Abstract

Aberrant gene activation driven by the histone acetyltransferases p300 and CREB binding protein (CBP) has been linked to several diseases, including cancers. Because of this, many efforts have been aimed toward the targeting of the closely related paralogues, p300 and CBP, but these endeavors have been exclusively directed toward noncovalent inhibitors. X-ray crystallography of A-485 revealed that both p300 and CBP possess a cysteine (C1450) near the active site, thus rendering covalent inhibition an attractive chemical approach. Herein we report the development of compound 2, an acrylamide-based inhibitor of p300/CBP that forms a covalent adduct with C1450. We demonstrated using mass spectrometry that compound 2 selectively targets C1450, and we also validated covalent binding using kinetics experiments and cellular washout studies. The discovery of covalent inhibitor 2 gives us a unique tool for the study of p300/CBP biology.
Keywords: p300, CBP, H3K27Ac, histone acetyltransferase, prostate cancer, covalent inhibitor
The two histone acetyltransferase (HAT) paralogues, p300 and CREB-binding protein (CREBBP or CBP), drive active gene expression and have been implicated in a variety of cellular processes involving cell-cycle regulation, differentiation and proliferation.1,2 Because deregulated gene expression is a hallmark of cancers, the biology of p300/CBP has attracted considerable attention in the past decades, and many reports suggest that p300/CBP inhibition could be therapeutically advantageous.3 As a result, many cancer drug discovery programs have been aimed at the discovery of catalytic inhibitors of p300/CBP.4,5 Indeed, the development of potent and selective dual inhibitors of p300/CBP with antitumor activity in animal models has recently been reported, thus generating considerable interest from the cancer research community.6−9
Covalent inhibitors are usually small molecules with an extended duration of interaction between the drug and its target, leading to the target’s inactivation. The prolonged target affinity that this modality confers can be of great benefit to patients because less than optimal pharmacokinetic properties are sometimes redressed because the pharmacodynamic properties of covalent inhibitors could extend beyond any measurable plasma concentration. As a result, the study and use of covalent inhibitors as drugs has had a long history in drug discovery, and today, more than 40 covalent drugs have entered the U.S. market.10 However, the covalent nature of these drugs has often been determined ex post facto rather than by careful design of the covalent mode of action, but over the past two decades, significant advancements have been made in the rational design of covalent drugs mainly in the fields of kinases and proteases.11−14 Herein we describe the first report of a successful structure-based drug design campaign that covalently targets a histone acetyltransferase that is p300/CBP.
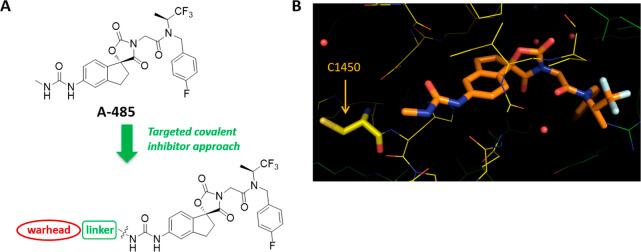
In a previous account, we described A-485, which is a potent (mid-nanomolar) and selective inhibitor of p300/CBP that emerged from an optimization campaign that began with a micromolar virtual ligand screening (VLS) hit.7,8 Subsequently, we established the binding mode and the specific interactions of A-485 through the generation of a high-resolution cocrystal structure (1.95 Å, PDB ID: 5KJ2) of the inhibitor bound to p300/CBP’s active site.9 The cocrystal structure obtained for A-485 also revealed that the cysteine 1450 (C1450) of p300 is favorably located within 7.27 Å, as measured by the distance between the methyl from the urea motif of the ligand and the sulfur atom of the cysteine (Figure 1). We noted that the urea moiety was solvent-exposed. suggesting that there should be no steric hindrance to reach the thiol group. Taken together, we believed that a potent covalent inhibitor of p300/CBP may potentially be generated through the design of linker warheads (i.e., an electrophilic functional group) appended to the urea moiety.
Figure 1.
Design of a covalent inhibitor targeting cysteine 1450 of p300/CBP. (A) Chemical structure of A-485 with the template for covalent inhibitor design. (B) X-ray of compound A-485 bound to the active site of p300/CBP with the cysteine residue displayed by a thick line. The distance between the sulfur and the carbon of the methyl group is 7.27 Å. PDB ID: 5KJ2.
