Abstract
The normal variability in breath size and frequency results in breath-to-breath variability of end-tidal PCO2 (PETCO2), the measured variable, and arterial partial pressure of carbon dioxide (PaCO2), the independent variable affecting cerebral blood flow (CBF). This study examines the effect of variability in PaCO2 on the pattern of resting-state functional MRI (rs-fMRI) connectivity. A region of interest (ROI)-to-ROI and Seed-to-Voxel first-level bivariate correlation, hemodynamic response function (hrf)-weighted analysis for measuring rs-fMRI connectivity was performed during two resting-state conditions: (a) normal breathing associated with breath-to-breath variation in PaCO2 (poikilocapnia), and (b) normal breathing with breath-to-breath variability of PETCO2 dampened using sequential rebreathing (isocapnia). End-tidal PCO2 (PETCO2) was used as a measurable surrogate for fluctuations of PaCO2. During poikilocapnia, enhanced functional connections were found between the cerebellum and inferior frontal and supramarginal gyrus (SG), visual cortex and occipital fusiform gyrus; and between the primary visual network (PVN) and the hippocampal formation. During isocapnia, these associations were not seen, rather enhanced functional connections were identified in the corticostriatal pathway between the putamen and intracalacarine cortex, supracalcarine cortex (SCC), and precuneus cortex. We conclude that vascular responses to variations in PETCO2, account for at least some of the observed resting state synchronization of blood oxygenation level-dependent (BOLD) signals.
Keywords: BOLD, end-tidal pressure of CO2, functional-connectivity, fMRI, resting-state
Introduction
The pioneering work of Biswal et al. (1995) showed that low-frequency (<0.1 Hz) fluctuations of EPI signal intensity were temporally synchronized across the non-contiguous motor cortex during resting-state functional MRI (rs-fMRI). This finding led to the development of a stimulus-free methodology for the assessment of functional brain networks. The correlated fluctuations within brain regions are thought to be synchronized variations in neuronal activity revealing functional connectivity between brain regions. However, one of the major challenges involved in the analysis of functional connectivity stems from problems separating the blood oxygenation level-dependent (BOLD) fMRI signals of neuronal stimulation, from those resulting from changes in arterial partial pressure of carbon dioxide (PaCO2). PaCO2 is a potent vasoactive molecule, which varies breath-to-breath due to differences in tidal volume during normal breathing (Wise et al., 2004; Birn et al., 2006; Madjar et al., 2012). Wise et al. (2004) found BOLD fMRI fluctuations in disparate brain regions and variability in the middle cerebral artery (MCA) flow velocity measured by transcranial Doppler that were correlated with changes in the end-tidal PCO2 (PETCO2), a non-invasive surrogate of PaCO2. The synchronous changes were widespread and bilaterally symmetrical, particularly in gray matter (GM) of the occipital, parietal, temporal, and cingulate cortex, and to a lesser extent in the white matter (WM) (Wise et al., 2004). PETCO2 fluctuations occur around 0.03 Hz in GM (Wise et al., 2004). This frequency of respiratory changes overlaps with resting-state brain activity frequency fluctuations of approximately 0.1 Hz, which are typically not filtered out by physiological noise correction routines and may confound resting-state neuronal functional connectivity measures. Eliminating PETCO2 fluctuations would dampen respiratory based synchronization leaving those of neuronal origin.
The relative contributions of neuronal and vascular factors comprising the BOLD signal during rs-fMRI are still unknown. In a pivotal study, Golestani et al. (2015) reported that spontaneous PETCO2 fluctuations act as strong modulators of the rs-fMRI signal contributing up to 15% of the total rs-fMRI signal variance based on a multi-regression model used to estimate the voxel-wise PETCO2 response functions. These CO2 fluctuations primarily affect fMRI signals in the GM, particularly in the occipital and temporal cortices in addition to subcortical structures including the precuneus, cingulate gyrus, and thalamus (Golestani et al., 2015).
Consequently, we studied BOLD signal synchronization in healthy volunteers under two conditions: (1) spontaneous breathing that is associated with normal PETCO2 variability (poikilocapnia), and (2) PETCO2 variability dampened by sequential gas delivery (isocapnia) (Fierstra et al., 2013; Fisher, 2016). We hypothesized that the regions showing synchronous BOLD fluctuations during poikilocapnia but not isocapnia are synchronized to PETCO2. The synchronized regions during isocapnia therefore likely reflect neuronal resting state functional connectivity under isocapnic breathing conditions.
Materials and Methods
Participants and Image Acquisition
This study conformed to the standards set by the latest revision of the Declaration of Helsinki and was approved by the Institution’s Research Ethics Board. Written informed consent was obtained from all participants. Ten healthy control participants with no history of neurological disorders [age range: 22–70; eight males; age mean (SD) 42.4 (18.68)] were recruited.
All images were acquired using a 3-Tesla GE MRI scanner (Signa HDx, GE Healthcare, Milwaukee, WI, United States), using an eight-channel phased array head coil. Participants had at least 10 min at rest in the scanner before the fMRI time series acquisition. High-resolution T1-weighted 3D spoiled gradient echo sequences were acquired with the following parameters: TR = 7.88 ms, TE = 3 ms, flip angle = 12°, 146 slices, voxel size = 0.85 × 0.85 × 1 mm, matrix size = 256 × 256, and field of view = 22 × 22 cm. Two BOLD fMRI echoplanar images were acquired, one for each resting-state condition with the following parameters: TR = 2400 ms, TE = 30 ms, flip angle = 70°, 250 volumes, 41 slices, isotropic voxel size = 3.5 mm, matrix size = 64 × 64, and field of view = 24 × 24 cm.
