Abstract
Summary
A growing body of evidence suggests that diet quality may predict muscle health. This study found that a “Traditional” dietary pattern predicted greater muscle mass, and an anti-inflammatory diet predicted greater muscle mass and better muscle function over 15 years. These findings reinforce the importance of optimising dietary behaviours for healthy ageing.
Introduction
Research investigating the roles of individual nutrients in muscle health fails to account for the synergistic relationships between foods and nutrients. This study aimed to investigate the predictive value of diet quality and dietary patterns for muscle mass and function in men over a 15-year period.
Methods
This longitudinal study was conducted in 522 men from the Geelong Osteoporosis Study with complete dietary and muscle mass or muscle function data at both baseline and 15-year follow-up assessments. Dietary exposures were extracted from food frequency questionnaires and included the Australian Recommended Food Score, the Dietary Inflammatory Index (DII®), and three a posteriori dietary patterns: Plant-focused, Western, and Traditional (Anglo-Australian). Outcome variables included dual-energy X-ray absorptiometry–derived skeletal muscle index (SMI) and muscle function measured with the timed up-and-go (TUG) test.
Results
An anti-inflammatory diet and higher scores on a Traditional dietary pattern both predicted greater SMI ((B: −0.04 (95%CI −0.08, −0.00) kg/m2) and (B: 0.12 (95%CI 0.04, 0.20) kg/m2), respectively), while a pro-inflammatory diet predicted slower TUG (B: 0.11 (95%CI 0.001, 0.21) sec) over the 15-year follow-up period. These associations remained significant following adjustment for confounding variables. There were no associations observed for other dietary exposures.
Conclusion
A Traditional dietary pattern higher in vegetables, wholegrain cereals, and animal protein was associated with greater skeletal muscle mass, and an anti-inflammatory diet, also rich in vegetables, fruit, and wholegrain cereals, was associated with greater skeletal muscle mass and better muscle function over 15 years.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00198-021-06012-3.
Keywords: Ageing, Diet quality, Dietary patterns, Muscle function, Muscle mass, Sarcopenia
Introduction
With advancements in modern medicine and public health, our global population is steadily ageing. In 2018, for the first time in history, the population aged over 65 years outnumbered those aged under 5 years [1]. Despite improvements in longevity, poor muscle health is associated with reduced quality of life and predicts mortality in ageing populations [2, 3]. Low muscle mass and poor muscle strength and function are key characteristics of poor muscle health. Low muscle mass predicts reduced mobility, and mobility—in turn—predicts mortality [4, 5]. While exercise training is a key health behaviour that can improve muscle health [6], identifying additional modifiable contributors to muscle health is imperative to guide intervention strategies.
Diet is a known correlate of muscle health [7]. Research investigating aspects of nutrition that relate to the risk for poor muscle health have historically focused on individual components of diet, such as protein and specific micronutrients. However, a focus on individual macro and micronutrients fails to consider synergistic relationships between foods and nutrients [8]. A priori diet quality scores, by contrast, provide a metric for assessing whole diet quality. These predefined indices are usually based on current nutrition guidelines or recommendations and can be used to quantify an individual’s overall diet quality. Examples include validated indices measuring adherence to a traditional Mediterranean diet (MD), such as the Mediterranean Diet Score (MDS), or to local guidelines, such as the Australian Recommended Food Score (ARFS) [9]. Another category of a priori diet quality scores focuses on the functional effect of the foods and nutrients—e.g. on ability to effect changes in blood glucose (e.g. the glycaemic index [10]) or inflammation (e.g. the Dietary Inflammatory Index [DII®])[11]. In contrast, data-driven a posteriori dietary patterns reflect the eating patterns specific to the study sample. These can be developed using principal component analysis, which cluster a study sample’s dietary patterns into distinct factors based on individuals’ frequency of consumption of specific foods.
Previously, studies investigating how diet quality scores relate to muscle mass and function in men longitudinally over 2 to 10 years have reported conflicting findings. The Baltic Sea Diet Score, considered the diet quality measure similar to the MDS for Nordic populations, did not predict either muscle mass or walking speed in Finnish men over 10 years [12, 13]. Similarly, in a sample of Chinese adults >65 years of age, neither the MDS nor data-driven dietary patterns provided any predictive value for sarcopenia risk over 4 years [14]. However, in an Italian cohort, higher scores on the MDS at baseline were associated with a lower risk of developing reduced walking speed over 6 years [15]. These conflicting findings may be due to the varying timespans and/or age groups studied, or to the application of indices measuring the MD in non-Mediterranean cultures. Due to the chronic inflammation associated with increases in age and decreases in physical functioning, the Dietary Inflammatory Index (DII®), a measure of the inflammatory potential of participants’ diets, has also has been utilised in muscle health research [16]. The relationship between the DII and muscle health has been investigated in Chinese children and Australian adults, with results from the latter suggesting an association between the DII and muscle health in Australian women, but not men, over 10 years of follow-up [17, 18].
Studies investigating the relationship between a posteriori dietary patterns and men’s muscle mass and function are scarcer. However, from the limited evidence, a Western dietary pattern was associated with poorer muscle function and a MD-style pattern associated with better muscle function in Spanish men aged 55–80 years in one cross-sectional study [19]. Furthermore, an investigation of culturally specific dietary patterns in UK men aged ≥ 85 years found that a diet high in meat, potato, and gravy predicted a slower timed up-and-go (TUG), which assesses muscle function via walking speed, balance, and mobility, than those who consumed less meat, over 5 years [20].
Findings from available studies addressing the role of diet in the maintenance of muscle mass and function are discordant in relation to age, muscle outcome measures, follow-up times, and ethnic groups. The diverse findings may reflect the need for measures of diet quality that are culturally specific to study samples or, at the other extreme, the need to describe diet quality in terms of physiological effects independent of culinary traditions and cultural foodways. The current study aimed to investigate the predictive value of various measures of diet quality and dietary patterns for muscle mass and function in Australian men over a 15-year period.
Methods
Participants
Study participants included 522 men from the baseline and 15-year follow-up of the Geelong Osteoporosis Study (GOS). The GOS, an ongoing prospective cohort study, has been collecting data on men since 2001. Participants were randomly selected from the electoral roll of the Barwon Statistical Division and invited to participate. Details of recruitment, data collection, and participation have been published elsewhere [21]. The GOS was approved by the Human Research Ethics Committee at Barwon Health, and written, informed consent is collected from all participants.
