Abstract
Gulf War Illness is a multisymptomatic condition which affects 30% of veterans from the 1991 Gulf War. While there is evidence for a role of peripheral cellular and humoral adaptive immune responses in Gulf War Illness, a potential role of the adaptive immune system in the central nervous system pathology of this condition remains unknown. Furthermore, many of the clinical features of Gulf War Illness resembles those of autoimmune diseases, but the biological processes are likely different as the etiology of Gulf War Illness is linked to hazardous chemical exposures specific to the Gulf War theatre. This review discusses Gulf War chemical–induced maladaptive immune responses and a potential role of cellular and humoral immune responses that may be relevant to the central nervous system symptoms and pathology of Gulf War Illness. The discussion may stimulate investigations into adaptive immunity for developing novel therapies for Gulf War Illness.
Keywords: Gulf war syndrome, gulf war chemicals, adaptive immunity, central nervous system, therapeutics
Significance Statement
Gulf War Illness (GWI) is possibly caused by exposure to hazardous chemicals and pesticides which may be capable of stimulating adaptive immune responses. In this review, we focus on how Gulf War chemical–induced maladaptive immune responses may contribute to the central nervous system (CNS) pathology in GWI.
Introduction
The 1990 to 1991 Persian Gulf War (GW) was a rapid response by the United States and allies to the invasion of Kuwait by Iraq. During the course of this war, almost 700 000 U.S. troops and military personnel from Australia, Canada, Denmark, France, and the United Kingdom were deployed to the Persian Gulf region. Although the war itself was brief with low combat casualties, nearly 30% of returning soldiers began reporting multiple symptoms which included fatigue, headaches, gastrointestinal discomfort, respiratory issues, chronic pain, and cognitive problems.1 This condition is now termed Gulf War Illness (GWI), and results from exposure to various hazards experienced by soldiers during the conflict, including chemicals such as pesticides, nerve agents, and prophylactics against these nerve agents (GW chemicals).1,2 This review will primarily focus on GW chemicals as triggers of adaptive immune responses that contribute to the central nervous system (CNS) symptoms observed in veterans with GWI. The objective is to identify underlying pathological processes which could be further investigated to identify novel therapeutic targets and biomarkers of GWI.
During the 1991 GW, soldiers were exposed to a variety of hazardous chemicals; smoke and combustion products from oil-well fires, multiple vaccines, pesticides, the acetylcholinesterase (AchE) inhibitor pyridostigmine bromide (PB) and nerve agents such as sarin gas.2 Given the types of symptoms reported by veterans, initial hypotheses around causal factors for GWI focused on vaccinations.3,4 Military personnel deployed to the Gulf region received multiple vaccinations including these against anthrax, tetanus, diphtheria, typhoid, and yellow fever.5 A potential role of multiple vaccination in GWI was initially promoted by Rook and Zumla, who suggested that large shifts away from normal immune responses after combined vaccination could have produced symptoms commonly reported by veterans with GWI.6 Several studies also suggested an association between soldiers receiving different combinations of vaccines and GWI onset.2,7,8 For instance, a cross-sectional study showed that GWI symptoms such as fatigue, psychological distress, and poor physical functioning were associated with multiple vaccines received during deployment.4 However, Steele et al9 showed no association between vaccines and GWI symptom reporting once analyses were adjusted for exposure to GW chemicals. Boyd et al10 showed that when comparing low versus high symptom self-reporting, the botulism vaccine was associated with increased reporting of symptoms whereas all other vaccines were not significant, particularly when analyzed with respect to PB use and chemical exposure. Concerns were raised against the use of pertussis and squalene as adjuvants for the anthrax vaccine without sufficient a priori research testing. Some studies have suggested that squalene in the anthrax vaccine may have also contributed to GWI, as antibodies against squalene were more prevalent among veterans with GWI compared to healthy GW veterans.11,12 Another study showed no association between the presence of squalene antibodies and the diagnosis of GWI.13 A recent case-control study of GWI showed that even though cases appeared to have a higher rate of vaccination compared to controls, adverse effects reported by GWI patients were more strongly associated with pesticide exposure.14 Both 2008 and 2016 reports by the Research Advisory Committee on GW Veterans’ Illnesses concluded that potential contributions of vaccines to GWI remain unconfirmed.1,2 Furthermore, in the absence of animal studies that evaluate GWI-related neurobehavioral changes and neuropathology following combined vaccine administration, a possible causal role of multiple vaccination in GWI pathogenesis remains to be determined.
Among GW chemical exposures, PB has been widely accepted as one of the key contributors to GWI. Soldiers were instructed to take PB tablets as an anti-nerve agent at a dose of 90 mg per day.2 However, variations in use occurred as some troops took up to 3 times more than the recommended dosage.2 Use of PB among GW veterans has also been associated with a higher rate of motor and cognitive impairment when compared to soldiers who did not consume PB.15 Some studies showed that an increase in the severity of symptom reporting by GW veterans was associated with an increase in days of PB consumption. A higher prevalence of GWI diagnosis was associated with consumption of ⩾21 pills of PB compared to those who consumed <21 pills.8,16 However, many of these studies were unable to detect an independent effect of PB alone and suggested potential interactions with stress and other GW chemicals as additional key contributors to the etiology of GWI.1,2
According to the 2003 Environmental Exposure Report by the Department of Defense (DoD), it was estimated that 15 different pesticides, including 13 insecticides used by GW soldiers, were of significant concern due to their overuse in the theatre.17 These included pyrethroids, DEET (N,N-diethyl-3-methylbenzamide) and organophosphate (OP) AChE inhibitors.17 Permethrin (PER), a pyrethroid, was either provided as a 0.5% spray or was imbedded in their uniforms.17 In addition to commonly available 33% DEET as cream, during the GW, soldiers were also provided a liquid form which contained 75% DEET.2 Among ground troops, 62% reported using PER or DEET ranging from 20 to 30 times per month. Gulf War veterans who reported using PER or DEET experienced symptoms consistent with GWI diagnosis compared to those who did not use these chemicals.2 Organophosphate pesticides (eg, chlorpyrifos [CPF], dichlorvos, and malathion) have also been described as contributors to GWI since these were used as fogs and sprays during GW by pesticide applicators who reported chronic health problems after returning from the conflict.2,18 In contrast to chronic GWI presentation, acute OP poisoning symptoms generally develop immediately after exposure and range from mild tremors to severe muscle contractions, dizziness, headaches, abdominal cramps, nausea, vomiting, and blurred vision.19 In some circumstances, OP-induced delayed neuropathy (OPIDN)2 develops several weeks later and consists of distal weakness and sensory loss.20 The DoD reported that munitions containing 8.5 metric tons of sarin/cyclosarin were destroyed by U.S personnel at Khamisiyah in Iraq and exposed about 20 000 individuals to low levels of OP gas.17 Among soldiers who witnessed the Khamisiyah demolition, some reported symptoms resembling those of acute OP poisoning1 and chronic cognitive deficits have been reported by GW veterans with predicted exposure to Khamisiyah plumes.21 Additionally, severity of symptom reporting by GW veterans worsened with longer durations of pesticide use,8 suggesting a dose-response relationship between exposure to GW chemicals and GWI symptomatology. As such, there is a high likelihood that exposure to these pesticides contributed to the symptoms experienced by ill GW veterans.