Control of Blood Gases During Spontaneous Breathing
The dampening of breath-to-breath PETCO2 and the end-tidal partial pressure of O2 (PETO2) was achieved using an automated gas blender that applies sequential gas delivery algorithms targeting resting PETCO2 (RespirAct™, Thornhill Research Inc., Canada; Slessarev et al., 2007; Fierstra et al., 2013; Fisher, 2016) thereby dampening physiologically significant breath-to-breath variation in PETCO2. Subjects breathed via a soft plastic mask sealed to their face using transparent dressing film (Tegaderm, 3M, St. Paul MN, United States) during poikilocapnia and isocapnia. For isocapnia, PETCO2 was controlled by targeting each subject’s resting PETCO2; average 38 mmHg (range 32–42 mmHg). PETO2 was targeted at 100 mmHg. During the poikilocapnic resting-state scan, participants breathed room air with no targeting of PETCO2 while PETO2 permitting physiological fluctuations as occurs in fMRI experiments. The sequence of isocapnia and poikilocapnia was randomized. All subjects indicated that they were unable to distinguish between the two resting-state conditions.
Data Preprocessing
Neuroimaging data were preprocessed and analyzed using SPM12 (The Wellcome Department of Cognitive Neurology, London, United Kingdom)1 running in Matlab v7.14 (The Mathworks Inc., United States). All functional volumes underwent slice-timing correction using sinc interpolation to temporally align the slices within each volume. Each volume was spatially realigned to the first volume acquired using a six-parameter rigid body transformation. Realignment motion parameters were set at 3 mm for translation and 1° for rotation relative to the first volume for exclusion criteria. Head movement was measured in three axes with 1.5 mm limits with all subjects meeting this criterion. High-resolution T1-weighted anatomical volumes were segmented into GM, WM, and cerebrospinal fluid (CSF), and were normalized to Montreal Neurological Institute (MNI) space using the normalized EPI image in SPM. Functional images were normalized into MNI space and smoothed with a spatial convolution 8 mm full-width half-maximum Gaussian kernel.
Functional connectivity measures were analyzed in Matlab using the CONN-fMRI functional connectivity toolbox v17f (Whitfield-Gabrieli and Nieto-Castanon, 2012).2 Data were de-noised following the anatomical CompCor approach (Behzadi et al., 2007). CompCor is advantageous in that it does not require external monitoring of physiological fluctuations (cardiac and respiratory) as compared to other noise correction routines. Anatomical CompCor factors in the signal from WM, ventricles, large vessels, and CSF to accurately model physiological fluctuations in GM areas and uses the five most significant principal components each from the WM and CSF as covariates in a general linear model (GLM) as an estimate for physiological noise (Behzadi et al., 2007). We additionally analyzed the resting-state data using CompCor and with CompCor turned off (i.e., removing WM and CSF covariates). We expected that isocapnia would mostly remove variations due to PETCO2 fluctuations, whereas CompCor would remove other sources of noise such as cardiac pulsations, in order to see how these two methods compared.
Global signal regression was not performed in order to bypass introduction of artifactually negative correlations into resulting connectivity measures (Chai et al., 2012). Instead, a number of confounds were regressed out via CompCor to further remove unwanted BOLD signal artifactual effects that were shown to improve sensitivity, specificity, and validity for subsequent functional connectivity analyses. In addition to the CompCor covariates, realignment motion regression (12 regressors comprised of six motion regressors and six first-order temporal derivatives), and functional outliers that were detected via the ART-based identification of outlier scans for scrubbing, thresholded at 0.9 mm for framewise displacement (Power et al., 2012) were also included. As recommended, band-pass filtering was also performed with a frequency window of 0.008–0.09Hz. This preprocessing step helps increase retest reliability, while reducing the effects of low frequency drift and high frequency noise (Weissenbacher et al., 2009). Linear detrending was performed as another de-noising parameter to remove linear trends within each functional scan.
PETCO2 and BOLD Correlation Analysis
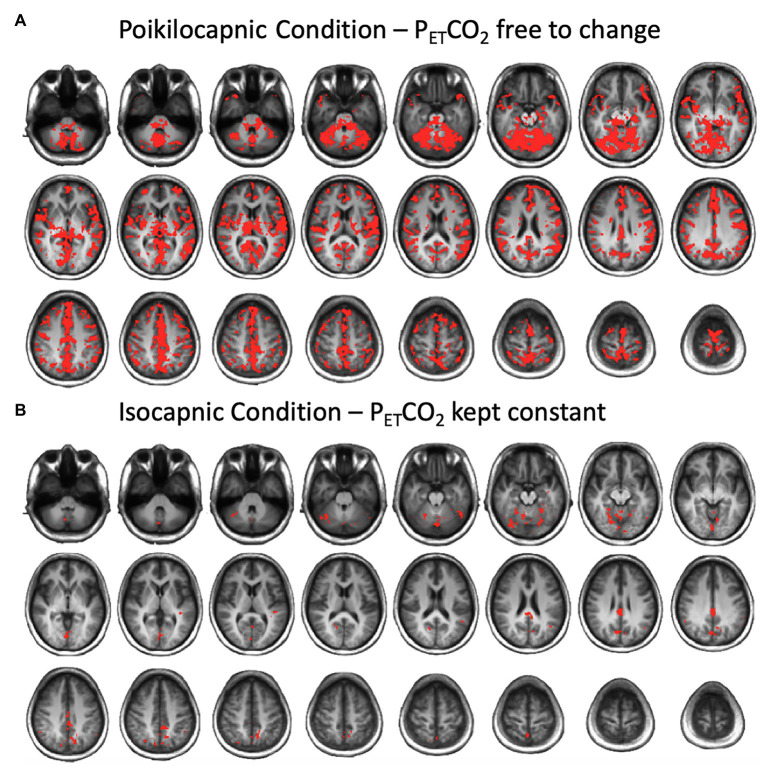
End-tidal PCO2 was shifted to the maximum correlation with the average BOLD signal using Matlab. Correlation maps between PETCO2 and the resting BOLD signal were computed for each resting-state condition using AFNI software (National Institutes of Health; Cox, 1996) with quadratic fitting to control for baseline and trend. Resulting images were reregistered to MNI space. After Fisher’s Z transformation of the correlation values, group analysis was computed for each group to compare correlations between the PETCO2 time course and BOLD signal during both resting-state conditions. Threshold-Free Cluster Enhancement (TFCE) was performed on the correlations between the BOLD signal and PETCO2 time course data. TFCE is an optimal method in enhancing cluster like structures that has been shown to provide better sensitivity than other methods throughout an extensive range of test signal shapes, signal-to-noise ratios (SNRs), and has been described in detail previously (Smith and Nichols, 2009). Permutation testing was then applied to the height of the maxima of the resulting statistic image, using the “randomize” permutation-based inference tool (Winkler et al., 2014) in FSL v.5.0.9 (FMRIB Library)3 that allowed for the maintenance of strong control over family-wise error (FWE). The effect of spontaneous breathing (poikilocapnia) and (isocapnia) were tested for multiple comparisons, where any significant regions at p < 0.05 were reported and illustrated (Figure 1).