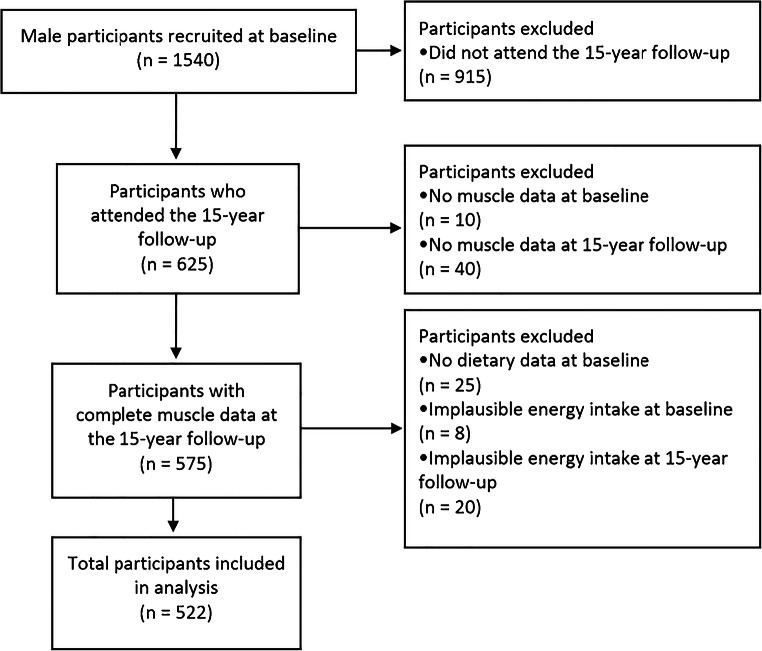
Participants included in the current study were those who had provided complete food frequency dietary data at both timepoints, in addition to complete dual-energy X-ray absorptiometry (DXA) scans and/or TUG data at both timepoints. Participants were excluded if energy intake was considered implausible, i.e. excessively high or low, defined as <3360 or >16,800 kJ/day [22] (Fig. 1).
Fig. 1.
Prisma diagram for GOS participant selection. Participants included were those who provided both muscle and diet data at 10- and 15-year men’s follow-ups of the Geelong Osteoporosis Study
Data collection
Anthropometry and body composition
Height was measured with a wall-mounted stadiometer and accurate to the nearest 0.1cm. Body composition was determined from whole body DXA using a densitometer (Lunar DPX-L and Lunar Prodigy-Pro, LUNAR Corporation, Madison, WI, USA). In the baseline cohort, the DPX-L was used on the initial n=544 and the ProdigyPro was used with the remaining n=996. However, no mean differences in bone mineral density were noted between the two measures [21]. From DXA scans, appendicular lean body mass (ALM) was calculated as the sum of lean mass for arms and legs.
Muscle mass and function
Skeletal muscle index (SMI), defined as ALM/height2 (kg/m2), was used as a surrogate measure of muscle mass. Muscle function was measured by the Timed Up and Go (TUG) test, which refers to the time taken (seconds) for a participant to rise from a chair, walk 3 m, turn around, walk back, and sit down, all without upper body assistance unless using a walking aid such as a walking frame [23]. Slower TUG is an indicator of poor muscle function. The cut-offs for low SMI and slow TUG in the current study were <7.0 kg/m2 and ≥20 s, respectively, as per recommendations from the revised European Working Group on Sarcopenia in Older People (EWGSOP2) [24].
Dietary data
Self-reported dietary data were collected using a food frequency questionnaire (FFQ), The Cancer Council Victoria’s Dietary Questionnaire for Epidemiological Studies, which has been validated in a similar population [9]. In brief, data relating to frequency of consumption of specific foods at designated serve sizes were collected for 74 foods, including milk, in addition to six alcoholic beverages, over the previous 12 months. The Cancer Council Victoria provided computed FFQ results which included both serves per day and grams per day.
Diet quality indices
FFQ data were used to calculate the ARFS and the DII scores. The ARFS is a validated diet quality index that measures an individual against adherence to the Australian Dietary Guidelines. Points are allocated based on serves of and/or frequency of consumption for the following: (i) vegetables, nuts, and beans, (ii) fruit, (iii) protein foods, (iv) grains, (v) dairy, (vi) fats, and (vii) alcohol, and points are totalled to obtain a score range of 0–74 [9].
The DII score measures dietary inflammatory potential and has been validated against various biomarkers in over 30 studies [25]. The theoretical DII score range is −8.87 to +7.98, with a positive score reflecting a pro-inflammatory diet and a negative score reflecting an anti-inflammatory diet. While the complete DII includes 45 food parameters associated with changes in inflammatory markers [11], as with most structured instruments, data from the FFQ were available for fewer (i.e. 22) food parameters.
A posteriori dietary patterns
Using a principal component analysis with orthogonal varimax rotation, three dietary patterns were identified from baseline FFQ data. Food components with a loading of <0.05 were excluded [26], resulting in a final list of 93 food components from the original 101 listed in the raw FFQ data. Factor loadings from principle component analysis were multiplied by participant food consumption data in grams per day to provide participant scores for each of the three dietary patterns.
Other potential confounders
Additional variables for consideration as potential confounders were collected at both baseline and 15-year follow-up assessment phases. These included age, current smoking, highest level of education ((i) never attended school, (ii) completed primary or some secondary school (including “primary school” and “secondary school”), (iii) completed secondary school or vocational training (including “completed secondary school” and “TAFE/trade/apprenticeship”), or (iv) tertiary education (including “university” and “other post-secondary training/qualification”)); employment status (either (i) working (including working full-time, part-time, voluntary work, and casually), (ii) not working (including retired and not retired), (iii) home duties, (iv) full-time or part-time student, (v) retired, (vi) unable to work/ill), or (vii) not applicable); ethnicity; total protein intake (g/day); total energy intake (kJ/day); and DXA-derived total body fat mass (g). Self-reported physical activity (PA) was measured using the Baecke Physical Activity Questionnaire [27], which uses a Likert scale to assess work, sport, and leisure time PA. Scores from these three domains were combined for a total PA score. A composite, dichotomised variable was developed of self-reported medications that may impact muscle. These included agents that affect the musculoskeletal system and endocrine and metabolic disorders. Medical conditions potentially affecting muscle also were dichotomised (yes/no) and included osteoarthritis, rheumatoid arthritis, thyroid conditions, type-1 and type-2 diabetes mellitus, hypoglycaemia, pernicious anaemia, asthma, emphysema, chronic bronchitis, and multiple sclerosis.
