Abstract
Long-term reliability of implantable biomedical devices is a critical issue for their practical usefulness and successful translation into clinical application. Reliability is particularly of great concern for recently demonstrated devices based on new materials typically relying on polymeric thin films and microfabrication process. While reliability testing protocol has been well-established for traditional metallic packages, common evaluation methods for polymer-based microdevices has yet to be agreed upon, even though various testing methods have been proposed. This article is aiming to summarize the evaluation methods on long-term reliability of emerging biomedical implants based on polymeric thin-films in terms of their theories and implementation with specific focus on difference from the traditional metallic packages.
Keywords: Long-term reliability, Aging test, Hermeticity, Accelerated aging, Polymer packaging
Introduction
Long-term reliability of implantable biomedical devices is of a critical concern for their successful regulatory approval and clinical application. Failed encapsulation of implanted devices can lead to ingress of conducting body fluid into electronics, thereby damaging circuit functionality. Also, toxic materials in circuit components are exposed to physiological environment, thus severely endangering the safety of the patients. Such packaging durability is particularly important for various modern biomedical implants based on emerging materials that have never cleared regulatory approval for use in implanted condition. Traditional implantable devices have commonly relied on metallic or ceramic packages to prevent gas or liquid penetration into moisture-sensitive electronics. Despite their hermeticity and impermeability to water, there remain several limitations for future applications. Traditional packages made of titanium, ceramic or glass are, first of all, bulky, rigid and heavy. They are not compatible with microfabrication or manufacturable batch process and also require feedthrough technology for electrically connecting inside and outside part of packages, all of which lead to need for time-consuming and labor-intensive manual assembly work as well as limited scalability for higher density stimulation or recording. As alternatives, MEMS-compatible thin-film based encapsulation technologies have been extensively demonstrated for use in chronic implantation, usually based on organic polymeric materials such as parylene-C, polyimide, silicon elastomers, liquid crystal polymer (LCP), cyclic olefin polymer (COC) [1–8].
Testing protocol for quantifying the packaging performance of traditional packages is well established by measuring helium leak rate through package walls which is outlined by a military standard and widely accepted in industry. The longevity evaluation of emerging polymeric devices, however, has not reached a common agreement in terms of theory, implementation, and failure criteria. This lack of settled protocol is considered to substantially impede the rapid translation of the new promising technologies into practical clinical applications.
Relatively short history of polymeric packages compared to that of metallic packages might be one reason of such absence, but another crucial hurdle is that leakage mechanisms of polymeric thin-film barrier is fundamentally different from leakage through metallic encapsulation both in terms of its structure (thin film laminar structure without feedthrough) and materials (gas permeable polymers) [9]. While gas transport in metallic seal takes place only through nanoscale leak channels that are randomly generated as defects of manufacturing process, molecular transportation in polymeric thin film occurs through bulk material surfaces through gas diffusion even in absence of any leak channels [10].
This review article is aiming to, therefore, summarize the existing various evaluation methodologies on long-term reliability of emerging biomedical implants based on polymeric thin-films. For that purpose, traditional hermeticity test protocol is first introduced to discuss its limitations to apply for polymeric devices. Considering water ingress as the primary concern, accelerated aging tests dedicated for polymer-encapsulated materials is reviewed in terms of basic theory and implementation in which devices are soaked in hot saline solution to accelerate the aging process in physiological environment.
Hermeticity tests for devices with metallic packages
Theory and procedure
The most widely accepted criteria for quantifying the hermeticity of a package in the industry is called fine leak test, in which a tracer gas is detected using a mass spectrometer to quantify how much the gas molecules have passed through the encapsulation barrier [11, 12]. Helium is the most commonly used as the tracer gas because it has small molecular weight, is non-toxic, inert, non-flammable, and rare in the atmosphere. Small molecular weight makes the tracer gas easily pass through leak channels for higher sensitivity, whereas rarity of helium gas in normal atmosphere contributes for higher selectivity. Inertness, non-toxicity, non-flammability of helium gas makes the testing procedure relatively simple and safe, but also does not chemically affect the device under test. This aspect makes helium gas more attractive than hydrogen gas, the smallest molecule. When compared to neon, the next noble gas, helium is relatively inexpensive and easily available in various size and purity. The leakage rate for helium can be simply converted into the equivalent leak rate L of another gas of interest. For example, the leak rate of H2O can be obtained from helium leak rate using the following relationship
| 1 |
where M is the molecular mass [13].
Most common and widely used helium fine leak test is described in military standard, MIL-STD-883, Methd 1014 [14]. The first step is to “bomb” the package with pressurized helium gas of pressure Pb, typically in the range of 3–10 atm, for period of time, tb, typically a few hours. Helium would ingress into the cavity as a result of pressure gradient through any existing leak channels connecting outside and inside of the cavity. Then, the specimen is taken out of the bombing chamber and placed into a mass spectrometer under vacuum to measure the rate at which the “bombed” helium leaks out of the package [10, 15–17].
As mentioned above, gas transport mechanism in metallic package is called gas conduction during which gas molecules penetrate through nanoscale channels randomly produced during sealing process [10]. In such gas conduction process, gas mass flux, J (kg/m2 s) can be modeled by gas conductance equation typically formed as [18]:
| 2 |
where ∆p is the difference in gas pressure (Pa) and F is the gas conductance (s/m) defined as
| 3 |
where dchannel is the diameter of a nanoscale leak channel, L is the path length for conduction, M is the gas molar mass, R0 is the universal gas constant, and T is the temperature (K). Gas molecules travelling through nanoscale channels in metallic packages can be described relatively simply compared to gas transport through polymeric devices because gas conduction occurs only through the leak channels of certain geometries and the gas pressure gradient is developed instantaneously.
