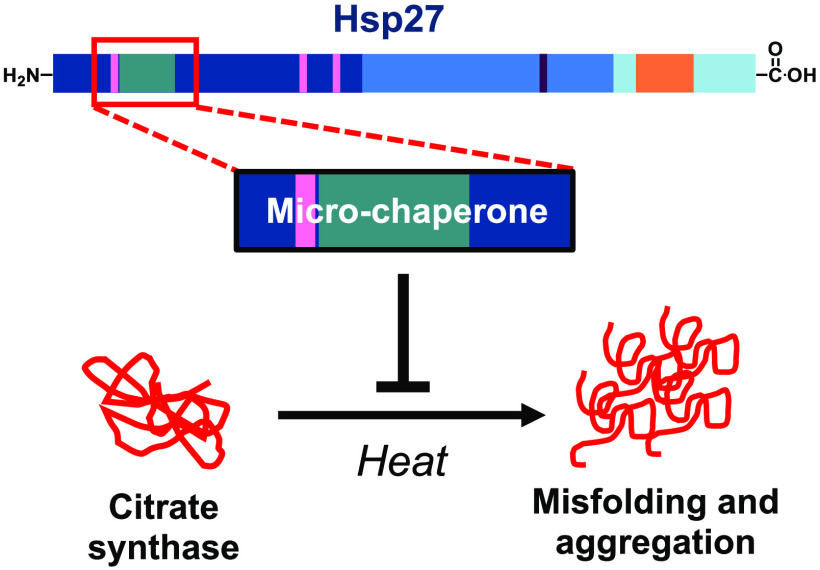
Abstract
We report the first small molecule peptides based on the N-terminal sequence of heat shock protein 27 (Hsp27, gene HSPB1) that demonstrates chaperone-like activity. The peptide, comprising the SWDPF sequence located at Hsp27’s amino (N)-terminal domain, directly regulates protein aggregation events, maintaining the disaggregated state of the model protein, citrate synthase. While traditional inhibitors of protein aggregation act via regulation of a protein that facilitates aggregation or disaggregation, our molecules are the first small peptides between 5 and 8 amino acids in length that are based on the N-terminus of Hsp27 and directly control protein aggregation. The presented strategy showcases a new approach for developing small peptides that control protein aggregation in proteins with high aggregate levels, making them a useful approach in developing new drugs.
Keywords: Hsp27, heat shock proteins, protein aggregation, citrate synthase, HSPB1
Many diseases result from an imbalance in proteostasis.1,2 Understanding how and why drugs that target protein aggregates associated with these diseases have failed is an ongoing challenge facing medicine.3 Small heat shock proteins (sHsps) function as molecular chaperones, which protect cellular proteins from aggregation or rescue them when they are misfolded.4−7 sHsps bind and hold onto misfolded substrate proteins until the protein folding machinery can refold them or transfer these misfolded proteins for degradation.8 Heat shock protein 27 (Hsp27) is the most abundantly expressed sHsp9 and is upregulated during the aging processes10 and cancer.11 Mutations in Hsp27 have also been linked to Charcot–Marie–Tooth (CMT) disease.12
Hsp27 exists as an equilibrium between large oligomers (sizes of 800 kDa, 30-mers) and smaller dissociated species, with the average size of the oligomers being 590 kDa (∼24-mers).13 The dissociated dimer of Hsp27 is thought to be responsible for its chaperone activity by interacting with aggregation-prone or misfolded proteins, subsequently transferring them to the proteasome for degradation or to other Hsps via BAG3 for refolding (Figure 1a).13−15 Defects resulting in inactivity of the Hsp27 chaperone protein are linked with many neuronal diseases.2,6,16 Moreover, controlling the oligomeric state of Hsp27, i.e., by driving the formation of dimers or large oligomers (Figure 1a), may represent a mechanism of targeting Hsp27 activity in the disease context. Successful phase I clinical trials used antisense oligonucleotides to manipulate the level of Hsp27 as a therapeutic approach for the treatment of castrate-resistant prostate cancer.17 This work demonstrates that “switching off” Hsp27 activity is a viable therapeutic approach in these diseases.17
Figure 1.