It is also reported that soldiers who were more frequently using multiple pesticides were also taking more PB pills.2 For instance, nearly 1 in 4 GW veterans reported using several pesticides ranging from 51 to 120 times in a month while taking 15 to 19 PB pills during the same time frame.2 Animal studies show that compared to a single GW chemical, combined exposure to several of these GW chemicals resulted in higher occurrences of neurobehavioral deficits consisting of cognitive impairment, fatigue, anxiety and neuropathological alterations suggesting activation of the CNS immune system (ie, astroglia and microglia activation and neuroinflammation).1,22−26 These studies are supported by neuroimaging work showing structural alterations in the brains of veterans with GWI consisting of neuroinflammation, neuritic loss and a disruption of white matter integrity.27−30 Collectively, these studies provide a strong rationale for a role of GW chemicals in the pathobiology of GWI and provide additional support that these chemicals may have triggered chronic and persistent health problems experienced by veterans with GWI.
Role of Immune System in GWI
As mentioned above, the clinical presentation of GWI suggests that ongoing immune dysfunction is one of the key drivers in the chronic pathology of this illness. Current literature suggests that both the innate and adaptive immune systems play a role in the chronic and persistent pathophysiology of GWI. While a role of innate immunity in GWI has been well-described and reviewed elsewhere,31,32 this review will focus primarily on the role of adaptive cellular and humoral immune responses (Figure 1) in the context of GW chemical exposure and discuss how such chemicals can promote long-lasting changes in the immune system. Discussions will also include how crosstalk between the brain’s innate immune system and the peripheral adaptive immune cells could contribute to the CNS pathology in GWI.
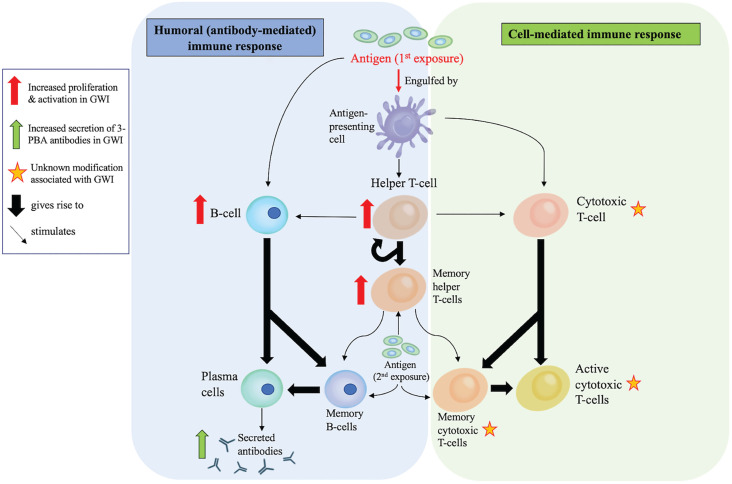
Figure 1.
Elements of the adaptive immune system that are observed to be altered in GWI: The adaptive immune system involves antigen-presenting cells, such as dendritic cells, monocytes and macrophages (cells that also have a role in innate immunity). The humoral responses are largely mediated by B-cells which are capable of directly responding to antigens and producing antibodies to neutralize them. The cellular responses are mediated by CD4 + T-helper cells and cytotoxic CD8 + T-cells which mount a highly specific response against antigens. Ultimately, these cells can generate memory T-cells that recruit B-cells to produce antibodies for neutralizing foreign antigens. The long-term memory T-cells and B-cells are responsible for producing long-lasting immunity. Thus far, observations of CD4-mediated immune responses and humoral responses have been observed in animal models and/or in blood of veterans with GWI. However, activation of CD8-mediated cytotoxic T-cell responses remains to be observed in GWI.
Adaptive Immune Responses to GW Chemicals in GWI
It was previously assumed that small chemicals (<1000 Daltons) can escape detection by the immune system. It is now known that many of these chemicals can become haptens by forming adducts with endogenous proteins, allowing recognition by the immune system as new antigens, thus causing an activation of adaptive immune responses.33,34 Classic examples of hapten-forming compounds include non-steroidal anti-inflammatory drugs (NSAID), penicillin and certain diuretics, all of which can trigger allergic immune responses.33 Certain of GW chemicals can also form protein adducts, suggesting that endogenous proteins modified by GW chemicals could serve as haptens. A study of CPF poisoning in humans showed that CPF, or its metabolite CPF-oxon CPO), can form adducts with tyrosine residues in human albumin.35 Sarin itself can also form adducts with serum albumin and butyrylcholinesterase, and such adducts have been detected in the tissue from a female fatally poisoned during the 2013 nerve gas attack in Syria.36 The major PER metabolite 3-phenoxybenzoic acid (3-PBA) undergoes glucuronidation in the liver prior to urinary excretion.37 These glucuronides can form adducts with specific amino groups on proteins.37 Protein adducts of 3-PBA have been measured in human plasma from farm workers and represent biomarkers of chronic PER exposure.38 Others have shown that protein adducts of 3-PBA can be detected a month after exposure to PER in guinea pigs (Noort pers. comm.). Consistent with these findings, Joshi et al39 showed that 3-PBA haptens can be detected up to one-month after exposure to PER in mice and in serum from individuals with chronic pyrethroid exposure. As with 3-PBA, it is possible that a common DEET metabolite, m-toluic acid, can undergo the same glucuronidation process and form adducts with endogenous proteins.40 However, this has not been evaluated yet and studies have yet to report whether PB can also form adducts with proteins.
