Abstract
We evaluated serum albumin as an index for predicting respiratory hospitalization in patients with bronchiectasis. We retrospectively reviewed the medical records of 177 patients with bronchiectasis, categorized them into low and normal albumin groups, and compared their clinical characteristics. The prediction of respiratory hospitalization by factors such as serum albumin level, bronchiectasis severity index (BSI), and FACED score (an acronym derived from five variables of forced expiratory volume in 1 s; FEV1, age, chronic colonization of Pseudomonas aeruginosa, extent of bronchiectasis, and dyspnea) was assessed. There were 15 and 162 patients categorized in the low and normal albumin groups, respectively. The low albumin group had lower body mass index and forced expiratory volume in 1 s, and higher age, frequency of previous respiratory hospitalization, percentage of Pseudomonas colonization, number of affected lobes, BSI and FACED scores, and C-reactive protein (CRP) level, than the normal albumin group. The areas under the receiver operating characteristic curve of serum albumin level and BSI and FACED scores for predicting respiratory hospitalization were 0.732 (95% confidence interval (CI), 0.647–0.816), 0.873 (95% CI, 0.817–0.928), and 0.708 (95% CI, 0.618–0.799), respectively. Albumin level, CRP, modified Medical Research Council score, and chronic Pseudomonas aeruginosa (and other organisms) colonization were independent risk factors for respiratory hospitalization. Low serum albumin level was associated with worse clinical condition, higher severity scores, and respiratory hospitalization in patients with bronchiectasis.
Keywords: Bronchiectasis, albumin, bronchiectasis severity index, FACED score, respiratory hospitalization
Introduction
Bronchiectasis has been defined as the presence of permanently dilated bronchi on high-resolution computed tomography (HRCT).1 In addition to structural changes, clinical features, such as cough, sputum production, and hemoptysis, have been reported for this condition.2,3 Although the etiologies of bronchiectasis are heterogeneous, most patients with people with bronchiectasis are susceptible to recurrent infection and chronic inflammation due to epithelial dysfunction, mucus hypersecretion, and ciliary dysfunction.4,5 These features lead to additional structural changes5; this vicious cycle adversely affects the patients’ respiratory prognosis. Patients with bronchiectasis experience frequent exacerbations and hospitalizations, as well as poor quality of life.6,7
It has been reported that the prevalence of bronchiectasis has increased by 40% in the past 10 years.8 As a result, the associated medical burden has increased, and more research on exacerbation and prognosis has been conducted in these patients. The bronchiectasis severity index (BSI) and FACED score (an acronym derived from five variables of forced expiratory volume in 1 s; FEV1, age, chronic colonization of Pseudomonas aeruginosa, extent of bronchiectasis, and dyspnea) were introduced as scoring systems to assess the severity of bronchiectasis.9,10 The BSI score combines age, body mass index (BMI), FEV1, previous hospitalization, exacerbation frequency, colonization status, and radiological appearance.9 These two scoring systems effectively predict morbidity in patients with bronchiectasis.11
However, these scores do not include serologic markers. As bronchiectasis is a respiratory disease characterized by chronic inflammation, multiple studies have investigated the usefulness of biomarkers of systemic inflammation for assessing severity and predicting prognosis in patients with bronchiectasis.12–14 Serum C-reactive protein (CRP) level has been associated with the severity of bronchiectasis.12,15 These studies were based on a concept of “spill-over” of inflammatory mediators from the pulmonary to the systemic circulation.16,17 Chronic systemic inflammation causes malnutrition subsequent to anorexia, decreased anabolic rate, and increased catabolic rate.18,19 Above all, bronchiectasis is a typical chronic respiratory disease associated with chronic inflammation and malnutrition. Serum albumin could be a useful indicator of bronchiectasis, reflecting both systemic inflammation and nutritional status.20,21 In a previous study, it was reported that presumably low serum albumin correlates with the BSI and FACED scores in patients with bronchiectasis,22 thus suggesting the use of the serum albumin level as an indicator of bronchiectasis severity.
We aimed to identify whether serum albumin level is related to respiratory hospitalization, which constitutes a crucial outcome in patients with bronchiectasis.
Materials and methods
Study design and sample
We retrospectively reviewed the medical records of clinically stable outpatients with bronchiectasis at the pulmonology department at Gyeongsang National University Hospital in Jinju, Korea, between July 2013 and June 2016. Bronchiectasis was diagnosed using HRCT in subjects with a compatible clinical presentation. The inclusion criteria were (1) a larger bronchial internal diameter than the accompanying pulmonary artery and (2) a lack of bronchial tapering in the lung periphery. We excluded patients with bronchiectasis attributable to non-cystic fibrosis bronchiectasis, non-tuberculous mycobacterial pulmonary disease, coexistent with active malignant disease, or active pulmonary tuberculosis.
