Abstract
Conventional wound dressing materials containing free antibiotics for bacterial wound infections are presented with several limitations, that is, lack of controlled and triggered release capabilities, and may often not be adequate to address the complex bacteria microenvironment of such infections. Additionally, the improper usage of antibiotics may also result in the emergence of drug resistant strains. While delivery systems (i.e., nanoparticles) that encapsulate antibiotics may potentially overcome some of these limitations, their therapeutic outcomes are still less than desirable. For example, premature drug release or unintended drug activation may occur, which would greatly reduce treatment efficacy. To address this, responsive nanoparticle-based antimicrobial therapies could be a promising strategy. Such nanoparticles can be functionalized to react to a single stimulus or multi stimulus within the bacteria microenvironment and subsequently elicit a therapeutic response. Such “intelligent” nanoparticles can be designed to respond to the microenvironment, that is, an acidic pH, the presence of specific enzymes, bacterial toxins, etc. or to an external stimulus, for example, light, thermal, etc. These responsive nanoparticles can be further incorporated into wound dressings to better promote wound healing. This review summarizes and highlights the recent progress on such intelligent nanoparticle-based dressings as potential wound dressings for bacteria-infected wounds, along with the current challenges and prospects for these technologies to be successfully translated into the clinic.
Keywords: antimicrobial, stimulus-responsive, intelligent nanoparticles, microenvironment, nanoparticle incorporated dressing, wound dressing, bacterial-infected wounds
1. Introduction
Wound infection is characterized by the colonization of bacteria and other microorganisms that can cause a delay in wound healing or worse, wound deterioration. Most cases of infected wounds are typically caused by bacteria contamination that originate from the skin, other parts of the body, or from the external environment. Intact skin has a three-layer structure, outer epidermis, dermis, and fatty subcutaneous layer, which acts as a protective barrier.1 However, once compromised, the disruption of the outer epidermal barrier, coupled with the denaturation of proteins and lipids, provides a fertile environment for bacteria growth.2−4 The result is an infection that triggers the immune system leading to inflammation and the retardation of healing. Although most wound infections usually resolve on their own, severe wounds that are untreated or inadequately treated may persist and become life-threatening. The goal in wound management is therefore to inhibit or eradicate pathogenic bacteria while promoting wound healing.
Conventionally, wound dressings have been employed as a passive, physical barrier to protect wounds from external contamination.5 Gradually, more advanced dressings that contain antibiotics or other antiseptic compounds were developed to replace conventional, nondrug-based dressings. Common antibiotics that are embedded into wound dressings are the tetracyclines, quinolones, aminoglycosides, and cephalosporins.6,7 These drugs work by altering protein and nucleic acid synthesis of the bacteria, leading to metabolic imbalances or by compromising bacterial cell wall integrity. However, improper usage and abuse of antibiotics may result in the emergence of drug resistant strains. While a long list of microorganisms has been observed to colonize wounds, the most abundant species is S. aureus.8−10 In fact, approximately 70% of wound colonizing bacteria, such as S. aureus and Klebsiella, have presented antibiotic resistance to at least one commonly prescribed agent, making such infections increasingly difficult to treat.6
To mitigate the emergence of antibiotic resistance strains, new drug-delivery technologies using nanoparticles have been explored to target pathogenic bacteria by delivering antimicrobial agents, or even codelivering with growth factors. Table 1 summarizes recent nanoenabled dressings (i.e., embedded with nanoparticles) for antimicrobial wound applications. Although antimicrobial nanoparticles from the literature have shown great potential, they do possess some inherent problems. For instance, premature drug release or unintended drug activation may occur, which would reduce treatment efficacy. To circumvent this, an effective strategy is to develop nanoparticle-based antimicrobial therapies that can respond to the microenvironment. While the literature is flooded with reports on responsive nanoparticles in targeting cancer,11−18 diabetes,19−23 and infectious diseases,24−32 to the best of our knowledge, currently there are few reviews relating to “stimulus-responsive” nanoparticles-based dressings specifically for skin wound infection.
Table 1. Recent In Vivo Antimicrobial Investigations of Nanoparticle-Embedded Dressingsa.
| nanoparticle-embedded dressings | outcomes on infected wound models |
|---|---|
| sericin/chitosan-capped silver nanoparticles (S/C-SNP) incorporated Carbopol hydrogel | S/C-SNPs hydrogel reduced the risk of bacterial infection, accelerated healing process and were found to be biocompatible when applied topically in a rat skin infection model.39 |
| DAPT-modified AuNPs decorated BC (BC-Au-DAPT nanocomposites) | The BC-Au-DAPT nanocomposites inhibited bacterial growth of E. coli or P. aeruginosa and promoted wound repair in a rat model.40 |
| nanosilver particles-collagen/chitosan hybrid scaffold (NAg-CCS) | NAg-CCS was bactericidal, anti-inflammatory, and promoted wound healing potentially by regulating fibroblast migration and macrophage activation in a rat model.41 |
| APA-coated AuNPs doped PCL/gelatin fibers | This wound scaffold demonstrated a striking ability to remedy an MDR E. coli wound infection and assisted the wound care for bacterial infections in a rat model.42 |
| plasma treated electrospun PCL scaffold was coated with AgNPs embedded gelatin (EsPCLGelAg membranes) | The multicoated EsPCLGelAg membrane was applied as first-aid dressing to protect the wound site against bacterial infection, accelerated wound healing process in mouse model.43 |
| MMT-capped AuNPs blending with gelatin (CS-Au@MMT/gelatin) | The CS-Au@MMT/gelatin dressing completely treated MRSA-associated wound infections and showed faster wound healing in rabbit model.44 |
| ciprofloxacin- and fluconazole-containing FNP-incorporated CS hydrogel (cFNPs+fFNPs-CH) bandages | The cFNPs+fFNPs-CH bandages showed a significant antimicrobial activity toward polymicrobial cultures of C. albicans, E. coli, and S. aureus in vitro and ex vivo. A significant reduction in microbial load was obtained upon application of bandages in vivo.45 |
| ZnO loaded Coll/CS nanofibrous | ZnO NPs loaded Coll/CS nanofibrous showed suitable antibacterial activity against S. aureus and E. coli in vitro studies; ZnO NPs loaded Coll/CS nanofibrous effectively quickened wound healing, expressed in the initial stage healing process in vivo studies.46 |
| PVA/CS nanofiber with carboxymethyl CS NPs encapsulating the antibacterial peptide OH-CATH30 (NP-30-NFs) | NP-30-NFs exhibited antibacterial properties against E. coli and S. aureus and promoted skin wound healing in mouse model.47 |
| CGA hydrogel containing liposomal MFX/DEX (CGA-Lipo-MFX/DEX) | CGA-Lipo-MFX/DEX inhibited pathogen microorganism growth and improved corneal wound healing in mouse model.48 |
Abbreviations: CS, chitosan; DAPT, 4,6-diamino-2-pyrimidinethiol; AuNPs, gold nanoparticles; BC, bacterial cellulose; APA, 6-aminopenicillanic acid; PCL, poly(ε-caprolactone); AgNPs, sliver nanoparticles; MMT, 2-mercapto-1-methylimidazole; FNP, fibrin nanoparticle; Coll, collagen; PVA, poly(vinyl alcohol); CGA, collagen/gelatin/alginate; DEX, dexamethasone; MFX, moxifloxacin.
One key risk factor for skin wound infection is diabetes. Diabetic foot ulcers are among the most severe complications of diabetes, leading to an increased risk of bacterial infections that will further impede wound healing.33,34 According to the latest Global Diabetes Map (ninth edition) released by the International Diabetes Federation, the number of diabetic patients worldwide in 2019 has reached 463 million and will approach to 700 million by 2045.35 The increasing cases and potential risk of bacterial infections are anticipated to boost the demand for advanced wound care dressings, thereby propelling its market growth. Moreover, the increasing cases of burns and a rapid rise in the geriatric population are both expected to further contribute to market growth of skin wound care products.36,37 According to a report from Grand View Research, the global advanced wound dressing market size is currently valued at USD 6.85 billion and is expected to register a compound annual growth rate (CAGR) of 4.3%.38 The expected growth of advanced wound care products therefore puts novel nanodrug delivery technologies, specifically for skin wounds, in a strategic position to improve how wounds are currently managed.
This review aims to provide a timely overview of recent research on nanoparticle-based dressings (NDs) that are designed to react or respond to the microenvironment of a wound infection. It aims to provide a comprehensive understanding of how such “intelligent” dressings can trigger the release of its payload under a specific environmental stimulus or itself becoming an antimicrobial agent in a microbial environment. Besides highlighting and summarizing innovative ideas in designing such wound dressings, this review will also provide a prospective of how such technologies can shape future research in technologies relating to wound dressings.
2. Recent Works of Intelligent Nanoparticles for Bacterial Wound Infection
In recent decades, the advent of nanotechnology through the exploitation of nanosized particles has shifted the paradigm of medical therapy, where on-demand delivery of therapeutics can be achieved using “intelligent” nanoparticles. By employing different strategies in functionalizing nanoparticles, intelligent nanoparticles can be designed to respond to a specific chemical, biochemical, or physical stimulus in a physiological environment. Unlike tumor-targeting nanoparticles, advances in antimicrobial nanoparticles’ come from an expanded knowledge of a wound infection microenvironment. Stimulus from such an environment is closely related to bacterial biological cues including bacterial toxins, hydrogen peroxide, the overexpression of specific enzymes, and an acidified environment. In response to these cues, nanoparticles can trigger the release of antimicrobial payloads or themselves eliciting antimicrobial properties. Examples of these cues could be as follows.
2.1. Bacterial Virulence Factors
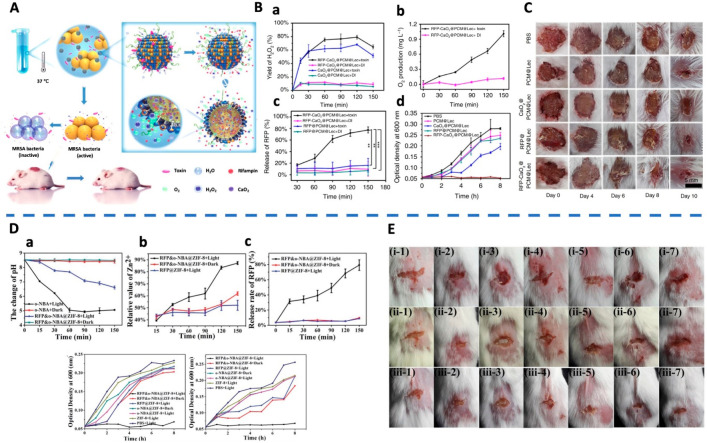
α-Toxin, one of the major cytotoxic agents secreted by S. aureus, is the first bacterial exotoxin identified as a pore former to disrupt cellular membranes.49 On the basis of this, a liposome-based nanoreactor can be formulated to target multidrug resistant (MDR) resistant bacterial infection (Figure 1A).50 This nanoreactor was made through a eutectic mixture, consisting of calcium peroxide and rifampicin, before coating with lecithin and DSPE-PEG3400. In a pathogenic environment, these nanoreactors can be “pierced” by α-toxins secreted by S. aureus to form pores. Water can then enter these nanoreactors through these pores that reacts with calcium peroxide to produce hydrogen peroxide (Figure 1B.a). Hydrogen peroxide would subsequently decompose into oxygen that drives the release of rifampicin (Figure 1B.b,c). These nanoreactors not only showed an enhanced anti-MRSA effect in vitro (Figure 1B.d) but also a significantly higher wound area closure rate than other control treatments in an in vivo model (Figure 1C).
