Abstract
Immunoglobulin G (IgG) glycosylation is studied in biological samples to develop clinical markers for precision medicine, for example, in autoimmune diseases and oncology. Inappropriate storage of proteins, lipids, or metabolites can lead to degradation or modification of biomolecular features, which can have a strong negative impact on accuracy and precision of clinical omics studies. Regarding the preservation of IgG glycosylation, the range of appropriate storage conditions and time frame is understudied. Therefore, we investigated the effect of storage on IgG Fc N-glycosylation in the commonly analyzed biofluids, serum and plasma. Short-term storage and accelerated storage stability were tested by incubating samples from three healthy donors under stress conditions of up to 50 °C for 2 weeks using −80 °C for 2 weeks as the reference condition. All tested IgG glycosylation features—sialylation, galactosylation, bisection, and fucosylation—remained unchanged up to room temperature as well as during multiple freeze–thaw cycles and exposure to light. Only when subjected to 37 °C or 50 °C for 2 weeks, galactosylation and sialylation subtly changed. Therefore, clinical IgG glycosylation analysis does not rely as heavily on mild serum and plasma storage conditions and timely analysis as many other omics analyses.
Keywords: immunoglobulin G glycosylation, serum and plasma storage, glycosylation stability, accelerated storage stability, glycoproteomics
Introduction
Immunoglobulin G (IgG) is the most common antibody class in blood. It has key roles not only in adaptive immunity but also in the dysregulation of immunity, which may result in autoimmune disease and contribute to cancer. IgGs are N-glycosylated in the Fc region of the heavy chain at Asp297. Additionally, the hypervariable domains may carry N-glycans. IgG Fc glycosylation has a critical role in protein stability and antibody effector functions as it affects the interaction with Fc gamma receptors and modulates complement activation.1
Glycosylation affects the safety and efficacy of IgG-based biopharmaceuticals.2 It is also studied as a clinical marker for early detection, (differential) diagnosis, and prognosis of pathologies,3,4 aiming at an earlier, more adequate treatment and thus improved outcomes for patients.5,6 IgG glycosylation clinical markers have high potential as, for example, sialylation and galactosylation are involved in the regulation of pro- and anti-inflammatory activities of IgG.7,8 IgG glycosylation has been investigated from various biological matrices, predominantly plasma and serum, for elucidating potential pathological roles and addressing diagnostic needs in autoimmune diseases, cancers, and infectious diseases. Examples include IgG glycosylation as a discriminator between Crohn’s disease and ulcerative colitis;3 as a potential marker for mortality in colorectal cancer;9 as a marker of epithelial ovarian cancer;10 and as a prognostic marker of HIV control and a predictor of efficient placental transfer of anti-HIV IgG.11,12 However, it is still largely unclear how IgG glycosylation may be connected to various disease pathophysiologies.13,14
The use of high-throughput (HT) glycomics and glycoproteomics methods enables the investigation of large sample cohorts in a rapid, efficient, and reproducible way.15,16 The duration of sample storage can vary from weeks to years. Instability of biomolecules upon storage may therefore affect the accuracy of their analysis. Furthermore, for studies on diseases with low prevalence or longitudinal studies, collection and storage of samples can take months or years with a large spread in individual storage times.17 Therefore, alteration of target biomolecular features may confound their analysis. Stability of proteins, lipids, and metabolites has been extensively studied concluding that these biomolecules are generally stable in frozen biofluids, specifically at −80 °C if stored for months.18−20 However, several freeze–thaw cycles can cause changes in metabolic profiles and certain metabolites are sensitive to light. Furthermore, proteases and other enzymes, such as phosphatases, can change the composition of the proteome, potentially requiring sample stabilization.21
In contrast, little information has been published on the impact of storage of biological samples on glycosylation. Storing dried blood spots at −80 °C for up to 6 weeks did not change their IgG N-glycosylation, while storage effects at room temperature (RT) for several months are disputed.22−24 However, systematic studies of the more common storage of plasma and serum in freezers are scarce.25 IgG glycosylation of biological samples could be altered during storage by chemical or biocatalytic reactions. Chemical hydrolysis of glycosidic bonds upon heat activation under acidic conditions is well established, especially involving sialic acids.26 However, under the neutral pH conditions of serum and plasma, a strong chemical hydrolysis would not be expected. In contrast, glycosyltransferases and glycosidases are present in circulation,27,28 suggesting a potential biocatalytic route to IgG N-glycan modification during plasma and serum sample storage. While knowing the stability of IgG glycosylation and the origin of modifications is important for planning the storage of biological samples, such information is hitherto largely lacking.
In this work, we studied short-term stability and accelerated storage stability of IgG Fc N-glycosylation in serum and plasma. We used a robust bottom-up liquid chromatography–mass spectrometry (LC–MS) method to analyze IgG Fc N-glycosylation in serum and plasma samples from three donors under different storage conditions and compared the results to a reference condition. Our study provides a better understanding of the stability of IgG glycosylation during sample storage.
Materials and Methods
Materials
CaptureSelect FcXL Affinity Matrix beads, phosphate-buffered saline (PBS, 10×, pH 7.4), and formic acid (FA, for MS, ∼98%) were purchased from Thermo Fischer Scientific (Landsmeer, Netherlands). From Orochem (Naperville, IL) were purchased 96-well Oro-Flex I Polypropylene filter plates with 10 μm PE frit. Sequencing-grade trypsin was purchased from Promega (Madison, WI). Clot tubes (VACUETTE TUBE 9 mL CAT Serum Clot Activator 16 × 100 red cap-black ring, nonridged), heparin tubes (VACUETTE TUBE 9 mL NH Sodium Heparin 16 × 100 green cap-green ring, nonridged), and V-bottom 96-well microplates were purchased from Greiner bio-one (Frickenhausen, Germany). Acetic acid (glacial), ammonium bicarbonate (ABC, >99.5%), and acetonitrile (ACN, for LC–MS) were purchased from Sigma-Aldrich (Steinheim, Germany). Trifluoroacetic acid (TFA, >99.7%) was purchased from Merck (Darmstadt, Germany). Deionized water was generated at 18.2 MΩ by using a Q-Gard 2 system (Millipore, Amsterdam, Netherlands).
