Abstract
Background:
3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) is used both recreationally and therapeutically. Little is known about the factors influencing inter- and intra-individual differences in the acute response to MDMA. Effects of other psychoactive substances have been shown to be critically influenced by personality traits and mood state before intake.
Methods:
We pooled data from 10 randomized, double-blind, placebo-controlled, cross-over studies performed in the same laboratory in 194 healthy subjects receiving doses of 75 or 125mg of MDMA. We investigated the influence of drug dose, body weight, sex, age, drug pre-experience, genetics, personality and mental state before drug intake on the acute physiological and psychological response to MDMA.
Results:
In univariable analyses, the MDMA plasma concentration was the strongest predictor for most outcome variables. When adjusting for dose per body weight, we found that (a) a higher activity of the enzyme CYP2D6 predicted lower MDMA plasma concentration, (b) a higher score in the personality trait “openness to experience” predicted more perceived “closeness”, a stronger decrease in “general inactivation”, and higher scores in the 5D-ASC (5 Dimensions of Altered States of Consciousness Questionnaire) scales “oceanic boundlessness” and “visionary restructuralization”, and (c) subjects with high “neuroticism” or trait anxiety were more likely to have unpleasant and/or anxious reactions.
Conclusions:
Although MDMA plasma concentration was the strongest predictor, several personality traits and mood state variables additionally explained variance in the response to MDMA. The results confirm that both pharmacological and non-pharmacological variables influence the response to MDMA. These findings may be relevant for the therapeutic use of MDMA.
Keywords: MDMA, prediction, safety, set and setting, individual differences
Introduction
3,4-methylenedioxymethamphetamine (MDMA) is known as the recreational drug “ecstasy”, but has recently gained interest as an adjunct in psychotherapy for post-traumatic stress disorder or other anxiety disorders (Danforth et al., 2018; Mithoefer et al., 2010, 2019; Oehen et al., 2013). The acute effects of MDMA typically include enhanced mood, openness, trust and enhanced empathy (Hysek et al., 2014a; Schmid et al., 2014). Accordingly, MDMA is classified as an “entactogen” and its effects differ from those of pure stimulants (Bershad et al., 2016a; Dolder et al., 2018; Nichols, 1986; Schmid et al., 2014).
Most of the MDMA effects were found to be dose dependent (Bedi and de Wit, 2011; Vizeli and Liechti, 2017) and overall comparable across different laboratories (Kirkpatrick et al., 2014). However, like any psychoactive drug, the response to MDMA is also influenced by non-pharmacological variables – also often referred to as set and setting (Hartogsohn, 2016; Kirkpatrick and de Wit, 2015). Set includes the personality, current mood state, preparation, expectation and intention of the person having the experience, whereas setting refers to the physical, social and cultural environment in which the experience takes place (Hartogsohn, 2016; Leary et al., 1963).
The influence of set and setting has been traditionally studied in the context of psychedelic drugs as responses to these drugs are thought to be particularly dependent on them (Carhart-Harris et al., 2018). Thus, several studies have demonstrated that – in addition to drug dose – personality traits, such as absorption and neuroticism, as well the mental state before drug intake shape the response to psychedelics (Carhart-Harris et al., 2018; Haijen et al., 2018; Studerus et al., 2012).
The psychoactive effects of MDMA and psychedelics, such as psilocybin, partly overlap (Holze et al., 2019). Both drugs are serotonergic substances interacting with the serotonin 5-HT2A receptor and serotonin transporter (Hysek et al., 2012c; Rickli et al., 2016) and are used at least in part for similar therapeutic indications (Mithoefer et al., 2016). It is therefore conceivable that responses to these substances are also at least in part similarly shaped by set and setting. However, this has not been systematically investigated. So far, few studies have investigated the relative contribution of pharmacological and non-pharmacological variables to the effects of MDMA. Among the studied predictors were sex (e.g., Liechti et al., 2001; Pardo-Lozano et al., 2012; Simmler et al., 2011), drug pre-experience (Bedi and de Wit, 2011; Kirkpatrick et al., 2014), social context (Kirkpatrick and de Wit, 2015), pharmacokinetics (Pardo-Lozano et al., 2012; Vizeli et al., 2017) and genetics (Bershad et al., 2016b; Schmid et al., 2016; Vizeli and Liechti, 2018; Vizeli et al., 2017, 2018a, 2018b). However, these studies each assessed only a small number of potential predictors, did not adjust for potentially confounding variables, and did not assess the importance of different variables. Additionally, the sample sizes were mostly rather small for such analyses.
In view of these methodological limitations and the current interest in MDMA research, including phase 3 trials (Mithoefer et al., 2019), investigations of predictor variables that may moderate MDMA effects are of high interest. Expanding the knowledge on such influencing variables could potentially not only increase the safety of the use of MDMA in research and psychotherapy, it could also inform treatment planning in MDMA-assisted psychotherapy. For example, it could help to set the environment and to prepare and select the patients in such a way that therapeutic effects are increased and the risk of adverse effects is minimized.
Thus, the present study investigated the relative effects of a large number of predictor variables, including age, sex, drug dose, body weight, previous drug experience, genetics, personality and mood before intake on the acute physiological and psychological response to MDMA. The present analysis is based on data of 10 controlled experimental studies with a total sample size of 194 healthy subjects tested in the same laboratory. This study is the first to evaluate potential predictors of the MDMA response covering a wide range of variables.
Methods
Study design
This is a pooled analysis of the raw data from 10 double-blind, placebo-controlled, crossover studies in healthy human subjects, of all of which have previously been described (Dolder et al., 2018; Holze et al., 2019; Hysek and Liechti, 2012; Hysek et al., 2011, 2012a, 2012b, 2012c, 2014b; Schmid et al., 2015a, 2015b). The studies were conducted at the University Hospital Basel from 2009 to 2018 and include a total of 194 healthy subjects. Seven studies each included 16 subjects (total of 112 subjects) who received 125 mg MDMA twice within four experimental sessions (MDMA alone, MDMA + pre-treatment with a medication, placebo and pre-treatment alone) (Hysek and Liechti, 2012; Hysek et al., 2011, 2012a, 2012b, 2012c, 2014b; Schmid et al., 2015b). In three additional studies, subjects received MDMA once within three or four experimental sessions (MDMA alone, placebo, and one or two other substances) (Dolder et al., 2018; Holze et al., 2019; Schmid et al., 2015a). Of these, one used an MDMA dose of 75 mg (n = 30) (Schmid et al., 2015a) and the others used 125 mg (n = 24 and n = 28) (Dolder et al., 2018; Holze et al., 2019). In the present analysis, only data from the MDMA-alone and placebo sessions were used. In all of the pooled studies, the washout periods between the single-dose administrations of MDMA were at least 7 days to exclude carry-over effects. The studies were all registered at ClinicalTrials.gov (NCT00886886, NCT00990067, NCT01136278, NCT01270672, NCT01386177, NCT01465685, NCT01771874, NCT01951508, NCT01616407, NCT03019822).
