Abstract

Borrowing hydrogen is a process that is used to diversify the synthetic utility of commodity alcohols. A catalyst first oxidizes an alcohol by removing hydrogen to form a reactive carbonyl compound. This intermediate can undergo a diverse range of subsequent transformations before the catalyst returns the “borrowed” hydrogen to liberate the product and regenerate the catalyst. In this way, alcohols may be used as alkylating agents whereby the sole byproduct of this one-pot reaction is water. In recent decades, significant advances have been made in this area, demonstrating many effective methods to access valuable products. This outlook highlights the diversity of metal and biocatalysts that are available for this approach, as well as the various transformations that can be performed, focusing on a selection of the most significant and recent advances. By succinctly describing and conveying the versatility of borrowing hydrogen chemistry, we anticipate its uptake will increase across a wider scientific audience, expanding opportunities for further development.
Short abstract
The borrowing hydrogen approach diversifies the synthetic utility of commodity alcohols. This outlook provides a perspective on the diversity of catalysts used and the various transformations enabled.
Introduction
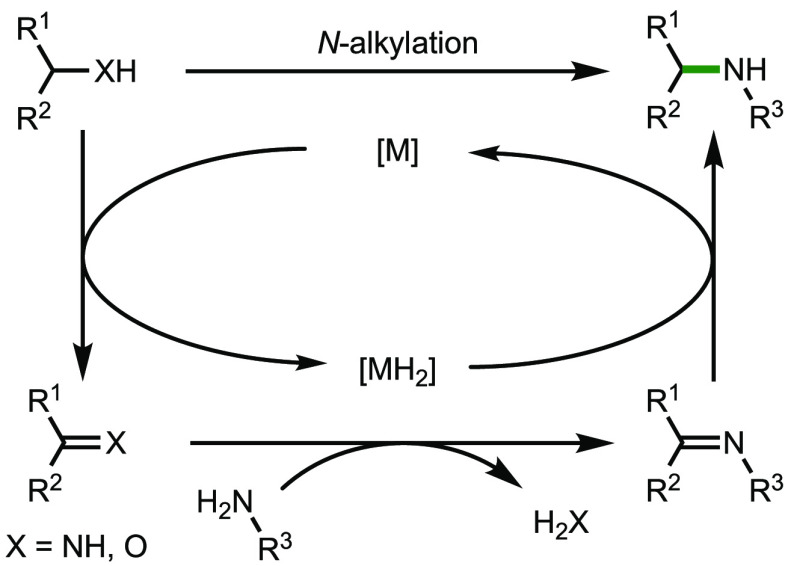
Hydrogenation is a ubiquitous transformation in chemistry with an enormous range of uses, from the synthesis of fine chemicals to the production of common margarine.1−3 An important subdivision of hydrogenation reactions is transfer hydrogenations, whereby hydrogen may be transferred from one molecule to another, rather than utilizing hydrogen gas.4 Borrowing hydrogen chemistry, also known as hydrogen autotransfer, operates under this regime but with a key difference; in a borrowing hydrogen reaction, a pair of transfer hydrogenations is coupled with an intermediate reaction on the in situ-generated reactive intermediate.5−9 The general pathway is shown (Scheme 1), as illustrated with amine N-alkylation.
Scheme 1. Generalized Transition-Metal-Catalyzed Borrowing Hydrogen Reaction.
The process begins with a transition-metal mediated dehydrogenation of an alcohol or amine to form a reactive carbonyl (or imine) intermediate. This unsaturated species can undergo a variety of subsequent transformations, including condensation with an amine. The resulting species can be reduced by [MH2], generated in the initial dehydrogenation step, to regenerate the active catalyst and liberate the product of the reaction (in this case, an N-alkylated amine), to complete the catalytic cycle.
Most commonly, the borrowing hydrogen approach enables the functionalization of alcohols, with the vast majority of transformations utilizing commodity alcohols directly as alkylating agents in a variety of C–N and C–C bond-forming processes. This is an appealing strategy in comparison to alternative alkylation approaches. For example, commonly employed strategies for N-alkylation include alcohol activation (e.g., alkyl halide/sulfonate formation) and subsequent substitution or alcohol oxidation (to the corresponding carbonyl compound) followed by reductive amination. Both approaches are multistep and generate stoichiometric waste products. The borrowing hydrogen approach is typically selective for monoalkylation, providing complementarity to many traditional alkylation methodologies. Furthermore, through the use of chiral catalysts, enantioselective borrowing hydrogen reactions have been developed.
The term “borrowing hydrogen” was coined in 2004 by Williams and co-workers;10 however, there are multiple examples of this approach being demonstrated decades earlier. An early example is the work of Winans and Adkins, who in 1932 reported the use of a supported nickel catalyst for the N-alkylation of anilines with alcohols.11 Other examples employing heterogeneous catalysis followed in the ensuing years, such as a report by Pratt and Frazza, where an alternative nickel catalyst was used to achieve the same transformation.12 By contrast, some of the earliest examples of homogeneous catalysis for borrowing hydrogen were not reported until the 1980s, when the works of Wantanabe and Grigg demonstrated the N-alkylation of anilines and acetonitrile derivatives with alcohols using ruthenium- and rhodium-based catalysts, respectively.13−15 These pioneering contributions demonstrated the potential of this approach and inspired many research groups to investigate further, including our own.
This outlook provides a perspective on the borrowing hydrogen approach, primarily focusing on advances since 2000. It features landmark contributions, the different types of transformations that can be realized, and the diversity of catalytic manifolds that can be employed, spanning heterogeneous, homogeneous, and biocatalytic systems. We will highlight the current capabilities, limitations, and applications of these methodologies, direct specialists to further reading, and stimulate further interest and research in this exciting field. This outlook will not cover related base-mediated alkylation processes, which have been reviewed previously.16
C–N Bond-Forming Processes
N-Alkylation processes are a key facet of borrowing hydrogen chemistry, enabling alcohols (and amines) to be employed directly as alkylating agents in a diverse array of C–N bond-forming reactions with various N-nucleophiles. The formation of N-benzylaniline from aniline and benzyl alcohol is the archetypal borrowing hydrogen reaction of this class and has been widely explored, with a plethora of literature examples. An overview of selected metal catalysts that have been employed for this transformation, alongside the reaction conditions and reaction yield, is shown chronologically in Scheme 2. Heterogeneous and homogeneous catalyst systems are highlighted in red and blue, respectively. While earlier works date back to the 1930s,11 the resurgence and subsequent popularity of borrowing hydrogen chemistry began in the early 2000s, where this outlook is focused. In 2003, Yamaguchi and co-workers utilized a homogeneous iridium-cyclopentadienyl complex in the N-benzylation of anilines–an excellent representative example.17 Williams and co-workers employed a ruthenium p-cymene dichloride dimer to perform the same reaction in 2009.18 The use of many alternative catalysts and reaction conditions was also reported in this year, with Satsuma19 and Ramón and Yus20 demonstrating the use of heterogeneous catalysts: supported silver and magnetite, respectively. Further studies showed the ability of many other metals to catalyze this transformation. In 2010, Sabater and co-workers reported the synthesis of N-benzylamine via a heterogeneous palladium-catalyzed borrowing hydrogen process.21 Other works utilizing gold catalysis also accomplished this transformation in the same year.22 In 2011, Ramón and co-workers reported a homogeneous palladium-catalyzed borrowing hydrogen reaction, using palladium(II) acetate.23 Gusev and co-workers reported an osmium-catalyzed borrowing hydrogen process utilizing an osmium PNP complex (1) in the same year,24 followed by another report using tin catalysis.25 In 2013, Kantam and co-workers reported a rhodium-catalyzed borrowing hydrogen formation of N-benzylaniline,26 and Satsuma and co-workers demonstrated a heterogeneous nickel-catalyzed transformation to the same product.27 Mishra and co-workers employed a heterogeneous copper catalyst immobilized on hydrotalcite (HT), further increasing the diversity of available catalysts for this transformation,28 and in 2014, further reports of borrowing hydrogen transformations were disclosed, where rhenium catalysis was employed to perform this reaction.29
Scheme 2. Selected Approaches for the Catalytic Synthesis of N-Benzylaniline via Borrowing Hydrogen.

At this time, early examples of earth-abundant transition-metal catalysis in borrowing hydrogen reactions were reported, inspiring much further research in the use of 3d-block transition-metals in this area. The groups of Kempe30 and Wills31 reported the use of cobalt and iron catalysis for this transformation in 2015. Both systems employed well-defined metal complexes (2 and 3). Beller and co-workers increased the range of earth-abundant metal catalysts available in 2016, reporting a manganese-catalyzed reaction using a PNP-pincer precatalyst (4).32 A year later, Banerjee and co-workers demonstrated the use of simple nickel(II) bromide as a precatalyst, using a phenanthroline ligand to create a homogeneous nickel catalyst.33 Homogeneous copper-catalyzed borrowing hydrogen (5) can also be affected, as demonstrated by Wang and co-workers in 2017.34 Most recently, Kempe and co-workers reported the use of a chromium PNP-pincer complex (6).35
The N-benzylation of aniline with benzyl alcohol is the archetypal C–N bond-forming borrowing hydrogen transformation. However, both the amine and alcohol can be varied extensively, encompassing a wide range of functional groups on both components. A selection of products that can be accessed from the methods described in Scheme 2 is shown in Figure 1, to highlight some of the functionalities that can be incorporated into products. Heterocyclic moieties are well tolerated, including the synthesis of N-benzyltryptamine (7), which also showcases the use of an alkyl amine nucleophile. Esters that could be susceptible to hydrogenation or amidation can also be tolerated in these processes (11). Product 12 shows good selectivity for the desired aniline being formed.35 Furthermore, the presence of halides rarely impedes these reactions and can serve as functional handles for further elaboration.36 These reactions typically show exquisite selectivity for mono-N-alkylation–an important distinction in the use of classical alkylating reagents, such as alkyl halides. The synthesis of compounds resembling active pharmaceutical ingredients (APIs), or the functionalization of biologically relevant molecules, has also been demonstrated. For example, Beller and co-workers reported the synthesis of molecules that bear structural resemblance to resveratrol (14),37 which finds use in the treatment of Alzheimer’s disease.38
Figure 1.
Examples showcasing product diversity.
Variations from aniline nucleophiles are possible and provide further breadth to this chemistry, as initially shown in Figure 1. For instance, aliphatic primary and secondary amines are shown as effective nucleophiles, with many literature reports.39−45 An interesting example can be found in the work of Newton and co-workers of AstraZeneca.46 The authors utilized ruthenium and iridium catalysis to provide alternative strategies in the synthesis of a variety of APIs. A range of compounds with piperazine moieties was synthesized on a multigram scale from primary and secondary amine nucleophiles. For example, key piperazine 16, used to synthesize API 17, was accessed in a one-pot fashion with a much simpler workup and impurity removal than the previous strategy (Scheme 3). This demonstrated the exciting opportunities for borrowing hydrogen in industry–in many cases, these reactions superseded the existing route by providing simpler workups or the avoidance of classical alkylating reagents. It is noteworthy that where aliphatic primary amines are employed, these reactions are often selective for the formation of tertiary amines, in contrast to the examples in Figure 1, where the formation of secondary anilines is most commonly observed.
Scheme 3. Routes Toward API Synthesis with Borrowing Hydrogen.
Further variation of the N-nucleophile has expanded the array of transformations possible using borrowing hydrogen. For example, the N-alkylation of sulfonamides has been widely reported with primary alcohols.47−49 Dong, Guan, and co-workers employed chiral nonracemic sulfinamides as nucleophiles in a diastereoselective N-alkylation with secondary alcohols, using Ru-Macho (19) as a borrowing hydrogen catalyst.50 Representative examples and reaction conditions are shown in Scheme 4, demonstrating excellent diastereocontrol across a range of substrates (20–23). A similar strategy was employed by Xia and co-workers, who used iridium catalysis to prepare two pharmaceutically relevant molecules.51 An excellent example of the application of this work is the synthesis of (S)-rivastigmine, an acetylcholinesterase inhibitor used in the treatment of dementia.52
Scheme 4. Diastereoselective N-Alkylation of Sulfinamides.
Multiple strategies for enantioselective N-alkylation have been explored within the field of borrowing hydrogen.53−58 An excellent example was reported by Zhao and co-workers in 2014.59 This reaction demonstrated the successful fusion of Brønsted acid organocatalysis with borrowing hydrogen catalysis–the combination of a chiral iridium catalyst (24) and a chiral phosphoric acid (CPA, 25) was used in the preparation of enantioenriched α-branched amines. The reaction conditions, catalysts, and proposed mechanism are shown in Scheme 5. A wide range of alcohols was employed as alkylating reagents, with the resulting products reported in up to 97% e.e., despite the elevated reaction temperature. The authors attribute this enantioselectivity to both the chiral iridium catalyst and the coordination of the chiral phosphate anion. This approach was later extended to the dynamic kinetic resolution of racemic alcohols into enantioenriched amines60 and to the enantioselective synthesis of tetralin- and indane-derived amines, as well as tetrahydroisoquinolines.61,62
Scheme 5. Enantioselective Alkylation of Amines.
