Abstract
The role of peripheral adenosine receptors in pain is a controversial issue and seems to be quite different from the roles of spinal and central adenosine receptors. The present study is aimed at clarifying the role of these receptors in peripheral nociception. To clarify this, studies were done on Swiss mice with adenosine receptor agonists and antagonists. Nociceptive behavior was induced by subcutaneous injection of glutamate (10 μmol) into the ventral surface of the hind paw of mice. Statistical analyses were performed by one-way ANOVA followed by the Student-Newman-Keuls post hoc test. Results showed that intraplantar (i.pl.) administration of N6-cyclohexyl-adenosine (CHA), an adenosine A1 receptor agonist, at 1 or 10 μg/paw significantly reduced glutamate-induced nociception (p<0.01 and p<0.001 vs. vehicle, respectively, n=8−10). In contrast, i.pl. injection of hydrochloride hydrate (CGS21680, an adenosine A2A receptor agonist) (1 μg/paw) induced a significant increase in glutamate-induced nociception compared to the vehicle (p<0.05, n=8), while 4-(-2-[7-amino-2-{2-furyl}{1,2,4}triazolo{2,3-a} {1,3,5}triazin-5-yl-amino]ethyl)phenol (ZM241385, an adenosine A2A receptor antagonist) (20 μg/paw) caused a significant reduction (p<0.05, n=7−8). There were no significant effects on i.pl. administration of four additional adenosine receptor drugs—8-cyclopentyl-1,3-dipropylxanthine (DPCPX, an A1 antagonist, 1–10 μg/paw), N(6)-[2-(3,5-dimethoxyphenyl)-2-(2-methylphenyl)-ethyl]adenosine (DPMA, an A2B agonist, 1–100 μg/paw), alloxazine (an A2B antagonist, 0.1–3 μg/paw), and 2-hexyn-1-yl-N(6)-methyladenosine (HEMADO) (an A3 agonist, 1–100 μg/paw) (p>0.05 vs. vehicle for all tests). We also found that prior administration of DPCPX (3 μg/paw) significantly blocked the anti-nociceptive effect of CHA (1 μg/paw) (p<0.05, n=7–9). Similarly, ZM241385 (20 μg/paw) administered prior to CGS21680 (1 μg/paw) significantly blocked CGS21680-induced exacerbation of nociception (p<0.05, n=8). Finally, inosine (10 and 100 μg/paw), a novel endogenous adenosine A1 receptor agonist recently reported by our research group, was also able to reduce glutamate-induced nociception (p<0.001 vs. vehicle, n=7–8). Interestingly, as an A1 adenosine receptor agonist, the inosine effect was significantly blocked by the A1 antagonist DPCPX (3 μg/paw) (p<0.05, n=7−9) but not by the A2A antagonist ZM241385 (10 μg/paw, p>0.05). In summary, these results demonstrate for the first time that i.pl administration of inosine induces an anti-nociceptive effect, similar to that elicited by CHA and possibly mediated by peripheral adenosine A1 receptor activation. Moreover, our results suggest that peripheral adenosine A2A receptor activation presents a pro-nociceptive effect, exacerbating glutamate-induced nociception independent of inosine-induced anti-nociceptive effects.
Keywords: Inosine, Anti-nociception, Adenosine receptor, Pain, Glutamate
Introduction
Adenosine receptors are widely distributed in mammals and are found in many organs and tissues of the central and peripheral nervous systems. Centrally, adenosine receptors are located in superficial layers of the dorsal horn of the spinal cord, at specific supraspinal pathways related to nociceptive signaling, pre- and post-synaptically on neurons, and on glial cells [1–8]. Interestingly, several studies have demonstrated the role of adenosine receptors in nociception. It is well established that spinal and supraspinal A1 adenosine receptor activation by classical selective agonists reduces nociception in a broad range of preclinical models [3, 9, 10]. We have previously demonstrated that the anti-nociceptive effect of inosine, a novel adenosine A1 receptor agonist, depends on A1 adenosine receptor activation in the spinal cord or supraspinally [11–14] to be effective in mouse chronic neuropathic or inflammatory pain models. Similarly, activation of A2A and A2B adenosine receptors appears to act on spinal glia to suppress nociceptive signaling and on immune cells to suppress inflammation, and, thus, this may be useful against inflammatory and neuropathic pain [3, 15–18]. In addition, A3 adenosine receptor-selective agonists promote anti-nociception when given systemically or spinally, with mechanistic actions on glial cells [19–22].
Despite their presence in peripheral nociceptive sensory nerve endings and on inflammatory and immune cells [8–14], the role of peripheral adenosine receptors in nociception has not been completely elucidated. Several animal studies have suggested that peripheral A1 adenosine receptors have an anti-nociceptive role, as adenosine and A1 adenosine receptor agonists injected into mouse paws induced an anti-nociceptive response [3, 23–26]. Other studies have proposed that peripheral A2 adenosine receptors have a pro-nociceptive effect, since A2 adenosine receptor agonists injected into rodent paws elicited hyperalgesia or nociceptive behaviors, which were then reduced by A2 adenosine receptor antagonists [17, 23]. Injections of A2 adenosine receptor agonists together with formalin also seem to exacerbate formalin-induced nociception [3, 15–18, 24, 25]. Taken together, these results suggest that adenosine may activate peripheral A1 adenosine receptors to relieve nociception but also activate A2 adenosine receptors to induce peripheral nociception. Thus, adenosine-induced peripheral pain does not seem to be a good strategy for investigating the role(s) of adenosine receptors, as it is not a classical nociceptive model. Further, it induces peripheral nociception only through activation of A2 adenosine receptors (A2A or A2B), hampering the identification of the roles played by A1 or A3 receptors.
Thus, in order to clarify the role of peripheral adenosine receptors in peripheral nociception, we used the well-established glutamate-induced nociception model. Glutamate is a major excitatory neurotransmitter that contributes to the development and maintenance of pain responsiveness and is directly involved in nociceptive signal generation and transmission in the nociceptive primary afferent neuron [27–29]. In addition, the glutamate induces nociceptive behavior of very short duration in the experimental animal [29], which is more ethical and acceptable when the objective is to study only peripheral and acute nociception. In addition to the classical adenosine receptor agonists and antagonists, we also used inosine, which we have already demonstrated acts via adenosine receptors to present anti-nociceptive effects in a glutamate-induced nociception model.
