Abstract
Background:
Statins have been proven to be cytotoxic to human cholangiocarcinoma cells by inhibiting cell division and inducing apoptosis. We aimed to determine the effect of statin use on the risk of cancer development and survival in patients with extrahepatic cholangiocarcinoma (ECC) including perihilar cholangiocarcinoma (pCCA) and distal cholangiocarcinoma (dCCA).
Methods:
394 patients with ECC and hyperlipidemia who received care at Mayo Clinic Rochester between 2005 and 2015 were matched by age, sex, race, ethnicity and residency to 788 controls with hyperlipidemia. Clinical and outcome data was abstracted. The odds ratios for risk and hazard ratios for outcomes were calculated.
Results:
The mean age and standard deviation (SD) for cases and controls was 65.6 years (13.8). The number of statin users in cases and controls were 73 (19%) and 403 (51%), respectively. Hepatitis C virus infection (OR=15.84, 95% CI 4.06–61.87; p<0.001) was the most significant risk factor for pCCA followed by inflammatory bowel disease and cirrhosis, whereas other liver diseases including biliary stone disease (OR=4.06, 2.24–7.36; p<0.001) was the only significant risk factor for dCCA. Statin use was associated with significantly reduced risk for all ECC (OR=0.22, 0.16–029) as well as for the subtypes pCCA (OR=0.3, 0.21–0.41) and dCCA (OR=0.06, 0.03–0.14) all p<0.0001. Moderate intensity dosage was found to decrease the risk of ECC (OR=0.48, 0.34–0.67, p<0.001). Comparing statin ever users to non-users, dCCA patients who used statins had significantly overall better survival (HR=0.53, 0.29–0.97, p=0.04).
Conclusion:
This case-control study suggests that statins decrease the risk of extrahepatic cholangiocarcinoma and may improve survival in patients with dCCA. Additional validation studies are warranted.
Keywords: bile duct cancer, cholangiocarcinoma, IBD, cholangitis, cirrhosis, Hepatitis C
Introduction:
Extrahepatic cholangiocarcinoma (ECC) is an aggressive malignant tumor arising from the biliary tree extending from the second order branches of the right and left hepatic ducts to the distal bile duct. It is further classified into two types 1) perihilar cholangiocarcinoma (pCCA) involving the confluence of right and left hepatic ducts up to the cystic duct insertion into the common hepatic duct 2) distal cholangiocarcinoma (dCCA) originating from common bile duct till its meeting point with pancreatic duct at the ampulla of water. pCCA and dCCA are now regarded as separate malignancies due to significant differences in their risk factors, presentation, severity, management and outcomes. Although the incidence of ECC has remained more or less stable, the overall mortality from cholangiocarcinoma in the US has increased by 36% over the past two decades. (1, 2) This calls for preventive measures as well as appropriate management of risk factors specific for each ECC subtype. IBD, cirrhosis, primary sclerosing cholangitis (PSC), cholecystitis and biliary cysts and biliary tract stones are the major risk factors for ECC in western population.(3) Due to the low incidence of Hepatitis B & C and parasitic infestation with liver flukes such as Clonorchis sinensis in the US population, precise evidence of their contribution to ECC in the US are not available.
Statins are lipid lowering drugs that decrease cholesterol production by the liver. Their main use is in prevention of cardiovascular events in high risk patients. Lately, statins have been extensively studied for their anti-inflammatory and anti-cancer properties. Molecular substrates downstream of the cholesterol pathway are reported to contribute to carcinogenesis. Statins prevent the synthesis of these intermediates by inhibiting the HMG-CoA reductase enzyme. Prospective and population based studies have suggested that statins reduce the incidences of many cancers. Clinical studies had shown that statins were associated with improved survival in patients with pancreatic cancer. (4–6) A nation-wide case-control study in Korea on type 2 diabetes patients with hepatocellular carcinoma (HCC) found dose-dependent reduction in development of HCC with statin use. (7) Similar findings were noted in a US study in which patients with low risk of HCC, who ever used statins had decreased risk of cancer. (8) Another nationwide study in Scotland showed reduced mortality in breast cancer patients who were on statins post-diagnosis. (9) Atorvastatin users with high risk of breast cancer had detectable levels of drug in breast tissue and decreased levels of C-reactive protein (CRP) detected on phase 1 biomarker modulation study. CRP is an independent biomarker predicting the risk of breast cancer in postmenopausal women. This concluded that atorvastatin reduced the risk of breast cancer. (10) Statin use before and after cancer diagnosis, has been shown to improve all-cause mortality in patients diagnosed with lung cancer. (11, 12) Population studies have shown that post diagnosis statin use had improved survival in ovarian cancer too. (13, 14)
These findings have been attributed to multiple pathways including activation of Liver kinase B1 (LKB-1) and inhibition of Ras-related C3 botulinum toxin substrate 1 (Rac1) activity. We therefore hypothesize that statin use reduces the risk of ECC. We devised a case-control study to test the association of statin use with risk of ECC in patients with hyperlipidemia and ECC. We also studied some of the common risk factors which are known to increase the risk of ECC in high risk patients and analyzed the factors separately for each subtype.
Patients and Methods
Study Population:
The current study was approved by the Mayo Clinic Institutional Review Board. All patients aged >18 years with ECC (Extrahepatic cholangiocarcinoma) diagnosed at the Mayo Clinic (Rochester, MN) between January 2005 and May 2015 were identified using the Mayo Clinic Advanced Cohort Exploratory tool. We searched for ECC cases using ICD-9 (International Classification of Diseases, Ninth revision) code “156.1”, ICD-10 code “C24.0” and/or the keywords “cholangiocarcinoma”, “bile duct cancer”, “extrahepatic cholangiocarcinoma”, “hilar cholangiocarcinoma”, “perihilar cholangiocarcinoma”, “distal cholangiocarcinoma”, “Klatskin’s tumor” and “carcinoma of extrahepatic bile duct” to identify all potential ECC patients (n=1,663). The diagnosis of ECC was confirmed by histopathology, and the anatomic location of the tumor was determined by review of radiology (computerized tomography, magnetic resonance imaging, or endoscopic retrograde cholangiopancreatography). ECCs were categorized as “perihilar” if the lesion was situated in the proximal right or left bile duct or between the biliary confluence and the cystic duct insertion or “distal” if the lesion arose from the cystic duct and the ampulla of Vater).