Statistical analyses
To account for the longitudinal nature of this study, generalised estimating equations (GEE) were employed. To assist in comparing beta coefficients between models, all diet quality scores were converted to z-scores. Potential confounders were first identified using trivariable models, through examining the p-values and inclusion if the potential confounder variable notably altered the B-coefficient of the exposure of interest. The final multivariable models were determined using a backwards stepwise variable selection strategy. In addition to the abovementioned data-driven confounder identification, PA, and age also were included in all adjusted models due to their widely accepted effects on muscle health [28]. Deviation from model assumptions, including normality of continuous dependent variables, was visually inspected and correlation coefficients were used to assess collinearity. In the final models, GEEs were employed to investigate associations between the dietary exposures and both SMI and TUG, after accounting for potential confounders. All variables considered as potential confounders were time-updated. GEE models included a nominal factor for measurement time (baseline, and 15-year follow-up), with time-updated diet measurement as the exposure of interest. Time-by-diet, age-by-diet, and PA-by-diet two-way interactions also were investigated. The time-by-diet interaction term examined the effect of diet change (from baseline to 15-year follow-up) on SMI and TUG. All analyses were conducted using Stata 16.0 (StataCorp LP. College Station, TX, USA) and the analysis plan was preregistered at Open Science Framework (https://osf.io/wxzpv).
Results
Study participant characteristics at baseline and 15-year follow-up are summarised in Table 1 and Fig. 2. Education levels and the number of participants who had retired increased, while median PA scores decreased over the 15-year period. A greater number of participants were taking medications and had a current medical condition at 15 years compared to baseline.
Table 1.
Participants characteristics at baseline and 15-year follow-up assessments
| Study participants (n=522) | ||
|---|---|---|
| Baseline | 15-year follow-up | |
| Age, years, median (IQR) | 50.0 (38.30, 59.70) | 64.0 (52.40, 73.60) |
| Education^* | ||
| • Never attended school , n (%) | 3 (0.6) | 0 |
| • Primary or some secondary school, n (%) | 212 (40.8) | 119 (23.0) |
| • Completed secondary or vocational training, n (%) | 159 (30.6) | 221 (42.6) |
| • Tertiary education, n (%) | 146 (28.0) | 178 (34.4) |
| Employment#* | ||
| • Working | 393 | 285 |
| • Not working | 13 | 10 |
| • Home duties | 0 | 5 |
| • Student | 6 | 3 |
| • Retired | 100 | 204 |
| • Unable to work | 6 | 5 |
| • Not applicable | 1 | 8 |
| PA score, median (IQR)** | 7.9 (7–9) | 7.0 (5–8) |
| Current smoker, n (%)* | 64 (12.3) | 38 (7.3) |
| Current use of medications, n (%)** | 64 (12.3) | 108 (20.8) |
| Medical conditions, n (%)** | 132 (25.4) | 224 (43.1) |
| BMI (kg/m2), median (IQR)** | 26.48 (24.16, 28.97) | 27.38 (24.94, 30.12) |
| Body fat (kg), median (IQR)** | 20.43 (15.25, 25.50) | 24.35 (18.76, 31.51) |
| SMI (kg/m2), mean (±SD)** | 8.67 (0.90) | 8.48 (1.00) |
| • Low SMI <7.0kg/m2, n (%)* | 6 (1.2) | 33 (6.4) |
| TUG (sec), median (IQR) ** | 7.10 (6.44, 8.00) | 8.43 (7.49, 9.80) |
| • Slow TUG ≥20 sec, n (%) | 0 | 10 (1.9) |
| ARFS, mean (±SD) | 30.56 (8.78) | 31.07 (8.90) |
| DII, median (IQR)** | −0.18 (−1.09, 0.69) | 0.18 (−0.75, 1.10) |
| Energy intake (kj/d), median (IQR)** | 8658.14 (6973.17, 10476.96) | 7679.27 (6123.96, 9510.77) |
PA = physical activity, PA score = Baecke Physical Activity Questionnaire, BMI = Body Mass Index, ARFS = Australian Recommended Food Score, DII = Dietary Inflammatory Index, SMI = Skeletal Muscle Index, TUG = Timed Up-and-Go. ^n=2 missing data at baseline, n=4 missing data at 15-year follow-up, #n=3 missing data at baseline, n=2 missing data at 15-year follow-up, *significant difference between baseline and 15-year follow-up values p<0.05, **significant difference between baseline and 15-year follow-up values p<0.001
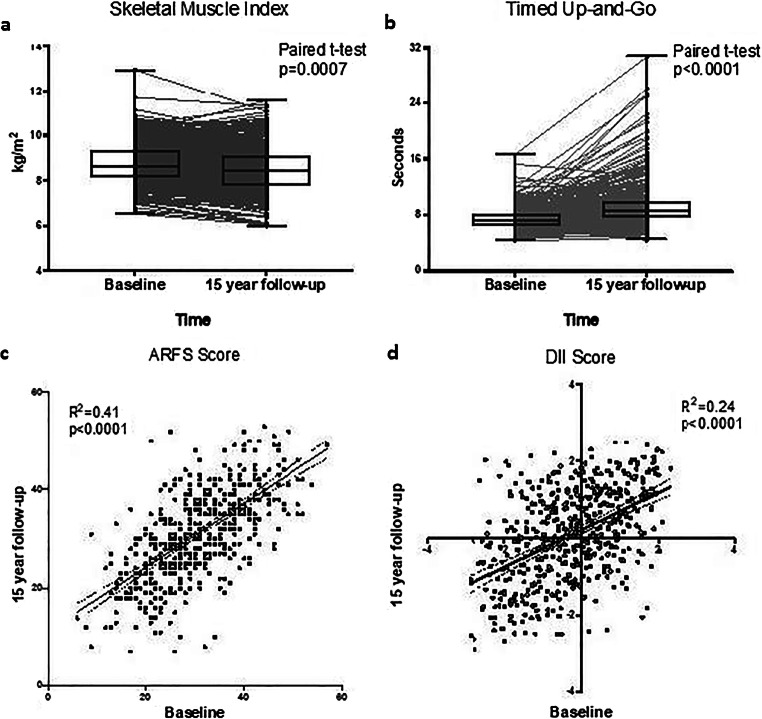
Fig. 2.