Clinical Samples and Ethical Statement
Human serum and heparin plasma samples from three donors (donor 1—male, 62 years old, donor 2—male, 27 years old, and donor 3—female, 26 years old) were kindly shared by Sanquin Research (Amsterdam, Netherlands). There they were collected in accordance with Dutch regulations and after approval from the Sanquin Ethical Advisory Board in accordance with the Declaration of Helsinki. For serum, blood was collected in clot tubes and allowed to clot by leaving it undisturbed at RT for 30 min, followed by centrifugation at 2000 × g for 10 min at 8 °C, serum harvesting, and immediate freezing at −20 °C. Plasma was collected in heparin tubes, centrifuged at 2000 × g for 15 min at 8 °C, followed by plasma harvest and immediate freezing at −20 °C. Samples were transported on dry ice to the research facilities and kept at −20 °C until the studied storage conditions were applied, which occurred 30 days after blood collection.
Sample Storage Conditions
Glycan stability was studied under diverse storage temperature, time of storage, exposure to light in normal laboratory conditions (sunlight and lamp light), and five freeze–thaw cycles; each cycle consisted of complete defrosting at RT for approximately 30 min and freezing again at −80 °C (Table 1). Frozen samples were considered at ultra-low temperature freezers for their widespread use in omics and −20 °C storage for additional accessibility to these freezers. Fridge conditions were considered for convenience and to study an alternative to freeze–thawing, as well as RT, which also resembles accidental sample storage. Storage at 37 and 50 °C allowed the study of accelerated storage stability conditions, as well as human enzymes’ optimum temperature. Four replicates of serum and plasma samples from donor 1 were evaluated, as well as one replicate from donors 2 and 3. Each replicate of 30 μL was stored in a 0.5 mL Eppendorf tube. After the last timepoint for all storage conditions, sample preparation took place for all samples simultaneously.
Table 1. Short-Term Storage and Accelerated Storage Stability Conditions Applied to Plasma and Seruma.
| time of storage (days) | temperature (oC) | exposure to light |
|---|---|---|
| 14 | –80 | − |
| 14 | –20 | − |
| 2 | 4 | − |
| 14 | 4 | − |
| 2 | RT | − |
| 2 | RT | Yes |
| 14 | RT | − |
| 14 | RT | Yes |
| 14 | 37 | − |
| 14 | 50 | − |
RT: room temperature.
Sample Preparation
Human serum and plasma samples were analyzed after being stored under different conditions according to a previously published method, albeit using FcXL beads for the affinity purification and sequencing-grade trypsin for the protease treatment.29 In short, 2 mL of FcXL beads was centrifuged in a 2 mL tube at 100 × g for 30 s, the supernatant was discarded, and the beads were resuspended in 1 mL of PBS and transferred into a 15 mL tube containing 5 mL of PBS, so that the beads were resuspended in a total of 6 mL of PBS (1×). 1 μL of sample, serum or plasma, was added to a preconditioned filter plate with 50 μL of resuspended FcXL beads, incubated on the filter plate for 1 h at 1000 rpm on a plate shaker (Titramax 100, Heidolph Instruments, Schwabach, Germany) to achieve IgG capturing, and washed three times with 200 μL of PBS (1×) and three times with 200 μL of deionized water using a vacuum manifold. Protein denaturation was performed by incubating in 100 mM FA for 5 min at RT at 1000 rpm. IgGs were eluted from the filter plate by centrifugation for 1 min at 100 × g into the Greiner V-bottom collection plate and then dried to complete dryness using a centrifugal vacuum concentrator (SpeedVac, RVC 2–33 CDPlus, Christ, Osterode am Harz, Germany) at 60 °C for 2 h. Dried glycoprotein was resuspended in 20 μL of freshly prepared 25 mM ABC. Sequencing-grade trypsin was dissolved in ice-cold 25 mM ABC to a concentration of 10 ng/μL. 20 μL of trypsin solution was added to the resuspended glycoprotein and the digest was incubated at 37 °C for 18 h. Afterward, the tryptic digest was stored at −20 °C until measured by LC–MS.
Nanoreversed-Phase Liquid Chromatography–Electrospray Ionization–Mass Spectrometry
The LC–MS conditions were the same as those previously reported.30 200 nL of tryptic digest was separated on a Dionex UltiMate 3000 nanoLC system (Thermo Fisher Scientific, Breda, Netherlands) by nanoreverse phase (RP)-LC. After trapping on an Acclaim PepMap 100 C18 5 mm × 300 μm trap column (Thermo Fisher Scientific), glycopeptides were separated on a nanoEase MZ Peptide BEH C18 column of 75 μm × 100 mm, featuring 130 Å pores and 1.7 μm particles (Waters, Milford, USA) at 45 °C, at 600 nL/min. The nanoRP-LC was hyphenated to an Impact HD quadrupole time-of-flight (q-TOF) mass spectrometer with a CaptiveSpray nanoBooster (Bruker, Bremen, Germany). The electrospray ionization MS (ESI-MS) parameters were as reported before, with a slight modification (see the Supporting Information).29
LC–MS Data Processing
The obtained spectra were transformed into mzXML files using MSConvertGUI (ProteoWizard). The data were aligned and calibrated, and analytes were quantified using LaCyTools (version 1.1.0 alpha) as previously described29,31 (see the Supporting Information). The list of alignment and calibration masses is shown in Tables S-1 and S-2, respectively. 136 glycopeptide compositions were targeted, of which 39 were successfully quantified after curation (Tables S-3 and S-4). The sum of the absolute glycopeptide intensities was normalized, and then, relative abundances of each glycopeptide were calculated per IgG subclass. Subsequently, derived traits were calculated separately for each IgG subclass: sialylation, galactosylation, fucosylation, and bisection, similar to previous reports30,32 (see the Supporting Information).
