Abstract
Introduction
Type 2 diabetes (T2D) is extremely burdensome to people with T2D and associated with impaired health-related quality of life. This study explores the impact of T2D and potentially relevant outcomes for new therapies using a unique approach to in-depth qualitative interviews where people with T2D are asked to think about their future with T2D.
Methods
A cross-sectional qualitative interview study among people with T2D from the USA and UK. Interviews explored their treatment journey, perceptions of their future with T2D, and the value of achieving normoglycemia (explored through presentation of two vignettes with hypothetical medications that reduced hemoglobin A1c [HbA1c] levels < 7% and < 5.7%).
Results
Patients with T2D (N = 50; US n = 25; UK n = 25) were 66.0% male, had a mean body mass index (BMI) of 30.8 ± 6.3 kg/m2, and had a mean of 13.0 ± 10.0 years since diagnosis. Current diabetes treatments included diet and exercise only (8.0%), oral medications only (62.0%), oral plus injections (24.0%), and insulin only (6.0%). Despite being treated, participants reported over 25 different unmet needs related to their T2D across a broad range of domains. The most common concerns were diet, diabetes-related complications, weight changes, and psychological and emotional issues. A large majority of participants indicated that achieving lower HbA1c values would change their life. When reflecting on the value of improved glycemic control, patients primarily anticipated physical improvements and improved psychological well-being. When presented with two hypothetical treatments, about 70% of participants preferred the < 5.7% treatment option over the < 7% HbA1c treatment option.
Conclusions
People with T2D have a high disease burden, a broad range of unmet needs, and extremely varied experiences and expectations on the impacts of T2D on their lives and future. Many patients indicated that achieving near normoglycemia would substantially change their lives primarily in terms of their physical and emotional health.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-021-01054-6.
Keywords: Diabetes, Disease burden, HbA1c, Normoglycemia, Type 2 diabetes, Unmet needs
Key Summary Points
| Why carry out this study? |
| As novel therapies are developed with potential for better outcomes, including the possibility of achieving near normoglycemia, it is important to understand how people with type 2 diabetes (T2D) currently view the burden of the disease and the benefits and risks associated with new treatment options |
| Most prior qualitative studies have explored disease burden based on the past and current experiences of individuals with T2D. The current study explores the impact of T2D and relevant outcomes for new therapies using a unique approach in which participants with T2D are asked to think about their future and verbalize what is most concerning and important to them. Through this approach, it was hoped that people with T2D could more easily prioritize and communicate the diabetes-related outcomes that are most meaningful to them |
| A key area of investigation in the current study was to explore how people with T2D might perceive achieving near normoglycemia, understand their perceptions of potential risk-benefit trade-offs (e.g., HbA1c, weight changes, side effects), and explore the specific benefits beyond HbA1c that were anticipated from the perspective of individuals with T2D |
| What was learned from the study? |
| The results of this study showed that a treatment that achieves near normoglycemia without increasing the risk of hypoglycemia would be a paradigm shift for many individuals with T2D and a highly valued option with many anticipated benefits. In addition to lowered HbA1c values, reductions in psychological and emotional distress and physical benefits would be the primary areas in which people with T2D would expect to experience meaningful improvements |
| There is still a substantial burden of disease for T2D that is not addressed by current treatments. New treatment options would be welcomed by people with T2D, though they would be evaluated by individual patients (and likely their physicians) in light of the associated risks, including side effects and, for some, the substantial weight loss |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14249138.
Introduction
Type 2 diabetes (T2D) is a serious condition, and the prevalence has risen dramatically over the past 3 decades in both developed and developing countries [1–3]. This number is projected to rise to 578 million, or 10.2% of the global population, by 2030 and to 700 million (10.9%) by 2045 [4]. Worsened by both an increasing elderly population and rising obesity rates [5–10], T2D now accounts for 90% of all diabetes cases [6, 11] and is currently the most common metabolic disorder worldwide.
People with T2D find their condition to be extremely burdensome, and it is often associated with impaired health-related quality of life (HRQoL) [12, 13]. Diabetes, especially with poor glycemic control, is associated with higher risks of serious complications and comorbidities, including coronary artery disease, stroke, peripheral vascular disease, retinopathy, nephropathy, and neuropathy [3, 14, 15].
Currently, there is a dearth of published qualitative research on the unmet needs of people with T2D. These types of studies can provide a patient-centered approach to gathering rich information on the nature, extent, and perceived importance of the continued challenges that people with T2D face. Poor psychological well-being, physical health status, lifestyle behavior, and social environments are several factors that have been previously found to be associated with diabetes [14, 16–18]. However, most prior qualitative studies have explored disease burden based on the past and current experiences of individuals with T2D. These retrospective and contemporaneous approaches yield a wealth of data but may make it more difficult to understand which aspects of their condition are the most important to patients and thus critical outcomes for the evaluation of novel therapies. The current study explores the impact of T2D and potentially relevant outcomes for new therapies using a unique approach in which participants with T2D are asked to think about their future with T2D and verbalize what is most concerning and important to them.
