Abstract
Following the advancements and diversification in synthetic strategies for porous covalent materials in the literature, the materials science community started to investigate the performance of covalent organic polymers (COPs) and covalent organic frameworks (COFs) in applications that require large surface areas for interaction with other molecules, chemical stability, and insolubility. Sensorics is an area where COPs and COFs have demonstrated immense potential and achieved high levels of sensitivity and selectivity on account of their tunable structures. In this review, we focus on those covalent polymeric systems that use fluorescence spectroscopy as a method of detection. After briefly reviewing the physical basis of fluorescence-based sensors, we delve into various kinds of analytes that have been explored with COPs and COFs, namely, heavy metal ions, explosives, biological molecules, amines, pH, volatile organic compounds and solvents, iodine, enantiomers, gases, and anions. Throughout this work, we discuss the mechanisms involved in each sensing application and aim to quantify the potency of the discussed sensors by providing limits of detection and quenching constants when available. This review concludes with a summary of the surveyed literature and raises a few concerns that should be addressed in the future development of COP and COF fluorescence-based sensors.
Keywords: fluorescence, sensors, covalent organic polymers, covalent organic frameworks, quenching, ions, explosives, amines, biological molecules, enantiomers
The idea of electron pair sharing between neighboring atoms, which is central to the definition of a covalent bond, was first introduced by Gilbert N. Lewis in 1916 when he presented the Lewis dot structures of covalent compounds.1,2 Eleven years later, the covalent bond was also defined quantum mechanically in terms of atomic orbital overlap by Walter Heitler and Fritz London.3 In the same decade, with the development of organic synthesis, the first covalently linked polymers were produced4,5 and the field of polymer science started to flourish. Unlike most electrostatic bonds, including coordination, hydrogen, and hydrophobic bonds, covalent bonds exhibit high bond strengths and can thus be applied in the synthesis of chemically more stable and robust materials. It is therefore not surprising that nylon, polyethylene, polystyrene, polyamide, and other products of covalent polymerizations found countless uses in everyday life.6
More recently, insoluble and porous covalent polymers have been praised for their extensive surface areas, which can interact favorably with other molecules through noncovalent interactions, a feature particularly useful in the fields of gas separation and storage, pollutant removal, drug delivery, catalysis, energy conversion, and sensing. These materials have been known under various names, but in the current review we primarily resort to the term covalent organic polymers (COPs).7 Typically, their backbones consist of lightweight elements such as C, H, N, O, F, and S. For specific applications that require metal ions, these can be introduced through coordination bonding. In 2005, these covalent polymeric materials were supplemented by covalent organic frameworks (COFs).8 The latter are characterized by the presence of dynamic covalent bonds, structural long-range order, and crystallinity.9 COFs diffract X-rays, which allows for the precise structure determination by the modeling of the experimental diffraction patterns. Such periodicity enables experimental and in silico molecular-level investigation of various phenomena, eliminates batch-to-batch variations in cross-linking density, and facilitates precise fine-tuning of the structure by postsynthetic modification.
Crystalline COFs and COPs can be designed in such a way that the extended π-conjugation in their backbone results in useful light-emitting properties. In comparison with traditional small-molecule chemosensors, these materials are insoluble in water and common organic solvents, which facilitate their separation, regeneration, repeated use, and incorporation into devices. Periodic structures allow introduction of specific target sites to increase the specificity. Furthermore, they possess large surface areas that can interact with targets, and their electronic and photophysical properties are tunable. While small-molecule chemosensors may also be noncovalently embedded into various matrices to enhance their stability, these systems may face the issue of leaching that COPs and COFs avoid on account of their covalent bonding. All of these properties make COPs and COFs promising sensors for a range of analytes, particularly those that interact with highly specialized environments such as enantiomers.10 These optical sensors can be based on different transduction mechanisms, including absorption, resonance, and fluorescence.11 Because fluorescence detection is significantly more sensitive than absorbance and can thus detect lower analyte concentrations,12 there is a push in the literature to design these kinds of materials for the detection of various analytes.
In this review, we first discuss the physical basis of fluorescence using the Jablonski diagram to illustrate the processes of excitation and emission. We also detail the various fluorescence mechanisms that are found in the existing fluorescence-based COP and COF sensors, including internal charge transfer (ICT), resonance energy transfer (RET), photoinduced electron transfer (PET), and aggregation-induced emission (AIE). In the next major section of the review, we describe the progress in the synthesis and performance of the relevant fluorescent sensors, primarily discussing the reports covering the past ten years. The synthetic approaches in these reports range from several kinds of C–C and C–N coupling reactions, boronic acid dehydration, azine, hydrazone, imine, imide and amide chemistries to triazine formation. We focus on various analytes, for instance explosives, metal cations, biological molecules, gases, amines, iodine, solvents, volatile organic compounds (VOCs), enantiomeric compounds, and anions. The current review concludes by summarizing the findings obtained herein and providing some future perspectives on the development of fluorescent COPs and COFs for sensing applications.
Physical Basis of Fluorescence and Its Detection
The phenomena of fluorescence and other types of radiative decay are commonly explained with the aid of the Jablonski diagram (Figure 1).13 As a molecule absorbs energy, it is promoted from the ground state (S0) to the excited state (S1). It is then subjected to various collisions with surrounding molecules, which force it to lose some energy and step down the ladder to the lowest vibrational state of the electronically excited state (S1).14 From there, the molecule returns to the electronic ground state S0 while emitting energy in the form of light as a fluorescent signal. Fluorescence usually occurs at longer wavelengths (higher frequencies) than the incident radiation because some energy is lost to the surroundings in the form of nonradiative decay. This observation is known as the Stokes shift. If a molecule contains a moderately heavy atom (which is sometimes the case in COPs and COFs), such an atom may induce strong spin–orbit coupling and force the molecule from the excited S1 state into the triplet T1 state in a process known as intersystem crossing (ISC). This state is significantly more stable and the molecule may remain trapped in it for several seconds or even minutes. However, it is still able to emit light weakly and gradually return to the ground state. This phenomenon of light emission is known as phosphorescence,14 but due to its long lifetime it is not generally used in sensors.
Figure 1.
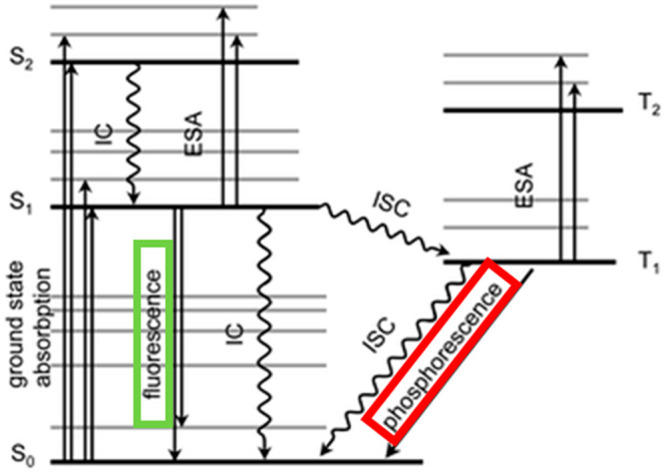
Jablonski diagram illustrating the phenomena of fluorescence and phosphorescence: IC, internal conversion; ESA, excited state absorption; ISC, intersystem crossing. Reproduced from ref (13) with permission from the PCCP Owner Societies. Copyright 2003.
Fluorescence Mechanisms
Multiple types of fluorescence mechanisms have been identified in donor and acceptor fluorophores in COPs and COFs sensors (Figure 2).15 In PET, the electron donor and acceptor form a charge transfer complex, which relaxes from the excited to the ground state with or without emission of a photon. In the former case, a fluorescence signal is observed and the extra electron on the acceptor is returned to the donor in the last step of the process (Figure 2a). This type of mechanism is commonly encountered in COP and COF sensors for heavy metal ions, where the excited electrons of the materials are transferred to the partly filled d-orbitals of the metal ions.16 In RET an initially excited donor molecule relaxes to the ground state while simultaneously transferring energy to the acceptor molecule, which is in turn promoted to the excited state (Figure 2b). This process requires that there be overlap between the emission spectrum of the donor fluorophore and the absorption spectrum of the acceptor and is commonly seen in DNA sensors.17 The term Förster resonance energy transfer (FRET) is often encountered in the literature,18 and it refers to the same kind of a physical process which occurs specifically at the Förster radius, a distance between the fluorophores where the energy transfer efficiency is 50%. In ICT, a single fluorophore must contain both an electron donating and an electron accepting group, such as a phenyl and a triazine ring, respectively. Photoinduced excitation can increase charge separation in the fluorophore, and subsequent events depend on the polarity of the solvent. A polar solvent stabilizes the species with the charges separated. In contrast, in a nonpolar solvent, the species without charge separation may have the lowest energy (Figure 2c).19 This type of the mechanism could be used in a fluorescent sensor, which contains electron donating and accepting groups, but its ICT is restricted by the presence of the analyte, which interacts with one of the two groups.20
Figure 2.
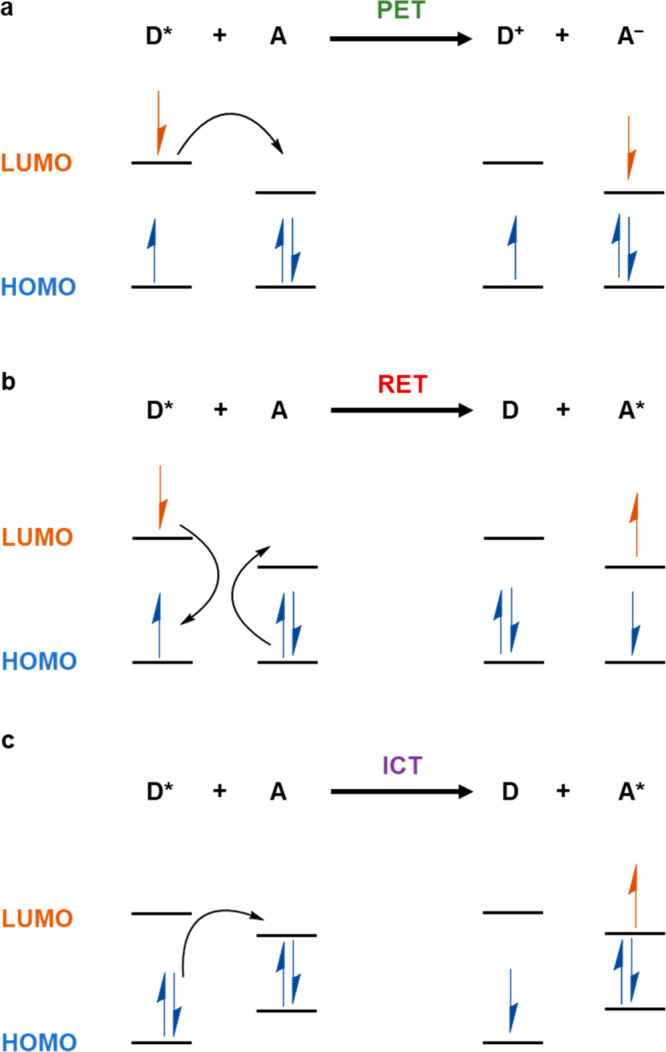
Schematic representation of the three main fluorescence mechanisms: photoinduced electron transfer (PET; a), resonance energy transfer (RET; b), and intramolecular charge transfer (ICT; c).21 D = donor; A = acceptor.
Fluorescence Spectroscopy of Covalent Polymeric Sensors: Quenching, Enhancement and Shifts
When an analyte is added to a fluorescence-based sensor, any of the above mechanisms may be triggered, which typically manifests in one of the three changes in the fluorescence spectrum of the fluorophore: (i) the intensity decreases which is referred to as turn-off fluorescence or quenching, (ii) the intensity increases, which is known as turn-on fluorescence or enhancement, or (iii) the maximum intensity shifts to a different wavelength.
The fluorescence quenching is a widely studied theory in the literature,22 which can occur as a result of diffusive encounter (i.e., dynamic quenching) or due to the formation of a complex (i.e., static quenching). These two processes can be distinguished on the basis of their lifetimes (τ). In static quenching, the fluorescence lifetime remains unchanged if the concentration of the quencher (e.g., an analyte) is increased because any remaining fluorophore moieties, which do not complex with the quencher, retain their original fluorescence lifetime. Thus, τ0/τ = 1. In dynamic quenching, by contrast, increasing concentration of the quencher reduces the fluorescence lifetime. This can be explained by the fact that the greater presence of the analyte increases the chance of a collisional encounter with the fluorophore. Therefore, τ0/τ = F0/F. Both types of quenching can be described by Stern–Volmer equations, which allow for the determination of the quenching constants. For further details on these constants, the reader is advised to refer to related reviews.23−25 These types of quenching as well as other mechanisms of fluorescence are further illustrated by specific examples in the next major section of the review.
The materials discussed herein have been divided into groups by the type of the analyte they can detect. Within each group, materials are compared to each other by several metrics, including the limits of detection (LODs; the lowest quantity of an analyte that can be distinguished from the absence of that analyte, or a blank) and Stern–Volmer quenching constants, where applicable. The materials are also evaluated and compared among each other in terms of their selectivity and recyclability.
Sensors for Heavy Metals
Numerous heavy metals are naturally present in the Earth’s crust, and many of them (e.g., Fe, Cu, Co, Mg, Mo, Cr, Se, Mn, Ni, and Zn) are required for the proper functioning of the human body. However, excess ingestion of these metals can have negative effects on human health. In addition, certain other metals, such as Hg, Cd, Pb, As, and Ag, are toxic to humans in even minute concentrations.26 Therefore, it is instrumental to design materials, which can detect the presence of these potentially dangerous metals in drinking water, food, and the environment. In this subsection, we comprehensively review fluorescence COPs and COFs for the detection of Hg2+, Fe3+, Cu2+, Cr3+, and a few mixed metal cation sensors. A typical mechanism involved in these types of sensors is an electron transfer from the excited material to the partially filled d-orbitals of the metal cations through the PET mechanism. Regardless of the metal, the strategy of having predesigned metal chelation sites in the structure almost always results in higher specificity and lower LODs.
Mercury
Mercury is a highly toxic metal, which is known to cause mercury poisoning and can even lead to the deadly Minamata disease. Thus, the maximum allowed concentration in drinking water is 2 ppb, 2 μg L–1, or ≈10 nM. Hg2+ ions are known to have an affinity for S-containing groups.27 While Hg2+ sensors without these moieties have also been reported, it is primarily those with free thiol, thioether, and carboxyhydrazide groups that reach lower detection limits and greater selectivity (Table 1). For example, the P1 COP sensor for Hg2+ based on the turn-on fluorescence was prepared by incorporating a small-molecule fluorophore 5,5-difluoro-1,3,7,9-tetramethyl-10-phenyl-5H-dipyrrolodiazaborinine (BODIPY) into a covalent polymer.28 The BODIPY dye is nonfluorescent in the original state on account of a rapid C=N isomerization that is perturbed when Hg2+ forms a coordination bond with two neighboring dye moieties. The initially non-emissive material starts to emit light in the presence of the metal cations; however, the detection limit for Hg2+ is rather high at 0.37 μM.
