Abstract
Background:
The relationship between the size of the primary tumor and the prognosis of patients with metastatic renal cell carcinoma (mRCC) is unclear. In this study, we aimed to investigate the significance of the size of the primary tumor in mRCC.
Methods:
We retrospectively reviewed the data of patients with mRCC who underwent cytoreductive nephrectomy (CN) from 2006 to 2013 in a Chinese center (n = 96) and those in the Surveillance, Epidemiology, and End Results (SEER) database (from 2004 to 2015, n = 4403). Tumors less than 4 cm in size were defined as small. Prognostic factors were analyzed using univariate and multivariate Cox proportional hazards regression analyses.
Results:
Patients with small tumors had a longer overall survival than other patients, both in the Chinese cohort (median, 30.0 vs 24.0 months, P = 0.026) and the SEER cohort (median, 43.0 vs 23.0 months, P < 0.001). After adjusting for other significant prognostic factors, small tumor size was still an independent protective factor in the Chinese cohort (adjusted hazard ratio [HR], 0.793; 95% confidence interval [CI]: 0.587–0.998, P = 0.043). In the SEER cohort, multivariate analysis showed that small tumor size was also an independent protective factor (HR, 0.880; 95% CI: 0.654–0.987, P = 0.008). In addition, as a continuous variable, a 1 cm elevation in tumor size translated into a 3.8% higher risk of death (HR, 1.038; 95% CI, 1.029–1.046; P < 0.001).
Conclusion:
Patients with small tumors may have a favorable prognosis after CN for mRCC. Although CN is not a standard protocol in mRCC, small tumor size may be a candidate when we are deciding to perform CN because of the potential benefit for OS.
Keywords: renal cell carcinoma, metastasis, primary tumor size, cytoreductive nephrectomy, prognosis
Introduction
Approximately 20%–30% of renal cell carcinomas (RCCs) are metastatic renal cell carcinoma (mRCC).1,2 Patients with metastases can be classified into different risk levels based on the Memorial Sloan Kettering Cancer Center (MSKCC) score or the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) score and receive appropriate systemic treatment, such as targeted therapy, immunotherapy, or combined therapies.3-5 Cytoreductive nephrectomy (CN), as surgical treatment, is not recommended in patients with intermediate- or poor-risk IMDC stratification based on several recent trials.6-8 However, the MSKCC and IMDC classifications mostly focus on the state of the performance status and laboratory values to predict outcomes, while the primary tumor status is not involved. Patients with a lower tumor burden may have a potential survival benefit by undergoing CN, which could be added to individual decision-making procedures.9
Although the primary tumor size was confirmed as a predictor of outcome in patients with localized disease, its role in predicting prognosis in patients with mRCC remains controversial.10 One study showed that early primary tumor size reduction is an independent predictor of improved survival outcomes in mRCC patients treated with targeted therapy.11 However, it is unclear whether the prognostic value of the primary tumor size remains after CN. Recently, DiNatale et al reported that a primary tumor size ≤ 4 cm was independently associated with improved overall survival (OS) in patients with metastatic clear cell RCC who had undergone surgery earlier.9 In the present study, we retrospectively examined the data of mRCC patients who had undergo CN retrieved from the database of a Chinese center and the Surveillance, Epidemiology, and End Results (SEER) program and aimed to determine how the size of the primary tumor affects the prognosis of mRCC patients.
Patients and Methods
With the approval of the Domain-Specific Review Board, records of patients with mRCC who underwent CN between 2006 and 2013 in a Chinese center were collected and retrospectively reviewed. We also identified 155,273 patients with renal tumors (International Classification of Disease for Oncology C64) between 2004 and 2015 in the SEER database. Of these, 97,951 patients with clear cell type, papillary type, and chromophobe type carcinomas were confirmed by histology. Among them, 8206 had metastatic disease. Patients with an unknown primary tumor size and an unclear survival status who were diagnosed by autopsy and those who underwent other local therapies or pre-surgical treatment were excluded. Finally, 4403 patients with mRCC treated with CN were eligible for the analysis. The process used to generate the analytic cohort is shown in Figure 1.
Figure 1.
The process of screening patients with metastatic renal cell carcinoma for analysis in the SEER cohort.