Achieving glycemic control is a crucial step to diabetes management and may reduce the risk of future complications. Current guidelines from the American Diabetes Association (ADA) recommend that people with T2D achieve a goal of < 7% (53 mmol/mol) hemoglobin A1c (HbA1c) [19]. Treatment in combination with lifestyle modifications is often recommended to achieve this goal [19], and advancements in the last 10 years have led to new approaches to therapy and made this glycemic goal easier to achieve [20]. More recently, research has shown that movement from above target HbA1c to normoglycemia (HbA1c < 5.7%), a blood glucose level similar to people without diabetes, may also be achieved therapeutically [21]. To date, achieving near normoglycemia has rarely been a focus, or even considered, by people with T2D or their treatment providers.
Little is known about what achieving normoglycemia would mean to people with T2D. Given the distinct possibility that this may now be achievable for at least some individuals with T2D, this study examined the perceived value of achieving near normoglycemia (HbA1c < 5.7%) among people with T2D. In the current study, participants with T2D were asked to imagine the future and anticipate how their lives could be different should they achieve near normoglycemia. Through this approach, it was hoped that people with T2D could more easily prioritize and communicate the diabetes-related outcomes that are most meaningful to them.
Methods
Sample
This was a cross-sectional qualitative study involving interviews with adult participants with self-reported T2D. Participants were recruited through newspaper and online advertisements in the USA and UK. Key inclusion criteria included age ≥ 18 years at the time of screening and self-reported diagnosis of T2D as diagnosed by a medical professional. Key exclusion criteria included diagnosis of type 1 diabetes mellitus or gestational diabetes, cognitive, physical, or psychological impairment, or insufficient knowledge of English that would interfere with their ability to provide consent or complete the interview.
Measures and Procedures
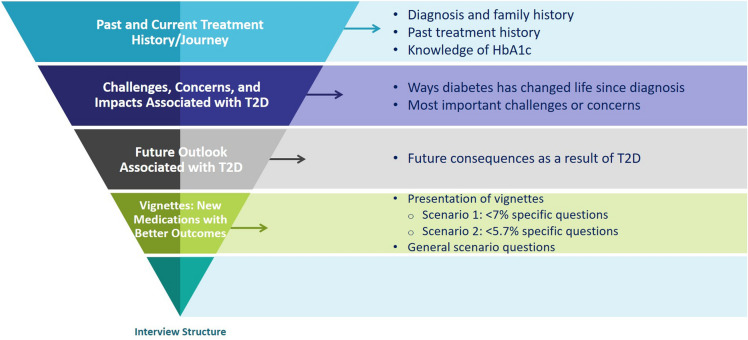
Interviews were conducted one on one either in person or by telephone/web conference using a standardized interview guide. The interview guide was developed based on key questions of interest and discussions with all members of the study team (see Supplemental material for example interview questions). Participants were asked about their T2D journey and treatment history, the challenges and changes they experienced as a result of their diagnosis, the ways in which diabetes has changed their lives, and perceptions of their future with T2D (Fig. 1). The interviews also included two vignettes (see Supplementary Figures S1a and S1b) that were used to understand the value of achieving normoglycemia (HbA1c target < 5.7%) from the perspective of people with T2D. These vignettes, developed based on results from a recent phase 2 trial [21], presented two hypothetical treatments with similar administration profiles; both treatments are non-insulin auto-injections to be taken once a week using a single-dose use pen. After 6 weeks of treatment, one treatment would lower HbA1c levels to about 7% (Figure S1a) with an average weight loss of 6 lbs and a 35% chance of gastrointestinal (GI) side effects. In contrast, the second treatment would lower HbA1c levels to 5.7% or lower with an average weight loss of 25 lbs and a 60% chance of GI side effects.
Fig. 1.
Interview flow
Participants were asked to complete sociodemographic and clinical questions at the end of the interview through a web-based application. All participants provided informed consent prior to the interviews and were remunerated for their participation. All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study protocol and all study procedures were approved by an institutional review board prior to initiating the study (Ethical and Independent Review Services, protocol #20010–01, v1.0 date 6 January 2020, approved 11 February 2020).
Analyses
All interviews were digitally recorded and transcribed. Qualitative data were analyzed using ATLAS.ti version 8.0 + [22], a data analysis software program commonly used to organize and categorize text in interview transcripts. Using a methodology described by Willis (2015) and Hsieh & Shannon (2005), the de-identified data were examined to evaluate the underlying structure of the qualitative data [23, 24]. A coding framework was developed based on the structure of the interview guide. The first several transcripts were independently coded, and the coding was compared for consistency. An iterative, comparative coding approach was used as coding continued between consecutive transcripts and new codes emerged [25]. Any new codes identified were subsequently added to the coding framework. Participant quotes were then grouped and summarized by thematic code, and coding outputs were generated based on each utilized code.
Descriptive statistics were used to characterize the sample. Means, standard deviations (SDs), and ranges were presented for continuous variables, and frequencies and percentages were presented for categorical variables. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results
A total of 50 people with T2D completed interviews, including 25 participants from the USA and 25 from the UK. Self-reported sociodemographic characteristics are presented in Table 1. The mean age of the participants was 63.7 years (SD: 11.3; range: 24–85), and 66% (n = 33) were male. The majority of the participants in each country identified as White (US: n = 21, 84%; UK: n = 14, 56%), with some representation of minorities in each country. In the US, most of the participants were retired (n = 15, 60%), while four reported full-time employment (16%). In the UK, close to one third of the participants were employed full-time (n = 8, 32%), and six were retired (24%). There was a good distribution of participants across the different education levels in each country.