An alternative strategy in the development of enantioselective borrowing hydrogen reactions is the use of biocatalysis, which has received much attention in recent years as a powerful technique for organic synthesis.63,64 Early work of Kroutil and co-workers spearheaded investigations into the biocatalytic N-alkylation of amines with alcohols.65 In general, an enzyme is used to oxidize secondary alcohols to ketones, and a second enzyme is used to perform a reductive amination of the ketone, returning the product amine. Often, a third enzyme is utilized to regenerate any required cosubstrates (such as adenosine triphosphate–ATP–or similar compounds) for either enzyme, thus allowing the catalytic cycle to continue. This was later reduced to two enzymes–an alcohol dehydrogenase and an amine dehydrogenase, an important advance reported by Turner and co-workers in 2015.66 However, despite good enantiomeric excesses of the formed primary amines, these reactions were limited to aqueous ammonia as nucleophile, returning primary amines as products. Two years later, Turner and co-workers reported a significant advance: the tolerance of primary amines as nucleophiles.67 Building on the earlier reported work, only two enzymes–an alcohol dehydrogenase and a reductive aminase (from the bacterium Aspergillus oryzae)–were required for this transformation. This design also allows for turnover of the cosubstrate required for the alcohol dehydrogenase (nicotinamide adenide dinucleotide phosphate, NADP+) from the reductive aminase. Representative examples and a simplified catalytic cycle are illustrated in Scheme 6. The yields and enantiomeric excesses (where applicable) were high, exceeding 95% in many cases (26–29). Additionally, the low temperature of this reaction (only 30 °C) demonstrates the power of this biocatalytic system, alongside the range of transformations possible. A year later, an exciting extension of this work was published by Mutti and co-workers, who demonstrated a heterogeneous approach to biocatalysis by immobilizing the required enzymes on resin beads.68 This allowed the authors to report this chemistry using nanomolar enzyme loading, with >99% enantiomeric excess in all examples.
Scheme 6. Biocatalytic Borrowing Hydrogen with Primary Amines.
Other variations in borrowing hydrogen N-alkylation chemistry come from employing alternative classes of electrophiles. For example, Sundararaju and co-workers reported the N-alkylation of amines with primary allylic alcohols.69 This transformation has the additional challenge of competing 1,2- vs 1,4-addition of the amine nucleophile to the in situ-generated α,β-unsaturated carbonyl compound, in addition to possible isomerization of the allylic alcohol. However, the authors exclusively observed the formation of N-allylated products derived from the 1,2-addition of various primary and secondary amines. When propylamine was employed as the nucleophile, exclusive dialkylation was observed.
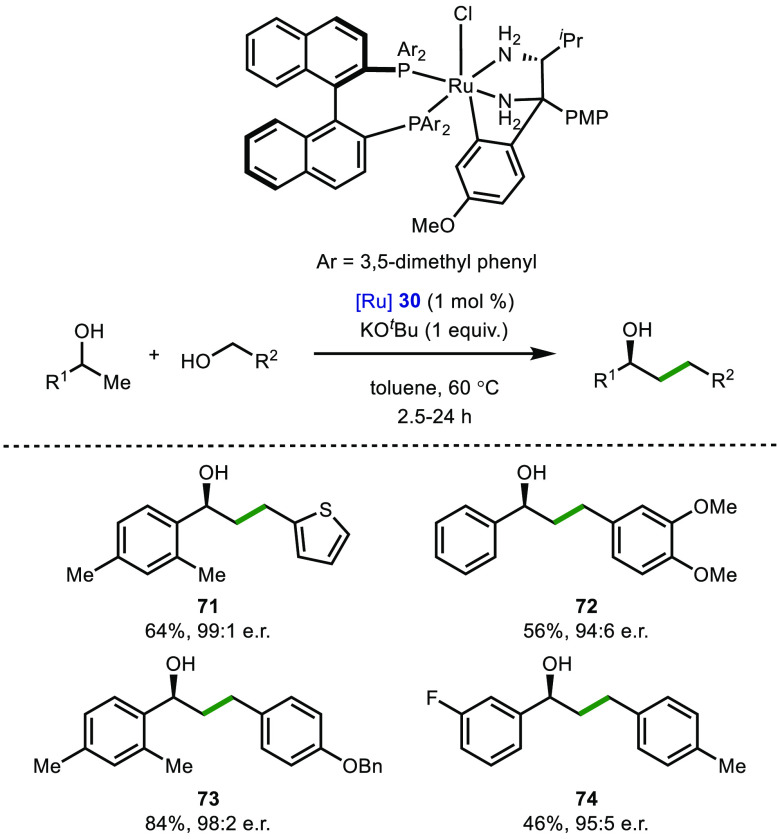
In the case of a 1,4-attack (γ-functionalization), the anti-Markovnikov hydroamination of secondary allylic alcohols has been reported employing ruthenium-70 and iron-based catalysts.71 More recently, a low temperature, stereoselective variant of this transformation was developed by Wang and co-workers, whereby a chiral ruthenium diamine-diphosphine complex (30) was employed to afford enantiomerically enriched γ-amino alcohols, bearing cyclic and acyclic tertiary amines (Scheme 7).72 Very high enantiomeric excesses were observed in almost every case, with the authors reporting an impressive 94% average e.e. in over 60 examples. The authors highlighted that this method could be applied to the synthesis APIs commonly used in the treatment of depression, such as (S)-fluoxetine (35).73 A similar ruthenium-catalyzed procedure for the γ-functionalization of allylic alcohols was reported shortly after by Xing and co-workers.74
Scheme 7. Enantioselective Hydroamination of Racemic Secondary Allylic Alcohols.
An important category of amine alkylation via borrowing hydrogen processes is methylations using methanol. These are challenging processes, partly due to the relatively high activation enthalpy of methanol dehydrogenation (ΔH = +84 kJ mol–1), as compared to other longer chain aliphatic alcohols, such as ethanol (ΔH = +68 kJ mol–1).75 An excellent example of borrowing hydrogen N-methylation procedures can be taken from the work of Beller and co-workers, who utilized a manganese PNP-pincer precatalyst (36) to effect selective mono-N-alkylation of anilines, tolerating a wide range of reducible functional groups (such as alkenes and ketones) and heterocycles. Scheme 8 shows the reaction conditions and representative examples.76 Other examples of methylation procedures include a range of precious and earth-abundant metal-catalyzed processes, reporting selective mono- or dimethylation of a variety of amines.77−79 Additionally, the gas phase formation of methylamine from ammonia and methanol has been reported using various heterogeneous zeolite-based catalysts.80,81 Further related reactions have been reported with the use of ethanol, as opposed to methanol.40,44,82,83
Scheme 8. Selective Mono-N-Methylation of Anilines.
Another example of electrophile variation is the N-alkylation of amines using amines as alkylating agents: a formal amine cross-coupling. This reaction, by contrast with those that employ alcohols as the electrophile, produces ammonia as the byproduct. Early examples of this work include that of Williams and co-workers, who demonstrated the use of secondary and tertiary amines as alkylating reagents, such as diisopropylamine, using iridium catalysis.84 Many other catalytic systems have also been reported for this process, predominantly using precious metal catalysts based on iridium, ruthenium, and platinum.85−87 In 2016, Zheng and Zhang reported an earth-abundant transition-metal-catalyzed reaction of this class, utilizing a cobalt PNP complex (41).88,89 A range of primary and secondary amines was employed as electrophiles. Interestingly, the nucleophilic amine was not limited to aromatic amines in this instance. A selection of amine homocouplings, from primary and secondary aliphatic amines, was also reported. Scheme 9 shows the reaction conditions, catalyst structure, and representative examples. Park and co-workers also further explored amine cross coupling, utilizing a bimetallic cobalt/rhodium catalyst to synthesize secondary and tertiary amines.89 This transformation has also been employed for the bulk production of secondary amines from primary amine feedstocks, using heterogeneous catalysis.90−92
Scheme 9. Amine Cross-Coupling Using Borrowing Hydrogen.
C–C Bond-Forming Processes
At the same time as the research into N-alkylation was performed, many authors also focused upon C-alkylation processes using the borrowing hydrogen approach. This reaction, while related, is a distinct and powerful tool in the formation of new C–C bonds. The archetypal reaction used to showcase these developments is the formation of dihydrochalcone from acetophenone and benzyl alcohol. This reaction can also be performed with a wide range of metal catalysts, shown in Scheme 10. As for N-alkylation, there are early, pioneering examples of borrowing hydrogen-catalyzed C-alkylation processes;13 however, this overview will focus on examples from 2000 and onward.
Scheme 10. Selected Approaches for the Catalytic Synthesis of Dihydrochalcone via Borrowing Hydrogen.

Early methods for this reaction utilized predominantly precious metal catalysts. For example, Chul Shim and co-workers employed a homogeneous ruthenium catalyst for the alkylation of acetophenones in 2002.93 Subsequent work demonstrated various other transition-metal-catalyzed examples. A homogeneous iridium-catalyzed solvent-free process was devised by Ishii and co-workers in 2004,94 while Cho and co-workers developed a heterogeneous palladium-catalyzed process soon after.95 Both authors incorporated hydrogen acceptors (1-dodecene and 1-decene) in their respective processes to suppress further reduction of the ketone product to the corresponding alcohol. The need for such an additive was superseded as catalysts in other media became more selective for the reduction of the intermediates over the products. In 2007, Yus and co-workers utilized nickel in the form of nanoparticles (Ni NPs) to catalyze this reaction.96 Investigations into new precious metal processes continued for several years, including reports of an osmium-catalyzed procedure from Yus and co-workers97 and a heterogeneous hydrotalcite supported copper-catalyzed approach from Mishra and co-workers, both in 2013.28 In the same year, Kantam and co-workers developed a rhodium-catalyzed procedure for alkylation of ketones with primary alcohols, albeit further reduction of the ketone products was observed.26 Another strategy employing rhodium, this time able to obtain ketone products, was later realized by Wang and co-workers in 2016.98 By this time, an increasing number of homogeneous earth-abundant transition-metal-catalyzed methods were emerging, including the works of Sortais and Darcel in 201599 and Beller and co-workers in 2016.100 The former demonstrated the iron-catalyzed α-alkylation of ketones with primary alcohols, including application to a Friedländer-type annulation, to synthesize quinolines from 2-aminobenzyl alcohols. On the other hand, Beller and co-workers employed a manganese catalyst (4) to alkylate not only acetophenones but also oxindoles with primary alcohols. Beller and co-workers later utilized the same PNP-pincer ligand within a rhenium complex (49) to catalyze the C-alkylation of acetophenone using similar reaction conditions.101 Soon after, a homogeneous cobalt-catalyzed process was reported in 2017 by Zhang.102 Other related examples include a homogeneous nickel-catalyzed method, which was demonstrated by Banerjee and co-workers in 2018. In this case, the synthesis of dihydrochalcone was not explicitly achieved due to a tendency for dialkylation at the α-position of unbranched acetophenones under the reported conditions.103
A selection of examples from these publications demonstrates the excellent tolerance these processes have for a variety of functional groups, including heterocyclic moieties (Figure 2). Likewise, they exemplify the potential applications of the method to the direct synthesis or late-stage modification of natural products, such as the synthesis of donepezil (56) and the alkylation of an estrone derivative (53). C-Alkylation via the borrowing hydrogen pathway is not limited to the alkylation of methyl ketones but also is available to a variety of other nucleophiles. Nitrile compounds are a strategically useful building block in organic synthesis,104 thus they are among the earliest13 and most explored nucleophiles for C-alkylation using precious metal catalysis,105,106 biocatalysis,107 and, more recently, earth-abundant metal catalysis.108−110 Similarly, functionalizing the α-position of esters and amides is possible but more challenging compared to the α-alkylation of ketones; the C–H acidity of esters and amides is comparably lower than ketones and aldehydes, while esters are also prone to undergo transesterification with alcohols. Early progress for the catalyzed α-alkylation of unactivated esters and amides with primary alcohols was made by Huang and Ishii, who both developed iridium-catalyzed processes.111−113 In 2016, Kempe reported the first earth-abundant metal-catalyzed α-alkylation of unactivated esters and amides using alcohols, via borrowing hydrogen. This reaction employed a homogeneous cobalt complex (57), shown along with representative examples (58–60) in Scheme 11.114 This chemistry was extended to manganese115,116 and nickel117 catalysis. Other C-nucleophiles include indoles,118,119 oxindoles,100,120 heteroarenes,121−123 napthols,124 sulfones,125 and thioamides,126 which can be functionalized in similar ways.
Figure 2.
Functional group tolerance and natural product modification in the α-alkylation of ketones.
Scheme 11. α-Alkylation of Esters and Amides.
Early C-alkylation works focused principally on the use of primary alcohols as alkylating agents, as the use of secondary alcohols was significantly more challenging.127 This was partly due to the issue of competing self-condensation of both the substrate and the ketone intermediate derived from the secondary alcohol. A major breakthrough was made by Donohoe and co-workers in 2017, whereby they addressed the self-condensation issue by employing a pentamethylphenyl group (Ph*) to sterically shield the carbonyl of the starting material (1-(pentamethylphenyl)ethan-1-one) from attack by enolates formed in situ.128,129 The Ph* group could be cleaved after workup by means of a retro-Friedel–Crafts acylation, to provide a series of β-branched esters and amides. Donohoe and co-workers also applied this methodology to the synthesis of (±)-3-methyl-5-phenylpentanol (63), a common fragrance additive used in cosmetics and toiletries.130Scheme 12 shows the reaction conditions for the borrowing hydrogen procedure and the subsequent retro-Friedel–Crafts reaction, with representative examples.128 In recent years, this approach with secondary alcohols has been extended to cobalt,131 iron,132 manganese,133 and transition-metal-free catalysis.134
Scheme 12. α-Alkylation of Ketones with Secondary Alcohols, with Second Stage Derivatization of Products.
