Abstract
BACKGROUD/OBJECTIVES
Evidence has suggested an association between serum vitamin D and metabolic syndrome (MetS), but prospective studies are very limited. The objective was to assess the dose-response association between serum vitamin D concentration and MetS risk using a systematic review and meta-analysis of updated observational studies.
MATERIALS/METHODS
Using MEDLINE, PubMed, and Embase, a systematic literature search was conducted through February 2020 and the references of relevant articles were reviewed. A random-effects model was used to estimate the summary odds ratio/relative risk and 95% confidence interval (CI). Heterogeneity among studies was evaluated with I2 statistic. In total, 23 observational studies (19 cross-sectional studies, and four cohort studies) were included in the meta-analysis.
RESULTS
The pooled estimates (95% CI) for MetS per 25-nmol/L increment in serum vitamin D concentration were 0.80 (95% CI, 0.76–0.84; I2 = 53.5) in cross-sectional studies, and 0.85 (95% CI, 0.72–0.98; I2 = 85.8) in cohort studies. Similar results were observed, irrespectively of age of study population, study location, MetS criteria, and adjustment factors. There was no publication bias for the dose-response meta-analysis of serum vitamin D concentrations and MetS.
CONCLUSIONS
Dose-response meta-analysis demonstrated that a 25-nmol/L increment in the serum vitamin D concentration was associated with 20% and 15% lower risks of MetS in cross-sectional studies and cohort studies, respectively.
Keywords: Metabolic syndrome, vitamin D, meta-analysis, systematic review
INTRODUCTION
Vitamin D deficiency is regarded as a global health issue over the world [1]. Previous studies have found high prevalence of vitamin D deficiency worldwide [2,3]. About 1 billion people have low vitamin D levels and this is found in all ethnicities and age groups [4].
Vitamin D plays a critical role in calcium metabolism, skeletal maintenance, immunity, and cell proliferation and differentiation [5]. Recently, vitamin D deficiency has been reported to have a strong relationship with increased risks of cardiovascular disease (CVD) and metabolic syndrome (MetS) [6,7]. MetS has been considered as a risk factor for CVD and type 2 diabetes mellitus [8,9]. MetS has rapidly increased with changing diets and lifestyle factors [10,11].
Numerous studies have showed the inverse relationship between vitamin D levels and the risk of developing MetS. In Beijing adults aged 21–97 years, the prevalence of MetS was at least three times greater in subjects with lower plasma 25-hydroxyvitamin D (25[OH]D) concentrations (< 10 ng/mL) than in those with higher plasma 25(OH)D concentrations (> 30 ng/mL) after adjustment for potential risk factors [12]. In a cross-sectional study of Australian adults aged 18–75 years, the prevalence of MetS was 65% lower in the highest tertile of serum vitamin D concentrations than in the lowest tertile [13]. Meta-analysis of cross-sectional studies also revealed an inverse association between serum vitamin D levels and MetS risk [14,15]. However, these studies failed to demonstrate a prospective association between serum vitamin D levels and MetS risk. Recently, more longitudinal studies have been published on the relationship between serum vitamin D levels and MetS risk. In longitudinal analysis of Preventive Health Program, an increase of serum 25(OH)D ≥75nmol/L was associated with a lower risk of MetS [16].
In this context, the dose-response association between serum vitamin D levels and MetS risk was explored through a systematic review and meta-analysis, combining data from updated observational studies including prospective studies.
MATERIALS AND METHODS
Literature search strategy
Two investigators (KL and JK) independently searched the literatures. Using MEDLINE, PubMed, and Embase, a systematic literature search was performed through February 2020. The following search terms were used: (‘vitamin D’ or ’25 hydroxyvitamin D’ or ‘25(OH)D’ or ‘cholecalciferol’) and (‘metabolic syndrome’ or ‘syndrome X’ or ‘insulin resistance syndrome’). Additionally, references of the articles searched and meta-analyses or review articles were reviewed to identify relevant studies.
Study eligibility
Observational studies were included, studies had to have vitamin D concentration as the exposure variable, have MetS as the outcome variable, and report odds ratio (OR) or relative risk (RR) and their confidence intervals (CIs) in adults. If duplication from the identical study were identified, the study with the largest number of subjects was selected. Titles and abstracts were checked out for first screening, and then full texts were reviewed. Publications written in non-English language were also included if the summary estimates and 95% CIs could be extracted from the table of full text. Two investigators (KL and JK) independently reviewed all studies, and discrepancies were discussed for agreement.
Data extraction
Two investigators (KL and JK) extracted the data from the original studies and further discussed the data to resolve any disagreements. The following data were extracted from individual study: publication year, first author's name, study design, study location, number and age of subjects, follow-up period for cohort studies, definition of MetS, OR or RR with 95% CI for MetS according to serum vitamin D, and adjusted variables. When multivariable adjustment models were presented, the most-adjusted model was selected. For consistency, data presented as ng/mL were converted to nmol/L with the conversion factor of 2.496. For studies that reported the 25(OH)D concentration as a range, the midpoint of the upper and lower bounds of the range was calculated. If the upper boundary for the highest category was not provided, the values were calculated based on the expectation that the boundary had the same amplitude as the adjacent category. When the lowest category was open-ended, the values were calculated through the computation of the midpoint.
Study quality assessment
Two investigators (KL and JK) independently assessed the quality of studies based on the modified Newcastle-Ottawa quality assessment scale [17] for the following criteria: justification of sample size, representativeness of the sample, comparability of subjects, ascertainment of the risk factor, non-respondents, and apparent description of the statistical analysis. The assessment scores ranged between 0 and 10. Total scores ≥ 8 (out of 10) are considered as high quality. Total scores < 8 indicated low quality. Disagreement in quality assessment between the 2 investigators were resolved by discussion. To avoid selection bias, we did not reject any study based on the quality criteria.
Statistical analysis
Random-effects model was used for the linear or non-linear dose-response analysis [18]. When a study reported separate estimates of the serum vitamin D concentration according to sex [19,20,21], the effect estimates in the same study for serum vitamin D concentration were combined in a random-effects model in the main analysis.
The dose-response association between the exposure (serum vitamin D) and the outcome (MetS) was examined in generalized least-squares trend estimation analysis. This analysis was used first to estimate the study-specific slope lines and then to derive an overall slope, which required the number of subjects and cases [18,22]. When the required numbers were not provided, variance-weighted least squares meta-regression analysis was applied to calculate the slopes [18,22]. For these two analyses, the mean or median value for each concentration category was used. For studies not providing these values in each category, the midpoint of the upper and lower boundary in each category was used as the average concentration.
To visualize and summarize the associations between exposure and outcome, the estimates from each study with pooled estimates are presented as forest plots. A P-value for nonlinearity was estimated by testing the null hypothesis that the coefficient of the second spline was equal to 0.
A subgroup and meta-regression analysis were performed to assess the sources of heterogeneity, including the study design (cross-sectional/cohort), study location (North America/Asia-Pacific/Europe), MetS criteria (National Cholesterol Education Program Adult Treatment Panel III [NCEP ATP III]/International Diabetes Federation/Joint interim statement/Chinese Diabetes Society)] and adjustment factors (age, alcohol intake, smoking, physical activity, education, body mass index [BMI], energy intake, dietary or supplemental calcium and vitamin D intake, serum parathyroid hormone [PTH] levels and season). For a sensitivity analysis, one study at a time was removed and the pooled estimates from remaining studies were calculated to assess the effect of the removed study. To identify heterogeneity and inconsistency, the Q test and I2 statistic were calculated [23,24]. The assumption of heterogeneity was considered valid for P-values < 0.10. The tau-squared statistic was calculated to estimate the between-study variance. Publication bias were evaluated by visual inspection of the funnel plot with a pseudo 95% CI and by Egger's regression asymmetry test and [25] and Begg's test [26]. The detection of publication bias was considered as P-value < 0.1. In addition, the Luis Furuya Kanamori (LFK) index was applied for the identification of publication bias [27]. The closer the value of the LFK index to zero, the more symmetrical plot would be and zero represents complete symmetry. The LFK values beyond ± 1 were deemed consistent with possible publication bias. Statistical analyses were conducted using Stata/SE 14.2 (STATA, College Station, TX, USA) and MetaXL version 5.3 (EpiGear International, Sunrise Beach, Australia).