The measured leak rate decreases over time after the spectrometer is turned on and this initial leak rate is called “apparent leak rate.” This initial leak rate is not only a function of the hermeticity of the specimen, but also depends on the volume of package and test parameters (i.e., bombing pressure, bombing time, dwell time between bombing and measurement) [10, 14]. Apparent leak rate therefore should be converted to equivalent “standard leak rate”, or “true leak rate”, which is defined as the leak rate when the package is exposed in 1 atm (760 mmHg) at the high-pressure side and 1 mmHg (nearly vacuum) at the low-pressure side. The standard leak rate represents the “true” hermeticity of the package depending only on the number and the geometries of leak channels, independent of the test parameters [10, 14]. Mathematical formulation on the relation of measured leak rate and the true leak test has been comprehensively derived in literature [10, 13, 15–17, 19, 20].
Other fine leak test methods are also suggested in MIL standard including leak detection with radioactive tracer gas and optical detection [13]. Mix of Kryton-85 and air is used as tracer gas in radioactive leak test and optical detection measures physical deflection of the package cap under pressurized gas [10, 13, 15].
Limitations and considerations for small packages
As mentioned above, determination of true helium leak rate of the device depends on volume of the internal cavity of the package. Conventional standards are typically based on relatively large volume of cavity with the lowest volume category in MIL-STD-883 being below 0.05 cm3. For internal cavities of volume less than 10−3 cm3, however, it has been shown that the traditional helium fine leak test standards are not applicable for measuring the hermeticity of such packages [10, 13, 16, 17, 21–23]. The reasons include inaccurate reading due to helium loss during dwell time between bombing and leak, undefined regime in leak rate spectrum as leak range of helium leak test and the bubble test do not overlap [16, 24]. Also, leak rate encountered in micropakcages can be lower than the lower detection limits of helium detectors [15, 16]. Various methods have been presented to estimate the true leak rate for small packages, especially focusing onto packaging schemes typically used in microelectromechanical systems (MEMS) with microscale packages [10, 13, 16, 17, 22–24].
These concerns are generally applied for the field of biomedical implants. Package miniaturization is one of the primary aims of the future technologies of implantable biomedical devices for reducing the inconvenience of the patients, risk of surgery and long-term stability. A series of new technologies for miniaturized implants have been developed by incorporating MEMS- or semiconductor technologies in the hermetic sealing of such devices. Considering hermeticity requirements need to be more stringent for the smaller packages as they are more susceptible to fine leakage [15], a new standard for quantifying the hermeticity of such miniaturized biomedical packages are required.
Difference between metallic package and polymeric packages
Metallic packages versus polymeric packages
Unlike traditional metal or ceramic packages typically manufactured by laser-welding to seal the boundaries of two or more pieces leaving a cavity inside, recently demonstrated implantable devices have increasingly relied on thin-film fabrication process especially based on polymeric materials and MEMS technologies. Such a transition imposes several limitations on helium leak test for being used to evaluate the hermeticity or long-term reliability of thin film based devices in terms of two aspects: material and structure. In material point of view, gas or liquid permeating mechanism in polymeric materials are different from that of metallic cases. In structural perspective, internal cavities of polymeric thin film devices fabricated from MEMS process is much smaller, or even zero (no cavity), than metallic packages. These fundamental discrepancies between traditional devices and emerging devices will be discussed in more detail in subsequent subchapters, followed by considerations on how such polymeric thin film based devices could be quantified for ensuring the durability in physiological condition.
Limitations of helium leak test
Various biocompatible polymers have been demonstrated as substrate and/or encapsulation materials of chronically implanted devices, including polyimide, parylene-C, silicone elastomers (such as polydimethylsiloxane (PDMS)), SU-8, COC, and LCP [1–7, 25]. Comparison and summarization on typical material properties and encapsulation characteristics of those materials can be found the previous literature [1–7, 26]. These materials are deposited by diverse technologies such as spin-coating, chemical vapor deposition (CVD), casting, film extrusion and thermal lamination, typically in the thickness range of hundreds of nanometers to tens of micrometers. Helium leak test can be misleading for polymeric thin-film devices for three major reasons: (1) gas penetrating mechanism is different; (2) helium adsorbed onto polymer surface during bombing is included leak rate reading; and (3) cavity volume is small or zero.
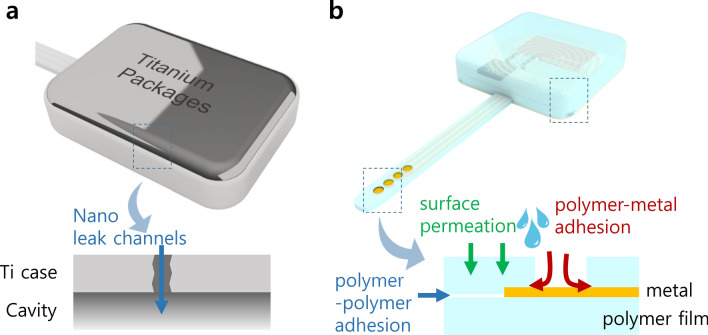
First, it is important to note that leakage mechanisms in polymeric encapsulation are completely different from metallic packages, thus involving another type of leakage that cannot be quantified by helium leak test [17] as illustrated in Fig. 1. Leakage in metallic package is gas transport through nanoscale leak channels, called gad conduction. In polymeric materials, however, permeation of gas and liquid can take place in the entire surface of the polymer films through a process called gas diffusion. Unlike metallic barriers that can be hermetic unless leak channels are formed as defects during manufacturing, polymer films have intrinsic leak rate by diffusion of molecules through bulk material, even in absence of any leak channels. This is why polymer encapsulation is not classified “hermetic”, but instead referred to as “near hermetic” or “semi hermetic” [17]. While gas conduction is a function of only the geometries of leak channels, bulk permeation depends on many parameters such as porosity of the materials, size and concentration of the gas molecules, and chemical affinity of leaking molecules and the barrier materials, rendering it more complicated to evaluate its barrier property by conventional test methods [9, 10, 17, 20, 27].