Table 1.
Self-reported sociodemographic and clinical characteristics
| Sociodemographic and clinical characteristics | Total N = 50 |
US N = 25 |
UK N = 25 |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 63.7 (11.3) | 68.9 (8.5) | 58.5 (11.5) |
| Gender, n (%) | |||
| Male | 33 (66%) | 17 (68%) | 16 (64%) |
| Racea n (%) | |||
| Asian | 7 (14%) | 2 (8%) | 5 (20%) |
| Black/African/Caribbean | 5 (10%) | 1 (4%) | 4 (16%) |
| White | 35 (70%) | 21 (84%) | 14 (56%) |
| Otherb,c | 3 (6%) | 1 (4%) | 2 (8%) |
| Employment status, n (%) | |||
| Employed, full-time | 12 (24%) | 4 (16%) | 8 (32%) |
| Employed, part-time | 5 (10%) | 2 (8%) | 3 (12%) |
| Retired | 21 (42%) | 15 (60%) | 6 (24%) |
| Otherd | 12 (24%) | 4 (16%) | 8 (32%) |
| Body mass index (BMI; kg/m2) | |||
| Mean (SD) | 30.8 (6.3) | 31.1 (6.5) | 30.5 (6.2) |
| Time since diagnosis (years) | |||
| Mean (SD) | 13.0 (10.0) | 16.2 (11.9) | 9.9 (6.6) |
| Most recent laboratory (HbA1c) level, n (%) | |||
| Below 6.5% (48 mmol/mol) | 13 (26%) | 5 (20%) | 8 (32%) |
| Between 6.5% and 7% (48 mmol/mol and 53 mmol/mol) | 9 (18%) | 6 (24%) | 3 (12%) |
| Between 7.1% and 8% (54 mmol/mol and 64 mmol/mol) | 12 (24%) | 10 (40%) | 2 (8%) |
| Between 8.1% and 9% (65 mmol/mol and 75 mmol/mol) | 3 (6%) | 2 (8%) | 1 (4%) |
| Higher than 9% (75 mmol/mol) | 5 (10%) | 2 (8%) | 3 (12%) |
| Do not know | 8 (16%) | 0 (0%) | 8 (32%) |
| Type of treatment currently receiving for diabetes by combination, n (%) | |||
| Diet and exercise only | 4 (8.0%) | 3 (12.0%) | 1 (4.0%) |
| Oral/tablets onlye | 31 (62.0%) | 9 (36.0%) | 22 (88.0%) |
| Insulin only e | 3 (6.0%) | 3 (12.0%) | 0 (0.0%) |
| Oral + insulin and/or non-insuline | 12 (24.0%) | 10 (40.0%) | 2 (8.0%) |
| Description of overall health, n (%) | |||
| Excellent | 2 (4%) | 0 (0%) | 2 (8%) |
| Very good | 7 (14%) | 7 (28%) | 0 (0%) |
| Good | 27 (54%) | 11 (44%) | 16 (64%) |
| Fair | 13 (26%) | 7 (28%) | 6 (24%) |
| Poor | 1 (2%) | 0 (0%) | 1 (4%) |
aNot mutually exclusive
bOther race (US only) includes: ‘Mexican’ (n = 1)
cOther race (UK only) includes: ‘Greek Cypriot’ (n = 1), ‘White and Black Caribbean’ (n = 1)
dOther employment includes: ‘disabled’ (n = 4), ‘homemaker/housewife’ (n = 2), ‘student’ (n = 1), ‘unemployed’ (n = 1), ‘carer’ (n = 1), ‘unpaid carer’ (n = 1), ‘COVID-19′ (n = 1), and ‘retired/self-employed’ (n = 1)
eIncludes participants who selected and did not select “diet and exercise”
Although almost all participants (92%) were currently being treated for T2D therapeutically, over 25 unmet needs related to their T2D were reported across a broad range of domains. Participants described how T2D affects their life, including challenges, changes, and concerns they have experienced because of T2D. The most common thematic responses described by participants were challenges, changes, or concerns related to diet (82%), health complications related to T2D (74%), and weight changes or control (68%) (Fig. 2). Close to 50% of the participants also brought up concepts in the domains of psychological and emotional burden (52%), activities of daily living (48%), and burden of additional monitoring (48%). When asked which of these was most important to them, participants most commonly selected complications (33%).
Fig. 2.
Challenges, changes, or concerns due to T2D. a Other includes: challenges of tiredness, fatigue, or lack of energy (n=5), concerns of taking too many tablets (n=5), fear of taking injections (n=2), risk of family members getting diabetes (n=2), concerns of keeping HbA1c low (n=2), frequency of administration (n=1), fear of worsening immune system (n=1), concerns of increased vulnerability (n=1), fear of worsening health (n=1), and fear of treatment side effects (n=1). b Percentages are based on the number of respondents for each question. N=50 responded to overall challenges, changes, or concerns due to T2D. N=46 responded to the most important challenge, change, or concern
As part of the interview, participants were presented with two scenarios describing two once-a-week non-insulin injectable medications. In the first medication profile scenario, participants would achieve HbA1c levels of about 7% and average weight loss was 6 lbs, and there was a 35% chance of mild-to-moderate GI side effects at the beginning of therapy. In the second medication profile scenario, participants would achieve HbA1c levels of 5.7% or lower and average weight loss was 25 lbs, and there was a 60% chance of mild-to-moderate GI side effects at the beginning of therapy. Participants were asked to provide feedback on whether achieving the HbA1c goal under the conditions stated in each scenario would change their perceptions of their future. Additionally, participants were asked whether the HbA1c levels would change their lives and, if so, how. For those participants who had already achieved the HbA1c levels mentioned in the scenarios, the interview questions were modified to ask how this had impacted their lives in the past tense. Most participants (83%) mentioned that both hypothetical treatments would change their perceptions of their future with diabetes (Fig. 3). More participants were optimistic about the future with the < 5.7% treatment scenario (80%) compared to the < 7% treatment scenario (66%).