Among the ECC cases, patients with a history of hyperlipidemia were identified using ICD-9-CM Diagnosis Code “272.4” and ICD-10 code “E78.5” and/or laboratory values from serum lipid profile. Hyperlipidemia was defined as a total cholesterol level of ˃200mg/dl and/or LDL level of ˃130mg/dl and/or HDL level ˂40mg/dl and/or triglyceride level ˃150mg/dl. Patients who ever had the above values on lipid profile were diagnosed as having hyperlipidemia. After review, 412 patients were confirmed to have the diagnosis of ECC and hyperlipidemia. This pool of patients was included in the study as cases. All cases had ECC as a primary tumor. ECC secondary to metastasis to liver or biliary ducts was excluded.
Control subjects were selected from the Mayo Clinic Biobank, which comprises patients receiving care at the Mayo Clinic who have agreed to participate in this clinic-based database. This database includes a large group of patients seen at the Mayo Clinic and is designed to provide control groups for studies performed at the Mayo Clinic, allowing selection of controls that are matched to cases by age, gender, ethnicity, and residence. Biobank participants provide a blood sample, complete a health questionnaire, and give authorization for use of their medical records in research. 394 cases were matched to two Mayo Clinic Biobank controls each by age (within 1–2years) at diagnosis of ECC, sex, ethnicity, and residence (Minnesota, Southern, Southwest, Northeast, Western, Pacific, Alaska and Midwest). 18 cases were unmatched due to discrepancies in parameters used for matching with controls. Of the unmatched cases, 6 were statin users. All controls had a history of hyperlipidemia but none had a history of cancer.
Clinical Parameters:
Additional information on each participant was abstracted from the electronic medical record (EMR) by manual review. This included: demographic data, clinical information, medications, laboratory results, and data on risk factors. Risk factors abstracted included baseline average body mass index (BMI), HCV, cirrhosis, Cholangitis, PSC, other liver diseases (non-alcoholic steatohepatitis, non-alcoholic fatty liver disease, alcoholic liver disease, autoimmune hepatitis, biliary stone diseases), diabetes mellitus (DM), IBD, coronary artery disease (CAD), and stroke.
We abstracted the results of tests for HCV infection for all cases and controls. HCV infection was defined as a positive HCV RNA. A diagnosis of HCV in the physician’s note was accepted as proof of viral infection. PSC was diagnosed by histopathology, physician diagnosis or clinical criteria as recommended by the PSC practice guidelines of the American Association for the Study of Liver Diseases. Cirrhosis was diagnosed by radiologic evidence of a nodular liver, caudate lobe hypertrophy, or portal hypertension (collateral vessels, varices, and splenomegaly). IBD including ulcerative colitis and Crohn’s disease was diagnosed by histopathology or typical endoscopic findings. History of DM, CAD and stroke was abstracted from physician notes.
Statin use was determined from medication lists and physicians’ notes, which were manually reviewed. For cases, we reviewed the medication list from the EMR until the date of last follow up to ensure that the statin was not withdrawn because of the diagnosis of malignancy. Similarly, for controls, we reviewed the medication list from the initial consultation at Mayo Clinic until the date of last follow up. Subjects were labelled as statin users if they ever used statins for more than 3 months. The intensity of statin use was classified as high, moderate or low based on the 2013 American Heart Association (AHA) Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults.(15) If the type and dose of statins were changed during the time period of usage, then the subjects were classified into the group with the higher intensity statin use among the different statin types and dosages.
Statistical Analysis:
We excluded hepatitis B virus (HBV) infection from the analysis because the numbers were too small in both the case and control groups. For baseline characteristics means and standard deviations were calculated for continuous data and t-tests were used to compare groups. Frequencies and percentages were computed for nominal data and Pearson’s chi-square or Fisher’s exact tests were used as appropriate to compare groups. Logistic regression was used to look at potential risk factors for being an ECC patient versus a control. Both univariate and multivariable Cox proportional hazards models were used to assess for potential risk factors for death after diagnosis among the ECC patients. All statistical tests were two-sided and p <0.05 was statistically significant. The analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary NC).
Results
Baseline characteristics:
412 cases and 788 controls were included in the analysis. The baseline characteristics of matched cases and controls are summarized in Supplemental Table 1. The mean ages of cases and controls were 65.7 and 65.6 years respectively. Male sex was predominant in cases and controls (68% and 68.3% respectively). The majority of study participants were white (96.4%). Statin use was significantly (P <0.001) higher in controls (51.1%) than in cases (19.1%). The prevalences of DM, IBD, PSC, cholangitis, HCV, cirrhosis and other liver diseases were all higher in cases than controls (P=0.015 for DM and P <0.001 for others). However, alcohol use was higher in controls (75.9%) than cases (53.3%) whereas the number of smokers is the same in both groups (47.7%).
Statin users versus statin non-users:
Statin use was reported in 79 of 412 cases (73 matched and 6 unmatched) and 403 of 786 controls. The baseline characteristics of statin users are shown in Table 1. Among both the cases and controls, statin users are predominantly males. The mean BMI of statin users (mean=29.1; SD 5.4 in cases, mean=29.5; SD 4.9 in controls) was higher than for statin non-users (mean=27.5; SD 6.1 in cases and mean=28.1; SD 5.5 in controls) in both cases and controls. Factors affecting the use of statins such as CAD and stroke were more prevalent in statin users in both cases and controls. The duration of statin use in statin users is shown in Supplemental Table 2. The difference between the numbers of statin users in cases and control with duration more than 5 years is large compared to other usage duration.
Table 1.