Figure 1.
Brain average maps displaying the correlations between the blood oxygenation level-dependent (BOLD) signal and end-tidal PCO2 (PETCO2) time course resting state conditions after multiple comparison correction using threshold free cluster enhancement (TFCE) in (A) the poikilocapnic condition, and (B) the isocapnic condition. Significant regions at p < 0.05 are shown.
Connectivity Analysis
An ROI-to-ROI and Seed-to-Voxel first-level bivariate correlation, hrf-weighted analysis was conducted using a functional connectivity (weighted GLM) model for measuring resting-state connectivity.
The first exploratory whole-brain ROI-to-ROI functional connectivity analysis was performed to examine an unbiased investigation of two types of ROIs within CONN tool. This consisted of a group of 132 ROIs and the second group of 31 ROIs across eight networks that were commonly assigned across subjects. The 132 ROI group was based on the FSL Harvard-Oxford Atlas including 91 cortical, 15 subcortical ROIs [developed at the Center for Morphometric Analysis (CMA), and distributed with the FMRIB Library in FSL], and 26 cerebellar parcellations from the Automated Anatomical Labeling (AAL) Atlas (Tzourio-Mazoyer et al., 2002). The 31 ROI group was derived across eight networks e.g., default mode network (DMN); medial prefrontal cortex (MPFC); posterior cingulate cortex (PCC); right lateral parietal (RLP); and left lateral parietal (LLP) cortex areas. For each ROI, the mean BOLD signal time series was extracted within the ROI voxels, and bivariate correlation coefficients were computed for each pair of ROIs that were Fisher z-transformed. For second-level analysis, a two-sided within-subject paired t-test for all subjects between-conditions contrast of isocapnia vs. poikilocapnia was applied using the false discovery rate (FDR) correction for multiple comparisons p < 0.05. Only significant ROI-to-ROI results are illustrated and reported.
The second hypothesis driven seed-to-voxel analysis was performed using 10 mm spheres positioned in ROIs that have been associated with respiration and rs-fMRI that included the cerebellum, insular/orbitofrontal/cingulate/precuneus/prefrontal cortex, putamen, caudate, superior temporal/supramarginal gyri (Birn et al., 2006; Chang and Glover, 2009), and visual cortex (Madjar et al., 2012). The mean time series were extracted in each seed region by averaging across all voxels for each participant. Bivariate correlation coefficients were computed between the seed time-course and with every other voxel in the brain. The subsequent whole-brain correlation maps were Fisher z-transformed producing z-value maps of voxel-wise functional connectivity for each seed ROI. For second-level analysis, a two-sided within-subject paired t-test for all subjects between-conditions contrast of isocapnia vs. poikilocapnia was applied for voxel-wise statistics throughout the whole brain at an uncorrected level (p < 0.001) before FWE correction was applied at the cluster level (p < 0.05) for multiple comparisons. Only significant seed-to-voxel results are illustrated and reported.
Results
Respiratory Data Results
The SD of the breath-to-breath spontaneous PETCO2 variability was significantly lower in the clamped condition 0.84 (0.46) mmHg compared to the unclamped condition 2.47 (0.96) mmHg [mean of variability (SD)], T(9) = 6.06, p = 0.0002 (Figure 2). The mean (SD) PETCO2 was higher in the isocapnic condition 37.53 (3.04) mmHg compared to the poikilocapnic condition 33.81 (3.3) mmHg, T(9) = 8.38, p < 0.0001. This is very unlikely to be of physiologic significance for the following reason. In the isocapnic condition, the PETCO2 is equal to the PaCO2 (Ito et al., 2008; Fisher et al., 2016). In healthy people PETCO2 is typically 2–4 mmHg less than PaCO2 due to a small volume of physiological alveolar deadspace (Ito et al., 2008).
Figure 2.
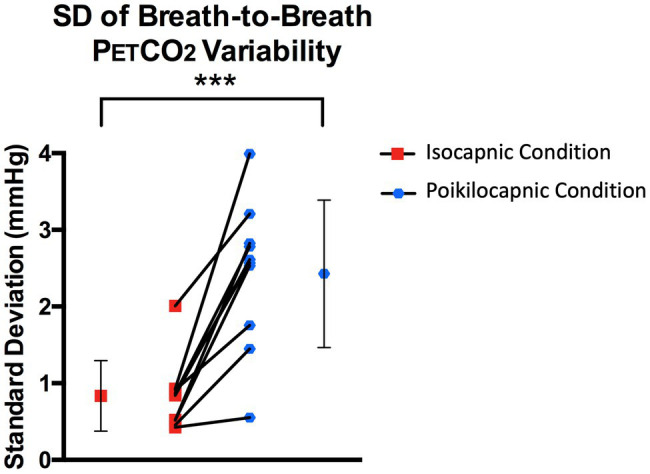
Respiratory data for both resting-state conditions. SD of breath-to-breath PETCO2 variability in the isocapnic spontaneous breathing condition (when PETCO2 was kept constant) denoted with red squares, and poikilocapnic spontaneous breathing condition (when PETCO2 was free to change) denoted with blue circles. Connecting lines in the middle of the graph associate each individual subject’s condition (the mean SD of breath-to-breath variability for the isocapnic and poikilocapnic conditions). The symbol and error bars beside each line graph side denotes the mean and SD of all 10 subjects for each condition. *** denotes p < 0.001.
