Abstract
Hyperleukocytosis in acute myeloid leukemia (AML) is associated with inferior outcomes. There is limited high quality evidence to support the benefits of leukapheresis. We retrospectively collected data from patients with newly-diagnosed AML who presented with a white cell count (WBC) >50×109/L to 12 centers in the United States and Europe from 2006–2017 and received intensive chemotherapy. Logistic regression models estimated odds ratios for 30-day mortality and achievement of composite complete remission (CRc). Cox proportional hazard models estimated hazard ratios for overall survival (OS). Among 779 patients, clinical leukostasis was reported in 27%, and leukapheresis was used in 113 patients (15%). Thirty-day mortality was 16.7% (95%CI:13.9–19.3%). Median OS was 12.6 months (95%CI:11.5–14.9) among all patients, and 4.5 months (95%CI: 2.7–7.1) among those ≥65 years. Use of leukapheresis did not significantly impact 30-day mortality, achievement of CRc, or OS in multivariate analysis based on available data or in analysis based on multiple imputation. Among patients with investigator-adjudicated clinical leukostasis, there were statistically significant improvements in 30-day mortality and OS with leukapheresis in unadjusted analysis, but not in multivariate analysis. Given the significant resource use, cost, and potential complications of leukapheresis, randomized studies are needed to evaluate its value.
Introduction
Acute myeloid leukemia (AML) is often a medical emergency necessitating hospital admission and prompt initiation of therapy, particularly in patients with high white blood cell (WBC) count. Hyperleukocytosis is usually defined as a WBC of >50 × 109/L or >100 × 109/L and is often seen in newly-diagnosed AML1. Hyperleukocytosis is associated with an increased risk of organ failure and early death secondary to leukostasis, tumor lysis syndrome (TLS) and disseminated intravascular coagulopathy (DIC)1–5. The organs most commonly affected by leukostasis are the lungs and the central nervous system (CNS), including the retina. However, the gastrointestinal (GI) and cardiovascular system may also be affected 6. Hyperleukocytosis, especially when associated with clinical leukostasis, is considered a medical emergency, and treatment options include urgent cytoreduction with leukapheresis, intensive chemotherapy (IC), and hydroxyurea 7, 8.
Limited data are available regarding the characteristics of newly-diagnosed AML patients who present with hyperleukocytosis, treatment patterns, short and long-term clinical outcomes, and the impact of leukapheresis on clinical outcomes. Retrospective studies show that leukapheresis is effective in decreasing the number of circulating blast cells 4, 9–17, but only for a very limited duration as the bulk of the disease is usually in the bone marrow. However, studies differ in their assessment of the impact of leukapheresis on short- and long-term clinical outcomes in AML patients with hyperleukocytosis, and most such studies were limited by single institution design and/or small cohort size. Indeed, a large internet-based survey study of Eastern Cooperative Oncology (ECOG) members demonstrated widespread variability in the management of hyperleukocytosis and perceptions regarding indications and outcomes related to the use of leukapheresis 18.
The objective of this study was to assess practice patterns for management of hyperleukocytosis among newly diagnosed patients with AML as well as the impact of leukapheresis use on short and long-term clinical outcomes in a large international patient cohort.
Patients and Methods
Data source and eligibility:
Data were retrospectively collected in the individual centers in the United States (US) and Europe (EU) and subsequently datasets were combined and analyzed at the coordinating center (Yale School of Medicine). Data were collected for patients who presented during the period of 2006 to 2017. Eligible patients had newly-diagnosed AML per the World Health Organization classification 19 as determined by participating centers and a WBC > 50 × 109/L at the time of presentation and subsequently received IC as defined by the local investigator. Patients with acute promyelocytic leukemia (APL) were not included. Responses to therapy were determined per the European Leukemia Network (ELN) response criteria 20 by the local investigators. The study was approved by the Yale institutional review board and was approved, acknowledged, or exempted by the other sites according to their local guidelines.
Patient characteristics:
Clinical and laboratory data were collected by local investigators in relationship to initial presentation and subsequent course and forwarded to be combined at the coordinating center. Cytogenetics were classified according to the Modified British Medical Research Council (MRC) classification 21, 22 and molecular data including mutations of fms-like tyrosine kinase 3 (FLT3) and nucleophosmin 1 (NPM1) genes were collected when available. Clinical data included the complete blood count (CBC) including WBC, hemoglobin (Hb) and platelet count, peripheral blood and bone marrow blast percentage and presence of leukostasis, TLS or DIC. TLS and DIC were designated by the investigator. Clinical evidence of leukostasis was defined as new onset hypoxia, chest pain, headache, focal neurological symptoms, priapism, intestinal ischemia or acute renal failure attributed to hyperleukocytosis by the primary team or local investigators. We recorded the management approach for hyperleukocytosis including: hydroxyurea administration followed by immediate or delayed initiation of IC, leukapheresis followed by immediate (within 24h of completion of leukapheresis) or delayed (after 24h of completion of leukapheresis) initiation of IC. Patients with hyperleukocytosis who did not receive IC were excluded from primary analysis and will be reported separately. Daytime (defined as presentation from 6 AM- 6 PM) vs. nighttime presentation and presentation on weekdays (Monday to Friday) vs. weekends (Saturday and Sunday) were recorded as well.
Response criteria and survival:
Best response to IC was reported by local investigators according to the 2003 revised International Working Group (IWG) AML criteria 23. The composite complete response rate (CRc) was defined as the combination of the complete response (CR) and complete response with incomplete count recovery (CRi) rate. Overall survival (OS) was calculated from time of presentation until death or end of follow-up.
Statistical analysis:
Wilcoxon rank sum test or t-test was used to compare continuous variables between treatment groups, whereas Fisher’s exact test was used to evaluate the association between treatment and categorical variables. Log rank test was used to compare overall survival between groups. Median OS time and its 95% confidence interval (CI) was calculated using Kaplan-Meier methods. Multivariable logistic regression models stratified by geographic location (EU vs. US) estimated odds ratios (OR) for death during induction (30-day mortality) and achievement of CRc. Multivariable Cox proportional hazard models stratified by geographic location (EU vs. US) estimated hazards ratios (HR) for OS. In multivariable analysis, we evaluated the impact of leukapheresis including age (≥ 55 years old vs < 55 years old with the median age of the population being 55 years), cytogenetic risk (poor vs. good/intermediate), WBC (> 100 × 109/L vs. ≤100 × 109/L), presence of clinical leukostasis, TLS and initiation of IC (beyond 48 hours vs. less than 48 hours) as covariates. Variables were excluded from multivariable regression analysis if they were highly correlated with variables selected into the models above or had p values greater than 0.10 in univariate analysis. The analysis of short- and long-term outcomes based on all available data was supplemented by a sensitivity analysis of these outcomes that included all patients and filled missing covariates with the use of multiple imputation 24. Ten imputed, complete data sets were generated and the analysis results from each dataset were pooled together by Rubin’s rules.