Fig. 3.
Anticipated changes in participants’ lives with lower HbA1c. <Emphasis Type="Bold">a</Emphasis> N=50 responded to anticipated changes (e.g., psychological and emotional). <Emphasis Type="Bold">b</Emphasis> Other includes: better convenience (e.g., fewer doctor visits, less time required for treatment administration; <5.7% n=7, <7% n=7), reduced treatment or frequency of treatment (e.g., fewer pills, once a week; <7% n=5), less monitoring (<5.7% n=2), increased complacency or recklessness (<5.7% n=1, <7% n=3), increased quality of life (<5.7% n=1), reduced likelihood of going on insulin (<7% n=1), better cognition (<7% n=1), and reduced doctor concerns (<7% n=1)
A large majority of participants indicated that achieving lower HbA1c values would change their life and their perception of their future with diabetes. Irrespective of the treatment scenario they discussed, the most commonly anticipated impacts on the participants’ lives and future perceptions were psychological and emotional in nature (86%), including an increased sense of security, better quality of life, better outlook for their future, feeling less worried or depressed, feeling a greater sense of happiness or optimism, having higher motivation and confidence, and anticipating better control over their lives (Table 2). Many participants also mentioned physical improvements (82%), including a lower risk of experiencing complications, less long-term damage to their bodies, weight loss, having more energy, bodies that were more physically healthy, having a stronger body, and better sleep. Sample quotes are provided below.
101-006 [<5.7%]: And I think I would feel like I have less fear of what might be future complications if I could get that HbA1c lower. Because that’s every diabetic’s fear, that if you don’t get your HbA1c under control or constant, you face the very real possibility of some alarming things down the road – stroke, heart attack, loss of vision, loss of feeling in your feet, that kind of thing.
202-002 [<5.7%]: The benefits being it would help my other conditions I’ve already got through diabetes, one being the eyesight, the retinopathy. I know the 5.7 will help me. Not so much the 7%. Having a low blood glucose level will definitely be the one I would ask for, a medication that will do that, if Scenario No. 2 would do that for me.
202-011 [<5.7%]: I just think, well, I think psychologically it’s going to have an effect because you know you’re within that range. If it’s now lower than 5.7, you know you’re going to be less at risk of heart attacks, strokes, etc. If it’s higher, your HbA1c is a high level, you know you’re more pushing toward that risk category. So I just think it’s, you know, the higher your HbA1c level is, the more stress and worry it is going to cause you.
301-004 [<5.7%]: I would be much more – my stress level would be much higher at 7%. I would be constantly worried about my blood sugar. At 7% I’d be thinking about it every single day, every time I’d look at a needle, it would be going through my mind. Because anything above 7% is really bad, so it’s like I’m on a ledge, you know. If it goes above 7%, I fall, you understand? To me, 7% is life-threatening. And I would worry about it, like I say, every single day.
Table 2.
Quotes on psychological/emotional and physical impacts of hypothetical treatments on participant’s lives and future perceptions
| Impacts | Quotes (bolding added for emphasis) |
|---|---|
| Psychological and emotional impact | |
| Increased sense of security |
402-008 [<5.7%]: Yeah, it would change my life significantly, I would say. It would—I’d basically be leading a life I led before I got the diagnosis, so, yeah, psychologically it would almost be as if my Type 2 diabetes was like benign or inactive and it wasn’t something that was like a problem or anything anymore. 101-041 [<5.7%]: I think emotionally it would make me feel normal…and I think it would make me feel healthier. 202-007 [<5.7%]: Psychologically you feel better knowing you’re not diabetic, albeit with medication |
| Better quality of life |
101-041 [<7%]: Well, definitely if I’m at 7% I would feel like my diabetes is under control, it would probably allow me to live my life a little bit more fully. And it’d probably make me feel better and think better and work better. 201-004 [<5.7%]: It would be amazing. Depending on where you are–wherever you are on the diabetes spectrum, anything that gives you better control or encourages you to have better control and change that lifestyle not on a temporary basis but for as long as you’ve got has to be a good thing. |
| Better future outlook |
201-001 [<7%]: Yeah, I think so, because like I said that I’m hoping that is a new medication and then the life expectancy will increase. 402-008 [<7%]: I think it would, yeah. It would give me, I guess, a bit more of a positive outlook regarding my health in the long term. 101-041 [<5.7%]: …if you do have it under control, yeah, I think it takes a lot of the fear away in terms of what your future health liability is going to be. 201-006 [<5.7%]: Yeah, you’d feel better about yourself, obviously. You know that you’re in that good range, basically, so you would feel more optimistic about the future, yeah. |
| Less worried or depressed |
202-010 [<7%]: Yeah, I think it would, again, make me feel a bit more confident about my health, a bit less concerned about the problems I’ve got. That would be the answer to that. 101-006 [<7%]: I think psychologically knowing I had a controllable HbA1c in the 7 range I’d worry less about complications down the road with things like vision, feelings in my feet and legs, extremities, all those complications that diabetics worry about – heart conditions, strokes. I’d be a lot less worried about that stuff because I knew my HbA1c was in a controllable range. 101-033 [<5.7%]: It just makes it easier, I think that once you lower your hemoglobin A1c of not – you keep taking that, the medicine, and continue with it, then I think you don’t have to worry about going up again. 301-004 [<5.7%]: It would take a lot of – tremendously lower my stress level because I wouldn’t be worried about the consequences, the progression and the consequences related to them, and I would be – I would think of myself as someone who was not a diabetic. |
| Greater sense of happiness or optimism |