Overview of Hsp27 structure and activity. (a) There is evidence that the dissociated forms of Hsp27 (dimers) are the chaperone active species and that the large oligomeric forms are inactive. Thus, a potential mechanism to activate Hsp27 in the context of disease is to develop a molecule that dissociates the oligomers or a mini-chaperone that can mimic Hsp27 activity. (b) Protein structure of Hsp27 with key regions containing residues critical for Hsp27 function as discussed.
In contrast, there are no therapeutic strategies that successfully mimic Hsp27 activity and facilitate client protein disaggregation. Peptides ranging from 11 to 27 amino acids and averaging 19 (∼2300 MW) have been reported as “mini-chaperones”.18−22 These relatively large peptides have chaperone-like activity when used at molar ratios of ∼10:1 (peptide/client protein) against the heat-induced amorphous aggregation of citrate synthase (CS) or alcohol dehydrogenase (ADH). The most effective peptides ranged from 19 to 27 amino acids and inhibited the aggregation of these two proteins by 50–90%. Each of these peptides were based on the highly conserved α-crystallin domain (ACD) of the αA-crystallin (HSPB4) and αB-crystallin (HSPB5) proteins.23 Although elegant in their discovery, these peptides are large molecules and thus are not feasible for conversion into small molecule drug leads. To date, no small peptide chaperones have been designed based on the sequence of Hsp27. Identifying Hsp27 sequences that facilitate inhibition of or promote protein aggregation is key to unravelling how this protein functions and will provide guidance for designing new peptides that can serve as mini-chaperones (>8 amino acids) or smaller peptides (4–8 amino acids).
Herein, we report the chaperone activity of small peptides that were derived from sequences within Hsp27.24−26 We started by synthesizing four peptides labeled A, B, C, and D, which were 10 amino acids in length (Figure 1b). These peptides were based on sequences that occurred across the entire Hsp27 protein. Structure–activity relationships (SARs) of these peptides pinpointed residues in an N-terminal amino acid sequence of Hsp27 that are critical for inhibiting the aggregation of the client protein, CS. By using small sections of the most active 10 amino acid structures, we identified two active peptides, a five amino acid and eight amino acid structure within region A, that directly inhibit CS aggregation
Synthesis of four regions provided structures covering the key sequences across Hsp27, where region A contained residues 13–22. This region contains the WDPF domain, which is highly conserved across species and enables the formation of large oligomers.14 The WDPF region is critical for forming tight binding interactions between monomers, thereby allowing the formation of large oligomers, which are the inactive state of Hsp27. Ser15, 78, and 82 act as the phosphorylation sites of Hsp27, the purpose of which is to control the oligomeric state of the protein.7 The more serines phosphorylated, the smaller the oligomers. Thus, region A contained the WDPF region and Ser15. Region B, incorporating residues 76–85, contained the remaining serines: Ser78 and 82. Region C, incorporating residues 133–142, is located within the ACD of Hsp27. Previously reported mini-chaperones were derived from the ACD of other sHsps.23 In Hsp27, this region also includes the highly conserved Cys137 in Hsp27, a key residue that forms an intermolecular disulfide bridge that links monomers into active dimers.6 Region D incorporates residues 176–185, including the IXI/v region near the C-terminus of Hsp27. This IXI/v region interacts with the β4−β8 groove in the ACD of other Hsp27 subunits to form oligomers.13
Starting from the sequences of each 10 amino acid region, peptides A–D and a negative control, scrambled peptide (SP, 10 random amino acids), were synthesized via solid phase peptide synthesis following our standard protocol (Supporting Information Schemes S1 and S2).27−29 Using 2-chlorotrityl chloride resin and sequential amino acid couplings, we generated the desired resin-bound linear peptides, whereupon the peptides were cleaved, deprotected, and purified via reverse phase HPLC to afford pure compound (Figure 2a).
Figure 2.
(a) Structure of the peptides A (GPSWDPFRDW), B (ALSRQLSSGV), C (YISRCFTRKY), D (SNEITIPVTF) and scrambled peptide (SP; SFNTTPEVLL). Effects of peptides A–D and SP on CS aggregation, as monitored by light scattering at 360 nm. CS (150 nM) was heated at 43 °C for 1 h in the presence of (b) 50 μM compound or (c) increasing concentrations of peptide A. Aggregation of CS with 1% DMSO was set to 100%, and Hsp27 (600 nM) reflects the impact of the chaperone on CS on the day that the experiments were run. All data and error bars represent mean ± SEM for at least three independent experiments.