Given the observed difference in baseline characteristics between patients receiving and not receiving leukapheresis, propensity score (PS) matching was used to identify a cohort of patients with similar baseline characteristics based on the imputed complete data. PS is the conditional probability of receiving leukapheresis and was estimated using a multivariable logistic regression model including age (≥ 55 years old vs < 55 years old), WBC (> 100 × 109/L vs. ≤100 × 109/L), presence of clinical leukostasis, cytogenetic risk, TLS and time to initiation of IC beyond 48 hours as predictors. Variables were excluded if they were colinear with variables that were already included in the propensity model. The 1:1 nearest neighbor matching algorithm with a fixed caliber width of 0.10 was used to match leukapheresis treated patients with control patients according to their propensity scores. Standardized differences were used to assess covariate balance between the matched patient groups. Absolute standardized differences of greater than 0.10 were regarded as meaningful imbalances. In the matched cohort, multivariable regression models were used to assess the impact of leukapheresis on 30-day mortality, the chance of achieving CRc, and OS.
Results
Study population:
Patient data were collected from 12 centers in the US and EU. Among 998 patients with AML and hyperleukocytosis whose data were collected, 779 were reported to have received IC and comprised the main cohort for this analysis. For patients who received IC, the median age was 55 years (interquartile range [IQR]: 41–66), and 51.1% were male (Table 1). Most patients (83.2%) presented on weekdays, whereas a similar percentage of patients presented during daytime (54.3%) and nighttime (45.7%). Median WBC at presentation was 110 × 109/L (IQR: 77–170) and 57% had WBC > 100 × 109/L. Median Hb was 9.2 g/dL (IQR, 7.8–10.9) and median platelet count was 31 × 109/L (IQR, 11–72). Favorable, intermediate, and poor risk karyotypes were present in 23.6%, 59.8% and 16.6% of patients, respectively. Clinical leukostasis, TLS and DIC were present in 27.2%, 28.2% and 18.2% of patients at time of presentation, respectively (Table 1). Organs affected by leukostasis were the lung, CNS, retina, kidney, heart and GI tract in 43.8%, 35.8%, 6.3%, 5.1%, 5.7% and 3.4%, respectively. Patients who received leukapheresis had a higher median WBC (175 × 109/L vs. 103 × 109/L, p<0.001), higher peripheral blood blast percentage (82.5% vs. 74.0%, p=0.004) and a higher rate of leukostasis (55.9% vs. 22.1%, p<0.001), but not TLS (p=0.090) or DIC (p=0.566) compared to patients who did not receive leukapheresis (Table 1).
Table 1:
Characteristics of the patients at baseline*
| Characteristics | N | All (N=779) | Without Leukapheresis (N=666) | Leukapheresis (N=113) | P |
|---|---|---|---|---|---|
| Median Age in years (IQR) | 778 | 55(41–66) | 55(42–66) | 55(38–65) | 0.459 |
| Female Sex | 779 | 381 (48.9%) | 325 (48.8%) | 56 (49.6%) | 0.919 |
| ECOG performance status <2 | 640 | 421 (65.8%) | 388 (66.9%) | 33 (55%) | 0.085 |
| WHO Type (%) | 278 | <0.001 | |||
| AML with recurrent genetic abnormalities | 89 (32%) | 75 (38.3%) | 14 (17.1%) | ||
| AML with myelodysplasia-related features | 23 (8.3%) | 16 (8.2%) | 7 (8.5%) | ||
| AML, not otherwise specified | 148 (53.2%) | 89 (45.4%) | 59 (72%) | ||
| Therapy-related AML | 18 (6.5%) | 16 (8.2%) | 2 (2.4%) | ||
| Cytogenetic/ molecular characteristics | 674 | <0.001 | |||
| Favorable cytogenetic risk | 155(23.6%) | 153(27.6%) | 2(2.0%) | ||
| Intermediate cytogenetic risk | 392(59.8%) | 314(56.5%) | 78(77.2%) | ||
| Unfavorable cytogenetic risk | 109(16.6%) | 88(15.9%) | 21(20.8%) | ||
| Complex Cytogenetics | 453 | 55 (12.1%) | 43 (11.4%) | 12 (16%) | 0.251 |
| Monosomy Karyotype | 398 | 17 (4.3%) | 15 (4.3%) | 2 (4.2%) | >0.999 |
| NPM1 mutation | 444 | 208 (46.8%) | 170 (45.2%) | 38 (55.9%) | 0.114 |
| FLT3 mutation | 518 | 264 (51%) | 198 (45.9%) | 66 (75.9%) | <0.001 |
| Complete Blood Count | |||||
| Median WBC (IQR) | 779 | 110(77–170) | 103(73–152) | 175(127–246) | <0.001 |
| Median HB (IQR) | 772 | 9.2(7.8–10.9) | 9.2(7.7–11) | 9.3(7.8–10.6) | 0.852 |
| Median Platelets (IQR) | 775 | 31(11–72) | 25(10.4–67) | 46.5(21.5–90) | <0.001 |
| Blast % | |||||
| Median Peripheral Blood Blast (IQR) | 512 | 75(41–90) | 74(37.2–88) | 82.5(61–93.8) | 0.004 |
| Median Bone Marrow Blast (IQR) | 438 | 83(64–91) | 82(61.4–91) | 85(76–91.8) | 0.034 |
| Clinical Presentation | |||||
| Leukostasis | 740 | 201 (27.2%) | 139 (22.1%) | 62 (55.9%) | <0.001 |
| TLS | 742 | 209 (28.2%) | 189 (29.3%) | 20 (20.8%) | 0.09 |
| DIC | 581 | 106 (18.2%) | 86 (17.8%) | 20 (20.4%) | 0.566 |
| Hyperleukocytosis Management | 754 | <0.001 | |||
| Hydroxyurea followed by IC | 304 (40.3%) | 301 (47%) | 3 (2.7%) | ||
| Immediate initiation of IC | 341 (45.2%) | 340 (53%) | 1 (0.9%) | ||
| Leukapheresis followed by delayed (after 24h) IC | 41 (5.4%) | 0 (0%) | 41 (36.3%) | ||
| Leukapheresis followed by immediate (within 24h) IC | 68 (9%) | 0 (0%) | 68 (60.2%) | ||
| Organs affected by Leukostasis | 176 | 0.272 | |||
| Pulmonary leukostasis | 77 (43.8%) | 34 (42.5%) | 43 (44.8%) | ||
| CNS Leukostasis | 63 (35.8%) | 28 (35%) | 35 (36.5%) | ||
| Retinal Leukostasis | 11 (6.3%) | 3 (3.75%) | 8 (8.3%) | ||
| Renal Failure | 9 (5.1%) | 4 (5%) | 5 (5.2%) | ||
| Chest Pain/MI | 10 (5.7%) | 8 (10%) | 2 (2.1%) | ||
| GI Leukostasis | 6 (3.4%) | 3 (3.75%) | 3 (3.1%) | ||
For continuous variables, t-test or Wilcoxon rank sum test was used to compare the difference between treatment groups, depending on the distribution of data. For categorical variables, Fisher’s exact test was used to examine the association with treatment groups. IQR denotes interquartile range.
Compared to patients without evidence of clinical leukostasis, patients with leukostasis had a poorer performance status (ECOG PS < 2 43.8% vs. 74.8%, p<0.001), a higher median WBC (168.1 vs. 101, p<0.001), a higher percentage of FLT3 mutations (62.7% vs. 47.7%, p=0.005) and higher incidence of TLS (49.2% vs. 22.4%, p<0.001) (Supplemental Table 1). Patients with leukostasis who underwent leukapheresis had a significantly higher median WBC (180.2 vs. 149, p=0.019), a higher median platelet count (59.5 vs. 35, p=0.001), a higher percentage of FLT3 mutations (81.8% vs. 53.5%, p=0.002) and a lower percentage of TLS (26.4% vs. 56.9%, p < 0.001) compared to patients with leukostasis who did not undergo leukapheresis (Supplemental Table 2).