Methods
Animals
A total of 244 Swiss mice (Mus musculus) (122 males and 122 females) from the Universidade Federal de Santa Catarina (Florianópolis, Brazil) were used for all experiments. Each of the various groups in the experimental design contained three to five mice. Mice (70 to 90 days old) were housed at 22±2 °C under a 12-h light/12-h dark cycle (lights on at 6:00 a.m.) and had access to food and water ad libitum. They were acclimatized to the laboratory room for at least 1 hour before testing and were used only once. Experiments were performed according to protocols approved by the Committee for Animal Research of the Universidade Federal de Santa Catarina (protocol number PP00484). All experiments were carried out in accordance with current guidelines for the care of laboratory animals and the ethical guidelines for investigations of experimental pain in conscious animals [30]. The number of animals and intensities of noxious stimuli used were the minimum necessary to demonstrate consistent effects. It is important to note that we did not keep track of mice sex (male or female) during the experiments where adenosine receptor agonists or antagonist were tested. At the end of each experiment, animals were euthanized with inhaled CO2 plus 10–50% O2
Drugs
All drugs were administered by the intraplantar (i.pl.) route. The maximum volume of each injection was 20 μL. When the same animal received both an antagonist and an agonist injection, the injections together had a total volume of 20 μL. The volume of glutamate injected was 20 μL. The interval between injections was 5 minutes. Inosine, L-glutamic acid hydrochloride (glutamate), N6-cyclohexyl-adenosine (CHA), and dimethylsulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, Missouri, USA). 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX), hydrochloride hydrate (GS21680), ZM241385, alloxazine, DPMA, and 2-hexyn-1-yl-N(6)-methyladenosine (HEMADO) were purchased from Tocris Bioscience (Tocris Cookson Ltd., Bristol, United Kingdom). Glutamate was prepared in sterile saline (0.9% NaCl), while all other drugs were dissolved in saline with 5% DMSO. The final concentration of DMSO did not exceed 5% and did not cause any effects per se.
Nociception test
We used the i.pl. glutamate injection as a model of peripheral pain. The procedure was similar to that described previously [29]. A volume of 20 μL of glutamate (10 μmol/paw) was injected intraplantarly into the ventral surface of the right hind paw. Animals were observed individually for 15 min after glutamate injection, as previous studies have suggested that the nociceptive response induced by glutamate at 10 μmol/paw is evident during the first 15 minutes after injection but nearly absent afterward [29]. The amount of time spent licking the injected paw was recorded with a chronometer and was considered indicative of nociception. In a separate group of animals, the vehicle used to prepare the glutamate injection (sterile saline, 0.9% NaCl) was injected into the paw; none of the mice exhibited a nociceptive response (data not shown). Therefore, to reduce the number of animals used, we did not include a vehicle control group for glutamate in the subsequent experimental protocols.
Evaluation of adenosine receptor involvement in inosine peripheral anti-nociception
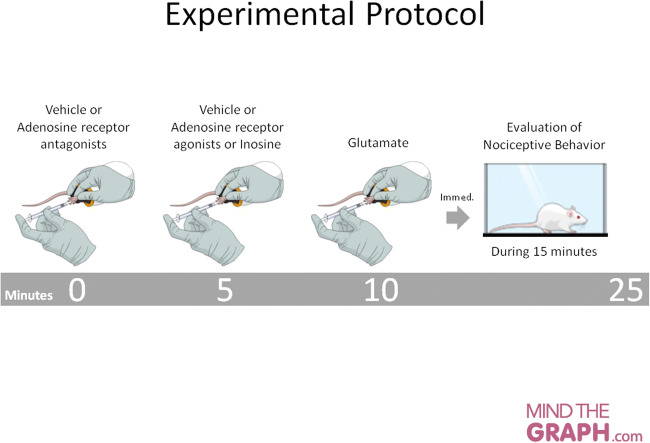
We used selective agonists and antagonists to evaluate the involvement of adenosine receptors on inosine peripheral anti-nociception. The sequence of injections to evaluate each receptor was depicted in Fig. 1. All intervals between injections were 5 minutes. This interval period was chosen in order to avoid paw swelling and/or redness and also changes in locomotor activity due to volume injection, which could prejudice behavior or hinder the assessment of the nociceptive response. The experimental protocol is summarized in Fig. 1. As there were very few data available in the literature to support dosage choices, we constructed dose-response curves for all agonists and antagonists to identify the dose that could inhibit or exacerbate glutamate-induced nociceptive behavior.
Fig. 1.
Schematic of the experimental protocol. Animals were first injected by the intraplantar route with vehicle or adenosine receptor antagonists; after 5 minutes, they received vehicle, adenosine receptor agonists, or inosine. After a 5-minute interval, glutamate (10 μmol/paw) was administered and nociceptive behavior was recorded for 15 minutes. The time spent licking the injected paw was considered indicative of nociception. The following sequences of drug administration were used: 1. Vehicle – vehicle – glutamate; 2. Vehicle – adenosine receptor agonist – glutamate; 3. Vehicle – inosine – glutamate; 4. Adenosine receptor antagonist – vehicle – glutamate; 5. Adenosine receptor antagonist – adenosine receptor agonist – glutamate; 6. Adenosine receptor antagonist – inosine – glutamate. n = 3−5 total male and female mice
Involvement of peripheral adenosine A1 receptor in glutamate-induced nociception
To evaluate the involvement of the peripheral adenosine A1 receptor in glutamate-induced nociception, we used CHA (0.1 to 10 μg/paw) and inosine (1 to 100 μg/paw), both of which are adenosine A1 receptor agonists, and the A1 receptor antagonist DPCPX (1 to 10 μg/paw). CHA and DPCPX have been shown to be selective to adenosine A1 receptors [30–33].