101-032 [<7%]: Yes, psychologically…I would be very happy, very good, and very positive and very open, you know, verbally to people and just letting them—no, I don’t see at all—psychologically it would be uplifting. 201-004 [<5.7%]: Yeah, it would make me think there’s a fighting chance, rather than the inevitability of all the issues. 101-032 [<5.7%]: Yes, it would probably give me literally a shot, so to speak, mentally and maybe physically to be more positive… a nice mental help on a positive note. 101-067 [<5.7%]: I would think it would be a miracle. I’d be living my life in a miracle. |
| Higher motivation and confidence |
202-010 [<7%]: Yeah, I think it would, again, make me feel a bit more confident about my health, a bit less concerned about the problems I’ve got. 101-012 [<5.7%]: Other than just not having to worry about it less and less. I don’t worry that much about it now, but it’s still in the back of my mind, yeah. Sometimes that’s good, it motivates me to go to the gym, motivates me to skip the donuts, whatever. 202-002 [<5.7%]: …having this control with this medication, certainly it will make me feel confident that, yeah, I’m sure I’d enjoy life a bit more, live longer. |
| Better control of life |
201-004 [<7%]: Yeah. It would free you up. I’m not sure whether I’m consciously thinking about it in the back of my mind all the time, but I know it’s there. 404-020 [<5.7%]: I think it would just give you some sense of control other than standing on quicksand. 101-009 [<5.7%]: Physically, if my weight was down, sweetie, first of all, I could shop wherever I wanted…I just think emotionally I’d be able to do more of what I want to do. |
| Physical impact | |
| Less risk of complications |
402-011 [<7%]: I mean, I think in terms of long-term effects, I’d probably feel a little more confident that I would not have the onset of more debilitating medical conditions. 101-006 [<5.7%]: And I think I would feel like I have less fear of what might be future complications if I could get that HbA1c lower. Because that’s every diabetic’s fear, that if you don’t get your HbA1c under control or constant, you face the very real possibility of some alarming things down the road – stroke, heart attack, loss of vision, loss of feeling in your feet, that kind of thing. So side effects. 202-002 [<5.7%]: I would suspect it would be because in my mind I’m thinking, well, you have less glucose going around in your body, less damage it’s going to do to your kidneys, to your eyes and anything else or risk of heart disease and all that. Yes, it’s beneficial. It’s a positive thing, yes, yes. |
| Weight loss |
101-009 [<7%]: Well, I’d like to – no, it just isn’t – weight loss of 6 pounds is not a lot. I mean, it’s better than nothing, but I would like it to be better than that. 101-008 [<5.7%]: One, sure, if I lose 25 pounds, I would lose a lot more than the other hypothetical medication, that would be wonderful. I mean I would feel a lot emotionally better and I would feel a lot more active and just would have a lot more spring in my step. 301-004 [<5.7%]: Yes, if it helps me lose weight, it would increase my mobility and stop the nerve damage progression or any negative consequences, it would halt it. |
| More energy |
202-010 [<7%]: It would improve the general condition, I think. I would expect to feel a bit more energetic, a bit less mental energy, concerned with what I’m eating. Yeah. I would say it would be an improvement, significant improvement, yeah. 101-026 [<7%]: Since this is a new medication that’s there, so maybe it would make me more active, [laughter] since it give me more energy to move. 101-026 [<5.7%]: Maybe I have more energy to exercise and walk. 101-006 [<5.7%]: Oh, I think it would. It would certainly – I think I would have more energy. The lower the HbA1c, the more energetic I would become, and I think I’d feel just healthier overall. |
| Physically healthier |
101-041 [<7%]: Well, I think it would give me the confidence that I would have the energy more to do my daily activity that I can do because if it’s better I would feel better and I’d probably be healthier. 402-003 [<7%]: Yes, I think it will make me more active, and then of course it’s more healthy if you’re more active, you know. 101-033 [<5.7%]: Just in your overall physical health, what you can do, what you’re able to do, and how you can enjoy it. |
| Stronger |
101-017 [<7%]: It might. It might. Might be able to get me the ability to endure a little more exercise, maybe get the muscles back to some reasonable shape, longer walks, that sort of thing. 301-022 [<7%]: The way I can—I feel more better, where I feel like sometime I might feel drowsy, lazy, don’t want to get out of bed. But I’d probably feel better, have more strength and just I’ll feel better. |
| Better sleep |
301-001 [<7%]: I’m sure when you lose the weight you have better sleep patterns. 301-011 [<5.7%]: Well, I thought it’d be wonderful, really, that kind of medication. Then there will be fewer diabetes, right? I don’t have any more diabetes, and I don’t have to worry about my weight, so that is wonderful to have this kind of medication in the future…then I won’t have to worry about having sleep apnea again. |
Participants were also asked which of the two medication scenarios they preferred. The majority of participants preferred the < 5.7% scenario (n = 34; 68%), fewer preferred < 7% (n = 8; 16%), some could not select a preference (n = 6; 12%), and two (4%) preferred either scenario (Fig. 4, top panel). The most common reasons mentioned by participants preferring the < 5.7% scenario were that they liked that the HbA1c would be significantly lowered and back to “normal” levels (n = 18). Many noted that the weight loss is significant (n = 13) and/or that the treatment would reduce the risk of complications and long-term damage (n = 10) (Fig. 4, bottom panel). Participants who preferred the < 7% treatment scenario felt the risk of side effects in the < 5.7% scenario was too high (n = 7). Several participants also mentioned that they did not want to lose too much weight (n = 4; 8%); two of these participants had BMIs within the normal range (18–25 kg/m²), while the other two had BMIs of 27 and 31, respectively. Among the participants who did not have a specific preference for either treatment, they felt that 7% would not have an impact on their life but also felt that 5.7% involved too much weight loss.
