Lin JT, Mathew P. Cancer pain management in prisons: a survey of primary care practitioners and inmates. J Pain Symptom Manage 2005;29:466–73. 10.1016/j.jpainsymman.2004.08.015 [DOI] [PubMed] [Google Scholar]
- 24.Binswanger IA, White MC, Pérez-Stable EJ, et al. Cancer screening among jail inmates: frequency, knowledge, and willingness. Am J Public Health 2005;95:1781–7. 10.2105/AJPH.2004.052498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hadden KB, Puglisi L, Prince L, et al. Health literacy among a formerly incarcerated population using data from the transitions clinic network. J Urban Health 2018;95:547–55. 10.1007/s11524-018-0276-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang EA, Wang Y, Krumholz HM. A high risk of hospitalization following release from correctional facilities in Medicare beneficiaries: a retrospective matched cohort study, 2002 to 2010. JAMA Intern Med 2013;173:1621–8. 10.1001/jamainternmed.2013.9008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Western B. Punishment and inequality in America. Russell Sage Foundation, 2006. [Google Scholar]
- 28.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. Wolters Kluwer Health/Lippincott Williams & Wilkins, 2008. [Google Scholar]
- 29.Markman M. Care of the incarcerated cancer patient. Curr Oncol Rep 2007;9:81–2. 10.1007/s11912-007-0001-8 [DOI] [PubMed] [Google Scholar]
- 30.Creswell JW, Plano Clark VL, Gutmann ML. Advanced mixed methods research designs. In: Tashakkori A, Teddlie C, Teddlie CB, eds. Handbook of mixed methods in social & behavioral research. Thousand Oaks, CA: SAGE Publications, 2003: 209–40. [Google Scholar]
- 31.Thomas EH, Wang EA, Curry LA, et al. Patients’ experiences managing cardiovascular disease and risk factors in prison. Health Justice 2016;4:4. 10.1186/s40352-016-0035-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guzman GG. Household income: 2019. American community survey Briefs. Washington DC: US Department of Commerce: US Census Bureau, 2020. [Google Scholar]
- 33.The Sentencing Project . The facts: state by state data. Washington, DC, 2020. Available: https://www.sentencingproject.org/the-facts/#rankings
- 34.Match*Pro Software [program]. 1.6.2 version. Rockville, MD: National Cancer Institute: Division of Cancer Control and Population Sciences: Surveillance Research Program 2020.
- 35.Fellegi IP, Sunter AB. A theory for record linkage. J Am Stat Assoc 1969;64:1183–210. 10.1080/01621459.1969.10501049 [DOI] [Google Scholar]
- 36.Singer JD, Willett JB. Modeling the days of our lives: using survival analysis when designing and analyzing longitudinal studies of duration and the timing of events. Psychol Bull 1991;110:268–90. 10.1037/0033-2909.110.2.268 [DOI] [Google Scholar]
- 37.Agency for Healthcare Research and Quality . 2007 national healthcare quality report. Rockville, MD: U.S. department of health and human services, agency for healthcare research and quality 2008.
- 38.Huff LS, Chang CA, Thomas JF, et al. Defining an acceptable period of time from melanoma biopsy to excision. Dermatol Reports 2012;4:2. 10.4081/dr.2012.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bardell T, Belliveau P, Kong W, et al. Waiting times for cancer surgery in Ontario: 1984-2000. Clin Oncol 2006;18:401–9. 10.1016/j.clon.2006.02.012 [DOI] [PubMed] [Google Scholar]
- 40.Bilimoria KY, Ko CY, Tomlinson JS, et al. Wait times for cancer surgery in the United States. Ann Surg 2011;253:779–85. 10.1097/SLA.0b013e318211cc0f [DOI] [PubMed] [Google Scholar]
- 41.Hanna TP, King WD, Thibodeau S, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ 2020;371:m4087. 10.1136/bmj.m4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang DD, Mahal BA, Muralidhar V, et al. Receipt of definitive therapy in elderly patients with unfavorable-risk prostate cancer. Cancer 2017;123:4832–40. 10.1002/cncr.30948 [DOI] [PubMed] [Google Scholar]
- 43.Reisch LM, Fosse JS, Beverly K, et al. Training, quality assurance, and assessment of medical record abstraction in a multisite study. Am J Epidemiol 2003;157:546–51. 10.1093/aje/kwg016 [DOI] [PubMed] [Google Scholar]
- 44.Bronson J, Sufrin C. Pregnant women in prison and jail don’t count: data gaps on maternal health and incarceration. Public Health Rep 2019;134:57S–62. 10.1177/0033354918812088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsieh FY, Bloch DA, Larsen MD. A simple method of sample size calculation for linear and logistic regression. Stat Med 1998;17:1623–34. [DOI] [PubMed] [Google Scholar]
- 46.Cohen J. Statistical power analysis. Curr Dir Psychol Sci 1992;1:98–101. 10.1111/1467-8721.ep10768783 [DOI] [Google Scholar]
- 47.Hsieh FY. Sample size tables for logistic regression. Stat Med 1989;8:795–802. 10.1002/sim.4780080704 [DOI] [PubMed] [Google Scholar]
- 48.Sandelowski M. Sample size in qualitative research. Res Nurs Health 1995;18:179–83. 10.1002/nur.4770180211 [DOI] [PubMed] [Google Scholar]
- 49.Curry LA, O’Cathain A, Clark VLP, et al. The role of group dynamics in mixed methods health sciences research teams. J Mix Methods Res 2012;6:5–20. 10.1177/1558689811416941 [DOI] [Google Scholar]
- 50.Corbin JM, Strauss A. Grounded theory research: procedures, canons, and evaluative criteria. Qual Sociol 1990;13:3–21. 10.1007/BF00988593 [DOI] [Google Scholar]
- 51.Glaser BG, Strauss AL. The discovery of grounded theory: strategies for qualitative research. Chicago, IL: Aldine de Gruyter, 1967. [Google Scholar]
- 52.Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res 2007;42:1758–72. 10.1111/j.1475-6773.2006.00684.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sbaraini A, Carter SM, Evans RW, et al. How to do a grounded theory study: a worked example of a study of dental practices. BMC Med Res Methodol 2011;11:128. 10.1186/1471-2288-11-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst 2002;94:490–6. 10.1093/jnci/94.7.490 [DOI] [PubMed] [Google Scholar]
- 55.Newman LA, Griffith KA, Jatoi I, et al. Meta-analysis of survival in African American and white American patients with breast cancer: ethnicity compared with socioeconomic status. J Clin Oncol 2006;24:1342–9. 10.1200/JCO.2005.03.3472 [DOI] [PubMed] [Google Scholar]
- 56.Mallik-Kane K, Liberman A, Dubay L. Using jail to enroll low-income men in medicaid. Washington DC: Urban Institute, Justice Policy Center, 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.