Involvement of peripheral adenosine A2A receptor in glutamate-induced nociception
To evaluate the involvement of the peripheral adenosine A2A receptor, we used the adenosine A2A receptor agonist CGS21680 (0.01 to 1 μg/paw) and the A2A receptor antagonist ZM241385 (1 to 20 μg/paw). Studies have suggested that both drugs are selective to adenosine A2A receptors [30, 33–36].
Involvement of peripheral adenosine A2B receptor in glutamate-induced nociception
To evaluate the involvement of the peripheral adenosine A2B receptor, we used the non-selective A2B receptor agonist DPMA (1 to 100 μg/paw) and the A2B receptor antagonist alloxazine (0.1 to 3 μg/paw). Studies have suggested that DPMA presents similar selectivity to A2 receptor subtypes [34, 35] and that alloxazine presents approximately 10-fold greater selectivity for A2B receptors than for A2A receptors [37].
Involvement of peripheral A3 receptor in glutamate-induced nociception
To evaluate the involvement of the peripheral adenosine A3 receptors, we used the adenosine A3 receptor agonist HEMADO (1 to 100 μg/paw). HEMADO has been shown to be selective to adenosine A3 receptors [30, 33, 38, 39].
Statistical analysis
The experimental results are presented as means ± the standard error of the mean (S.E.M). No data were excluded and were analyzed by one-way ANOVA followed by Student-Newman-Keuls post hoc test. p values less than 0.05 were considered indicative of significance. The statistical software used was Prism 4.0 (GraphPad Software, San Diego, California, USA).
Results
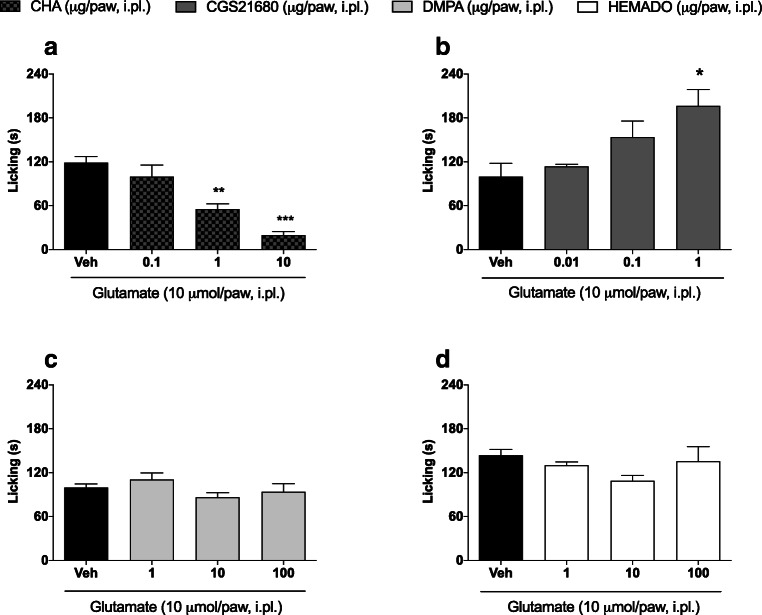
We first evaluated whether peripherally administered adenosine receptor agonists could reduce glutamate-induced nociception. Figure 2a shows that compared to the vehicle, two doses of CHA (1 and 10 μg/paw) reduced glutamate-induced nociception (119.1±8.14 vs. 55.33±7.32; p<0.01 and 119.1±8.14 vs. 19.71±5.05; p<0.001, respectively). On the other hand, as shown in Fig. 2b, i.pl. administration of CGS21680 (1 μg/paw) exacerbated glutamate-induced nociception (196.3±22.66 vs. 99.80±18.13; p<0.05). The effects of DPMA (1–100 μg/paw) and HEMADO (1–100 μg/paw) were not significantly different from that induced by the vehicle at any of the tested doses (Figs. 2c, d, respectively).
Fig. 2.
Effect of adenosine receptor agonists in a glutamate-induced nociception model. Dose-response curve of (a) CHA—an adenosine A1 receptor agonist, (b) CGS21680—an adenosine A2a receptor agonist, (c) DPMA—an adenosine A2b receptor agonist, and (d) HEMADO—an adenosine A3 receptor agonist. CHA, CGS21680, DPMA, or HEMADO were administered by the intraplantar route 5 minutes before glutamate injection. Data are presented as mean±S.E.M. Statistical analysis was performed using one-way ANOVA followed by Student-Newman-Keuls post hoc test. s, seconds. *p<0.05, **p<0.01, and ***p<0.001 for comparisons of the effects of agonists and antagonists with those of vehicle. n = 2−3 total male and female mice
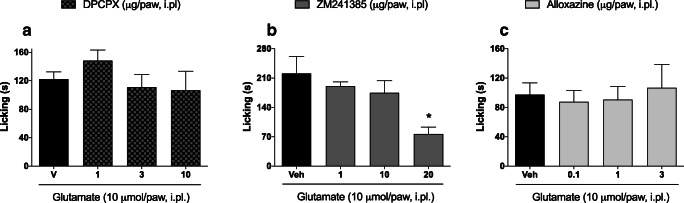
Next, we evaluated whether peripherally administered adenosine receptor antagonists could reduce glutamate-induced nociception. Figure 3a shows that i.pl. administration of DPCPX (1–10 μg/paw) did not significantly change nociception at any of the tested doses. However, ZM241385 (20 μg/paw) significantly reduced glutamate-induced nociception (77.00±16.26 vs. 221.3±40.69; p<0.05) (Fig. 3b). Interestingly, the mice receiving ZM241385 (Fig. 3b) spent more time licking their glutamate-injected paw than did the mice receiving DPCPX or alloxazine (Figs. 3a, c). We were not able to identify the specific variable(s) affecting the amount of time spent paw licking, but all of the experimental groups represented in Fig. 3 appear to have been affected in a similar way. Although not common, this kind of variation is possible when considering animal behavior; Therefore, we believe that this observation neither impairs the interpretation of the results nor the conclusion that ZM241385 (20 μg/paw) significantly reduced glutamate-induced nociception. Alloxazine did not have an effect on nociception at any of the doses tested (Fig. 3c).