A total of 177 patients were enrolled. We checked the age, sex, BMI, and smoking status (Former smoker, current smoker, and never smoker) at baseline. Former smokers were defined as patients who stopped smoking 3 months prior to the first outpatient visit. Information about comorbid diseases, including chronic obstructive pulmonary disease (COPD), bronchial asthma, and a previous history of tuberculosis, hypertension, diabetes mellitus, cardiovascular disease, and depression was obtained from medical records. We retrieved spirometry parameters, including FEV1, forced vital capacity (FVC), and FEV1/FVC ratio. Pulmonary function tests were conducted in accordance with criteria published by the American Thoracic Society and the European Respiratory Society.23 We collected information on the serum white blood cell and platelet counts and hemoglobin, total protein, albumin, total bilirubin, uric acid, and CRP levels. Chronic colonization with Pseudomonas aeruginosa or other organisms was verified using sputum culture. All data were collected at the outpatient clinic with the patients in a stable condition without any exacerbation or complications such as infection.
Eligible participants were categorized into the low (<3.5 g/dL) or normal (≥3.5 g/dL) serum albumin groups.24 We measured the degree of breathlessness using the modified Medical Research Council (mMRC) dyspnea scale and checked the history of respiratory hospitalizations, acute exacerbations of respiratory symptoms associated with bronchiectasis (worsening cough, increasing volume/purulence of sputum, hemoptysis, and increases in breathlessness), and number of exacerbations in the previous year.
We reviewed chest HRCT findings to determine radiological severity using the modified Reiff score, which includes the number of affected lobes (right upper, right middle, right lower, upper division of left upper, lingular division of the left upper, and left lower lobes) and patterns of bronchiectasis (cylindrical, varicose, and cystic).25
Bronchiectasis severity was determined using the BSI and FACED scores. The BSI grades were classified as low (0–4 points), intermediate (5–8 points), and high (9–26 points), while the FACED scores were classified as low (0–2 points), intermediate (3–4 points), and high (5–7 points).
We identified episodes of respiratory hospitalization by reviewing medical records in December 2018. Respiratory hospitalizations were defined as unscheduled hospitalizations or emergency department visits for exacerbations or complications.
This study was approved by the institutional review board of the Gyeongsang National University Hospital (approval GNUH2016-02-0050) and the requirement for obtaining informed consent from the patients was waived.
Statistical analysis
Data are presented as means ± standard deviations or as frequencies and percentages. Categorical variables were compared using Pearson’s χ2 test. Comparison of continuous variables between two and three groups was performed using the unpaired t-test and one-way analysis of variance with Bonferroni post hoc testing, respectively. Predictive factors independently associated with respiratory hospitalization were analyzed using multivariate binary logistic regression. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. Only variables with statistical significance in the univariate analysis were included in the multivariate analysis. The ability of serum albumin, BSI, and FACED scores to predict respiratory hospitalization in patients with bronchiectasis was compared through receiver operating characteristic (ROC) curve analysis. P-values <0.05 were considered statistically significant for all analyses. An additional sensitivity analysis was performed due to the large difference in the number of subjects between two groups. Statistical analyses were performed using IBM SPSS version 21.0 (IBM Corp., Armonk, NY, USA) and R statistical software 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
Of the 177 patients, 15 and 162 were categorized in the low and normal albumin groups, respectively, with corresponding mean serum albumin values of 3.1 ± 0.3 g/dL and 4.3 ± 0.3 g/dL. Table 1 shows the baseline characteristics of the participants according to their serum albumin level. Patients in the low albumin group were older (69 ± 10 vs. 63 ± 10 years, p = 0.037) and had lower BMI (19.6 ± 3.6 vs. 22.1 ± 3.3 kg/m2, p = 0.005) and FEV1 (45.7 ± 22.1 vs. 67.3 ± 21.6%, p < 0.001) than those in the normal albumin group. The mean serum CRP level of the low albumin group was more than twice as high as that of the normal albumin group (14.3 ± 13.6 mg/L vs. 6.8 ± 9.5 mg/L, p = 0.005). There were no significant differences in sex, smoking status, comorbidities, and mMRC dyspnea score between groups. Chronic bacterial colonization was found on sputum culture in 78 patients (44.1%). Pseudomonas aeruginosa was the most common pathogen, being present in 40 patients (22.6%), and the incidence of colonization by this pathogen was higher in the low albumin group than in the normal albumin group (46.7% vs. 20.4%, p = 0.020). Chronic colonization by organisms other than Pseudomonas aeruginosa was found in 38 patients (21.5%), and its incidence was similar between the two albumin level groups. The mean number of affected lobes was three. The low albumin group had nearly one more affected lobe on average than the normal albumin group (3.8 ± 1.6 vs. 3.0 ± 1.4, p = 0.037). There were no significant differences in the bronchiectasis patterns between the groups, but half of the patients in the low albumin group had cystic bronchiectasis.