Statistical Analysis
Derived traits for each studied storage condition were compared to the reference condition—–80 °C for 2 weeks—with a parametric, unpaired t-test for donor 1. Relative abundances of each individual glycopeptide for each studied storage condition were also compared to the reference condition in the same way. Bonferroni’s multiple comparison correction was applied. Statistical analysis was performed using GraphPad Prism version 8.1.1 for Windows (GraphPad Software, San Diego, CA).
Results and Discussion
Stability of biomolecules is an important factor impacting their utility as clinical markers.20,33 The impact of storage of biofluids on the stability of IgG glycosylation has not been studied. Using samples collected from three donors, we studied the short-term stability and accelerated storage stability of IgG Fc N-glycosylation in serum and plasma. A range of short-term storage and accelerated storage stability conditions was investigated (Table 1) and compared to the reference condition. This included exposure to temperatures from −80 to 50 °C for up to 2 weeks, as well as the exposure to light at RT and the impact of five freeze–thaw cycles. All conditions were compared to the coldest commonly available storage at −80 °C. The three donors showed different IgG Fc glycosylations under the reference condition, in line with literature knowledge on interindividual variation of IgG Fc glycosylation patterns under homeostasis.34,35
Short-Term Storage Stability
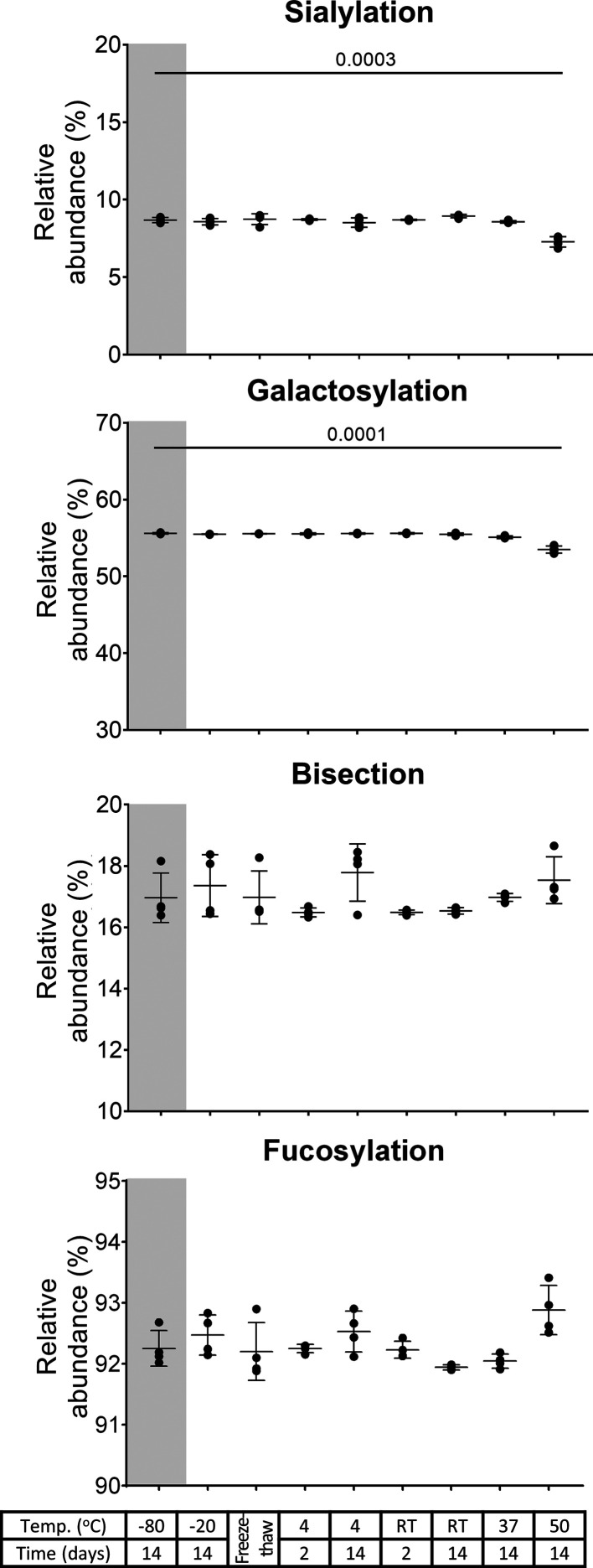
Oftentimes, serum and plasma samples are stored at −80 or −20 °C between collection and glycomics analysis. Such conditions did not show any changes in the glycosylation features after 2 weeks for any of the IgG subclasses. This was true for sialylation, galactosylation, bisection, and fucosylation (Figures 1, S-1, and S-2). Also, at 4 °C as well as at RT, IgG glycosylation was stable. Exposing the samples to multiple freeze–thaw cycles did not cause any changes either. IgG glycosylation did not change under light exposure at RT. These results were consistent among the three donors (Figures S-3 and S-4). The lack of glycosylation changes observed in all IgG subclasses across all these storage conditions, which was also observed at the individual glycopeptide level (Table S-6), illustrates a remarkable thermal- and photostability of IgG N-glycans. Thus, plasma or serum samples may even be briefly stored refrigerated or at RT before IgG glycosylation analysis.