Changes in muscle health measures and diet quality indices between baseline and 15-year follow-up. a A significant decrease was observed in participants’ skeletal muscle index between baseline and 15 years. b Timed up-and-go scores were significantly slower at 15 years compared to baseline. c Participants’ Australian Recommended Food Scores remained consistent over the 15-year period with R2=0.41 between the two time points. d Minor changes were observed between Dietary Inflammatory Index scores between baseline and 15 years with R2=0.24
SMI reduced over 15 years while the TUG scores increased (i.e. slower times) (Fig. 2A and B). The proportion of participants with low SMI and slow TUG also increased (Table 1). The ARFS dietary scores at 15 years correlated with the baseline time point (r2=0.41, p<0.0001) (Fig. 2C) and did not change over time (Table 1). The DII score was also moderately correlated between the two time points (r2=0.24, p<0.0001) (Fig. 2D) and a paired Wilcoxon rank sum test indicated that the scores increased slightly over 15 years (p<0.0001), indicating the diets became marginally more pro-inflammatory (Table 1). Dietary energy intake decreased over the 15 years and BMI and body fat increased between follow-up assessments (Table 1).
Participants who were lost to follow-up were older than study sample participants at baseline, with a median age of 67 years. These participants had a similar median SMI to the current study sample, but a greater percentage had a low SMI (6.76%). Median TUG at baseline was also slower (7.91 s) with a greater proportion having a slow TUG of ≥ 20 s (8.0%) compared to the study sample.
Diet quality indices and a posteriori dietary patterns
DII scores ranged from −2.99 to +2.53 and, prior to z-score conversion, ARFS scores ranged from 6 to 57.
In addition, three a posteriori dietary patterns at baseline were identified using principle component analysis (Online Resource 1). These were designated (i) Plant-focused, which reflected higher consumption of vegetables, fruit, yoghurt, muesli, multigrain bread, and tofu, and lower consumption of sugar and discretionary foods, full-fat dairy products, white bread and margarine, red, white, and processed meats, and alcohol; (ii) Western, reflecting higher consumption of foods such as pizza, meat pies, hamburgers, and alcohol, with negative factor loadings suggesting lower consumption of vegetables, jam, tinned fruit, desserts, and cereals; and (iii) Traditional (Anglo-Australian pattern), characterised by greater consumption of red, white, and processed meats, unprocessed-fish, fruits and vegetables, wholegrain cereals, nuts, and discretionary foods such as cakes and biscuits.
Dietary factors and skeletal muscle index
There was no relationship between ARFS and SMI. However, in both unadjusted and adjusted models, the DII was inversely associated with SMI, suggesting that consumption of a less inflammatory diet was associated with a smaller decrease in SMI over 15 years (Table 2). Higher scores on the Traditional dietary pattern also predicted smaller reductions SMI over 15 years, both before and after adjustment for confounders. There was some evidence that higher scores on a Western dietary pattern were associated with smaller reductions in SMI in unadjusted models (Table 2); however, this association was lost following adjustment for age, physical activity, and fat mass, with age being the variable responsible for attenuation. In the unadjusted model, there was weak evidence that a Plant-focused diet was inversely associated with SMI but, like the Western dietary pattern, this association was attenuated after adjustments (Table 3).
Table 2.
Generalised Estimating Equation results for Skeletal Muscle Index (SMI)
| β | 95% CI | |
|---|---|---|
| Diet quality indices | ||
| Australian Recommended Food Score | ||
| Model 1# | 0.03 | −0.02, 0.08 |
| Model 2^ | 0.01 | −0.034, 0.06 |
| ^Model adjusted for PA, age and smoking | ||
| Dietary Inflammatory Index | ||
| Model 1# | −0.05* | −0.09, −0.01 |
| Model 2^ | −0.04* | −0.08, −0.004 |
| ^Model adjusted for PA and age, *p<0.05 | ||
| Dietary patterns | ||
| Plant-focused | ||
| Model 1# | −0.09 | −0.18, 0.00 |
| Model 2^ | 0.05 | −0.04, 0.13 |
| ^Model adjusted for PA, age, and fat mass | ||
| Western | ||
| Model 1# | 0.14* | 0.04, 0.24 |
| Model 2^ | 0.00 | −0.10, 0.10 |
| ^Model adjusted for PA, age, and fat mass | ||
| Traditional | ||
| Model 1# | 0.19** | 0.10, 0.28 |
| Model 2^ | 0.12* | 0.04, 0.20 |
#Model unadjusted, ^Model adjusted for PA, age, and Fat mass, PA = physical activity, *p<0.05, **p<0.001, n=522
Table 3.
Generalised Estimating Equation results for Timed Up-and-Go (TUG)
| β | 95% CI | |
|---|---|---|
| Diet quality indices | ||
| Australian Recommended Food Score | ||
| Model 1# | −0.17 | −0.34, 0.01 |
| Model 2^ | −0.06 | −0.22, 0.10 |
| ^Model adjusted for PA, education, age | ||
| Dietary Inflammatory Index | ||
| Model 1# | 0.19* | 0.06, 0.32 |
| Model 2^ | 0.11* | 0.001, 0.21 |
| ^Model adjusted for PA and age, *p<0.05 | ||
| Dietary patterns | ||
| Plant-focused | ||
| Model 1# | 0.12 | −0.05, 0.28 |
| Model 2^ | −0.14 | −0.30, 0.02 |
| ^Model adjusted for PA, age, and education | ||
| Western | ||
| Model 1# | −0.77** | −1.06, −0.48 |
| Model 2^ | −0.18 | −0.42, 0.06 |
| ^Model adjusted for PA, age, and education | ||
| Traditional | ||
| Model 1# | −0.18 | −0.37, 0.02 |
| Model 2^ | 0.05 | −0.11, 0.21 |
#Model unadjusted, ^Model adjusted for PA, age, and education, *p<0.05, **p<0.001, n=522
Dietary factors and timed up-and-go
The DII was positively associated with the TUG measure (Table 3), suggesting that a pro-inflammatory diet was associated with slower TUG time. While the beta-coefficient was reduced after adjustment, the model retained significance, indicating that the association between a pro-inflammatory diet and slower TUG persisted after accounting for differences in age and PA. Higher scores on the Western dietary pattern also predicted slower TUG time. However, the association disappeared after adjustment for PA, age, and education. Concordant with the SMI results, age was the variable that explained the association. Neither the ARFS diet quality score, Plant-focused, or Traditional dietary patterns were associated with TUG time.