CN was defined as any nephrectomy performed in patients diagnosed with metastasis. Tumor size was defined as the maximum diameter of the primary tumor (cm) in the pathology review. A cutoff value of 4 cm was used for the primary tumor analysis based on current staging definitions, categorizing patients as having small (≤ 4 cm) or large tumors.10,12 Patient clinical information was obtained from the electronic medical record inquiry system and the SEER database. Variables including age at diagnosis, sex, ethnicity, marital status, year of diagnosis, tumor location, tumor size, MSKCC risk factors, tumor grade, T stage, N stage, and histological type were used for analysis. To be consistent with the SEER database regarding the tumor grading system, we reviewed our pathological slices. The SEER cohort did not cover the MSKCC risk factors and the subsequent treatment schedules because of unavailability of laboratory and drug information. The primary outcome of the study was OS.
Means, medians, and ranges are reported for continuous variables. Relationships between the groups were compared using the chi-squared test, Fisher’s exact test, or Student’s t-test. Univariate and multivariate Cox regression analyses were performed. Factors that were significant in the univariate analysis were included in a multivariate model. Statistical analyses were performed using the R software environment for statistical computing and graphics (version 4.0.0). R packages including readxl, survminer, ggplot2, ggpubr, and rms were used to analyze and visualize the data. Differences were considered statistically significant at P < 0.05.
Results
Patient Characteristics
A total of 96 patients from the Chinese cohort were included in this study, including 80 (83.3%) male and 16 (16.7%) female patients. The median primary tumor size was 7.0 cm (interquartile range [IQR]: 5.3–8.0 cm). Histologically, 90 (93.8%) patients had clear cells, and 6 (6.2%) had papillary histological types. There were 19 (19.8%), 16 (16.6%), 38 (39.6%), and 23 (24.0%) patients in the pT1, pT2, pT3, and pT4 stages, respectively. Sixty-three (65.6%) patients were in the intermediate-risk group, and 33 (34.4%) patients were in the poor-risk group. The metastatic sites were the lungs (n = 74, 77.1%), bones (n = 34, 35.4%), adrenal glands (n = 4, 4.2%), liver (n = 2, 2.1%), and spleen (n = 1, 1.0%). All patients received first-line targeted therapy after CN (51 patients received sunitinib and 45 patients received sorafenib). Eighty-seven (90.6%) patients exhibited progression, and 76 (79.2%) patients received second-line targeted therapies, including pazopanib, everolimus, or axitinib. Another 11 (11.5%) patients declined second-line therapies and received the best supportive care. Thirteen (13.5%) patients had small renal tumors (≤ 4 cm), and 83 (86.5%) patients had large renal tumors (> 4 cm). Patients in the tumor size ≤ 4 cm group were less likely to exhibit G3/G4 tumor grade (30.8% vs. 72.3%, P = 0.008), T3–T4 stage (23.1% vs. 69.9%, P = 0.003), and N1 stage (7.7% vs. 41.0%, P = 0.044). In addition, patients with small tumor size also had fewer metastatic sites (7.7% vs. 42.2%, P = 0.038). The patient characteristics are summarized in Table 1.
Table 1.
Association Between Tumor Size and the Clinicopathological Features of the Cohort of Chinese Center.