Baseline characteristics of matched statin users and statin non-user in all ECC cases and all controls
| Variable | All ECC | All controls | ||||
|---|---|---|---|---|---|---|
| Statin user (N=73) | Statin non-user (N=321) | p-value | Statin user (N=403) | Statin non-user (N=385) | p-value | |
| Age in years, mean (SD) | 69.3 (10.9) | 64.9 (14.3) | 0.014 | 65.9 (12.4) | 65.2 (15.0) | 0.98 |
| Sex, male | 57 (78.1%) | 211 (65.7%) | 0.041 | 297 (73.7%) | 241 (62.6%) | <0.001 |
| Race | 0.082 | 0.015 | ||||
| White, n (%) | 73 (100.0%) | 307 (95.6%) | 395 (98.0%) | 365 (94.8%) | ||
| Non-white, n (%) | 0 (0.0%) | 14 (4.4%) | 8 (2.0%) | 20 (5.2%) | ||
| BMI; mean (SD) | 29.1 (5.4) | 27.5 (6.1) | 0.005 | 29.5 (4.9) | 28.1 (5.5) | <0.001 |
| DM, n (%) | 39 (53.4%) | 84 (26.2%) | <0.001 | 209 (51.9%) | 94 (24.4%) | <0.001 |
| IBD, n (%) | 2 (2.7%) | 39 (12.1%) | 0.017 | 7 (1.7%) | 4 (1.0%) | 0.40 |
| Cirrhosis, n (%) | 8 (11.0%) | 67 (20.9%) | 0.068 | 34 (8.4%) | 25 (6.5%) | 0.30 |
| Cholangitis, n (%) | 27 (37%) | 86 (26.8%) | 0.087 | 1 (0.2%) | 0 (0.0%) | 0.33 |
| PSC, n (%) | 11 (15.1%) | 114 (35.5%) | 0.005 | 0 (0.0%) | 0 (0.0%) | 1.00 |
| HCV, n (%) | 2 (2.7%) | 19 (5.9%) | 0.39 | 5 (1.2%) | 1 (0.3%) | 0.11 |
| Other liver disease, n (%) | 3 (4.1%) | 36 (11.2%) | 0.081 | 11 (2.7%) | 10 (2.6%) | 0.91 |
| CAD, n (%) | 44 (60.3%) | 69 (21.5%) | <0.001 | 209 (51.9%) | 49 (12.7%) | <0.001 |
| Stroke, n (%) | 6 (8.2%) | 1 (0.3%) | <0.001 | 105 (26.1%) | 27 (7.0%) | <0.001 |
| Smoking, n (%) | 16 (5.71%) | 58 (20.7%) | 0.07 | 199 (25.4%) | 175 (22.3%) | 0.27 |
| Alcohol use, n (%) | 25 (8.93%) | 101 (36.1%) | 0.04 | 309 (39.4%) | 287 (36.6%) | 0.38 |
Risk factors for ECC:
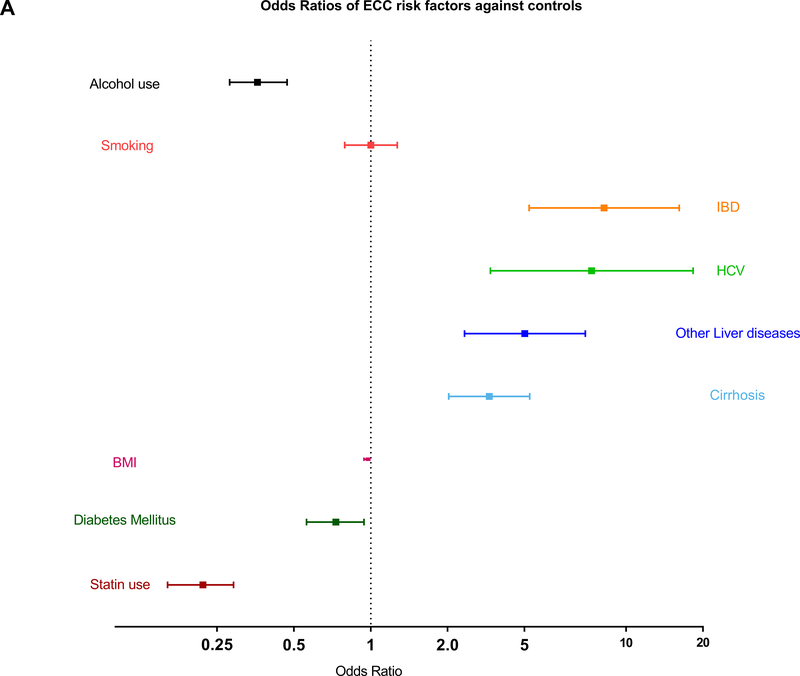
The risk factors for ECC are shown in Table 2. IBD (OR=8.2, 95% CI 4.17–16.15; p<0.0001), cirrhosis (OR=2.91 95% CI 2.02–4.19; p<0.0001), HCV (OR=7.33, 95% CI 2.94–18.31; p<0.0001) and other liver diseases (OR=4.01, 95% CI 2.33–6.92; p<0.0001) were associated with increased risk of ECC. Smoking had not shown to increase the risk of ECC. Alcohol use was associated with lower risk of ECC and its subtypes owning to the fact that controls had higher percentage of usage compared to cases (Figure 1A). Odds Ratios for cholangitis and PSC were not computable as none of the controls had these risk factors. DM was associated with slightly a reduced risk of ECC (OR=0.73, 95% CI 0.56–0.94; p=0.015). Moderate intensity statin therapy (OR=0.48, 95% CI 0.34–0.67; p<0.001) and statin use for a duration of less than a year (OR=0.38, 95% CI 0.22–0.67; p=0.001) or 1–3 years (OR=0.51, 95% CI 0.29–0.90); p=0.02) had statistically significant decrease in risk of ECC (Table 3). Use of statins for 3–5 years or >5 years was not associated with a decrease in cancer risk. The number of observations in statin use for less than one year, 1 to 3 years, 3 to 5 years and more than 5 years were 10, 10, 10 and 26 in matched cases versus 7, 58, 33 and 283 in controls respectively, suggesting that we were not able to show a difference due to small numbers.
Table 2.