Discussion
The current study investigated the relationships between a priori dietary indices, the ARFS and DII, and a posteriori dietary patterns derived from raw data, with changes in both lean muscle mass and function in men over a 15-year period. Both an “anti-inflammatory” dietary pattern and a “Traditional” dietary pattern, characterised by a greater consumption of vegetables, fruits, nuts, unprocessed-fish, wholegrain cereals, and both red and white meats, were associated with greater SMI, even after adjustment for age and measures of physical activity. Similarly, an anti-inflammatory diet was associated with faster TUG, even when adjusted for age and physical activity. However, neither a dietary pattern that was also higher in plant foods but lower in animal proteins, nor a Western-style dietary pattern, was associated with measures of muscle health. Moreover, a diet quality score that measures adherence to Australian Dietary Guidelines, the ARFS, was not observed to predict muscle mass or function over time.
Our finding that the DII was a significant independent predictor of SMI and TUG is discordant with results from a similar Australian cohort assessed over 10 years, wherein researchers reported that an association between the DII and muscle mass and quality was attenuated when controlled for—among other variables—steps-per-day, age, and body fat [18]. While Cervo et al. also used DXA-derived fat mass to measure body fat, our current study used the Baecke Physical Activity Questionnaire to measure PA which is, arguably, a more comprehensive measure than the steps-per-day measure of PA used by Cervo et al., and may therefore provide a more accurate reflection of the impact of PA on the DII-muscle relationship. While there are no prior studies investigating the ARFS diet quality score and muscle health, the ARFS has been previously utilised in body weight research in Australians, finding no associations with weight gain over 6 years [29]. This may be due to some limitations of this dietary measure. Although the ARFS is based on types of foods consumed per day, it does not take portion sizes into account and does not penalise for excessive consumption of discretionary foods. Furthermore, the ARFS is based on the 2003 Australian Dietary Guidelines, which would have been current at baseline in this study but outdated for the 15-year follow-up. For example, points are awarded for consumption of margarine but not olive oil, the health benefits of which have been well established since 2003 [9, 30] .
A Traditional dietary pattern was associated with SMI, but not TUG, even after adjustment for PA, age, and fat mass. The standardised beta coefficient for the Traditional dietary pattern and SMI of 0.12 kg/m2 was the largest observed in this study and translates to an increase of 120g of lean mass per m2 for every unit increase of the Traditional dietary pattern score. Therefore, an individual of standard height who improves their diet from the lowest to the highest score of the Traditional dietary pattern would increase their lean mass by 1.5kg which may provide a clinically relevant increase in lean mass [24].
In a previous study assessing the relationship between dietary patterns and TUG in participants aged ≥85 years, researchers reported that a dietary pattern high in fruits, vegetables, grains, and animal products, and low in processed foods, was associated with faster TUG time compared to a dietary pattern comparable to the current study’s Western dietary pattern [20]. Similar findings have been reported longitudinally over a 28-year period, showing a diet similar to our Traditional dietary pattern predicted healthier physical functioning at 60–64 years old [31]. However, in a 9-year longitudinal French study, which utilised subjective physical functioning measures, no such association was observed [32]. While a trend was observed between the Traditional dietary pattern and faster TUG in the current study, this was attenuated with adjustment for PA, age, and education. The discrepancy between results for the Traditional dietary pattern and SMI and TUG outcomes may be due to age-related loss of muscle mass preceding deterioration of functional ability [33]. Furthermore, this variability in results may also reflect individual differences in nutrient status [34]. For example, a Traditional dietary pattern consumed by a participant who is protein deficient due to anabolic resistance may have a different effect on muscle health than on an individual who is protein replete. Collection of detailed baseline biological data, in addition to nutrition intake data, to establish participants’ baseline nutritional status may help to elucidate this potential determinant in future research.
Of the three dietary patterns identified in this study, the Traditional dietary pattern was characterised by greater consumption of wholegrains and nuts, and a wider variety of plant foods and animal foods including non-processed fish, compared to the Western and Plant-focused dietary patterns. The association observed between the Traditional dietary pattern and SMI may therefore reflect the important role of intake of a high diversity of foods, which is more likely to provide a range of micronutrients and other dietary components. While literature assessing the association of dietary diversity with muscle health is scarce, a recent Korean study investigated dietary diversity and sarcopenia, reporting that, cross-sectionally, poor dietary diversity was associated with sarcopenia in women, but not men. Future muscle health research may benefit from investigation of the longitudinal dietary diversity-muscle health relationship. In addition to diversity, the Traditional dietary pattern also included greater consumption of nuts and non-processed fish, compared to the Plant-focused and Western patterns. Therefore, fat type in the Traditional dietary pattern may include greater quantities of mono- and poly-unsaturated fats from these sources. Although not captured by the FFQ, if fresh fish includes oily fish such as salmon, the Traditional dietary pattern may provide greater levels of omega-3 fatty acids which have been directly associated with improved muscle metabolism in older adults when taking in supplemental form [35]. The quantity of high-quality protein provided by animal products in the Traditional pattern may also contribute to muscle metabolism by providing a rich amino acid pool to fuel muscle protein synthesis. In addition to quantity and source of protein, timing of its consumption is also an important consideration for muscle anabolism which may be a useful FFQ component for future diet-muscle research.
Finally, while higher scores on the Western dietary pattern were associated with higher SMI and faster TUG in unadjusted analyses, suggesting a non-intuitive beneficial impact of a highly processed dietary pattern on muscle health, these associations were driven by age, as Western dietary pattern scores were higher in younger males (data not shown).
The biological mechanisms through which healthier dietary habits may impact muscle health are numerous and complex; however, one such mechanism is the potential role of nutrition in attenuating systemic inflammation. Higher consumption of certain foods such as vegetables, nuts, olive oil, and foods high in anti-oxidants have been associated with favourable changes in inflammatory biomarkers [36–38]. In contrast, pro-inflammatory diets which are often low in wholefoods and micronutrients, and high in fats and refined carbohydrates, have been associated with increased levels of pro-inflammatory cytokines including IL-6, CRP, and TNF-α [11, 39]. Dysregulation of these cytokines has been implicated in muscle health research, with higher IL-6 and CRP levels associated with a greater loss of muscle mass longitudinally over 5 years, and muscle power and strength cross-sectionally [40, 41]. In addition, chronically elevated pro-inflammatory cytokines contribute to oxidative stress which is a hallmark of age-related inflammation [42]. Although the precise mechanisms by which dysregulated cytokines affect muscle health are still unclear, cytokines such IL-6 and TNF--α are involved in regulating signalling pathways for muscle metabolism [43]. A potential mechanism of action may therefore be a pro-inflammatory diet increasing oxidative stress which interrupts healthy muscle metabolism signalling.