Fig. 1.
Illustration of leaking mechanisms in conventional metallic packages (a) and polymer-based devices (b)
The second reason is adsorption onto polymer surface. Gas permeation through polymer walls can be explained by three steps. Molecules first are adsorbed onto the surface of the polymer surface, followed by slow diffusion into and through the bulk package material [10, 17]. These molecules are then desorbed from the inner surface of the package into the cavity [28]. Mass spectroscopic measurement of helium leak test, therefore, detects helium molecules from two different origins: i) helium that permeated into the cavity during bombing and permeates out in the reverse process in mass spectroscopy; and ii) helium adsorbed on and desorbed off the outer surface of polymer films without having permeated through the barrier [17, 29]. Even though some studies have calibrated the leak rate by adding a control sample of solid polymer block, it is fundamentally impossible for helium detectors to differentiate helium molecules coming from leak channels, bulk permeation or surface adsorption [30].
Small volume of thin film based polymeric packages makes it more difficult to directly apply conventional test method primarily due to the lowest detectable limits of helium detectors, that is, small amount of helium leaking from small-volume packages may not be detected [15, 16]. Literature commonly sets the limit of the cavity volume 10−3 cm3, below which helium leak test may not be reliably applicable [10, 13, 15, 17, 21, 23]. Most cutting-edge devices based on MEMS technology marginally fall into this regime. Furthermore, active components are conformally encapsulated by CVD, ALD, or casting, thus without leaving any cavity inside the package.
For these reasons, in addition to relatively short history of investigation on polymer-based packaging of active biomedical implants to overcome the limitations of metallic packages, generally agreed testing protocol does not yet exist. Expected lifetime, or equivalent “leak rate”, must still be quantified to assess the long-term durability of thin film encapsulation under body condition. Following chapter introduces such efforts.
Long-term reliability tests for thin film-based devices
Focusing on water ingress
Conventional helium leak standard is intended for quantifying transportation of gas molecules to evaluate hermeticity of general packages. On the other hand, polymer materials can be degraded in physiological condition as a result of hydrolysis (reaction with water in tissue), oxidation (reaction with oxidants produced by tissue), enzymatic degradation, and physical degradation [31]. With regard to biomedical implants, therefore, the primary concern is ingress of water vapor or liquid water from physiological environment into the devices. Typical polymeric devices having multilayered structure fabricated from thin film MEMS technologies are highly susceptible to water ingress through the interfaces between layers, resulting in delamination and eventual device failure. Opening windows of electrode arrays with exposed metal-polymer interfaces are also weak points against water penetration and delamination.
Polymer materials cannot be ideally hermetic against helium gas. However, the polymer-based devices could be considered “practically” or “sufficiently” reliable, if it is shown through relevant evaluation methods that they can prevent water ingress at least for the expected lifetime of devices ranging from a few years up to several decades depending on applications [9]. In that respect, water penetrating in polymer-based devices has been categorized into three pathways: (1) surface permeation, (2) polymer–polymer adhesion, and (3) polymer-metal adhesion [9], as shown in Fig. 1b. The reliability of all-polymer devices can be quantified by applying relevant testing protocols for each leak pathway, for example, analytic approach for computing surface permeation, and employing specially designed test samples having polymer–polymer adhesion and/or polymer-metal adhesion [9]. The test method also can be applied for emerging inorganic thin film encapsulation such as Al2O3 [32, 33], HfO2 [34], SiC [35] and Diamond [36, 37].
Accelerated aging test: theory
One of the most common method for evaluating durability of implantable devices in aqueous condition is accelerated aging test. Specimen is soaked in saline solution of elevated temperature to accelerate the aging process during which relevant properties of devices are monitored in terms of, for example, leakage current, impedance spectroscopy, or functionality [9, 26]. The lifetime of the package, so called “mean time to failure (MTTF)”, at certain accelerating temperature can be translated into equivalent lifetime at body temperature using the Arrheinus reaction rate function. It analyses the temperature-dependence of chemical reaction rate, k, as [9, 38, 39]:
| 4 |
where A is a constant, Ea is activation energy, R is the gas constant, and T is the temperature (K) [9, 26]. The accelerating factor, k1/k2, of the raised test temperature, T1, with respect to body temperature T2 can be estimated as [38, 39]:
| 5 |
Once the activation energy can be found by using MTTF or reaction rates from two or more different temperatures, the lifetime of the devices at body temperature can be extrapolated. The “10-degree rule” is commonly accepted as an approximation of Arrhenius relationship, which explains the chemical reaction rate is doubled for every temperature increment of 10 °C, given assumption that the accelerated aging process is first-order or pseudo first-order [38, 39]. The Eq. (5) can be simplified as:
| 6 |
Accelerating temperature is often recommended to be kept below 60 °C for two reasons: (1) the first-order assumption for approximation of Arrhenius relationship becomes inaccurate with increasing deviation from the reference body temperature; and (2) high temperature can cause additional polymer degradation phenomena that would not occur at body temperature [38, 39]. However, considering the 10-degree rule provides a conservative prediction over a wide range of temperatures, it can be still useful for estimating lifetime of polymeric packages including the worst-case scenarios [38–40].