Certain haptens have been detected in lymph nodes where they are displayed on the major histocompatibility complex (MHC) protein by dendritic cells (DC)41,42 and other antigen presenting cells (APC). In the lymphatic system, these APCs display MHCII-bound haptens to naive T-cells, resulting in the generation of antigen-specific effector and memory T-cells and subsequent activation of B-cells, finally resulting in antibody production against the haptenated proteins.41,43 Although this has not been studied extensively in the context of GW chemicals, an ex vivo murine blood culture study showed that CD19+ and CD27+ B-cells were increased after administration of 3-PBA-albumin compared to free 3-PBA, albumin, or control treatments.39 Additionally, effector T-cells (CD3+ and CD4+ T-helper [TH] cells) were elevated after 3-PBA-albumin treatment compared to all other treatments.39 These studies suggest that GW chemicals or their metabolites can activate peripheral adaptive immune responses. This led us to hypothesize that exposure to GW chemicals that can form adducts with proteins and become haptens that may then elicit immune changes that persist for decades after exposure to such chemicals (Figures 2 and 3). Furthermore, once the adaptive immune system becomes maladaptive, chronicity of GWI can be explained by skewed cellular, and humoral immune responses observed in veterans with GWI that are summarized below.
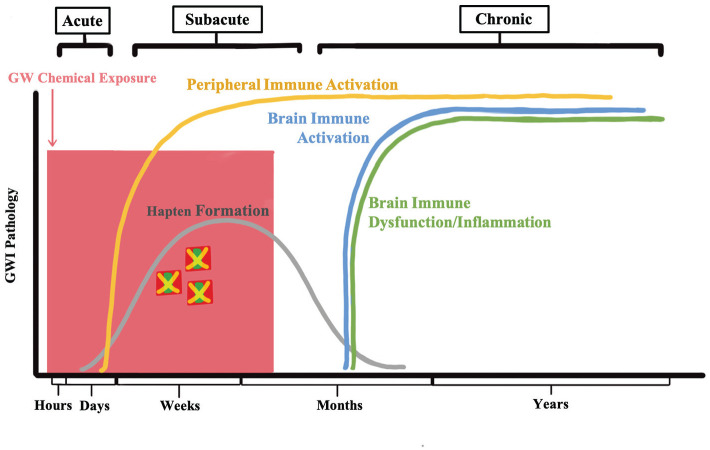
Figure 2.
A hypothetical timeline of GW pesticide-induced immune dysfunction. This figure shows how the initial exposure to GW chemicals may give rise to chronic and persistent immune dysfunction associated with GWI.
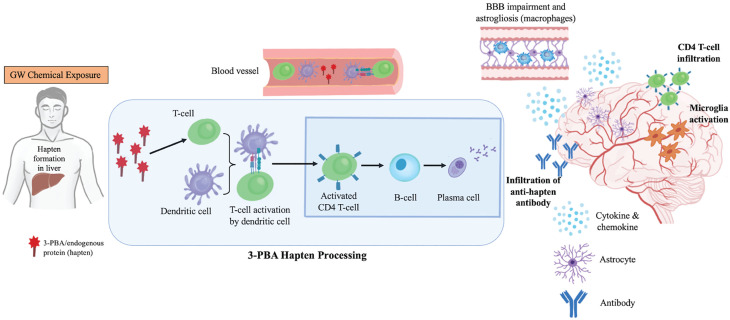
Figure 3.
Schematics of GW pesticide-induced immune dysfunction hypothesis. Pesticide/pesticide metabolite-protein formation in the liver is followed by antigen presentation by APC to T-cells in the periphery followed by B-cell activation results in autoantibody production. These autoantibodies or the peripheral immune cells enter the brain after transient opening of the BBB and cross-react with brain proteins that are similar to haptenated proteins and contribute to the activation of brain immune cells, including microglia and astroglia, and inflammation. Antigen presentation by brain macrophages that are part of the adaptive immune responses is also possible.
Activation of Cellular Adaptive Immunity in GWI
Veterans with GWI have elevated levels of lymphocytes and monocytes.39,44,45 Increases in B-cells and memory B-cells (CD19+ and CD27+) have been observed in the blood of veterans with GWI39,44 and in a mouse model of GWI.39 A study conducted in early 2000 showed an increase in CD4+/CD8+ T-cell ratios in the blood of veterans with GWI compared to controls.44 Whistler et al46 observed lower ratios of CD4/CD8 in GWI compared to controls but an absolute CD4+ and CD8+ count did not differ between these 2 groups. Elevated CD3+ CD4+ but no change in CD8+ T-cells corresponded with higher ratios of CD4/CD8 cells in veterans with GWI and in mice exposed to GW chemicals.39 Zhang et al47 showed elevated levels of CD3+ CD4+ T-cells among GW veterans with chronic fatigue syndrome (CFS) compared to healthy GW veterans. The inconsistency of CD4/CD8 ratios may be due to the timing of these studies in relation to the chronicity of GWI or possible methodological differences where a generalized CD8 would not differentiate between cytotoxic-T-cells and contributions from other cells.48 For instance, CD8 is expressed not just on T-cells but it can also be found on the natural killer (NK) and dendritic cells48 and, therefore, if studies did not select specifically for CD3+ T-cells, the ratios could represent contributions of CD8 from NK and dendritic cells as well. Since CD4+ T-cells initially differentiate into pro-inflammatory TH1 and anti-inflammatory TH2 lineage, many studies have focused on these immune responses in GWI.49−53 Rook and Zumla proposed that multiple vaccinations may have contributed to a shift in a TH2 immune response, presenting with increases in anti-inflammatory cytokines.6 However, most studies conducted to date do not support a strictly TH1 or TH2 type immune response. For example, Skowera et al50 showed increases in CD4+ T-cells which produce TH1 skewed pro-inflammatory cytokines, such as interleukin (IL)-2, IL-1β, interferon gamma (IFN-γ), and Tumor Necrosis Factor alpha (TNF-α) among ill compared to healthy GW veterans. Elevated plasma levels of pro-inflammatory cytokines, such as IL-6, IFN-γ, and TNF-α and chemokines CX3CL1, CCL5 and CXCL8 are also reported in GWI compared to controls.39,52,54−56 Low TNF-α levels have also been reported in veterans with GWI compared to healthy controls.52 A mouse model of GWI shows changes in IL-6, IL-1β and IFN-γ levels; many of these cytokines are chronically elevated in blood of mice exposed to GW chemicals compared to control mice.39,57−59
Interleukin-4 and IL-10 are generally associated with TH2 responses to suppress inflammation.60 In GWI veterans, CD4+ T-cells expressing IL-4 were elevated in comparison to healthy GW veterans.50 While IL-10 expressing CD4+ T-cells did not differ at baseline, these cells increased IL-10 expression in GWI compared to controls upon stimulation.50 However, plasma levels of IL-4 are lower and no changes are observed in IL-10 in GWI compared to healthy controls.52,55 Other studies have shown elevated TH17 cytokines in GWI compared to control GW veterans, also supporting an activation of pro-inflammatory responses in GWI52,61,62 (see Table 1 for a summary of TH immune response in GWI). Hence, reported variations across these studies with respect to TH1 and TH2 responses may be due to the timing of these studies in relation to GW chemical exposure and the chronicity of this illness. Despite these differences, collectively, these studies suggest mixed TH1 and TH2 response as well as TH17 responses often observed in patients with autoimmune diseases, such as Graves’ disease and Rheumatoid Arthritis,63,64 and may also suggest an autoimmune component to GWI. In most autoimmune disorders, however, dysregulated cellular immune responses are secondary to reduced immunological self-tolerance,65 which has not yet been reported in GWI.