Fig. 4.
Participants’ preferences for medication scenarios and rationale
Discussion
As novel therapies are developed with potential for better outcomes, including the possibility of achieving near normoglycemia [21], it is important to understand how people with T2D view the benefits and risks associated with new treatment options. The results of the current study and prior studies suggest that participants have extremely varied experiences and perspectives in terms of their diagnostic and treatment histories and their perceptions of the impact of T2D on their lives [26–29]. However, to date, there has been very little research on the perceptions and expectations of people with diabetes about their future with the condition. The aim of the current study was to gather these perspectives directly from individuals with T2D through interviews that focused on how people with diabetes view their future. It was anticipated that this approach might help to highlight the outcomes and concerns that are most important to them. These findings may help identify currently unmet needs of people with T2D.
Despite the broad range of options, participants on currently available treatments for T2D continue to report a high disease burden and many unmet needs. A recent study suggests that both emotional burden and regimen-related distress are very high, particularly among people with T2D [30]. In the current study, the most common challenges and concerns described by participants were related to diet, complications, and weight changes or control, which was expected. However, more than half of the participants also highlighted psychological and emotional issues as a significant challenge, with this domain ranking second after complications in terms of what was most important to them. Consistent with this finding, it has previously been shown that T2D individuals with higher HbA1c levels have increased psychological and emotional distress [31–33]. In addition, prior studies have also found that individuals with T2D who show improvements in clinical measures through diabetes treatment also report improvements in psychological health [34–36].
A key area of investigation in the current study was to explore how people with T2D might perceive achieving near normoglycemia, understand their perceptions of potential risk–benefit trade-offs (e.g., HbA1c, weight changes, side effects), and explore the specific benefits beyond HbA1c that were anticipated from the patient perspective. When presented with two hypothetical treatments, the majority of participants (about 70%) preferred the < 5.7% HbA1c treatment option over the < 7% treatment option. Many of the participants noted that they would be “back to normal” in terms of HbA1c, and the weight loss was often perceived as a significant benefit. Participants anticipated a reduced risk of complications and long-term physical damage to their bodies, which was often associated with expected decreases in psychological or emotional distress. Not all participants preferred the treatment that achieved near normoglycemia. Almost universally, participants who did not prefer the < 5.7% treatment option cited the risk of side effects as too high to justify the reduction in HbA1c. In addition, some participants, half of whom had BMI within the normal range (18.5–25 kg/m2), also felt that the weight loss associated with this treatment option was excessive (Fig. 4, lower panel). These results suggest that achieving normoglycemia would be highly valued by a majority of participants with T2D, and often preferred, despite a much larger risk of treatment side effects as compared with alternative options that offered more modest reductions in HbA1c. Regardless of the preferred treatment option, participants most frequently mentioned that they anticipated changes to their lives that were positive including 80% anticipating benefits that were psychological, emotional, and/or physical. The anticipated benefits that were described by participants in the areas of psychological or emotional functioning suggest that future studies of treatments with the potential to achieve near normoglycemia might be best poised to identify relevant benefits by including measures that are designed to capture concepts such as levels of security, worry, depression, optimism, motivation, confidence, and control.
Limitations
The results of this study should be interpreted with consideration of the following limitations. The population recruited for this study was a convenience sample; participants were recruited through newspaper and online advertisements, and participants were older. Fewer participants than planned in the protocol were interviewed in-person due to COVID-19 restrictions; however, during the analyses no important differences were noted between those who completed telephone interviews versus in-person interviews. All participants in the study were included based on a self-reported diagnosis of their T2D. All participants were deemed sufficiently knowledgeable of T2D during the interviews to provide confidence in this diagnosis; however, no confirmation of diagnosis from a clinician or medication prescription was required. Lastly, the study design, which involved interviews to explore the impact of T2D and potentially relevant outcomes for new therapies, may have resulted in some selection bias. It is unknown to what extent those who volunteered to participate in the interviews represent the population of T2D more generally.