Accelerated aging test: measurables
The subject of accelerated soak test can be either the fully functional devices or separately prepared test samples specially designed for analyzing the failure process. Depending on the specimen, several variables can be measured during accelerated aging test including leakage current, electrochemical impedance spectroscopy (EIS), and functionality of working devices, as will be detailed in the following subchapters.
Functionality
Fully functional devices such as stimulating/recording electrode arrays, wired or wireless neural recording system, or wireless neural interfacing chiplets can be used as test vehicles in accelerated aging test for evaluating the long-term reliability in aqueous condition. In this case, there is no need to prepare the specially designed sample only for aging test. Also, the MTTF results from aging of the functional devices can provide the most straightforward prediction on the lifetime of the devices compared to specially designed test samples [41]. On the other hand, such test method cannot provide quantitatively detailed information regarding how the encapsulation has failed but a binary reading whether the device is functional or not. Additionally, the result can be misleading when the degradation of solely encapsulation needs to be identified, because the loss of functionality of the full system is not only the result of encapsulation failure, but also associated with a number of factors consisting of the full system. In this regard, accelerated aging of full system would be useful in the final stage of the device development for confirming the durability of the full system, rather than for specific interest of the encapsulation performance of packages.
Long-term stability of Al2O3-parylene bilayer coating for cortically implanted Utah electrode array (UEA) was investigated by Xie et al. [42]. Functionality of UEA wireless neural recording system subject to 57 °C was measured by monitoring RF signal strength from the device. Sub-millimeter-sized tiny neural interfacing chiplets (“Neurograin”) encapsulated by ALD-deposited HfO2-SiO2 multistack was wirelessly interrogated from external transmitter [34]. The wirelessly backscattered RF signal include pre-defined 31-bit data such that functionality in terms of bit error rate (BER) in addition to signal strength could be wirelessly monitored. Thermally grown SiO2 layer transferred to flexible electronics such as resistors, capacitors, diodes, and transistors was evaluated in accelerated aging at 90 °C while the electrical characteristics of those components are monitored via wired interconnection [43]. Not a functional system, but comb-like capacitors replacing the circuit board of a full system can be used to form a LC-tank, such that the resonance frequency change due to moisture ingress in accelerated aging can be detected from external coils [44].
Leakage current
Leakage current under DC bias is usually measured between a pair of interdigitated electrodes (IDEs) encapsulated by packaging material. IDE consists of two individually addressable comb-like electrode structure [45]. The initial leakage current before soaking in the range of pico-ampere (usually a few to tens of pA) gradually increases as conductive saline solution begins to penetrate through the encapsulation barrier, followed by a catastrophic failure when liquid water shorts two IDEs as indicated by abrupt soaring of leakage current above micro-ampere. The IDEs are fabricated and encapsulated by the packaging materials of interest based on specially designed test samples for aging test, the structure of which vary from simple slide glass to the same form factor as the full system. Leakage current can provide the greatest sensitivity for detecting the gradual ingress of moisture. Also, IDEs are easy and inexpensive to fabricate. For these advantages, IDEs are also widely used in the field of biological and chemical sensors [45].
A wide range of packaging materials in varying applications have been evaluated by measuring leakage current from IDE samples, including 10 μm-thick polyimide and parylene-C at 75 °C [46], 6 μm-thick polyimide at 57 to 67 °C [32, 47], 25 μm-thick LCP at 75–87 °C [46, 48, 49], Al2O3 (52 nm) + parylene-C (6 μm) bilayer at 60–80 °C [32, 33, 47], and 100 nm-thick HfO2 or HfO2/SiO2 bilayer at 87 °C [34].
Impedance spectroscopy
Electrochemical impedance spectroscopy (EIS) is another set of variables measured from accelerated soak test which is usually acquired from analyzing impedance between encapsulated IDEs. Despite lower sensitivity to moisture ingress than measuring leakage current in pico-ampere range, EIS data has important advantage that can provide more comprehensive analysis in the course of the degradation of packages by fitting the impedance data to equivalent circuit model of the encapsulation interfaces [26, 50, 51]. Variation of each circuit components implies the water penetrating paths and failure mechanisms of, for example, dissolution, blistering, ion transport, or pore formation [50, 33]. Among varying representations of equivalent circuit models depending on the moisture penetration into the encapsulation and finally to IDE electrode, one representative model is presented in [50]. Intact encapsulation can be modeled as coating capacitance (Cc) in parallel to shunt resistance (Rc). Moisture diffusion into the coating increases Cc while decreasing Rc. When liquid water presents at surface of the IDE electrode, an electrode–electrolyte interface is formed between water and metal electrode which can be modeled as a double layer capacitance (constant phase element, CPE) and a charge transfer resistance Rct. Finally when condensed water liquid electrically connects two adjacent IDE electrode, lateral current path dominates the current through coating [50].
Long-term stability of Al2O3-parylene bilayer coating for cortically implanted Utah electrode array (UEA) was investigated by Caldwell et al. [52]. The authors measured EIS change upon accelerated aging for with and without exposure of electrode site at the tip of shanks. A wide range of packaging materials in varying applications have been evaluated by EIS analyses, including 10 μm-thick polyimide at 60 °C [53, 54], silicone elastomer (5 mm) + parylene-C (40 μm) at 85–97 °C [55], and 650 nm-thick silicon carbide (SiC) at 90 °C [56].