Table 1. COPs and COFs Used As Heavy Metal Ion Sensors.
| material | metal ion | sensing mechanism | KSV (M–1) | LOD | ref |
|---|---|---|---|---|---|
| COF-LZU8 | Hg2+ | quenching | n/a | 25 ppb | (29) |
| TNPP | Hg2+ | quenching | 3.78 × 105 | 22.8 ppb | (30) |
| NOP-28 | Hg2+ | PET (quenching) | 3.7 × 104 | 12 ppb | (16) |
| TFPPy-CHYD | Hg2+ | PET (static quenching) | n/a | 17 nM | (31) |
| P1 | Hg2+ | turn-on fluorescence | n/a | 0.37 μM | (28) |
| COP-401-COOH | Fe3+ | PET (quenching) | 8.4 × 104 | n/a | (26) |
| COP-64 | Fe3+ | PET (quenching) | 1.10 × 105 | n/a | (27) |
| COP-9 | Fe3+ | ACQ | 1.7 × 104 | mM range | (34) |
| COP-1 | Fe3+ | PET (static quenching) | n/a | 0.42 μM | (33) |
| TPA-COP | Fe3+ | chelation-induced turn-on fluorescence | n/a | 0.43 μM | (37) |
| COP-100 | Fe3+ | collisional quenching | 2.97 × 104 | 0.245 μM | (42) |
| Bth-Dma COF | Fe3+ | quenching | 2.3 × 104 | 0.17 μM | (38) |
| COF-JLU3 | Cu2+ | PET (quenching) | 3.8 × 104 | 0.31 μM | (40) |
| COPs-DT | Cu2+ | PET (quenching) | n/a | 0.076 μM | (41) |
| Salen-COP | Cu2+ | CHEQ | n/a | 0.545 μM | (43) |
| PI nanonsheets | Cr3+ | Quenching | n/a | n/a | (44) |
| TFPT-BTAN-AO | UO22+ | PET (quenching) | n/a | 6.7 nM | (32) |
| CorMeO–COF | Cr3+ | turn-on fluorescence | n/a | 0.85 μM | (45) |
| Salen-COP | Al3+ | turn-on fluorescence, PET | n/a | 0.248 μM | (43) |
| Salen-COP | Fe3+ | CHEQ | n/a | 0.140 μM | (43) |
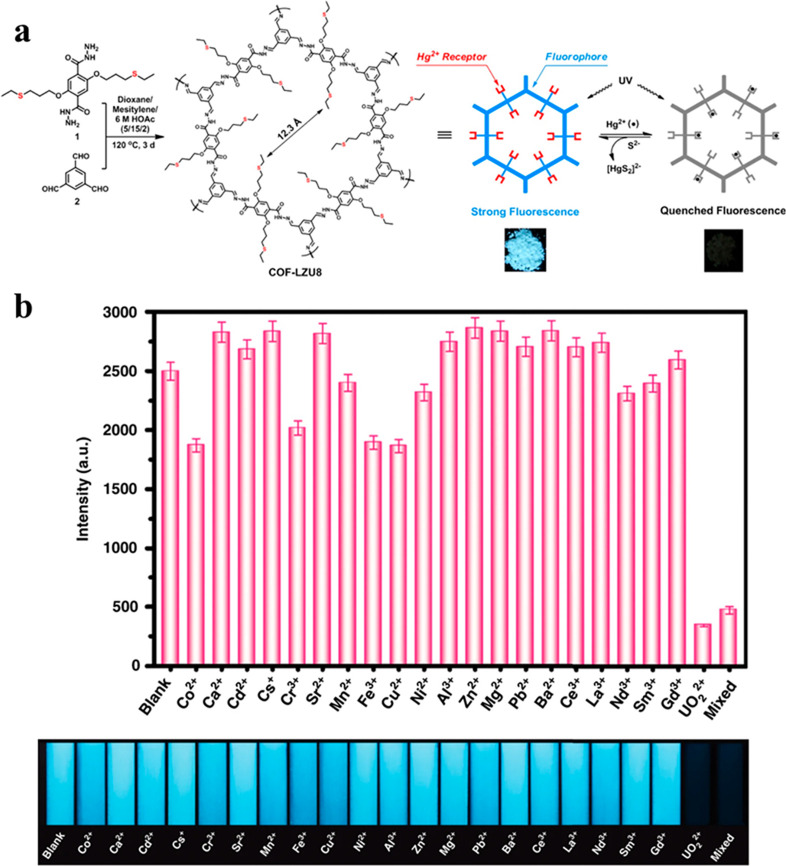
The very first manuscript on fluorescent COFs for metal ion detection was published by Wang et al. in 2016, and it triggered the development of diverse COF-based detection systems. In this work, the authors constructed COF-LZU8 with thioether functionalities on the starting hydrazide, which produced a polymeric structure with thioethers pointing both toward and away from the pore interior (Figure 3a).29 The COF exhibited a strong fluorescence signal upon excitation at 390 nm, which was quenched by the addition of Hg2+ ions in acetonitrile. The COF possessed good selectivity as it preferentially removed Hg2+ even in the presence of 2 equiv of various competitive metal ions. The LOD was 25 ppb. A similar LOD (22.8 ppb) was also observed for a triarylamine-based COP synthesized through Suzuki coupling and postsynthetically modified with thiosemicarbazone to introduce Hg2+ chelation sites.30
Figure 3.
Sensing of heavy metal ions. (a) Design strategy for COF-LZU8 and the fluorescence quenching mechanism triggered by the addition of Hg2+ ions. Reproduced with permission from ref (29). Copyright 2016 American Chemical Society. (b) Selectivity of TFPT-BTAN-AO for UO22+. Reproduced with permission from ref (32). Copyright 2020 The Authors under Creative Commons Attribution 4.0 International license (https://creativecommons.org/licenses/by/4.0/).
Reversibility of Hg2+ binding, a feature integral to reuse of the sensor, was not considered in COF-LZU8 (Figure 3a),29 but a later manuscript found it to be nearly irreversible.31 It was therefore urgent to design materials that will serve not only as chemodosimeters but rather as reversible Hg2+ sensors. Sulfide-bridged polytriazine nanospheres NOP-28 were fully reversible and retained specificity among 11 different metal cations.16 The metal ion detection was based on fluorescence quenching at 415 nm ascribed to the electron transfer from the COP to the formed guest metal complexes, or metal ion-induced polymer chain aggregation. Their LOD in ethanol was still rather high at 12 ppb.
The issue of reversibility was also addressed in the TFPPy-CHYD COF where the thiol chelation sites were replaced by carbohydrazide linkages.31 This material reached a low LOD of 17 nM or 3.4 ppb, which is almost sufficient for sensing Hg2+ in drinking water. The pyrene–carboxyhydrazine conjugate was also praised for its fast response time (2 s), selectivity for Hg2+ in the presence of other cations and anions at five times higher concentrations in DMF, and facile regeneration ability with Na2S. This work thus represents a monumental step in the direction of sensitive, reversible Hg2+ COF sensors.
A few aspects of COPs and COFs mercury sensors remain unaddressed. First, organic solvents are used to dissolve Hg2+ while sensing in water would be the most relevant in environmental and health applications. This is likely due to the interference of highly polar water molecules in the fluorescence properties of the synthesized materials. Second, all of the discussed examples focus on inorganic Hg2+ detection. Although this species is toxic, it is the organic mercury such as methyl mercury, or [CH3Hg]+, that is even more hazardous. These species are formed when Hg2+ comes into contact with bacteria in water or soil, and its toxicity is particularly problematic due to its bioaccumulation. Therefore, future sensor design should not neglect [CH3Hg]+, although its handling and storage in a laboratory setting may be challenging.
Iron
Iron is an essential element in various metabolic processes, including oxygen uptake into red blood cells and its transfer, and DNA synthesis. However, if present in higher amounts in biological organisms, it can lead to disease, so iron detection is of importance. The maximum allowed concentration of Fe3+ in drinking water is 5.4 μM, or ≈302 ppb. Similar to Hg2+ sensors, Fe3+ sensors also commonly incorporate metal cation chelation sites that lower the LODs.33 For example, Yamamoto cross-coupling produced a series of COPs (COP6–9) with LODs in the mM range. These materials were interesting for the absorption competition quenching (ACQ) mechanism for Fe3+ sensing.34 In DMF, both the COP and the Fe3+ ions absorb light in the 250–400 nm window, so upon excitation the two species compete for the light energy. For that reason, the presence of Fe3+ reduces the excitation of the COP and quenches the fluorescence signal. Interestingly, other divalent metal cations exhibit smaller absorption spectral overlap with the COP, so they are less able to quench the fluorescent signal, ultimately leading to selectivity for Fe3+.
A range of chelation sites can be incorporated into polymeric materials to make more efficient Fe3+ sensors. For instance, -COOH and -SO2Cl functionalities introduced into conjugated COPs synthesized through Yamamoto cross-coupling (e.g., COP-401-COOH and COP-64) have been reported for Fe3+ sensing.35,36 These modifications resulted in dynamic Stern–Volmer constants of 8.4 × 104 and 4.3 × 104 M–1, respectively, which is an order of magnitude higher than the parent, nonfunctionalized COP. In another study, Sonogashira–Hagihara37 cross-coupled TPA-COP contained the tridentate Schiff base sites able to selectively coordinate Fe3+ ions. Similar to the Hg2+ turn-on sensor discussed above,28 Fe3+ induces rigidity and suppresses PET from the imine N to the excited moiety, turning on the fluorescence. The authors refer to this phenomenon as chelation-induced turn-on fluorescence.
A particularly innovative report constitutes the Bth-Dma COF, the first example of a luminescent COF with predesigned O–N–O′ chelation site for the detection of metal ions in water. The O–N–O′ chelation sites in this hydrazone-linked COF participate in fluorescence quenching upon addition of Fe3+ ions.38 XPS studies on the Fe 2p and N 1s orbitals further confirmed the chelation process. The LOD in this COF is low at 0.17 μM, and the Stern–Volmer quenching constant is KSV = 2.3 × 104 M–1, which is comparable to other COPs, COFs, and MOFs. Importantly, these values were obtained in water rather than in an organic solvent. Therefore, the results of this report may be more applicable to real-life sensing applications in environmental, food, and health sciences.
Copper
Copper is an essential element for the human body. Enzymes utilize it in energy generation, neurotransmitter and pigment synthesis, and epigenetic modification. Its hyper-accumulation in biological fluids and tissues can, however, lead to diseases such as Wilson and Meknes.39 An azine-linked COF-JLU3 with a fluorescence half-life of 1.5 ns was utilized for selective detection of Cu2+ in THF.40 The mechanism involved PET from the COF to the d-orbitals of the Cu2+ ions that coordinated to the framework through Lewis acid–base interactions with the OH side functional groups and azine linkages. The LOD of this system was 0.31 μM, and the Stern–Volmer quenching constant was calculated to be 3.8 × 104 M–1. In another study, four times lower LOD (0.076 μM) was achieved with imine-linked COPs-DT sensors with the same kind of sensing mechanism.41 Given that the surface areas of the two materials are comparable (456 and 540 m2 g–1), better performance of COPs-DT might be attributed to the solvent (isopropanol), or the strength of chelation. Notably, this material exhibited a good level of selectivity among 16 metal cations tested and could be regenerated by adding a strong competitive metal chelating agent (EDTA) without compromising the sensitivity. COPs and COFs for sensing Cu2+ in water are yet to be explored in greater detail and so are those for sensing copper species with oxidation states other than 2+.
Uranium
With increasing dependence on nuclear power plants for the generation of electricity, the issue of radioactive waste is more significant than ever before. Materials are being developed to detect uranium and its oxides, such as the TFPT-BTAN-AO COF generated through the Knoevenagel condensation and postsynthetically modified by amidoximation (Figure 3b).32 This work is truly remarkable for several reasons: (1) it utilizes Knoevenagel condensation, a lesser-known chemistry in the COF synthesis, (2) postsynthetic amidoximation introduces a large number of -OH and -NH2 functional groups into the network, which serve as exclusive binding sites for UO22+ among 21 metal cations tested, and (3) upon excitation at 270 nm in water, the LOD is 6.7 nM, which is well below the WHO contamination limit for UO22+ in drinking water (63 nM). XPS results show that UO22+ binds to the N atoms of the NH2 groups and the O atoms (O 1s and N 1s peaks shift 0.20 and 0.22 eV to higher energies, respectively, and new U–N and U–O peaks appear at 401.1 and 531.3 eV, respectively). As such, this work presents a COF sensor of the highest merit: it is operational in water, exhibits high selectivity and LOD below the allowed concentration in water, and demonstrates an innovative synthetic approach. The challenge now lies in scaling up the synthesis of the material and optimizing its production cost.
Groups of Metal Cations with Common Physical Properties
Some sensors for heavy metal cations were not specific to a particular metal such as the ones presented above but rather exhibited selectivity based on common properties of metal ions, such as valency and/or oxidation number,43,45 atomic mass,46 and/or metals with filled d-orbitals.47 The utility of these systems depends on their purpose; they might be very helpful when the presence of any kind of heavy metal is of interest but less so when our interest is in a particular metal ion. In most cases, the materials contain main-chain or side functional groups that form different interactions with metal cations depending on the type of metal. For instance, an azo-linked conjugated COP exhibited fluorescence quenching when exposed to heavy metals but showed no response to light metals such as Na+ and Ca2+. This difference was rationalized by the hard acid nature of Na+, K+, and Ca2+, which leads to a low affinity for aromatic systems and azo groups.46
One of the most interesting examples of COFs showing distinct fluorescence responses to different heavy metal ions is the corrole-based CorMeO–COF.45 This material can be used as a rather general heavy metal sensor, as it exhibits turn-on fluorescence in the presence of trivalent metal ions (Al3+, Cr3+, Ga3+, and Fe3+) in THF. The 2D COF had strong interlayer π–π stacking interactions, which were loosened in the presence of these trivalent metal ions, enhancing the fluorescence signal. By observing that an analogous porphyrin-based COF with a similar skeleton does not exhibit enhanced fluorescence in the presence of these metals, the authors conclude that the fluorescence phenomenon is a result of aggregation-induced quenching, which is common in bulk 2D-COF materials. The LODs were found to be in the low-μM concentration, which is within the range of maximum allowed limits. It is important to note that some trivalent heavy metals (Tb3+, La3+, Eu3+, and Pr3+) among those tested induce only a little fluorescence enhancement.
In general, studies aiming at detecting groups of metal cations should be supplemented by an investigation on the response to mixtures of heavy metals. It would be expected that the sensitivity for each individual metal would decrease in the presence of other metal ions, but the extent of the reduction would need to be carefully evaluated.
Sensors for Explosives
For safety reasons, effective sensors for explosives are currently high in demand. It is therefore not surprising that a plethora of COPs and COFs sensors were designed for compounds such as picric acid or 2,4,6-trinitrophenol (TNP), 2,4,6-trinitrotoluene (TNT), 2,6-dinitrophenol (DNP), 2,6-dinitrotoluene (DNT), 2-nitrophenol (NP), and 2-nitrotoluene (NT; Table 2).48 Diverse chemical reactions have been utilized for the synthesis of nitroaromatics sensors, including nitrile trimerization into triazines,49 Sonogashira–Hagihara coupling,50,51 Yamamoto coupling,52,53 Suzuki coupling,54 Buchwald–Hartwig coupling,55 and imine56 and azo bond formations.57
Table 2. COPs Used as Nitro Explosive Sensors.
| material | explosive | sensing mechanism | KSV (M–1) | LOD | ref |
|---|---|---|---|---|---|
| MAEC-PMA | TNP | static quenching | 2.95 × 104 | 93.3 nM | (59) |
| CMP-LS1 | TNP | PET, RET | 5.05 × 104 | n/a | (54) |
| CMP-LS2 | TNP | PET, RET | 3.70 × 104 | n/a | (54) |
| COP-612 | TNP | static quenching | 2.5 × 105 | n/a | (52) |
| COP-401 | TNP | PET (dynamic quenching) | 8.3 × 104 | <1 ppm | (53) |
| COP-301 | TNP | PET (dynamic quenching) | 2.6 × 105 | <1 ppm | (53) |
| SNW-1 | TNP | quenching | 9.4 × 104 | 50 nM | (58) |
| A-NS | TNP | static quenching | 8 × 105 | 90 nM | (56) |
| PrTAPB-Azo-COP | TNP | static quenching | 1.1 × 104 | n/a | (57) |
| COP-3 | TNP | PET (static quenching) | 1.45 × 104 | <1 ppm | (60) |
| DTF | TNP | quenching | 2.08 × 103 | 0.722 μM | (51) |
| LMOP-15 | TNP | PET (quenching) | 2.6 × 104 | 0.33 μM | (61) |
| LPCMP2 | TNT | PET | 4.15 × 103 | 3.64 μM | (55) |
| PCPDI | o-NP | ACQ, IFE | 1.74 × 105 | 17.2 pM | (49) |
| DP2A2 | o-NP | PET (static quenching) | 2.00 × 104 | 1.5 nM | (50) |
A range of COPs have been used for detecting nitro explosives, and this section only discusses the highlights of the literature that exists in the area. While some materials serve as sensors for nitro explosives in general,58 others are tailored to specific explosive compounds.49,53 Nitrophenols contain a polar hydroxyl group able to form hydrogen bonds that is lacking in nitrotoluenes, often leading to selectivity. For instance, the PCPDI triazine framework formed through trimerization of aromatic nitriles of N,N′-di(4-cyanophenyl)-3,4,9,10-tetracarboxylic diimide was selective for o-NP to a very low level of 17.2 pM.49 Other explosives, including DNT, p-NP, m-NP, and NT exhibited negligent quenching effect. This selectivity was explained by the formation of hydrogen bonds between o-NP, and O- and N-rich PCPDI and the absorption spectra overlap between the two. The hydrogen bonding is also responsible for the ACQ mechanism dominating the fluorescence response. Similarly, in a mixture of TNP, NT, DNT, m-DNB, p-DNB, and NP, Yamamoto-coupled conjugated COP-612 showed a strong preference for TNP.52
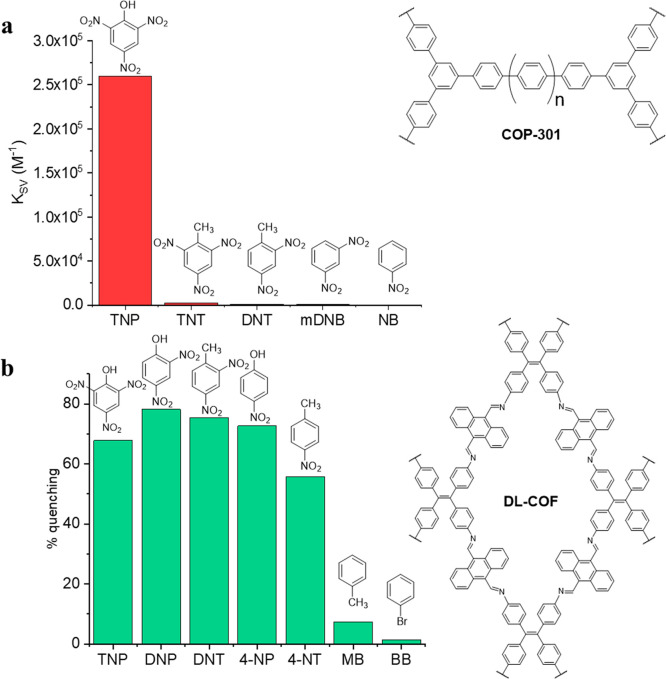
A detailed study on the mechanism of explosives detection in COP-301, another Yamamoto-coupled COP, has found two major contributions to the selectivity for TNP: (i) the relative positions of HOMO and LUMO and (ii) the electron withdrawing effects of -NO2 functional groups (Figure 4a).53 The COP and the nitro explosives are aromatic, so they interact through π–π stacking interactions. Upon excitation, electrons in the conduction band of the COP are transferred to the LUMO of TNP, which results in quenching. Because of electron withdrawing effect of the nitro groups, TNP has the lowest LUMO, so its quenching ability is the highest.
