Abstract
Structure–activity relationship studies directed toward the replacement of the fused phenyl ring of the lead hexahydrobenzoindole RORγt inverse agonist series represented by 1 with heterocyclic moieties led to the identification of three novel aza analogs 5–7. The hexahydropyrrolo[3,2-f]quinoline series 5 (X = N, Y = Z=CH) showed potency and metabolic stability comparable to series 1 but with improved in vitro membrane permeability and serum free fraction. This structural modification was applied to the hexahydrocyclopentanaphthalene series 3, culminating in the discovery of 8e as a potent and selective RORγt inverse agonist with an excellent in vitro profile, good pharmacokinetic properties, and biologic-like in vivo efficacy in preclinical models of rheumatoid arthritis and psoriasis.
Keywords: RORγt, RORc, inverse agonist, aza substitution, acanthosis, collagen-induced arthritis
The orphan receptor RORγt (RORc in humans) belongs to the retinoic acid orphan receptor family of nuclear hormone receptors. There are three members of the family (RORα, RORβ, and RORγ) which are widely expressed in many tissues and organs and have roles in varied cell functions.1 In contrast, the expression of RORγt, a shortened splice variant of RORγ, is limited to cells of the lymphoid lineage. This receptor has important effects in the immune response. In particular, it controls the differentiation of CD4+ T cells into Th17 cells, regulating the production and release of pro-inflammatory cytokines such as IL-17, IL-22, and GM-CSF, which have been implicated in diseases such as psoriasis, rheumatoid arthritis, Crohn’s disease, and multiple sclerosis.2−4 This has been confirmed by the clinical efficacy of marketed anti-IL-17 monoclonal antibodies such as secukinumab and ixekizumab.5 The success of these antibodies has led to significant efforts to discover orally active small molecules which down-regulate the production of these cytokines via pharmacological inhibition of RORγt.6−8
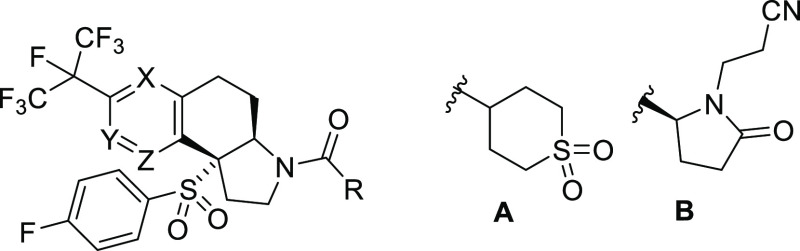
Previous reports from our laboratories have described novel inverse agonists of RORγt based on the 2,3,3a,4,5,9b-hexahydro-1H-benzo[e]indole core 1 shown in Figure 1.9−11 The carboxylic acid derivative BMS-986251 (1a) showed excellent potency and oral bioavailability and was efficacious in mouse models of skin inflammation, leading to selection of this molecule as a candidate for clinical evaluation.10 Additional work sought to identify nonacidic analogs in an effort to avoid potential issues due to acyl glucuronide formation,12,13 leading to analogs such as sulfone 1b and the carbinol-substituted pyroglutamide 1c,11 both of which also possessed good preclinical profiles and oral efficacy. More recent work has identified the carbocyclic 2,3,3a,4,5,9b-hexahydro-1H-cyclopenta[a]naphthalene series as an interesting alternative to the hexahydrobenzoindole core.14 Compounds 2 and 3a are leading examples from this series, and 3a (BMS-986313) was an advanced preclinical candidate.15
Figure 1.
Previously reported RORγt inverse agonists with tricyclic cores (EC50 values shown).
Heteroatom replacement (O or N) at the benzylic position of the saturated ring in structure 1 had been previously examined and was found to have negligible effect on potency,9 despite a reduction in lipophilicity. For example, the calculated log P(16) of chromane 4 (2.38; Figure 2) is an order of magnitude lower than that of 1b (3.18). Since the tricyclic motif binds in a largely lipophilic site of RORγt, many of the best analogs were very lipophilic (clog P values for other nonacid analogs shown in Figure 1 range from 4.45 for 1c to 5.67 for 2). Reduction in lipophilicity is, in general, a desirable goal, since high log P values have been associated with a higher potential for liabilities including poor solubility, promiscuity, and toxicities such as drug-induced liver injury.17−19
Figure 2.
Heterocyclic variants of tricyclic RORγt inverse agonists.
We were interested in further exploring the effect of heteroatom substitution, specifically conversion of the fused benzene ring of 1–3 into a pyridine ring, both on RORγt inverse agonist activity and on the overall profile of the molecules. Calculations on model compounds represented by 5–7 (Figure 2) indicated that the log P value would show a desirable decrease between 0.84 (for 6 and 7) and 0.99 (for 5) relative to the corresponding benzene analogs 1. Such aza analogs were not anticipated to possess significant basicity due to the electron-withdrawing effect of the perfluoroisopropyl group as well as the presence of the p-fluorophenylsulfone substituent at the quaternary benzylic position of the embedded tetrahydroquinoline or tetrahydroisoquinoline ring system. Calculations20 indicated that the pKa values for the nitrogen atoms of 5–7 would be in the range of 1.0 to 2.5, compared to 6.01 and 6.14 for tetrahydroquinoline and tetrahydroisoquinoline, respectively.