Voltage waveform
Monitoring of voltage transient between two adjacent channels of functional electrode arrays can be used to detect water ingression through the polymer-metal interfaces around the site opening [9]. The amplitude of voltage waveform across two channels during bipolar stimulation abruptly decreases as a result of ingression of saline solution which eventually shorts the two interconnection lines of the stimulating channels.
Others
Other metrics in accelerated aging test have been utilized to quantify the encapsulation performance of varying materials. Measurement of film thickness can be used to obtain dissolution rate of the packaging layer in physiological condition typically for inorganic materials. Thermally grown silicon dioxide or chemically deposited silicon carbide layers were soaked in saline of elevated temperature 87–90 °C to find the dissolution rate to find out how long it takes for the entire coating dissolves away [35, 56, 57]. Water vapor transmission rate (WVTR) is another common industrial standard for barrier performance of films against water vapor in the dimension of mg/m2∙day, which was adopted for characterization of multistack ALD encapsulation of PI/HfO2/Al2O3/HfO2/PI [58]. Measuring mechanical properties of organic film in course of accelerated aging is a simple but useful approach as polymeric materials undergo property changes of, for example, Young’s modulus, fracture energy, and stress at break [59].
Reactive accelerated aging (RAA)
Reactive accelerated aging (RAA) protocol has been proposed to complement the accelerated aging test and further accelerate the aging by using harsher conditions than physiological environment [60, 61]. For rapid simulation of in vivo degradation in RAA, hydrogen peroxide (H2O2) is introduced to saline solution to mimic aggressive chemical environment that is created by activated immune cells, which release digestive enzymes and reactive oxygen species (ROS) [61, 62]. Consequently, the devices are subject to two types of stress in RAA for better mimic the physiological condition to which the real implanted devices are exposed, H2O2 acting like attack from immune system, and high temperature accelerating the chemical reactions. The failure modes of intracortical implants from chronic animal experiments have been found very similar to the results from 7 days of RAA [63, 64]. Temperature and ROS are considered to have different effects on packaging degradation, with increased temperature causing insulation loss from the electrode microwires, and ROS concentration correlating with metal dissolution [61]. It is also noted that RAA results provide qualitative insight for in vivo device degradation, rather than quantitative prediction on the in vivo lifetime of the devices [60, 61].
Discussion and summary
Evaluation methods of hermeticity or long-term reliability of biomedical implantable devices in the physiological implanted conditions were reviewed with a specific focus on the recently demonstrated devices relying on polymer-based thin film encapsulation. For that purpose, traditional hermeticity test has been introduced followed by the limitations making it difficult to identically apply the helium leak test for polymer-based packages. Then, accelerated aging test was discussed as the most widely accepted testing protocol for polymeric encapsulation. The main interest in this article were the basic theory and implementations in terms of how to measure and what to measure to quantify the aging process including functionality, leakage current and EIS.
Even though this article has mainly focused on the integrality of packaging as one of the most critical issues for biomedical implants, other technological and physiological hurdles impeding chronic implantation also need to be addressed to ensure safe and effective applications of emerging biomedical therapeutic and diagnostic technologies. For example, minimization of immune reaction is an important issue for chronic usage of the implant, as formation of fibrosis and gliosis as a result of immune reaction significantly degrade the efficiency of the electronics-tissue interfaces even without device failure.
Not discussed in the article, but it is also noteworthy that electrical bias during wired or wireless interrogation of the specimen in the aging test may cause electrical stress that the existing aging test is not designed to quantify. Electrical stress can initiate or accelerate a sort of new failure modes in the polymeric encapsulation as voltage gradient or current flow can prompt electrochemical process such as corrosion, ion movement, and water electrolysis [32, 54]. This effect has not yet been extensively investigated, but is a critical part considering that most of the implantable devices are utilizing electrical power. Another interesting approach regarding polymer-based implantable devices, which may be a kind of “reverse approach” to long-term reliability is use of bioresorbable materials for biomedical implants [65, 66]. In these approaches, drug delivery or neural regeneration devices have been demonstrated using special materials that can be dissolved away in physiological condition at a controlled manner after having remained functional for certain period of time required for therapeutics.
Lastly, but not least, establishment of general agreement on testing protocol for microscale polymer-based or thin-film-based packaging technologies is of a paramount importance for regulatory approval and practical applications of such emerging biomedical implantable devices.
Funding
This work was supported by a 2-year Research Grant of Pusan National University and by BK21 FOUR from National Research Foundation of Korea.
Declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Scholten K, Meng E. Materials for microfabricated implantable devices: a review. Lab Chip. 2015;15(22):4256–4272. doi: 10.1039/c5lc00809c. [DOI] [PubMed] [Google Scholar]
- 2.Fattahi P, Yang G, Kim G, Abidian MR. A review of organic and inorganic biomaterials for neural interfaces. Adv Mater. 2014;26(12):1846–1885. doi: 10.1002/adma.201304496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onuki Y, Bhardwaj U, Papadimitrakopoulos F, Burgess DJ. A review of the biocompatibility of implantable devices: current challenges to overcome foreign body response. J Diabetes Sci Technol. 2008;2(6):1003–1015. doi: 10.1177/193229680800200610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim C, Jeong J, Kim SJ. Recent progress on non-conventional microfabricated probes for the chronic recording of cortical neural activity. Sensors. 2019;19(5):1–23. doi: 10.3390/s19051069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ordonez J, Schuettler M, Boehler C, Boretius T, Stieglitz T. Thin films and microelectrode arrays for neuroprosthetics. MRS Bull. 2012;37(6):590–598. [Google Scholar]
- 6.Hassler C, Boretius T, Stieglitz T. Polymers for neural implants. J Polym Sci Part B Polym Phys. 2011;49(1):18–33. [Google Scholar]
- 7.Kim BJ, Meng E. Review of polymer MEMS micromachining. J Micromech Microeng. 2015;26(1):013001. [Google Scholar]
- 8.Gwon TM, Kim C, Shin S, Park JH, Kim JH, Kim SJ. Liquid crystal polymer (LCP)-based neural prosthetic devices. Biomed Eng Lett. 2016;6(3):148–163. [Google Scholar]
- 9.Jeong J, Bae SH, Seo J-M, Chung H, Kim SJ. Long-term evaluation of a liquid crystal polymer (LCP)-based retinal prosthesis. J Neural Eng. 2016;13(2):25004. doi: 10.1088/1741-2560/13/2/025004. [DOI] [PubMed] [Google Scholar]
- 10.Han B. Measurements of true leak rates of MEMS packages. Sensors. 2012;12(3):3082–3104. doi: 10.3390/s120303082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knarr OM. Industrial gaseous leak detection manual, McGraw Hill; 1998.
- 12.Hablanian MH. High-vacuum technology: a practical guide, 2nd ed. CRC Press, 1997.
- 13.Vanhoestenberghe A, Donaldson N. The Limits of Hermeticity Test Methods for Micropackages. Artif Organs. 2011;35(3):242–244. doi: 10.1111/j.1525-1594.2011.01222.x. [DOI] [PubMed] [Google Scholar]
- 14.MIL-STD-883F Method 1014.12, Test Method Standard Microcircuits, Department of Defense: Arlington; 2004.
- 15.Goswami A, Han B. On ultra-fine leak detection of hermetic wafer level packages. IEEE Trans Adv Packag. 2008;31(1):14–21. [Google Scholar]
- 16.Tao Y, Malshe AP. Theoretical investigation on hermeticity testing of MEMS packages based on MIL-STD-883E. Microelectron Reliab. 2005;45(3–4):559–566. [Google Scholar]
- 17.Costello S, Desmulliez MPYY, McCracken S. Review of test methods used for the measurement of hermeticity in packages containing small cavities. IEEE Trans Comp Packag Manuf Technol. 2012;2(3):430–438. [Google Scholar]
- 18.Dushman S. Scientific foundations of vacuum technique, 2nd ed. Wiley; 1949.
- 19.Howl DA, Mann CA. The back-pressurising technique of leak-testing. Vacuum. 1965;15(7):347–352. [Google Scholar]
- 20.Greenhouse H. Hermeticity of electronic packages, 2nd ed. William Andrew Publishing; 2011.
- 21.Goswami A, Han B. On the applicability of MIL-Spec-based helium fine leak test to packages with sub-micro liter cavity volumes. Microelectron Reliab. 2008;48(11–12):1815–1821. [Google Scholar]
- 22.Millar S, Desmulliez MPY, McCracken S. Leak detection methods for glass capped and polymer sealed MEMS packaging. Microsyst Technol. 2011;17(4):677–684. [Google Scholar]
- 23.Millar S, Desmulliez M. MEMS ultra low leak detection methods: a review. Sens Rev. 2009;29(4):339–344. [Google Scholar]
- 24.Jourdain A, De Moor P, Pamidighantam S, Tilmans HAC. Investigation of the hermeticity of BCB-sealed cavities for housing (RF-) MEMS devices. Tech. Dig. MEMS 2002 IEEE international conference fifteenth micro electron mechanical systems (Cat. No. 02CH37266), IEEE, 2002. p. 677–80.