In addition, a growing body of research supports a “gut-muscle axis”, one aspect of which is the role of the gut microbiome in regulating inflammation both locally in the gut and systemically [44]. Indeed, high DII diet quality and Traditional dietary pattern scores would also represent dietary intakes that are higher in fibre and healthy fats, known to lead to greater abundances of beneficial bacteria in the human gut [45, 46]. Furthermore, while greater intakes of fibre and unsaturated fats have been associated with higher concentrations of anti-inflammatory metabolites such as butyrate [47], greater consumption of saturated fat has been implicated in increased gut permeability, which is theorised to contribute to systemic inflammation [47]. Therefore, both an anti-inflammatory diet and a Traditional diet may be beneficial for muscle health through maintaining favourable gut microbiome composition and function.
Strengths and limitations
One important strength of this study is the number of participants with data at two time points, 15 years apart. Also, due to the extensive data collection conducted in GOS, we were able to account for a number of medical and lifestyle variables. One such variable was the robust Baecke Physical Activity Questionnaire measure of PA, which accounts for leisure, work, and sport PA. This allowed for adequate adjustment of participants’ PA, which explained several observed associations. Assessment of diet using validated instruments facilitated rigorous multidimensional measures of diet quality and patterns, and DXA-derived lean mass and TUG provided objective measures of muscle health.
Despite its strengths, the study has several weaknesses. The GOS recruitment was based on a random sampling technique, which was representative of the European-Australian population and may not be generalisable to other populations including men of other ethnicities, nor women. Results from the current study also may reflect those of a healthier cohort of European-Australian participants, due to volunteer bias, which is broadly problematic in human research. Moreover, differential loss to follow-up in association with poor muscle health may have biased our results. Due to the limited 22 nutritional DII components available from the FFQ data, the DII scores in the current study may not have been as robust had we had access to all 45 components in the complete DII formula. Indeed, the range of DII values was narrower than those reported previously [25]. Furthermore, longitudinal diet data was based on baseline and 15-year time points, from which the GEE models calculated a mean value for analyses. More frequent diet data points may have provided a more accurate measure of diet changes over the 15-years; however, these values appear to be relatively stable of this time period.
Conclusions
A healthy “Traditional” dietary pattern predicted greater muscle mass, and an anti-inflammatory diet predicted greater muscle mass and better muscle function over 15 years in Australian men. These findings reinforce the importance of optimising dietary behaviours for healthy ageing. Future longitudinal research including women and assessing additional muscle strength outcomes may generate insights directly applicable to clinical recommendations for preventing and/or treating sarcopenia. Although methodologically challenging, long-term dietary interventions would also yield insights into the potential and relative importance of dietary improvement for optimising muscle health in older adults.
Supplementary Information
(DOCX 23 kb)
Acknowledgements
The authors would like to thank the Geelong Osteoporosis Study participants and staff and acknowledge the support of the Deakin University IMPACT Institute. We thank Professor Graham Giles of the Cancer Epidemiology Centre of The Cancer Council Victoria, for permission to use the Dietary Questionnaire for Epidemiological Studies (Version 2), Melbourne: The Cancer Council Victoria 1996.
Funding
This research was funded by the National Health and Medical Research Council (NHMRC) Australia (projects 299831, 628582).
Availability of data and material
Data are available from the Geelong Osteoporosis Study data steering committee upon reasonable request. Please direct enquiries to the corresponding author.
Code availability
Not applicable
Declarations
Ethical statement
This study has been approved by Barwon Health and Deakin University research ethics committees and has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study
Conflicts of interest
Jessica A Davis is supported by an Australian Government Research Training Program Scholarship. Amy Loughman is supported by Deakin Dean’s Postdoctoral Research Fellowship and Heidi Staudacher is supported by an Alfred Deakin Postdoctoral Research Fellowship. Dr. James R. Hébert owns controlling interest in Connecting Health Innovations LLC (CHI), a company that has licensed the right to his invention of the dietary inflammatory index (DII®) from the University of South Carolina in order to develop computer and smart phone applications for patient counseling and dietary intervention in clinical settings. Dr. Nitin Shivappa is an employee of CHI. The subject matter of this paper will not have any direct bearing on that work, nor has that activity exerted any influence on this project. Felice Jacka has received (1) competitive Grant/Research support from the Brain and Behaviour Research Institute, the National Health and Medical Research Council (NHMRC), Australian Rotary Health, the Geelong Medical Research Foundation, the Ian Potter Foundation, The University of Melbourne; (2) industry support for research from Meat and Livestock Australia, Woolworths Limited, the A2 Milk Company, Be Fit Foods; (3) philanthropic support from the Fernwood Foundation, Wilson Foundation, the JTM Foundation, the Serp Hills Foundation, the Roberts Family Foundation, the Waterloo Foundation and; (4) travel support and speakers honoraria from Sanofi-Synthelabo, Janssen Cilag, Servier, Pfizer, Network Nutrition, Angelini Farmaceutica, Eli Lilly and Metagenics. Felice Jacka has written two books for commercial publication. Julie A Pasco has received speaker fees from Amgen, Eli Lilly and Sanofi-Aventis and funding from the NHMRC and MRFF, Barwon Health, Deakin University, Australia and New Zealand Bone and Mineral Society (ANZBMS), Amgen-GSK OA-ANZBMS, Amgen Australia, the BUPA Foundation, Osteoporosis Australia, the Geelong Community Foundation, the Western Alliance, the Norman Beischer Foundation and the Victorian COVID-19 Research Fund. Mohammadreza Mohebbi has received Grant/research support from NHMRC, Deakin University School of Medicine, Deakin Biostatistics Unit, Institute for Mental and Physical Health and Clinical Translation, and Medibank Health Research Fund. Fiona Collier has no disclosures to report.