Figure 4.
Selectivity of sensors for nitro explosives: (a) COP-301 sensor for a specific nitro explosive, TNP;53 (b) DL-COF, a general sensor for nitro explosives.65
In the realm of COFs, one should highlight the very first example of a sensor constructed from this class of materials. This was an azine-linked chemosensor for TNP reported in 2013, which was simultaneously the first COF linked by azine bonds (Table 3).62 The fluorescence signal of Py-azine COF at 522 nm (λex = 470 nm) was quenched by the addition of up to 70 ppm TNP due to the static quenching phenomenon. For the same reasons of hydrogen bond formation by nitrophenols but not nitrotoluenes, Py-azine COF along with imide-linked TfpBDH COF,63 and PI-COF64 showed selectivity for TNP. One of the most potent COFs used for the detection of general nitro explosives is DL-COF (Figure 4b).65 This material was not selective for a particular explosive as it exhibited high Stern–Volmer constants for TNP, DNP, DNT, 4-NP, and 4-NT (Table 3). Selectivity experiments demonstrated that non-explosives (e.g., toluene or bromobenzene) induced very little fluorescence quenching.
Table 3. COFs Used as Nitro Explosive Sensors.
| material | explosive | sensing mechanism | KSV (M–1) | LOD | ref |
|---|---|---|---|---|---|
| Py-azine COF | TNP | static quenching | 7.8 × 104 | n/a | (62) |
| TfpBDH | TNP | PET (dynamic quenching) | 2.6 × 104 | n/a | (63) |
| TFPC-NDA COF | TNP | static and dynamic quenching | 2.48 × 105 | n/a | (66) |
| PI-COF | TNP | PET, IFE | 1 × 107 | 0.25 μM | (64) |
| 3D-Py-COF | TNP | quenching | 3.1 × 104 | n/a | (67) |
| TAPB-TFPB | TNP | static quenching | 5.9 × 104 | n/a | (68) |
| DL-COF | TNP | static quenching | 2.24 × 106 | 57.31 nM | (65) |
| DL-COF | DNP | dynamic quenching | 4.28 × 106 | 46.50 nM | (65) |
| DL-COF | DNT | dynamic quenching | 3.71 × 106 | 57.32 nM | (65) |
| DL-COF | 4-NP | dynamic quenching | 3.18 × 106 | 37.05 nM | (65) |
| DL-COF | 4-NT | dynamic quenching | 1.56 × 106 | 50.52 nM | (65) |
Given the plethora of COP and COF sensors for explosives, this class of analytes is probably the most suitable to compare the relative merits of the two classes of materials. Almost all of the reports present quenching of the fluorescence signal upon the addition of the analytes, so a comparison is even fitter. The data in Tables 2 and 3 suggest that both COPs and COFs can reach very low LODs, down to nM or pM levels. A major difference that we observe, however, is in the magnitude of the Stern–Volmer quenching constants. We note that the COFs can exhibit KSV values on the order of 106 to 107, whereas most COPs reach a maximum of 105. This suggests that long-range order in COFs contributes to greater degrees of quenching for a given analyte concentration and initial fluorescence signal. It is therefore expected that the concentration of the analyte would be more accurately calculated from an experimental fluorescence measurement in the case of COFs.
Sensors for Biological Molecules
As has been noted with other analytes, the structures of COPs and COFs can be adapted to form favorable interactions with disease markers (methylglyoxal, a marker for diabetes mellitus,69 the anthrax biomarker,70 or sialic acid, an ovarian cancer biomarker71), reactive oxygen species (hydroxyl radicals, •OH),72 and antibiotics.73 In TpPa-1@LE, the imine linkages were deprotonated, which generated anionic N– centers that could interact with methylglyoxal.69 The latter induced a shift in emission from 476 to 525 nm. The mechanism of fluorescence involved exciplexes (heterodimeric short-lived species generated in the excited state) that formed when the excited state TpPa-1@LE collided with methylglyoxal molecules and charge transfer occurred to form a luminescent intermediate. •OH radicals, in contrast, transfer an electron to the COF-TpMA framework, forming an aggregate through π–π stacking and inducing fluorescence quenching.72 The FRET mechanism is involved in sensing tetracycline with Suzuki-coupled CMP-LS7 and CMP-LS8.73 These materials and tetracycline form π–π interactions through aromatic rings and hydrogen bonds.
COF sensors for biological molecules often take advantage of incorporating metal ions that actively participate in the sensing process in various ways. First, the metal centers can act as electron acceptors. A sensor for levofloxacin used a COF as a solid support system for Eu3+ ions, which can bind strongly to β-diketone in levofloxacin through coordination bonds.74 Upon irradiation, the β-diketone substructure of the antibiotic enters the triplet state, from which an electron is transferred to the excited-state energy level of Eu3+. The light is emitted by the relaxation from that excited state. This example illustrates that the ordered nature makes COFs useful solid supports into which metal ions can be introduced in a controlled manner. The COFs thus prevent metal ion aggregation, facilitate separation from solution, and prevent leaching. The overall effect is a reproducible sensing behavior (Table 4). Second, the metal centers can give up their hydration to coordinate the analyte molecules (e.g., dipicolinic acid). This “antenna effect” results in turn-on fluorescence that originates from the metal center.70 Third, metal ions can participate in the indicator displacement assay (Figure 5a).71 Here, the metal center serves as a receptor of the analyte (sialic acid) and the dye-functionalized TpPa-1 COF as a turn-on fluorescence indicator.
Table 4. COP and COF Sensors for Biological Molecules.
| material | analyte | sensing mechanism | KSV (M–1) | LOD | ref |
|---|---|---|---|---|---|
| CMP-LS7 | tetracycline | IFE (quenching) | 1.92 × 104 | 0.94 μM | (73) |
| CMP-LS8 | tetracycline | IFE (quenching) | 8.86 × 104 | 0.22 μM | (73) |
| Eu@TpPa-1 | levofloxacin | PET, turn-on fluorescence | n/a | 0.2 μM | (74) |
| Tb-COP | dipicolinic acid | turn-on fluorescence, antenna effect | n/a | 13.5 nM | (70) |
| TpPa-1 | sialic acid | turn-on fluorescence | n/a | 7.08 nM | (71) |
| PF-DNA CPN | DNA | FRET | n/a | nM range | (17) |
| TpTta | DNA | FRET, turn-on fluorescence | n/a | 3.7 nM | (75) |
| TPA-COF NSs | DNA | turn-on fluorescence | n/a | 20 pM | (76) |
| COF-TpMA | •OH | static quenching | 0.8281 | n/a | (72) |
| TpPa-1@LE | methylglyoxal | exciplex formation | n/a | 109.6 nM | (69) |
Figure 5.
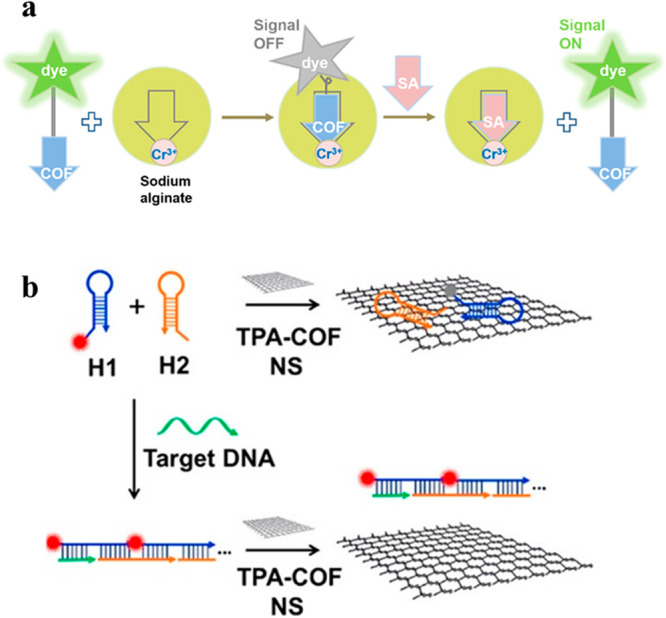
Operating principles of biomarker and DNA sensing platforms. (a) TpPa-1 turn-on sensor for sialic acid biomarker present in ovarian cancer. Reproduced with permission from ref (71). Copyright 2020 American Chemical Society. (b) TpTta COF sensor for DNA. Reproduced with permission from ref (75). Copyright 2016 American Chemical Society.
DNA sensing has also been investigated with fluorescent COPs and COFs.17,75,76 In most cases, the sensors are attached covalently or noncovalently to the DNA strands complementary to the target DNA. Because of these complementary strands, the selectivity of these systems is very high, but it cannot be attributed to the COF. Similar to the Eu3+ ions in the antibiotic sensing,74 the role of the COF is primarily to serve as support or anchor point. For example, carboxylic acid-functionalized polyfluorenes were conjugated with a specific oligonucleotide and the thus-obtained system was used as a FRET sensor for specific complementary chains of DNA.17 In the presence of the target DNA and a FRET signal enhancer, the PicoGreen dye, quenching of the signal was observed at 426 nm and a new emission peak appeared at 530 nm. None of these changes were observed in the presence of noncomplementary DNA chains. In addition to being a solid support, COFs in DNA sensing can also be the quenchers of the DNA probe’s fluorescence (Figure 5b). In this case the probe is composed of a DNA strand complementary to the target and a fluorescent dye such as carboxyfluorescein72 or Texas red.73 Upon the addition of the target DNA, the probe detaches from the COF, interacts with the target DNA, and re-emits fluorescence through the FRET mechanism.75,76
In summary, some sensors for small biological molecules resemble the operating principles of explosives and heavy metals sensors by taking advantage of their own fluorescent nature and formation of noncovalent interactions with the analytes. However, several systems utilize COPs or COFs as support systems or anchor points, so that the actual sensing process happens at another moiety, e.g., transition metal ion, or DNA probe. This is an interesting approach scarcely seen in the detection of other analytes, likely because of the need for extreme levels of specificity in biological systems. These examples demonstrate that covalent polymeric materials can serve various purposes in sensing systems. In addition to being direct responders to analytes, they also have other favorable properties such as porosity, structural robustness, and ability to form noncovalent interactions and can therefore serve as support, anchoring points, or quenchers.
Sensors for pH
pH sensing is important in biomedical, clinical, environmental, and industrial process control.77 The most accurate method of measuring pH of a solution is undoubtedly using a well-calibrated pH electrode, but alternative methods of less accurate but faster and cheaper detection are of interest to the scientific community. In this section, we discuss COP- and COF-based pH sensors. Most of them contain a specific moiety, such as an imidazole, a hydroxyquinoline, or a ketoenamine unit, which can be reversibly protonated in acidic pH. Typically, the protonation prevents the electron transfer and induces a change in the fluorescence properties of the materials. Two strategies of pH sensor design prevail the literature: (i) known small-molecule pH-responsive molecules are integrated into polymeric scaffolds, or (ii) specific protonatable chemical functionalities within the framework are utilized.
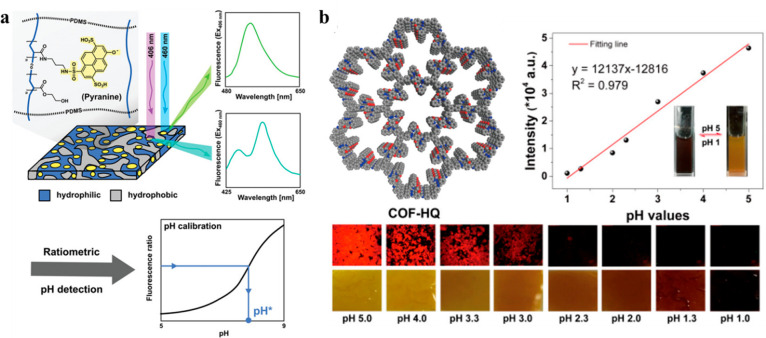
COPs that serve as pH sensors commonly incorporate a fluorescent monomer that can be reversibly protonated, thereby changing its fluorescence signal. Two such moieties were coumarin-based dyes78 and pyranine, a known photostable ratiometric pH sensing small molecule.79 Coumarin COPs exhibited a decrease in the fluorescence intensity with an increasing pH in the range from 10 to 13.6. This can be rationalized with the structures of the coumarin dyes, which contain an imidazole ring. In neutral and near neutral environments, this ring is protonated and the material exhibits strong fluorescence. As the pH starts to increase in the highly basic level, the proton is gradually lost and the fluorescence signal diminishes. Once deprotonated, the imidazole anion is able to quench the coumarin fluorescence by transferring an electron to the coumarin through PET. In contrast, pyranine in PyrAPCN has two excitation wavelengths (406 and 460 nm) that respond differently to the changes in pH. With increasing pH levels, the emission of the 406 nm excitation increases, whereas that of the 460 nm excitation decreases. Using the ratio of the emission intensities at the two wavelengths enables more precise determination of pH in the range of 5–9 pH units, which is particularly relevant to biological environments (Figure 6a). Increasing pH gradually deprotonates the phenolic hydroxyl and sulfonic acid groups in the pyranine molecule, which are, similar to the imidazole anion above, now able to transfer an electron to the pyrene core through PET.
Figure 6.
(a) Preparation of ratiometric pH sensor based on PyrAPCN with dual fluorescence. Adapted with permission from ref (79). Copyright 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (b) Structure of COF-HQ, its pH calibration curve, and fluorescence and optical images of the material after exposure to acidic solutions with specific pH levels. Reproduced with permission from ref (82). Copyright 2018 American Chemical Society.