RESULTS
Literature search
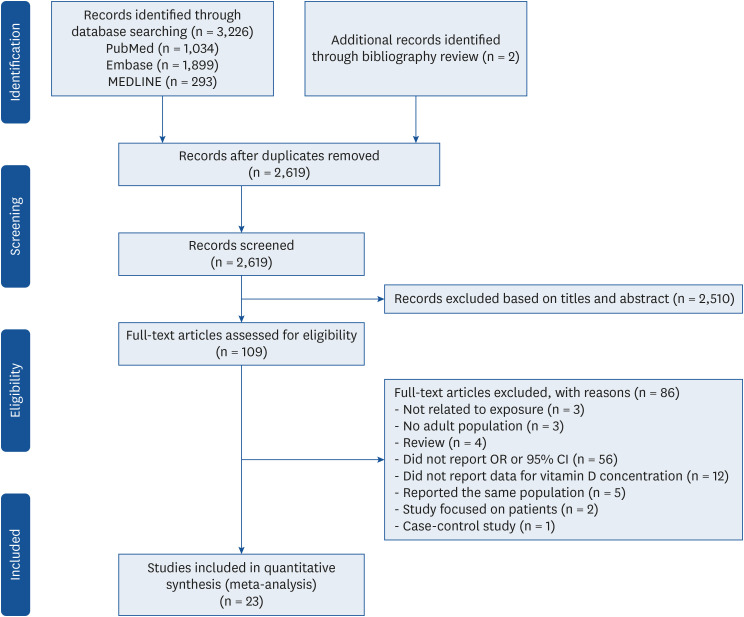
A total of 3,226 papers were searched and 609 were excluded due to duplication. Additionally, two articles were included from reference review. During title and abstract screening, 2,510 articles were excluded. The following 86 articles were further excluded from full text review: 3 not related to exposure, 4 reviews, 3 studies that did not conduct in adults, 56 studies that did not report OR or 95% CI, 12 studies that did not report data for vitamin D concentration, 5 studies reported in the same population, 2 study that focused on patients with particular diseases, and 1 case-control study. Ultimately, 23 observational studies [12,13,16,19,20,21,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44] were included in the meta-analysis including 19 cross-sectional studies, and four cohort studies (Fig. 1).
Fig. 1. Flow chart of the selection process according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement.
Study characteristics
A dose response meta-analysis on the association between serum vitamin D level and MetS risk included 23 studies, including 19 cross-sectional studies [12,13,20,21,28,30,31,32,33,34,35,36,37,38,40,41,42,43,44], and four cohort studies [16,19,29,39] (Table 1). Six studies [16,21,30,31,32,38] were conducted in North America, eleven studies [12,19,20,28,29,33,35,37,41,43,44] were conducted in Asia-Pacific and 6 studies [13,34,36,39,40,42] were conducted in Europe. The follow-up period of the cohort studies was from 1.1 to 6.8 years. Eleven studies [20,21,30,32,33,34,35,36,37,38,39] defined MetS according to the guidelines of the NCEP ATP III, while twelve studies [13,16,19,28,29,31,33,40,41,42,43,44] defined MetS according to the Joint interim statement, or International Diabetes Federation, or Chinese Diabetes Society criteria. Most studies were adjusted for sex, age, alcohol intake, smoking, and physical activity. Nine studies [13,32,34,37,38,39,40,41,43] provided the OR/RR adjusted for education. Eight studies [20,28,29,31,33,34,35,41] provided the OR/RR adjusted for BMI. Three studies [31,32,38] reported the OR/RR adjusted for vitamin D intake and supplement use. Five studies [13,28,30,31,35] reported the OR/RR adjusted for calcium intake and supplement use. In the quality assessment of the studies, the quality scores for the studies were between 5 and 10, with a mean score of 7.6.
Table 1. Characteristics of studies on the association between serum vitamin D status and metabolic syndrome.
| Author, year | Study design (follow up period) | Location | Age (yrs) | No. of subjects | Criteria for metabolic syndrome | Serum vitamin D concentration (nmol/L) | OR or RR (95% CI) | Adjustments | Overall quality | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cross-sectional studies | ||||||||||
| Ford et al., 2005 [32] | Cross-sectional | US | ≥ 20 | 8,421 | Modified NCEP ATP III | Q1 (≤ 48.4) | 1.00 | Age, sex, race/ethnicity, education, smoking status, cotinine concentration, total cholesterol concentration, C-reactive protein concentration, alcohol use, physical activity, fruit and vegetable intake, vitamin or supplement use, and season of study participation | 6 | |
| Q2 (48.5–63.4) | 0.82 (0.60–1.10) | |||||||||
| Q3 (63.5–78.1) | 0.75 (0.55–1.02) | |||||||||
| Q4 (78.2–96.3) | 0.60 (0.44–0.83) | |||||||||
| Q5 (≥ 96.4) | 0.46 (0.32–0.67) | |||||||||
| Reis et al., 2007 [21] | Cross-sectional | US | 44–96 | 1,070 | Modified NCEP ATP III | Men | Men | Age, current smoking, alcohol use, exercise, season of study participation, and hormone therapy (in women) | 9 | |
| I (< 87.5) | 1.00 | |||||||||
| II (87.5–97.4) | 0.83 (0.39–1.73) | |||||||||
| III (97.5–110) | 0.68 (0.32–1.43) | |||||||||
| IV (110.1–126.2) | 0.65 (0.32–1.34) | |||||||||
| V (≥ 126.3) | 0.57 (0.26–1.25) | |||||||||
| Women | Women | |||||||||
| I (< 77.5) | 1.00 | |||||||||
| II (77.5–92.4) | 0.96 (0.48–1.90) | |||||||||
| III (92.5–103.7) | 0.96 (0.51–1.79) | |||||||||
| IV (103.8–119.9) | 1.33 (0.69–2.57) | |||||||||
| V (≥ 120) | 0.88 (0.43–1.80) | |||||||||
| Hyppönen et al., 2008 [34] | Cross-sectional | UK | 42–46 | 6,810 | Modified NCEP ATP III | Lowest third (9–45) | 1.00 | Sex, month, hour of measurement, and insulin-like growth factor-I | 7 | |
| Middle third (46–67) | 0.58 (0.48–0.72) | |||||||||
| Highest third (68–231) | 0.33 (0.26–0.42) | |||||||||
| Lee et al., 2009 [36] | Cross-sectional | UK | 40–79 men | 3,069 | Modified NCEP ATP III | Q1 (< 35.7) | 1.00 | Age, smoking, alcohol consumption, physical activity, season and center, PTH, and HOMA-IR | 7 | |
| Q2 (35.7–49.4) | 0.94 (0.62–1.43) | |||||||||
| Q3 (49.5–65.1) | 0.78 (0.56–1.08) | |||||||||
| Q4 (65.2–85.9) | 0.61 (0.36–1.04) | |||||||||
| Q5 (> 85.9) | 0.60 (0.47–0.78) | |||||||||