The functional activity of the peptides A–D and SP were evaluated using a CS aggregation assay. CS is a commonly used model protein, as it is readily misfolded at high temperatures and subsequently aggregates.30,31 The CS assay involves heating the peptides to 43 °C in buffer whereupon the CS is added as soon as the temperature has equilibrated. The aggregation of CS is monitored via the change in light scattering over time. In the presence of Hsp27, thermal aggregation of CS is suppressed, leading to lower light scattering compared to the control in which no Hsp27 was added (Figure 2b). Each peptide, A–D and SP, was individually incubated with CS at 43 °C. Monitoring the aggregation of CS via light scattering enabled the identification of compounds that were capable of inhibiting CS aggregation, with peptide A being the most effective (Figure 2b) compared to peptides B–D or SP.
Treating CS with increasing concentrations of peptide A resulted in a concentration-dependent decrease in the amount of CS aggregation (Figure 2c), demonstrating that peptide A behaved as a micro-chaperone and inhibited CS aggregation. In contrast, the negative control SP had no effect on CS aggregation even at the highest concentration tested (200 μM, Supporting Information Figure S1). Thus, peptide A contains residues that impact CS, suggesting that this region of Hsp27 potentially interacts and inhibits CS aggregation.
As peptide A contains the highly conserved WDPF region in Hsp27, it was hypothesized that this region was responsible for the activity of A. In order to explore this, additional peptides containing this sequence were synthesized: Peptide 1, consisting only of the WDPF, and peptides 2 and 3, which were five amino acid structures that included residues on either side of the WDPF region (Figure 3a). These molecules were then assessed for their ability to inhibit CS aggregation. Peptide 2 (SWDPF) proved to be highly effective at suppressing CS aggregation (Figure 3b) and behaved in a concentration-dependent manner. Indeed, high concentrations of 2 almost completely suppressed CS aggregation, making it significantly more effective than peptide A despite having half the number of amino acids.
Figure 3.
(a) Structures of peptides 1–3. (b) Effects of 1–3 on the CS aggregation at 43 °C, as monitored by light scattering at 360 nm. All peptides were tested at 200 μM unless otherwise indicated. Aggregation of CS (150 nM) with 1% DMSO was set to 100% and Hsp27 (600 nM). All data and error bars represent mean ± SEM for at least three independent experiments.
In contrast, the WDPF region alone (1) was not effective at inhibiting CS aggregation, nor was the structurally similar peptide 3 (WDPFR). These data strongly support the hypothesis that both the WDPF region and the serine are important for inhibiting CS aggregation. Our hypothesis of why the smaller peptide 2 (5 amino acids) was more effective at inhibiting CS aggregation than the larger peptide A (10 amino acids) is that the side chains of the SWDPF region are more accessible in the shorter sequence (i.e., 2) than the longer peptide (A).
Peptide 2 and A appear to be potent against CS, where A exhibited no chaperone activity against aggregates formed by κ-casein (Supporting Information Figure S2), while 2 was relatively active mimicking Hsp27 behavior at 8-fold higher concentration (Figure S2c). The difference in activity between the compounds acting on CS versus κ-casein is likely due to the different types of protein aggregates formed, where CS forms amorphous aggregates and κ-casein forms amyloid fibrils. Hence, the lower activity observed against κ-casein aggregation is likely due to the different aggregates formed or the sequence of the peptides favoring binding to CS than κ-casein.
An alanine scan of 2 was performed to identify the residues critical for the chaperone-like activity of the peptide. In total, five compounds (4–8) were made (Figure 4a). None of the alanine substituted molecules were active compared to 2 (Figure 4b). These data, coupled with the lack of chaperone-like activity observed with peptide 1, indicate that the SWDPF is the minimum sequence required for the inhibition of CS aggregation.
Figure 4.
(a) Structure of peptides 4–8. (b) Effects of peptides 4–8. (c) Structure of peptides 9 and 10. (d) Effects of 9 and 10 on CS aggregation. (e) Structure of peptides 11–13. (f) Effects of peptides 11–13 on CS aggregation. All peptides were tested at 200 μM unless otherwise indicated. CS aggregation was monitored by light scattering at 360 nm. Aggregation of CS (150 nM) with 1% DMSO was set to 100%, and Hsp27 (600 nM) reflects the impact of the chaperone on CS on the day that the experiments were run. All data and error bars represent mean ± SEM for at least two independent experiments.