Patterns of hyperleukocytosis management including leukapheresis:
Leukapheresis was used in 113 patients (15%) (Table 1). In four treatment centers none of the patients underwent leukapheresis. Leukapheresis was administered in 31% of patients with clinical leukostasis and in 9% of patients without evidence of leukostasis (p<0.001). In patients who did not receive leukapheresis, hyperleukocytosis was managed either by immediate initiation of IC (n=340, 53%) or by the administration of hydroxyurea followed by IC (n=301, 47%). Patients managed with leukapheresis received either immediate induction of IC (n=68, 60.2%) or delayed induction of IC (n=41, 36.3%) after completion of leukapheresis. In a multivariable analysis, a WBC > 100 × 109/L (OR 3.53, p<0.001), the presence of clinical leukostasis (OR 6.06, p<0.001), and unfavorable cytogenetic risk (OR 2.05, p=0.028) predicted increased odds of receiving leukapheresis, whereas the presence of TLS (OR=0.27, p<0.001) was associated with decreased odds of receiving leukapheresis (Supplemental Figure 1). Median time from presentation to initiation of IC was 48 hours (Interquartile range [IQR], 24–96 hours).
Short and Long-term clinical outcomes:
The 30-day mortality was 16.7% (95%CI: 13.9–19.3%); 19.8%, 11.0% and 14.2% of patients were admitted to the ICU, underwent hemodialysis, or required mechanical ventilation, respectively (Table 2). After initiation of chemotherapy, 50.4% of patients had a complete remission (CR), 13.7% had a complete remission with incomplete count recovery (CRi) and 3.8% achieved a partial remission (PR), whereas 32.1% had no response to therapy (Table 2). Response to IC lasted a median of 202 days and 42.6% of patients experienced a relapse of their disease; 31.1% of patients underwent allogeneic hematopoietic stem cell transplant (HSCT). Median OS for all patients was 12.6 months (95%CI: 11.5–14.9) (Table 2). The 2-year, 3-year and 5-year survival rates were 35% (95%CI: 32–39%), 31% (95%CI: 28–35%) and 28% (95%CI: 25–32%), respectively. Patients receiving leukapheresis were significantly more likely to be admitted to the ICU (72.2% vs. 10.7%, p<0.001), undergo mechanical ventilation (33.3% vs. 7.7% p<0.001) and had an increased risk for relapse (63.8% vs. 38.8%, p<0.001) compared to patients, who did not receive leukapheresis (Table 2).
Table 2:
Characteristics of clinical outcomes in all the patients and by treatment groups*.
| Outcomes | N | All | Without Leukapheresis | Leukapheresis | P value |
|---|---|---|---|---|---|
| Response | 766 | 0.036 | |||
| CR | 386 (50.4%) | 339 (51.5%) | 47 (43.5%) | ||
| CRi | 105 (13.7%) | 84 (12.8%) | 21 (19.4%) | ||
| No Response | 246 (32.1%) | 214 (32.5%) | 32 (29.6%) | ||
| PR | 29 (3.8%) | 21 (3.2%) | 8 (7.4%) | ||
| Death in the first 30 days | 755 | 126 (16.7%) | 112 (17.3%) | 14 (13.2%) | 0.329 |
| ICU Admission | 484 | 96 (19.8%) | 44 (10.7%) | 52 (72.2%) | <0.001 |
| Hemodialysis Required | 621 | 68 (11%) | 56 (10.6%) | 12 (12.8%) | 0.59 |
| Mechanical Ventilation Required | 226 | 32 (14.2%) | 13 (7.7%) | 19 (33.3%) | <0.001 |
| Relapse after initial response | 453 | 193 (42.6%) | 149 (38.8%) | 44 (63.8%) | <0.001 |
| Hematopoietic Stem Cell Transplant | 505 | 157 (31.1%) | 124 (29.5%) | 33 (39.3%) | 0.093 |
| Median Duration of CR in months (IQR) | 247 | 202(114–363) | 208(133–368) | 171(78–280) | 0.192 |
| Median Overall Survival in months (95% CI) | 12.6(11.5,14.9) | 12.0(10.4, 13.9) | 18.8(13.3,32.5) | 0.07 | |
For categorical variables, the comparisons between treatment groups were based on Fisher’s exact test. For continuous variables, the comparisons were based on Wilcoxon rank sum test. Log rank test was used to compare the overall survival between two groups.
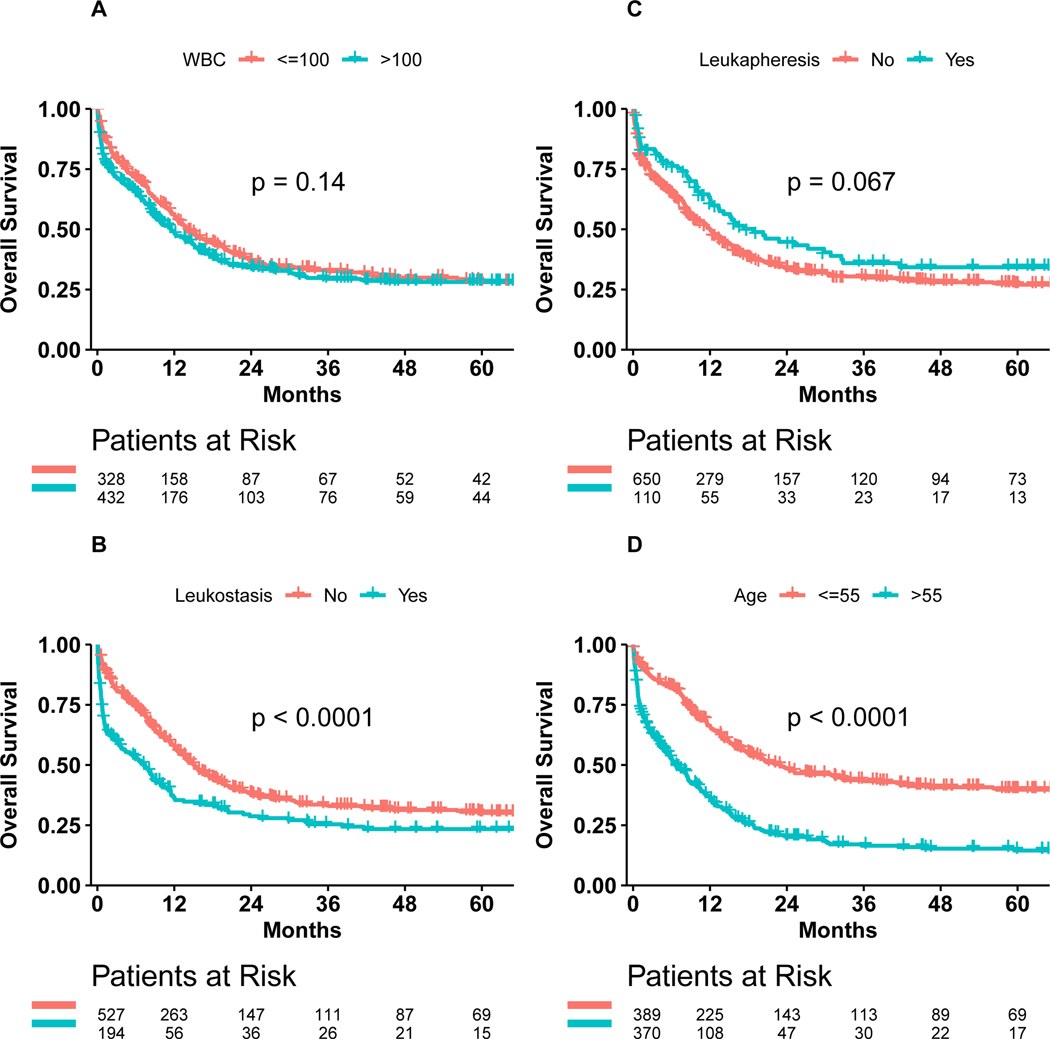
Median OS was 14.0 months (95%CI: 12.1–18.3) for patients with a WBC <100 × 109/L versus 11.5 months (95%CI: 9.3–14.1) for patients with a WBC >100 × 109/L (p=0.14) (Figure 1A). Median OS was 15.1 months (95%CI: 13.4–17.4) for patients without clinical leukostasis, which was significantly longer than the median OS of 7.4 months (95%CI: 3.9–9.8) for patients with symptoms or signs of leukostasis (p<0.0001) (Figure 1B). Median OS was 12.0 months (95%CI: 10.4–13.9) for patients not receiving leukapheresis versus 18.8 months (95%CI: 13.3–32.5) for patients receiving leukapheresis (p=0.067) (Figure 1C).