Functional Connectivity Results
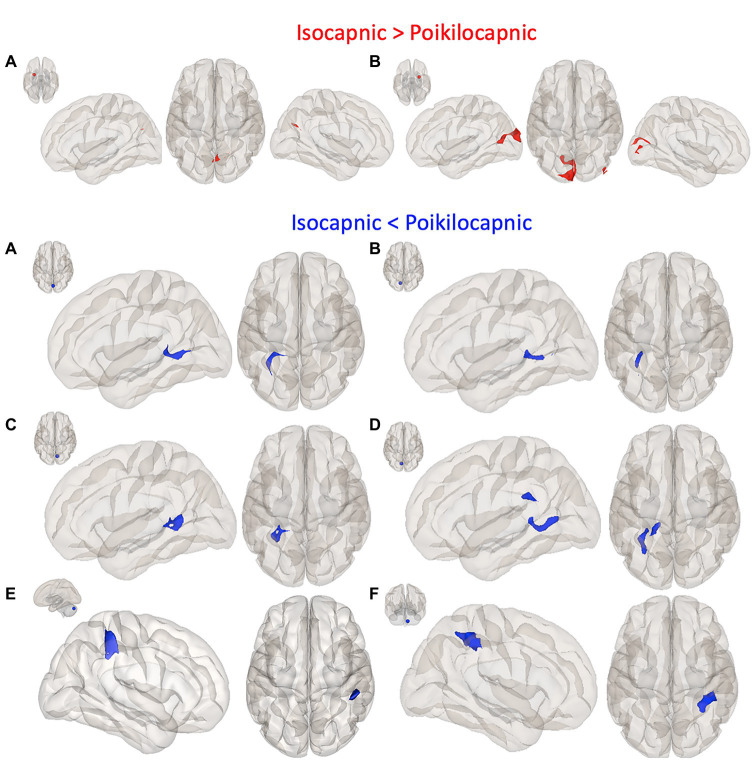
Exploratory whole-brain functional connectivity assessment within a whole-brain parcellated network of 132 ROIs and 42 networks revealed significant differences between the two resting state conditions. The controlled breathing isocapnic condition had enhanced functional connectivity between the right putamen with the left intracalcarine cortex (ICC) and the right putamen with the right supracalcarine cortex (SCC) (Figure 3A; Table 1). The poikilocapnic spontaneous breathing condition had enhanced functional connectivity between the right opercular part of the inferior frontal gyrus (IFG) with the left Cerebellar area 1 and the right opercular part of the IFG with the right Cerebellar area 9 (Figure 3B; Table 1). There were no differences between turning CompCor on or off for ROI-to-ROI results.
Figure 3.
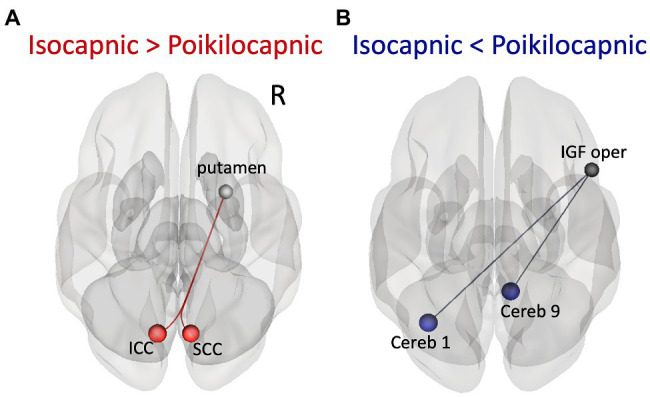
ROI-to-ROI functional connectivity results. (A) Superior axial view of significantly increased functional connectivity in the isocapnic condition (when PETCO2 was kept constant) compared to the poikilocapnic condition (when PETCO2 was free to change) between the right putamen ROI seed and left intracalcarine cortex (ICC) and right supracalcarine cortex (SCC). (B) Superior axial view of significantly increased functional connectivity in the poikilocapnic condition compared to the isocapnic condition between the right opercular part of the inferior frontal gyrus (IFG) seed and left Cerebellum region 1 and right Cerebellum region 9.
Table 1.
ROI-to-ROI functional connectivity results within a whole-brain parcellations of 174 ROIs.
| ROI 1 | ROI 2 | T Score | Beta | p-unc | p-FDR |
|---|---|---|---|---|---|
| Contrast: Isocapnia > Poikilocapnia Figure 5A (red) | |||||
| Right putamen | Left intracalcarine cortex | 5.93 | 0.11 | 0.0002 | 0.0307 |
| Right supracalcarine cortex | 5.51 | 0.15 | 0.0004 | 0.0307 | |
| Left intracalarine cortex | Right putamen | 5.93 | 0.11 | 0.0002 | 0.0360 |
| Contrast: Isocapnia > Poikilocapnia Figure 5B (blue) | |||||
| Right inferior frontal gyrus | Left cerebellum crus 1 | 5.84 | 0.15 | 0.0002 | 0.0256 |
| Right cerebellum 9 | 5.65 | 0.13 | 0.0003 | 0.0256 | |
| Left cerebellum crus 1 | Right inferior frontal gyrus | 5.84 | 0.15 | 0.0002 | 0.0407 |
Significant connections at a p < 0.05 false discovery rate (FDR) corrected threshold are reported.
In addition, a hypothesis-based seed-to-voxel analysis for contrasts comparing isocapnic to poikilocapnic conditions was calculated using second-level GLM analyses. Reported clusters were thresholded at p < 0.05, FWE-corrected, T maxima = 4.78. Enhanced functional connectivity in the isocapnic condition was found between the left putamen seed and a cluster in the precuneus cortex as well as the right putamen seed with a cluster in the left occipital pole and cluster in the inferior division of the right lateral occipital cortex (Figure 4; Table 2). Enhanced functional connectivity in the poikilocapnic condition was found between the primary visual network (PVN) seed and the left hippocampal formation; the left ICC and right SCC seeds with clusters in the left hippocampus; the left SCC seed with clusters in the left hippocampus/lingual gyrus, and callosal body; the posterior cerebellar network (PCN) seed with a cluster in the right supramarginal gyrus (SG); and the cerebellum region 9 seed with the a cluster in the right superior parietal lobule (SPL) (Figure 4; Table 2).
Figure 4.