The Ph* group was also used in an iridium-catalyzed (5 + 1) annulation strategy to synthesize cyclohexanes using 1,5-diols as alkylating agents by the same authors.135 It also was later incorporated for further stereoselective studies, utilizing a chiral phosphine ligand to control the facial selectivity of hydride deposition to the enone intermediate, resulting in enantioenriched products as shown in Scheme 13 (65–67).136,137 The diastereoselective dialkylation of methyl ketones using diols to obtain cycloalkanes has also been performed with manganese138,139 and iron catalysis.140 A recent study from Gunanathan and co-workers successfully demonstrated the alkylation of unsubstituted and unhindered acetophenone compounds with secondary alcohols by employing Ru-Macho as the catalyst and, contrary to previous reports, using a catalytic amount of base.141
Scheme 13. α-Alkylation of Ketones with Diols.
The research discussed so far is predominantly limited to methyl ketone substrates using benzyl or long chain n-alkyl alcohols as alkylating agents. The ability to perform methylation to form α-branched products via the borrowing hydrogen method remained a challenge for many years due to the same reasons discussed earlier for N-methylation.76 In 2014, Donohoe made a breakthrough using a rhodium catalyst, while utilizing methanol as both the methyl source and the solvent.142 This work also showcased double α-methylation of simple methyl ketones: a limitation in the interest of monoselectivity. Reaction conditions and representative examples are shown in Scheme 14. α-Methylation procedures were later established with earth-abundant metal catalysts, containing cobalt,143 iron,144 and manganese.145,146
Scheme 14. α-Methylation of Ketones.
While the works described thus far have focused on the alkylation of ketones, the formal alkylation of alcohols is also possible via borrowing hydrogen catalysis. The β-alkylation of secondary alcohols with alcohols (alcohol cross-coupling) is much like the alkylation of methyl ketones, except an additional transfer hydrogenation sequence is required to generate a nucleophilic species and return an alcohol product. As a result, many of the catalysts discussed in Scheme 10 are capable of both transformations; therefore, several one-pot alcohol cross-coupling procedures have been accomplished throughout the borrowing hydrogen era, employing a range of metals under both homogeneous and heterogeneous catalysis.147−155 Other notable reports showcasing β-alkylation of secondary alcohols include transformations related to those already discussed, stereoselective cycloalkane synthesis with diols,139 and methylation. The β-methylation of secondary alcohols has been accomplished using heterogeneous iridium156 and palladium catalysis,157 as well as homogeneous ruthenium,158 iron,159,160 and, most recently, manganese catalysis.161,162
Recently, Zhao and co-workers demonstrated a significant improvement for alcohol β-alkylation–the iridium-catalyzed β-alkylation of secondary alcohols with primary alcohols at room temperature.163 This was a remarkable feat, given the high temperatures typically required for borrowing hydrogen reactions. 3-Pentanone is used as a hydrogen acceptor to promote the reaction. The authors went on to report an enantioselective ruthenium-catalyzed alkylation of secondary alcohols with primary alcohols, obtaining enantiomeric excesses as high as 92%. Very few enantioselective reports with respect to C–C bond formation via a borrowing hydrogen cycle had been disclosed prior to this work.164−166 Almost simultaneously, Wang and co-workers reported a closely related process, also employing a chiral ruthenium complex as the catalyst (30).167 While this process occurred at 60 °C, there was no requirement for a promotor. A large number of examples were shown with products formed in up to 98% e.e., such as those highlighted in Scheme 15.
Scheme 15. Enantioselective β-Alkylation of Alcohols.
There are other applications of the alkylation of alcohols, beyond the shown examples. As the world seeks to replace fossil fuels with more sustainable alternatives, alcohol-based fuels have emerged as a viable option.168,169n-Butanol as a biofuel has advantages over bioethanol, namely its higher energy density.170 However, its main source of production is as a byproduct from the Acetone-Ethanol-Butanol (ABE) fermentation of biomass, a lengthy, inefficient process.171 An alternative chemical pathway to n-butanol is the homocoupling of lower alcohols, a method which has been established for over a century since the original Guerbet reaction–which featured the coupling of aliphatic n-butanol to form 2-ethylhexanol.172,173 Classically, this method requires an alkali metal hydroxide and Raney-nickel as a hydrogen transfer catalyst. Recent efforts have sought to apply other catalysts for the Guerbet reaction, with much interest surrounding the production of n-butanol via the homocoupling of ethanol. The vast majority of these processes employs a precious metal catalyst and requires high reaction temperatures (≤110 °C).174−178 The first example of upgrading ethanol into higher alcohols using a homogeneous nonprecious metal catalyst was reported by Liu and co-workers in 2017.179 Utilizing ppm levels of a PNP-pincer precatalyst (4) (8 ppm), they were able to achieve a very high TON (114,120), surpassing many precious metal-catalyzed examples, while also maintaining good selectivity for 1-butanol (92%), albeit still requiring high temperatures. Reaction conditions and a catalytic cycle are shown in Scheme 16.
Scheme 16. Upgrading of Ethanol to n-Butanol.
As previously discussed in C–N bond-forming reactions, there is much potential in borrowing hydrogen chemistry for powerful, dual-catalytic systems. In 2013, Quintard and Rodriguez combined an iron-catalyzed borrowing hydrogen cycle with secondary amine organocatalysis.165 The authors were able to perform asymmetric γ-functionalization of simple allylic alcohols to obtain γ-functionalized alcohols using mild reaction conditions. The mechanism of this reaction is shown in Scheme 17. The reaction begins with catalyst activation with Me3NO. The typical borrowing hydrogen reaction then occurs, but the intermediate enal can be intercepted by the secondary amine organocatalyst (79), resulting in an enantioselective Michael addition of the nucleophile (a β-keto ester, in this case) to the formed iminium ion. This is followed by hydrolysis and chemoselective reduction, regenerating the active iron dehydrogenation complex and generating the γ-functionalized alcohol product. The authors later extended this reaction to a one-pot synthesis of enantioenriched spiro-δ-lactones.166
Scheme 17. Dual-Catalytic System Combining Borrowing Hydrogen Activation and Enantioselective Organocatalysis.

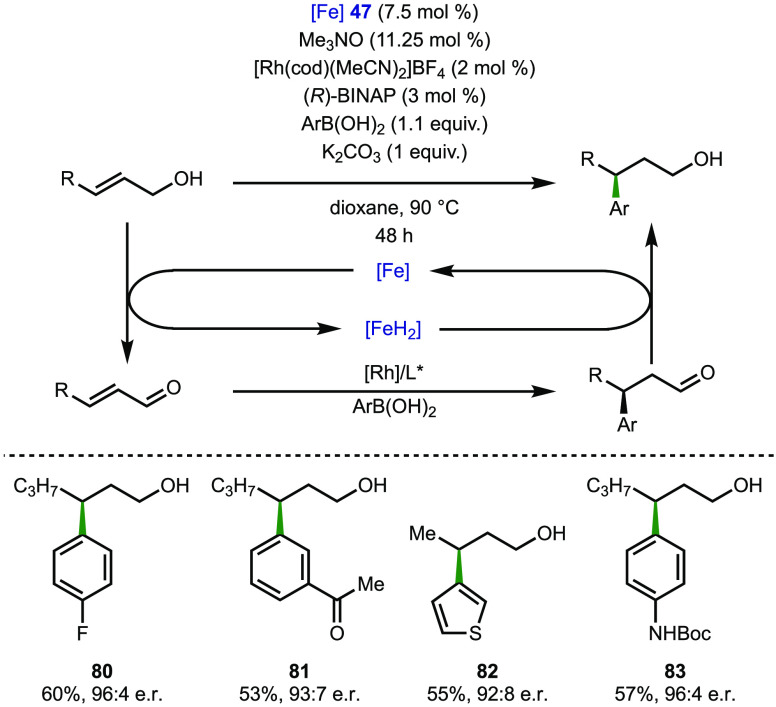
In 2019, Dydio and co-workers combined borrowing hydrogen reactions with transition-metal-catalyzed functionalization to devise one-pot dual-catalytic systems.180 One system combined a ruthenium-catalyzed borrowing hydrogen process with palladium-catalyzed arylation to generate β-aryl alcohols from primary alcohol substrates. A second transformation combined a borrowing hydrogen process with rhodium-catalyzed hydroarylation, ultimately to access enantioenriched γ-aryl alcohol products from primary allylic alcohols. In this study, both ruthenium and iron complexes were explored as a hydrogen transfer catalyst. Scheme 18 shows the reaction conditions and representative examples (80–83) when employing an iron complex (47) as a hydrogen transfer catalyst.
Scheme 18. Dual-Catalytic Transition-Metal System to Access Enantioenriched γ-Aryl Alcohols from Allylic Alcohols.
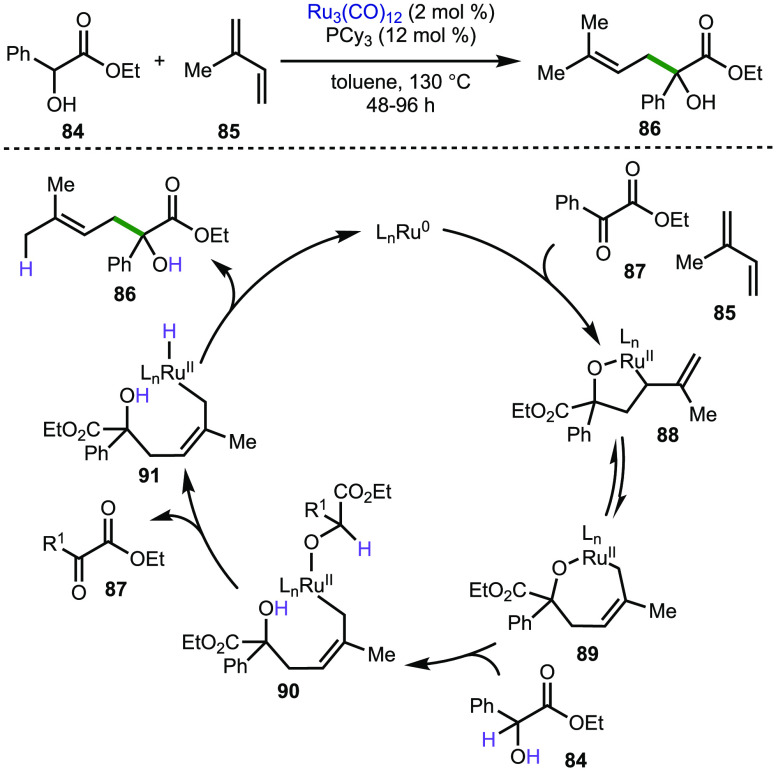
The coupling of olefins with alcohols via hydrogen autotransfer is a transformation demonstrated by many in recent years, as summarized in a review by Kirsche and co-workers.181 They themselves have demonstrated ruthenium-catalyzed redox coupling of α-hydroxyesters and dienes. Using Ru3(CO)12/PCy3 as a catalyst system, α-hydroxyester (84), and an excess of isoprene as olefin,182 the postulated mechanism proceeds via oxidative coupling of isoprene (85) and the in situ generated ketone (87), as illustrated in Scheme 19. The resulting five-membered ruthenium(II) oxametallacycle (88) isomerizes to the seven-membered variant (89), followed by protonation of the oxametallocycle forming the ruthenium(II) alkoxide species (90). Subsequent β-hydride elimination to the ruthenium(II) hydride species (91), followed by reductive elimination, delivers the product (86) and a ruthenium (0) species, completing the catalytic cycle. Similar ruthenium-catalyzed coupling processes from Krische and co-workers have been applied to heteroaryl substituted secondary alcohols,183 as well as 3-hydroxy-2-indoles.184
Scheme 19. Ruthenium-Catalyzed Redox Coupling of α-Hydroxyesters and Dienes.