Risk factors for all ECC cases, its subtypes pCCA and dCCA and respective controls
| Risk factors | All ECC vs Controls | pCCA vs Controls | dCCA vs Controls | |||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | p-value | Odds Ratio (95% CI) | p-value | Odds Ratio (95% CI) | p-value | |
| Statin use | 0.22 (0.16–0.29) | <0.0001 | 0.3 (0.21–0.41) | <0.0001 | 0.06 (0.03–0.14) | <0.0001 |
| BMI | 0.97 (0.94–0.99) | 0.004 | 0.95 (0.92–0.98) | <0.001 | 1.01 (0.97–1.05) | 0.77 |
| DM | 0.73 (0.56–0.94) | 0.015 | 0.63 (0.46–0.87) | 0.005 | 0.94 (0.61–1.44) | 0.77 |
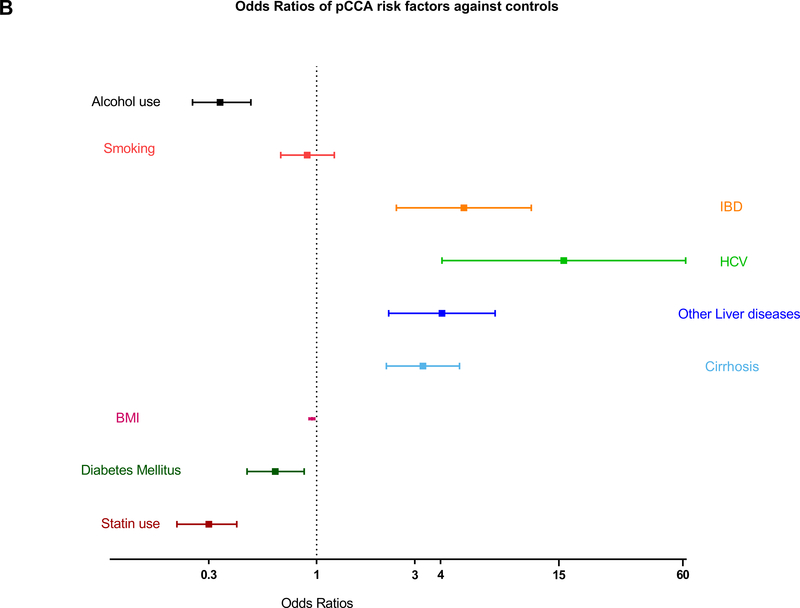
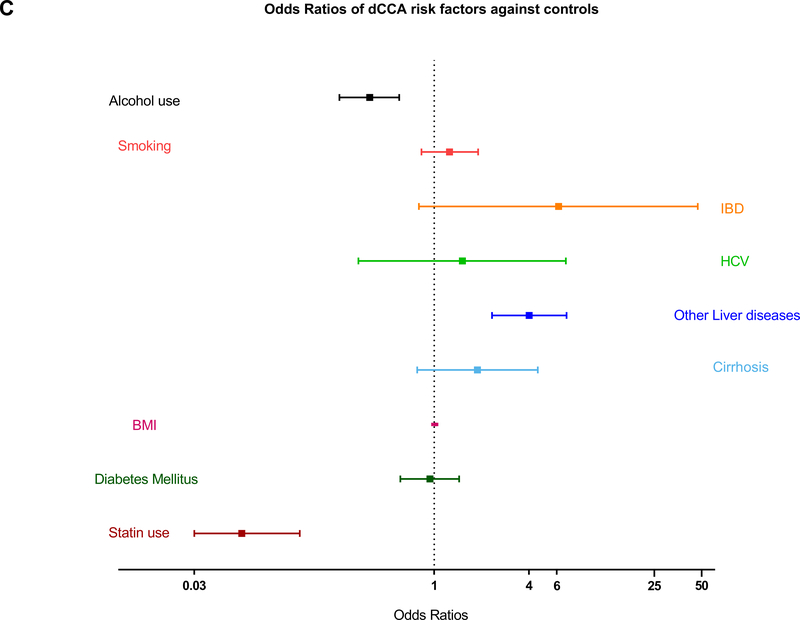
| IBD | 8.2 (4.17–16.15) | <0.0001 | 5.19 (2.44–11.02) | <0.001 | 6.16 (0.80–47.13) | 0.080 |
| Cirrhosis | 2.91 (2.02–4.19) | <0.0001 | 3.28 (2.18–4.94) | <0.001 | 1.88 (0.78–4.55) | 0.16 |
| HCV | 7.33 (2.94–18.31) | <0.0001 | 15.84 (4.06–61.87) | <0.001 | 1.51 (0.33–6.85) | 0.59 |
| Other liver disease | 4.01 (2.33–6.92) | <0.0001 | 4.06 (2.24–7.36) | <0.001 | 4.01 (2.33–6.92) | <0.001 |
| Smoking, n (%) | 1.00 (0.79–1.27) | 0.99 | 0.9 (0.67–1.22) | 0.49 | 1.25 (0.83–1.9) | 0.29 |
| Alcohol use, n (%) | 0.36 (0.28–0.47) | <0.0001 | 0.34 (0.25–0.48) | <0.0001 | 0.39 (0.25–0.6) | <0.0001 |
FIG. 1.
Forest plots showing ORs of risk factors in patients with ECC and its subtypes against their controls. (A,B) Patients with IBD, HCV, cirrhosis, and other liver diseases had significantly higher risk of ECC and pCCA, whereas patients with diabetes and history of statin use had lower risk of these cancers. (C) Patients with other liver diseases had significantly higher risk of dCCA, and those with a history of statin use had lower risk of dCCA.
Table 3.
Comparing different doses of statins and different statins among matched statin users in cases and controls
| Statin therapy | All ECC | *All controls | p-value | pCCA | pCCA controls | p-value | dCCA | dCCA controls | p-value |
|---|---|---|---|---|---|---|---|---|---|
| High intensity | 9 (13.4%) | 29 (10.5%) | 0.01 | 5 (11.6%) | 17 (8.6%) | 0.017 | 4 (16.7%) | 12 (15.0%) | 0.48 |
| Moderate intensity | 38 (56.7%) | 206 (74.4%) | 24 (55.8%) | 150 (76.1%) | 14 (58.3%) | 56 (70.0%) | |||
| Low intensity | 20 (29.9%) | 42 (15.2%) | 14 (32.6%) | 30 (15.2%) | 6 (25.0%) | 12 (15.0%) |
Risk factors for ECC subtypes:
Of the ECC cases, 259 were pCCA and 135 were dCCA. HCV is the most significant risk factor for pCCA (OR=15.84, 95% CI 4.06–61.87; p<0.001). IBD (OR=5.19, 95% CI 2.44–11.02; p<0.001), cirrhosis (OR=3.28, 95% CI 2.18–4.94; p<0.001) and other liver diseases including biliary tract stones (OR=4.06, 95% CI 2.24–7.36; p<0.001) also increased the risk of pCCA. Any statin use was associated with a 78% decrease in risk of ECC (OR=0.22, 95% CI 0.16–0.29; p<0.0001). Moderate intensity statin therapy use (OR=0.43, 95% CI 0.28–0.67; p<0.001) decreased the risk of pCCA (Table 4). Statin use for the duration of less than a year (OR=0.45, 95% CI 0.23–0.90; p=0.023) and 1–3 years (OR=0.45, 95% CI 0.22–0.93; p=0.032) also showed decreased risk of pCCA. Statin use for less than one year was associated with reduced risk of dCCA (OR= 0.27, 95% CI 0.10–0.73; p=0.010) and other liver diseases (OR=4.01, 95% CI 2.33–6.92; p<0.001) was the only significant risk factor causing increased risk. The difference in the magnitude of effect of risk factors for pCCA and dCCA and the accuracy of the study may also be affected by the number of cases in the study population and variations in pathogenesis of each subtype (Figures 1B & 1C).
Table 4.