Fig. 3.
Effect of adenosine receptor antagonists in glutamate-induced nociception model. Dose-response curves of (a) DPCPX—an adenosine A1 receptor antagonist, (b) ZM241385—an adenosine A2a antagonist, and (c) alloxazine—an adenosine A2b receptor antagonist. DPCPX, ZM241385, or alloxazine were administered by the intraplantar route 5 minutes before glutamate injection. Data are presented as mean±S.E.M. Statistical analysis was performed using one-way ANOVA followed by Student-Newman-Keuls post hoc test. s, seconds. *p<0.05 for the comparisons of the effects of agonists and antagonists with those of vehicle. n = 3−5 total male and female mice
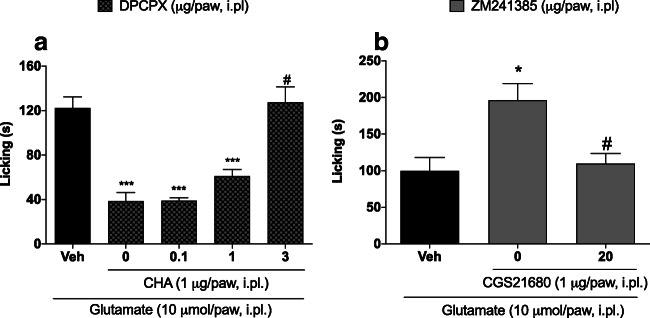
The anti-nociceptive effect of CHA (1 μg/paw) was significantly reduced by prior injection of the A1 receptor antagonist DPCPX (3 μg/paw) (38.50±7.79 vs. 127.50±14.05; p<0.05) (Fig. 4a). In addition, prior administration of ZM241385 (20 μg/paw) reduced the exacerbation of nociception by CGS21680 (1 μg/paw) (196.3±22.66 vs. 109.6±14.04; p<0.05).
Fig. 4.
Effect of adenosine receptor antagonists administered after adenosine receptor agonists in glutamate-induced nociception. (a) DPCPX—an adenosine A1 antagonist—inhibited the anti-nociceptive effect of CHA. (b) ZM241385—an adenosine A2a antagonist-reduced CGS21680-induced increases in glutamate-induced nociception. DPCPX or ZM241385 was administered by the intraplantar route 5 minutes before CHA or CGS21680, respectively. CHA or CGS21680 was administered by the intraplantar route 5 minutes before glutamate injection. Data are presented as mean±S.E.M. Statistical analysis was performed using one-way ANOVA followed by Student-Newman-Keuls post hoc test. s, seconds. *p<0.05 and ***p<0.001 for comparisons of the effects of agonists and antagonists with those of vehicle. # denotes a statistically significant difference compared with CHA (1 μg/paw) or CGS21680 (1 μg/paw) groups in the absence of DPCPX or ZM241385, respectively. n = 3−5 total male and female mice
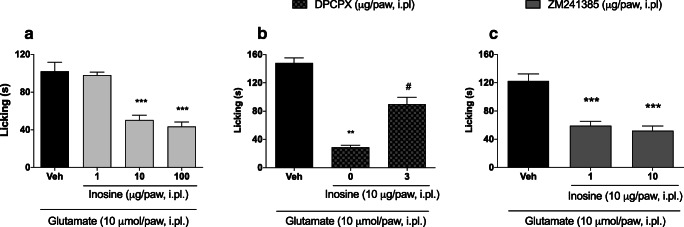
Finally, previous studies by our research group have shown that inosine is able to directly activate adenosine A1 receptors to promote anti-nociceptive effects. Here, we confirm and extend these previous findings. Figure 5a demonstrates that nociception induced by glutamate was significantly reduced by inosine at 10 and 100 μg/paw (102±9.76 vs. 50.43±5.25 and 102±9.76 vs. 43.43±4.82, respectively; p<0.001 for both comparisons). Moreover, the peripheral anti-nociceptive effect induced by inosine (10 μg/paw) was significantly reduced by a prior injection of DPCPX (3 μg/paw) (Fig. 5b). Finally, as shown in Fig. 5c, prior injection of ZM241385 (10 μg/paw, a dose that did not present an anti-nociceptive effect) was not able to counteract the anti-nociceptive effect of inosine (10 μg/paw) (59.20±6.18 vs. 52.00±6.67; p>0.05).
Fig. 5.
The anti-nociceptive effect of inosine in a glutamate-induced nociception model is mediated by adenosine A1 receptors. (a) Inosine dose-response curve. (b) DPCPX—an adenosine A1 antagonist—inhibited the anti-nociceptive effect of inosine. (c) ZM241385—an adenosine A2a antagonist—did not affect the anti-nociceptive effect of inosine. Inosine was administered by the intraplantar route 5 minutes before glutamate injection. DPCPX or ZM241385 was administered by the intraplantar route 5 minutes before inosine. Data are presented as mean±S.E.M. Statistical analysis was performed using one-way ANOVA followed by Student-Newman-Keuls post hoc test. s, seconds. *p<0.05, **p<0.01, and ***p<0.001 for comparisons of the effects of agonists and antagonists with those of vehicle. # denotes significant statistical difference compared with inosine (10 μg/paw) group in absence of DPCPX. n = 3−5 total male and female mice
It is important to point out that there were no significant differences in average licking times between male and female mice (120.8±25.48 s and 117±31.62 s, respectively; p=0.83 by Student’s t-test). However, it should be noted that we did not keep track the mice sex (male or female) during the experiments where the adenosine receptor agonists or antagonists were tested.
Discussion
The results of the present study demonstrate an anti-nociceptive effect of peripheral adenosine A1 receptor activation and a pro-nociceptive effect of peripheral adenosine A2A receptor activation in the glutamate-induced nociception model. These findings were obtained using adenosine receptor agonists and antagonists with high levels of specificity for their respective receptors. CHA presents an approximately 540-fold greater selectivity for A1 receptors than A2 [31], while CGS21680 exhibits an approximately 140-fold selectivity for A2A receptors compared with the A1 receptor [34]. The antagonist DPCPX presents a 10- to 1,000-fold greater selectivity for the A1 receptor than other receptors [33], and the A2A receptor antagonist ZM-241385 exhibits a 50- to 500-fold greater selectivity for A2A receptors [35, 36].