Miscellaneous Processes
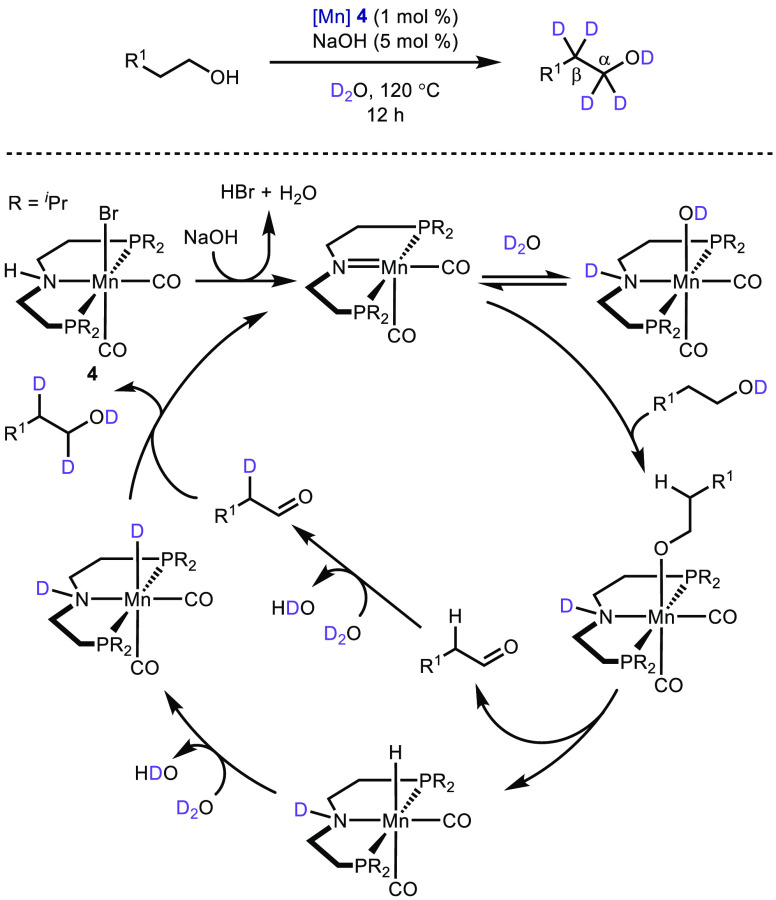
Almost all borrowing hydrogen works involve the formation of new C–C or C–N bonds. However, there are other useful applications of this methodology.185 Deuterium labeled compounds are useful as internal standards for mass spectrometry, as solvents for NMR spectroscopy, and in medicinal chemistry for clarifying biosynthetic pathways.186 In 2018, Prakesh and co-workers reported an effective strategy for the regioselective deuteration of primary alcohols.187 An iron catalyst could selectively deuterate the α-position, while a manganese catalyst was able to deuterate at both the α- and β-positions. The postulated mechanism of the manganese-catalyzed transformation is shown in Scheme 20.
Scheme 20. Deuteration of Alcohols.
Summary and Perspective
Within this outlook, we have provided an overview of the borrowing hydrogen approach and its application in synthesis. We have discussed the most significant advances across a variety of important C–N and C–C bond-forming processes. Several landmark advancements have been made using precious metal, earth-abundant metal, and biocatalysis across both heterogeneous and homogeneous systems, demonstrating the versatility of this chemistry. Despite a considerable increase in the number of publications in the area over the past decade, there remain several challenges and opportunities, and there is no doubt that advances will continue to be made. In accordance with the increasing global demand to preserve the finite resources on Earth, many earlier existing precious metal-catalyzed transformations have now been translated to the use of earth-abundant metal catalysts–a collective aim for researchers in the area. Thus, more elaborate and novel developments are to be expected in the coming years in this area. There remains an absence of biocatalysis for borrowing hydrogen C-alkylation processes, and asymmetric processes are poorly represented with respect to earth-abundant metal catalysis. Further collective targets that will continue to be sought are milder reaction conditions and lower catalyst loadings. We also anticipate more efforts targeting the design of new, more active catalysts for various processes as well as further implementation of the borrowing hydrogen methodology in dual-catalysis systems. Can alternative transformations and cascades be incorporated into borrowing hydrogen processes to create powerful novel one-pot transformations?188 It is inevitable that many new and exciting borrowing hydrogen transformations will be discovered via these various avenues in the coming years. To close this outlook, we hope this article has conveyed the importance and usefulness of borrowing hydrogen for organic synthesis, and we envision a bright future for the area.
Acknowledgments
We gratefully acknowledge the School of Chemistry, Cardiff University for generous support, the EPSRC Doctoral Training Grant (B.G.R-B., EP/M507842/1), the EPSRC-funded Bath/Bristol/Cardiff Catalysis Centre for Doctoral Training (D.E.L., EP/L016443/1), and the TETFund (M.B.D) for Ph.D. studentships.
Author Contributions
# B.G.R.-B. and D.E.L. contributed equally to this work.
The authors declare no competing financial interest.
References
- Andersson P. G., Munslow I. J., Eds.; Modern Reduction Methods; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, 2008; 10.1002/9783527622115. [DOI] [Google Scholar]
- Freeman I. P.; Melnikov S. M.. Margarines. Ullmann’s Encyclopedia of Industrial Chemistry; 2015; pp 1–24, 10.1002/14356007.a16_145.pub2. [DOI] [Google Scholar]
- Bonrath W.; Medlock J.; Schütz J.; Wüstenberg B.; Netscher T.. Hydrogenation in the Vitamins and Fine Chemicals Industry – An Overview. In Hydrogenation; Karamé I., Ed.; IntechOpen: 2012; pp 69–90, 10.5772/48751. [DOI] [Google Scholar]
- Wang D.; Astruc D. The Golden Age of Transfer Hydrogenation. Chem. Rev. 2015, 115 (13), 6621–6686. 10.1021/acs.chemrev.5b00203. [DOI] [PubMed] [Google Scholar]
- Shimizu K. I. Heterogeneous Catalysis for the Direct Synthesis of Chemicals by Borrowing Hydrogen Methodology. Catal. Sci. Technol. 2015, 5 (3), 1412–1427. 10.1039/C4CY01170H. [DOI] [Google Scholar]
- Corma A.; Navas J.; Sabater M. J. Advances in One-Pot Synthesis through Borrowing Hydrogen Catalysis. Chem. Rev. 2018, 118 (4), 1410–1459. 10.1021/acs.chemrev.7b00340. [DOI] [PubMed] [Google Scholar]
- Irrgang T.; Kempe R. 3d-Metal Catalyzed N- and C-Alkylation Reactions via Borrowing Hydrogen or Hydrogen Autotransfer. Chem. Rev. 2019, 119 (4), 2524–2549. 10.1021/acs.chemrev.8b00306. [DOI] [PubMed] [Google Scholar]
- Reed-Berendt B. G.; Polidano K.; Morrill L. C. Recent Advances in Homogeneous Borrowing Hydrogen Catalysis Using Earth-Abundant First Row Transition Metals. Org. Biomol. Chem. 2019, 17 (7), 1595–1607. 10.1039/C8OB01895B. [DOI] [PubMed] [Google Scholar]
- Kwok T.; Hoff O.; Armstrong R. J.; Donohoe T. J. Control of Absolute Stereochemistry in Transition-Metal-Catalysed Hydrogen-Borrowing Reactions. Chem. - Eur. J. 2020, 26 (57), 12912–12926. 10.1002/chem.202001253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. G.; Jazzar R. F. R.; Paine B. M.; Shermer D. J.; Whittlesey M. K.; Williams J. M. J.; Edney D. D. Borrowing Hydrogen: A Catalytic Route to C–C Bond Formation from Alcohols. Chem. Commun. 2004, 4 (1), 90–91. 10.1039/B312162C. [DOI] [PubMed] [Google Scholar]
- Winans C. F.; Adkins H. The Alkylation of Amines as Catalyzed by Nickel. J. Am. Chem. Soc. 1932, 54 (1), 306–312. 10.1021/ja01340a046. [DOI] [Google Scholar]
- Pratt E. F.; Frazza E. J. Disproportionative Condensations. II. The N-Alkylation of Anilines with Primary Alcohols. J. Am. Chem. Soc. 1954, 76 (23), 6174–6179. 10.1021/ja01652a091. [DOI] [Google Scholar]
- Grigg R.; Mitchell T. R. B.; Sutthivaiyakit S.; Tongpenyai N. Oxidation of Alcohols by Transition Metal Complexes Part V. Selective Catalytic Monoalkylation of Arylacetonitriles by Alcohols. Tetrahedron Lett. 1981, 22 (41), 4107–4110. 10.1016/S0040-4039(01)82078-5. [DOI] [Google Scholar]
- Watanabe Y.; Tsuji Y.; Ohsugi Y. The Ruthenium Catalyzed N-Alkylation and N-Heterocyclization of Aniline Using Alcohols and Aldehydes. Tetrahedron Lett. 1981, 22 (28), 2667–2670. 10.1016/S0040-4039(01)92965-X. [DOI] [Google Scholar]
- Watanabe Y.; Tsuji Y.; Ige H.; Ohsugi Y.; Ohta T. Ruthenium-Catalyzed N-Alkylation and N-Benzylation of Aminoarenes with Alcohols. J. Org. Chem. 1984, 49 (18), 3359–3363. 10.1021/jo00192a021. [DOI] [Google Scholar]
- Porcheddu A.; Chelucci G. Base-Mediated Transition-Metal-Free Dehydrative C–C and C–N Bond-Forming Reactions from Alcohols. Chem. Rec. 2019, 19 (12), 2398–2435. 10.1002/tcr.201800170. [DOI] [PubMed] [Google Scholar]
- Fujita K. I.; Li Z.; Ozeki N.; Yamaguchi R. N-Alkylation of Amines with Alcohols Catalyzed by a Cp*Ir Complex. Tetrahedron Lett. 2003, 44 (13), 2687–2690. 10.1016/S0040-4039(03)00371-X. [DOI] [Google Scholar]
- Hamid M. H. S. A.; Allen C. L.; Lamb G. W.; Maxwell A. C.; Maytum H. C.; Watson A. J. A.; Williams J. M. J. Ruthenium-Catalyzed -Alkylation of Amines and Sulfonamides Using Borrowing Hydrogen Methodology. J. Am. Chem. Soc. 2009, 131 (5), 1766–1774. 10.1021/ja807323a. [DOI] [PubMed] [Google Scholar]
- Shimizu K.; Nishimura M.; Satsuma A. γ-Alumina-Supported Silver Cluster for N-Benzylation of Anilines with Alcohols. ChemCatChem 2009, 1 (4), 497–503. 10.1002/cctc.200900209. [DOI] [Google Scholar]
- Martínez R.; Ramón D. J.; Yus M. Selective N-Monoalkylation of Aromatic Amines with Benzylic Alcohols by a Hydrogen Autotransfer Process Catalyzed by Unmodified Magnetite. Org. Biomol. Chem. 2009, 7 (10), 2176–2181. 10.1039/b901929d. [DOI] [PubMed] [Google Scholar]
- Corma A.; Ródenas T.; Sabater M. J. A Bifunctional Pd/MgO Solid Catalyst for the One-Pot Selective N-Monoalkylation of Amines with Alcohols. Chem. - Eur. J. 2010, 16 (1), 254–260. 10.1002/chem.200901501. [DOI] [PubMed] [Google Scholar]
- He L.; Lou X. B.; Ni J.; Liu Y. M.; Cao Y.; He H. Y.; Fan K. N. Efficient and Clean Gold-Catalyzed One-Pot Selective N-Alkylation of Amines with Alcohols. Chem. - Eur. J. 2010, 16 (47), 13965–13969. 10.1002/chem.201001848. [DOI] [PubMed] [Google Scholar]
- Martínez-Asencio A.; Yus M.; Ramón D. J. Palladium(II) Acetate as Catalyst for the N-Alkylation of Aromatic Amines, Sulfonamides, and Related Nitrogenated Compounds with Alcohols by a Hydrogen Autotransfer Process. Synthesis 2011, 2011, 3730–3740. 10.1055/s-0030-1260238. [DOI] [Google Scholar]
- Bertoli M.; Choualeb A.; Lough A. J.; Moore B.; Spasyuk D.; Gusev D. G. Osmium and Ruthenium Catalysts for Dehydrogenation of Alcohols. Organometallics 2011, 30 (13), 3479–3482. 10.1021/om200437n. [DOI] [Google Scholar]
- He W.; Wang L.; Sun C.; Wu K.; He S.; Chen J.; Wu P.; Yu Z. Pt-Sn/γ-Al2O3-Catalyzed Highly Efficient Direct Synthesis of Secondary and Tertiary Amines and Imines. Chem. - Eur. J. 2011, 17 (47), 13308–13317. 10.1002/chem.201101725. [DOI] [PubMed] [Google Scholar]
- Satyanarayana P.; Reddy G. M.; Maheswaran H.; Kantam M. L. Tris(Acetylacetonato)Rhodium(III)-Catalyzed α-Alkylation of Ketones, β-Alkylation of Secondary Alcohols and Alkylation of Amines with Primary Alcohols. Adv. Synth. Catal. 2013, 355 (9), 1859–1867. 10.1002/adsc.201300061. [DOI] [Google Scholar]
- Shimizu K. I.; Imaiida N.; Kon K.; Hakim Siddiki S. M. A.; Satsuma A. Heterogeneous Ni Catalysts for N-Alkylation of Amines with Alcohols. ACS Catal. 2013, 3 (5), 998–1005. 10.1021/cs4001267. [DOI] [Google Scholar]
- Dixit M.; Mishra M.; Joshi P. A.; Shah D. O. Clean Borrowing Hydrogen Methodology Using Hydrotalcite Supported Copper Catalyst. Catal. Commun. 2013, 33, 80–83. 10.1016/j.catcom.2012.12.027. [DOI] [Google Scholar]