| Lu et al., 2009 [37] | Cross-sectional | China | 50–70 | 3,262 | Modified NCEP ATP III | Q1 (≤ 28.7) | 1.00 | Age, sex, geographic location, residential region, visit date, education, physical activity, smoking, alcohol drinking, family history of CVD and diabetes, self-reported coronary heart disease and stroke, and inflammatory factors | 7 | |
| Q2 (28.8–36.8) | 0.94 (0.76–1.17) | |||||||||
| Q3 (36.9–45.5) | 0.71 (0.57–0.88) | |||||||||
| Q4 (45.6–57.6) | 0.58 (0.47–0.72) | |||||||||
| Q5 (≥ 57.7) | 0.40 (0.32–0.50) | |||||||||
| Kim et al., 2010 [35] | Cross-sectional | Korea | ≥ 40 | 1,330 | Modified NCEP ATP III | Q1 (10–29.7) | 1.00 | Age, gender, BMI, season of blood draw, smoking, drinking, exercise, total energy, Ca and sodium intake, PTH, and serum Ca levels | 9 | |
| Q2 (30–39.2) | 0.72 (0.47–1.09) | |||||||||
| Q3 (39.4–49.4) | 0.72 (0.46–1.12) | |||||||||
| Q4 (49.7–61.2) | 0.55 (0.35–0.89) | |||||||||
| Q5 (61.4–116.8) | 0.34 (0.21–0.58) | |||||||||
| Chacko et al., 2011 [31] | Cross-sectional | US | 50–79 women | 292 | Joint interim statement | T1 (< 35) | 1.00 | Age, race/ethnicity, month of blood draw, geographic region, case-control status, smoking status, alcohol intake, physical activity, supplemental vitamins (vitamin D, Ca, magnesium, multivitamins with minerals), and BMI | 7 | |
| T2 (35–51) | 0.43 (0.20–0.93) | |||||||||
| T3 (≥ 52) | 0.38 (0.16–0.91) | |||||||||
| Majumdar et al., 2011 [20] | Cross-sectional | India | 18–75 | 441 | Modified NCEP ATP III | Men | Men | Age, BMI, and smoking habits | 7 | |
| Q1 (< 28.2) | 1.00 | |||||||||
| Q2 (28.2–38.0) | 0.3 (0.1–0.9) | |||||||||
| Q3 (38.1–47.0) | 0.8 (0.3–2.0) | |||||||||
| Q4 (47.1–57.8) | 0.9 (0.3–2.3) | |||||||||
| Q5 (> 57.8) | 0.6 (0.2–1.7) | |||||||||
| Women | Women | |||||||||
| Q1 (< 25.2) | 1.00 | |||||||||
| Q2 (25.2–34.2) | 1.1 (0.4–3.4) | |||||||||
| Q3 (34.3–42.9) | 1.1 (0.4–3.4) | |||||||||
| Q4 (43.0–53.5) | 1.5 (0.5–4.9) | |||||||||
| Q5 (> 53.5) | 1.2 (0.4–3.6) | |||||||||
| Maki et al., 2012 [38] | Cross-sectional | US | ≥ 20 | 3,529 | Modified NCEP ATP III | Q1 (7.5–44.9) | 1.00 | Age, sex, race/ethnicity, education, smoking status, serum cotinine, C-reactive protein, alcohol use, physical activity, sum of total fruit and vegetable Healthy Eating Index scores, and daily intake of vitamin D from dietary supplements | 6 | |
| Q2 (45–59.9) | 0.75 (0.54–1.03) | |||||||||
| Q3 (60–74.9) | 0.69 (0.49–0.96) | |||||||||
| Q4 (75–215) | 0.40 (0.27–0.59) | |||||||||
| Bea et al., 2015 [30] | Cross-sectional | US | Mean 65 | 2,096 | Modified NCEP ATP III | Deficient (< 50) | 1.00 | Age, race/ethnicity, supplemental Ca, waist-hip ratio and sex | 9 | |
| Inadequate (50–75) | 0.70 (0.54–0.92) | |||||||||
| Adequate (≥ 75) | 0.47 (0.35–0.63) | |||||||||
| Huang et al., 2015 [33] | Cross-sectional | Taiwan | 22–39 | 355 | Modified NCEP ATP III | T1 (21.5–58.8) | 1.00 | Age, sex, smoking status, alcohol consumption, physical activity, BMI, and HOMA-IR | 8 | |
| T2 (58.9–79.4) | 0.81 (0.19–3.40) | |||||||||
| T3 (79.5–218.2) | 0.64 (0.14–2.89) | |||||||||
| Lu et al., 2015 [12] | Cross-sectional | China | 21–97 | 3,275 | International Diabetes Federation | < 25 | 1.00 | Age, sex, BMI, waist circumference, FPG, triglyceride, HDL-C, low-density lipoprotein, systolic blood pressure, and diastolic blood pressure | 7 | |
| 25–50 | 0.70 (0.46–1.06) | |||||||||
| 50–75 | 0.27 (0.15–0.46) | |||||||||
| ≥ 75 | 0.16 (0.06–0.38) | |||||||||
| Vitezova et al., 2015 [40] | Cross-sectional | Netherlands | ≥ 55 | 3,240 | Joint interim statement | < 50 | 1.00 | Age, sex, physical activity, diet quality score, family history of cardiometabolic diseases, baseline cardiometabolic diseases, smoking, education, income, season of blood draw, and year of blood draw | 8 | |
| 50–75 | 0.70 (0.58–0.84) | |||||||||
| ≥ 75 | 0.61 (0.49–0.77) | |||||||||
| Akter et al., 2017 [28] | Cross-sectional | Japan | 18–69 | 1,790 | Joint interim statement | < 50 | 1.00 | Age, sex, energy intake, smoking status, alcohol intake, physical activity, night or rotating shift work, Ca intake and BMI | 9 | |
| 50–75 | 0.79 (0.55–1.15) | |||||||||
| ≥ 75 | 0.52 (0.25–1.04) | |||||||||
| Pannu et al., 2017 [13] | Cross-sectional | Australia | 18–75 | 3,404 | Joint interim statement | Low (33) | 1.00 | Age, gender, country of birth, income, education, smoking, season, energy intake, physical activity level, body weight, alcohol, dietary fiber, magnesium, Ca, and retinol | 10 | |
| Medium (54) | 0.77 (0.58–1.04) | |||||||||
| High (77) | 0.35 (0.26–0.48) | |||||||||
| Huang et al., 2019 [41] | Cross-sectional | China | 49–86 women | 616 | International Diabetes Federation | Deficient (< 50) | 1.00 | Age, years after menopause, BMI, education, season of blood sampling, exercise, PTH, estradiol (pg/mL) | 7 | |
| Insufficient (50–75) | 0.76 (0.52–1.11) | |||||||||
| Sufficient (≥ 75) | 0.38 (0.22–0.66) | |||||||||
| Ganji et al., 2020 [43] | Cross-sectional | Qatar | 20–80 women | 700 | International Diabetes Federation | Q1 (< 32.5) | 1.00 | Age, income, education, and menopause | 8 | |