In the realm of COFs, materials typically do not incorporate moieties that are pH sensors on the monomer level but rather rely on reversible protonation of the linkages that join the building blocks together. For instance, the first example of a pH sensor based on COFs used a Schiff base reaction along with β-ketoenamine formation in COF-JLU4.80 This material served as a pH sensor for a broad pH range from 0.9 to 13.0 and exploited its protonatable nitrogen atoms in the β-ketoenamine linkages to induce changes in the fluorescence signal at 428 nm. Above pH 9, the fluorescence intensity gradually decreased due to β-ketoenamine nitrogen deprotonation, whereas below pH 4.5, it blue-shifted and increased with the lowering of the pH as a result of nitrogen protonation. Similarly, COF-TP relied on the protonation of its imine linkages to cause fluorescence quenching with pH decreasing from 6 to 0.81
In contrast, 8-hydroxyquinoline-decorated imine COF-HQ relied on a simple protonation of the pyridine unit of 8-hydroxyquinoline. In the protonated state, 8-hydroxyquinoline blocked the π–π* charge transfer interaction from the electron-rich phenol ring to the electron-deficient pyridine and quenched the fluorescence signal (Figure 6b).82 This sensor could detect pH in the range from 1 to 5 by the means of fluorescence quenching with decreasing pH, as well as colorimetrically. COF-HQ is as reversible as COF-JLU4 since its fluorescent and colorimetric modes of detection can be restored for at least five cycles.
Integrating known pH sensing molecules into covalent networks contributes to easy recovery and regeneration of the sensor compared to free small molecules. However, these moieties may be difficult to functionalize with groups that can participate in polymerization reactions or may contain functionalities that require protecting groups before polymerization can be carried out. Therefore, strategies that rely on the reversible protonation of COP/COF linkages or protonatable functionalities in the pores may be easier and cheaper to implement. The environment in which these materials are to be used as pH sensors must also be carefully evaluated. It is possible that ions other than hydrogen protons, for instance metal ions, would bind to these sites and potentially hamper the efficiency of the pH sensors.
Sensors for Solvents and Volatile Organic Compounds
VOCs are atmospheric contaminants released in industrial processes and from transportation vehicles and present in paints, rubber, plastics, solvents, and lubricants. They have been associated with different kinds of irritations and even cancer, so sensors for their detection are highly desired. The examples from the literature in this subsection can be divided into three categories, (i) general VOC sensors, (ii) sensors for specific VOCs, and (iii) water sensors.
A general sensor able to distinguish nitro group-containing VOCs from other types of VOCs was composed of triazine and spirobifluorene subunits and was synthesized by simultaneous Friedel–Crafts and Scholl coupling with different ratios of the reacting monomers.83 Sbf-TMP@4:2 was probed for the detection of 20 different volatile organic solvents, and the emission peak at ∼590 nm was quenched in the presence of nitro compounds (e.g., nitromethane) or enhanced upon the addition of other VOCs (e.g., 1,4-dioxane, toluene, acetone, and chloroform). This difference could be explained by the relative positions of the conduction band of Sbf-TMP@4:2 and the LUMO of the analytes. The material’s conduction band is higher than the LUMO of the electron-deficient nitro compounds, so electrons flow from the COP to the analyte, which causes quenching. In contrast, the LUMOs of the other analytes are higher than the conduction band of the COP, so the electrons flow in the opposite direction and the fluorescence signal is enhanced (Table 5). Another COF composed of electron-deficient triazine moieties and electron-rich phenyl and fused phenyl rings was able to distinguish between electron-deficient solvents and the electron-rich ones.66 The electron donating VOCs (e.g., toluene, chlorobenzene, and mesitylene) caused red-shifting of the exciplex emission by forming a charge transfer complex, in which electrons were donated by the COF to the VOC.
Table 5. COPs and COFs Used as VOC and Solvent Sensors.
| material | solvent analyte | sensing mechanism | LOD | ref |
|---|---|---|---|---|
| Sbf-TMP@4:2 | nitro compounds | quenching | n/a | (83) |
| TFPC-NDA COF | electron-donating VOCs | exciplex and CT complex formation | n/a | (66) |
| TB-TZ-COP | 1,4-dioxane | ICT (enhancement) | 22.2 ppm | (84) |
| DhaTab-COF-EuIL | acetone | PET (quenching) | 1% | (85) |
| PTMSDPA | water | deswelling-induced quenching | n/a | (86) |
| TzDa | water | ICT, ESIPT | 0.006–0.085% | (87) |
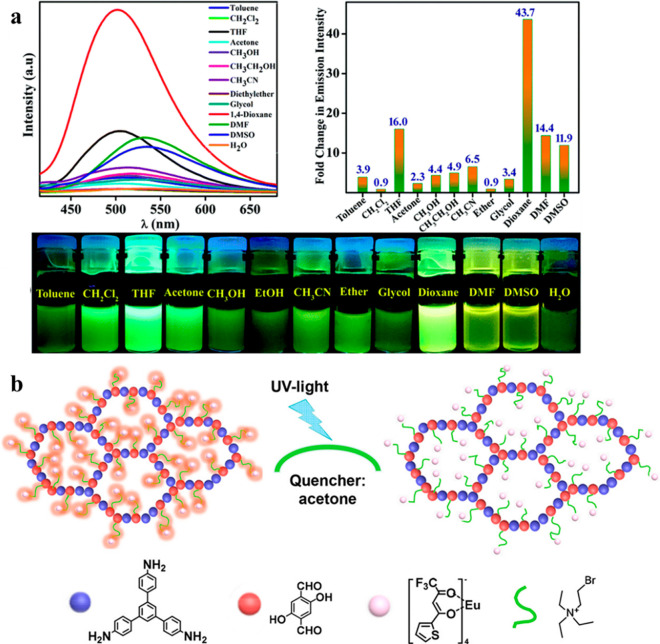
COPs and COFs have been investigated as sensors for specific volatile solvents such as 1,4-dioxane84 and acetone.85 An acetone sensor TB-TZ-COP (Figure 7a) was constructed through imide chemistry based on the amino-1,8-naphthalimide Tröger’s base, a molecule known for its fluorescence due to the ICT mechanism. TB-TZ-COP dispersed in 1,4-dioxane showed a dramatic 44% enhancement of fluorescence upon excitation at 360 nm, while the other solvents showed only modest enhancement (THF, 16%) or caused red-shifting due to polarity of the solvent that stabilized the excited fluorophore. This effect was explained computationally by an electron transfer from the high-energy LUMO of 1,4-dioxane to the lower-energy LUMO of the sensor. The acetone sensor DhaTab-COF-EuIL was likewise selective for the target analyte among 8 tested solvents as its UV spectrum only overlapped with that of acetone. Upon excitation, a competition for adsorption between the two components arises and PET from the material to the solvent and subsequent quenching result (Figure 7b).85
Figure 7.
Sensors for various solvents. (a) Emission spectra (λex = 360 nm) of TB-TZ-COP in different solvents and the corresponding fold change in emission intensity with respect to its emission in H2O along with optical photographs of TB-TZ-COP dispersed in different solvents taken under UV light. Reproduced from ref (84) with permission from The Royal Society of Chemistry. Copyright 2019. (b) Structure and acetone sensing operating principle of DhaTab-COF-EuIL. Reproduced with permission from ref (85). Copyright 2019 American Chemical Society.
Finally, in addition to sensing organic solvents, some applications require an efficient sensor for water, for instance the production of dry solvents needed in organic synthesis. Such sensors have been developed on the basis of a range of principles. For example, poly[1-phenyl-2-(p-trimethylsilyl)phenylacetylene] (PTMSDPA) swells in an organic solvent such as ethanol as a result of van der Waals interactions.86 When even a small amount of water is added to the system, ethanol forms a hydrogen bond with water and dissociates from PTMSDPA. This causes deswelling of the material, ultimately leading to fluorescence quenching. The stronger the ability of the organic solvent to form hydrogen bonding with water, the more pronounced is the deswelling-induced quenching effect. In contrast, TzDa COF contains the triazine and 2,5-dihydroxyterephthalaldehyde subunits, which both form hydrogen bonding with trace water.87 Once this interaction forms, ICT from the phenyl to the triazine core is prevented, resulting in the fluorescence quenching at 500 nm. Furthermore, the hydroxyl group on the terephthalaldehyde no longer forms a hydrogen bond with the imine bond of the COF but rather with a water molecule, thereby preventing the excited-state intramolecular proton transfer (ESIPT) and causing signal enhancement at 590 nm. Similar behavior was obtained for various organic solvents (isopropanol, acetone, THF, ethyl acetate, and ethanol), with LODs between 0.006 and 0.085%.
As previously mentioned for pH sensors, the environment in which the sensor is meant to operate is of immense importance. While the majority of the literature reports delineate the mechanisms of sensor operation and their performance, they rarely look at the specific potential uses of the materials. The use of TB-TZ-COP as a 1,4-dioxane sensor, however, was also studied in the presence of ethylene glycol, which is the starting material for the synthesis of 1,4-dioxane. Therefore, this sensor could be used specifically to study the conversion of ethylene glycol into 1,4-dioxane in an industrial setting. TB-TZ-COP was able to detect 1,4-dioxane in ethylene glycol even at the very low concentration of 22.2 ppm. In future sensor studies, similar considerations of potential interferences should be investigated.
Iodine
Radioactive iodine is a volatile solid produced in nuclear power plants as a byproduct of the fission of uranium and in detonation of atomic weapons. It is a carcinogenic molecule with radioactive isotopes I129 and I131 with half-lives of up to 15.7 million years.88 Detecting leakages of such toxic species is essential to ensure a safe energy supply.89 Several COPs have been developed for remediation of iodine emissions90−92 as well as for its detection through fluorescence. Friedel–Crafts93−95 and related coupling reactions96 have often been used in generating COP sensors for iodine because they can easily generate fully sp2-conjugated systems that are fluorescent in nature and possess a high affinity for iodine (dipole–dipole interactions, ionic interactions with polyiodides). Triazine,97 tetraphenyl ethylene,98 and pyrazine99 moieties have all been utilized in the synthesis of COPs through Friedel–Crafts coupling (Table 6). The materials exhibited strong fluorescence in specific solvents, which was quenched upon the addition of I2 through the PET mechanism: the excitation light promoted the electron in the COP from the HOMO to the LUMO orbital, from where it was transferred to the LUMO of electron-deficient iodine. The limits of detection were generally the lowest in tetraphenyl ethylene-based systems (2.98 pM and 0.296 pM),98 followed by the pyrazine (24.7 pM and 0.136 nM)99 and triazine-based systems (80.5 pM and 1.56 nM).97 The Stern–Volmer quenching constants were likewise the highest for the tetraphenyl ethylene systems (1.53 × 105 M–1 and 9.07 × 104 M–1),98 and on the order of 103 M–1 for the pyrazine- and triazine-containing COPs.97,99
Table 6. COPs Used as Iodine Sensors.
| material | mechanism | KSV (M–1) | LOD | ref |
|---|---|---|---|---|
| TS-TAD | PET | 5.76 × 103 | 80.5 pM | (97) |
| TS-TADP | PET | 5.59 × 103 | 1.56 nM | (97) |
| TTTAT | PET | 1.53 × 105 | 2.98 pM | (98) |
| TTDAT | PET | 9.07 × 104 | 0.296 pM | (98) |
| TDPz | PET | 3.76 × 103 | 24.7 pM | (99) |
| TTDPz | PET | 1.10 × 103 | 0.136 nM | (99) |
| PTThP-2 | static and dynamic quenching | 1.99 × 103 | 75.4 nM | (96) |
| PTThP-3 | static and dynamic quenching | 5.09 × 103 | 29.5 nM | (96) |
| PCPP | quenching | 1.4 × 105 | 0.314 pM | (100) |
| TDPA | PET | 1.85 × 104 | 16.2 pM | (94) |
| TTPBTA | PET | 6.56 × 104 | 6.86 pM | (94) |
| TDPDB | static and dynamic quenching | 5.83 × 104 | 2.57 pM | (93) |
| TTPA | PET | 2.38 × 104 | 32.2 pM | (95) |
| TTDATA | PET | 4.33 × 102 | n/a | (95) |
| TTMDATA | PET | 7.31 × 102 | n/a | (95) |
An alternative to Friedel–Crafts reaction, trimerization leading to the formation of the triazine ring from the nitrile-group-containing starting materials yielded the best material reported so far for iodine sensing in terms of the LOD (0.314 pM) and the KSV constant (1.4 × 105 M–1).100 The mechanism for this adsorption stems from the large energy difference of 2.926 eV between the LUMO of I2 and the LUMO of PCPP. Despite the plethora of COP-based iodine sensors, to the best of our knowledge, no fluorescent COF-based sensor has been developed for iodine.
Enantiomers
Enantiomeric sensing is of high interest for monitoring production processes of various biologically relevant molecules as well as for medical diagnostics and biotechnology.101 The disaster with thalidomide, a drug often taken by pregnant women with morning sickness, that resulted in thousands of babies born with limb malformations between 1957 and 1961, has drawn attention to chirality and its effects in drug molecules.102 We note two strategies for synthesizing covalent network sensors for enantiomers. The first one takes advantage of the different abilities of the d- and l-enantiomers of the analytes to from interactions with the synthesized COPs without using chiral building blocks for the material synthesis.103 If an intermolecular interaction is formed, the fluorescence signal is altered. An alternative strategy to enantiomeric sensing in COFs is the incorporation of chiral building blocks into the fluorescent material, which then naturally responds differently to the d- and l-forms of the analytes.
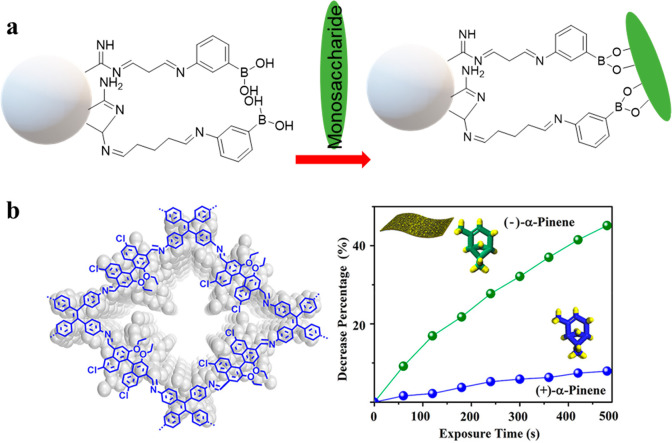
The first of the two strategies was utilized in monosaccharide enantiomeric sensing in polyacrylonitrile nanoparticles.103 To achieve enantioselectivity, these nanoparticles were postsynthetically modified in three steps: first, amidine groups were introduced through the Pinner synthesis; second, Schiff base was synthesized by adding glutaraldehyde to the amidine-terminated nanoparticles; third, the Schiff base was reacted with 4-aminophenylboronic acid to create boronic functionalities at the outer layer (Figure 8a). The modified B-PAN nanoparticles had a dramatically enhanced fluorescence upon excitation at 300 nm, likely as a result of PET between the Schiff base and the phenylboronic acid (Table 7). The material was exposed to different d- and l-monosaccharides and it was observed that the d-enantiomers induced a more pronounced enhancement of the fluorescence signal in the case of glucose and galactose. This enantioselectivity stems from the intramolecular hydrogen bonding, which develops between the imine and amine groups of B-PAN. Further, the protonated tertiary nitrogen and the Lewis acidic ring oxygen form a hydrogen bond, leading to enantiomeric specificity in a material that contains no chiral centers.
Figure 8.
Sensors for chiral molecules. (a) Mechanism of B-PAN for sensing d- and l-monosaccharides.103 (b) Structure of CCOF7 and a graph showing its selective (−)-α-pinene detection over (+)-α-pinene. Reproduced with permission from ref (104). Copyright 2019 American Chemical Society.
Table 7. COPs and COFs Employed in Enantiomer Sensing.
| material | analyte | sensing mechanism | KSV (M–1) | enantioselectivity | ref |
|---|---|---|---|---|---|
| B-PAN | monosaccharides | PET | n/a | n/a | (103) |
| CCOF7 | (−)-(α)-pinene | static quenching | 1348 | 3.49 | (104) |
| CCOF17 | d-phenylglycinol | static enhancement | n/a | 14.72 | (105) |
| CCOF17 | d-phenylalaninol | static enhancement | n/a | 12.85 | (105) |
| CCOF17 | d-tryptophanol | static quenching | 820.5 | 2.41 | (105) |
The second strategy was implemented in two recent reports of COFs. One of them contained 1,1′-bi-2-naphthol (BINOL), which is a frequently used source of chirality in organic synthesis and materials science as well as a fluorophore (Figure 8b).104 The imine-linked CCOF7 was exfoliated into sheets with a thickness of ∼4 nm. These nanosheets with chiral pores and free ethoxy groups were then investigated for enantiomeric sensing of d- and l-forms of various organic vapors (α-pinene, limonene, fenchone, carvone, and terpinen-4-ol). The addition of any of the chiral vapors induced static quenching, generally with the (−)-enantiomers exhibiting more pronounced and faster quenching. For example, (−)-(α)-pinene quenched the fluorescence faster than the corresponding (+)-enantiomer with KSV values of 1348 and 395 M–1, respectively. The reported quenching ratios [QR = Ksv(−)/Ksv(+)] were in the range of 1.2–3.49, indicating enantioselectivity for all five tested organic vapors.