- Abdukader A.; Jin H.; Cheng Y.; Zhu C. Rhenium-Catalyzed Amination of Alcohols by Hydrogen Transfer Process. Tetrahedron Lett. 2014, 55 (30), 4172–4174. 10.1016/j.tetlet.2014.05.068. [DOI] [Google Scholar]
- Rösler S.; Ertl M.; Irrgang T.; Kempe R. Cobalt-Catalyzed Alkylation of Aromatic Amines by Alcohols. Angew. Chem., Int. Ed. 2015, 54 (50), 15046–15050. 10.1002/anie.201507955. [DOI] [PubMed] [Google Scholar]
- Rawlings A. J.; Diorazio L. J.; Wills M. C-N Bond Formation between Alcohols and Amines Using an Iron Cyclopentadienone Catalyst. Org. Lett. 2015, 17 (5), 1086–1089. 10.1021/ol503587n. [DOI] [PubMed] [Google Scholar]
- Elangovan S.; Neumann J.; Sortais J. B.; Junge K.; Darcel C.; Beller M. Efficient and Selective N-Alkylation of Amines with Alcohols Catalysed by Manganese Pincer Complexes. Nat. Commun. 2016, 7, 12641. 10.1038/ncomms12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellakkaran M.; Singh K.; Banerjee D. An Efficient and Selective Nickel-Catalyzed Direct N-Alkylation of Anilines with Alcohols. ACS Catal. 2017, 7 (12), 8152–8158. 10.1021/acscatal.7b02817. [DOI] [Google Scholar]
- Xu Z.; Wang D. S.; Yu X.; Yang Y.; Wang D. Tunable Triazole-Phosphine-Copper Catalysts for the Synthesis of 2-Aryl-1H-Benzo[d]Imidazoles from Benzyl Alcohols and Diamines by Acceptorless Dehydrogenation and Borrowing Hydrogen Reactions. Adv. Synth. Catal. 2017, 359 (19), 3332–3340. 10.1002/adsc.201700179. [DOI] [Google Scholar]
- Kallmeier F.; Fertig R.; Irrgang T.; Kempe R. Chromium-Catalyzed Alkylation of Amines by Alcohols. Angew. Chem., Int. Ed. 2020, 59 (29), 11789–11793. 10.1002/anie.202001704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayogu J. I.; Onoabedje E. A. Recent Advances in Transition Metal-Catalysed Cross-Coupling of (Hetero)Aryl Halides and Analogues under Ligand-Free Conditions. Catal. Sci. Technol. 2019, 9 (19), 5233–5255. 10.1039/C9CY01331H. [DOI] [Google Scholar]
- Baur J. A.; Sinclair D. A. Therapeutic Potential of Resveratrol: The in Vivo Evidence. Nat. Rev. Drug Discovery 2006, 5, 493–506. 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Lu C.; Guo Y.; Yan J.; Luo Z.; Luo H.; Yan M.; Huang L.; Li X. Design, Synthesis, and Evaluation of Multitarget-Directed Resveratrol Derivatives for the Treatment of Alzheimer’s Disease. J. Med. Chem. 2013, 56 (14), 5843–5859. 10.1021/jm400567s. [DOI] [PubMed] [Google Scholar]
- Yan T.; Feringa B. L.; Barta K. Direct N-Alkylation of Unprotected Amino Acids with Alcohols. Sci. Adv. 2017, 3 (12), eaao6494. 10.1126/sciadv.aao6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T.; Feringa B. L.; Barta K. Iron Catalysed Direct Alkylation of Amines with Alcohols. Nat. Commun. 2014, 5 (5602), 5602. 10.1038/ncomms6602. [DOI] [PubMed] [Google Scholar]
- García Ruano J. L.; Parra A.; Alemán J.; Yuste F.; Mastranzo V. M. Monoalkylation of Primary Amines and N-Sulfinylamides. Chem. Commun. 2009, 4, 404–406. 10.1039/B816846F. [DOI] [PubMed] [Google Scholar]
- Labes R.; Mateos C.; Battilocchio C.; Chen Y.; Dingwall P.; Cumming G. R.; Rincón J. A.; Nieves-Remacha M. J.; Ley S. V. Fast Continuous Alcohol Amination Employing a Hydrogen Borrowing Protocol. Green Chem. 2019, 21 (1), 59–63. 10.1039/C8GC03328E. [DOI] [Google Scholar]
- Lamb G. W.; Watson A. J. A.; Jolley K. E.; Maxwell A. C.; Williams J. M. J. Borrowing Hydrogen Methodology for the Conversion of Alcohols into N-Protected Primary Amines and in Situ Deprotection. Tetrahedron Lett. 2009, 50 (26), 3374–3377. 10.1016/j.tetlet.2009.02.129. [DOI] [Google Scholar]
- Enyong A. B.; Moasser B. Ruthenium-Catalyzed N-Alkylation of Amines with Alcohols under Mild Conditions Using the Borrowing Hydrogen Methodology. J. Org. Chem. 2014, 79 (16), 7553–7563. 10.1021/jo501273t. [DOI] [PubMed] [Google Scholar]
- Dang T. T.; Ramalingam B.; Shan S. P.; Seayad A. M. An Efficient Palladium-Catalyzed N-Alkylation of Amines Using Primary and Secondary Alcohols. ACS Catal. 2013, 3 (11), 2536–2540. 10.1021/cs400799n. [DOI] [Google Scholar]
- Leonard J.; Blacker A. J.; Marsden S. P.; Jones M. F.; Mulholland K. R.; Newton R. A Survey of the Borrowing Hydrogen Approach to the Synthesis of Some Pharmaceutically Relevant Intermediates. Org. Process Res. Dev. 2015, 19 (10), 1400–1410. 10.1021/acs.oprd.5b00199. [DOI] [Google Scholar]
- Qu P.; Sun C.; Ma J.; Li F. The N-Alkylation of Sulfonamides with Alcohols in Water Catalyzed by the Water-Soluble Iridium Complex {Cp*[6,6’-(OH)2bpy](H2O)}[OTf]2. Adv. Synth. Catal. 2014, 356 (2–3), 447–459. 10.1002/adsc.201300711. [DOI] [Google Scholar]
- Shi F.; Tse M. K.; Cui X.; Gördes D.; Michalik D.; Thurow K.; Deng Y.; Beller M. Copper-Catalyzed Alkylation of Sulfonamides with Alcohols. Angew. Chem., Int. Ed. 2009, 48 (32), 5912–5915. 10.1002/anie.200901510. [DOI] [PubMed] [Google Scholar]
- Shi F.; Tse M. K.; Zhou S.; Pohl M. M.; Radnik J.; Hübner S.; Jähnisch K.; Brückner A.; Beller M. Green and Efficient Synthesis of Sulfonamides Catalyzed by Nano-Ru/Fe3O4. J. Am. Chem. Soc. 2009, 131 (5), 1775–1779. 10.1021/ja807681v. [DOI] [PubMed] [Google Scholar]
- Oldenhuis N. J.; Dong V. M.; Guan Z. From Racemic Alcohols to Enantiopure Amines: Ru-Catalyzed Diastereoselective Amination. J. Am. Chem. Soc. 2014, 136 (36), 12548–12551. 10.1021/ja5058482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi X.; Li Y.; Wang G.; Xu G.; Shang L.; Zhang Y.; Xia L. Iridium-Catalyzed Diastereoselective Amination of Alcohols with Chiral: Tert-Butanesulfinamide by the Use of a Borrowing Hydrogen Methodology. Org. Biomol. Chem. 2019, 17 (33), 7651–7654. 10.1039/C9OB01417A. [DOI] [PubMed] [Google Scholar]
- Khoury R.; Rajamanickam J.; Grossberg G. T. An Update on the Safety of Current Therapies for Alzheimer’s Disease: Focus on Rivastigmine. Ther. Adv. Drug Saf. 2018, 9 (3), 171–178. 10.1177/2042098617750555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eka Putra A.; Oe Y.; Ohta T. Ruthenium-Catalyzed Enantioselective Synthesis of β-Amino Alcohols from 1,2-Diols by “Borrowing Hydrogen.. Eur. J. Org. Chem. 2013, 2013, 6146–6151. 10.1002/ejoc.201300692. [DOI] [Google Scholar]
- Peña-López M.; Neumann H.; Beller M. (Enantio)Selective Hydrogen Autotransfer: Ruthenium-Catalyzed Synthesis of Oxazolidin-2-Ones from Urea and Diols. Angew. Chem., Int. Ed. 2016, 55 (27), 7826–7830. 10.1002/anie.201600698. [DOI] [PubMed] [Google Scholar]
- Yang P.; Zhang C.; Ma Y.; Zhang C.; Li A.; Tang B.; Zhou J. S. Nickel-Catalyzed N-Alkylation of Acylhydrazines and Arylamines Using Alcohols and Enantioselective Examples. Angew. Chem., Int. Ed. 2017, 56 (46), 14702–14706. 10.1002/anie.201708949. [DOI] [PubMed] [Google Scholar]
- Yang L. C.; Wang Y. N.; Zhang Y.; Zhao Y. Acid-Assisted Ru-Catalyzed Enantioselective Amination of 1,2-Diols through Borrowing Hydrogen. ACS Catal. 2017, 7 (1), 93–97. 10.1021/acscatal.6b02959. [DOI] [Google Scholar]
- Zhang J.; Wang J. Atropoenantioselective Redox-Neutral Amination of Biaryl Compounds through Borrowing Hydrogen and Dynamic Kinetic Resolution. Angew. Chem., Int. Ed. 2018, 57 (2), 465–469. 10.1002/anie.201711126. [DOI] [PubMed] [Google Scholar]
- Xu G.; Yang G.; Wang Y.; Shao P. L.; Yau J. N. N.; Liu B.; Zhao Y.; Sun Y.; Xie X.; Wang S.; et al. Stereoconvergent, Redox-Neutral Access to Tetrahydroquinoxalines through Relay Epoxide Opening/Amination of Alcohols. Angew. Chem., Int. Ed. 2019, 58 (40), 14082–14088. 10.1002/anie.201906199. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Lim C. S.; Boon Sim D. S.; Pan H. J.; Zhao Y. Catalytic Enantioselective Amination of Alcohols by the Use of Borrowing Hydrogen Methodology: Cooperative Catalysis by Iridium and a Chiral Phosphoric Acid. Angew. Chem., Int. Ed. 2014, 53 (5), 1399–1403. 10.1002/anie.201307789. [DOI] [PubMed] [Google Scholar]
- Rong Z. Q.; Zhang Y.; Chua R. H. B.; Pan H. J.; Zhao Y. Dynamic Kinetic Asymmetric Amination of Alcohols: From a Mixture of Four Isomers to Diastereo- and Enantiopure α-Branched Amines. J. Am. Chem. Soc. 2015, 137 (15), 4944–4947. 10.1021/jacs.5b02212. [DOI] [PubMed] [Google Scholar]
- Lim C. S.; Quach T. T.; Zhao Y. Enantioselective Synthesis of Tetrahydroquinolines by Borrowing Hydrogen Methodology: Cooperative Catalysis by an Achiral Iridacycle and a Chiral Phosphoric Acid. Angew. Chem., Int. Ed. 2017, 56 (25), 7176–7180. 10.1002/anie.201703704. [DOI] [PubMed] [Google Scholar]
- Rong Z.-Q.; Yu Z.; Weng C.; Yang L. C.; Lu S.; Lan Y.; Zhao Y. Dynamic Kinetic Asymmetric Amination of Alcohols Assisted by Microwave: Stereoconvergent Access to Tetralin- and Indane-Derived Chiral Amines. ACS Catal. 2020, 10 (16), 9464–9475. 10.1021/acscatal.0c02468. [DOI] [Google Scholar]
- Bommarius A. S. Biocatalysis: A Status Report. Annu. Rev. Chem. Biomol. Eng. 2015, 6 (1), 319–345. 10.1146/annurev-chembioeng-061114-123415. [DOI] [PubMed] [Google Scholar]
- Devine P. N.; Howard R. M.; Kumar R.; Thompson M. P.; Truppo M. D.; Turner N. J. Extending the Application of Biocatalysis to Meet the Challenges of Drug Development. Nat. Rev. Chem. 2018, 2 (12), 409–421. 10.1038/s41570-018-0055-1. [DOI] [Google Scholar]
- Sattler J. H.; Fuchs M.; Tauber K.; Mutti F. G.; Faber K.; Pfeffer J.; Haas T.; Kroutil W. Redox Self-Sufficient Biocatalyst Network for the Amination of Primary Alcohols. Angew. Chem., Int. Ed. 2012, 51 (36), 9156–9159. 10.1002/anie.201204683. [DOI] [PubMed] [Google Scholar]
- Mutti F. G.; Knaus T.; Scrutton N. S.; Breuer M.; Turner N. J. Conversion of Alcohols to Enantiopure Amines through Dual-Enzyme Hydrogen-Borrowing Cascades. Science 2015, 349 (6255), 1525–1529. 10.1126/science.aac9283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery S. L.; Mangas-Sanchez J.; Thompson M. P.; Aleku G. A.; Dominguez B.; Turner N. J. Direct Alkylation of Amines with Primary and Secondary Alcohols through Biocatalytic Hydrogen Borrowing. Angew. Chem., Int. Ed. 2017, 56 (35), 10491–10494. 10.1002/anie.201705848. [DOI] [PubMed] [Google Scholar]