| Q2 (32.5–45) | 1.37 (0.88–2.15) | |||||||||
| Q3 (45–62.5) | 1.34 (0.85–2.13) | |||||||||
| Q4 (≥ 62.5) | 0.50 (0.29–0.85) | |||||||||
| Yeap et al., 2020 [42] | Cross-sectional | Australia | Mean 58.1 | 4,858 | International Diabetes Federation | < 50 | Males | Unadjusted | 5 | |
| 50–100 | 1.00 | |||||||||
| > 100 | 0.56 (0.37–0.86) | |||||||||
| 0.24 (0.15–0.86) | ||||||||||
| Females | ||||||||||
| 1.00 | ||||||||||
| 0.61 (0.46–0.81) | ||||||||||
| 0.37 (0.46–0.81) | ||||||||||
| Weldegiorgis et al., 2020 [44] | Cross-sectional | China | > 50 | 2,764 | Joint interim statement | Q1 (≤ 24.6) | 1.00 | Age, sex, cigarette status, alcohol consumption, physical activity, total serum cholesterol, low-density lipoprotein, and creatinine | 7 | |
| Q2 (24.6–35) | 0.76 (0.55–1.06) | |||||||||
| Q3 (35–48.7) | 0.74 (0.53–1.03) | |||||||||
| Q4 (≥ 48.8) | 0.67 (0.45–0.90) | |||||||||
| Cohort studies | ||||||||||
| Gagnon et al., 2012 [39] | Cohort (5 yrs) | Australia | ≥ 25 | 11,247 | Modified NCEP ATP III | Q1 (< 45) | 1.00 | Age, sex, ethnicity, season, latitude, smoking, family history of type 2 diabetes, physical activity, education, epidermal growth factor receptor, and HOMA-IR | 9 | |
| Q2 (45–57.5) | 1.26 (0.95–1.65) | |||||||||
| Q3 (60–67.5) | 0.87 (0.65–1.17) | |||||||||
| Q4 (70–82.5) | 0.91 (0.68–1.21) | |||||||||
| Q5 (85–232.5) | 0.72 (0.53–0.98) | |||||||||
| Amirbaigloo et al., 2013 [29] | Cohort (6.8 yrs) | Iran | ≥ 20 | 644 | Joint interim statement | < 50 | 1.00 | BMI, waist circumference, FPG, blood pressure, triglyceride, HDL-C, and smoking status | 8 | |
| 50–75 | 0.96 (0.66–1.39) | |||||||||
| > 75 | 1.01 (0.66–1.55) | |||||||||
| Pham et al., 2015 [16] | Cohort (1.1 yrs) | Canada | Mean 51 | 6,682 | Joint interim statement | < 50 | 1.00 | Gender, baseline age, season at baseline, season at follow-up, tobacco smoking status, alcohol drinking status, physical activity at baseline, and physical activity change during follow-up | 7 | |
| 50–75 | 0.78 (0.60–1.01) | |||||||||
| 75–100 | 0.49 (0.37–0.64) | |||||||||
| 100–125 | 0.37 (0.27–0.52) | |||||||||
| > 125 | 0.24 (0.16–0.34) | |||||||||
| Gao et al., 2017 [19] | Cohort (4 yrs) | China | Mean 46 | 474 | Chinese Diabetes Society | Men | Men | Age, physical activity, smoking, alcohol consumption, family history of obesity, diabetes, hypertension, hyperlipidemia and CVD, baseline weight, FPG, 2-hour postprandial glucose level, triglyceride, HDL-C, systolic blood pressure, and diastolic blood pressure | 7 | |
| Q1 (13.93–32.6) | 1.00 | |||||||||
| Q2 (32.61–39.15) | 1.00 (0.38–2.62) | |||||||||
| Q3 (39.16–45.15) | 0.88 (0.32–2.40) | |||||||||
| Q4 (45.15–64.14) | 0.29 (0.06–1.30) | |||||||||
| Women | Women | |||||||||
| Q1 (15.42–36.57) | 1.00 | |||||||||
| Q2 (36.58–41.71) | 0.82 (0.26–2.58) | |||||||||
| Q3 (41.72–49.49) | 0.66 (0.19–2.23) | |||||||||
| Q4 (49.5–80.3) | 0.33 (0.07–1.58) | |||||||||
OR, odds ratio; RR, relative risk; CI, confidence interval; NCEP ATP III, National Cholesterol Education Program Adult Treatment Panel III; PTH, parathyroid hormone; HOMA-IR, homeostasis model assessment of insulin resistance; CVD, cardiovascular disease; BMI, body mass index; Ca, calcium; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol.
Dose-response meta-analysis between serum vitamin D concentration and MetS
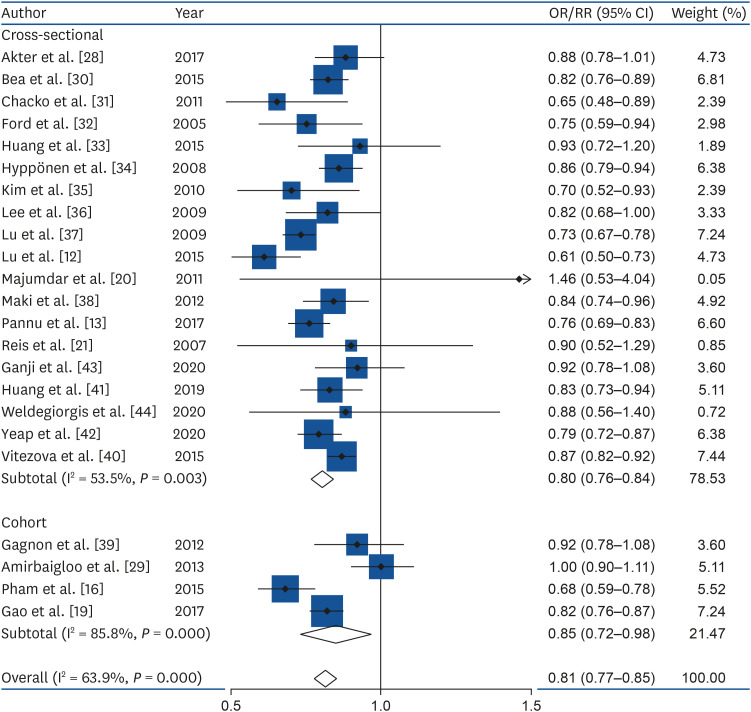
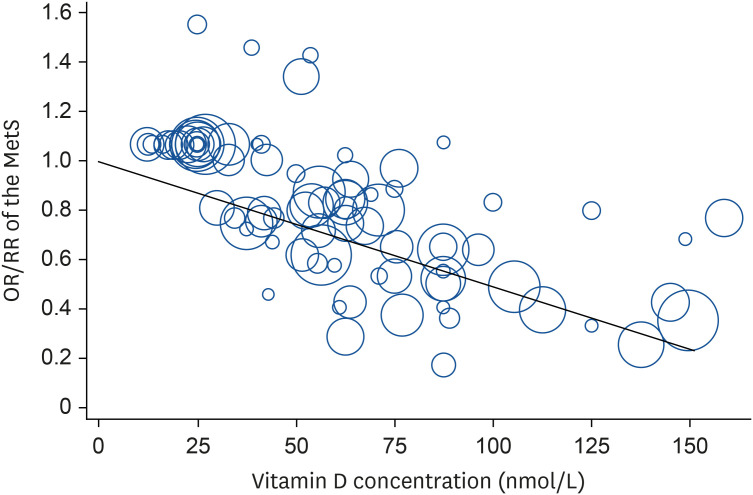
Totally, 23 studies involving 15,540 cases and 70,369 participants were investigated. In a dose-response meta-analysis, a 25-nmol/L increase in the serum vitamin D concentration was associated with a 20% lower risk of MetS (OR = 0.80; 95% CI, 0.76–0.84; I2 = 53.5%; τ2 = 0.003; Z = 41.52, P < 0.001) in cross-sectional studies, a 15% lower risk of MetS (RR = 0.85; 95% CI, 0.72–0.98; I2 = 85.8%; τ2 = 0.014; Z = 13.29, P < 0.001) in cohort studies, and an 19% lower risk of MetS in all studies (OR = 0.81; 95% CI, 0.77–0.85; I2 = 63.9%; τ2 = 0.0042; Z = 42.59, P < 0.001) (Fig. 2). Compared with low concentration of serum vitamin D, the estimated OR/RR of MetS was 0.81 (95% CI, 0.77–0.85) for 25 nmol/L, 0.67 (95% CI, 0.59–0.74) for 50 nmol/L, 0.54 (95% CI, 0.45–0.63) for 75 nmol/L, 0.35 (95% CI, 0.26–0.44) for 125 nmol/L, and 0.27 (95% CI, 0.19–0.35) for 150 nmol/L. However, there was no non-linear association between serum vitamin D and the MetS (P non-linearity = 0.10); therefore, a weighted linear regression model was applied (Fig. 3).
Fig. 2. Forest plot for the linear dose-response relationship between serum vitamin D status (per 25-nmol/L increment) and metabolic syndrome.
OR, odds ratio; RR, relative risk; CI, confidence interval.
Fig. 3. Linear dose-response regression model on the relationship between serum vitamin D and the risk of MetS in observational studies. The solid line represents the weighted regression line, with weights proportional to the precision of the OR/RR.
MetS, metabolic syndrome; OR, odds ratio; RR, relative risk.