| Characteristics | Overall (n = 96) | ≤ 4 cm (n = 13) | > 4 cm (n = 83) | P value |
|---|---|---|---|---|
| Age at diagnosis, mean ± SD (year) | 60.3 ± 9.8 | 61.9 ± 11.0 | 60.1 ± 9.6 | 0.527 |
| Sex, n (%) | 0.789 | |||
| Male | 80 (83.3) | 10 (76.9) | 70 (84.3) | |
| Female | 16 (16.7) | 3 (23.1) | 13 (15.7) | |
| Marital status, n (%) | 0.632 | |||
| Married | 94 (97.9) | 12 (92.3) | 82 (98.8) | |
| Not Married | 2 (2.1) | 1 (7.7) | 1 (1.2) | |
| Year of diagnosis, n (%) | 0.151 | |||
| 2006-2010 | 58 (60.4) | 5 (38.5) | 53 (91.4) | |
| 2011-2013 | 38 (39.6) | 8 (61.5) | 30 (81.6) | |
| Tumor location, n (%) | 0.635 | |||
| Left | 57 (59.4) | 9 (69.2) | 48 (57.8) | |
| Right | 39 (40.6) | 4 (30.8) | 35 (42.2) | |
| MSKCC score, n (%) | 0.984 | |||
| Intermediate | 63 (65.6) | 8 (61.5) | 55 (66.3) | |
| Poor | 33 (34.4) | 5 (38.5) | 28 (33.7) | |
| Tumor grade, n (%) | 0.008 | |||
| G1/G2 | 32 (33.3) | 9 (69.2) | 23 (27.7) | |
| G3/G4 | 64 (66.7) | 4 (30.8) | 60 (72.3) | |
| T stage, n (%) | 0.003 | |||
| T1-T2 | 35 (36.5) | 10 (76.9) | 25 (30.1) | |
| T3-T4 | 61 (63.5) | 3 (23.1) | 58 (69.9) | |
| N stage, n (%) | 0.044 | |||
| N0 | 61 (63.5) | 12 (92.3) | 49 (59.0) | |
| N1 | 35 (36.5) | 1 (7.7) | 34 (41.0) | |
| Number of metastatic sites, n (%) | 0.038 | |||
| Solitary | 60 (62.5) | 12 (92.3) | 48 (57.8) | |
| Multiple | 36 (37.5) | 1 (7.7) | 35 (42.2) | |
| Histological types, n (%) | 0.397 | |||
| Clear cell | 90 (93.8) | 11 (84.6) | 79 (95.2) | |
| Papillary | 6 (6.2) | 2 (15.4) | 4 (4.8) | |
Abbreviation: MSKCC, Memorial Sloan Kettering Cancer Center.
Similarly, in the SEER cohort, 4403 patients included 3081 (70.0%) men and 1322 (30.0%) women with 3964 (90.0%) clear cells, 353 (8.0%) papillary, and 86 (2.0%) chromophobe histological types. The median primary tumor size was 9.0 cm (IQR: 6.5–11.5 cm). The number of patients with small and large renal tumors was 343 (7.8%) and 4060 (92.2%), respectively. Patients in the tumor size ≤ 4 cm group were less likely to present with G3/G4 tumor grade (47.2% vs. 70.6%, P < 0.001), T3-T4 stage (32.4% vs. 71.8%, P < 0.001), and N1 stage (14.6% vs. 24.0%, P < 0.001). Comparisons between tumor size and clinical and pathological features in the SEER cohort are presented in Table 2.
Table 2.
Association Between Tumor Size and the Clinicopathological Features of SEER Database.
| Characteristics | Overall (n = 4403) | ≤ 4 cm (n = 343) | > 4 cm (n = 4060) | P value |
|---|---|---|---|---|
| Age at diagnosis, mean ± SD (cm) | 61.0 ± 10.7 | 62.5 ± 11.5 | 60.9 ± 10.6 | 0.051 |
| Sex, n (%) | 0.669 | |||
| Male | 3081 (70.0) | 244 (71.1) | 2837 (69.9) | |
| Female | 1322 (30.0) | 99 (28.9) | 1223 (30.1) | |
| Marital status, n (%) | 0.513 | |||
| Married | 2934 (66.6) | 219 (63.8) | 2715 (66.9) | |
| Not Married | 593 (13.5) | 51 (14.9) | 542 (13.3) | |
| Others | 876 (19.9) | 73 (21.3) | 803 (19.8) | |
| Ethnicity, n (%) | 0.055 | |||
| White | 3761 (85.4) | 288 (84.0) | 3473 (85.5) | |
| Black | 307 (7.0) | 34 (9.9) | 273 (6.7) | |
| Others | 335 (7.6) | 21 (6.1) | 314 (7.8) | |
| Year of diagnosis, n (%) | 0.060 | |||