- Böhmer W.; Knaus T.; Mutti F. G. Hydrogen-Borrowing Alcohol Bioamination with Coimmobilized Dehydrogenases. ChemCatChem 2018, 10 (4), 731–735. 10.1002/cctc.201701366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emayavaramban B.; Roy M.; Sundararaju B. Iron-Catalyzed Allylic Amination Directly from Allylic Alcohols. Chem. - Eur. J. 2016, 22 (12), 3952–3955. 10.1002/chem.201505214. [DOI] [PubMed] [Google Scholar]
- Nakamura Y.; Ohtaa T.; Oe Y. A Formal Anti-Markovnikov Hydroamination of Allylic Alcohols via Tandem Oxidation/1,4-Conjugate Addition/1,2-Reduction using a Ru Catalyst. Chem. Commun. 2015, 51 (35), 7459–7462. 10.1039/C5CC01584G. [DOI] [PubMed] [Google Scholar]
- Ma W.; Zhang X.; Fan J.; Liu Y.; Tang W.; Xue D.; Li C.; Xiao J.; Wang C. Iron-Catalyzed Anti-Markovnikov Hydroamination and Hydroamidation of Allylic Alcohols. J. Am. Chem. Soc. 2019, 141 (34), 13506–13515. 10.1021/jacs.9b05221. [DOI] [PubMed] [Google Scholar]
- Xu R.; Wang K.; Liu H.; Tang W.; Sun H.; Xue D.; Xiao J.; Wang C. Anti-Markovnikov Hydroamination of Racemic Allylic Alcohols to Access Chiral γ-Amino Alcohols. Angew. Chem., Int. Ed. 2020, 59 (49), 21959–21964. 10.1002/anie.202009754. [DOI] [PubMed] [Google Scholar]
- Cipriani A.; Furukawa T. A.; Salanti G.; Chaimani A.; Atkinson L. Z.; Ogawa Y.; Leucht S.; Ruhe H. G.; Turner E. H.; Higgins J. P. T.; Egger M.; Takeshima N.; Hayasaka Y.; Imai H.; Shinohara K.; Tajika A.; Ioannidis J. P. A.; Geddes J. R. Comparative Efficacy and Acceptability of 21 Antidepressant Drugs for the Acute Treatment of Adults with Major Depressive Disorder: A Systematic Review and Network Meta-analysis. Lancet 2018, 391 (10128), 1357–1366. 10.1016/S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y.; You Y.; He D.; Chen F.; Chang X.; Yu M. J.; Xing X. Asymmetric Synthesis of γ-Secondary Amino Alcohols via a Borrowing-Hydrogen Cascade. Org. Lett. 2020, 22 (18), 7278–7283. 10.1021/acs.orglett.0c02614. [DOI] [PubMed] [Google Scholar]
- Lin W. H.; Chang H. F. A Study of Ethanol Dehydrogenation Reaction in a Palladium Membrane Reactor. Catal. Today 2004, 97 (2–3), 181–188. 10.1016/j.cattod.2004.03.068. [DOI] [Google Scholar]
- Neumann J.; Elangovan S.; Spannenberg A.; Junge K.; Beller M. Improved and General Manganese-Catalyzed N-Methylation of Aromatic Amines Using Methanol. Chem. - Eur. J. 2017, 23 (23), 5410–5413. 10.1002/chem.201605218. [DOI] [PubMed] [Google Scholar]
- Dang T. T.; Ramalingam B.; Seayad A. M. Efficient Ruthenium-Catalyzed N-Methylation of Amines Using Methanol. ACS Catal. 2015, 5 (7), 4082–4088. 10.1021/acscatal.5b00606. [DOI] [Google Scholar]
- Liu Z.; Yang Z.; Yu X.; Zhang H.; Yu B.; Zhao Y.; Liu Z. Efficient Cobalt-Catalyzed Methylation of Amines Using Methanol. Adv. Synth. Catal. 2017, 359 (24), 4278–428. 10.1002/adsc.201701044. [DOI] [Google Scholar]
- Deng D.; Hu B.; Yang M.; Chen D. Methylation of Amines and Ketones with Methanol Catalyzed by an Iridium Complex Bearing a 2-Hydroxypyridylmethylene Fragment. Organometallics 2018, 37 (19), 3353–3359. 10.1021/acs.organomet.8b00575. [DOI] [Google Scholar]
- Fujita T.; Ogura K.; Niwa K.; Fukatsu M.; Production of methylamines. Eur. Pat. Office 0763519 A2, September 11, 1996.
- Bosch M.; Eberhardt J.; Röttger R.; Krug T.; Melder J.-P.. Method for the continuous synthesis of methylamines. World Int. Pat. Office 2005/123658 A1, June 16, 2005.
- Vayer M.; Morcillo S. P.; Dupont J.; Gandon V.; Bour C. Iron-Catalyzed Reductive Ethylation of Imines with Ethanol. Angew. Chem., Int. Ed. 2018, 57 (12), 3228–3232. 10.1002/anie.201800328. [DOI] [PubMed] [Google Scholar]
- Lator A.; Gaillard S.; Poater A.; Renaud J. L. Well-Defined Phosphine-Free Iron-Catalyzed N-Ethylation and N-Methylation of Amines with Ethanol and Methanol. Org. Lett. 2018, 20 (19), 5985–5990. 10.1021/acs.orglett.8b02080. [DOI] [PubMed] [Google Scholar]
- Saidi O.; Blacker A. J.; Farah M. M.; Marsden S. P.; Williams J. M. J. Selective Amine Cross-Coupling Using Iridium-Catalyzed “Borrowing Hydrogen” Methodology. Angew. Chem., Int. Ed. 2009, 48 (40), 7375–7378. 10.1002/anie.200904028. [DOI] [PubMed] [Google Scholar]
- Miyazawa A.; Saitou K.; Tanaka K.; Gädda T. M.; Tashiro M.; Prakash G. K. S.; Olah G. A. Reaction of Primary Amines with Pt/C Catalyst in Water under Microwave Irradiation: A Convenient Synthesis of Secondary Amines from Primary Amines. Tetrahedron Lett. 2006, 47 (9), 1437–1439. 10.1016/j.tetlet.2005.12.075. [DOI] [Google Scholar]
- Hollmann D.; Bähn S.; Tillack A.; Beller M. A General Ruthenium-Catalyzed Synthesis of Aromatic Amines. Angew. Chem., Int. Ed. 2007, 46 (43), 8291–8294. 10.1002/anie.200703119. [DOI] [PubMed] [Google Scholar]
- Zou Q.; Wang C.; Smith J.; Xue D.; Xiao J. Alkylation of Amines with Alcohols and Amines by a Single Catalyst under Mild Conditions. Chem. - Eur. J. 2015, 21 (27), 9656–9661. 10.1002/chem.201501109. [DOI] [PubMed] [Google Scholar]
- Yin Z.; Zeng H.; Wu J.; Zheng S.; Zhang G. Cobalt-Catalyzed Synthesis of Aromatic, Aliphatic, and Cyclic Secondary Amines via a “Hydrogen-Borrowing” Strategy. ACS Catal. 2016, 6 (10), 6546–6550. 10.1021/acscatal.6b02218. [DOI] [Google Scholar]
- Chung H.; Han S.; Chung Y. K.; Park J. H. Conversion of Primary Amines to Symmetrical Secondary and Tertiary Amines Using a Co-Rh Heterobimetallic Nanocatalyst. Adv. Synth. Catal. 2018, 360 (6), 1267–1272. 10.1002/adsc.201701522. [DOI] [Google Scholar]
- Frauenkron M.; Krug T.; Evers H.; Melder J.-P.; Röttger R.; Siegert M.; Gerlach T.; Nouwen J.; Dahlhoff E.; Miller C.. Method for producing ethylene-amines. World Int. Pat. Office 2005/12223 A1, February 10, 2005.
- Melder J.-P.; Krug T.. Method for producing bis-[(3-dimethylamino)propyl]amine (BISDMAPA). World Int. Pat. Office 2006/082202 A1, August 10, 2005.
- van Cauwenberge G.; Melder J.-P.; Evers H.; Gerlach T.; Kiesslich F.; Schwab E.; Hoffer B. W.. Process for producing ethyleneamines. U.S. Patent 7,696,384, April 13, 2010.
- Cho C. S.; Kim B. T.; Kim T. J.; Chul Shim S. Ruthenium-Catalyzed Regioselective α-Alkylation of Ketones with Primary Alcohols. Tetrahedron Lett. 2002, 43 (44), 7987–7989. 10.1016/S0040-4039(02)01625-8. [DOI] [Google Scholar]
- Taguchi K.; Nakagawa H.; Hirabayashi T.; Sakaguchi S.; Ishii Y. An Efficient Direct α-Alkylation of Ketones with Primary Alcohols Catalyzed by [Ir(cod)Cl]2/PPh3/KOH System without Solvent. J. Am. Chem. Soc. 2004, 126 (1), 72–73. 10.1021/ja037552c. [DOI] [PubMed] [Google Scholar]
- Cho C. S. A Palladium-Catalyzed Route for α-Alkylation of Ketones by Primary Alcohols. J. Mol. Catal. A Chem. 2005, 240 (1–2), 55–60. 10.1016/j.molcata.2005.06.043. [DOI] [Google Scholar]
- Alonso F.; Riente P.; Yus M. The α-Alkylation of Methyl Ketones with Primary Alcohols Promoted by Nickel Nanoparticles under Mild and Ligandless Conditions. Synlett 2007, 2007, 1877–1880. 10.1055/s-2007-984522. [DOI] [Google Scholar]
- Buil M. L.; Esteruelas M. A.; Herrero J.; Izquierdo S.; Pastor I. M.; Yus Y. Osmium Catalyst for the Borrowing Hydrogen Methodology: α-Alkylation of Arylacetonitriles and Methyl Ketones. ACS Catal. 2013, 3 (9), 2072–2075. 10.1021/cs4005375. [DOI] [Google Scholar]
- Yu X.; Wang Q. Y.; Wu Q. J.; Wang D. W. Rhodium-Catalyzed Alkylation of Ketones and Alcohols with Alcohols. Russ. J. Gen. Chem. 2016, 86 (1), 178–183. 10.1134/S107036321601028X. [DOI] [Google Scholar]
- Elangovan S.; Sortais J. B.; Beller M.; Darcel C. Iron-Catalyzed α-Alkylation of Ketones with Alcohols. Angew. Chem., Int. Ed. 2015, 54 (48), 14483–14486. 10.1002/anie.201506698. [DOI] [PubMed] [Google Scholar]
- Peña-López M.; Piehl P.; Elangovan S.; Neumann H.; Beller M. Manganese-Catalyzed Hydrogen-Autotransfer C–C Bond Formation: α-Alkylation of Ketones with Primary Alcohols. Angew. Chem., Int. Ed. 2016, 55 (48), 14967–14971. 10.1002/anie.201607072. [DOI] [PubMed] [Google Scholar]
- Piehl P.; Peña-López M.; Frey A.; Neumann H.; Beller M. Hydrogen Autotransfer and Related Dehydrogenative Coupling Reactions using a Rhenium(I) Pincer Catalyst. Chem. Commun. 2017, 53 (22), 3265–3268. 10.1039/C6CC09977G. [DOI] [PubMed] [Google Scholar]
- Zhang G.; Wu J.; Zeng H.; Zhang S.; Yin Z.; Zheng S. Cobalt-Catalyzed α-Alkylation of Ketones with Primary Alcohols. Org. Lett. 2017, 19 (5), 1080–1083. 10.1021/acs.orglett.7b00106. [DOI] [PubMed] [Google Scholar]
- Das J.; Singh K.; Vellakkaran M.; Banerjee D. Nickel-Catalyzed Hydrogen-Borrowing Strategy for α-Alkylation of Ketones with Alcohols: A New Route to Branched gem-Bis(alkyl) Ketones. Org. Lett. 2018, 20 (18), 5587–5591. 10.1021/acs.orglett.8b02256. [DOI] [PubMed] [Google Scholar]
- Fleming F. F.; Yao L.; Ravikumar P. C.; Funk L.; Shook B. C. Nitrile-Containing Pharmaceuticals: Efficacious Roles of the Nitrile Pharmacophore. J. Med. Chem. 2010, 53 (22), 7902–7917. 10.1021/jm100762r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buil M. L.; Esteruelas M. A.; Herrero J.; Izquierdo S.; Pastor I. S.; Yus M. Osmium Catalyst for the Borrowing Hydrogen Methodology: α-Alkylation of Arylacetonitriles and Methyl Ketones. ACS Catal. 2013, 3 (9), 2072–2075. 10.1021/cs4005375. [DOI] [Google Scholar]
- Thiyagarajan S.; Gunanathan C. Facile Ruthenium(II)-Catalyzed α-Alkylation of Arylmethyl Nitriles Using Alcohols Enabled by Metal-Ligand Cooperation. ACS Catal. 2017, 7 (8), 5483–5490. 10.1021/acscatal.7b01427. [DOI] [Google Scholar]
- Smallridge A. J.; Ten A.; Trewhella M. A. Enzymatic Alkylation of α-Cyanoketones by Bakers Yeast. Tetrahedron Lett. 1998, 39 (28), 5121–5124. 10.1016/S0040-4039(98)00945-9. [DOI] [Google Scholar]