All of the studies were approved by the local ethics committee and conducted in accordance with the Declaration of Helsinki. The use of MDMA was authorized by the Swiss Federal Office for Public Health (BAG), Bern, Switzerland. Written informed consent was obtained from all of the participants. All of the subjects were paid for their participation. Detailed pharmacokinetic and safety data from these studies have been published elsewhere (Schmid et al., 2016; Vizeli and Liechti, 2017; Vizeli et al., 2017).
Test sessions were conducted in a quiet hospital research ward with no more than two research subjects present per session. The participants were comfortably lying in hospital beds and were mostly listening to music and not engaging in physical activities. MDMA was administered without food in the fasting state in the morning at 8:00−9:00 a.m. A small standardized lunch was served at 12:00−1:00 p.m.
Subjects
A total of 194 (97 female) healthy subjects, aged 18–45 years (mean ± SD = 25.1 ± 4 years), were recruited from the University of Basel campus and participated in the study. One genotyping sample was missing, and three participants did not give consent for genotyping. The mean ± SD body weight was 69 ± 10 kg (range: 46–97 kg). Exclusion criteria included a history of psychiatric disorders, physical illness, a lifetime history of illicit drug use more than 10 times (with the exception of past cannabis use), illicit drug use within the past 2 months and illicit drug use during the study. Drug screens were conducted before the test sessions as reported in detail elsewhere (Hysek and Liechti, 2012; Hysek et al., 2012a, 2012b, 2012c). Seventy-five subjects had prior illicit substance experiences (1–8 times), of which 41 subjects had previously used MDMA (1–5 times), 18 subjects had previously used amphetamine or methamphetamine (1–2 times), 15 subjects had previously used cocaine (1–4 times), 10 subjects had previously used lysergic acid diethylamide (1–2 times), and 15 subjects had previously used psilocybin (1–4 times). Compared to a normative sample, the included subjects scored significantly higher in the personality trait of “openness to experience” as measured by the NEO Five Factor Inventory (NEO-FFI) (33.2 ± 6.3 v. 24.5 ± 5.5; t = 19.7, p < 0.001; Körner et al., 2008).
Study drug
(±)MDMA hydrochloride (Lipomed AG, Arlesheim, Switzerland) was administered orally at a single dose of 75 or 125 mg prepared as gelatin capsules (25 or 100 mg, Bichsel Laboratories, Interlaken, Switzerland). Male and female subjects received the same doses of MDMA irrespective of their body weight as it is done in therapeutic studies (Mithoefer et al., 2010; Oehen et al., 2013). The dose per body weight (mean ± SD) was 1.7 ± 0.4 mg/kg (range: 0.8–2.7 mg/kg).
Predictor variables
Effects of MDMA are dose- and body-weight-dependent (Vizeli and Liechti, 2017; Schmid et al., 2014). Therefore, dose divided by body weight was included as covariate in the analysis. This also accounted for the higher mg/kg dose of MDMA in females compared with males due to the lower body weight in women compared to men.
From the socio-demographic predictor variable domain, we included sex and age as predictors. Sex was included because sex differences in the MDMA experience were reported in several controlled studies even after adjusting for differences in dosing (Bedi and de Wit, 2011; Liechti et al., 2001; Simmler et al., 2011; Vizeli and Liechti, 2017). Age was included since younger age was associated with more unpleasant acute effects of psilocybin (Studerus et al., 2012), while no data is available on MDMA.
Individual metabolic differences in the enzymes metabolizing MDMA influence the exposure to MDMA and thereby its acute effects. Specifically, the activity of cytochrome P450 enzymes has been shown to alter MDMA concentrations and concomitant subjective and cardiovascular responses (de la Torre et al., 2012; Schmid et al., 2016; Vizeli et al., 2017). Thus, we included the CYP2D6 genetic activity score (Hicks et al., 2013; Schmid et al., 2016) as an additional predictor variable. We did not include measures of other CYP enzyme activity as these have been shown to have no or only very small effects on the response to MDMA (Vizeli et al., 2017). Likewise, other potential pharmacogenetic predictors were not included, because they also showed no or only minimal effects on the acute response to MDMA (Bershad et al., 2016b; Vizeli and Liechti, 2018; Vizeli et al., 2018a, 2018b).
Although all subjects had no or only very limited previous experiences with psychoactive substances (0–5 times), the number of MDMA consumptions prior to participation was included as a continuous predictor variable in the analysis, since MDMA effects have been reported to change with long-term use (Parrott, 2005) and more experienced users experienced smaller drug effects than inexperienced persons (Kirkpatrick et al., 2014).
Mood states prior to the administration of a psychoactive substance influence its response as previously shown for psilocybin in a similar study (Studerus et al., 2012). Therefore, we included ratings on the Adjective Mood Rating Scale (AMRS) (Janke and Debus, 1978) to assess mood states prior to the MDMA administration. Sixty adjectives were rated on 4-point Likert scales and items were grouped into six main scales: “Performance-Related Activity”, “General Inactivation”, “Extraversion-Introversion”, “General Well-Being”, “Emotional Excitability” and “Anxiety-Depressiveness”, “Extraversion” and “Introversion” were analyzed separately.
Personality traits were assessed using the NEO-FFI (Borkenau and Ostendorf, 2008) which contains 60 self-referent statements rated on a 5-point Likert scale. The NEO-FFI covers the personality traits “Neuroticism”, “Extraversion”, “Openness to experience”, “Agreeableness” and “Conscientiousness”. Subjects completed the questionnaire as part of the screening procedure at the start of the study. Finally, the trait scale of the State-Trait Anxiety Inventory (STAI-T) was included (Spielberger et al., 1970). This self-assessment questionnaire contains 20 statements describing anxiety as a stable personality trait.
Response variables
Blood samples for the pharmacokinetic response were collected in lithium heparin tubes 0, 0.33, 0.67, 1, 1.5, 2, 3, 4 and 6 h after administration of MDMA or placebo and immediately centrifuged. Plasma was stored at –20°C until analysis. Plasma concentrations of MDMA were determined as previously described (Hysek et al., 2012c). The area under the concentration–time curve (AUC) from 0 to 6 h after dosing was calculated following the trapezoidal rule as a measure of total exposure to MDMA.
Blood pressure, heart rate, and body temperature were assessed repeatedly before and 0, 0.33, 0.67, 1, 1.5, 2, 2.5, 3, 4, 5 and 6 h after MDMA or placebo administration. Systolic and diastolic blood pressure and heart rate were measured using an automatic oscillometric device (OMRON Healthcare Europe NA, Hoofddorp, Netherlands). The measurements were performed in duplicate and after a resting time of at least 5 min. The averages were calculated for analysis. Core (tympanic) temperature was measured using a GeniusTM 2 ear thermometer (Tyco Healthcare Group LP, Watertown, NY, USA). The mean arterial pressure (MAP) was calculated as diastolic blood pressure + (systolic blood pressure – diastolic blood pressure)/3. For the different autonomic response measures, we used the highest values (Emax) as outcome variable for the analysis because high cardiovascular stimulation or body temperature are the clinically relevant potentially adverse outcomes associated with MDMA use (Liechti, 2014; Liechti et al., 2005; Vizeli and Liechti, 2017).