Figure 1.
IgG1 glycosylation features—sialylation, galactosylation, bisection, and fucosylation—of serum from donor 1 (mean, SD, four full technical replicates). A decrease in sialylation and galactosylation is observed after sample storage at 50 °C for 2 weeks (p-values of t-test findings are displayed). The gray background highlights the reference condition. RT: room temperature.
Some instability might have been expected to occur at −20 °C in the month between sampling and experimentation.36 However, any effects would have been likely to continue during the studied storage period, likely increasing in effect size with storage at temperatures of 4 °C and RT. Consequently, the observed stability at −20 °C for 2 (additional) weeks suggests no impact of prior sample storage, though it cannot be fully excluded.
Accelerated Storage Stability
The impact of long-term storage on IgG Fc N-glycosylation was assessed via an accelerated storage study. Samples were exposed to elevated temperatures to simulate long-term storage of frozen samples. This approach is common in the biopharmaceutical industry to study the long-term stability of monoclonal antibodies.37 Samples were stored at 37 and 50 °C for 2 weeks, and results were compared to the reference condition.
Serum samples did not show any changes in glycosylation after being stored at 37 °C for 2 weeks for any of the IgG subclasses, except a minor decrease in galactosylation of 0.27 ± 0.05% in IgG2/3 (Figures 1 and S-1). Plasma samples showed the same stability for all IgG subclasses except for a marginal decrease in sialylation of 0.32 ± 0.05% for IgG2/3 and a decrease in galactosylation of 1.1 ± 0.2% in IgG4 (Figure S-2). Storage at 50 °C for 2 weeks reduced sialylation and galactosylation for serum IgG1 by 1.4 ± 0.2 and 2.1 ± 0.2%, respectively (Figure 1); plasma IgG1 sialylation and galactosylation were also decreased by 1.5 ± 0.1 and 2.0 ± 0.2%, respectively (Figures 2 and S-2). The same effects were observed for IgG2/3 and IgG4 in serum and plasma samples (Figures 3, S-1, and S-2), and results were consistent among the three donors (Figures 4, S-3, and S-4). This decrease in sialylation and galactosylation was also visible at the individual glycopeptide level, such as the sialylated, galactosylated glycoforms H4N4F1S1, H5N4F1S1, H4N5F1S1, H5N5F1S1, and H5N4F1S2 (Figures 5, S-5, and S-6, Table S-6). A lack of alterations in the level of bisected glycoforms is also present (Table S-6), as it is shown in the stability of bisection under these accelerated storage stability conditions for all IgG subclasses in serum and plasma (Figures 1, S-1, and S-2). Fucosylation remained unchanged, except for a minor increase at 50 °C for plasma IgG1 of 0.87 ± 0.12% and for serum IgG2/3 of 0.20 ± 0.04% (Figures 1, S-1, and S-2). Just for this extreme condition, a trend toward increased fucosylation could also be observed for plasma IgG2/3 and serum IgG1 as well as for the other donors in most instances (Figure 4). However, the change was always minimal (below 0.9%). Thus, we conclude that fucosylation does not notably change. Generally, sialic acids and galactoses appear to be more chemically labile than other monosaccharides in IgG glycosylation.30
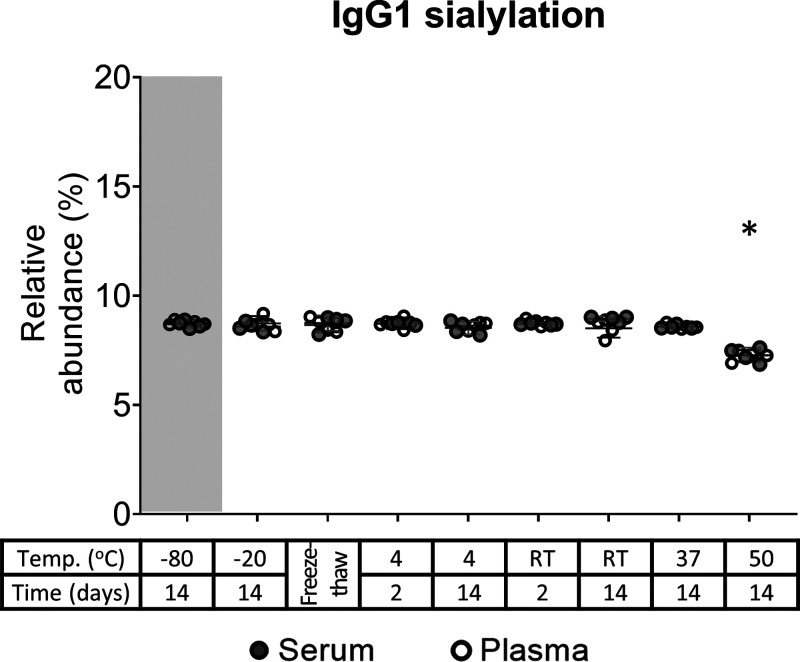
Figure 2.
IgG1 sialylation of serum and plasma from donor 1. All findings in the serum of donor 1 (Figure 1) are also observed in plasma and serum, exemplified for IgG1 sialylation here. * Changes correspond to p-values of 3 × 10–4 and 3 × 10–5 in serum and plasma, respectively. RT: room temperature.
Figure 3.