- Jana A.; Reddy C. B.; Maji B. Manganese Catalyzed α-Alkylation of Nitriles with Primary Alcohols. ACS Catal. 2018, 8 (10), 9226–9231. 10.1021/acscatal.8b02998. [DOI] [Google Scholar]
- Ma W.; Cui S.; Sun H.; Tang W.; Xue D.; Li C.; Fan J.; Xiao J.; Wang C. Iron-Catalyzed Alkylation of Nitriles with Alcohols. Chem. - Eur. J. 2018, 24 (50), 13118–13123. 10.1002/chem.201803762. [DOI] [PubMed] [Google Scholar]
- Bera S.; Bera A.; Banerjee D. Nickel-Catalyzed Hydrogen-Borrowing Strategy: Chemoselective Alkylation of Nitriles with Alcohols. Chem. Commun. 2020, 56 (50), 6850–6853. 10.1039/D0CC02261F. [DOI] [PubMed] [Google Scholar]
- Iuchi Y.; Obora Y.; Ishii Y. Iridium-Catalyzed α-Alkylation of Acetates with Primary Alcohols and Diols. J. Am. Chem. Soc. 2010, 132 (8), 2536–2537. 10.1021/ja9106989. [DOI] [PubMed] [Google Scholar]
- Guo L.; Liu Y.; Yao W.; Leng X.; Huang Z. Iridium-Catalyzed Selective α-Alkylation of Unactivated Amides with Primary Alcohols. Org. Lett. 2013, 15 (5), 1144–1147. 10.1021/ol400360g. [DOI] [PubMed] [Google Scholar]
- Guo L.; Ma X.; Fang H.; Jia X.; Huang Z. A General and Mild Catalytic α-Alkylation of Unactivated esters using Alcohols. Angew. Chem., Int. Ed. 2015, 54 (13), 4023–4027. 10.1002/anie.201410293. [DOI] [PubMed] [Google Scholar]
- Deibl N.; Kempe R. General and Mild Cobalt-Catalyzed C-Alkylation of Unactivated Amides and Esters with Alcohols. J. Am. Chem. Soc. 2016, 138 (34), 10786–10789. 10.1021/jacs.6b06448. [DOI] [PubMed] [Google Scholar]
- Chakraborty S.; Daw P.; Ben David Y.; Milstein D. Manganese-Catalyzed α-Alkylation of Ketones, Esters, and Amides Using Alcohols. ACS Catal. 2018, 8 (11), 10300–10305. 10.1021/acscatal.8b03720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang Y. K.; Krückel T.; Rueping M.; El-Sepelgy O. Sustainable Alkylation of Unactivated Esters and Amides with Alcohols Enabled by Manganese Catalysis. Org. Lett. 2018, 20 (24), 7779–7783. 10.1021/acs.orglett.8b03184. [DOI] [PubMed] [Google Scholar]
- Midya S.; Rana J.; Pitchaimani J.; Nandakumar A.; Madhu V.; Balaraman E. Ni-Catalyzed α-Alkylation of Unactivated Amides and Esters with Alcohols by Hydrogen Auto-Transfer Strategy. ChemSusChem 2018, 11 (22), 3911–3916. 10.1002/cssc.201801443. [DOI] [PubMed] [Google Scholar]
- Seck C.; Mbaye M. D.; Gaillard S.; Renaud J. L. Bifunctional Iron Complexes Catalyzed Alkylation of Indoles. Adv. Synth. Catal. 2018, 360 (23), 4640–4645. 10.1002/adsc.201800924. [DOI] [Google Scholar]
- Bains A. K.; Biswas A.; Adhikari D. Nickel-Catalysed Chemoselective C-3 Alkylation of Indoles with Alcohols through a Borrowing Hydrogen Method. Chem. Commun. 2020, 56 (98), 15442–15445. 10.1039/D0CC07169B. [DOI] [PubMed] [Google Scholar]
- Dambatta M. B.; Polidano K.; Northey A. D.; Williams J. M. J.; Morrill L. C. Iron-Catalyzed Borrowing Hydrogen C-Alkylation of Oxindoles with Alcohols. ChemSusChem 2019, 12 (11), 2345–2349. 10.1002/cssc.201900799|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellakkaran M.; Das J.; Bera S.; Banerjee D. Nickel-Catalysed Alkylation of C(sp3)–H bonds with Alcohols: Direct Access to Functionalised N-heteroaromatics. Chem. Commun. 2018, 54 (87), 12369–12372. 10.1039/C8CC06370B. [DOI] [PubMed] [Google Scholar]
- Mishra A.; Dwivedi A. D.; Shee S.; Kundu S. Cobalt-Catalyzed Alkylation of Methyl-substituted N-heteroarenes with Primary Alcohols: Direct Access to Functionalized N-heteroaromatics. Chem. Commun. 2020, 56 (2), 249–252. 10.1039/C9CC08448G. [DOI] [PubMed] [Google Scholar]
- Kabadwal L. M.; Bera S.; Banerjee D. Iron-Catalysed Alkylation of 2-methyl and 4-methyl Azaarenes with Alcohols via C–H Bond Activation. Chem. Commun. 2020, 56 (35), 4777–47804. 10.1039/D0CC01593H. [DOI] [PubMed] [Google Scholar]
- Mastalir M.; Pittenauer E.; Allmaier G.; Kirchner K. Manganese-Catalyzed Aminomethylation of Aromatic Compounds with Methanol as a Sustainable C1 Building Block. J. Am. Chem. Soc. 2017, 139 (26), 8812–8815. 10.1021/jacs.7b05253. [DOI] [PubMed] [Google Scholar]
- Waiba S.; Das A.; Barman M. K.; Maji B. Base Metal-Catalyzed Direct Olefinations of Alcohols with Sulfones. ACS Omega 2019, 4 (4), 7082–7087. 10.1021/acsomega.9b00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P.; Wang X.; Ma Y.; Sun Y.; Zhang L.; Yue J.; Fu K.; Zhou J. S.; Tang B. Nickel-Catalyzed C-Alkylation of Thioamide, Amides and Esters by Primary Alcohols through a Hydrogen Autotransfer Strategy. Chem. Commun. 2020, 56 (90), 14083–14086. 10.1039/D0CC06468H. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T.; Obora Y. Iridium-catalyzed α-Alkylation of Acetonitrile with Primary and Secondary Alcohols. Chem. Lett. 2011, 40 (9), 1055–1057. 10.1246/cl.2011.1055. [DOI] [Google Scholar]
- Akhtar W. M.; Cheong C. B.; Frost J. R.; Christensen K. E.; Stevenson N. G.; Donohoe T. J. Hydrogen Borrowing Catalysis with Secondary Alcohols: A New Route for the Generation of β-Branched Carbonyl Compounds. J. Am. Chem. Soc. 2017, 139 (7), 2577–2580. 10.1021/jacs.6b12840. [DOI] [PubMed] [Google Scholar]
- Frost J. R.; Cheong C. B.; Akhtar W. M.; Caputo D. F. J.; Stevenson N. G.; Donohoe T. J. Strategic Application and Transformation of ortho-Disubstituted Phenyl and Cyclopropyl Ketones to Expand the Scope of Hydrogen Borrowing Catalysis. J. Am. Chem. Soc. 2015, 137 (50), 15664–15667. 10.1021/jacs.5b11196. [DOI] [PubMed] [Google Scholar]
- Scognamiglio J.; Jones L.; Letizia C. S.; Api A. M. Fragrance Material Review on 3-Methyl-5-Phenylpentanol. Food Chem. Toxicol. 2012, 50 (2), S215–S219. 10.1016/j.fct.2011.10.026. [DOI] [PubMed] [Google Scholar]
- Chakraborty P.; Gangwar M. K.; Emayavaramban B.; Manoury E.; Poli R.; Sundararaju B. α-Alkylation of Ketones with Secondary Alcohols Catalyzed by Well-Defined Cp*CoIII-Complexes. ChemSusChem 2019, 12 (15), 3463–3467. 10.1002/cssc.201900990. [DOI] [PubMed] [Google Scholar]
- Bettoni L.; Gaillard S.; Renaud J. L. Iron-Catalyzed α-Alkylation of Ketones with Secondary Alcohols: Access to β-Disubstituted Carbonyl Compounds. Org. Lett. 2020, 22 (5), 2064–2069. 10.1021/acs.orglett.0c00549. [DOI] [PubMed] [Google Scholar]
- Waiba S.; Jana S. K.; Jati A.; Jana A.; Maji B. Manganese complex-catalysed α-alkylation of ketones with secondary alcohols enables the synthesis of β-branched carbonyl compounds. Chem. Commun. 2020, 56 (60), 8376–8379. 10.1039/D0CC01460E. [DOI] [PubMed] [Google Scholar]
- Dambatta M. B.; Santos J.; Bolt R.; Morrill L. M. Transition Metal Free α-C-alkylation of Ketones Using Secondary Alcohols. Tetrahedron 2020, 76 (45), 131571. 10.1016/j.tet.2020.131571. [DOI] [Google Scholar]
- Akhtar W. M.; Armstrong R. J.; Frost J. R.; Stevenson N. G.; Donohoe T. J. Stereoselective Synthesis of Cyclohexanes via an Iridium Catalyzed (5 + 1) Annulation Strategy. J. Am. Chem. Soc. 2018, 140 (38), 11916–11920. 10.1021/jacs.8b07776. [DOI] [PubMed] [Google Scholar]
- Armstrong R. J.; Akhtar W. M.; Young T. A.; Duarte F.; Donohoe T. J. Catalytic Asymmetric Synthesis of Cyclohexanes by Hydrogen Borrowing Annulations. Angew. Chem., Int. Ed. 2019, 58 (36), 12558–12562. 10.1002/anie.201907514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheang D. M. J.; Armstrong R. J.; Akhtar W. M.; Donohoe T. J. Enantioconvergent Alkylation of Ketones with Racemic Secondary Alcohols: Via Hydrogen Borrowing Catalysis. Chem. Commun. 2020, 56 (24), 3543–3546. 10.1039/D0CC00767F. [DOI] [PubMed] [Google Scholar]
- Kaithal A.; Gracia L.-L.; Camp C.; Quadrelli E. A.; Leitner W. Direct Synthesis of Cycloalkanes from Diols and Secondary Alcohols or Ketones Using a Homogeneous Manganese Catalyst. J. Am. Chem. Soc. 2019, 141 (44), 17487–17492. 10.1021/jacs.9b08832. [DOI] [PubMed] [Google Scholar]
- Jana A.; Das K.; Kundu A.; Thorve P. R.; Adhikari D.; Maji B. A Phosphine-Free Manganese Catalyst Enables Stereoselective Synthesis of (1 + n)-Membered Cycloalkanes from Methyl Ketones and 1,n-Diols. ACS Catal. 2020, 10 (4), 2615–2626. 10.1021/acscatal.9b05567. [DOI] [Google Scholar]
- Bettoni L.; Gaillard S.; Renaud J. L. A phosphine-free iron complex-catalyzed synthesis of cycloalkanes via the borrowing hydrogen strategy. Chem. Commun. 2020, 56 (85), 12909–12912. 10.1039/D0CC05840H. [DOI] [PubMed] [Google Scholar]
- Thiyagarajan S.; Vijaya Sankar R.; Gunanathan C. Ruthenium-Catalyzed α-Alkylation of Ketones Using Secondary Alcohols to β-Disubstituted Ketones. Org. Lett. 2020, 22 (20), 7879–7884. 10.1021/acs.orglett.0c02787. [DOI] [PubMed] [Google Scholar]
- Chan L. K. M.; Poole D. L.; Shen D.; Healy M. P.; Donohoe T. J. Rhodium-catalyzed Ketone Methylation using Methanol under Mild Conditions: Formation of α-Branched Products. Angew. Chem., Int. Ed. 2014, 53 (3), 761–765. 10.1002/anie.201307950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.; Yang Z.; Yu X.; Zhang H.; Yu B.; Zhao Y.; Liu Z. Methylation of C(sp3)–H/C(sp2)–H Bonds with Methanol Catalyzed by Cobalt System. Org. Lett. 2017, 19 (19), 5228–5231. 10.1021/acs.orglett.7b02462. [DOI] [PubMed] [Google Scholar]
- Polidano K.; Allen B. D. W.; Williams J. M. J.; Morrill L. C. Iron-Catalyzed Methylation Using the Borrowing Hydrogen Approach. ACS Catal. 2018, 8 (7), 6440–6445. 10.1021/acscatal.8b02158. [DOI] [Google Scholar]
- Bruneau-Voisine A.; Pallova L.; Bastin S.; César V.; Sortais J. B. Manganese Catalyzed α-Methylation of Ketones with Methanol as a C1 Source. Chem. Commun. 2019, 55 (3), 314–317. 10.1039/C8CC08064J. [DOI] [PubMed] [Google Scholar]
- Sklyaruk J.; Borghs J. C.; El-Sepelgy O.; Rueping M. Catalytic C1 Alkylation with Methanol and Isotope-Labeled Methanol. Angew. Chem., Int. Ed. 2019, 58 (3), 775–779. 10.1002/anie.201810885. [DOI] [PubMed] [Google Scholar]
- Cho C. S.; Kim B. T.; Kim H. S.; Kim T. J.; Shim S. C. Ruthenium-catalyzed one-pot β-alkylation of secondary alcohols with primary alcohols. Organometallics 2003, 22 (17), 3608–3610. 10.1021/om030307h. [DOI] [Google Scholar]