Disclaimer
The funding organisation played no role in the design or conduct of the study, in the collection, management, analysis, and interpretation of the data, nor in the preparation, review, and approval of the manuscript.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
J. A. Pasco and F. N. Jacka are both joint senior authors.
References
- 1.United Nations, Department of Economic and Social Affairs PD (2019) World population prospects: The 2019 Revision
- 2.Pasco JA, Mohebbi M, Holloway KL, Brennan-Olsen SL, Hyde NK, Kotowicz MA. Musculoskeletal decline and mortality: prospective data from the Geelong Osteoporosis Study. J Cachexia Sarcopenia Muscle. 2017;8:482–489. doi: 10.1002/jcsm.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li R, Xia J, Zhang X, Gathirua-Mwangi WG, Guo J, Li Y, McKenzie S, Song Y. Associations of muscle mass and strength with all-cause mortality among US older adults. Med Sci Sports Exerc. 2018;50:458–467. doi: 10.1249/MSS.0000000000001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 5.Bergland A, Jørgensen L, Emaus N, Strand BH. Mobility as a predictor of all-cause mortality in older men and women: 11.8 year follow-up in the Tromsø study. BMC Health Serv Res. 2017;17:22. doi: 10.1186/s12913-016-1950-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montero-Fernández N, Serra-Rexach JA. Role of exercise on sarcopenia in the elderly. Eur J Phys Rehabil Med. 2013;49:131–143. [PubMed] [Google Scholar]
- 7.Mithal A, Bonjour J-P, Boonen S, Burckhardt P, Degens H, El Hajj Fuleihan G, Josse R, Lips P, Morales Torres J, Rizzoli R, Yoshimura N, Wahl DA, Cooper C, Dawson-Hughes B, Group for the ICNW Impact of nutrition on muscle mass, strength, and performance in older adults. Osteoporos Int. 2013;24:1555–1566. doi: 10.1007/s00198-012-2236-y. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs DR, Steffen LM. Nutrients, foods, and dietary patterns as exposures in research: A framework for food synergy. Am J Clin Nutr. 2003;78:508s–513s. doi: 10.1093/ajcn/78.3.508s. [DOI] [PubMed] [Google Scholar]
- 9.Collins CE, Burrows TL, Rollo ME, Boggess MM, Watson JF, Guest M, Duncanson K, Pezdirc K, Hutchesson MJ. The comparative validity and reproducibility of a diet quality index for adults: the Australian Recommended Food Score. Nutrients. 2015;7:785–798. doi: 10.3390/nu7020785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brouwer-Brolsma EM, Berendsen AAM, Sluik D, van de Wiel AM, Raben A, de Vries JHM, Brand-Miller J, Feskens EJM (2019) The glycaemic index-food-frequency questionnaire: development and validation of a food frequency questionnaire designed to estimate the dietary intake of glycaemic index and glycaemic load: An effort by the PREVIEW consortium. Nutrients 11. 10.3390/nu11010013 [DOI] [PMC free article] [PubMed]
- 11.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2013;17:1689–1696. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perälä MM, Von Bonsdorff MB, Männistö S, Salonen MK, Simonen M, Kanerva N, Rantanen T, Pohjolainen P, Eriksson JG. The healthy Nordic diet predicts muscle strength 10 years later in old women, but not old men. Age Ageing. 2017;46:588–594. doi: 10.1093/ageing/afx034. [DOI] [PubMed] [Google Scholar]
- 13.Perälä MM, Von Bonsdorff M, Männistö S, Salonen MK, Simonen M, Kanerva N, Pohjolainen P, Kajantie E, Rantanen T, Eriksson JG. A healthy Nordic diet and physical performance in old age: findings from the longitudinal Helsinki Birth Cohort Study. Br J Nutr. 2016;115:878–886. doi: 10.1017/S0007114515005309. [DOI] [PubMed] [Google Scholar]
- 14.Chan R, Leung J, Woo J. A prospective cohort study to examine the association between dietary patterns and sarcopenia in Chinese community-dwelling older people in Hong Kong. J Am Med Dir Assoc. 2016;17:336–342. doi: 10.1016/j.jamda.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Talegawkar SA, Bandinelli S, Bandeen-Roche K, Chen P, Milaneschi Y, Tanaka T, Semba RD, Guralnik JM, Ferrucci L. A higher adherence to a mediterranean-style diet is inversely associated with the development of frailty in community-dwelling elderly men and women. J Nutr. 2012;142:2161–2166. doi: 10.3945/jn.112.165498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calder PC, Bosco N, Bourdet-Sicard R, Capuron L, Delzenne N, Doré J, Franceschi C, Lehtinen MJ, Recker T, Salvioli S, Visioli F. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res Rev. 2017;40:95–119. doi: 10.1016/J.ARR.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Kwame Amakye W, Zhang Z, Wei Y, Shivappa N, Hebert JR, Wang J, Su Y, Mao L. The relationship between dietary inflammatory index (DII) and muscle mass and strength in Chinese children aged 6-9 years old. Asia Pac J Clin Nutr. 2018;27:1315–1324. doi: 10.6133/apjcn.052018.10. [DOI] [PubMed] [Google Scholar]
- 18.Cervo MM, Shivappa N, Hebert JR, Oddy WH, Winzenberg T, Balogun S, Wu F, Ebeling P, Aitken D, Jones G, Scott D. Longitudinal associations between dietary inflammatory index and musculoskeletal health in community-dwelling older adults. Clin Nutr. 2020;39:516–523. doi: 10.1016/j.clnu.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 19.del Mar Bibiloni M, Julibert A, Argelich E, Aparicio-Ugarriza R, Palacios G, Pons A, Gonzalez-Gross M, Tur JA (2017) Western and mediterranean dietary patterns and physical activity and fitness among Spanish older adults. Nutrients 9. 10.3390/nu9070704 [DOI] [PMC free article] [PubMed]
- 20.Granic A, Jagger C, Davies K, Adamson A, Kirkwood T, Hill TR, Siervo M, Mathers JC, Sayer AA. Effect of dietary patterns on muscle strength and physical performance in the very old: findings from the Newcastle 85+ Study. PLoS One. 2016;11:e0149699. doi: 10.1371/journal.pone.0149699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasco JA, Nicholson GC, Kotowicz MA. Cohort profile: Geelong Osteoporosis Study. Int J Epidemiol. 2012;41:1565–1575. doi: 10.1093/ije/dyr148. [DOI] [PubMed] [Google Scholar]
- 22.Willett W (2013) Nutritional Epidemiology. Oxford Scholarship Online