Table 1.
Baseline characteristics of the patients with bronchiectasis according to the serum albumin level.
| Characteristic | All patients (n = 177) | Low albumin group (n = 15) | Normal albumin group (n = 162) | p-value |
|---|---|---|---|---|
| Age, years | 64 ± 10 | 69 ± 10 | 63 ± 10 | 0.037 |
| Female | 92 (52.0) | 7 (46.7) | 85 (52.5) | 0.667 |
| Body mass index, kg/m2 | 21.9 ± 3.3 | 19.6 ± 3.6 | 22.1 ± 3.3 | 0.005 |
| Smoking status | 0.986 | |||
| Current smoker | 12 (6.8) | 1 (6.7) | 11 (6.8) | |
| Former smoker | 44 (24.9) | 4 (26.7) | 40 (24.7) | |
| Never smoker | 121 (68.4) | 10 (66.7) | 111 (68.5) | |
| Comorbidities | ||||
| COPD | 94 (53.1) | 11 (73.3) | 83 (51.2) | 0.101 |
| Bronchial asthma | 40 (22.6) | 1 (6.7) | 39 (24.1) | 0.123 |
| Previous history of tuberculosis | 39 (22.0) | 5 (33.3) | 34 (21.0) | 0.270 |
| Hypertension | 41 (23.2) | 2 (13.3) | 39 (24.1) | 0.346 |
| Diabetes | 26 (14.7) | 2 (13.3) | 24 (14.8) | 0.877 |
| Cardiovascular disease | 6 (3.4) | 1 (6.7) | 5 (3.1) | 0.464 |
| Depression | 6 (3.4) | 1 (6.7) | 5 (3.1) | 0.464 |
| mMRC dyspnea score | 0.4 ± 0.6 | 0.6 ± 0.7 | 0.4 ± 0.6 | 0.171 |
| FEV1/FVC, % | 67.0 ± 13.2 | 60.1 ± 15.2 | 67.7 ± 12.8 | 0.032 |
| FEV1, L | 1.7 ± 0.7 | 1.1 ± 0.5 | 1.8 ± 0.7 | <0.001 |
| FEV1, % predicted | 65.5 ± 22.4 | 45.7 ± 22.1 | 67.3 ± 21.6 | <0.001 |
| Laboratory findings | ||||
| WBC, 103/mm3 | 7.2 ± 2.2 | 8.2 ± 2.2 | 7.1 ± 2.2 | 0.065 |
| Neutrophil, % | 59.3 ± 10.4 | 67.3 ± 14.7 | 58.5 ± 9.6 | 0.037 |
| Lymphocyte, % | 30.2 ± 9.4 | 23.3 ± 13.5 | 30.8 ± 8.7 | 0.003 |
| Hemoglobin, g/dL | 12.8 ± 1.5 | 11.3 ± 1.8 | 12.9 ± 1.4 | <0.001 |
| Platelet, 103/mm3 | 257.7 ± 74.0 | 292.4 ± 96.1 | 254.3 ± 71.0 | 0.135 |
| Total protein, g/dL | 7.2 ± 0.6 | 7.1 ± 4.0 | 7.2 ± 0.5 | 0.718 |
| Albumin, g/dL | 4.2 ± 0.5 | 3.1 ± 0.3 | 4.3 ± 0.3 | <0.001 |
| Total bilirubin, mg/dL | 0.5 ± 0.3 | 0.6 ± 0.6 | 0.5 ± 0.3 | 0.386 |
| Uric acid, mg/dL | 4.7 ± 1.6 | 4.4 ± 1.2 | 4.7 ± 1.6 | 0.335 |
| C-reactive protein, mg/L | 7.4 ± 10.1 | 14.4 ± 13.6 | 6.8 ± 9.5 | 0.005 |
| Chronic colonization | ||||
| Pseudomonas aeruginosa | 40 (22.6) | 7 (46.7) | 33 (20.4) | 0.020 |
| Other organisms | 38 (21.5) | 3 (20.0) | 35 (21.6) | 0.885 |
| Number of affected lobes | 3.1 ± 1.5 | 3.8 ± 1.6 | 3.0 ± 1.4 | 0.037 |
| Patterns of bronchiectasis | 0.353 | |||
| Cylindrical | 97 (54.8) | 7 (46.7) | 90 (55.6) | |
| Varicose | 11 (6.2) | 0 (0) | 11 (6.8) | |
| Cystic | 69 (39.0) | 8 (53.3) | 61 (37.7) | |
| History of acute exacerbation | 118 (66.7) | 13 (86.7) | 105 (64.8) | 0.086 |
| History of respiratory hospitalization | 76 (42.9) | 11 (73.3) | 65 (40.1) | 0.013 |
| Number of exacerbations in the previous year | 0.9 ± 0.9 | 1.3 ± 1.0 | 0.9 ± 0.9 | 0.097 |
| BE severity score | ||||
| FACED score | 2.0 ± 1.7 | 3.6 ± 1.4 | 1.9 ± 1.6 | <0.001 |
| BSI score | 8.2 ± 4.0 | 12.6 ± 3.5 | 7.8 ± 3.8 | <0.001 |
Data are presented as mean ± SD or number (%).
