Abstract
Lung cancer is a global disease and a major cause of cancer-related mortality worldwide. Accumulated studies have confirmed the essential role of long non-coding RNAs (lncRNAs) in the occurrence and development of cancers. Meanwhile, there have been reports concerning the role of Small Nucleolar RNA Host Gene 3 (SNHG3) in various cancers. However, there are so far few studies on the function and mechanism of SNHG3 in lung cancer. In the present study, SNHG3 was found to be highly expressed in lung cancer tissues and cells. Downregulation of SNHG3 could inhibit cell proliferation, migration, invasion and epithelial-mesenchymal transition (EMT) process. In addition, SNHG3 was found to have the ability to bind to miR-515-5p. Furthermore, Small Ubiquitin Like Modifier 2 (SUMO2) was identified to be the downstream target of miR-515-5p, which was negatively correlated with miR-515-5p expression. SNHG3 could positively regulate SUMO2 expression by sponging miR-515-5p. In addition, the rescue experiment showed that simultaneous transfection of miR-515-5p or SUMO2 siRNA could reverse the effect of SNHG3 expression on cell proliferation and metastasis. Collectively, our study demonstrates that SNHG3 can act on miR-515-5p in the form of competitive endogenous RNA (ceRNA) to regulate SUMO2 positively and thus affect the proliferation and metastasis of NSCLC cells. Findings in our study support that SNHG3/miR-515-5p/SUMO2 regulatory axis may become a potential therapeutic target for lung cancer.
Keywords: lncRNA, SNHG3, miR-515-5p, SUMO2, NSCLC
Induction
The morbidity and mortality of lung cancer, a malignancy, rank the first in the world,1 which are also in the first place in China.2 Non-small cell lung cancer (NSCLC) accounts for approximately 80% of lung cancer, among which adenocarcinoma (AD) and squamous cell carcinoma (SCC) are the primary causes of cancer-related death. Progress has been made in chemotherapy and molecular targeted therapy of NSCLC.3 However, the 5-year survival rate of NSCLC is still less than 20% due to limited therapeutic methods as well as tumor metastasis and recurrence.4 In this regard, it is of great significance to explore the molecular mechanism of the occurrence and development of lung cancer and find new therapeutic targets, so as to improve the clinical diagnosis and treatment for this type of cancer. Recent studies have proven that long non-coding RNA (lncRNAs) plays an important regulatory role in the occurrence and development of lung cancer.5-7 They can affect the curative effect of NSCLC, and some lncRNAs can also regulate drug resistance and radiosensitivity.8,9 Hence, lncRNAs can be regarded as potential therapeutic targets for the prognosis and curative effect of lung cancer.
Small nucleolar RNA host gene 3 (SNHG3), also known as U17 Host Gene (U17HG), was for the first time discovered by Professor Pelczar and Filipowicz in 1998, which is located at 1q35.3.10 Previous bioinformatics analysis have shown that SNHG3 may play a key role in regulating RNA splicing, tRNA processing, signal transduction, cell adhesion, transcription and apoptosis.11 Besides, recent experimental studies have found that SNHG3 functions significantly in the occurrence and development of many malignant tumors. For example, SNHG3 can promote the malignant progression of glioma by silencing KLF2 and p21.12 SNHG3 was up-regulated in ovarian cancer as an oncogene and affected cell proliferation and invasion by regulating GSK3β/β-catenin signaling activity.13 In addition, SNHG was also associated with drug resistance.14 Moreover, SNHG3 was also highly expressed in colorectal cancer, and it could upregulate c-myc and its target genes by acting as the ceRNA of miR-182-5p, thus promoting disease progression.15 Besides, LncRNA SNHG3, as the ceRNA of miRNA-151a-3p, could upregulate RAB22A expression and promote tumor invasion and migration in osteosarcoma.16 In addition, SNHG3 was also reported to be related to the prognosis of malignant tumors, and can be used as a potential biomarker to determine the prognosis.17 Simultaneously, it has been revealed that SNHG3 was also up-regulated in NSCLC cells.11,18 However, it remains unclear with respect to the specific mechanism.
In the present study, experiments were carried out with the confirmation of the main cytoplasmic localization of SNHG3 in NSCLC cells, the promoting effect of SNHG3 on the proliferation and metastasis of lung cancer cells, and the positive regulatory effect of SNHG3 on SUMO2 by acting on miR-515-5p as ceRNA. It eventually verifies the possibility of SNHG3/miR-515-5p/SUMO2 regulatory axis as a potential therapeutic target for lung cancer.
Materials and Methods
Patients and Clinical Samples
A total of 15 pairs of NSCLC tissues and adjacent normal tissues were collected from patients at the Sixth Medical Center of PLA General Hospital. Each patient has previously signed an informed consent. The study obtained the approval of the Research Ethics Committee of the Sixth Medical Center of PLA General Hospital (No. HZKY-YJ-2019-2).
Cell Culture and Transfection
The human NSCLC cell lines (A549, HCC827, H2170, and H520) were obtained from the Cell Resource Center, Peking Union Medical College. Airway epithelial cell line (BEAS-2B) was purchased from Shanghai Institutes for Biological Sciences, China Academy of Science (Shanghai, China). All cell lines were cultured in RPMI-1640 (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (Life Technologies, Carlsbad, CA, USA) maintained at 37°C in a humidified incubator with 5% CO2.