Aided by molecular modeling, covalent inhibitors were designed and synthesized with the aim of assessing both the electrophilic warhead and the linker spacing group. An acrylamide warhead coupled to a phenyl spacer was used as the initial template for the identification of covalent inhibitors. It was found that substitution of the phenyl at the meta position placed the electrophile at the optimal locality. This led to the discovery of acrylamide 2, which displayed potent inhibition of the p300 HAT domain (IC50 = 166 nM) and showed significantly better inhibition of histone substrate acetylation in cells compared with lead compound A-485, as indicated by a decrease in the levels of H3K27Ac of PC-3 cells (EC50 = 37 nM; N = 3 in a 3 h H3K27Ac assay). In addition, compound 2 was evaluated for its antiproliferative effect against androgen receptor (AR)-positive LnCaP-FGC cells and showed potent activity (EC50 = 87 nM; N = 2 in a 5 day cell and gene therapy (CGT) assay) while displaying significantly less activity in the AR-negative cell line DU-145 (EC50 = 1.37 μM; N = 4 in a 5 day CTG assay), an expected outcome resulting from p300 inhibition. Moreover, compound 2 maintained selectivity in both kinome and CEREP panels. (See Figures S4 and S5.) Following these results, additional structure–activity relationship (SAR) studies were conducted, and we saw that flexible linker variations such as a saturated cyclic analog or an alkyl chain variant (5 and 7, Table 1) were tolerated but did not afford substantial potency gains in terms of cellular activity. Switching the warhead from acrylamide to the more reactive sulfonamide or sulfone led to a decrease in potency, and attempts to optimize the position of the sulfonamide warhead did not improve inhibition of p300 HAT domain activity (3, 4, and 8, Table 1). On the basis of these results, we chose compound 2 for further functional characterization.
Table 1. Comparative Profile of A-485 and Covalent Analogs.
Mass spectrometry was used to confirm that compound 2 forms a covalent bond with C1450 of p300. A truncated p300 HAT protein containing the C1450 site was incubated with compound 2 for 30 min and then quenched with acetonitrile that contained 0.1% formic acid. It was next subjected to intact mass spectrometry analysis in which the protein was observed as two mass peaks at 40 329 and 40 999 Da. (See Figure S1.)15,16 The increased molecular weight was equal to the molecular weight of compound 2 (668 g/mol), and peak integration showed 51% covalent adduct formation. In contrast, mass spectrometry of the protein fragment with a cysteine → alanine or cysteine → serine mutation failed to display the same mass addition when we incubated with compound 2, thus suggesting that the covalent adduct between p300 and compound 2 is formed specifically at C1450. Moreover, we tested compound 2 for unspecific cysteine reactivity using the protein-based method ALARM NMR and found compound 2 to be nonreactive toward unspecific cysteines.17,18
Genetic studies have shown that p300/CBP preferentially catalyzes the acetylation of H3K27 in prostate adenocarcinoma PC-3 cells.2 Our previous studies have shown that treatment with a p300/CBP inhibitor such as A-485 results in the dose-dependent inhibition of H3K27Ac in PC-3 cells (EC50 = 103 nM).9 To demonstrate the covalent mode of binding in cells, we did a comparative analysis of the reversibility of the inhibition of H3K27Ac with compound A-485 and compound 2. PC-3 cells were incubated with A-485 or with our covalent compound for 24 h. The cells were washed and then treated with a new medium that was free of compound before being further incubated. (See Table 2.) Inhibition of H3K27Ac was then determined at the indicated time points (ranging from 1 to 48 h) postwashout. As expected, inhibition of H3K27Ac was completely lost 1 h following washout of the cells treated with A-485 (from EC50 = 0.227 μM to EC50 ≥ 10 μM). In contrast, cells treated with compound 2 sustained inhibition of H3K27Ac for much longer times following washout, with close to maximal inhibition of H327Ac (as measured by EC50) being sustained for at least 4 h following washout. (See Figures S2 and S3 for dose–response curves.) The sustained inhibitory effect is most likely due to the covalent attachment of compound 2 onto p300/CBP.
Table 2. Examination of the Reversibility of H3K27Ac Inhibition upon Treatment with A-485 or Compound 2a.
|
A-485 |
compound 2 |
|||
|---|---|---|---|---|
| washout (WO) | EC50 (μM) | fold shift EC50 | EC50 (μM) | fold shift EC50 |
| 0 h | 0.227 | N/A | 0.011 | N/A |
| 1 h WO | >10 | >44 | 0.015 | 1.3 |
| 2 h WO | >10 | >44 | 0.017 | 1.5 |
| 4 h WO | >10 | >44 | 0.024 | 2.1 |
| 8 h WO | >10 | >44 | 0.129 | 11.3 |
| 24 h WO | >10 | >44 | 0.544 | 47.8 |
| 48 h WO | >10 | >44 | 0.656 | 57.7 |
| 48 h no WO | 0.253 | 1.11 | 0.021 | 1.8 |
Inhibition of H3K27Ac is rapidly reversible in the case of A-485 but is significantly less reversible in the case of compound 2, pointing to the prolonged inhibition of the target by the drug. See Figures S2 and S3 for dose–response curves.