BE, bronchiectasis; BSI, bronchiectasis severity index; COPD, chronic obstructive pulmonary disease; FACED, an acronym for FEV1, age, chronic colonization, extension and dyspnea; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; Hb, hemoglobin; mMRC, modified Medical Research Council; NLR, neutrophil-lymphocyte ratio; WBC, white blood cell.
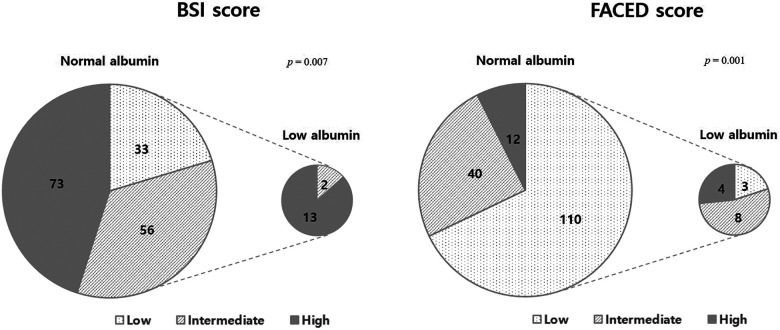
More than half of the patients had a history of acute exacerbation, and the average number of exacerbations was approximately one per year. There was no difference in the history and number of acute exacerbations in the previous year between the low and normal albumin groups, but the low albumin group had a higher percentage of patients with history of respiratory hospitalization than the normal albumin group (73.3% vs. 40.1%, p = 0.013). Additionally, the low albumin group had significantly higher FACED (3.6 ± 1.4 vs. 1.9 ± 1.6, p < 0.001) and BSI scores (12.6 ± 3.5 vs. 7.8 ± 3.8, p < 0.001) than the normal albumin group. The proportion of patients in low albumin group increased with the increase in bronchiectasis severity (Figure 1). Most of the patients in the low albumin group had intermediate or high BSI (p = 0.007) and FACED scores (p = 0.001). None of the patients in the low albumin group had a low BSI score, and only three patients (20%) had a low FACED score.
Figure 1.
Distribution of bronchiectasis severity grades in normal and low albumin groups in patients with bronchiectasis.
Predictors of respiratory hospitalization
Forty-seven (26.6%) of the 177 patients, 11 patients in low albumin group and 36 patients in normal albumin group, experienced at least one respiratory hospitalization during the follow-up period. The median follow-up duration was 50.5 months. The percentage of patients who experienced respiratory hospitalization was higher in the low albumin group than in the normal albumin group (73.3% vs. 22.2%, p < 0.001, Figure 2).
Figure 2.
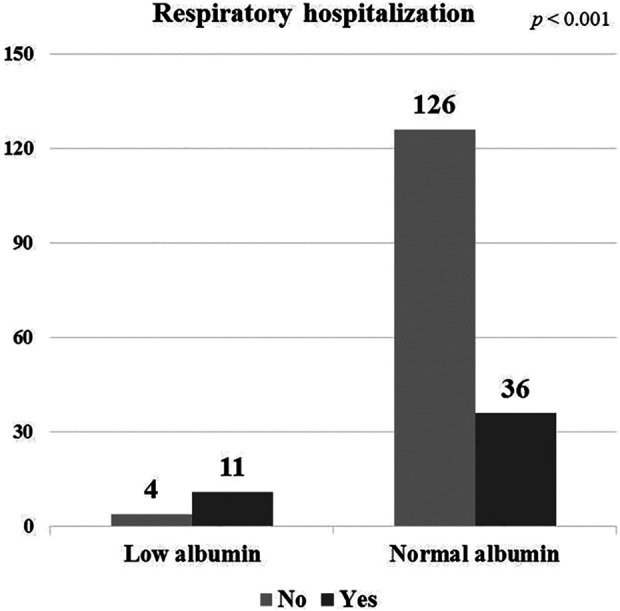
Number of patients with respiratory hospitalization in low and normal albumin groups.