Conclusions
A treatment that achieves near normoglycemia without increasing the risk of hypoglycemia would be a paradigm shift for many individuals with T2D and a highly valued option with many anticipated benefits. In addition to lowered HbA1c values, reductions in psychological and emotional distress and physical benefits would be the primary areas in which people with T2D would expect to experience meaningful improvements. These treatment options would be welcomed by people with T2D, though they would be evaluated by individual patients (and likely their physicians) in light of the associated risks, including side effects and, for some, the substantial weight loss.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Cristina Abel, Fanyang Zeng, Ella Brookes, and Myrto Trapali for their assistance in executing the study. The authors would like to thank Fritz Hamme, Kawthar Nakayima, Amara Tiebout, Dawn Ri’chard, and Emily Sargent from Evidera for providing editing and production support throughout the study. The authors would also like to thank Amara Tiebout from Evidera for providing editing and production support during the writing of this article. All editing and production support services were funded by Eli Lilly and Company. Lastly, the authors would like to thank the participants of the study.
Funding
The study was funded by Eli Lilly and Company. Eli Lilly and Company also funded this journal’s Rapid Service Fee.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
All authors participated in the design of the study. Heather Gelhorn and Zaneta Balantac participated in the execution of the study. All authors participated in the interpretation of data, review, and approval of the manuscript.
Disclosures
Heather Gelhorn and Zaneta Balantac are employees of Evidera, an independent research organization that received support from Eli Lilly to conduct this work. Kristina S. Boye, Vivian Thuyanh Thieu, and Shraddha Shinde were employees and shareholders of Eli Lilly and Company during the time this work was conducted and analyzed.
Compliance with Ethics Guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study protocol and all study procedures were approved by an institutional review board prior to initiating the study (Ethical and Independent Review Services, protocol #20010-01, v1.0 date 06Jan2020, approved 11Feb2020). Informed consent was obtained from all individual participants included in the study.
Data Availability
Data are not publicly available but may be provided upon request.
Contributor Information
Heather Gelhorn, Email: Heather.Gelhorn@evidera.com.
Zaneta Balantac, Email: zaneta.balantac@evidera.com.
Shraddha Shinde, Email: shinde_shraddha@lilly.com.
Vivian Thuyanh Thieu, Email: thieu_vivian_thuyanh@lilly.com.
Kristina S. Boye, Email: boye_kristina_secnik@lilly.com
References
- 1.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world: A growing challenge. N Engl J Med. 2007;356(3):213–215. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 2.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diab Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO). Diabetes: Global report on diabetes Geneva, Switzerland; 2016 [October 13, 2020]. 88 pgs. Available from: http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf?ua=1.
- 4.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 5.Bhupathiraju SN, Hu FB. Epidemiology of obesity and diabetes and their cardiovascular complications. Circ Res. 2016;118(11):1723–1735. doi: 10.1161/CIRCRESAHA.115.306825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389(10085):2239–2251. doi: 10.1016/S0140-6736(17)30058-2. [DOI] [PubMed] [Google Scholar]
- 7.Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diab Care. 2011;34(6):1249–1257. doi: 10.2337/dc11-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaacks LM, Vandevijvere S, Pan A, McGowan CJ, Wallace C, Imamura F, et al. The obesity transition: stages of the global epidemic. Lancet Diabetes Endocrinol. 2019;7(3):231–240. doi: 10.1016/S2213-8587(19)30026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalyani RR, Golden SH, Cefalu WT. Diabetes and aging: unique considerations and goals of care. Diab Care. 2017;40(4):440–443. doi: 10.2337/dci17-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tabish SA. Is diabetes becoming the biggest epidemic of the twenty-first century? Int J Health Sci (Qassim). 2007;1(2):V–VIII. [PMC free article] [PubMed]
- 11.Goldstein BJ. Insulin resistance: from benign to type 2 diabetes mellitus. Rev Cardiovasc Med. 2003;4(Suppl 6):S3–10. [PubMed] [Google Scholar]
- 12.Ogunmodede AJ, Abiodun O, Makanjuola AB, Olarinoye JK, Ogunmodede JA, Buhari OI. Burden of care and psychological distress in primary caregivers of patients with type -2 diabetes mellitus in A tertiary hospital in Nigeria. Ethiop J Health Sci. 2019;29(6):697–708. doi: 10.4314/ejhs.v29i6.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubin RR, Peyrot M. Quality of life and diabetes. Diabetes Metab Res Rev. 1999;15(3):205–218. doi: 10.1002/(SICI)1520-7560(199905/06)15:3<205::AID-DMRR29>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 14.Cannon A, Handelsman Y, Heile M, Shannon M. Burden of illness in type 2 diabetes mellitus. J Manag Care Spec Pharm. 2018;24(9-a Suppl):S5–S13. [DOI] [PMC free article] [PubMed]
- 15.Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26(2):77–82. doi: 10.2337/diaclin.26.2.77. [DOI] [Google Scholar]
- 16.Beverly EA, Ritholz MD, Brooks KM, Hultgren BA, Lee Y, Abrahamson MJ, et al. A qualitative study of perceived responsibility and self-blame in type 2 diabetes: reflections of physicians and patients. J Gen Intern Med. 2012;27(9):1180–1187. doi: 10.1007/s11606-012-2070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cimo A, Dewa CS. Symptoms of mental illness and their impact on managing type 2 diabetes in adults. Can J Diabetes. 2018;42(4):372–381. doi: 10.1016/j.jcjd.2017.08.256. [DOI] [PubMed] [Google Scholar]
- 18.Peyrot M, Rubin RR, Lauritzen T, Snoek FJ, Matthews DR, Skovlund SE. Psychosocial problems and barriers to improved diabetes management: results of the Cross-National Diabetes Attitudes, Wishes and Needs (DAWN) Study. Diabet Med. 2005;22(10):1379–1385. doi: 10.1111/j.1464-5491.2005.01644.x. [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association (ADA). Standards of medical care in diabetes—2020 abridged for primary care providers. Clin Diabetes. 2020;38(1):10–38. [DOI] [PMC free article] [PubMed]
- 20.Artasensi A, Pedretti A, Vistoli G, Fumagalli L. Type 2 diabetes mellitus: a review of multi-target drugs. Molecules. 2020;25(8). [DOI] [PMC free article] [PubMed]
- 21.Frias JP, Nauck MA, Van J, Kutner ME, Cui X, Benson C, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018;392(10160):2180–2193. doi: 10.1016/S0140-6736(18)32260-8. [DOI] [PubMed] [Google Scholar]
- 22.Friese S, Ringmayr T. ATLAS.ti 8 User Guide and Reference. Berlin: ATLAS.ti Scientific Software Development GmBH; 2020.