Figure 1: Overall Survival.
A: Patients with WBC > 100.000 vs ≤ 100.000
B: Patients with and without evidence of leukostasis
C: Patients receiving and not receiving leukapheresis
D: Patients > 55 years vs. ≤ 55 years old
Importantly, the median OS for patients older than 55 years (6.6 months, 95%CI: 5.4–8.6) was significantly shorter than for patients younger than 55 years (23 months, 95%CI: 17.8–32.8, p<0.0001) (Figure 1D, Supplemental Table 3). Patients older than 65 years with hyperleukocytosis had a dismal OS with a median of only 4.5 months (95%CI: 2.7–7.1), which was significantly shorter than for patients younger than 65 years with hyperleukocytosis (16.2 months, 95%CI: 14.1–20.6, p<0.0001) (Supplemental Figure 2, Supplemental Table 3). Similarly, the 30-day survival probability was significantly less for patients older than 55 (> 55 vs. ≤55 years: 0.745 vs. 0.918, p < 0.0001) and 65 years of age (> 65 vs. ≤65 years: 0.692 vs. 0.885, p < 0.0001) compared to patients younger than 55 and 65 years, respectively (Supplemental Table 3).
Predictors of clinical outcomes including impact of leukapheresis:
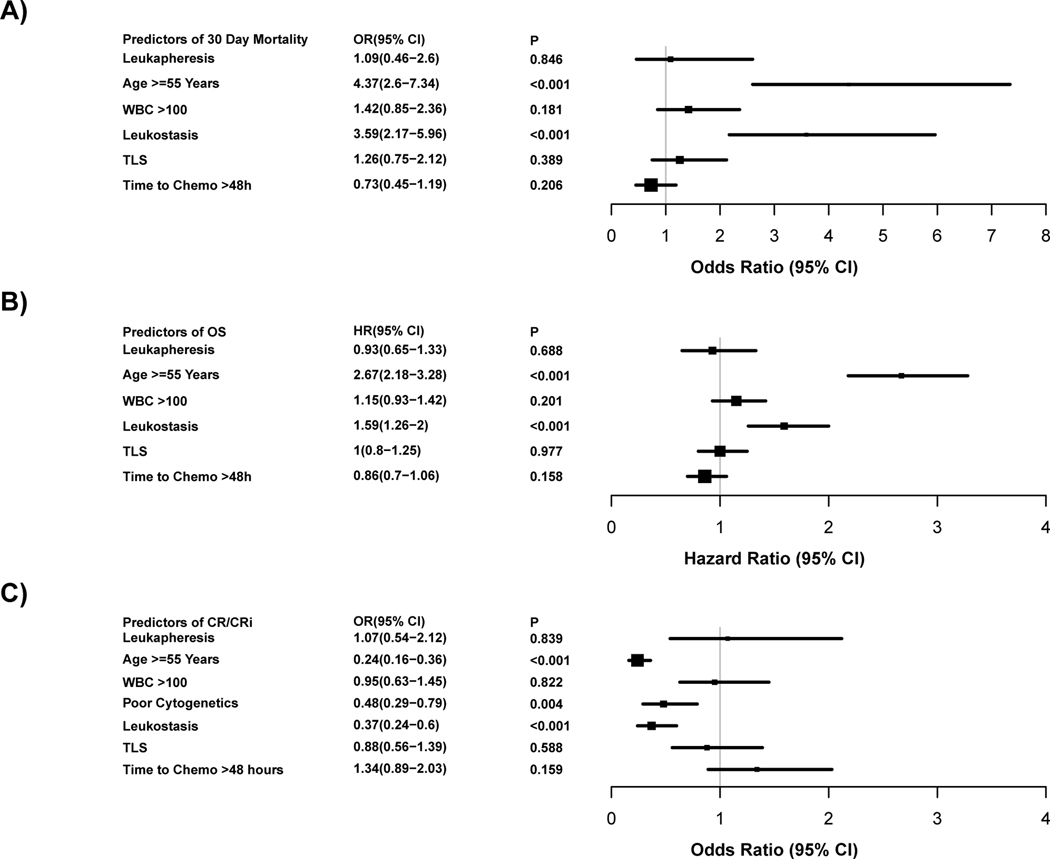
In multivariable regression analysis (n=619), higher odds of 30-day mortality was associated with age ≥ 55 years (OR 4.37, p<0.001) and presence of clinical leukostasis (OR 3.59, p<0.001) (Figure 2A). Neither WBC count > 100 × 109/L, presence of TLS, initiation of IC beyond 48 hours of presentation nor use of leukapheresis significantly affected the odds of 30-day mortality.
Figure 2: Forest plot of multivariable analysis.
The grey vertical line represents the odds ratio (Fig 2 A+C), and hazard ratio (Fig 2 B) of no effect. The box sizes are proportional to the precision of the estimates with large boxes indicating a great degree of precision. OR denotes odds ratio and HR denotes hazard ratio.
A. Predictors of 30-day mortality by multivariable logistic regression analysis (N=619).
B. Predictors of overall survival by multivariable Cox regression analysis (N=623).
C. Predictors of CRc by multivariable logistic regression (N=531).
In multivariable regression analyses of OS (n=623), age ≥ 55 years (HR 2.67, p<0.001) and presence of clinical leukostasis (HR=1.59, p<0.001) predicted inferior OS (Figure 2B). Neither WBC, TLS nor initiation of IC beyond 48 hours had a significant impact on OS. The use of leukapheresis was associated with a non-significant trend towards improved OS in unadjusted analysis (p=0.067) (Figure 1C), but this was not statistically significant in multivariable analysis (Figure 2B).