| 2004-2010 | 2347 (53.3) | 200 (58.3) | 2147 (52.9) | |
| 2011-2015 | 2056 (46.7) | 143 (41.7) | 1913 (47.1) | |
| Tumor location, n (%) | 0.564 | |||
| Left | 2273 (51.6) | 183 (53.4) | 2090 (51.5) | |
| Right | 2124 (48.2) | 159 (46.3) | 1965 (48.4) | |
| Bilateral | 6 (0.2) | 1 (0.3) | 5 (0.1) | |
| Tumor grade, n (%) | < 0.001 | |||
| G1/G2 | 981 (22.3) | 135 (39.4) | 846 (20.8) | |
| G3/G4 | 3028 (68.8) | 162 (47.2) | 2866 (70.6) | |
| GX | 394 (8.9) | 46 (13.4) | 348 (8.6) | |
| T stage, n (%) | < 0.001 | |||
| T1-T2 | 1366 (31.0) | 231 (67.3) | 1135 (28.0) | |
| T3-T4 | 3025 (68.7) | 111 (32.4) | 2914 (71.8) | |
| T0-TX | 12 (0.3) | 1 (0.3) | 11 (0.2) | |
| N stage, n (%) | < 0.001 | |||
| N0 | 3146 (71.5) | 271 (79.0) | 2875 (70.8) | |
| N1 | 1024 (23.3) | 50 (14.6) | 974 (24.0) | |
| NX | 233 (5.2) | 22 (6.4) | 211 (5.2) | |
| Histological types, n (%) | < 0.001 | |||
| Clear cell | 3964 (90.0) | 282 (82.2) | 3682 (90.7) | |
| Papillary | 353 (8.0) | 51 (14.9) | 302 (7.4) | |
| Chromophobe | 86 (2.0) | 10 (2.9) | 76 (1.9) |
Abbreviation: MSKCC, Memorial Sloan Kettering Cancer Center.
Correlation Between Primary Tumor Size and Survival Outcome
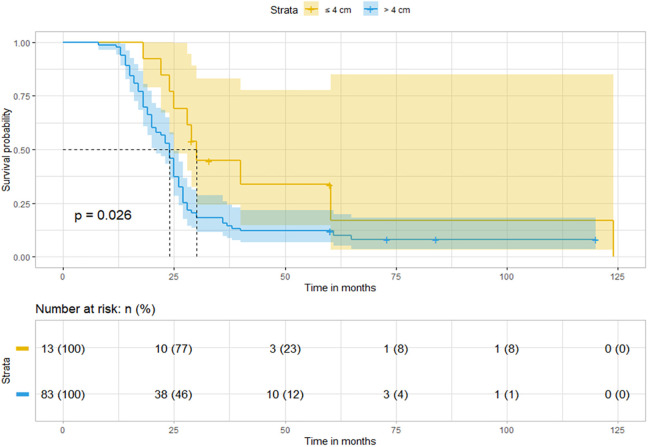
In the Chinese cohort, the median follow-up period was 73.0 months (IQR, 60.1–84.0 months). The 2- and 5-year OS rates were 57.3% and 14.9%, respectively. Compared to patients in the tumor size > 4 cm group, patients in the tumor size ≤4 cm group experienced longer OS (median, 30.0 vs. 24.0 months, P = 0.026) (Figure 2). In contrast, the median follow-up period in the SEER cohort was 75.0 months (IQR, 39.0-109.0 months). The 2- and 5-year OS rates were 50.4% and 25.3%, respectively. Patients in the tumor size ≤ 4 cm group achieved significantly longer OS (median, 43.0 vs. 23.0 months, P < 0.001) than patients in the tumor size > 4 cm group (Figure 3A).
Figure 2.
Patients with tumor size ≤ 4 cm achieved longer OS than those with tumor size > 4 cm in the Chinese cohort.
Figure 3.
Survival curves indicate differences in tumor sizes between different histological types in the SEER cohort. A, All histologies; (B) Clear cell; (C) Papillary; (D) Chromophobe.
Subsequently, we stratified the patients into different histological types to evaluate the significance of a small tumor size. Given the limited number of cases in the Chinese cohort, we only analyzed histologic types in the cohort of the SEER database. In the clear cell subgroup, patients in the tumor size ≤ 4 cm group (n = 282, 7.1%) had longer OS (median, 43.0 vs 24.0 months, P < 0.001) than patients in the tumor size > 4 cm group (n = 3682, 92.9%) (Figure 3B). In the papillary subgroup, patients in the tumor size ≤ 4 cm group (n = 51, 14.4%) had longer OS (median, 22.0 vs 14.0 months, P = 0.043) than patients in the tumor size > 4 cm group (302, 85.6%) (Figure 3C). In the chromophobe subgroup, the survival difference between patients in the tumor size ≤ 4 cm group (n = 10, 11.6%) and patients in the tumor size > 4 cm group (n = 76, 88.4%) was not statistically significant (median, 35.0% vs. 36.0%, P = 0.710) (Figure 3D).