Table 1.
Summary of TH1, TH2, and TH17 cytokine profiles in veterans with GWI compared to control veterans.
| TH responses | Cytokines | GWI vs. controls | Author | Summary |
|---|---|---|---|---|
| TH1 | IL-2 | Upregulated | Zhang et al47 | GW veterans with CFS had increased mRNA expression of these cytokines in peripheral blood lymphocytes. |
| IFN-g | ||||
| TNF-a | ||||
| TH2 | IL-10 | Upregulated | ||
| TH1 | IFN-g | Downregulated | Everson et al49 | IFN-g was lower in unstimulated cell supernatant of symptomatic GW compared to asymptomatic veterans. |
| IL-6 | No change | |||
| TNF-a | No change | |||
| TH1 | IFN-g | Upregulated | Skowera et al50 | Both TH1 and TH2 cytokines on CD4+ T-cells were elevated in ill compared to healthy GW veterans. Upon activation, CD4+ T-cells expressing IL-10 increased in ill GW veterans. |
| IL-2 | ||||
| TH2 | IL-4 | Upregulated | ||
| IL-10 | ||||
| TH2 | IL-5 | Upregulated | Smylie et al51 | Sex bias was observed at peak effort with female veterans with GWI showing elevated plasma IL-5 levels compared to healthy female GW veterans. IL-13 was only elevated in plasma from male veterans with GWI compared to healthy male veterans. |
| IL-13 | ||||
| TH1 | IL-1b | Upregulated | Parkitny et al53 | Apart from IL-1b, most TH1, TH2 and TH17 cytokines in serum did not differ between the groups. Both IL-1b and IL-15 were correlated with daily fatigue severity. |
| IL-15 | No change | |||
| TH2 | IL-10 | No change | ||
| TH17 | IL-17 | No change | ||
| TH1 | IFN-g | Upregulated | Khaiboullina et al52 | In serum, 14 (17.7%) out of 77 cytokines were significantly different between GWI and control subjects. |
| TNF-a | Downregulated | |||
| TH17 | IL-17A | Upregulated | ||
| IL-17F | ||||
| TH2 | IL-33 | Upregulated | ||
| IL-5 | ||||
| TH2 | IL-13 | Downregulated | ||
| IL-25 | ||||
| IL-4 | ||||
| TH2 | IL-6 | Upregulated | Broderick et al54 | Plasma IL-6 was elevated in GWI compared to controls. In stimulated PBMC, IL-5 was elevated at before exercise but IFN-g increased post-exercise in stimulated cells. |
| IL-5 | ||||
| IL-10 | ||||
| TH1 | IFN-g | Upregulated | ||
| TNF-a | No difference | |||
| IL-6 | No difference | |||
| TH1 | IL-6 | Upregulated | Butterick et al55 | Plasma IL-6 was marginally increased in GWI and correlated with plasma CRP. |
| TH1 | IFN-g | Upregulated | Joshi et al39 | Plasma TH1 cytokines IFN-g and IL-1b were significantly elevated and TNF-a and IL-6 being marginally elevated in GWI compared to controls. |
| IL-6 | ||||
| IL-1b | ||||
| TNF-a | ||||
| TH2 | IL-5 | No difference | ||
| TH1 | IL-6 | Upregulated | Alshelh et al56 | These cytokines were marginally increased in GWI compared to control subjects. |
| IL-1b | ||||
| TNF-a |
Antibody Mediated Adaptive Immune Responses in Veterans with GWI
Evidence for abnormal humoral immune responses in GWI is evidenced by increases in immunoglobulin G (IgG) and immunoglobulin M (IgM) in the blood of those with GWI compared to healthy GW veterans.44 The presence of anti-nuclear antibodies (ANA), which target nuclear envelop proteins in autoimmune diseases,66 have also been detected in serum of pyrethroid-exposed individuals.67 Skowera et al68 studied this in the context of GWI, but serum ANA levels did not differ between GWI as compared to healthy GW veterans. Presence of antibody-antigen complexes which engage the complement system is also a feature of systemic lupus erythematosus (SLE)-type autoimmune diseases.69 To assess the presence of immune complexes, Vojdani et al44 examined anti-C1q levels which were elevated in veterans with GWI compared to controls. A pilot study examining circulating immune complex (CIC) associated with complement C3d showed that their levels did not differ between veterans with GWI compared to healthy GW veterans (Abdullah, unpublished data).
A recent study focusing on GW pesticide exposure detected antibodies against 3-PBA-albumin in blood from veterans with GWI and in a mouse model of GWI.39 Importantly, antibodies from veterans with GWI, and GW chemical-exposed mice did not cross-react with albumin alone.39 These studies suggest that humoral responses in GWI do not resemble those of typical autoimmune diseases and that there may be humoral responses specific to GW chemical exposure. While peripheral antibodies may be of diagnostic value, particularly those that are specific to GW chemicals, their role in the chronic pathology of GWI (if any) has yet to be determined.
Crosstalk between Innate and Adaptive Immunity in the CNS Pathology of GWI
Under normal physiological conditions, immune surveillance of the brain by leukocytes, T-cells, B-cells and monocytes, is largely restricted to the choroid plexus (CP) and the meninges by the presence of the blood brain barrier (BBB).70 The meningeal lymphatic system lies beneath the dural sinuses and allows transport of surveying immune cells from the CNS to the lymphoid organs.71 Under pathological conditions, immune cells in the extra-parenchymal compartments secrete cytokines that alter BBB permeability, allowing these leukocytes to gain access to the brain parenchyma where they play a role in the ongoing brain pathology.70 Microglia are viewed as the resident innate immune cells of the brain and play a key role in modulating immune responses within the brain parenchyma. While astroglia are not traditional immune cells, they do participate in both the innate and the adaptive immune responses in the CNS.72 Activation of these cells has been reported in animal models of GWI23−25,39 and Translocator protein, a marker of microglia, and astroglia activation, is upregulated in the brains of veterans with GWI.56 In addition to these cells, infiltrating blood monocytes and lymphocytes also contribute to brain inflammation in certain autoimmune conditions like Multiple Sclerosis (MS).70 In MS, CNS immune dysfunction is associated with the failure of regulatory mechanisms which suppress autoreactivity and maintain self-tolerance.70 However, it is unclear if self-tolerance mechanisms of the adaptive immune system have become abnormal in GWI. Furthermore, GWI does not appear to have an MS-type clinical presentation and no evidence exists of an increased risk of MS in veterans with GWI.