Association between duration and intensity of statin use and risk of ECC
| Variable | All ECC vs Controls | pCCA vs Controls | dCCA vs Controls | |||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | p-value | Odds Ratio (95% CI) | p-value | Odds Ratio (95% CI) | p-value | |
| Statin use | ||||||
| None | reference | reference | reference | |||
| <1year | 0.38 (0.22–0.67) | 0.001 | 0.45 (0.23–0.90) | 0.023 | 0.27 (0.10–0.73) | 0.010 |
| 1–3years | 0.51 (0.29–0.90) | 0.020 | 0.45 (0.22–0.93) | 0.032 | 0.67 (0.26–1.72) | 0.41 |
| 3–5years | 1.14 (0.63–2.09) | 0.66 | 1.10 (0.51–2.36) | 0.80 | 1.21 (0.45–3.26) | 0.71 |
| >5years | 1.43 (0.94–2.19) | 0.097 | 1.19 (0.69–2.06) | 0.52 | 2.01 (0.99–4.08) | 0.053 |
| Statin therapy | ||||||
| None | reference | reference | reference | |||
| High intensity | 0.80 (0.45–1.43) | 0.45 | 0.79 (0.36–1.71) | 0.55 | 0.79 (0.32, 1.94) | 0.68 |
| Moderate intensity | 0.48 (0.34–0.67) | <0.001 | 0.43 (0.28–0.67) | <0.001 | 0.59 (0.33, 0.95) | 0.077 |
| Low intensity | 1.23 (0.78–1.92) | 0.38 | 1.25 (0.72–2.17) | 0.55 | 1.18 (0.53, 2.64) | 0.61 |
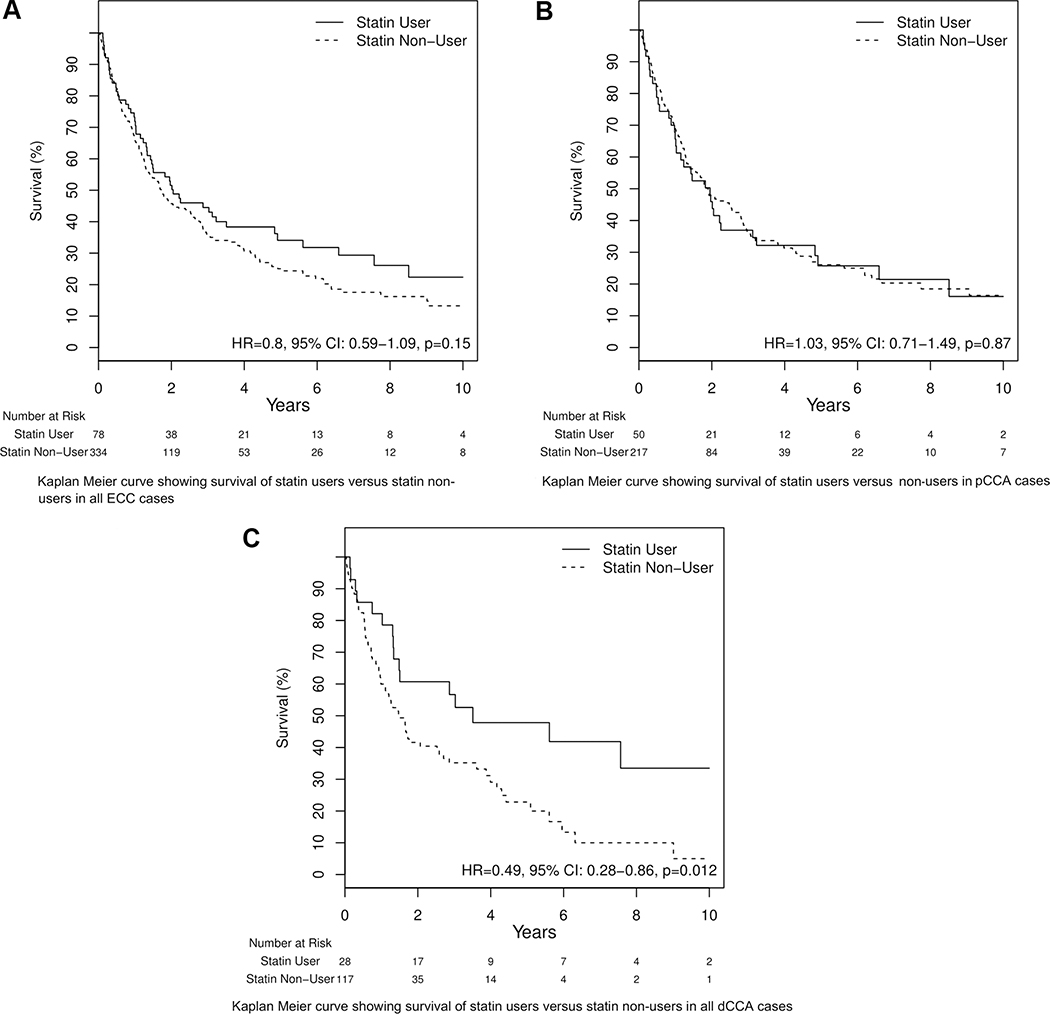
The effect of statin on cancer survival:
Significant improvement in overall survival with statin use was seen only in dCCA cases (HR=0.49, 95% CI 0.28−0.86, p=0.012) Figure 2(A), 2(B) & 2(C). Advanced age (HR=1.01, 95% CI 1–1.02; p=0.01) and diagnosis in the late stage of disease (HR=2.66 95% CI 1.28–5.52; p=0.009) are the two variables proven to have a negative impact on survival in all the pCCA cases. IBD, Cirrhosis, PSC, HCV and other liver diseases appeared to improve survival in pCCA patients in univariate analysis. Cirrhosis and HCV remained protective in pCCA patients after adjusting for other risk factors. Advanced age and cholangitis after cancer diagnosis late stage of cancer showed adverse effects on survival of dCCA patients and males and statin users with dCCA showed a tendency to better survival (HR=0.64, 95% CI 0.39–1.03; p=0.06). On multivariate analysis, any statin use was associated with overall better survival (HR=0.53, 95% CI 0.29–0.97; p=0.04) (Table 5).
FIG. 2.
Kaplan Meier curves showing survival in ECC and its subtypes for statin users against statin nonusers. (A-C) Number at risk represents the number of patients at risk of death because of cancer at time points 2, 4, 6, 8, and 10 years from the diagnosis of cancer; (C) patients with dCCA with a history of statin use had significantly lower risk of cancer compared with statin nonusers.
Table 5.