Adenosine receptors, particularly the A1 and A2 receptors, are located on peripheral nociceptive sensory nerve endings [3, 25, 26, 40–44] and modulate pain thresholds when they are activated or blocked. Many studies have used the peripheral injection of adenosine receptor agonists and antagonists to study the involvement of these receptors in peripheral nociception [3, 23–25, 42, 44]. Our findings reinforce the theory that adenosine receptors are located on peripheral nociceptive sensory nerve endings. Glutamate injections directly activate the glutamate receptors found on nociceptive sensory nerve endings, causing depolarization mainly mediated by NMDA and AMPA receptors [27, 45]. Considering that adenosine receptors co-exist with glutamate receptors in peripheral nociceptive sensory nerve endings, local activation or blockage of the receptors may be modulating the response induced by glutamate.
The adenosine A1 receptor is a classical Gi protein-coupled receptor in which intracellular signaling involves inhibition of cyclic AMP/PKA, blockage of Ca2+ channels, and the opening of K+ channels [2, 3, 46]. In this sense, peripheral activation of adenosine A1 receptors reduces neuronal excitability and consequently the transmission of action potentials evoked by the nociceptive stimulus. Interestingly, some studies have suggested that peripheral adenosine A1 receptors are involved in the anti-nociceptive effects of important drugs such as amitriptyline, acetaminophen, and tramadol [47–49]. Additionally, it is well established that adenosine A1 receptor agonists present anti-nociceptive properties when administered peripherally and that these effects can be reduced by an adenosine A1 receptor antagonist [24]. When we administered CHA, a classical adenosine A1 receptor agonist, prior to injecting glutamate, we observed that it reduced glutamate-induced nociception. Furthermore, when the A1 receptor antagonist DPCPX was administered before CHA, it was able to reduce the CHA-induced anti-nociceptive effects. Similarly, the novel A1 receptor agonist inosine reduced glutamate-induced nociception, and its effect was in turn reduced by DPCPX. We have previously demonstrated that inosine acts as an adenosine A1 receptor agonist and that its anti-nociceptive effect is blocked by A1 receptor antagonists and in adenosine A1 receptor knockout mice [11, 12]. Results of the current study extend the knowledge base on inosine’s anti-nociceptive effects, suggesting that the effects observed after intraperitoneal injection [11, 13], could be due, at least in part, to its interaction with A1 receptors in the peripheral nociceptor terminals. Our findings clearly indicate that activation of peripheral adenosine A1 receptors induces an anti-nociceptive effect in a glutamate-induced nociceptive behavior.
Adenosine A2A receptors are expressed peripherally in inflammatory and immune cells, but also in sensory nerve endings [3, 23]. They are classical Gs-coupled receptors in which intracellular signaling involves activation of cyclic AMP/PKA [2, 23, 46]. The role of A2A receptors in nociception processing has been ambiguous, although a broad range of evidence suggests a peripheral pro-nociceptive action [17, 23–25, 30]. Our data show that previous administration of the A2A receptor agonist CGS21680 was not able to reduce glutamate-induced nociception; rather, at the highest dose tested (1 μg/paw), it exacerbated the glutamate-induced nociceptive behavior. The A2A receptor antagonist ZM241385 (20 μg/paw) given prior to glutamate did, however, present an anti-nociceptive effect. These results suggest that peripheral adenosine A2A receptors contribute in some fashion to glutamate-induced nociception and that previous activation of these receptors facilitates and increases nociception. To confirm these findings, we administered ZM241385 (20 μg/paw) to paws previously injected with CGS21680 and glutamate. From this experiment, we determined that ZM241385 was able to counteract CGS21680 exacerbation of glutamate-induced nociception, reducing the nociceptive behavior to levels seen in the controls. Together, these results strongly suggest that in the glutamate-induced pain model, the activation of peripheral adenosine A2A receptors plays a pro-nociceptive role. Moreover, ZM241385 (10 μg/paw, a dose that did not present an anti-nociceptive effect) did not reduce the anti-nociceptive effects of inosine, suggesting that peripheral adenosine A2A receptors are not involved in peripheral inosine anti-nociception.
Our results have demonstrated that peripheral adenosine A2B receptors do not present anti- or pro-nociceptive effects in a glutamate-induced nociceptive behavior. Similarly, neither DPMA, an A2B agonist, nor alloxazine, an A2B antagonist, promoted changes in nociceptive behavior at any of the doses tested. Recent studies of adenosine A3 receptors have demonstrated that their agonists exhibit anti-nociceptive effects, especially when administered systemically, spinally, or supraspinally [19, 22, 50]. In our study, previous administration of different dosages of HEMADO (an A3 receptor agonist) did not reduce glutamate-induced nociception. This suggests that in this acute pain model, peripheral activation of adenosine A3 receptors does not present an anti-nociceptive effect.
Potential study limitations include the lack of blinding in the experimental design. However, the interpretation of nociceptive behavior in the glutamate-induced nociception is not subjective, which will mitigate any experimenter bias. We also cannot rule out the possibility that the effects of agonists, antagonists, and inosine could have been due partly to their effects on the central nervous system (CNS), although doses administered to the paw are unlikely to reach the CNS in sufficient amounts to induce an anti-nociceptive effect. Further, the adenosine receptor agonists and antagonists that we have used are highly selective for specific receptor subtypes. However, we have to consider the possibility that at the doses used in the present study, these agonists and antagonists may also be activating or antagonizing receptor subtypes for which they have very low affinity. We examined dose-response relationships for all of the agents in order to define a dose that could affect glutamate-induced nociceptive behaviors. However, we cannot rule out the possibility of effects from different dosages, possibly larger than the ones we tested. This may be particularly true for the agonists and antagonists that neither inhibited nor exacerbated glutamate-induced nociception.