The subjective response to MDMA was assessed using psychometric scales. Visual Analog Scales (VASs) (Hysek et al., 2011; Hysek et al., 2012c) were used before and 0.33, 0.67, 1, 1.5, 2, 2.5, 3, 4, 5 and 6 h after MDMA or placebo administration. VASs for “any drug effect”, “good drug effect”, “bad drug effect”, “high mood”, “drug liking” and “stimulated” were presented as 100-mm horizontal lines (0–100%), marked from “not at all” on the left to “extremely” on the right. The VASs “closeness”, “openness” and “talkative” were bidirectional (±50%). Additionally, the AMRS was administered 1.25, 2 and 5 h after administration of MDMA or placebo. The response on each VAS and AMRS subscale was included into the analysis as area under the effect-time-curve (AUEC) value, reflecting the overall response throughout the study day.
The 5D-ASC (altered state of consciousness) questionnaire (Dittrich et al., 2010) was additionally administered 6 h after drug administration to retrospectively assess alterations in waking consciousness induced by MDMA. The 5D-ASC consists of 94 visual analog scale items and measures three etiology-independent and two etiology-dependent dimensions of altered states of consciousness. To reduce multiple testing, we only included the etiology-independent dimensions (i.e., Oceanic Boundlessness, Dread of Ego Dissolution, and Visionary Restructuralization). Furthermore, since these three dimensions are heterogenous constructs (Studerus et al., 2010) and since we were most interested in adverse drug reactions, we additionally included the more homogenous “impaired control and cognition” and “anxiety” subscales constructed by Studerus et al. (2010).
Statistical analyses
All data were analyzed using the R language and environment for statistical computing (R Core Team, 2019). Since some of the predictor and response variables contained missing data (see Supplementary Table 1), we first performed multiple imputation (MI) using the Multivariate Imputation via Chained Equations (MICE) package in R (Buuren and Groothuis-Oudshoorn, 2010). We chose this method because it yields unbiased parameter estimates and standard errors under a “missing at random” (MAR) or “missing completely at random” (MCAR) missing data mechanism and maximizes statistical power by using all available information (Enders, 2010). The assumption of MAR was plausible in this study because the missing data mostly resulted from different study designs among the pooled studies. We generated 20 imputations of the missing values such that 20 completed datasets were obtained to avoid a potential power falloff from an insufficient number of imputations (Graham et al., 2007). The analyses of interest were then conducted in each completed data set and parameter estimates were pooled according to Rubin’s rules (Little and Rubin, 2019), except for the LASSO models (see below).
To account for the clustering in our data arising from pooling across studies, we used linear mixed effects models in which the intercepts were allowed to vary randomly across studies. For each combination of predictor and response variable, an adjusted and unadjusted model was fitted using the R package nlme (Pinheiro et al., 2019). In the unadjusted model, only the predictor of interest was included in the fixed effects part of the model, whereas in the adjusted model “dose per body weight” was additionally included. Predictor and response variables were z-transformed before inclusion in the models, such that the estimated regression coefficients were fully standardized and comparable across predictors and responses. In each model, the amount of variance explained by each fixed effects predictor was determined by calculating the semi-partial R2 using r2beta function in r2glmm package with the Kenward–Roger approach (Jaeger et al., 2017). To account for multiple testing, p-values were corrected across all significance tests using the Benjamini–Hochberg procedure (Benjamini and Hochberg, 1995).
To identify the best subset of predictors for each response variable and to determine relative importance of these predictors, we applied the least absolute shrinkage and selection operator (LASSO) using the R package penalized (Goeman, 2018). LASSO conducts both variable selection and regularization (i.e., shrinkage of regression coefficients) in order to optimize the predictive accuracy and interpretability of the model. It has been shown that variable selection with the LASSO is often more accurate than with traditional methods, such as stepwise methods (Tibshirani, 1997).
For each response variable, a LASSO model was developed according to the following procedure. First, the optimal shrinkage parameter of each model was determined by performing grid search. For each lambda in the grid, bootstrapping with 50 iterations was performed and the average predictive performance (i.e., mean squared error) across all out-of-bag samples was calculated using the machine learning in R (mlr) package (Bischl et al., 2016). Second, the lambda value producing the highest out-of-bag predictive performance was chosen as the optimal lambda value and used for the final LASSO model fitted on the whole sample. Since it is currently unclear how to combine LASSO models across multiply imputed datasets and since the amount of missing data in our data set was relatively small, only single imputation was used for the LASSO models. Furthermore, for simplicity, we did not account for a potential clustering in our data in these analyses.
Results
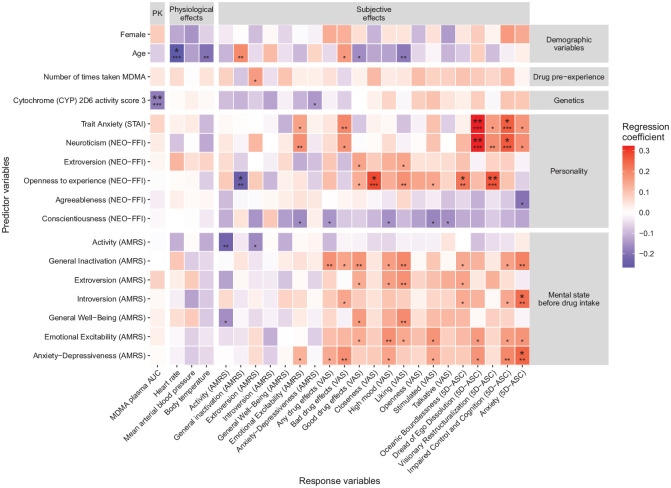
The size of the fully standardized regression coefficients and statistical significance of each predictor variable for each outcome variable in the unadjusted and adjusted analyses are shown in Supplementary Figure 1 and Figure 1, respectively.
Figure 1.
Standardized regression coefficients and statistical significance of each predictor variable in the linear mixed effects models adjusting for drug dose per body weight. Smaller asterisks show the uncorrected statistical significance. Bigger asterisks show the significance after correction for multiple testing across all 20 × 25 = 500 significance tests using the Benjamini–Hochberg procedure (Benjamini and Hochberg, 1995). *p < 0.05, **p < 0.01, ***p < 0.001. AUC, area under curve; AMRS, Adjective Mood Rating Scale; ASC, altered state of consciousness; NEO-FFI, NEO Five Factor Inventory; STAI, State-Trait Anxiety Inventory; VAS, Visual Analog Scale.