Figure 1.
(A) Schematic of endogenous stimulus-powered antibiotic release from RFP-CaO2@PCM@Lec nanoreactors for bacterial infection therapy. (B) Endogenous stimulus-triggered release from the nanoreactors including the (a) ratio of H2O2 production to theoretical yield, (b) O2 production, and (c) Rifampin. (d) Growth curve of MRSA incubated with different materials. (C) Photographs of MRSA-infected wounds with various treatments. Reproduced from Wu et al., 2019.50 Copyright 2019 Springer Nature. (D) pH change, the release efficiency of Zn2+, and the release of antibiotic after UV light treatment for different periods of time. The optical density at 600 nm of ampicillin-resistant E. coli and MRSA after treatment with various materials. (E) Photographs of MRSA-infected wound with various treatments of (1) PBS + Light, (2) ZIF-8 + Light, (3) o-NBA@ZIF-8 + Dark, (4) o-NBA@ZIF-8 + Light, (5) RFP@ZIF-8 + Light, (6) RFP and o-NBA@ZIF-8 + Dark, (7) RFP and o-NBA@ZIF-8 + Light. (i) 0 d, (ii) 1 d, (iii) 3 d. Reproduced from Song et al., 2018.52 Copyright 2018 WILEY-VCH Verlag GmbH and Co. KGaA, Weinheim.
2.2. pH
The bacterial microenvironment is usually acidic. As such, Hassan et al. formulated pH-responsive lipid-polymer hybrid nanovesicles, loaded with vancomycin (VM-OLA-LPHVs1) for treating bacterial infections.51 A novel oleylamine-based zwitterionic lipid was first synthesized before formulating it into chitosan-based pH-responsive nanovesicles. With its surface-charge switching from negative (at neutral pH) to positive (at acidic pH), VM-OLA-LPHVs1 presented a more rapid release of vancomycin with a 97% release after 72 h, leading to a lower minimum inhibitory concentration (MIC). They observed a 52.9-fold increase in antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA) and a significantly higher percentage of biofilm eradication. In subsequent in vivo studies, the pH-responsive VM-OLA-LPHVs1 revealed a 95-fold lower MRSA burden compared to the free vancomycin group in a mouse-infected skin model, suggesting its potential for bacterial wound infection treatment.
2.3. Light
Song et al. developed a new strategy to combine the antibiotic delivery and light-responsive metal–organic frameworks (MOFs).52 In their work, the zeolitic imidazolate framework (ZIF) was formed and modified with a pH-jump reagent (2-nitrobenzaldehyde, NBA). Under the stimulation of light at a specific wavelength (365 nm), the decomposition of the NBA was triggered to produce hydrogen ions that causes a pH change inside the ZIF (Figure 1D.a). This further promoted the decomposition of ZIF that releases rifampicin (Figure 1D.b,c). Here, the porous nanoparticulate ZIF acts as a gatekeeper, enabling light-triggered nanoparticles to inhibit bacterial infection, through a switchable and synergistic antibacterial effect (Figure 1D.d,e). With synergistic treatments of MOFs and light, it is shown that the area of wound decreased approximately 80% quicker than other treatments (Figure 1E).
2.4. Bacterial Enzymes
Many kinds of Gram-positive pathogens secrete hyaluronidase.53 RuNP-based antibacterial nanosystems (AA@Ru@HA-MoS2) for combined chemo-photothermal therapy against MDR bacteria were thus developed.54 These mesoporous RuNPs were first encapsulated with ascorbic acid (AA) and covered with hyaluronic acid (HA). Nanoparticles were subsequently coated with molybdenum disulfide (MoS2), which was modified with bacteria-targeting ciprofloxacin. At the infection site, HA was decomposed by hyaluronidase secreted by bacteria, resulting in AA release, which could be directly catalyzed by MoS2 to generate hydroxyl radicals. By taking advantage of the excellent photothermal therapy performance of RuNPs, the nanosystem in a skin-infected model exhibits potent antibacterial activity, which inhibits the bacteria and prevents the formation of biofilms.
The studies above show how responsive nanoparticles can effectively and selectively be delivered on-demand to the bacterial infection site, under the stimulation of a specific cue. These strategies can improve the therapeutic effect of antibiotics, minimize its dosage and frequency of administration while minimizing the side effects of the drugs.55−58 However, nanoparticle delivery of antibiotics for the treatment of bacterial infected wounds has limitations. For example, how can biofilm formation be overcome and prevent the emergence of multidrug resistant bacteria? By introducing intelligent antimicrobial nanoparticles into wound dressings, more effective treatments can be realized that meets current clinical demands.
3. Intelligent Nanoparticle-Based Dressings for Bacterial Wound Infection
Overcoming the above-mentioned limitations therefore requires the development of intelligent antimicrobial nanoparticles. Depending to the complexity of the response process, intelligent nanoparticle-based dressings can be classified as single-stimulus or multiple-stimulus responsive nanoparticle-based dressings.
3.1. Single-Stimulus Responsive Nanoparticle-Based Dressings
3.1.1. pH-Responsive
pH-responsive nanoparticles are one of the most widely studied and reported responsive systems. On the basis of the microscopic perspective of the well-established pH in different cellular compartments, the acidification of endosomes (pH 5.5–6.5) and subsequent fusion with lysosomes (pH 4.5–4.7), during nanoparticle uptake, provides an acidic environmental gradient for drug release.59−61 From a macroscopic perspective, other types of pH gradients in physiological environments, such as acidification of pathogenic infectious wounds, can also be exploited by pH-responsive nanoparticle-based dressings.62,63 The hypoxic environment in infection sites causes anaerobic glycolysis, which results in ion channel turbulence and acid production. Lactic acid as a byproduct of the host immune response will further lower the local pH of the pathogenic site. In addition, the pH of the microenvironment is different whether it is acute wounds, chronic wounds, or different stages of the wound healing process. For example, the pH of acute and chronic wounds is acidic during the healing process, while the pH of chronic wounds is alkaline.109,110 On the basis of this, various pH-responsive nanoparticle-based dressings have been developed.
Cellulose has been extensively explored as some of the most promising biomaterial and is considered a suitable template for the encapsulation of metal nanoparticles through its three-dimensional, porous structure with specific nanopore size distribution. Bacterial cellulose (BC)-Ag nanocomposites can be prepared by inducing silver nanoparticles into bacterial cellulose microfibrils.64 The authors pointed out that the BC-Ag nanocomposites presented pH-responsive controlled release behaviors that resulted in a significantly higher and faster release of Ag ions in an acidic environment. Furthermore, the excellent biocompatibility and excellent antimicrobial effects on E. coli, S. aureus, B. subtilis, and C. albicans suggested this nanocomposite to have a strong potential as wound dressings.
More recently, MOFs are novel porous materials, constructed from inorganic metal ions and connected by organic linkers. Their intrinsic advantages, such as biodegradability, biocompatibility, pH-responsive behavior, possesses a wide range of pore size and high porosity, and high surface area, make them promising materials for clinical applications.65 Mazloom-Jalali et al. developed a chitosan-polyethylene glycol nanocomposite film containing ZIF-8 nanoparticles and the antibiotic cephalexin.66 The nanocomposite films showed a pH-responsive release of cephalexin in an acidic solution. Notably, the release of cephalexin abruptly increased to over 65% within approximately 3 h due to the rapid swelling of the film and fast degradation of MOFs in acidic condition compared to a neutral (release over 50% in 8 h) and an alkaline (10 h) media. Moreover, the film presented remarkable antibacterial performances against B. cereus, S. aureus, and E. coli. In addition to the presence of the antibiotic, the presence of the cationic Zn2+ metal ions from the degraded MOFs also plays a role. Zn2+ was internalized into the bacterial cells through their damaged cell membranes and reacted with the DNA and enzymes, leading to the dysfunction of the cells. In addition, these nanocomposite films are shown to preserve cell viabilities, indicating their biocompatibility.
For a chronic wound microenvironment, such as a diabetic wound, it has a more alkaline pH as compared to that of healthy tissues. To prevent bacterial infection, or to eradicate pathogens, Ca-alginate hydrogel loaded with protamine NPs (cationic antimicrobial peptide) were prepared.67 The hydrogel presents pH-responsive behaviors providing a much more rapid and complete drug release in a mimicked diabetic wound microenvironment with a pH value of 8.0. Such an environment facilitated a faster release of protamine NPs to enhance their antibacterial activity.
3.1.2. Bacterial Enzymes-Responsive
Pathogenic bacteria, such as S. aureus and P. aeruginosa, secrete various virulence factors, such as toxins and enzymes (such as lipase, phosphatase, phospholipase, and hyaluronidase), which enable them to thrive in their environment.68,69 Among these virulence factors, enzymes play a vital role due to their high specificity and catalytic ability in cellular metabolisms. Pathogen-induced enzyme abnormalities can also become a target of intelligent nanoparticle-based dressings. For example, the ester bond in materials can target phosphatase, intracellular acid hydrolase, and several other esterases. By taking advantage of these overly expressed enzymes at most bacterial infection sites, nanoparticle-based dressings that respond to these cues could be developed.