In the univariate analysis, low albumin level (<3.5g/dL), CRP level, BMI, FEV1 (%), mMRC dyspnea score, number of exacerbations in the previous year, number of affected lobes, chronic colonization with Pseudomonas aeruginosa or other organisms, and cystic pattern were significantly associated with respiratory hospitalization. Finally, we identified the following independent predictive factors of respiratory hospitalization: low albumin level (OR 5.814, p = 0.028), chronic colonization with Pseudomonas aeruginosa (OR 3.466, p = 0.011) or other organisms (OR 3.428, p = 0.023), mMRC dyspnea score (OR 2.583, p = 0.014), and CRP level (OR 1.053, p = 0.016) in the multivariate analysis (Table 2).
Table 2.
Factors predicting respiratory hospitalization in patients with bronchiectasis.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Albumin | ||||||
| ≥3.5 g/dL | 1 | 1 | ||||
| <3.5 g/dL | 9.625 | 2.891–32.05 | <0.001 | 5.814 | 1.209–27.955 | 0.028 |
| C-reactive protein, (mg/L) | 1.061 | 1.026–1.097 | <0.001 | 1.053 | 1.010–1.099 | 0.016 |
| Body mass index (kg/m2) | 0.843 | 0.755–0.941 | 0.002 | 0.921 | 0.802–1.058 | 0.245 |
| FEV1 (% predicted) | 0.963 | 0.946–0.980 | <0.001 | 0.992 | 0.967–1.017 | 0.522 |
| mMRC dyspnea score | 2.549 | 1.513–4.292 | <0.001 | 2.583 | 1.208–5.527 | 0.014 |
| Number of exacerbations in the previous year | 2.082 | 1.392–3.113 | <0.001 | 1.474 | 0.898–2.420 | 0.125 |
| Chronic colonization with Pseudomonas aeruginosa | 5.476 | 2.563–11.696 | <0.001 | 3.466 | 1.334–9.006 | 0.011 |
| Chronic colonization with other organisms | 2.941 | 1.380–6.267 | 0.005 | 3.428 | 1.187–9.896 | 0.023 |
| Number of affected lobes | 1.547 | 1.210–1.977 | 0.001 | 1.247 | 0.868–1.790 | 0.232 |
| Cystic bronchiectasis patterna | 2.731 | 1.378–5.413 | 0.004 | 1.135 | 0.433–2.976 | 0.796 |
CI, confidence interval; FEV1, forced expiratory volume in 1 second; mMRC, modified Medical Research Council; OR, odds ratio.
a Compared with both cylindrical and varicose patterns.
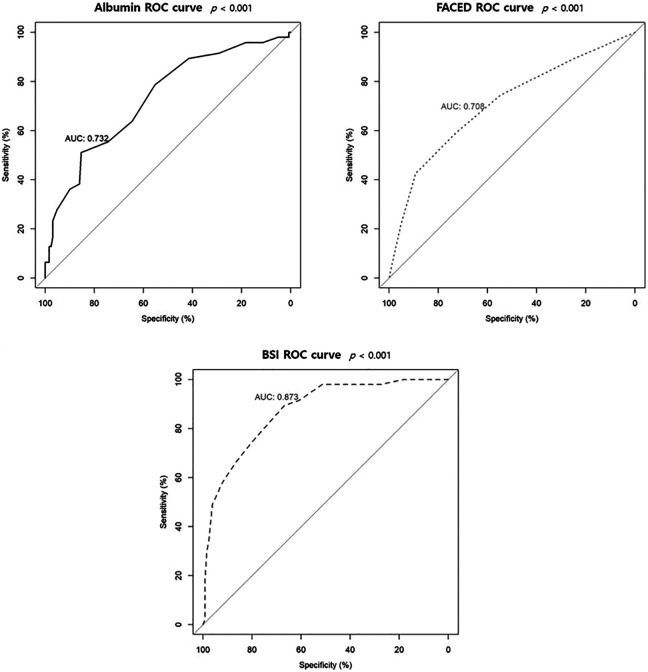
Figure 3 shows the ROC curves of albumin level, BSI and FACED scores for predicting respiratory hospitalization. The area under the ROC curves (AUCs) for respiratory hospitalization were 0.732 (95% CI 0.647–0.817, p < 0.001) for the albumin level, 0.873 (95% CI 0.817–0.928, p < 0.001) for the BSI score, and 0.708 (95% CI 0.618–0.798, p < 0.001) for the FACED score.
Figure 3.
Receiver operating characteristic curves of serum albumin, FACED score, and bronchiectasis severity index to predict respiratory hospitalization.