We have to highlight that we used animals from both sexes in our study. It is clear that there is a difference in sensitivity, interpretation, or pain threshold between sexes and a series of studies corroborate this [51–53]. Also, some studies found male and female pain threshold differences when used glutamate to induce pain behavior [54, 55]. However, these studies used different animal species and tissues to induce pain in comparison with our study. Further, even though there are several hypotheses for this difference of pain perception [56], also in many situations or pain models, this difference is not observed or significant [56, 57] as in our present study. Considering that in the present study we did not keep track mice sex (male or female) during the experiments, we are not able to conclude whether or not there are sex differences in the effects of the adenosine receptor agonists and antagonists.
In conclusion, our results confirm that the activation of peripheral adenosine A1 receptors by classical agonists or inosine presents an anti-nociceptive effect on a glutamate-induced nociceptive behavior, suggesting that these receptors may be an important peripheral target for analgesic drug development. At the same time, our findings support those of other studies suggesting that peripheral A2A receptor activation induces nociceptive behavior.
Acknowledgments
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNpQ) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its additional files.
S. J. Macedo-Júnior
Pharmacist graduated from the Federal University of Santa Catarina (2011). Master (2014) and Doctor (2018) in Pharmacology by the Graduate Program in Pharmacology at the Federal University of Santa Catarina. Post-doctorate (2018-2020) at the Center for Innovation and Pre-Clinical Trials (CIEnP) in Florianópolis, focusing on non-clinical studies for drug development. He has experience in the area of toxicity induced by heavy metals, studying the protective potential of endogenous purines against the toxic effects of methylmercury in rodents. He also has experience in the field of pain pharmacology, studying the involvement of TRPs receptors in animal models of neuropathic pain and pain caused by toxins. He is currently a Junior Researcher at CIEnP where he is the Director of Efficacy Studies.

Funding
This study was supported by grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Declaration
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This study was authorized by the Committee for Animal Research of the Universidade Federal de Santa Catarina.
Informed consent
Not applicable
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Borea PA, Varani K, Vincenzi F, et al. The A3 adenosine receptor: history and perspectives. Pharmacol Rev. 2014;67:74–102. doi: 10.1124/pr.113.008540. [DOI] [PubMed] [Google Scholar]
- 2.Borea PA, Gessi S, Merighi S, Vincenzi F, Varani K. Pharmacology of adenosine receptors: the state of the art. Physiol Rev. 2018;98:1591–1625. doi: 10.1152/physrev.00049.2017. [DOI] [PubMed] [Google Scholar]
- 3.Sawynok J. Adenosine receptor targets for pain. Neuroscience. 2016;3:1–18. doi: 10.1016/j.neuroscience.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 4.Schulte G, Robertson B, Fredholm BB, DeLander GE, Shortland P, Molander C. Distribution of antinociceptive adenosine A1 receptors in the spinal cord dorsal horn, and relationship to primary afferents and neuronal subpopulations. Neuroscience. 2003;121:907–916. doi: 10.1016/s0306-4522(03)00480-9. [DOI] [PubMed] [Google Scholar]
- 5.Ribeiro JA, Sebastiao AM, Mendoca A. Adenosine receptors in the nervous system: pathophysiological implications. Prog Neurobiol. 2003;68:377–392. doi: 10.1016/s0301-0082(02)00155-7. [DOI] [PubMed] [Google Scholar]
- 6.Boison D, Chen JF, Fredholm BB. Adenosine signaling and function in glial cells. Cell Death Differ. 2010;17:1071–1082. doi: 10.1038/cdd.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Popoli P, Pepponi R. Potential therapeutic relevance of adenosine A2B and A2A receptors in the central nervous system. CNS Neurol Disord Drug Targets. 2012;11:644–674. doi: 10.2174/187152712803581100. [DOI] [PubMed] [Google Scholar]
- 8.Gomes C, Ferreira R, George J, Sanches R, Rodrigues DI, Gonçalves N, Cunha RA (2013) Activation of microglial cells triggers a release of brain-derived neurotrophic factor (BDNF) inducing their proliferation in an adenosine A2A receptor-dependent manner: A2A receptor blockade prevents BDNF release and proliferation of microglia. J Neuroinflammation 10. 10.1186/1742-2094-10-16 [DOI] [PMC free article] [PubMed]
- 9.Maione S, de Novellis V, Cappellacci L, Palazzo E. The antinociceptive effect of 2-chloro-2’-C-methyl-N6-cyclopentyladenosine (2’-Me-CCPA), a highly selective adenosine A1 receptor agonist, in the rat. Pain. 2007;131:281–292. doi: 10.1016/j.pain.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Gong QJ, Li YY, Xin WJ, Wei XH, Cui Y, Wang J, Liu Y, Liu CC, Li YY, Liu XG. Differential effects of adenosine A1 receptor on pain-related behavior in normal and nerve-injured rats. Brain Res. 2010;1361:23–30. doi: 10.1016/j.brainres.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 11.Nascimento FP, Figueredo SM, Marcon R, Martins DF, Macedo SJ, Jr, Lima DAN, Almeida RC, Ostroski RM, Rodrigues ALS, Santos ARS. Inosine reduces pain-related behavior in mice: involvement of adenosine A1 and A2A receptor subtypes and protein kinase C pathways. J Pharmacol Exp Ther. 2010;334:590–598. doi: 10.1124/jpet.110.166058. [DOI] [PubMed] [Google Scholar]
- 12.Nascimento FP, Macedo-Júnior SJ, Pamplona FA, et al. Adenosine A1 receptor-dependent antinociception induced by inosine in mice: pharmacological, genetic and biochemical aspects. Mol Neurobiol. 2014;51:1368–1378. doi: 10.1007/s12035-014-8815-5. [DOI] [PubMed] [Google Scholar]