- 25.Jeong J, Bae SH, Min KS, Seo JM, Chung H, Kim SJ. A miniaturized, eye-conformable, and long-term reliable retinal prosthesis using monolithic fabrication of liquid crystal polymer (LCP) IEEE Trans Biomed Eng. 2015;62(3):982–989. doi: 10.1109/TBME.2014.2377197. [DOI] [PubMed] [Google Scholar]
- 26.Ahn S-H, Jeong J, Kim SJ. Emerging encapsulation technologies for long-term reliability of microfabricated implantable devices. Micromachines. 2019;10(8):508. doi: 10.3390/mi10080508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Traeger R. Nonhermeticity of polymeric lid sealants. IEEE Trans Parts Hybrids Packag. 1977;13(2):147–152. [Google Scholar]
- 28.Schjølberg-Henriksen K, Poppe E, Moe S, Storås P, Taklo MMV, Wang DT, Jakobsen H. Anodic bonding of glass to aluminium. Microsyst Technol. 2006;12(5):441. [Google Scholar]
- 29.Pham A. Packaging with liquid crystal polymer. IEEE Microw Mag. 2011;66:83–91. [Google Scholar]
- 30.McGrath MP, Aihara K, Chen MJ, Chen C, Pham A-V. Liquid crystal polymer for RF and millimeter-wave multi-layer hermetic packages and modules. RF Microw Microelectron Packag. 2010;66:91–113. [Google Scholar]
- 31.Lyu S, Untereker D. Degradability of polymers for implantable biomedical devices. Int J Mol Sci. 2009;10(9):4033–4065. doi: 10.3390/ijms10094033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie X, Rieth L, Caldwell R, Diwekar M, Tathireddy P, Sharma R, Solzbacher F. Long-term bilayer encapsulation performance of atomic layer deposited Al2O3 and parylene c for biomedical implantable devices. IEEE Trans Biomed Eng. 2013;60(10):2943–2951. doi: 10.1109/TBME.2013.2266542. [DOI] [PubMed] [Google Scholar]
- 33.Minnikanti S, Diao G, Pancrazio JJ, Xie X, Rieth L, Solzbacher F, Peixoto N. Lifetime assessment of atomic-layer-deposited Al2O3-Parylene C bilayer coating for neural interfaces using accelerated age testing and electrochemical characterization. Acta Biomater. 2014;10(2):960–967. doi: 10.1016/j.actbio.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 34.Jeong J, Lee J, Ritasalo R, Pudas M, Laiwalla F, Leung V, Larson L, Nurmikko A. Conformal hermetic sealing of wireless microelectronic implantable chiplets by multilayered atomic layer deposition (ALD) Adv Funct Mater. 2018;1806440:1806440. [Google Scholar]
- 35.Lei X, Kane S, Cogan S, Lorach H, Galambos L, Huie P, Mathieson K, Kamins T, Harris J, Palanker D. SiC protective coating for photovoltaic retinal prosthesis. J Neural Eng. 2016;13(4):046016. doi: 10.1088/1741-2560/13/4/046016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen YC, Tsai CY, Lee CY, Lin IN. In vitro and in vivo evaluation of ultrananocrystalline diamond as an encapsulation layer for implantable microchips. Acta Biomater. 2014;10(5):2187–2199. doi: 10.1016/j.actbio.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 37.Ganesan K, Garrett DJ, Ahnood A, Shivdasani MN, Tong W, Turnley AM, Fox K, Meffin H, Prawer S. An all-diamond, hermetic electrical feedthrough array for a retinal prosthesis. Biomaterials. 2014;35(3):908–915. doi: 10.1016/j.biomaterials.2013.10.040. [DOI] [PubMed] [Google Scholar]
- 38.Hukins DWL, Mahomed A, Kukureka SN. Accelerated aging for testing polymeric biomaterials and medical devices. Med Eng Phys. 2008;30(10):1270–1274. doi: 10.1016/j.medengphy.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Hemmerich KJ. General aging theory and simplified protocol for accelerated aging of medical devices. Med Plast Biomater. 1998;5:16–23. [Google Scholar]
- 40.Lambert BJ, Tang FW. Rationale for practical medical device accelerated aging programs in AAMI TIR 17. Radiat Phys Chem. 2000;57(3–6):349–353. [Google Scholar]
- 41.Jeong J, Hyun Bae S, Seo J-M, Chung H, June KS. Long-term evaluation of a liquid crystal polymer (LCP)-based retinal prosthesis. J Neural Eng. 2016;13(2):025004. doi: 10.1088/1741-2560/13/2/025004. [DOI] [PubMed] [Google Scholar]
- 42.Xie X, Rieth L, Williams L, Negi S, Bhandari R, Caldwell R, Sharma R, Tathireddy P, Solzbacher F. Long-term reliability of Al2O3 and Parylene C bilayer encapsulated Utah electrode array based neural interfaces for chronic implantation. J Neural Eng. 2014;6:35. doi: 10.1088/1741-2560/11/2/026016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fang H, Zhao J, Yu KJ, Song E, Farimani AB, Chiang CH, Jin X, Xue Y, Xu D, Du W, Seo KJ. Ultrathin, transferred layers of thermally grown silicon dioxide as biofluid barriers for biointegrated flexible electronic systems—SI. Proc Natl Acad Sci. 2016;113(42):11682–7. doi: 10.1073/pnas.1605269113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang X, Denprasert PM, Zhou L, Vest AN, Kohan S, Loeb GE. Accelerated life-test methods and results for implantable electronic devices with adhesive encapsulation. Biomed Microdevices. 2017;19(3):46. doi: 10.1007/s10544-017-0189-9. [DOI] [PubMed] [Google Scholar]
- 45.Mazlan NS, Ramli MM, Abdullah MMAB, Halin DSC, Isa SSM, Talip LFA, Danial NS, Murad SAZ. Interdigitated electrodes as impedance and capacitance biosensors: a review. AIP Conf Proc. 2017;1885(1):20276. [Google Scholar]
- 46.Lee SW, Min KS, Jeong J, Kim J, Kim SJ. Monolithic encapsulation of implantable neuroprosthetic devices using liquid crystal polymers. IEEE Trans Biomed Eng. 2011;58(8):2255–2263. [Google Scholar]
- 47.Caldwell R, Mandal H, Sharma R, Solzbacher F, Tathireddy P, Rieth L. Analysis of Al2O3—Parylene C bilayer coatings and impact of microelectrode topography on long term stability of implantable neural arrays. J Neural Eng. 2017;14(4):46011. doi: 10.1088/1741-2552/aa69d3. [DOI] [PubMed] [Google Scholar]
- 48.Gwon TM, Kim JH, Choi GJ, Kim SJ. Mechanical interlocking to improve metal-polymer adhesion in polymer-based neural electrodes and its impact on device reliability. J Mater Sci. 2016;51(14):1–16. [Google Scholar]
- 49.Jeong J, Lee SW, Min KS, Shin S, Jun SB, Kim SJ. Liquid crystal polymer (LCP), an Attractive substrate for retinal implant. Sens Mater. 2012;24(4):189–203. [Google Scholar]
- 50.Chun W, Chou N, Cho S, Yang S, Kim S. Evaluation of sub-micrometer parylene C films as an insulation layer using electrochemical impedance spectroscopy. Prog Org Coat. 2014;77(2):537–547. [Google Scholar]
- 51.Caldwell R, Rieth L, Xie X, Sharma R, Solzbacher F, Tathireddy P. Failure mode analysis of Al2O2-parylene c bilayer encapsulation for implantable devices and application to penetrating neural arrays. In 2015 Transducers—2015 18th international conference solid-state sensors, actuators microsystems, TRANSDUCERS 2015 2015:1747–50.