There is also a possibility that peripheral immune dysregulation in GWI could alter the inflammatory milieu in the brain. For instance, during systemic inflammation, peripheral cytokines can increase matrix metalloproteinase 9 (MMP9) activity at the BBB and enhance the transmigration process, allowing peripheral immune cells to infiltrate the CNS.73 At 7-months post-exposure to PB and PER in mice, BBB disruption was evident through significant increases in MMP9 and IgG but showed decreases in the tight junction protein occludin in the brains of exposed mice.39 Hence, these studies suggest that BBB disturbances may allow peripheral immune cells to infiltrate the CNS. Circulating monocytes are capable of replenishing long-lived brain macrophages that reside within the leptomeningeal compartments of the brain.74 Animal studies suggest that under inflammatory conditions, astroglia can release chemokines, such as Chemokine (C-C motif) ligand 2 (CCL2), which recruit these macrophages and/or peripheral monocytes to the CNS.70 Release of CCL2 by astrocytes in the brain promotes the egress of blood monocytes into the parenchymal space in mice.71 Using a mouse model of GWI, Joshi et al58 showed that C-C chemokine receptor (CCR2) and its ligand CCL2 were elevated in the brains of GW chemical exposed mice. Increases in CCL2 mRNA was also detected in the brains of mice exposed to corticosterone and a sarin surrogate Diisopropyl fluorophosphate (DFP).75 In GW chemical exposed mice, increases of blood CD11b+ and CD115+ monocytes corresponded with increases in macrophages (CD11b+, CD206+, and Ly6C+)39 that are derived from monocytes capable of infiltrating the brain,76,77 which are distinct from brain-resident microglia.77 These studies suggest that a communication between peripheral leukocytes and resident immune cells in the brain may be integral to the ongoing brain pathology associated with GWI. This process is unlikely to be specific to the initial pathogenic drivers of GWI, but nevertheless suggests their role in the chronic CNS pathology of GWI.
When there is a breakdown of self-tolerance with impaired tolerogenic responses,65 peripheral APC and lymphocytes entering the brain parenchyma following BBB disruptions may perceive brain proteins as foreign antigens and promote antibody production against brain proteins.78 Alternatively, brain proteins leaking into the periphery may be seen as foreign antigens by the peripheral immune cells which may then give rise to antibodies against brain proteins.79 In either circumstance, antibodies could be raised against brain proteins via activation of humoral immune responses. In that regard, a recent study showed that autoantibodies against brain proteins, such as glial fibrillary acidic protein (GFAP), myelin basic protein (MBP) and tau are elevated in veterans with GWI compared to controls.80 These brain-specific autoantibodies have been detected in individuals with chronic fatigue syndrome (CFS) and in individuals exposed to OP pesticides.81,82 Elevation of brain protein-specific autoantibodies has also been seen in other conditions, such as MS, traumatic brain injury (TBI), and Alzheimer’s disease (AD).83,84 These occurrences appear to accompany a wide range of neurological illnesses71 and may represent secondary aspects following a variety of brain insults. However, mechanisms that contribute to humoral responses against brain proteins in GWI remain unknown, as currently there is no evidence for an infiltration of peripheral lymphocytes in the brains or leakage of brain proteins in the periphery for either veterans with GWI or in current rodent models following GW chemical exposure.
It is thought that antibodies against peripheral antigens may enter the brain through the circumventricular organs or when the BBB is compromised. For example, Sydenham’s chorea (SC), a neurological manifestation of rheumatic fever, is an illness where antibodies against Group A β-hemolytic streptococcus cross-react with neuronal proteins.85 In this regard, SC represents a good example of a humoral immune response to an environmental antigen that results in cross reactivity to self-proteins and subsequently causes damage to the brain. In SLE, N-methyl-D-aspartate (NMDA) receptors are targeted by peripheral autoantibodies that were originally generated against self-antigens (ie, double-stranded DNA) due to reduced immunological tolerance and inadequate clearance of apoptotic debris.65,86 In SLE, pathologic autoantibodies are thought to interfere with neurotransmission, alter cellular signaling and promote neuroinflammation.87 For instance, anti-NMDA antibodies, isolated from patients with SLE and administered to mice, are shown to cause neuronal damage by activating the microglial complement component C1q.71 Interestingly, anti-NMDA antibodies isolated from patients with psychosis reduced synaptic transmission compared to antibodies from control subjects.88 These studies suggest that antibodies against foreign or self-antigens are capable of disturbing the neuroimmune homeostasis in the brain. However, at present, the role of the adaptive immune system in the CNS pathology of GWI and the communication between the peripheral adaptive immune cells and the CNS resident immune cells remains unclear in GWI.
Conclusions and Future Directions for Understanding the Molecular Mechanisms of GW Chemical-Induced Adaptive Immune Dysregulation Associated with the Chronic CNS Pathology of GWI
Clinical similarities between GWI and other autoimmune disorders initially suggests a presence of comparable underlying adaptive immune responses. Cellular immune responses in GWI appear to be closely related to RA, but a lack of complement cascade activation, ANA and anti-phospholipid antibodies suggests GWI is unlikely to be share features of classic autoimmune diseases. Hence, drawing parallels between GWI and autoimmune diseases may be somewhat misleading given that many biological features of GWI appear to be inherent to GW chemical exposures and do not follow the classic clinical patterns of aforementioned autoimmune diseases. Interestingly, the presence of 3-PBA hapten antibodies and CNS immune activation supports further investigation into the mechanisms associated with GW chemical-induced disturbances in adaptive immunity. There exist several key outstanding questions with respect to GW chemical-induced immune dysfunction which require further attention. For instance, there is no evidence that GW chemical adducts and any haptens (ie, 3-PBA-albumin) generated from these chemicals play a causal role in GWI. It is also unclear whether anti-3-PBA-albumin antibodies are able to enter the brain and contribute to the brain pathology in GWI. While evidence of peripheral autoantibodies entering the brain and causing brain damage in SC and SLE do reveal such possibilities, this has not been demonstrated in GWI. Furthermore, it is unclear whether peripheral adaptive immune responses could trigger changes in adaptive immune cells or whether innate immunity may be associated with different triggers in GWI. In conclusion, additional research into the role of adaptive immune responses in GWI is warranted to better understand the chronic pathobiology of GWI and develop therapies for this illness, which after 30 years, clearly remains an outstanding need of veterans with GWI.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Department of defense - Congressionally directed medical research programs (DoD/CDMRP) funding [GW200027].