- 13.Macedo-Junior SJ, Nascimento FP, Luiz-Cerutti M, Santos AR. Role of pertussis toxin-sensitive G-protein, K+ channels, and voltage-gated Ca2+ channels in the antinociceptive effect of inosine. Purinergic Signal. 2013;9:51–58. doi: 10.1007/s11302-012-9327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Oliveira ED, Schallenberger C, Böhmer AE, et al. Mechanisms involved in the antinociception induced by spinal administration of inosine or guanine in mice. Eur J Pharmacol. 2016;5:71–82. doi: 10.1016/j.ejphar.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 15.Loram LC, Harrison JA, Sloane EM, Hutchinson MR, Sholar P, Taylor FR, Berkelhammer D, Coats BD, Poole S, Milligan ED, Maier SF, Rieger J, Watkins LR. Enduring reversal of neuropathic pain by a single intrathecal injection of adenosine 2A receptor agonists: a novel therapy for neuropathic pain. J Neurosci. 2009;29:14015–14025. doi: 10.1523/JNEUROSCI.3447-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loram LC, Taylor FR, Strand KA, Harrison JA, RzasaLynn R, Sholar P, Rieger J, Maier SF, Watkins LR. Intrathecal injection of adenosine 2A receptor agonists reversed neuropathic allodynia through protein kinase (PK)A/PKC signaling. Brain Behav Immun. 2013;33:112–122. doi: 10.1016/j.bbi.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li L, Hao JX, Fredholm BB. Peripheral adenosine A2A receptors are involved in carrageenan-induced mechanical hyperalgesia in mice. Neuroscience. 2010;170:923–928. doi: 10.1016/j.neuroscience.2010.07.045. [DOI] [PubMed] [Google Scholar]
- 18.Hussey MJ, Clarke GD, Ledent C, Kitchen I, Hourani SMO. Genetic deletion of the adenosine A2A receptor in mice reduces the changes in spinal cord NMDA binding and glucose uptake caused by a nociceptive stimulus. Neurosci Lett. 2010;479:297–301. doi: 10.1016/j.neulet.2010.05.084. [DOI] [PubMed] [Google Scholar]
- 19.Ford A, Castonguay A, Cottet M, Little JW, Chen Z, Symons-Liguori AM, Doyle T, Egan TM, Vanderah TW, de Koninck Y, Tosh DK, Jacobson KA, Salvemini D. Engagement of the GABA to KCC2 signaling pathway contributes to the analgesic effects of A3AR agonists in neuropathic pain. J Neurosci. 2015;35:6057–6067. doi: 10.1523/JNEUROSCI.4495-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janes K, Esposito E, Doyle T. A3 adenosine receptor agonist prevents the development of paclitaxel-induced neuropathic pain by modulating spinal glial-restricted redox-dependent signaling pathways. Pain. 2014;155:2560–2567. doi: 10.1016/j.pain.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janes K, Wahlman C, Little JW, Doyle T, Tosh DK, Jacobson KA, Salvemini D. Spinal neuroimmune activation is independent of T-cell infiltration and attenuated by A3adenosine receptor agonists in a model of oxaliplatin-induced peripheral neuropathy. Brain Behav Immun. 2015;44:91–99. doi: 10.1016/j.bbi.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Little JW, Ford A, Symons-Ligouri AM, et al. Endogenous adenosine A3 receptor activation selectively alleviates persistent pain states. Brain. 2015;138:28–35. doi: 10.1093/brain/awu330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taiwo YO, Levine JD. Direct cutaneous hyperalgesia induced by adenosine. Neuroscience. 1990;38:757–762. doi: 10.1016/0306-4522(90)90068-f. [DOI] [PubMed] [Google Scholar]
- 24.Karlsten R, Gordh T, Post C. Local antinociceptive and hyperalgesic effects in the formalin test after peripheral administration of adenosine analogues in mice. Pharmacol Toxicol. 1992;70:434–438. doi: 10.1111/j.1600-0773.1992.tb00503.x. [DOI] [PubMed] [Google Scholar]
- 25.Doak GJ, Sawynok J. Complex role of peripheral adenosine in the genesis of the response to subcutaneous formalin in the rat. Eur J Pharmacol. 1995;281:311–318. doi: 10.1016/0014-2999(95)00257-l. [DOI] [PubMed] [Google Scholar]
- 26.Lima FO, Souza GR, Verri WA, et al. Direct blockade of inflammatory hypernociception by peripheral A1 adenosine receptors: involvement of the NO/cGMP/PKG/KATP signaling pathway. Pain. 2010;15:506–515. doi: 10.1016/j.pain.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 27.Millan MJ. The induction of pain: an integrative review. Prog Neurobiol. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- 28.Fundytus ME. Glutamate receptors and nociception: implications for the drug treatment of pain. CNS Drugs. 2001;15:29–58. doi: 10.2165/00023210-200115010-00004. [DOI] [PubMed] [Google Scholar]
- 29.Beirith A, Santos AR, Calixto JB. Mechanisms underlying the nociception and paw oedema caused by injection of glutamate into the mouse paw. Brain Res. 2002;924:219–228. doi: 10.1016/s0006-8993(01)03240-1. [DOI] [PubMed] [Google Scholar]
- 30.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;3:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daly JW, Padgett WL, Secunda SI, Thompson RD, Olsson RA. Structure activity relationships for 2-substituted adenosines at A1 and A2 adenosine receptors. Pharmacology. 1993;46:91–100. doi: 10.1159/000139033. [DOI] [PubMed] [Google Scholar]
- 32.Bruns RF. Adenosine receptor activation in human fibroblasts: nucleoside agonists and antagonists. Can J Physiol Pharmacol. 1980;58:673–691. doi: 10.1139/y80-110. [DOI] [PubMed] [Google Scholar]