Real-time binding measurements by surface plasmon resonance (SPR) were used to characterize A-485 and compound 2 binding to p300. In contrast with the noncovalent inhibitor A-485, which showed 14 nM KD and a moderately slow off rate (kd) of 1.3 × 10–3 s–1, compound 2 showed an apparent slower and biphasic off rate that plateaus (the initially slow dissociation becomes much slower) on long time scales, as shown in Figure 2. The biphasic dissociation observed for compound 2 demonstrates an increase in the duration of the interaction between the drug and the target, thus pointing to covalent bond formation. In addition, the resonance unit (RU) level remaining suggests that ∼50% of the p300 remains bound to compound 2, which is consistent with the amount of covalent adduct formation observed by MS (Figure S1b) and is indicative of a slow rate of covalent association of the protein–ligand binary complex.19
Figure 2.
Single-cycle p300 kinetics by SPR of A-485 and compound 2. (A) Binding kinetics of reversible inhibitor A-485. (B) Sensorgram showing the binding and dissociation of the covalent inhibitor (compound 2). The dissociation plateaus (becomes much slower) on long time scales, consistent with covalent inhibition. Note that each step is an injection of higher concentration from low to high. The experiment has been performed stepwise in five steps with brief dissociation times between each step and one long dissociation time after the fifth injection.
The preparation of compound 2 is depicted in Scheme 1. The commercially available compound 9 was converted to amide 11 after treatment with acryloyl chloride. Following Boc protecting group removal under standard conditions, the obtained compound underwent urea formation with the previously described aniline 12(7) using triphosgene to yield compound 2.
Scheme 1. Synthesis of Compound 2.
Reagents and conditions: (a) DIPEA, CH2Cl2, 0 °C → rt, 71%; (b) TFA, CH2Cl2; (c) compound 12, triphosgene, triethylamine, rt, 16 h.
In summary, the work we outlined constitutes the first example of an inhibitor that covalently binds to p300/CBP. We have described the use of a structure-guided rational design strategy to introduce an acrylamide warhead to covalently target a cysteine residue. Using mass spectrometry analysis, we validated that the obtained acrylamide forms a covalent adduct with C1450. We also demonstrated that the inhibition of p300/CBP in cells is sustainable after washout, whereas the reversible inhibitor completely loses activity following washout of the cells. In addition, kinetics studies showed a lack of dissociation consistent with a covalent adduct. Although the pharmacokinetics properties of our covalent inhibitor are less than optimal,20 we believe that the covalent nature of compound 2 makes it a unique compound tool for studying the biology of the histone acetyltransferase p300/CBP.
Glossary
Abbreviations
- HAT
histone acetyl transferase
- CBP
CREB-binding protein
- VLS
virtual ligand screening
- ALARM NMR
a La assay to detect reactive molecules by nuclear magnetic resonance
- SPR
surface plasmon resonance
- Boc
butyloxycarbonyl
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.0c00654.
Molecular modeling protocols, biological assays conditions and protocols, conditions and procedures for mass spectrometry studies and kinetics experiments, kinome and CEREP selectivity heatmaps, synthetic procedures, analytical data for all compounds, and full characterization data for compound 2 (PDF)
A.M., C.L., E.D., D.B.R., L.M.L., K.D.B., V.M., B.S., M.A., M.J.P., C.C.S., S.R., A.L., and M.R.M. are employees of AbbVie at the time of the study. W.J.M. and D.M. were employees of AbbVie at the time of the study. The design, study conduct, and financial support for this research were provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the publication.
The authors declare no competing financial interest.