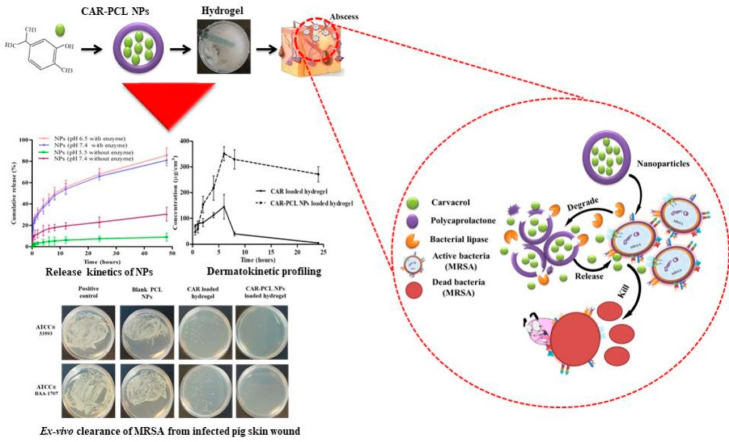
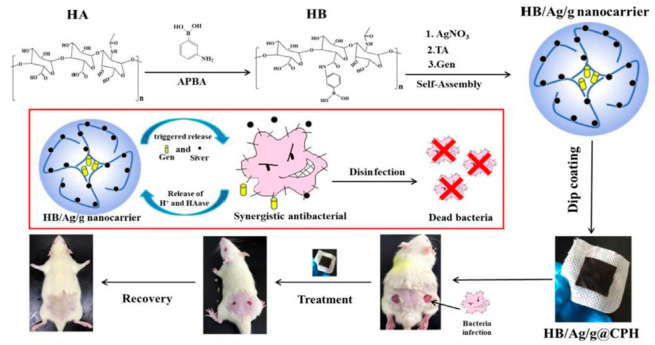
Mir et al. formulated a poly(ε-caprolactone) nanoparticles loaded with carvacrol, which was effective against MRSA.70 By encapsulating into nanoparticles and incorporating them into a hydrogel matrix, the anti-MRSA activity of carvacrol was increased 2-fold higher than its free form. In the presence of bacterial lipase, the nanoparticle-embedded hydrogel exhibited a significantly higher release of carvacrol, highlighting its potential as a triggered delivery system. Furthermore, the ex vivo study revealed that this formulation exhibited productive antimicrobial activity against MRSA induced skin infections (Figure 2). Hyaluronidase, also widely found in MRSA secretions, breaks down hyaluronic acid and helps spread the bacterial cells.71,72 On the basis of this, a nanocarrier-based chitin hydrogel containing hyaluronan, AgNPs, and gentamicin was also prepared (Figure 3).73 With bacterial hyaluronidase, this hydrogel can be triggered to provide a controlled release of gentamicin and AgNPs, thereby bringing about synergistic in vitro and in vivo bactericidal activity.
Figure 2.
Schematic of preparing carvacrol-loaded poly(ε-caprolactone) nanoparticles. Reproduced from Mir et al., 2019.70 Copyright 1996–2020 MDPI.
Figure 3.
Schematic of preparing HB/Ag/g nanocarrier and designed synergetic antibacterial hydrogel for wound disinfection. Reproduced from Yu et al., 2020.73 Copyright 2019 Elsevier B.V.
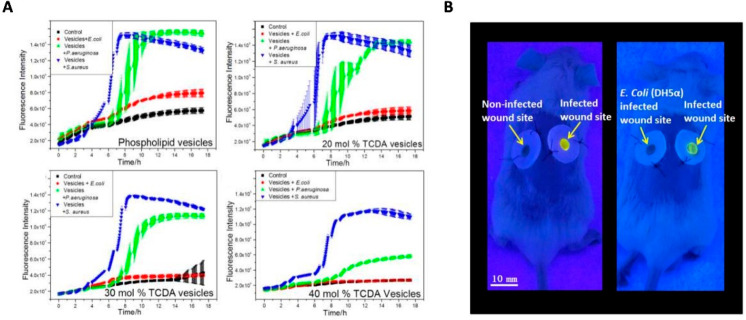
In addition to infection therapy, the instant detection of wound bacterial infection can also be explored as a potential benefit of the intelligent nanoparticle-based dressings. Typical microbial quantification methods for the detection of bacteria have a detection limit of theoretically one organism per analyzed portion of the sample. However, using intelligent nanoparticles, the detection accuracy could be further improved. To achieve this, lipid vesicles containing self-quenching fluorescent dyes and antimicrobial agents embedded in a methacrylated gelatin were developed.74 In the presence of bacterial lipase, the gelatin would be triggered to release fluorescein to show a color change as well as to release antibiotics as an on-demand antibacterial action to an infected wound caused by S. aureus and P. aeruginosa (Figure 4A). This intelligent dressing offered a method to provide early warnings of wound infections by “sensing” the wound (Figure 4B).
Figure 4.
(A) Kinetics of the interaction of bacteria with phospholipid or TCDA vesicles. Toxins secreted by P. aeruginosa (green) and S. aureus (blue) induced vesicles permeabilization as revealed by the increased fluorescence; E. coli (red) as control did not permeabilize vesicles. (B) Representative imaging of colorimetric sensing property of the prototype wound dressing in Balb/c model. Reproduced from Zhou et al., 2018.74 Copyright 2018 Elsevier Ltd.
3.1.3. Photo- and Photothermal-Responsive
Light of different wavelengths, such as ultraviolet (UV), visible, and near-infrared (NIR), have been widely used as a trigger to design responsive nanoparticles. Although numerous research articles have applied this approach for cancer,75−78 the penetration of visible and UV light may limit their in vivo applications. This is due to the strong scattering characteristics of ultraviolet/visible light by soft tissues. On the contrary, there is no such problem for topical skin management, especially in treating skin wound infections.
In this regard, a photosensitive nanogel containing AgNPs immobilized on the surface of poly(ε-caprolactone) nanofibers mats was developed.79 It can undergo structural changes under UV light stimulation. Upon irradiation (405 nm), the nanogel collapsed, and AgNPs were released from the nanogel but dispersed into the nanofiber mats. Simultaneously, the plasmonic band of the AgNPs was excited, effectively controlling the propagation of silver ions, leading to excellent antibacterial effects against both S. aureus and E. coli.
In addition to directly trigger the payload release, antimicrobial photodynamic therapies (APDTs) and antimicrobial photothermal therapies (APTTs) based on nanoparticles with photosensitizers were developed as noninvasive strategies for treating bacterial wound infections.80−82 Incorporated with photosensitizers, nanoparticle-based dressings can either produce reactive oxygen species (ROS) (photodynamic effects) or convert light photons into heat under NIR irradiation (photothermal effects) to destroy microorganisms.
3.1.3.1. APDT
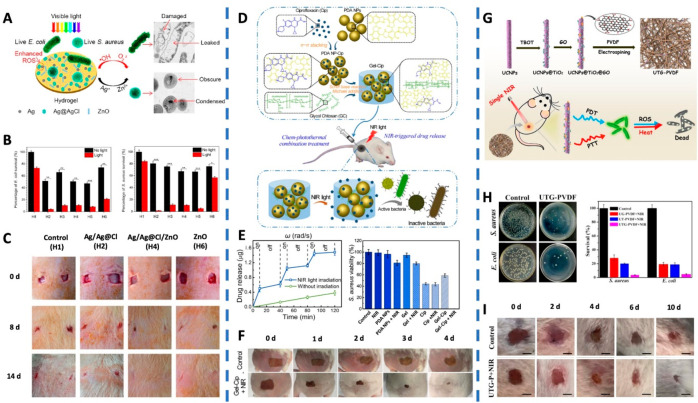
A hybrid hydrogel containing Ag/Ag@AgCl/ZnO nanostructures had also been prepared as a potential strategy for wound management.83 In this hydrogel, the Ag/Ag@AgCl nanostructures were assembled through UV light chemical reduction followed by the incorporation of ZnO nanostructures. It is reported that upon exposure to visible light, the Ag/Ag@AgCl nanostructures enhanced the photocatalytic of ZnO, resulting in the deposition of Ag/Ag@AgCl, which generated enhanced production of ROS such as singlet oxygen and hydroxyl radical (Figure 5A). Because of the synergistic effect of ROS by photoexcitation of ZnO and the antimicrobial ions (Ag+ and Zn3+) released from the nanocomposite, the bacteria exposed to the hydrogels showed significant decrease of microbial (killing 95.95% of E. coli and 98.49% of S. aureus) after exposure to stimulated sunlight (Figure 5B). Furthermore, the in vivo assessments showed that after 14-day treatment, the wounds treated with Ag/Ag@AgCl/ZnO nanostructures revealed complete closure but not the other controls. This suggests that this nanocomposite hydrogel can accelerate wound healing (Figure 5C).
Figure 5.
(A) Schematic of the visible light triggered photodynamic therapy for Ag/Ag@Cl/ZnO hydrogel for bacterial inactivation. (B) Ability of the hydrogels in killing E. coli and S. aureus under simulated sunlight. (C) In vivo study on the effects of treatment of S. aureus-induced wound infections by hydrogels and the corresponding wound photographs of the rats at days 0, 8, and 14. (H1, control hydrogel; H2, Ag/Ag@AgCl hydrogel; H3, H4, and H5: Ag/Ag@AgCl/ZnO hydrogels; H6, ZnO hydrogel). Reproduced from Mao et al., 2017.83 Copyright 2017 American Chemical Society. (D) Schematics of the synthetic route of Gel-Cip and NIR light irradiation-triggered Cip release from Gel-Cip for bacterial inactivation. (E) NIR light-triggered Cip release from Gel-Cip. The corresponding statistical diagram of S. aureus colonies with various treatments. (F) Photographs of S. aureus-infected wound of mice after treated with Gel-Cip and NIR light irradiation. Reproduced from Gao et al., 2019.84 Copyright 2018 Elsevier Ltd. (G) Schematic of the synthesis of UTG-PVDF nanocomposite membrane and the bactericidal activities of UTG-PVDF membrane upon NIR Light Illumination. (H) Photographs of S. aureus and E. coli colonies on the UTG-PVDF membrane upon NIR irradiation. Histogram showing the relative bacterial survival. (I) Photographs of wounds on the mice during the therapeutic process. Reproduced from Sun et al., 2019.88 Copyright 2019 American Chemical Society.
3.1.3.2. APTT
Gao et al. developed a NIR light-triggered hydrogel-based drug reservoir by mixing ciprofloxacin-loaded polydopamine nanoparticles with glycol chitosan to form a hydrogel (Figure 5D).84 This hydrogel-based drug reservoir was able to be controlled to release antibiotics upon NIR irradiation. Meanwhile, NIR irradiation activated the photothermal polydopamine nanoparticles and generated local hyperthermia to inactivate the bacteria in a synergistic manner (Figure 5E). In vivo healing ability of the synergistic strategy was assessed on S. aureus-infected mice revealed that the wound, after treatment with Gel-Cip plus NIR irradiation, almost recovered on the fourth day; that is, only 6.4% of wound area remaining, compared to the various controls (Figure 5F). Similarly, gold nanorods and an antimicrobial peptide (IK8) were coencapsulated in liposomes, and then incorporated into PEG hydrogel, which was able to release IK8 against P. aeruginosa and S. aureus upon laser irradiation at 860 nm.85 By increasing the laser intensity, a thermal enhancement of the antimicrobial peptide bactericidal activity could also be achieved. The photothermal triggered release and enhancement of photothermal efficacy indicated that the therapeutic gel has the potential to treat pathogenic bacteria. Recently, scholars focused on NIR-responsive materials with antimicrobial photothermal properties. Such as gallic acid functional silver nanoparticles embedded polysaccharide hydrogels and Prussian blue nanoparticles embedded chitosan hydrogels.86,87 The silver nanoparticles and MOFs in the hydrogels were both capable of effectively and controllably converting 808 nm NIR light into heat. The gradual temperature increase damages the bacterial membrane and subsequently denatures the protein, resulting in a bacteria-killing effect.