Similarly, age ≥ 55 years (OR 0.24, p<0.001), poor cytogenetic risk group (OR 0.48, p=0.004) and leukostasis (OR 0.37, p<0.001) were each associated with decreased odds of achieving CRc in multivariable analysis of CRc (n=531). However, initiation of IC beyond 48 hours of presentation, and use of leukapheresis did not significantly impact odds of achieving CRc (Figure 2C).
Of note, day of presentation (weekdays vs. weekends) was not associated with OS (HR=0.99, p=0.94), 30-day mortality (OR=1.46, P=0.12), or CRc (OR=0.97, P=0.89) in unadjusted analysis. Similarly, time of presentation (daytime vs. evening) was not significantly associated with OS (HR=0.81, p=0.257), 30-day mortality (OR=0.58, p=0.347), or CRc (OR=0.94, p=0.84) in unadjusted analysis. Therefore, day and time of presentation were not included in the multivariable analysis in Figure 2. ECOG status was not included for multivariate analysis because of its correlation with age, WBC, leukostasis, and TLS. The 48-hour cutoff from time of presentation to initiation of IC was chosen in the abovementioned models because it represented the median time to IC initiation. When using time to initiation of IC as a continuous variable or using alternative timepoint cutoffs from presentation to initiation of IC in sensitivity analyses including the 25th percentile (24 hours) and the 75th percentile (96 hours), there were no significant association with short term outcomes (odds of 30-day mortality or achieving CRc) or long-term outcomes (OS) in the adjusted multivariable models.
Subgroup and sensitivity analyses:
In a subgroup analysis limited to patients with investigator-adjudicated clinical leukostasis, use of leukapheresis was associated with statistically significant improvements in 30-day mortality (19.3% vs. 36.1%, p=0.026) and OS (median: 12 months vs. 4.4 months, p=0.044) in unadjusted analyses (Supplemental Table 4). However, in multivariable regression analyses stratified by geographic location (US vs. EU), including age (≥ 55 years vs. < 55 years), cytogenetic risk (poor vs. good/normal), WBC (> 100 × 109/L vs. ≤100 × 109/L), TLS and initiation of IC (beyond 48 hours vs. less than 48 hours), the use of leukapheresis was not associated with improved 30-day mortality (p=0.345), OS (p=0.323) or CRc rate (p=0.729) in this patient subpopulation.
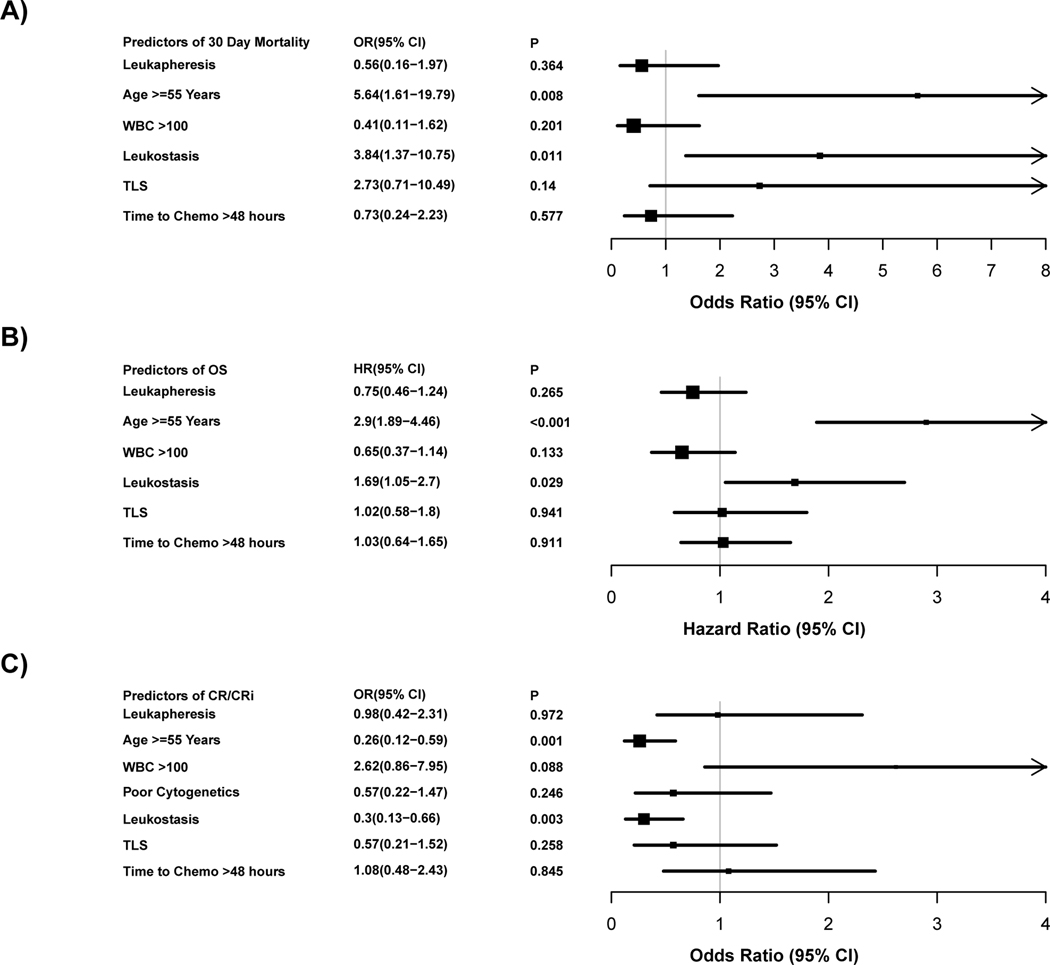
In a multivariable regression sensitivity analysis using imputed data (n=761), leukapheresis was not significantly associated with improved 30-day mortality, OS or CRc (Supplemental Figure 3A-C). In a PS-matched cohort (98 controls and 98 leukapheresis-treated), multiple regression analyses controlling for age (≥55 vs <55 years), WBC (>100 vs. ≤100), presence of leukostasis, TLS, time to initiation of IC beyond 48 hours and stratified by centers (US vs. EU) showed that the use of leukapheresis did not improve 30-day mortality (p=0.364), OS (p=0.265) or the chance of achieving CRc (p=0.972) as shown in Figure 3A-C.
Figure 3: Forest plot of propensity score matched multivariate analysis.
The grey vertical line represents the odds ratio (Fig 3 A+C), and hazard ratio (Fig 3 B) of no effect. The box sizes are proportional to the precision of the estimates with large boxes indicating a great degree of precision. OR denotes odds ratio and HR denotes hazard ratio.
A: Predictors of 30-day mortality by multivariable logistic regression analysis (N=196).
B: Predictors of overall survival by using multivariable Cox regression analysis (N=196).
C: Predictors of CRc by multivariable logistic regression analysis (N=196).
Discussion
The management of hyperleukocytosis in AML as well as the indications and benefits of leukapheresis remain controversial with extensive variability observed between providers, centers, and guidelines as demonstrated in a large US web-based survey 18. To our knowledge, we report one of the largest, if not the largest, cohorts of patients with AML presenting with hyperleukocytosis. Given the size of the patient cohort, we were able to make several important observations regarding the characteristics of patients presenting with hyperleukocytosis and analyze the impact of hyperleukocytosis, leukostasis and leukapheresis on short-term (30-day mortality and CRc) and long-term (OS) clinical outcomes. We also evaluated factors such as time to initiation of IC and evening/weekend presentation on short and long-term outcomes.