In the unadjusted (i.e., univariable) analyses, MDMA plasma concentration was the strongest predictor of the physiological and psychological response to MDMA. It was significantly associated with 16 of 24 outcome variables when not correcting for multiple testing and with eight variables after the correction. Among these, the most statistically significant association was observed with the VAS scale “any drug effect” (standardized regression coefficient β = 0.48, corrected p < 0.001, semi-partial R2 = 0.35), followed by mean arterial blood pressure (β = 0.35, corrected p < 0.001, semi-partial R2 = 0.25), VAS “liking” (β = 0.36, corrected p < 0.001, semi-partial R2 = 0.17), VAS “good drug effects” (β = 0.35, corrected p < 0.001, semi-partial R2 = 0.19), VAS “stimulated” (β = 0.3, corrected p = 0.001, semi-partial R2 = 0.44), VAS “high mood” (β = 0.29, corrected p = 0.002, semi-partial R2 = 0.43), ARMS “introversion” (β = 0.24, corrected p = 0.032, semi-partial R2 = 0.21) and the 5D-ASC scale “oceanic boundlessness” (β = 0.25, corrected p = 0.045, semi-partial R2 = 0.06). Furthermore, MDMD plasma concentration was the strongest predictor of the VAS scale “any drug effect”. However, despite its superior predictive power, we did not use this variable as a covariate in the adjusted analysis because we wanted to predict the response to MDMA already at the time of drug intake, when MDMA plasma concentration is not yet known. Instead, we used the drug dose per kg body as a covariate, as drug dose and body weight were both strong determinants of the MDMA plasma concentration (see Supplementary Figure 1).
After adjusting for drug dose per body weight and correcting for multiple testing, sex was no longer predictive for the MDMA plasma concentration. However, a genetically determined low CYP2D6 activity still predicted a larger MDMA plasma concentration (β = −0.19, corrected p < 0.001, semi-partial R2 = 0.1). Additionally, older age was still significantly associated with a smaller change in heart rate in response to MDMA (β = −0.27, corrected p = 0.019, semi-partial R2 = 0.07). More “openness to experience” in the NEO-FFI predicted a stronger decrease in “general inactivation” (β = −0.25, corrected p = 0.042, semi-partial R2 = 0.06), larger positive changes in the VAS rating of “closeness” (β = 0.28, corrected p = 0.019, semi-partial R2 = 0.08) and larger 5D-ASC ratings of “oceanic boundlessness” (β = 0.22, corrected p = 0.042, semi-partial R2 = 0.06) and “visionary restructuralization” after MDMA (β = 0.27, corrected p = 0.006, semi-partial R2 = 0.09). Subjects who scored higher in “neuroticism” in the NEO-FFI or “trait anxiety” in the STAI-T were more likely to experience “dread of ego dissolution” (β = 0.32 and 0.32, respectively; corrected p = 0.001 and 0.004, respectively, semi-partial R2 = 0.12 and 0.11, respectively) and “impaired control and cognition” (β = 0.26 and 0.26, respectively; corrected p = 0.014 and 0.042, respectively, semi-partial R2 = 0.08 and 0.07, respectively) as measured by the 5D-ASC. More “anxiety-depressiveness” (ARMS) and “introversion” immediately before drug intake predicted higher scores in the 5D-ASC “anxiety” subscale (β = 0.25 and 0.23, respectively; corrected p = 0.042 and 0.042, respectively, semi-partial R2 = 0.06 and 0.05, respectively).
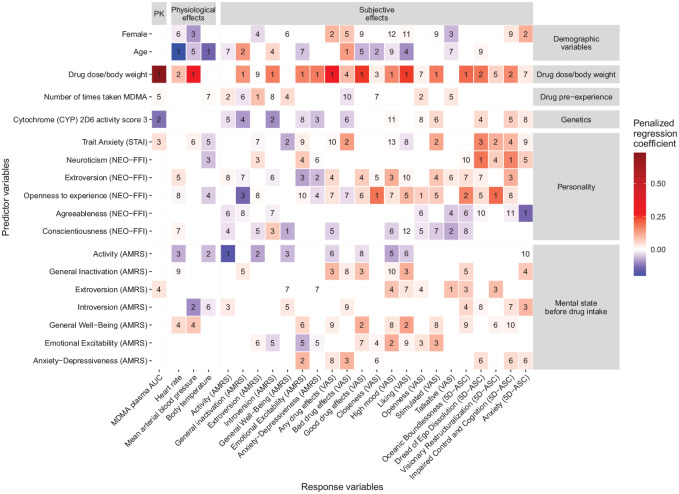
The regression coefficients of the LASSO models are shown in Figure 2. On average, 8.6 predictors (range: 5–13) were selected for each response variables. Dose per body weight was the most frequently selected predictor variable (i.e., it was identified as an important predictor for 21 of 25 response variables). For 12 response variables, it also had the largest absolute standardized regression coefficient and thus was the most important predictor. However, while it was the most important predictor for the VAS scales “any drug effects”, “good drug effects”, “high mood”, “stimulated” and “liking”, as well as “oceanic boundlessness” in the 5D-ASC, it was less important for the prediction of unpleasant or anxious reactions to MDMA. Specifically, the most important predictor for the VAS scale “bad drug effects” was older age, followed by “trait anxiety” in the STAI-T and ”anxiety-depressiveness” in the ARMS immediately before drug intake. Furthermore, “neuroticism” in the NEO-FFI was the most important predictor for the 5D-ASC scales “dread of ego dissolution” and “impaired control and cognition”.
Figure 2.
Size of the penalized regression coefficients and rank of importance of the predictor variables in the LASSO models. As one Lasso model was developed for each response variable, each column in the tile plot reports the results of one Lasso model. The rank of importance of each predictor for each outcome was determined by ranking the predictor variables according to their absolute size of the regression coefficients in each Lasso model. AUC, area under curve; AMRS, Adjective Mood Rating Scale; ASC, altered state of consciousness; NEO-FFI, NEO Five Factor Inventory; STAI, State-Trait Anxiety Inventory; VAS, Visual Analog Scale.
Discussion
This study investigated the influence of 20 predictor variables on the physiological and psychological response to MDMA in healthy humans. We found that physiological as well as most psychological effects were most strongly dependent on MDMA plasma levels, which in turn was most strongly dependent on drug dose and body weight. When adjusted for drug dose per body weight and corrected for multiple testing, only age and the genetically determined activity of the enzyme CYP2D6 had an influence on the physiological response to MDMA. Specifically, younger subjects responded to MDMA with a stronger increase in heart rate than older subjects and a higher activity of the enzyme CYP2D6 predicted lower MDMA plasma concentration. With regard to psychological effects, subjects with a high score in “openness to experience” responded with more “closeness”, a stronger decrease in “general inactivation” and higher scores in the 5D-ASC scales “oceanic boundlessness” and “visionary restructuralization” in response to MDMA, whereas subjects with high “neuroticism” or trait anxiety experienced more “anxious ego dissolution” and “impaired control and cognition”. Furthermore, being more anxious-depressive or introverted immediately before MDMA intake was associated with more anxiety in response to MDMA.