To explore the effects of these changes on the biological profile of the series, we undertook the synthesis of the three azatricyclic ring systems 5–7 and prepared two amide examples of each, to compare with the corresponding nonaza analogs in the series represented by 1. We then extended this design concept to the hexahydrocyclopentaquinoline series 8.
We examined two nonacidic R substituents in each of the aza series 5, 6, and 7, using substituents of previous interest in the original hexahydropyrrolonaphthalene series 1. Compounds were evaluated in a primary assay comprised of a Gal4-Luc reporter assay in Jurkat cells which were transfected to overexpress RORγt (GAL4). Compounds showing reasonable inverse agonistic potency were further evaluated as inhibitors of IL-17A production in human whole blood (hWB) stimulated with CD3 and CD28. We also measured the amount of compound remaining after 10 min of incubation with human and mouse liver microsomes (LM) as a measure of metabolic stability. The results are shown in Table 1 along with the results for the same analogs in the nonaza series for comparison.
Table 1. In Vitro Data for Aza Substitutions in the Hexahydropyrrolonaphthalene Seriesa.
| cmpd | X | Y | Z | R | RORγt GAL4 EC50 (nM) | IL-17 hWB EC50 (nM) | LM h, m (% rem)b | Caco-2 A-B (efflux)c | PB % free (h, m)d | rCYP 2C8, 3A4 IC50 (μM)e |
|---|---|---|---|---|---|---|---|---|---|---|
| 5a | N | CH | CH | A | 15 ± 3.9 | 67 ± 33 | 100, 91 | 230 (0.7) | nd, 5.0 | 16, >20 |
| 5b | N | CH | CH | B | 24 ± 17 | 31 ± 16 | 100, 83 | 190 (1.7) | 7.1, 5.1 | >20, 19 |
| 6a | CH | N | CH | A | 17 ± 3.3 | 23 ± 11 | 92, 49 | nd | nd, nd | nd |
| 6b | CH | N | CH | B | 11 | 27 ± 5.2 | 100, 69 | 120 (3.7) | 12, 6.8 | >20, 12 |
| 7a | CH | CH | N | A | 71 ± 2.9 | nd | 100, 96 | nd | nd, nd | nd |
| 7b | CH | CH | N | B | 65 ± 27 | 86 ± 26 | 83, 82 | 94 (5.8) | 20, 16 | >20, >20 |
| 1d | CH | CH | CH | A | 35 ± 22 | 51 ± 4 | 93, 100 | 130 (0.6) | 0.8, 0.8 | 9.4, 13 |
| 1e | CH | CH | CH | B | 18 ± 11 | 30 ± 15 | 92, 85 | 170 (0.9) | 1.8, 1.7 | 13, 10 |
See the Supporting Information for assay protocols. Values are the means of two or more experiments. SDs are provided for means of three or more experiments. nd = not determined.
Percent remaining after 10 min of incubation with human or mouse LM.
Pc in nm/s in the apical to basal direction in Caco-2 cells. Values in parentheses are the efflux ratios (BA/AB).
Protein binding, % free in human or mouse serum.
Inhibition of recombinant cytochrome P450 isoforms. Isoforms not shown >20 μM.
The first aza-substituted compounds in the hexahydropyrrolo[3,2-f]quinoline series, 5a and 5b, were equipotent to the corresponding nonaza analogs 1d and 1e in both the GAL4 and hWB assays. This was also true for the hexahydropyrrolo[2,3-h]isoquinoline analogs 6a and 6b. However, the hexahydropyrrolo[2,3-h]quinoline analogs 7a and 7b were significantly less potent, losing 2- to 3-fold in GAL4 potency. The decrease in potency might be explained by consideration of our previously reported X-ray cocrystal structure of 1c in the ligand binding domain (LBD) of RORγt.11 Replacement of C-9 carbon with a nitrogen atom would place the ring nitrogen lone pair electrons in 7 in close proximity with the hydrophobic face of helix 3 and ∼3.5 Å away from the carbonyl oxygen of Cys320. In addition, repulsion between the pyridine ring nitrogen lone pair of 7 and one of the oxygen atoms of the sulfonyl group (distance ∼3.0 Å in the bound conformation of compounds in the benzo-fused series) may raise the energy of this conformation and further disfavor binding. In contrast, the ring nitrogen atoms in 5 and 6 are not predicted to make any close contacts with residues of the LBD.