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Laila Abdullah, Aurore Nkiliza and Utsav Joshi wrote the paper with support from Megan Parks and Ghania Ait-Ghezala in generating figures and table. All authors provided critical feedback and helped shape the manuscript
ORCID iDs: Aurore Nkiliza  https://orcid.org/0000-0002-6102-1142
https://orcid.org/0000-0002-6102-1142
Utsav Joshi  https://orcid.org/0000-0001-5220-5639
https://orcid.org/0000-0001-5220-5639
Bibliography
- 1. White RF, Steele L, O’Callaghan JP, et al. Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: effects of toxicant exposures during deployment. Cortex. 2016;74:449-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Binns J, Barlow C, Bloom FE, et al. Research Advisory Committee on Gulf War Veterans’ Illnesses: Gulf War Illness and the Health of Gulf War Veterans: Scientific Findings and Recommendations. U.S. Government Printing Office; 2008. [Google Scholar]
- 3. Unwin C, Blatchley N, Coker W, et al. Health of UK servicemen who served in Persian Gulf War. Lancet. 1999;353(9148):169-178. [DOI] [PubMed] [Google Scholar]
- 4. Hotopf M, David A, Hull L, Ismail K, Unwin C, Wessely S. Role of vaccinations as risk factors for ill health in veterans of the Gulf war: cross sectional study. BMJ. 2000;320(7246):1363-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mawson AR, Croft AM. Gulf War illness: unifying hypothesis for a continuing health problem. Int J Environ Res Public Health. 2019;16(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rook GA, Zumla A. Gulf War syndrome: is it due to a systemic shift in cytokine balance towards a Th2 profile? Lancet. 1997;349(9068):1831-1833. [DOI] [PubMed] [Google Scholar]
- 7. Hotopf M. Reanalysis of Gulf war vaccination data does not contradict findings. BMJ. 2000;321(7263):761-762. [PMC free article] [PubMed] [Google Scholar]
- 8. Cherry N, Creed F, Silman A, et al. Health and exposures of United Kingdom Gulf war veterans. Part II: the relation of health to exposure. Occup Environ Med. 2001;58(5):299-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Steele L, Sastre A, Gerkovich MM, Cook MR. Complex factors in the etiology of Gulf War illness: wartime exposures and risk factors in veteran subgroups. Environ Health Perspect. 2012;120(1):112-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boyd KC, Hallman WK, Wartenberg D, Fiedler N, Brewer NT, Kipen HM. Reported exposures, stressors, and life events among Gulf War registry veterans. J Occup Environ Med. 2003;45(12):1247-1256. [DOI] [PubMed] [Google Scholar]
- 11. Asa PB, Cao Y, Garry RF. Antibodies to squalene in Gulf War syndrome. Exp Mol Pathol. 2000;68(1):55-64. [DOI] [PubMed] [Google Scholar]
- 12. Asa PB, Wilson RB, Garry RF. Antibodies to squalene in recipients of anthrax vaccine. Exp Mol Pathol. 2002;73(1):19-27. [DOI] [PubMed] [Google Scholar]
- 13. Phillips CJ, Matyas GR, Hansen CJ, Alving CR, Smith TC, Ryan MA. Antibodies to squalene in US Navy Persian Gulf War veterans with chronic multisymptom illness. Vaccine. 2009;27(29):3921-3926. [DOI] [PubMed] [Google Scholar]
- 14. Golomb BA, Nguyen E, Dinkeloo E. Radiation exposure predicts reported vaccine adverse effects in veterans with Gulf War illness. Int J Environ Res Public Health. 2020;17(19):7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Golomb BA. Acetylcholinesterase inhibitors and Gulf War illnesses. Proc Natl Acad Sci USA. 2008;105(11):4295-4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spencer PS, McCauley LA, Lapidus JA, Lasarev M, Joos SK, Storzbach D. Self-reported exposures and their association with unexplained illness in a population-based case-control study of Gulf War veterans. J Occup Environ Med. 2001;43(12):1041-1056. [DOI] [PubMed] [Google Scholar]
- 17. Winkenwerder W. Environmental Exposure Report. US Department of Defense; 2003. [Google Scholar]
- 18. Sullivan K, Krengel M, Bradford W, et al. Neuropsychological functioning in military pesticide applicators from the Gulf War: effects on information processing speed, attention and visual memory. Neurotoxicol Teratol. 2018;65:1-13. [DOI] [PubMed] [Google Scholar]
- 19. Blain PG. Organophosphorus poisoning (acute). BMJ Clin Evid. 2011;2011:2102. [PMC free article] [PubMed] [Google Scholar]
- 20. Stephens R, Sreenivasan B. Neuropsychological effects of long-term low-level organophosphate exposure in orchard sprayers in England. Arch Environ Health. 2004;59(11):566-574. [DOI] [PubMed] [Google Scholar]
- 21. Chao LL, Raymond MR, Leo CK, Abadjian LR. Evidence of hippocampal structural alterations in Gulf War veterans with predicted exposure to the Khamisiyah plume. J Occup Environ Med. 2017;59(10):923-929. [DOI] [PubMed] [Google Scholar]
- 22. Abdel-Rahman A, Shetty AK, Abou-Donia MB. Disruption of the blood-brain barrier and neuronal cell death in cingulate cortex, dentate gyrus, thalamus, and hypothalamus in a rat model of Gulf-War syndrome. Neurobiol Dis. 2002;10(3):306-326. [DOI] [PubMed] [Google Scholar]
- 23. Abdullah L, Crynen G, Reed J, et al. Proteomic CNS profile of delayed cognitive impairment in mice exposed to Gulf War agents. Neuromolecular Med. 2011;13(4):275-288. [DOI] [PubMed] [Google Scholar]
- 24. Parihar VK, Hattiangady B, Shuai B, Shetty AK. Mood and memory deficits in a model of Gulf War illness are linked with reduced neurogenesis, partial neuron loss, and mild inflammation in the hippocampus. Neuropsychopharmacology. 2013;38(12):2348-2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Locker AR, Michalovicz LT, Kelly KA, Miller JV, Miller DB, O’Callaghan JP. Corticosterone primes the neuroinflammatory response to Gulf War illness-relevant organophosphates independently of acetylcholinesterase inhibition. J Neurochem. 2017;142(3):444-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Naughton SX, Terry AV., Jr. Neurotoxicity in acute and repeated organophosphate exposure. Toxicology. 2018;408:101-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chao LL, Rothlind JC, Cardenas VA, Meyerhoff DJ, Weiner MW. Effects of low-level exposure to sarin and cyclosarin during the 1991 Gulf War on brain function and brain structure in US veterans. Neurotoxicology. 2010;31(5):493-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Van Riper SM, Alexander AL, Koltyn KF, et al. Cerebral white matter structure is disrupted in Gulf War veterans with chronic musculoskeletal pain. Pain. 2017;158(12):2364-2375. [DOI] [PubMed] [Google Scholar]