Survival analysis for all pCCA and dCCA cases (includes the unmatched cases)
| pCCA | dCCA | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Univariate | Multivariate | Univariate | Multivariate | ||||
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age | 1.04 (1.02–1.05) | <0.001 | 1.01 (1–1.02) | <0.03 | 1.03 (1.01–1.05) | 0.003 | 1.03 (1–1.05) | 0.01 |
| Sex, male | 0.94 (0.68–1.29) | 0.69 | - | - | 0.61 (0.39–0.95) | 0.027 | 0.73 (0.45–1.17) | 0.19 |
| BMI | 1.0 (0.98–1.03) | 0.68 | - | - | 1.0 (0.95–1.04) | 0.94 | - | - |
| Statin use | 1.03 (0.71–1.49) | 0.87 | - | - | 0.49 (0.28–0.86) | 0.01 | 0.53 (0.29–0.97) | 0.04 |
| DM | 0.95 (0.68–1.34) | 0.78 | - | - | 0.92 (0.59–1.42) | 0.92 | - | - |
| IBD | 0.50 (0.31–0.82) | 0.006 | 0.62 (0.3–1.29) | 0.19 | 0.58 (0.23–1.44) | 0.24 | - | - |
| Cirrhosis | 0.41 (0.28–0.60) | <0.001 | 0.56 (0.36–0.89) | 0.01 | 1.26 (0.58–2.73) | 0.56 | - | - |
| Cholangitis after diagnosis | 1.07 (0.78–1.48) | 0.68 | - | - | 1.76 (1.04–2.96) | 0.035 | 1.61 (0.93–2.79) | 0.09 |
| PSC | 0.48 (0.34–0.67) | <0.001 | 0.84 (0.54–1.31) | 0.44 | 0.80 (0.48–1.31) | 0.37 | - | - |
| HCV | 0.38 (0.19–0.79) | 0.009 | 0.34 (0.15–0.81) | 0.01 | 2.03 (0.50–8.32) | 0.32 | - | - |
| Other liver disease | 0.47 (0.28–0.78) | 0.003 | 1.17 (0.63–2.18) | 0.61 | 2.41 (0.87–6.65) | 0.091 | 2.53 (0.9–7.06) | 0.08 |
| Smoking, n (%) | 1.28 (0.82–2.01) | 0.27 | - | - | 0.86 (0.41–1.82) | 0.69 | - | - |
| Alcohol use, n (%) | 1.1 (0.69–1.75) | 0.69 | - | - | 0.83 (0.39–1.81) | 0.65 | - | - |
| Stage of cancer | ||||||||
| Early | Reference | - | Reference | - | Reference | - | Reference | - |
| Late | 1.73 (1.26–2.37) | <0.001 | 1.39 (1–1.93) | 0.048 | 1.83(0.97–3.46) | 0.063 | 2.66 (1.28–5.52) | 0.009 |
Discussion
We report a hospital/clinic based case control study evaluating the effect of statins on the risk of ECC development and survival and the risk factors in patients with hyperlipidemia who eventually progressed to ECC. DM, IBD, cirrhosis, HCV and other liver diseases were found to increase the risk of ECC at varying degrees, although their effects differed significantly in the two subtypes pCCA and dCCA thereby supporting the hypothesis that the two diseases have distinct pathogenesis and pathophysiology. The contributions of PSC and cholangitis in ECC development could not be determined as none of the controls had the diseases.
Prior studies have shown that DM increases the risk of both intrahepatic and extrahepatic cholangiocarcinoma. Population-based studies in Taiwan had investigated the effect of DM in ECC.(16, 17) They demonstrated that DM increases the risk of ECC and intrahepatic cholangiocarcinoma in women, with adjusted odds ratios of 1.48 and 1.22 respectively (17). However, in our cohort DM was associated with significantly decreased risks of ECC (OR=0.73, 95% CI 0.56–0.94; p=0.015) and pCCA (OR=0.63, 95% CI 0.46–0.87; p=0.005). No association could be established between DM and dCCA. Previous history of DM had no influence on the survival of ECC or its subtypes.
As expected, IBD was significantly associated with all types of ECC. 39 out of 41 matched cases and all five unmatched cases with IBD had coexisting PSC. As such, it was undetermined whether IBD is independently associated with increased risk of ECC or only in association with PSC. In a retrospective study, Gulamhusein et al., identified prolonged duration of IBD as an independent risk factor for cholangiocarcinoma.(18) An epidemiological review in Germany concluded that IBD and PSC, either alone or in association, increase the risk of Cholangiocarcinoma.(3) However, contradictory results have been shown in other studies in which it was unclear whether IBD was an independent risk factor or acts via association with PSC.(19) A US population based study on cholangiocarcinoma using the SEER-Medicare database hypothesized that a common pathway of chronic inflammation causes risk factors like IBD to increase risk of all types of cholangiocarcinoma.(19, 20)
Studies of the association of HCV with risk of cholangiocarcinoma have shown controversial results.(3, 19) Li et al., 2015 have shown that HCV infection is associated with a greater risk of intrahepatic cholangiocarcinoma (ICC) than ECC.(21) A population based study from Taiwan has reported increase in risk of cholangiocarcinoma with prior exposure to HCV, not differentiating between ICC and ECC.(16) By comparison, a hospital based case-control study had found significant association of HCV with ICC but not ECC.(22) Contrary to these results, another study showed a strong association of HCV infection with ECC and pCCA, but not with dCCA. (23) In our current study, the univariate Cox Proportional Hazard models showed that IBD, PSC, cirrhosis, HCV and other liver diseases are associated with improved survival in the pCCA subgroup. Cirrhosis and HCV remained protective after correcting for other significant risk factors. The differences are possibly related to increased surveillance in patients having known risk factors, leading to diagnosing the cancer at an early stage. This in turn might have led to patients being eligible to more suitable management options and better survival.