We showed that clinical evidence of leukostasis was present in about a quarter of patients with hyperleukocytosis, and that it was associated with worse 30-day mortality (OR 2.6, p=0.004) and a non-significant trend for inferior OS (HR 1.3 p=0.085) in multivariable analysis. We also demonstrated that older patients presenting with hyperleukocytosis have poor short-term outcomes (30 days survival probability 0.75 and 0.69 for patients > 55 and > 65 years old, respectively) and dismal long-term survival (median OS 6.6 and 4.5 months for patients > 55 and > 65 years old, respectively) despite undergoing IC.
Most patients in our study were managed with IC alone, and leukapheresis was only used in a small subgroup of patients (15%). Leukapheresis was used more commonly in patients with a WBC > 100 × 109/L, in the presence of clinical leukostasis, and those with unfavorable cytogenetic risk AML. While 31% of patients with clinical leukostasis underwent leukapheresis, only 10% of patients without clinical leukostasis received leukapheresis (p<0.001). These findings are consistent with our prior survey study, in which providers indicated that the preferred management of hyperleukocytosis differed based on whether patients show clinical evidence of leukostasis 18.
Some studies have suggested a reduction of early mortality and possibly increased CR rate with the use of leukapheresis 10–12, 17. Other studies have not found a meaningful effect of leukapheresis on early mortality in AML patients with hyperleukocytosis 4, 13–15. Importantly, even after controlling for potential confounders such as age, degree of hyperleukocytosis, and the presence of leukostasis, there appeared to be no significant mortality benefit for leukapheresis 15. Additionally, a meta-analysis failed to show a beneficial effect on early mortality for either leukapheresis or hydroxyurea/low-dose chemotherapy-mediated leukoreduction 13.
In our study, leukapheresis was not significantly associated with 30-day mortality or achieving CRc in unadjusted and multivariable regression analysis in the entire sample. However, it should be noted we only included patients who received IC, that patients with clinical leukostasis constituted a small proportion of the cohort, and that most patients in this cohort received IC within 4 days of presentation.
Prior studies have not found a beneficial effect of leukapheresis on OS 10, 11, 14. In our study, use of leukapheresis showed a trend towards improved OS with borderline statistical significance in unadjusted analysis (HR 0.77, 95%CI: 0.6–1.0; p=0.052), but not in multivariable analysis using imputed complete data (HR 0.75, 95%CI: 0.55–1.03; p=0.075). Those results were confirmed in multivariable analysis using the non-imputed data set (HR 0.93, 95%CI: 0.65–1.33; p=0.688) and in additional sensitivity analysis using a PS-matched cohort of 98 control patient and 98 leukapheresis treated patients (HR 0.75, 95%CI: 0.46–1.24; p=0.265).
Among patients with investigator-adjudicated clinical leukostasis, there were statistically significant improvements in 30-day mortality and OS with leukapheresis in unadjusted analysis but not in multivariate analysis. Importantly, in our study, leukostasis was defined by the local investigators based on clinical symptoms as there is no definite diagnostic test for leukostasis apart from pathological evaluation, which is either obtained post-mortem or, for those living, precluded by deep cytopenias and the need for expedited therapy based on provider clinical suspicion. Therefore, it is possible that patients were assigned the attribute of leukostasis but in fact their symptoms might have been explained by an alternative disease process and not leukostasis. Due to this issue and associated selection issues, as well as the small proportion of patients with clinical leukostasis, we were unable to evaluate whether there was a definite benefit of leukapheresis among this patient population. Therefore, it is possible that leukapheresis might improve outcomes in some patients with clinical leukostasis.
In addition to use of leukapheresis, we evaluated the impact of time from presentation to initiation of IC as well as the impact of time of presentation (day vs night, weekend vs weekday). It is generally an accepted practice that AML patients presenting with hyperleukocytosis are considered emergencies and that IC should be started as soon as feasible in those patients who are eligible for IC 1. As expected, in our cohort the median time to start of IC was 48 hours, with 75% of patients starting IC within 96 hours of presentation which might explain why we did not observe an impact of time to initiation of IC on short- or long-term clinical outcomes.
A related issue is the time of presentation for medical conditions that require urgent evaluation and intervention. There are data for patients suffering from a myocardial infarction 25, 26 or stroke 27, 28 demonstrating inferior outcomes when presenting at night or during weekends. In our cohort, we did not observe a negative impact of presentation time on short- or long-term outcomes. This may reflect the level of expertise and resources available in large academic centers such as the ones that participated in this study and might not apply to smaller or community-based settings.
The dogma for older fit AML patients with “proliferative” AML and hyperleukocytosis is that hypomethylating agents are not effective to control the disease and that IC should be always deployed whenever possible. However, we noted in our study that patients older than 65 years who presented with hyperleukocytosis had exceptionally poor outcomes (30-day mortality of 31% and median OS of only 4.5 months [95%CI: 2.7–7.1]) despite receiving IC. Our data argue for the exploration of alternative approaches in clinical trials for those patients (e.g. cytoreduction with hydroxyurea followed by azacitidine-venetoclax combination).
As with all retrospective studies, selection bias can result from the treating physician’s choice to administer leukapheresis based on age and performance status of the patient and potential contraindications for leukapheresis including life-threatening complications. Furthermore, we cannot exclude that local-regional guidelines regarding the use of leukapheresis had an impact on physicians’ decision to employ leukapheresis. We attempted to reduce potential selection bias by examining the impact of leukapheresis on short and long-term outcomes in multivariable and propensity score-matched analysis controlling for age, ECOG PS, WBC, presence of leukostasis and continent of practice (US vs. EU). As we did not record the exact dosing of the induction chemotherapy administered, we were unable to evaluate the potential value of higher dose cytarabine as induction therapy. Of note, patients with APL were not included in our study. However, a recent retrospective study demonstrated that leukapheresis did not improve the CR rate or 3-year OS in APL patients with hyperleukocytosis (defined as WBC > 50 × 109/L) 29.
In summary, we were unable to detect a benefit of leukapheresis on short and long-term clinical outcomes in a large cohort of AML patients presenting with hyperleukocytosis and receiving IC with a median of 48 hours of presentation at large tertiary centers. Clinical outcomes were generally poor especially among older patients. Due to limitations of the sample size and definition of clinical leukostasis, we could not reach definitive conclusions in this patient subset and it possible that leukapheresis might improve outcomes in some patients with clinical leukostasis. Given its associated risks and costs, only a randomized controlled trial can ultimately evaluate what impact leukapheresis has on clinical outcomes for AML patients who present with hyperleukocytosis. However, given the rarity of the condition and urgency of initiating therapy, especially among patients with clinical leukostasis, it is unlikely that such a trial can be feasibly undertaken.
Supplementary Material
Acknowledgements:
Amer Zeidan is a Leukemia and Lymphoma Society Scholar in Clinical Research and is also supported by a NCI’s Cancer Clinical Investigator Team Leadership Award (CCITLA). Research reported in this publication was in part supported by the National Cancer Institute of the National Institutes of Health under Award Number P30 CA016359. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would like to acknowledge all the patients who contributed data for this study, and the Frederick A. DeLuca Foundation for supporting the statistical analyses.