In the second report by the same group, two crown ether-based COFs, CCOF17 and CCOF18, synthesized through Knoevenagel condensation were turned chiral following the reduction of the double bonds in the -C=C–CN linkages.105 The cavity of the macrocycles in the core could accommodate the amino groups of the three tested chiral amino alcohols. Phenylglycinol and phenylalaninol enhanced the fluorescence emission by forming a crown ether–amino alcohol adduct, which weakened the π–π interactions between the COF layers. In contrast, tryptophanol formed not only the adduct with crown ether groups but also π–π interactions and hydrogen bonds with the COFs through its indole rings and indole NH groups, respectively. This led to the quenching of the fluorescence signal.
While enantiomeric sensing is likely one of the most challenging kinds of chemical sensing, the tunable nature of COFs has proven particularly beneficial. The preparation of these sensors is a daunting task that either requires multiple postsynthetic modifications or a challenging preparation of the chiral monomers. Nonetheless, the alternatives are few and are based on biological macromolecules that pose additional challenges in handling, storing, and utilization.106 For these reasons, COFs are possibly some of the most promising types of enantiomeric sensors currently available. However, their direct utilization for compounds of interest to pharmaceutical and other industries is currently lacking.
Sensors for Ammonia and Amines
Amines are used in numerous industries, including chemical, dye, pharmaceutical, military, and food industry.107 While low molecular weight amines, such as ethylamine, are harmful for the aquatic environment but only mildly toxic to humans, higher order amines have been linked to respiratory, neural, and cardiovascular damage in humans or to cancer. Colorimetric COP sensors for ammonia and amines have been studied in the past,108−110 with some sensor materials changing color in response to the chemical reduction induced by ammonia,109 or as a result of charge transfer interaction between the electron-deficient COP and the electron-rich amines.110 However, more recently fluorescence-based sensors have been prioritized on account of their sensitivity. Fluorescent COPs and COFs can detect amines in three different ways: (i) by reacting with free NH2 groups of the primary amines,111 (ii) by forming hydrogen bonding with hydrogen and/or nitrogen atoms of the amine groups,112 or (iii) by accepting an electron pair directly from the electron-rich amine groups (doping).113,114
The Suzuki-coupled P7-COP sensor contained aliphatic side chains and aldehyde and trifluoroacetyl side functional groups.111 These reticularly chosen aldehyde groups chemically reacted with primary amines through Schiff base formation while the trifluoroacetyl groups formed zwitterions or hemiaminals with organic amines. As a result, P7 responded differently to different types of amines: primary alkyl amines blue-shifted the emission from 525 to 435 nm, secondary alkyl amines blue-shifted the emission to 475 nm, and aromatic amines quenched the fluorescence at 525 nm. The same behavior was also observed in mixtures of amine vapors. The lowest LOD was determined for o-toluidine in the ppt range (Table 8).
Table 8. COPs and COFs Used as Sensors for Various Amines and Ammonia.
| material | amine analyte | sensing mechanism | LOD | KSV (M–1) | ref. |
|---|---|---|---|---|---|
| F-CTF-3 | phenylamine | static quenching | 11.7 nM | 8.01 × 105 | (112) |
| F-CTF-3 | p-phenylenediamine | static quenching | 1.47 nM | 6.36 × 106 | (112) |
| F-CTF-3 | 1-naphthylamine | static quenching | 26.2 nM | 3.57 × 105 | (112) |
| P7-COP | n-propyl amine | PET | 114 ppm | n/a | (111) |
| P7-COP | n-hexylamine | PET | 4.0 ppm | n/a | (111) |
| P7-COP | diethylamine | PET | 190 ppm | n/a | (111) |
| P7-COP | dipropylamine | PET | 0.036 ppb | n/a | (111) |
| P7-COP | aniline | PET | 0.52 ppb | n/a | (111) |
| P7-COP | o-toluidine | PET | 0.55 ppt | n/a | (111) |
| COP-1 | NH3 | ICT | 5.9 × 10–4 | n/a | (116) |
| P1 | p-phenylenediamine | PET (quenching) | 171 nM | n/a | (115) |
| P2 | aniline | PET (quenching) | 81 nM | n/a | (115) |
| P2 | triethylamine | turn-on fluorescence | 43 nM | n/a | (115) |
| TPE-Ph COF | NH3 | AIE | n/a | 4.44 × 105 | (117) |
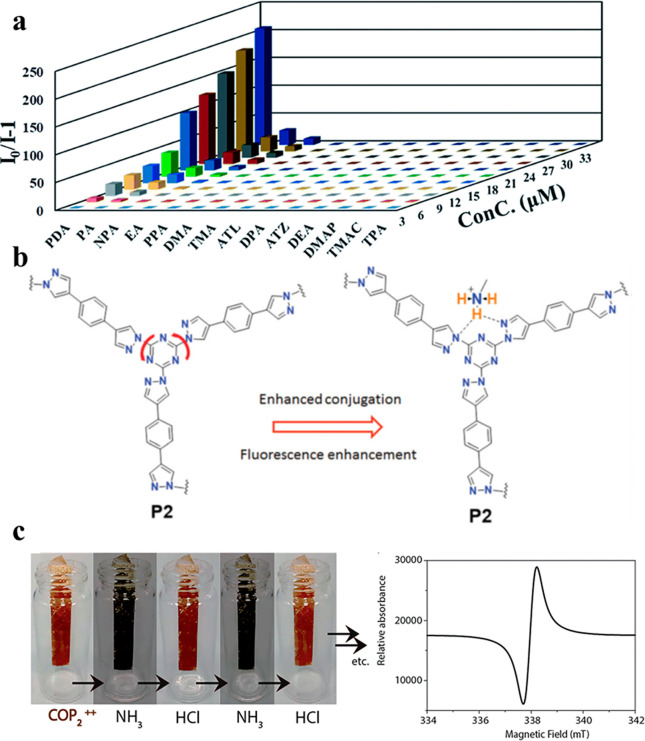
Amines contain nitrogen and hydrogen atoms that can form hydrogen bonds with a range of functionalities in covalent polymeric materials, either primary linkages, nonreacting functional groups, or postsynthetically introduced moieties. The development of such a network of hydrogen bonds affects the fluorescence properties and is useful in sensing applications. For example, in thiadiazole-based covalent triazine nanosheets F-CTF-3 the main mechanism at play is static quenching that stems from the ground-state nonfluorescent complex formed through hydrogen bonding between the polymer’s thiazoles and the analyte’s NH2 groups. This hydrogen bonding interaction is particularly pronounced with p-phenylenediamine, and control experiments show that the higher number of amino groups induce more effective fluorescence quenching (Figure 9a).112 F-CTF-3 was not only remarkably selective, but it also exhibited record-low LOD for phenylamine and p-phenylenediamine in water. A similar mechanism was also observed in the conjugated polymer P1 synthesized from triazine and pyrazole–benzothiadiazole–pyrazole subunits.115 Insensitivity to aliphatic amines was explained by the protonation state of the amines. Aliphatic amines are typically protonated at neutral pH, while aromatic amines are not. Therefore, only the latter can transfer the photoinduced electron to the excited state of the polymer and induce fluorescence quenching.
Figure 9.
(a) Stern–Volmer plots of 14 different amines tested as analytes for F-CTF-3. PDA = p-phenylenediamine; PA = phenylamine; NPA = 1-naphthylamine; EA = ethylamine; PPA = n-propylamine; DMA = dimethylamine; TMA = trimethylamine; ATL = amitrole; DPA = diphenylamine; ATZ = 5-amino-1H-tetrazol; DEA = diethylamine; DMAP = 4-dimethylaminopyridine; TMAC = tetramethylammonium chloride; TPA = triphenylamine. Reproduced from ref (112) with permission from The Royal Society of Chemistry. Copyright 2020. (b) Sensing mechanism between protonated aliphatic amines and pyrazole rings of P2 leads to conjugation enhancement and turns on the fluorescence signal. Reproduced from ref (115) with permission from The Royal Society of Chemistry. Copyright 2020. (c) Reversibility of NH3 sensing with COP2++. Sorption of NH3 induces the formation of radical cationic viologen species in the COP, as demonstrated by an EPR spectrum. Reproduced from ref (109) with permission from The Royal Society of Chemistry. Copyright 2016.
The formation of such a hydrogen bonding network can also have a conjugative effect, leading to turn-on fluorescence. Primary aliphatic amines, which are typically protonated at neutral pH, can extend the conjugative effect on pyrazole rings in P2 (Figure 9b).115 Aromatic primary amines are not able to do so at neutral pH because they have lower pKa values than aliphatic amines. Instead, they prefer to engage in PET by transferring an electron from the lone pair on the amino group to the polymer.
Finally, NH3 and amines contain a free electron pair on the nitrogen atom that can be attracted to an electron-deficient moiety in a COP or COF such as a triazine ring. The electron pair can be directly transferred to the electron-deficient unit through doping. Further, n−π interactions can develop between the lone pair of electrons in NH3 and the π-electron cloud in the electron-deficient triazine (Figure 9c).113,114 An aldol condensation reaction was used to synthesize an olefin-linked microporous fluorescent COP-1 with a triazine core, which was synthesized in bulk as well as on a quartz surface in the form of thin films.116 COP-1 was then used as a sensor for HCl and ammonia gases. Upon exposure to HCl, the fluorescence signal was quenched and red-shifted. The addition of NH3 reversed the change and caused an enhancement of the fluorescence signal upon excitation at 453 nm. In the realm of COFs, one of the highly emissive boronate-linked materials served as an ammonia sensor, the first COF to operate by the principle of aggregation-induced emission (AIE).117 In such materials, the intralayer covalent and interlayer noncovalent interactions restrict rotation and prevent energy dissipation. The boronate linkages of the COF acted as Lewis acids and NH3 served as a Lewis base in such a way that the addition of NH3 quenched the fluorescence signal upon excitation in toluene.
The detection of amines is mainly needed for environmental applications (e.g., sensing of pollutants in air) and in the biomedical field (sensing inside the cells).118 Depending on which avenue the sensor is being developed for, it must meet a different set of requirements. For any kind of biological sensing, the materials would need to undergo a thorough investigation on biocompatibility, particle size distribution, and membrane permeability, etc. For environmental measurements, fixing the materials into membranes or other kinds of supports would need to be attempted. In the current state-of-the-art, basic research has been mostly focused on investigating the mechanisms of amine sensing and has not yet matured into fully fledged sensing platforms for specific uses. The promising early studies with good selectivity and reversibility in most sensors, and increasingly fast response times, however, indicate a huge potential of these amine sensors.
Sensors for Gases
Porous covalent networks have been primarily studied in the contexts of gas adsorption, storage, or conversion, but a select number of examples also discussed gas sensing. The property of gas acidity can be exploited to cause protonation of various chemical functionalities in the materials and lead to a change in the fluorescence signal. Hydrogen chloride gas (HCl), for instance, is known to interact favorably with nitrogen centers of organic molecules by reversibly protonating them. This means that sensors reported for this gas operate on a similar principle as the pH sensors discussed above. An imine-linked COF TATF-COM that contained triazine subunits in both of its building blocks has been utilized as a chromogenic and fluorescence-based sensor for HCl.119 HCl induced quenching of the fluorescence signal observed at 550 nm with the LOD of 2.38 ppb (Table 9). FTIR measurements indicated that the gas interacted with both triazine and imine nitrogen atoms. Similarly, the imine linkages of a carbazole-containing COF BCTB-BCTA became protonated by HCl.120 This enhanced the ICT from one carbazole subunit to the imine and resulted in a red shift in fluorescence from 472 to 495 nm. Further addition of HCl also protonated the carbazole nitrogen atoms, which impeded the ICT and produced the quenching of the fluorescence signal at 495 nm, reaching the LOD of 10 nM.
Table 9. COPs and COF Used as Sensors for Various Gases.
Carbon dioxide (CO2) is also mildly acidic, so its sensing can operate on a similar principle. A fluorescence-based Polym-H7 sensor has been developed to detect CO2 down to 1.3% (v/v) levels.121 This commercially available polymer is composed of fluorescein o-acrylate (a pH indicator), methyl methacrylate (MMA) and hydroxymethyl methacrylate (HEMA) and can be incorporated into films by spin-coating. If present, CO2 reacts with tetraoctylammonium hydroxide (TONOH) to form anionic bicarbonate and cationic TON+. The fluorescein o-acrylate pH indicator in the polymer exhibits two fluorescence spectra, one for its protonated native state and the other for its negatively charged state complexed with TON+. On the basis of the ratio of the two, the concentration of CO2 can be determined.
From an industrial point of view, the global gas sensors market is estimated at 2.19 billion USD in 2019 with most sensors used for the detection of oxygen, carbon dioxide, and nitric oxide.122 However, most of CO2 sensors, for instance, rely on parameters other than fluorescence, including capacitance and resistivity, and are based on inorganic materials.118 Given that these materials reach good levels of detection and can be synthesized at lower costs than COPs and COFs, it becomes fathomable why few of the latter have been investigated for the sensing of common gases. They may be better suited for the sensing of more intricate chemicals in the gaseous phase, such as chiral α-pinene vapors.
Sensors for Anions
Among various anions, oxoanions such as CrO42–, Cr2O72–, and MnO4– are commonly used oxidants in laboratories and in industry. However, these anions contain heavy metals which can be toxic, so their presence needs to be closely monitored.109 For this purpose, the development of sensors for oxoanions is of great importance, although the current survey of the literature suggests that oxoanion removal is more commonly studied than oxoanion sensing.
One strategy of designing heavy metal oxoanion sensors is to synthesize COFs with UV absorption spectra that overlap with those of oxoanions. Such materials are also likely to be selective because nonheavy metal containing oxoanions will absorb light at higher energies. A recently reported 3D TT-COF with a bis(tetraoxacalix[2]arene[2]triazine) core was found to be an efficient sensor for CrO42–, Cr2O72–, and MnO4– while not being sensitive to 16 other anions upon excitation at 490 nm.123 The LODs for all three anions were ∼0.3 mM, and the Stern–Volmer quenching constants were ∼1.4 × 104 M–1 (Table 10). The UV–vis absorbance spectra of TT-COF overlapped in several regions with the spectra of the three anions, so the mechanism of fluorescence quenching was found to be based on absorption competition. When the analytes absorb the excitation energy, they suppress excitation energy transfer to an organic ligand of COF-TT, thereby decreasing the emission intensity or quenching it.
Table 10. COPs and COF Used as Anion Sensors.
Another strategy of designing anion sensors is to incorporate specific linkers, such as guanidine that can donate a proton to an anion serving as a Bronsted–Lowry base, into the backbone of the COFs. Pal et al. developed a cationic guanidine-based fluorescent sensor, DATGCl, which responded to strongly basic anions, primarily F–, but also OH–, SO42–, and CO32–.124 The guanidine linker and, specifically, its protonation state play a major role in the process of detection. Fluoride anion is small, polarizable, and basic enough to abstract a proton from guanidine, which becomes neutral and is now able to transfer an electron to the fluorophore aldehyde in the backbone of DATGCl. DFT calculations on the system showed that the abstraction of a guanidine proton increases the HOMO–LUMO energy so that an electron can be easily transferred to the fluorophore aldehyde via the PET mechanism. Impressively, the LOD for fluoride anions of this system is 5 ppb, which is the lowest ever reported for F– in any class of materials.
Conclusion and Future Perspectives
In summary, this review discussed the physical basis of fluorescence in covalent polymeric materials and explained how fluorescence spectroscopy can be used to design covalent polymeric sensor materials. It detailed the sensing properties of COPs and COFs for various analytes, including heavy metal ions, explosives, biological molecules, pH, solvents and VOCs, iodine, enantiomers, ammonia and amines, gases, and anions. After a thorough analysis, we conclude that the most common mechanism of fluorescence sensing involves PET, in which a photoexcited electron is transferred from the HOMO or LUMO of the donor to the LUMO of the acceptor, either of which can be the fluorophore. In most reports, the fluorescence intensity is quenched by the addition of the analyte, although several cases of turn-on fluorescence have also been reported and discussed herein. Low LODs for specific analytes in some discussed materials demonstrate the potential of these types of materials for sensing applications; for instance, the most sensitive sensor for iodine among all classes of materials is the PCPP COP.100 Moreover, certain COPs and COFs have extraordinarily short response times of as little as 2 s,31,32 in addition to being insoluble in water and common organic solvents, which makes separation, purification, and recycling far more efficient than for soluble materials.10
In spite of the major advantages of the two classes of materials as sensors, there are also several areas where improvements are needed.