We performed sensitivity analysis with the value of albumin 4.0 g/dL using the lowest tertial/quartile of albumin level. Forty-seven subjects were included in lower albumin group (<4.0 g/dL) and 130 subjects were included in higher albumin group (≥4.0 g/dL). In a multivariate analysis, lower albumin level (<4.0 g/dL) increased the risk of respiratory hospitalization (OR 3.775, p = 0.008) and other variables such as chronic colonization, mMRC, and CRP, also indicated statistically significant factors for respiratory hospitalization (see Supplementary Table S1). These results were consistent with those based on an albumin value of 3.5 g/day.
Discussion
In the present study, only 8.5% of patients with bronchiectasis were classified into the low albumin group, but they had significantly higher risk of respiratory hospitalization than patients in the normal albumin group. This demonstrates that low albumin level was an independent and significant predictor of respiratory hospitalization in patients with bronchiectasis. Low albumin level was comparable to BSI and FACED scores, which are severity scores and predictors of outcome of bronchiectasis, in terms of predicting respiratory hospitalization. Albumin level is not included in the existing bronchiectasis severity scores, but it may be a prognostic factor in patients with bronchiectasis.
The etiology of bronchiectasis is diverse, and it has been regarded as an orphan disease.2 However, bronchiectasis has become one of the major chronic respiratory diseases since it is characterized by a lifelong risk of exacerbation; moreover, as life expectancy has become longer, quality of life is of increasing importance. Therefore, bronchiectasis severity scoring systems such as the BSI and FACED scores were introduced to more effectively understand and manage bronchiectasis.9,10 The BSI score is more useful than the FACED score in predicting clinically relevant aspects such as future exacerbations, hospital admissions, quality of life, and death.9,26 The FACED score is superior in terms of predicting long-term outcomes (15-year mortality) compared to the BSI score (AUC 0.82 vs. 0.69, p = 0.0495),11 although more items are required for calculating the former. The E-FACED score, which added the occurrence of exacerbations to the previous FACED score, improves the prediction of future annual exacerbations.27
However, these scores do not include biomarkers and comorbidities that are highly relevant to the prognosis of patients with chronic respiratory disease. Cano et al. showed that patients with bronchiectasis had the highest CRP level and the lowest BMI, albumin, and prealbumin levels among patients with various chronic respiratory diseases.28 This could mean that bronchiectasis is a respiratory disease closely related to chronic inflammation and malnutrition.18 Previous studies have shown that CRP is a useful marker of inflammation and a prognostic factor of clinical outcomes in patients with bronchiectasis.12,13,29 CRP level is also significantly correlated with the BSI and FACED scoring systems.15
Serum albumin is another marker of inflammation and nutrition, but there are only a few studies showing that albumin level is associated with severity and prognosis in patients with bronchiectasis.20,22 It has been reported that serum albumin levels are correlated with disease severity and modified Reiff scores in patients with bronchiectasis, and that it is a better indicator than prealbumin and BMI.22 Serum albumin levels are also correlated with lung function decline and Bhalla score after controlling for age, BMI, and energy intake in patients with bronchiectasis.20
Our results are relatively consistent with the notion that serum albumin is associated with the clinical features of patients with bronchiectasis. Although the number of patients in the low albumin group was relatively small, they had clinically unfavorable factors such as older age, lower BMI, lower lung function, higher CRP level, and more affected lobes compared with patients in the normal albumin group. As a result, these clinical factors corresponded with the BSI and FACED scores. Low albumin level (<3.5 g/dL) was identified as the greatest risk factor for respiratory hospitalization (OR 5.184, p = 0.028). Two scoring systems did not consider any laboratory findings as variables. If serum albumin is added to the existing BSI and FACED scoring systems, they may be able to better assess severity and predict prognosis, including the risk of respiratory hospitalization.
A number of comorbidities can potentially influence the clinical outcomes of patients with bronchiectasis; indeed, systemic inflammation has been proposed as a link between bronchiectasis and comorbid conditions.30,31 Patients with bronchiectasis have increased arterial stiffness, which is associated with a high risk of cardiovascular events, reduced exercise capacity, and bone thinning.30 Gale et al. suggested that this increased risk was related to systemic inflammation. Another study reported COPD and rheumatoid arthritis as comorbidities of bronchiectasis.31 This originates from an inflammation “double hit”. In subsequent studies, both inflammatory markers and comorbidities should be evaluated to better predict prognosis in patients with bronchiectasis.