- 33.Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Müller CE. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors--an update. Pharmacol Rev. 2011;63:1–34. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao ZG, Blaustein J, Gross AS, Melman N, Jacobson KA. N6–substituted adenosine derivatives: selectivity, efficacy, and species differences at A3 adenosine receptors. Biochem Pharmacol. 2003;65:1675–1684. doi: 10.1016/s0006-2952(03)00153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bridges AJ, Bruns RF, Ortwine DF, Priebe SR, Szotek DL, Trivedi BK. N6-[2-(3, 5-dimethoxyphenyl)-2-(2-methylphenyl)ethyl]adenosine and its uronamide derivatives. Novel adenosine agonists with both high affinity and high selectivity for the adenosine A2 receptor. J Med Chem. 1988;31:1282–1285. doi: 10.1021/jm00402a004. [DOI] [PubMed] [Google Scholar]
- 36.Palmer TM, Poucher SM, Jacobson KA, Stiles GL. 125I-4-(2-[7-amino-2-{furyl}{1,2,4}triazolo{2,3-a} {1,3,5}triazin-5-ylaminoethyl)phenol] (125I-ZM241385), a high affinity antagonist radioligand selective for the A2A adenosine receptor. Mol Pharmacol. 1996;48:970–974. [PMC free article] [PubMed] [Google Scholar]
- 37.Brackett LE, Daly JW. Functional characterization of the A2b adenosine receptor in NIH 3T3 fibroblasts. Biochem Pharmacol. 1994;47:801–814. doi: 10.1016/0006-2952(94)90480-4. [DOI] [PubMed] [Google Scholar]
- 38.Volpini R, Costanzi S, Lambertucci C, Taffi S, Vittori S, Klotz KN, Cristalli G. N6-alkyl-2-alkynyl derivatives of adenosine as potent and selective agonists at the human adenosine A3 receptor and a starting point for searching A2B ligands. J Med Chem. 2002;45:3271–3279. doi: 10.1021/jm0109762. [DOI] [PubMed] [Google Scholar]
- 39.Klotz KN, Falgner N, Kachler S, Lambertucci C, Vittori S, Volpini R, Cristalli G. [3 H]HEMADO—a novel tritiated agonist selective for the human adenosine A3 receptor. Eur J Pharmacol. 2007;556:14–18. doi: 10.1016/j.ejphar.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 40.Sawynok J, Liu XJ. Adenosine in the spinal cord and periphery: release and regulation of pain. Prog Neurobiol. 2003;69:313–340. doi: 10.1016/s0301-0082(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 41.Zylka MJ. Pain-relieving prospects for adenosine receptors and ectonucleotidases. Trends Mol Med. 2011;17:188–196. doi: 10.1016/j.molmed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khasar SG, Wang JF, Taiwo YO, Heller PH, Green PG, Levine JD. Mu-opioid agonist enhancement of prostaglandin-induced hyperalgesia in the rat: a G-protein beta gamma subunit-mediated effect? Neuroscience. 1995;67:189–195. doi: 10.1016/0306-4522(94)00632-f. [DOI] [PubMed] [Google Scholar]
- 43.Sawynok J. Adenosine receptor activation and nociception. Eur J Pharmacol. 1998;347:1–11. doi: 10.1016/s0014-2999(97)01605-1. [DOI] [PubMed] [Google Scholar]
- 44.Katz NK, Ryals JM, Wright DE. Central or peripheral delivery of an adenosine A1 receptor agonist improves mechanical allodynia in a mouse model of painful diabetic neuropathy. Neuroscience. 2015;285:312–323. doi: 10.1016/j.neuroscience.2014.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bleakman D, Alt A, Nisenbaum ES. Glutamate receptors and pain. Semin Cell Dev Biol. 2006;17:592–604. doi: 10.1016/j.semcdb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 46.Chen JF, Lee CF, Chern Y. Adenosine receptor neurobiology: overview. Int Rev Neurobiol. 2014;119:1–49. doi: 10.1016/B978-0-12-801022-8.00001-5. [DOI] [PubMed] [Google Scholar]
- 47.Liu J, Reid AR, Sawynok J. Spinal serotonin 5-HT7 and adenosine A1 receptors, as well as peripheral adenosine A1 receptors, are involved in antinociception by systemically administered amitriptyline. Eur J Pharmacol. 2013;698:213–219. doi: 10.1016/j.ejphar.2012.10.042. [DOI] [PubMed] [Google Scholar]
- 48.Liu J, Reid AR, Sawynok J. Antinociception by systemically-administered acetaminophen (paracetamol) involves spinal serotonin 5-HT7 and adenosine A1 receptors, as well as peripheral adenosine A1 receptors. Neurosci Lett. 2013;536:64–68. doi: 10.1016/j.neulet.2012.12.052. [DOI] [PubMed] [Google Scholar]
- 49.Sawynok J, Reid AR, Liu J. Spinal and peripheral adenosine A1 receptors contribute to antinociception by tramadol in the formalin test in mice. Eur J Pharmacol. 2013;714:373–378. doi: 10.1016/j.ejphar.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 50.Chen Z, Janes K, Chen C. Controlling murine and rat chronic pain through A3 adenosine receptor activation. FASEB J. 2012;5:1855–1865. doi: 10.1096/fj.11-201541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chanda ML, Mogil JS. Sex differences in the effects of amiloride on formalin test nociception in mice. Am J Phys Regul Integr Comp Phys. 2006;291:R335–R342. doi: 10.1152/ajpregu.00902.2005. [DOI] [PubMed] [Google Scholar]
- 52.Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain. 2004;8:397–411. doi: 10.1016/j.ejpain.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Rosen S, Ham B, Mogil JS. Sex differences in neuroimmunity and pain (2017) J Neurosci Res. 2017;95:500–508. doi: 10.1002/jnr.23831. [DOI] [PubMed] [Google Scholar]
- 54.Cairns BE, Hu JW, Arendt-Nielsen L, Sessle BJ, Svensson P. Sex-related differences in human pain and rat afferent discharge evoked by injection of glutamate into the masseter muscle. J Neurophysiol. 2001;86:782–791. doi: 10.1152/jn.2001.86.2.782. [DOI] [PubMed] [Google Scholar]
- 55.Castrillon EE, Cairns BE, Wang K, Arendt-Nielsen L, Svensson P. Comparison of glutamate-evoked pain between the temporalis and masseter muscles in men and women. Pain. 2012;153:823–829. doi: 10.1016/j.pain.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 56.Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13:859–866. doi: 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- 57.Racine M, Tousignant-Laflamme Y, Kloda LA, Dion D, Dupuis G, Choinière M. A systematic literature review of 10 years of research on sex/gender and experimental pain perception - part 1: are there really differences between women and men? Pain. 2012;153:602–618. doi: 10.1016/j.pain.2011.11.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article and its additional files.