Short interfering RNA (siRNA) specifically targeting SNHG3 (NR_036473), SUMO2 (NM_006937.4) and negative control (NC) were designed and synthesized by GenePharma (GenePharma Co., Ltd., Shanghai, China). SNHG3 si#1: 5′-GGGCACTTCGTAAGGTTTAAA-3′; SNHG3 si#2: 5′-GACCAATAGGACCGTAAGTCT-3′; SUMO2 si: 5′-GACTGAGAACAACGATCATAT-3′. The miR-515-5p and NC miRNA mimics were from Thermo Fisher (Thermo Fisher Scientific, MA, USA). The pCMV/SNHG3-WT vector contained a full-length wild-type sequence of SNHG3. pCMV/SNHG3-MUT1-2 contained SNHG3 mutation sequence, with deletion of “TTTGGAGA” at 106-112nt and 280-287nt, respectively. The luciferase reporter vector pGL3/SNHG3-WT contains the entire wild type sequence of SNHG3, while pGL3/SNHG3-MUT1, pGL3/SNHG3-MUT2 and pGL3/SNHG3-MUT1-2 had “TTTGGAGA” sequence deletions at 106-112nt or 280-287nt, or both. The pGL3/SUMO2-WT vector has SUMO2 3′UTR wild type full-length sequence, while pGL3/SUMO2-MUT contains SUMO2 3′UTR mutation sequence (“TTTGGAGAA” deletion at 413-420nt). All the vectors were designed and constructed by Hitrobio (Hitrobio.tech, Beijing, China). The transfection was carried out by lipofectamine 2000 (Invitrogen, Carlsbad, CA) following the manufacturer’s protocols.
Luciferase Assay
The firefly luciferase reporter plasmids containing wild-type or mutated 3′UTRs (100 ng), the control renilla luciferase plasmid (pRL-CMV, 5 ng) (Promega, Madison, WI, USA) and miRNA mimics (100 nM) were co-transfected into cells using Lipofectamine 2000, according to the manufacturer’s protocol. After 48 hours, luciferase activities were measured using the Dual-Luciferase Reporter Assay System (Promega). Firefly luciferase was normalized to renilla luciferase activity.
Cell Viability Assay
The proliferation of cells was measured using the Cell Counting Kit-8 (CCK-8, Dojindo, Japan). Briefly, 2 × 103 cells were seeded into 96-well plates in triplicate and cultured for various time points at 37°C. The absorbance values of 450 nm wavelength were determined by enzyme labeling instrument (Multimode Reader, PerkinElmer, USA).
Quantitative RT-PCR (qRT-PCR)
Trizol kit (Invitrogen, MA, USA) was employed to extract total RNA from cells or tissues. Reverse transcription was conducted with a commercially available kit (Takara, Dalian, China). The SYBR Green MasterMix (Takara, Dalian, China) was used for qPCR reaction on ABI 7900 system. The expression of SNHG3 was calculated using 2−ΔΔCt method, and endogenous GAPDH or U6 was used for internal reference. Primers for RT-qPCR sequences are listed in Supplementary Table S1.
Protein Extraction and Western Blot Analysis
Total cell lysates from cells were prepared using ProteoJET™ Mammalian Cell Lysis Reagent (Thermo Scientific, Waltham, MA, USA). BCA Protein Assay Kit (Thermo Scientific) was used to quantify protein concentration. Proteins (50 μg) were separated using SDS-PAGE (10%), transferred to nitrocellulose membranes (162-0115; Bio-Rad Laboratories), and blocked in Tris-buffered saline with Tween-20 (TBST) containing 5% non-fat milk. The membranes were probed with the specific primary antibodies. After being washed in TBST 3 times, the membranes were incubated with corresponding horseradish peroxidase-conjugated secondary antibody (ab205718 or ab6728, 1:5000). Finally, protein bands were visualized using the enhanced chemiluminescent substrate, according to the manufacturer’s protocol (Thermo Scientific). The specific antibodies to E-cadherin (1:1000, ab76055), N-cadherin (1:1000, ab18203), Vimentin (1:1000, ab8978), SUMO2 (1:1000, ab233222), SUMO3 (1:1000, ab203570) and GAPDH (1:2000, ab245357) were purchased from Abcam (Cambridge, UK).
Transwell Assay
Transwell plates (BD Biosciences) were used to analyze cellular migration ability. Cells in serum-free DMEM were added to the upper chamber, and DMEM with 20% FBS was applied to the lower chamber. After 18 h, residual cells were removed from the upper chamber. Cells were then fixed with methanol and stained with with 4′,6-diamidino-2-phenylindole (DAPI) (Thermo Fisher Science). After washing with PBS, the number of cells passing through the membrane was counted under an inverted microscope (IX71, Olympus, Japan). Three random microscopic fields were selected for statistical analysis. Cell invasion assay was performed similarly using transwell upper chamber coated with Matrigel (BD Biosciences).
Microscopy
Cells were fixed in 4% paraformaldehyde for 10 min at room temperature. Cells were then washed with PBS twice before additional fixation and permeabilization with 100% methanol for 10 min at room temperature. For RNA FISH, the methanol was aspirated, and RNA probes in RNA FISH buffer were directly added to cells. FITC-labeled RNA probe for SNHG3 was designed and synthesized by RiboBio (RiboBio Co., Ltd. Guangzhou, China). Ten ng of the probe was diluted in RNA FISH buffer consisting of 2×SSC, 10% formamide, and 10% dextran sulfate. Probes were incubated with samples overnight at 37°C in a humidified chamber. Samples were then washed 6 times with 2×SSC. Cells were also stained with Alexa Fluor® 594 phalloidin (Thomo Fisher) to label F-actin and DAPI to label nuclei. Finally, cells were mounted in ProLong® Gold antifade reagent and visualized using a fluorescence microscope (IX71, Olympus, Japan).