3.1.3.3. Combination of Both Therapies
Recently, significant research has been focused on the combination of APDT and APTT in a single nanoparticle-based dressing. For example, Sun et al. hierarchically structured the up-conversion nanoparticles (UCNPs) as the core and followed by coating them with TiO2 nanoparticles as the shell.88 These core–shell UCNPs@TiO2 nanoparticles were doped with a photothermal agent, graphene oxide, to obtain a mixture called UTG. Thereafter, the mixture in poly(vinylidene) fluoride (PVDF) was electrospun to generate the nanoparticle-incorporated membrane (UTG-PVDF) (Figure 5G). Upon NIR irradiation, the UTG-PVDF membrane could generate ROS, and a rise in temperature simultaneously occurred. This triggered synergistic antibacterial effects against Gram-positive and Gram-negative bacteria (Figure 5H). The APDT/APTT synergistic therapeutics of this nanoparticle-bound membrane on open infected wounds were also investigated, in which the UTG-PVDF membrane after NIR irradiation could efficiently prevent wound infection and promote wound healing (Figure 5I). Similarly, in Cui and colleagues’ work, a conjugated polymer PDPP with high photothermal conversion efficiency was fabricated into nanoparticles, which were further grafted with a cell-penetrating peptide on the surface, achieving one of the composites, CPNs-TAT.89 By physically mixing polyisocyanides hydrogel, polythiophene, and CPNs-TAT, an intelligent nanoparticle-based dressing with APDT and APTT properties was obtained. The hydrogel can regulate the dispersity of polythiophene and improve ROS production. Meanwhile, the CPNs-TAT can be uniformly scattered in the hydrogel, thereby achieving a consistent temperature increase to enhance the therapeutic effect of PTT. When exposed to white and NIR light sequentially, synergistic PDT and PTT presented more substantial antimicrobial effects than PDT or PTT alone.
3.2. Multiple-Stimuli Responsive Nanoparticle-Based Dressings
In reality, a single nanoparticle system that responses to a single stimulus may not be sufficient to efficiently deliver therapeutic agents or elicit antibacterial effects at the site of the infected wound. For multistimulus nanoparticle-based dressings, such dressings could react to a range of cues in the targeted microenvironment to elicit a response, that is, therapeutic or diagnostic.
Qiao et al. developed a smart nanoparticle-based wound dressing, which was capable of monitoring bacterial infection and offering an on-demand treatment.90 This intelligent hydrogel consisted of four parts: physically cross-linked of poly(vinyl alcohol), a UV-cleavable poly prodrug (GS-Linker-MPEG), Cyanine3- and Cyanine5-modified silica nanoparticles (SNP-Cy3/Cy5), and up-conversion nanoparticles (UCNPs). Within a bacterial microenvironment, a pH-responsive fluorescence resonance energy transfer (FRET) transition between Cy3 and Cy5 was activated. Thereby, the SNP-Cy3/Cy5, acting as a pH-responsive fluorescent probe, was able to detect bacterial infection. Moreover, upon the irradiation with NIR, UCNPs were able to cleave the linker and release GS from the poly prodrug. This intelligent nanoparticle-composited hydrogel also presented great water absorptivity, outstanding mechanical properties, and excellent biocompatibility.
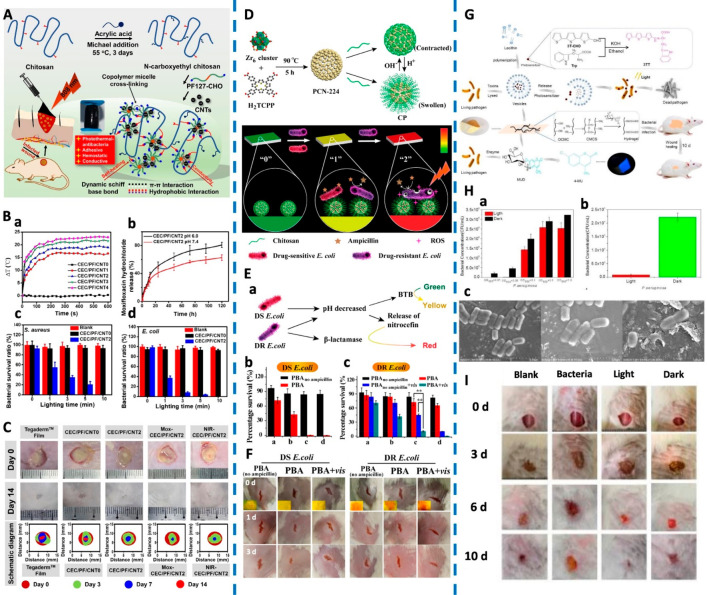
He et al. developed a series of conductive self-healing and adhesive nanocomposite hydrogels based on N-carboxyethyl chitosan (CEC) and benzaldehyde-terminated Pluronic F127/carbon nanotubes (PF127/CNT) (Figure 6A).91 Because of photosensitive composites in the hydrogel, such as CNTs, polyaniline, and polypyrrole, the hydrogel exhibited photothermal behavior upon the exposure to NIR (Figure 6B.a). More than 80% of the S. aureus and E. coli were killed under the NIR irradiation within 5 min, which was further improved to 100% eradication if exposure is prolonged to 10 min (Figure 6B.c,d). Moreover, after loading the antibiotic moxifloxacin, the hydrogel showed a faster release in acidic conditions (pH = 6.0) than under a typical physiological environment (pH = 7.4), revealing pH-sensitive release characteristics (Figure 6B.b). The moxifloxacin-loaded nanocomposites hydrogel promoted healing even on a full-thickness skin infected model, indicating that this hydrogel with multistimulus responsive ability has excellent potential as a drug carrier for treating bacteria-infected wounds (Figure 6C).
Figure 6.
(A) Schematic of the CEC/PF/CNT hydrogel. (B) Diagrams of ΔT-NIR irradiation time. Drug release behavior. Bacterial survival ratios of S. aureus and E. coli. (C) Representative photographs of a wound infection model with various treatments and the schematic diagram of wound closure on the 3rd, 7th, and 14th day. Reproduced from He et al., 2020.91 Copyright 2020 Elsevier B.V. (D) Schematics of preparation routes of CP- and pH-responsive transformation between contracted state and swollen state of chitosan. Schematic Illustration of PBA (“0”, green) for sensing bacterial infection (“1”, yellow) and drug resistance (“2”, red), and the implementing antibiotic-based chemotherapy and PCN-224-based PDT, respectively. (E) Schematic diagram of PBA for sensing bacterial infection and drug resistance. Viability of DS E. coli and DR E. coli incubated on PBA or PBAno ampicillin with or without light irradiation. (F) Photographs of wounds on the mice. Inset images in the first row revealed the color on PBA. Reproduced from Sun et al., 2020.92 Copyright 2019 American Chemical Society. (G) Schematic of development of an intelligent wound dressing and the mode of action following infection. (H) Vesicle photodynamic against P. aeruginosa. Wound dressing against P. aeruginosa. SEM images of P. aeruginosa after treatments with vesicles containing photosensitizer (Left: P. aeruginosa; Middle: P. aeruginosa with vesicles, in the dark; Right: P. aeruginosa with irradiated vesicles). (I) Photographs of the wound healing model. Reproduced from Zhou et al., 2020.93 Copyright 2020 Wiley-VCH Verlag GmbH and Co. KGaA, Weinheim.
Sun et al. developed a multistimulus responsive, multitherapeutic delivery, and multifunctionalized paper-based band-aid (PBA) for sensing and treating drug-resistant bacteria (Figure 6D).92 In this PBA, bromothymol blue (BTB) and nitrocefin were used as bacterial indicators and chitosan-coated ampicillin-loaded porphyrin-based MOFs (denoted as CP) as the therapeutic agent. BTB responded to an acid microenvironment at infectious sites, which was accompanied by a green to yellow color change. Meanwhile, the acid pH triggered the release of ampicillin, thereby killing drug-sensitive (DS) bacteria. For a drug-resistant (DR) bacterium, nitrocefin changed from yellow to red because of the β-lactamase, secreted by many drug-resistant bacteria (Figure 6E.a). For the DS E. coli, the survival rates decreased with the increase in the amount of CP (Figure 6E.b). For the DR E. coli, upon light irradiation, ROS produced by MOFs caused significant damage to bacteria and weakened their resistance. There was a considerable synergistic effect between APDT (45.3%) and chemotherapy (72.4%) in eliminating the DR bacteria (Figure 6E.c). Under a combination of light and PBA, the wound infected with DR E. coli healed better and a nascent epidermal layer was observed on the wound surface (Figure 6F). This dressing has both diagnosis and treatment functionalities, which can be used to detect the existence of drug-sensitive or drug-resistant bacteria according to the color change, and treating them on-demand.
In another work reported by Zhou et al., tryptophan-modified trithiophene aldehyde (3TT) as a new photosensitizer was loaded into vesicles and incorporated into a gel scaffold to obtain a wound dressing with detection and antibacterial functionalities.93 The vesicles could be lysed by cytotoxins produced by pathogenic bacteria, thereby releasing the photosensitive antibacterial agent. This achieved a simultaneous intelligent detection and treatment of pathogenic bacteria (Figure 6G). The 3TT vesicles in the light-irradiated group presented nearly complete bactericidal effect on low density of P. aeruginosa (Figure 6H.a). Meanwhile, the photodynamic bactericidal effect of the wound dressing on P. aeruginosa showed that released of 3TT could inactivate P. aeruginosa after light irradiation (Figure 6H.b). As shown in Figure 6H.c, damage to the bacterial cell-wall was observed, which was attributed by the ROS generated by the photosensitive antibacterial agents. Similarly, the wounds treated with the 3TT vesicles incorporated dressing with light healed much faster than the other groups (Figure 6I).
Multiple-stimuli responsive nanoparticle-based dressings show great promise. One of the major advantages is the controlled release of antimicrobials from carriers at an infected wound, which can be achieved by photo, photothermal, bacterial, and pH stimulus. Moreover, this approach seems better than existing approaches due to multiple-functionalization that can provide a point-of-care monitoring at the infected site.
4. Challenges and Perspectives
The extensive literature on stimulus-responsive nanoparticle-based dressings indicates that these systems hold great promise for this purpose. However, there are still challenges involved that impedes the translation of these technologies into the clinic, which would be discussed.