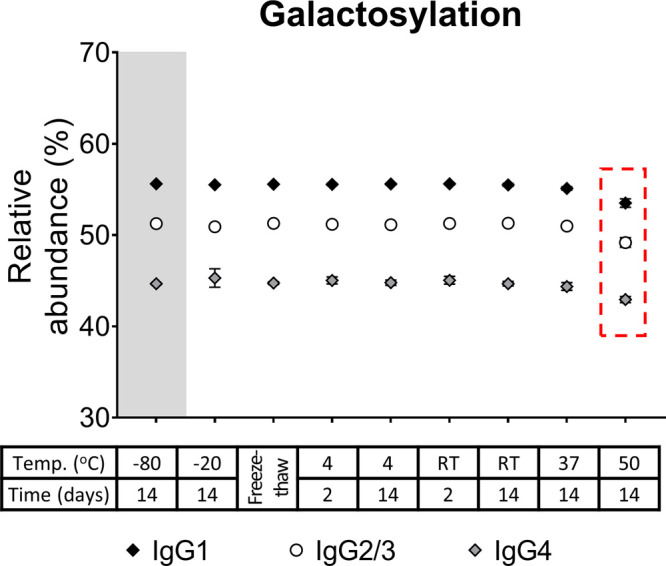
IgG galactosylation of the three subclasses (IgG1, IgG2/3, and IgG4) from the serum of donor 1 (mean, SD, N = 4). All IgG1 findings were also observed for the other subclasses, exemplified for serum galactosylation of donor 1 here. The box highlights changes corresponding to p-values = 1 × 10–4, 2 × 10–4, and 6 × 10–5 for IgG1, IgG2/3, and IgG4, respectively. RT: room temperature.
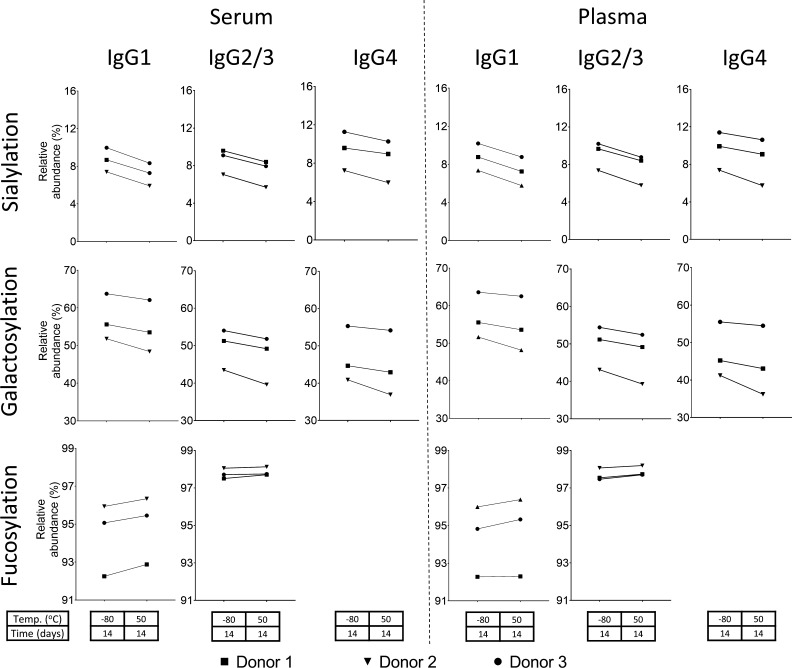
Figure 4.
Changes of IgG glycosylation features (sialylation, galactosylation, and fucosylation) from the reference storage condition—–80 °C for 2 weeks—to the most extreme accelerated storage stability condition—50 °C for 2 weeks—of serum and plasma for the three donors at each IgG subclass (IgG1, IgG2/3, and IgG4). The significant findings for donor 1 are also observed as trends for donors 2 and 3.
Figure 5.
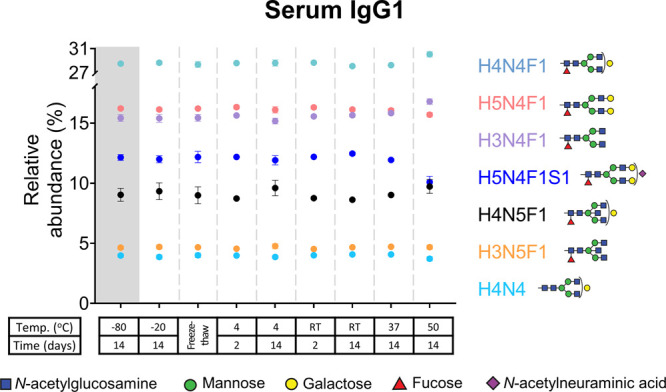
Serum IgG1 glycopeptides from donor 1 (mean, SD) after exposure to different storage conditions. Glycoforms with a relative abundance above 2.5% are depicted. A decrease in the sialylated, galactosylated glycoform H5N4F1S1 is observed after storage at 50 °C for 2 weeks. The bisected forms, H3N5F1 and H4N5F1, were unchanged across all studied conditions. RT: room temperature.
The observed changes in serum and plasma IgG glycosylation suggest the hydrolysis of glycosidic bonds. Biocatalytic activity of human enzymes should show the largest effects at 37 °C, while chemical hydrolysis would be expected to increase with temperature. The loss of galactosylation and sialylation was only observed at the highest temperature, supporting a chemically driven degradation of the glycan structures rather than an enzymatically catalyzed one. The observed changes are not likely to result from peptide modifications. A differential impact of glycosylation on the propensity for peptide modification is rare, and only in such a scenario, glycosylation-relative abundances would be affected. In contrast, the hydrolysis of glycosidic bonds, especially involving sialic acids, is well established. The changes in the glycosylation-derived traits were as expected for the proposed glycosidic bond hydrolysis. A substrate–product relationship was visible at the individual glycan level, such as H5N4F1S1, H4N4F1S1, and H4N4F1, further confirming this interpretation (Figures 5, S-5, and S-6, Table S-6).
