Abstract
Among the various chemicals that are commonly used as pesticides, organophosphates (OPs), and to a lesser extent, carbamates, are most frequently associated with adverse long-term neurological consequences. OPs and the carbamate, pyridostigmine, used as a prophylactic drug against potential nerve agent attacks, have also been implicated in Gulf War Illness (GWI), which is often characterized by chronic neurological symptoms. While most OP- and carbamate-based pesticides, and pyridostigmine are relatively potent acetylcholinesterase inhibitors (AChEIs), this toxicological mechanism is inadequate to explain their long-term health effects, especially when no signs of acute cholinergic toxicity are exhibited. Our previous work suggests that a potential mechanism of the long-term neurological deficits associated with OPs is impairment of axonal transport (AXT); however, we had not previously evaluated carbamates for this effect. Here we thus evaluated the carbamate, physostigmine (PHY), a highly potent AChEI, on AXT using an in vitro neuronal live imaging assay that we have previously found to be very sensitive to OP-related deficits in AXT. We first evaluated the OP, diisopropylfluorophosphate (DFP) (concentration range 0.001-10.0 µM) as a reference compound that we found previously to impair AXT and subsequently evaluated PHY (concentration range 0.01-100 nM). As expected, DFP impaired AXT in a concentration-dependent manner, replicating our previously published results. In contrast, none of the concentrations of PHY (including concentrations well above the threshold for impairing AChE) impaired AXT. These data suggest that the long-term neurological deficits associated with some carbamates are not likely due to acute impairments of AXT.
Keywords: Pesticide, insecticide, organophosphate, carbamate, gulf war illness, agriculture
Introduction
Pesticides are used effectively worldwide to improve farming productivity and to combat vector borne illnesses. Their widespread use has become an environmental concern, however, since a variety of deleterious neurodevelopmental effects and long-term neurological symptoms have been associated with repeated exposures to pesticides.1-3 Among the various chemicals that are currently used as pesticides, organophosphates (OPs) are most commonly associated with adverse long-term health consequences, although other pesticide classes such as carbamates (particularly carbofuran) have also been implicated.4 One case where a large number of people are known to have been exposed to both OPs and carbamates is the first gulf war, which is associated with a host of chronic symptoms now collectively known as “Gulf War Illness” (GWI). GWI symptoms include fatigue, headaches, respiratory difficulties, musculoskeletal pain, gastrointestinal distress, skin rashes, and a variety of psychiatric and neurological symptoms such as mood alterations, attentional deficits, and learning and memory impairments.5,6
While the etiology of GWI symptoms may be multifactorial and potentially related to excessive heat, stress, vaccinations, smoke from oil well fires, infectious organisms, etc., 1 plausible explanation for the neurological symptoms is exposure to 1 or more acetylcholinesterase (AChE) inhibitors.7 It was estimated that at least 41 000 military personnel were exposed to insecticides that contained either carbamate or OP-based AChEIs.8 In addition to OP-based pesticides, exposures to OP-nerve agents may have also been a contributing factor to GWI since as many as 100 000 soldiers may have been exposed to low (ie, non-acutely toxic) levels of sarin/cyclosarin following the destruction of an Iraqi munitions storage complex at Khamisiyah, Iraq, in March 1991.9 Finally, up to 250 000 soldiers received the carbamate AChEI pyridostigmine bromide as a prophylactic measure against potential nerve agent exposure.