4.1. Prerequisite of Wound Dressing Materials
There are many prerequisites for the choice of wound healing materials. For example, they should aid in wound healing, be biocompatible, and not cause any skin allergies or irritations, should possess sufficiently good adhesive properties while still allowing easy removal, and the list goes on. Unfortunately, a wound dressing that meets to all these criteria still requires extensive research. There is currently no one dressing material that can be applied for all types of wounds and over a prolong period. For example, the Comfeel Plus Transparent hydrocolloid dressings are not suitable for wounds with large amounts of exudate and may cause skin damage during removal. Therefore, it is important to evaluate the wound periodically, to familiarize with the properties of the various dressings and their scope of application, type, and stage of healing, before selecting the best dressing.
In addition to the selection of the types of dressing, another major challenge lies in the safety prerequisite of wound dressing materials, to ensure that the materials used and processing methods are physiologically compatible, and all toxicity-related issues have been carefully addressed, that is, cytotoxicity, systemic toxicity, and immunological rejection. The complexities involved in fabricating sophisticated, multifunctional devices often exacerbate safety and biocompatibility issues. While stimulus-responsive nanoparticles often require multistep processes, designing them through simpler and reproducible processes is often preferred. This would reduce costs and enable an easier and more economical approach in fabricating these nanoparticles through good manufacturing practices (GMP).
In terms of nanoparticle-based wound dressing materials, of which nanoparticles are the most important components, their biocompatibility must be considered as a priority. Recently, MOFs have received widespread attention in the field of drug delivery because of their ultrahigh porosity, specific surface area, structural diversity, and designability. However, inorganic nanoparticles are generally made from metallic materials, and their biodegradability and biocompatibility are still questionable. If the metabolic pathways of these MOFs could be understood and their possible toxicity could be mitigated, MOFs would have tremendous promise as drug delivery vehicles for this purpose. As an organic material, biodegradable PLGA nanoparticles have been validated as safe for clinical applications. However, the safety of these highly functionalized, stimulus-responsive PLGA nanoparticles is yet to be investigated. For any device that will be used in intimate contact with human tissues, safety is certainly paramount.
4.2. Combinational Use of Growth Factors for Skin Reconstruction
There are four phases in a normal wound healing process, including hemostasis, inflammation, proliferation, and remodeling, whereby each phase occurs sequentially. To promote wound healing, bioactive molecules, such as growth factors, can be introduced into a wound dressing matrix. Several approved medications containing growth factors are currently available for external usage in the form of gels, creams, and solutions such as Regranex Gel (rhPDGF), Fiblast Spary (rhbFGF), Heberprot-P (rhEGF), etc.94 However, such topical medications have shown limited success, especially in chronic wounds.95 The complex microenvironment and healing process do influence the possible outcomes of these growth factors. For instance, activated proteases in a wound bed may degrade both endogenous and exogenous growth factors.96,97 Thus, current wound healing creams may not provide a sufficiently long residence time for growth factors, such as epidermal growth factor (EGF)-like growth factors in epithelialization, to remain bioactive within a wound bed. This requires high dosages or repeated administrations, which may lead to other serious side effects, such as oncogenesis.98 Delivery of growth factors therefore requires an encapsulation system to provide more effective and safe treatments. However, much has yet to be explored when codelivering antimicrobial therapeutics along with bioactive molecules in an intelligent nanoparticle-based dressing. Embedded within a multiresponse or multifunctionalization wound dressing matrix, the release of growth factors could be achieved in a desired manner to boost skin regeneration. Ideally, a continuous release of antimicrobial agents at the infected wound site is first required, followed by the delivery of growth factors to promote skin reconstruction. Responsive delivery systems that can promote sequential release would be useful for such an application.
4.3. Biofilm Barriers
Even if nanoparticle-based dressings are adequately designed, the presence of certain factors in the complex infected wound may influence the performance of stimulus-responsive nanoparticle-based dressings. For instance, the physical barrier of biofilm limits the ability of drugs in eradicating pathogenic cells. Developing nanoparticle-based dressings that can penetrate biofilms, destroy biofilms, or even prevent its formation are promising approaches for the future. Our previous work has shown that cationic lipid polymer hybrid nanoparticles (LPNs) can penetrate the biofilms and even bacterial cell membranes to deliver payloads to efficiently inactivate bacteria.99 LPNs are core–shell nanoparticulate structures comprising polymer cores and lipid shells. Another example would be the use of Janus particles (JPs) to prevent the formation of biofilms.100 JPs are asymmetric structured particles with two or more distinct compositions. A combination of LPNs or JPs with dressing matrix could be a robust approach to target biofilm effectively. More importantly, the ability to carry out multiencapsulation can also give rise to multifunctional systems, such as therapeutic and diagnostic functions, would be advantageous. Such a strategy would be an exciting proposition in designing nanoparticle-based dressings for wound infection.
4.4. Artificial Intelligence
In the future, nanoparticle-based dressings can also integrate their functionality with artificial intelligence (AI).101−105 In cancer research, AI has been developed based on a convolutional neural network to rapidly identify various tumor cells from normal mammalian cells with 96% accuracy.106 A novel technology named “quantitative live cell histology” has been reported using AI to identify melanoma cells and predict the spread of melanoma.107 Similar technologies could be developed in the area of antibacterial research. Using both established database and machine learning, different types of bacterial pathogens in a wound can be recognized and tracked. Hence, a combination antimicrobial therapy targeting different bacterial pathogens can be developed to provide a more personalized treatment. In this aspect, AI could also help to optimize the combination therapy in a highly efficient manner. For instance, an AI platform, CURATE.AI, has been used to prospectively guide the combination dose of a bromodomain inhibitor and enzalutamide to a patient with prostate cancer.108 An integrated diagnostic and therapeutic stimulus-responsive wound dressing could open up new directions for the development of skin-related biomedicine through responsive nanosystems. While challenges remain for the development of the next generation of stimulus-responsive wound dressings, it can be expected that nanoparticle-based therapies will poise themselves to be the next frontier in wound infection management.
5. Conclusion
This review summarizes and highlights the recent progress on “intelligent” nanoparticles that can augment wound dressings for bacteria-infected wounds. While such nanoparticles have potential for this application, they are not without limitations and challenges. Overcoming these challenges will open avenues for these nanoparticles to be translated into a commercially viable technology that can be used clinically. We envisage that such intelligent nanoparticle-based dressings will be a novel and efficient platform for the treatment of bacterial wound infections.
Acknowledgments
The authors would like to acknowledge the financial support from the Singapore Centre for Environmental Life Sciences Engineering (SCELSE) (MOE/RCE: M4330019.C70), Ministry of Education AcRF-Tier 1 grant (RG19/18), Agri-Food & Veterinary Authority of Singapore (APF LCK102), Biomedical Research Council (BMRC) - Therapeutics Development Review (TDR-G- 004-001), NTU-HSPH grant (NTU-HSPH 17002), and the Bill and Melinda Gates Foundation (OPP1199116). The authors would also like to acknowledge the assistance of Dr. Tan Kei Xian, Dr. Lim Yi Guang Jerome, and Li Wenrui in proof-reading and providing their invaluable insights on this manuscript.
The authors declare no competing financial interest.
References
- The Layers of Your Skin. Healthline, 2018. https://www.healthline.com/health/layers-of-skin (accessed 20th Aug 2020).
- Sevgi M.; Toklu A.; Vecchio D.; Hamblin M. R. Topical antimicrobials for burn infections - an update. Recent Pat. Anti-Infect. Drug Discovery 2014, 8 (3), 161–197. 10.2174/1574891X08666131112143447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin M. R. Novel pharmacotherapy for burn wounds: what are the advancements. Expert Opin. Pharmacother. 2019, 20 (3), 305–321. 10.1080/14656566.2018.1551880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cefalu J. E.; Barrier K. M.; Davis A. H. Wound Infections in Critical Care. Critical Care Nursing Clinics 2017, 29 (1), 81–96. 10.1016/j.cnc.2016.09.009. [DOI] [PubMed] [Google Scholar]