To further evaluate the value of the primary tumor size in clinical outcomes, cutoff values of 4 cm, 7 cm, and 10 cm were used for the analysis of primary tumors based on the current TNM staging definitions. Patients were divided into 4 subgroups, including patients with tumor size ≤ 4 cm, 4 cm < tumor size ≤ 7 cm, 7 cm < tumor size ≤ 10 cm, and tumor size > 10 cm. Kaplan–Meier analysis showed that the survival curves of patients with tumor size > 7 cm were close and had shorter OS than other patients in both cohorts (Figure 4). The median OS for patients with tumor size ≤ 4 cm, 4 cm < tumor size ≤ 7 cm, 7 cm < tumor size ≤ 10 cm, and tumor size > 10 cm was 30.0, 25.0, 20.0, and 16.0 months, respectively, in the Chinese cohort, and 43.0, 30.0, 23.0, and 19.0 months, respectively, in the SEER cohort.
Figure 4.
Survival difference in patients with different tumor sizes based on the staging system. A, Chinese cohort; (B) SEER cohort.
Univariate and multivariate regression models were also constructed (Table 3). In the Chinese cohort, univariate survival analysis revealed that poor MSKCC risk (P < 0.001), tumor grade III/IV (P < 0.001), tumor size ≤ 4 cm (P = 0.006), T3/4 stage (P < 0.001), lymph node metastasis (P = 0.007), and clear cell pathological type (P < 0.001) were significant prognostic factors for OS. Similar results were observed in the SEER cohort: tumor grade III/IV (P < 0.001), tumor size ≤ 4 cm (P < 0.001), T3/4 stage (P < 0.001), and lymph node metastasis (P < 0.001). Moreover, age (P < 0.001) and marital status (P < 0.001) were also associated with prognosis. In addition, we also fit a univariate Cox model, including tumor size as a continuous variable, in the SEER cohort. The results revealed that a 1 cm elevation in tumor size translated into a 3.8% higher risk of death (hazard ratio [HR], 1.038; 95% confidence interval [CI], 1.029–1.046–; P < 0.001).
Table 3.
Univariate and Multivariate Cox Regression Analysis of Clinical Factors in Patients With Metastatic Renal Cell Carcinoma.
| Chinese center | SEER database | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||||||
| Variable | HR | 95% CI | P | Adjusted HR | 95% CI | P | HR | 95% CI | P | Adjusted HR | 95% CI | P |
| Age, continuous | 0.989 | 0.967 -1.013 | 0.374 | 1.008 | 1.005 -1.012 | < 0.001 | 1.012 | 1.008 -1.015 | < 0.001 | |||
| Sex, male | 1.253 | 0.673-2.334 | 0.466 | 0.937 | 0.869 -1.010 | 0.090 | ||||||
| Marital status, married | 3.854 | 0.534-27.821 | 0.181 | 0.868 | 0.807-0.934 | < 0.001 | 0.858 | 0.798-0.923 | < 0.001 | |||
| Tumor location, left | 1.085 | 0.699 -1.685 | 0.715 | 1.034 | 0.965 -1.108 | 0.340 | ||||||
| MSKCC score, poor | 2.458 | 1.358-4.475 | < 0.001 | 1.581 | 1.274-3.358 | 0.004 | ||||||
| Tumor grade, III/IV | 3.172 | 1.948-5.166 | < 0.001 | 1.410 | 0.852-2.334 | 0.082 | 1.495 | 1.385 -1.614 | < 0.001 | 1.318 | 1.217 -1.427 | < 0.001 |
| Tumor size, ≤ 4 cm | 0.475 | 0.237-0.952 | 0.006 | 0.793 | 0.587-0.998 | 0.043 | 0.652 | 0.567-0.749 | < 0.001 | 0.880 | 0.654-0.987 | 0.008 |
| T stage, T3/4 | 6.670 | 3.530-12.600 | < 0.001 | 4.272 | 1.958-9.323 | < 0.001 | 1.520 | 1.408 -1.641 | < 0.001 | 1.240 | 1.142 -1.346 | < 0.001 |
| N stage, N1 | 1.996 | 1.232-3.233 | 0.007 | 1.476 | 1.138-2.165 | 0.015 | 1.865 | 1.724-2.016 | < 0.001 | 1.723 | 1.590 -1.866 | < 0.001 |
| Pathology type, Clear cell | 9.548 | 2.960-30.800 | < 0.001 | 3.412 | 0.928-12.542 | 0.065 | 1.053 | 0.762 -1.954 | 0.130 | |||
Abbreviation: MSKCC, Memorial Sloan Kettering Cancer Center.