- 29. Cheng CH, Koo BB, Calderazzo S, et al. Alterations in high-order diffusion imaging in veterans with Gulf War illness is associated with chemical weapons exposure and mild traumatic brain injury. Brain Behav Immun. 2020;89:281-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang Y, Avery T, Vakhtin AA, et al. Brainstem atrophy in Gulf War illness. Neurotoxicology. 2020;78:71-79. [DOI] [PubMed] [Google Scholar]
- 31. Dickey B, Madhu LN, Shetty AK. Gulf War illness: mechanisms underlying brain dysfunction and promising therapeutic strategies. Pharmacol Ther. 2020;220:107716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Trageser KJ, Sebastian-Valverde M, Naughton SX, Pasinetti GM. The innate immune system and inflammatory priming: potential mechanistic factors in mood disorders and Gulf War illness. Front Psychiatry. 2020;11:704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Warrington R. Drug allergy: causes and desensitization. Hum Vaccin Immunother. 2012;8(10):1513-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mokarizadeh A, Faryabi MR, Rezvanfar MA, Abdollahi M. A comprehensive review of pesticides and the immune dysregulation: mechanisms, evidence and consequences. Toxicol Mech Methods. 2015;25(4):258-278. [DOI] [PubMed] [Google Scholar]
- 35. Li B, Eyer P, Eddleston M, Jiang W, Schopfer LM, Lockridge O. Protein tyrosine adduct in humans self-poisoned by chlorpyrifos. Toxicol Appl Pharmacol. 2013;269(3):215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. John H, van der Schans MJ, Koller M, et al. Fatal sarin poisoning in Syria 2013: forensic verification within an international laboratory network. Forensic Toxicol. 2018;36(1):61-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Noort D, van Zuylen A, Fidder A, van Ommen B, Hulst AG. Protein adduct formation by glucuronide metabolites of permethrin. Chem Res Toxicol. 2008;21(7):1396-1406. [DOI] [PubMed] [Google Scholar]
- 38. Thiphom S, Prapamontol T, Chantara S, et al. Determination of the pyrethroid insecticide metabolite 3-PBA in plasma and urine samples from farmer and consumer groups in northern Thailand. J Environ Sci Health B. 2014;49(1):15-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Joshi U, Pearson A, Evans JE, et al. A permethrin metabolite is associated with adaptive immune responses in Gulf War illness. Brain Behav Immun. 2019;81:545-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Abu-Qare AW, Abou-Donia MB. In vitro metabolism and interactions of pyridostigmine bromide, N,N-diethyl-m-toluamide, and permethrin in human plasma and liver microsomal enzymes. Xenobiotica. 2008;38(3):294-313. [DOI] [PubMed] [Google Scholar]
- 41. Karlsson I, Samuelsson K, Simonsson C, et al. The fate of a Hapten - from the skin to modification of macrophage migration inhibitory factor (MIF) in lymph nodes. Sci Rep. 2018;8(1):2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gefen T, Vaya J, Khatib S, et al. The effect of haptens on protein-carrier immunogenicity. Immunology. 2015;144(1):116-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314(6011):537-539. [DOI] [PubMed] [Google Scholar]
- 44. Vojdani A, Thrasher JD. Cellular and humoral immune abnormalities in Gulf War veterans. Environ Health Perspect. 2004;112(8):840-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Johnson GJ, Slater BC, Leis LA, Rector TS, Bach RR. Blood biomarkers of chronic inflammation in Gulf War illness. PLoS One. 2016;11(6):e0157855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Whistler T, Fletcher MA, Lonergan W, et al. Impaired immune function in Gulf War illness. BMC Med Genomics. 2009;2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang Q, Zhou XD, Denny T, et al. Changes in immune parameters seen in Gulf War veterans but not in civilians with chronic fatigue syndrome. Clin Diagn Lab Immunol. 1999;6(1):6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Campbell JP, Guy K, Cosgrove C, Florida-James GD, Simpson RJ. Total lymphocyte CD8 expression is not a reliable marker of cytotoxic T-cell populations in human peripheral blood following an acute bout of high-intensity exercise. Brain Behav Immun. 2008;22(3):375-380. [DOI] [PubMed] [Google Scholar]
- 49. Everson MP, Shi K, Aldridge P, Bartolucci AA, Blackburn WD. Immunological responses are not abnormal in symptomatic Gulf War veterans. Ann N Y Acad Sci. 2002;966:327-342. [DOI] [PubMed] [Google Scholar]
- 50. Skowera A, Hotopf M, Sawicka E, et al. Cellular immune activation in Gulf War veterans. J Clin Immunol. 2004;24(1):66-73. [DOI] [PubMed] [Google Scholar]
- 51. Smylie AL, Broderick G, Fernandes H, et al. A comparison of sex-specific immune signatures in Gulf War illness and chronic fatigue syndrome. BMC Immunol. 2013;14:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Khaiboullina SF, DeMeirleir KL, Rawat S, et al. Cytokine expression provides clues to the pathophysiology of Gulf War illness and myalgic encephalomyelitis. Cytokine. 2015;72(1):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Parkitny L, Middleton S, Baker K, Younger J. Evidence for abnormal cytokine expression in Gulf War illness: a preliminary analysis of daily immune monitoring data. BMC Immunol. 2015;16:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Broderick G, Fletcher MA, Gallagher M, Barnes Z, Vernon SD, Klimas NG. Exploring the diagnostic potential of immune biomarker coexpression in Gulf War illness. Methods Mol Biol. 2012;934:145-164. [DOI] [PubMed] [Google Scholar]
- 55. Butterick TA, Trembley JH, Hocum Stone LL, Muller CJ, Rudquist RR, Bach RR. Gulf War illness-associated increases in blood levels of interleukin 6 and C-reactive protein: biomarker evidence of inflammation. BMC Res Notes. 2019;12(1):816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Alshelh Z, Albrecht DS, Bergan C, et al. In-vivo imaging of neuroinflammation in veterans with Gulf War illness. Brain Behav Immun. 2020;87:498-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zakirova Z, Reed J, Crynen G, et al. Complementary proteomic approaches reveal mitochondrial dysfunction, immune and inflammatory dysregulation in a mouse model of Gulf War illness. Proteomics Clin Appl. 2017;11(9-10):1600190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Joshi U, Evans JE, Joseph R, et al. Oleoylethanolamide treatment reduces neurobehavioral deficits and brain pathology in a mouse model of Gulf War illness. Sci Rep. 2018;8(1):12921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Joshi U, Evans JE, Pearson A, et al. Targeting sirtuin activity with nicotinamide riboside reduces neuroinflammation in a GWI mouse model. Neurotoxicology. 2020;79:84-94. [DOI] [PubMed] [Google Scholar]