- 23.Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 24.Willis GB. Analysis of the Cognitive Interview in Questionnaire Design. Understanding Qualitative Research. New York, NY: Oxford University Press; 2015.
- 25.Boeije H. A purposeful approach to the constant comparative method in the analysis of qualitative interviews. Qual Quant. 2002;36(4):391–409. doi: 10.1023/A:1020909529486. [DOI] [Google Scholar]
- 26.Litterbach E, Holmes-Truscott E, Pouwer F, Speight J, Hendrieckx C. 'I wish my health professionals understood that it's not just all about your HbA1c !'. Qualitative responses from the second Diabetes MILES - Australia (MILES-2) study. Diabetic Med. 2020;37(6):971–81. [DOI] [PubMed]
- 27.Pal K, Dack C, Ross J, Michie S, May C, Stevenson F, et al. Digital health interventions for adults with type 2 diabetes: qualitative study of patient perspectives on diabetes self-management education and support. J Med Internet Res. 2018;20(2):e40. doi: 10.2196/jmir.8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.St George SM, Pulgaron ER, Ferranti D, Agosto Y, Toro MI, Ramseur KC, et al. A qualitative study of cognitive, behavioral, and psychosocial challenges associated with pediatric type 2 diabetes in ethnic minority parents and adolescents. Diabetes Educ. 2017;43(2):180–189. doi: 10.1177/0145721717691146. [DOI] [PubMed] [Google Scholar]
- 29.Tanenbaum ML, Kane NS, Kenowitz J, Gonzalez JS. Diabetes distress from the patient's perspective: Qualitative themes and treatment regimen differences among adults with type 2 diabetes. J Diabetes Complications. 2016;30(6):1060–1068. doi: 10.1016/j.jdiacomp.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wardian JL, Tate J, Folaron I, Graybill S, True M, Sauerwein T. Who's distressed? A comparison of diabetes-related distress by type of diabetes and medication. Patient Educ Couns. 2018;101(8):1490–1495. doi: 10.1016/j.pec.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Jeong M, Reifsnider E. Associations of diabetes-related distress and depressive symptoms with glycemic control in Korean Americans with type 2 diabetes. Diabetes Educ. 2018;44(6):531–540. doi: 10.1177/0145721718807443. [DOI] [PubMed] [Google Scholar]
- 32.Mishra A, Podder V, Modgil S, Khosla R, Anand A, Nagarathna R, et al. Higher perceived stress and poor glycemic changes in prediabetics and diabetics among Indian population. J Med Life. 2020;13(2):132–137. doi: 10.25122/jml-2019-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parsa S, Aghamohammadi M, Abazari M. Diabetes distress and its clinical determinants in patients with type II diabetes. Diabetes Metab Syndr. 2019;13(2):1275–1279. doi: 10.1016/j.dsx.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Ascher-Svanum H, Zagar A, Jiang D, Schuster D, Schmitt H, Dennehy EB, et al. Associations between glycemic control, depressed mood, clinical depression, and diabetes distress before and after insulin initiation: An exploratory. Post Hoc Anal Diabetes Ther. 2015;6(3):303–316. doi: 10.1007/s13300-015-0118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J, Fan L, Peng X, Ilag L, Ly T, Johnson J. Patient-reported outcomes in a study of human regular U-500 insulin delivered by continuous subcutaneous insulin infusion or multiple daily injections in patients with type 2 diabetes. Diabetes Obes Metab. 2020. [DOI] [PMC free article] [PubMed]
- 36.Zhu L, Chandran SR, Tan WB, Xin X, Goh SY, Gardner DSL. Persistent anxiety is associated with higher glycemia post-transition to adult services in Asian Young adults with diabetes. Diabetes Metab J. 2020. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are not publicly available but may be provided upon request.