The majority of reports utilize sensors in organic solvents rather than in water. This might be due to better analyte solubility in organic solvents, better dispersibility in nonaqueous media, or better efficiency of the electron transfer. It is, however, beyond doubt that water is the most common liquid medium in which detection of pollutants, toxic compounds, and other analytes is of interest in practical applications. Therefore, more emphasis should be placed on designing sensors with efficient operation in aqueous media.
A sensor is only efficient if it can be used multiple times. This ultimately requires developing a procedure for detaching the target analyte, which does not harm the structure and function of the materials, so that their selectivity and sensitivity to the target are preserved and the sensor can be reused. Currently, many reported materials have not been tested for regenerability, or partly or fully lost their detection potency after regeneration. Yet it is essential to explore this aspect of any material considered to be a sensor in real-life applications.
It is far easier to detect the appearance of a bright signal against a dark background than to detect the disappearance of a bright signal. Therefore, emphasis should be placed on developing the turn-on fluorescence sensors rather than relying primarily on the turn-off materials. This can be achieved in 2D materials whose individual layers are stacked, so no fluorescence is observed, but the addition of the analyte relieves the aggregation-caused quenching.45 Alternatively, rapid isomerization may prevent fluorescence of the materials, but the addition of the analyte prevents such isomerization, ultimately turning-on the fluorescence response.28
The majority of the referenced examples detect analytes in suspensions. Few efforts have been made to investigate how they can be used as sensors in the solid state although this approach can afford very promising results. For instance, when an imide-linked COF was tested for nitroaromatics detection in suspension, its fluorescence was quenched by the analytes. However, when the same material was used in a solid-state sensor, the addition of TNP enhanced the fluorescence signal.63
Finally, COPs and COFs are typically composed of anisotropic particles of various dimensions, which can interfere with reproducibility of the observations. Therefore, protocols should be developed to minimize such variations among particles by mechanically grinding them, incorporating them into membranes, or coating them on solid supports.
This analysis of the current state-of-the-art in sensing applications demonstrates that the recent developments and the impressive performance of COPs and COFs sensors justify their further investigation and optimization. It also shows that, in the majority of cases, research has focused on understanding the interplay between the materials’ and the analytes’ structures and their performance in sensorics. Many mechanistic studies and theoretical modeling have also aimed at gaining a thorough understanding of the mechanisms of these fluorescence sensors. What has commonly been overlooked, and where the greatest opportunities await in the future, is tailoring these sensors to very specific applications and investigating all of the external factors that may influence the sensor effectiveness. This involves performing various competition and interference studies, optimizing the physical parameters such as temperature, pressure, and humidity, etc., and building full sensor setups.
Glossary
Vocabulary
- Covalent organic polymer
a mostly insoluble, often porous polymer with building blocks linked to each other through covalent bonds
- Covalent organic framework
a crystalline porous extended structure characterized by light elements and dynamic covalent bonding between the building blocks
- Fluorescence spectroscopy
an analytical technique that detects the light emission from a sample promoted from the ground electronic to the excited electronic state by the incident excitation light
- Analyte
a substance being identified (in the context of sensorics, often the moiety that induces changes in the fluorescence spectrum of the sensor)
- Fluorescence quenching
a common type of fluorescence mechanism where the fluorescence intensity of a substance or material decreases as a result of transferring energy to another molecule
Glossary
Abbreviations
- ACQ
aggregation-caused quenching
- AIE
aggregation-induced emission
- ATL
amitrole
- ATZ
5-amino-1H-tetrazol
- BINOL
1,1′-bi-2-naphthol
- BODIPY
5,5-difluoro-1,3,7,9-tetramethyl-10-phenyl-5H-dipyrrolodiazaborinine
- CHEQ
chelation enhanced quenching
- COF
covalent organic framework
- COP
covalent organic polymer
- DEA
diethylamine
- DMA
dimethylamine
- DMAP
4-(dimethylamino)pyridine
- DMF
N,N-dimethylformamide
- DNA
deoxyribonucleic acid
- DNP
2,6-dinitrophenol
- DNT
2,6-dinitrotoluene
- DPA
diphenylamine
- EA
ethylamine
- EDTA
ethylenediaminetetraacetic acid
- EE
electron exchange
- EPR
electron paramagnetic resonance
- ESA
excited-state absorption
- ESIPT
excited-state intramolecular proton transfer
- FRET
Förster resonance energy transfer
- FTIR
Fourier transform infrared
- HEMA
hydroxymethyl methacrylate
- HOMO
highest occupied molecular orbital
- IC
internal conversion
- ICT
internal charge transfer
- IFE
inner filter effect
- ISC
intersystem crossing
- KSV
Stern–Volmer constant
- LOD
limit of detection
- LUMO
lowest unoccupied molecular orbital
- MMA
methyl methacrylate
- MOF
metal–organic framework
- NMR
nuclear magnetic resonance
- NP
2-nitrophenol
- NPA
1-naphthylamine
- NT
2-nitrotoluene
- PA
phenylamine
- PDA
p-phenylenediamine
- PET
photoelectron transfer
- PPA
n-propylamine
- RET
resonance energy transfer
- RNA
ribonucleic acid
- THF
tetrahydrofuran
- TMA
trimethylamine
- TMAC
tetramethylammonium chloride
- TNP
2,4,6-trinitrophenol
- TNT
2,4,6-trinitrotoluene
- TPA
triphenylamine
- UV
ultraviolet
- VOCs
volatile organic compounds
- WHO
World Health Organization
- XPS
X-ray photoelectron spectroscopy
Author Contributions
The manuscript was written by T.S. and reviewed by D.S. and M.V. All authors have given approval to the final version of the manuscript.
The authors acknowledge the financial support from the Slovenian Research Agency (Research Core Funding No. P-0412).
The authors declare no competing financial interest.
References
- Lewis G. N.Valence and the Structure of Atoms and Molecules; Dover: New York, 1966. [Google Scholar]
- Lewis G. N. The Atom and the Molecule. J. Am. Chem. Soc. 1916, 38 (4), 762–785. 10.1021/ja02261a002. [DOI] [Google Scholar]
- Heitler W.; London F. Wechselwirkung Neutraler Atome Und Homöopolare Bindung Nach Der Quantenmechanik. Eur. Phys. J. A 1927, 44 (6), 455–472. 10.1007/BF01397394. [DOI] [Google Scholar]
- Staudinger H. Über Polymerisation. Ber. Dtsch. Chem. Ges. B 1920, 53 (6), 1073–1085. 10.1002/cber.19200530627. [DOI] [Google Scholar]
- Frey H.; Johann T. Celebrating 100 Years of “Polymer Science”: Hermann Staudinger’s 1920 Manifesto. Polym. Chem. 2020, 11 (1), 8–14. 10.1039/C9PY90161B. [DOI] [Google Scholar]
- Peplow M. The Plastics Revolution: How Chemists Are Pushing Polymers to New Limits. Nature 2016, 536 (7616), 266–268. 10.1038/536266a. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Aguila B.; Ma S. Potential Applications of Functional Porous Organic Polymer Materials. J. Mater. Chem. A 2017, 5 (35), 18896–18896. 10.1039/C7TA90189E. [DOI] [Google Scholar]
- Cote A. P.; Benin A. I.; Ockwig N. W.; O’keeffe M.; Matzger A. J.; Yaghi O. M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310 (5751), 1166–1170. 10.1126/science.1120411. [DOI] [PubMed] [Google Scholar]
- Feng X.; Ding X.; Jiang D. Covalent Organic Frameworks. Chem. Soc. Rev. 2012, 41 (18), 6010–6022. 10.1039/c2cs35157a. [DOI] [PubMed] [Google Scholar]
- Škorjanc T.; Shetty D.; Olson M. A.; Trabolsi A. Design Strategies and Redox-Dependent Applications of Insoluble Viologen-Based Covalent Organic Polymers. ACS Appl. Mater. Interfaces 2019, 11 (7), 6705–6716. 10.1021/acsami.8b20743. [DOI] [PubMed] [Google Scholar]
- De Acha N.; Elosua C.; Matias I.; Arregui F. J. Luminescence-Based Optical Sensors Fabricated by Means of the Layer-by-Layer Nano-Assembly Technique. Sensors 2017, 17 (12), 2826. 10.3390/s17122826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowicz J. R.Fluorescence Sensing. In Principles of Fluorescence Spectroscopy; Lakowicz J. R., Ed.; Springer: Boston, MA, USA, 2006; pp 623–673, 10.1007/978-0-387-46312-4_19. [DOI] [Google Scholar]
- Zimmermann J.; Zeug A.; Röder B. A Generalization of the Jablonski Diagram to Account for Polarization and Anisotropy Effects in Time-Resolved Experiments. Phys. Chem. Chem. Phys. 2003, 5 (14), 2964–2969. 10.1039/B303138A. [DOI] [Google Scholar]
- Atkins P.; de Paula J.. Atkins’ Physical Chemistry; Oxford University Press: Oxford, U.K., 2010. [Google Scholar]
- Li X.; Gao Q.; Wang J.; Chen Y.; Chen Z.-H.; Xu H.-S.; Tang W.; Leng K.; Ning G.-H.; Wu J.; Xu Q.-H.; Quek S. Y.; Lu Y.; Loh K. P. Tuneable near White-Emissive Two-Dimensional Covalent Organic Frameworks. Nat. Commun. 2018, 9 (1), 2335. 10.1038/s41467-018-04769-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y.; Yu W.; Zhang W.; Huang Q.; Yan J.; Pan C.; Yu G. Sulfur-Rich Covalent Triazine Polymer Nanospheres for Environmental Mercury Removal and Detection. Polym. Chem. 2018, 9 (30), 4125–4131. 10.1039/C8PY00419F. [DOI] [Google Scholar]
- Bao B.; Ma M.; Zai H.; Zhang L.; Fu N.; Huang W.; Wang L. Conjugated Polymer Nanoparticles for Label-Free and Bioconjugate-Recognized DNA Sensing in Serum. Adv. Sci. 2015, 2 (3), 1400009. 10.1002/advs.201400009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medintz I. L., Hildebrandt N., Eds. FRET-Förster Resonance Energy Transfer: From Theory to Applications; John Wiley & Sons: Weinheim, Germany, 2013; 10.1002/9783527656028. [DOI] [Google Scholar]
- Jana D.; Jana S. Donor-Pyrene-Acceptor Distance-Dependent Intramolecular Charge-Transfer Process: A State-Specific Solvation Preferred to the Linear-Response Approach. ACS Omega 2020, 5 (17), 9944–9956. 10.1021/acsomega.0c00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y.; Li B.; Li W.; Zhao J.; Sun S.; Pang Y. ICT-Not-Quenching” near Infrared Ratiometric Fluorescent Detection of Picric Acid in Aqueous Media. Chem. Commun. 2013, 49 (42), 4764–4766. 10.1039/c3cc41994k. [DOI] [PubMed] [Google Scholar]
- Sasaki S.; Drummen G. P. C.; Konishi G. Recent Advances in Twisted Intramolecular Charge Transfer (TICT) Fluorescence and Related Phenomena in Materials Chemistry. J. Mater. Chem. C 2016, 4 (14), 2731–2743. 10.1039/C5TC03933A. [DOI] [Google Scholar]
- Lakowicz J. R.Mechanisms and Dynamics of Fluorescence Quenching. In Principles of fluorescence spectroscopy; Springer: New York, NY, USA, 2006; Vol. 3, pp 331–351, 10.1007/978-0-387-46312-4_9. [DOI] [Google Scholar]
- Sun X.; Wang Y.; Lei Y. Fluorescence Based Explosive Detection: From Mechanisms to Sensory Materials. Chem. Soc. Rev. 2015, 44 (22), 8019–8061. 10.1039/C5CS00496A. [DOI] [PubMed] [Google Scholar]
- Zu F.; Yan F.; Bai Z.; Xu J.; Wang Y.; Huang Y.; Zhou X. The Quenching of the Fluorescence of Carbon Dots: A Review on Mechanisms and Applications. Microchim. Acta 2017, 184 (7), 1899–1914. 10.1007/s00604-017-2318-9. [DOI] [Google Scholar]
- Zheng P.; Wu N. Fluorescence and Sensing Applications of Graphene Oxide and Graphene Quantum Dots: A Review. Chem. - Asian J. 2017, 12 (18), 2343–2353. 10.1002/asia.201700814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaishankar M.; Tseten T.; Anbalagan N.; Mathew B. B.; Beeregowda K. N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7 (2), 60–72. 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty D.; Boutros S.; Eskhan A.; De Lena A. M.; Skorjanc T.; Asfari Z.; Traboulsi H.; Mazher J.; Raya J.; Banat F.; Trabolsi A. Thioether-Crown-Rich Calix[4]Arene Porous Polymer for Highly Efficient Removal of Mercury from Water. ACS Appl. Mater. Interfaces 2019, 11 (13), 12898–12903. 10.1021/acsami.9b02259. [DOI] [PubMed] [Google Scholar]
- Haldar U.; Lee H. BODIPY-Derived Polymeric Chemosensor Appended with Thiosemicarbazone Units for the Simultaneous Detection and Separation of Hg(II) Ions in Pure Aqueous Media. ACS Appl. Mater. Interfaces 2019, 11 (14), 13685–13693. 10.1021/acsami.9b00408. [DOI] [PubMed] [Google Scholar]
- Ding S.-Y.; Dong M.; Wang Y.-W.; Chen Y.-T.; Wang H.-Z.; Su C.-Y.; Wang W. Thioether-Based Fluorescent Covalent Organic Framework for Selective Detection and Facile Removal of Mercury (II). J. Am. Chem. Soc. 2016, 138 (9), 3031–3037. 10.1021/jacs.5b10754. [DOI] [PubMed] [Google Scholar]