The in vitro screening results shown in Table 1 indicate additional differences between the series. The two hexahydropyrrolo[2,3-h]isoquinolines 6a and 6b show significantly reduced metabolic stability in mouse LM relative to the corresponding analogs 5 or 7 and to the carbon analogs 1, although no difference was seen in human LM. This was of particular importance because the mouse was the preclinical species for in vivo efficacy evaluation. Both 6b and 7b showed reduced membrane permeability in the apical to basal direction as measured in Caco-2 cells relative to either 1 or 5, with markedly increased efflux ratios. However, 5a showed significantly improved A-B permeability relative to its carbon analog 1d. Of particular note is the significantly reduced serum protein binding (increased free fraction) for all three aza-substituted series relative to 1, especially the hexahydropyrrolo[2,3-h]quinoline compound 7b. Finally, slightly decreased inhibition of two recombinant cytochrome P450 isoforms was observed for analogs 5–7, relative to 1.
Based on these structure–activity relationships, the hexahydropyrrolo[3,2-f]quinoline series 5 emerged as the most interesting of the three aza-substituted series examined. Although 5a and 5b were approximately equipotent in the human whole blood assay compared to the nonaza analogs 1d and 1e, they showed marginal improvements in the Caco-2 and CYP inhibition assays while significantly improving the free fraction in human and mouse serum.
Having selected 5 as representing the favorable position for aza substitution, attention was turned to the subsequently discovered hexahydro-1H-cyclopenta[a]naphthalene series represented by 2 and 3. Although 3a was a very appealing compound,15 we speculated whether further improvements could be made by incorporation of the ring nitrogen to provide the corresponding hexahydro-cyclopentaquinoline core. In particular, we hoped to improve the hWB potency of the former series, while maintaining or improving the in vitro profile with respect to metabolic stability, bioavailability, and selectivity.
Three analogs of this new core, 8a–8c (Figure 2) were initially prepared and evaluated to allow a matched-pair comparison with the previously described series represented by 3a–3c.15 Results of in vitro screening are shown in Table 2. As was seen for the hexahydropyrrolo[3,2-f]quinoline series 5, introduction of a nitrogen atom at position X of the fused benzene ring resulted in a significant increase in the free fraction in serum and a generally modest decrease in CYP inhibition. However, there was no significant effect of this molecular edit on metabolic stability. Similarly, the EC50 values in the GAL4 and hWB assays were essentially unchanged from the already potent values observed with 3a–3c. While this was disappointing, the increases in membrane permeability and serum free fraction observed with 8a compared to 3a were viewed as encouraging.
Table 2. Comparison of Hexahydro-5H-cyclopenta[f]quinolines 8 and Hexahydro-1H-cyclopenta[a]naphthalenes 3a.
| cmpd | X | R | RORγt GAL4 EC50 (nM) | IL-17 hWB EC50 (nM) | LM h, m (% rem)b | Caco-2 Pc, nm/s (efflux)c | PB % free (h, m)d | rCYP 2C8, 2C9, 2C19, 3A4 IC50 (μM)e |
|---|---|---|---|---|---|---|---|---|
| 8a | N | A | 3.9 | 67 ± 13 | 100, 100 | 180 (0.5) | nd, 2.3 | 8.3, 11, >20, >20 |
| 8b | N | B | 6.0 ± 2.7 | 101 ± 45 | 100, 87 | 120 (0.5) | nd, 1.8 | 6.3, 8.5, 17, 9.1 |
| 8c | N | C | 8.4 ± 3.8 | 67 ± 15 | 100, 82 | 81 (2.2) | nd, 2.2 | 16, 9.4, 16, >20 |
| 8d | N | D | 12 ± 4.8 | 260 ± 120 | 97, 100 | nd | nd | nd |
| 8e | N | E | 2.5 ± 1.7 | 26 ± 13 | 89, 98 | 110 (1.2) | 2.2, 1.5 | 7.6, >20, >20, >20 |
| 3a | CH | A | 3.7 ± 2.3 | 50 ± 14 | 100, 92 | 106 (0.5) | 0.5, 0.6 | 12, 12, 19, 11 |
| 3b | CH | B | 2.5 ± 2.1 | 54 ± 20 | 97, 86 | 150 (0.5) | 0.2, 0.3 | 6.5, nd, 7.7, 5.8 |
| 3c | CH | C | 8.7 | 50 ± 25 | 100, 95 | 73 (1.4) | nd, 0.2 | 13, 5.5, >20, >20 |
| 3d | CH | D | 5.5 | 130 ± 32 | 89, 83 | 76 (0.9) | nd, 0.4 | 5.0, 9.2, 15, 0.25 |
| 3e | CH | E | 2.5 ± 0.80 | 39 ± 23 | 98, 84 | 64 (0.5) | nd, 0.4 | 3.2, 12, 15, >20 |
See the Supporting Information for assay protocols. Values are the means of two or more experiments. SDs are provided for means of three or more experiments. nd = not determined.
Percent remaining after 10 min of incubation with human or mouse LM.
Pc in nm/s in the apical to basal direction in Caco-2 cells. Values in parentheses are the efflux ratios (BA/AB).
Protein binding, % free in human or mouse serum.
Inhibition of recombinant cytochrome P450 isoforms. Isoforms not shown are >20 μM.