Subgroup, meta-regression and sensitivity analysis
The results of the subgroup and meta-regression analyses are presented in Table 2. The subgroup analysis revealed no differences according to age group. For study design, cross-sectional studies (OR = 0.80; 95% CI, 0.76–0.84) [12,13,20,21,28,30,31,32,33,34,35,36,37,38,40,41,42,43,44] and cohort studies (RR = 0.85; 95% CI, 0.72–0.98) [16,19,29,39] showed significant inverse associations. Subgroup analyses showed no difference in study design, study location and MetS criteria (P > 0.1). Study quality (P = 0.010), and adjustment for alcohol intake (P = 0.045) contributed to heterogeneity. Regarding to adjustment factors such as smoking, physical activity, education, BMI, energy intake, calcium intake or supplement use, vitamin D intake or supplement use, serum PTH levels, and season, no differences were found (P > 0.1). By study design, study quality contributed to heterogeneity (P = 0.092) across cross-sectional studies and study quality (P = 0.001) and adjustment for alcohol intake (P = 0.001) contributed to heterogeneity across cohort studies.
Table 2. Subgroup analysis of studies on the association of serum vitamin D status with the risk of MetS in observational studies.
| Study | No. of studies | OR or RR (95% Cl) | I2 (%) | P value for heterogeneity | P* | ||
|---|---|---|---|---|---|---|---|
| All studies | 23 | 0.81 (0.77–0.85) | 63.9 | 0.000 | |||
| Age | 0.475 | ||||||
| Total | 10 | 0.82 (0.74–0.91) | 73.5 | 0.000 | |||
| Middle | 4 | 0.81 (0.72–0.89) | 70.0 | 0.019 | |||
| Elderly | 9 | 0.80 (0.76–0.85) | 52.3 | 0.032 | |||
| Study design | 0.374 | ||||||
| Cross-sectional | 19 | 0.80 (0.76–0.84) | 53.5 | 0.003 | |||
| Cohort | 4 | 0.85 (0.72–0.98) | 85.8 | 0.000 | |||
| Study location | 0.290 | ||||||
| North America | 6 | 0.77 (0.70–0.84) | 43.7 | 0.114 | |||
| Asia-Pacific | 11 | 0.82 (0.75–0.90) | 74.0 | 0.000 | |||
| Europe | 6 | 0.83 (0.78–0.88) | 46.6 | 0.096 | |||
| MetS criteria | 0.840 | ||||||
| NCEP ATP-III | 11 | 0.81 (0.77–0.86) | 34.3 | 0.124 | |||
| Others | 12 | 0.81 (0.75–0.86) | 75.6 | 0.000 | |||
| Quality assessment | 0.010 | ||||||
| High (≥ 8) | 10 | 0.86 (0.81–0.92) | 55.2 | 0.017 | |||
| Low (< 8) | 13 | 0.77 (0.73–0.82) | 55.5 | 0.008 | |||
| Adjustment for confounders | |||||||
| Alcohol | 0.045 | ||||||
| Yes | 13 | 0.78 (0.74–0.82) | 34.3 | 0.108 | |||
| No | 10 | 0.84 (0.79–0.90) | 40.6 | 0.000 | |||
| Smoking | 0.798 | ||||||
| Yes | 17 | 0.82 (0.77–0.86) | 64.6 | 0.000 | |||
| No | 6 | 0.80 (0.74–0.87) | 68.5 | 0.007 | |||
| Physical activity | 0.500 | ||||||
| Yes | 17 | 0.82 (0.78–0.87) | 70.3 | 0.000 | |||
| No | 6 | 0.83 (0.73–0.93) | 77.9 | 0.000 | |||
| Education | 0.626 | ||||||
| Yes | 9 | 0.82 (0.77–0.87) | 63.9 | 0.005 | |||
| No | 14 | 0.80 (0.74–0.86) | 66.1 | 0.000 | |||
| BMI | 0.477 | ||||||
| Yes | 9 | 0.82 (0.72–0.91) | 74.3 | 0.000 | |||
| No | 14 | 0.80 (0.77–0.84) | 53.9 | 0.009 | |||
| Energy intake | 0.640 | ||||||
| Yes | 3 | 0.79 (0.70–0.88) | 47.0 | 0.152 | |||
| No | 20 | 0.81 (0.77–0.86) | 66.3 | 0.000 | |||
| Calcium (dietary intake or supplement use) | 0.442 | ||||||
| Yes | 5 | 0.79 (0.73–0.85) | 37.2 | 0.173 | |||
| No | 18 | 0.82 (0.77–0.87) | 68.4 | 0.000 | |||
| Vitamin D (dietary intake or supplement use) | 0.480 | ||||||
| Yes | 3 | 0.77 (0.67–0.88) | 28.0 | 0.249 | |||
| No | 20 | 0.82 (0.78–0.86) | 67.1 | 0.000 | |||
| Serum PTH | 0.782 | ||||||
| Yes | 3 | 0.81 (0.73–0.89) | 0.0 | 0.534 | |||
| No | 20 | 0.81 (0.77–0.85) | 68.1 | 0.000 | |||
| Season | 0.536 | ||||||
| Yes | 9 | 0.80 (0.74–0.86) | 57.1 | 0.017 | |||
| No | 14 | 0.82 (0.77–0.87) | 69.2 | 0.000 | |||
MetS, metabolic syndrome; OR, odds ratio; RR, relative risk; CI, confidence interval; NCEP ATP-III, National Cholesterol Education Program Adult Treatment Panel III; BMI, body mass index; PTH, parathyroid hormone.
*P values for heterogeneity between subgroups in meta-regression analysis.
In a sensitivity analysis, the pooled estimates were in the range of 0.80 (95% CI, 0.77–0.85) to 0.82 (95% CI, 0.79–0.86). When 2 studies [12,29] were excluded, the heterogeneity decreased (I2 = 50.8%) with the different result (OR = 0.83; 95% CI, 0.80–0.86).
Publication bias
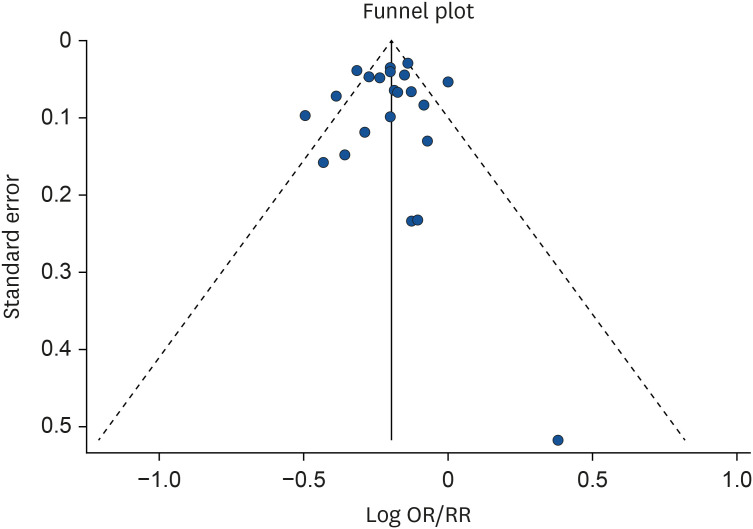
Publication bias for the dose-response meta-analysis of serum vitamin D concentrations and MetS risk was not observed (Begg's P = 0.94, Egger's P = 0.78, LFK index= 0.53). Funnel plot for publication bias was presented in Fig. 4.
Fig. 4. Funnel plot for publication bias in the relationship between serum vitamin D and the risk of metabolic syndrome in observational studies.
OR, odds ratio; RR, relative risk.
DISCUSSION
This meta-analysis revealed a linear inverse association between serum vitamin D concentration and the risk of MetS in both cross-sectional and cohort studies. In a dose-response meta-analysis, a 25-nmol/L increase in serum vitamin D concentration was associated with a 20% lower risk of MetS in cross-sectional studies and a 15% lower risk of MetS in cohort studies. A 25-nmol/L increment in serum vitamin D concentration was associated with a 19% lower risk of MetS when all the studies were combined. Study quality contributed to heterogeneity among cross-sectional study whereas study location, study quality, adjustment for alcohol intake, physical activity, and BMI contributed to heterogeneity among cohort study.