Disclosure of conflicts of interest:
Maximilian Stahl: No relevant financial relationship(s) to disclose
Rory Michael Shallis: No relevant financial relationship(s) to disclose
Wei Wei: No relevant financial relationship(s) to disclose
Pau Montesinos:
Daiichi Sankyo: Consultancy and Speakers Bureau
Novartis: Research Funding and Speakers Bureau
Etienne Lengline: No relevant financial relationship(s) to disclose
Judith Neukirchen: No relevant financial relationship(s) to disclose
Vijaya R. Bhatt:
Consulting fees: CSL Behring, Agios, Abbvie, Partner therapeutics and Incyte
Research funding: Jazz, Incyte, Tolero Pharmaceuticals, Inc, and National Marrow Donor Program.
Mikkael A. Sekeres: No relevant financial relationship(s) to disclose
Amir T. Fathi:
Consulting/advisory board – Celgene, Takeda, Abbvie, Forty seven, NewLink, Trovagene, Pfizer, Daiichi Sankyo, Astellas, Amphivena
Clinical trial funding – Celgene and Agios
Heiko Konig: No relevant financial relationship(s) to disclose
Selina Luger: No relevant financial relationship(s) to disclose
Irum Khan:
Teva: Speakers Bureau
Gail J. Roboz:
AbbVie: Consultancy
Amphivena Therapeutics: Consultancy
Argenx: Consultancy
Astex Pharmaceuticals: Consultancy
Bayer: Consultancy
Celgene Corporation: Consultancy
Celltrion: Consultancy
Daiichi Sankyo: Consultancy
Bayer: Consultancy
Eisai: Consultancy
Janssen Pharmaceuticals: Consultancy
Jazz Pharmaceuticals: Consultancy
Novartis: Consultancy
Daiichi Sankyo: Consultancy
Orsenix: Consultancy
Otsuka: Consultancy
Eisai: Consultancy
Pfizer: Consultancy
Roche/Genentech: Consultancy
Sandoz: Consultancy
Cellectis: Research Funding
Orsenix: Consultancy
Thomas Cluzeau:
Celgene: Consultancy, Membership on an entity’s Board of Directors or advisory committees and Speakers
Bureau
Menarini: Consultancy
Jazz Pharma: Consultancy, Membership on an entity’s Board of Directors or advisory committees and Speakers Bureau
Amgen: Membership on an entity’s Board of Directors or advisory committees and Speakers Bureau
AbbVie: Membership on an entity’s Board of Directors or advisory committees and Speakers Bureau
Sanofi: Speakers Bureau
Pfizer: Speakers Bureau
David Martínez-Cuadron: No relevant financial relationship(s) to disclose
Emmanuel Raffoux: No relevant financial relationship(s) to disclose
Ulrich Germing:
Celgene: Honoraria, Research Funding and Consultancy
Novartis: Honoraria and Research Funding
Janssen: Honoraria
Jayadev Manikkam Umakanthan: No relevant financial relationship(s) to disclose
Sudipto Mukherjee:
Aplastic Anemia & MDS International Foundation in Joint Partnership with Cleveland Clinic Taussig Cancer Institute: Honoraria
BioPharm Communications: Consultancy
Bristol Myers Squib: Honoraria and Speakers Bureau
LEK Consulting: Consultancy and Honoraria
Novartis: Consultancy, Membership on an entity’s Board of Directors or advisory committees and Research Funding
Pfizer: Honoraria
Projects in Knowledge: Honoraria
Takeda: Membership on an entity’s Board of Directors or advisory committees
Andrew M. Brunner:
Takeda: Research Funding
Celgene: Consultancy and Research Funding
Novartis: Research Funding
Adam M. Miller: No relevant financial relationship(s) to disclose
Christine M. McMahon: No relevant financial relationship(s) to disclose
Ellen K. Ritchie:
Incyte: Consultancy and Speakers Bureau
Celgene: Consultancy, Other: Travel, Accommodations, Expenses and Speakers Bureau
Pfizer: Consultancy and Research Funding
Novartis: Consultancy, Other: Travel, Accommodations, Expenses, Research Funding and Speakers Bureau
ARIAD Pharmaceuticals: Speakers Bureau
Astellas Pharma: Research Funding
Bristol Myers Squibb: Research Funding
NS Pharma: Research Funding
Rebeca Rodríguez Veiga: No relevant financial relationship(s) to disclose
Raphael Itzykson: No relevant financial relationship(s) to disclose
Blanca Boluda: No relevant financial relationship(s) to disclose
Florence Rabian: No relevant financial relationship(s) to disclose
Mar Tormo: No relevant financial relationship(s) to disclose
Evelyn Gloria Acuna Cruz: No relevant financial relationship(s) to disclose
Emma Rabinovich: No relevant financial relationship(s) to disclose
Brendan Yoo: No relevant financial relationship(s) to disclose
Isabel Cano: No relevant financial relationship(s) to disclose
Nikolai A. Podoltsev:
Agios Pharmaceuticals: Consultancy and Honoraria
Astellas Pharma: Research Funding
Blueprint Medicines: Consultancy and Honoraria
Incyte: Consultancy and Honoraria
Novartis: Consultancy and Honoraria
Boehringer Ingelheim: Research Funding
Daiichi Sankyo: Research Funding
Sunesis Pharmaceuticals: Research Funding
Celator: Research Funding
Pfizer: Research Funding, Consultancy and Honoraria
Astex Pharmaceuticals: Research Funding
Celgene: Research Funding, Consultancy and Honoraria
Genentech: Research Funding
AI Therapeutics: Research Funding
Samus Therapeutics: Research Funding
Arog Pharmaceuticals: Research Funding
Kartos Therapeutics: Research Funding
Jan Philipp Bewersdorf: No relevant financial relationship(s) to disclose
Steven D. Gore:
Celgene: Consultancy and Research Funding
Amer M. Zeidan:
A.M.Z. received research funding from Celgene, Acceleron, Abbvie, Otsuka, Pfizer, Medimmune/AstraZeneca, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Novartis, Aprea, Astex, and ADC Therapeutics. A.M.Z had a consultancy with and received honoraria from AbbVie, Otsuka, Pfizer, Celgene, Jazz, Incyte, Agios, Boehringer-Ingelheim, Novartis, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Seattle Genetics, BeyondSpring, Trovagene, Takeda, Ionis, and Epizyme. A.M.Z received travel support for meetings from Pfizer, Novartis, and Trovagene. None of these relationships were related to the development of this manuscript.