Consideration of the X-ray crystal structure of 3a in the LBD of RORγt (Figure 3a)15 indicated the potential for additional binding interactions in the right-hand side of the LBD pocket, either through lipophilic interactions with the side chains of Leu287 and Gln286 or by engaging polar interactions with the guanidine moieties of Arg367 or Arg364. Substitution of one of the methyl groups of the dimethyl carbinol of 3a was therefore contemplated. In the event, the methylsulfonyl-substituted analog of 8a was prepared and resolved into the diastereomers 8d and 8e (Table 2). Gratifyingly, the (S)-isomer 8e showed a significant reduction in the EC50 value in the hWB assay (26 ± 13 nM compared to 67 ± 13 nM for 8a). However, the (R)-isomer 8d was less potent in both the GAL4 and hWB assays. In contrast, the corresponding carbocyclic analog 3e showed similar potency to 3a in the GAL4 and hWB assays. Interestingly, 3e showed significant ion channel inhibitory activity in a hERG patch clamp assay compared to 8e (IC50 = 2.5 μM and 16 μM, respectively). Also of interest was the increased potency associated with 8e as an inhibitor of CD3/CD28 stimulated IL-17A release in mouse whole blood (mWB), the species used for preclinical efficacy studies. In this assay, 8e was significantly more potent (EC50 = 68 ± 36 nM) than both 3a (EC50 = 470 ± 200 nM) and the first clinical candidate 1a (EC50 = 170 ± 48 nM).
Figure 3.
(A) Co-crystal structure of 3a with the LBD of RORγt (PDB ID: 7KQJ). (B) Co-crystal structure of 8e with the LBD of RORγt (PDB ID: 7LUK).
An X-ray co-crystal structure of 8e bound in the LBD of RORγt revealed that additional interactions with the receptor were indeed established (Figure 3B). The overall binding mode is very similar to that of 3a, with the amide NH forming an intramolecular hydrogen-bond to one of the phenylsulfonyl oxygen atoms. The tertiary carbinol is positioned to form an intermolecular hydrogen-bond either to the backbone carbonyl of Phe377, similar to that observed with 3a, or possibly to one of the methylsulfone oxygen atoms. Due to the altered positioning of the carbinol, water-mediated hydrogen-bonding to the backbone NH of Glu379 is also possible, which was not observed for 3a. The sulfone methyl is positioned to form favorable hydrophobic interactions with the side chains of Leu287 and, possibly, Gln286, as predicted, while the remaining methylsulfone oxygen projects toward solvent.
Additional in vitro evaluation of 8e indicated good specificity and selectivity for RORγt. The compound showed no significant inhibition of the related nuclear hormone receptors RORα, RORβ, PXR, LXRα, and LXRβ (EC50 > 10 μM). As noted in Table 2, the only detectable CYP inhibition was weak activity against the 2C8 isoform (IC50 7.6 μM). Inhibition in the HepG2 cell proliferation assay was weak (IC50 = 54 μM), suggesting a low likelihood for drug-induced liver toxicity. Ion channel inhibition in the hERG patch clamp assay was likewise weak (IC50, 16 μM), suggestive of a low potential for cardiac liabilities.
Since the in vitro metabolic stability was uniformly good (t1/2 > 120 min) across species, the oral pharmacokinetic properties of 8e were evaluated in rodents, dogs, and cynomolgus monkeys, with the results summarized in Table 3. Exposure after oral dosing was excellent in all species, with low clearances, long half-life values, low peak to trough ratios, and good oral bioavailabilities.
Table 3. Pharmacokinetics of Compound 8e.
| Species | Mousea | Ratb | Dog | Cyno |
|---|---|---|---|---|
| IV dose, mg/kgc | 2 | 2 | 1 | 1 |
| CL, mL/min/kg | 2.1 | 1.9 ± 0.2 | 0.22 ± 0.02 | 2.8 ± 0.4 |
| Vss, L/kg | 2.2 | 2.5 ± 0.5 | 1.3 ± 0.4 | 2.7 ± 0.5 |
| t1/2, h | 11 | 13 ± 2.7 | 67 ± 12 | 12 ± 0.3 |
| AUCtot, μM·h | 22.7 | 26.3 ± 3.7 | 114 ± 8.8 | 9.0 ± 1.5 |
| PO dose, mg/kgc | 4 | 4 | 2 | 2 |
| tmax, h | 2 | 4 | 7 | 7 |
| Cmax, nM | 2800 | 1600 ± 700 | 1700 ± 100 | 600 ± 200 |
| Peak/trough | 5 | 2 | 1.4 | 3 |
| AUCtot, μM·h | 45.9 | 44.9 ± 4.2 | 163 ± 14 | 13.2 ± 4.1 |
| %F | 101 | 85 | 72 | 73 |
The excellent in vitro profile and good pharmacokinetic properties associated with 8e prompted evaluation of the compound in a mouse model of psoriasis: acanthosis induced by IL-23.21,22 In this model, skin inflammation was induced by injection of recombinant human IL-23 into the ear of C57BL/6 female mice every other day. A baseline measurement of ear thickness was made on day 0, and the thickness was measured every other day prior to injection with IL-23, with a final measurement on day 10. Compound 8e was administered orally twice daily at doses of 5, 10, or 20 mg/kg, with antihuman IL-23 adnectin delivered subcutaneously serving as a positive control and blank dosing vehicle as a negative control. The results shown in Figure 4 illustrate that a dose-dependent reduction in ear thickening was observed. The trough blood concentration on day 10 in mice receiving the highest dose exceeded the mWB IC90 by a factor of 4.9 and provided essentially the same degree of protection (77% inhibition of ear thickening) as the anti-IL-23 adnectin which served as the positive control (Figure 4 A, B). These data are also consistent with the histology score (Figure 4C) and the reduction in gene expression profiles for proinflammatory cytokines IL-17a, IL-6, and lipocalin-2 (LCN2), a proinflammatory mediator which is expressed in higher concentrations in psoriasis patients (Figure 4D). The drug was well tolerated, as indicated by the lack of significant weight loss at any of the doses.