Statistical Analysis
SPSS 24.0 and GraphPad Prism7 statistical software were used for statistical analysis. Statistical description of measurement data was expressed as mean ± SD, and multiple sets were analyzed by one-way ANOVA. For pairwise comparison between groups, if the variance was homogeneous, the least significant difference (LSD) was used for analysis, and if the variance was not homogeneous, the Tamhane test was used for analysis. The statistical significance was set at P < 0.05.
Results
High Expression of SNHG3 in Lung Cancer Tissues and Cells
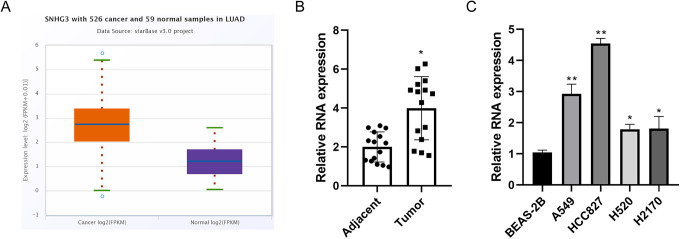
In the first step, our study conducted an online analysis on the relative content of SNHG3 mRNA in lung adenocarcinoma and corresponding normal tissues by Starbase (http://starbase.sysu.edu.cn/). The results showed that SNHG3 was highly expressed in lung adenocarcinoma (Figure 1A). The abnormal high expression of SNHG3 was confirmed in 15 pairs of NSCLC tissues and corresponding adjacent tissues by quantitative PCR (Figure 1B). Subsequently, SNHG3 expression was compared in normal lung epithelial cells (BEAS-2B) and a variety of NSCLC cells (A549, HCC827, H2170 and H520). Similarly as those in tissues, the relative content of SNHG3 in lung cancer cells was significantly higher than that in BEAS-2B cells (Figure 1C).
Figure 1.
Abnormal high expression of SNHG3 in lung cancer tissues and cells. A, Online analysis of the difference of SNHG3 mRNA expression in lung adenocarcinoma and normal tissues using Starbase database. B, Quantitative PCR analysis of SNHG3 in 15 pairs of NSCLC tissues and corresponding adjacent tissues. C, Quantitative analysis of the relative expression of SNHG3 in various cells using PCR. Data are the means ± SD of 3 independent experiments. *P < 0.05, **P < 0.01 compared with adjacent tissues group or BEAS-2B cell group.
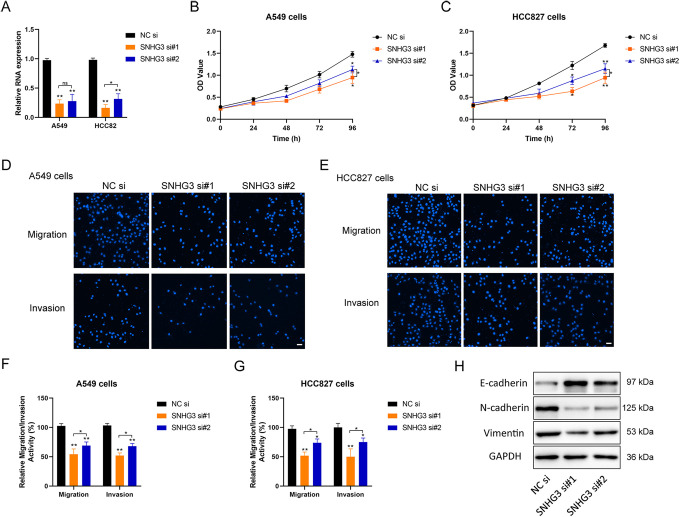
Inhibitory Effect of SNHG3 Knockdown on the Proliferation and Migration of Lung Cancer Cells
A549 and HCC827 cells with relatively high SNHG3 expression were screened for the subsequent experiment, and the relative content of SNHG3 was reduced by using siRNA (Figure 2A). There existed a significant decrease in the proliferation (Figure 2B, C), migration and invasion of lung cancer cells (Figure 2D-G) along with the decrease of SNHG3 content. Meanwhile, according to the results of Western blot, the knockdown of lncRNA SNHG3 could inhibit the expression of EMT related proteins N-cadherin and Vimentin, and enhanced the expression of E-cadherin (Figure 2H). Therefore, lncRNA-SNHG3 may exert a promoting role in the malignant phenotype of lung cancer cells in vitro.
Figure 2.
Effect of SNHG3 in the regulation of proliferation and metastasis of lung cancer cells. A, Quantitative PCR detection of the silencing effect of siRNA on SNHG3 in A549 and HCC827 cells. B and C, Detection of the proliferation of tumor cells transfected with siRNA using CCK-8. D and E, Detection of the migration and invasion ability of tumor cells transfected with siRNA using Transwell cell migration assay. The cells were stained with DAPI and observed under fluorescence microscope (Magnification, 200×; Scale bar, 50 µm). F and G, Quantification of the relative migration and invasion ability of cells in each group by histogram through counting under different visual fields. H, Analysis of the relative expression of EMT related proteins after siRNA transfection using western blot; GAPDH was used as the internal reference. si, siRNA; si#1 and si#2, two different siRNA duplexes against SNHG3. One-way ANOVA with Tukey post hoc analysis. *P < 0.05 and **P < 0.01.