Seed-to-voxel functional connectivity results. The first panel shows greater connectivity in the isocapnic condition (red) compared to the poikilocapnic condition; seed regions (red 10 mm ROIs) are depicted by the small axial brain images (top left), and red cluster regions shown in left sagittal, superior axial, and right sagittal views. (A) Left putamen seed, precuneus cortex cluster; (B) Right putamen seed, left occipital pole and right lateral occipital cortex clusters. Panels 2–4 show greater connectivity in the poikilocapnic condition (blue) compared to the isocapnic condition; seed regions (blue 10 mm ROIs), and blue cluster regions in the left sagittal and superior axial view. (A) primary visual network (PVN) seed, hippocampal formation cluster; (B) ICC seed, Hippocampus (Hi) cluster; (C) left SCC seed, Hi cluster; (D) right SCC seed, Hi/lingual gyrus cluster; (E) posterior cerebellar seed; supramarginal gyrus (SG) cluster; (F) Cerebellum region 9 seed; superior parietal lobule (SPL) cluster. Only significant family-wise error (FWE) corrected p < 0.05 are reported.
Table 2.
Seed-to-voxel results, brain regions showing altered functional connectivity between seed and corresponding clusters within the two different resting state condition in controls comparing with CompCor and without CompCor.
| Seed | Cluster | MNI | MNI | K | K | Beta | Beta | T | T | P | P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CompCor | -CompCor | CompCor | -CompCor | CompCor | -CompCor | CompCor | -CompCor | CompCor | -CompCor | |||
| Isocapnia > Poikilocapnia (Figure 3, red) | ||||||||||||
| (A) | L putamen | Precuneus cortex | −02 -72 +28 | none | 107 | none | 0.13 | none | 7.19 | none | 0.0227 | none |
| (B) | R putamen | L occipital pole | +00–92 +16 | +00–94 +14 | 550 | 518 | 0.14 | 0.14 | 9.88 | 9.72 | < 0.00001 | < 0.00001 |
| R iLOC | +46–84 +08 | +46–84 +08 | 83 | 78 | 0.19 | 0.20 | 7.17 | 7.75 | 0.034 | 0.047 | ||
| Poikilocapnia > Isocapnia (Figure 3, blue) | ||||||||||||
| (A) | PVN 2, −79, 12 | L Hippocampus | −28 -40 +02 | −28 -40 +02 | 169 | 154 | 0.18 | 0.18 | 12.66 | 11.96 | 0.0015 | 0.027 |
| (B) | L ICC | L Hippocampus | −28 -40 +00 | −28 -40 +00 | 136 | 134 | 0.19 | 0.18 | 12.26 | 12.05 | 0.0051 | 0.0056 |
| (C) | R SCC | L Hippocampus | −28 -54 +10 | −28 -54 +10 | 191 | 181 | 0.16 | 0.16 | 13.85 | 13.34 | 0.0007 | 0.0009 |
| (D) | L SCC | L Hippocampus | −30 -66 +04 | −32 -62 +04 | 233 | 124 | 0.18 | 0.19 | 11.85 | 11.96 | 0.0011 | 0.0098 |
| L Callosal Body | −18 -36 +24 | none | 121 | none | 0.15 | none | 11.11 | none | 0.0394 | none | ||
| (E) | PCN 0, −79, −32 | R SG | +54–36 +60 | +50–32 +44 | 272 | 255 | 0.20 | 0.19 | 9.01 | 8.81 | 0.00003 | 0.00006 |
| (F) | R Cerebellum 9 | R SPL | +32–52 +52 | +32–52 +52 | 397 | 379 | 0.16 | 0.16 | 8.80 | 8.91 | < 0.00001 | < 0.00001 |
Cluster location, Montreal Neurological Institute (MNI) peak cluster co-ordinates (x,y,z) and are shown in (mm), k = number of contiguous voxels in cluster (voxel size = 2 mm3); CompCor = using CompCor, CompCor = without CompCor; Beta = effect sizes, which represent the magnitude of connectivity (Fisher-transformed correlation coefficients) and shows the difference in means of groups (isocapnic and poikilocapnic); T = test statistic (two-sided), p = family-wise error (FWE) corrected cluster-level significance set at p < 0.05; L, left; R, right; PVN, primary visual network; iLOC, Lateral Occipital Cortex, inferior division. ICC, intracalcarine cortex; SCC, supracalcarine cortex; PCN, posterior cerebellar network; SG, supramarginal gyrus – posterior division; SPL, superior parietal lobule.
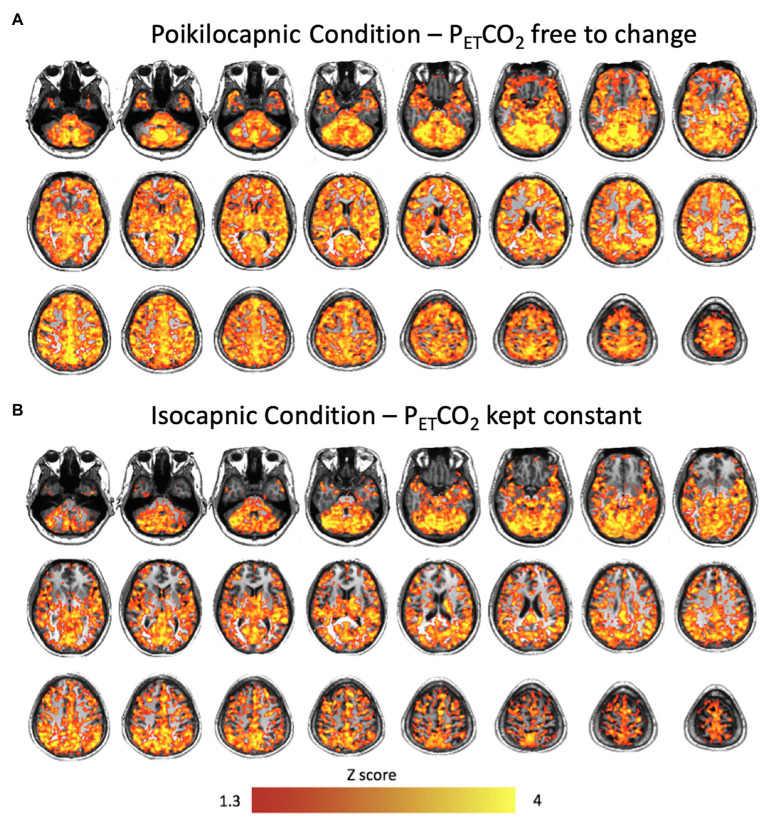
Correlation maps between the PETCO2 and the resting BOLD revealed stronger correlations in the poikilocapnic resting state condition, where PETCO2 was allowed to vary spontaneously as compared with the isocapnic PETCO2 resting state condition. These regions were found predominantly in cerebellar, frontal, and occipital regions (Figure 5). In addition, statistical testing using TFCE for multiple comparison correction p < 0.05 was applied, where significant regions of correlations between PETCO2 with resting BOLD are shown in red (Figure 1). Spontaneous breathing during the poikilocapnic condition showed significantly more PETCO2 and BOLD correlations in GM cerebellar, frontal, parietal, temporal, and occipital regions (Figure 1A) compared to the isocapnic resting state condition (Figure 1B). There were minor differences when using CompCor compared without CompCor in resting state seed-to-voxel analyses.