- 52.Caldwell R, Mandal H, Sharma R, Solzbacher F, Tathireddy P, Rieth L. Analysis of Al2O3—parylene C bilayer coatings and impact of microelectrode topography on long term stability of implantable neural arrays. J Neural Eng. 2017;14(4):046011. doi: 10.1088/1741-2552/aa69d3. [DOI] [PubMed] [Google Scholar]
- 53.Palopoli-Trojani K, Woods V, Chiang C-H, Trumpis M, Viventi J. In vitro assessment of long-term reliability of low-cost μΕCoG arrays. In 2016 38th annual international conference IEEE engineering medical biological society, IEEE; 2016. p. 4503–6. [DOI] [PubMed]
- 54.Woods V, Trumpis M, Bent B, Palopoli-Trojani K, Chiang C-H, Wang C, Yu C, Insanally M, Froemke RC, Viventi J. Long-term recording reliability of liquid crystal polymer µECoG arrays. J Neural Eng. 2018;15(6):66024. doi: 10.1088/1741-2552/aae39d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jeong J, Chou N, Kim S. Long-term characterization of neural electrodes based on parylene-caulked polydimethylsiloxane substrate. Biomed Microdevices. 2016;18:42. doi: 10.1007/s10544-016-0065-z. [DOI] [PubMed] [Google Scholar]
- 56.Hsu JM, Tathireddy P, Rieth L, Normann AR, Solzbacher F. Characterization of a-SiCx: H thin films as an encapsulation material for integrated silicon based neural interface devices. Thin Solid Films. 2007;516(1):34–41. doi: 10.1016/j.tsf.2007.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cogan SF, Edell DJ, Guzelian AA, Ping Liu Y, Edell R. Plasma-enhanced chemical vapor deposited silicon carbide as an implantable dielectric coating. J Biomed Mater Res Part A. 2003;67(3):856–867. doi: 10.1002/jbm.a.10152. [DOI] [PubMed] [Google Scholar]
- 58.de Beeck MO, Verplancke R, Schaubroeck D, Cuypers D, Cauwe M, Vandecasteele B, O'Callaghan J, Braeken D, Andrei A, Firrincieli A, Ballini M. Ultra-thin biocompatible implantable chip for bidirectional communication with peripheral nerves. In 2017 IEEE biomedical circuits systems conference, IEEE, 2017. p. 1–4.
- 59.Rubehn B, Stieglitz T. In vitro evaluation of the long-term stability of polyimide as a material for neural implants. Biomaterials. 2010;31(13):3449–3458. doi: 10.1016/j.biomaterials.2010.01.053. [DOI] [PubMed] [Google Scholar]
- 60.Street MG, Welle CG, Takmakov PA. Automated reactive accelerated aging for rapid in vitro evaluation of neural implant performance. Rev Sci Instrum. 2018;89(9):094301. doi: 10.1063/1.5024686. [DOI] [PubMed] [Google Scholar]
- 61.Takmakov P, Ruda K, Scott Phillips K, Isayeva IS, Krauthamer V, Welle CG. Rapid evaluation of the durability of cortical neural implants using accelerated aging with reactive oxygen species. J Neural Eng. 2015;12(2):026003. doi: 10.1088/1741-2560/12/2/026003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patrick E, Orazem ME, Sanchez JC, Nishida T. Corrosion of tungsten microelectrodes used in neural recording applications. J Neurosci Methods. 2011;198(2):158–171. doi: 10.1016/j.jneumeth.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barrese JC, Rao N, Paroo K, Triebwasser C, Vargas-Irwin C, Franquemont L, Donoghue JP. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J. Neural Eng. 2013;5:6. doi: 10.1088/1741-2560/10/6/066014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prasad A, Sanchez JC. Quantifying long-term microelectrode array functionality using chronic in vivo impedance testing. J Neural Eng. 2012;27:145. doi: 10.1088/1741-2560/9/2/026028. [DOI] [PubMed] [Google Scholar]
- 65.Choi YS, Hsueh YY, Koo J, Yang Q, Avila R, Hu B, Xie Z, Lee G, Ning Z, Liu C, Xu Y. Stretchable, dynamic covalent polymers for soft, long-lived bioresorbable electronic stimulators designed to facilitate neuromuscular regeneration. Nat Commun. 2020;11(1):1–14. doi: 10.1038/s41467-020-19660-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koo J, Kim SB, Choi YS, Xie Z, Bandodkar AJ, Khalifeh J, Yan Y, Kim H, Pezhouh MK, Doty K, Lee G. Wirelessly controlled, bioresorbable drug delivery device with active valves that exploit electrochemically triggered crevice corrosion. Sci Adv. 2020;6(35):eabb1093. doi: 10.1126/sciadv.abb1093. [DOI] [PMC free article] [PubMed] [Google Scholar]