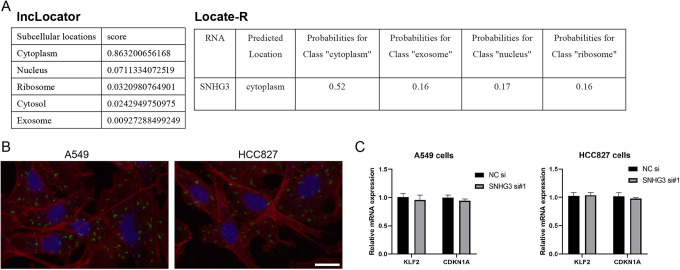
Subcellular Localization of SNHG3 in Lung Cancer Cells
SNHG3 localization in lung cancer cells has not been verified in detail although it was named small nucleolar RNA host gene 3. lncLocator (http://www.csbio.sjtu.edu.cn/bioinf/lncLocator/) and Locate-R (http://locate-r.azurewebsites.net/) were searched for online prediction in our study, both of which indicated that SNHG3 was mainly located in cytoplasm (Figure 3A). RNA FISH further showed that SNHG3 was mainly distributed in the cytoplasm of both A549 and HCC827 cells (Figure 3B), which was consistent with the above predicted results. Fei et al reported that SNHG3 could inhibit the transcription of KLF2 and CDKN1A by recruiting EZH2 to KLF2 and CDKN1A (p21) promoters in the nucleus of glioma cells. However, knockdown of SNHG3 in lung cancer cells resulted in no significant change in the transcription levels of KLF2 and CDKN1A (Figure 3C). These results suggest that the function of SNHG3 in lung cancer cells may exhibit an intimate association with the cytoplasmic localization.
Figure 3.
Subcellular localization analysis of SNHG3 in lung cancer cells. A, Online prediction of SNHG3 localization by lncLocator and Locate-R. B, Detection of the localization of SNHG3 in A549 and HCC827 lung cancer cells using FISH assay (Green: SNHG3; Blue: DAPI; Red: F-actin; Scale bar, 20 µm). C, Quantitative PCR analysis of the relative content of KLF2 and CDKN1A mRNA after siRNA transfection. *P < 0.05, **P < 0.01 compared with NC siRNA group.
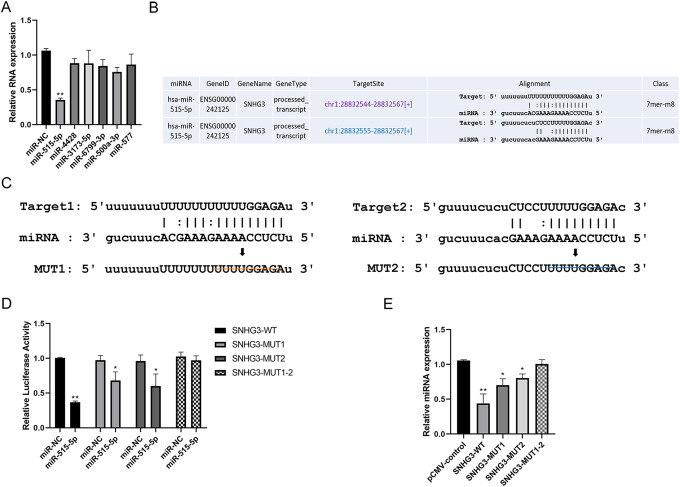
Interaction Between miR-515-5p and SNHG3 in Lung Cancer Cells
There might be an interaction with miRNA in view of the localization of SNHG3 in the cytoplasm of lung cancer. According to the online analysis using Starbase, our experiment obtained the top miRNAs (miR-515-5p, miR-4428, miR-3173-5p, miR-6799-3p, miR-500a-3p and miR-577) that could potentially target SNHG3. In HCC827 lung cancer cells, following the overexpression of all miRNA screened, only miR-515-5p showed the most significant down-regulatory effect on the relative content of SNHG3 (Figure 4A). Starbase predicted that there were at least 2 target sites for lncRNA-SNHG3 interacting with miR-515-5p seed sequence (Figure 4B). Subsequently, the 2 target sites (WT1 and WT2) were mutated separately and simultaneously (Figure 4C) respectively to construct dual luciferase reporter gene vectors (SNHG3-MUT1, SNHG3-MUT2 and SNHG3-MUT1-2). The results of dual luciferase reporter gene assay showed that miR-515-5p could significantly inhibit the luciferase activity of SNHG3-WT group (Figure 4D). Corresponding inhibitory effect was significantly down-regulated in SNHG3-MUT1 group and MUT2 group, yet no similar result was found in SNHG3-MUT1-2 group (Figure 4D). On the other hand, SNHG3-WT and SNHG3-MUT were overexpressed in H2170 cells with relatively low expression of SNHG3, respectively. The results revealed that the relative content of miR-515-5p could be downregulated significantly by overexpressing SNHG3-WT than SNHG3-MUT (Figure 4E). These results suggest that SNHG3-WT, as a ceRNA, acts on the core binding site of miR-515-5p in the form of sponges.
Figure 4.
The sponge effect of SNHG3 on miR-515-5p. A, Quantitative PCR detection of the regulatory effect of the overexpression of various miRNAs on SNHG3 in HCC827 cells. B, Online analysis of potential targets of SNHG3 and miR-515-5p using Starbase database. C, Construction of the wild-type and mutant-type luciferase reporter gene vectors containing the predicted target SNHG3 of miR-515-5p; and the schematic diagram of potential targets of SNHG3 with deletion mutation and miR-515-5p. D, Detection of the direct targeting effect of miR-515-5p on SNHG3 by luciferase activity assay. E, Quantitative PCR detection of the regulatory effect of overexpressing SNHG3-WT or SNHG3-MUT on the relative content of miR-515-5p in HCC827 cells. *P < 0.05, **P < 0.01 versus control.