Figure 4.
Oral efficacy of compound 8e in mouse acanthosis.
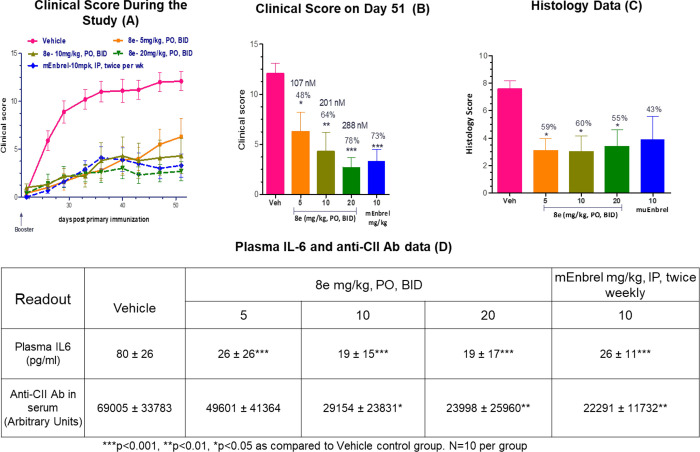
The observation that IL-17 is present in the synovial fluid of rheumatoid arthritis (RA) patients suggests that it may play a prominent role in the pathogenesis of RA. Moreover, double immunofluorescence studies have shown that RORγt colocalizes with both IL-17a and IL-17f, suggesting a key role for Th17 cells as a source of the local production of these cytokines.23 The efficacy and safety of the anti-IL-17 antibody secukinumab was recently evaluated in a Phase III trial where a dose of 150 mg resulted in improved symptoms and reduced disease activity in patients with active RA who had an inadequate response to TNF inhibitors.24 Because of these promising results, we studied 8e in mouse collagen-induced arthritis, a well-accepted preclinical model for rheumatoid arthritis.25,26 Male DBA/1 mice were primed with bovine type II collagen in adjuvant, with a booster given 21 days after the primary injection. Animals were administered 8e by oral gavage twice daily at doses of 5, 10, or 20 mg/kg. Disease activity was monitored and scored using standard criteria. The results shown in Figure 5 illustrate a dose-dependent reduction in the clinical score. At the highest dose tested, 8e provided essentially the same arthritic score reduction as a murine equivalent of the marketed TNF decoy receptor etanercept (mEnbrel) which served as the positive control (Figure 5A, B) and showed a trend toward reduction in gene expression profiles for the proinflammatory cytokine IL-6 and antibodies against type II collagen (anti-CII) (Figure 5D). The lack of dose response in the histology data (Figure 5C) compared to the clinical score (Figure 5B) may be due to the limitation of histological analysis to only hind paws, whereas clinical scoring considered all four paws. It is clear, however, that 8e was effective in reducing arthritis both clinically and at the joint tissue level.
Figure 5.
Oral efficacy of 8e in mouse collagen-induced arthritis.
The synthesis of compounds 5–8 generally followed routes previously reported,9,15,27 starting from commercially available materials, with the synthesis of 8e shown in Scheme 1. (Complete details for the preparation of this and all other compounds are provided in the Supporting Information.) 2-Hydroxy-7,8-dihydro-5(6H)-quinolinone (9) was protected as the 2,6-dichlorobenzyl ether. (The simple benzyl ether of 9 caused problems in the oxidation step (d) en route to the preparation of 11, giving the epoxysulfone instead of the vinylsulfone in a low-yielding reaction.) Acid-catalyzed conversion of the ketone to the thioketal, reported previously for the corresponding carbocyclic core,9 failed; however, conversion to the vinyl triflate 10, followed by palladium-catalyzed coupling with 4-fluorobenzenethiol, provided, after oxidation, the vinyl sulfone 11 in good overall yield. The previously reported 3-carbon annelation protocol14 was followed by conversion of the benzyl ether of 12 to the bromide 13 in 3 steps. Copper-catalyzed incorporation of the perfluoroisopropyl group was followed by saponification of the ester, providing 14. A Curtius rearrangement and trapping of the intermediate isocyanate as the trimethylsilylethyl carbamate followed by SFC resolution of the resulting diastereomeric mixture gave the desired carbamate 15 as a single, enantiomerically pure diastereomer. Deprotection and amide formation with enantiomerically pure 17b then provided 8e. The absolute configurations of both components used in the final coupling reaction were confirmed by the X-ray cocrystal structure discussed above (Figure 3B).