- He Y.; Wang X.; Wang K.; Wang L. A Triarylamine-Based Fluorescent Covalent Organic Framework for Efficient Detection and Removal of Mercury (II) Ion. Dyes Pigm. 2020, 173, 107880. 10.1016/j.dyepig.2019.107880. [DOI] [Google Scholar]
- Cui W.-R.; Jiang W.; Zhang C.-R.; Liang R.-P.; Liu J.; Qiu J.-D. Regenerable Carbohydrazide-Linked Fluorescent Covalent Organic Frameworks for Ultrasensitive Detection and Removal of Mercury. ACS Sustainable Chem. Eng. 2020, 8 (1), 445–451. 10.1021/acssuschemeng.9b05725. [DOI] [Google Scholar]
- Cui W. R.; Zhang C. R.; Jiang W.; Li F. F.; Liang R. P.; Liu J.; Qiu J. D. Regenerable and Stable sp2 Carbon-Conjugated Covalent Organic Frameworks for Selective Detection and Extraction of Uranium. Nat. Commun. 2020, 11 (1), 436. 10.1038/s41467-020-14289-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Zhou J.; Shan G.-G.; Li G.-F.; Sun C.-Y.; Cui D.-X.; Wang X.-L.; Su Z.-M. A Tetraphenylethylene-Based Covalent Organic Polymer for Highly Selective and Sensitive Detection of Fe3+ and as a White Light Emitting Diode. Chem. Commun. 2019, 55 (82), 12328–12331. 10.1039/C9CC06337D. [DOI] [PubMed] [Google Scholar]
- Guo L.; Zeng X.; Lan J.; Yun J.; Cao D. Absorption Competition Quenching Mechanism of Porous Covalent Organic Polymer as Luminescent Sensor for Selective Sensing Fe3. ChemistrySelect 2017, 2 (3), 1041–1047. 10.1002/slct.201602076. [DOI] [Google Scholar]
- Guo L.; Zeng X.; Cao D. Porous Covalent Organic Polymers as Luminescent Probes for Highly Selective Sensing of Fe3+ and Chloroform: Functional Group Effects. Sens. Actuators, B 2016, 226, 273–278. 10.1016/j.snb.2015.11.108. [DOI] [Google Scholar]
- Guo L.; Cao D. Color Tunable Porous Organic Polymer Luminescent Probes for Selective Sensing of Metal Ions and Nitroaromatic Explosives. J. Mater. Chem. C 2015, 3 (33), 8490–8494. 10.1039/C5TC01649E. [DOI] [Google Scholar]
- Guo B.; Liu Q.; Su Q.; Liu W.; Ju P.; Li G.; Wu Q. A Triphenylamine-Functionalized Fluorescent Organic Polymer as a Turn-on Fluorescent Sensor for Fe 3+ Ion with High Sensitivity and Selectivity. J. Mater. Sci. 2018, 53 (22), 15746–15756. 10.1007/s10853-018-2726-1. [DOI] [Google Scholar]
- Chen G.; Lan H.-H.; Cai S.-L.; Sun B.; Li X.-L.; He Z.-H.; Zheng S.-R.; Fan J.; Liu Y.; Zhang W.-G. Stable Hydrazone-Linked Covalent Organic Frameworks Containing O, N, O′-Chelating Sites for Fe (III) Detection in Water. ACS Appl. Mater. Interfaces 2019, 11 (13), 12830–12837. 10.1021/acsami.9b02640. [DOI] [PubMed] [Google Scholar]
- Lee S.; Barin G.; Ackerman C. M.; Muchenditsi A.; Xu J.; Reimer J. A.; Lutsenko S.; Long J. R.; Chang C. J. Copper Capture in a Thioether-Functionalized Porous Polymer Applied to the Detection of Wilson’s Disease. J. Am. Chem. Soc. 2016, 138 (24), 7603–7609. 10.1021/jacs.6b02515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.; Zhang Y.; Xia H.; Mu Y.; Liu X. A Robust and Luminescent Covalent Organic Framework as a Highly Sensitive and Selective Sensor for the Detection of Cu 2+ Ions. Chem. Commun. 2016, 52 (39), 6613–6616. 10.1039/C6CC01476C. [DOI] [PubMed] [Google Scholar]
- Cui C.; Wang Q.; Xin C.; Liu Q.; Deng X.; Liu T.; Xu X.; Zhang X. Covalent Organic Framework Nanofiber with Bidentate Ligand as Enhanced Fluorescent Sensor for Cu2+. Microporous Mesoporous Mater. 2020, 299, 110122. 10.1016/j.micromeso.2020.110122. [DOI] [Google Scholar]
- Özdemir E.; Thirion D.; Yavuz C. T. Covalent Organic Polymer Framework with C–C Bonds as a Fluorescent Probe for Selective Iron Detection. RSC Adv. 2015, 5 (84), 69010–69015. 10.1039/C5RA10697D. [DOI] [Google Scholar]
- Ju P.; Su Q.; Liu Z.; Li X.; Guo B.; Liu W.; Li G.; Wu Q. A Salen-Based Covalent Organic Polymer as Highly Selective and Sensitive Fluorescent Sensor for Detection of Al 3+, Fe 3+ and Cu 2+ Ions. J. Mater. Sci. 2019, 54 (1), 851–861. 10.1007/s10853-018-2821-3. [DOI] [Google Scholar]
- Xiao J.-D.; Qiu L.-G.; Yuan Y.-P.; Jiang X.; Xie A.-J.; Shen Y.-H. Ultrafast Microwave-Enhanced Ionothermal Synthesis of Luminescent Crystalline Polyimide Nanosheets for Highly Selective Sensing of Chromium Ions. Inorg. Chem. Commun. 2013, 29, 128–130. 10.1016/j.inoche.2012.12.028. [DOI] [Google Scholar]
- Li Y.; Chen M.; Han Y.; Feng Y.; Zhang Z.; Zhang B. Fabrication of a New Corrole-Based Covalent Organic Framework as a Highly Efficient and Selective Chemosensor for Heavy Metal Ions. Chem. Mater. 2020, 32 (6), 2532–2540. 10.1021/acs.chemmater.9b05234. [DOI] [Google Scholar]
- El-Kadri O. M.; Tessema T.-D.; Almotawa R. M.; Arvapally R. K.; Al-Sayah M. H.; Omary M. A.; El-Kaderi H. M. Pyrene Bearing Azo-Functionalized Porous Nanofibers for CO2 Separation and Toxic Metal Cation Sensing. ACS Omega 2018, 3 (11), 15510–15518. 10.1021/acsomega.8b01920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R.; Zhao W.; Qiu Q.; Xie A.; Cheng S.; Jiao Y.; Pan X.; Dong W. Fluorescent Conjugated Microporous Polymer (CMP) Derived Sensor Array for Multiple Organic/Inorganic Contaminants Detection. Sens. Actuators, B 2020, 320, 128448. 10.1016/j.snb.2020.128448. [DOI] [Google Scholar]
- Gao Q.; Li X.; Ning G. H.; Leng K.; Tian B.; Liu C.; Tang W.; Xu H.-S.; Loh K. P. Highly Photoluminescent Two-Dimensional Imine-Based Covalent Organic Frameworks for Chemical Sensing. Chem. Commun. 2018, 54 (19), 2349–2352. 10.1039/C7CC09866A. [DOI] [PubMed] [Google Scholar]
- Geng T.; Chen G.; Zhang C.; Ma L.; Zhang W.; Xia H. A Superacid-Catalyzed Synthesis of Fluorescent Covalent Triazine Based Framework Containing Perylene Tetraanhydride Bisimide for Sensing to o-Nitrophenol with Ultrahigh Sensitivity. J. Macromol. Sci., Part A: Pure Appl.Chem. 2019, 56 (11), 1004–1011. 10.1080/10601325.2019.1640064. [DOI] [Google Scholar]
- Geng T.-M.; Li D.-K.; Zhu Z.-M.; Zhang W.-Y.; Ye S.-N.; Zhu H.; Wang Z.-Q. Fluorescent Conjugated Microporous Polymer Based on Perylene Tetraanhydride Bisimide for Sensing O-Nitrophenol. Anal. Chim. Acta 2018, 1011, 77–85. 10.1016/j.aca.2018.01.002. [DOI] [PubMed] [Google Scholar]
- Geng T. M.; Ye S. N.; Wang Y.; Zhu H.; Wang X.; Liu X. Conjugated Microporous Polymers-Based Fluorescein for Fluorescence Detection of 2,4,6-Trinitrophenol. Talanta 2017, 165, 282–288. 10.1016/j.talanta.2016.12.046. [DOI] [PubMed] [Google Scholar]
- Guo L.; Cao D.; Yun J.; Zeng X. Highly Selective Detection of Picric Acid from Multicomponent Mixtures of Nitro Explosives by Using COP Luminescent Probe. Sens. Actuators, B 2017, 243, 753–760. 10.1016/j.snb.2016.12.060. [DOI] [Google Scholar]
- Sang N.; Zhan C.; Cao D. Highly Sensitive and Selective Detection of 2, 4, 6-Trinitrophenol Using Covalent-Organic Polymer Luminescent Probes. J. Mater. Chem. A 2015, 3 (1), 92–96. 10.1039/C4TA04903A. [DOI] [Google Scholar]
- Wang S.; Liu Y.; Yu Y.; Du J.; Cui Y.; Song X.; Liang Z. Conjugated Microporous Polymers Based on Biphenylene for CO 2 Adsorption and Luminescence Detection of Nitroaromatic Compounds. New J. Chem. 2018, 42 (12), 9482–9487. 10.1039/C8NJ01306C. [DOI] [Google Scholar]
- Pan L.; Liu Z.; Tian M.; Schroeder B. C.; Aliev A. E.; Faul C. F. J. Luminescent and Swellable Conjugated Microporous Polymers for Detecting Nitroaromatic Explosives and Removing Harmful Organic Vapors. ACS Appl. Mater. Interfaces 2019, 11 (51), 48352–48362. 10.1021/acsami.9b16767. [DOI] [PubMed] [Google Scholar]
- Jiang S.; Meng L.; Ma W.; Qi Q.; Zhang W.; Xu B.; Liu L.; Tian W. Morphology Controllable Conjugated Network Polymers Based on AIE-Active Building Block for TNP Detection. Chin. Chem. Lett. 2020, 10.1016/j.cclet.2020.09.019. [DOI] [Google Scholar]
- Kaleeswaran D.; Murugavel R. Picric Acid Sensing and CO2 Capture by a Sterically Encumbered Azo-Linked Fluorescent Triphenylbenzene Based Covalent Organic Polymer. J. Chem. Sci. 2018, 130 (1), 1. 10.1007/s12039-017-1403-2. [DOI] [Google Scholar]
- Zhang W.; Qiu L.-G.; Yuan Y.-P.; Xie A.-J.; Shen Y.-H.; Zhu J.-F. Microwave-Assisted Synthesis of Highly Fluorescent Nanoparticles of a Melamine-Based Porous Covalent Organic Framework for Trace-Level Detection of Nitroaromatic Explosives. J. Hazard. Mater. 2012, 221, 147–154. 10.1016/j.jhazmat.2012.04.025. [DOI] [PubMed] [Google Scholar]
- Hu Y.-J.; Tan S.-Z.; Shen G.-L.; Yu R.-Q. A Selective Optical Sensor for Picric Acid Assay Based on Photopolymerization of 3-(N-Methacryloyl) Amino-9-Ethylcarbazole. Anal. Chim. Acta 2006, 570 (2), 170–175. 10.1016/j.aca.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Xiang Z.; Cao D. Synthesis of Luminescent Covalent–Organic Polymers for Detecting Nitroaromatic Explosives and Small Organic Molecules. Macromol. Rapid Commun. 2012, 33 (14), 1184–1190. 10.1002/marc.201100865. [DOI] [PubMed] [Google Scholar]
- Cui Y.; Liu Y.; Liu J.; Du J.; Yu Y.; Wang S.; Liang Z.; Yu J. Multifunctional Porous Tröger’s Base Polymers with Tetraphenylethene Units: CO2 Adsorption, Luminescence and Sensing Properties. Polym. Chem. 2017, 8 (33), 4842–4848. 10.1039/C7PY00856B. [DOI] [Google Scholar]
- Dalapati S.; Jin S.; Gao J.; Xu Y.; Nagai A.; Jiang D. An Azine-Linked Covalent Organic Framework. J. Am. Chem. Soc. 2013, 135 (46), 17310–17313. 10.1021/ja4103293. [DOI] [PubMed] [Google Scholar]
- Das G.; Biswal B. P.; Kandambeth S.; Venkatesh V.; Kaur G.; Addicoat M.; Heine T.; Verma S.; Banerjee R. Chemical Sensing in Two Dimensional Porous Covalent Organic Nanosheets. Chem. Sci. 2015, 6 (7), 3931–3939. 10.1039/C5SC00512D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.; Zhang S.; Yan Y.; Xia F.; Huang A.; Xian Y. Highly Fluorescent Polyimide Covalent Organic Nanosheets as Sensing Probes for the Detection of 2,4,6-Trinitrophenol. ACS Appl. Mater. Interfaces 2017, 9 (15), 13415–13421. 10.1021/acsami.6b16423. [DOI] [PubMed] [Google Scholar]
- Faheem M.; Aziz S.; Jing X.; Ma T.; Du J.; Sun F.; Tian Y.; Zhu G. Dual Luminescent Covalent Organic Frameworks for Nitro-Explosive Detection. J. Mater. Chem. A 2019, 7 (47), 27148–27155. 10.1039/C9TA09497K. [DOI] [Google Scholar]
- Das P.; Mandal S. K. A Dual-Functionalized, Luminescent and Highly Crystalline Covalent Organic Framework: Molecular Decoding Strategies for VOCs and Ultrafast TNP Sensing. J. Mater. Chem. A 2018, 6 (33), 16246–16256. 10.1039/C8TA05070H. [DOI] [Google Scholar]
- Lin G.; Ding H.; Yuan D.; Wang B.; Wang C. A Pyrene-Based, Fluorescent Three-Dimensional Covalent Organic Framework. J. Am. Chem. Soc. 2016, 138 (10), 3302–3305. 10.1021/jacs.6b00652. [DOI] [PubMed] [Google Scholar]
- Kaleeswaran D.; Vishnoi P.; Murugavel R. [3 + 3] Imine and β-Ketoenamine Tethered Fluorescent Covalent-Organic Frameworks for CO2 Uptake and Nitroaromatic Sensing. J. Mater. Chem. C 2015, 3 (27), 7159–7171. 10.1039/C5TC00670H. [DOI] [Google Scholar]
- Wang J.; Yan B. Improving Covalent Organic Frameworks Fluorescence by Triethylamine Pinpoint Surgery as Selective Biomarker Sensor for Diabetes Mellitus Diagnosis. Anal. Chem. 2019, 91 (20), 13183–13190. 10.1021/acs.analchem.9b03534. [DOI] [PubMed] [Google Scholar]
- Qu S.; Song N.; Xu G.; Jia Q. A Ratiometric Fluorescent Probe for Sensitive Detection of Anthrax Biomarker Based on Terbium-Covalent Organic Polymer Systems. Sens. Actuators, B 2019, 290, 9–14. 10.1016/j.snb.2019.03.110. [DOI] [Google Scholar]
- Wang J.; Zhao L.; Yan B. Indicator Displacement Assay inside Dye-Functionalized Covalent Organic Frameworks for Ultrasensitive Monitoring of Sialic Acid, an Ovarian Cancer Biomarker. ACS Appl. Mater. Interfaces 2020, 12 (11), 12990–12997. 10.1021/acsami.0c00101. [DOI] [PubMed] [Google Scholar]
- Liu W.; Cao Y.; Wang W.; Gong D.; Cao T.; Qian J.; Iqbal K.; Qin W.; Guo H. Mechanochromic Luminescent Covalent Organic Frameworks for Highly Selective Hydroxyl Radical Detection. Chem. Commun. 2019, 55 (2), 167–170. 10.1039/C8CC07783E. [DOI] [PubMed] [Google Scholar]
- Wang S.; Hu Q.; Liu Y.; Meng X.; Ye Y.; Liu X.; Song X.; Liang Z. Multifunctional Conjugated Microporous Polymers with Pyridine Unit for Efficient Iodine Sequestration, Exceptional Tetracycline Sensing and Removal. J. Hazard. Mater. 2020, 387, 121949. 10.1016/j.jhazmat.2019.121949. [DOI] [PubMed] [Google Scholar]
- Wang J.-M.; Lian X.; Yan B. Eu3+-Functionalized Covalent Organic Framework Hybrid Material as a Sensitive Turn-on Fluorescent Switch for Levofloxacin Monitoring in Serum and Urine. Inorg. Chem. 2019, 58 (15), 9956–9963. 10.1021/acs.inorgchem.9b01106. [DOI] [PubMed] [Google Scholar]
- Li W.; Yang C. X.; Yan X. P. A Versatile Covalent Organic Framework-Based Platform for Sensing Biomolecules. Chem. Commun. 2017, 53 (83), 11469–11471. 10.1039/C7CC06244C. [DOI] [PubMed] [Google Scholar]
- Peng Y.; Huang Y.; Zhu Y.; Chen B.; Wang L.; Lai Z.; Zhang Z.; Zhao M.; Tan C.; Yang N.; et al. Ultrathin Two-Dimensional Covalent Organic Framework Nanosheets: Preparation and Application in Highly Sensitive and Selective DNA Detection. J. Am. Chem. Soc. 2017, 139 (25), 8698–8704. 10.1021/jacs.7b04096. [DOI] [PubMed] [Google Scholar]