If exposures to carbamate or OP-based chemicals contributed to the etiology of GWI, the underlying mechanism for the long-term symptoms is unclear since there were no reports of widespread cholinergic-based symptoms in soldiers that would normally be associated with AChE inhibition after acute toxic exposures. OPs can affect hundreds of enzymes in addition to AChE, as well as neurotransmitter receptors, and other proteins10 and they can influence multiple neurobiological processes including inflammation,11,12 oxidative stress,13,14 and autoimmunity.15-17 Likewise, some carbamates (eg, carbofuran) can negatively affect multiple protein targets in addition to AChE and elicit neurophysiological and neurobehavioral deficits4 as well as oxidative stress, endocrine disruption, and immunotoxicity.18
In both neuronal culture and animal studies in our laboratory,19-22 we have identified OP-related axonal transport (AXT) deficits as a potential non-cholinesterase mechanism for the long-term deleterious neurological effects of OPs. These observations may be important given the fundamental nature of AXT to neuronal health and its impairment in multiple neurologic and neurodegenerative illnesses, including diseases where cognitive impairments are observed. The efficient transport of a variety of cargoes (eg, synaptic vesicles, neurofilaments, cytosolic proteins) along axons in the anterograde direction, that is, from the cell body to synaptic terminals, in the opposite (retrograde) direction (eg, signaling endosomes, autophagosomes, injury signals, growth factors) and bi-directionally (eg, mitochondria, some endosomal populations, lysosomes, mRNAs) is essential to neuronal survival and function.23 To date, we have not evaluated the effects of carbamates on AXT, however. The notion that pyridostigmine would serve as a source of GWI symptoms,5 especially the neurological symptoms, is somewhat perplexing given its charged quaternary ammonium structure which would be expected to prevent it from readily crossing the blood brain barrier (BBB). There is evidence, however, to suggest that exposure to stress can increase BBB permeability24 and accordingly, it has been hypothesized that combat stress may have facilitated central penetration of pyridostigmine in Gulf War soldiers. However, experimental studies on the central effects of combined exposure to stress and pyridostigmine in rodents are conflicting with some studies demonstrating increases in BBB permeability and inhibition of brain AChE activity (associated with pyridostigmine) while others have not confirmed this observation.25-27 It is also important to note that carbamate-based AChEIs including pyridostigmine, neostigmine, and rivastigmine have been used therapeutically for many years for conditions including myasthenia gravis and Alzheimer’s disease,28 and to our knowledge, GWI-like symptoms have not been observed in these patients. Thus, 1 purpose of the experiments described in this report was to evaluate a different carbamate, physostigmine (PHY) on AXT using an in vitro live imaging assay that we have previously found to be very sensitive to OP-related deficits in AXT. PHY is potent AChEI and a lipid-soluble tertiary amine that readily crosses the BBB from peripheral administration.29,30 These experiments would be expected to address the question of whether carbamate-based AChE inhibitors impair AXT without encountering the controversies surrounding BBB penetration.
Materials and methods
Chemicals and reagents
Diisopropylfluorophosphate (DFP) and eserine hemisulfate (PHY) were purchased from Sigma-Aldrich (St. Louis, MO). To maintain stability of DFP, the colorless liquid concentrate was stored in a freezer at −70°C. The PHY salt was stored in a refrigerator in a desiccator at 4°C. Cell culture reagents were purchased from Fisher Scientific (Pittsburgh, PA) unless otherwise stated.
Cell culture
Primary cortical neurons were harvested from pregnant Sprague-Dawley rats on gestation day E18 as described previously.19,20 Cortices were incubated at 37°C for 15 minutes in 0.25% Trypsin (Life Technologies, Carlsbad, CA) in the presence of DNase (Sigma #D4513), with gentle agitation after every 5 minutes. Tissues first rinsed in HBSS underwent trituration with a glass fire-polished pipette in neurobasal media (Gibco, Gaithersburg, MD) with 2% B27 and 10% fetal bovine serum (FBS) in the presence of DNase. Dissociated cells were then spun into a pellet in a centrifuge for 8 minutes at 25°C at 200G. The cells were then re-suspended in neurobasal media with 2% B27, 10% FBS, and 100 U/mL penicillin-streptomycin (Life Technologies, Grand Island, NY) before final plating at a density of 500 000 cells/mL. Two hours after plating, media was replaced with serum-free media comprised of neurobasal media with 2% B27, 100 U/mL penicillin-streptomycin and 0.5mM GlutaMAX™ Supplement (l-Alanyl-l-glutamine) was added to provide a stable source of l-glutamine. Media was changed after 4 days and 0.5 μM AraC (cytarabine) was added overnight at 3 days in vitro (DIV) to inhibit glial cell proliferation and penicillin-streptomycin was removed before transfection. Cells were maintained with 5% CO2 at 37°C with in a standard cell culture incubator.
Transfection and drug treatments
All transfections occurred at DIV5-6 using the plasmid pEGFP-n1-APP (Addgene #69924). Cells were transfected using 2 μg cDNA and 2 μL Lipofectamine® 2000 (ThermoFisher) per coverslip. Cells were treated on DIV5-6 with drugs (DFP or PHY), or vehicle (ultrapure water) for 24 hours prior to imaging. Both DFP and PHY solutions were made up fresh just before each experiment (ie, dissolved in ultrapure water and immediately added to the neuronal cultures to begin the 24 hour incubation period).