Our finding that the MDMA plasma concentration – and indirectly MDMA dose per body weight – is the most important predictor for the response to MDMA is in line previous dose-response studies (Bedi and de Wit, 2011; Harris et al., 2002; Kolbrich et al., 2008; Kuypers et al., 2017). However, as can be seen in Supplementary Figure 1, there were also several outcome variables that were not or only weakly predicted by MDMA plasma concentration. In general, MDMA plasma level tended to be most predictive for positive or neutral MDMA effects, as measured by the VAS scales “any drug effects”, “good drug effects”, “liking” and “stimulated” or the 5D-ASC scale “oceanic boundlessness”, and less so for negatively experienced MDMA effects, such as the VAS scale “bad drug effects” or the 5D-ASC scales “anxiety”, “impaired control and cognition” or “anxious ego dissolution”. This supports the study of Kolbrich et al. (2008), which found that MDMA dose correlated with subjective “energy level”, “feelings of closeness to others”, “mind racing”, “heightened senses” and “high”, but not with “ability to concentrate”. We also found that the MDMA plasma concentration was positively associated with mean arterial blood pressure and heart rate, but not with body temperature, which is again in line with the study of Kolbrich et al. (2008).
To investigate the effects of all other predictors adjusted for the amount of drug, we used drug dose per body weight rather than MDMA plasma concentration as a covariate since the latter is not known in advance and unlikely to be determined in the clinical setting. Drug dose per body weight was shown to be a good proxy for MDMA plasma concentration, because, when adjusting for drug dose per body weight, only the genetically-determined enzyme CYP2D6 activity contributed to the prediction of MDMA plasma concentration. In line with this finding, CYP2D6 poor metabolizers have previously been shown to have higher blood plasma concentrations than extensive/normal metabolizers (de la Torre et al., 2005; Schmid et al., 2016).
After adjusting for drug dose per body weight, sex did not significantly influence the effects of MDMA indicating that the stronger response in women as shown in the unadjusted analyses was due to lower body weight and correspondingly higher drug dose per body weight in women. Thus, this study could not confirm earlier studies reporting sex-differences in acute physiological and subjective responses to MDMA even after adjusting for body weight (for review, see Allott and Redman, 2007). For example, Liechti et al. (2001) reported that women experienced both more intense positive and negative subjective drug effects, but particularly perceptive changes, thought disturbances, and fear of loss of body control, whereas men showed a higher increase in blood pressure in response to MDMA. Other studies suggested that women may be particularly vulnerable to acute negative subjective and cardiovascular (Bedi and de Wit, 2011; Pardo-Lozano et al., 2012; Vizeli and Liechti, 2017), acute biological (Simmler et al., 2011) and subacute negative effects of MDMA (Verheyden et al., 2002). On the other hand, the largest study to date including 220 individuals (44% female) from three different laboratories, who had received MDMA in controlled experiments (Kirkpatrick et al., 2014), could not detect any gender differences in acute cardiovascular and subjective responses to MDMA in line with the current study.
A more consistent effect was observed for age, which inversely correlated with the MDMA-induced heart rate elevation. This finding might be explained by an age-associated decrease of adrenergic receptor sensitivity and density in cardiac muscle (Xiao and Lakatta, 1992) and supports the theory of an adrenergic receptor mediated cardiovascular effect of MDMA that can be inhibited by carvedilol (Hysek et al., 2012b). However, we do not think that this relationship is of high clinical relevance or use in practice. Our data also suggested that older age was associated with a lower increase in body temperature, less “liking”, “good drug effects” and decrease in “general inactivation” and more “bad drug effects”. An increase in “bad drug effects” with older age would be in contrast to the response to the psychedelic drug psilocybin, which has been shown to be more often challenging in younger age (Studerus et al., 2012). However, these associations should be interpreted cautiously as they did not withstand correction for multiple testing and result from a rather limited dataset in terms of age variation. Individuals over 45 were excluded from the study and could react differently, especially since comorbidities also increase with age.
Previous MDMA experience showed no moderating effect on the response to MDMA in our study, which was rather surprising since recreational MDMA users frequently report experiencing the strongest effects the first time they ever tried MDMA (Davison and Parrott, 1997; Solowij et al., 1992) and developing tolerance to the positive subjective effects of MDMA over time (Parrott, 2005; Verheyden et al., 2003). Accordingly, one laboratory based multicenter study has found modest evidence that greater prior use of MDMA is associated with lesser ratings of feeling any drug effect (Kirkpatrick et al., 2014). However, this association was not consistently observed across all study centers and a further laboratory-based study could also not detect it (Bedi and de Wit, 2011). It is important to note that in the present study, 79% of subjects were MDMA naïve and the others had a maximum of only five previous MDMA experiences. Therefore, the influence of heavier past MDMA use could not be assessed in the present study. Notably, patients in clinical trials using MDMA will, similar to the present study population, likely have no to little experience in using MDMA, enhancing the relevance of the present data for the clinical situation. In contrast, the majority of other controlled studies using MDMA in healthy subjects has been conducted in persons with considerably greater MDMA use in the past.
Regarding the influence of personality, we found that subjects with more “openness to experience” experienced more “closeness”, a stronger decrease in “general inactivation” and more “oceanic boundlessness” and “visionary restructuralization” in response to MDMA. “Oceanic boundlessness”, as measured by the 5D-ASC questionnaire, describes happiness-inducing aspects of the experience and includes experiences of oneness with the self and the world and liberation from the restrictive aspects of space and time, whereas “visionary restructuralization” covers phenomena of altered perception and meaning (Dittrich et al., 2010). Our finding that “openness to experience” is positively associated with these subjective effects of MDMA is consistent with its conceptual overlap with the personality traits of “absorption” (Glisky et al., 1991), which is associated with differential responsivity to various ASC induction procedures, including hypnosis, meditation, cannabis and electromyograph biofeedback (Pekala et al., 1985). Accordingly, “absorption” has also shown to be one of the most important predictors of pleasant and “mystical-type” experiences in response to psychedelic drugs (Haijen et al., 2018), including psilocybin (Studerus et al., 2012) and ayahuasca (Bresnick and Levin, 2006).
While more “openness to experience” seemed to intensify pleasant and prosocial effects of MDMA, we found that more pronounced “neuroticism” or “trait anxiety” led to more “dread of ego dissolution” and “impaired control and cognition” in response to MDMA. Furthermore, a higher score in “anxiety-depressiveness” or “introversion” immediately before MDMA intake increased the likelihood of anxious responses to MDMA. This is again consistent with the responsivity to psychedelic drugs, since a higher score in “neuroticism” trait or “emotional excitability” before drug administration were found to forecast more “challenging experiences” after taking psychedelic substances (Barrett et al., 2017; Haijen et al., 2018; Studerus et al., 2012). However, it should be noted that anxiety scores in response to MDMA relative to other subjective response measures are rather small and only 7% of subjects reported anxiety as an acute adverse effect in the high dose condition (i.e., 125mg) (Vizeli and Liechti, 2017). Thus, challenging experiences are considered less likely to occur after MDMA than after high dose of psychedelics.