Scheme 1. Synthesis of Compound 8e.

Reagents and conditions: (a) 2,6-dichlorobenzyl bromide, Cs2CO3, MeCN, rt, 85%; (b) (CF3SO2)2NPh, KHMDS, −78 °C, 99%; (c) 4-fluorobenzenethiol, Pd2(dba)3, xantphos, i-PrNEt2, dioxane, 120 °C; (d) mCPBA, DCM, 0 °C to rt, 79–89% (2 steps); (e) ethyl 4-chlorobutanoate, LiHMDS, THF, −78 °C, 64%; (f) HCl, dioxane, DCE, 50 °C, 18 h; (g) (CF3SO2)O, pyridine, DCM; (h) LiBr, TsOH, DCM, 50 °C, 16 h, 72% (3 steps); (i) activated Cu, IC(CF3)2F, DMF, 120 °C, ∼100%; (j) LiOH, THF-EtOH-H2O, rt, ∼100%; (k) DPPA, Et3N, toluene, 0 °C to rt; 2-(trimethylsilyl)ethan-1-ol, 80 °C, 90 min, 98%; (m) SFC, (R,R)Whelk-O1 column, 31%; (n) HCl, dioxane, DCE, 50 °C, 3 h, 99%; (p) 17b, HATU, i-PrNEt2, DMF, rt, 72%.
In summary, the incorporation of a nitrogen atom into the fused benzene ring of potent, selective hexahydro-1H-benzo[e]indole-based inverse agonists of RORγt was explored. Aza substitution at two of the three available positions provided compounds which were equipotent with the all-carbon prototypes. The series based on the hexahydropyrrolo[3,2-f]quinoline 5 showed improved cell permeability, increased serum free fraction, and reduced CYP inhibition relative to the parent analogs 1. Incorporation of this structural modification into the hexahydro-1H-cyclopenta[a]naphthalene core represented by 3 provided similarly improved properties, although a targeted improvement in human whole blood potency was not seen. However, improved profiles were seen in a rationally designed sulfone analog 8e which maintained the desirable properties while showing improvement in whole blood potency compared to 3a and its analog 3e. Moreover, 8e demonstrated good pharmacokinetic profiles in several preclinical species and dose-dependent efficacy in a mouse acanthosis model of psoriasis, data which prompted evaluation in the collagen-induced arthritis model, where excellent efficacy, equivalent to that seen with a TNF decoy receptor, was observed.
Glossary
Abbreviations
- AUC
area under the curve
- CL
clearance
- Cmax
maximum concentration
- dba
dibenzylideneacetone
- DPPA
diphenylphosphoryl azide
- EC50
50% efficacious concentration
- F
bioavailability
- GAL4
GAL4-luciferase reporter assay
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HATU
hexafluorophosphate azabenzotriazole tetramethyl uronium
- HMDS
hexamethyldisilazide
- hWB
human whole blood IL-17A assay
- IL
interleukin
- LBD
ligand binding domain
- LM
liver microsomes
- LXR
liver X receptor
- mCPBA
m-chloroperoxy-benzoic acid
- mWB
mouse whole blood IL-17A assay
- PXR
pregnane X receptor
- rCyP
recombinant cytochrome P450
- ROR
retinoic acid related orphan receptor
- SFC
supercritical fluid chromatography
- Vss
volume of distribution
- xantphos
4,5-bis(diphenylphosphino)-9,9-dimethylxanthene
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.1c00112.