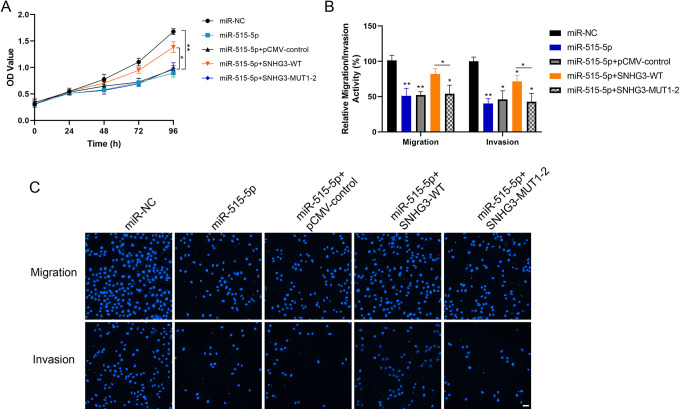
Overexpression of miR-515-5p could inhibit cell proliferation, migration and invasion (Figure 5A-C). While rescue experiment revealed that simultaneous overexpression of SNHG3-WT could weaken the inhibitory effect of miR-515-5p on the malignant performance of cells (Figure 5A-C).
Figure 5.
Inhibitory effect of miR-515-5p on the proliferation and metastasis of lung cancer cells. A, Detection of cell proliferation ability in each group using CCK-8 following the rescue experiment by overexpressing miR-515-5p and simultaneous transfection of SNHG3 in HCC827 cells. B and C, Detection of the ability of cell migration and invasion by Transwell assay (Magnification, 200×; Scale bar, 50 µm). *P < 0.05, **P < 0.01 versus control.
Positive Regulatory Effect of LncRNA SNHG3 on SUMO2 Expression by Sponging miR-515-5p
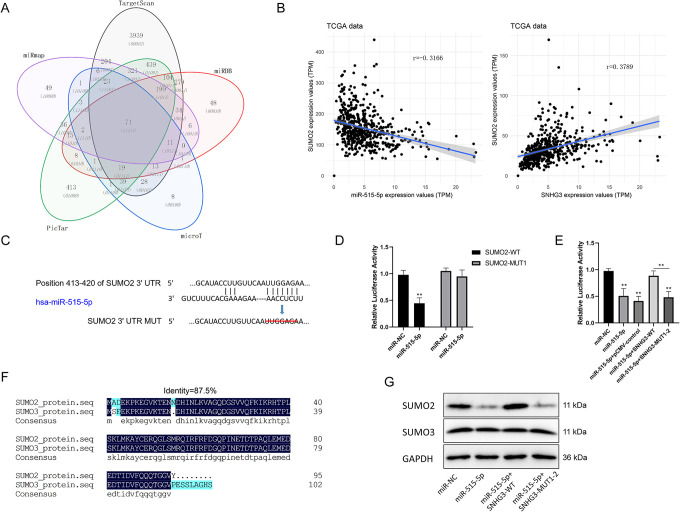
For further determination of the potential downstream target genes of miR-515-5p, PITA, miRmap, microT, PicTar and TargetScan databases were searched for bioinformatics analysis. The results showed that a total of 71 candidate genes might be the potential targets of miR-515-5p (Figure 6A). TCGA database was further applied to study the correlation between miR-515-5p and several genes. Statistical analysis of relevant data revealed a negative correlation of miR-515-5p with SUMO2 expression, while a positive correlation of lncRNA SNHG3 expression with SUMO2 expression in lung cancer tissues (Figure 6B). In addition, bioinformatics analysis predicted that there was a binding site with miR-515-5p in the 3′UTR sequence of SUMO2 (Figure 6C). Consequently, dual luciferase reporter gene assay was conducted and confirmed that miR-515-5p had a direct effect on SNHG3-WT mRNA 3′UTR to inhibit luciferase activity, but not UTR-MUT of the target site (Figure 6D). Additional dual luciferase reporter gene assay indicated that SNHG3 could weaken the effect of miR-515-5p on SUMO2 mRNA 3′UTR by competitive binding with miR-515-5p, and thus upregulate luciferase activity (Figure 6E). Furthermore, in lung cells HCC827, overexpressing miR-515-5p could inhibit the protein expression of SUMO2 (Figure 6G), and the proposed inhibitory effect could be eliminated by simultaneous expression of SNHG3-WT (Figure 6G). Interestingly, miR-515-5p produced no significant effect on SUMO3 expression, despite a high similarity in the protein sequences of SUMO3 and SUMO2 (Figure 6F). Undoubtedly, these results indicate that LncRNA SNHG3 can regulate SUMO2 expression positively by sponging miR-515-5p in lung cancer cells.
Figure 6.
Positive regulation of LncRNA SNHG3 on the expression of SUMO2 by sponging miR-515-5p. A, Prediction of the potential targets of miR-515-5p by PITA, RNA22, miRmap, microT, PicTar and TargetScan. B, Analysis of the correlation of miR-515-5p and SNHG3 with SUMO2 based on data from TCGA. C, Diagrammatic sketch of SUMO2 mRNA 3′UTR possessing the target site of miR-515-5p and its target deletion mutation. D, Detection of the direct targeting effect of miR-515-5p on SUMO2 3′UTR using luciferase activity assay. E, Luciferase activity assay was used to detect the effect of SNHG3 on miR-515-5p targeting of SUMO2. F, Sequence similarity of SUMO2 and SUMO3 proteins. G, Western blot detection of the effect of overexpressing miR-515-5p and SNHG3 on the relative expression of SUMO2. *P < 0.05, **P < 0.01 versus control.