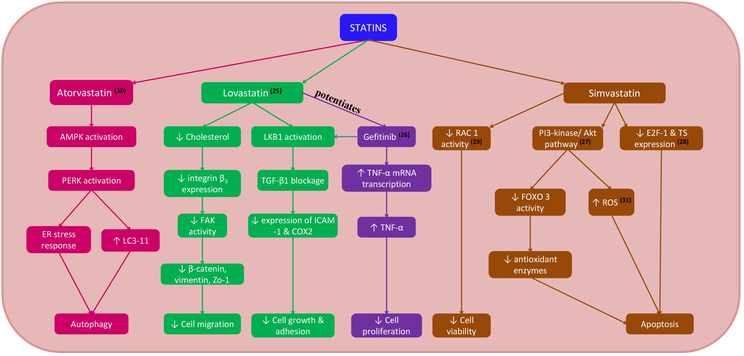
Statin use was significantly associated with a 4-fold reduction in risk of ECC. The risk reduction was observed among the two subtypes of ECC to varying degrees; 3-fold in pCCA and 16-fold in dCCA. Several lines of evidence point to the potential mechanic basis of these observations (Figure 3). These findings are supported by in vitro studies in which statins have been shown to inhibit cell proliferation and induce apoptosis of human cholangiocarcinoma cell lines. Atorvastatin induced apoptosis and autophagy through various mechanisms such as formation of autophagosomes in the cytoplasm, activation of AMP-activated protein kinase (AMPK), and through activated endoplasmic reticulum stress response. (24) Lovastatin suppressed the cell proliferation and migration in cholangiocarcinoma cell lines by reducing the expression of integrin ß3 and via activation of LKB1 - a tumor suppressor. (25) Combined treatment with lovastatin and gefitinib increased the expression of TNF-a mRNA and regulated cell cycle arrest via LKB1 activation in human cholangiocarcinoma cells. Gefitinib potentiated the anti-proliferative properties of lovastatin through enhancing TNF-a expression. (26) Similarly, Simvastatin induced cell death by necrosis in murine colon carcinoma cells. Treatment of colon carcinoma cells with simvastatin released reactive oxygen species (ROS), causing oxidative stress. ROS enhanced activation of Akt which further induced the generation of intracellular ROS. (27) Simvastatin also enhanced the cytotoxicity and apoptotic activity of 5-fluorouracil (5-FU) on bile duct cancer cells by downregulating the expression of E2F transcription factor 1 (E2F-1) and thymidylate synthase (TS), thereby sensitizing bile duct cancer cells to 5- FU. (28) The pleotropic effects of Simvastatin are further established by downregulation of Rac1 activity and disruption of Rac1 localization in lipid rafts through reduction of cholesterol levels in human cholangiocarcinoma cell lines. (29) Pitavastatin induced morphological changes such as decreased cellular diameter and reduced pseudopod formation in human ICC and ECC cell lines. The anti-proliferative activity of gemcitabine, 5-FU and cisplatin on human extrahepatic cholangiocarcinoma cell lines was potentiated with prior exposure to pitavastatin. (30) Although some of these effects are dose-dependent, our study showed that moderate intensity statin use has been significantly associated with decreased risk of ECC and its subtypes compared to low or high intensity statin use.
FIG. 3.
Intracellular pathways affected by statins in cholangiocarcinoma cells. Abbreviations: AMPK, adenosine monophosphate–activated protein kinase; COX2, cyclooxygenase-2; E2F-1, E2F transcription factor 1; ER, endoplasmic reticulum; FAK, focal adhesion kinase; FOXO, forkhead box O3; ICAM-1, intercellular adhesion molecule 1; LC3–11, light chain3–11; PERK, protein kinase R-like endoplasmic reticulum kinase; PI3, phosphatidylinositol 3-kinase; TGF-β1, transforming growth factor; TS, thymidylate synthase.
Our study showed that dCCA patients who ever used statins had better overall survival. This observation was not seen in pCCA patients. The beneficial effects with statin use were not significant when duration (<5years or >5 years) of usage was compared among different groups of ECC. However, multivariate analysis showed improved survival with any statin use in dCCA cases (HR= 0.53, 95% CI 0.29–0.97).
Limitations exist in our study despite the evidence on statin use and ECC prevention and the supportive data from above cancer studies. 67 cases out of 79 statin users and 277 out of 403 controls have data on statin dosage and duration of use. There is some data missing on the drug use which limited the ability to firmly establish the associations with cancer risk. Each subject reported using multiple statins for different duration of periods with different dosages. This had been another major challenge in our analysis. We tried to overcome that by regrouping the patients based on the intensity of statins to balance the effect of overall statin use. However, the combined effect of drug dosage and duration was not investigated. Intensity of statin was used alone to determine the association between the risk of ECC and statin use. Duration of statin use was used in survival analysis to limit the number of variables to corresponding events. Our cases had more than one risk factor that significantly conferred increase in risk of ECC. Therefore, associations with individual risk factors have to be studied in detail for confirmation. A randomized clinical trial to study the effect of statins in individuals with high risk factors separately for pCCA and dCCA like IBD, PSC and cirrhosis can further substantiate our findings and be a breakthrough in the cancer prevention. Despite the limitations, our study was the first to propose the association of statins and ECC in a large hospital based cohort.
In conclusion, statins reduce risk of ECC development and its subtypes. They also improve survival in dCCA patients. Since most of our study population was Caucasian, these findings have to be validated in other races and ethnicities as well.
Supplementary Material
Acknowledgments
Financial support: This publication was supported by Grant Numbers CA186566, CA 221205 and CA 210964 from the National Cancer Institute (NCI) and by The Cholangiocarcinoma Foundation to L.R. Roberts.
List of abbreviations:
- ECC
extrahepatic cholangiocarcinoma
- SD
standard deviation
- HCV
Hepatitis C virus
- pCCA
perihilar cholangiocarcinoma
- IBD
inflammatory bowel disease
- dCCA
distal cholangiocarcinoma
- PSC
primary sclerosing cholangitis
- LKB1
Liver kinase B1
- Rac1
Ras-related C3 botulinum toxin substrate 1
- ICD
International Classification of Diseases
- EMR
electronic medical records
- BMI
body mass index
- DM
diabetes mellitus
- CAD
coronary artery disease
- HBV
Hepatitis B Virus
- AHA
American Heart Association
- ICC
intrahepatic cholangiocarcinoma
- AMPK, AMP
activated protein kinase
- ERK
extracellular signal–regulated kinase
- 5-FU
5-fluorouracil
- E2F-1
E2F transcription factor 1
- TS
thymidylate synthase
- mTOR
mechanistic target of rapamycin
- RhoB
Rho-related GTP-binding protein
- HCC
hepatocellular carcinoma
- CRP
C-reactive protein
References