- Downie F.; Egdell S.; Bielby A.; Searle R. Barrier dressings in surgical site infection prevention strategies. Br J. Nurs 2010, 19 (20), S42–6. 10.12968/bjon.2010.19.Sup10.79693. [DOI] [PubMed] [Google Scholar]
- Negut I.; Grumezescu V.; Grumezescu A. M. Treatment Strategies for Infected Wounds. Molecules 2018, 23 (9), 2392. 10.3390/molecules23092392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões D.; Miguel S. P.; Ribeiro M. P.; Coutinho P.; Mendonça A. G.; Correia I. J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. 10.1016/j.ejpb.2018.02.022. [DOI] [PubMed] [Google Scholar]
- Scudiero O.; Brancaccio M.; Mennitti C.; Laneri S.; Lombardo B.; De Biasi M. G.; De Gregorio E.; Pagliuca C.; Colicchio R.; Salvatore P.; Pero R. Human Defensins: A Novel Approach in the Fight against Skin Colonizing Staphylococcus aureus. Antibiotics (Basel, Switz.) 2020, 9 (4), 198. 10.3390/antibiotics9040198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival S. L.; Emanuel C.; Cutting K. F.; Williams D. W. Microbiology of the skin and the role of biofilms in infection. International Wound Journal 2012, 9 (1), 14–32. 10.1111/j.1742-481X.2011.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ki V.; Rotstein C. Bacterial skin and soft tissue infections in adults: A review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can. J. Infect Dis Med. Microbiol 2008, 19 (2), 173–184. 10.1155/2008/846453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar S.; Sharma D.; Kalia K.; Tekade R. K. Tumor microenvironment targeted nanotherapeutics for cancer therapy and diagnosis: A review. Acta Biomater. 2020, 101, 43–68. 10.1016/j.actbio.2019.09.009. [DOI] [PubMed] [Google Scholar]
- Uthaman S.; Huh K. M.; Park I.-K. Tumor microenvironment-responsive nanoparticles for cancer theragnostic applications. Biomaterials Research 2018, 22 (1), 22. 10.1186/s40824-018-0132-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulzar A.; Xu J.; Wang C.; He F.; Yang D.; Gai S.; Yang P.; Lin J.; Jin D.; Xing B. Tumour microenvironment responsive nanoconstructs for cancer theranostic. Nano Today 2019, 26, 16–56. 10.1016/j.nantod.2019.03.007. [DOI] [Google Scholar]
- Chen B.; Dai W.; He B.; Zhang H.; Wang X.; Wang Y.; Zhang Q. Current Multistage Drug Delivery Systems Based on the Tumor Microenvironment. Theranostics 2017, 7 (3), 538–558. 10.7150/thno.16684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surendran S. P.; Moon M. J.; Park R.; Jeong Y. Y. Bioactive Nanoparticles for Cancer Immunotherapy. Int. J. Mol. Sci. 2018, 19 (12), 3877. 10.3390/ijms19123877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y.; Tang H.; Radosz M.; Van Kirk E.; Murdoch W. J.. pH-Responsive Nanoparticles for Cancer Drug Delivery. In Drug Delivery Systems; Jain K. K., Ed.; Humana Press: Totowa, NJ, 2008; pp 183–216. [DOI] [PubMed] [Google Scholar]
- Tang H.; Zhao W.; Yu J.; Li Y.; Zhao C. Recent Development of pH-Responsive Polymers for Cancer Nanomedicine. Molecules 2019, 24 (1), 4. 10.3390/molecules24010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y.; Wan J.; Zhou L.; Ma W.; Yang Y.; Luo W.; Yu Z.; Wang H. Stimuli-responsive nanotherapeutics for precision drug delivery and cancer therapy. WIREs Nanomedicine and Nanobiotechnology 2019, 11 (1), e1527. 10.1002/wnan.1527. [DOI] [PubMed] [Google Scholar]
- Lin Y.-J.; Mi F.-L.; Lin P.-Y.; Miao Y.-B.; Huang T.; Chen K.-H.; Chen C.-T.; Chang Y.; Sung H.-W. Strategies for improving diabetic therapy via alternative administration routes that involve stimuli-responsive insulin-delivering systems. Adv. Drug Delivery Rev. 2019, 139, 71–82. 10.1016/j.addr.2018.12.001. [DOI] [PubMed] [Google Scholar]
- Luo Y. Y.; Xiong X. Y.; Tian Y.; Li Z. L.; Gong Y. C.; Li Y. P. A review of biodegradable polymeric systems for oral insulin delivery. Drug Delivery 2016, 23 (6), 1882–1891. 10.3109/10717544.2015.1052863. [DOI] [PubMed] [Google Scholar]
- Wong C. Y.; Al-Salami H.; Dass C. R. Potential of insulin nanoparticle formulations for oral delivery and diabetes treatment. J. Controlled Release 2017, 264, 247–275. 10.1016/j.jconrel.2017.09.003. [DOI] [PubMed] [Google Scholar]
- Zhao L.; Wang L.; Zhang Y.; Xiao S.; Bi F.; Zhao J.; Gai G.; Ding J. Glucose Oxidase-Based Glucose-Sensitive Drug Delivery for Diabetes Treatment. Polymers (Basel, Switz.) 2017, 9 (7), 255. 10.3390/polym9070255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souto E. B.; Souto S. B.; Campos J. R.; Severino P.; Pashirova T. N.; Zakharova L. Y.; Silva A. M.; Durazzo A.; Lucarini M.; Izzo A. A.; Santini A. Nanoparticle Delivery Systems in the Treatment of Diabetes Complications. Molecules 2019, 24 (23), 4209. 10.3390/molecules24234209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makabenta J. M. V.; Nabawy A.; Li C.-H.; Schmidt-Malan S.; Patel R.; Rotello V. M., Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2020. 10.1038/s41579-020-0420-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.; Li F.; Hu X.; Lu J.; Sun X.; Gao J.; Ling D. Responsive Assembly of Silver Nanoclusters with a Biofilm Locally Amplified Bactericidal Effect to Enhance Treatments against Multi-Drug-Resistant Bacterial Infections. ACS Cent. Sci. 2019, 5 (8), 1366–1376. 10.1021/acscentsci.9b00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo R. R.; Lozano D.; González B.; Manzano M.; Izquierdo-Barba I.; Vallet-Regí M. Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery: an update. Expert Opin. Drug Delivery 2019, 16 (4), 415–439. 10.1080/17425247.2019.1598375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devnarain N.; Osman N.; Fasiku V. O.; Makhathini S.; Salih M.; Ibrahim U. H.; Govender T. Intrinsic stimuli-responsive nanocarriers for smart drug delivery of antibacterial agents-An in-depth review of the last two decades. Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol. 2020, 1, e1664. 10.1002/wnan.1664. [DOI] [PubMed] [Google Scholar]
- Yeh Y.-C.; Huang T.-H.; Yang S.-C.; Chen C.-C.; Fang J.-Y. Nano-Based Drug Delivery or Targeting to Eradicate Bacteria for Infection Mitigation: A Review of Recent Advances. Front. Chem. 2020, 8 (286), 1–22. 10.3389/fchem.2020.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K.; Li C.; Chen D.; Pan Y.; Tao Y.; Qu W.; Liu Z.; Wang X.; Xie S. A review on nanosystems as an effective approach against infections of Staphylococcus aureus. Int. J. Nanomed. 2018, 13, 7333–7347. 10.2147/IJN.S169935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W.; Thamphiwatana S.; Angsantikul P.; Zhang L. Nanoparticle approaches against bacterial infections. WIREs Nanomedicine and Nanobiotechnology 2014, 6 (6), 532–547. 10.1002/wnan.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zazo H.; Colino C. I.; Lanao J. M. Current applications of nanoparticles in infectious diseases. J. Controlled Release 2016, 224, 86–102. 10.1016/j.jconrel.2016.01.008. [DOI] [PubMed] [Google Scholar]
- Liang J.; Peng X.; Zhou X.; Zou J.; Cheng L. Emerging Applications of Drug Delivery Systems in Oral Infectious Diseases Prevention and Treatment. Molecules 2020, 25 (3), 516. 10.3390/molecules25030516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan M. A.; Tamer T. M.; Rageh A. A.; Abou-Zeid A. M.; Abd El-Zaher E. H. F.; Kenawy E.-R. Insight into multidrug-resistant microorganisms from microbial infected diabetic foot ulcers. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 2019, 13 (2), 1261–1270. 10.1016/j.dsx.2019.01.044. [DOI] [PubMed] [Google Scholar]
- Lipsky B. A.; Senneville É.; Abbas Z. G.; Aragón-Sánchez J.; Diggle M.; Embil J. M.; Kono S.; Lavery L. A.; Malone M.; van Asten S. A.; Urbančič-Rovan V.; Peters E. J. G.; Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes/Metab. Res. Rev. 2020, 36 (S1), e3280. 10.1002/dmrr.3280. [DOI] [PubMed] [Google Scholar]
- Global Diabetes Map; International Diabetes Federation, 2019. https://www.diabetesatlas.org/en/ (accessed 26th Aug 2020).
- Burns; WHO, 2018. https://www.who.int/news-room/fact-sheets/detail/burns (accessed 26th Aug 2020).
- Ageing and Health; WHO, 2018. https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed 31st Aug 2020).
- Advanced Wound Dressing Market Size, Share & Trends Analysis Report By End Use (Hospitals, Home Healthcare), By Application (Chronic, Acute Wounds), By Product (Foam, Film, Hydrogel), And Segment Forecasts, 2019–2026; Grand View Research, 2019. https://www.grandviewresearch.com/industry-analysis/advanced-wound-care-dressing-market.
- Verma J.; Kanoujia J.; Parashar P.; Tripathi C. B.; Saraf S. A. Wound healing applications of sericin/chitosan-capped silver nanoparticles incorporated hydrogel. Drug Delivery Transl. Res. 2017, 7 (1), 77–88. 10.1007/s13346-016-0322-y. [DOI] [PubMed] [Google Scholar]
- Li Y.; Tian Y.; Zheng W.; Feng Y.; Huang R.; Shao J.; Tang R.; Wang P.; Jia Y.; Zhang J.; Zheng W.; Yang G.; Jiang X. Composites of Bacterial Cellulose and Small Molecule-Decorated Gold Nanoparticles for Treating Gram-Negative Bacteria-Infected Wounds. Small 2017, 13 (27), 1700130. 10.1002/smll.201700130. [DOI] [PubMed] [Google Scholar]
- You C.; Li Q.; Wang X.; Wu P.; Ho J. K.; Jin R.; Zhang L.; Shao H.; Han C. Silver nanoparticle loaded collagen/chitosan scaffolds promote wound healing via regulating fibroblast migration and macrophage activation. Sci. Rep. 2017, 7 (1), 10489. 10.1038/s41598-017-10481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.; Yang J.; Wang L.; Ran B.; Jia Y.; Zhang L.; Yang G.; Shao H.; Jiang X. Pharmaceutical Intermediate-Modified Gold Nanoparticles: Against Multidrug-Resistant Bacteria and Wound-Healing Application via an Electrospun Scaffold. ACS Nano 2017, 11 (6), 5737–5745. 10.1021/acsnano.7b01240. [DOI] [PubMed] [Google Scholar]