Serum and plasma storage at 37 °C for 2 weeks did not induce any relevant changes in IgG Fc N-glycosylation. Consequently, one may conclude that storage for several years in the freezer, especially at −80 °C, will have no noticeable influence on subsequent IgG Fc N-glycosylation analysis. IgG glycosylation is also likely to be unchanged when technical issues with refrigeration systems during sample transportation and storage temporarily prevent the desired storage conditions. Even though minor changes were observed at 50 °C after 2 weeks, differences are only likely to manifest if different samples in the same cohort are stored for vastly different time periods. Moreover, other studies in the glycosylation stability of optimized storage of dried blood spots and plasma showed similar observations to our findings, demonstrating that glycosylation remains stable under a variety of storage conditions including long-term studies.22−25 Hence, the profound stability of IgG Fc N-glycosylation observed here under various accelerated storage stability conditions allows us to rule out conventional storage conditions as an important confounder of IgG Fc N-glycosylation studies. Exceptions may be found in studies with a large spread in individual storage times and a focus on assessing minute glycosylation changes, such as 1% differences in specific glycosylation-derived traits.38
Conclusions
IgG Fc N-glycosylation is highly stable in frozen serum and plasma samples. Even temporary exposure to RT, visible light, or repeated freeze–thaw cycles is tolerated. Our study indicates that the sample storage for IgG glycosylation analysis demands less efforts than that for other omics studies. This makes IgG glycosylation studies feasible for cohorts with suitably extensive consent that no longer allow the unbiased detection of less stable biomolecular features, for example, due to repeated freeze–thaw cycles. Also, in situations where samples cannot be processed in a very fast and highly controlled manner, or the cold chain cannot be warranted for sample shipment and storage, IgG glycosylation analysis is not likely to suffer reduced accuracy and precision. The low effort to warrant IgG glycosylation stability adds to its attractiveness as a clinical marker.
Acknowledgments
The authors thank Gestur Vidarsson for supplying the healthy donor samples.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jproteome.1c00148.
Experimental details and supporting figures: Figure S-1, serum IgG glycosylation features from donor 1; Figure S-2, plasma IgG glycosylation features from donor 1; Figure S-3, serum IgG glycosylation features from three donors; Figure S-4, plasma IgG glycosylation features from three donors; Figure S-5, serum IgG glycopeptides from donor 1; and Figure S-6, plasma IgG glycopeptides from donor 1 (PDF)
Table S-1, alignment list; Table S-2, calibration list; Table S-3, analyte list; Table S-4, extracted mass spectrometric features; Table S-5, relative abundances; and Table S-6, individual glycopeptides (XLSX)
Author Contributions
All authors designed the experiments. M.A.M. performed and evaluated the experiments, supervised by D.F. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
M.A.M. received funding from the European Union as part of a Marie Curie Innovative Training Network (Analytics for Biologics project, grant agreement no. 765502). David Falck received funding from the Dutch Research Council (NWO) in the framework of the ENW PPP Fund for the top sectors (project Proteoform-resolved pharmacokinetics of biopharmaceuticals, no. 019.012).
The authors declare the following competing financial interest(s): Manuela Amez Martín is employed by Ludger Ltd.
Notes
The MS data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the MassIVE repository with the dataset identifier PXD025074 and MSV000087037 (https://doi.org/doi:10.25345/C5P225), respectively.
Supplementary Material
References
- Yamaguchi Y.; Barb A. W. A synopsis of recent developments defining how N-glycosylation impacts immunoglobulin G structure and function. Glycobiology 2020, 30, 214–225. 10.1093/glycob/cwz068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P.; Woen S.; Wang T.; Liau B.; Zhao S.; Chen C.; Yang Y.; Song Z.; Wormald M. R.; Yu C.; Rudd P. M. Challenges of glycosylation analysis and control: an integrated approach to producing optimal and consistent therapeutic drugs. Drug Discovery Today 2016, 21, 740–765. 10.1016/j.drudis.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Šimurina M.; de Haan N.; Vučković F.; Kennedy N. A.; Štambuk J.; Falck D.; Trbojević-Akmačić I.; Clerc F.; Razdorov G.; Khon A.; Latiano A.; D’Incà R.; Danese S.; Targan S.; Landers C.; Dubinsky M.; McGovern D. P. B.; Annese V.; Wuhrer M.; Lauc G. Glycosylation of Immunoglobulin G Associates With Clinical Features of Inflammatory Bowel Diseases. Gastroenterology 2018, 154, 1320–1333.e10. 10.1053/j.gastro.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempers A. C.; Hafkenscheid L.; Scherer H. U.; Toes R. E. M. Variable domain glycosylation of ACPA-IgG: A missing link in the maturation of the ACPA response?. Clin. Immunol. 2018, 186, 34–37. 10.1016/j.clim.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Budach V.; Tinhofer I. Novel prognostic clinical factors and biomarkers for outcome prediction in head and neck cancer: a systematic review. Lancet Oncol. 2019, 20, e313–e326. 10.1016/s1470-2045(19)30177-9. [DOI] [PubMed] [Google Scholar]