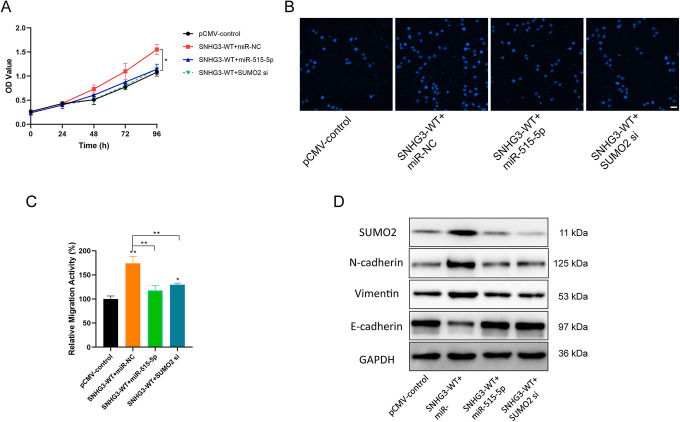
Besides, overexpressing SNHG3 in H1270 cells resulted in a highly increase in cell proliferation and migration (Figure 7A-C). If there was a simultaneous overexpression of miR-515-5p or SUMO2 siRNA, the malignant performance of SNHG3 was obviously inhibited (Figure 7A-C), accompanied by significant upregulation of the expression of EMT related proteins N-cadherin and Vimentin, while decreased E-cadherin expression (Figure 7D). These results indicate that SNHG3/miR-515-5p/SUMO2 pathway can exert a regulatory effect on the proliferation and metastasis of lung cancer cells.
Figure 7.
Regulatory effect of LncRNA SNHG3 on the proliferation and metastasis of lung cancer cells through miR-515-5p/SUMO2. A, CCK-8 detection of the inhibitory effect of miR-515-5p or SUMO2 siRNA on the cell proliferation-promoting of overexpressing SNHG3 in lung cancer H1270 cells. B and C, Transwell detection of the inhibitory effect of miR-515-5p or SUMO2 siRNA on the cell migration-promoting of overexpressing SNHG3 in lung cancer H1270 cells (Magnification, 200×; Scale bar, 50 µm). D, Western blot detection of the effect of overexpressing SNHG3 and simultaneous transfection of miR-515-5p or SUMO2 siRNA on the expression of EMT related proteins in lung cancer H1270 cells. *P < 0.05, **P < 0.01 versus control.
Discussion
LncRNAs are identified to have a wide distribution in cells, and corresponding location may effect their molecular function usually.19,20 Generally, lncRNAs in the nucleus are associated primarily with chromatin modification or transcriptional regulation; while those located in the cytoplasm are generally involved in the regulation of mRNA stability and protein expression, and can also be regarded as a ceRNA to regulate protein expression.21 SNHG3 was first named as small nucleolar RNA.22 However, recent studies have found that besides a location in the nucleus, SNHG3 is also located in the cytoplasm.23 For example, SNHG3 located in the nucleus could regulate the methylation of adjacent MED18 gene by binding with the enhancer of EZH2, thus promoting the progress of gastric cancer24; it could enhance the malignant behaviors of glioma by recruiting EZH2 into KLF2 and p21 promoters to silence their transcription.12 At the same time, SNHG3 located in the cytoplasm could upregulate the expression of Ras related protein 22a (rab22a) by sponging miR-151a-3p as a ceRNA, thus increasing the migration and invasion of osteosarcoma cells16; and it was also involved in energy metabolism by regulating eukaryotic translation initiation factor 4A3 (EIF4A3) mRNA in ovarian cancer.14 The aforementioned studies also suggest the heterogeneity localization and function of SNHG3 in tumor cells. Similarly, the results of RNA FISH in our study showed that SNHG3 was mainly distributed in the cytoplasm of lung cancer cells, suggesting a higher possibility of SNHG3 as a ceRNA to play its biological functions in the cytoplasm. Actually, the results of our study revealed that SNHG3 served as a ceRNA by sponging miR-515-5p.
Initially, miR-515-5p was recognized as a placental specific miRNA that was involved in regulating fetal growth. Recent studies, however, have found its role as a tumor suppressor. For instance, Pardo et al reported that miR-515-5p inhibited breast cancer and lung cancer cell migration by downregulating MARK4.25 Nevertheless, no evidence of miR-515-5p targeting MARK4 was found by using multiple bioinformatics analysis software. It indicated that the down-regulatory effect of miR-515-5p on MARK4 was an indirect regulatory effect, and MARK4 might not be the direct target of miR-515-5p. In this study, on the basis of bioinformatics analysis and experimental verification, mir-515-5p was verified to target SUMO2 in lung cancer cells. Despite a high similarity in amino acid sequences between SUMO2 and SUMO3, miR-515-5p produced no effect on the expression of SUMO3, revealing the specificity of miR-515-5p/SUMO2 regulatory pathway. Furthermore, the enhanced cell proliferation and migration induced by overexpressing SNHG3 could be inhibited by miR-515-5p or SUMO2 siRNA, suggesting the association between SNHG3/miR-515-5p/SUMO2 regulatory axis and the malignant performance of lung cancer cells. Recent studies have also found that SNHG3 promotes the proliferation and metastasis of non-small cell lung cancer cells by competing with other miRNAs (miR-340-5p and miR-216a-5p).26,27 These data also confirm the important regulation of SNHG3 in non-small cell lung cancer cells, and the SNHG3/miR-515-5p/SUMO2 we found may be only the tip of the iceberg of SNHG3 regulatory network.
SUMO2 is a member of the small ubiquitin-related modifier (SUMO) family.28 SUMO is a class of highly conserved proteins in eukaryotes, with a size of about 12 kD. At present, there are 4 subtypes of SUMO in mammals, including SUMO1, SUMO2, SUMO3 and SUMO4. SUMO 1-3 are expressed in a variety of human tissues, while SUMO4 mRNA is detected only in kidney, spleen and lymph nodes.29 SUMO2 and SUMO3 share similarity in amino acid sequences and are often co-written as SUMO2/3. As a protein similar to ubiquitin in molecular structure, SUMO is involved in the post-translational modification of protein. While there exists difference in the surface charge distribution between SUMO and ubiquitin, indicating the presence of difference in their functions.30,31 There is a likeness in the process between SUMOylation and ubiquitination cycles, but they are totally dissimilar in the function.32 To be specific, the target protein modified by ubiquitination is to realize the recognition and degradation of the target protein by proteasome, while the protein modified by SUMOylation exhibits better stability. Significantly, SUMO protein can modify target protein reversibly by covalent binding to involved in its post-translational modification, and hence regulate multiple biological behaviors including target protein stability, subcellular localization and its function.