Live imaging and analysis
Live imaging experiments were performed as previously described.19,20,31 Coverslips were placed in a specialized live imaging chamber and media was changed to clear Neurobasal media immediately before live imaging experiments began. All experiments were performed 24 to 36 hours after transfection on a Zeiss LSM780 inverted microscope. The microscope was equipped with an environmental chamber which maintained the cells at 37°C with 5% CO2 for the duration of all experiments. Neurons expressing green fluorescent amyloid precursor protein (GFP-APP) were identified 63X magnification (1.42 numerical aperture) and axons were identified using morphological criteria defined by Kriegstein and Dichter (finest, longest cell processes with no spines).32 Videos of individual axons were captured at a rate of 1 frame every 2 seconds for 3 minutes (90 frames total) using Zen image capture software (Carl Zeiss). Image processing and analysis were conducted using FIJI/ImageJ 1.52b. Prior to analysis, we used the bleach correction tool in FIJI/ImageJ to help control for the effects of photobleaching; additionally, the “straighten curved objects” plugin was used to straighten individual axons. Kymographs were generated using the “Kymograph Action Tool” plugin and analyzed using the KymoAnlyzer plugin toolkit V1.01.33 The following settings were used in KymoAnalyzer: Cmin = 3, cminRV = 3, pixel size = 0.307, frame rate = 0.5, factor = 0.33/frame. Anterograde and retrograde cargos were defined as having moved more than 3 μm away from (anterograde) or towards (retrograde) the cell body. Reversing cargos were defined as having traveled a total distance greater than 3 μm and having changed directions. Cargos were considered stationary if they traveled less than 3 μm. Pauses were defined as periods where a motile cargo’s speed reduced to ⩽0.15 μm/s. Density was calculated as the total number of cargos (anterograde, retrograde, stationary, and reversing) per micron across the entire length of axon.
Measurement of AChE activity
AChE activity was determined by the method of Ellman with minor modifications to accommodate 96 well microplate format19,20 using purified eel acetylcholinesterase (CAS # 9000-81-1, Sigma-Aldrich, St. Louis, MO, USA). The reaction mixture was prepared in 1.0 mM sodium phosphate buffer (pH 7.0 ± 0.05) and contained the following concentrations for each chemical (Sigma-Aldrich, St. Louis, MO): 0.48 mM acetylthiocholine, 0.070 mM tetraisopropyl pyrophosphoramide (iso-OMPA, a butyryl-cholinesterase inhibitor) and 0.52 mM 5,5ʹ-dithiobis(2-nitrobenzoic acid). Reaction product formation was monitored by measuring absorbance values at 412 nm every 2 minutes for 16 minutes (Mx synergy Microplate Spectrophotometer, BioTek Instruments Inc., Winooski, VT, USA). The rate of AChE activity was then calculated for each individual time point of measurement using the formula (change in absorbance/min)/(1.36 × 104), before normalization to the intra-experiment vehicle-treated control. Velocity was expressed as micromoles of substrate hydrolyzed per minute for every milligram of protein. All assays were performed at least 2 or 3 times. The IC50 values (concentration causing a half-maximal inhibition of the control response) were determined by nonlinear regression analysis of the concentration-response curves generated.
Statistical analyses
All statistical analyses were performed using GraphPad Prism Version 8.4.3 (GraphPad Software, San Diego, CA). Analysis of variance (ANOVA) was used to compare the concentration-dependent effects of chemical treatments to vehicle-treated controls and the method of Dunnett was used to examine post hoc differences when indicated. Statistical significance was assessed using an alpha level of 0.05. Values depicted in the histogram figures reflect the mean ± s.e.m. The number of independent experiments conducted for each drug evaluation and the number of replicates per drug concentration are indicated in the figure legends.
Results
GFP-APP transfection and live imaging
Twenty-four hours following transfection, cultured rat cortical neurons exhibited clear expression of GFP-APP in the soma and axons (Figure 1A). For imaging, individual GFP-APP -labeled membrane bound organelles (MBOs) in axons were identified as distinct (green fluorescent) structures with a circular or tubular shaped appearance (see the arrows in Figure 1A). Definitive proximal and distal axonal regions of each axon were identified and corresponding kymographs (see Figure 1B for an example) demonstrate that many MBOs were highly mobile, moving in both the anterograde (A) and retrograde (R) directions, while others remained stationary (S). The arrows in Figure 1C indicate individual MBOs moving along the axon at various stages in the anterograde and retrograde direction.
Figure 1.

Methods for testing DFP and PHY for their ability to affect AXT in vitro: (A) representative image demonstrating successful transfection with pEGFP-n1-APP in rat primary cortical neurons. Arrows indicate MBOs, scale bar = 20 µm, (B) kymograph generated from images captured at a rate of 1 frame every 2 s for 3 min demonstrating movement of pEGFP-n1-APP labeled MBOs. MBOs are categorized in 1 of 4 ways: anterograde (A), retrograde (R), stationary (S), or reversal (RV), and (C) representative frames demonstrating progression of MBOs moving in the anterograde and retrograde directions.