- Nabbout R.; Kuchenbuch M. Impact of predictive, preventive and precision medicine strategies in epilepsy. Nat. Rev. Neurol. 2020, 16, 674–688. 10.1038/s41582-020-0409-4. [DOI] [PubMed] [Google Scholar]
- Kaneko Y.; Nimmerjahn F.; Ravetch J. V. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 2006, 313, 670–673. 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- Lin S.; Wang Y.; Wang X.; Yan B.; Lou W.; Di W. Serum immunoglobulin G N-glycome: a potential biomarker in endometrial cancer. Ann. Transl. Med. 2020, 8, 748. 10.21037/atm-20-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoratou E.; Thaci K.; Agakov F.; Timofeeva M. N.; Stambuk J.; Pucic-Bakovic M.; Vuckovic F.; Orchard P.; Agakova A.; Din F. V.; Brown E.; Rudd P. M.; Farrington S. M.; Dunlop M. G.; Campbell H.; Lauc G. Glycosylation of plasma IgG in colorectal cancer prognosis. Sci. Rep. 2016, 6, 28098. 10.1038/srep28098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieczorek M.; Braicu E. I.; Oliveira-Ferrer L.; Sehouli J.; Blanchard V. Immunoglobulin G Subclass-Specific Glycosylation Changes in Primary Epithelial Ovarian Cancer. Front. Immunol. 2020, 11, 654. 10.3389/fimmu.2020.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman M. E.; Crispin M.; Yu X.; Baruah K.; Boesch A. W.; Harvey D. J.; Dugast A.-S.; Heizen E. L.; Ercan A.; Choi I.; Streeck H.; Nigrovic P. A.; Bailey-Kellogg C.; Scanlan C.; Alter G. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J. Clin. Invest. 2013, 123, 2183–2192. 10.1172/jci65708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D. R.; Fong Y.; Li S. H.; Yang F.; Jennewein M. F.; Weiner J. A.; Harrell E. A.; Mangold J. F.; Goswami R.; Seage G. R. 3rd; Alter G.; Ackerman M. E.; Peng X.; Fouda G. G.; Permar S. R. Fc Characteristics Mediate Selective Placental Transfer of IgG in HIV-Infected Women. Cell 2019, 178, 190–201.e11. 10.1016/j.cell.2019.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb B. A. The history of IgG glycosylation and where we are now. Glycobiology 2020, 30, 202–213. 10.1093/glycob/cwz065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudelj I.; Lauc G.; Pezer M. Immunoglobulin G glycosylation in aging and diseases. Cell. Immunol. 2018, 333, 65–79. 10.1016/j.cellimm.2018.07.009. [DOI] [PubMed] [Google Scholar]
- Momčilović A.; de Haan N.; Hipgrave Ederveen A. L.; Bondt A.; Koeleman C. A. M.; Falck D.; de Neef L. A.; Mesker W. E.; Tollenaar R.; de Ru A.; van Veelen P.; Wuhrer M.; Dotz V. Simultaneous Immunoglobulin A and G Glycopeptide Profiling for High-Throughput Applications. Anal. Chem. 2020, 92, 4518–4526. 10.1021/acs.analchem.9b05722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.-Y.; Dong M.; Yang G.; Zhou Y.; Clark D. J.; Lih T. M.; Schnaubelt M.; Liu Z.; Zhang H. Glycans, Glycosite, and Intact Glycopeptide Analysis of N-Linked Glycoproteins Using Liquid Handling Systems. Anal. Chem. 2020, 92, 1680–1686. 10.1021/acs.analchem.9b03761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneveld M. E.; Natunen S.; Sainio S.; Koeleman C. A. M.; Holst S.; Dekkers G.; Koelewijn J.; Partanen J.; van der Schoot C. E.; Wuhrer M.; Vidarsson G. Glycosylation pattern of anti-platelet IgG is stable during pregnancy and predicts clinical outcome in alloimmune thrombocytopenia. Br. J. Haematol. 2016, 174, 310–320. 10.1111/bjh.14053. [DOI] [PubMed] [Google Scholar]
- Tworoger S. S.; Hankinson S. E. Collection, processing, and storage of biological samples in epidemiologic studies: sex hormones, carotenoids, inflammatory markers, and proteomics as examples. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1578–1581. 10.1158/1055-9965.epi-06-0629. [DOI] [PubMed] [Google Scholar]
- Burla B.; Arita M.; Arita M.; Bendt A. K.; Cazenave-Gassiot A.; Dennis E. A.; Ekroos K.; Han X.; Ikeda K.; Liebisch G.; Lin M. K.; Loh T. P.; Meikle P. J.; Orešič M.; Quehenberger O.; Shevchenko A.; Torta F.; Wakelam M. J. O.; Wheelock C. E.; Wenk M. R. MS-based lipidomics of human blood plasma: a community-initiated position paper to develop accepted guidelines. J. Lipid Res. 2018, 59, 2001–2017. 10.1194/jlr.s087163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens V. L.; Hoover E.; Wang Y.; Zanetti K. A. Pre-Analytical Factors that Affect Metabolite Stability in Human Urine, Plasma, and Serum: A Review. Metabolites 2019, 9, 156. 10.3390/metabo9080156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson M.; Borén M.; Sköld K.; Fälth M.; Sjögren B.; Andersson M.; Svenningsson P.; Andrén P. E. Heat stabilization of the tissue proteome: a new technology for improved proteomics. J. Proteome Res. 2009, 8, 974–981. 10.1021/pr8006446. [DOI] [PubMed] [Google Scholar]