- 1.Yao KJ, Jabbour S, Parekh N, Lin Y, Moss RA. Increasing mortality in the United States from cholangiocarcinoma: an analysis of the National Center for Health Statistics Database. BMC Gastroenterol. 2016;16(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirstein MM, Vogel A. Epidemiology and Risk Factors of Cholangiocarcinoma. Visc Med. 2016;32(6):395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardinale V, Semeraro R, Torrice A, Gatto M, Napoli C, Bragazzi MC, et al. Intra-hepatic and extra-hepatic cholangiocarcinoma: New insight into epidemiology and risk factors. World journal of gastrointestinal oncology. 2010;2(11):407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.E JY, Lu SE, Lin Y, Graber JM, Rotter D, Zhang L, et al. Differential and Joint Effects of Metformin and Statins on Overall Survival of Elderly Patients with Pancreatic Adenocarcinoma: A Large Population-Based Study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2017;26(8):1225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang BZ, Chang JI, Li E, Xiang AH, Wu BU. Influence of Statins and Cholesterol on Mortality Among Patients With Pancreatic Cancer. Journal of the National Cancer Institute. 2017;109(5). [DOI] [PubMed] [Google Scholar]
- 6.Kozak MM, Anderson EM, von Eyben R, Pai JS, Poultsides GA, Visser BC, et al. Statin and Metformin Use Prolongs Survival in Patients With Resectable Pancreatic Cancer. Pancreas. 2016;45(1):64–70. [DOI] [PubMed] [Google Scholar]
- 7.Kim G, Jang SY, Han E, Lee YH, Park SY, Nam CM, et al. Effect of statin on hepatocellular carcinoma in patients with type 2 diabetes: A nationwide nested case-control study. International journal of cancer. 2017;140(4):798–806. [DOI] [PubMed] [Google Scholar]
- 8.McGlynn KA, Divine GW, Sahasrabuddhe VV, Engel LS, VanSlooten A, Wells K, et al. Statin use and risk of hepatocellular carcinoma in a U.S. population. Cancer epidemiology. 2014;38(5):523–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mc Menamin UC, Murray LJ, Hughes CM, Cardwell CR. Statin use and breast cancer survival: a nationwide cohort study in Scotland. BMC cancer. 2016;16:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arun BK, Gong Y, Liu D, Litton JK, Gutierrez-Barrera AM, Jack Lee J, et al. Phase I biomarker modulation study of atorvastatin in women at increased risk for breast cancer. Breast cancer research and treatment. 2016;158(1):67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang WY, Li CH, Lin CL, Liang JA. Long-term statin use in patients with lung cancer and dyslipidemia reduces the risk of death. Oncotarget. 2016;7(27):42208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardwell CR, Mc Menamin U, Hughes CM, Murray LJ. Statin use and survival from lung cancer: a population-based cohort study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015;24(5):833–41. [DOI] [PubMed] [Google Scholar]
- 13.Vogel TJ, Goodman MT, Li AJ, Jeon CY. Statin treatment is associated with survival in a nationally representative population of elderly women with epithelial ovarian cancer. Gynecologic oncology. 2017;146(2):340–5. [DOI] [PubMed] [Google Scholar]
- 14.Couttenier A, Lacroix O, Vaes E, Cardwell CR, De Schutter H, Robert A. Statin use is associated with improved survival in ovarian cancer: A retrospective population-based study. PLoS One. 2017;12(12):e0189233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889–934. [DOI] [PubMed] [Google Scholar]
- 16.Chang JS, Tsai CR, Chen LT. Medical risk factors associated with cholangiocarcinoma in Taiwan: a population-based case-control study. PLoS One. 2013;8(7):e69981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang YJ, Wu AT, Chiou HY, Chuang MT, Meng TC, Chien LN, et al. Interactive role of diabetes mellitus and female sex in the risk of cholangiocarcinoma: A population-based nested case-control study. Oncotarget. 2017;8(4):6642–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gulamhusein AF, Eaton JE, Tabibian JH, Atkinson EJ, Juran BD, Lazaridis KN. Duration of Inflammatory Bowel Disease Is Associated With Increased Risk of Cholangiocarcinoma in Patients With Primary Sclerosing Cholangitis and IBD. The American journal of gastroenterology. 2016;111(5):705–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54(1):173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrick JL, Yang B, Altekruse SF, Van Dyke AL, Koshiol J, Graubard BI, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: A population-based study in SEER-Medicare. PLoS One. 2017;12(10):e0186643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, Hu B, Zhou ZQ, Guan J, Zhang ZY, Zhou GW. Hepatitis C virus infection and the risk of intrahepatic cholangiocarcinoma and extrahepatic cholangiocarcinoma: evidence from a systematic review and meta-analysis of 16 case-control studies. World J Surg Oncol. 2015;13:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaib YH, El-Serag HB, Nooka AK, Thomas M, Brown TD, Patt YZ, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a hospital-based case-control study. The American journal of gastroenterology. 2007;102(5):1016–21. [DOI] [PubMed] [Google Scholar]
- 23.Choi J, Ghoz HM, Peeraphatdit T, Baichoo E, Addissie BD, Harmsen WS, et al. Aspirin use and the risk of cholangiocarcinoma. Hepatology. 2016;64(3):785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang PM, Liu YL, Lin YC, Shun CT, Wu MS, Chen CC. Inhibition of autophagy enhances anticancer effects of atorvastatin in digestive malignancies. Cancer research. 2010;70(19):7699–709. [DOI] [PubMed] [Google Scholar]
- 25.Yang SH, Lin HY, Changou CA, Chen CH, Liu YR, Wang J, et al. Integrin beta3 and LKB1 are independently involved in the inhibition of proliferation by lovastatin in human intrahepatic cholangiocarcinoma. Oncotarget. 2016;7(1):362–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang SH, Lin HY, Chang VH, Chen CC, Liu YR, Wang J, et al. Lovastatin overcomes gefitinib resistance through TNF-alpha signaling in human cholangiocarcinomas with different LKB1 statuses in vitro and in vivo. Oncotarget. 2015;6(27):23857–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qi XF, Kim DH, Yoon YS, Kim SK, Cai DQ, Teng YC, et al. Involvement of oxidative stress in simvastatin-induced apoptosis of murine CT26 colon carcinoma cells. Toxicology letters. 2010;199(3):277–87. [DOI] [PubMed] [Google Scholar]
- 28.Cai JP, Chen W, Hou X, Liang LJ, Hao XY, Yin XY. Simvastatin enhances the chemotherapeutic efficacy of S-1 against bile duct cancer: E2F-1/TS downregulation might be the mechanism. Anti-cancer drugs. 2013;24(10):1020–9. [DOI] [PubMed] [Google Scholar]
- 29.Miller T, Yang F, Wise CE, Meng F, Priester S, Munshi MK, et al. Simvastatin stimulates apoptosis in cholangiocarcinoma by inhibition of Rac1 activity. Dig Liver Dis. 2011;43(5):395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamigaki M, Sasaki T, Serikawa M, Inoue M, Kobayashi K, Itsuki H, et al. Statins induce apoptosis and inhibit proliferation in cholangiocarcinoma cells. Int J Oncol. 2011;39(3):561–8. [DOI] [PubMed] [Google Scholar]
- 31.Buranrat B, Senggunprai L, Prawan A, Kukongviriyapan V. Simvastatin and atorvastatin as inhibitors of proliferation and inducers of apoptosis in human cholangiocarcinoma cells. Life Sci. 2016;153:41–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.