AXT velocity
The velocity of GFP-APP labeled MBO movements in cortical axons observed in this study under control conditions, –1.1 to 1.6 µm/sec in the anterograde, and −0.7 to 1.0 µm/sec in retrograde direction, is similar to that observed in our previously published studies using APPDendra2-labeled APP as well as GFP-APP labeled MBOs.19,20,31 Moreover, the range of velocities fits within the range (0.5 to 3.0 µm/sec) that is considered “fast AXT.”34
Effects of DFP on AXT velocity
In the current study, we evaluated DFP as a reference compound in concentrations ranging from 0.001 to 10 µM. As expected, DFP incubated in neuronal culture for 24 hours, depending on the concentration, decreased the velocity of both anterograde and retrograde AXT (see Figure 2A and B, respectively) as we have observed in our previously published results.19,31 Statistical analysis indicated a significant main effect of concentration on anterograde velocity, F(3, 573) = 9.545, P < .0001; and retrograde velocity, F(3, 288) = 5.248, P = .0015. Post hoc analysis indicated that the 0.1 and 1.0 µM concentration of DFP significantly (P < .05) decreased anterograde velocity and the 0.1 µM concentration significantly decreased retrograde velocity compared to vehicle control. Please see Gao et al.19 for a more extensive analysis of additional effects of DFP on other AXT-related measurements such as the percentage of all particles that moved in the anterograde or retrograde direction or remained stationary, reversals, pauses, etc.
Figure 2.
Effects of DFP exposure for 24 h across a range of 3 concentrations on AXT in primary cortical neurons: (A) velocities of APP containing membrane bound organelles (MBOs) in the anterograde direction and (B) velocities of APP containing MBOs in the retrograde direction. Bars depict the mean ± SEM of all MBOs measured (ie, 54-201 MBOs) per drug concentration obtained from 14 to 21 individual neurons from 2 to 3 independent experiments.
##P < .01. ###P < .001 = significant decrease compared vehicle control conditions.
Effects of PHY on AChE activity
As expected, PHY was found to strongly inhibit purified eel AChE with an IC50 of 0.02 μM as determined by a modification of the Ellman assay (see Figure 3). These data are in agreement with other previously published in vitro studies35,36 where IC50 values in the low nM range for AChE inhibition were reported. In the subsequent culture experiments (see Results below), we evaluated a wide range of concentrations to cover levels that were below and well-above above the threshold for AChE inhibition.
Figure 3.
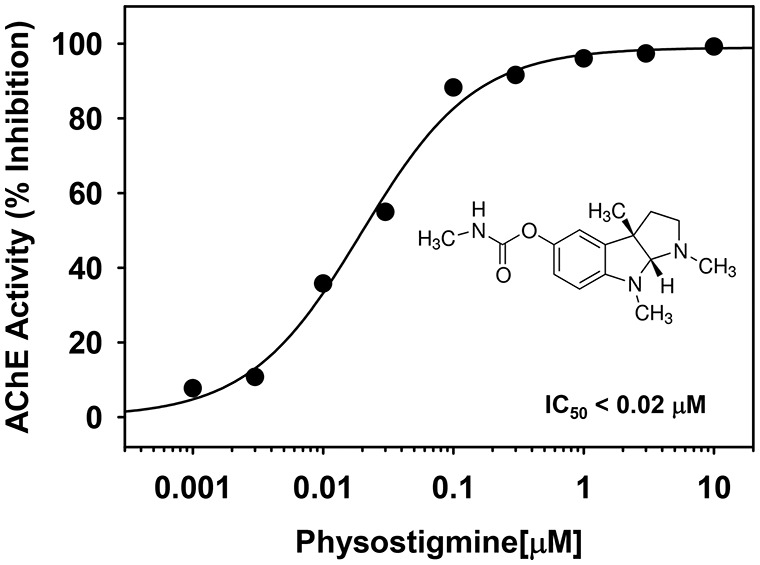
Effects of a range of concentrations of PHY on purified eel acetylcholinesterase (AChE) activity in vitro. Each symbol represents the mean from 2 to 3 independent experiments and 4 replicates per concentration. The IC50 value (concentration causing a half-maximal inhibition of the control response) was determined by nonlinear regression analysis of the concentration-response curve generated.