Furthermore, SUMOylation is a dynamic balance, while its imbalance may result in the occurrence and development of a series of benign and malignant diseases. In terms of its function, SUMOylation may be partially responsible for the occurrence and development of tumors by modifying oncoprotein, DNA damage repair, growth promotion, drug resistance and metastasis.33 While SENPs can regulate the cell cycle, aging, angiogenesis, invasion, metastasis and other behaviors of tumor cells through deSUMOylation.34 This study revealed the regulatory effect of SNHG3/miR-515-5p/SUMO2 regulatory axis on the proliferation and metastasis of lung cancer cells. Nevertheless, it is still not clear with regard to the downstream effector molecules modified by SUMO2 specifically, which requires to be further explored.
Collectively, the present study confirms the primary cytoplasmic localization of SNHG3 in NSCLC cells. It identifies the role of SNHG3 in promoting the proliferation and metastasis of lung cancer cells, and proves that SNHG3 can act on miR-515-5p as the ceRNA to regulate SUMO2 positively. It suggests that SNHG3/miR-515-5p/SUMO2 regulatory axis may be a potential therapeutic target for lung cancer.
Supplemental Material
Supplemental Material, sj-pdf-1-tct-10.1177_15330338211019376 for LncRNA SNHG3 Promotes Proliferation and Metastasis of Non-Small-Cell Lung Cancer Cells Through miR-515-5p/SUMO2 Axis by Yongqun Li, Lipin Gao, Caiyun Zhang and Jiguang Meng in Technology in Cancer Research & Treatment
Supplemental Material, sj-pdf-2-tct-10.1177_15330338211019376 for LncRNA SNHG3 Promotes Proliferation and Metastasis of Non-Small-Cell Lung Cancer Cells Through miR-515-5p/SUMO2 Axis by Yongqun Li, Lipin Gao, Caiyun Zhang and Jiguang Meng in Technology in Cancer Research & Treatment
Abbreviations
- lncRNA
long non-coding RNA
- SNHG3
small nucleolar RNA host gene 3
- EMT
epithelial-mesenchymal transition
- SUMO2
small ubiquitin like modifier 2
- ceRNA
competitive endogenous RNA
- qRT-PCR
quantitative reverse transcriptase PCR.
Footnotes
Authors’ Note: Yongqun Li, MD, and Lipin Gao, BM, contributed equally to this work. YL and JM conceived and designed the experiments. YL analyzed and interpreted the results of the experiments, YL, LG and CZ performed the experiments. All data generated or analyzed during this study are included in this published article. Written informed consents were obtained from all the participants.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Health Care Foundation of Logistics Support Department of Central Military Commission (grant no. 17BJZ04).
Supplemental Material: Supplemental material for this article is available online.
ORCID iD: Jiguang Meng, MD  https://orcid.org/0000-0001-8018-7255
https://orcid.org/0000-0001-8018-7255
References
- 1. Nokin MJ, Ambrogio C, Nadal E, Santamaria D. Targeting infrequent driver alterations in non-small cell lung cancer. Trends Cancer. 2020;7(5):410–429. [DOI] [PubMed] [Google Scholar]
- 2. Sun D, Li H, Cao M, et al. Cancer burden in China: trends, risk factors and prevention. Cancer Biol Med. 2020;17(4):879–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Imyanitov EN, Iyevleva AG, Levchenko EN. Molecular testing and targeted therapy for non-small cell lung cancer: current status and perspectives. Crit Rev Oncol Hematol. 2020;157:103194. [DOI] [PubMed] [Google Scholar]
- 4. Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non-small cell lung cancer: a review. JAMA. 2019;322(8):764–774. [DOI] [PubMed] [Google Scholar]
- 5. Herrera-Solorio AM, Armas-López L, Arrieta O, Zúñiga J, Piña-Sánchez P, Ávila-Moreno F. Histone code and long non-coding RNAs (lncRNAs) aberrations in lung cancer: implications in the therapy response. Clin Epigenetics. 2017;9(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Loewen G, Jayawickramarajah J, Zhuo Y, Shan B. Functions of lncRNA HOTAIR in lung cancer. J Hematol Oncol. 2014;7(1):1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peng Y, Tang D, Zhao M, Kajiyama H, Kikkawa F, Kondo Y. Long non-coding RNA: a recently accentuated molecule in chemoresistance in cancer. Cancer Metastasis Rev. 2020;39(3):825–835. [DOI] [PubMed] [Google Scholar]
- 8. Liu X, Lu X, Zhen F, et al. Linc00665 induces acquired resistance to gefitinib through recruiting Ezh2 and activating PI3K/AKT pathway in NSCLC. Mol Ther Nucleic Acids. 2019;16:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xiao H, Liu Y, Liang P, et al. Tp53tg1 enhances cisplatin sensitivity of non-small cell lung cancer cells through regulating mir-18a/PTEN axis. Cell Biosci. 2018;8(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pelczar P, Filipowicz W. The host gene for intronic U17 small nucleolar RNAs in mammals has no protein-coding potential and is a member of the 5’-terminal oligopyrimidine gene family. Mol Cell Biol. 1998;18(8):4509–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu L, Ni J, He X. Upregulation of the long noncoding RNA SNHG3 promotes lung adenocarcinoma proliferation. Dis Markers. 2018;2018:5736716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fei F, He Y, He S, et al. LncRNA SNHG3 enhances the malignant progress of glioma through silencing KLF2 and p21. Biosci Rep. 2018;38(5):BSR20180420. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13. Hong L, Chen W, Wu D, Wang Y. Upregulation of SNHG3 expression associated with poor prognosis and enhances malignant progression of ovarian cancer. Cancer Biomark. 2018;22(3):367–374. [DOI] [PubMed] [Google Scholar]