- Tra Thanh N.; Ho Hieu M.; Tran Minh Phuong N.; Do Bui Thuan T.; Nguyen Thi Thu H.; Thai V. P.; Do Minh T.; Nguyen Dai H.; Vo V. T.; Nguyen Thi H. Optimization and characterization of electrospun polycaprolactone coated with gelatin-silver nanoparticles for wound healing application. Mater. Sci. Eng., C 2018, 91, 318–329. 10.1016/j.msec.2018.05.039. [DOI] [PubMed] [Google Scholar]
- Lu B.; Ye H.; Shang S.; Xiong Q.; Yu K.; Li Q.; Xiao Y.; Dai F.; Lan G. Novel wound dressing with chitosan gold nanoparticles capped with a small molecule for effective treatment of multiantibiotic-resistant bacterial infections. Nanotechnology 2018, 29 (42), 425603. 10.1088/1361-6528/aad7a7. [DOI] [PubMed] [Google Scholar]
- Thattaruparambil Raveendran N.; Mohandas A.; Ramachandran Menon R.; Somasekharan Menon A.; Biswas R.; Jayakumar R. Ciprofloxacin- and Fluconazole-Containing Fibrin-Nanoparticle-Incorporated Chitosan Bandages for the Treatment of Polymicrobial Wound Infections. ACS Applied Bio Materials 2019, 2 (1), 243–254. 10.1021/acsabm.8b00585. [DOI] [PubMed] [Google Scholar]
- Sun L.; Han J.; Liu Z.; Wei S.; Su X.; Zhang G. The facile fabrication of wound compatible anti-microbial nanoparticles encapsulated Collagenous Chitosan matrices for effective inhibition of poly-microbial infections and wound repairing in burn injury care: Exhaustive in vivo evaluations. J. Photochem. Photobiol., B 2019, 197, 111539. 10.1016/j.jphotobiol.2019.111539. [DOI] [PubMed] [Google Scholar]
- Zou P.; Lee W.-H.; Gao Z.; Qin D.; Wang Y.; Liu J.; Sun T.; Gao Y. Wound dressing from polyvinyl alcohol/chitosan electrospun fiber membrane loaded with OH-CATH30 nanoparticles. Carbohydr. Polym. 2020, 232, 115786. 10.1016/j.carbpol.2019.115786. [DOI] [PubMed] [Google Scholar]
- Chang M.-C.; Kuo Y.-J.; Hung K.-H.; Peng C.-L.; Chen K.-Y.; Yeh L.-K. Liposomal dexamethasone-moxifloxacin nanoparticle combinations with collagen/gelatin/alginate hydrogel for corneal infection treatment and wound healing. Biomedical Materials 2020, 15 (5), 055022. 10.1088/1748-605X/ab9510. [DOI] [PubMed] [Google Scholar]
- Bhakdi S.; Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol. Rev. 1991, 55 (4), 733–751. 10.1128/MMBR.55.4.733-751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.; Song Z.; Wang H.; Han H. Endogenous stimulus-powered antibiotic release from nanoreactors for a combination therapy of bacterial infections. Nat. Commun. 2019, 10 (1), 4464. 10.1038/s41467-019-12233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan D.; Omolo C. A.; Fasiku V. O.; Mocktar C.; Govender T. Novel chitosan-based pH-responsive lipid-polymer hybrid nanovesicles (OLA-LPHVs) for delivery of vancomycin against methicillin-resistant Staphylococcus aureus infections. Int. J. Biol. Macromol. 2020, 147, 385–398. 10.1016/j.ijbiomac.2020.01.019. [DOI] [PubMed] [Google Scholar]
- Song Z.; Wu Y.; Cao Q.; Wang H.; Wang X.; Han H. pH-Responsive, Light-Triggered on-Demand Antibiotic Release from Functional Metal–Organic Framework for Bacterial Infection Combination Therapy. Adv. Funct. Mater. 2018, 28 (23), 1800011. 10.1002/adfm.201800011. [DOI] [Google Scholar]
- Tyner H.; Patel R. Hyaluronidase in Clinical Isolates of Propionibacterium acnes. Int. J. Bacteriol. 2015, 2015, 218918–218918. 10.1155/2015/218918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Lin A.; Liu J.; Chen X.; Zhu X.; Gong Y.; Yuan G.; Chen L.; Liu J. Enzyme-Responsive Mesoporous Ruthenium for Combined Chemo-Photothermal Therapy of Drug-Resistant Bacteria. ACS Appl. Mater. Interfaces 2019, 11 (30), 26590–26606. 10.1021/acsami.9b07866. [DOI] [PubMed] [Google Scholar]
- Moreno-Sastre M.; Pastor M.; Esquisabel A.; Pedraz J. L., The Use of Nanoparticles for Antimicrobial Delivery. In New Weapons to Control Bacterial Growth; Villa T. G., Vinas M., Eds.; Springer International Publishing: Cham, 2016; pp 453–487. [Google Scholar]
- Hussain S.; Joo J.; Kang J.; Kim B.; Braun G. B.; She Z.-G.; Kim D.; Mann A. P.; Mölder T.; Teesalu T.; Carnazza S.; Guglielmino S.; Sailor M. J.; Ruoslahti E. Antibiotic-loaded nanoparticles targeted to the site of infection enhance antibacterial efficacy. Nature Biomedical Engineering 2018, 2 (2), 95–103. 10.1038/s41551-017-0187-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira M. C.; Sanchez-Lopez E.; Espina M.; Calpena A. C.; Silva A. M.; Veiga F. J.; Garcia M. L.; Souto E. B. Chapter 9 - Advances in antibiotic nanotherapy: Overcoming antimicrobial resistance. In Emerging Nanotechnologies in Immunology; Shegokar R., Souto E. B., Eds.; Elsevier: Boston, 2018; pp 233–259. [Google Scholar]
- Ritsema J. A.; der Weide H. v.; te Welscher Y. M; Goessens W. H.; van Nostrum C. F; Storm G.; Bakker-Woudenberg I. A.; Hays J. P Antibiotic-nanomedicines: facing the challenge of effective treatment of antibiotic-resistant respiratory tract infections. Future Microbiol. 2018, 13 (15), 1683–1692. 10.2217/fmb-2018-0194. [DOI] [PubMed] [Google Scholar]
- Wang C.; Zhao T.; Li Y.; Huang G.; White M. A.; Gao J. Investigation of endosome and lysosome biology by ultra pH-sensitive nanoprobes. Adv. Drug Delivery Rev. 2017, 113, 87–96. 10.1016/j.addr.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.; Deng X.; Ding J.; Zhou W.; Zheng X.; Tang G. Mechanisms of drug release in pH-sensitive micelles for tumour targeted drug delivery system: A review. Int. J. Pharm. 2018, 535 (1), 253–260. 10.1016/j.ijpharm.2017.11.003. [DOI] [PubMed] [Google Scholar]
- Liu G.; Zhao X.; Zhang Y.; Xu J.; Xu J.; Li Y.; Min H.; Shi J.; Zhao Y.; Wei J.; Wang J.; Nie G. Engineering Biomimetic Platesomes for pH-Responsive Drug Delivery and Enhanced Antitumor Activity. Adv. Mater. 2019, 31 (32), 1900795. 10.1002/adma.201900795. [DOI] [PubMed] [Google Scholar]
- Rippke F.; Berardesca E.; Weber T. M. pH and Microbial Infections. Curr. Probl Dermatol 2018, 54, 87–94. 10.1159/000489522. [DOI] [PubMed] [Google Scholar]
- Chen H.; Jin Y.; Wang J.; Wang Y.; Jiang W.; Dai H.; Pang S.; Lei L.; Ji J.; Wang B. Design of smart targeted and responsive drug delivery systems with enhanced antibacterial properties. Nanoscale 2018, 10 (45), 20946–20962. 10.1039/C8NR07146B. [DOI] [PubMed] [Google Scholar]
- Shao W.; Liu H.; Wu J.; Wang S.; Liu X.; Huang M.; Xu P. Preparation, antibacterial activity and pH-responsive release behavior of silver sulfadiazine loaded bacterial cellulose for wound dressing applications. J. Taiwan Inst. Chem. Eng. 2016, 63, 404–410. 10.1016/j.jtice.2016.02.019. [DOI] [Google Scholar]
- Cui Y.; Li B.; He H.; Zhou W.; Chen B.; Qian G. Metal-Organic Frameworks as Platforms for Functional Materials. Acc. Chem. Res. 2016, 49 (3), 483–493. 10.1021/acs.accounts.5b00530. [DOI] [PubMed] [Google Scholar]
- Mazloom-Jalali A.; Shariatinia Z.; Tamai I. A.; Pakzad S.-R.; Malakootikhah J. Fabrication of chitosan-polyethylene glycol nanocomposite films containing ZIF-8 nanoparticles for application as wound dressing materials. Int. J. Biol. Macromol. 2020, 153, 421–432. 10.1016/j.ijbiomac.2020.03.033. [DOI] [PubMed] [Google Scholar]
- Wang T.; Zheng Y.; Shi Y.; Zhao L. pH-responsive calcium alginate hydrogel laden with protamine nanoparticles and hyaluronan oligosaccharide promotes diabetic wound healing by enhancing angiogenesis and antibacterial activity. Drug Delivery Transl. Res. 2019, 9 (1), 227–239. 10.1007/s13346-018-00609-8. [DOI] [PubMed] [Google Scholar]
- Sharma A. K.; Dhasmana N.; Dubey N.; Kumar N.; Gangwal A.; Gupta M.; Singh Y. Bacterial Virulence Factors: Secreted for Survival. Indian J. Microbiol. 2017, 57 (1), 1–10. 10.1007/s12088-016-0625-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virulence Factors of Bacterial and Viral Pathogens; Lumen, 2020. https://courses.lumenlearning.com/microbiology/chapter/virulence-factors-of-bacterial-and-viral-pathogens/.
- Mir M.; Ahmed N.; Permana A. D.; Rodgers A. M.; Donnelly R. F.; Rehman A. u. Enhancement in Site-Specific Delivery of Carvacrol against Methicillin Resistant Staphylococcus aureus Induced Skin Infections Using Enzyme Responsive Nanoparticles: A Proof of Concept Study. Pharmaceutics 2019, 11 (11), 606. 10.3390/pharmaceutics11110606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibberson C. B.; Jones C. L.; Singh S.; Wise M. C.; Hart M. E.; Zurawski D. V.; Horswill A. R. Staphylococcus aureus hyaluronidase is a CodY-regulated virulence factor. Infect. Immun. 2014, 82 (10), 4253–4264. 10.1128/IAI.01710-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera Starr C.; Engleberg N. C. Role of Hyaluronidase in Subcutaneous Spread and Growth of Group A Streptococcus. Infect. Immun. 2006, 74 (1), 40. 10.1128/IAI.74.1.40-48.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu N.; Wang X.; Qiu L.; Cai T.; Jiang C.; Sun Y.; Li Y.; Peng H.; Xiong H. Bacteria-triggered hyaluronan/AgNPs/gentamicin nanocarrier for synergistic bacteria disinfection and wound healing application. Chem. Eng. J. 2020, 380, 122582. 10.1016/j.cej.2019.122582. [DOI] [Google Scholar]
- Zhou J.; Yao D.; Qian Z.; Hou S.; Li L.; Jenkins A. T. A.; Fan Y. Bacteria-responsive intelligent wound dressing: Simultaneous In situ detection and inhibition of bacterial infection for accelerated wound healing. Biomaterials 2018, 161, 11–23. 10.1016/j.biomaterials.2018.01.024. [DOI] [PubMed] [Google Scholar]