Under cellular stress, Hsp27 can be phosphorylated on Ser15, 78, and 82, a process that drives the dissociation of large oligomers into active dimers (Figure 1a).7 A mutant of Hsp27 that is often used to identify the impact of phosphorylation at these sites on Hsp27 structure and activity is Hsp27-3D. In this mutant, the three Ser15, 78, and 82 are substituted with Asp in order to mimic the negative charge that occurs as a result of phosphorylation. We assessed the ability of Hsp27-3D to inhibit the aggregation of CS and found that it was more effective at inhibiting CS aggregation than Hsp27, which is in agreement with previous studies (Supporting Information Figure S3).7 We synthesized the Ser-to-Asp mutated version of 2, peptide 9 (i.e., SWDPF to DWDPF), and found that it was not active in the CS aggregation assay (Figure 4d). Thus, similar to the case of compounds 1, 3, and 4 where the serine is not present, the peptide loses its chaperone-like activity, indicating that this serine in 2 must play a key role in suppressing the aggregation of CS. The increased activity of Hsp27-3D compared to Hsp27 is most likely due to the combination of all three Ser-to-Asp mutations and perhaps a structural change that is present in the mutant. These likely provide additional interactions with CS that are not present with Hsp27 alone or in compounds 2 or 9.
A significant concern with developing peptides for in vivo studies is their susceptibility to protease degradation. d-Amino acids are unrecognizable by proteases and as such would be resistant to degradation. Thus, we synthesized peptide 2 with d-amino acids (10). Evaluation of this peptide in the CS assay indicated that, unlike its enantiomer 2, peptide 10 does not display chaperone-like activity (Figure 4d). Thus, it is concluded that peptide 2 inhibits the aggregation of CS in a stereospecific manner.
Linear peptides form 3-D structures, and typically, the longer the peptide, the more robust the structure. In light of this, we created peptides 11–13, which included the five amino acid SWDPF sequence with extended sequences of six, seven, and eight amino acids (Figure 4e). Peptide 11 was inactive (Figure 4f), indicating that Arg inhibited the interaction between the peptide and CS, where it is likely that the positively charged Arg and the negatively charged Asp may prefer to interact with each other rather than CS.
While 12 had significant activity (Figure 4f), the most surprising result was that of 13, which was highly effective at inhibiting CS aggregation (Figure 4f). The activity of 13 was also shown to be concentration dependent (Supporting Information Figure S4). These data indicate that not only is the SWDPF region critical for the chaperone-like activity of these peptides, but also the additional amino acids in 13 likely offer additional interactions for binding to CS compared to 2. In summary, the most effective peptides were 2, 12, and 13 (Table 1).
Table 1. Summary of the Percent of CS Aggregation That Is Inhibited When Treated by Peptides A and 1–13a.
Asterisk (*) indicates d-amino acid.
An alternative method commonly used to evaluate CS aggregation involves chemical denaturation conditions. Using a method described by Garcia et al.,32 the activity of peptides 2, 12, and 13 was evaluated in a chemically denatured CS assay (Figure 5). In this assay, CS was denatured by guanidine chloride (Gdn-Cl) and dithiothreitol (DTT) at room temperature, whereupon a small aliquot was added to a refolding buffer. Similar to the thermal CS assays, the CS aggregates were monitored by light scattering, where the value of the 1% DMSO control after 1 h was set to 100% aggregation. Both chemical denaturation and heat denaturation gave similar results. Specifically, compound 13 was highly effective at inhibiting the aggregation of refolding CS and completely suppressed aggregation in the chemical denaturation assay. The consistency of these results between the chemical and thermal CS aggregation assays provides strong evidence that compound 13 is highly effective at inhibiting aggregation.
Figure 5.
Effect of Hsp27 and peptides 2, 12, and 13 on CS aggregation, as monitored by light scattering at 320 nm. Chemically denatured CS (150 nM) was monitored for 1 h in the presence of Hsp27 (600 nM) and peptides 2, 12, and 13 (200 μM). Aggregation of CS with 1% DMSO was set to 100%, and Hsp27 (600 nM) reflects the impact of the chaperone on CS on the day the experiments were run. All data and error bars represent mean ± SEM for at least two independent experiments.