- 60. Chen X, Zhang J, Song Y, et al. Deficiency of anti-inflammatory cytokine IL-4 leads to neural hyperexcitability and aggravates cerebral ischemia-reperfusion injury. Acta Pharm Sin B. 2020;10(9):1634-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kugyelka R, Kohl Z, Olasz K, et al. Enigma of IL-17 and Th17 cells in rheumatoid arthritis and in autoimmune animal models of arthritis. Mediators Inflamm. 2016;2016:6145810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Matsui K, Sano H. T Helper 17 cells in primary Sjogren’s syndrome. J Clin Med. 2017;6(7):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8(3):223-246. [PubMed] [Google Scholar]
- 64. Rydzewska M, Jaromin M, Pasierowska IE, Stozek K, Bossowski A. Role of the T and B lymphocytes in pathogenesis of autoimmune thyroid diseases. Thyroid Res. 2018;11:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pozsgay J, Szekanecz Z, Sarmay G. Antigen-specific immunotherapies in rheumatic diseases. Nat Rev Rheumatol. 2017;13(9):525-537. [DOI] [PubMed] [Google Scholar]
- 66. Grygiel-Gorniak B, Rogacka N, Puszczewicz M. Antinuclear antibodies in healthy people and non-rheumatic diseases - diagnostic and clinical implications. Reumatologia. 2018;56(4):243-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rosenberg AM, Semchuk KM, McDuffie HH, et al. Prevalence of antinuclear antibodies in a rural population. J Toxicol Environ Health A. 1999;57(4):225-236. [DOI] [PubMed] [Google Scholar]
- 68. Skowera A, Stewart E, Davis ET, et al. Antinuclear autoantibodies (ANA) in Gulf War-related illness and chronic fatigue syndrome (CFS) patients. Clin Exp Immunol. 2002;129(2):354-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Browning JL. B cells move to centre stage: novel opportunities for autoimmune disease treatment. Nat Rev Drug Discov. 2006;5(7):564-576. [DOI] [PubMed] [Google Scholar]
- 70. Prinz M, Priller J. The role of peripheral immune cells in the CNS in steady state and disease. Nat Neurosci. 2017;20(2):136-144. [DOI] [PubMed] [Google Scholar]
- 71. Pape K, Tamouza R, Leboyer M, Zipp F. Immunoneuropsychiatry - novel perspectives on brain disorders. Nat Rev Neurol. 2019;15(6):317-328. [DOI] [PubMed] [Google Scholar]
- 72. Priego N, Valiente M. The potential of astrocytes as immune modulators in brain tumors. Front Immunol. 2019;10:1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Song J, Wu C, Korpos E, et al. Focal MMP-2 and MMP-9 activity at the blood-brain barrier promotes chemokine-induced leukocyte migration. Cell Rep. 2015;10(7):1040-1054. [DOI] [PubMed] [Google Scholar]
- 74. Garre JM, Yang G. Contributions of monocytes to nervous system disorders. J Mol Med (Berl). 2018;96(9):873-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. O’Callaghan JP, Kelly KA, Locker AR, Miller DB, Lasley SM. Corticosterone primes the neuroinflammatory response to DFP in mice: potential animal model of Gulf War illness. J Neurochem. 2015;133(5):708-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953-964. [DOI] [PubMed] [Google Scholar]
- 77. Jakubzick CV, Randolph GJ, Henson PM. Monocyte differentiation and antigen-presenting functions. Nat Rev Immunol. 2017;17(6):349-362. [DOI] [PubMed] [Google Scholar]
- 78. Diamond B, Huerta PT, Mina-Osorio P, Kowal C, Volpe BT. Losing your nerves? Maybe it’s the antibodies. Nat Rev Immunol. 2009;9(6):449-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hromadkova L, Ovsepian SV. Tau-reactive endogenous antibodies: origin, functionality, and implications for the pathophysiology of Alzheimer’s disease. J Immunol Res. 2019;2019:7406810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Abou-Donia MB, Conboy LA, Kokkotou E, et al. Screening for novel central nervous system biomarkers in veterans with Gulf War illness. Neurotoxicol Teratol. 2017;61:36-46. [DOI] [PubMed] [Google Scholar]
- 81. Thrasher JD, Madison R, Broughton A. Immunologic abnormalities in humans exposed to chlorpyrifos: preliminary observations. Arch Environ Health. 1993;48(2):89-93. [DOI] [PubMed] [Google Scholar]
- 82. El Rahman HAA, Salama M, Gad El-Hak SA, El-Harouny MA, ElKafrawy P, Abou-Donia MB. A panel of autoantibodies against neural proteins as peripheral biomarker for pesticide-induced neurotoxicity. Neurotox Res. 2018;33(2):316-336. [DOI] [PubMed] [Google Scholar]
- 83. Levin EC, Acharya NK, Han M, et al. Brain-reactive autoantibodies are nearly ubiquitous in human sera and may be linked to pathology in the context of blood-brain barrier breakdown. Brain Res. 2010;1345:221-232. [DOI] [PubMed] [Google Scholar]
- 84. Wang KK, Yang Z, Yue JK, et al. Plasma anti-glial fibrillary acidic protein autoantibody levels during the acute and chronic phases of traumatic brain injury: a transforming research and clinical knowledge in traumatic brain injury pilot study. J Neurotrauma. 2016;33(13):1270-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Diamond B, Honig G, Mader S, Brimberg L, Volpe BT. Brain-reactive antibodies and disease. Annu Rev Immunol. 2013;31:345-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Omdal R, Brokstad K, Waterloo K, Koldingsnes W, Jonsson R, Mellgren SI. Neuropsychiatric disturbances in SLE are associated with antibodies against NMDA receptors. Eur J Neurol. 2005;12(5):392-398. [DOI] [PubMed] [Google Scholar]
- 87. Ludwig RJ, Vanhoorelbeke K, Leypoldt F, et al. Mechanisms of autoantibody-induced pathology. Front Immunol. 2017;8:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Jezequel J, Johansson EM, Dupuis JP, et al. Dynamic disorganization of synaptic NMDA receptors triggered by autoantibodies from psychotic patients. Nat Commun. 2017;8(1):1791. [DOI] [PMC free article] [PubMed] [Google Scholar]