Figure 5.
Brain average maps displaying the correlations between the BOLD signal and PETCO2 time course during resting states in (A) the poikilocapnic condition, and (B) the isocapnic condition.
Discussion
Main Findings
To the best of our knowledge, this study is the first to compare functional connectivity between poikilocapnic and isocapnic resting-state conditions using whole-brain ROI-to-ROI and seed-to-voxel analysis. The main findings of this study are that implementing isocapnia reveals neuronal functional connectivity within: (1) the corticostriatal pathway between the right putamen and ICC as well as the SCC; and (2) the left putamen and the precuneus cortex. These results are similar to those of Madjar et al. (2012) who found enhanced connectivity between the PCC and occipital regions when PETCO2 was controlled during the resting-state acquisition. Although Madjar et al. (2012) only focused on the PCC as their seed of interest, our findings reveal other resting-state networks that were induced by respiratory changes in PCO2.
PaCO2-Induced Synchrony of BOLD Signal in Vasoactive Tissue
Frontal, temporal, and occipital GM and cerebellum are characterized by large BOLD signal changes in response to a CO2 challenge (Rostrup et al., 2000; Wise et al., 2004) and therefore may also show the greatest synchronization of BOLD signals to changes in PaCO2 and hence to each other during poikilocapnia. Functional connections were found between the cerebellum and the IFG and SPL; the cerebellar network with the SG; the visual cortex with the occipital fusiform gyrus; the PVN with the hippocampal formation; and the ICC/SCC with the hippocampus. That these regions are synchronized by PETCO2 fluctuation is shown by loss of synchronization with isocapnia (Figures 1, 5). These findings are consistent with those of Bright et al. (2020) who reported a significant relationship between the more vascularized visual networks during a working memory task under poikilocapnia that was not observed during isocapnia. This finding also supports the notion of a vascular driver for this functional network system as the negative BOLD signal changes were time-locked to the working memory task, whereas visual activation during the working memory task resulted in positive BOLD signal changes.
In addition, a previous study investigating the effects of speech tasks reported decreased CO2 pressure in the blood (hypocapnia), mainly accounting for the measured changes in oxygenation and cerebral hemodynamics. Their findings suggest the need to monitor PETCO2 during speech tasks as PaCO2 is still underestimated and a potential confounder in functional brain studies (Scholkmann et al., 2013).
Unmasking Neuronal Synchronization With Isocapnia
The percentage of BOLD signal change per increment in PETCO2 is lowest in the striatum and thalamus (Posse et al., 1999; Wise et al., 2004), and the basal ganglia (Kastrup et al., 1999). Increased synchronization of the right putamen with the ICC during isocapnia may therefore be explained by withdrawal of confounding vasoactive stimulation, leaving undistorted neuronal synchronization. Thus, vascular responses to changes in PETCO2 that cease with isocapnia are most likely added noise in resting-state functional connectivity analyses and therefore limit sensitivity.
Isocapnia vs. CompCor Noise Correction
We compared resting-state analyses with and without CompCor noise correction. We found that the significant clusters from seed-to-voxel analysis were slightly larger in volume using CompCor. These results may be indicative of how isocapnic and CompCor operations remove correlated components. We expected isocapnia to mostly remove variations due to PETCO2 fluctuations, whereas CompCor would remove other sources of noise including, for example, those due to cardiac pulsations. Tellingly, isocapnic data were unaffected by application of CompCor. It may be that isocapnia predominantly removes artifactual correlations between highly vasoactive regions, while CompCor predominantly removes artifacts from different spatial locations, revealing when they are actually operating independently. Such post processing should reduce false positives and true positives, whereas isocapnia should not reduce true positives. With control fluctuations of PaCO2, CompCor may be used to account for respiratory motion and cardiac pulsations, which persist with isocapnia.
Stimulus Free Cerebrovascular Reactivity From Poikilocapnic PETCO2 Variability
It would seem reasonable to regress out the variation due to breath-to-breath variability in PETCO2 and generate cerebrovascular reactivity data expressed as Δ BOLD/Δ PETCO2. Unfortunately, the general correlation between PaCO2 and PETCO2 breaks down at the scale of small breath-to-breath PETCO2 fluctuations. A small reduction in tidal volume, for example, may result in an incomplete sampling of alveolar gas at the sensor resulting in an erroneously low PETCO2. However, the same breath also results in reduced alveolar ventilation causing an increase in PaCO2. This breath-by-breath divergence of PaCO2 from PETCO2 confounds calculations of CVR from changes in PETCO2, while breathing at rest. Moreover, Golestani and Chen (2020) sought to quantify the difference between rs-fMRI measures during isocapnia, poikilocapnia, and poikilocapnia after PETCO2 correction using a CO2 response function (HRFCO2) (Golestani and Chen, 2020). Their findings revealed that clamping PETCO2 during isocapnia did not significantly affect respiratory volume variability and cardiac-rate variation, but significantly reduced functional connectivity as calculated with voxel seeds and reduced inter-subject variability in functional connectivity. In addition, applying the PETCO2 correction during poikilocapnia showed to increase inter-subject variability in functional connectivity (Golestani and Chen, 2020).