In conclusion, the two most effective molecules, peptides 2 and 13, suppressed heat-induced CS aggregation to 46% and 13%, respectively, and chemically induced CS aggregation to 63% and 0%, respectively. This unique discovery highlights how small peptides are capable of inhibiting protein aggregation and suggests an approach that may be useful in regulating proteins involved in diseases.
We have established that these peptides are sequence specific, where the SWDPF region is responsible for the chaperone-like activity of the peptide, suggesting that this region plays a critical role in the interaction of Hsp27 with aggregation-prone CS. These findings are supported by data showing that the relative derivatives of peptide 2 including alanine mutants (4–8), serine-to-aspartic acid mutant (9), and the d-amino acid version (10) are inactive. Finally, our data indicates that although the core sequence of peptide 2, SWDPF, is critical for preventing aggregation, binding can be enhanced, by extending from five amino acids to eight amino acids (peptide 2 to 13 respectively). The enhancement of activity in 13 must be due to the sequence as well as the more developed 3-D structure of the peptide.
Thus, these peptides provide the first proof of principle that it is possible to develop small molecules with chaperone-like activity based on the amino terminus of Hsp27, whereas previous peptides labeled as “mini-chaperones” were based on the ACD region. To date, these are also the smallest protein-based peptides reported to disrupt aggregation, where previously reported mini-chaperones were significantly larger than the peptides reported in this study (∼19 amino acids versus 5–8 amino acids). By demonstrating that small peptides with a “druglike size” between five and eight amino acids can impact protein aggregation, we provide a viable strategy for future peptidomimetic structures to be developed. Development of these peptides for other clients of Hsp27 such as Tau and α-synuclein represent a new approach that may successfully lead to therapeutic structures that directly control protein aggregation in Alzheimer’s and Parkinson’s models, respectively.
Glossary
Abbreviations
- Abs
absorbance
- ACH
α-crystallin domain
- ADH
alcohol dehydrogenase
- Ala (A)
alanine
- Arg (R)
arginine
- BAG3
BAG family molecular chaperone regulator 3
- CS
citrate synthase
- Asp (D)
aspartic acid
- EDT
1,2-ethanedithiol
- Glu (E)
glutamic acid
- HSP
heat shock protein
- kDa
kilodalton
- NMR
nuclear magnetic resonance
- Phe (F)
phenylalanine
- Pro (P)
proline
- SAR
structure–activity relationship
- Ser (S)
serine
- sHsp
small heat shock protein
- SPPS
solid-phase peptide synthesis
- Trp (W)
tryptophan
- UV
ultraviolet
- Val (V)
valine
- GdnCl
guanidinium chloride
- DTT
dithiothreitol
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.0c00609.
Materials and methods, synthetic schemes, biological assay results, and κ-casein aggregation data; full characterization of each compound (peptides A–D, SP, and 1–13); LC/MS data, proton and 2-D NMR, showing our compounds have purities of ≥95% (PDF)
Author Contributions
The manuscript was written through contributions of all authors.
We thank the Australian Government and UNSW for support of J.K. and P.C.P. We thank UNSW for financial support for this work. We thank the Frederick Family Trust (HBF001) for partial funding of this project. We thank the staff of the Mark Wainwright Analytical Centre. The authors acknowledge the significant contributions by the UNSW Recombinant Products Facility for their assistance in the production and purification of proteins used in this study, and specifically Helene Lebhar and Christopher Marquis for their critical support of this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Balch W. E.; Morimoto R. I.; Dillin A.; Kelly J. W. Adapting proteostasis for disease intervention. Science 2008, 319, 916–919. 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- Chiti F.; Dobson C. M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006, 75, 333–366. 