Effects of PHY on AXT velocity and directional movements of MBOs
PHY incubated in neuronal culture for 24 hours across a range of concentrations (0.01-100 nM) was not associated with any changes in the anterograde velocities of GFP-APP tagged MBOs compared to control (Figure 4A), main effect of concentration, F(5, 1751) = 1.207, P = .30. Interestingly, the highest concentration of PHY was associated with a significant increase in the velocity of MBOs moving in the retrograde direction (Figure 4B) compared to vehicle control, main effect of concentration, F(5, 1028) = 3.782, P = .0021 and post hoc analysis indicated a significant (P < .05) difference between the 100 nM concentration and vehicle control. Another interesting observation was that the 10 nM concentration of PHY was associated with an increase in the number of MBOs moving in the anterograde direction, while in contrast, the 100 nM concentration of PHY was associated with a significant decrease in the number of MBOs moving in the anterograde direction (Figure 4C). Statistical analysis indicated a main effect of concentration, F(5, 206) = 6.442, P < .0001, and post hoc analysis indicated significant differences (P < .05) between the 10 and 100 nM concentrations and vehicle control. The analysis of the number of MBOs moving in the retrograde direction (Figure 4D) indicated the following, main effect of concentration, F(5, 206) = 2.363, P = .04 and post hoc analysis indicated a non-significant trend (P = .06) toward an increase associated with lowest PHY concentration (0.01 nM). The analysis of the number of stationary MBOs (Figure 4E) indicated the following, main effect of concentration, F(5, 206) = 9.080, P < .0001. Post hoc analysis indicated that the 100 nM concentration of PHY was associated with a significant increase in the number of stationary MBOs.
Figure 4.
PHY exposure for 24 h across a range of concentrations on AXT in primary cortical neurons: (A) velocities of APP containing membrane bound organelles (MBOs) moving in the anterograde direction, (B) velocities of APP containing MBOs moving in the retrograde direction, (C) percentage of all MBOs (particles) moving in the anterograde direction, (D) percentage of all MBOs (particles) moving in the retrograde direction, and (E) percentage of all MBOs (particles) remaining stationary. ANT = anterograde; RET = retrograde; STAT = stationary. Bars depict the mean ± SEM of all MBOs measured (ie, 140-380 MBOs) per drug concentration obtained from 12 to 20 individual neurons from 3 to 4 independent experiments.
*P < .05. ***P < .001 = significant increase from vehicle control; #P < .05 = significant decrease from vehicle control.
Effects of PHY on additional AXT-related movements of MBOs
Table 1 provides a summary of the effects of PHY on several additional AXT-related measurements including the density of cargos, the percentage of particles that reversed direction, the pause frequency, and pause duration. The following observations were made: when compared to vehicle control, 1) 3 concentrations of PHY (0.01, 0.10, and 1.0 nM) were associated with a significant (P < .05) decrease in the number of MBOs/mm, 2) 1 concentration of PHY (0.10 nM) was associated with a significant decrease in the percentages of particles that reversed direction, 3) the highest concentration of PHY (100 nM) significantly increased the number of pauses, and 4) with the exception of the highest concentration of PHY, all other concentrations slightly (but significantly) decreased the pause duration.
Table 1.
Additional axonal transport-related measurements.
| Compound | Concentration | APP particles | Reversals | Pause frequency | Pause duration |
|---|---|---|---|---|---|
| nM | # of MBOs/mm | % of all particles | #Pauses/min | s | |
| PHY | 0.0 | 0.24 ± 0.02 | 7.64 ± 1.21 | 0.95 ± 0.05 | 45.34 ± 1.58 |
| 0.01 | 0.15 ± 0.01# | 6.12 ± 1.15 | 0.82 ± 0.08 | 35.33 ± 1.94# | |
| 0.10 | 0.17 ± 0.01# | 3.39 ± 0.61# | 0.79 ± 0.05 | 39.45 ± 1.65# | |
| 1.0 | 0.18 ± 0.01# | 7.14 ± 1.56 | 0.79 ± 0.06 | 38.37 ± 1.79# | |
| 10.0 | 0.19 ± 0.01 | 6.11 ± 1.07 | 0.77 ± 0.06 | 33.62 ± 1.73# | |
| 100.0 | 0.31 ± 0.02* | 5.03 ± 1.06 | 1.20 ± 0.07* | 50.77 ± 2.06 |
Each value represents the mean ± s.e.m obtained from 12 to 20 individual neurons from 3 to 4 separate experiments and 4 to 5 replicates/concentration.
Significantly (P < .05) higher than vehicle control (0.0 concentration).
Significantly (P < .05) lower than vehicle control (0.0 concentration) conditions.