- 14. Li N, Zhan X. Anti-parasite drug ivermectin can suppress ovarian cancer by regulating lncRNA-EIF4a3-mRNA axes. EPMA J. 2020;11(2):289–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang W, Tian Y, Dong S, et al. The long non-coding RNA SNHG3 functions as a competing endogenous RNA to promote malignant development of colorectal cancer. Oncol Rep. 2017;38(3):1402–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zheng S, Jiang F, Ge D, et al. LncRNA SNHG3/miRNA-151a-3p/RAB22A axis regulates invasion and migration of osteosarcoma. Biomed Pharmacother. 2019;112:108695. [DOI] [PubMed] [Google Scholar]
- 17. Xu J, Wu M, Sun Y, Zhao H, Wang Y, Gao J. Identifying dysregulated lncRNA-associated ceRNA network biomarkers in CML based on dynamical network biomarkers. Biomed Res Int. 2020;2020:5189549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shi J, Li J, Yang S, et al. LncRNA SNHG3 is activated by E2F1 and promotes proliferation and migration of non-small-cell lung cancer cells through activating TGF-β pathway and IL-6/JAK2/STAT3 pathway. J Cell Physiol. 2020;235(3):2891–2900. [DOI] [PubMed] [Google Scholar]
- 19. Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172(3):393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sullenger BA, Nair S. From the RNA world to the clinic. Science. 2016;352(6292):1417–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yao RW, Wang Y, Chen LL. Cellular functions of long noncoding RNAs. Nat Cell Biol. 2019;21(5):542–551. [DOI] [PubMed] [Google Scholar]
- 22. Kato L, Begum NA, Burroughs AM., et al. Nonimmunoglobulin target loci of activation-induced cytidine deaminase (AID) share unique features with immunoglobulin genes. Proc Natl Acad Sci U S A. 2012;109(7):2479–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gong J, Li Y, Liu CJ, et al. A pan-cancer analysis of the expression and clinical relevance of small nucleolar RNAs in human cancer. Cell Rep. 2017;21(7):1968–1981. [DOI] [PubMed] [Google Scholar]
- 24. Xuan Y, Wang Y. Long non-coding RNA SNHG3 promotes progression of gastric cancer by regulating neighboring MED18 gene methylation. Cell Death Dis. 2019;10(10):694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pardo OE, Castellano L, Munro CE, et al. miR-515-5p controls cancer cell migration through MARK4 regulation. EMBO Rep. 2016;17(4):570–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. He WW, Ma HT, Guo X, Wu WM, Gao EJ, Zhao YH. LncRNA SNHG3 accelerates the proliferation and invasion of non-small cell lung cancer by downregulating miR-340-5p. J Biol Regul Homeost Agents. 2020;34(6):2017–2027. [DOI] [PubMed] [Google Scholar]
- 27. Zhao S, Gao X, Zhong C, Li Y, Wang M, Zang S. SNHG3 knockdown suppresses proliferation, migration and invasion, and promotes apoptosis in non-small cell lung cancer through regulating miR-216a/ZEB1 axis. Onco Targets Ther. 2020;13:11327–11336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Flotho A, Melchior F. Sumoylation: a regulatory protein modification in health and disease. Annu Rev Biochem. 2013;82:357–385. [DOI] [PubMed] [Google Scholar]
- 29. Bohren KM, Nadkarni V, Song JH, Gabbay KH, Owerbach D. A m55 V polymorphism in a novel SUMO gene (SUMO-4) differentially activates heat shock transcription factors and is associated with susceptibility to type I diabetes mellitus. J Biol Chem. 2004;279(26):27233–27238. [DOI] [PubMed] [Google Scholar]
- 30. Chang HM, Yeh E. SUMO: from bench to bedside. Physiol Rev. 2020;100(4):1599–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mevissen T, Komander D. Mechanisms of deubiquitinase specificity and regulation. Annu Rev Biochem. 2017;86:159–192. [DOI] [PubMed] [Google Scholar]
- 32. Swatek KN, Komander D. Ubiquitin modifications. Cell Res. 2016;26(4):399–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Seeler JS, Dejean A. SUMO and the robustness of cancer. Nat Rev Cancer. 2017;17(3):184–197. [DOI] [PubMed] [Google Scholar]
- 34. Shin EJ, Shin HM, Nam E, et al. Desumoylating isopeptidase: a second class of SUMO protease. EMBO Rep. 2012;13(4):339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-tct-10.1177_15330338211019376 for LncRNA SNHG3 Promotes Proliferation and Metastasis of Non-Small-Cell Lung Cancer Cells Through miR-515-5p/SUMO2 Axis by Yongqun Li, Lipin Gao, Caiyun Zhang and Jiguang Meng in Technology in Cancer Research & Treatment
Supplemental Material, sj-pdf-2-tct-10.1177_15330338211019376 for LncRNA SNHG3 Promotes Proliferation and Metastasis of Non-Small-Cell Lung Cancer Cells Through miR-515-5p/SUMO2 Axis by Yongqun Li, Lipin Gao, Caiyun Zhang and Jiguang Meng in Technology in Cancer Research & Treatment