While this study suggests that personality traits such as “openness to experience” and “neuroticism” influence the MDMA experience, there is some indication that the effect goes in the opposite direction too and that this may potentially act as a therapeutic mechanism of change. For example, it has been found that MDMA-assisted psychotherapy in patients with Post-Traumatic Stress Disorder (PTSD) led to long-lasting increased “openness to experience” and decreased “neuroticism” and that changes in “openness to experience” but not “neuroticism” played a moderating role in the relationship between reduced PTSD symptoms and MDMA treatment (Wagner et al., 2017). A similar persisting effect on personality traits was also observed after the ingestion of psilocybin or LSD (Erritzoe et al., 2018; Lebedev et al., 2016; MacLean et al., 2011). Thus, the finding that subjects who take MDMA with an “open mind” display more potentially therapeutic beneficial effects could suggest that patients progressively benefit from multiple MDMA-assisted psychotherapy sessions, as they likely become more open to the experience over time.
Strengths and limitations
Besides the present work, we are not aware of any other study investigating the predictors of the physiological and psychological response to MDMA to a similar extent in a controlled setting. There are a few studies with a small number of potential predictors, but their results were not adjusted for potentially confounding variables and did not display the importance of different variables.
Limitations of the present study include, first of all, the young, mostly MDMA-naïve, healthy study population. Thus, the findings only partly translate to patients with psychiatric disorders showing clearly greater psychopathology and presumably a greater likelihood of adverse psychological responses to MDMA. Furthermore, even though this study population mirrors the general population in illicit drug experience better than most previous studies with MDMA, it still includes more illicit drug experiences than one would expect within the general population (European Monitoring Centre for Drugs and Drug Addiction, 2020). Another deviation between our study sample and the general population is that our clinical trials might have attracted people with higher ratings in “openness to experience”. Second, only one of two different doses of MDMA was administered (i.e., 75 or 125 mg) and the vast majority of subjects (85%) received the higher dose, which is also commonly used in clinical trials with MDMA. It is therefore conceivable that with more varied drug doses the relative contribution of MDMA plasma levels to the response to MDMA would have been even higher. Third, the influence of the “physical and social environment” which is an important moderator of the effects of psychedelics (Hartogsohn, 2016; Leary et al., 1963), and possibly also of MDMA (Kirkpatrick and de Wit, 2015), could not be studied. For example, effects of MDMA may be different in a therapeutic setting with high engagement of the therapist or in a large party setting etc. However, this study was conducted in a highly standardized research setting with little variation, leaving minimal scope for research on this potential predictor. Finally, while the present study is informative on the acute effects of MDMA, little can be extrapolated to address longer-term effects.
Conclusions
Taken together, we could demonstrate that both pharmacological and non-pharmacological variables play a role in the effects of MDMA. While the response to MDMA was strongly dependent on drug dose and largely unaffected by age, sex and previous use of MDMA, we found that a high score in the personality trait “openness to experience” increased the intensity of pleasant and prosocial effects of MDMA, whereas having high scores in the personality traits of “neuroticism” and state anxiety and being anxious or depressive immediately before drug intake increased the likelihood of unpleasant or anxious reactions to MDMA. These associations are strikingly similar to those previously observed in psychedelics (Haijen et al., 2018; Studerus et al., 2012) and could potentially inform the planning of MDMA-assisted psychotherapy.
Supplemental Material
Supplemental material, sj-docx-1-jop-10.1177_0269881121998322 for Prediction of MDMA response in healthy humans: a pooled analysis of placebo-controlled studies by Erich Studerus, Patrick Vizeli, Samuel Harder, Laura Ley and Matthias E Liechti in Journal of Psychopharmacology
Supplemental material, sj-docx-2-jop-10.1177_0269881121998322 for Prediction of MDMA response in healthy humans: a pooled analysis of placebo-controlled studies by Erich Studerus, Patrick Vizeli, Samuel Harder, Laura Ley and Matthias E Liechti in Journal of Psychopharmacology
Footnotes
Author contributions: ES and PV contributed equally to this work.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MEL is a consultant for Mind Medicine, Inc. The other authors declare no conflicts of interests. Knowhow and data associated with this work and owned by the University Hospital Basel were licensed by Mind Medicine, Inc. after study completion. Mind Medicine, Inc. had no role in financing, planning, or conducting the present study or the present publication.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Erich Studerus  https://orcid.org/0000-0003-4233-0182
https://orcid.org/0000-0003-4233-0182
Matthias E. Liechti  https://orcid.org/0000-0002-1765-9659
https://orcid.org/0000-0002-1765-9659
Supplemental material: Supplemental material for this article is available online.
References
- Allott K, Redman J. (2007) Are there sex differences associated with the effects of ecstasy/3,4-methylenedioxymethamphetamine (MDMA)? Neurosci Biobehav Rev 31: 327–347. [DOI] [PubMed] [Google Scholar]
- Barrett FS, Johnson MW, Griffiths RR. (2017) Neuroticism is associated with challenging experiences with psilocybin mushrooms. Pers Individ Dif 117: 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, de Wit H. (2011) Individual differences in acute responses to MDMA in humans: Effects of sex and past ecstasy use. Open Addict J 4: 6–7. [Google Scholar]
- Benjamini Y, Hochberg Y. (1995) Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc B 57: 289–300. [Google Scholar]
- Bershad AK, Miller MA, Baggott MJ, et al. (2016. a) The effects of MDMA on socio-emotional processing: does MDMA differ from other stimulants? J Psychopharmacol 30: 1248–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershad AK, Weafer JJ, Kirkpatrick MG, et al. (2016. b) Oxytocin receptor gene variation predicts subjective responses to MDMA. Soc Neurosci 11: 592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischl B, Lang M, Kotthoff L, et al. (2016) mlr: Machine Learning in R. J Mach Learn Res 17: 5938–5942. [Google Scholar]
- Borkenau P, Ostendorf F. (2008) NEO-Fünf-Faktoren-Inventar (NEO-FFI) nach Costa und McCrae. 2nd ed. Göttingen: Hogrefe. [Google Scholar]
- Bresnick T, Levin R. (2006) Phenomenal qualities of ayahuasca ingestion and its relation to fringe consciousness and personality. J Conscious Stud 13: 5–24. [Google Scholar]