- Martínez R.; Ramón D. J.; Yus M. RuCl2(DMSO)4 Catalyzes the β-Alkylation of Secondary Alcohols with Primary Alcohols through a Hydrogen Auto-Transfer Process. Tetrahedron 2006, 62 (38), 8982–8987. 10.1016/j.tet.2006.07.012. [DOI] [Google Scholar]
- Shimizu K. I.; Sato R.; Satsuma A. Direct C-C Cross-Coupling of Secondary and Primary Alcohols Catalyzed by a γ-Alumina-Supported Silver Subnanocluster. Angew. Chem., Int. Ed. 2009, 48 (22), 3982–3986. 10.1002/anie.200901057. [DOI] [PubMed] [Google Scholar]
- Kose O.; Saito S. Cross-Coupling Reaction of Alcohols for Carbon-Carbon Bond Formation Using Pincer-Type NHC/Palladium Catalysts. Org. Biomol. Chem. 2010, 8 (4), 896–900. 10.1039/B914618K. [DOI] [PubMed] [Google Scholar]
- Yang J.; Liu X.; Meng D.-L.; Chen H. Y.; Zong Z. H.; Feng T.-T.; Sun K. Efficient Iron-Catalyzed Direct β-Alkylation of Secondary Alcohols with Primary Alcohols. Adv. Synth. Catal. 2012, 354 (2–3), 328–334. 10.1002/adsc.201000907. [DOI] [Google Scholar]
- Liao S.; Yu K.; Li Q.; Tian H.; Zhang Z.; Yu X.; Xu Q. Copper-Catalyzed C-Alkylation of Secondary Alcohols and Methyl Ketones with Alcohols Employing the Aerobic Relay Race Methodology. Org. Biomol. Chem. 2012, 10 (15), 2973–2978. 10.1039/c1ob06739g. [DOI] [PubMed] [Google Scholar]
- Freitag F.; Irrgang T.; Kempe R. Cobalt-Catalyzed Alkylation of Secondary Alcohols with Primary Alcohols via Borrowing Hydrogen/Hydrogen Autotransfer. Chem. - Eur. J. 2017, 23 (50), 12110–12113. 10.1002/chem.201701211. [DOI] [PubMed] [Google Scholar]
- Liu T.; Wang L.; Wu K.; Yu Z. Manganese-Catalyzed β-Alkylation of Secondary Alcohols with Primary Alcohols under Phosphine-Free Conditions. ACS Catal. 2018, 8 (8), 7201–7207. 10.1021/acscatal.8b01960. [DOI] [Google Scholar]
- Thiyagarajan S.; Gunanathan C. Catalytic Cross-Coupling of Secondary Alcohols. J. Am. Chem. Soc. 2019, 141 (9), 3822–3827. 10.1021/jacs.9b00025. [DOI] [PubMed] [Google Scholar]
- Oikawa K.; Itoh S.; Yano H.; Kawasaki H.; Obora Y. Preparation and Use of DMF-Stabilized Iridium Nanoclusters as Methylation Catalysts Using Methanol as the C1 Source. Chem. Commun. 2017, 53 (6), 1080–1083. 10.1039/C6CC09279A. [DOI] [PubMed] [Google Scholar]
- Siddiki S. M. A. H.; Touchy A. S.; Jamil Md. A.R.; Toyao T.; Shimizu K. I. C-Methylation of Alcohols, Ketones, and Indoles with Methanol Using Heterogeneous Platinum Catalysts. ACS Catal. 2018, 8 (4), 3091–3103. 10.1021/acscatal.7b04442. [DOI] [Google Scholar]
- Li Y.; Li H.; Junge H.; Beller M. Selective Ruthenium-Catalyzed Methylation of 2-Arylethanols using Methanol as C1 Feedstock. Chem. Commun. 2014, 50 (95), 14991–14994. 10.1039/C4CC06933A. [DOI] [PubMed] [Google Scholar]
- Polidano K.; Williams J. M. J.; Morrill L. C. Iron-Catalyzed Borrowing Hydrogen β-C(sp3)-Methylation of Alcohols. ACS Catal. 2019, 9 (9), 8575–8580. 10.1021/acscatal.9b02461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettoni L.; Gaillard S.; Renaud J. L. Iron-Catalyzed β-Alkylation of Alcohols. Org. Lett. 2019, 21 (20), 8404–8408. 10.1021/acs.orglett.9b03171. [DOI] [PubMed] [Google Scholar]
- Kaithal A.; van Bonn P.; Hölscher M.; Leitner W. Manganese(I)-Catalyzed β-Methylation of Alcohols Using Methanol as C1 Source. Angew. Chem., Int. Ed. 2020, 59 (1), 215–220. 10.1002/anie.201909035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlagbauer M.; Kallmeier F.; Irrgang T.; Kempe R. Manganese-Catalyzed β-Methylation of Alcohols by Methanol. Angew. Chem., Int. Ed. 2020, 59 (4), 1485–1490. 10.1002/anie.201912055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T. W.; Liao G.; Lau K. K.; Pan H.-J; Zhao Y. Room-Temperature Guerbet Reaction with Unprecedented Catalytic Efficiency and Enantioselectivity. Angew. Chem., Int. Ed. 2020, 59 (28), 11384–11389. 10.1002/anie.202004758. [DOI] [PubMed] [Google Scholar]
- Shermer D. J.; Slatford P. A.; Edney D. D.; Williams J. M.J. Borrowing Hydrogen in an Indirect Asymmetric Wittig Reaction. Tetrahedron: Asymmetry 2007, 18 (24), 2845–2848. 10.1016/j.tetasy.2007.11.019. [DOI] [Google Scholar]
- Quintard A.; Constantieux T.; Rodriguez J. An Iron/Amine-Catalyzed Cascade Process for the Enantioselective Functionalization of Allylic Alcohols. Angew. Chem., Int. Ed. 2013, 52 (49), 12883–12887. 10.1002/anie.201307295. [DOI] [PubMed] [Google Scholar]
- Quintard A.; Roudier M.; Rodriguez J. Multicatalytic Enantioselective Borrowing Hydrogen δ-Lactonization Strategy from β-Keto Esters and Allylic Alcohols. Synthesis 2018, 50 (04), 785–792. 10.1055/s-0036-1588547. [DOI] [Google Scholar]
- Wang K.; Zhang L.; Tang W.; Sun H.; Xue D.; Lei M.; Xiao J.; Wang C. Asymmetric Guerbet Reaction to Access Chiral Alcohols. Angew. Chem., Int. Ed. 2020, 59 (28), 11408–11415. 10.1002/anie.202003104. [DOI] [PubMed] [Google Scholar]
- Çelebi Y.; Aydın H. An Overview on the Light Alcohol Fuels in Diesel Engines. Fuel 2019, 236, 890–911. 10.1016/j.fuel.2018.08.138. [DOI] [Google Scholar]
- Trindade W. R. da S.; Santos R. G. d. Review on the Characteristics of Butanol, its Production and use as Fuel in Internal Combustion Engines. Renewable Sustainable Energy Rev. 2017, 69, 642–651. 10.1016/j.rser.2016.11.213. [DOI] [Google Scholar]
- Veza I.; Said M. F. M.; Latiff Z. A. Progress of Acetone-Butanol-Ethanol (ABE) as Biofuel in Gasoline and Diesel Engine: A Review. Fuel Process. Technol. 2019, 196, 106179. 10.1016/j.fuproc.2019.106179. [DOI] [Google Scholar]
- Mužíková Z.; Šimáček P.; Pospíšil M.; Šebor G. Density, Viscosity and Water Phase Stability of 1-Butanol-Gasoline Blends. J. Fuels 2014, 2014, 459287. 10.1155/2014/459287. [DOI] [Google Scholar]
- Guerbet M. C. Action de l’Alcool Amylique de Fermentation sur son Dérivé Sodé. R. Acad. Sci. Paris 1899, 128, 1002–1008. [Google Scholar]
- Guerbet M. C. Condensation de l’Alcool Isopropylique avec son Dérivé Sodé; Formation du Méthylisobutylcarbinol et du Diméthyl-2,4-heptanol-6R. Acad. Sci. Paris 1909, 149, 129–132. [Google Scholar]
- Matsu-Ura T.; Sakaguchi S.; Obora Y.; Ishii Y. Guerbet Reaction of Primary Alcohols Leading to β-Alkylated Dimer Alcohols Catalyzed by Iridium complexes. J. Org. Chem. 2006, 71 (21), 8306–8308. 10.1021/jo061400t. [DOI] [PubMed] [Google Scholar]
- Obora Y.; Anno Y.; Okamoto R.; Matsu-Ura T.; Ishii Y. Iridium-Catalyzed Reactions of ω-Arylalkanols to α,ω-Di-arylalkanes. Angew. Chem., Int. Ed. 2011, 50 (37), 8618–8622. 10.1002/anie.201104452. [DOI] [PubMed] [Google Scholar]
- Tseng K. T.; Lin S.; Kampf J. W.; Szymczak N. K. Upgrading Ethanol to 1-Butanol with a Homogeneous Air-Stable Ruthenium Catalyst. Chem. Commun. 2016, 52 (14), 2901–2904. 10.1039/C5CC09913G. [DOI] [PubMed] [Google Scholar]
- Xie Y.; Ben-David Y.; Shimon L. J. W.; Milstein D. Highly Efficient Process for Production of Biofuel from Ethanol Catalyzed by Ruthenium Pincer Complexes. J. Am. Chem. Soc. 2016, 138 (29), 9077–9080. 10.1021/jacs.6b05433. [DOI] [PubMed] [Google Scholar]
- Aitchison H.; Wingad R. L.; Wass D. F. Homogeneous Ethanol to Butanol Catalysis - Guerbet Renewed. ACS Catal. 2016, 6 (10), 7125–7132. 10.1021/acscatal.6b01883. [DOI] [Google Scholar]
- Fu S.; Shao Z.; Wang Y.; Liu Q. Manganese-Catalyzed Upgrading of Ethanol into 1-Butanol. J. Am. Chem. Soc. 2017, 139 (34), 11941–11948. 10.1021/jacs.7b05939. [DOI] [PubMed] [Google Scholar]
- Lichosyt D.; Zhang Y.; Hurej K.; Dydio P. Dual-Catalytic Transition Metal Systems for Functionalization of Unreactive Sites of Molecules. Nat. Catal. 2019, 2, 114–122. 10.1038/s41929-018-0207-1. [DOI] [Google Scholar]
- Nguyen K. D.; Park B. Y.; Luong T.; Sato H.; Garza V. J.; Krische M. J. Metal-catalyzed reductive coupling of olefin-derived nucleophiles: Reinventing carbonyl addition. Science 2016, 354 (6310), aah5133. 10.1126/science.aah5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J. C.; Geary L. M.; Chen T.-Y.; Zbieg J. R.; Krische M. J. Direct, Redox-Neutral Prenylation and Geranylation of Secondary Carbinol C–H Bonds: C4-Regioselectivity in Ruthenium- Catalyzed C–C Couplings of Dienes to α-Hydroxy Esters. J. Am. Chem. Soc. 2012, 134 (38), 15700–15703. 10.1021/ja3075049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B. Y.; Montgomery T. P.; Garza V. J.; Krische M. J. Ruthenium Catalyzed Hydrohydroxyalkylation of Isoprene with Heteroaromatic Secondary Alcohols: Isolation and Reversible Formation of the Putative Metallacycle Intermediate. J. Am. Chem. Soc. 2013, 135 (44), 16320–16323. 10.1021/ja4087193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.-Y.; Krische M. J. Regioselective Ruthenium Catalyzed Hydrohydroxyalkylation of Dienes with 3-Hydroxy-2- oxindoles: Prenylation, Geranylation, and Beyond. Org. Lett. 2013, 15 (12), 2994–2997. 10.1021/ol401184k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corma A.; Navas J.; Rõdenas T.; Sabater M. J. One-Pot Palladium-Catalyzed Borrowing Hydrogen Synthesis of Thioethers. Chem. - Eur. J. 2013, 19 (51), 17464–17471. 10.1002/chem.201302226. [DOI] [PubMed] [Google Scholar]
- Pirali T.; Serafini M.; Cargnin S.; Genazzani A. A. Applications of Deuterium in Medicinal Chemistry. J. Med. Chem. 2019, 62 (11), 5276–5297. 10.1021/acs.jmedchem.8b01808. [DOI] [PubMed] [Google Scholar]
- Kar S.; Goeppert A.; Sen R.; Kothandaraman J.; Surya Prakash G. K. Regioselective Deuteration of Alcohols in D2O Catalysed by Homogeneous Manganese and Iron Pincer Complexes. Green Chem. 2018, 20 (12), 2706–2710. 10.1039/C8GC01052H. [DOI] [Google Scholar]
- Wübbolt S.; Cheong C. B.; Frost J. R.; Christensen K. E.; Donohoe T. J. A Vinyl Cyclopropane Ring Expansion and Iridium-Catalyzed Hydrogen Borrowing Cascade. Angew. Chem., Int. Ed. 2020, 59 (28), 11339–11344. 10.1002/anie.202003614. [DOI] [PMC free article] [PubMed] [Google Scholar]