Study Limitations
The small sample size may have limited our ability to detect additional regions masked by respiratory effects. However, that we have found a consistent difference in synchronization of specific brain territories with and without isocapnia despite the inter-subject variability and our small sample size suggests that the effect size of poikilocapnia is large.
Changes in perfusion pressure can affect cerebral blood flow (CBF) during hypercapnia (Aaslid et al., 1989; Perry et al., 2014). An increase in mean arterial pressure (MAP) can alter CBF in the absence of cerebral micro-vessel vasodilation (Claassen et al., 2007). The importance of accounting for MAP would help solve for the magnitude of cerebral vasodilatory response, which is important for adequately matching cerebral perfusion with neuronal metabolic demand. However, MAP cannot be measured breath-to-breath with a non-invasive blood pressure cuff, which would confound the matching of blood pressure to breath-to-breath changes in PETCO2 and BOLD signal. In our study, we did not measure MAP. A common relationship in previous studies showed elevated MAP responses to hypercapnia in older adults compared to young adults (Claassen et al., 2007; Coverdale et al., 2017; Miller et al., 2018). However, in our studies hypercapnia was not applied and there was no reason to suspect synchrony of blood pressure with respiration. Additionally, there may be other mechanisms hampering rs-fMRI brain activity arising from other non-neuronal sources such as contributions from sympathetic vascular innervation, which may affect the fMRI signal through its connection with CBF regulation (Özbay et al., 2019) that warrant further investigation. A theoretical possibility is that eliminating the breath-to-breath variability of arterial PCO2 would affect the intrinsic connectivity pattern. However, the actual finding in this paper remains that the pattern seen with poikilocapnia includes false synchrony due to vasoactivity. As such, reports of connectivity data collected under poikilocapnia would need to address this issue.
Summary
We found that isocapnia decreases or ceases the synchronization in some brain regions previously considered part of the connectome. This suggests that fluctuating PETCO2, during the acquisition of resting-state functional data generate CO2-synchronized changes that would be considered false positive results for neuronal based connectivity. This artifact is particularly prominent in regions that are highly vascularized and have high vasoactive responses to PaCO2 such as the visual, cerebellar, and frontal regions. In those, and possibly other regions, the magnitude of the BOLD signal changes resulting from vasoactive stimulation may overwhelm those due to resting state neurovascular coupling, such as in the putamen and calcarine cortex, resulting in false negative connectivity readings in those regions. The findings indicate that resting state acquisitions would benefit from isocapnia in identifying neuronal resting state functional connectivity.
Conclusion
The important finding in this paper remains that the pattern seen with poikilocapnia includes false synchrony due to vasoactivity. Armed with this, the ability to assess how other conditions or diseases alter DMN activity can be more accurately identified and quantitated. This has the added benefit of reducing the sample size in studies trying to determine how experimental conditions, drugs, or diseases influence activity in the DMN.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Research Ethics Board of the University Health Network and conformed to the standards set by the latest revision of the Declaration of Helsinki. The participants provided their written informed consent to participate in this study.
Author Contributions
LM, KS, AC, JF, and DM designed the study. LM, KS, OS, and JP collected the data. LM analyzed the data and wrote the manuscript. LM, JP, AC, OS, JF, and DM interpreted the data for the work. LM, KS, JP, AC, OS, LV, JD, JF, and DM contributed to the manuscript revision and reviewed and approved the final submission. All authors contributed to the article and approved the submitted version.
Conflict of Interest
JF and DM are among the developers of the RespirAct™ for MRI studies at the University Health Network, part of the University of Toronto. Thornhill Research Inc. (TRI) is a for-profit biomedical manufacturing company that was spun off from UHN. It assembles the RespirAct™, on a non-profit basis to enable MRI research at UHN and around the world. JF receives income for work done for TRI and DM holds a minor equity position in TRI.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Abby Skanda for her assistance in subject recruitment and the late Eugen Hlasny for his assistance in data acquisition. This research study was supported by CIHR. This work was supported by the Holt-Hornsby and Andreae Vascular Dementia Research Unit in the Joint Department of Medical Imaging at the Toronto Western Hospital and the University Health Network.