Experimental details of the preparation and characterization of all target compounds and details of the in vivo evaluation of 8e (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Cook D. N.; Kang H. S.; Jetten A. M. Retinoic acid-related orphan receptors (RORs): regulatory functions in immunity, development, circadian rhythm, and metabolism. Nucl. Receptor Res. 2015, 2, 2. 10.11131/2015/101185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I. I.; McKenzie B. S.; Zhou L.; Tadokoro C. E.; Lepelley A.; Lafaille J. J.; Cua D. J.; Littman D. R. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006, 126, 1121. 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Korn T.; Bettelli E.; Oukka M.; Kuchroo V. K. IL-17 and Th17 cells. Annu. Rev. Immunol. 2009, 27, 485. 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Lee J. S.; Cua D. J. The emerging landscape of RORγt biology. Immunity 2014, 40, 451. 10.1016/j.immuni.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Balato A.; Scala E.; Balato N.; Caiazzo G.; Di Caprio R. D.; Monfrecola G.; Raimondo A.; Lembo S.; Ayala F. Biologics that inhibit the TH17 pathway and related cytokines to treat inflammatory disorders. Expert Opin. Biol. Ther. 2017, 17, 1363. 10.1080/14712598.2017.1363884. [DOI] [PubMed] [Google Scholar]
- Dhar T. G. M.; Zhao Q.; Markby D. W. Targeting the nuclear hormone receptor RORγt for the treatment of autoimmune and inflammatory disorders. Annu. Rep. Med. Chem. 2013, 48, 169. 10.1016/B978-0-12-417150-3.00012-0. [DOI] [Google Scholar]
- Fauber B. P.; Magnuson S. Modulators of the nuclear receptor retinoic acid receptor-related orphan receptor-c (RORγ or RORc). J. Med. Chem. 2014, 57, 5871. 10.1021/jm401901d. [DOI] [PubMed] [Google Scholar]
- Pandya V. B.; Kumar S.; Sachchidanand; Sharma R.; Desai R. C. Combating autoimmune diseases with retinoic acid receptor-related orphan receptor-γ (RORγ or RORc) inhibitors: hits and misses. J. Med. Chem. 2018, 61, 10976. 10.1021/acs.jmedchem.8b00588. [DOI] [PubMed] [Google Scholar]
- Marcoux D.; Duan J. J.-W.; Shi Q.; Cherney R. J.; Srivastava A. S.; Cornelius L.; Batt D. G.; Liu Q.; Beaudoin-Bertrand M.; Weigelt C. A.; Khandelwal P.; Vishwakrishnan S.; Selvakumar K.; Karmakar A.; Gupta A. K.; Basha M.; Ramlingam S.; Manjunath N.; Vanteru S.; Karmakar S.; Maddala N.; Vetrichelvan M.; Gupta A.; Rampulla R. A.; Mathur A.; Yip S.; Li P.; Wu D.-R.; Khan J.; Ruzanov M.; Sack J. S.; Wang J.; Yarde M.; Cvijic M. E.; Li S.; Shuster D. J.; Borowski V.; Xie J. H.; McIntyre K. W.; Obermeier M. T.; Fura A.; Stefanski K.; Cornelius G.; Hynes J.; Tino J. A.; Macor J. E.; Salter-Cid L.; Denton R.; Zhao Q.; Carter P. H.; Dhar T. G. M. Rationally designed, conformationally constrained inverse agonists of RORγt — identification of a potent, selective series with biologic-like in vivo efficacy. J. Med. Chem. 2019, 62, 9931. 10.1021/acs.jmedchem.9b01369. [DOI] [PubMed] [Google Scholar]
- Cherney R. J.; Cornelius L. A. M.; Srivastava A.; Weigelt C. A.; Marcoux D.; Duan J. J.-W.; Shi Q.; Batt D. G.; Liu Q.; Yip S.; Wu D.-R.; Ruzanov M.; Sack J.; Khan J.; Wang J.; Yarde M.; Cvijic M. E.; Mathur A.; Li S.; Shuster D.; Khandelwal P.; Borowski V.; Xie J.; Obermeier M.; Fura A.; Stefanski K.; Cornelius G.; Tino J. A.; Macor J. E.; Salter-Cid L.; Denton R.; Zhao Q.; Carter P. H.; Dhar T. G. M. Discovery of BMS-986251: a clinically viable, potent, and selective RORγt inverse agonist. ACS Med. Chem. Lett. 2020, 11, 1221. 10.1021/acsmedchemlett.0c00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q.; Batt D. G.; Weigelt C. A.; Yip S.; Wu D.-R.; Ruzanov M.; Sack J. S.; Wang J.; Yarde M.; Li S.; Shuster D. J.; Xie J. H.; Sherry T.; Obermeier M. T.; Fura A.; Stefanski K.; Cornelius G.; Khandelwal P.; Tino J. A.; Macor J. E.; Salter-Cid L.; Denton R.; Zhao Q.; Dhar T. G. M. Novel tricyclic pyroglutamide derivatives as potent RORγt inverse agonists identified using a virtual screening approach. ACS Med. Chem. Lett. 2020, 11, 2510. 10.1021/acsmedchemlett.0c00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walles M.; Brown A. P.; Zimmerlin A.; End P. New perspectives on drug-induced liver injury risk assessment of acyl glucoronides. Chem. Res. Toxicol. 2020, 33, 1551. 10.1021/acs.chemrestox.0c00131. [DOI] [PubMed] [Google Scholar]
- Shipkova M.; Armstrong V. W.; Oellerich M.; Wieland E. Acyl glucuronide drug metabolites: toxicological and analytical implications. Ther. Drug Monit. 2003, 25, 1. 10.1097/00007691-200302000-00001. [DOI] [PubMed] [Google Scholar]
- Marcoux D.; Beaudoin Bertrand M.; Weigelt C. A.; Yip S.; Galella M.; Park H.; Wu D.-R.; Wang J.; Yarde M.; Cvijic M. E.; Li S.; Hynes J.; Tino J. A.; Zhao Q.; Dhar T. G. M. Annulation reaction enables the identification of an exocyclic amide tricyclic chemotype as retinoic acid receptor-related orphan receptor gamma (RORg/RORc) inverse agonists. Bioorg. Med. Chem. Lett. 2020, 30, 127466. 10.1016/j.bmcl.2020.127466. [DOI] [PubMed] [Google Scholar]
- Yang M. G.; Beaudoin Bertrand M.; Xiao Z.; Marcoux D.; Weigelt C. A.; Yip S.; Wu D.-R.; Ruzanov M.; Sack J. S.; Wang J.; Yarde M.; Li S.; Shuster D. J.; Xie J. H.; Sherry T.; Obermeier M. T.; Fura A.; Stefanski K.; Cornelius G.; Khandelwal P.; Karmakar A.; Basha M.; Babu V.; Gupta A. K.; Mathur A.; Salter-Cid L.; Denton R.; Zhao Q.; Dhar T. G. M. Tricyclic-carbocyclic RORγt inverse agonists – discovery of BMS-986313. J. Med. Chem. 2021, 64, 2714. 10.1021/acs.jmedchem.0c01992. [DOI] [PubMed] [Google Scholar]
- ChemAxon, https://docs.chemaxon.com/display/docs/LogP_and_logD_calculations.md.