- Buuren Sv, Groothuis-Oudshoorn K. (2010) mice: Multivariate imputation by chained equations in R. J Stat Softw 45: 1–68. [Google Scholar]
- Carhart-Harris RL, Roseman L, Haijen E, et al. (2018) Psychedelics and the essential importance of context. J Psychopharmacol 32: 725–731. [DOI] [PubMed] [Google Scholar]
- Danforth AL, Grob CS, Struble C, et al. (2018) Reduction in social anxiety after MDMA-assisted psychotherapy with autistic adults: A randomized, double-blind, placebo-controlled pilot study. Psychopharmacology (Berl) 235: 3137–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison D, Parrott AC. (1997) Ecstasy (MDMA) in recreational users: Self-reported psychological and physiological effects. Hum Psychopharmacol 12: 221–226. [Google Scholar]
- de la Torre R, Farre M, Mathuna BO, et al. (2005) MDMA (ecstasy) pharmacokinetics in a CYP2D6 poor metaboliser and in nine CYP2D6 extensive metabolisers. Eur J Clin Pharmacol 61: 551–554. [DOI] [PubMed] [Google Scholar]
- de la Torre R, Yubero-Lahoz S, Pardo-Lozano R, et al. (2012) MDMA, methamphetamine, and CYP2D6 pharmacogenetics: What is clinically relvant? Front Genet 3: 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich A, Lamparter D, Maurer M. (2010) 5D-ASC: Questionnaire for the Assessment of Altered States of Consciousness. A Short Introduction. Zurich: PSIN PLUS. [Google Scholar]
- Dolder PC, Muller F, Schmid Y, et al. (2018) Direct comparison of the acute subjective, emotional, autonomic, and endocrine effects of MDMA, methylphenidate, and modafinil in healthy subjects. Psychopharmacology (Berl) 235: 467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK. (2010) Applied Missing Data Analysis. New York: Guilford Press. [Google Scholar]
- Erritzoe D, Roseman L, Nour MM, et al. (2018) Effects of psilocybin therapy on personality structure. Acta Psychiatr Scand 138: 368–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction (2020) European Drug Report 2020: Key Issues. Luxembourg: Publications Office of the European Union. [Google Scholar]
- Glisky ML, Tataryn DJ, Tobias BA, et al. (1991) Absorption, openness to experience, and hypnotizability. J Pers Soc Psychol 60: 263–272. [DOI] [PubMed] [Google Scholar]
- Goeman JJ. (2018) Penalized R package, version 0.9-51. [Google Scholar]
- Graham JW, Olchowski AE, Gilreath TD. (2007) How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci 8: 206–213. [DOI] [PubMed] [Google Scholar]
- Haijen E, Kaelen M, Roseman L, et al. (2018) Predicting responses to psychedelics: A prospective study. Front Pharmacol 9: 897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DS, Baggott M, Mendelson JH, et al. (2002) Subjective and hormonal effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology (Berl) 162: 396–405. [DOI] [PubMed] [Google Scholar]
- Hartogsohn I. (2016) Set and setting, psychedelics and the placebo response: An extra-pharmacological perspective on psychopharmacology. J Psychopharmacol 30: 1259–1267. [DOI] [PubMed] [Google Scholar]
- Hicks JK, Swen JJ, Thorn CF, et al. (2013) Clinical pharmacogenetics implementation consortium guideline for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants. Clin Pharmacol Ther 93: 402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holze F, Vizeli P, Mueller F, et al. (2019) Distinct acute effects of LSD, MDMA, and D-amphetamine in healthy subjects. Neuropsychopharmacology 45: 462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Brugger R, Simmler LD, et al. (2012. a) Effects of the alpha2-adrenergic agonist clonidine on the pharmacodynamics and pharmacokinetics of 3,4-methylenedioxymethamphetamine in healthy volunteers. J Pharmacol Exp Ther 340: 286–294. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Liechti ME. (2012) Effects of MDMA alone and after pretreatement with reboxetine, duloxetine, clonidine, carvedilol, and doxazosin on pupillary light reflex. Psychopharmacology (Berl) 224: 363–376. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Schmid Y, Rickli A, et al. (2012. b) Carvedilol inhibits the cardiostimulant and thermogenic effects of MDMA in humans. Br J Pharmacol 166: 2277–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Schmid Y, Simmler LD, et al. (2014. a) MDMA enhances emotional empathy and prosocial behavior. Soc Cogn Affect Neurosci 9: 1645–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Simmler LD, Ineichen M, et al. (2011) The norepinephrine transporter inhibitor reboxetine reduces stimulant effects of MDMA (“ecstasy”) in humans. Clin Pharmacol Ther 90: 246–255. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Simmler LD, Nicola V, et al. (2012. c) Duloxetine inhibits effects of MDMA (“ecstasy”) in vitro and in humans in a randomized placebo-controlled laboratory study. PLoS One 7: e36476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Simmler LD, Schillinger N, et al. (2014. b) Pharmacokinetic and pharmacodynamic effects of methylphenidate and MDMA administered alone and in combination. Int J Neuropsychopharmacol 17: 371–381. [DOI] [PubMed] [Google Scholar]
- Jaeger BC, Edwards LJ, Das K, et al. (2017) An R2 statistic for fixed effects in the generalized linear mixed model. J Appl Stat 44: 1086–1105. [Google Scholar]
- Janke W, Debus G. (1978) Die Eigenschaftswörterliste. Göttingen: Hogrefe. [Google Scholar]
- Kirkpatrick MG, Baggott MJ, Mendelson JE, et al. (2014) MDMA effects consistent across laboratories. Psychopharmacology (Berl) 231: 3899–3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, de Wit H. (2015) MDMA: A social drug in a social context. Psychopharmacology 232: 1155–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbrich EA, Goodwin RS, Gorelick DA, et al. (2008) Physiological and subjective responses to controlled oral 3,4-methylenedioxymethamphetamine administration. J Clin Psychopharmacol 28: 432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner A, Drapeau M, Albani C, et al. (2008) Deutsche Normierung des NEO-Fünf-Faktoren-Inventars (NEO-FFI). Zeitschrift für Medizinische Psychologie 17: 133–144. [Google Scholar]
- Kuypers KPC, Dolder PC, Ramaekers JG, et al. (2017) Multifaceted empathy of healthy volunteers after single doses of MDMA: A pooled sample of placebo-controlled studies. J Psychopharmacol 31: 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary T, Litwin GH, Metzner R. (1963) Reactions to psilocybin administered in a supportive environment. J Nerv Ment Dis 137: 561–573. [DOI] [PubMed] [Google Scholar]
- Lebedev AV, Kaelen M, Lövdén M, et al. (2016) LSD-induced entropic brain activity predicts subsequent personality change. Hum Brain Mapp 37: 3203–3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME. (2014) Effects of MDMA on body temperature in humans. Temperature 1: 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Gamma A, Vollenweider FX. (2001) Gender differences in the subjective effects of MDMA. Psychopharmacology (Berl) 154: 161–168. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Kunz I, Kupferschmidt H. (2005) Acute medical problems due to Ecstasy use: Case-series of emergency department visits. Swiss Med Wkly 135: 652–657. [DOI] [PubMed] [Google Scholar]