Footnotes
References
- Aaslid R., Lindegaard K. F., Sorteberg W., Nornes H. (1989). Cerebral autoregulation dynamics in humans. Stroke 20, 45–52. 10.1161/01.STR.20.1.45, PMID: [DOI] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T. T. (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage 37, 90–101. 10.1016/j.neuroimage.2007.04.042, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn R. M., Diamond J. B., Smith M. A., Bandettini P. A. (2006). Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. NeuroImage 31, 1536–1548. 10.1016/j.neuroimage.2006.02.048, PMID: [DOI] [PubMed] [Google Scholar]
- Biswal B., Yetkin F. Z., Haughton V. M., Hyde J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 34, 537–541. 10.1002/mrm.1910340409, PMID: [DOI] [PubMed] [Google Scholar]
- Bright M. G., Whittaker J. R., Driver I. D., Murphy K. (2020). Vascular physiology drives functional brain networks. NeuroImage 217:116907. 10.1016/j.neuroimage.2020.116907, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X. J., Castañón A. N., Ongür D., Whitfield-Gabrieli S. (2012). Anticorrelations in resting state networks without global signal regression. NeuroImage 59, 1420–1428. 10.1016/j.neuroimage.2011.08.048, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Glover G. H. (2009). Relationship between respiration, end-tidal CO2, and BOLD signals in resting-state fMRI. NeuroImage 47, 1381–1393. 10.1016/j.neuroimage.2009.04.048, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen J. A. H. R., Zhang R., Fu Q., Witkowski S., Levine B. D. (2007). Transcranial Doppler estimation of cerebral blood flow and cerebrovascular conductance during modified rebreathing. J. Appl. Physiol. 102, 870–877. 10.1152/japplphysiol.00906.2006, PMID: [DOI] [PubMed] [Google Scholar]
- Coverdale N. S., Badrov M. B., Shoemaker J. K. (2017). Impact of age on cerebrovascular dilation versus reactivity to hypercapnia. J. Cereb. Blood Flow Metab. 37, 344–355. 10.1177/0271678X15626156, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 29, 162–173. 10.1006/cbmr.1996.0014, PMID: [DOI] [PubMed] [Google Scholar]
- Fierstra J., Sobczyk O., Battisti-Charbonney A., Mandell D. M., Poublanc J., Crawley A. P., et al. (2013). Measuring cerebrovascular reactivity: what stimulus to use? J. Physiol. 591, 5809–5821. 10.1113/jphysiol.2013.259150, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J. A. (2016). The CO2 stimulus for cerebrovascular reactivity: fixing inspired concentrations vs. targeting end-tidal partial pressures. J. Cereb. Blood Flow Metab. 36, 1004–1011. 10.1177/0271678X16639326, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J. A., Iscoe S., Duffin J. (2016). Sequential gas delivery provides precise control of alveolar gas exchange. Respir. Physiol. Neurobiol. 225, 60–69. 10.1016/j.resp.2016.01.004, PMID: [DOI] [PubMed] [Google Scholar]
- Golestani A. M., Chang C., Kwinta J. B., Khatamian Y. B., Jean Chen J. (2015). Mapping the end-tidal CO2 response function in the resting-state BOLD fMRI signal: spatial specificity, test-retest reliability and effect of fMRI sampling rate. NeuroImage 104, 266–277. 10.1016/j.neuroimage.2014.10.031, PMID: [DOI] [PubMed] [Google Scholar]
- Golestani A. M., Chen J. J. (2020). Controlling for the effect of arterial-CO2 fluctuations in resting-state fMRI: comparing end-tidal CO2 clamping and retroactive CO2 correction. NeuroImage 216:116874. 10.1016/j.neuroimage.2020.116874, PMID: [DOI] [PubMed] [Google Scholar]
- Ito S., Mardimae A., Han J., Duffin J., Wells G., Fedorko L., et al. (2008). Non-invasive prospective targeting of arterial P(CO2) in subjects at rest. J. Physiol. 586, 3675–3682. 10.1113/jphysiol.2008.154716, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastrup A., Li T. Q., Glover G. H., Moseley M. E. (1999). Cerebral blood flow-related signal changes during breath-holding. AJNR Am. J. Neuroradiol. 20, 1233–1238. PMID: [PMC free article] [PubMed] [Google Scholar]
- Madjar C., Gauthier C. J., Bellec P., Birn R. M., Brooks J. C. W., Hoge R. D. (2012). Task-related BOLD responses and resting-state functional connectivity during physiological clamping of end-tidal CO2. NeuroImage 61, 41–49. 10.1016/j.neuroimage.2012.02.080, PMID: [DOI] [PubMed] [Google Scholar]
- Miller K. B., Howery A. J., Harvey R. E., Eldridge M. W., Barnes J. N. (2018). Cerebrovascular reactivity and central arterial stiffness in habitually exercising healthy adults. Front. Physiol. 9:1096. 10.3389/fphys.2018.01096, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özbay P. S., Chang C., Picchioni D., Mandelkow H., Chappel-Farley M. G., van Gelderen P., et al. (2019). Sympathetic activity contributes to the fMRI signal. Commun. Biol. 2, 1–9. 10.1038/s42003-019-0659-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry B. G., Lucas S. J. E., Thomas K. N., Cochrane D. J., Mündel T. (2014). The effect of hypercapnia on static cerebral autoregulation. Phys. Rep. 2:e12059. 10.14814/phy2.12059, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posse S., Wiese S., Gembris D., Mathiak K., Kessler C., Grosse-Ruyken M. L., et al. (1999). Enhancement of BOLD-contrast sensitivity by single-shot multi-echo functional MR imaging. Magn. Reson. Med. 42, 87–97. , PMID: [DOI] [PubMed] [Google Scholar]
- Power J. D., Barnes K. A., Snyder A. Z., Schlaggar B. L., Petersen S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 59, 2142–2154. 10.1016/j.neuroimage.2011.10.018, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostrup E., Law I., Blinkenberg M., Larsson H. B., Born A. P., Holm S., et al. (2000). Regional differences in the CBF and BOLD responses to hypercapnia: a combined PET and fMRI study. NeuroImage 11, 87–97. 10.1006/nimg.1999.0526, PMID: [DOI] [PubMed] [Google Scholar]
- Scholkmann F., Gerber U., Wolf M., Wolf U. (2013). End-tidal CO2: an important parameter for a correct interpretation in functional brain studies using speech tasks. NeuroImage 66, 71–79. 10.1016/j.neuroimage.2012.10.025, PMID: [DOI] [PubMed] [Google Scholar]
- Slessarev M., Han J., Mardimae A., Prisman E., Preiss D., Volgyesi G., et al. (2007). Prospective targeting and control of end-tidal CO2 and O2 concentrations. J. Physiol. 581, 1207–1219. 10.1113/jphysiol.2007.129395, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S., Nichols T. (2009). Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 44, 83–98. 10.1016/j.neuroimage.2008.03.061, PMID: [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 15, 273–289. 10.1006/nimg.2001.0978, PMID: [DOI] [PubMed] [Google Scholar]
- Weissenbacher A., Kasess C., Gerstl F., Lanzenberger R., Moser E., Windischberger C. (2009). Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. NeuroImage 47, 1408–1416. 10.1016/j.neuroimage.2009.05.005, PMID: [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2, 125–141. 10.1089/brain.2012.0073, PMID: [DOI] [PubMed] [Google Scholar]
- Winkler A. M., Ridgway G. R., Webster M. A., Smith S. M., Nichols T. E. (2014). Permutation inference for the general linear model. NeuroImage 92, 381–397. 10.1016/j.neuroimage.2014.01.060, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R. G., Ide K., Poulin M. J., Tracey I. (2004). Resting fluctuations in arterial carbon dioxide induce significant low frequency variations in BOLD signal. NeuroImage 21, 1652–1664. 10.1016/j.neuroimage.2003.11.025 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.