- Hughes J. D.; Blagg J.; Price D. A.; Bailey S.; DeCrescenzo G. A.; Devraj R. V.; Ellsworth E.; Fobian Y. M.; Gibbs M. E.; Gilles R. W.; Greene N.; Huang E.; Krieger-Burke T.; Loesel J.; Wager T.; Whiteley L.; Zhang Y. Physicochemical drug properties associated with in vivo toxicological outcomes. Bioorg. Med. Chem. Lett. 2008, 18, 4872. 10.1016/j.bmcl.2008.07.071. [DOI] [PubMed] [Google Scholar]
- Waring M. J.; Arrowsmith J.; Leach A. R.; Leeson P. D.; Mandrell S.; Owen R. M.; Pairaudeau G.; Pennie W. D.; Pickett S. D.; Wang J.; Wallace O.; Weir A. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat. Rev. Drug Discovery 2015, 14, 475. 10.1038/nrd4609. [DOI] [PubMed] [Google Scholar]
- Meanwell N. A. Improving drug design: an update on recent applications of efficiency metrics, strategies for replacing problematic elements, and compounds in nontraditional drug space. Chem. Res. Toxicol. 2016, 29, 564. 10.1021/acs.chemrestox.6b00043. [DOI] [PubMed] [Google Scholar]
- ChemAxon, https://docs.chemaxon.com/display/docs/pka-calculation.md.
- Zheng Y.; Danilenko D. M.; Valdez P.; Kasman I.; Eastham-Anderson J.; Wu J.; Ouyang W. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 2007, 445, 648. 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- Chan J. R.; Blumenschein W.; Murphy E.; Diveu C.; Wiekowski M.; Abbondanzo S.; Lucian L.; Geissler R.; Brodie S.; Kimball A. B.; Gorman D. M.; Smith K.; de Wall Malefyt R.; Kastelein R. A.; McClanahan T. K.; Bowman E. P. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J. Exp. Med. 2006, 203, 2577. 10.1084/jem.20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert M.; Miossec P. IL-17 in rheumatoid arthritis and precision medicine: From synovitis expression to circulating bioactive levels. Front. Med. 2019, 5, 364. 10.3389/fmed.2018.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco F. J.; Moricke R.; Dokoupilova E.; Codding C.; Neak J.; Andersson M.; Rohrer S.; Richards H. Secukinumab in active rheumatoid arthritis. A phase III randomized, double-blind, active comparator- and placebo-controlled study. Arthritis Rheumatol. 2017, 69, 1144. 10.1002/art.40070. [DOI] [PubMed] [Google Scholar]
- Chang M. R.; Lyda B.; Kamenecka T. M.; Griffin P. R. Pharmacologic repression of retinoic acid receptor–related orphan nuclear receptor γ is therapeutic in the collagen-induced arthritis experimental model. Arthritis Rheumatol. 2014, 66, 579. 10.1002/art.38272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X.; Soroosh P.; Leon-Tabaldo A. D.; Luna-Roman R.; Sablad M.; Rozenkrants N.; Yu J.; Castro G.; Banie H.; Fung-Leung W.-P.; Santamaria-Babi L.; Schlueter T.; Albers M.; Leonard K.; Budelsky A.; Fourie A. Pharmacologic modulation of RORγt translates to efficacy in preclinical and translational models of psoriasis and inflammatory arthritis. Sci. Rep. 2016, 6, 37977. 10.1038/srep37977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q.; Greenwood N. S.; Meehan M. C.; Park H.; Galella M.; Sandhu B.; Khandelwal P.; Coombs J. R.; Gallagher W. P.; Guerrero C. A.; Hynes J.; Dhar T. G. M.; Gonzalez Bobes F.; Marcoux D. One-step diastereoselective pyrrolidine synthesis using a sulfinamide annulating reagent. Org. Lett. 2019, 21, 9198. 10.1021/acs.orglett.9b03560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.