Our study has several limitations. First, we evaluated a small number of outpatients with bronchiectasis, especially in the low albumin group. However, this number represents regularly followed up patients in a relatively stable clinical condition. Thus, this may be demonstrative of a real-world group of patients with bronchiectasis. But there might be selection bias in the patients recruited from outpatients. Second, we identified patients’ exacerbations or respiratory hospitalization using interviews and medical records; therefore, information bias may have occurred. Since it is difficult to verify all events accurately, exacerbations and hospitalizations may be underestimated in our study. Further prospective, well-designed observational studies of bronchiectasis, including albumin measurements, should be carried out. Third, infection is a common etiology for bronchiectasis, due to the high incidence of pulmonary tuberculosis in Korea. Previous studies of the BSI and FACED scores have been conducted in Europeans with bronchiectasis from different backgrounds, but these scoring systems have also been proven to be useful predictors of prognosis in Asians.32,33
In conclusion, patients with bronchiectasis with low serum albumin level had poor clinical outcome and experienced severe disease. Serum albumin level was independently associated with respiratory hospitalization in patients with bronchiectasis.
Supplemental material
Supplemental Material, sj-docx-1-crd-10.1177_14799731211017548 for Serum albumin is a predictor of respiratory hospitalization in patients with bronchiectasis by Sunmi Ju, Jong Hwan Jeong, Manbong Heo, I Re Heo, Tae Hoon Kim, Ho Cheol Kim, Jung-Wan Yoo, Yu Ji Cho, Yi Yeong Jeong, Jong Deog Lee and Seung Jun Lee in Chronic Respiratory Disease
Supplemental Material, sj-docx-2-crd-10.1177_14799731211017548 for Serum albumin is a predictor of respiratory hospitalization in patients with bronchiectasis by Sunmi Ju, Jong Hwan Jeong, Manbong Heo, I Re Heo, Tae Hoon Kim, Ho Cheol Kim, Jung-Wan Yoo, Yu Ji Cho, Yi Yeong Jeong, Jong Deog Lee and Seung Jun Lee in Chronic Respiratory Disease
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research was funded by the biomedical research institute fund (GNUHBRIF-2021-0007) from Gyeongsang National University Hospital.
ORCID iD: Sunmi Ju  https://orcid.org/0000-0003-1474-1064
https://orcid.org/0000-0003-1474-1064
Supplemental material: Supplemental material for this article is available online.
References
- 1. Grenier P, Maurice F, Muss D, et al. Bronchiectasis: assessment by thin section CT. Radiology 1986; 161: 95–99. [DOI] [PubMed] [Google Scholar]
- 2. Barker AF. Bronchiectasis. N Engl J Med 2002; 346: 1383–1393. [DOI] [PubMed] [Google Scholar]
- 3. Polverino E, Goeminne PC, McDonnell MJ, et al. European respiratory society guidelines for the management of adult bronchiectasis. Eur Respir J 2017; 50: 1700629. [DOI] [PubMed] [Google Scholar]
- 4. Flume PA, Chalmers JD, Olivier KN. Advances in bronchiectasis: endotyping, genetics, microbiome, and disease heterogeneity. Lancet 2018; 392: 880–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boucher RC. On the pathogenesis of acute exacerbations of mucoobstructive lung diseases. Ann Am Thorac Soc 2015; 12: S160–S163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Finch S, Laska IF, Abo-Leyah H, et al. Validation of the COPD assessment test (CAT) as an outcome measure in bronchiectasis. Chest 2020; 157: 815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McCullough AR, Tunney MM, Quittner AL, et al. Treatment adherence and health outcomes in patients with bronchiectasis. BMC Pulm Med 2014; 14: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quint JK, Millett ER, Joshi M, et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population-based cohort study. Eur Respir J 2016; 47: 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med 2014; 189: 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martinez-Garcia MA, de Gracia J, Relat MV, et al. Multidimensional approach to non-cystic fibrosis bronchiectasis: the FACED score. Eur Respir J 2014; 43: 1357–1367. [DOI] [PubMed] [Google Scholar]
- 11. Ellis HC, Cowman S, Fernandes M, et al. Predicting mortality in bronchiectasis using bronchiectasis severity index and FACED scores: a 19-year cohort study. Eur Respir J 2016; 47: 482–489. [DOI] [PubMed] [Google Scholar]
- 12. Martinez-Garcia MA, Soler-Cataluna JJ, Perpina-Tordera M, et al. Factors associated with lung function decline in adult patients with stable non-cystic fibrosis bronchiectasis. Chest 2007; 132: 1565–1572. [DOI] [PubMed] [Google Scholar]