After adjustment for the MSKCC prognostic factors (P = 0.004), tumor grade (P = 0.082), T stage (P < 0.001), N stage (P = 0.015), and pathological type (P = 0.065), multivariate Cox regression analysis showed that tumor size ≤ 4 cm (adjusted HR, 0.793; 95% CI: 0.587–0.998, P = 0.043) was an independent protective factor in the Chinese cohort. In contrast, after adjusting for age (P < 0.001), marital status (P < 0.001), tumor grade (P < 0.001), T stage (P < 0.001), and N stage (P < 0.001), in the SEER cohort, tumor size ≤ 4 cm (adjusted HR, 0.880; 95% CI: 0.654–0.987, P = 0.008) was also found to be an independent protective factor.
Discussion
Although CN is a selective approach for mRCC, its value for survival remains controversial. Primary tumor size, an important clinical feature, is ignored in terms of the prognosis of mRCC patients. In this study, we investigated the survival outcomes of mRCC patients with small tumors (≤ 4 cm) undergoing CN in a Chinese center and the SEER database. The results revealed that a small primary tumor size was associated with improved OS and was found to be an independent protective factor in the multivariate analysis after adjustment. We consider that primary tumor size should be carefully considered when preparing individual treatment schedules.
The primary tumor size is of marked importance in localized kidney cancer13-15 and is also one of the most essential factors in the TNM stage,16 but its value in mRCC is neglected. DiNatale et al investigated the impact of primary tumor size on survival in patients with mRCC in MSKCC and IMDC cohorts who underwent CN.9 The results showed that in both the MSKCC and IMDC cohorts, patients with tumor sizes ≤ 4 cm had longer OS than patients with tumor sizes > 4 cm (129 vs. 37 months, P = 0.004; 77 vs. 38 months, P = 0.004, respectively). Similarly, in this study, we reviewed and analyzed mRCC patients who had small tumor sizes (≤ 4 cm) and had undergone CN in a Chinese cohort. We found that patients in the tumor size ≤ 4 cm group experienced longer OS (median, 30.0 vs. 24.0 months, P = 0.026) than those in the tumor size > 4 cm group. To confirm these findings, we conducted a similar analysis in the SEER cohort with 4403 patients who had undergone CN in the US. Finally, the results demonstrated that patients with tumor sizes ≤ 4 cm had longer OS (median, 43.0 vs. 23.0 months, P < 0.001) than patients with tumor sizes > 4 cm. In addition, in the subgroup of different histological types, patients with clear cell and papillary mRCC achieved a long survival time in the tumor size ≤ 4 cm group, while this difference was not significant in chromophobe RCC patients. In the multivariate analysis, tumor size ≤ 4 cm was confirmed as a protective factor. Based on these results, we consider that a small tumor size could constitute a new candidate for the selection of CN when appropriate. To further evaluate the value of the primary tumor size, we applied 4 cutoff values to estimate the survival difference. The results showed that the survival curve was close in patients with tumor sizes > 7 cm who experienced shorter survival time, which suggested that patients with tumor sizes > 7 cm undergoing CN may have limited benefit. We also analyzed the tumor size as a continuous variable in the SEER cohort and found that elevation of tumor size was associated with a high risk of death.
In the targeted era, some scholars believe that a portion of patients benefit from CN,17 while most scholars support the idea that targeted therapy alone is sufficient in mRCC patients based on the recently published CARMENA trial, showing that the median OS in a sunitinib-alone group was longer than that in a nephrectomy-sunitinib group (18.4% vs. 13.9%) in patients with intermediate-risk or poor-risk disease.6 However, this trial was based on the MSKCC model and failed to include all related factors. Many surgeons consider that CN should not be excluded, and the use of CN should be decided on a per person basis and follow individualized treatment schemes.18 Other factors, especially tumor-related factors, should be considered more comprehensively. Treatment decisions cannot be based only on the risk prediction of the IMDC or MSKCC models. In the Chinese cohort, we found that small tumor sizes were associated with fewer metastatic sites, indicating that these patients may have a lower tumor burden. Therefore, given that a small tumor size carries a lower tumor burden and is associated with improved OS, we hypothesize that it would be possible for tumor size to be part of the MSKCC or IMDC score to screen for eligible patients for CN or systemic treatment.