Discussion
The results of these experiments can be summarized as follows: (1) as expected, depending on concentration, DFP significantly impaired both anterograde and retrograde AXT of APP-labeled MBOs in rat primary cortical neurons as we have observed previously, (2) also as expected, the carbamate PHY was a potent inhibitor of AChE in vitro, (3) PHY did not significantly affect anterograde AXT across a wide range of concentrations including a concentration (100 nM) that was associated with near maximal (asymptotic) levels of AChE inhibition. The 100 nM concentration of PHY was, however, associated with some ostensibly negative effects including a decrease in the percentage of MBOs moving in the anterograde direction, an increase in the % of stationary particles, and an increase in the number of pauses.
There were other interesting observations regarding the effects of PHY that might be interpreted as positive or negative. For example, the 10 nM concentration of PHY was associated with an increase in the percentages of MBOs moving in the anterograde direction, the 0.1 nM concentration was associated with a decrease in the percentage of MBOs that reversed directions, and, with the exception of the highest concentration of PHY (100 nM), all of the concentrations of PHY were associated with a decrease in the pause duration. The observation that the highest concentration of PHY was associated with an increase in the velocity of MBOs moving in the retrograde direction is difficult to interpret since it decreased the % of MBOs moving in the anterograde direction, increased the % of stationary particles and the number of pauses. Finally, the observation that concentrations of PHY ranging from 0.01 to 1.0 nM decreased the density of APP particles (MBOs/mm) while the highest concentration (100 nM) increased particle density is also perplexing.
Given the suggestion that carbamate-based pesticides and therapeutic agents like pyridostigmine might lead to long-term neurological deficits like those observed in GWI, we were interested to learn if (similar to OPs) another potent class of AChE inhibitors, carbamates, might impair AXT. Like pyridostigmine, PHY is considered safe as a therapeutic agent when used at the appropriate doses and it is currently prescribed for the treatment of glaucoma and anticholinergic toxicity such as that occasionally encountered after accidental overdoses with antihistamines, atypical antipsychotics, and tricyclic antidepressants.28,37 The most common acute side effects of PHY are associated with cholinergic overstimulation.38,39 In fact, the mechanism of the acute toxicity of carbamates like PHY is similar to that of OPs (AChE inhibition), and the symptoms of acute carbamate poisoning are indistinguishable from OP toxicity, and include miosis, urination, diarrhea, salivation, muscle fasciculation, bradycardia, and CNS effects. However, AChE inhibition induced by carbamates is transient and rapidly reversible, since there is rapid reactivation of the carbamylated enzyme, and carbamylated AChE does not undergo the aging reaction that is often observed with OPs.10 While the mechanism of the OP effects on AXT are currently unknown, we hypothesized that the reversible nature of the carbamylation enzyme reaction might be relevant to long-term effects on non-cholinesterase targets. Here it is important to note that our previous studies with OPs (chlorpyrifos oxon and diisopropylfluorophosphate) indicate that their ability to inhibit AXT in vitro occurs well below the threshold for AChE inhibition, whereas in the current study concentrations of PHY that clearly impaired AChE activity did not impair anterograde or retrograde AXT. In the previous work with OPs, we also found that the OP effects on AXT were not blocked by nicotinic or muscarinic receptor antagonists.19,20 These previous observations along with the current observations with PHY further bolster the argument that AXT effects (of potent AChE inhibitors) and impairments of AChE activity can be distinguished.
In conclusion, the results of these in vitro studies indicate that the carbamate PHY does not impair anterograde or retrograde AXT across a range of concentrations. These data suggest that the long-term neurological deficits that have been attributed to carbamate-based AChEIs are not likely due to acute impairments of AXT. However, it is important to note, that while we have previously shown that the OPs, chlorpyrifos and DFP (in addition to their negative effects in vitro) also impair AXT in vivo in rats,21,22 we have not yet evaluated the effects of PHY on AXT in vivo. These experiments may be necessary in order for us to make more conclusive statements about PHY and AXT.
Acknowledgments
The authors thank Ms. Ashley Davis for administrative assistance in preparing this article.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work described in this manuscript was supported by the Congressionally Directed Medical Research Programs (CDMRP), specifically, the Gulf War Illness Research Program (GWIRP), grant numbers W81XWH-12-1-0536 (Terry) and W81XWH-18-1-0750 (Baas).
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: SXN, WDB, and ZW performed experiments and analyzed data. All of the authors contributed to the writing and editing of the manuscript. All authors also reviewed and gave approval of the intellectual content of the final manuscript.