- 13. Hsieh MH, Fang YF, Chen GY, et al. The role of the high-sensitivity C-reactive protein in patients with stable non-cystic fibrosis bronchiectasis. Pulm Med 2013; 2013: 795140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chalmers JD, Smith MP, McHugh BJ, et al. Short- and long-term antibiotic treatment reduces airway and systemic inflammation in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 2012; 186: 657–665. [DOI] [PubMed] [Google Scholar]
- 15. Coban H, Gungen AC. Is there a correlation between new scoring systems and systemic inflammation in stable bronchiectasis? Can Respir J 2017; 2017: 9874068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. He Z, Chen Y, Chen P, et al. Local inflammation occurs before systemic inflammation in patients with COPD. Respirology 2010; 15: 478–484. [DOI] [PubMed] [Google Scholar]
- 17. Tkacova R. Systemic inflammation in chronic obstructive pulmonary disease: may adipose tissue play a role? Review of the literature and future perspectives. Mediators Inflamm 2010; 2010: 585989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jensen GL, Mirtallo J, Compher C, et al. Adult starvation and disease-related malnutrition: a proposal for etiology-based diagnosis in the clinical practice setting from the international consensus guideline committee. JPEN J Parenter Enteral Nutr 2010; 34: 156–159. [DOI] [PubMed] [Google Scholar]
- 19. Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial 2004; 17: 432–437. [DOI] [PubMed] [Google Scholar]
- 20. Olveira G, Olveira C, Gaspar I, et al. Fat-free mass depletion and inflammation in patients with bronchiectasis. J Acad Nutr Diet 2012; 112: 1999–2006. [DOI] [PubMed] [Google Scholar]
- 21. Boussoffara L, Bouchareb S, Boudawara NK, et al. Nutritional status in patients with non-cystic fibrosis bronchiectasis. EC Pulmonol Respir Med 2018; 7: 121–125. [Google Scholar]
- 22. Li L, Li Z, Bi J, et al. The association between serum albumin/prealbumin level and disease severity in non-CF bronchiectasis. Clin Exp Pharmacol Physiol 2020; 47: 1537–1544. [DOI] [PubMed] [Google Scholar]
- 23. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. [DOI] [PubMed] [Google Scholar]
- 24. Weaving G, Batstone GF, Jones RG, et al. Age and sex variation in serum albumin concentration: an observational study. Ann Clin Biochem 2016; 53: 106–111. [DOI] [PubMed] [Google Scholar]
- 25. Reiff DB, Wells AU, Carr DH, et al. CT findings in bronchiectasis: limited value in distinguishing between idiopathic and specific types. Am J Radiol 1995; 165: 261–267. [DOI] [PubMed] [Google Scholar]
- 26. McDonnell MJ, Aliberti S, Goeminne PC, et al. Multidimensional severity assessment in bronchiectasis: an analysis of seven European cohorts. Thorax 2016; 71: 1110–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martinez-Garcia MA, Athanazio RA, Giron R, et al. Predicting high risk of exacerbations in bronchiectasis: the E-FACED score. Int J Chron Obstruct Pulmon Dis 2017; 12: 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cano NJ, Pichard C, Roth H, et al. C-reactive protein and body mass index predict outcome in end-stage respiratory failure. Chest 2004; 126: 540–546. [DOI] [PubMed] [Google Scholar]
- 29. Courtney JM, Kelly MG, Watt A, et al. Quality of life and inflammation in exacerbations of bronchiectasis. Chron Respir Dis 2008; 5: 161–168. [DOI] [PubMed] [Google Scholar]
- 30. Gale NS, Bolton CE, Duckers JM, et al. Systemic comorbidities in bronchiectasis. Chron Respir Dis 2012; 9: 231–238. [DOI] [PubMed] [Google Scholar]
- 31. McDonnell MJ, Aliberti S, Goeminne PC, et al. Comorbidities and the risk of mortality in patients with bronchiectasis: an international multicentre cohort study. Lancet Respir Med 2016; 4: 969–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang H, Ji XB, Li CW, et al. Clinical characteristics and validation of bronchiectasis severity score systems for post-tuberculosis bronchiectasis. Clin Respir J 2018; 12: 2346–2353. [DOI] [PubMed] [Google Scholar]
- 33. Lee SJ, Kim HJ, Kim JY, et al. Serum albumin and disease severity of non-cystic fibrosis bronchiectasis. Respir Care 2017; 62: 1075–1084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-docx-1-crd-10.1177_14799731211017548 for Serum albumin is a predictor of respiratory hospitalization in patients with bronchiectasis by Sunmi Ju, Jong Hwan Jeong, Manbong Heo, I Re Heo, Tae Hoon Kim, Ho Cheol Kim, Jung-Wan Yoo, Yu Ji Cho, Yi Yeong Jeong, Jong Deog Lee and Seung Jun Lee in Chronic Respiratory Disease
Supplemental Material, sj-docx-2-crd-10.1177_14799731211017548 for Serum albumin is a predictor of respiratory hospitalization in patients with bronchiectasis by Sunmi Ju, Jong Hwan Jeong, Manbong Heo, I Re Heo, Tae Hoon Kim, Ho Cheol Kim, Jung-Wan Yoo, Yu Ji Cho, Yi Yeong Jeong, Jong Deog Lee and Seung Jun Lee in Chronic Respiratory Disease