In this study, we also found that patients in the tumor size ≤ 4 cm group were less likely to exhibit high tumor grade, T stage, and N stage. According to the literature, tumors are commonly accompanied by intratumor heterogeneity,19,20 and the mechanism of early metastasis of small tumors is unclear. This may serve as a possible reason for CN, that is, to further clarify the specific extent of tumor malignancy in the specimen. However, further exploration of this mechanism is required. Interestingly, we also found that marital status was a protective factor for OS in patients who had undergone CN in Western populations from the SEER, which has also been reported in other published papers.21,22 We considered that being single could be indicative of poor quality of care. The medical community should pay particular attention to the vulnerability of single patients to assess the need for further treatment and research.
The present study had some limitations: First, its retrospective nature and the fact that we included patients from different time spans contributed to potential selection biases. Second, the SEER database does not allow stratification according to the MSKCC and IMDC classifications. The variables of specific metastases, laboratory values, adjuvant therapy, and treatment of recurrent disease were not complete or available in the SEER database. The major strength of this study was the large sample size of patients with mRCC who underwent CN. To the best of our knowledge, this was the largest study to evaluate the impact of tumor size on the OS of mRCC patients who have undergone CN.
In conclusion, mRCC patients with a primary tumor size ≤ 4 cm undergoing CN may have a favorable prognosis. Elevation in tumor size may also be associated with a high risk of death. Small tumor size may be a candidate when deciding to perform CN.
Abbreviations
- CI
Confidence Interval
- CN
Cytoreductive Nephrectomy
- HR
Hazard Ratio
- IMDC
International Metastatic Renal Cell Carcinoma Database Consortium
- IQR
Interquartile Range
- mRCC
Metastatic Renal Cell Carcinoma
- MSKCC
Memorial Sloan Kettering Cancer Center
- OS
Overall Survival
- RCC
Renal Cell Carcinoma
- SEER
Surveillance, Epidemiology, and End Results
Footnotes
Author Contributions: All authors listed in this manuscript contributed significantly to the study. Jiang WX contributed to data analysis and drafted the manuscript. Wen L contributed to update the data of clinical information. Zhang HJ and Zheng S contributed to reviewing the pathology slices. Li CL and Ma JH contributed to the study design and reviewed the manuscript. Shou JZ and Shi HZ contributed to critical revision of manuscript and final approval of the version to be submitted for critical revisions. All authors read and approved the final manuscript.
Availability of Data and Materials: The datasets used and/or analyzed data in the current study are available from the corresponding author on reasonable request.
Patient Consent for Publication: Patient study consent was not required due to the study’s retrospective nature.
Ethics Statement: The SEER data were released from an online publicly available database, and a data agreement form was submitted to the SEER administration. The research of Chinese cohort was reviewed and approved by the Domain-Specific Review Board, Cancer Hospital Chinese Academy of Medical Sciences (ID Num: NCC2016XQ-22). The authors state that they have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Doctoral Innovation Fund of Peking Union Medical College [2019-1002-61].