ORCID iD: Alvin V Terry  https://orcid.org/0000-0003-2071-4767
https://orcid.org/0000-0003-2071-4767
References
- 1. London L, Beseler C, Bouchard MF, et al. Neurobehavioral and neurodevelopmental effects of pesticide exposures. Neurotoxicology. 2012;33:887-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rauh VA, Margolis AE. Research review: environmental exposures, neurodevelopment, and child mental health - new paradigms for the study of brain and behavioral effects. J Child Psychol Psychiatry. 2016;57:775-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gunnarsson LG, Bodin L. Occupational exposures and neurodegenerative diseases-a systematic literature review and meta-analyses. Int J Environ Res Public Health. 2019;16:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seth B, Yadav A, Tandon A, Shankar J, Chaturvedi RK. Carbofuran hampers oligodendrocytes development leading to impaired myelination in the hippocampus of rat brain. Neurotoxicology. 2019;70:161-179. [DOI] [PubMed] [Google Scholar]
- 5. White RF, Steele L, O’Callaghan JP, et al. Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: effects of toxicant exposures during deployment. Cortex. 2016;74:449-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sullivan K, Krengel M, Bradford W, et al. Neuropsychological functioning in military pesticide applicators from the Gulf War: effects on information processing speed, attention and visual memory. Neurotoxicol Teratol. 2018;65:1-13. [DOI] [PubMed] [Google Scholar]
- 7. Golomb BA. Acetylcholinesterase inhibitors and Gulf War illnesses. Proc Natl Acad Sci U S A. 2008;105:4295-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Winkenwerder W. Environmental Exposure Report: Pesticides Final Report. U.S. Department of Defense, Office of the Special Assistant to the Undersecretary of Defense (Personnel and Readiness) for Gulf War Illnesses Medical Readiness and Military Deployments; 2003. [Google Scholar]
- 9. Berardocco D. DoD, CIA release Khamisiyah modeling data. GulfNEWS, 1997;1. [Google Scholar]
- 10. Costa LG. Organophosphorus compounds at 80: some old and new issues. Toxicol Sci. 2018;162:24-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koo BB, Michalovicz LT, Calderazzo S, et al. Corticosterone potentiates DFP-induced neuroinflammation and affects high-order diffusion imaging in a rat model of Gulf War Illness. Brain Behav Immun. 2018;67:42-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mohammadzadeh L, Hosseinzadeh H, Abnous K, Razavi BM. Neuroprotective potential of crocin against malathion-induced motor deficit and neurochemical alterations in rats. Environ Sci Pollut Res Int. 2018;25:4904-4914. [DOI] [PubMed] [Google Scholar]
- 13. Eftekhari A, Ahmadian E, Azami A, Johari-Ahar M, Eghbal MA. Protective effects of coenzyme Q10 nanoparticles on dichlorvos-induced hepatotoxicity and mitochondrial/lysosomal injury. Environ Toxicol. 2018;33(2):167-177. [DOI] [PubMed] [Google Scholar]
- 14. Abolaji AO, Ojo M, Afolabi TT, Arowoogun MD, Nwawolor D, Farombi EO. Protective properties of 6-gingerol-rich fraction from Zingiber officinale (Ginger) on chlorpyrifos-induced oxidative damage and inflammation in the brain, ovary and uterus of rats. Chem Biol Interact. 2017;270:15-23. [DOI] [PubMed] [Google Scholar]
- 15. Abou-Donia MB, Abou-Donia MM, ElMasry EM, Monro JA, Mulder MF. Autoantibodies to nervous system-specific proteins are elevated in sera of flight crew members: biomarkers for nervous system injury. J Toxicol Environ Health A. 2013;76:363-380. [DOI] [PubMed] [Google Scholar]
- 16. Abou-Donia MB, Conboy LA, Kokkotou E, et al. Screening for novel central nervous system biomarkers in veterans with Gulf War Illness. Neurotoxicol Teratol. 2017;61:36-46. [DOI] [PubMed] [Google Scholar]
- 17. El Rahman HAA, Salama M, Gad El-Hak SA, El-Harouny MA, ElKafrawy P, Abou-Donia MB. A Panel of Autoantibodies against neural proteins as peripheral biomarker for pesticide-induced neurotoxicity. Neurotox Res. 2018;33:316-336. [DOI] [PubMed] [Google Scholar]
- 18. Dhouib I, Jallouli M, Annabi A, et al. Carbamates pesticides induced immunotoxicity and carcinogenicity in human: a review. J Appl Biomed. 2016;14:85-90. [Google Scholar]