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- Mehta D.; Jackson R.; Paul G.; Shi J.; Sabbagh M. Why do trials for Alzheimerʼs disease drugs keep failing? A discontinued drug perspective for 2010–2015. Expert Opin. Invest. Drugs 2017, 26, 735–739. 10.1080/13543784.2017.1323868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia T. D.; Craig E. A. Four small Drosophila heat shock proteins are related to each other and to mammalian R-Crystallin. Proc. Natl. Acad. Sci. U. S. A. 1982, 79, 2360–2364. 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara H.; Inaguma Y.; Goto S.; Inagaki T.; Kato K. RB Crystallin and HSP28 are enhanced in the cerebral cortex of patients with Alzheimer’s disease. J. Neurol. Sci. 1993, 119, 203–208. 10.1016/0022-510X(93)90135-L. [DOI] [PubMed] [Google Scholar]
- Hochberg G. K. A.; Ecroyd H.; Liu C.; Cox D.; Cascio D.; Sawaya M. R.; Collier M. P.; Stroud J.; Carver J. A.; Baldwin A. J.; Robinson C. V.; Eisenberg D. S.; Benesch J. L. P.; Laganowsky A. The structured core domain of αB-Crystallin can prevent amyloid fibrillation and associated toxicity. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, E1562–E1570. 10.1073/pnas.1322673111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovcevski B.; Kelly M. A.; Rote A. P.; Berg T.; Gastall H. Y.; Benesch J. L. P.; Aquilina J. A.; Ecroyd H. Phosphomimics Destabilize Hsp27 Oligomeric Assemblies and Enhance Chaperone Activity. Chem. Biol. 2015, 22, 186–195. 10.1016/j.chembiol.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Haslbeck M.; Buchner J. Chaperone function of sHSPs. Prog. Mol. Subcell. Biol. 2002, 28, 37–59. 10.1007/978-3-642-56348-5_3. [DOI] [PubMed] [Google Scholar]
- Vos M. J.; Kanon B.; Kampinga H. H. HSPB7 is a SC35 speckle resident small heat shock protein. Biochim. Biophys. Acta, Mol. Cell Res. 2009, 1793, 1343–1353. 10.1016/j.bbamcr.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Weindruch R.; Prolla T. A.; Lee C.-K. Gene-expression profile of the ageing brain in mice. Nat. Genet. 2000, 25, 294–297. 10.1038/77046. [DOI] [PubMed] [Google Scholar]
- Ciocca D. R.; Calderwood S. K. Heat shock proteins in cancer: diagnostic, prognostic, predictive and treatment implications. Cell Stress Chaperones 2005, 10, 86–103. 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evgrafov O. V.; Mersiyanova I.; Irobi J.; Van Den Bosch L.; Dierick I.; Leung C. L.; Schagina O.; Verpoorten N.; Van Impe K.; Fedotov V.; et al. Mutant small heat-shock protein 27 causes axonal Charcot-Marie-Tooth disease and distal hereditary motor neuropathy. Nat. Genet. 2004, 36, 602–606. 10.1038/ng1354. [DOI] [PubMed] [Google Scholar]
- Rauch J. N.; Tse E.; Freilich R.; Mok S.-A.; Makley L. N.; Southworth D. R.; Gestwicki J. E. BAG3 is a modular, scaffolding protein that physically links heat shock protein 70 (Hsp70) to the small heat shock protiens. J. Mol. Biol. 2017, 429, 128–141. 10.1016/j.jmb.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald E. T.; Bortolus M.; Koteiche H. A.; McHaourab H. S. Sequence, structure, and dynamic determinants of Hsp27 (HspB1) equilibrium dissociation are encoded by the N-terminal domain. Biochemistry 2012, 51, 1257–1268. 10.1021/bi2017624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes D.; Napoli V.; Mazurkie A.; Stafford W. F.; Graceffa P. Phosphorylation dependence of hsp27 multimeric size and molecular chaperone function. J. Biol. Chem. 2009, 284, 18801–18807. 10.1074/jbc.M109.011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlden H.; Laura M.; Wavrant-De Vrieze F.; Blake J.; Wood N.; Reilly M. M. Mutations in the HSP27 (HSPB1) gene cause dominant, recessive, and sporadic distal HMN/CMT type 2.. Neurology 2008, 71, 1660–1668. 10.1212/01.wnl.0000319696.14225.67. [DOI] [PubMed] [Google Scholar]
- Chi K. N.; Yu E. Y.; Jacobs C.; Bazov J.; Kollmannsberger C.; Higano C. S.; Mukherjee S. D.; Gleave M. E.; Stewart P. S.; Hotte S. J. A phase I dose-escalation study of apatorsen (OGX-427), an antisense inhibitor targeting heat shock protein 27 (Hsp27), in patients with castration-resistant prostate cancer and other advanced cancers. Ann. Oncol 2016, 27, 1116–1122. 10.1093/annonc/mdw068. [DOI] [PubMed] [Google Scholar]