These findings are line with the results of previous meta-analyses. A 25-nmol/L increment in serum vitamin D concentration was associated with a 13% lower risk of MetS in 16 cross-sectional studies [14]. A meta-analysis of eight cross-sectional studies found that the highest group of serum vitamin D levels was associated with a 51% lower risk of MetS than the lowest group [15]. A meta-analysis of prospective studies reported that individuals in the top third of vitamin D level had a 14% lower risk of MetS compared with those in the bottom third of vitamin D [45].
Numerous studies have supported inverse relationships between vitamin D status and the components of MetS as well as MetS itself. In a clinical trial of overweight and obese women aged 20–40 years, 6-week supplementation with 50,000 IU of vitamin D/week reduced the waist circumference in the intervention group [46]. A vitamin D intervention of 50,000 IU/week for four months reduced triglyceride levels in an Iranian population aged 30–50 years [47]. In a clinical trial of diabetic patients, fasting plasma glucose levels decreased in the intervention group supplemented with 50,000 IU of vitamin D/week for eight weeks [48]. A 14-year follow-up study in US women aged 22–44 years revealed that individuals with vitamin D insufficiency had a greater risk of hypertension after adjustment for potential confounders [49]. Given these results, the protective effects of a higher vitamin D status on the components of MetS might ultimately reduce the risk of MetS.
Potential mechanisms have been suggested to explain the inverse association between serum vitamin D concentration and the risk of MetS. Hypovitaminosis D has long been suspected to increase the risk of glucose intolerance by influencing insulin sensitivity or β cell function. Chiu et al. reported that 25(OH)D concentration was positively correlated with insulin sensitivity and hypovitaminosis D was negatively related to β cell function in glucose-intolerant subjects [50]. Vitamin D is an essential nutrient for insulin secretion. Indeed, insulin secretion was found to be impaired in subjects with vitamin D-deficiency [50]. Vitamin D not only accelerates the biosynthetic capacity of β cells, but also facilitates the insulin synthesis from proinsulin [50].
Vitamin D is closely linked to atherogenic lipid profile. Vitamin D deficiency causes adipogenesis and hyperlipidemia through the decrease of sirtuin (SIRT)-1, which stimulate lipolysis and inhibits adipogenesis by deacetylation of peroxisome proliferator-activated receptor gamma [51]. Moreover, SIRT-1 plays a critical role in lipid metabolism through the control of the secretion and action of insulin [52]. Chang et al reported that SIRT-1 activity decreased significantly in obese rats fed vitamin D-insufficient diet [53].
Low vitamin D levels may elevate blood pressure by activating the renin-angiotensin system via the vitamin D receptor (VDR) [54]. Li et al. reported that renin activity and circulating plasma angiotensin II concentrations were significantly elevated in VDR knock-out mice [55]. VDR liganded with 1,25-dihydroxyvitamin D, the active form of vitamin D, suppresses renin gene expression, and VDR agonists have exhibited protective effects on blood pressure and cardiac tissue [55].
Vitamin D deficiency is associated with reduced calcium absorption in intestine, leading to low serum calcium concentrations. Low serum calcium levels stimulates PTH secretion to ensure adequate serum calcium levels; however, the resulting secondary hyperparathyroidism has several deleterious effects [56]. Epidemiological studies have demonstrated that elevated PTH levels are associated with higher risk of cardiometabolic factors [57]. A cohort study of adults aged 55–85 years in the Netherlands demonstrated that higher PTH levels were associated with higher blood pressure levels after adjustment for potential confounders [58]. Ahlstrom et al. found that plasma PTH levels were positively correlated with and waist circumference in a Swedish population aged 70 years [59]. Taken together, these results indicate that the impacts of the serum vitamin D concentration on individual components of MetS might influence the risk of MetS.
In addition, vitamin D has been suggested to play important roles in inflammation [60]. For instance, 1,25-dihydroxyvitamin D regulates the production of pro-inflammatory cytokines including tumor necrosis factor-α, interleukin (IL)-1, IL-6, IL-8, and in immune system cells [61,62]. In patients aged 48–81 years with type 2 diabetes, 1,25-dihydroxyvitamin D downregulated cytokines in monocytes, whereas this did not occur in the control group [62].
To the best of our knowledge, the present study is the first meta-analysis including prospective studies to reveal a dose response inverse relationship between serum vitamin D status and the risk of MetS. Previous meta-analysis failed to examine prospective association between vitamin D and MetS because they mostly included cross-sectional studies. This meta-analysis included studies with good quality and the studies adjusted for potential confounders of MetS such as sociodemographic and lifestyle factors.
Our results may be hampered by heterogeneity. This heterogeneity could be attributable to differences in study quality and adjustment factor such as alcohol intake across cohort studies. Nevertheless, it is clear that there is a majority agreement among the study results and an overall consensus regarding the inverse association between vitamin D and MetS when evaluated as combined and as separate outcomes as well.
This study has a few limitations. Small number of cohort studies were included in the meta-analysis. The possibility of unmeasured confounding factors might exist in this study. However, a small E-value indicates no unmeasured confounding is needed to explain away the observed association [63]. The effect size could be overestimated because the estimates were pooled from OR and RR. Also, the results should be interpreted cautiously because of the evidence of heterogeneity across the studies in this analysis. The possibility for ecological bias may exist because meta-analyses use aggregated data rather than analyzing individual data.
In conclusion, this dose-response meta-analysis demonstrated that a 25-nmol/L increment in the serum vitamin D concentration was associated with 20% and 15% lower risks of MetS in cross-sectional studies and cohort studies, respectively. In the subgroup analysis, study quality and adjustment for alcohol intake contributed to heterogeneity. Also, vitamin D status was inversely associated with risk of MetS regardless of race- or geographic region. A linear association between serum vitamin D and MetS risk suggest that maintaining the proper vitamin D level may reduce the public health burden for MetS in general population. The importance of vitamin D status should be emphasized for the higher risk groups such as chronic alcohol drinker. Further well-designed clinical trials are required to evaluate the causal association between vitamin D status and MetS risk and determine the benefit of vitamin D supplementation for the prevention of MetS.
Footnotes
Funding: This research was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science, and Technology (NRF2017 R1D1A1B03931307 and NRF2018R1D1A1B07045558). The NRF had no role in the study design, data analysis, or writing of this article.
Conflict of Interest: The authors declare no potential conflicts of interests.
- Conceptualization: Kim J.
- Formal analysis: Lee K, Kim J.
- Funding acquisition: Kim J.
- Methodology: Kim J.
- Supervision: Kim J.
- Writing - original draft: Lee K.
- Writing - review & editing: Kim J.