ORCID iD: Jianzhong Shou, MD  https://orcid.org/0000-0002-7984-1966
https://orcid.org/0000-0002-7984-1966
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 2. Capitanio U, Bensalah K, Bex A, et al. Epidemiology of renal cell carcinoma. Eur Urol. 2019;75(1):74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol 2019;30(5):706–720. [DOI] [PubMed] [Google Scholar]
- 4. Heng DYC, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27(34):5794–5799. [DOI] [PubMed] [Google Scholar]
- 5. Powles T, Albiges L, Staehler M, et al. Updated European Association of Urology guidelines: recommendations for the treatment of first-line metastatic clear cell renal cancer. Eur Urol. 2018;73(3):311–315. [DOI] [PubMed] [Google Scholar]
- 6. Méjean A, Ravaud A, Thezenas S, et al. Sunitinib alone or after nephrectomy in metastatic renal-cell carcinoma. N Engl J Med. 2018;379(5):417–427. [DOI] [PubMed] [Google Scholar]
- 7. Larcher A, Wallis CJD, Bex A, et al. Individualised indications for cytoreductive nephrectomy: which criteria define the optimal candidates? Eur Urol Oncol 2019;2(4):365–378. [DOI] [PubMed] [Google Scholar]
- 8. Bex A, Mulders P, Jewett M, et al. Comparison of immediate vs deferred cytoreductive nephrectomy in patients with synchronous metastatic renal cell carcinoma receiving sunitinib: the SURTIME randomized clinical trial. JAMA Oncol. 2019;5(2):271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. DiNatale RG, Xie W, Becerra MF, et al. The association between small primary tumor size and prognosis in metastatic renal cell carcinoma: insights from two independent cohorts of patients who underwent cytoreductive nephrectomy. Eur Urol Oncol. 2020;3(1):47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thompson RH, Hill JR, Babayev Y, et al. Metastatic renal cell carcinoma risk according to tumor size. J Urol. 2009;182(1):41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abel EJ, Culp SH, Tannir NM, Tamboli P, Matin SF, Wood CG. Early primary tumor size reduction is an independent predictor of improved overall survival in metastatic renal cell carcinoma patients treated with sunitinib. Eur Urol 2011;60(6):1273–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Motzer RJ, Jonasch E, Michaelson MD, et al. NCCN guidelines insights: kidney cancer, Version 2.2020. J Natl Compr Canc Netw 2019;17(11):1278–1285. [DOI] [PubMed] [Google Scholar]
- 13. Schiavina R, Borghesi M, Chessa F, et al. The prognostic impact of tumor size on cancer-specific and overall survival among patients with pathologic T3a renal cell carcinoma. Clin Genitourin Cancer. 2015;13(4):e235-e241. [DOI] [PubMed] [Google Scholar]
- 14. Brookman-May SD, May M, Wolff I, et al. Evaluation of the prognostic significance of perirenal fat invasion and tumor size in patients with pT1-pT3a localized renal cell carcinoma in a comprehensive multicenter study of the CORONA project. Can we improve prognostic discrimination for patients with stage pT3a tumors? Eur Urol. 2015;67(5):943–951. [DOI] [PubMed] [Google Scholar]
- 15. Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade, and necrosis: the SSIGN score. J Urol. 2002;168(6):2395–2400. [DOI] [PubMed] [Google Scholar]
- 16. Dunnick NR. Renal cell carcinoma: staging and surveillance. Abdom Radiol (NY) 2016;41(6):1079–1085. [DOI] [PubMed] [Google Scholar]
- 17. Heng DY, Wells JC, Rini BI, et al. Cytoreductive nephrectomy in patients with synchronous metastases from renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol 2014;66(4):704–710. [DOI] [PubMed] [Google Scholar]
- 18. Bhindi B, Abel EJ, Albiges L, et al. Systematic review of the role of cytoreductive nephrectomy in the targeted therapy era and beyond: an individualized approach to metastatic renal cell carcinoma. Eur Urol. 2019;75(1):111–128. [DOI] [PubMed] [Google Scholar]
- 19. McGranahan N, Swanton C. Biological and therapeutic impact of intratumor heterogeneity in cancer evolution. Cancer Cell. 2015;27(1):15–26. [DOI] [PubMed] [Google Scholar]
- 20. Beksac AT, Paulucci DJ, Blum KA, Yadav SS, Sfakianos JP, Badani KK. Heterogeneity in renal cell carcinoma. Urol Oncol. 2017;35(8):507–515. [DOI] [PubMed] [Google Scholar]
- 21. Rosiello G, Knipper S, Palumbo C, et al. Unmarried status is a barrier for access to treatment in patients with metastatic renal cell carcinoma. Int Urol Nephrol 2019;51(12):2181–2188. [DOI] [PubMed] [Google Scholar]
- 22. Zhang SL, Sun HT, Li ZM, et al. A real-world 1:1 propensity-matched study revealed unmarried status was independently associated with worse survival for patients with renal clear cell carcinoma. J Cancer 2019;10(16):3767–3777. [DOI] [PMC free article] [PubMed] [Google Scholar]