Supplementary Material
References
- Wang L.; Tang Y.; Cole P. A.; Marmorstein R. Structure and chemistry of the p300/CBP and Rtt109 histone acetyltransferases: implications for histone acetyltransferase evolution and function. Curr. Opin. Struct. Biol. 2008, 18 (6), 741–747. 10.1016/j.sbi.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q.; Yu L. R.; Wang L.; et al. Distinct roles of GCN5/PCAF-mediated H3K9ac and CBP/p300-mediated H3K18/27ac in nuclear receptor transactivation. EMBO J. 2011, 30 (2), 249–262. 10.1038/emboj.2010.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrowsmith C. H.; Bountra C.; Fish P. V.; Lee K.; Schapira M. Epigenetic protein families: a new frontier for drug discovery. Nat. Rev. Drug Discovery 2012, 11 (5), 384–400. 10.1038/nrd3674. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Zhang R.; Li Z.; et al. Discovery of Highly Potent, Selective, and Orally Efficacious p300/CBP Histone Acetyltransferases Inhibitors. J. Med. Chem. 2020, 63 (3), 1337–1360. 10.1021/acs.jmedchem.9b01721. [DOI] [PubMed] [Google Scholar]
- Wei J.; Yang Y.; Lu M.; et al. Recent Advances in the Discovery of HIF-1α-p300/CBP Inhibitors as Anti-Cancer Agents. Mini-Rev. Med. Chem. 2018, 18 (4), 296–309. 10.2174/1389557516666160630124938. [DOI] [PubMed] [Google Scholar]
- Wu F.; Hua Y.; Kaochar S.; et al. Discovery, Structure-Activity Relationship, and Biological Activity of Histone-Competitive Inhibitors of Histone Acetyltransferases P300/CBP. J. Med. Chem. 2020, 63 (9), 4716–4731. 10.1021/acs.jmedchem.9b02164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelides M. R.; Kluge A.; Patane M.; et al. Discovery of Spiro Oxazolidinediones as Selective, Orally Bioavailable Inhibitors of p300/CBP Histone Acetyltransferases. ACS Med. Chem. Lett. 2018, 9 (1), 28–33. 10.1021/acsmedchemlett.7b00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelides M.; Hansen T.; Dai Y.; Zhu G.; Frey R.; Gong J.; Penning T.; Curtin M.; McClellan W.; Clark R.; Torrent M.; Mastracchio A.; Kesicki E. A.; Kluge A. F.; Patane M. A.; Van Drie J. H. Jr.; Ji Z.; Lai C.; Wang L.. Spirocyclic Compounds as HAT Inhibitors and Their Preparation. WO2016044770A1, 2016-03-24.
- Lasko L. M.; Jakob C. G.; Edalji R. P.; et al. Discovery of a selective catalytic p300/CBP inhibitor that targets lineage-specific tumours [published correction appears in Nature. 2018 May 16]. Nature 2017, 550 (7674), 128–132. 10.1038/nature24028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer R. A. Covalent inhibitors in drug discovery: from accidental discoveries to avoided liabilities and designed therapies. Drug Discovery Today 2015, 20 (9), 1061–1073. 10.1016/j.drudis.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Gehringer M.; Laufer S. A. Emerging and Re-Emerging Warheads for Targeted Covalent Inhibitors: Applications in Medicinal Chemistry and Chemical Biology. J. Med. Chem. 2019, 62 (12), 5673–5724. 10.1021/acs.jmedchem.8b01153. [DOI] [PubMed] [Google Scholar]
- Bachovchin D. A.; Cravatt B. F. The pharmacological landscape and therapeutic potential of serine hydrolases. Nat. Rev. Drug Discovery 2012, 11 (1), 52–68. 10.1038/nrd3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J.; Petter R. C.; Kluge A. F. Targeted covalent drugs of the kinase family. Curr. Opin. Chem. Biol. 2010, 14 (4), 475–480. 10.1016/j.cbpa.2010.06.168. [DOI] [PubMed] [Google Scholar]
- Powers J. C.; Asgian J. L.; Ekici O. D.; James K. E. Irreversible inhibitors of serine, cysteine, and threonine proteases. Chem. Rev. 2002, 102 (12), 4639–4750. 10.1021/cr010182v. [DOI] [PubMed] [Google Scholar]
- 10 μM concentration of both compound 2 and p300 was used.
- Please note that the mutated cysteine is cysteine 165 in the abbreviated form being expressed, and this corresponds to cysteine 1450 in the full sequence according to ProteinWeb.
- Huth J. R.; Mendoza R.; Olejniczak E. T.; Johnson R. W.; Cothron D. A.; Liu Y.; Lerner C. G.; Chen J.; Hajduk P. J. ALARM NMR: a rapid and robust experimental method to detect reactive false positives in biochemical screens. J. Am. Chem. Soc. 2005, 127 (1), 217–24. 10.1021/ja0455547. [DOI] [PubMed] [Google Scholar]
- Dahlin J. L.; Cuellar M.; Singh G.; Nelson K. M.; Strasser J.; Rappe T.; Xia Y.; Veglia G.; Walters M. A. ALARM NMR for HTS triage and chemical probe validation. Curr. Protoc Chem. Biol. 2018, 10 (1), 91–117. 10.1002/cpch.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The SPR and mass spectrometry experiments suggest a two-stage dissociation state whereby the ligand binds to the active site of the protein (stage 1) and then a covalent adduct is formed (stage 2). It is our belief that a longer incubation time during both the SPR and mass spectra experiments would result in higher covalent adduct formation, but we did not explore this further. The ∼50% dissociation that we observed thus reflects an insufficient time of covalent bond formation.
- Mouse PK: IV (1 mg/kg) CLp = 0.41 L/h/kg; t1/2 = 1.77 h; F = 1% (1 mg/kg (PO)).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.