- Raju M.; Santhoshkumar P.; Sharma K. K. aA-Crystallin–Derived Mini-Chaperone Modulates Stability and Function of Cataract Causing aAG98R-Crystallin. PLoS One 2012, 7, e44077 10.1371/journal.pone.0044077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju M.; Santhoshkumar P.; Sharma K. K. Alpha-Crystallin-derived peptides as therapeutic chaperones. Biochim. Biophys. Acta, Gen. Subj. 2016, 1860, 246–251. 10.1016/j.bbagen.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh J. G.; Estrada M. R.; Clark J. I. Interactive domains for chaperone activity in the small heat shock protein, human alphaB Crystallin. Biochemistry 2005, 44, 14854–14869. 10.1021/bi0503910. [DOI] [PubMed] [Google Scholar]
- Raju M.; Santhoshkumar P.; Xie L.; Sharma K. K. Addition of αA-Crystallin Sequence 164–173 to a Mini-Chaperone DFVIFLDVKHFSPEDLT Alters the Conformation but Not the Chaperone-like Activity. Biochemistry 2014, 53, 2615–2623. 10.1021/bi4017268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya J.; Udupa E. G. P.; Wang J.; Sharma K. K. Mini-RB-Crystallin: A Functional Element of RB-Crystallin with Chaperone-like Activity. Biochemistry 2006, 45, 3069–3076. 10.1021/bi0518141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee P. R.; Pande A.; Shekhtman A.; Pande J. Molecular Mechanism of the Chaperone Function of Mini-α-Crystallin, a 19-Residue Peptide of Human α-Crystallin. Biochemistry 2015, 54, 505–515. 10.1021/bi5014479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehle S.; Vollmar B. S.; Bardiaux B.; Dove K. K.; Rajagopal P.; Gonen T.; Oschkinat H.; Klevit R. E. N-terminal domain of alphaB-Crystallin provides a conformational switch for multimerization and structural heterogeneity. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 6409–6414. 10.1073/pnas.1014656108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelj-Garolla B.; Mauk A. G. Roles of the N- and C-terminal sequences in Hsp27 self-association and chaperone activity. Protein Sci. 2012, 21, 122–133. 10.1002/pro.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslbeck M.; Ignatiou A.; Saibil H.; Helmich S.; Frenzl E.; Stromer T.; Buchner J. A domain in the N-terminal part of Hsp26 is essential for chaperone function and oligomerization. J. Mol. Biol. 2004, 343, 445–455. 10.1016/j.jmb.2004.08.048. [DOI] [PubMed] [Google Scholar]
- Buckton L. K.; Wahyudi H.; McAlpine S. R. The first report of direct inhibitors that target the C-terminal MEEVD region on heat shock protein 90. Chem. Commun. 2016, 52, 501–504. 10.1039/C5CC03245H. [DOI] [PubMed] [Google Scholar]
- Rahimi M. N.; Buckton L. K.; Zaiter S. S.; Kho J.; Chan V.; Guo A.; Konesan J.; Kwon S.; Lam L.; Lawler M. F.; Leong M.; Moldovan G.; Neale D.; Thornton G.; McAlpine S. R. Synthesis and structure activity relationships of inhibitors that target the C-terminal MEEVD region on heat shock protein 90. ACS Med. Chem. Lett. 2018, 9, 73–77. 10.1021/acsmedchemlett.7b00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaiter S. S.; Huo Y.; Tiew F. Y.; Gestwicki J. E.; McAlpine S. R. Regulating protein-protein interactions via de-novo small molecule design. J. Med. Chem. 2019, 62, 742–761. 10.1021/acs.jmedchem.8b01436. [DOI] [PubMed] [Google Scholar]
- Jakob U.; Gaestel M.; Engel K.; Buchner J. Small heat shock proteins are molecular chaperones. J. Biol. Chem. 1993, 268, 1517–1520. 10.1016/S0021-9258(18)53882-5. [DOI] [PubMed] [Google Scholar]
- Buchner J.; Grallert H.; Jakob U. Analysis of chaperone function using citrate synthase as nonnaitve substrate protein. Methods Enzymol. 1998, 290, 323–338. 10.1016/S0076-6879(98)90029-5. [DOI] [PubMed] [Google Scholar]
- Garcia V. M.; Rowlett V. W.; Margolin W.; Morano K. A. Semi-Automated Microplate Monitoring of Protein Polymerization and Aggregation. Anal. Biochem. 2016, 508, 9–11. 10.1016/j.ab.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.