- Zhou M.; Wang Z.; Wang X.. Carbon Nanotubes for Sensing Applications. In Industrial Applications of Carbon Nanotubes; Peng H., Li Q., Chen T., Eds.; Micro and Nano Technologies; Elsevier: Boston, MA, USA, 2017; Chapter 5, pp 129–150, 10.1016/B978-0-323-41481-4.00005-8G. [DOI] [Google Scholar]
- Nguyen T. H.; Sun T.; Grattan K. T. V. Novel Coumarin-Based PH Sensitive Fluorescent Probes for the Highly Alkaline pH Region. Dyes Pigm. 2020, 177, 108312. 10.1016/j.dyepig.2020.108312. [DOI] [Google Scholar]
- Ulrich S.; Osypova A.; Panzarasa G.; Rossi R. M.; Bruns N.; Boesel L. F. Pyranine-Modified Amphiphilic Polymer Conetworks as Fluorescent Ratiometric PH Sensors. Macromol. Rapid Commun. 2019, 40 (21), 1900360. 10.1002/marc.201900360. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Shen X.; Feng X.; Xia H.; Mu Y.; Liu X. Covalent Organic Frameworks as PH Responsive Signaling Scaffolds. Chem. Commun. 2016, 52 (74), 11088–11091. 10.1039/C6CC05748A. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Wang K.; Hu X.; Shi P.; Guo Z.; Zhan H. Novel One-Dimensional Covalent Organic Framework as a H+ Fluorescent Sensor in Acidic Aqueous Solution. ACS Appl. Mater. Interfaces 2021, 13 (1), 1145–1151. 10.1021/acsami.0c16116. [DOI] [PubMed] [Google Scholar]
- Chen L.; He L.; Ma F.; Liu W.; Wang Y.; Silver M. A.; Chen L.; Zhu L.; Gui D.; Diwu J.; et al. Covalent Organic Framework Functionalized with 8-Hydroxyquinoline as a Dual-Mode Fluorescent and Colorimetric pH Sensor. ACS Appl. Mater. Interfaces 2018, 10 (18), 15364–15368. 10.1021/acsami.8b05484. [DOI] [PubMed] [Google Scholar]
- Gu S.; Guo J.; Huang Q.; He J.; Fu Y.; Kuang G.; Pan C.; Yu G. 1, 3, 5-Triazine-Based Microporous Polymers with Tunable Porosities for CO2 Capture and Fluorescent Sensing. Macromolecules 2017, 50 (21), 8512–8520. 10.1021/acs.macromol.7b01857. [DOI] [Google Scholar]
- Shanmugaraju S.; Umadevi D.; González-Barcia L. M.; Delente J. M.; Byrne K.; Schmitt W.; Watson G. W.; Gunnlaugsson T. Turn-on” Fluorescence Sensing of Volatile Organic Compounds Using a 4-Amino-1, 8-Naphthalimide Tröger’s Base Functionalised Triazine Organic Polymer. Chem. Commun. 2019, 55 (81), 12140–12143. 10.1039/C9CC05585A. [DOI] [PubMed] [Google Scholar]
- Zuo H.; Li Y.; Liao Y. Europium Ionic Liquid Grafted Covalent Organic Framework with Dual Luminescence Emissions as Sensitive and Selective Acetone Sensor. ACS Appl. Mater. Interfaces 2019, 11 (42), 39201–39208. 10.1021/acsami.9b14795. [DOI] [PubMed] [Google Scholar]
- Han D.; Jin Y.; Lee J.; Kim S.; Kim H.; Song K.; Kwak G. Environment-Specific Fluorescence Response of Microporous, Conformation-Variable Conjugated Polymer Film to Water in Organic Solvents: On-line Real-Time Monitoring in Fluidic Channels. Macromol. Chem. Phys. 2014, 215 (11), 1068–1076. 10.1002/macp.201400088. [DOI] [Google Scholar]
- Qian H.-L.; Dai C.; Yang C.-X.; Yan X.-P. High-Crystallinity Covalent Organic Framework with Dual Fluorescence Emissions and Its Ratiometric Sensing Application. ACS Appl. Mater. Interfaces 2017, 9 (29), 24999–25005. 10.1021/acsami.7b08060. [DOI] [PubMed] [Google Scholar]
- Skorjanc T.; Shetty D.; Sharma S. K.; Raya J.; Traboulsi H. M.; Han D. S.; Lalla J.; Newlon R.; Jagannathan R.; Kirmizialtin S.; Olsen J.-C.; Trabolsi A. Redox-Responsive Covalent Organic Nanosheets from Viologens and Calix[4]Arene for Iodine and Toxic Dye Capture. Chem. - Eur. J. 2018, 24 (34), 8648–8655. 10.1002/chem.201800623. [DOI] [PubMed] [Google Scholar]
- Valdes-García J.; Rosales-Vázquez L. D.; Bazany-Rodríguez I. J.; Dorazco-González A. Recent Advances in Luminescent Recognition and Chemosensing of Iodide in Water. Chem. - Asian J. 2020, 15 (19), 2925–2938. 10.1002/asia.202000758. [DOI] [PubMed] [Google Scholar]
- Das G.; Skorjanc T.; Sharma S. K.; Prakasam T.; Platas-Iglesias C.; Han D. S.; Raya J.; Olsen J.-C.; Jagannathan R.; Trabolsi A. Morphological Diversity in Nanoporous Covalent Organic Materials Derived from Viologen and Pyrene. ChemNanoMat 2018, 4 (1), 61–65. 10.1002/cnma.201700242. [DOI] [Google Scholar]
- Shetty D.; Raya J.; Han D. S.; Asfari Z.; Olsen J.-C.; Trabolsi A. Lithiated Polycalix [4] Arenes for Efficient Adsorption of Iodine from Solution and Vapor Phases. Chem. Mater. 2017, 29 (21), 8968–8972. 10.1021/acs.chemmater.7b03449. [DOI] [Google Scholar]
- Das G.; Skorjanc T.; Sharma S. K.; Gándara F.; Lusi M.; Shankar Rao D. S.; Vimala S.; Krishna Prasad S.; Raya J.; Han D. S.; Jagannathan R.; Olsen J.-C.; Trabolsi A. Viologen-Based Conjugated Covalent Organic Networks via Zincke Reaction. J. Am. Chem. Soc. 2017, 139 (28), 9558–9565. 10.1021/jacs.7b02836. [DOI] [PubMed] [Google Scholar]
- Geng T.; Liu M.; Zhang C.; Hu C.; Xia H. Y. The Preparation of Covalent Triazine-Based Framework via Friedel-Crafts Reaction of 2,4,6-Trichloro-1,3,5-Triazine with N,N′-Diphenyl-N,N′-Di(m-Tolyl)Benzidine for Capturing and Sensing to Iodine. Polym. Adv. Technol. 2020, 31 (6), 1388–1394. 10.1002/pat.4868. [DOI] [Google Scholar]
- Geng T.; Liu M.; Zhang C.; Hu C.; Xu H. Synthesis of Secondary Amine-Based Fluorescent Porous Organic Polymers via Friedel–Crafts Polymerization Reaction for Adsorbing and Fluorescent Sensing Iodine. J. Appl. Polym. Sci. 2020, 137 (41), 49255. 10.1002/app.49255. [DOI] [Google Scholar]
- Geng T.; Zhang W.; Zhu Z.; Kai X. Triazine-Based Conjugated Microporous Polymers Constructing Triphenylamine and Its Derivatives with Nitrogen as Core for Iodine Adsorption and Fluorescence Sensing I2. Microporous Mesoporous Mater. 2019, 273, 163–170. 10.1016/j.micromeso.2018.07.004. [DOI] [Google Scholar]
- Geng T.; Ma L.; Chen G.; Zhang C.; Zhang W.; Xia H.; Zhu H. Poly [1,3,6,8-tetra(2-thiophenyl)pyrene] and Poly[1,3,6,8-tetra(3-thiophenyl)pyrene] Conjugated Microporous Polymers for Reversible Adsorbing and Fluorescent Sensing Iodine. J. Polym. Res. 2019, 26 (5), 113. 10.1007/s10965-019-1766-9. [DOI] [Google Scholar]
- Geng T.; Chen G.; Ma L.; Zhang C.; Zhang W.; Xu H. The Spirobifluorene-Based Fluorescent Conjugated Microporous Polymers for Reversible Adsorbing Iodine, Fluorescent Sensing Iodine and Nitroaromatic Compounds. Eur. Polym. J. 2019, 115, 37–44. 10.1016/j.eurpolymj.2019.02.047. [DOI] [Google Scholar]
- Geng T.; Zhang C.; Chen G.; Ma L.; Zhang W.; Xia H. Synthesis of Tetraphenylethylene-Based Fluorescent Conjugated Microporous Polymers for Fluorescent Sensing and Adsorbing Iodine. Microporous Mesoporous Mater. 2019, 284, 468–475. 10.1016/j.micromeso.2019.04.036. [DOI] [Google Scholar]
- Geng T.; Ma L.; Chen G.; Zhang C.; Zhang W.; Niu Q. Fluorescent Conjugated Microporous Polymers Containing Pyrazine Moieties for Adsorbing and Fluorescent Sensing of Iodine. Environ. Sci. Pollut. Res. 2020, 27 (16), 20235–20245. 10.1007/s11356-019-06534-8. [DOI] [PubMed] [Google Scholar]
- Geng T. M.; Zhang C.; Hu C.; Liu M.; Fei Y. T.; Xia H. Y. Synthesis of 1,6-Disubstituted Pyrene-Based Conjugated Microporous Polymers for Reversible Adsorption and Fluorescence Sensing of Iodine. New J. Chem. 2020, 44 (6), 2312–2320. 10.1039/C9NJ05509F. [DOI] [Google Scholar]
- Kasper M.; Busche S.; Gauglitz G.. Optical Sensing of Enantiomers. In Frontiers in Chemical Sensors: Novel Principles and Techniques; Orellana G., Moreno-Bondi M. C., Eds.; Springer: Berlin, Heidelberg, 2005; pp 323–341, 10.1007/3-540-27757-9_11. [DOI] [Google Scholar]
- Vargesson N. Thalidomide-Induced Teratogenesis: History and Mechanisms. Birth Defects Res., Part C 2015, 105 (2), 140–156. 10.1002/bdrc.21096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh W.-K.; Jeong Y. S.; Lee K. J.; Jang J. Fluorescent Boronic Acid-Modified Polymer Nanoparticles for Enantioselective Monosaccharide Detection. Anal. Methods 2012, 4 (4), 913–918. 10.1039/c2ay05800f. [DOI] [Google Scholar]
- Wu X.; Han X.; Xu Q.; Liu Y.; Yuan C.; Yang S.; Liu Y.; Jiang J.; Cui Y. Chiral BINOL-Based Covalent Organic Frameworks for Enantioselective Sensing. J. Am. Chem. Soc. 2019, 141 (17), 7081–7089. 10.1021/jacs.9b02153. [DOI] [PubMed] [Google Scholar]
- Yuan C.; Fu S.; Yang K.; Hou B.; Liu Y.; Jiang J.; Cui Y. Crystalline C - C and C=C Bond-Linked Chiral Covalent Organic Frameworks. J. Am. Chem. Soc. 2021, 143, 369–381. 10.1021/jacs.0c11050. [DOI] [PubMed] [Google Scholar]
- Li Z.; Mo Z.; Meng S.; Gao H.; Niu X.; Guo R. The Construction and Application of Chiral Electrochemical Sensors. Anal. Methods 2016, 8 (46), 8134–8140. 10.1039/C6AY02431A. [DOI] [Google Scholar]
- Zhao Y.; Sui Z.; Chang Z.; Wang S.; Liang Y.; Liu X.; Feng L.; Chen Q.; Wang N. A Trifluoromethyl-Grafted Ultra-Stable Fluorescent Covalent Organic Framework for Adsorption and Detection of Pesticides. J. Mater. Chem. A 2020, 8, 25156–25164. 10.1039/D0TA07708A. [DOI] [Google Scholar]
- Gräfe A.; Haupt K.; Mohr G. J. Optical Sensor Materials for the Detection of Amines in Organic Solvents. Anal. Chim. Acta 2006, 565 (1), 42–47. 10.1016/j.aca.2006.02.034. [DOI] [Google Scholar]
- Das G.; Prakasam T.; Nuryyeva S.; Han D. S.; Abdel-Wahab A.; Olsen J.-C.; Polychronopoulou K.; Platas-Iglesias C.; Ravaux F.; Jouiad M.; Trabolsi A. Multifunctional Redox-Tuned Viologen-Based Covalent Organic Polymers. J. Mater. Chem. A 2016, 4 (40), 15361–15369. 10.1039/C6TA06439F. [DOI] [Google Scholar]
- Kim K.; Buyukcakir O.; Coskun A. Diazapyrenium-Based Porous Cationic Polymers for Colorimetric Amine Sensing and Capture from CO2 Scrubbing Conditions. RSC Adv. 2016, 6 (81), 77406–77409. 10.1039/C6RA16714D. [DOI] [Google Scholar]
- Fu Y.; Yao J.; Xu W.; Fan T.; He Q.; Zhu D.; Cao H.; Cheng J. Reversible and “Fingerprint” Fluorescence Differentiation of Organic Amine Vapours Using a Single Conjugated Polymer Probe. Polym. Chem. 2015, 6 (12), 2179–2182. 10.1039/C4PY01793E. [DOI] [Google Scholar]
- Tang Y.; Huang H.; Peng B.; Chang Y.; Li Y.; Zhong C. A Thiadiazole-Based Covalent Triazine Framework Nanosheet for Highly Selective and Sensitive Primary Aromatic Amine Detection among Various Amines. J. Mater. Chem. A 2020, 8 (32), 16542–16550. 10.1039/C9TA14252E. [DOI] [Google Scholar]
- Tao L.-M.; Niu F.; Zhang D.; Wang T.-M.; Wang Q.-H. Amorphous Covalent Triazine Frameworks for High Performance Room Temperature Ammonia Gas Sensing. New J. Chem. 2014, 38 (7), 2774–2777. 10.1039/c4nj00476k. [DOI] [Google Scholar]
- Niu F.; Shao Z.-W.; Tao L.-M.; Ding Y. Covalent Triazine-Based Frameworks for NH3 Gas Sensing at Room Temperature. Sens. Actuators, B 2020, 321, 128513. 10.1016/j.snb.2020.128513. [DOI] [Google Scholar]
- Yu J.; Zhang C. Fluorescent Sensing for Amines with a Low Detection Limit Based on Conjugated Porous Polymers. J. Mater. Chem. C 2020, 8, 16463–16469. 10.1039/D0TC02592E. [DOI] [Google Scholar]
- Xu N.; Wang R.-L.; Li D.-P.; Zhou Z.-Y.; Zhang T.; Xie Y.-Z.; Su Z.-M. Continuous Detection of HCl and NH 3 Gases with a High-Performance Fluorescent Polymer Sensor. New J. Chem. 2018, 42 (16), 13367–13374. 10.1039/C8NJ02344A. [DOI] [Google Scholar]
- Dalapati S.; Jin E.; Addicoat M.; Heine T.; Jiang D. Highly Emissive Covalent Organic Frameworks. J. Am. Chem. Soc. 2016, 138 (18), 5797–5800. 10.1021/jacs.6b02700. [DOI] [PubMed] [Google Scholar]
- Klockow J. L.; Hettie K. S.; Glass T. E.. Fluorescent Sensors for Amines. In Comprehensive Supramolecular Chemistry II; Elsevier: Amsterdam, 2017; pp 447–467, 10.1016/B978-0-12-409547-2.12630-7G. [DOI] [Google Scholar]
- Subodh; Prakash K.; Masram D. T. Chromogenic Covalent Organic Polymer-Based Microspheres as Solid-State Gas Sensor. J. Mater. Chem. C 2020, 8 (27), 9201–9204. 10.1039/D0TC02129F. [DOI] [Google Scholar]
- EL-Mahdy A. F. M.; Lai M.-Y.; Kuo S.-W. Highly Fluorescent Covalent Organic Framework as Hydrogen Chloride Sensor: Roles of Schiff Base Bonding and π-Stacking. J. Mater. Chem. C 2020, 8 (28), 9520–9528. 10.1039/D0TC01872D. [DOI] [Google Scholar]
- Contreras-Gutierrez P. K.; Medina-Rodríguez S.; Medina-Castillo A. L.; Fernandez-Sanchez J. F.; Fernandez-Gutierrez A. A New Highly Sensitive and Versatile Optical Sensing Film for Controlling CO2 in Gaseous and Aqueous Media. Sens. Actuators, B 2013, 184, 281–287. 10.1016/j.snb.2013.04.074. [DOI] [Google Scholar]
- Gas sensor market size, share, industry analysis report, 2027:
- Li M.; Cui Z.; Pang S.; Meng L.; Ma D.; Li Y.; Shi Z.; Feng S. Luminescent Covalent Organic Framework as a Recyclable Turn-off Fluorescent Sensor for Cations and Anions in Aqueous Solution. J. Mater. Chem. C 2019, 7 (38), 11919–11925. 10.1039/C9TC03265G. [DOI] [Google Scholar]
- Singh H.; Devi M.; Jena N.; Iqbal M. M.; Nailwal Y.; De Sarkar A.; Pal S. K. Proton-Triggered Fluorescence Switching in Self-Exfoliated Ionic Covalent Organic Nanosheets for Applications in Selective Detection of Anions. ACS Appl. Mater. Interfaces 2020, 12 (11), 13248–13255. 10.1021/acsami.9b20743. [DOI] [PubMed] [Google Scholar]