References
- 1.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 2.Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, El-Hajj Fuleihan G, Josse RG, Lips P, Morales-Torres J IOF Committee of Scientific Advisors (CSA) Nutrition Working Group. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009;20:1807–1820. doi: 10.1007/s00198-009-0954-6. [DOI] [PubMed] [Google Scholar]
- 3.Wahl DA, Cooper C, Ebeling PR, Eggersdorfer M, Hilger J, Hoffmann K, Josse R, Kanis JA, Mithal A, Pierroz DD, Stenmark J, Stöcklin E, Dawson-Hughes B. A global representation of vitamin D status in healthy populations. Arch Osteoporos. 2012;7:155–172. doi: 10.1007/s11657-012-0093-0. [DOI] [PubMed] [Google Scholar]
- 4.Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol. 2014;144(Pt A):138–145. doi: 10.1016/j.jsbmb.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim M, Na W, Sohn C. Correlation between vitamin D and cardiovascular disease predictors in overweight and obese Koreans. J Clin Biochem Nutr. 2013;52:167–171. doi: 10.3164/jcbn.12-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson JL, May HT, Horne BD, Bair TL, Hall NL, Carlquist JF, Lappé DL, Muhlestein JB Intermountain Heart Collaborative (IHC) Study Group. Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol. 2010;106:963–968. doi: 10.1016/j.amjcard.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 7.Botella-Carretero JI, Alvarez-Blasco F, Villafruela JJ, Balsa JA, Vázquez C, Escobar-Morreale HF. Vitamin D deficiency is associated with the metabolic syndrome in morbid obesity. Clin Nutr. 2007;26:573–580. doi: 10.1016/j.clnu.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Wilson PW, D'Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 9.Wu SH, Liu Z, Ho SC. Metabolic syndrome and all-cause mortality: a meta-analysis of prospective cohort studies. Eur J Epidemiol. 2010;25:375–384. doi: 10.1007/s10654-010-9459-z. [DOI] [PubMed] [Google Scholar]
- 10.Borch-Johnsen K. The metabolic syndrome in a global perspective. The public health impact--secondary publication. Dan Med Bull. 2007;54:157–159. [PubMed] [Google Scholar]
- 11.Lee SE, Han K, Kang YM, Kim SO, Cho YK, Ko KS, Park JY, Lee KU, Koh EH Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association. Trends in the prevalence of metabolic syndrome and its components in South Korea: findings from the Korean National Health Insurance Service Database (2009–2013) PLoS One. 2018;13:e0194490. doi: 10.1371/journal.pone.0194490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu Y, Liu M, Pei Y, Li J, Tian H, Cheng X, Fang F, Sun B, Xiao H, Li N, Miao X, Li C. Low levels of serum 25-hydroxyvitamin D and risk of metabolic syndrome in China. Int J Clin Exp Med. 2015;8:13790–13796. [PMC free article] [PubMed] [Google Scholar]
- 13.Pannu PK, Zhao Y, Soares MJ, Piers LS, Ansari Z. The associations of vitamin D status and dietary calcium with the metabolic syndrome: an analysis of the Victorian Health Monitor survey. Public Health Nutr. 2017;20:1785–1796. doi: 10.1017/S1368980016001609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ju SY, Jeong HS, Kim DH. Blood vitamin D status and metabolic syndrome in the general adult population: a dose-response meta-analysis. J Clin Endocrinol Metab. 2014;99:1053–1063. doi: 10.1210/jc.2013-3577. [DOI] [PubMed] [Google Scholar]
- 15.Parker J, Hashmi O, Dutton D, Mavrodaris A, Stranges S, Kandala NB, Clarke A, Franco OH. Levels of vitamin D and cardiometabolic disorders: systematic review and meta-analysis. Maturitas. 2010;65:225–236. doi: 10.1016/j.maturitas.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 16.Pham TM, Ekwaru JP, Setayeshgar S, Veugelers PJ. The effect of changing serum 25-hydroxyvitamin D concentrations on metabolic syndrome: a longitudinal analysis of participants of a preventive health program. Nutrients. 2015;7:7271–7284. doi: 10.3390/nu7095338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, Perruolo E, Parati G ESH Working Group on CV Risk in Low Resource Settings. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PLoS One. 2016;11:e0147601. doi: 10.1371/journal.pone.0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 19.Gao Y, Ran X, Ren Y, Chen T, Zheng T, Zhong L, Tian H. Relationship between vitamin D level and the incidence of metabolic syndrome in adults in Chengdu city: a prospective cohort study. Chin J Evid-Based Med. 2017;17:1121–1126. [Google Scholar]
- 20.Majumdar V, Nagaraja D, Christopher R. Vitamin D status and metabolic syndrome in Asian Indians. Int J Obes. 2011;35:1131–1134. doi: 10.1038/ijo.2010.232. [DOI] [PubMed] [Google Scholar]
- 21.Reis JP, von Mühlen D, Kritz-Silverstein D, Wingard DL, Barrett-Connor E. Vitamin D, parathyroid hormone levels, and the prevalence of metabolic syndrome in community-dwelling older adults. Diabetes Care. 2007;30:1549–1555. doi: 10.2337/dc06-2438. [DOI] [PubMed] [Google Scholar]
- 22.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J. 2006;6:40–57. [Google Scholar]
- 23.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 27.Furuya-Kanamori L, Barendregt JJ, Doi SA. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int J Evid-Based Healthc. 2018;16:195–203. doi: 10.1097/XEB.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 28.Akter S, Eguchi M, Kurotani K, Kochi T, Kashino I, Ito R, Kuwahara K, Tsuruoka H, Kabe I, Mizoue T. Serum 25-hydroxyvitamin D and metabolic syndrome in a Japanese working population: the Furukawa Nutrition and Health Study. Nutrition. 2017;36:26–32. doi: 10.1016/j.nut.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 29.Amirbaigloo A, Hosseinpanah F, Sarvghadi F, Tohidi M, Eskandary PS, Azizi F. Absence of association between vitamin D deficiency and incident metabolic syndrome: Tehran Lipid and Glucose Study. Metab Syndr Relat Disord. 2013;11:236–242. doi: 10.1089/met.2012.0121. [DOI] [PubMed] [Google Scholar]
- 30.Bea JW, Jurutka PW, Hibler EA, Lance P, Martínez ME, Roe DJ, Sardo Molmenti CL, Thompson PA, Jacobs ET. Concentrations of the vitamin D metabolite 1,25(OH)2D and odds of metabolic syndrome and its components. Metabolism. 2015;64:447–459. doi: 10.1016/j.metabol.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chacko SA, Song Y, Manson JE, Van Horn L, Eaton C, Martin LW, McTiernan A, Curb JD, Wylie-Rosett J, Phillips LS, Plodkowski RA, Liu S. Serum 25-hydroxyvitamin D concentrations in relation to cardiometabolic risk factors and metabolic syndrome in postmenopausal women. Am J Clin Nutr. 2011;94:209–217. doi: 10.3945/ajcn.110.010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ford ES, Ajani UA, McGuire LC, Liu S. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care. 2005;28:1228–1230. doi: 10.2337/diacare.28.5.1228. [DOI] [PubMed] [Google Scholar]
- 33.Huang CY, Chang HH, Lu CW, Tseng FY, Lee LT, Huang KC. Vitamin D status and risk of metabolic syndrome among non-diabetic young adults. Clin Nutr. 2015;34:484–489. doi: 10.1016/j.clnu.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Hyppönen E, Boucher BJ, Berry DJ, Power C. 25-hydroxyvitamin D, IGF-1, and metabolic syndrome at 45 years of age: a cross-sectional study in the 1958 British Birth Cohort. Diabetes. 2008;57:298–305. doi: 10.2337/db07-1122. [DOI] [PubMed] [Google Scholar]
- 35.Kim MK, Il Kang M, Won Oh K, Kwon HS, Lee JH, Lee WC, Yoon KH, Son HY. The association of serum vitamin D level with presence of metabolic syndrome and hypertension in middle-aged Korean subjects. Clin Endocrinol (Oxf) 2010;73:330–338. doi: 10.1111/j.1365-2265.2010.03798.x. [DOI] [PubMed] [Google Scholar]