References
- 1.Rollig C, Ehninger G. How I treat hyperleukocytosis in acute myeloid leukemia. Blood 2015. May 21; 125(21): 3246–3252. [DOI] [PubMed] [Google Scholar]
- 2.Greenwood MJ, Seftel MD, Richardson C, Barbaric D, Barnett MJ, Bruyere H, et al. Leukocyte count as a predictor of death during remission induction in acute myeloid leukemia. Leuk Lymphoma 2006. July; 47(7): 1245–1252. [DOI] [PubMed] [Google Scholar]
- 3.Marbello L, Ricci F, Nosari AM, Turrini M, Nador G, Nichelatti M, et al. Outcome of hyperleukocytic adult acute myeloid leukaemia: a single-center retrospective study and review of literature. Leukemia research 2008. August; 32(8): 1221–1227. [DOI] [PubMed] [Google Scholar]
- 4.Porcu P, Danielson CF, Orazi A, Heerema NA, Gabig TG, McCarthy LJ. Therapeutic leukapheresis in hyperleucocytic leukaemias: lack of correlation between degree of cytoreduction and early mortality rate. Br J Haematol 1997. August; 98(2): 433–436. [DOI] [PubMed] [Google Scholar]
- 5.De Santis GC, de Oliveira LC, Romano LG, Almeida Prado Bde P Jr., Simoes BP, Rego EM, et al. Therapeutic leukapheresis in patients with leukostasis secondary to acute myelogenous leukemia. J Clin Apher 2011; 26(4): 181–185. [DOI] [PubMed] [Google Scholar]
- 6.Porcu P, Cripe LD, Ng EW, Bhatia S, Danielson CM, Orazi A, et al. Hyperleukocytic leukemias and leukostasis: a review of pathophysiology, clinical presentation and management. Leuk Lymphoma 2000. September; 39(1–2): 1–18. [DOI] [PubMed] [Google Scholar]
- 7.Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010. January 21; 115(3): 453–474. [DOI] [PubMed] [Google Scholar]
- 8.Zuckerman T, Ganzel C, Tallman MS, Rowe JM. How I treat hematologic emergencies in adults with acute leukemia. Blood 2012. September 6; 120(10): 1993–2002. [DOI] [PubMed] [Google Scholar]
- 9.Bruserud O, Liseth K, Stamnesfet S, Cacic DL, Melve G, Kristoffersen E, et al. Hyperleukocytosis and leukocytapheresis in acute leukaemias: experience from a single centre and review of the literature of leukocytapheresis in acute myeloid leukaemia. Transfus Med 2013. December; 23(6): 397–406. [DOI] [PubMed] [Google Scholar]
- 10.Bug G, Anargyrou K, Tonn T, Bialleck H, Seifried E, Hoelzer D, et al. Impact of leukapheresis on early death rate in adult acute myeloid leukemia presenting with hyperleukocytosis. Transfusion 2007. October; 47(10): 1843–1850. [DOI] [PubMed] [Google Scholar]
- 11.Giles FJ, Shen Y, Kantarjian HM, Korbling MJ, O’Brien S, Anderlini P, et al. Leukapheresis reduces early mortality in patients with acute myeloid leukemia with high white cell counts but does not improve long- term survival. Leuk Lymphoma 2001. June; 42(1–2): 67–73. [DOI] [PubMed] [Google Scholar]
- 12.Thiebaut A, Thomas X, Belhabri A, Anglaret B, Archimbaud E. Impact of pre-induction therapy leukapheresis on treatment outcome in adult acute myelogenous leukemia presenting with hyperleukocytosis. Annals of hematology 2000. September; 79(9): 501–506. [DOI] [PubMed] [Google Scholar]
- 13.Oberoi S, Lehrnbecher T, Phillips B, Hitzler J, Ethier MC, Beyene J, et al. Leukapheresis and low-dose chemotherapy do not reduce early mortality in acute myeloid leukemia hyperleukocytosis: a systematic review and meta-analysis. Leuk Res 2014. April; 38(4): 460–468. [DOI] [PubMed] [Google Scholar]
- 14.Berber I, Kuku I, Erkurt MA, Kaya E, Bag HG, Nizam I, et al. Leukapheresis in acute myeloid leukemia patients with hyperleukocytosis: A single center experience. Transfus Apher Sci 2015. October; 53(2): 185–190. [DOI] [PubMed] [Google Scholar]
- 15.Choi MH, Choe YH, Park Y, Nah H, Kim S, Jeong SH, et al. The effect of therapeutic leukapheresis on early complications and outcomes in patients with acute leukemia and hyperleukocytosis: a propensity score-matched study. Transfusion 2018. January; 58(1): 208–216. [DOI] [PubMed] [Google Scholar]
- 16.Chang MC, Chen TY, Tang JL, Lan YJ, Chao TY, Chiu CF, et al. Leukapheresis and cranial irradiation in patients with hyperleukocytic acute myeloid leukemia: no impact on early mortality and intracranial hemorrhage. Am J Hematol 2007. November; 82(11): 976–980. [DOI] [PubMed] [Google Scholar]
- 17.Inaba H, Fan Y, Pounds S, Geiger TL, Rubnitz JE, Ribeiro RC, et al. Clinical and biologic features and treatment outcome of children with newly diagnosed acute myeloid leukemia and hyperleukocytosis. Cancer 2008. August 1; 113(3): 522–529. [DOI] [PubMed] [Google Scholar]
- 18.Stahl M, Pine A, Hendrickson JE, Litzow MR, Luger SM, Stone RM, et al. Beliefs and practice patterns in hyperleukocytosis management in acute myeloid leukemia: a large U.S. web-based survey(). Leuk Lymphoma 2018. April 18: 1–4. [DOI] [PubMed] [Google Scholar]
- 19.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016. May 19; 127(20): 2391–2405. [DOI] [PubMed] [Google Scholar]
- 20.Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017. January 26; 129(4): 424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood 2010. July 22; 116(3): 354–365. [DOI] [PubMed] [Google Scholar]
- 22.Breems DA, Van Putten WL, De Greef GE, Van Zelderen-Bhola SL, Gerssen-Schoorl KB, Mellink CH, et al. Monosomal karyotype in acute myeloid leukemia: a better indicator of poor prognosis than a complex karyotype. J Clin Oncol 2008. October 10; 26(29): 4791–4797. [DOI] [PubMed] [Google Scholar]
- 23.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol 2003. December 15; 21(24): 4642–4649. [DOI] [PubMed] [Google Scholar]
- 24.Stef van Buuren KG-O. mice: Multivariate Imputation by Chained Equations in R. Journal of Statistical Software 2011. [Google Scholar]
- 25.Sorita A, Ahmed A, Starr SR, Thompson KM, Reed DA, Prokop L, et al. Off-hour presentation and outcomes in patients with acute myocardial infarction: systematic review and meta-analysis. BMJ 2014. January 21; 348: f7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kostis WJ, Demissie K, Marcella SW, Shao YH, Wilson AC, Moreyra AE, et al. Weekend versus weekday admission and mortality from myocardial infarction. N Engl J Med 2007. March 15; 356(11): 1099–1109. [DOI] [PubMed] [Google Scholar]
- 27.Janszky I, Ahnve S, Ljung R. Weekend versus weekday admission and stroke outcome in Sweden from 1968 to 2005. Stroke 2007. September; 38(9): e94; author reply e95. [DOI] [PubMed] [Google Scholar]
- 28.Reeves MJ, Smith E, Fonarow G, Hernandez A, Pan W, Schwamm LH, et al. Off-hour admission and in-hospital stroke case fatality in the get with the guidelines-stroke program. Stroke 2009. February; 40(2): 569–576. [DOI] [PubMed] [Google Scholar]
- 29.Daver N, Kantarjian H, Marcucci G, Pierce S, Brandt M, Dinardo C, et al. Clinical characteristics and outcomes in patients with acute promyelocytic leukaemia and hyperleucocytosis. Br J Haematol 2015. March; 168(5): 646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.