- 23.Richardson S. The timed “up & go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 24.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M, Bautmans I, Baeyens J-P, Cesari M, Cherubini A, Kanis J, Maggio M, Martin F, Michel J-P, Pitkala K, Reginster J-Y, Rizzoli R, Sánchez-Rodríguez D, Schols J. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips CM, Chen L-W, Heude B, Bernard JY, Harvey NC, Duijts L, Mensink-Bout SM, Polanska K, Mancano G, Suderman M, Shivappa N, Hébert JR. Dietary Inflammatory Index and non-communicable disease risk: a narrative review. Nutrients. 2019;11:1873. doi: 10.3390/nu11081873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsunaga M. How to factor-analyze your data right: do’s, don’ts, and how-to’s. Int J Psychol Res. 2010;3:97–110. doi: 10.21500/20112084.854. [DOI] [Google Scholar]
- 27.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 28.Bloom I, Shand C, Cooper C, Robinson S, Baird J (2018) Diet quality and sarcopenia in older adults: a systematic review. Nutrients 10. 10.3390/nu10030308 [DOI] [PMC free article] [PubMed]
- 29.Aljadani HMA, Sibbritt D, Patterson A, Collins C. The Australian Recommended Food Score did not predict weight gain in middle-aged Australian women during six years of follow-up. Aust N Z J Public Health. 2013;37:322–328. doi: 10.1111/1753-6405.12079. [DOI] [PubMed] [Google Scholar]
- 30.Nocella C, Cammisotto V, Fianchini L, D’Amico A, Novo M, Castellani V, Stefanini L, Violi F, Carnevale R. Extra virgin olive oil and cardiovascular diseases: benefits for human health. Endocr Metab Immune Disord Drug Targets. 2017;18:4–13. doi: 10.2174/1871530317666171114121533. [DOI] [PubMed] [Google Scholar]
- 31.Robinson SM, Westbury LD, Cooper R, Kuh D, Ward K, Syddall HE, Sayer AA, Cooper C. Adult lifetime diet quality and physical performance in older age: findings from a British birth cohort. J Gerontol A Biol Sci Med Sci. 2018;73:1532–1537. doi: 10.1093/gerona/glx179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pilleron S, Pérès K, Jutand M-A, Helmer C, Dartigues J-F, Samieri C, Féart C. Dietary patterns and risk of self-reported activity limitation in older adults from the Three-City Bordeaux Study. Br J Nutr. 2018;120:549–556. doi: 10.1017/S0007114518001654. [DOI] [PubMed] [Google Scholar]
- 33.Doherty TJ, Vandervoort AA, Taylor AW, Brown WF. Effects of motor unit losses on strength in older men and women. J Appl Physiol. 1993;74:868–874. doi: 10.1152/jappl.1993.74.2.868. [DOI] [PubMed] [Google Scholar]
- 34.Heaney RP. Vitamin D — Baseline status and effective dose. N Engl J Med. 2012;367:77–78. doi: 10.1056/nejme1206858. [DOI] [PubMed] [Google Scholar]
- 35.Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJ, Mittendorfer B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr. 2011;93:402–412. doi: 10.3945/ajcn.110.005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crane TE, Kubota C, West JL, Kroggel MA, Wertheim BC, Thomson CA. Increasing the vegetable intake dose is associated with a rise in plasma carotenoids without modifying oxidative stress or inflammation in overweight or obese postmenopausal women. J Nutr. 2011;141:1827–1833. doi: 10.3945/jn.111.139659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Estruch R (2020) Dietary strategies to prevent and mitigate inflammatory diseases Anti-inflammatory effects of the Mediterranean diet: the experience of the PREDIMED study. In: 3rd International Immunonutrition Workshop Session 4
- 38.Valtueña S, Pellegrini N, Franzini L, Bianchi MA, Ardigò D, Del Rio D, Piatti P, Scazzina F, Zavaroni I, Brighenti F. Food selection based on total antioxidant capacity can modify antioxidant intake, systemic inflammation, and liver function without altering markers of oxidative stress 1-3. Am J Clin Nutr. 2008;87:1290–1297. doi: 10.1093/ajcn/87.5.1290. [DOI] [PubMed] [Google Scholar]
- 39.Cavicchia PP, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Hébert JR. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J Nutr. 2009;139:2365–2372. doi: 10.3945/jn.109.114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alemán H, Esparza J, Ramirez FA, Astiazaran H, Payette H. Longitudinal evidence on the association between interleukin-6 and C-reactive protein with the loss of total appendicular skeletal muscle in free-living older men and women. Age Ageing. 2011;40:469–475. doi: 10.1093/ageing/afr040. [DOI] [PubMed] [Google Scholar]
- 41.Cesari M, Penninx BWJH, Pahor M, Lauretani F, Corsi AM, Rhys Williams G, Guralnik JM, Ferrucci L (2004) Inflammatory markers and physical performance in older persons: the InCHIANTI Study. J Gerontol 59 [DOI] [PubMed]
- 42.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Leung KS, Chow SKH, Cheung WH. Inflammation and age-associated skeletal muscle deterioration (sarcopaenia) J Orthop Transl. 2017;10:94–101. doi: 10.1016/j.jot.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Longman RS, Littman DR. The functional impact of the intestinal microbiome on mucosal immunity and systemic autoimmunity. Curr Opin Rheumatol. 2015;27:381–387. doi: 10.1097/BOR.0000000000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.So D, Whelan K, Rossi M, Morrison M, Holtmann G, Kelly JT, Shanahan ER, Staudacher HM, Campbell KL. Dietary fiber intervention on gut microbiota composition in healthy adults: a systematic review and meta-analysis. Am J Clin Nutr. 2018;107:965–983. doi: 10.1093/ajcn/nqy041. [DOI] [PubMed] [Google Scholar]
- 46.Watson H, Mitra S, Croden FC, Taylor M, Wood HM, Perry SL, Spencer JA, Quirke P, Toogood GJ, Lawton CL, Dye L, Loadman PM, Hull MA. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut. 2018;67:1974–1983. doi: 10.1136/gutjnl-2017-314968. [DOI] [PubMed] [Google Scholar]
- 47.Bach Knudsen K, Lærke H, Hedemann M, Nielsen T, Ingerslev A, Gundelund Nielsen D, Theil P, Purup S, Hald S, Schioldan A, Marco M, Gregersen S, Hermansen K. Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients. 2018;10:1499. doi: 10.3390/nu10101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 23 kb)
Data Availability Statement
Data are available from the Geelong Osteoporosis Study data steering committee upon reasonable request. Please direct enquiries to the corresponding author.
Not applicable