- 36.Lee DM, Rutter MK, O'Neill TW, Boonen S, Vanderschueren D, Bouillon R, Bartfai G, Casanueva FF, Finn JD, Forti G, Giwercman A, Han TS, Huhtaniemi IT, Kula K, Lean ME, Pendleton N, Punab M, Silman AJ, Wu FC European Male Ageing Study Group. Vitamin D, parathyroid hormone and the metabolic syndrome in middle-aged and older European men. Eur J Endocrinol. 2009;161:947–954. doi: 10.1530/EJE-09-0496. [DOI] [PubMed] [Google Scholar]
- 37.Lu L, Yu Z, Pan A, Hu FB, Franco OH, Li H, Li X, Yang X, Chen Y, Lin X. Plasma 25-hydroxyvitamin D concentration and metabolic syndrome among middle-aged and elderly Chinese individuals. Diabetes Care. 2009;32:1278–1283. doi: 10.2337/dc09-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maki KC, Fulgoni VL, 3rd, Keast DR, Rains TM, Park KM, Rubin MR. Vitamin D intake and status are associated with lower prevalence of metabolic syndrome in U.S. adults: National Health and Nutrition Examination Surveys 2003–2006. Metab Syndr Relat Disord. 2012;10:363–372. doi: 10.1089/met.2012.0020. [DOI] [PubMed] [Google Scholar]
- 39.Gagnon C, Lu ZX, Magliano DJ, Dunstan DW, Shaw JE, Zimmet PZ, Sikaris K, Ebeling PR, Daly RM. Low serum 25-hydroxyvitamin D is associated with increased risk of the development of the metabolic syndrome at five years: results from a national, population-based prospective study (the Australian Diabetes, Obesity and Lifestyle Study: AusDiab) J Clin Endocrinol Metab. 2012;97:1953–1961. doi: 10.1210/jc.2011-3187. [DOI] [PubMed] [Google Scholar]
- 40.Vitezova A, Zillikens MC, van Herpt TT, Sijbrands EJ, Hofman A, Uitterlinden AG, Franco OH, Kiefte-de Jong JC. Vitamin D status and metabolic syndrome in the elderly: the Rotterdam Study. Eur J Endocrinol. 2015;172:327–335. doi: 10.1530/EJE-14-0580. [DOI] [PubMed] [Google Scholar]
- 41.Huang H, Guo J, Chen Q, Chen X, Yang Y, Zhang W, Liu Y, Chen X, Yang D. The synergistic effects of vitamin D and estradiol deficiency on metabolic syndrome in Chinese postmenopausal women. Menopause. 2019;26:1171–1177. doi: 10.1097/GME.0000000000001370. [DOI] [PubMed] [Google Scholar]
- 42.Yeap BB, Dedic D, Budgeon CA, Murray K, Knuiman MW, Hunter M, Zhu K, Cooke BR, Lim EM, Mulrennan S, Walsh JP, Green DJ. U-shaped association of vigorous physical activity with risk of metabolic syndrome in men with low lean mass, and no interaction of physical activity and serum 25-hydroxyvitamin D with metabolic syndrome risk. Intern Med J. 2020;50:460–469. doi: 10.1111/imj.14379. [DOI] [PubMed] [Google Scholar]
- 43.Ganji V, Sukik A, Alaayesh H, Rasoulinejad H, Shraim M. Serum vitamin D concentrations are inversely related to prevalence of metabolic syndrome in Qatari women. Biofactors. 2020;46:180–186. doi: 10.1002/biof.1572. [DOI] [PubMed] [Google Scholar]
- 44.Weldegiorgis TZ, Hidru TH, Yang XL, Xia YL, Ma L, Li HH. Association between serum 25-hydroxyvitamin D concentrations and metabolic syndrome in the middle-aged and elderly Chinese population in Dalian, northeast China: a cross-sectional study. J Diabetes Investig. 2020;11:184–191. doi: 10.1111/jdi.13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khan H, Kunutsor S, Franco OH, Chowdhury R. Vitamin D, type 2 diabetes and other metabolic outcomes: a systematic review and meta-analysis of prospective studies. Proc Nutr Soc. 2013;72:89–97. doi: 10.1017/S0029665112002765. [DOI] [PubMed] [Google Scholar]
- 46.Khosravi ZS, Kafeshani M, Tavasoli P, Zadeh AH, Entezari MH. Effect of vitamin D supplementation on weight loss, glycemic indices, and lipid profile in obese and overweight women: a clinical trial study. Int J Prev Med. 2018;9:63. doi: 10.4103/ijpvm.IJPVM_329_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salekzamani S, Mehralizadeh H, Ghezel A, Salekzamani Y, Jafarabadi MA, Bavil AS, Gargari BP. Effect of high-dose vitamin D supplementation on cardiometabolic risk factors in subjects with metabolic syndrome: a randomized controlled double-blind clinical trial. J Endocrinol Invest. 2016;39:1303–1313. doi: 10.1007/s40618-016-0507-8. [DOI] [PubMed] [Google Scholar]
- 48.Talaei A, Mohamadi M, Adgi Z. The effect of vitamin D on insulin resistance in patients with type 2 diabetes. Diabetol Metab Syndr. 2013;5:8. doi: 10.1186/1758-5996-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Griffin FC, Gadegbeku CA, Sowers MR. Vitamin D and subsequent systolic hypertension among women. Am J Hypertens. 2011;24:316–321. doi: 10.1038/ajh.2010.226. [DOI] [PubMed] [Google Scholar]
- 50.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 51.Liu HX, Wang YM, Hu JP, Huang LY, Fang NY. Adipocyte differentiation is regulated by mitochondrial trifunctional protein α-subunit via sirtuin 1. Exp Cell Res. 2017;357:271–281. doi: 10.1016/j.yexcr.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 52.Ye X, Li M, Hou T, Gao T, Zhu WG, Yang Y. Sirtuins in glucose and lipid metabolism. Oncotarget. 2017;8:1845–1859. doi: 10.18632/oncotarget.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang E, Kim Y. Vitamin D decreases adipocyte lipid storage and increases NAD-SIRT1 pathway in 3T3-L1 adipocytes. Nutrition. 2016;32:702–708. doi: 10.1016/j.nut.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 54.Vaidya A, Williams JS. The relationship between vitamin D and the renin-angiotensin system in the pathophysiology of hypertension, kidney disease, and diabetes. Metabolism. 2012;61:450–458. doi: 10.1016/j.metabol.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li YC. Vitamin D regulation of the renin-angiotensin system. J Cell Biochem. 2003;88:327–331. doi: 10.1002/jcb.10343. [DOI] [PubMed] [Google Scholar]
- 56.Pilz S, Tomaschitz A, März W, Drechsler C, Ritz E, Zittermann A, Cavalier E, Pieber TR, Lappe JM, Grant WB, Holick MF, Dekker JM. Vitamin D, cardiovascular disease and mortality. Clin Endocrinol (Oxf) 2011;75:575–584. doi: 10.1111/j.1365-2265.2011.04147.x. [DOI] [PubMed] [Google Scholar]
- 57.Pilz S, Tomaschitz A, Drechsler C, Ritz E, Boehm BO, Grammer TB, März W. Parathyroid hormone level is associated with mortality and cardiovascular events in patients undergoing coronary angiography. Eur Heart J. 2010;31:1591–1598. doi: 10.1093/eurheartj/ehq109. [DOI] [PubMed] [Google Scholar]
- 58.Snijder MB, Lips P, Seidell JC, Visser M, Deeg DJ, Dekker JM, van Dam RM. Vitamin D status and parathyroid hormone levels in relation to blood pressure: a population-based study in older men and women. J Intern Med. 2007;261:558–565. doi: 10.1111/j.1365-2796.2007.01778.x. [DOI] [PubMed] [Google Scholar]
- 59.Ahlström T, Hagström E, Larsson A, Rudberg C, Lind L, Hellman P. Correlation between plasma calcium, parathyroid hormone (PTH) and the metabolic syndrome (MetS) in a community-based cohort of men and women. Clin Endocrinol (Oxf) 2009;71:673–678. doi: 10.1111/j.1365-2265.2009.03558.x. [DOI] [PubMed] [Google Scholar]
- 60.Garbossa SG, Folli F. Vitamin D, sub-inflammation and insulin resistance. A window on a potential role for the interaction between bone and glucose metabolism. Rev Endocr Metab Disord. 2017;18:243–258. doi: 10.1007/s11154-017-9423-2. [DOI] [PubMed] [Google Scholar]
- 61.Neve A, Corrado A, Cantatore FP. Immunomodulatory effects of vitamin D in peripheral blood monocyte-derived macrophages from patients with rheumatoid arthritis. Clin Exp Med. 2014;14:275–283. doi: 10.1007/s10238-013-0249-2. [DOI] [PubMed] [Google Scholar]
- 62.Giulietti A, van Etten E, Overbergh L, Stoffels K, Bouillon R, Mathieu C. Monocytes from type 2 diabetic patients have a pro-inflammatory profile. 1,25-dihydroxyvitamin D(3) works as anti-inflammatory. Diabetes Res Clin Pract. 2007;77:47–57. doi: 10.1016/j.diabres.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 63.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167:268–274. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]