- Vreeker G. C. M.; Bladergroen M. R.; Nicolardi S.; Mesker W. E.; Tollenaar R. A. E. M.; van der Burgt Y. E. M.; Wuhrer M. Dried blood spot N-glycome analysis by MALDI mass spectrometry. Talanta 2019, 205, 120104. 10.1016/j.talanta.2019.06.104. [DOI] [PubMed] [Google Scholar]
- Simunovic J.; Vilaj M.; Trbojevic-Akmacic I.; Momcilovic A.; Vuckovic F.; Gudelj I.; Juric J.; Nakic N.; Lauc G.; Pezer M. Comprehensive N-glycosylation analysis of immunoglobulin G from dried blood spots. Glycobiology 2019, 29, 817–821. 10.1093/glycob/cwz061. [DOI] [PubMed] [Google Scholar]
- Choi N. Y.; Hwang H.; Ji E. S.; Park G. W.; Lee J. Y.; Lee H. K.; Kim J. Y.; Yoo J. S. Direct analysis of site-specific N-glycopeptides of serological proteins in dried blood spot samples. Anal. Bioanal. Chem. 2017, 409, 4971–4981. 10.1007/s00216-017-0438-z. [DOI] [PubMed] [Google Scholar]
- Hu Y.; Ferdosi S.; Kapuruge E. P.; Diaz de Leon J. A.; Stücker I.; Radoï L.; Guénel P.; Borges C. R. Diagnostic and Prognostic Performance of Blood Plasma Glycan Features in the Women Epidemiology Lung Cancer (WELCA) Study. J. Proteome Res. 2019, 18, 3985–3998. 10.1021/acs.jproteome.9b00457. [DOI] [PubMed] [Google Scholar]
- Chuanxiang W.; Lian X.; Lijie L.; Fengli Q.; Zhiwei S.; Xianen Z.; Jinmao Y. A sensitive and efficient method for determination of N-acetylhexosamines and N-acetylneuraminic acid in breast milk and milk-based products by high-performance liquid chromatography via UV detection and mass spectrometry identification. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2016, 1011, 14–23. 10.1016/j.jchromb.2015.12.032. [DOI] [PubMed] [Google Scholar]
- Yang W. H.; Aziz P. V.; Heithoff D. M.; Mahan M. J.; Smith J. W.; Marth J. D. An intrinsic mechanism of secreted protein aging and turnover. Proc. Natl. Acad. Sci. U.S.A. 2015, 112, 13657–13662. 10.1073/pnas.1515464112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Sundlov M. M.; Ashline D. J.; Hanneman A. J.; Grozovsky R.; Reinhold V. N.; Hoffmeister K. M.; Lau J. T. Circulating blood and platelets supply glycosyltransferases that enable extrinsic extracellular glycosylation. Glycobiology 2017, 27, 188–198. 10.1093/glycob/cww108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falck D.; Jansen B. C.; de Haan N.; Wuhrer M. High-Throughput Analysis of IgG Fc Glycopeptides by LC-MS. Methods Mol. Biol. 2017, 1503, 31–47. 10.1007/978-1-4939-6493-2_4. [DOI] [PubMed] [Google Scholar]
- Amez-Martín M.; Wuhrer M.; Falck D. Immunoglobulin G Glycoprofiles are Unaffected by Common Bottom-Up Sample Processing. J. Proteome Res. 2020, 19, 4158–4162. 10.1021/acs.jproteome.0c00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen B. C.; Falck D.; de Haan N.; Hipgrave Ederveen A. L.; Razdorov G.; Lauc G.; Wuhrer M. LaCyTools: A Targeted Liquid Chromatography-Mass Spectrometry Data Processing Package for Relative Quantitation of Glycopeptides. J. Proteome Res. 2016, 15, 2198–2210. 10.1021/acs.jproteome.6b00171. [DOI] [PubMed] [Google Scholar]
- Fokkink W. J. R.; Falck D.; Santbergen T. C. M.; Huizinga R.; Wuhrer M.; Jacobs B. C. Comparison of Fc N-Glycosylation of Pharmaceutical Products of Intravenous Immunoglobulin G. PLoS One 2015, 10, e0139828 10.1371/journal.pone.0139828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi H.; Guo Z.; Jia X.; Liu H.; Ma L.; Xue L. The key points in the pre-analytical procedures of blood and urine samples in metabolomics studies. Metabolomics 2020, 16, 68. 10.1007/s11306-020-01666-2. [DOI] [PubMed] [Google Scholar]
- Bondt A.; Selman M. H. J.; Deelder A. M.; Hazes J. M. W.; Willemsen S. P.; Wuhrer M.; Dolhain R. J. E. M. Association between galactosylation of immunoglobulin G and improvement of rheumatoid arthritis during pregnancy is independent of sialylation. J. Proteome Res. 2013, 12, 4522–4531. 10.1021/pr400589m. [DOI] [PubMed] [Google Scholar]
- Liu J.; Dolikun M.; Štambuk J.; Trbojević-Akmačić I.; Zhang J.; Wang H.; Zheng D.; Zhang X.; Peng H.; Zhao Z.; Liu D.; Sun Y.; Sun Q.; Li Q.; Zhang J.; Sun M.; Cao W.; Momčilović A.; Razdorov G.; Wu L.; Russell A.; Wang Y.; Song M.; Lauc G.; Wang W. The association between subclass-specific IgG Fc N-glycosylation profiles and hypertension in the Uygur, Kazak, Kirgiz, and Tajik populations. J. Hum. Hypertens. 2018, 32, 555–563. 10.1038/s41371-018-0071-0. [DOI] [PubMed] [Google Scholar]
- Hubel A.; Spindler R.; Skubitz A. P. N. Storage of human biospecimens: selection of the optimal storage temperature. Biopreserv. Biobanking 2014, 12, 165–175. 10.1089/bio.2013.0084. [DOI] [PubMed] [Google Scholar]
- International Council for Harmonisation . Q5C Quality of Biotechnological Products: Stability Testing of Biotechnological/Biological Products; European Medicines Agency—EMEA: London, 1996. [Google Scholar]
- Wang J.-R.; Gao W.-N.; Grimm R.; Jiang S.; Liang Y.; Ye H.; Li Z.-G.; Yau L.-F.; Huang H.; Liu J.; Jiang M.; Meng Q.; Tong T.-T.; Huang H.-H.; Lee S.; Zeng X.; Liu L.; Jiang Z.-H. A method to identify trace sulfated IgG N-glycans as biomarkers for rheumatoid arthritis. Nat. Commun. 2017, 8, 631. 10.1038/s41467-017-00662-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.