- Sánchez-Ramírez D. R.; Domínguez-Ríos R.; Juárez J.; Valdés M.; Hassan N.; Quintero-Ramos A.; del Toro-Arreola A.; Barbosa S.; Taboada P.; Topete A.; Daneri-Navarro A. Biodegradable photoresponsive nanoparticles for chemo-, photothermal- and photodynamic therapy of ovarian cancer. Mater. Sci. Eng., C 2020, 116, 111196. 10.1016/j.msec.2020.111196. [DOI] [PubMed] [Google Scholar]
- Wang X.; Xuan Z.; Zhu X.; Sun H.; Li J.; Xie Z. Near-infrared photoresponsive drug delivery nanosystems for cancer photo-chemotherapy. J. Nanobiotechnol. 2020, 18 (1), 108. 10.1186/s12951-020-00668-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas G.; Jena B. C.; Maiti S.; Samanta P.; Mandal M.; Dhara D. Photoresponsive Block Copolymer Prodrug Nanoparticles as Delivery Vehicle for Single and Dual Anticancer Drugs. ACS Omega 2017, 2 (10), 6677–6690. 10.1021/acsomega.7b00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C.; Wu M.; Zhang C.; Lin X.; Wei Z.; Zheng Y.; Da Z.; Zhang Z.; Liu X. Photoresponsive lipid-polymer hybrid nanoparticles for controlled doxorubicin release. Nanotechnology 2017, 28 (25), 255101. 10.1088/1361-6528/aa702a. [DOI] [PubMed] [Google Scholar]
- Ballesteros C. A. S.; Correa D. S.; Zucolotto V. Polycaprolactone nanofiber mats decorated with photoresponsive nanogels and silver nanoparticles: Slow release for antibacterial control. Mater. Sci. Eng., C 2020, 107, 110334. 10.1016/j.msec.2019.110334. [DOI] [PubMed] [Google Scholar]
- Kim M.-H. Nanoparticle-Based Therapies for Wound Biofilm Infection: Opportunities and Challenges. IEEE Trans Nanobioscience 2016, 15 (3), 294–304. 10.1109/TNB.2016.2527600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam S.; Shai S.; Shahane A.; Kaushik K. S. Recent Advances in Non-Conventional Antimicrobial Approaches for Chronic Wound Biofilms: Have We Found the ’Chink in the Armor’?. Biomedicines 2019, 7 (2), 35. 10.3390/biomedicines7020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.; Liu X.; Tan L.; Cui Z.; Yang X.; Li Z.; Zheng Y.; Yeung K. W. K.; Chu P. K.; Wu S. Noninvasive rapid bacteria-killing and acceleration of wound healing through photothermal/photodynamic/copper ion synergistic action of a hybrid hydrogel. Biomater. Sci. 2018, 6 (8), 2110–2121. 10.1039/C8BM00499D. [DOI] [PubMed] [Google Scholar]
- Mao C.; Xiang Y.; Liu X.; Cui Z.; Yang X.; Yeung K. W. K.; Pan H.; Wang X.; Chu P. K.; Wu S. Photo-Inspired Antibacterial Activity and Wound Healing Acceleration by Hydrogel Embedded with Ag/Ag@AgCl/ZnO Nanostructures. ACS Nano 2017, 11 (9), 9010–9021. 10.1021/acsnano.7b03513. [DOI] [PubMed] [Google Scholar]
- Gao G.; Jiang Y.-W.; Jia H.-R.; Wu F.-G. Near-infrared light-controllable on-demand antibiotics release using thermo-sensitive hydrogel-based drug reservoir for combating bacterial infection. Biomaterials 2019, 188, 83–95. 10.1016/j.biomaterials.2018.09.045. [DOI] [PubMed] [Google Scholar]
- Moorcroft S. C. T.; Roach L.; Jayne D. G.; Ong Z. Y.; Evans S. D. Nanoparticle-Loaded Hydrogel for the Light-Activated Release and Photothermal Enhancement of Antimicrobial Peptides. ACS Appl. Mater. Interfaces 2020, 12 (22), 24544–24554. 10.1021/acsami.9b22587. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Li F.; Guo Z.; Xiao Y.; Zhang Y.; Sun X.; Zhe T.; Cao Y.; Wang L.; Lu Q.; Wang J. Silver nanoparticle-embedded hydrogel as a photothermal platform for combating bacterial infections. Chem. Eng. J. 2020, 382, 122990. 10.1016/j.cej.2019.122990. [DOI] [Google Scholar]
- Han D.; Li Y.; Liu X.; Li B.; Han Y.; Zheng Y.; Yeung K. W. K.; Li C.; Cui Z.; Liang Y.; Li Z.; Zhu S.; Wang X.; Wu S. Rapid bacteria trapping and killing of metal-organic frameworks strengthened photo-responsive hydrogel for rapid tissue repair of bacterial infected wounds. Chem. Eng. J. 2020, 396, 125194. 10.1016/j.cej.2020.125194. [DOI] [Google Scholar]
- Sun J.; Song L.; Fan Y.; Tian L.; Luan S.; Niu S.; Ren L.; Ming W.; Zhao J. Synergistic Photodynamic and Photothermal Antibacterial Nanocomposite Membrane Triggered by Single NIR Light Source. ACS Appl. Mater. Interfaces 2019, 11 (30), 26581–26589. 10.1021/acsami.9b07037. [DOI] [PubMed] [Google Scholar]
- Cui Q.; Yuan H.; Bao X.; Ma G.; Wu M.; Xing C. Synergistic Photodynamic and Photothermal Antibacterial Therapy Based on a Conjugated Polymer Nanoparticle-Doped Hydrogel. ACS Applied Bio Materials 2020, 3 (7), 4436–4443. 10.1021/acsabm.0c00423. [DOI] [PubMed] [Google Scholar]
- Qiao B.; Pang Q.; Yuan P.; Luo Y.; Ma L. Smart wound dressing for infection monitoring and NIR-triggered antibacterial treatment. Biomater. Sci. 2020, 8 (6), 1649–1657. 10.1039/C9BM02060H. [DOI] [PubMed] [Google Scholar]
- He J.; Shi M.; Liang Y.; Guo B. Conductive adhesive self-healing nanocomposite hydrogel wound dressing for photothermal therapy of infected full-thickness skin wounds. Chem. Eng. J. 2020, 394, 124888. 10.1016/j.cej.2020.124888. [DOI] [Google Scholar]
- Sun Y.; Zhao C.; Niu J.; Ren J.; Qu X. Colorimetric Band-aids for Point-of-Care Sensing and Treating Bacterial Infection. ACS Cent. Sci. 2020, 6 (2), 207–212. 10.1021/acscentsci.9b01104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q.; Wang J.; Liang Y.; Yang H.; Li Q.; Li Q.; Liao D.; Liu Y.; Liu H.-B. Development of an Intelligent Photosensitive Antibacterial Wound Dressing: Simultaneous Detection and Treatment of Bacterial Infection for Accelerated Wound Healing. ChemNanoMat 2020, 6 (4), 516–523. 10.1002/cnma.202000042. [DOI] [Google Scholar]
- Gainza G.; Villullas S.; Pedraz J. L.; Hernandez R. M.; Igartua M. Advances in drug delivery systems (DDSs) to release growth factors for wound healing and skin regeneration. Nanomedicine 2015, 11 (6), 1551–73. 10.1016/j.nano.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Wieman T. J. Clinical efficacy of becaplermin (rhPDGF-BB) gel. Becaplermin Gel Studies Group. Am. J. Surg. 1998, 176 (2A Suppl), 74s–79s. 10.1016/S0002-9610(98)00185-8. [DOI] [PubMed] [Google Scholar]
- Park J. W.; Hwang S. R.; Yoon I.-S. Advanced Growth Factor Delivery Systems in Wound Management and Skin Regeneration. Molecules 2017, 22 (8), 1259. 10.3390/molecules22081259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mast B. A.; Schultz G. S. Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair Regen 1996, 4 (4), 411–20. 10.1046/j.1524-475X.1996.40404.x. [DOI] [PubMed] [Google Scholar]
- Huang J. S.; Huang S. S. Role of Growth Factors in Oncogenesis: Growth Factor-Proto-Oncogene Pathways of Mitogenesis. Ciba Foundation Symposium 116 - Growth Factors in Biology and Medicine 2008, 46–65. 10.1002/9780470720974.ch4. [DOI] [PubMed] [Google Scholar]
- Baek J.-S.; Tan C. H.; Ng N. K. J.; Yeo Y. P.; Rice S. A.; Loo S. C. J. A programmable lipid-polymer hybrid nanoparticle system for localized, sustained antibiotic delivery to Gram-positive and Gram-negative bacterial biofilms. Nanoscale Horizons 2018, 3 (3), 305–311. 10.1039/C7NH00167C. [DOI] [PubMed] [Google Scholar]
- Kirillova A.; Marschelke C.; Friedrichs J.; Werner C.; Synytska A. Hybrid Hairy Janus Particles as Building Blocks for Antibiofouling Surfaces. ACS Appl. Mater. Interfaces 2016, 8 (47), 32591–32603. 10.1021/acsami.6b10588. [DOI] [PubMed] [Google Scholar]
- Ho D.; Wang P.; Kee T. Artificial intelligence in nanomedicine. Nanoscale Horizons 2019, 4 (2), 365–377. 10.1039/C8NH00233A. [DOI] [PubMed] [Google Scholar]
- Feigl C. A.; Motevalli B.; Parker A. J.; Sun B.; Barnard A. S. Classifying and predicting the electron affinity of diamond nanoparticles using machine learning. Nanoscale Horizons 2019, 4 (4), 983–990. 10.1039/C9NH00060G. [DOI] [Google Scholar]
- Ho D.; Teo G. Digital Medicine - The New Frontier for AI in Healthcare. Advanced Therapeutics 2020, 3 (4), 2000015. 10.1002/adtp.202000015. [DOI] [Google Scholar]
- Wilson B.; KM G. Artificial intelligence and related technologies enabled nanomedicine for advanced cancer treatment. Nanomedicine 2020, 15 (5), 433–435. 10.2217/nnm-2019-0366. [DOI] [PubMed] [Google Scholar]
- Adir O.; Poley M.; Chen G.; Froim S.; Krinsky N.; Shklover J.; Shainsky-Roitman J.; Lammers T.; Schroeder A. Integrating Artificial Intelligence and Nanotechnology for Precision Cancer Medicine. Adv. Mater. 2020, 32 (13), 1901989. 10.1002/adma.201901989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toratani M.; Konno M.; Asai A.; Koseki J.; Kawamoto K.; Tamari K.; Li Z.; Sakai D.; Kudo T.; Satoh T.; Sato K.; Motooka D.; Okuzaki D.; Doki Y.; Mori M.; Ogawa K.; Ishii H. A Convolutional Neural Network Uses Microscopic Images to Differentiate between Mouse and Human Cell Lines and Their Radioresistant Clones. Cancer Res. 2018, 78 (23), 6703–6707. 10.1158/0008-5472.CAN-18-0653. [DOI] [PubMed] [Google Scholar]
- Quantitative Live Cell Histology; Ben-Gurion University of the Negev, 2018. https://www.assafzaritsky.com/research (accesssed 31st Aug 2020).
- Pantuck A. J.; Lee D.-K.; Kee T.; Wang P.; Lakhotia S.; Silverman M. H.; Mathis C.; Drakaki A.; Belldegrun A. S.; Ho C.-M.; Ho D. Modulating BET Bromodomain Inhibitor ZEN-3694 and Enzalutamide Combination Dosing in a Metastatic Prostate Cancer Patient Using CURATE.AI, an Artificial Intelligence Platform. Advanced Therapeutics 2018, 1 (6), 1800104. 10.1002/adtp.201800104. [DOI] [Google Scholar]
- Schneider L. A.; Korber A.; Grabbe S.; Dissemond J. Influence of pH on wound-healing: a new perspective for wound-therapy?. Arch. Dermatol. Res. 2007, 298, 413–420. 10.1007/s00403-006-0713-x. [DOI] [PubMed] [Google Scholar]
- Surber C.; Abels C.; Maibach H. pH of the Skin: Issues and Challenges. Curr. Probl Dermatol 2018, 54, 87–94. 10.1159/000489512. [DOI] [PubMed] [Google Scholar]