- Little RJ, Rubin DB. (2019) Statistical Analysis with Missing Data. New York: Wiley. [Google Scholar]
- MacLean KA, Johnson MW, Griffiths RR. (2011) Mystical experiences occasioned by the hallucinogen psilocybin lead to increases in the personality domain of openness. J Psychopharmacol 25: 1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithoefer MC, Feduccia AA, Jerome L, et al. (2019) MDMA-assisted psychotherapy for treatment of PTSD: Study design and rationale for phase 3 trials based on pooled analysis of six phase 2 randomized controlled trials. Psychopharmacology(Berl) 236: 2735–2745. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mithoefer MC, Grob CS, Brewerton TD. (2016) Novel psychopharmacological therapies for psychiatric disorders: Psilocybin and MDMA. Lancet Psychiatry 3: 481–488. [DOI] [PubMed] [Google Scholar]
- Mithoefer MC, Wagner MT, Mithoefer AT, et al. (2010) The safety and efficacy of ±3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: The first randomized controlled pilot study. J Psychopharmacol 25: 439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE. (1986) Differences between the mechanism of action of MDMA, MBDB, and the classic hallucinogens. Identification of a new therapeutic class: Entactogens. J Psychoactive Drugs 18: 305–313. [DOI] [PubMed] [Google Scholar]
- Oehen P, Traber R, Widmer V, et al. (2013) A randomized, controlled pilot study of MDMA (±3,4-methylenedioxymethamphetamine)-assisted psychotherapy for treatment of resistant, chronic post-traumatic stress disorder (PTSD). J Psychopharmacol 27: 40–52. [DOI] [PubMed] [Google Scholar]
- Pardo-Lozano R, Farre M, Yubero-Lahoz S, et al. (2012) Clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”): The influence of gender and genetics (CYP2D6, COMT, 5-HTT). PLoS One 7: e47599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott AC. (2005) Chronic tolerance to recreational MDMA (3,4-methylenedioxymethamphetamine) or Ecstasy. J Psychopharmacol 19: 71–83. [DOI] [PubMed] [Google Scholar]
- Pekala RJ, Wenger CF, Levine RL. (1985) Individual differences in phenomenological experience: States of consciousness as a function of absorption. J Pers Soc Psychol 48: 125–132. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, et al. (2019) nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-140. Available from https://CRAN.R-project.org/package=nlme
- R Core Team (2019) R: A Language and Environment for Statistical Computing. [Google Scholar]
- Rickli A, Moning OD, Hoener MC, et al. (2016) Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. Eur Neuropsychopharmacol 26: 1327–1337. [DOI] [PubMed] [Google Scholar]
- Schmid Y, Hysek CM, Preller KH, et al. (2015. a) Effects of methylphenidate and MDMA on appraisal of erotic stimuli and intimate relationships. Eur Neuropsychopharmacol 25: 17–25. [DOI] [PubMed] [Google Scholar]
- Schmid Y, Hysek CM, Simmler LD, et al. (2014) Differential effects of MDMA and methylphenidate on social cognition. J Psychopharmacol 28: 847–856. [DOI] [PubMed] [Google Scholar]
- Schmid Y, Rickli A, Schaffner A, et al. (2015. b) Interactions between bupropion and 3,4-methylenedioxymethamphetamine in healthy subjects. J Pharmacol Exp Ther 353: 102–111. [DOI] [PubMed] [Google Scholar]
- Schmid Y, Vizeli P, Hysek CM, et al. (2016) CYP2D6 function moderates the pharmacokinetics and pharmacodynamics of 3,4-methylene-dioxymethamphetamine in a controlled study in healthy individuals. Pharmacogenet Genom 26: 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler LD, Hysek CM, Liechti ME. (2011) Sex differences in the effects of MDMA (ecstasy) on plasma copeptin in healthy subjects. J Clin Endocrinol Metab 96: 2844–2850. [DOI] [PubMed] [Google Scholar]
- Solowij N, Hall W, Lee N. (1992) Recreational MDMA use in Sydney: a profile of ‘Ecstacy’ users and their experiences with the drug. Br J Addict 87: 1161–1172. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RC, Lusheme RE. (1970) Manual for the Stait Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Studerus E, Gamma A, Kometer M, et al. (2012) Prediction of psilocybin response in healthy volunteers. PLoS One 7: e30800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studerus E, Gamma A, Vollenweider FX. (2010) Psychometric evaluation of the altered states of consciousness rating scale (OAV). PLoS One 5: e12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibshirani R. (1997) The lasso method for variable selection in the Cox model. Stat Med 16: 385–395. [DOI] [PubMed] [Google Scholar]
- Verheyden SL, Hadfield J, Calin T, et al. (2002) Sub-acute effects of MDMA (+/-3,4-methylenedioxymethamphetamine, “ecstasy”) on mood: Evidence of gender differences. Psychopharmacology (Berl) 161: 23–31. [DOI] [PubMed] [Google Scholar]
- Verheyden SL, Henry JA, Curran HV. (2003) Acute, sub-acute and long-term subjective consequences of ‘ecstasy’ (MDMA) consumption in 430 regular users. Hum Psychopharmacol 18: 507–517. [DOI] [PubMed] [Google Scholar]
- Vizeli P, Liechti ME. (2017) Safety pharmacology of acute MDMA administration in healthy subjects. J Psychopharmacol 31: 576–588. [DOI] [PubMed] [Google Scholar]
- Vizeli P, Liechti ME. (2018) Oxytocin receptor gene variations and socio-emotional effects of MDMA: A pooled analysis of controlled studies in healthy subjects. PLoS One 13: e0199384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizeli P, Meyer Zu, Schwabedissen HE, Liechti ME. (2018. a) No major role of norepinephrine transporter gene variations in the cardiostimulant effects of MDMA. Eur J Clin Pharmacol 74: 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizeli P, Meyer Zu Schwabedissen HE, Liechti ME. (2018. b) Role of serotonin transporter and receptor gene variations in the acute effects of MDMA in healthy subjects. ACS Chem Neurosci. Epub ahead of print 27 December 2018. DOI: 10.1021/acschemneuro.8b00590. [DOI] [PubMed] [Google Scholar]
- Vizeli P, Schmid Y, Prestin K, et al. (2017) Pharmacogenetics of ecstasy: CYP1A2, CYP2C19, and CYP2B6 polymorphisms moderate pharmacokinetics of MDMA in healthy subjects. Eur Neuropsychopharmacol 27: 232–238. [DOI] [PubMed] [Google Scholar]
- Wagner MT, Mithoefer MC, Mithoefer AT, et al. (2017) Therapeutic effect of increased openness: Investigating mechanism of action in MDMA-assisted psychotherapy. J Psychopharmacol 31: 967–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao RP, Lakatta EG. (1992) Deterioration of beta-adrenergic modulation of cardiovascular function with aging. Ann N Y Acad Sci 673: 293–310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jop-10.1177_0269881121998322 for Prediction of MDMA response in healthy humans: a pooled analysis of placebo-controlled studies by Erich Studerus, Patrick Vizeli, Samuel Harder, Laura Ley and Matthias E Liechti in Journal of Psychopharmacology
Supplemental material, sj-docx-2-jop-10.1177_0269881121998322 for Prediction of MDMA response in healthy humans: a pooled analysis of placebo-controlled studies by Erich Studerus, Patrick Vizeli, Samuel Harder, Laura Ley and Matthias E Liechti in Journal of Psychopharmacology