- 19. Gao J, Naughton SX, Wulff H, et al. Diisopropylfluorophosphate impairs the transport of membrane-bound organelles in rat cortical axons. J Pharmacol Exp Ther. 2016;356:645-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gao J, Naughton SX, Beck WD, et al. Chlorpyrifos and chlorpyrifos oxon impair the transport of membrane bound organelles in rat cortical axons. Neurotoxicology. 2017;62:111-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hernandez CM, Beck WD, Naughton SX, et al. Repeated exposure to chlorpyrifos leads to prolonged impairments of axonal transport in the living rodent brain. Neurotoxicology. 2015;47:17–26. [DOI] [PubMed] [Google Scholar]
- 22. Naughton SX, Hernandez CM, Beck WD, et al. Repeated exposures to diisopropylfluorophosphate result in structural disruptions of myelinated axons and persistent impairments of axonal transport in the brains of rats. Toxicology. 2018;406-407:92-103. [DOI] [PubMed] [Google Scholar]
- 23. Olenick MA, Holzbaur ELF. Dynein activators and adaptors at a glance. J Cell Sci. 2019;132:jcs227132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Esposito P, Chandler N, Kandere K, et al. Corticotropin-releasing hormone and brain mast cells regulate blood-brain-barrier permeability induced by acute stress. J Pharmacol Exp Ther. 2002;303:1061-1066. [DOI] [PubMed] [Google Scholar]
- 25. Friedman A, Kaufer D, Shemer J, Hendler I, Soreq H, Tur-Kaspa I. Pyridostigmine brain penetration under stress enhances neuronal excitability and induces early immediate transcriptional response. Nat Med. 1996;2:1382-1385. [DOI] [PubMed] [Google Scholar]
- 26. Park D, Jeon JH, Shin S, et al. Debilitating stresses do not increase blood-brain barrier permeability: Lack of the involvement of corticosteroids. Environ Toxicol Pharmacol. 2008;26:30-37. [DOI] [PubMed] [Google Scholar]
- 27. Amourette C, Lamproglou I, Barbier L, et al. Gulf War illness: effects of repeated stress and pyridostigmine treatment on blood-brain barrier permeability and cholinesterase activity in rat brain. Behav Brain Res. 2009;203:207-214. [DOI] [PubMed] [Google Scholar]
- 28. Pope C, Karanth S, Liu J. Pharmacology and toxicology of cholinesterase inhibitors: uses and misuses of a common mechanism of action. Environ Toxicol Pharmacol. 2005;19:433-446. [DOI] [PubMed] [Google Scholar]
- 29. Somani SM, Khalique A. Pharmacokinetics and pharmacodynamics of PHY in the rat after intravenous administration. Drug Metab Dispos. 1987;15:627-633. [PubMed] [Google Scholar]
- 30. Scremin OU, Scremin AM, Somani SM, Giacobini E. Brain regional distribution of PHY and its relation to cerebral blood flow following intravenous administration in rats. J Neurosci Res. 1990;26:188-195. [DOI] [PubMed] [Google Scholar]
- 31. Naughton SX, Beck WD, Wei Z, Wu G, Terry AV., Jr. Multifunctional compounds lithium chloride and methylene Blue attenuate the negative effects of diisopropylfluorophosphate on axonal transport in rat cortical neurons. Toxicology. 2020;431:152379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kriegstein AR, Dichter MA. Morphological classification of rat cortical neurons in cell culture. J Neurosci. 1983;3:1634-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Neumann S, Chassefeyre R, Campbell GE, Encalada SE. KymoAnalyzer: a software tool for the quantitative analysis of intracellular transport in neurons. Traffic. 2017;18:71-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maday S, Twelvetrees AE, Moughamian AJ, Holzbaur EL. Axonal transport: cargo-specific mechanisms of motility and regulation. Neuron. 2014;84:292-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Atack JR, Yu QS, Soncrant TT, Brossi A, Rapoport SI. Comparative inhibitory effects of various PHY analogs against acetyl- and butyrylcholinesterases. J Pharmacol Exp Ther. 1989;249:194-202. [PubMed] [Google Scholar]
- 36. Jackisch R, Förster S, Kammerer M, et al. Inhibitory potency of choline esterase inhibitors on acetylcholine release and choline esterase activity in fresh specimens of human and rat neocortex. J Alzheimers Dis. 2009;16:635-647. [DOI] [PubMed] [Google Scholar]
- 37. Watkins JW, Schwarz ES, Arroyo-Plasencia AM, Mullins ME, Toxicology Investigators, Consortium investigators. The use of PHY by toxicologists in anticholinergic toxicity. J Med Toxicol. 2015;11:179-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Arens AM, Shah K, Al-Abri S, Olson KR, Kearney T. Safety and effectiveness of PHY: a 10-year retrospective review. Clin Toxicol (Phila). 2018;56:101-107. [DOI] [PubMed] [Google Scholar]
- 39. Arens AM, Kearney T. Adverse effects of PHY. J Med Toxicol. 2019;15:184-191. [DOI] [PMC free article] [PubMed] [Google Scholar]




